Summary
Background
Current available therapeutic options for Coronavirus Disease-2019 (COVID-19) are primarily focused on treating hospitalized patients, and there is a lack of oral therapeutic options to treat mild to moderate outpatient COVID-19 and prevent clinical progression. Raloxifene was found as a promising molecule to treat COVID-19 due to its activity to modulate the replication of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and act as an immunomodulator to decrease proinflammatory cytokines.
Methods
This was a phase 2 multicenter, randomized, placebo-controlled trial to evaluate the efficacy and safety of raloxifene in adult patients with mild to moderate COVID-19 between October 2020 to June 2021 in five centers located in Italy. This was a planned 2/3 adaptive study, but due to operational difficulties, the study was discontinued during the phase 2 study segment. Participants were randomized 1:1:1 to receive oral placebo, raloxifene 60 mg, or raloxifene 120 mg by self-administration for a maximum of two weeks. The primary outcomes were the proportion of patients with undetectable SARS-CoV-2 via nasopharyngeal swabs at day 7 and the proportion of patients who did not require supplemental oxygen therapy or mechanical ventilation on day 14. Safety was assessed. The trial is registered (EudraCT 2021–002,476–39, and ClinicalTrials.gov: NCT05172050).
Findings
A total of 68 participants were enrolled and randomized to placebo (n = 21), raloxifene 60 mg (n = 24), and raloxifene 120 mg (n = 23). The proportion of participants with undetectable SARS-CoV-2 after seven days of treatment with raloxifene 60 mg [36.8%, 7/19 vs. 0.0%, 0/14] and 120 mg [22.2%, 4/18 vs. 0.0%, 0/14] was better compared to placebo, [risk difference (RD) = 0·37 (95% C.I.:0·09–0·59)] and [RD = 0·22 (95% C.I.: -0·03–0·45)], respectively. There was no evidence of effect for requirement of supplemental oxygen and/or mechanical ventilation with effects for raloxifene 60 mg and raloxifene 120 mg over placebo, [RD = 0·09 (95% C.I.: -0·22–0·37)], and [RD = 0·03 (95% C.I.: -0·28–0·33)], respectively. Raloxifene was well tolerated at both doses, and there was no evidence of any difference in the occurrence of serious adverse events.
Interpretation
Raloxifene showed evidence of effect in the primary virologic endpoint in the treatment of early mild to moderate COVID-19 patients shortening the time of viral shedding. The safety profile was consistent with that reported for other indications. Raloxifene may represent a promising pharmacological option to prevent or mitigate COVID-19 disease progression.
Funding
The study was funded by Dompé Farmaceutici SpA and supported by the funds from the European Commission – Health and Consumers Directorate General, for the Action under the Emergency Support Instrument- Grant to support clinical testing of repurposed medicines to treat SARS-COV-2 patients (PPPA-ESI-CTRM-2020-SI2.837140), and by the COVID-2020–12,371,675 Ricerca finalizzata and line 1 Ricerca Corrente COVID both funded by Italian Ministry of Health.
Keywords: Raloxifene, COVID-19, SARS-CoV-2, Estrogen, Selective estrogen receptor modulator (SERM)
Research in context.
Evidence before this study
Searching PubMed, from 2019 to 2021, utilizing terms including “raloxifene” and “viral infections”, we found in vitro studies suggesting raloxifene to have high pulmonary distribution and be an effective molecule against SARS-CoV-2 when compared to commonly used antivirals such as remdesivir. Raloxifene has previously been shown to have antiviral activity against influenza A, Ebola, hepatitis C, and Flaviviruses including the Zika virus, dengue virus, and West Nile virus. However, there has been no previous randomized controlled trial performed to evaluate the role of raloxifene in mild to moderate COVID-19.
Added value of this study
To the best of our knowledge, this is the first multicenter, randomized, placebo-controlled trial evaluating the efficacy and safety of raloxifene in adult patients with mild to moderate COVID-19. The proportion of patients with undetectable SARS-CoV-2 after seven days of treatment with raloxifene 60 mg was greater compared to placebo, while maintaining a good safety profile.
Implications of all the available evidence
Raloxifene represents a promising pharmacological option to prevent or mitigate COVID-19 disease progression and has the potential to reduce the current burden on healthcare systems. Further studies are required to confirm these results.
Alt-text: Unlabelled box
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2 emerged in December 2019 and it quickly spread globally to cause the coronavirus disease 2019 (COVID-19) pandemic.1 An estimated 80% of cases are classified as mild to moderate COVID-19 disease, and patients may display symptoms such as fever, fatigue, dry cough, dyspnea, headache, and muscle aches.1,2 However, some patients may quickly deteriorate to severe or critical disease caused by complications of respiratory failure, septic shock, or single or multiple organ dysfunction.1,2 Risk factors for COVID-19 complications may include the elderly, cardiovascular disease, malignancy, diabetes, lung disease, and obesity.3 Hyperactivation of the immune system by SARS-CoV-2 is hypothesized to be responsible for the rapid deterioration of patients with COVID-19 to acute lung injury (ALI), acute respiratory distress syndrome (ARDS), and multiple organ system dysfunction.4
SARS-CoV-2 enters host cells via the angiotensin-converting enzyme 2 (ACE-2) receptor which triggers an inflammatory response to produce proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) leading to cytokine release syndrome (CRS) or “cytokine storm”.5 Current available therapeutic options are primarily focused on treating hospitalized patients with severe disease requiring supplemental oxygen, or outpatients at high risk of disease progression (i.e. elderly, obesity, chronic conditions, immunosuppressive disease or treatment).6 There is a current lack of oral therapeutic options to treat mild to moderate COVID-19, and prevent/attenuate the clinical progression, with many medications under investigation including platform trials evaluating repurposing medications, or platform trials such as the AGILE-ACCORD evaluating new, multiple, candidate therapies at the same time.
A gender disparity exists in male patients with COVID-19 in which they are more likely to have symptomatic COVID-19 and progress to severe disease.7 The rationale for these current disparities may involve the role of estrogen in COVID-19 disease. Estrogen and estrogen receptor (ER) signaling pathways play a crucial role in both innate and adaptive immune responses in which estrogen has protective effects to increase production of anti-inflammatory cytokines and decrease production of proinflammatory cytokines, increase immune tolerance and antibody production, and suppress the migration of macrophages and monocytes. Conversely, testosterone can suppress immune cell activity, together, supporting more rapid viral recognition and clearance in women than men.8 Additionally, estradiol can downregulate mRNA expression of the SARS-CoV-2 receptor, ACE2, which facilitates viral entry.9
There is a need for COVID-19 treatment to reduce the impact on healthcare systems, and their resources, by preventing progression to severe or critical disease, as well as shortening the time of viral shedding to curb infectivity and reduce the number of cases. Developing new medications is a time-consuming process, therefore, repurposing existing medications for other indications may prove to be a viable solution. Repurposing of existing medications is an efficient means to facilitate use of new COVID-19 treatments since pharmacokinetic and safety data are already available and validated for such agents. Exscalate4CoV (E4C) is a multinational public-private consortium supported by the European Commission's Horizon 2020 program for countering the coronavirus pandemic and improving the management and care of patients. The core of E4C is an artificial intelligence platform capable of screening large libraries of molecules to identify promising agents that may be effective against SARS-CoV-2 infections.10 Raloxifene was identified in silico among 400,000 candidate molecules and was preselected to proceed with in vitro testing among 7,000 molecules. Thereafter, raloxifene was found to be the most promising molecule to treat COVID-19 due to its activity to modulate the replication and activity of SARS-CoV-2 and act as an immunomodulator to decrease proinflammatory cytokines.
Raloxifene is a second-generation selective estrogen receptor modulator (SERM) that is already approved in the United States and Europe to treat and prevent osteoporosis in postmenopausal women and reduce the risk of invasive breast cancer in postmenopausal women.11 Raloxifene shares structural similarities to 17β-estradiol, which allows it to bind to the ligand binding domain of ERs and affect downstream gene expression. It acts as an estrogen agonist in nonreproductive tissues (eg, bone) and as an antagonist in other, reproductive tissues (eg, endometrium and breast) contributing towards its beneficial safety profile by avoiding widespread adverse effects.12
The aim of this study was to evaluate the efficacy and safety of raloxifene in outpatient managed patients with mild to moderate COVID-19.
Methods
Study design
This was originally planned as a phase 2/3 adaptive, multicenter, randomized, placebo-controlled, double-blind, parallel-group trial to evaluate the efficacy and safety of raloxifene compared to placebo in adult patients with mild to moderate COVID-19. The study evaluated two different doses of raloxifene compared to placebo with no comparison between the raloxifene arms. This study was planned to be performed at 10 sites including Italy (6), France (3), and Spain (1). The clinical study protocol v3.0 was approved by the local Ethics Committees and Regulatory Competent Authorities and their approvals were obtained before the study started (EudraCT 2021–002,476–39, ClinicalTrials.gov:NCT05172050).
The study was subject to the pandemic trend as well as operational difficulties due to the target outpatient population of the trial. Despite the Sponsor's adoption of alternative solutions, in line with the protocol, the dilution of the study due to the flattening of the pandemic curve and the lack of suitable patients for enrollment did not allow enrollment to be completed as planned. Considering all these aspects, the Sponsor has decided to discontinue the study globally during the phase 2 study segment. This study has been conducted according to the CONSORT guidelines.
Trial population
Patients aged 40 years or older with microbiologically confirmed SARS-CoV-2 infection by an approved molecular polymerase chain reaction (PCR) test in Europe within 10 days of symptoms could be enrolled if they presented at least one of the following symptoms: fever, dyspnea, headache, cough, dysgeusia, conjunctivitis, vomiting, diarrhea, anosmia, muscle or body aches or other symptoms which in the opinion of the investigator were part of the COVID-19 clinical picture. Patients were excluded if they were completely asymptomatic at the screening time or required supplemental oxygen therapy or mechanical ventilation, if had a history of stroke, venous thromboembolism (VTE), chronic kidney disease stage 3 or higher, Child-Pugh Class A or higher liver disease, known hypoalbuminemia, endometrial bleeding, endometrial cancer, breast cancer, autoimmune disease receiving therapy, and extended periods of immobilization. Eligible patients could not have a presumptive hypersensitivity to the active principles of raloxifene or excipients, or taking home medications including cholestyramine, warfarin or any other drug that is known to interact with raloxifene. Pregnant or lactating woman, and patients with a positive or missing pregnancy test before day one could not take part in the trial. The full list of the inclusion and exclusion criteria can be found within the protocol. All participants provided written, informed, consent.
Randomization
The randomization list was created by an independent statistician not involved in the conduction of the study. Randomization was stratified by site and gender to ensure balanced assignment across treatment groups. It was generated with a computer procedure, randomizing an excess of subjects to allow competitive recruitment within each center. After confirmation of SARS-COV-2 infection, patients meeting the inclusion criteria for this study were randomized (1:1:1) to 1 of 3 double blind treatment groups and instructed by a physician on the correct self-administration for a maximum two weeks and completion of their daily diaries. A period of 2 weeks of follow-up was guaranteed. The allocation was performed by an interactive response system (IRS), that was part of the electronic-case report form (eCRF) and contained the functionality to allow breaking the code only in cases of urgent or emergency medical need.
Procedures
On day one of treatment, two oral doses were administered (one dose in the morning and one dose in the evening). Thereafter, a single daily oral dose was administered by patients: raloxifene 60 mg, raloxifene 120 mg or placebo for a maximum 14 days with a median follow-up of 30 days for all arms. Details of the management of blinding are provided in the protocol. A matching placebo to raloxifene was identical to appearance including packaging and labeling. Each dose administered contained two capsules of either 60 mg of raloxifene or placebo as follows: group 1 received one capsule of raloxifene 60 mg and one capsule of placebo, group 2 received two capsules of raloxifene 60 mg, and the control group received two capsules of placebo.
Due to the requirement of home confinement for COVID-19 positive patients, the study was conducted using the decentralized clinical trial approach through the implementation of a telemedicine platform complemented with regular weekly home visits performed by qualified and duly trained study health care workers (HCWs) nurses. A digital platform, with an electronic patient diary, was dispensed to ensure a telemedicine consult as an easy point of contact and communication between investigators and patients and for monitoring the patients (including safety assessments).
Through appropriate medical devices connected with Genius ROSA™ (Remote Omnichannel Study Assistant), a private, study specific, fully encrypted, regulatory validated and compliant digital platform, patients were able to measure daily vital parameters like blood pressure, heart rate, oxygen saturation, and body temperature and filled this data into an electronic diary; all recorded values were automatically captured in real-time into the study database via the tele-medicine platform.
Ethics approval and consent to participate
At study entry, all patients gave a written informed consent. The protocol and protocol amendment, together with all required clinical trial documentation, were approved by the independent ethics committee (IEC) of each investigational study site before the trial was initiated.
Outcomes
The primary virologic outcome was the proportion of participants with undetectable SARS-CoV-2 by nasopharyngeal swabs at day 7. The primary clinical outcome was the proportion of participants who do not require supplemental oxygen therapy and/or mechanical ventilation at day 14 after randomization.
Secondary efficacy outcomes included the proportion of participants with undetectable SARS-CoV-2 by nasopharyngeal swabs at days 14 and 28, proportion of participants who do not require supplemental oxygen therapy and/or mechanical ventilation at days 7 and 28, proportion of participants in each National Early Warning Score (NEWS) category at days 7, 14 and 28, proportion of survivors at day 7, 14, and 28, and the mean variation of value of the following biomarkers: hepatic function, coagulation, and inflammatory markers from baseline to days 7, 14, 21 and 28.
Safety outcomes included the proportion of participants with any adverse event with grade ≤ 2 according to the Common Terminology Criteria for Adverse Events (CTCAE) at days 7, 14 and 28, and the proportion of participants with any severe adverse events (grade ≥ 3 according to CTCAE) at days 7, 14 and 28.
Sample size
The sample size was based on the following virologic and clinical assumptions: an increase from 25 to 50% (absolute difference 25%) in the proportion of participants with undetectable SARS-CoV-2 in the upper respiratory tract at day 7 of raloxifene treatment and an increase from 50 to 75% (absolute difference 25%) in the proportion of participants who recover without mechanical ventilation and/or supplemental oxygen by day 14 of raloxifene treatment. Based on these assumptions, the overall sample size was adaptively determined to achieve a power greater than 80% to show superiority of raloxifene vs placebo in terms of either one of the primary endpoints with a one-sided alpha below 0·025. Afterwards, an interim analysis was planned at 150 patients (50 per arm) with an additional 174 patients expected to be randomized (total planned sample size 324). The study was not powered to perform comparison between raloxifene arms (Appendix).
Statistical analysis
Descriptive statistics were utilized according to the nature of variables (quantitative summaries for continuous data, frequency distributions and percentages for categorical data). Comparison of proportions were performed through Fisher's exact test (descriptive in nature). For time-to-event variables, cumulative freedom from event was evaluated using the Kaplan-Meier method. The degree of uncertainty was expressed with 95% confidence limits (calculated per the method proposed by Greenwood). Comparison of curves among arms was performed with the log-rank test. Unless otherwise specified, the significance level used for statistical testing was 0.05 and two-sided tests were used.
The proportion of participants with undetectable SARS-CoV-2 at day 7 after randomization, and the proportion of participants who did not require mechanical ventilation and/or supplemental oxygen therapy (NEWS < 2) at day 14 after randomization (primary endpoints) were analyzed and compared by treatment (raloxifene vs placebo) by means of a binary logistic regression model. This was performed by treatment group and pre-defined baseline factors (center, age, status, and their interaction effects with treatment group). Odds ratios (OR) and risk differences (RD) were computed for comparisons of each active treatment group versus placebo. i.e., raloxifene 60 mg versus placebo and raloxifene 120 mg versus placebo. All secondary endpoints were analyzed at each available timepoint by means of descriptive statistics and by appropriate parametric tests.
An interim analysis was planned for when half of the evaluable patients had assessments available for each of the co-primary endpoints, at which time treatment arm continuation and/or reassessment of the sample size was determined to achieve a power of >80% for superiority of raloxifene vs placebo. Pocock's spending functions were planned to be used to control type I and II errors for analysis of the primary endpoints (nominal type I error at interim equal to 0·0035). Based on Pocock's boundaries and conditional power at the interim analysis, each treatment was to be classified and ranked as “Effective”, “Favorable”, “Promising”, or “Unfavorable”. According to the most effective ranked arm, a decision on the continuation of the study and on the reassessment of sample size was to be taken (Appendix).
Additionally, to maintain the integrity of the study, the analysis was to be conducted by an independent statistician and results were to be reviewed by the Data and Safety Monitoring Board (DSMB). Study enrollment was to cease in the case of efficacy in at least one arm or futility in both arms. However, due to premature termination of the study from lack of patient enrollment, neither an interim analysis nor reassessment of the sample size was ever performed.
The safety and the full analysis set (FAS) population consisted of all patients who were randomized and received at least one dose of the investigational product. The safety population was analyzed according to the actual treatment received. The FAS population was analyzed according to the intention-to-treat (ITT) analysis i.e. by treatment allocation. Primary and secondary efficacy analyses were conducted on the FAS population while the safety analysis set (SAF) population were used for the safety analyses.
All statistical analyses and data processing were performed using Statistical Analysis Systems (SAS®) Software (release 9.4).
Data management
Data management of the eCRFs, electronic diaries and protocol-deviation forms was performed by the contract research organization (CRO) appointed by Dompé. All data were verified in a timely manner for missing information, inconsistencies, and for any necessary medical clarifications. Queries arising from the edit checks (either programmed or manual) were sent to the Investigator for a response. Once all data queries had been resolved, and comments/changes arisen from the Data Review Meeting incorporated, the study data were declared to be “clean”, and the study database was locked ready for analysis. A Data Management Plan was issued, detailing the flow of data handling from entry into the eCRF to final database audit.
As integration of the quality control was performed by the appointed monitor, and an audit visit was performed on May 18, 2021 by the Quality Assurance Department of the CRO to verify compliance with GCP and study procedures in the coordinating site.
Role of the funding sources
The funders were responsible for study design, data collection, data analysis, data interpretation, and writing of the manuscript. All authors had access to the included study data and all authors agreed with the final decision to submit for publication.
Results
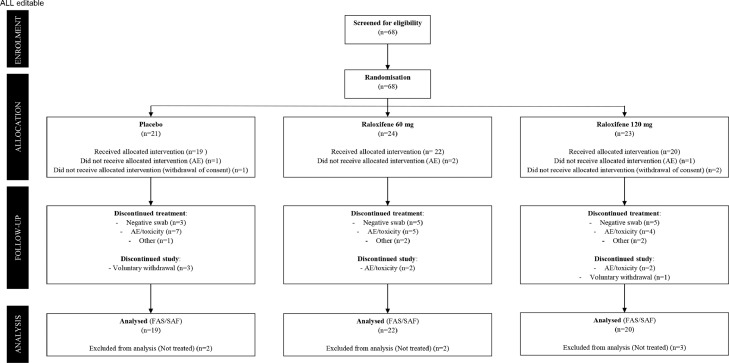
Between October 2020 to June 2021, after a process of selection and screening, 68 participants were enrolled and randomized to placebo (N = 21), raloxifene 60 mg (N = 24), and raloxifene 120 mg (N = 23). Among these patients, 16 patients completed with study within the placebo group, 20 patients completed the study within the raloxifene 60 mg group, and 17 patients completed the study within the raloxifene 120 mg group as noted in Figure 1. Baseline characteristics are shown in Table 1. The overall population were similar among all three groups with the average age of the cohort being 56·7 ± 10·1 years old, 33 (54·1%) patients were male, and the average time from first symptoms to enrollment being 11 ± 4·3 days. Within the entire cohort, 40 patients (65·6%) were never smokers, 16 patients (26·2%) were affected by hypertension, thyroid dysfunction was known in 6 patients (9·8%), while only 4 patients (0·66%) were affected by diabetes mellitus and dyslipidemia. Cough (45 patients, 73·8%), fever (39 patients, 63·9%), and muscle aches (37 patients, 60·7%) were the most common reported symptoms. There were no clinically relevant differences between treatment groups (Table 1).
Figure 1.
Trial profile.
All consented patients were eligible to be enrolled in the trial. The Safety (SAF) and the Full Analysis Set (FAS) populations consist of all randomized patients who received at least one dose of the investigational product. The SAF population was analyzed according to the actual treatment received, while the FAS population according to ITT principle, i.e. by treatment allocation.
AE: Adverse Event.
Table 1.
Patient baseline characteristics – FAS/SAF population.
| Placebo (n = 19) | Raloxifene 60 mg (n = 22) | Raloxifene 120 mg (n = 20) | |
|---|---|---|---|
| Centers* | |||
| Humanitas Milan, Italy | 0 (0% [0–17·6])) | 1 (4·5% [0·1–22·8]) | 0 (0 [0–16·8]) |
| IRCCS Lazzaro Spallanzani, Rome, Italy | 8 (42·1% [20·3–66·5]) | 9 (40·9% [20·7–63·7]) | 9 (45·0% [23·1–68·5]) |
| Ospedale Monaldi, Naples, Italy | 1 (5·3% [0·1–26·0]) | 2 (9·1% [1·1–29·2]) | 3 (15·0% [3·2–37·9]) |
| Citta della salute e della Scienza–Presidio Molinette, Turin, Italy | 7 (36·8% [16·3 – 61·6]) | 5 (22·7% [7·8–45·4]) | 6 (30·0% [11·9–54·3]) |
| Ospedale San Salvatore, L'Aquila, Italy | 3 (15·8% [3·4–39·6]) | 5 (22·7% [7·8–45·4]) | 2 (10·0% [1·2–31·7]) |
| Age, years | 54·6 ± 9·3 | 55·1 ± 10·9 | 58·9 ± 10·0 |
| Male, Sex | 10 (52·6% [28·9–75·6]) | 11 (50·0% [28·2–71·8]) | 12 (60·0% [36·1–80·9]) |
| Body Mass Index, kg/m2 | 28·5 ± 4·1 | 25·8 ± 3·1 | 26·6 ± 4·1 |
| Race, White | 19 (100% [82·4–100]) | 22 (100% [84·6–100]) | 20 (100% [83·2–100]) |
| Sp02,% | 96·3 ± 1·60 | 96·4 ± 1·95 | 96·6 ± 1·73 |
| Smoking status | |||
| Current smoker | 3 (15·8% [3·4–39·6]) | 2 (9·1% [1·1–29·2]) | 5 (25·0% [8·7–49·1]) |
| Ex-smoker | 1 (5·3% [0·1–26·0]) | 5 (22·7% [7·8–45·4]) | 5 (25·0% [8·7–49·1]) |
| Never smoker | 15 (78·9% [54·4–94·0]) | 15 (68·2% [45·1–86·1]) | 10 (50·0% [27·2–72·8]) |
| Medical History | |||
| Thyroid Dysfunction | 1 (5·3% [0·1–26·0]) | 1 (4·5% [0·1–22·8]) | 4 (20·0% [5·7–43·6]) |
| Diabetes Mellitus | 1 (5·3% [0·1–26·0]) | 1 (4·5% [0·1–22·8]) | 2 (10·0% [1·2–31·7]) |
| Dyslipidemia | 3 (15·8% [3·4–39·6]) | 0 (0% [0–15·4]) | 1 (5·0% [0·1–24·9]) |
| Asthma | 0 (0% [0–17·6])) | 0 (0% [0–15·4])) | 1 (5·0% [0·1–24·9]) |
| Hypertension | 7 (36·8% [16·3 – 61·6]) | 2 (9·1% [1·1–29·2]) | 7 (35·0% [15·4–59·2]) |
| Time from first symptoms, days | 9·6 ± 2·8 | 10·7 ± 4·6 | 12·9 ± 4·9 |
| Symptoms at Enrollment | |||
| Fever | 13 (68·4% [43·5–87·4]) | 14 (63·6% [40·7–82·8]) | 12 (60·0% [36·1–80·9]) |
| Dyspnea | 0 (0% [0–17·6]) | 3 (13·6% [2·9–34·9]) | 1 (5·0% [0·1–24·9]) |
| Headache | 6 (31·6% [8·4–16·7]) | 11 (50·0% [28·2–71·8]) | 8 (40·0% [19·1–64·0]) |
| Cough | 13 (68·4% [43·5–87·4]) | 17 (77·3% [54·6–92·2]) | 15 (75·0% [50·9–91·3]) |
| Muscle or body aches | 10 (52·6% [28·9–75·6]) | 15 (68·2% [45·1–86·1]) | 12 (60·0% [36·1–80·9]) |
| Concomitant Medications (n) | |||
| Anticoagulantsα | 2 (10·5 [1·3 – 33·1]) | 3 (13·6% [2·9–34·9]) | 2 (10·0% [1·2–31·7]) |
| Antibiotics∞ | 1 (5·3% [0·1–26·0]) | 1 (4·5% [0·1–22·8]) | 2 (10·0% [1·2–31·7]) |
| Corticosteroids§ | 2(10·5 [1·3 – 33·1]) | 4 (18·2% [5·2–40·3]) | 3 (15·0% [3·2–37·9]) |
| Remdesivir | 1 (5·3% [0·1–26·0]) | 0 (0% [0–15·4])) | 0 (0 [0–16·8]) |
| Progesterone and Estrogens | 0 (0% [0–17·6]) | 1 (4·5% [0·1–22·8]) | 0 (0 [0–16·8]) |
Data are presented for the FAS as number, proportion (%) with 95% exact C.I. or mean ± SD (standard deviation) Sp02:Oxygen Saturation.
All centers were located in Italy.
Anticoagulants included heparin and enoxaparin.
Antibiotics include pencillins, fluroquinolones, and macrolides.
Corticosteroids included dexamethasone and prednisone,
This clinical trial was impacted due to the COVID-19 pandemic, and experienced operational difficulties for patient enrollment. The following modifications were made to the original patient population to increase patient recruitment: inclusion of additional centers, lowering the age of the inclusion criteria from 50 to 40 years of age, increasing the time window of SARS-CoV-2 positive results from 7 to 10 days, and deleting a time cut-off of when initial COVID-19 related symptoms were experienced by patients (fever, dyspnea, headache, cough, dysgeusia, conjunctivitis, vomiting, diarrhea, anosmia, muscle or body aches or other symptoms).
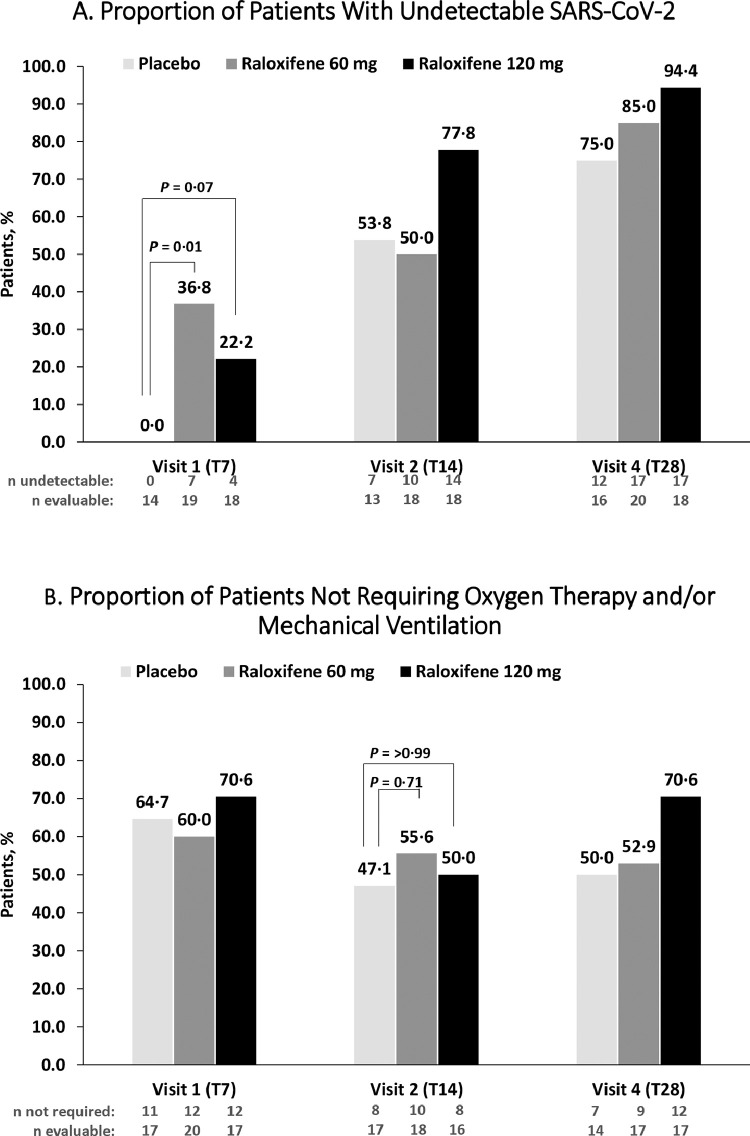
The proportion of patients with undetectable SARS-CoV-2 after seven days of treatment with raloxifene 60 mg was greater compared to placebo [36·8%, N = 7 vs. 0·0%, N = 0, OR = 9·99 (95% C.I.: 1·78–NE), RD = 0·37 (95% C.I.:0·09–0·59)] (Table 2). A favorable trend for raloxifene 120 mg compared to placebo was also reported, respectively [22·2%, N = 4 vs. 0·0%, N = 0, OR = 5·41 (95% C.I.: 0·86–NE), RD = 0·22 (95% C.I.: −0·03–0·45)] (Figure 2). A post-hoc analysis for the proportion of patients with undetectable SARS-CoV-2 of the pooled raloxifene population compared to placebo was notably greater at day 7 [28·9%, N = 11 vs. 0·0%, N = 0] and had a favorable trend, at each subsequent assessment visit (Figure A-1 Appendix). After 14 and 28 days a favorable outcome for both doses of raloxifene was maintained. (Figure A-1 Appendix)
Table 2.
Efficacy endpoints evaluation – FAS population.
| Patients with an undetectable SARS-CoV-2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Raloxifene 60mg |
Raloxifene 120mg |
|||||||
| Odds Ratio (OR) | 95% CI | Risk Difference (RD) | 95% CI | Odds Ratio (OR) | 95% CI | Risk Difference (RD) | 95% CI | |
| Day 7* | 9·99 | 1·78–NE | 0·37 | 0·09–0·59 | 5·41 | 0·86–NE | 0·22 | −0·03–0·45 |
| Day 14 | 1·11 | 0·22–5·71 | 0·02 | −0·30–0·33 | 3·20 | 0·54–22·11 | 0·24 | −0·09–0·52 |
| Day 28 | 2·16 | 0·29–18·53 | 0·10 | −0·15–0·36 | 7·22 | 0·59–413·50 | −0·14 | −0·06–0·44 |
| Patients who do not require supplemental oxygen support (NEWS ≤ 2) | ||||||||
| Day 7 | 0·97 | 0·19–4·91 | −0·05 | −0·33–0·25 | 1·16 | 0·20–6·82 | 0·06 | −0·24–0·34 |
| Day 14* | 1·66 | 0·34–9·05 | 0·09 | −0·22–0·37 | 0·96 | 0·19–4·98 | 0·03 | −0·28–0·33 |
| Day 28 | 1·07 | 0·21–5·48 | 0·03 | −0·29–0·34 | 2·07 | 0·37–12·58 | 0·21 | −0·13–0·49 |
NE definition: not evaluable.
Primary Endpoints
The FAS population consists of all randomized participants who received at least one dose of study drug, analyzed according to ITT principle. Figure 2 represents Fisher's exact tests, and are based on evaluable patients only with percentages calculated based on undetectable/number of evaluable participants.
Figure 2.
Endpoint analysis of raloxifene population
The primary virological endpoint was the proportion of patients with undetectable SARS-CoV-2 at Day 7 and the primary clinical endpoint was the proportion of patients not requiring oxygen therapy and/or mechanical ventilation at Day 14 within the FAS population. The figure depicts raloxifene 120 mg as the black bar, raloxifene 60 mg as the dark gray bar, and placebo as the light gray color bar. Undetectable was defined as the proportion of participants with undetectable SARS-CoV-2 infection by an approved molecular polymerase chain reaction (PCR) test. Evaluable was defined as the number of participants that were included within the analysis at that time point. Reasons for discontinuing treatment are provided in Fig. 1. All p-values are descriptive in nature with significance defined as a p-value <0.05. A. The raloxifene 60 mg group demonstrated benefit for the primary virological endpoint. Whereas a favorable trend for raloxifene was maintained at day 14 for raloxifene 120 mg, and at day 28 for raloxifene 60 mg and 120 mg. B. The lower dose of raloxifene demonstrated some benefit, but no relevant differences were reached for the primary clinical endpoint. At day 28, there was a trend towards a favorable improvement for the raloxifene 120 mg group, compared to placebo.
T7: 7 days; T14: 14 days; T28: 28 days.
A sub analysis was performed in a subgroup of female patients that showed a significant increase in participants with undetectable SARS-CoV-2 in nasopharyngeal swabs at day 7 in the raloxifene 60 mg group compared to placebo (Figure A-2 Appendix). Within the male population, a favorable outcome for raloxifene was achieved at the higher dose of 120 mg and only after three weeks of treatment (Figure A-3 Appendix). Additionally, when evaluating the efficacy in the subpopulation of patients not treated with corticosteroids, raloxifene 60 mg achieved a favorable outcome of undetectable SARS-CoV-2 nasopharyngeal swabs at day 7 compared to placebo (Figure A-4 Appendix).
Raloxifene 60 mg demonstrated some benefit compared to placebo, but no relevant differences were reached when evaluating patients that did not require supplemental oxygen therapy (NEWS ≤ 2) and/or mechanical ventilation at day 14 [55·6%, N = 10 vs. 47·1%, N = 8, OR = 1·66 (95% C.I.: 0·34–9·05), RD = 0·09 (95% C.I.: −0·22–0·37)] (Figure 2). At day 28, there was a trend towards a favourable improvement for the raloxifene 120 mg group compared to placebo when comparing the proportion of patients not requiring oxygen support or mechanical ventilation [70·6%, N = 12 vs. 50·0%, N = 7]. No differences were noted between the two arms at 7, 14, and 28 days regarding the average NEWS data (Figure 2).
Exploring further secondary endpoints, laboratory parameters were assessed as changes from baseline at day 7, 14, 21 and 28 as seen in the Appendix. Most notable are that patients within the raloxifene 60 mg group demonstrated a minor increase of total cholesterol at day 21, a minor increase of white blood cells from baseline at day 21 and 28, and a lack of increase of neutrophils (%) at day 28, but an associated minor increase at day 28. Lastly, we underline the lack of d-dimer elevations from baseline to day 28 in both raloxifene dose groups and no increase in hepatic and renal markers (Appendix).
Table 3 summarizes treatment-emergent adverse events (TEAEs): 10 patients (52·6%) experienced any TEAE within the placebo group compared to 8 patients (36·4%) within the raloxifene 60 mg group, and 10 patients (50·0%) within the raloxifene 120 mg group. Of all the TEAEs that occurred in the three arms, 17·6% were considered related to treatment in the placebo group, compared to 10·5% in the raloxifene 60 mg group, and 40·0% within the raloxifene 120 mg group. Treatment-emergent serious adverse events (TESAEs) leading to hospitalization occurred more frequently within the placebo group (26·3%) compared to both the raloxifene 60 mg group (13·6%) and raloxifene 120 mg group (10·0%,), but in no cases was an intensive care admission required. Overall, the most frequently reported TEAEs leading to hospitalization were gastrointestinal disorders including abdominal pain, gastric disorder, nausea, and dyspepsia that occurred in 10·5% of patients in the placebo group, compared to 22·7% in the raloxifene 60 mg group, and 15% in the raloxifene 120 mg group. The most frequent TESAEs leading to hospitalization were respiratory symptoms (dyspnea, pneumonia, cough, respiratory failure) with a similar occurrence in all three groups: 10·5% within the placebo group, 9·1% within the raloxifene 60 mg group, and 10% within the raloxifene 120 mg group. None of the patients in any treatment group were admitted to the intensive care unit after randomization at any timepoint, and there were also no deaths in any treatment or placebo group.
Table 3.
Summary of Adverse Events – SAF population.
| Placebo (N = 19) | Raloxifene 60 mg (N = 22) | Raloxifene 120 mg (N = 20) | |
|---|---|---|---|
| Number of TEAEs | 17 | 19 | 20 |
| Number of patients with TEAEs | 10 (52·6 [28·9–75·6]) | 8 (36·4 [17·2–59·3]) | 10 (50·0 [27·2–72·8]) |
| Number of TESAEs | 6 | 4 | 3 |
| Number of patients with TESAEs | 5 (26·3 [9·2–51·2]) | 3 (13·6 [2·9–34·9]) | 2 (10·0 [1·2–31·7]) |
| Number of patients who discontinued study drug due to Adverse Event | 7 (36·8 [16·3 – 61·6]) | 5 (22·7 [7·8–45·4]) | 4 (20·0 [5·7–43·6]) |
| Gastrointestinal Discomfort | 2 (10·5 [1·3 – 33·1]) | 2 (9·1 [1·1–29·2]) | 2 (10·0 [1·2–31·7]) |
| COVID-19 Pneumonia | 2 (10·5 [1·3 – 33·1]) | 1 (4·5 [0·1–22·8]) | 0 (0 [0–16·8]) |
| D-Dimer Increases | 2 (10·5 [1·3 – 33·1]) | 0 (0 [0–15·4]) | 0 (0 [0–16·8]) |
| Respiratory Symptoms (cough, dyspnea respiration failure) | 2 (10·5 [1·3 – 33·1]) | 2 (9·1 [1·1–29·2]) | 2 (10·0 [1·2–31·7]) |
| Any adverse event (Grade ≤ 2) | |||
| Gastrointestinal discomfort | 1 (5·3 [0·1–26·0]) | 3 (13·6 [2·9–34·9]) | 2 (10·0 [1·2–31·7]) |
| D-dimer Increased | 2 (10·5 [1·3 – 33·1]) | 0 (0 [0–15·4]) | 0 (0 [0–16·8]) |
| Hypertriglyceridemia | 1 (5·3 [0·1–26·0]) | 0 (0 [0–15·4]) | 0 (0 [0–16·8]) |
| Muscle Spasms | 0 (0 [0–7·6]) | 2 (9·1 [1·1–29·2]) | 1 (5·0 [0·1–24·9]) |
| Confusion | 0 (0 [0–7·6]) | 1 (4·5 [0·1–22·8]) | 0 (0 [0–16·8]) |
| Cough | 0 (0 [0–7·6]) | 1 (4·5 [0·1–22·8]) | 0 (0 [0–16·8]) |
| Respiratory Failure | 1 (5·3 [0·1–26·0]) | 0 (0 [0–15·4]) | 1 (5·0 [0·1–24·9]) |
| Flushing | 0 (0 [0–7·6]) | 0 (0 [0–15·4]) | 1 (5·0 [0·1–24·9]) |
| Any adverse event (Grade ≥3) | |||
| Gastrointestinal discomfort | 1 (5·3 [0·1–26·0]) | 2 (9·1 [1·1–29·2]) | 1 (5·0 [0·1–24·9]) |
| COVID-19 Pneumonia | 2 (10·5 [1·3 – 33·1]) | 1 (4·5 [0·1–22·8]) | 0 (0 [0–6·8]) |
| Dyspnea | 0 (0 [0–7·6]) | 1 (4·5 [0·1–22·8]) | 1 (5·0 [0·1–24·9]) |
| Hypertension | 0 (0 [0–7·6]) | 1 (4·5 [0·1–22·8]) | 0 (0 [0–6·8]) |
| Adverse Events of Special Interest (AESI) | |||
| Venous Thromboembolic events | 0 (0 [0–7·6]) | 0 (0 [0–5·4]) | 0 (0 [0–6·8]) |
| Arterial thromboembolic reactions | 0 (0 [0–7·6]) | 0 (0 [0–5·4]) | 0 (0 [0–6·8]) |
| Thrombocytopenia | 0 (0 [0–7·6]) | 0 (0 [0–5·4]) | 0 (0 [0–6·8]) |
All data are presented as number of patients, proportion (%) with 95% exact C.I.
Abbreviations: TEAE: treatment-emergent adverse events, TESAE: Treatment-emergent serious adverse events.
Discussion
In this small randomized controlled trial, more outpatients with mild to moderate COVID-19 had undetectable SARS-CoV-2 after seven days of treatment with raloxifene 60 mg when compared to placebo. These results allow us to hypothesize an ability of raloxifene to induce an early and sustained reduction in viral load, with a positive impact on the diffusive capacity of the virus, leading to reduced contagiousness and decreasing the number of new cases in the susceptible population.
This evidence is in line with in vitro data that showed raloxifene to have high pulmonary distribution and be an effective molecule against SARS-CoV-2 when compared to commonly used antivirals such as remdesivir.10 This is not surprising considering the data on raloxifene's antiviral activity against influenza A, Ebola, hepatitis C, and Flaviviruses including the Zika virus, dengue virus, and West Nile virus.13 Several mechanisms have been proposed to explain raloxifene's antiviral activity. Raloxifene has been shown to inhibit viral entry into host cells by interacting with viral spike proteins and blocking SARS-CoV-2 replication and transcription by inhibiting viral proteases.14 Additionally, raloxifene acts as an immunomodulator to decrease proinflammatory cytokines (ie, IL-6, TNF-a) release and regulates bradykinin, which are crucial in preventing CRS.15 Lastly, raloxifene's modulation of the ER pathways may be useful in COVID-19 by reducing tissue injury through increasing vasodilation, nitric oxide, and prostacyclin and decreasing endothelin-1, directly affecting the inflammatory response, and protecting against ALI and ARDS.10,16 The virologic outcome was not achieved with the higher dose of raloxifene 120 mg. It can be hypothesized that lower doses of raloxifene act as an ER agonist, by acting as an anti-inflammatory and antiviral agent, but higher doses of raloxifene act as an antagonist. As mentioned above, it is well known that the drug has a double receptor activity, either as an estrogen agonist in some tissues or as an estrogen antagonist in others, such as endometrium and breast.12 Doses above 60 mg are often utilized in women seeking estrogen receptor antagonism in the oncological patient population.17
Interestingly, the primary outcome with raloxifene 60 mg remained significant in the sub analysis of females. These results support current literature that underlines the role of estradiol and estrogen receptors in COVID-19 disease.9 In contrast, males displayed a favorable virologic profile only after 28 days of raloxifene 120 mg. This suggests the possibility that males may need to be treated with higher doses and for a longer period due to lower concentrations of estrogen, differences in pharmacological metabolism, and because a more severe form of COVID-19 disease is often experienced by males compared to females.18, 19–20 There remains a continued need for effective, available, therapeutic options in early infection that modulates the cytokine cascade dysregulation.21 Common immomodulators such as tocilizumab and baricitinib have shown benefit in clinical outcomes in more severe COVID-19 patients requiring hospitalization and oxygen support through noninvasive and invasive ventilation, whereas systemic corticosteroid therapy improves clinical outcomes and reduces mortality in hospitalized patients with COVID-19 who require supplemental oxygen.6
There is a global, urgent, medical need to find an oral antiviral option that is easily administered by patients, and safe and effective for COVID-19 treatment in the non-hospitalized patient population. Currently, molnupiravir, an oral repurposed antiviral medication able to inhibit the RNA-dependent RNA polymerase (RdRp) enzyme, and nirmatrelvir/ritonavir, protease inhibitor oral antiviral therapy, are showing promise and have recently received emergency use authorization for treatment for mild-to-moderate COVID 19, yet this is only within the high-risk population. Our results appear in line with a recent trial in the outpatient setting by Weinreich et al. in which REGN-COV2, a neutralizing antibody cocktail of casirivimab plus indevimab, reduced the viral load within 7 days.22 However, like other utilized monoclonal antibodies for COVID-19 there are limitations such as their intravenous administration route, lack of use in the non-high-risk population, and healthcare facilities overload including personnel, resources, and space. In contrast, raloxifene may be an easy-to-administer oral therapeutic option that patients can self-administer at home in both urban and rural settings. The Food and Drug Administration (FDA) has granted emergency use authorization (EUA) status for several monoclonal antibodies including bamlanivimab plus etesevimab, casirivimab and imdevimab, and sotrovimab. Most recently, both bamlanivimab monotherapy, and bamlanivimab plus etesevimab combination therapy are no longer recommended due to their reduced susceptibility to emerging SARS-CoV-2 variants (i.e.beta, delta, gamma, zeta).23 In further support of raloxifene, a recent systematic analysis demonstrated raloxifene to be efficacious on the most common viral variants currently circulating in Europe, United Kingdom, Brazil, South Africa, and India.24 However, the efficacy of raloxifene against emerging variants needs to be further validated by performing genomic sequencing of the virus.
Raloxifene has a well-established post-marketing safety profile in its current indications, and this was maintained in patients with mild to moderate COVID-19, with both treatment-emergent adverse events and treatment-emergent serious adverse event occurring more rarely than in the placebo group. Common adverse reactions from previous clinical trials are minor and include hot flashes, leg cramps, flu syndrome, peripheral edema, arthralgia, and sweating.11 This is comparable to the mild side effects that were also noted in this study including gastrointestinal discomfort as the most reported side effect. Raloxifene has also been investigated for use in males for an extended period at both 60 mg/day and 120 mg/day for evaluation of lipid, hormone, and bone turnover markers. Within these studies, men tolerated raloxifene well with no severe side effects, dropouts, and no thromboembolic events. The most common side effects compared to placebo included hot flushes, and muscle cramps.25,26 It is important to note that the risk of cardiovascular events have been extensively studied already with prolonged follow-up periods.27 However, patients with a history of stroke and VTE were excluded from this study. Of the patients who enrolled in this study, none experienced a cardiovascular event. The lack of risk of VTE is reflected by current recommendations that do not support the use of anticoagulants and antiplatelet therapy in non-hospitalized patients with COVID-19.28 Within this study, there was no increase in d-dimer levels noted, even with active or previous smokers being a significant characteristic within our baseline population.29 It is important to note some biochemistry data such as the limited increase of total cholesterol in patients treated with raloxifene 60 mg, that could be correlated with the medication's agonistic action at ERs within adipose tissues, leading to protection against a possible increased risk for vascular accidents.30
This study presents several limitations that deserve acknowledgement. Despite the promising results obtained for the virologic outcome, the study was stopped early for practical reasons related to low enrollment and therefore future larger studies are needed to confirm these results. The evaluation of clinical effectiveness in patients who do not require supplemental oxygen therapy and/or mechanical ventilation was not met which may be explained by our low sample size, reduction in hospital complications in patients with COVID-19, and lower COVID-19 cases during the summer in temperate countries.31 Therefore, it is unknown if raloxifene has benefit only in the early phase of the disease during viral replication. Additionally, the study population was characterized by limited diversity in demographics from the geographic location of clinical sites and therefore only Caucasians were enrolled. Outside of the mild to moderate COVID-19 patient population, it is unknown if raloxifene has a role in other patient groups such as for postexposure prophylaxis, or within hospitalized patients. Patients with a high risk of VTE events were excluded, and therefore the safety is unknown in this population. Likewise, patients included in this study had few comorbidities, and therefore it is unknown if the results can be extrapolated to patients with severe comorbidities and high-risk factors for COVID-19 complications. Additionally, the benefit of raloxifene within the obese population, including pharmacokinetic data, is unknown at this time.
Raloxifene, with its pharmacological activity at very low circulating concentrations (micromolar range), a wide distribution within the lungs and good safety profile, showed evidence of effect in the primary virologic endpoint of this trial in patients with mild to moderate COVID-19 with further studies required to confirm these results. The modulation of estrogen-regulated signaling provides a novel host-mediated mechanism of action that should maintain activity against emerging viral variants, and it may represent a promising pharmacological option to prevent or mitigate the disease progression and COVID-19 associated complications.
Contributors
MA, FM and AB conceived and designed the study; EN, FM, EP,ET, FR, GF, LS, SV and Raloxifene Territorial Health COVID19 STUDY GROUP collected the study data; MA, FM and FV supervised the study; GG and FM analyzed the data; MDP and GT were responsible for project administration for the study; EG and CM drafted the manuscript; MA and EN have verified the underlying data. All authors reviewed and edited the manuscript. All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication. All authors read and approved the final version of the manuscript. EN and MA accessed and verified data. MA and FM were responsible for the decision to submit the manuscript.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
The study was funded by Dompé Farmaceutici SpA and supported by the funds from the European Commission – Health and Consumers Directorate General, for the Action under the Emergency Support Instrument- Grant to support clinical testing of repurposed medicines to treat SARS-COV-2 patients (PPPA-ESI-CTRM-2020-SI2.837140), and by the COVID-2020–12,371,675 Ricerca finalizzata and line 1 Ricerca Corrente COVID both funded by Italian Ministry of Health.
Declaration of interests
Marcello Allegretti, Giovanni Goisis, Maria De Pizzol, Flavio Mantelli, Carolina Marsiglia, Giuseppe Terpolilli and Andrea R. Beccari are full time Dompé Farmaceutici SpA employees. Elizabeth Marie Gavioli is a full time employee of Dompe, U.S., Inc. Emanuele Pivetta declared a loan from the Butterfly network for ultrasound machines for research purpose. Emanuele Nicastri declared training fees and medical boards of Gilead Science, SOBI, Eli Lilly, and Roche. Andrea Rosario Beccari declared EP20173318.5 compounds for the treatment of COVID-19. Elena Torri, Franco Marinangeli, Francesco Reggiani, Francesco Vaia, Giuseppe Fiorentino, Scorzolini Laura and Serena Vettori have not declared any conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101450.
Appendix. Supplementary materials
References
- 1.World Health Organization. Listings of WHO's response to COVID-19. Accessed August 17, 2021. Available at: https://www.who.int/news/item/29-06-2020-covidtimeline.
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.CDC . CDC; 2021. People with Certain Medical Conditions.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Updated May 13. Accessed 10 August 2021. [Google Scholar]
- 4.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/ Accessed 27 August 2021. [PubMed]
- 7.Peckham H., de Gruijter N.M., Raine C., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Jerkic M., Slutsky A.S., Zhang H. Molecular mechanisms of sex bias differences in COVID-19 mortality. Crit Care. 2020;24(1):405. doi: 10.1186/s13054-020-03118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stelzig K.E., Canepa-Escaro F., Schiliro M., Berdnikovs S., Prakash Y.S., Chiarella S.E. Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2020;318(6):L1280–L12l1. doi: 10.1152/ajplung.00153.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allegretti M., Cesta M.C., Zippoli M., et al. Repurposing the estrogen receptor modulator raloxifene to treat SARS-CoV-2 infection. Cell Death Differ. 2021 doi: 10.1038/s41418-021-00844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EVISTA (raloxifene hydrochloride). Package insert. Lilly USA, LLC, Indianapolis, IN; 1997. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020815s034lbl.pdf. Accessed 5 May 2020
- 12.Patel H.K., Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018;186:1–24. doi: 10.1016/j.pharmthera.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Hong S., Chang J., Jeong K., Lee W. Raloxifene as a treatment option for viral infections. J Microbiol. 2021;59(2):124–131. doi: 10.1007/s12275-021-0617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyre N.S., Kirby E.N., Anfiteatro D.R., et al. Identification of estrogen receptor modulators as inhibitors of flavivirus infection. Antimicrob Agents Chemother. 2020;64(8) doi: 10.1128/AAC.00289-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gianni W., Ricci A., Gazzaniga P., et al. Raloxifene modulates interleukin-6 and tumor necrosis factor-alpha synthesis in vivo: results from a pilot clinical study. J Clin Endocrinol Metab. 2004;89(12):6097–6099. doi: 10.1210/jc.2004-0795. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5(1):293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis J.S., Jordan V.C. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat Res. 2005;591(1–2):247–263. doi: 10.1016/j.mrfmmm.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Fuentes N., Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135–170. doi: 10.1016/bs.apcsb.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess R.A., Cooke P.S. Estrogen in the male: a historical perspective. Biol Reprod. 2018;99(1):27–44. doi: 10.1093/biolre/ioy043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baiardo Redaelli M., Landoni G., Di Napoli D., et al. Novel coronavirus disease (COVID-19) in Italian patients: gender differences in presentation and severity. Saudi J Med Med Sci. 2021;9(1):59–62. doi: 10.4103/sjmms.sjmms_542_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solinas C., Perra L., Aiello M., Migliori E., Petrosillo N. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine Growth Factor Rev. 2020;54:8–23. doi: 10.1016/j.cytogfr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2020;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U. S. Food and drug administration. Corona virus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. April 16, 2021.
- 24.Iaconis D., Talarico C., Manelfi C., et al. Characterization of raloxifene as potential pharmacological agent against SARS-CoV-2 and its variants. bioRxiv. 2021 doi: 10.1038/s41419-022-04961-z. 2021.10.22.465294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doran P.M., Riggs B.L., Atkinson E.J., Khosla S. Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res. 2001;16(11):2118–2125. doi: 10.1359/jbmr.2001.16.11.2118. [DOI] [PubMed] [Google Scholar]
- 26.Duschek E.J., Gooren L.J., Netelenbos C. Effects of raloxifene on gonadotrophins, sex hormones, bone turnover and lipids in healthy elderly men. Eur J Endocrinol. 2004;150(4):539–546. doi: 10.1530/eje.0.1500539. [DOI] [PubMed] [Google Scholar]
- 27.Adomaityte J., Farooq M., Qayyum R. Effect of raloxifene therapy on venous thromboembolism in postmenopausal women. A meta-analysis. Thromb Haemost. 2008;99(2):338–342. [PubMed] [Google Scholar]
- 28.Moores L.K., Tritschler T., Brosnahan S., et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enga K.F., Braekkan S.K., Hansen-Krone I.J., le Cessie S., Rosendaal F.R., Hansen J.B. Cigarette smoking and the risk of venous thromboembolism: the Tromsø study. J Thromb Haemost JTH. 2012;10(10):2068–2074. doi: 10.1111/j.1538-7836.2012.04880.x. [DOI] [PubMed] [Google Scholar]
- 30.Hernández E., Valera R., Alonzo E., et al. Effects of raloxifene on bone metabolism and serum lipids in postmenopausal women on chronic hemodialysis. Kidney Int. 2003;63(6):2269–2274. doi: 10.1046/j.1523-1755.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 31.Ciceri F., Ruggeri A., Lembo R., Puglisi R., Landoni G., Zangrillo A., COVID-BioB Study Group Decreased in-hospital mortality in patients with COVID-19 pneumonia. Pathog Glob Health. 2020;114(6):281–282. doi: 10.1080/20477724.2020.1785782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.