Abstract
This review compares the main brain abnormalities in schizophrenia (SZ), bipolar disorder (BD), major depressive disorder (MDD), and 22q11.2 Deletion Syndrome (22q11DS) determined by ENIGMA (Enhancing Neuro Imaging Genetics through Meta Analysis) consortium investigations. We obtained ranked effect sizes for subcortical volumes, regional cortical thickness, cortical surface area, and diffusion tensor imaging abnormalities, comparing each of these disorders relative to healthy controls. In addition, the studies report on significant associations between brain imaging metrics and disorder-related factors such as symptom severity and treatments. Visual comparison of effect size profiles shows that effect sizes are generally in the same direction and scale in severity with the disorders (in the order SZ>BD>MDD). The effect sizes for 22q11DS, a rare genetic syndrome that increases the risk for psychiatric disorders, appear to be much larger than for either of the complex psychiatric disorders. This is consistent with the idea of generally larger effects on the brain of rare compared to common genetic variants. Cortical thickness and surface area effect sizes for 22q11DS with psychosis compared to 22q11DS without psychosis are more similar to those of SZ and BD than those of MDD; a pattern not observed for subcortical brain structures and fractional anisotropy effect sizes. The observed similarities in effect size profiles for cortical measures across the psychiatric disorders mimic those observed for shared genetic variance between these disorders reported based on family and genetic studies and are consistent with shared genetic risk for SZ and BD and structural brain phenotypes.
Keywords: ENIGMA, Schizophrenia, Bipolar Disorder, Major Depressive Disorder, Velocardiofacial, 22q, meta-analysis, mega-analysis, subcortical, thickness, surface area, fractional anisotropy
Introduction
Neuroimaging studies have been subject to scrutiny with regard to reproducibility, and numerous publications have described a crisis in replicable findings in psychiatry and neuroscience1–5. Differences in samples (e.g,. inclusion/exclusion criteria), data acquisition (e.g., scanner hardware, field strength, sequence etc.), and image analysis methods (e.g., segmentation protocols) across studies and small sample sizes can result in false-positive and false-negative findings in neuroimaging research6. Collaborative, large-scale, coordinated data analyses offer an approach to tackle the crisis in reproducibility and increase the robustness of findings based on already collected data6. A key to the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) consortium’s approach is to conduct meta- and mega-analyses, that harmonize image analysis, quality assurance, and statistical analyses procedures across contributing samples, mitigating errors and biases produced by individual studies. This approach maximizes statistical power by pooling data through collaborative analysis efforts. Development and sharing of high-quality image analysis pipelines, by ENIGMA (http://enigma.ini.usc.edu/protocols) and others7,8, that can be used by the research community also greatly facilitates replicable findings. Complementary approaches include conducting well-powered, large-scale prospective studies; methods employed by projects such as the ABCD study9,10 and UK Biobank (UKB)11–13. All these efforts are enhanced by the development of tools and data warehouses that enable open access data sharing to promote reproducible neuroimaging science (e.g., www.repronim.org, nidm.nidash.org, nda.nih.gov, etc.)14–17.
The ENIGMA project started in 2009 with the initial aim of aggregating brain imaging and genetic data across research centers in order to identify the predominantly small effects of common genetic variations in the brain. ENIGMA’s first imaging genetics publication yielded the first genome-wide significant genetic loci associated with hippocampal and intracranial volumes based on data obtained from 21,151 and 15,782 individuals, in collaboration with the CHARGE (Cohorts of Heart and Aging Research in Genomic Epidemiology) consortium18. A subsequent study, examining subcortical brain structures, identified genome-wide significant loci associated with putamen and caudate volumes in data obtained from 30,717 individuals19. A top-up study analysis of hippocampal volumes, in collaboration with the CHARGE consortium, identified 6 genome-wide significant loci, based on data obtained from 33,536 individuals19. A subsequent analysis of the seven subcortical structures (excluding the hippocampus), combining ENIGMA, CHARGE and also UKB data, found 48 loci (40 novel)20. More recent analyses, based on merging ENIGMA-CHARGE-UKB samples (N>55,000 & N>66,000) identified more than 400 and 553 genome-wide significant genetic loci for subcortical structures, respectively21,22.
Finally, ENIGMA’s most recent imaging genetics study identified 175 genome-wide significant loci associated with cortical surface area and 46 loci associated with cortical thickness23 based on data from 33,992 individuals along with a replication sample of 14,792 individuals24. Together, these studies exemplify a success story of bringing together scientists globally to answer questions about the brain that are difficult, if not impossible, to address by any single research group in the world. Similar to the case-control GWAS (genome-wide association study) findings reported by the Psychiatric Genomics Consortium25,26, ENIGMA is generating imaging-genetics findings that replicate previously identified genetic loci and yield new loci with ongoing increases in sample size18,24.
In 2014, the ENIGMA project was funded by a National Institutes of Health (NIH) Big Data to Knowledge (BD2K) award and grew to include 12 methods Development Working groups and 9 disorder Working Groups. The objectives of the disorder Working Groups were to compare findings across disorders, rank the magnitude of case-control (disorder-related) effect sizes of brain measures, and investigate factors that moderate these effect sizes. The collaboration has produced some of the largest neuroimaging studies in psychiatric disorders to date, and has grown to include more than 50 Working Groups and more than 2,000 researchers from more than 400 institutions from over 45 countries across the globe6.
The ENIGMA consortium has produced several reviews detailing its progress6,27–37. This review summarizes the main findings from the schizophrenia, bipolar disorder, major depressive disorder, and 22q11DS Working Groups published to date and performs some initial cross-disorder comparisons based on the published effect sizes. More formal, direct comparisons based on cross-disorder mega-analyses are underway, and will enable the determination of disorder-related and disorder-specific brain abnormalities to advance our understanding of psychiatric disorder pathogenesis and physiology.
The ENIGMA Schizophrenia (SZ), Bipolar Disorder (BD), Major Depressive Disorder (MDD) and 22q11DS Working Groups coordinate analyses using harmonized image analysis, quality assurance, and statistical analysis procedures. The T1-weighted image analyses have examined subcortical volumes as well as both global and regional measures of cortical thickness and surface area obtained from Desikan-Killiany atlas regions of interest (ROIs)38. ENIGMA opted to study cortical thickness and surface area, rather than cortical volume, which is their product, given that they are thought to be influenced by separate sets of genes39,40, during different stages of development41–43, and may thus be differentially affected in different disorders. The white matter microstructural analyses, based on diffusion tensor imaging (DTI) data, have examined fractional anisotropy (FA), as well as axial (AD), radial (RD), and mean diffusivity (MD) obtained from JHU atlas regions44 using the ENIGMA DTI analysis protocol45. FA represents the degree to which diffusion is anisotropic and MD characterizes the magnitude of the water diffusion irrespective of directionality42. AD is the rate of diffusion in the principal diffusion direction and RA is the rate of diffusion perpendicular to the principal diffusion direction43. The underlying pathology associated with each of these imaging measures is multifactorial, may differ by disorder, and continues to be an active area of research; for detailed review of these measures, see44.
For their initial studies, the SZ, BD, and MDD groups used the meta-analytic method, where each site analyses their own data and a leading site conducts the meta-analysis based on each site’s results. The 22q11DS group was able to conduct mega-analyses, which pooled data from all sites for combined analyses while statistically adjusting for effects of scanner (site)46–49. Both analysis approaches, also referred to as two-stage and one-stage meta-analyses in the statistical literature50, have advantages and disadvantages; for a review and a comparison, see29.
This review largely follows the chronological order in which the groups have produced their findings, starting with findings on subcortical brain structures, followed by cortical thickness and surface area, diffusion tensor imaging, and the most recent subcortical structure shape analysis findings; details of reported studies are summarized in Table 1. After reviewing the findings, cross disorder effect size profiles and cross disorder effect size correlations are compared.
Table 1.
Studies of ENIGMA SZ, BD, MDD, and 22q11DS Disorder Working Groups
| Working Group | Study (author, year) | Cohort | N | Age Range (Years) | Imaging Modality | Main Findings |
|---|---|---|---|---|---|---|
| SZ | van Erp et al. 201651 | 15 | SZ:2028 HC:2540 |
SZ:23–42 HC:22–43 |
sMRI(FreeSurfer subcortical volume) | SZ had smaller hippocampus (Cohen’s d = −0.46), amygdala (d = −0.31), thalamus (d = −0.31), accumbens (d = −0.25) and intracranial volumes (d = −0.12), as well as larger pallidum (d = 0.21) and lateral ventricle volumes (d = 0.37). |
| Kelly et al. 201773 | 29 | SZ:1963 HC:2359 |
SZ:18–77 HC:18–86 |
DTI (FA, RD, MD and AD) | SZ had a widespread, significant reduction in FA. Effect size was the greatest for the entire WM skeleton (d = 0.42), anterior corona radiata (d = 0.40) and corpus callosum (d = 0.39). This study shows robust and widespread WM changes in schizophrenia. |
|
| Walton et al. 201765 | 17 | SZ:1987 | SZ:28–43 | sMRI(FreeSurfer superior temporal gyrus thickness) | Severity of positive symptoms was negatively associated with superior temporal gyrus thickness of both hemispheres(ßstd = −0.052; P = 0.021; right: ßstd= −0.073; P = 0.001), controlling for age, sex, and site. | |
| Walton et al 201866 | 17 | SZ:1985 | SZ:28–43 | sMRI(FreeSurfer medial orbitofrontal cortex thickness) | Severity of negative symptoms was negatively associated with medial orbitofrontal cortex thickness(βstd = −0.075; p = 0.019), controlling for age, sex, and site | |
| van Erp et al. 201864 | 39 | SZ:4474 HC:5098 |
SZ:11–78 HC:10–87 |
sMRI(FreeSurfer cortical thickness and surface area) | SZ had a widespread reduction in cortical thickness (left/right hemisphere:Cohen’s d = −0.530/−0.516) and surface area(left/right hemisphere:Cohen’s d = −0.251/−0.254) with the greatest effect sizes for both in frontal and temporal lobe regions. | |
| Holleran et al. 2020105 | 11 | SZ:760 HC:957 |
NA | DTI(gFA, LA-gFA) | gFA explained a significant amount of cognitive variation and similar effect sizes were observed in both patients (effect size = 0.20) and healthy participant groups (effect size = 0.32). Comparable patterns of association were also observed between LA-gFA and cognition. However, this association was unrelated to diagnosis. | |
| BD | Hibar et al. 201655 | 20 | BD: 1710 HC: 2594 | NA | sMRI(FreeSurfer subcortical volume and intracranial volume) | BD had a significant volume reduction for mean hippocampus (Cohen’s d = −0.232) and thalamus(d = −0.148) and enlarged lateral ventricles (d = −0.260). No significant differences in volume between bipolar subtypes with greater magnitude differences for BDI with controls. Lithium treatment related to larger thalamic volumes. |
| Hibar et al 2018.67 | 28 | BD:2447 ( adult BD: 1837) HC:4056 ( HC: 2582) |
NA | sMRI (FreeSurfer cortical thickness and surface area)) | BD had a thinner cortex in frontal, temporal, parietal regions in both hemispheres with greatest effect sizes for left pars opercularis(cohen d = −0.293), left fusiform gyrus(cohen d = −0.288) and left rostral middle frontal cortex (cohen d = −0.276). A longer duration of illness was associated with a decrease in cortical thickness in the frontal, medial parietal and occipital regions. Lithium intake was associated with increased cortical thickness and had the largest effects in the left paracentral gyrus and the left and right superior parietal gyrus. | |
| Favre et al. 201947 | 26 | BD:1482 HC:1551 |
18–65 for both groups | DTI (FA) | BD had a significantly lower FA in 29 regions with greatest effect for the corpus callosum (R2 = 0.041, Pcorr < 0.001) and cingulum (right: R2 = 0.041, left: R2 = 0.040, Pcorr < 0.001). Lithium medication, later onset and shorter disease duration were related to higher FA. | |
| Nunes et al. 2020106 | 13 | BD:853 HC:2167 |
NA | sMRI (regional cortical thickness, surface area, subcortical volumes) | Applying machine learning to structural MRI data for differentiating bipolar disorders, accuracies ranged from 45.23% to 81.07% according to sites. Aggregate subject-level analyses showed the highest accuracy(65.23%).There was a substantive agreement between the best performing sites and regions that helped identify BD participants in aggregated datasets (Cohen’s Kappa = 0.83) | |
| Haukvik et al. 202056 | 23 | BD:1472 HC:3226 |
NA | sMRI(FreeSurfer hippocampal subfield segmentation algorithm) | BD had smaller hippocampus (Cohen’s d = −0.20), cornu ammonis (CA)1 (d = −0.18), CA2/3(d = −0.11), CA4 (d = −0.19), molecular layer (d = −0.21), granule cell layer of dentate gyrus (d = −0.21), hippocampal tail (d = −0.10), subiculum (d = −0.15), presubiculum(d = −0.18), and hippocampal amygdala transition area (d = −0.17). Lithium use was associated with larger volumes whereas antipsychotic or antiepileptic medication use was associated with smaller subfield volumes. | |
| McWhinney et al. 202157 | 17 | BD:1134 HC: 1601 | NA | sMRI(Free surfer subcortical volume, lateral ventricles volume) | BD had higher body mass index(BMI), larger lateral ventricular volume, and smaller volumes of amygdala, hippocampus, pallidum, caudate, and thalamus. BMI was positively associated with ventricular and amygdala and negatively with pallidal volumes. 18.4 % of the total association between BD and ventricular volume was related to the higher body mass index (BMI) in BD (Z = 2.73, p = 0.006). Other subcortical areas were robustly associated with BD even after controlling BMI, and there was no interaction between BD and BMI in relation to subcortical brain volume. | |
| MDD | Schmaal et al. 201658 | 15 | MDD:1728 HC:7199 |
NA | sMRI (FreeSurfer subcortical volume) | MDD had a reduced hippocampal volume(Cohen’s d = −0.14, % difference = −1.24). This effect is largely attributed to recurrent depression(Cohen’s d = −0.17, % difference = −1.44) not first onset MDD. Earlier onset age was associated with smaller hippocampal (Cohen’s d = −0.20, % difference = −1.85) and amygdala volume (Cohen’s d = −0.11, % difference = −1.23) and larger lateral ventricle volume (Cohen’s d = 0.12, % difference = 5.11). |
| Schmaal et al. 201768 | 20 | MDD:2148 HC:7957 |
NA | sMRI(FreeSurfer cortical thickness and surface area) | MDD had a reduced cortical volume in the orbitofrontal cortex, anterior and posterior cingulate, insula and temporal lobes (Cohen’s d effect sizes: −0.10 to −0.14). Patients with the first episode and adult onset showed more pronounced cortical thinning in these areas. There was no difference in cortical thickness between adolescent patients and controls. The total surface area of the left and right hemispheres was smaller in adolescent major depressive disorder. | |
| Frodl et al. 201746 | 9 | MDD:958 HC:2078 |
NA | sMRI(FreeSurfer subcortical gray matter volume,lateral ventricles, and total intracranial volume) | Significant interactions between childhood adversity, MDD diagnosis, gender, and site were apparent. The caudate volume is lower in females, independent of MDD, which was associated with increased exposure to adversity in childhood(right caudate: F=10.7, p< 0.001, Bonferroni correction: pcorr<0.007; left caudate F=13.4, p<0.001, pcorr <0.005). All subcategories of childhood adversity were negatively associated with the caudate volume, in females, especially emotional and physical negligence (regardless of age, ICV, imaging site, MDD diagnosis). | |
| Renteria et al. 201759 | MDD with suicide :451 MDD no suicide:650 HC:1996 |
NA | sMRI (FreeSurfer subcortical gray matter volume, lateral ventricles, and total intracranial volume) | MDD with suicidal symptoms had a reduced intracranial volume (P = 4.12 × 10−3) or a 2.87% volume reduction (Cohen’s d = −0.284) compared to controls. | ||
| van Velzen et al 2018.74 | 20 | MDD:1305 HC:1602 |
12–88 for both groups | DTI (FA, RD) | MDD had a widespread lower FA in 16 out of 25 WM tracts of interest (Cohen’s d: 0.12 to 0.26). The most contributing regions were corona radiata and corpus callosum regions. Depressive patients also had a widespread higher RD (Cohen’s d: 0.12 to 0.18). These effects are largely explained by recurrent depression and adult onset depression. | |
| Tozzi et al 202049 | 12 | MDD:1284 HC:2588 | 13–89 for both groups | sMRI (FreeSurfer cortical thickness and surface area) | Childhood maltreatment was inversely related to cortical thickness in the banks of the superior temporal sulcus (Wald χ2 = 14.583, p FDR = 0.033, B = −0.001) and supramarginal gyrus (Wald χ2= 8.889, p FDR = 0.049, B = −0.001). A significant negative effect on the surface area of the middle temporal lobe was also reported (Wald χ2 = 12.368, p FDR = 0.015, B = −1.504). Individuals with a history of both childhood neglect and abuse were associated with reduced cortical thickness in the inferior parietal lobe (Wald χ2 = 15.273, p FDR = 0.023), middle temporal lobe (Wald χ2 = 15.273, p FDR = 0.023) and precuneus (Wald χ2 = 15.325, p FDR = 0.023). | |
| Han et al. 2020107 | 19 | MDD:2675 HC:4314 |
18–75 for both groups | sMRI(FreeSurfer cortical thickness, surface area, lateral ventricles and total intracranial volume) | MDD had a higher brain-predicted age difference (brain PAD) of +1.08 (SE 0.22) years (Cohen’s d = 0.14, 95% CI 0.08–0.20). | |
| de Kovel et al. 2019 108 | 31(Cortical regions), 32(Subcortical regions) | Cortical regions-MDD:2256 HC:3504 Subcortical regions-MDD: 2540 HC:4230 | NA | sMRI(FreeSurfer subcortical volumes, cortical thickness and surface area) | No significant effects of MDD diagnosis were found for any of the cortical thickness, cortical surface, or subcortical volume asymmetry index (AI) after correction for multiple testing. The strongest effect of diagnosis involved a Cohen’s d value of 0.085 for the superior temporal gyrus thickness AI, which was not significant after adjustment for multiple testing. | |
| Ho et al. 202061 | 10 | MDD:1781 HC: 2953 |
NA | sMRI (shape metrics (thickness and surface area) on the surface of seven bilateral subcortical structures: nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus) | Adolescent-onset MDD (≤21 years) had a reduced thickness and surface area of hippocampal ammonis cornea (CA) 1 and basolateral amygdala (Cohen’s d = −0.164 to −0.180). Patients with recurrent MDD had lower hippocampal CA1 and basolateral amygdala thickness and surface area (Cohen’s d = −0.173 to −0.184) compared to the first episode of MDD. | |
| Leerssen et al. 202072 | 15 | MDD:1053 HC:2108 BD(clinical control):260 | 13–79 | sMRI(cortical thickness, surface area and subcortical volumes) | MDD patients with more severe insomnia had a smaller surface area of the right insula( f2= 0.02, ΔR2 = 1.5%, pcorr= 0.031), left inferior frontal gyrus pars triangularis (f2 = 0.02, ΔR2 = 1.8%, pcorr = 0.018), the left frontal pole (f2 = 0.01, ΔR2 =0.6%, pcorr = 0.031), right superior parietal cortex (f2 = 0.02, ΔR2 = 1.6%, pcorrected = 0.026), right medial OFC (f2 = 0.02, ΔR2 = 1.3%, pcorr = 0.031), and the right supramarginal gyrus (f2 = 0.02, ΔR2 = 1.3%, pcorrected = 0.031). Associations were specific for insomnia severity and MDD diagnosis (not for overall depression severity, HC, BD) | |
| Opel et al. 202071 | 28 | MDD:2901 HC:3519 | NA | sMRI(total and regional cortical thickness and surface area and measures of subcortical and intracranial volumes) | Obesity was associated with lower cortical thickness (most pronounced in the temporo-frontal lobe, maximum Cohen ´s d (left fusiform gyrus) = −0.33), regionally specific cortical surface area alterations, and increased subcortical volume in the amygdala, the thalamus and the nucleus accumbens. | |
| Campos et al. 202160 | 18 | MDD:6448 (of whom, attempted suicide:694) HC:12477 | NA | sMRI(regional cortical thickness and surface area and measures of subcortical, lateral ventricular, and intracranial volumes) | Depressed suicidal attempters had smaller volumes in the left and right thalamus and right pallidum compared with both clinical (Cohen’s d = −0.13, −0.14, and −0.12, respectively) and healthy control. Attempeters also had smaller surface area in the left inferior parietal lobe after multiple correction when compared with clinical control(Cohen’s d = −0.12). | |
| 22q11DS | Sun et al. 201870 | 10 | 22q11DS:474 HC:315 |
NA | sMRI(FreeSurfer cortical thickness and surface area) | 22q11DS had thicker cortical gray matter overall (left/right hemispheres: Cohen’s d = 0.61/0.65), but thinner temporal and cingulate cortex. 22q11DS had a smaller surface area (left/right hemispheres: d = −1.01/−1.02). These neuroanatomic patterns could differentiate 22q11DS with 93.8% accuracy. 22q11DS subjects with psychosis showed thinner cortex compared to those without psychosis and 22q11DS-psychosis and idiopathic schizophrenia showed significant convergence of affected brain regions, especially in the frontotemporal cortex. Results showed a strong effect of deletion size on local cortical surface area, most notably in the frontal and parietal lobes. |
| Villalón-Reina et al. 201948 | 10 | 22q11DS:334 HC:260 |
6–52 for both groups | DTI(FA, MD, RD, AD) | 22q11DS had widespread smaller MD, RD, AD, most prominent in areas with major cortico-cortical and cortico-thalamic fibers (Cohen’s d= −0.9 to −1.3). 22q11DS showed higher mean partial anisotropy (FA) in the corpus callosum and projection fibers (internal capsule and corona radiata) compared to controls, but lower FA than controls primarily in areas with associative fibers. Psychosis of 22q11DS was associated with a more substantial reduction in diffusion coefficient in multiple regions. | |
| Ching et al. 202063 | 11 | 22q11DS: 533 HC: 330 |
6–56 for both groups | sMRI(gross volume and subcortical shape morphometry) | 22q11DS had a lower intracranial volume (ICV), thalamus, putamen, hippocampus, and amygdala volume and larger lateral ventricles, caudate nucleus, and nucleus accumbens (Cohen’s d=−0.90–0.93). Results showed widespread subcortical structure changes, affected by deletion size and psychotic illness. 22q11DS with psychosis demonstrated significant convergence with idiopathic schizophrenia, and other severe neuropsychiatric illnesses. |
N, Number; SZ, Schizophrenia; BD, bipolar disorder; MDD, major depressive disorder; 22q11.2 Deletion Syndrome, 22q11DS; HC, Healthy Control; NA, not available; DTI, diffusion tensor imaging; FA, fractional anisotropy; RD, radial diffusivity; MD, mean diffusivity; AD, axial diffusivity; gFA, global fractional anisotropy component; LA-gFA, fractional anisotropy component for six long association tracts.
Subcortical Structures
Subcortical structures in schizophrenia
Van Erp and colleagues’ 2016 meta-analysis reported the rank order of case-control effect sizes for deep brain structure volumes comparing data from 2,028 individuals with schizophrenia with 2,540 healthy controls obtained from 15 centers worldwide51. They found significantly smaller hippocampus, amygdala, thalamus, nucleus accumbens, and intracranial volumes and larger pallidum and lateral ventricle volumes in individuals with schizophrenia compared to controls (Figure 1A). Meta-regressions also showed positive associations between putamen and pallidum volumes and duration of illness and age at scan, while hippocampal volumes were negatively associated with the proportion of unmedicated patients (assessed for each cohort).
Figure 1.
Maps of Case-control Effect Sizes by Disorder
A. Subcortical structures, B. Cortical Thickness, C. Cortical Surface Area.
SZ = Schizophrenia, BD = Bipolar Disorder, MDD = Major Depressive Disorder, 22q = 22q11.2 deletion syndrome, 22qP = 22q11.2 deletion syndrome with Psychosis.
In addition to the reported case-control effect sizes, Franke and colleagues evaluated genome-wide overlap between genetic effects on schizophrenia and subcortical structure volumes by integrating genetic results from (common variant) a case-control GWAS of schizophrenia, from a meta-analysis by the Psychiatric Genomics Consortium (33,636 cases, 43,008 controls), and a GWAS of the volumes of brain structures that show abnormalities in schizophrenia, from a meta-analyses by the ENIGMA consortium (at the time, the total sample size was 11,840 subjects)52. They reported no significant genetic correlations at the level of overall common variant genetic architecture or for single genetic markers. The lack of overlap in genetic influences on schizophrenia and deep brain structure volumes found by Franke and colleagues52 was surprising and also found by a second study11. However, a study with a larger imaging sample size (N>26,000) did find polygenic overlap between schizophrenia and hippocampus, putamen and intracranial volume53. In addition, a more recent SNP effect concordance analysis (SECA) also found overlap in genetic risk for cross-disorder vulnerability to mental disorders and genetic risk for subcortical brain volume54. These findings may suggest that the earlier GWAS subcortical findings may not have been sufficiently robust to detect the more recently observed genetic overlap based on the larger samples.
Subcortical structures in bipolar disorder
Hibar and colleagues’ 2016 meta-analysis reported the rank order of case-control effect sizes for deep brain structure volumes comparing data from 1,710 individuals with bipolar disorder to data from 2,594 healthy controls obtained from 15 centers worldwide55. They reported significantly lower hippocampus and thalamus volumes and higher lateral ventricle volumes in individuals with bipolar disorder compared to controls (Figure 1A). Direct comparisons of deep brain volumes between individuals with Bipolar I disorder (BDI; n=1,100) and Bipolar II disorder (BDII; n=360) showed no significant differences between the subtypes; though only individuals with BDI showed lower hippocampal and amygdala volumes and higher ventricle volumes when compared to controls and the the magnitude of differences between the patient and the control group was larger for BDI (d=−0.203, −0.117, and 0.251, respectively) than for BDII (d=−0.134, −0.012, and 0.096, respectively). The study also found possible opposing effects of anti-epileptic and lithium medication treatments on structural brain measures. More specifically, patients taking anti-epileptics had significantly smaller hippocampal volumes than patients not taking anti-epileptic medications, while patients not taking lithium had significantly smaller hippocampal volumes than patients taking lithium.
Haukvik and colleagues’ 2020 mega-analysis reported the rank order of case-control effect sizes for hippocampal subfields comparing data from 1472 individuals with BD and 3226 healthy controls from 23 countries worldwide56. They found that BD had widespread smaller hippocampal subfield volumes (in nine of twelve regions). Here also, lithium was associated with protective effects, whereas antipsychotics and antiepileptics were associated with smaller hippocampal subfield volumes.
McWhinney and colleagues investigated the effects of obesity on subcortical volumes from 1134 individuals with BD and 1601 healthy controls from 13 countries worldwide57. They found that 18.4 % of the total association between BD and ventricular volume was explained by a higher body mass index (BMI) in BD. Other subcortical regions, including the hippocampus, caudate and thalamus, were robustly associated with BD even after controlling BMI, and there was no interaction between BD and BMI in predicting subcortical brain volumes.
Subcortical structures in major depressive disorder
Schmaal and colleagues’ 2016 meta-analysis reported the rank order of case-control effect sizes for deep brain structure volumes comparing data from the 1,728 MDD patients and 7,199 controls from 15 research samples worldwide58. Individuals with MDD showed slightly lower hippocampal volume compared to controls (Figure 1A). Of note, this effect was present in recurrent depressive disorder but was not present in first onset depressive disorder. Moreover, smaller hippocampal and amygdala volumes and larger lateral ventricle volumes were observed in those with early onset age (≤21 years) but not adult onset age (>21 years). In addition to the effects of MDD on deep brain structures, two subsequent studies evaluated the effects of suicidal thoughts and behaviors and childhood maltreatment on subcortical volumes in MDD.
Renteria and colleagues examined the effects of suicidal ideation or behaviour in a meta-analysis of 3,097 participants including 1,101 individuals with MDD (451 of whom reported suicidal ideation or behaviour) and 1,996 healthy controls based on 20 samples worldwide59. MDD patients who reported suicide plans or attempted suicide had smaller intracranial volume compared to controls. No significant differences were reported between MDD patients with and without suicidal symptoms for one of the deep brain structural volumes. However, more recently Campos and colleagues pooled subcortical, cortical thickness and surface area data from 18,925 individuals, of which 694 individuals with MDD and a history of suicide attempts, 5754 individuals with MDD without a history of suicide attempts, and 12,377 healthy comparison subjects from 18 samples worldwide60. In this much larger study, they found smaller right and left thalamus and right pallidum volumes in MDD with versus without suicide attempts. These findings underscore the possible involvement of the thalamus, a widely connected relay center of the brain, and the pallidum, an area involved in reward, motivation and positive affect.
Frodl and colleagues conducted a mega-analysis investigating the effect of childhood adversity and its interactions with MDD diagnosis and sex on deep brain structure volumes in 958 MDD patients and 2,078 healthy controls46. The severity of adversity in childhood was significantly associated with smaller left and right caudate volumes in women but not in men. No diagnosis by childhood adversity interaction effect was found for any of the subcortical structure volumes. Hence the study did not observe a hypothesized more notable effect of childhood adversity on brain volume in MDD patients compared to healthy individuals. MDD is a heterogeneous disorder, and many efforts have been made to find subtypes that will help predict disease progression and plan treatment. Overall, the results suggest that smaller hippocampal volume may be due to stress-induced mechanisms directly related to MDD, whereas caudate nucleus volume may be related to exposure to stress during sensitive period development common across individuals with and without MDD.
More recently, Ho and colleagues performed a meta-analysis of subcortical shape in the ENIGMA MDD Working Group. Their study included 1,781 individuals with MDD and 2,953 healthy controls61. Individuals with early-onset MDD (≤ 21 years) had thinner cortex and surface area of the subiculum, cornu ammonis (CA) 1 of the hippocampus and basolateral amygdala, compared to healthy controls. Consistent with, and in addition to the volumetric findings, individuals with recurrent MDD had lower hippocampal CA1 and basal amygdala thickness and surface area compared with individuals with first episode MDD. These findings are consistent with those reported for total deep brain structure volumes and may provide more specific anatomical localization of the observed effects.
In addition to suicide and childhood adversity, the ENIGMA MDD Working Group also investigated associations between brain structure and obesity; a physical condition that is highly prevalent in neuropsychiatric disorder and has a significant impact on neurophysiology. Opel and colleagues examined structural MRI data from 6,420 participants (3,519 healthy controls and 2,901 MDD patients from 28 sites) along with genetic data from 3,907 participants62. This study found that obesity, independent from MDD diagnosis, was associated with larger amygdala, thalamus, and nucleus accumbens volumes. However, a polygenic risk score for obesity was not found to be associated with any of the subcortical volumes.
Subcortical structures in 22q11DS
Ching and colleagues63 reported on subcortical volumes and shape in a sample of 533 individuals with 22q11DS and 330 matched healthy controls. Compared to the control group, the 22q11DS group showed smaller thalamus, putamen, hippocampus, amygdala, and intracranial volume (ICV) and larger lateral ventricle, caudate, and accumbens volumes (Figure 1A). Subcortical shape analysis showed complex local morphometry differences between 22q11DS participants and healthy participants covering the majority of the subcortical areas of interest; differences that cannot be identified using traditional total volume analysis. The authors found extensive abnormalities in subcortical brain structure, influenced by deletion size and psychosis. Compared to subcortical results from other neuropsychiatric disorders studied by the ENIGMA consortium, significant overlap was found between 22q11DS-related psychosis (Figure 1A), idiopathic schizophrenia, and other severe neuropsychiatric disorders63.
Cortical Thickness
Cortical thickness in schizophrenia
Van Erp and colleagues’ 2018 meta-analysis reported the rank order of case-control effect sizes for cortical thickness comparing data from 4,474 individuals with schizophrenia compared to 5,098 healthy controls obtained from 39 centers worldwide64. Individuals with schizophrenia showed thinner cortex compared to healthy volunteers, with maximum effect sizes in frontal and temporal lobe regions (Figure 1B). Cortical thickness abnormalities in schizophrenia were found to be regionally specific based on an analysis that controlled for mean thickness. In addition, the effect sizes for thinner cortex in schizophrenia scaled with antipsychotic medication treatment. Compared to the unmedicated group, effect sizes were about 2 to 3 times higher for the group on second-generation and first-generation antipsychotic drugs, respectively. The negative correlation between age and bilateral temporal pole thickness was higher in individuals with schizophrenia than healthy participants. Insula thickness had positive correlation with onset age and negative correlation with illness duration. Regional cortical thickness also showed significant negative correlations symptom severity, and with standardized medication dosage; the latter even when controlling with for duration of illness and severity of symptoms. Two additional studies specifically focused on relationships between cortical thickness and severity of negative and positive symptoms in schizophrenia.
Walton and colleagues’ 2017 meta-analysis, including 1,987 individuals with schizophrenia from 17 centers, reported that the severity of positive symptoms was negatively associated with bilateral superior temporal gyrus thickness when adjusted for age, sex, and scanner65. This effect remained significant in the model, even when accounting for potentially confounding covariates such as duration of the illness, antipsychotics treatment or handedness. The superior temporal gyrus has been implicated in auditory processing, language comprehension and self-monitoring. Altered superior temporal gyrus neural activity has been related to positive symptoms such as hallucinations and delusions.
Walton and colleagues’ 2018 meta-analysis, including 1,985 individuals with schizophrenia from 17 research groups around the world, found that left but not right hemisphere medial orbitofrontal cortex thickness was significantly associated with the severity of negative symptoms, when statistically adjusting for age, sex, and scanner66. This effect remained stable in a model that included overall symptom severity. Using these large samples and the meta-analytic approach, their findings show an association between prefrontal cortex thickness in schizophrenia and the severity of negative symptoms. The discovery of a negative association between left medial orbitofrontal cortex thickness and negative symptoms underscores the significance of this region in motivational and executive function, which are known areas of deficiency in individuals of schizophrenia61.
The sample sizes for both symptom association studies were 10 times larger than any prior study. This made it possible to identify these relatively small effects and investigate contributions of potentially confounding variables. The findings provide insight into the neurobiology of schizophrenia symptoms.
Cortical thickness in bipolar disorder
Hibar and colleagues performed a meta-analysis of cortical gray matter thickness obtained from 6,503 individuals with BD, which included 1,837 adults with BD and 2,582 healthy controls67. They reported a significant and universal pattern of thinner cortex in BD (Figure 1B). Thinner cortex was observed in bilateral frontal, temporal and parietal regions, with maximum effects in the left cerebral hemisphere, left fusiform gyrus, and left rostral prefrontal cortex. No significant differences in cortical thickness were reported between BDI and BDII subtypes. Thinner cortex in frontal, medial parietal and occipital regions was associated with longer duration of illness. Similar to the pattern observed for some of the deep brain structures, thicker cortex was observed in bipolar disorder patients taking lithium compared to those not taking lithium, and thinner cortex was observed in bipolar disorder patients who were taking anti-epileptic medications compared to those not taking antiepileptic medications. Lithium showed the largest effect in the left paracentral gyrus and the left and right superior parietal gyrus and antiepileptics showed the largest effects in the left and right lateral occipital gyrus and the right paracentral gyrus.
Cortical thickness in major depressive disorder
Schmaal and colleagues’ 2017 meta-analysis evaluated cortical structural alterations in MDD from 2,148 MDD patients and 7,957 healthy controls at 20 sites around the world68. Compared to controls, they found significantly thinner orbitofrontal cortex (OFC), anterior and posterior cingulate, insula and temporal lobes in adult individuals with MDD (age > 21 years; Figure 1B) but not adolescent individuals with MDD (age<=21). Consistent with no cortical thickness abnormalities in adolescent patients with MDD, only patients with adult-onset MDD, but not adults with adolescent onset MDD showed thinner cortex. It is important to note that thinner cortex was observed in adult individuals with MDD who were taking antidepressants when compared to controls but not in those not taking antidepressants compared to controls. This could in part reflect the severity of depression - but also suggests that treatment effects may contribute.
Tozzi and colleagues conducted a mega-analysis investigating associations between the type (no, abuse only, neglect only, or both) and severity (total score of childhood trauma questionnaire) of childhood maltreatment and brain structural abnormalities in a sample of 3,872 individuals with MDD49. They found that only individuals with a history of both childhood neglect and abuse had thinner cortex in the banks of the superior temporal sulcus, inferior parietal lobe, middle temporal lobe, precuneus, and supramarginal gyrus, compared with participants with no history of childhood maltreatment. In addition, severity of childhood maltreatment was associated with thinner cortex of the banks of the superior temporal sulcus and supramarginal gyrus. They also reported significant interactions between childhood maltreatment and age in predicting cortical thickness in several frontal, temporal, and posterior parietal regions. These findings suggest that childhood maltreatment may affect brain maturation with age, especially in areas relevant to default mode network, perception and theory of mind.
A mega-analysis by Han and colleagues’ (2020) compared estimated ‘brain age’ between individuals with MDD and healthy volunteers69. Brain age is the chronological age as predicted by brain imaging data and was in this study based on a model derived from subcortical and intracranial brain volumes, cortical thickness, and surface area data. They found that individuals with MDD had a higher brain-predicted age difference (brain age - chronological age) of +1.08 years when compared to controls.
In addition to effects on subcortical volumes, Opel and colleagues’ (2021) reported that obesity was associated with thinner cortex, predominantly in the temporal and frontal lobes, while statistically controlling for antidepressant medication and mean cortical thickness62. The effect of obesity also interacted with age in predicting cortical thickness with a larger effect in older compared to younger individuals. Obesity may be a significant contributor to brain structural abnormalities in psychiatric disorders including depression, and warrants further investigation.
Cortical thickness in 22q11DS
Sun and colleagues investigated cortical abnormalities from 474 subjects with 22q11DS and 315 typically developing and matched controls from 10 centers worldwide70. Compared to healthy controls, individuals with 22q11DS showed an extensively thicker cortex bilaterally, with the exception of thinner cortex in the superior temporal, cingulate and parahippocampal regions (Figure 1B). Of note, 22q11DS participants with psychosis had significantly thinner cortex compared to participants without psychosis, with strongest effects in the frontotemporal region, similar to that observed in idiopathic psychosis (Figure 1B).
Surface Area
Surface area in schizophrenia
Van Erp and colleagues analyzed surface area abnormalities using data from 4,474 participants with schizophrenia and 5,098 healthy volunteers obtained with harmonized methods from 39 centers worldwide64. Participants with schizophrenia showed widespread smaller regional cortical surface areas compared to healthy volunteers with maximum effect size in both the frontal and temporal lobe regions (Figure 1C). The effect sizes for surface area were about half those observed for cortical thickness and no regional specificity was found for surface area based on an analysis that statistically controlled for total surface area. In spite of the highly powered study’s ability to find small effects, antipsychotic treatment and other clinical variables were not significantly related to cortical surface area. These results suggest that genetic association studies of schizophrenia employing cortical surface area as a quantitative trait, in contrast to those employing cortical thickness, may not be confounded by the effects of antipsychotic treatments.
Surface area in bipolar disorder
Hibar and colleagues performed meta-analysis on surface area measurements from 6,503 individuals with BD, which included 1,837 adults with BD and 2,582 healthy controls67. They found no significant differences in cortical surface area between individuals with BD and healthy controls (Figure 1C) andno significant surface area differences between BDI and BDII subtypes. Smaller cortical surface area in BD patients was associated with a history of psychosis, but not with mood state at the time of scanning. They also found larger left paracentral lobule surface area in individuals with BD on lithium treatment compared to those not on lithium treatment. In addition, larger cortical surface area was associated with typical antipsychotic treatment in the left middle temporal gyrus, left inferior parietal gyrus and right temporal pole, while lower cortical surface area was associated with atypical antipsychotic treatment in the right rostral middle frontal gyrus and right superior frontal gyrus. In contrast to the findings on deep brain structures and cortical thickness, no effects of anti-epileptic medications on surface area were observed.
Surface area in major depressive disorder
Schmaal and colleagues performed a meta-analysis on regional cortical surface area measurements from 2,148 MDD patients and 7,957 healthy controls68. The total surface area of the left and right hemispheres was smaller in adolescent and young adult patients with MDD (age ≤ 21 years) compared to adolescent controls - with higher effect sizes than those observed for cortical thickness in adult patients with MDD. Lower surface area was found in medial OFC and superior frontal gyrus as well as primary and higher-order visual, somatosensory and motor areas. The strongest effects were found in recurrent adolescent patients. No surface area abnormalities were observed in adult MDD patients (Figure 1C) or adult MDD patients with an adolescent or young adulthood age of onset. Of note, across all ENIGMA MDD Working Group papers, cortical thickness findings are more pronounced in adults/advanced aging, while surface area and subcortical volumes are more predominant in young people. These findings suggest that cortical thickness may be more reflective of an adult onset MDD subtype and chronicity, while smaller cortical surface area may reflect an early developmental subtype of depressive disorder, induced by genetic factors or early life adversity29. We speculative that the observed presence of cortical thickness deficiencies in the adult but not adolescent MDD sample may be due to the older mean age of the adult than adolescent MDD sample, and may be suggestive of a more pronounced effect of aging on cortical thickness in MDD versus controls69; though longitudinal studies are needed to test the hypothesis72.
In addition to the cortical thickness effects, the ENIGMA MDD Working Group studies found that childhood maltreatment severity was associated with smaller middle temporal lobe surface area49, that individuals with MDD who attempted suicide versus those who did not had smaller left inferior parietal lobe surface area60, and that a higher polygenic risk score for obesity correlates significantly with a smaller occipital lobe surface area71.
Leerssen and colleagues investigated whether insomnia severity was associated with global and regional differences in cortical thickness, cortical surface areas, and volumes of subcortical regions in a sample of 1,053 individuals with MDD from 15 cohorts worldwide72. Only cortical surface area, and neither subcortical volumes or cortical thickness, was predictive of insomnia severity in MDD. Regionally, insomnia severity was associated with smaller right insula, left inferior frontal gyrus pars triangularis, left frontal pole, right superior parietal cortex, right medial orbitofrontal cortex, and right supramarginal gyrus surface area.
Surface area in 22q11DS
Sun and colleagues collected imaging data from 474 subjects with 22q11DS and 315 typically developing, matched controls from 10 centers worldwide and investigated distinct neuroanatomic signatures of 22q11DS70. Compared to healthy controls, individuals with 22q11DS showed widespread smaller cortical surface area that was almost twice the effect size of that observed for cortical thickness; with the largest effect sizes observed in the parieto-occipital and anterior cingulate regions (Figure 1C). Larger deletion size was associated with significantly smaller cortical surface area.
To examine how precisely 22q11DS subjects could be differentiated from controls based upon cortical measures, a machine-learning based classification analysis was also performed. Cases and controls with 22q11DS were classified with an accuracy of 93.8% based on neuroanatomical patterns; the top five features contributing to the classification were surface area in the left caudal anterior cingulate, precentral gyrus, and bilateral cuneus, and cortical thickness in the left insula.
White Matter
Diffusion tensor imaging in schizophrenia
Kelly and colleagues performed a meta-analysis of white matter (WM) abnormalities based on diffusion tensor imaging (DTI) data from 1,963 individuals with schizophrenia and 2,359 healthy controls based on 29 international samples73. Individuals with schizophrenia showed widespread lower fractional anisotropy (FA) compared to healthy controls; with the largest effect size for global average FA, followed by the anterior corona radiata, the whole corpus callosum (CC) and its body (BCC) and genu (GCC). Regional specificity was examined by statistically controlling for mean FA in the entire TBSS skeleton, mean FA in JHU (Johns Hopkins University) atlas ROIs (core), and mean FA outside the JHU atlas ROIs (periphery). When statistically controlling for mean FA in the entire TBSS skeleton or mean FA in the periphery, individuals with schizophrenia no longer showed significantly lower FA that survived multiple comparisons in any of the JHU ROIs. These findings suggest predominantly global rather than regional effects of schizophrenia diagnosis on FA. Compared to controls, individuals with schizophrenia showed significantly higher mean diffusivity (MD) and significantly higher radial diffusivity (RD) overall. The observed effects were greater and most widespread for RD. No significant association was observed between FA and age of onset, severity of symptoms, or medication dosage.
Diffusion tensor imaging in bipolar disorder
Favre and colleagues reported on a mega- and meta-analysis of white matter (WM) abnormalities based on diffusion tensor imaging (DTI) data from 1,482 individuals with bipolar disorder (BD) and 1551 healthy controls from 26 samples worldwide47. The mega-analysis found significantly lower FA in individuals with BD compared to healthy controls in 29 out of 43 JHU atlas ROIs with the largest effect sizes in the whole corpus callosum (CC), followed by the body and genu of the CC and the bilateral cingula. The meta-analysis found very similar effects but with somewhat lower effect sizes. Within the patients, lithium intake, later onset, and shorter disease duration were associated with higher FA in multiple ROIs.
Diffusion tensor imaging in major depressive disorder
Van Velzen and colleagues investigated white matter alterations in 1,305 MDD patients and 1,602 healthy controls from 20 cohorts worldwide74. This analysis included both adults and adolescents with MDD. They reported subtle, but distributed, lower fractional anisotropy (FA) in adult MDD patients compared with healthy volunteers in 16 out of 25 regions of interest. The regions that contributed most strongly to the overall effect of the lower FA were the corona radiata and corpus callosum. Widespread higher radial diffusivity (RD) was also found. The WM abnormalities in adult MDD appeared to be driven by patients with multiple depressive episodes and adult age of onset. After false discovery rate (FDR) correction, no significant differences were found in FA, axial diffusivity (AD), mean diffusivity (MD), or RD between adolescents with MDD and healthy volunteers.
Diffusion tensor imaging in 22q11DS
Villalón-Reina and colleagues investigated WM microstructure alterations in 22q11DS using a meta- and mega- analysis based on ENIGMA’s harmonized analysis protocol in a sample of 334 participants with 22q11DS and 260 healthy volunteers48. The mega- and meta-analysis showed almost identical results for overall 22q11DS-related abnormalities in FA, MD, AD and RD. Compared to healthy controls, individuals with 22q11DS had lower MD, AD, and RD, with the largest effect sizes in regions with major cortico-cortical and cortico-thalamic fibers: the corona radiata, corpus callosum, superior longitudinal fasciculus, posterior thalamic radiations, and sagittal stratum. Higher AD in patients with 22q11DS was detected only in the posterior limbs of the internal capsule (IC). Patients with 22q11DS had higher mean FA in the corpus callosum and projection fibers (IC and corona radiata) compared to controls, but lower FA than controls primarily in areas with associative fibers. Of note, the WM microstructural changes in 22q11DS with psychosis had a pattern opposite to that seen in idiopathic schizophrenia, with predominantly higher FA (not lower) and lower diffusivities (rather than higher). No consistent effect of deletion size on WM architecture was detected. Also, the relationship between WM microstructure and IQ was similar between the 22q11DS participants and healthy volunteers.
Cross Disorder Comparisons
The use of the same analysis methods across the different disorder Working groups in ENIGMA allows for the comparison of the patterns of brain abnormalities across disorders. These comparisons can be performed by comparing meta-analysis findings as well as by pooling individual subject-level data across disorders for cross disorder mega-analyses.
Family and genetic studies have shown larger overlap between schizophrenia and bipolar disorder than between schizophrenia or bipolar disorder and major depressive disorder75–77. In addition, a 22q11DS diagnosis is associated with a 20–30% increased risk for psychosis78. Based on these findings, one may predict more similar brain abnormalities between schizophrenia and bipolar disorder, and schizophrenia and 22q11DS, than between schizophrenia, bipolar disorder, or 22q11DS and major depressive disorder in brain imaging measures that share genetic liability for these disorders.
Here we graphically represent the overlap in patterns of brain abnormalities between the disorders - using profile plots, similar to those used for neuropsychological profiles79. The presented case-control Cohen’s d effect size profile plots are sorted based on the observed effect sizes in schizophrenia. All plots were produced separately for subcortical volumes, regional cortical thickness, surface area, and fractional anisotropy. We also present cross disorder Spearman rank correlations for each of these brain measures separately. Given that cortical data is non-independent, due to surface-based smoothing, the significance of the between disorder Spearman rank correlations was tested using the spin test80, while the significance of deep brain structure volumes and regional FA were tested using the ‘shuf’ test (1,000 rotations or permutations each); both implemented in the ENIGMA Toolbox81. For comparison purposes, permutation testing was only completed between groups with complete effect size data for all regions of interest (i.e., correlations with 22q11DS FA were excluded from permutation testing).
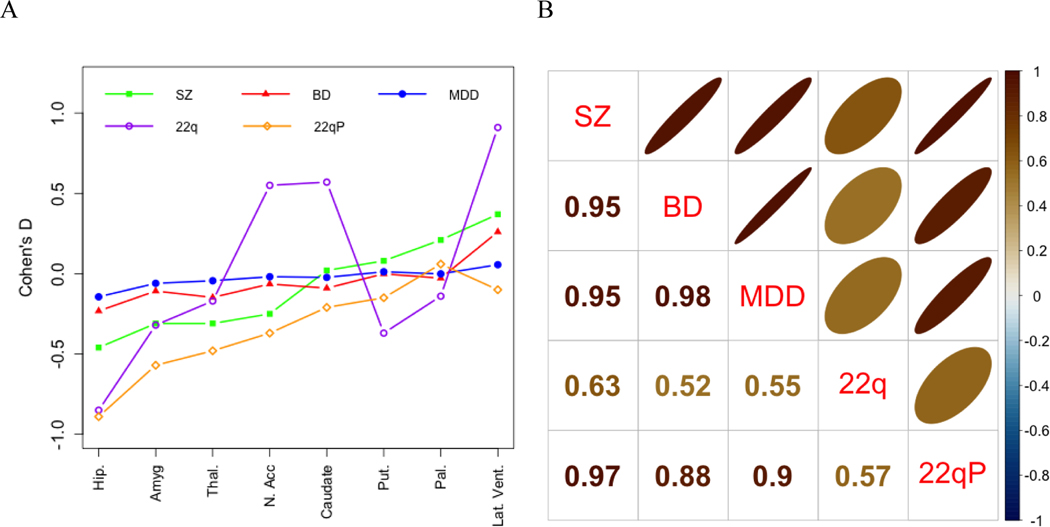
In general, the effect size profiles of deep brain structure abnormalities show larger effects for SZ than for BD and MDD. In contrast to MDD, both schizophrenia and bipolar disorder are associated with larger lateral ventricle volume. The 22q11DS profile shows a substantially different profile from the other groups with significantly larger nucleus accumbens and caudate volumes that were not observed in any of the other groups, as well as a very large effect size for the lateral ventricles. However, the profile of 22q11DS subjects with psychosis (22qP) versus those without psychosis (22qNP) is more severe but highly similar to that of schizophrenia, with the exception of the lateral ventricles which were not significantly larger in 22q11DS subjects with versus without psychosis. In terms of Spearman rank order correlations, SZ, BD, MDD and 22qP showed very high correlations (r range: 0.88–0.98, all p=0.001) while correlations between all the groups with 22q were moderate (r range: 0.52–0.63; p(22q-22qP)=0.049, all other p> 0.05; see Figure 2).
Figure 2.
Profile and Spearman Correlation Plots for Case-control Subcortical Volume Effect Sizes across Disorders
A. Effect size profiles by disorder; B. Between disorder Spearman rank correlations. SZ = Schizophrenia, BD = Bipolar Disorder, MDD = Major Depressive Disorder, 22q = 22q11.2 deletion syndrome, 22qP = 22q11.2 deletion syndrome with Psychosis. Effect sizes for mean subcortical volumes were reported for SZ, BD, and MDD. The BD and MDD studies did not report effect sizes by hemisphere. The 22q11DS study only reported effect sizes for the left and right hemispheres separately and these were averaged.
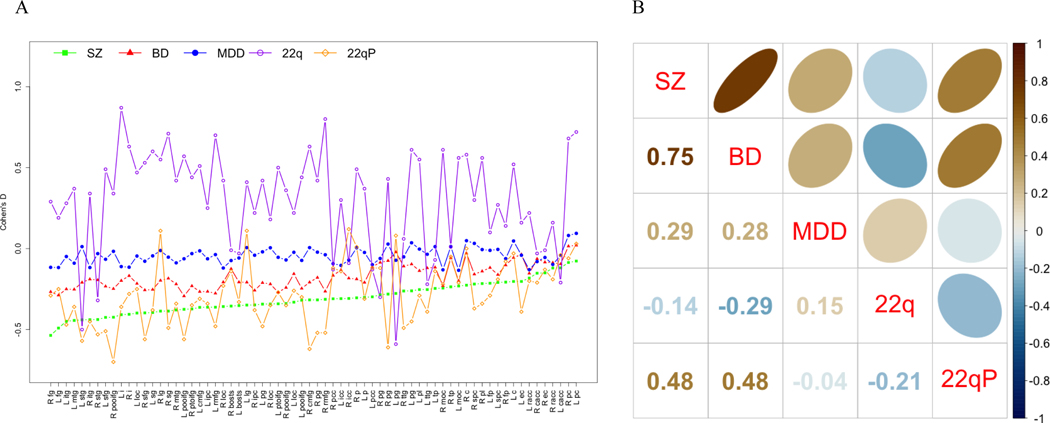
The effect size profiles of cortical thickness abnormalities also show more severe effects for schizophrenia than for bipolar disorder and major depressive disorder. Again, the 22q11DS (22q) profile shows a substantially different profile from all the other groups, while the profile of 22qP, is most similar to that of SZ and BD. In terms of Spearman rank order correlations, the profiles of SZ and BD show a more than two times higher correlation (r=0.75; p=0.001) than those between SZ and MDD and BD and MDD (r=0.29, p=0.045 and 0.28, p=0.026, respectively). The correlations between 22q11DS and the other groups range from −0.29 to 0.15 [p(22q-BD=0.019, all other p>0.05]. 22qP was positively correlated with both SZ and BD (both r=0.48, p=0.003 and 0.005, respectively) but not MDD (r=−0.4; p>0.05; see Figure 3).
Figure 3.
Profile and Spearman Correlation Plots for Case-control Cortical Thickness Effect Sizes across Disorders
A. Effect size profiles by disorder; B. Between disorder Spearman rank correlations. SZ = Schizophrenia, BD = Bipolar Disorder, MDD = Major Depressive Disorder, 22q = 22q11.2 deletion syndrome, 22qP = 22q11.2 deletion syndrome with Psychosis.
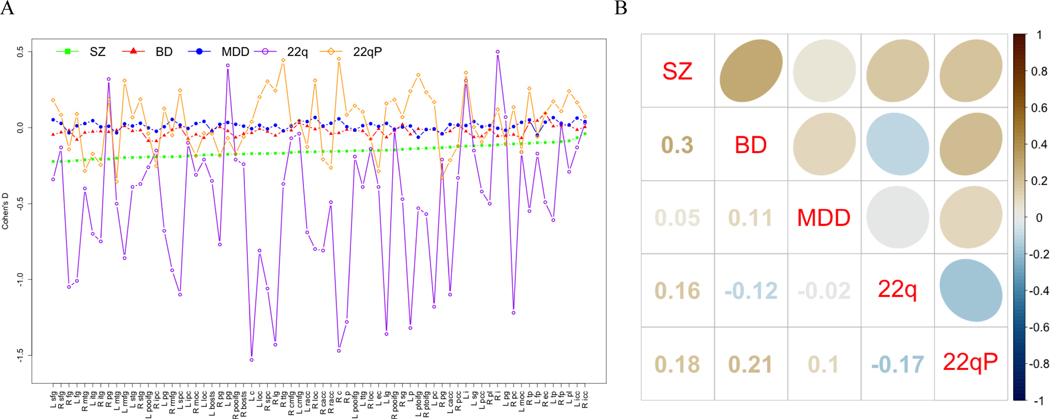
The effect size profiles for cortical surface abnormalities also show more severe effects for SZ than for BD and MDD. The effect size profiles for 22q11DS (22q) are highly variable and those for 22qP also show more variability than those for SZ, BD, and MDD. The Spearman correlation between the SZ and BD profile (r=0.3; p=0.006) is 6 times higher than between the SZ and MDD profiles (r=0.05; p>0.05) and almost 3 times higher than between the BD and MDD profiles (r=0.11; p>0.05). The correlations between 22q11DS and the other groups range from −0.17 to 0.16 (p>0.05). The 22qP profile showed small positive correlations with those of SZ and BD (r=0.18; p>0.05 and 0.21; p=0.0485, respectively) that, while not statistically significant, are almost twice as large as the correlation with the MDD profile (r=0.10; p>0.05; see Figure 4).
Figure 4.
Profile and Spearman Correlation Plots for Case-control Surface Area Effect Sizes across Disorders
A. Effect size profiles by disorder; B. Between disorder Spearman rank correlations. SZ = Schizophrenia, BD = Bipolar Disorder, MDD = Major Depressive Disorder, 22q = 22q11.2 deletion syndrome.
The effect size profiles of fractional anisotropy abnormalities show more severe effects for schizophrenia than for major depressive disorder with more variable effect sizes in bipolar disorder; some of the effect sizes in bipolar disorder are similar or stronger than those in schizophrenia, while others are similar or less severe than those reported in MDD. In terms of Spearman rank correlations the SZ effect size profile showed an almost 3 times higher correlation with BD and MDD (r=0.66; p=0.001, and 0.72; p=0.001, respectively) than between BD and MDD (r=0.24, p>0.05). The higher variable profile for 22q11DS showed near 0 correlations with all three disorders (r range: −0.06–0.02; no shuf test was performed due to missing data for some regions of interest; see Figure 5).
Figure 5.
Profile and Spearman Correlation Plots for Case-control Fractional Anisotropy Effect Sizes across Disorders
A. Effect size profiles by disorder; B. Between disorder Spearman rank correlations. SZ = Schizophrenia, BD = Bipolar Disorder, MDD = Major Depressive Disorder, 22q = 22q11.2 deletion syndrome. Effect sizes for several regions of interest were not computed in the 22q11.2 deletion syndrome study.
Discussion
In this cross-disorder review of ENIGMA findings, we separately compared effect size profiles for subcortical volumes, cortical thickness, cortical surface area, and regional fractional anisotropy across three neuropsychiatric disorders and one rare genetic disorder that is associated with increased risk for several neuropsychiatric illnesses; in particular psychosis. We found that the severity of brain abnormalities generally scales across psychiatric disorders (in the order SZ>BD>MDD) and is the largest in 22q11DS. Most case-control effect sizes are in the same direction, with the notable exception of the predominantly thicker cortex in 22q11DS. While perhaps somewhat surprising given that 22q11DS is associated with increased risk for several neuropsychiatric disorders, thicker cortex is observed in several other severe developmental disorders with a genetic basis82–84. Though, influences on the brain are multifactorial, and for example in 22q11DS also include deleterious effects of disorder-related congenital heart disease on cortical thickness85. Moreover, the disorders studied here show varying levels of similarity in the pattern of regional distribution. More specifically, while subcortical volumes are more affected in SZ>BD>MDD, their cross-region effect sizes show very high correlations within these three disorders and even moderate correlations with those of 22q11DS. The fractional anisotropy effect size profiles show that BD is similar to SZ for some regions and similar to MDD in other regions. In addition, the pattern of fractional anisotropy effect sizes appears more similar between SZ and MDD, than either one of these disorders and BD, and for each of these disorders is very different from those reported for 22q11DS. Regional cortical thickness and surface area show between a 2.6 to 6 times higher correlation between SZ and BD than between SZ or BD and MDD (see Figures 3b & 4b). Likewise, the effect sizes of cortical thickness and surface area of 22q11DS with psychosis compared to 22q11DS without psychosis were more comparable to the effect sizes of cortical thickness and surface area of SZ and BD than MDD. Both these findings are consistent with the substantial genetic overlap between SZ and BD reported in large-scale psychiatric genomics studies76. The studies reviewed also suggest significant medication effects, with lithium generally associated with larger regional volumes and thicker cortex, antipsychotics and anticonvulsants associated with smaller volumes and cortical thickness, and antidepressants associated with minimal effects on brain structure; though none of these findings can be interpreted as direct effects as they are based on cross sectional data and await confirmation via randomized clinical trials. Taken together, these novel insights have been obtained from the systematic application of standardized analytical protocols established through the ENIGMA project.
Comparisons of within-disorder case-control effect sizes from independent studies have reported high correlations. For instance, correlations of schizophrenia versus control deep brain structure effect size profiles based on three independent reports -the ENIGMA Schizophrenia Working Group51, Cognitive Genetics Collaborative Research Organization (COCORO)86,87, and a third independent cohort11- showed correlations of about 0.98; for review see88. Similarly, case-control fractional anisotropy effect sizes based on ENIGMA47,73,74 and COCORO89 studies showed high (schizophrenia=0.94; bipolar disorder=0.79) to moderate correlations (MDD r=0.47); for review see32. These findings suggest that large meta- and mega-analyses can generate reproducible structural image profiles for neuropsychiatric disorders.
Several studies have compared effect sizes for neuroanatomical abnormalities between disorders6,63,70,90. Similar to the current review, Opel and colleagues (2020) performed a cross-disorder analysis of brain structure differences between healthy volunteers and individuals with a psychiatric disorder based upon regional effect sizes. In their cross disorder analysis, they computed correlations between combined effect sizes of both regional cortical thickness and subcortical volumes acquired from mega- and meta-analyses published by the ENIGMA consortium90; though they combined subcortical volumes and cortical thickness and the type of correlation reported (e.g., Pearson or Spearman) is unclear. They found that brain structural abnormalities in BD, SZ and obsessive-compulsive disorder were highly correlated. The most pronounced correlations observed were similar to those observed in our cross-disorder analysis, namely between BD and SZ. Our review adds to the findings by Opel and colleagues (2020) in that it reports separate Spearman correlations for deep brain structures and cortical thickness as well as for surface area and fractional anisotropy and that it included effect sizes for 22q11DS versus controls and 22q11DS with versus without psychosis. More recently, Koshiyama et al.89 reported cross-disorder analysis of white matter abnormalities. Consistent with our between-disorder comparisons of case-control group differences in effect sizes in fractional anisotropy, they found that SZ and BD showed more severe effects than MDD when compared to controls. However, our Spearman rank correlations suggest more overlap in the overall brain pattern of white matter structural abnormalities between SZ and MDD than between BD and either SZ or MDD; perhaps because some of the regions of interest in BD show similar effect sizes to those observed in SZ, while others show similar effect sizes to those observed in MDD.
Overall, our findings suggest that mean multivariate patterns of brain abnormalities in psychiatric disorders provide additional information beyond the comparisons of individual brain regions between disorders. For instance, the pattern of higher similarity in cortical thickness and surface area between SZ and BD than between either disorder and MDD mimics the level of genetic overlap between these three disorders. This overlap is consistent with prior findings that have directly tested genetic overlap in risk for SZ and brain structure. Direct assessments of pleiotropy for disorders and brain structure have found evidence for the shared genetic basis of SZ and regional cortical thickness and surface area91,92 and are starting to examine such contributions based on cross disorder data92. However, it is unknown whether group-level multivariate patterns of brain abnormalities are informative with regard to identifying individuals or clusters of individuals who may be more similar, either genetically, with regard to symptom profiles, treatment, or prognosis.
Several studies have started assessing individual subject level multivariate structural brain patterns either based on the modeling of data of individual samples93–97 or by making use of expected patterns of abnormalities reported in meta- and mega-analyses32,98. What these studies seem to suggest is that while mean patterns within a disorder appear to be highly replicable, there is substantial heterogeneity among patients within each of these diagnostic categories. More work is needed to identify the most informative multivariate brain pattern measures for different brain imaging modalities or whether multivariate brain patterns can help identify new genetic risk loci. In addition, more work is needed to determine whether multivariate brain patterns map onto Research Domain Criteria (RDoC) dimensions that may move us away from treating categorical diagnosis to treating dimensions of psychopathology99,100. Our ability to ultimately make individual level predictions of outcomes or prognosis, is particularly relevant in the area of clinical high risk for psychosis101. This type of individual-level predictions will require resolution of this heterogeneity either through identification of clusters of patients with similar genetic, behavioral, and/or brain profiles or the use of more advanced models. Those models will ideally make use of objective data; possibly including genomic and multi-modal imaging data and also taking advantage of developments in classification and predictive capabilities provided by machine and deep learning, and Bayesian statistics.
One strength of the study is that the effect sizes used for the cross disorder comparisons are based on some of the largest meta- and mega- analyses published to date, which have shown highly replicable results. Another strength is that the effect sizes of each disorder were obtained using similar imaging, quality assurance, and statistical methods and that the figures presented are all on the same scale, which facilitates comparisons between groups. Finally, this study compared the effect sizes from all the structural phenotypes for these four disorders from ENIGMA meta- and mega-analyses published to date.
Several weaknesses should be noted. First, given that we set out to rank order effect sizes of brain abnormalities within each disorder, we based our current between-disorder comparisons on visual review of the effect size patterns and Spearman rank correlations. The advantage of Spearman rank correlations is that they avoid assumptions of normal distribution of data. However, comparisons using Pearson’s correlations, intraclass correlations (consistency, or absolute agreement), or similarity/dissimilarity matrices could yield additional insights and should be explored in future work. Second, the effect sizes compared in the current review are mostly based on individuals with disorders who were medicated and thus the findings may be confounded by treatment. More reports on effect sizes for unmedicated or mediation-naive patient groups are needed in order to compare morphological similarities and differences between disorders independent of treatment effects. Third, this study does not take into account possible overlap in controls between some of the ENIGMA studies. Though, an ongoing cross-disorder mega-analyses is removing any overlapping controls from its analysis. Fourth, the profiles reported in this review are based on data from regions of interest (ROI) and it is likely that profiles based on voxel wise or vertex wise analysis (e.g., cortical or deep brain structure shape, once completed by all Working Groups) will provide additional information. Finally, a limitation of the ENIGMA studies thus far has been that the analysis pipelines have predominantly relied on FreeSurfer102 and FSL’s TBSS103,104. These analysis platforms produce highly reliable brain measures but replication of findings using additional imaging methods could guard against possible biases in image analysis software.
In conclusion, this study reviewed the patterns of effect sizes for structural brain abnormalities in psychiatric disorders as well as influences of illness characteristics (e.g., recurrent depressive episodes, symptom severity) and their treatments; sometimes in opposing directions. While deep brain structure volumes are more affected in SZ>BD>MDD, their cross-region effect sizes rankings are very highly correlated within these three disorders and even moderately correlated with those of 22q11DS. The ranking of fractional anisotropy effect sizes appears more similar between schizophrenia and MDD, than either one of these disorders and bipolar disorder, and for each of these disorders is very different from those reported for 22q11DS. Finally, regional cortical thickness and surface area show higher correlation between SZ (idiopathic and 22q11DS psychosis) and BD, than between SZ or BD and MDD, consistent with shared genetic or environmental influences for SZ and BD.
Acknowledgments
The research studies produced by the ENIGMA Working Groups would not be possible without the contributions of many researchers across the globe and the authors of this review thank all scientists who contribute to making this work possible. A full list of ENIGMA Consortium current and past members can be found here http://enigma.ini.usc.edu/ongoing/members/. The authors acknowledge the NIH Big Data to Knowledge (BD2K) award for foundational support and consortium development (U54 EB020403 to PMT) and support from NIMH R01MH116147 (PMT), NIMH R01MH121246 (JAT, TGMvE, VDC), NIMH R01MH117601 (NJ, LS), NIMH R01MH085953 (CEB), NIMH R21MH116473 (CEB), NIMH 1U01MH119736 (CEB). For a complete list of ENIGMA-related grant support please see here: http://enigma.ini.usc.edu/about-2/funding.
Footnotes
Disclosure Statement
Authors CRKC, NJ, and PMT received partial research support from Biogen, Inc., for research unrelated to this manuscript. OAA is a consultant to HealthLytix, and has received speaker’s honorarium from Lundbeck and Sunovion. None of the other authors have any conflicts or competing interests to report.
References
- 1.Ioannidis JPA. Why Science Is Not Necessarily Self-Correcting. Perspectives on Psychological Science. 2012; 7: 645–54. [DOI] [PubMed] [Google Scholar]
- 2.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci 2017; 18: 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014; 505: 612–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005; 2: e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci 2013; 14: 365–76. [DOI] [PubMed] [Google Scholar]
- 6.Thompson PM, Jahanshad N, Ching CRK, Salminen LE, Thomopoulos SI, Bright J, et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry 2020; 10: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 2019; 16: 111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorgolewski KJ, Alfaro-Almagro F, Auer T, Bellec P, Capotă M, Chakravarty MM, et al. BIDS apps: Improving ease of use, accessibility, and reproducibility of neuroimaging data analysis methods. PLoS Comput. Biol 2017; 13: e1005209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy MA, Dufour SC, Gray JC. The association between child alcohol sipping and alcohol expectancies in the ABCD study. Drug Alcohol Depend. 2021; 221: 108624. [DOI] [PubMed] [Google Scholar]
- 10.Ohi K, Ochi R, Noda Y, Wada M, Sugiyama S, Nishi A, et al. Polygenic risk scores for major psychiatric and neurodevelopmental disorders contribute to sleep disturbance in childhood: Adolescent Brain Cognitive Development (ABCD) Study. Transl. Psychiatry 2021; 11: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alnæs D, Kaufmann T, van der Meer D, Córdova-Palomera A, Rokicki J, Moberget T, et al. Brain Heterogeneity in Schizophrenia and Its Association With Polygenic Risk. JAMA Psychiatry. 2019; 76: 739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Principles Stranger M. and Practice in Biobank Governance. Routledge; 2016. [Google Scholar]
- 13.Littlejohns TJ, Holliday J, Gibson LM, Garratt S, Oesingmann N, Alfaro-Almagro F, et al. The UK Biobank imaging enhancement of 100,000 participants: rationale, data collection, management and future directions. Nat. Commun 2020; 11: 2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gau R, Noble S, Heuer K, Bottenhorn KL, Bilgin IP, Yang Y-F, et al. Brainhack: Developing a culture of open, inclusive, community-driven neuroscience. Neuron. 2021; 109: 1769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandrowski AE, Martone ME. RRIDs: A Simple Step toward Improving Reproducibility through Rigor and Transparency of Experimental Methods. Neuron. 2016; 90: 434–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maumet C, Auer T, Bowring A, Chen G, Das S, Flandin G, et al. Sharing brain mapping statistical results with the neuroimaging data model. Sci Data. 2016; 3: 160102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koslow SH. Should the neuroscience community make a paradigm shift to sharing primary data? Nat. Neurosci 2000; 3: 863–5. [DOI] [PubMed] [Google Scholar]
- 18.Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat. Genet 2012; 44: 552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015; 520: 224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satizabal CL, Adams HHH, Hibar DP, White CC, Knol MJ, Stein JL, et al. Genetic architecture of subcortical brain structures in 38,851 individuals. Nat. Genet 2019; 51: 1624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renteria M. ENIGMA and the PGC: Converging Discoveries From Psychiatric and Neuroimaging Genomics: Insights Into the Shared Genetic Architecture of Subcortical Brain Structures and Complex Human Traits. 2021; Available from https://www.biologicalpsychiatryjournal.com/article/S0006-3223(21)00184-0/pdf
- 22.Campos A, Rabinowitz J, Jahanshad N, Thompson P, Medland S, Renteria M. Polygenic Prediction of Subcortical Volumes and Cross ancestry Validation. In: Abstract Book of the 27th annual meeting of the Organization for Human Brain Mapping. 2021. p. 70. [Google Scholar]
- 23.Erratum for the Research Article “The genetic architecture of the human cerebral cortex,” by Grasby KL et al. Science. 2021; 374. Available from 10.1126/science.abm7211 [DOI] [PubMed] [Google Scholar]
- 24.Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, et al. The genetic architecture of the human cerebral cortex. Science. 2020; 367: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013; 381: 1371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hettema JM, Verhulst B, Chatzinakos C, Bacanu S-A, Chen C-Y, Ursano RJ, et al. Genome-wide association study of shared liability to anxiety disorders in Army STARRS. Am. J. Med. Genet. B Neuropsychiatr. Genet 2020; 183: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014; 8: 153–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson PM, Andreassen OA, Arias-Vasquez A, Bearden CE, Boedhoe PS, Brouwer RM, et al. ENIGMA and the individual: Predicting factors that affect the brain in 35 countries worldwide. Neuroimage. 2017; 145: 389–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmaal L, Pozzi E, C Ho T, van Velzen LS, Veer IM, Opel N, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl. Psychiatry 2020; 10: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bearden CE, Thompson PM. Emerging Global Initiatives in Neurogenetics: The Enhancing Neuroimaging Genetics through Meta-analysis (ENIGMA) Consortium. Neuron. 2017; 94: 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ching CRK, Hibar DP, Gurholt TP, Nunes A, Thomopoulos SI, Abé C, et al. What we learn about bipolar disorder from large-scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Hum. Brain Mapp 2020; Available from 10.1002/hbm.25098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochunov P, Hong LE, Dennis EL, Morey RA, Tate DF, Wilde EA, et al. ENIGMA-DTI: Translating reproducible white matter deficits into personalized vulnerability metrics in cross-diagnostic psychiatric research. Hum. Brain Mapp 2020; Available from 10.1002/hbm.24998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoogman M, van Rooij D, Klein M, Boedhoe P, Ilioska I, Li T, et al. Consortium neuroscience of attention deficit/hyperactivity disorder and autism spectrum disorder: The ENIGMA adventure. Hum. Brain Mapp 2020; Available from 10.1002/hbm.25029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong X-Z, Postema MC, Guadalupe T, de Kovel C, Boedhoe PSW, Hoogman M, et al. Mapping brain asymmetry in health and disease through the ENIGMA consortium. Hum. Brain Mapp 2020; Available from 10.1002/hbm.25033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sønderby IE, Ching CRK, Thomopoulos SI, van der Meer D, Sun D, Villalon-Reina JE, et al. Effects of copy number variations on brain structure and risk for psychiatric illness: Large-scale studies from the ENIGMA working groups on CNVs. Hum. Brain Mapp 2021; Available from 10.1002/hbm.25354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sisodiya SM, Whelan CD, Hatton SN, Huynh K, Altmann A, Ryten M, et al. The ENIGMA-Epilepsy working group: Mapping disease from large data sets. Hum. Brain Mapp 2020; Available from 10.1002/hbm.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Heuvel OA, Boedhoe PSW, Bertolin S, Bruin WB, Francks C, Ivanov I, et al. An overview of the first 5 years of the ENIGMA obsessive-compulsive disorder working group: The power of worldwide collaboration. Hum. Brain Mapp 2020; Available from 10.1002/hbm.24972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- 39.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 2009; 19: 2728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010; 53: 1135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, et al. Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cereb. Cortex 2015; 25: 2204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, et al. Differential Longitudinal Changes in Cortical Thickness, Surface Area and Volume across the Adult Life Span: Regions of Accelerating and Decelerating Change. Journal of Neuroscience. 2014; 34: 8488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Norbom LB, Ferschmann L, Parker N, Agartz I, Andreassen OA, Paus T, et al. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: Integrating macro- and microstructural MRI findings. Prog. Neurobiol 2021; 204: 102109. [DOI] [PubMed] [Google Scholar]
- 44.Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PCM. Three-Dimensional Atlas of Brain White Matter Tracts. 2005. [Google Scholar]
- 45.Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA–DTI working group. NeuroImage. 2013; 81: 455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frodl T, Janowitz D, Schmaal L, Tozzi L, Dobrowolny H, Stein DJ, et al. Childhood adversity impacts on brain subcortical structures relevant to depression. J. Psychiatr. Res 2017; 86: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abé C, et al. Correction: Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology. 2019; 44: 2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villalón-Reina JE, Martínez K, Qu X, Ching CRK, Nir TM, Kothapalli D, et al. Altered white matter microstructure in 22q11.2 deletion syndrome: a multisite diffusion tensor imaging study. Mol. Psychiatry 2020; 25: 2818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol. Med 2020; 50: 1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat. Med 2017; 36: 855–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 2016; 21: 547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franke B, Stein JL, Ripke S, Anttila V, Hibar DP, van Hulzen KJE, et al. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat. Neurosci 2016; 19: 420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smeland OB, Wang Y, Frei O, Li W, Hibar DP, Franke B, et al. Genetic Overlap Between Schizophrenia and Volumes of Hippocampus, Putamen, and Intracranial Volume Indicates Shared Molecular Genetic Mechanisms. Schizophr. Bull 2018; 44: 854–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Campbell M, Jahanshad N, Mufford M, Choi KW, Lee P, Ramesar R, et al. Overlap in genetic risk for cross-disorder vulnerability to mental disorders and genetic risk for altered subcortical brain volumes. J. Affect. Disord 2021; 282: 740–56. [DOI] [PubMed] [Google Scholar]
- 55.Hibar DP, Westlye LT, van Erp TGM, Rasmussen J, Leonardo CD, Faskowitz J, et al. Subcortical volumetric abnormalities in bipolar disorder. Mol. Psychiatry 2016; 21: 1710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haukvik UK, Gurholt TP, Nerland S, Elvsåshagen T, Akudjedu TN, Alda M, et al. In vivo hippocampal subfield volumes in bipolar disorder-A mega-analysis from The Enhancing Neuro Imaging Genetics through Meta-Analysis Bipolar Disorder Working Group. Hum. Brain Mapp 2020; Available from 10.1002/hbm.25249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McWhinney SR, Abé C, Alda M, Benedetti F, Bøen E, Del Mar Bonnin C, et al. Association between body mass index and subcortical brain volumes in bipolar disorders-ENIGMA study in 2735 individuals. Mol. Psychiatry 2021; Available from 10.1038/s41380-021-01098-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmaal L, Veltman DJ, van Erp TGM, Sämann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry 2016; 21: 806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rentería ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl. Psychiatry 2017; 7: e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campos AI, Thompson PM, Veltman DJ, Pozzi E, van Veltzen LS, Jahanshad N, et al. Brain Correlates of Suicide Attempt in 18,925 Participants Across 18 International Cohorts. Biol. Psychiatry 2021; Available from 10.1016/j.biopsych.2021.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho TC, Gutman B, Pozzi E, Grabe HJ, Hosten N, Wittfeld K, et al. Subcortical Shape Alterations in Major Depressive Disorder: Findings from the ENIGMA Major Depressive Disorder Working Group. Hum. Brain Mapp 2020; Available from 10.1101/534370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Opel N, Thalamuthu A, Milaneschi Y, Grotegerd D, Flint C, Leenings R, et al. Correction: Brain structural abnormalities in obesity: relation to age, genetic risk, and common psychiatric disorders. Mol. Psychiatry 2021; Available from 10.1038/s41380-021-01191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ching CRK, Gutman BA, Sun D, Villalon Reina J, Ragothaman A, Isaev D, et al. Mapping Subcortical Brain Alterations in 22q11.2 Deletion Syndrome: Effects of Deletion Size and Convergence With Idiopathic Neuropsychiatric Illness. Am. J. Psychiatry 2020; 177: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol. Psychiatry 2018; 84: 644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walton E, Hibar DP, van Erp TGM, Potkin SG, Roiz-Santiañez R, Crespo-Facorro B, et al. Positive symptoms associate with cortical thinning in the superior temporal gyrus via the ENIGMA Schizophrenia consortium. Acta Psychiatr. Scand 2017; 135: 439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walton E, Hibar DP, van Erp TGM, Potkin SG, Roiz-Santiañez R, Crespo-Facorro B, et al. Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol. Med 2018; 48: 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry 2018; 23: 932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 2017; 22: 900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol. Psychiatry 2020; Available from 10.1038/s41380-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun D, Ching CRK, Lin A, Forsyth JK, Kushan L, Vajdi A, et al. Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size. Mol. Psychiatry 2020; 25: 2818–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Opel N, Thalamuthu A, Milaneschi Y, Grotegerd D, Flint C, Leenings R, et al. Brain structural abnormalities in obesity: relation to age, genetic risk, and common psychiatric disorders : Evidence through univariate and multivariate mega-analysis including 6420 participants from the ENIGMA MDD working group. Mol. Psychiatry 2020; Available from 10.1038/s41380-020-0774-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leerssen J, Blanken TF, Pozzi E, Jahanshad N, Aftanas L, Andreassen OA, et al. Brain structural correlates of insomnia severity in 1053 individuals with major depressive disorder: results from the ENIGMA MDD Working Group. Translational Psychiatry. 2020; 10. Available from 10.1038/s41398-020-01109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry 2017; 23: 1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatry 2020; 25: 1511–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cardno AG, Owen MJ. Genetic Relationships Between Schizophrenia, Bipolar Disorder, and Schizoaffective Disorder. Schizophrenia Bulletin. 2014; 40: 504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet 2013; 45: 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Zwarte SMC, Brouwer RM, Agartz I, Alda M, Aleman A, Alpert KI, et al. The Association Between Familial Risk and Brain Abnormalities Is Disease Specific: An ENIGMA-Relatives Study of Schizophrenia and Bipolar Disorder. Biol. Psychiatry 2019; 86: 545–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree MBM, et al. Psychiatric Disorders From Childhood to Adulthood in 22q11.2 Deletion Syndrome: Results From the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. American Journal of Psychiatry. 2014; 171: 627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Erp TGM, Preda A, Turner JA, Callahan S, Calhoun VD, Bustillo JR, et al. Neuropsychological profile in adult schizophrenia measured with the CMINDS. Psychiatry Res. 2015; 230: 826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alexander-Bloch AF, Shou H, Liu S, Satterthwaite TD, Glahn DC, Shinohara RT, et al. On testing for spatial correspondence between maps of human brain structure and function. Neuroimage. 2018; 178: 540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lariviere S, Paquola C, Park B-Y, Royer J, Wang Y, Benkarim O, et al. The ENIGMA Toolbox: Cross-disorder integration and multiscale neural contextualization of multisite neuroimaging datasets. Cold Spring Harbor Laboratory. 2020; : 2020.12.21.423838. [Cited 2021 Jan 26] Available from https://www.biorxiv.org/content/10.1101/2020.12.21.423838v2.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Writing Committee for the ENIGMA-CNV Working Group, van der Meer D, Sønderby IE, Kaufmann T, Walters GB, Abdellaoui A, et al. Association of Copy Number Variation of the 15q11.2 BP1-BP2 Region With Cortical and Subcortical Morphology and Cognition. JAMA Psychiatry. 2020; 77: 420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee NR, Adeyemi EI, Lin A, Clasen LS, Lalonde FM, Condon E, et al. Dissociations in Cortical Morphometry in Youth with Down Syndrome: Evidence for Reduced Surface Area but Increased Thickness. Cereb. Cortex 2016; 26: 2982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manning KE, Tait R, Suckling J, Holland AJ. Grey matter volume and cortical structure in Prader-Willi syndrome compared to typically developing young adults. Neuroimage Clin. 2018; 17: 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fountain DM, Schaer M, Mutlu AK, Schneider M, Debbané M, Eliez S. Congenital heart disease is associated with reduced cortical and hippocampal volume in patients with 22q11.2 deletion syndrome. Cortex. 2014; 57: 128–42. [DOI] [PubMed] [Google Scholar]
- 86.Okada N, Fukunaga M, Yamashita F, Koshiyama D, Yamamori H, Ohi K, et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 2016; 21: 1460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koshiyama D, Miura K, Nemoto K, Okada N, Matsumoto J, Fukunaga M, et al. Neuroimaging studies within Cognitive Genetics Collaborative Research Organization aiming to replicate and extend works of ENIGMA. Hum. Brain Mapp 2020; Available from 10.1002/hbm.25040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kochunov P, Thompson PM, Hong LE. Toward High Reproducibility and Accountable Heterogeneity in Schizophrenia Research. JAMA Psychiatry. 2019; 76: 680–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koshiyama D, Fukunaga M, Okada N, Morita K, Nemoto K, Usui K, et al. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol. Psychiatry 2020; 25: 883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Opel N, Goltermann J, Hermesdorf M, Berger K, Baune BT, Dannlowski U. Cross-Disorder Analysis of Brain Structural Abnormalities in Six Major Psychiatric Disorders: A Secondary Analysis of Mega- and Meta-analytical Findings From the ENIGMA Consortium. Biological Psychiatry. 2020; 88: 678–86. [DOI] [PubMed] [Google Scholar]
- 91.Cheng W, Frei O, van der Meer D, Wang Y, O’Connell KS, Chu Y, et al. Genetic Association Between Schizophrenia and Cortical Brain Surface Area and Thickness. JAMA Psychiatry. 2021; Available from 10.1001/jamapsychiatry.2021.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee PH, Baker JT, Holmes AJ, Jahanshad N, Ge T, Jung J-Y, et al. Partitioning heritability analysis reveals a shared genetic basis of brain anatomy and schizophrenia. Mol. Psychiatry 2017; 22: 1224. [DOI] [PubMed] [Google Scholar]
- 93.Doucet GE, Moser DA, Rodrigue A, Bassett DS, Glahn DC, Frangou S. Person-Based Brain Morphometric Similarity is Heritable and Correlates With Biological Features. Cereb. Cortex 2019; 29: 852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doucet GE, Lin D, Du Y, Fu Z, Glahn DC, Calhoun VD, et al. Personalized estimates of morphometric similarity in bipolar disorder and schizophrenia. NPJ Schizophr. 2020; 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Janssen J, Díaz-Caneja CM, Alloza C, Schippers A, de Hoyos L, Santonja J, et al. Dissimilarity in Sulcal Width Patterns in the Cortex can be Used to Identify Patients With Schizophrenia With Extreme Deficits in Cognitive Performance. Schizophr. Bull 2021; 47: 552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lv J, Di Biase M, Cash RFH, Cocchi L, Cropley VL, Klauser P, et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. Mol. Psychiatry 2020; Available from 10.1038/s41380-020-00882-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wolfers T, Doan NT, Kaufmann T, Alnæs D, Moberget T, Agartz I, et al. Mapping the Heterogeneous Phenotype of Schizophrenia and Bipolar Disorder Using Normative Models. JAMA Psychiatry. 2018; 75: 1146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kochunov P, Fan F, Ryan MC, Hatch KS, Tan S, Jahanshad N, et al. Translating ENIGMA schizophrenia findings using the regional vulnerability index: Association with cognition, symptoms, and disease trajectory. Hum. Brain Mapp 2020; Available from 10.1002/hbm.25045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014; 13: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krueger RF, DeYoung CG. The RDoC initiative and the structure of psychopathology. Psychophysiology. 2016; 53: 351–4. [DOI] [PubMed] [Google Scholar]
- 101.ENIGMA Clinical High Risk for Psychosis Working Group, Jalbrzikowski M, Hayes RA, Wood SJ, Nordholm D, Zhou JH, et al. Association of Structural Magnetic Resonance Imaging Measures With Psychosis Onset in Individuals at Clinical High Risk for Developing Psychosis: An ENIGMA Working Group Mega-analysis. JAMA Psychiatry. 2021; 78: 753–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fischl B. FreeSurfer. Neuroimage. 2012; 62: 774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31: 1487–505. [DOI] [PubMed] [Google Scholar]
- 104.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat. Protoc 2007; 2: 499–503. [DOI] [PubMed] [Google Scholar]
- 105.Holleran L, Kelly S, Alloza C, Agartz I, Andreassen OA, Arango C, et al. The Relationship Between White Matter Microstructure and General Cognitive Ability in Patients With Schizophrenia and Healthy Participants in the ENIGMA Consortium. Am. J. Psychiatry 2020; 177: 537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nunes A, Schnack HG, Ching CRK, Agartz I, Akudjedu TN, Alda M, et al. Using structural MRI to identify bipolar disorders - 13 site machine learning study in 3020 individuals from the ENIGMA Bipolar Disorders Working Group. Mol. Psychiatry 2020; 25: 2130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Website. [Cited 2021 Apr 2] Available from Han, M LK et al. Brain aging in major depressive disorder: results from the ENIGMA Major Depressive Disorder working group. bioRxiv. 10.1101/560623 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Kovel CGF, Aftanas L, Aleman A, Alexander-Bloch AF, Baune BT, Brack I, et al. No Alterations of Brain Structural Asymmetry in Major Depressive Disorder: An ENIGMA Consortium Analysis. Am. J. Psychiatry 2019; 176: 1039–49. [DOI] [PubMed] [Google Scholar]