Abstract
Background:
Phlebotomy-induced anemia (PIA) is universal and variable in degree among preterm infants and may contribute to neurodevelopmental risk. In mice, PIA causes brain tissue hypoxia, iron deficiency, and long-term sex-dependent neurobehavioral abnormalities. The neuroregulatory molecular pathways disrupted by PIA underlying these effects are unknown.
Methods:
Male and female pups were phlebotomized daily from postnatal day (P)3-P14 via facial venipuncture to target hematocrits of 25% (moderate, mPIA) and 18% (severe, sPIA). P14 hippocampal RNA from non-bled control and PIA mice was sequenced by Next-Generation Sequencing to identify differentially-expressed-genes (DEGs) that were analyzed using Ingenuity Pathway Analysis.
Results:
mPIA females showed the least DEGs (0.5% of >22,000 genes) whereas sPIA females had the most (8.6%), indicating a dose-dependent effect. mPIA and sPIA males showed similar changes in gene expression (5.3% and 4.7%, respectively), indicating a threshold effect at mPIA. The pattern of altered genes induced by PIA indicate sex-specific and anemia-dose-dependent effects with increased pro-inflammation in females and decreased neurodevelopment in males.
Conclusion:
These gene-expression changes may underlie the reduced recognition memory function in male and abnormal social-cognitive behavior in female adult mice following neonatal PIA. These results parallel clinical studies demonstrating sex-specific behavioral outcomes as a function of neonatal anemia.
Introduction
Preterm infants are at risk for lifelong neurodevelopmental deficits.1–3 The vulnerability of the preterm infant’s brain can be attributed in part to its high metabolic demands during rapid development after 24 weeks post-conceptional age.4 This exponential growth, characterized by neuronal differentiation, spinogenesis, and synaptogenesis,1 is highly dependent on the delivery of substrates such as oxygen and iron that support mitochondrial energy production.5
Phlebotomy-induced anemia (PIA) is nearly universal in extremely preterm infants. A wide range of anemia is tolerated by clinicians and not all neonates are affected equally by it, making it difficult to ascertain the hematocrit levels at which to maintain preterm infants without risk to neurodevelopment. Frequent iatrogenic phlebotomy in neonates can account for loss of 15–30% of total blood volume every week for the first four postnatal weeks leading to reduced substrate availability for the developing brain because of compromised iron and oxygen delivery.6 PIA in a developmentally-timed preclinical mouse model induces hippocampal iron deficiency and hypoxia and alters the activity of the mTOR signaling pathway.7 mTOR signaling regulates neuronal actin polymerization, autophagy, and RNA translation rate during neural development.7,8 Compromised hippocampal oxidative metabolism during this critical developmental period is detrimental to overall neuronal growth and differentiation9 and mitochondrial motility,10 in turn altering neural networks and connectivity,11 and resulting in short- and long-term neurobehavioral abnormalities.12
Beyond reducing critical substrates, anemia has also been shown to alter tissue inflammatory responses.13,14 PIA in the developing mouse pup increases intestinal inflammation and alters macrophage function in the gut, thereby creating a self-sustaining pro-inflammatory environment.13,14 Whether PIA also induces a similar pro-inflammatory environment in the developing brain is unknown. As such, premature infants’ brains may be at higher risk of anemia-induced pro-inflammatory states because they have a compromised innate anti-inflammatory response to counter neonatal pro-inflammatory diseases.15 Inflammation is toxic to both gray and white matter development and integrity in preterm infants.16 Thus, we investigated whether PIA induces a pro-inflammatory phenotype in the neonatal brain as it does in the intestine.13,14
In this study, we compared transcriptomic data from the hippocampus of moderately anemic (mPIA) mice phlebotomized to a hematocrit of 25% to those with a more severe anemia of 18% (sPIA) to assess dose-dependent responses to degree of neonatal anemia. These hematocrit levels mimic those found in preterm infants,17,18 providing a translational significance for the model. The objectives of this study were to determine whether neonatal PIA alters neuroinflammatory and neurodevelopmental pathways in the developing hippocampus in a dose- and sex-dependent manners and whether such changes in pattern of gene expression can account for the long-term social and memory behavioral deficits previously documented in this model.12
Methods
Animals
The study was conducted with the approval of the Institutional Animal Care and Use Committee at the University of Minnesota. C57Bl6/J mice were used for all the experiments. Pregnant and lactating dams were fed standard chow containing 200 ppm of iron (Envigo; Indianapolis, IN). They were given access to food and water ad libitum and maintained on a 14 h light/10 h dark cycle with the humidity and environmental temperature controlled per Research Animal Resources guidelines. Litters were culled to 9 pups at P2 or in the case of smaller litters, combined to maintain a similar numbers. This was done to reduce variability in growth rates resulting in disproportionate weight gain and rate of blood volume expansion.
Phlebotomy-Induced Anemia
Neonatal mice were phlebotomized from P3 to P14 via facial venipuncture using a micropipette as described previously.7 Pups were weighed daily to determine the volume of blood to be drawn. For both 25% and 18% hematocrit groups, mice were bled 5.25 μL/g twice daily until the desired hematocrit threshold was reached and then bled 3.5 μL/g daily to maintain target hematocrit levels. Hematocrit levels were measured daily by centrifugation of glass microhematocrit tubes quantification by hematocrit card reader. Non-bled controls (NBCs) received non-phlebotimizing needle prick to the nape of the neck each day. Pups from each litter were randomly chosen for the study groups in order to prevent litter effects of the outcome variables. A total of 60 pups from 22 dams were utilized in the study. All the pups within a litter spent an equal amount of time away from the nest and each litter was comprised of pups from both control and phlebotomized groups. All pups were handled similarly in order to minimize variability in separation effects.
Hippocampal RNA Isolation and Sequencing
Animals were euthanized with a fatal overdose of sodium pentobarbital at P14 and immediately decapitated per IACUC approved protocol. The hippocampus was isolated, flash-frozen in liquid nitrogen, and stored at −80°C until further use. Total hippocampal RNA was isolated from one lobe using the RNAqueous RNA Isolation kit (Ambion; Austin, TX) and quantified by Nanodrop P1000 (Thermo Scientific; Waltham, MA). For RNAseq analysis, 4 mice per experimental group were studied. The sample size was determined based upon previously described power calculations to optimize detection of differentially-expressed genes.19,20 RNA sequencing was performed by the University of Minnesota Genomics Center after quality check by RiboGreen RNA quantification (Invitrogen; Carlsbad, CA), and an Agilent 2100 Bioanalyzer (Agilent; Santa Clara, CA; Supplemental Table 1). RNA samples with RIN values ≥ 9.2 were used for library construction and sequenced at 50b paired-end (PE) at >25M reads/sample. For Real-time PCR validation, RNA was isolated from hippocampi in the same way as above (n=6/group) from a separate set of experimental mice. Selected mRNAs were quantified using Taqman gene expression probes (Supplemental Table 2) on a ThermoFisher QuantStudio 3 Real-Time PCR system. Tbp, which was not affected by iron deficiency,10 was used as a control gene.
Analysis of RNA-Sequencing Data
FastQ PE reads for samples (n=28.4M reads/sample) were trimmed using Trimmomatic (v 0.33) enabled with the optional “−q” option: 3bp sliding-window trimming 3’ requiring minimum Q30. Quality control on raw sequence data for each sample was performed using FastQC and read mapping was done with Hisat2 (v2.1.0) using the mouse genome (mm10) as reference. Gene quantification was done via Feature Counts for raw read counts. Finally, differentially-expressed genes (DEGs) were identified using the EdgeR feature in CLCGWB (Qiagen; Redwood City, CA) using raw read counts with p-value <0.05 and false discovery rate (FDR) <0.05 to adjust for multiple comparisons.
Ingenuity Pathway Analysis
The identified DEGs were analyzed using the knowledge-based Ingenuity Pathway Analysis (IPA) database (Qiagen), which has >7.8 million findings, to identify networks, canonical pathways, molecular and cellular functions, upstream and downstream regulators, and affected diseases that are altered by anemia relative to controls as previously described.20 Briefly, IPA core analyses were performed using cutoff criteria that include, P <0.05, FDR < 0.05 to account for multiple comparisons and eliminate false positives, absolute fold change of 1.3, and only direct relationships to functionally annotate the transcriptomic changes between NBCs and experimental groups (mPIA and sPIA of both sexes). Statistical significance of genes mapping onto a given pathway, regulator, function, or disease was determined by Fisher’s exact test;20 a significant p value (p<0.05) for each network indicates that the mapping was unlikely if, in fact, no association existed.
Statistical Analysis
Weight and hematocrit data for males and females were analyzed using a two-way ANOVA (Graphpad Prism v9; La Jolla, CA). qPCR data were analyzed using a two-factor analysis of variance (ANOVA, SAS version 9.4; Cary, NC), estimating the sex-specific levels of expression as compared to controls. Correlation analysis of inflammatory gene expression and synaptic plasticity gene expression was assessed using SAS version 9.4.
Data Sharing Statement
Original data are available upon request to corresponding author (tgisslen@umn.edu). RNAseq data have been deposited in GEO with acquisition number GSE179877.
Results
Hematocrit levels
Animals in the mPIA group reached the 25% hematocrit threshold at P7-P8 whereas animals in the sPIA group reached the target hematocrit of 18% at P10-P11 (Fig. 1A). Hematocrit levels between the two anemia groups were significantly different from each other by P9. On P14, both PIA groups had significantly lower mean hematocrits compared to NBCs that had a mean ± SEM hematocrit of 34.9% ± 0.3 (mPIA 22.0% ± 0.2; sPIA 17.3% ± 0.2; p<0.0001).
Figure 1: Hematocrit and Weight trajectories of mPIA and sPIA animals and distribution of novel and common dysregulated genes.
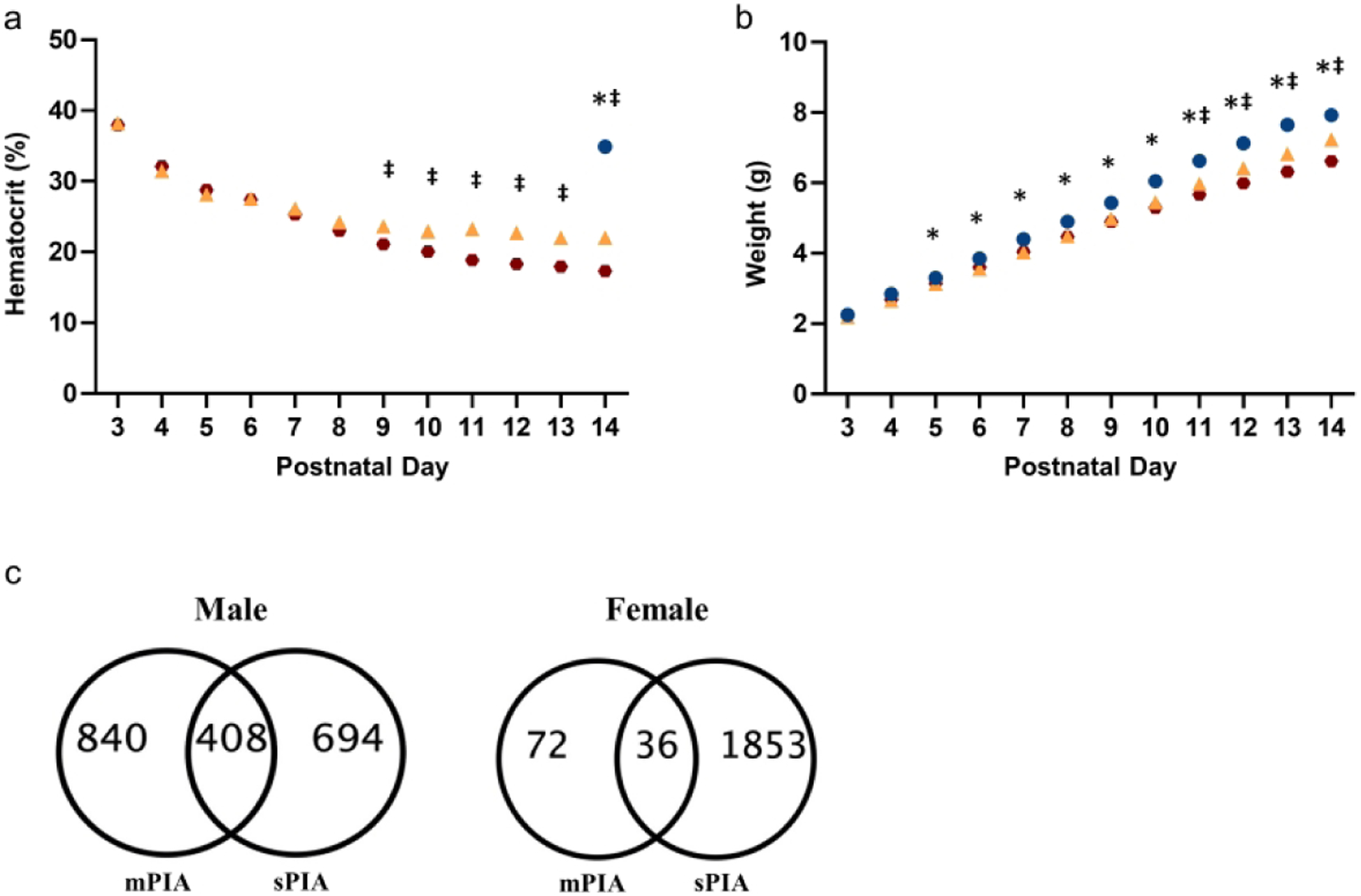
(A) Daily hematocrit values for each group. (B) Daily weight values for each group. Blue circles = NBC; Yellow triangles = mPIA; Red circles = sPIA. *p< 0.05 NBC vs mPIA and sPIA; ‡p<0.05 mPIA vs sPIA. Graphs represent males and females combined (n= 64–92). (C) mPIA and sPIA males experienced a similar degree of dysregulation with mPIA resulting in dysregulation of 840 genes unique to the mPIA phenotype and 694 genes unique to the sPIA phenotype. 408 genes were common to both cohorts. Females experienced relatively minor gene dysregulation at mPIA but were severely affects at sPIA with 1853 unique dysregulated genes.
PIA impairs weight gain velocity
PIA resulted in a dose-dependent slower growth rate (Fig. 1B). Starting at P7, the total group of phlebotomized pups had lower weights compared to NBCs. From P11, weights of phlebotomized pups diverged between the two groups; sPIA pups had lower weights than mPIA pups. By P14, the mPIA pups weighed 7.6% less than the NBCs (p=0.0003) and the sPIA pups weighed 15.3% less than NBCs (p<0.0001). Growth velocity for NBCs was 0.52 ± 0.007 g/day. mPIA and sPIA rates were lower at 0.46 ± 0.008 g/day and 0.42 ± 0.007 g/day, respectively. No significant differences in weight loss were observed between males and the females.
PIA induces distinct transcriptomic changes in male and female mouse hippocampus
Hippocampal transcriptome data obtained from mPIA and sPIA males and females were compared to those obtained from age-matched NBCs to determine dose- and sex-dependent effects. Final analysis included 4 animals per experimental group except for NBC males in which we removed one outlier based on principal component analysis (PCA) (Supplemental Fig. 1). Male and female mPIA and sPIA groups clustered according to sex and treatment within the dendrogram analysis (Supplemental Fig. 2), but male and female NBCs had more gene variability in this physiologic model than other groups.
Compared to NBCs, both mPIA and sPIA male pups had similar magnitudes of gene dysregulation (5.3% and 4.7% respectively of 23,221 genes), showing a threshold effect for gene disruption at 25% hematocrit (Table 1). Further analysis revealed a distinct pattern of gene expression with 840 vs 694 uniquely dysregulated genes in mPIA and sPIA respectively, with 408 genes in common between them (Fig. 1C). In contrast, female pups showed a lower magnitude of dysregulated genes (0.5%, 108 DEGs) at 25% hematocrit compared to males and a larger magnitude of dysregulated genes (8.6%, 1889 DEGs) at 18% hematocrit (Table 1). Thus, females demonstrated an anemia-dose dependent response as well as a lower hematocrit threshold response than males. While 36 DEGs were common between female anemia groups, anemia to 18% caused additional dysregulation of 1853 novel genes (Fig. 1C).
Table 1:
Differentially expressed genes (DEGs) in mPIA and sPIA males and females.
| mPIA | sPIA | mPIA | sPIA | |
|---|---|---|---|---|
| Genes sequenced | 23221 | 23221 | 23221 | 21,957 |
| DEGs | 1248 (5.3%) | 1102 (4.7%) | 108 (0.5%) | 1889 (8.6%) |
| Upregulated | 388 (31% DEGs) | 389 (35.3% DEGs) | 49 (45.3% DEGs) | 882 (46.6% DEGs) |
| Downregulated | 860 (69% DEGs) | 713 (64.7% DEGs) | 59 (54.7% DEGs) | 1007 (53.4% DEGs) |
Severe PIA increased neuroinflammatory signaling pathways in the female hippocampus
Analysis of the hippocampal transcriptome by IPA mapped PIA-induced gene expression changes onto functional networks that indicate disrupted activity of inflammatory regulators. These canonical pathways, which included neuroinflammation signaling and its clustered pathways, were upregulated in sPIA females (Fisher’s exact test p-value <0.05, z-score >1.3) (Fig. 2). Similarly, several upstream regulators important for inflammatory signaling including Interferon Regulatory Factors (IRFs), STAT1, NFATC2, and REL (Table 2) had z-scores > 2, indicating increased activation. These pathways were unchanged in mPIA females, confirming the dose-dependent effect in females seen in the overall transcriptomic analysis. In contrast, DEGs of both mPIA and sPIA male groups implicated downregulation of neuroinflammatory signaling and its clustered canonical pathways and a lack of activation of inflammatory upstream regulators at P14, indicating a sex-specific effect of PIA.
Figure 2: Comparison analysis of dysregulated canonical pathways in PIA males and females.
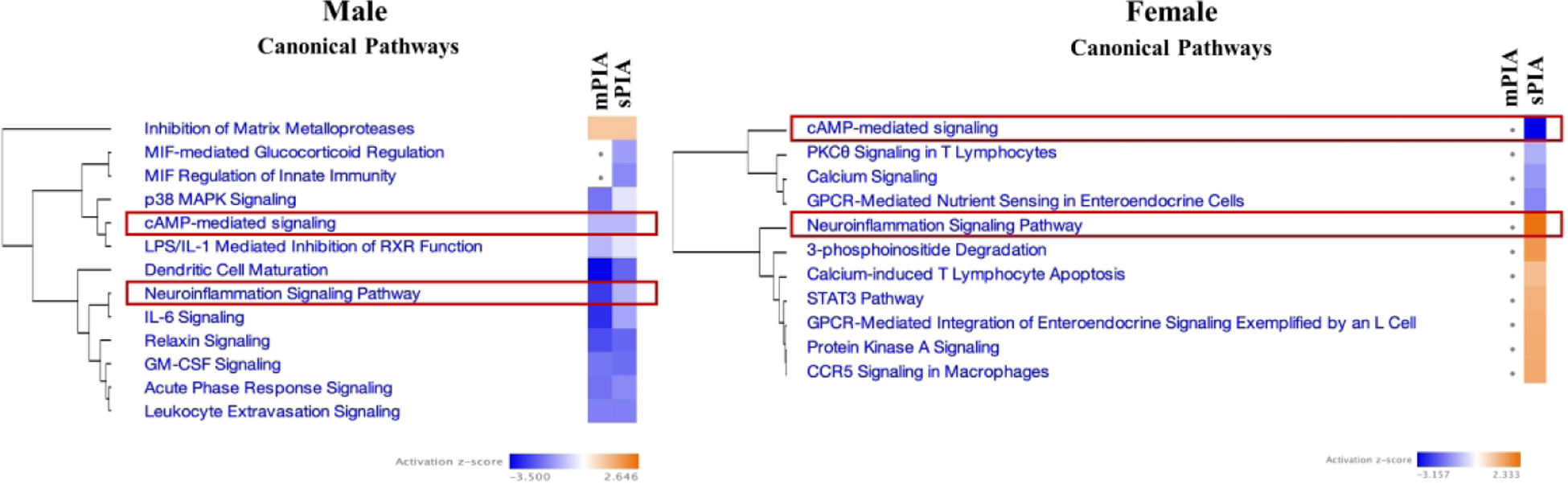
(A) Males showed similar downregulation of pathways regardless of degree of anemia whereas females (B) showed dysregulation following sPIA only. Both sexes showed dysregulation of neuroinflammatory and cAMP-mediated signaling; directionality was sex-dependent for neuroinflammatory signaling, downregulation predicted for males and upregulation predicted for females.
Table 2:
Up and downregulated upstream regulators with z-score ≥2 in mPIA and sPIA animals
| Upregulated Genes | Downregulated Genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| sPIA Males | sPIA Females | sPIA Males | sPIA Females | ||||||||
| Upstream Regulator | Activation z-score | Genes affected | Upstream Regulator | Activation z-score | Genes affected | Upstream Regulator | Activation z-score | Genes affected | Upstream Regulator | Activation z-score | Genes affected |
| HOXA10 | 2.11 | 11 | IRF7 | 3.741 | 19 | CREB1 | −3.385 | 37 | CREB1 | −3.819 | 93 |
| NEUROG1 | 2.0 | 4 | EPAS1 | 3.227 | 32 | CEBPB | −3.347 | 28 | TRIM24 | −3.208 | 17 |
| IRF3 | 3.212 | 20 | ESR1 | −3.181 | 84 | SOX1 | −3.162 | 10 | |||
| ESR1 | 3.123 | 117 | HTT | −3.046 | 47 | SOX3 | −3.162 | 10 | |||
| STAT1 | 3.093 | 44 | SIX5 | −2.828 | 8 | GMNN | −2.525 | 11 | |||
| STAT4 | 2.984 | 32 | ID1 | −2.777 | 8 | NKX2–1 | −2.43 | 21 | |||
| SPI1 | 2.63 | 24 | CREBBP | −2.578 | 34 | RUNX3 | −2.389 | 13 | |||
| EGR2 | 2.598 | 20 | SRF | −2.57 | 23 | RUNX1 | −2.384 | 17 | |||
| PARP1 | 2.409 | 16 | LHX1 | −2.496 | 13 | IRF4 | −2.359 | 14 | |||
| NFATC2 | 2.403 | 22 | CEBPA | −2.441 | 29 | TFAP2A | −2.348 | 8 | |||
| IRF1 | 2.384 | 17 | JUNB | −2.401 | 7 | NEUROG1 | −2.309 | 12 | |||
| HMGB1 | 2.379 | 7 | FOXO3 | −2.347 | 18 | NUPR1 | −2.271 | 38 | |||
| KLF4 | 2.273 | 36 | CEBPE | −2.335 | 8 | SS18 | −2.236 | 6 | |||
| HDAC9 | 2.236 | 5 | THRA | −2.232 | 10 | HOXA10 | −2.231 | 19 | |||
| REL | 2.22 | 28 | PLAG1 | −2.177 | 8 | HHEX | −2.211 | 8 | |||
| NFAT5 | 2.219 | 8 | HNF4A | −2.11 | 80 | KLF5 | −2.2 | 5 | |||
| NFKBIB | 2.157 | 12 | MRTFA | −2.045 | 11 | NR3C1 | −2.109 | 62 | |||
| HNF1B | 2.137 | 11 | NR3C2 | −2.03 | 10 | ||||||
| KDM5A | 2.132 | 9 | SATB1 | −2.025 | 11 | ||||||
| CTNNB1 | 2.068 | 84 | SIM1 | −2 | 21 | ||||||
| IRF5 | 2.038 | 11 | |||||||||
| RSF1 | 2 | 4 | |||||||||
| TGFB1I1 | 2 | 4 | |||||||||
STAT1 is a transcription factor involved in interferon receptor (IRF) signaling and was highly influenced by sex and PIA dose in our model. The patterns of gene expression changes indicate a switch in STAT1 signaling from predicted inhibition in mPIA males to predicted activation in sPIA males (Fig. 3). DEGs suggest increased STAT1 signaling in both female groups, but significantly fewer dysregulated pathway genes in mPIA females (5 genes, z-score <1) compared to sPIA females (131 genes, z-score >3), further corroborating the dose-dependent effect of PIA. There was direct involvement of IRF1, IRF3, IRF5, and IRF7 in the upregulation of STAT1 in sPIA females with overlap of downstream targets between STAT1 and IRF family genes including CXCL10 and IL-6 (Supplemental Fig. 3). To validate the changes observed in the transcriptomes, expression of CXCL10 and IL-6, both integral components of the STAT1 signaling pathway (Fig. 4A),21 was independently quantified by qPCR (Fig. 4C). Both genes showed increased expression in sPIA males compared to NBC males, confirming the indicated outcomes based on RNAseq data.
Figure 3: STAT1 dysregulation across PIA severity in males and females.
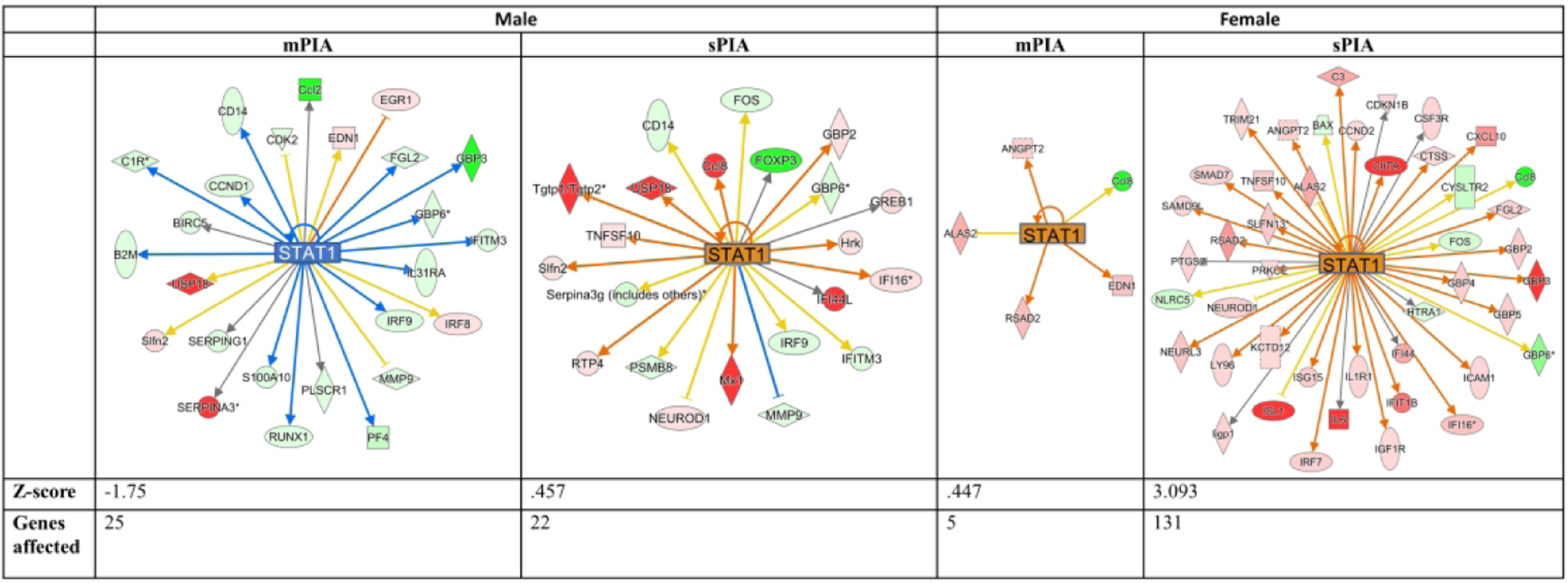
sPIA females are predicted to have a significant upregulation of the STAT1 pathway. sPIA and mPIA males showed a similar dysregulation but opposite directionality. Red- and green-filled shapes indicate up- and downregulation of genes, respectively, with brighter color indicating larger differences. Orange lines indicate activation; blue lines indicate inhibition; yellow lines indicate findings inconsistent with the state of downstream activity; gray lines indicate that the effect is not predicted.
Figure 4: Inflammatory and synaptic plasticity genes affected by PIA.
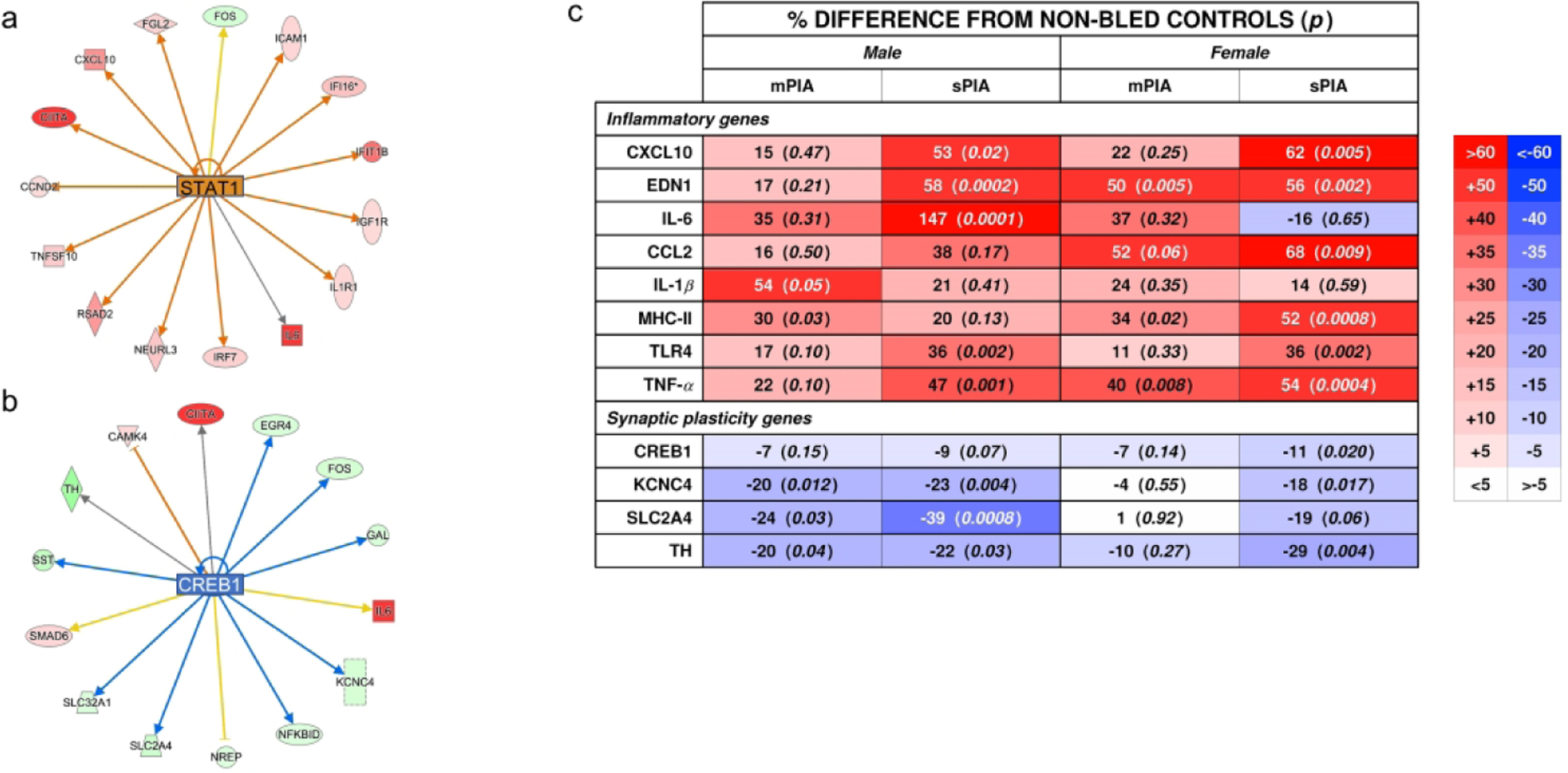
Selected genes from the (A) STAT1 pathway and (B) CREB1 pathway were selected for confirmatory qPCR analyses along with other common inflammatory genes. Percentage difference from NBCs and p values are summarized in panel (C). Color intensity increases for increased difference. There was increased dysregulation after 25%, indicating a threshold response for males. Females showed a dose-dependent response to hematocrit for most genes at 18%.
To further investigate inflammation within the hippocampus, expression of common inflammatory markers was assessed (Fig. 4C). qPCR results confirmed the upregulation of pro-inflammatory markers across both anemia groups in males and females. Most of these genes showed a hematocrit dose-dependent transcriptional response in both males and females. In contrast, expression of MHCII and IL-1β exhibited a threshold response in mPIA males. Expression of CCL2, MHCII, and TNF-α showed a threshold response in mPIA females and was further upregulated in sPIA females. PIA upregulated IL-6 expression in a dose-dependent manner in males, but induced a blunted response in females regardless of degree of anemia.
PIA altered neurodevelopmental pathways regulating memory and behavioral functions
We previously demonstrated that neonatal PIA negatively affects learning, memory, and social behavior in adulthood and does so differently between males and females.12 In the current study, large-scale dysregulation in gene pathways and networks important for neurodevelopment was present in mPIA and sPIA males and sPIA females. Both mPIA and sPIA males showed a similar degree of dysregulation of genes critical for embryonic, organismal, and tissue development in the hippocampus (Supplementary Table 3). In contrast, compared to mPIA females, sPIA females had a greater number of gene expression changes in gene networks regulating organismal development, nervous system development, and behavior, predicating more severe neurodevelopmental deficits. Further, the gene expression changes in sPIA females indicate decreased activity of multiple upstream regulators of neurodevelopment such as CREB1, NEUROG1, and S0X2 (Table 2, Fisher’s exact test p-value <0.001).
Given the importance of cAMP response element-binding protein (CREB) signaling in neurodevelopment, long-term memory formation, and neuronal cell survival via the regulation of reactive oxygen species (ROS) detoxification,22 transcriptomic changes in the CREB signaling were further assessed. Both sPIA males and females showed gene expression changes indicating a decreased CREB1 signaling (z-scores < −3, Fig. 5). sPIA females exhibited a greater number of gene expression changes (93 genes) compared to sPIA males (36 genes) in this canonical pathway. Hippocampal expression of genes within the CREB signaling pathway [CREB1, Tyrosine hydroxylase (TH), KCNC4 and SLC2A4] were further analyzed by qPCR (Fig. 4C). Decreased expression of these genes occurred upon reaching the 25% threshold in males but only occurred at the 18% threshold for females. No significant expression changes were found in mPIA female pups.
Figure 5: CREB1 dysregulation in sPIA males and females.
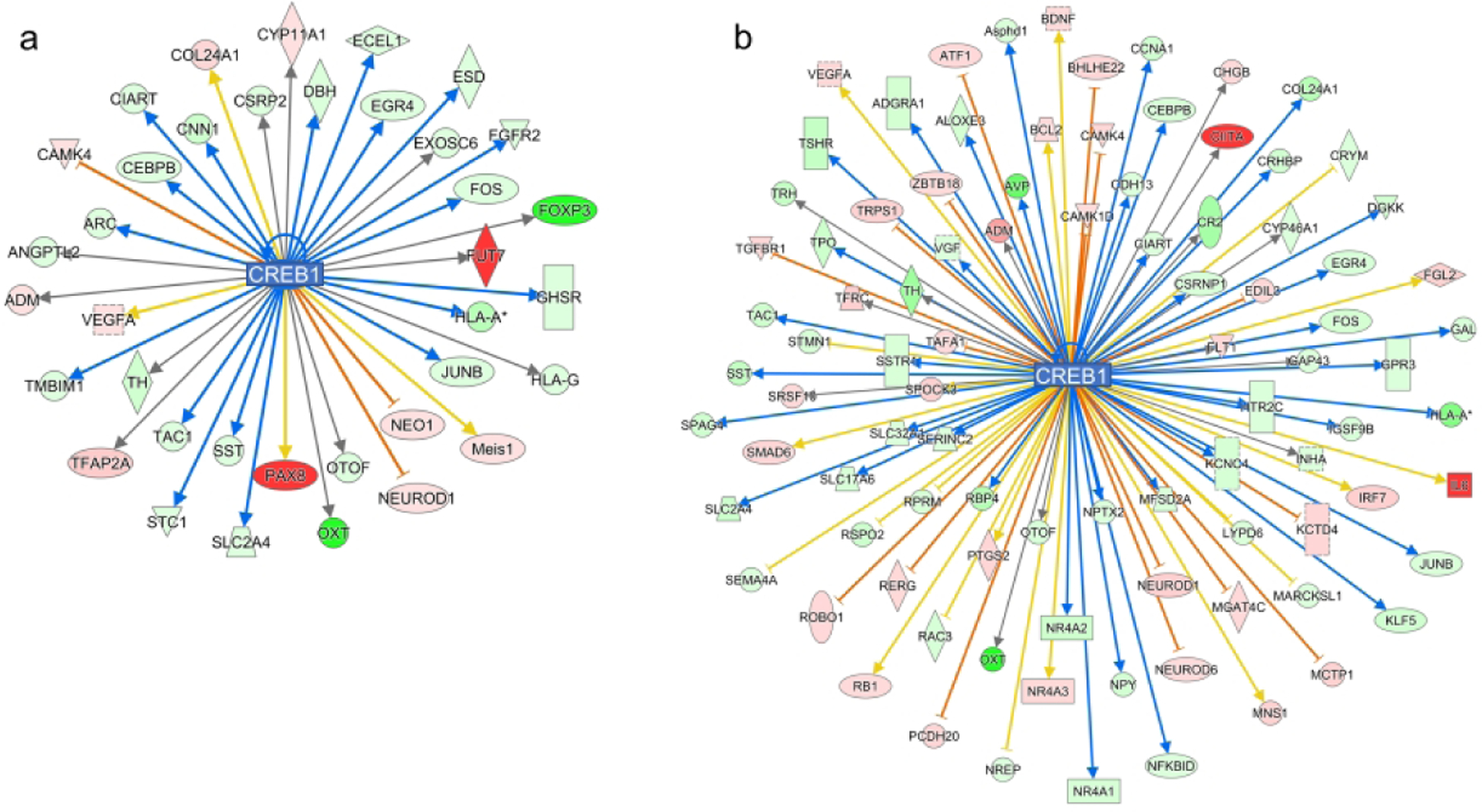
CREB1 expression is predicted to be downregulated in both sPIA males (z score −3.38) and females (z-score −3.82) with more downstream genes being affected in females (36 v 93 genes). Red- and green-filled shapes indicate up- and downregulation of genes, respectively, with brighter color indicating larger differences. Orange lines indicate activation; blue lines indicate inhibition; yellow lines indicate findings inconsistent with the state of downstream activity; gray lines indicate that the effect is not predicted.
A correlative analysis of the synaptic plasticity genes (CREB1, TH) as a function of neuroinflammatory genes (IL-1β, TNF- α) showed a negative correlation between IL-1β and CREB1 (p=0.03), IL-1β and TH (p=0.014), and TNF- α and TH (p=0.01) in PIA males. PIA females lacked significant directionality for these correlations. This analysis provides further evidence supporting differential response pattern of synaptic plasticity to neuroinflammation due to PIA in males vs females (Fig. 6).
Figure 6: Regression analysis of synaptic plasticity genes as a function of neuroinflammation genes.
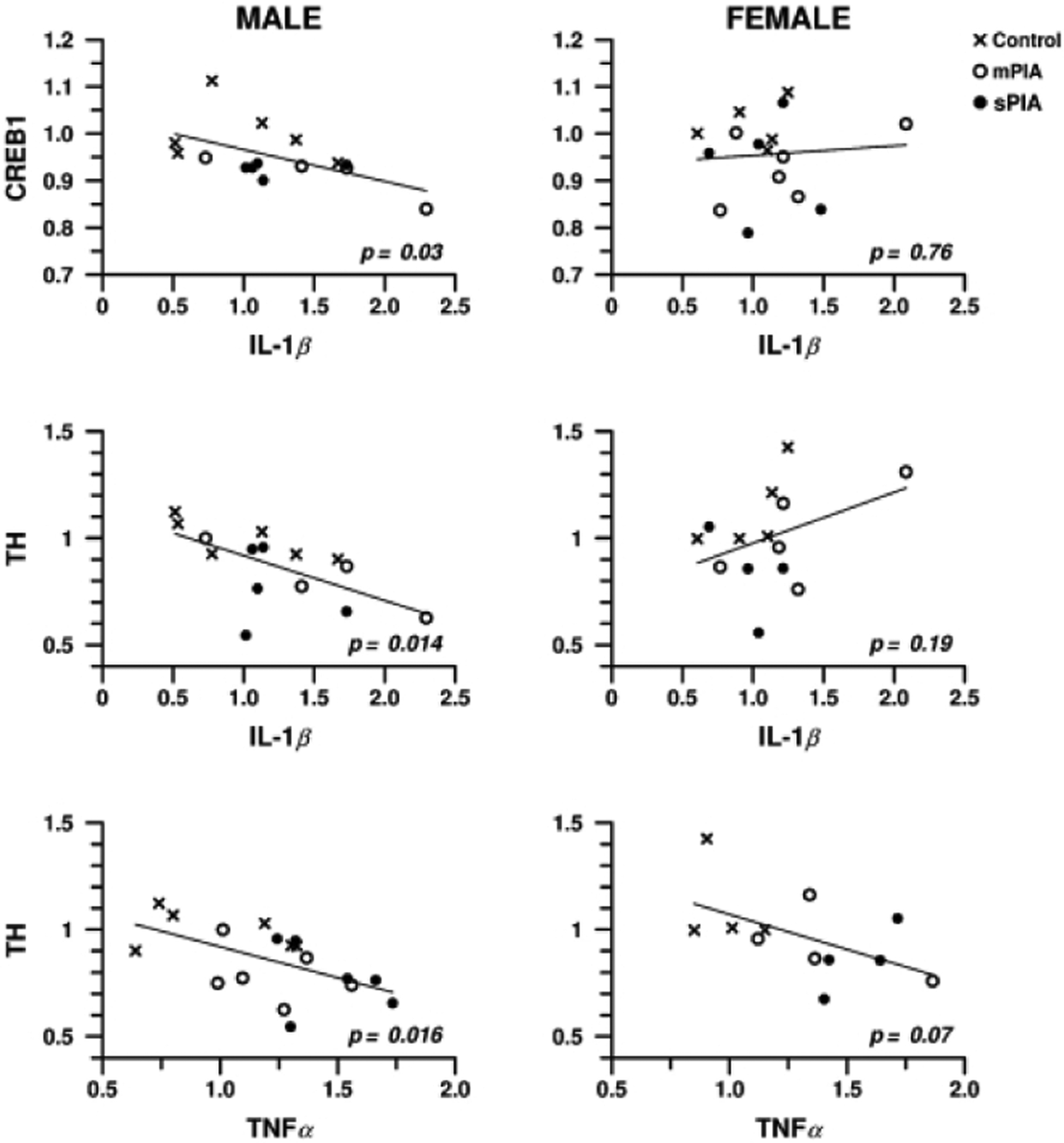
A negative correlation was observed for CREB1 as a function of IL-1β and TH as a function of Tnf-α and IL-1β in males. Females did not show a significant correlation.
Discussion
PIA in a developmentally appropriate model of preterm human brain development revealed dose- and sex-dependent effects on pro-inflammatory and synaptic plasticity pathways. The finding that anemia alters brain inflammatory gene expression is unique and concerning if translatable to human infants because of the deleterious effect of inflammation on the developing brain. The findings of the study provide several potential mechanisms to explain the sex-dependent differences in neurobehavioral performance reported in this model12 and in clinical studies of anemic preterm infants.23–25
Multiple factors that affect preterm infant neurodevelopment are not controllable by clinicians, but the degree of anemia and therapies to treat anemia are. The level of anemia in preterm neonates tolerated by clinicians varies considerably. While recent randomized trials show that small variances in anemia thresholds for red cell transfusion do not affect major neurodevelopmental outcomes (eg, Bayley Scales of Infant Development),17,26 other studies demonstrate dose- and sex-dependent effects on more specific developmental outcomes (eg, verbal fluency).25 Moreover, animal models of neonatal PIA confirm significant long-term effects on learning and memory as well as social-cognitive behaviors where sPIA males were more impaired than females.12
Given these findings, using an a priori approach we sought to examine the transcriptome of the developing hippocampus, a brain region that is crucial to memory, cognition, and social behavior. By imposing a clinically relevant model of PIA severity that parallels the 50% percent reduced hematocrit typically observed in NICU patients in an appropriately neurodevelopmentally timed model, we demonstrated differential sex-specific responses to degree of anemia based on the global transcriptomic changes. From a model perspective, the transcriptomic changes mapped onto functional (mTOR signaling) and neurobehavioral (cognition and social behavior) deficits previously identified in this model7,12 and in preterm infants.24,27 The findings provide testable molecular bases that may underlie these neurobehavioral effects.
While the males had a threshold response to PIA at 25% hematocrit based on a similar number of DEGs at both 18% and 25%, the female transcriptome was relatively unaffected until the hematocrit reached 18%. Severely anemic females had the largest changes, indicating a robust response once a threshold PIA severity was reached. This sex-specific pattern of transcriptional responses could be due to a breakdown of compensatory pathways in response to mPIA in females, which was absent in males. Another possibility is that sex hormone differences can confer neuroprotection from early-life adverse events, including anemia, in developing brains of females compared to male pups because the brains have already masculinized or feminized by P3 in mice.28
PIA-dysregulated genes mapped onto canonical pathways and functions pertaining to neuroinflammation and neurodevelopment, indicating a likely increase in pro-inflammatory function and a decrease in synaptic plasticity in the developing hippocampus. These novel findings revealed the neuroinflammatory nature of PIA, adding the brain as an organ where anemia alters inflammatory gene expression.14 It is difficult to ascertain whether the neuroinflammatory gene response observed in the brain was a localized response to a cytokine imbalance, a secondary response to immune mediators activated by peripheral inflammation crossing the blood brain barrier (BBB), or a combination of the two. Inflammation triggers the induction of hepcidin in the brain as well as increases transport of systemic hepcidin through the BBB.29,30 In this regard, the presence of significant inflammatory activity could alter brain iron availability. Additional comparative analysis of plasma and hippocampal cytokine levels may help better understand whether inflammation of the two organ systems occur independently of each other or simultaneously due to signal trafficking across the BBB.
The sex differences in our findings were striking and underscore the importance of studying neonatal events in a sex-specific manner. Sex differences in long-term outcomes as a function of neonatal hematocrit level have been reported in preterm neonates.23,24 In our preclinical model, regardless of the degree of anemia, male mouse pups displayed downregulation of neuroinflammatory pathways (Fig. 2). This is suggestive of either a lesser pro-inflammatory response or more likely, an increased compensatory anti-inflammatory response in PIA males, resulting in the downregulation of acute phase response and regulatory cytokine signaling pathways (eg. IL-6, GM-CSF, Fig. 2). This potentially protective response was diminished as the animals were subjected to a higher degree of anemia at sPIA. This interpretation is supported by less downregulation of the neuroinflammation and IL-6 signaling pathways (Fig. 2) and results of qPCR analysis of pro-inflammatory markers IL-6 and TNF-α where male pups showed a significant response at sPIA. In contrast, we showed evidence of an upregulated pro-inflammatory state in female pups indicated by increased activity of neuroinflammatory pathways and a dose-dependent increased expression of specific pro-inflammatory genes. In the context of early postnatal brain development, white matter and gray matter development are sexually dimorphic with male development taking longer than for females.31 In the case of PIA, males have transcriptomic dysregulation earlier, upon reaching the 25% threshold, during an already developmentally critical time-period. Further, synaptic plasticity gene are increasingly downregulated as inflammation increases (Fig. 6). The underlying mechanisms driving the differential response by sex remain unknown and require further exploration in light of the parallel findings in humans.23,24
Apart from their classical immunomodulatory role, cytokines and chemokines are also neuroregulatory. During the normal course of neurodevelopment, the low basal level of both pro-inflammatory and anti-inflammatory cytokines maintains the bidirectional neuroglial communication that modulates synaptogenesis and synaptic transmission.32 Long-term potential (LTP) and long-term depression (LTD), both forms of synaptic plasticity crucial for memory formation and retention, are dependent on synaptic activation of post-synaptic N-methyl-d-aspartate receptors (NMDARs), post-synaptic Ca2+ entry, and signal transductions involving α-calcium–calmodulin-dependent protein kinase II (CaMKII), mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK).33 All of these factors are influenced by cytokines like IL-1β, TNF-α, and IL-6 or reactive oxygen species. These molecules are overexpressed during a neuroinflammatory event and negatively influence the induction of LTP.34–36 PIA induces an upregulation of IL-6 and IL-1β expression in the hippocampus, which would likely inhibit MAPK and Ca2+ signaling, thereby hindering LTP formation. LTP is a cellular form of memory function and this mechanism may explain the cognitive impairment previously observed in this model.
The upregulation of inflammatory genes suggests the activation of microglia, the main immunocompetent cells in the immature CNS. Under normal non-pathologic circumstances, microglia aid in vasculature development and are essential in the establishment of the blood brain barrier.37 Activation of microglia during neuroinflammation caused by PIA may cause a change to their homeostatic functions, such as synaptic pruning.38,39 This impairment occurring during an important period of synaptogenesis would provide a potential cellular basis for a poor synaptic transmission in this model.
We previously showed that moderate PIA induces hippocampal iron deficiency, hypoxia, and acidosis.40 In this study, at the same and at a more severe degree of anemia, we show that PIA alters expression of inflammatory pathway genes, a potentially injurious result within the hippocampus during this neurodevelopmentally significant period. Such injury is suggested by the performance of PIA animals on the Novel Object Recognition task in adulthood, where their behaviors are consistent with impaired hippocampal function long after the resolution of anemia.12 Impairments in sociability and social novelty preference are also present in adulthood following neonatal PIA.12 This finding is of interest because of the increased risk of autism documented in children who were born with extreme prematurity and the role that neuroinflammation is thought to play in autism pathogenesis.41,42 Further, the sex-dependent divergence of inflammatory pathway expression in PIA could explain why male preterm infants are more neurodevelopmentally susceptible than females to certain adverse conditions such as Autism Spectrum Disorders (ASD) and Attention Deficit/Hyperactivity Disorder (ADHD).31,38,41,42
The translational value of our findings is clinically relevant as they may provide an insight to the appropriate method and timing of treatment to minimize long-term deficits. In the present study, there were noticeable sex differences, with the PIA males predicted to have a downregulation of neurodevelopmentally significant genes at a higher hematocrit threshold. Such dysregulation has been associated with memory and behavioral deficits. Our findings are consistent with human studies that show that males have worse outcomes following hypoxicischemic injury or neonatal stroke, where inflammation plays an important role in injury.43–45 Human studies show females have poorer neurodevelopmental outcomes following transfusion at higher thresholds.23,24 It should be noted that the effect of anemia without transfusion has not been assessed in these human studies. Thus, the pro-inflammatory nature of red cell transfusion which further aggravates the inflammatory conditions caused by anemia,13 rather than anemia alone, may account for the findings in human females and therefore the difference between our model and the clinical literature. Importantly, the divergent sex responses to PIA could be indicative of the neurodevelopmental abnormalities in morphology and function of synapse components mediated by different pathophysiological processes between the sexes (ie. hyperactive inflammation in the females and stalled synaptogenesis in the males). Thus, these differential effects have significant implications in the treatment thresholds and the modality of treatment for anemia for males and females.
Supplementary Material
Impact:
Phlebotomy-induced anemia (PIA) in neonatal mice results in an altered hippocampal transcriptome and the severity of changes are dependent upon degree of anemia and sex of neonatal mice.
The reported findings provide context to the sex-specific outcomes that have been reported in transfusion threshold clinical trials of preterm infants and therefore may inform treatment strategies that may be based on sex.
These data advance the field by showing that consequences of PIA may be based in sex-specific transcriptomic alterations. Such changes may also result from other causes of neonatal anemia that also affect term infants.
Financial support:
This study was supported by grant 5P01HL046925-22 from the National Institutes of Health, USA.
Footnotes
Disclosure statement: The authors declare no financial competing interests.
References
- 1.Bouyssi-Kobar M, et al. Third Trimester Brain Growth in Preterm Infants Compared With In Utero Healthy Fetuses. Pediatrics. 2016;138:e20161640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356:1162–1163. [DOI] [PubMed] [Google Scholar]
- 3.Smyser CD, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex. 2010;20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol. 1998;Suppl 27:177–209. [DOI] [PubMed] [Google Scholar]
- 5.Bastian TW, von Hohenberg WC, Mickelson DJ, Lanier LM, Georgieff MK. Iron Deficiency Impairs Developing Hippocampal Neuron Gene Expression, Energy Metabolism, and Dendrite Complexity. Dev Neurosci. 2016;38:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widness JA. Treatment and Prevention of Neonatal Anemia. Neoreviews. 2008;9:526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallin DJ, et al. Neonatal mouse hippocampus: phlebotomy-induced anemia diminishes and treatment with erythropoietin partially rescues mammalian target of rapamycin signaling. Pediatr Res. 2017;82:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. [DOI] [PubMed] [Google Scholar]
- 9.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr. 2011;2:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastian TW, von Hohenberg WC, Georgieff MK, Lanier LM. Chronic Energy Depletion due to Iron Deficiency Impairs Dendritic Mitochondrial Motility during Hippocampal Neuron Development. J Neurosci. 2019;39:802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tran PV, et al. Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency-Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus. J Nutr. 2016;146:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matveeva TM, Singh G, Gisslen TA, Gewirtz JC, Georgieff MK. Sex differences in adult social, cognitive, and affective behavioral deficits following neonatal phlebotomy-induced anemia in mice. Brain Behav. 2021;11:e01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MohanKumar K, et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat Commun. 2019;10:3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arthur CM, et al. Anemia induces gut inflammation and injury in an animal model of preterm infants. Transfusion. 2019;59:1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz C, et al. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallard C, Tremblay ME, Vexler ZS. Microglia and Neonatal Brain Injury. Neuroscience. 2019;405:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirpalani H, et al. Higher or Lower Hemoglobin Transfusion Thresholds for Preterm Infants. N Engl J Med. 2020;383:2639–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirpalani H, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. [DOI] [PubMed] [Google Scholar]
- 19.Ching T, Huang S, Garmire LX. Power analysis and sample size estimation for RNA-Seq differential expression. RNA. 2014;20:1684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barks A, Fretham SJB, Georgieff MK, Tran PV. Early-Life Neuronal-Specific Iron Deficiency Alters the Adult Mouse Hippocampal Transcriptome. J Nutr. 2018;148:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao Y, et al. Synergistic activation of inflammatory cytokine genes by interferon-γ-induced chromatin remodeling and toll-like receptor signaling. Immunity. 2013;39:454–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nopoulos PC, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011;165:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benavides A, et al. Long-term outcome of brain structure in female preterm infants: possible associations of liberal versus restrictive red blood cell transfusions. J Matern Fetal Neonatal Med. 2021;34:3292–3299.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCoy TE, et al. The relationship between brain structure and cognition in transfused preterm children at school age. Dev Neuropsychol. 2014;39:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whyte RK, et al. ; PINTOS Study Group. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207–13. [DOI] [PubMed] [Google Scholar]
- 27.McCoy TE, et al. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 2011;17:347–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Driscoll DN, McGovern M, Greene CM, Molloy EJ. Gender disparities in preterm neonatal outcomes. Acta Paediatr. 2018;107:1494–1499. [DOI] [PubMed] [Google Scholar]
- 29.Sakamori R, et al. STAT3 signaling within hepatocytes is required for anemia of inflammation in vivo. J Gastroenterol. 2010;45:244–248 [DOI] [PubMed] [Google Scholar]
- 30.Hepcidin Vela D. an emerging and important player in brain iron homeostasis. J Transl Med. 2018;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koolschijn PC, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szepesi Z, Manouchehrian O, Bachiller S, Deierborg T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front Cell Neurosci. 2018;12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. [DOI] [PubMed] [Google Scholar]
- 34.Lynch MA. Neuroinflammatory changes negatively impact on LTP: A focus on IL-1β. Brain Res. 20154;1621:197–204. [DOI] [PubMed] [Google Scholar]
- 35.Tancredi V, et al. The inhibitory effects of interleukin-6 on synaptic plasticity in the rat hippocampus are associated with an inhibition of mitogen-activated protein kinase ERK. J Neurochem. 2000;75:634–43. [DOI] [PubMed] [Google Scholar]
- 36.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. [DOI] [PubMed] [Google Scholar]
- 37.Muldoon LL, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab. 2013;33:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016;160:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. [DOI] [PubMed] [Google Scholar]
- 40.Wallin DJ, et al. Phlebotomy-induced anemia alters hippocampal neurochemistry in neonatal mice. Pediatr Res. 2015;77:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ardalan M, Chumak T, Vexler Z, Mallard C. Sex-Dependent Effects of Perinatal Inflammation on the Brain: Implication for Neuro-Psychiatric Disorders. Int J Mol Sci. 2019;20:2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal S, Rao SC, Bulsara MK, Patole SK. Prevalence of Autism Spectrum Disorder in Preterm Infants: A Meta-analysis. Pediatrics. 2018;142:e20180134. [DOI] [PubMed] [Google Scholar]
- 43.Charriaut-Marlangue C, Besson VC, Baud O. Sexually Dimorphic Outcomes after Neonatal Stroke and Hypoxia-Ischemia. Int J Mol Sci. 2017;19:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu YW, et al. Nighttime delivery and risk of neonatal encephalopathy. Am J Obstet Gynecol. 2011;204:37.e1–6. [DOI] [PubMed] [Google Scholar]
- 45.Golomb MR, et al. Neonatal arterial ischemic stroke and cerebral sinovenous thrombosis are more commonly diagnosed in boys. J Child Neurol. 2004;19:493–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


