Abstract
Minimally invasive direct coronary artery bypass (MIDCAB) surgery and percutaneous coronary intervention (PCI) are both well-established minimally invasive revascularization strategies in patients with proximal left anterior descending (LAD) lesions. We aimed to evaluate the 20-years’ experience by performing a systematic review and meta-analysis comparing MIDCAB versus PCI in adults with proximal LAD disease. We searched MEDLINE, EMBASE and Cochrane on October 1st, 2021 for articles published in the year 2000 or later. The primary outcome was all-cause mortality. Secondary outcomes included cardiac mortality, repeat target vessel revascularization (rTVR), myocardial infarction (MI), and cerebrovascular accident (CVA). Outcomes were analysed at short-term, mid-term, and long-term follow-up. Random effects meta-analyses were performed. Events were compared using risk ratios (RR) with 95% confidence intervals (CI). Our search yielded 17 studies pooling 3847 patients. At short-term follow-up, cardiac mortality was higher with MIDCAB than with PCI (RR 7.30, 95% CI: 1.38 to 38.61). At long-term follow-up, MIDCAB showed a decrease in all-cause mortality (RR 0.66, 95% CI: 0.46 to 0.93). MIDCAB showed a decrease in rTVR at mid-term follow-up (RR 0.16, 95% CI: 0.11 to 0.23) and at long-term follow-up (RR 0.25, 95% CI: 0.17 to 0.38). MI and CVA comparisons were not significant. In conclusion, in patients with proximal LAD lesions, MIDCAB showed a higher short-term mortality in the RCTs, but the cohort studies suggested a lower all-cause mortality at long-term follow-up. We confirm a decreased rTVR at mid-term follow-up in the RCTs and long-term follow-up in the cohort studies.
Keywords: Minimally invasive direct coronary artery bypass, Percutaneous coronary intervention, Proximal LAD lesion, Meta-analysis
Abbreviations: BMS, bare metal stent; CABG, coronary artery bypass grafting; CI, confidence interval; CVA, cerebrovascular accident; DES, drug eluting stent; LAD, left anterior descending; LITA, left internal thoracic artery; (RA)-MIDCAB, (robotic assisted) minimally invasive direct coronary artery bypass; MAC(C)E, Major Adverse Cardiac (and Cerebrovascular) Events; MI, myocardial infarction; NNT, number needed to treat; PCI, percutaneous coronary intervention; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; RR, risk ratio; rTVR, repeat target vessel revascularization
1. Introduction
The most recent European Guidelines for myocardial revascularization recommend both coronary artery bypass surgery (CABG) and percutaneous coronary intervention (PCI) for patients with isolated proximal left anterior descending (LAD) disease [1], [2], [3], [4], [5], [6], [7], [8], [9].
In favor of PCI is the less invasive nature of the treatment, while in favor of CABG is the long-term survival benefit offered by the left internal thoracic artery (LITA) to LAD and a decrease in the occurrence of repeat revascularization [10], [11], [12], [13]. Over the past two decades there has been an increased adoption of a minimally invasive direct coronary artery bypass (MIDCAB) strategy to perform the LITA-LAD conduit through a small left thoracotomy. It has been shown that MIDCAB has a similar safety and efficacy profile when compared to conventional CABG [4], [8], [14], [15]. However, it is not clear whether the long-term survival benefit that has been demonstrated with conventional CABG also applies to MIDCAB when compared to PCI and MIDCAB is currently not included in the ESC/EACTS guidelines for revascularization [1].
In this meta-analysis, we aimed to aggregate and critically evaluate the best evidence from the past 20 years on long-term outcomes after MIDCAB or PCI in adults with isolated proximal LAD disease.
2. Materials and methods
2.1. Search strategy
We queried MEDLINE via Pubmed, EMBASE and the Cochrane database on October 1st, 2021, using variations and synonyms of the search terms: minimally invasive direct coronary artery bypass surgery, percutaneous coronary intervention and proximal left anterior descending artery lesions (Appendix 1 for full search strings). The review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and aimed to find all published reports comparing MIDCAB and PCI as a revascularization strategy for proximal LAD lesions in adults and was performed in duplicate by two researchers (MG and ARJ) [16]. We also performed a cross-reference check.
2.2. Inclusion and exclusion criteria
Cohort studies and randomized controlled trials (RCT) comparing the treatment of adult patients with isolated proximal LAD lesion who underwent MIDCAB or PCI as the primary procedure were included. For inclusion the studies had to be written in English, reporting original data and published in or after the year 2000. For inclusion at least one of the following outcomes of interest had to be reported: of all cause and cardiac mortality, repeat target vessel revascularization (rTVR), myocardial infarction (MI) and cerebrovascular accident (CVA). We excluded studies reporting non isolated LAD lesion treatment, or papers without definition of primary intervention strategy. Papers including a cohort of patients who underwent a primary full sternotomy, were excluded. We excluded papers reporting no original data or papers, without definition of primary intervention strategy and/or the outcomes of interest. Additionally, papers were assessed for their quality using the Risk of Bias 2 (RoB 2) Cochrane tool for randomized trials, whereas the ROBINS-I tool was used for cohort studies [17], [18]. Articles with a high risk of bias were excluded from the analysis.
2.3. Data extraction
After the search was performed, two independent reviewers (MG and ARJ) reviewed all articles. Discrepancies and were addressed and solved by a third reviewer (KJ). Data extractions were performed independently by three reviewers (MG, ARJ and HFN). When the same author published multiple studies we extracted patients’ characteristics from the first study and outcomes of interest at subsequent follow-ups from later studies. When two studies by the same institution reported the same outcomes at similar follow-up periods, we included either the higher quality or the most informative publication. Articles assessment was performed with the Cochrane Risk of Bias tool by the three aforementioned reviewers (Appendix 2).
2.4. Outcomes
The primary outcome was all-cause mortality at three different timeframes: < 30 days (short-term), 30 days – 1 year (mid-term), and > 3 years (long-term) of follow-up. All-cause mortality was defined as any cause of death.
Secondary outcomes were cardiac mortality, repeat target vessel revascularization (rTVR), myocardial infarction (MI) and cerebrovascular accident (CVA) at < 30 days, 30 days – 1 year, and > 3 years of follow-up. Cardiac mortality was defined as a primary cardiac cause of death. rTVR was defined as repeat revascularization of the target vessel from the original procedure. MI was defined following the definition of the original article. CVA did not include transient ischemic attacks.
2.5. Statistical analysis
Statistical analyses were performed using Review Manager (Version 5.3.5 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) and the statistical program R (version 4.0.3. 2020 The R Foundation for Statistical Computing). We used random effects models (Mantel-Haenszel method) instead of fixed effects for a more robust and conservative risk ratio (RR). The RR was calculated for categorical variables as the effect estimate for all outcomes. The results were presented as a forest plot, depicting the individual RR from each study as well as the overall composite effect estimate. An RR with its 95% confidence interval (95% CI) <1 would favor MIDCAB. In the inverse weighted model, each study contributed to a percentage of the final pooled estimate, and was presented in each forest plot under the column of weight [19]. According to Bate’s correction, 0.1 was added to each cell of the two-by-two table in case the study or control arm had zero events [20]. We calculated the risk difference with the number needed to treat (NNT).
The I2 statistic and its corresponding p-value were calculated to test for heterogeneity. We additionally re-analyzed the data using fixed-effects models. All data were stratified by study design at short-term (<30 days follow-up), mid-term (30 days – 1 year), and long-term (>3 years) follow-up. Publication bias was assessed using visual inspection of the contour-enhanced funnel plot symmetry and with Egger’s test. A p-value of 0.05 or less was considered statistically significant.
3. Results
3.1. Selection and characteristics of included studies
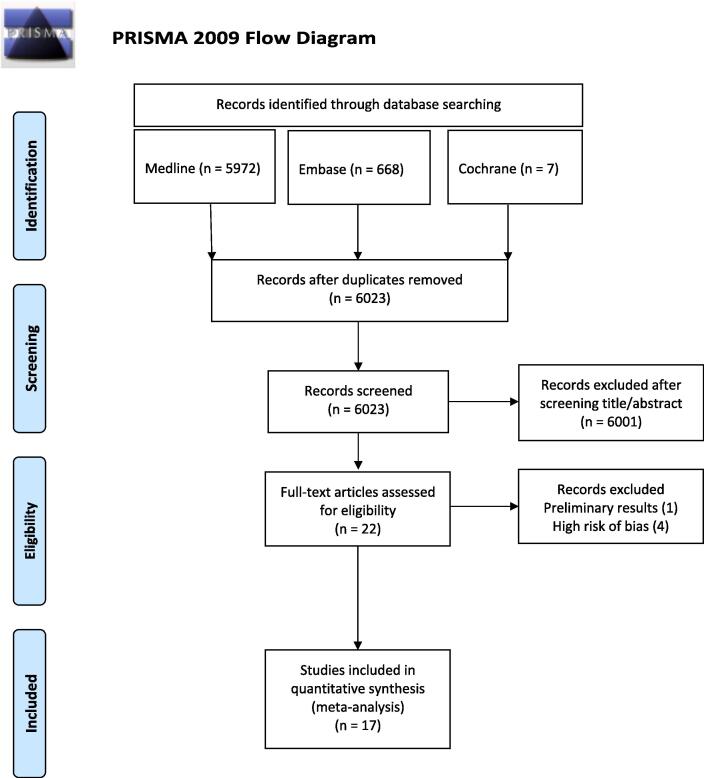
The literature search yielded a total of 6647 papers. Following data deduplication, 624 papers were excluded. Only 22 of the remaining 6023 papers matched our inclusion criteria and were included for full text screening. After critical appraisal we excluded five papers: three cohort studies papers presented a high risk of bias due to confounding, and one RCT paper presented a high risk of bias arising from the randomization process [21], [22], [23], [24] and one published preliminary results which were irrelevant to our research question [25] (see Appendix 2). Seventeen published papers fulfilling our inclusion criteria were included in this meta-analysis [2], [3], [9], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. The PRISMA flow diagram is presented in Fig. 1.
Fig. 1.
Flow diagram of selected studies.
We included six original RCTs, that were described in nine articles, with a total of 376 patients in the MIDCAB group and 376 patients in the PCI group [2], [3], [9], [34], [35], [36], [37], [38], [39]. Eight cohort studies were included with a total of 1283 patients in the MIDCAB group and 1812 patients in the PCI group [26], [27], [28], [29], [30], [31], [32], [33]. For both RCTs and cohort studies, follow-up varied from six months to ten years. The study design and characteristics of the studies included are summarized in Table 1.
Table 1.
Summary of selected studies RCT.
| Author, year of publication, country | Design RCT | Study period | Procedure | Population (N) | PCI (N) | MIDCAB (N) | Follow-up | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Diegeler et al. [2002] Germany Thiele et al. [2005] Germany Blazek et al. [2013] Germany |
Open label single center randomized controlled trial | June 1997 – June 2001 | MIDCAB under direct vision PCI with BMS |
220 |
110 |
110 | 30 days 6-months 5 years 10 years |
Low Low Low |
| Thiele et al. [2009] Germany Blazek et al. [2015] Germany |
Open label single center randomized controlled trial | January 2003 – October 2007 | MIDCAB under direct vision PCI with DES |
130 | 65 | 65 | 30 days 1 year 7 years |
Low Low |
| Cisowski et al. [2002] Poland |
Open label single center randomized controlled trial | 2000–2001 | MIDCAB thoracoscopic assistance PCI with BMS |
100 |
50 | 50 | 30 days 6-months 1 year |
Low |
| Drenth et al. [2002] The Netherlands |
Open label single center randomized controlled trial | March 1997 – September 1999 | MIDCAB under direct vision PCI with BMS |
102 |
51 | 51 | 30 days 6-months |
Moderate |
| Kim et al. [2005] South Korea |
Open label single center randomized controlled trial | January 2000 – December 2001 | MIDCAB under direct vision and mini-sternotomy PCI with BMS |
100 |
50 | 50 | 30 days 1 year |
Low |
| Reeves et al. [2004] United Kingdom |
Open label multicenter randomized controlled trial | November 1999 – December 2001 | MIDCAB under direct vision or thoracoscopic assistance PCI with BMS |
100 |
50 | 50 | 30 days 12 months |
Low |
| Summary of selected cohort studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, year of publication, country | Design cohort | Study period | Procedure | Population (N) | PCI (N) | MIDCAB (N) | Follow-up | Risk of bias |
| Benedetto et al. [2014] United Kingdom |
Retrospective single center prospective propensity score-matched comparison | April 2001 - May 2013 | MIDCAB under direct vision or thoracoscopic assistance PCI with DES |
1033 (before matching) |
303 | 303 | 30 days 1 year 5 years 10 years |
Moderate |
| Choi et al. [2019] South Korea |
Retrospective single center prospective propensity score-matched comparison | September 2007 – June 2017 | MIDCAB under direct vision PCI with DES |
154 |
77 | 77 | 3 years | Moderate |
| Etienne et al. [2013] Belgium |
Retrospective multicenter study prospective propensity score-matched comparison | 1997–2011 | MIDCAB under direct vision PCI with DES |
456 |
196 | 260 | 30 days 5 years |
Moderate |
| Iakovou et al. [2002] United States of America |
Retrospective single center prospective propensity score-matched comparison | June 1996 – December 1999 | MIDCAB under direct vision PCI with BMS |
560 | 441 | 119 | 30 days 1 year |
Moderate |
| Li et al. [2021] China |
Retrospective single center prospective propensity score-matched comparison | July 2007 – November 2011 |
RA-MIDCAB PCI with DES |
719 (before matching) | 108 | 108 | 4 years | Moderate |
| Merkle et al. [2019] Germany |
Retrospective single center matched comparison | 2006–2012 | MIDCAB harvesting under direct vision or using thoracoscopic assistance PCI with DES |
206 |
100 | 106 | 1 year 6 years 10 years |
Moderate |
| Shirai et al. [2004] United States of America |
Retrospective single center matched comparison | February 1990 – October 1999 | MIDCAB unknown technique PCI with BMS |
581 | 429 | 152 | 30 days 6 months |
Moderate |
| Patel et al. [2020] United States of America |
Retrospective single center prospective propensity score-matched comparison | January 2008 –December 2016 | RA-MIDCAB PCI with DES |
531 (before matching) | 158 | 158 | 9 years | Moderate |
Abbreviations: BMS: bare metal stent, DES: drug eluting stent, RA-MIDCAB: robotic assisted minimally invasive direct coronary artery bypass, PCI: percutaneous coronary intervention, RCT: randomized controlled trial.
4. Study outcomes
4.1. Primary outcome
4.1.1. All-cause mortality RCT and cohort studies
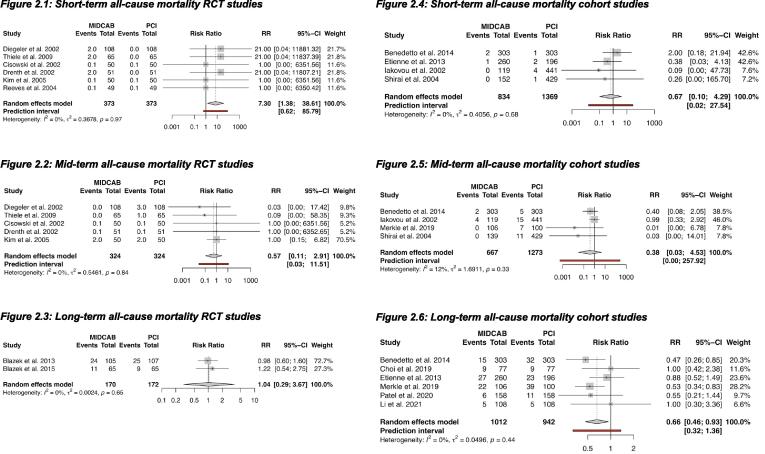
At short-term follow-up, all six RCTs showed a higher all-cause mortality in the MIDCAB group (RR 7.30, 95% CI: 1.38 to 38.61) (NNT = 100) [9], [34], [36], [37], [38], [39]. Five RCTs reported mid-term all-cause mortality and two trials reported long-term all-cause mortality [2], [3], [9], [34], [36], [37], [38], [39] with no difference in these 2 follow-up periods. (Fig. 2).
Fig. 2.
All-cause mortality. RR <1 is in favor of MIDCAB. Legend: Forest plots, short-term, mid-term and long-term all-cause mortality in RCTs and cohort studies. CI: confidence interval, MIDCAB: minimally invasive direct coronary artery bypass, PCI: percutaneous coronary interventions, RCT: randomized controlled trial, RR: risk ratio.
Four cohort studies reported short-term and mid-term all-cause mortality, with no difference between the two intervention groups [26], [28], [29], [31], [33]. At long-term follow-up, a benefit of MIDCAB over PCI was demonstrated (RR 0.66, 95% CI: 0.46 to 0.93) (NNT = 25) (Fig. 2) [26], [27], [28], [30], [31], [32].
5. Secondary outcomes
5.1. Cardiac mortality RCT and cohort studies
Six RCTs showed an increase in cardiac mortality after MIDCAB compared to PCI at short-term follow-up, which was significant (RR 7.30, 95% CI: 1.38 to 38.61) (NNT = 100) [9], [34], [36], [37], [38], [39]. At mid-term and long-term follow-up, no difference in cardiac mortality was found [9], [34], [36], [37], [38].
Short-term and long-term cardiac mortality were reported in two cohort studies [28], [29], [32] but we could not draw any conclusions because of the low numbers of events [2], [3]. No cohort studies reported mid-term cardiac mortality (Appendix 3).
5.2. rTVR RCT and cohort studies
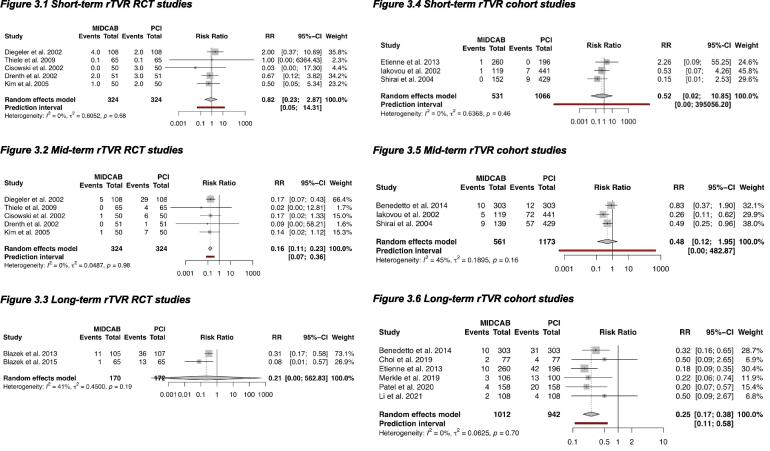
In the RCTs at short-term no difference was found in rTVR between MIDCAB and PCI [9], [34], [36], [37], [38]. At mid-term follow-up a significant benefit of MIDCAB over PCI was demonstrated (RR 0.16, 95% CI: 0.11 to 0.23) (NNT = 10) [9], [34], [36], [37], [38]. At long-term follow-up only two RCTs reported rTVR which resulted in unstable estimates because of the low numbers of events [2], [3].
In the cohort studies at short- and mid-term follow-up no difference was found in rTVR [28], [29], [33]. At long-term follow-up a decreased rTVR in the MIDCAB group over the PCI group was found (RR 0.25, 95% CI: 0.17 to 0.38) (NNT = 13) (Fig. 3) [26], [27], [28], [30], [31], [32].
Fig. 3.
Repeat target vessel revascularization. RR <1 is in favor of MIDCAB. Legend: Forest plots, short-term, mid-term and long-term cardiac mortality in RCTs and cohort studies. CI: confidence interval, MIDCAB: minimally invasive direct coronary artery bypass, PCI: percutaneous coronary interventions, RCT: randomized controlled trial, RR: risk ratio, rTVR: repeat vessel revascularization.
5.3. MI RCT and cohort studies
No difference in MI was found at short-term, mid-term or long-term follow-up in both RCTs and cohort studies. (Appendix 4) [9], [28], [29], [33], [34], [36], [37], [38], [39].
5.4. CVA RCTs and cohort studies
No differences in CVAs was found in either the RCTs or the cohort studies when comparing MIDCAB and PCI (Appendix 5) [26], [27], [28], [29], [30], [33], [37], [38].
5.5. Heterogeneity and bias
Funnel plot asymmetry could be visually suspected in the RCTs for all-cause mortality and cardiac mortality at short-term and mid-term follow-up. Egger’s test also detected possible publication bias for these outcomes. In the cohort group funnel plot asymmetry was found for all-cause mortality at mid-term follow-up and Egger’s test detected publication bias (Appendix 6).
Analysing outcomes using fixed effects models showed no significant difference in pooled effect estimates when compared to the random-effects’ models.
Heterogeneity was considered as I2 > 50% and moderate heterogeneity was present at mid-term follow-up rTVR in the RCT group.
6. Discussion
Our meta-analysis comparing MIDCAB with PCI suggests that for isolated lesions of the LAD, MIDCAB had a higher mortality risk at short-term follow-up. Short-term all-cause mortality was fully driven by cardiac mortality. At long-term follow-up, MIDCAB showed a survival benefit in the cohort studies. In addition, MIDCAB decreased rTVR at mid-term and long-term follow-up. No difference between MIDCAB and PCI in terms of MI and CVA risk was found.
Previous meta-analyses reported MIDCAB and PCI being both effective strategies for isolated LAD stenosis. They have shown similar clinical outcomes, but did not report a survival benefit in the MIDCAB group at long-term follow-up even though a decrease in mortality could be expected based on the proven survival benefit of the LITA-LAD conduit in conventional CABG [4], [40], [41]. We did find differences in mortality between the two treatments. In the RCTs we found at short-term follow-up an increase in all-cause mortality, which was fully driven by cardiac causes, in favor of PCI. Nevertheless, these results showed possible publication bias as assessed by Egger’s test and a wide confidence interval. The increased 30-day mortality after MIDCAB might be driven by the more invasive character of the procedure, the early-stage technique and little experience available at the time of the RCTs.
At long-term follow-up, the cohort studies showed a decreased all-cause mortality for MIDCAB when compared to PCI. This was not present in the RCTs, perhaps because long-term all-cause mortality was reported for only 2 RCTs with a small sample-size. Alternatively the difference may be caused by the moderate selection bias in the cohort studies (Appendix 2). However, an all-cause mortality benefit of conventional CABG over PCI has been confirmed by the SYNTAX trial for patients with a high (≥32) and intermediate (23–32) SYNTAX-score. The presence of the LITA graft has shown to be an independent predictor of survival and contributes significantly to superior long-term survival [10], [12], [13]. The LITA graft produces nitric oxide, inducing a vasodilator response in LAD protecting against atherosclerosis and thereby prevents MI and cardiac death in the long term [43]. Moreover, most patients undergoing MIDCAB have low SYNTAX-score (0–22). Hence, we expect the potential survival benefit of the use of the LITA also in patients receiving MIDCAB. This might explain the long-term survival of MIDCAB in the cohort studies.
In our analysis we confirmed that MIDCAB offers a decreased TVR at mid-term and long-term follow-up. MI and CVA rates were not different between MIDCAB and PCI, even though there was only limited experience with MIDCAB when the RCTs and cohort studies were conducted. We hypothesize that with increasing surgeons’ experience in this technique, fewer complications such as these will occur.
Only a small number of studies reported cardiac mortality, myocardial infarction and CVA. Several meta-analyses reported the incidence of composite outcomes such as Major Adverse Cardiac (and Cerebrovascular) Events (MAC(C)E). We excluded these as an outcome because of the variety of definitions for MAC(C)E used. We recommended the adoption of standard definition to allow adequate comparisons of future results.
The most recent European Guidelines for myocardial revascularization classified both CABG and PCI as class I, level A evidence for the management of proximal LAD disease [1]. However the optimal revascularization strategy for coronary artery disease is under constant debate because of the continuing development of surgical techniques and stent technology. PCI techniques have improved over the last years moving from BMS to third-generation DES. Secondary cardiovascular management changed with the introduction of more effective anti-thrombotic medications for better stent protection [44], [45], [46], [47]. In the past two decades, the adoption of MIDCAB for isolated proximal LAD lesions or in combination with PCI (hybrid coronary revascularization) increased worldwide. Nowadays LITA robotic-assisted harvesting induces minimal tissue damage optimizing the operation quality and reducing complications [42], [48], [49], [50]. Moreover, CABG and PCI differ substantially in revascularization mechanisms. CABG provides alternative vascularization routes addressing existing and future atherosclerotic lesions. PCI, in contrast, treats only existing lesions. Therefore, it has been observed that only CABG increases long-term survival in patients with stable coronary artery disease by providing “surgical collateralization” [51], [52], [53]. Our analysis confirmed long-term survival benefit in the MIDCAB group, though we do not know whether this is the result of lower cardiac mortality or of MI.
We acknowledge that this study has some limitations. Firstly, the number of included patients and the number of events were small across all studies. Secondly, moderate risk of bias was detected in all the cohort studies and possible publication bias was found in the primary outcome at short- and mid-term follow-up. The detected bias in the cohort studies was mainly because of selection and confounding. Selection bias could have distorted the published estimates of the articles. Our stated RR might therefore be over- or underestimated when it comes to the reported outcomes, according to the direction of distortion due to bias. The same applies for publication bias, being a type of selection bias. The possible presence of residual confounding in the included studies could have resulted in the unstable estimates pooled, hence the wide confidence intervals. Thus, our demonstrated short- and long-term outcomes should be interpreted with caution, unlike the more robust outcomes at long-term follow-up. Thirdly, we did not include considerations on LAD anatomy and its potential influence on SYNTAX-scores. Furthermore, MIDCAB is a technically demanding procedure and has therefore a long learning curve. We did not correct for differences between surgeons’ and centres expertise. Finally, because of a limited number of studies, we were not able to differentiate between LITA harvesting techniques or between different stents.
7. Conclusion
We did a meta-analysis of evidence from the past 20 years to compare MIDCAB with PCI in patients with proximal LAD lesions. The RCT data suggested that MIDCAB was associated with a higher short-term mortality, although a level A evidence, these analyses may be limited by possible publication bias. In contrast, in the cohort studies, a level B evidence, MIDCAB appeared to offer a long-term survival benefit. A decreased mid-term rTVR was demonstrated by the RCTs and cohort studies showed a decrease in rTVR rates in the long term. MIDCAB might therefore be considered an adequate first treatment option for proximal isolated LAD disease in selected patients. Multicenter RCTs with long-term follow-up that have adequate statistical power are required to confirm these results and to investigate if increased experience with MIDCAB has reduced the associated short-term mortality.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2022.101046.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Neumann F.-J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 2.Blazek S., Holzhey D., Jungert C., et al. Comparison of bare-metal stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 10-year follow-up of a randomized trial. JACC Cardiovasc Interv. 2013;6(1):20–26. doi: 10.1016/j.jcin.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Blazek S, Rossbach C, Borger MA, et al. Comparison of sirolimus-eluting stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 7-year follow-up of a randomized trial. JACC Cardiovasc. Interv. 2015;8(1 Pt A):30–38. doi:10.1016/j.jcin.2014.08.006. [DOI] [PubMed]
- 4.Aziz O., Rao C., Panesar S.S., et al. Meta-analysis of minimally invasive internal thoracic artery bypass versus percutaneous revascularisation for isolated lesions of the left anterior descending artery. BMJ. 2007;334(7594):617. doi: 10.1136/bmj.39106.476215.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S., Zucker D., Peduzzi P., et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet Lond Engl. 1994;344(8922):563–570. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 6.Dzavik V., Ghali W.A., Norris C., et al. Long-term survival in 11,661 patients with multivessel coronary artery disease in the era of stenting: a report from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Am. Heart J. 2001;142(1):119–126. doi: 10.1067/mhj.2001.116072. [DOI] [PubMed] [Google Scholar]
- 7.Hannan E.L., Zhong Y., Walford G., et al. Coronary Artery Bypass Graft Surgery Versus Drug-Eluting Stents for Patients With Isolated Proximal Left Anterior Descending Disease. J. Am. Coll. Cardiol. 2014;64(25):2717. doi: 10.1016/j.jacc.2014.09.074. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor J.R., Gienger A.L., Ardehali R., et al. Isolated disease of the proximal left anterior descending artery comparing the effectiveness of percutaneous coronary interventions and coronary artery bypass surgery. JACC Cardiovasc. Interv. 2008;1(5):483–491. doi: 10.1016/j.jcin.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Thiele H., Neumann-Schniedewind P., Jacobs S., et al. Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J. Am. Coll. Cardiol. 2009;53(25):2324–2331. doi: 10.1016/j.jacc.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Loop F.D., Lytle B.W., Cosgrove D.M., et al. Influence of the Internal-Mammary-Artery Graft on 10-Year Survival and Other Cardiac Events. N. Engl. J. Med. 1986;314(1):1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 11.Willerson J.T. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. Circulation. 1996;94(6):1194. doi: 10.1161/01.cir.94.6.1193. [DOI] [PubMed] [Google Scholar]
- 12.Serruys P.W., Morice M.-C., Kappetein A.P., et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N. Engl. J. Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 13.BARI investigators. The final 10-year follow-up results from the BARI randomized trial. J. Am. Coll. Cardiol. 2007;49(15):1600–1606. doi:10.1016/j.jacc.2006.11.048. [DOI] [PubMed]
- 14.Mack M.J. Minimally Invasive and Robotic Surgery. JAMA. 2001;285(5):568–572. doi: 10.1001/jama.285.5.568. [DOI] [PubMed] [Google Scholar]
- 15.Diegeler A., Walther T., Metz S., et al. Comparison of MIDCAP versus conventional CABG surgery regarding pain and quality of life. Heart Surg. Forum. 1999;2(4):290–295. discussion 295–296. [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 18.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 20Accessed March 8, 2020. https://training.cochrane.org/handbook/current.
- 20.Friedrich J.O., Adhikari N.K.J., Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med. Res. Method. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S.J., Lim D.-S., Seo H.S., et al. Percutaneous coronary intervention with drug-eluting stent implantation vs. minimally invasive direct coronary artery bypass (MIDCAB) in patients with left anterior descending coronary artery stenosis. Catheter Cardiovasc. Interv. Off J. Soc. Card. Angiogr. Interv. 2005;64(1):75–81. doi: 10.1002/ccd.20238. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Gal Y., Mohr R., Braunstein R., et al. Revascularization of left anterior descending artery with drug-eluting stents: comparison with minimally invasive direct coronary artery bypass surgery. Ann. Thorac. Surg. 2006;82(6):2067–2071. doi: 10.1016/j.athoracsur.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Forouzandeh F., Stahl E., Patel S., et al. Percutaneous Coronary Intervention Versus Robotic-Assisted Coronary Artery Bypass for Left Anterior Descending Artery Chronic Total Occlusion. JACC Cardiovasc Interv. 2018;11(15):1542–1544. doi: 10.1016/j.jcin.2018.01.255. [DOI] [PubMed] [Google Scholar]
- 24.Fraund S., Herrmann G., Witzke A., et al. Midterm follow-up after minimally invasive direct coronary artery bypass grafting versus percutaneous coronary intervention techniques. Ann. Thorac. Surg. 2005;79(4):1225–1231. doi: 10.1016/j.athoracsur.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 25.Diegeler A., Spyrantis N., Matin M., et al. The revival of surgical treatment for isolated proximal high grade LAD lesions by minimally invasive coronary artery bypass grafting. Eur. J. Cardio-Thorac. Surg. Off J. Eur. Assoc. Cardio-Thorac. Surg. 2000;17(5):501–504. doi: 10.1016/s1010-7940(00)00400-0. [DOI] [PubMed] [Google Scholar]
- 26.Benedetto U., Raja S.G., Soliman R.F.B., et al. Minimally invasive direct coronary artery bypass improves late survival compared with drug-eluting stents in isolated proximal left anterior descending artery disease: a 10-year follow-up, single-center, propensity score analysis. J. Thorac. Cardiovasc. Surg. 2014;148(4):1316–1322. doi: 10.1016/j.jtcvs.2013.12.062. [DOI] [PubMed] [Google Scholar]
- 27.Choi W., Chang H.W., Kang S.-H., et al. Comparison of Minimally Invasive Direct Coronary Artery Bypass and Percutaneous Coronary Intervention Using Second-Generation Drug-Eluting Stents for Coronary Artery Disease - Propensity Score-Matched Analysis. Circ J Off J Jpn Circ Soc. 2019;83(7):1572–1580. doi: 10.1253/circj.CJ-18-1330. [DOI] [PubMed] [Google Scholar]
- 28.Etienne PY, D’hoore W, Papadatos S, et al. Five-year follow-up of drug-eluting stents implantation vs minimally invasive direct coronary artery bypass for left anterior descending artery disease: a propensity score analysis. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 2013;44(5):884-890. doi:10.1093/ejcts/ezt137. [DOI] [PubMed]
- 29.Iakovou I., Dangas G., Mehran R., et al. Minimally invasive direct coronary artery bypass (MIDCAB) versus coronary artery stenting for elective revascularization of the left anterior descending artery. Am. J. Cardiol. 2002;90(8):885–887. doi: 10.1016/s0002-9149(02)02715-7. [DOI] [PubMed] [Google Scholar]
- 30.Li S, Zhang H, Xiao C, Wang R, Wu Y. Robotically assisted coronary artery bypass graft surgery versus drug-eluting stents for patients with stable isolated proximal left anterior descending disease. J Card Surg. Published online February 18, 2021. doi:10.1111/jocs.15433. [DOI] [PubMed]
- 31.Merkle J., Zeriouh M., Sabashnikov A., et al. Minimally invasive direct coronary artery bypass graft surgery versus percutaneous coronary intervention of the LAD: costs and long-term outcome. Perfusion. 2019;34(4):323–329. doi: 10.1177/0267659118820771. [DOI] [PubMed] [Google Scholar]
- 32.Patel NC, Hemli JM, Seetharam K, et al. Minimally invasive coronary bypass versus percutaneous coronary intervention for isolated complex stenosis of the left anterior descending coronary artery. J. Thorac. Cardiovasc. Surg. Published online May 29, 2020. doi:10.1016/j.jtcvs.2020.04.171. [DOI] [PubMed]
- 33.Shirai K., Lansky A.J., Mehran R., et al. Minimally invasive coronary artery bypass grafting versus stenting for patients with proximal left anterior descending coronary artery disease. Am. J. Cardiol. 2004;93(8):959–962. doi: 10.1016/j.amjcard.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 34.Diegeler A., Thiele H., Falk V., et al. Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N. Engl. J. Med. 2002;347(8):561–566. doi: 10.1056/NEJMoa013563. [DOI] [PubMed] [Google Scholar]
- 35.Thiele H., Oettel S., Jacobs S., et al. Comparison of bare-metal stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: a 5-year follow-up. Circulation. 2005;112(22):3445–3450. doi: 10.1161/CIRCULATIONAHA.105.578492. [DOI] [PubMed] [Google Scholar]
- 36.Cisowski M., Drzewiecki J., Drzewiecka-Gerber A., et al. Primary stenting versus MIDCAB: preliminary report-comparision of two methods of revascularization in single left anterior descending coronary artery stenosis. Ann. Thorac. Surg. 2002;74(4):S1334–S1339. doi: 10.1016/s0003-4975(02)03971-1. [DOI] [PubMed] [Google Scholar]
- 37.Drenth D.J., Winter J.B., Veeger N.J.G.M., et al. Minimally invasive coronary artery bypass grafting versus percutaneous transluminal coronary angioplasty with stenting in isolated high-grade stenosis of the proximal left anterior descending coronary artery: six months’ angiographic and clinical follow-up of a prospective randomized study. J. Thorac. Cardiovasc. Surg. 2002;124(1):130–135. doi: 10.1067/mtc.2002.122525. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.W., Lim D.S., Sun K., Shim W.J., Rho Y.M. Stenting or MIDCAB using ministernotomy for revascularization of proximal left anterior descending artery? Int. J. Cardiol. 2005;99(3):437–441. doi: 10.1016/j.ijcard.2004.08.045. [DOI] [PubMed] [Google Scholar]
- 39.Reeves B.C., Angelini G.D., Bryan A.J., et al. A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery. Health Technol Assess Winch Engl. 2004;8(16):1–43. doi: 10.3310/hta8160. [DOI] [PubMed] [Google Scholar]
- 40.Kinnaird T., Kwok C.S., Narain A., et al. Meta-Analysis of Percutaneous Coronary Intervention With Drug-Eluting Stent Versus Coronary Artery Bypass Grafting for Isolated Proximal Left Anterior Descending Coronary Disease. Am. J. Cardiol. 2016;118(8):1171–1177. doi: 10.1016/j.amjcard.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 41.Patel A.J., Yates M.T., Soppa G.K.R. What is the optimal revascularization technique for isolated disease of the left anterior descending artery: minimally invasive direct coronary artery bypass or percutaneous coronary intervention? Interact. Cardiovasc. Thorac. Surg. 2014;19(1):144–148. doi: 10.1093/icvts/ivu076. [DOI] [PubMed] [Google Scholar]
- 42.Head S.J., Davierwala P.M., Serruys P.W., et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur. Heart J. 2014;35(40):2821–2830. doi: 10.1093/eurheartj/ehu213. [DOI] [PubMed] [Google Scholar]
- 43.Tarr F.I., Sasvári M., Tarr M., Rácz R. Evidence of nitric oxide produced by the internal mammary artery graft in venous drainage of the recipient coronary artery. Ann. Thorac. Surg. 2005;80(5):1728–1731. doi: 10.1016/j.athoracsur.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Shen L., Hu S., Wang H., et al. One-stop hybrid coronary revascularization versus coronary artery bypass grafting and percutaneous coronary intervention for the treatment of multivessel coronary artery disease: 3-year follow-up results from a single institution. J. Am. Coll. Cardiol. 2013;61(25):2525–2533. doi: 10.1016/j.jacc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Halkos M.E., Vassiliades T.A., Douglas J.S., et al. Hybrid Coronary Revascularization Versus Off-Pump Coronary Artery Bypass Grafting for the Treatment of Multivessel Coronary Artery Disease. Ann. Thorac. Surg. 2011;92(5):1695–1702. doi: 10.1016/j.athoracsur.2011.05.090. [DOI] [PubMed] [Google Scholar]
- 46.Puskas J.D., Williams W.H., Mahoney E.M., et al. Off-pump vs conventional coronary artery bypass grafting: early and 1-year graft patency, cost, and quality-of-life outcomes: a randomized trial. JAMA. 2004;291(15):1841–1849. doi: 10.1001/jama.291.15.1841. [DOI] [PubMed] [Google Scholar]
- 47.Balacumaraswami L., Taggart D.P. Intraoperative imaging techniques to assess coronary artery bypass graft patency. Ann. Thorac. Surg. 2007;83(6):2251–2257. doi: 10.1016/j.athoracsur.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 48.Hammal F., Nagase F., Menon D., Ali I., Nagendran J., Stafinski T. Robot-assisted coronary artery bypass surgery: a systematic review and meta-analysis of comparative studies. Can J. Surg. J. Can. Chir. 2020;63(6):E491–E508. doi: 10.1503/cjs.013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong W., Cai J., Wang Z., et al. Robot-assisted coronary artery bypass grafting improves short-term outcomes compared with minimally invasive direct coronary artery bypass grafting. J Thorac Dis. 2016;8(3):459–468. doi: 10.21037/jtd.2016.02.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groh M.A., Sutherland S.E., Burton H.G., 3rd, Johnson A.M., Ely S.W. Port-access coronary artery bypass grafting: technique and comparative results. Ann. Thorac. Surg. 1999;68(4):1506–1508. doi: 10.1016/s0003-4975(99)00949-2. [DOI] [PubMed] [Google Scholar]
- 51.Doenst T., Haverich A., Serruys P., et al. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019;73(8):964–976. doi: 10.1016/j.jacc.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 52.Palmerini T., Biondi-Zoccai G., Della Riva D., et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet Lond. Engl. 2012;379(9824):1393–1402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 53.Jeon C., Candia S.C., Wang J.C., et al. Relative spatial distributions of coronary artery bypass graft insertion and acute thrombosis: A model for protection from acute myocardial infarction. Am. Heart J. 2010;160(1):195–201. doi: 10.1016/j.ahj.2010.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.