Abstract
A novel facultatively psychrophilic bacterium, strain S-1, which exhibits extraordinarily high catalase activity was isolated from the drain pool of a fish product processing plant that uses H2O2 as a bleaching and microbicidal agent. The catalase activity of the isolate was 1 or 2 orders of magnitude higher than those of Corynebacterium glutamicum, Staphylococcus aureus, Pseudomonas fluorescens, and five other species tested in this study. The strain seemed to possess only one kind of catalase, according to the results of polyacrylamide gel electrophoresis of the cell extract. The optimum temperature for catalase activity was about 30°C, which was about 20°C lower than that for bovine catalase activity. Electron microscopic observation revealed that the surface of the microorganism was covered by blebs. Although the isolate was nonflagellated, its taxonomic position on the basis of physiological and biochemical characteristics and analysis of 16S rRNA sequence and DNA-DNA relatedness data indicated that strain S-1 is a new species belonging to the genus Vibrio. Accordingly, we propose the name Vibrio rumoiensis. The type strain is S-1 (FERM P-14531).
The response of bacteria to oxidative stress has been extensively studied for enteric bacteria (7). Most studies dealt with the response to oxidative stress or to an inducer of oxidative stress (3, 5, 7, 13, 14, 21). The gene regulation systems involved in the response to oxidative stress have also been studied (7). However, those studies investigated relatively short-term responses to oxidative stress in limited microorganisms, such as enteric bacteria. Few studies are available on the mechanisms involved in adaptation to relatively long-term oxidative stress. For example, little is known about microorganisms living in oxidative environments. Thus, we initiated studies to understand how a bacterium adapts to oxidative stress in a highly oxidative environment and why such an adaptable bacterium exists in such an environment. In addition, studies on such microorganisms will give us more opportunities to detect the proteins or genes concerned with oxidative stress. A bacterium exhibiting high catalase activity was isolated from the drain pool of a fishery product processing plant that uses hydrogen peroxide as a bleaching agent (33), and we think that the isolate might be a good candidate for studying the mechanisms of bacterial adaptation to oxidative stress.
Although there are few examples of industrial applications of cold-adapted microorganisms, there may be great potential for such applications. Hydrogen peroxide is used as a bleaching or microbicidal agent in the paper, food, textile, and semiconductor industries. In wastewater treatment in such industries, H2O2 should be removed before activated-sludge treatment, because H2O2 damages microorganisms present in the treatment system. If a cold-adapted bacterium that decomposes H2O2 effectively could be applied, industrial wastewater could be treated even at low temperatures. The procedure will be useful for low-energy wastewater treatment, especially in countries with cold climates. Thus, another reason for the study of such microorganisms is their possible industrial applications at low temperatures.
In the present study, we determined the taxonomic position of the isolate that exhibits high catalase activity, compared the catalase activities of several kinds of bacteria, and analyzed the catalase activity in a cell extract of the isolate.
MATERIALS AND METHODS
Bacterial strains and cultivation.
The strain that we examined was strain S-1 (33). The organism was cultivated aerobically until the late logarithmic growth phase at 27°C in PYS-2 medium (pH 7.5), unless otherwise stated. The PYS-2 medium contained (per liter of deionized water) 8.0 g of polypeptone (Nihon Pharmaceuticals, Tokyo, Japan), 3.0 g of yeast extract (Kyokuto, Tokyo, Japan), 5.0 g of NaCl, and 15 g of agar (when necessary). For the comparative study of catalase activity, Alcaligenes faecalis (laboratory strain), Corynebacterium glutamicum IAM 12432, Staphylococcus aureus IAM 12544T, Aeromonas hydrophila subsp. hydrophila JCM 1027T, Escherichia coli IAM 1264, Pseudomonas fluorescens JCM 5963T, Bacillus subtilis IAM 1026, and Vibrio parahaemolyticus JCM 2147 were used. These microorganisms were also grown in PYS-2 medium until the stationary growth phase. For DNA-DNA hybridization, Vibrio halioticoli IAM 14596T, Vibrio alginolyticus V477 (a kind gift from the Tokyo Metropolitan Research Laboratory of Public Health), Vibrio pelagius ATCC 25916T, Vibrio campbellii ATCC 25926T, Vibrio fischeri ATCC 7744T, Vibrio splendidus ATCC 33125T, V. parahaemolyticus JCM 2147, Vibrio harveyi NCMB 1280T, Photobacterium leiognathi NCMB 391, and Photobacterium phosphoreum IAM 12085 were used. These microorganisms were grown in ZoBell 2216E broth (26) without FeSO4 · 7H2O.
Phenotypic characterization of strain S-1.
For phenotypic characterization, PYS-2 medium was used as the basal medium, the culture was incubated at 27°C for 2 weeks unless otherwise stated, and the experiment was performed more than twice. Morphological, physiological, and biochemical tests were performed as described previously (2). Carbohydrate metabolism was tested by the method of Leifson (22). The results were checked daily until 2 weeks after inoculation. Alginase activity was determined by 10 days of culture on an agar plate containing alginic acid and overlaid with ethanol. Sensitivity to the vibriostatic compound O/129 (2,4-diamino-6,7-di-iso-propylpteridine phosphate) was determined after agar plate culture for 1 week by using diagnostic discs (10 and 150 μg) (Oxoid Ltd., Basingstoke, Hampshire, England). Determination of the utilization of the substrate as the sole carbon and energy source was performed in US medium (pH 7.5) containing 1% substrate, 2 g of NH4Cl, 2 g of Na2HPO4, 1 g of KH2PO4, 0.1 g of MgSO4 · 7H2O, 0.05 g of CaCl2 · 2H2O, and 1 ml of trace minerals. The trace minerals included (per 100 ml) 1.8 g of EDTA · 2Na, 5.0 g of ZnSO4 · 7H2O, 5.0 g of FeSO4 · 7H2O, 1.5 g of MnSO4 · 4H2O, 0.4 g of CuSO4 · 5H2O, 0.25 g of Co(NO3)2 · 6H2O, and 0.1 g of H3BO3.
Electron microscopy.
Cells grown on PYS-2 agar slants were suspended in physiological saline. A small drop of the suspension was placed on a carbon-coated copper grid and negatively stained with 1% phosphowolframic acid for observation with a transmission electron microscope (Hitachi H-800).
16S rRNA sequencing.
The 16S rRNA gene was amplified by PCR. The sequences of the primers used for amplification were 5′-AGAGTTTGATCCTGGCT-3′ and 5′-AAGGAGGTGATCCAGCCGCA-3′, corresponding to positions 8 to 24 and 1521 to 1540, respectively, in the 16S rRNA sequence of E. coli (4). The 1.5-kb PCR product (positions 29 to 1520 in the 16S rRNA sequence of E. coli) was directly sequenced by the dideoxynucleotide chain termination method with a DNA sequencer (model 377; Applied Biosystems, Inc.). Multiple alignments of the sequence were performed, nucleotide substitution rates (Knuc values) were calculated, and a neighbor-joining phylogenetic tree (18, 28) was constructed by using the CLUSTAL W program (31) with the determined 1494-bp sequence. The similarity values of the sequences were calculated by using the GENETYX computer program (Software Development Co., Ltd., Tokyo, Japan).
Reference sequences.
The accession numbers for the sequences used as references are as follows: Salinivibrio costicola ATCC 35508T, X74699; Photobacterium angustum ATCC 25915T, X74685; P. leiognathi ATCC 25521T, X74686; P. phosphoreum ATCC 11040T, X74687; V. fischeri ATCC 7744T, X74702; Vibrio logei ATCC 15832, X74708; Vibrio cholerae ATCC 14035T, X74695; Vibrio vulnificus ATCC 27562T, X76333; V. splendidus ATCC 33125T, X74724; Vibrio anguillarum ATCC 43313, X74719; Vibrio fluvialis ATCC 33809T, X74703; Vibrio orientalis ATCC 33934T, X74714; Vibrio nereis ATCC 25917T, X74716; Vibrio proteolyticus ATCC 15338T, X74723; V. pelagius ATCC 25916T, X74722; V. campbellii ATCC 25920T, X74692; V. parahaemolyticus CIP 73.30, X74721; V. alginolyticus ATCC 17749T, 74706; V. harveyi ATCC 14126T, X74706; Vibrio cincinnatiensis ATCC 35912T, X74698; Vibrio gazogenes ATCC 29988T, X74705; and V. halioticoli IAM 14596T, AB000390.
DNA base composition and DNA-DNA hybridization.
DNA was prepared from bacterial cells by the method of Marmur (25). The G+C content of the DNA was determined by the method of Tamaoka and Komagata (30). The prepared DNA was digested with nuclease P1 (Yamasa Shoyu, Choshi, Japan). The resulting nucleotides were analyzed by high-pressure liquid chromatography (HPLC) with a 4.6- by 250-mm Inertsil C4 column (GL Science) at room temperature. The HPLC system used consisted of a solvent delivery pump (model CCPM-II; Tosoh) and a UV spectrophotometer as the detector (model UV-8020; Tosoh) at 235 nm. An equimolar mixture of four deoxyribonucleotides (Yamasa Shoyu) was used as the standard.
Levels of DNA-DNA relatedness were determined fluorometrically by the method of Ezaki et al. (6) with photobiotin-labeled DNA probes and microplates.
Growth conditions and preparation of cell extracts.
Cells for catalase activity determination were incubated in a 2,000-ml flask containing 800 ml of PYS-2 broth medium, which was set on a rotary shaker (100 rpm · min−1) and maintained at 27°C for 48 h. For comparison, cells of other strains were also incubated under the same conditions. A cell extract was obtained as follows. The cells were harvested by centrifugation at 10,000 × g for 15 min and suspended in a buffer containing 20 mM MgSO4 and 50 mM Tris-HCl, pH 8.0 (buffer A). The cells were disrupted by passage through a French pressure cell (SLM-AMINCO) at 20,000 lb/in2 at 4°C. The suspension was then centrifuged at 10,000 × g for 15 min to remove unbroken cells. The resulting supernatant was used as a cell extract. The protein content was determined by the method of Lowry et al. (24), with bovine serum albumin as a standard.
Enzyme assay conditions.
Catalase activity was measured spectrophotometrically by monitoring the decrease in absorbance at 240 nm caused by the disappearance of H2O2, using a Hitachi U-3210 spectrophotometer. The ɛ value at 240 nm for H2O2 was assumed to be 43.6 M−1 cm−1 (16). The standard reaction mixture for the assay contained 50 mM potassium phosphate buffer (pH 7.0), 30 mM H2O2, and 3 μl of a catalase-containing solution, in a total volume of 1.0 ml. The amount of enzyme activity that decomposed 1 μmol of H2O2 per min was defined as 1 U of activity. Enzyme activity was estimated more than four times for each sample, using at least two independent samples.
Catalase activity staining.
The cell extract was centrifuged at 105,000 × g for 1 h. The resulting supernatant was separated by native gel electrophoresis with a 12.5% polyacrylamide gel according to the method of Laemmli (20). Staining for catalase activity was performed as follows (12): the electrophoresed gel was soaked in 50 mM potassium phosphate buffer (pH 7.4) containing 1 mg of 3,3-diaminobenzidine tetrachloride per ml and 10 U of horseradish peroxidase per ml for 45 min in the dark, and then 30% H2O2 was added to the reaction mixture.
Nucleotide sequence accession number.
The nucleotide sequence data determined in this study have been deposited in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB013297.
RESULTS
Morphology.
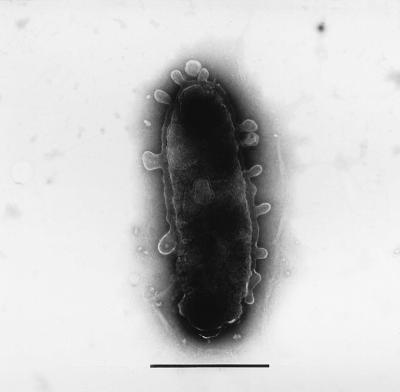
After incubation at 27°C on PYS-2 agar medium, colonies of strain S-1T were circular and colorless, and cells appeared as nonpigmented, nonflagellated rods 0.5 to 0.9 by 0.7 to 2.1 μm in size. Spore formation was absent, and Gram staining was negative. In electron microscopic analysis, numerous blebs were observed on the cell surface (Fig. 1). Microscopic observations of other strains (controls) were also performed. However, there were no blebs on the cell surfaces of the other strains.
FIG. 1.
Electron micrograph of a negatively stained cell of V. rumoiensis S-1T, showing blebs on the cell surface. Bar, 1 μm.
Cultural characteristics.
The microorganism can grow at temperatures ranging from 2 to 34°C, with an optimal temperature of around 30°C (data not shown). The growth rate under optimal conditions gave a doubling time of about 100 min (data not shown).
Phenotypic characteristics.
Strain S-1T exhibited the following physiological and biochemical characteristics. It was positive for oxidase and catalase. It fermented d-glucose, l-arabinose, d-fructose, d-maltose, d-mannose, sucrose, d-xylose, d-mannitol, and d-galactose but not myo-inositol and l-rhamnose. No growth was observed in the absence of NaCl in the culture medium; in contrast, prolific growth was observed in the medium supplemented with 3, 4, or 6% NaCl. Susceptibility to vibriostatic compound O/129 (10 and 150 μg) was observed. The strain was positive for methyl red, citrate utilization, and reduction of NO3 to NO2 but negative for the Voges-Proskauer test, arginine dihydrolase, and indole and H2S production. It hydrolyzed chitin, starch, DNA, and Tweens 20, 40, 60, and 80 but not casein, gelatin, or alginic acid. The strain utilized l-arabinose, d-fructose, d-glucose, glycerol, lactose, and d-gluconate as the sole carbon and energy source for growth but not melibiose, raffinose, and d-sorbitol.
DNA base composition.
The G+C content of strain S-1T was 43.2%.
16S rRNA sequence analysis.
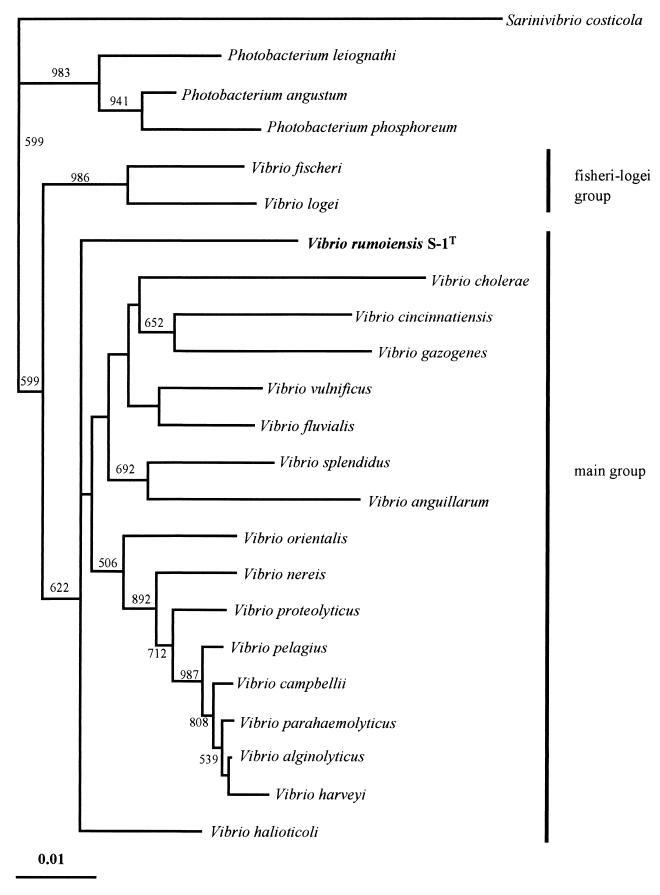
The almost-complete 16S rRNA sequence of strain S-1T, which consists of 1,494 nucleotides, was found to have 92.4 to 95.5% similarity to the 16S rRNA sequences of Vibrio and Photobacterium strains. In contrast, its similarity to the 16S rRNA sequences of Pseudoalteromonas, Shewanella, Moritella, Alteromonas, Escherichia, Pasteurella, Aeromonas, and Colwellia strains was determined to be 83.6 to 90.1%. A phylogenetic tree constructed by the neighbor-joining method showed that strain S-1T was part of the cluster of the genus Vibrio. However, strain S-1T existed as an independent branch between the V. fischeri-V. logei group and the main Vibrio group (Fig. 2). Strain S-1T showed similarities of 89.5, 93.0 to 94.3, 93.3 to 93.8, and 92.4 to 95.5% to S. costicola, Photobacterium strains, the V. fischeri-V. logei group, and the main Vibrio group (27), respectively. Recently, a nonmotile Vibrio strain, V. halioticoli, was isolated from abalone gut. Strain S-1T showed a similarity of 94.8% to V. halioticoli.
FIG. 2.
Phylogenetic tree of V. rumoiensis S-1T, other Vibrio strains, and other related strains derived from 16S rRNA sequence data, using the neighbor-joining method for calculation. Numbers indicate bootstrap values of greater than 500. Bar, 0.01 Knuc unit.
DNA-DNA hybridization.
According to 16S rRNA sequence analysis, strain S-1T is closely related to the genus Vibrio. The levels of DNA-DNA relatedness were estimated by using strain S-1T and representative strains from the genus Photobacterium, the V. fischeri-V. logei group, and the main Vibrio group (27). The levels of DNA-DNA relatedness between strain S-1T and type or reference strains of the 10 species tested were significantly low (Table 1). Although the 16S rRNA sequence similarity between strain S-1T and V. campbellii was 95.5%, which was the highest similarity value among the strains shown in Fig. 2, the level of DNA-DNA relatedness was only 8.2%.
TABLE 1.
Levels of relatedness of V. rumoiensis S-1T DNA and DNAs of other Vibrio strains and related strains
| Strain | % Homology |
|---|---|
| V. rumoiensis S-1T | 100 |
| V. halioticoli IAM 14596T | 3.2 |
| V. alginolyticus V477 | 8.9 |
| V. pelagius biovar I ATCC 25916T | 4.0 |
| V. campbellii biovar I ATCC 25920T | 8.2 |
| V. fischeri ATCC 7744T | 8.2 |
| V. splendidus biovar I ATCC 33125T | 7.1 |
| V. parahaemolyticus JCM 2147 | 9.0 |
| V. harveyi NCMB 1280T | 8.8 |
| P. leiognathi NCMB 391 | 5.8 |
| P. phosphoreum IAM 12085 | 6.4 |
Characterization of catalase activity.
To compare the catalase activity of strain S-1T with those of other strains, we estimated the catalase activities in cell extracts of bacterial strains that were incubated under the culture conditions described in Materials and Methods. It was found that the catalase activity of strain S-1T was 1 or 2 orders of magnitude higher than those of the other tested strains (Table 2).
TABLE 2.
Catalase activities in cell extracts of V. rumoiensis S-1T and other strainsa
| Strain or species | Catalase activity (U/mg of protein)b |
|---|---|
| V. rumoiensis S-1T | 4,092.4 ± 408.2 |
| A. faecalis | 500.7 ± 121.2 |
| C. glutamicum | 368.8 ± 46.2 |
| S. aureus | 347.1 ± 85.0 |
| A. hydrophila | 144.9 ± 8.3 |
| E. coli | 65.8 ± 7.4 |
| P. fluorescens | 44.5 ± 25.0 |
| B. subtilis | 16.4 ± 1.1 |
| V. parahaemolyticus | 14.2 ± 0.8 |
The catalase activities in cell extracts prepared from cells obtained under the same culture conditions were assayed. The catalase activity was measured as described in the text.
Results are averages and standard deviations from at least nine experiments with at least two independent preparations of cell extracts. Statistical analysis was done by Student’s t test at P = 0.05.
To determine whether the catalase of strain S-1T is an intracellular or an extracellular enzyme, we estimated the catalase activity of strain S-1T in both culture medium and cell extract. Only 6 U/mg of protein was detected in the 29-h culture medium. Based on this value, it was determined that the total amount of extracellular catalase activity is 1.8% of the sum of the total intracellular and extracellular catalase activities (data not shown).
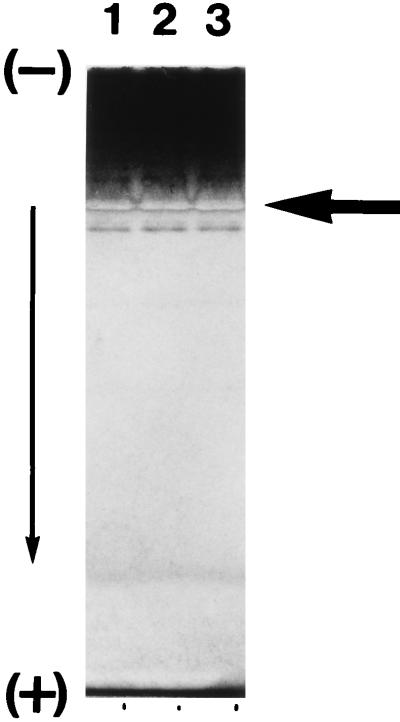
It was reported that several kinds of catalase exist in cells of several microorganisms and that they are induced in different fashions (7, 10, 15, 17, 19, 23). E. coli, for example, has two kinds of catalase, hydroperoxidase I (HP I) and HP II, which are encoded by two separate genes, katG and katE, respectively. HP I exists in the periplasmic space, and its level increases gradually to about twofold that of HP II during the logarithmic growth phase but ceases to increase during the stationary phase. On the other hand, HP II exists in the cytoplasmic space, and its level, which is initially lower than that of HP I, increases 10-fold during growth to the stationary phase (7, 23). Therefore, we investigated whether strain S-1T has more than one kind of catalase. To this end, the cell extract was ultracentrifuged, and the supernatant was subjected to polyacrylamide gel electrophoresis. The results showed only one band representing catalase activity (Fig. 3). During the first purification step, anion-exchange chromatography, in which a crude soluble fraction was loaded into the column, only one peak representing catalase was detected (data not shown).
FIG. 3.
Identification of catalase activity of V. rumoiensis S-1T at different growth phases. The arrow indicates staining parts of catalase activities. Cell extracts were obtained from cells grown for 24 h (late exponential growth phase) (lane 1), 48 h (mid-stationary phase) (lane 2), or 72 h (late stationary phase) (lane 3).
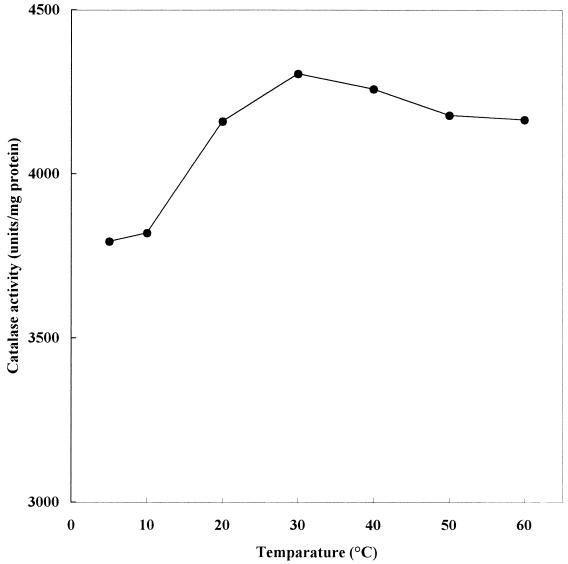
The temperature dependence of the catalase activity of the cell extract, which was obtained from cells grown at 27°C, was estimated and is shown in Fig. 4. Compared with that of most enzymes, the temperature dependence of the catalase activity was not great. In the case of bovine liver catalase also, the activity was weakly dependent on the temperature (data not shown). Recently, a thermostable catalase from the culture broth of the thermophilic fungus Thermoascus aurantiacus was purified and characterized. The catalase activity of the fungus was weakly dependent on temperature (32). From the facts described above, it is considered that the weak temperature dependence of the activity is a common feature among the catalases. We estimated that the optimum temperature for enzyme activity was about 30°C. The stability of strain S-1T catalase activity was examined by incubating a cell extract at a predetermined temperature for 15 min or 1 h (data not shown). Catalase activity was stable at a 40°C; however, it was almost completely eliminated at 60°C (in the case of bovine catalase, activity was almost completely eliminated at 65°C). After incubation at 50°C for 1 h, the remaining catalase activity of strain S-1T was only 29.2% whereas the remaining bovine Aspergillus niger, and T. aurantiacus catalase activities were 80, 100, and 100%, respectively (32).
FIG. 4.
Catalase activities in cell extracts of V. rumoiensis S-1T at different temperatures. The microorganism was grown at 27°C.
DISCUSSION
Strain S-1T was isolated from the drain pool of a fish product processing plant that uses H2O2 as a bleaching agent, and its preliminary characteristics were studied (33). In the present study, we attempted to identify the microorganism to the species level and to characterize its crude catalase in the cell extract.
Electron microscopic observation revealed the presence of blebs on the cell surface. Similar membrane structures were also reported for other gram-positive and gram-negative bacteria (1, 8, 9, 11). Although the function of the blebs of strain S-1T is not known, these structures could be associated with its ability to grow in an H2O2-containing environment. For example, the surface vesicles of strain S-1T may contain hydrogen peroxide-decomposing enzymes such as catalase and peroxidase.
Based on our phenotypic and phylogenetic characterization, strain S-1T was identified as a member of the genus Vibrio, although it has no flagella. Recently, a nonflagellated Vibrio strain, Vibrio halioticoli, was isolated from the gut of abalone (29). Strain S-1T was isolated from the drain pool of a herring egg processing plant. It requires NaCl for growth and is able to decompose chitin. Therefore, it is considered that the origin of strain S-1T is probably the intestine of herring. Although at present we know little about the intestinal microflora of marine organisms, other unknown nonflagellated Vibrio strains may exist in the intestines of marine organisms. Interestingly, the phylogenetic 16S rRNA sequence analysis demonstrated that strain S-1T occupies a distinct position, similar to the case for nonmotile V. halioticoli. However, their phylogenetic positions differed, and the level of DNA homology between strain S-1T and V. halioticoli was only 3.2%. Although the phylogenetic positions of these nonmotile strains are unique, bootstrap analysis indicates that they are in the main Vibrio group. In conclusion, on the basis of phenotypic characteristics, phylogenetic analysis, and DNA-DNA hybridization experiments, strain S-1T is confirmed to be a new species, and the name Vibrio rumoiensis is proposed.
In our previous study, we compared the catalase activity of strain S-1T with those of E. coli, B. subtilis, V. parahaemolyticus, and Micrococcus luteus and found that the catalase activity of strain S-1T was as high as that of M. luteus, which is well known for its high catalase activity, and 1 or 2 orders of magnitude higher than those of E. coli, B. subtilis, and V. parahaemolyticus (33). In the present experiments, for a more comprehensive comparative study, we added five species for comparison and performed experiments on a total of eight reference species. Our results confirm that the catalase activity of strain S-1T is much higher than those of other bacterial species.
The optimum temperature for strain S-1T catalase activity in the cell extract was around 30°C (Fig. 4), which was 20°C lower than that for the activity of purified catalase from bovine liver (data not shown). In the case of T. aurantiacus, the optimum temperature for catalase activity was 70°C. From comparison of optimum temperatures for catalases from another origins, it is considered that the catalase of strain S-1T is more adaptable to cold environments than other known catalases.
Description of Vibrio rumoiensis sp. nov.
Vibrio rumoiensis (ru.moi.en′sis. L. adj. rumoiensis, from Rumoi, the place where the microorganism was isolated). Cells are rod shaped (0.5 to 0.9 by 0.7 to 2.1 μm), gram negative, and nonflagellated, and numerous blebs exist on the cell surface. Colonies are white. Catalase and oxidase reactions are positive. The organism ferments d-glucose, l-arabinose, d-fructose, d-maltose, d-mannose, sucrose, d-xylose, and d-mannitol. No growth is observed in the absence of NaCl in the culture medium; however, growth is prolific in medium supplemented with 3, 4, or 6% NaCl. Susceptibility to vibriostatic compound O/129 (10 and 150 μg) is observed. Growth occurs between 2 and 34°C. The organism is positive for methyl red, citrate utilization, and reduction of NO3 to NO2 but negative for the Voges-Proskauer test, arginine dihydrolase, and indole and H2S production. It hydrolyzes chitin, starch, DNA, and Tweens 20, 40, 60, and 80 but not casein, gelatin, or alginic acid. The organism utilizes l-arabinose, d-fructose, d-glucose, glycerol, lactose, and d-gluconate as the sole carbon and energy source for growth but not melibiose, raffinose, and d-sorbitol. The G+C content of the DNA is 43.2 mol% (determined by HPLC). The type strain V. rumoiensis S-1 has been deposited in the Patent Microorganism Depository, National Institute of Bioscience and Human Technology (Tsukuba, Japan), as strain FERM P-14531.
ACKNOWLEDGMENT
We thank Y. Nodasaka (Hokkaido University) for his help with electron microscopy.
REFERENCES
- 1.Antranikian G, Herzberg C, Mayer F, Gottschalk G. Changes in the cell envelope structure of Clostridium sp. strain EM1 during massive production of α-amylase and pullulanase. FEMS Microbiol Lett. 1987;41:193–197. [Google Scholar]
- 2.Barrow G L, Feltham R K A. Cowan and Steel’s manual for the identification of medical bacteria. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 3.Bishai W R, Smith H O, Barcak G J. A peroxide/ascorbate-inducible catalase from Haemophilus influenzae is homologous to the Escherichia coli katE gene product. J Bacteriol. 1994;176:2914–2921. doi: 10.1128/jb.176.10.2914-2921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosius J, Palmer J L, Kennedy J P, Noller H F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown S M, Howell M L, Vasil M L, Anderson A J, Hassett D J. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J Bacteriol. 1995;177:6537–6544. doi: 10.1128/jb.177.22.6536-6544.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezaki T, Hashimoto Y, Yabuuchi E. Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution well as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int J Syst Bacteriol. 1989;39:224–229. [Google Scholar]
- 7.Farr S B, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsberg C W, Beveridge T J, Hellstrom A. Cellulase and xylanase release from Bacteroides succinogenes and its importance in the rumen environment. Appl Environ Microbiol. 1981;42:886–896. doi: 10.1128/aem.42.5.886-896.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauthire M J, Lafay B, Christen R, Fernandez L, Acquaviva M, Bonin P, Bertrand J-C. Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. Int J Syst Bacteriol. 1992;42:568–576. doi: 10.1099/00207713-42-4-568. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg I, Hochman A. Three different types of catalases in Klebsiella pneumoniae. Arch Biochem Biophys. 1989;268:124–128. doi: 10.1016/0003-9861(89)90572-9. [DOI] [PubMed] [Google Scholar]
- 11.González J M, Mayer F, Moran M A, Hodoson R E, Whitman W B. Microbulbifer hydrolyticus gen. nov., sp. nov., and Marinobacterium georgiense gen. nov., sp. nov., two marine bacteria from a lignin-rich pulp mill waste enrichment community. Int J Syst Bacteriol. 1997;47:369–376. doi: 10.1099/00207713-47-2-369. [DOI] [PubMed] [Google Scholar]
- 12.Gregory E M, Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1978;58:57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- 13.Hartford O M, Dowds B C A. Isolation and characterization of a hydrogen peroxide resistant strain of Bacillus subtilis. Microbiology. 1994;140:297–304. doi: 10.1099/13500872-140-2-297. [DOI] [PubMed] [Google Scholar]
- 14.Hérouart D, Sigaud S, Moreau S, Frendo P, Touati D, Puppo A. Cloning and characterization of the katA gene of Rhizobium meliloti encoding a hydrogen peroxide-inducible catalase. J Bacteriol. 1996;178:6802–6809. doi: 10.1128/jb.178.23.6802-6809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks D B. Purification of three catalase isozymes from facultatively alkaliphilic Bacillus firmus OF4. Biochim Biophys Acta. 1995;1299:347–355. doi: 10.1016/0005-2728(95)00016-c. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrandt A G, Roots I. Reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent formation and breakdown of hydrogen peroxide during mixed function oxidation reaction in liver microsomes. Arch Biochem Biophys. 1975;171:385–397. doi: 10.1016/0003-9861(75)90047-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Lee J S, Hah Y C, Roe J H. Characterization of the major catalase from Streptomyces coelicolor ATCC 10147. Microbiology. 1994;140:3391–3397. doi: 10.1099/13500872-140-12-3391. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M. A simple method for estimating evolutionary rates of base substitution through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 19.Klots M G, Hutcheson S W. Multiple periplasmic catalases in phytopathogenic strains of Pseudomonas syringae. Appl Environ Microbiol. 1992;58:2468–2473. doi: 10.1128/aem.58.8.2468-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee J S, Hah Y C, Roe J H. The induction of oxidative enzymes in Streptomyces coelicolor upon hydrogen peroxide treatment. J Gen Microbiol. 1993;139:1013–1018. [Google Scholar]
- 22.Leifson E. Determination of carbohydrate metabolism of marine bacteria. J Bacteriol. 1963;85:1183–1184. doi: 10.1128/jb.85.5.1183-1184.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewen P C, Switala J, Triggs-Raine B L. Catalases HPI and HPII of Escherichia coli are induced independently. Arch Biochem Biophys. 1985;243:144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Marmur J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 26.Oppenheimer C H, ZoBell C E. The growth and viability of sixty-three species of marine bacteria as influenced by hydrostatic pressure. J Mar Res. 1952;11:10–18. [Google Scholar]
- 27.Ruimy R, Breittmayer V, Elbaze P, Lafay B, Boussemart O, Gauthier M, Christen R. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int J Syst Bacteriol. 1994;44:416–426. doi: 10.1099/00207713-44-3-416. [DOI] [PubMed] [Google Scholar]
- 28.Satiou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 29.Sawabe T, Sugimura I, Ohtsuka M, Nakano K, Tajima K, Ezura Y, Christen R. Vibrio halioticoli sp. nov., a nonmotile alginolytic marine bacterium isolated from gut of abalone Haliotis diacus hannai. Int J Syst Bacteriol. 1998;48:573–580. doi: 10.1099/00207713-48-2-573. [DOI] [PubMed] [Google Scholar]
- 30.Tamaoka J, Komagata K. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol Lett. 1984;25:125–128. [Google Scholar]
- 31.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence weighing, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;76:4350–4354. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, H., Y. Tokusige, H. Shinoyama, T. Fujii, and T. Urakami. Purification and characterization of thermostable catalase from culture broth of Thermoascus aurantiacus. J. Ferment. Bioeng. 85:169–173.
- 33.Yumoto I, Yamazaki K, Kawasaki K, Ichise N, Morita N, Hoshino T, Okuyama H. Isolation of Vibrio sp. S-1 exhibiting extraordinarily high catalase activity. J Ferment Bioeng. 1998;85:113–116. [Google Scholar]