Abstract
Objectives:
Main aim of the study was to explore the association between genetic polymorphisms in ACTN3 and bruxism. Secondary objectives included masseter muscle phenotypes assessment between bruxers and non-bruxers and according to genetic polymorphisms in ACTN3.
Materials and Methods:
Fifty-four patients undergoing orthognathic surgery for correction of their malocclusion were enrolled. Self-reported bruxism and temporomandibular disorders status were preoperatively recorded. Saliva samples were used for ACTN3 genotyping. Masseter muscle samples were collected bilaterally at the time of orthognathic surgery to explore the muscle fiber characteristics.
Results:
There were significant differences in genotypes for rs1815739 (R577X nonsense) (p = 0.001), rs1671064 (Q523R missense) (p = 0.005), and rs678397 (intronic variant) (p = 0.001) between bruxers and non-bruxers. Patients with self-reported bruxism presented a larger mean fiber area for types IIA (p = 0.035). The mean fiber areas in individuals with the wild-type CC genotype for rs1815739 (R577X) were significantly larger for type IIA fibers (1394.33 μm2 [572.77 μm2]) than in those with the TC and TT genotypes (832.61 μm2 [602.43 μm2] and 526.58 μm2 [432.21 μm2] [p = 0.014]). Similar results for Q523R missense and intronic variants.
Conclusions:
ACTN3 genotypes influence self-reported bruxism in patients with dentofacial deformity through specific masseter muscle fiber characteristics.
Keywords: ACTN3 protein, human, bruxism, malocclusion, masseter muscle, sleep bruxism, temporomandibular joint disorders
1 |. INTRODUCTION
Dentofacial deformities often develop as a complex trait condition that is influenced by a combination of genes acting on bone, teeth, and skeletal muscle (Sciote et al., 2013). Indeed, previous studies have shown that the masticatory muscle genotype and phenotype contribute greatly to variations in the vertical dimension of facial growth (Ringqvist, 1974; Rowlerson et al., 2005; Sciote et al., 2012) or mandibular asymmetry (Raoul et al., 2011), through changes in the size and proportion of masseter muscle fibers. One gene of interest which is well documented to influence muscle performance and the proportion of fiber types is ACTN3 (Vincent et al., 2007). It encodes alpha-actinin-3, which is a Z-disc structural protein found only in type II muscle fibers (North & Beggs, 1996), functions to enhance the force, speed, and strength of skeletal muscle contraction. A common nonsense mutation, R577X, was identified in the ACTN3 gene and results in a lack of protein expression due to the production of a stop codon at residue 577 (North et al., 1999). The absence of alpha-actinin-3 protein due to this polymorphism has been shown to change fiber type proportion, skeletal muscle metabolism, bone mineral density, and is associated with class II and deep-bite skeletal malocclusions (Zebrick et al., 2014). This mutated genotype also results in significantly smaller diameters of fast type II fibers in masseter muscles. Patients with facial asymmetry consisting of a longer ramus but dimensionally smaller condyles are more likely to have self-reported temporomandibular disorder (TMD) symptoms and significantly more common clinical diagnosis of TMD, with masticatory myalgia most prominent (Nicot et al., 2020). Genotyping revealed two significant associations of these conditions with the ACTN3 polymorphisms rs1671064 (Q523R missense) and rs678397 (intronic single nucleotide polymorphism [SNP]) and one allele rs1815739 (R577X nonsense) (Nicot et al., 2020). In addition, the rs678397, rs1671064, and rs1815739 polymorphisms in ACTN3 have been reported to be associated with bruxism in children (Calvano Küchler et al., 2020).
Bruxism is a complex oral condition leading to a number of clinical problems, including orofacial pain, tooth wear, and failure of dental restorative treatments. According to international expert consensus, bruxism was defined as (Lobbezoo et al., 2013) “a repetitive jaw muscle activity characterized by clenching or grinding of the teeth and/or by bracing or thrusting of the mandible, having two distinct circadian manifestations. It can occur during sleep (indicated as sleep bruxism) or during wakefulness (indicated as awake bruxism).” Since 2018, experts have defined awake bruxism as “a masticatory muscle activity during wakeful-ness that is characterized by repetitive or sustained tooth contact and/or by bracing or thrusting of the mandible and is not a movement disorder in otherwise healthy individuals,” while sleep bruxism is defined as “a masticatory muscle activity during sleep that is characterized as rhythmic (phasic) or nonrhythmic (tonic) and is not a movement disorder or a sleep disorder in otherwise healthy individuals.”(Lobbezoo et al., 2018; Manfredini et al., 2019, 2021) The prevalence of sleep bruxism is reported to be about 16% in the adult population, while the prevalence of awake bruxism is 24% (Lobbezoo et al., 2012). Both types occur due to the contraction of jaw muscles. Awake bruxism occurs mainly in the form of tooth clenching or mandible bracing. This represents prolonged isometric contractions of the masticatory muscles that differ from motor activity with respect to the phasic, isotonic, and the sudden-onset contraction of rhythmic masticatory muscle activity that occurs in sleep bruxism episodes (Manfredini et al., 2021). Currently, there is a paradigm shift in relation to bruxism from a pathology to a behavior, albeit with some clinical links to TMD (Manfredini et al., 2021), and a genetic component (Calvano Küchler et al., 2020; Vieira et al., 2020) Nevertheless, its place among the onset of pain conditions is particularly complex and varies according to age. A recent meta-analysis (de Oliveira Reis et al., 2019) focusing on the pediatric population showed that children with bruxism have a greater chance of developing TMD (de Oliveira Reis et al., 2019). Conversely, evidence provided in the systematic review of the adult population (Jiménez–Silva et al., 2017) was inconclusive, but suggested that bruxism is associated with TMD (Jiménez-Silva et al., 2017). Similarly, the systematic review by Baad–Hansen et al., in 2019 did not support a direct linear causal relationship between bruxism and musculoskeletal pain symptoms, pointing more in the direction of a multifaceted relationship dependent on the presence of other risk factors (Baad-Hansen et al., 2019). On the other hand, bruxism and dysfunctional oral habits were shown to be risk factors for the presence of TMD symptoms after combined orthodontic and surgical treatment in a population with dentofacial deformities (Bruguiere et al., 2019).
As ACTN3 genotypes have been associated with bone growth-skeletal malocclusion characteristics and muscle fiber types—masticatory functional differences in patients undergoing surgical treatment for dentofacial deformity, we hypothesized that variations in these genotypes could contribute to bruxism through specific patterns of motor unit recruitment and fiber type. Therefore, the main aim of this study was to explore the association between genetic polymorphisms in ACTN3 and bruxism in a cohort of young adults with dentofacial deformities. In addition, we investigated the masseter muscle histomorphometry between bruxers and non-bruxers. Finally, as alpha-actinin-3 loss has been shown to be associated with changes in muscle fiber characteristics, we evaluated the association between genotypes in ACTN3 variants altering the protein structure and masseter muscle histomorphometry to determine how the relationships between genetics and physiology might underly bruxism in adults. Given the potential role of bruxism in the onset of TMD, we extended these investigations to TMDs.
2 |. MATERIAL AND METHODS
2.1 |. Participants
Patients undergoing orthognathic surgery for correction of malocclusion were recruited from the University of Lille Department of Oral and Maxillofacial Surgery (France). All patients with a neurological or systemic condition, with developmental disorders of the temporomandibular joint (TMJ) that might influence TMD, or with medication influencing bruxism development, were excluded from the study.
All procedures performed in the study were in accordance with the ethical standards of the Helsinki declaration. All participants provided written informed consent, and the research protocol was validated by a French Independent Ethics Committee (CPP12/44), the Temple University (Certificate 13438), and the University of Pittsburgh (Certificate PRO12080373) IRB Committees.
Age, sex, and bruxism status, as well as TMJ symptoms, were recorded during preoperative examination. Cephalometric analysis was performed to determine the craniofacial morphologic diagnosis. Saliva samples collected from all subjects were stored in Oragene® kits (DNA Genotek) and used for DNA extraction and posterior genotyping. Masseter muscle samples were collected bilaterally from the area of the deep anterior masseter muscle, by using an intraoral approach at the time of orthognathic surgery. Indeed, all included patients had a bilateral sagittal split osteotomy by Epker surgical procedure, which permits to easily perform a masseter biopsy without additional surgical incision. Masseter muscle samples were then snap-frozen in isopentane cooled with liquid nitrogen to ensure optimal preservation and avoid any disintegration of any tissue parts, and stored at −80°C prior to histologic evaluation and gene expression analysis.
2.2 |. Bruxism status and TMD status
Bruxism status was recorded during the preoperative interview and clinical examination. According to international expert consensus (Lobbezoo et al., 2013, 2018), bruxism was diagnosed based on non-instrumental approaches (self-reporting and clinical examination). Each patient was asked whether he/she engaged in grinding or clenching his/her teeth, and in bracing or thrusting of the mandible. Bruxism dental signs were clinically collected (attrition; study wear facets; abfractions; chipping, breaking, cracks and fractures; periodontal recession and bone loss; tooth mobility; etc.)
According to the diagnostic criteria for TMDs (DC/TMD) (Dworkin & LeResche, 1992; Reiter et al., 2012; Schiffman et al., 2014), TMD status was classified into five types of symptoms: myalgia, arthralgia, headache attributed to TMD, disc displacement with reduction, and disc displacement without reduction. As defined in the DC/TMD, myalgia was diagnosed based on history and clinical examination as a pain of muscular origin that is affected by jaw movement, function, or parafunction; replication of this pain occurs with provocation testing of masticatory muscles.
2.3 |. Cephalometric analysis
Cephalometric data were assessed and classified according to Delaire analysis (Delaire et al., 1981). This allowed us to precisely determine the exact craniofacial morphotype of each patient. Patients were then classified into one of six craniofacial morphologic groups that included a variation of sagittal skeletal jaw malocclusion (Class II or Class III), and a variation of vertical skeletal occlusal relationship (open bite, deep bite, or normal bite). Anterior facial asymmetry was identified by measuring the deviation of the mandibular dental midline, and asymmetry was defined as a deviation ≥2 mm.
2.4 |. Genotyping
Three SNPs in ACTN3 (rs1815739 [R577X nonsense], rs1671064 [Q523R missense], and rs678397 [intronic variant]) were selected for genotyping of all subjects. To determine if specific allelic variants were overrepresented in patients with bruxism, TMD subtypes, and muscle fiber types, we analyzed these SNPs using TaqMan chemistry and end-point analysis in an automatic sequence-detection instrument (ABI Prism 7900HT, Applied Biosystems), as described previously (Zucchero et al., 2004).
2.5 |. Analysis of masseter muscles
Serial sections (10 μm thickness) were prepared from frozen muscles. The sections were mounted on glass microscope slides for immunostaining with antibodies specific for myosin heavy chain (MyHC) isoforms as described previously (Sciote et al., 1994). The antibodies used were as follows: Anti-I (BA-F8), Anti-fast (BF-35), Anti-IIA (SC-71), Anti-neonatal, and Anti-atrial. We then classified masseter fibers into the following six groups: type I, type hybrid (containing both type I and II MyHC), type IIA, type IIX, neonatal type, and atrial type (Sciote et al., 1994). Neonatal and atrial types both contained the neonatal and/or α–cardiac MyHC in combination with other type I and II isoforms (Korfage et al., 2005a). Type I fibers are slow-contracting and fatigue-resistant, and function most commonly to produce the jaw posture freeway space that maintains the airway. Type II fibers are fast contracting and are either fatigue-resistant or fatigable. Hybrid type fibers, which are a very unusual and distinctive group, are found in masseter muscles, which combine slow and fast contractile properties. Hybrid fibers can be found in certain states of skeletal muscle pathology, but are almost never present in normal limb muscle. For fiber type classification, only tissue section series with consistent antibody reactions for all stains and acceptable morphology of muscle fibers that were clear in transverse section were used. All fibers within the selected areas were type-classified and their cross-sectional areas were analyzed with Image Image J software. Tests for measurement error included intra-rater reliability in determination of fiber area (by repeating morphometric tracing of all fiber areas in one biopsy by one examiner), which resulted in an R2 value of 0.94. Listed histomorphometric data included fiber type, the number of each fiber, their percentage of occupancy, and their mean surface area.
2.6 |. Statistical analysis
Patient characteristics were analyzed using the usual rules for descriptive statistics: frequencies and percentages for categorical variables, and mean and standard deviation for quantitative variables. After ensuring compliance with Hardy–Weinberg equilibrium, allelic frequencies between bruxers and non-bruxers were compared using the χ2 test or Fisher’s exact test in a dominant model. One-way analysis of variance (ANOVA) was used for comparisons of the phenotypic characteristics of participants (bruxism status, TMD subtypes, or muscle phenotypes) in various ACTN3 genotypes. The Bonferroni correction method was used to correct the p-values in multiple testing, with a p < 0.017 (p = 0.05/3, where 3 is the number of SNPs included in this study) considered to indicate statistical significance. To evaluate the effect of ACTN3 genotypes on phenotype, multinomial logistic regression was used to adjust for the association of these SNPs with age, sex and biometrics, and the related odds ratio (OR) values were determined. Muscle phenotype quantitative variables between bruxers and non-bruxers were analyzed using Student’s t-test. When the distribution of the variable was not normal, the nonparametric Wilcoxon test was performed. All statistical analysis was performed using SPSS software (SAS Institute), and p values < 0.05 were considered to indicate statistical significance.
3 |. RESULTS
We recruited 54 orthognathic surgery patients who were systemically healthy and without genetic craniofacial syndromes, other growth disturbances, or reported trauma. This sample included a larger proportion of women 39 (72.22%), young people (18 years [16; 29.75]), and Class II dentofacial deformity patients (74.07%). Details of the age, sex, and biometric characteristics of the study population are listed in Table 1. There was no significant difference between the two groups (bruxers and non-bruxers) regarding age, sex, or biometric characteristics of patients, suggesting that the two populations were similar. Regarding TMD diagnoses, there was no difference in the proportion of myalgia (p = 0.67) or of disc displacement with reduction (p = 0.34) using Fisher’s exact between bruxers and non-bruxers.
TABLE 1.
Description of the study population
| Characteristics of the study population | n = 54 |
|---|---|
| Age md (Q1;Q3) | 18 (16;29.75) |
| Females n (%) | 39 (72.22) |
| Biometrical characteristics: | |
| Sagittal n (%) | |
| Class I | 1 (1.85) |
| Class II | 40 (74.07) |
| Class III | 13 (24.07) |
| Vertical n (%) | |
| Normal bite | 19 (35.18) |
| Open bite | 25 (46.30) |
| Deep bite | 10 (18.52) |
| Mandibular asymmetry n (%) | |
| No asymmetry | 33 (61,11) |
| Mandibular asymmetry >2 mm | 21 (38,89) |
| Bruxism n (%) | 10 (18.52) |
| Myalgia n (%) | 9 (16.67) |
| Arthralgia n (%) | 4 (7.41) |
| Headache attributed to TM D n (%) | 2 (3.70) |
| Disc displacement with reduction n (%) | 9 (16.67) |
| Disc displacement without reduction n (%) | 1 (1.85) |
Abbreviations: md, median; n (%), number of observation (percentage); n, number of observations; Q1;Q3, interquartile.
3.1 |. Association between bruxism and ACTN3 genotypes or alleles
There were significant differences in genotypes for SNPs rs1815739 (R577X nonsense) (p = 0.001), rs1671064 (Q523R missense) (p = 0.005), and rs678397 (intronic variant) (p = 0.001) between bruxers and non-bruxers. There were also significant differences in alleles for rs1815739 (R577X nonsense) (p < 0.002) rs1671064 (Q523R missense) (p = 0.013), and rs678397 (intronic variant) (p = 0.000) between the two groups (Table 2).
TABLE 2.
Comparison of allelic and genotypic frequencies between bruxers and non-bruxers for rs1815739 (R577X nonsense), rs1671064 (Q523R missense), and rs678397 (intronic variant)
| rs1815739 (R577X) |
p-value genotype/allele |
rs1671064 (Q523R) |
p-value genotype/allele |
rs678397 (Intronic) |
p-value genotype/allele |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | TC | TT | AA | GA | GG | CC | TC | TT | ||||
| Bruxers | 5 (50.0) | 5 (50.0) | 0 (0) | p = 0.001/0.002 | 4 (40.0) | 6 (60.0) | 0 (0) | p = 0.005/0.013 | 6 (66.7) | 2 (22.2) | 1 (11.1) | p = 0.001/0.000 |
| Non-bruxers | 4 (9.1) | 28 (63.6) | 12 (27.3) | 4 (9.1) | 28 (63.6) | 12 (27.3) | 4 (9.8) | 23 (56.1) | 14 (34.1) | |||
3.2 |. Association between masseter fiber types and bruxers
Patients with self-reported bruxism presented a larger mean fiber area for types IIA, atrial, and neonatal. The mean area of type IIA fibers was 1227.29 μm2 (508.27 μm2) for bruxers and 774.33 μm2 (614.24 μm2) for non-bruxers (p = 0.035), while the mean fiber area of the atrial type was 564.67 μm2 (517.04 μm2) for bruxers and 258.33 μm2 (406.50 μm2) for non-bruxers (p = 0.046) and the mean fiber area of the neonatal type was 74838.51 μm2 (235827.73 μm2) for bruxers and 246.52 μm2 (466.19 μm2) for non-bruxers (p = 0.035) (Table 3).
TABLE 3.
Association between masseter fiber types and bruxer status
| Bruxers (n = 10) | Non-bruxers (n = 44) | p | |
|---|---|---|---|
| Muscle fiber mean area (μm2) | |||
| Type I | 2010.24 (640.52) | 1695.14 (881.33) | 0.292 |
| Type hybrid | 1171.52 (487.33) | 1265.23 (735.93) | 0.704 |
| Type IIA | 1227.29 (508.27) | 774.33 (614.24) | 0.035 |
| Type IIX | 195.13 (617.07) | 489.37 (955.30) | 0.358 |
| Atrial | 564.67 (517.04) | 258.33 (406.50) | 0.046 |
| Neonatal | 74838.51 (235827.73) | 246.52 (466.19) | 0.035 |
| Muscle fiber percent occupancy (%) | |||
| Type I | 38.72 (19.46) | 43.46 (15.18) | 0.401 |
| Type hybrid | 19.47 (13.14) | 27.24 (17.80) | 0.199 |
| Type IIA | 23.66 (22.13) | 17.59 (12.99) | 0.253 |
| Type IIX | 0.36 (1.13) | 7.33 (16.16) | 0.181 |
| Atrial | 8.02 (14.33) | 2.52 (5.03) | 0.042 |
| Neonatal | 9.77 (29.47) | 1.86 (3.75) | 0.085 |
| Number of fibers | |||
| Type I | 42.55 (20.93) | 41.17 (26.18) | 0.877 |
| Type hybrid | 34.85 (24.82) | 30.09 (22.83) | 0.561 |
| Type IIA | 33.85 (20.20) | 35.67 (38.11) | 0.885 |
| Type IIX | 0.70 (2.21) | 7.65 (16.36) | 0.189 |
| Atrial | 14.15 (27.46) | 3.24 (5.74) | 0.016 |
| Neonatal | 1.40 (2.96) | 2.97 (6.27) | 0.447 |
Abbreviation: N, number of observation. All variables are expressed as means (standard deviations).
Bold values are statistically significant p-values.
There was also a significant difference in the percent occupancy for the atrial type fibers, with a larger percent occupancy in the bruxer group than in the non-bruxer group (8.02% [14.33%] vs. 2.52% [5.03%]; [p = 0.042]). For neonatal type fibers, there was a tendency for a larger percent occupancy in the bruxer group than in the non-bruxer group (9.77% [29.47%] vs. 1.86% [3.75%]), although this difference did not reach the level of statistical significance (p = 0.085). Finally, bruxers had significantly more atrial fibers than non-bruxers (14.15 [27.46] vs. 3.24 [5.74]; p = 0.016).
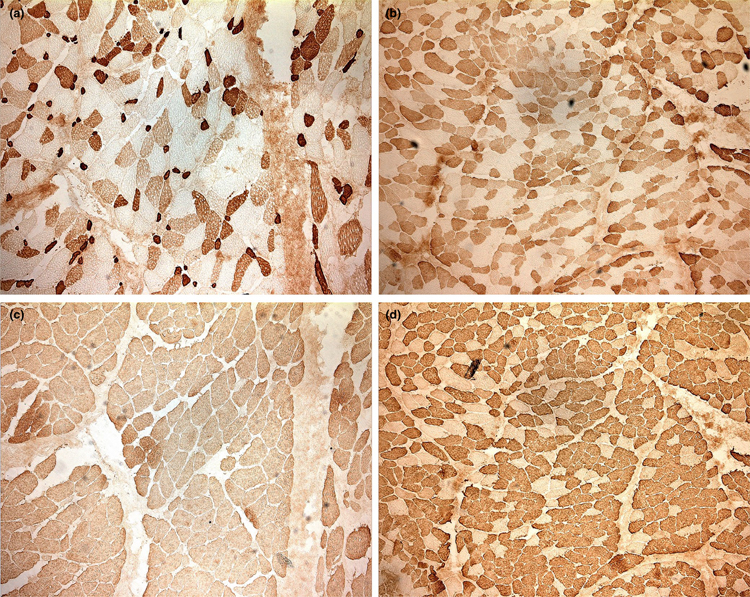
Figure 1 shows four low power (10×) immunohistochemical staining images of fast and slow myosin in masseter fibers of a patient with homozygous mutated ACTN3 genotypes for rs1815739 (R577X nonsense), rs1671064 (Q523R missense), and rs678397 (intronic variant), compared with the fibers of a patient with the wild-type genotypes for all three positions. Differences in overall fast versus slow myosin-fiber composition of masseter fibers are highlighted depending on the ACTN3 genotype.
FIGURE 1.
Low power (10×) immunohistochemical staining images of fast (a) and slow (c) myosin in masseter fibers of a patient with homozygous mutated ACTN3 genotypes for rs1815739 (R577X nonsense), rs1671064 (Q523R missense), and rs678397 (intronic variant), compared with the fast (b) and slow (d) myosin in fibers of a patient with the wild-type genotypes for all three positions
3.3 |. Association between ACTN3 genotypes or alleles and masseter fiber type characteristics
For rs1815739 (R577X nonsense leading to absence of alpha-actinin-3), there were significant differences in the mean fiber areas among the genotypes for type IIA (p = 0.014) and neonatal type (p < 0.0001) and alleles for types I (p = 0.014) and IIA (p = 0.003). The mean fiber areas in individuals with the wild-type CC genotype were significantly larger for type IIA fibers (1394.33 μm2 [572.77 μm2]) than in those with the TC and TT genotypes (832.61 μm2 [602.43 μm2] and 526.58 μm2 [432.21 μm2], respectively). There were also significant differences in the percent occupancy among the genotypes for type I (p < 0.011) and neonatal type (p < 0.0001) fibers. Finally, there were significantly fewer type I/II hybrid fibers in patients with genotype CC than in patients with either of the other genotypes (p = 0.009) (Tables 4–6).
TABLE 4.
Muscle fiber mean area depending on ACTN3 genotypes and alleles
| rs1815739 (R577X) |
p-value Genotype/allele |
rs1671064 (Q523R) |
p-value Genotype/allele |
rs678397 (intronic) |
p-value Genotype/allele |
||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle fiber mean area in μm2: my (SD) | |||||||||
| Type I | CC (N = 9) | 2374.72 (909.48) | 0.112/0.014 | AA (8) | 2341.98 (966.59) | 0.112/0.032 | CC (10) | 2412.88 (865.91) | 0.112/0.004 |
| TC (33) | 1591.90(671.20) | GA (34) | 1622.62 (684.81) | TC (25) | 1527.64 (647.01) | ||||
| TT (12) | 1731.97 (1067.18) | GG (12) | 1731.97 (1067.18) | TT (15) | 1652.15 (966.67) | ||||
| Type I/II hybrid | CC (9) | 1337.94 (351.12) | 0.713/0.674 | AA (8) | 1341.21 (375.22) | 0.713/0.684 | CC (10) | 1340.74 (331.16) | 0.713/0.535 |
| TC (33) | 1202.85 (688.74) | GA (34) | 1206.05 (678.49) | TC (25) | 1211.85 (609.99) | ||||
| TT (12) | 1304.16(912.23) | GG (12) | 1304.16(912.23) | TT (15) | 1167.82 (881.97) | ||||
| Type IIA | CC (9) | 1394.23 (572.77) | 0.014/0.003 | AA (8) | 1344.86 (591.49) | 0.014/0.014 | CC (10) | 1369.62 (545.59) | 0.014/0.000 |
| TC (33) | 832.61 (602.43) | GA (34) | 860.75 (615.50) | TC (25) | 728.59 (576.77) | ||||
| TT (12) | 526.58 (432.21) | GG (12) | 526.58 (432.21) | TT (15) | 547.11 (385.48) | ||||
| Type IIX | CC (9) | 310.84 (675.85) | 0.312/0.657 | AA (8) | 105.78 (299.18) | 0.312/0.269 | CC (10) | 279.76 (644.74) | 0.312/0.544 |
| TC (33) | 354.75 (661.34) | GA (34) | 401.70 (706.47) | TC (25) | 409.68 (701.42) | ||||
| TT (12) | 748.16 (1483.10) | GG (12) | 748.17 (1483.10) | TT (15) | 598.53(1350.63) | ||||
| Atrial type | CC (9) | 437.67 (549.14) | 0.115/0.365 | AA (8) | 356.62 (526.36) | 0.115/0.775 | CC (10) | 479.14 (534.08) | 0.115/0.219 |
| TC (33) | 346.75 (435.13) | GA (34) | 368.50(446.85) | TC (25) | 389.69 (446.48) | ||||
| TT (12) | 135.95 (337.29) | GG (12) | 135.95 (337.29) | TT (15) | 108.76 (304.23) | ||||
| Neonatal type | CC (9) | 588.04 (670.96) | <0.0001/0.667 | AA (8) | 463.62 (596.05) | <0.0001/0.685 | CC (10) | 75130.67 (235725.34) | <0.0001/0.043 |
| TC (33) | 22777.42 (129834.71) | GA (34) | 22151.15 (127904.03) | TC (25) | 198.36 (366.05) | ||||
| TT (12) | 198.66(557.65) | GG (12) | 198.66(557.65) | TT (15) | 197.76 (510.39) | ||||
Abbreviations: my, mean; SD, standard deviation.
Bold values are statistically significant p-values.
TABLE 6.
Muscle fiber number depending on ACTN3 genotypes and alleles
| rs1815739 (R577X) |
p-value genotype/allele |
rs1671064 (Q523R) |
p-value genotype/allele |
rs678397 (intronic) |
p-value genotype/allele |
||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle fiber percent occupancy in %: my (SD) | |||||||||
| Type I | CC (N = 9) | 39.17 (21.80) | 0.708/0.771 | AA (8) | 36.63 (21.84) | 0.708/0.563 | CC (10) | 42.50 (23.10) | 0.708/0.738 |
| TC (33) | 42.68 (23.21) | GA (34) | 43.18 (23.04) | TC (25) | 40.42 (18.77) | ||||
| TT (12) | 39.67 (33.37) | GG (12) | 39.67 (33.37) | TT (15) | 38.63 (29.83) | ||||
| Type I/II hybrid | CC (9) | 27.00 (17.71) | 0.009/0.576 | AA (8) | 22.56 (12.48) | 0.009/0.267 | CC (10) | 32.90 (25.04) | 0.009/0.940 |
| TC (33) | 35.49 (26.47) | GA (34) | 36.28 (26.47) | TC (25) | 38.98 (25.27) | ||||
| TT (12) | 21.54 (11.06) | GG (12) | 21.54 (11.06) | TT (15) | 21.10 (12.69) | ||||
| Type IIA | CC (9) | 33.94 (13.31) | 0.614/0.899 | AA (8) | 31.44 (11.73) | 0.614/0.739 | CC (10) | 31.85 (14.19) | 0.614/0.681 |
| TC (33) | 30.05 (21.50) | GA (34) | 30.75 (21.56) | TC (25) | 29.78 (21.46) | ||||
| TT (12) | 50.92 (64.74) | GG (12) | 50.92 (64.74) | TT (15) | 49.53 (58.30) | ||||
| Type IIX | CC (9) | 2.22 (4.66) | 0.869/0.370 | AA (8) | 1.63 (4.60) | 0.869/0.339 | CC (10) | 2.00 (4.45) | 0.869/0.327 |
| TC (33) | 7.33 (17.11) | GA (34) | 7.32 (16.85) | TC (25) | 8.40 (18.65) | ||||
| TT (12) | 6.79 (14.22) | GG (12) | 6.79 (14.22) | TT (15) | 5.43 (12.92) | ||||
| Atrial type | CC (9) | 6.06 (10.03) | 0.713/0.845 | AA (8) | 6.69 (10.53) | 0.713/0.743 | CC (10) | 5.75 (9.51) | 0.713/0.949 |
| TC (33) | 6.61 (15.77) | GA (34) | 6.44 (15.56) | TC (25) | 8.24 (17.81) | ||||
| TT (12) | 0.96 (3.32) | GG (12) | 0.96 (3.32) | TT (15) | 0.77 (2.97) | ||||
| Neonatal type | CC (9) | 1.50 (2.72) | 0.801/0.511 | AA (8) | 1.69 (2.84) | 0.801/0.607 | CC (10) | 2.10 (3.19) | 0.801/0.646 |
| TC (33) | 3.39 (6.93) | GA (34) | 3.29 (6.85) | TC (25) | 3.90 (7.72) | ||||
| TT (12) | 1.58 (3.72) | GG (12) | 1.58 (3.72) | TT (15) | 1.73 (3.65) | ||||
Abbreviations: my, mean; SD, standard deviation.
Bold values are statistically significant p-values.
Similarly, for rs1671064 (Q523R missense), there were significant differences in mean fiber area in genotypes for type IIA (p = 0.014) and neonatal type (p < 0.0001) fibers and alleles for type I (p = 0.032) and IIA (p = 0.014) fibers. The mean fiber area for type IIA fibers of individuals with the wild-type AA genotype (1344.86 μm2 [591.49 μm2]) was significantly larger than that of individuals with the GA and GG genotypes (860.75 μm2 [615.50 μm2] and 526.58 μm2 [432.21 μm2], respectively). There were also significant differences in the percent occupancy among genotypes for type I (p = 0.011) and neonatal type (p < 0.0001) fibers. Type I/II hybrid fibers were also significantly fewer in individuals with the AA genotype than in those with either of the other genotypes (p = 0.009).
Finally, for rs678397 (intronic SNP), there were significant differences in the mean fiber area among the genotypes for type IIA (p = 0.014) and neonatal type (p < 0.0001) fibers and alleles for type I (p = 0.004), IIA (p = 0.000) and neonatal type (p = 0.043) fibers. The mean fiber area of type IIA fibers was significantly larger in participants with the wild-type CC genotype (1369.62 μm2 [545.59 μm2]) than that among individuals with the TC and TT genotypes (728.59 μm2 [576.77 μm2] and 547.11 μm2 [385.48 μm2], respectively). However, there were also significant differences in the percent occupancy of type I and neonatal type fibers (p = 0.011 and p < 0.0001, respectively). Similarly, type I/II hybrid fibers were also significantly fewer in participants with the CC genotype than in those with either of the other genotypes (p = 0.009).
Using multinomial logistic regression, we showed that carriers of TC/TT genotypes of ACTN3 R577X were at significantly lower risk of bruxism (OR = 0.128, 95%CI = 0.017–0.945; p = 0.044 and OR = 0.026, 95%CI = 0.001–0.531; p = 0.018, respectively) compared to individuals with the CC genotype. Carriers of the GG genotype of ACTN3 Q523R were at significantly lower risk of bruxism (OR = 0.025, 95%CI = 0.001–0.617; p = 0.024) compared to individuals with the AA genotype. Finally, carriers of the TC/TT genotypes of ACTN3 intronic variant were also at significantly lower risk of bruxism (OR = 0.087, 95%CI = 0.011–0.718; p = 0.023 and OR = 0.046, 95%CI = 0.004–0.547; p = 0.015, respectively) compared to individuals with the CC genotype. Multinomial logistic regression showed no relevant associations regarding the muscle phenotypes (data not shown).
3.4 |. Association between ACTN3 genotypes or alleles, masseter fiber types, and subtypes of TMD
No associations were identified among the different TMD subtypes, ACTN3 genotypes, and the different phenotypic characteristics of the muscle (data not detailed).
In particular, there was no association between myalgia and genotype/alleles in alpha-actinin-3 altering variants rs1815739 (R577X nonsense) (p = 0.143/p = 1) and rs1671064 (Q523R missense) (p = 0.168/p = 1). There was no significant difference in the mean fiber area for all fiber types (all p values >0.2 except for neonatal type [p = 0.135]), in the percent occupancy for all fiber types (all p values >0.2 except for neonatal type [p = 0.103]), and in the number of each fiber (all p values >0.2 except for neonatal type [p = 0.132]).
In addition, there was no association between disc displacement with reduction and genotype/alleles in alpha-actinin-3 altering variants rs1815739 (R577X nonsense) (p = 0.143/p = 1) and rs1671064 (Q523R missense) (p = 0.168/p = 1). There was no significant difference in the mean fiber area for all fiber types (all p values >0.2 except for neonatal type [p = 0.135]), in the percent occupancy for all fiber types (all p values >0.2 except for type I [p = 0.145] and neonatal [p = 0.103]), and in the number of each fiber (all p values >0.2 except for neonatal types [p = 0.132]).
4 |. DISCUSSION
Alpha-actinin-3, which is a cytoskeletal protein encoded by the ACTN3 gene, binds actin filaments in skeletal muscle (North et al., 1999). In contrast to alpha-actinin-2, alpha-actinin-3 protein is present only in type II fast contracting fiber types, where they cross-link actin filaments with dense bodies located in the Z-disk of the sarcomere. This interaction helps to order the myofibril array, which is important in coordinating sarcomere contraction (Vincent et al., 2007). The ACTN3 R577X polymorphism (rs1815739) consists in a cytosine to thymine mutation at nucleotide 1586 in exon 16, which converts the arginine at position 577 to a stop codon, and produces three genotypes: CC (normal), CT (heterozygote), and TT (no alpha-actinin-3 protein) (North et al., 1999). Approximately 18% of the European population is homozygous for this common nonsense mutation (Yang et al., 2003).
ACTN3 genotypes have been widely studied in human elite athletic population, mainly through ACTN3 R577X variations, since they represent a natural experimental model of a nonsense mutation contributing to muscle performance (Ma et al., 2013; Tharabenjasin et al., 2019). Compared with X allele carriers, studies have indicated that the RR genotype and R allele carriers have greater muscle size and strength (Broos et al., 2015; Kikuchi & Nakazato, 2015; Walsh et al., 2008), faster sprint times (Moran et al., 2007), and a higher proportion of fast-twitch muscle fibers (Ahmetov et al., 2011; Vincent et al., 2007). Reciprocally, elite power sports athletes have been reported to have a higher frequency of the RR +RX genotype in their fast-twitch skeletal muscle compared to controls (Yang et al., 2003). Nevertheless, a recent meta-analysis by Tharabenjasin et al., in 2019 showed a significant association of the R allele with female elite power sports athletes, while no such association was identified for their male counterparts (Tharabenjasin et al., 2019). In a histomorphometric study focusing on the vastus lateralis muscle in young adult males, individuals carrying the RR genotype had type IIX, fast contracting-fatigable fibers, which were significantly larger in size and greater in number compared to individuals carrying the mutated XX genotype (Vincent et al., 2007). In this study, alpha-actinin-3 protein content was systematically higher in type IIX compared with type IIA fibers. Therefore, we propose that the ACTN3 variant could be one of the genes that contributes to the heritability of fiber type distribution through its interaction with calcineurin. Alpha-actinin-3 also binds to calsarcin family signaling proteins located at the Z-disc (Frey & Olson, 2002). These, in turn, bind to the signaling protein calcineurin, which has a key role in determining fiber type and size through activation fiber type-specific gene expression pathways (Swoap et al., 2000). Calcineurin activity is increased when alpha-actinin-3 expression is lost, explaining the slower metabolic, physiological, and functional phenotypes associated with alpha-actinin-3 deficiency.
Increased calcineurin activity is associated with increased muscle plasticity, as demonstrated by an enhanced adaptive response to endurance training in ACTN3 knockout mice and an increased switch in muscle fiber type from fast-twitch glycolytic fibers (type IIX) toward fast-twitch oxidative fibers (type IIA) (Seto et al., 2013). Myofibers are known to undergo a progressive transition due to changes in muscle activity, usually changing from type IIX to type IIA to type I when muscles are loaded (Yamada et al., 2020). Variation in the pellet hardness of a rat diet is an experimental model of this masseter fiber type transition. Studies have shown a reduction in fiber volume (Kawai et al., 2010; Kiliaridis et al., 1988; Kiliaridis & Shyu, 1988; Miehe et al., 1999) and a significant increase in type IIB fibers in the deep masseter fibers after consuming soft food compared to those on a hard food diet (Saito et al., 2002). Based on this model, Saito et al. suggested that similar changes that might be induced by a soft diet in human masseter muscle could result in a phenotypic transition from type I/IIA fibers, which are predominant under normal conditions, to type IID/X or even to IIB’ (Saito et al., 2002). More recently, Takasu et al. reported evidence in support of previous results in a rabbit model, showing that a liquid diet caused a reduction in the diameter of fast-twitch muscle fibers and increased the proportion of fast-twitch fibers (Takasu et al., 2019).
In our study, we showed a significant association of participants with self-reported bruxism with wild-type genotypes and alleles for SNPs rs1671064 (Q523R missense), rs1815739 (R577X nonsense), and rs678397 (intronic SNP). In addition, these participants had a larger mean fiber area for type IIA and an increased number of atrial/neonatal type fibers. Type I fibers are characterized by low force, power, and speed production and high endurance, while type IIX fibers are characterized by high force, power, and speed production and low endurance, with type IIA with intermediate characteristics (Korfage et al., 2005a). Therefore, our results are consistent with previous reports and the relevant experimental models showing a specific pattern of muscle phenotype characterized by larger fast-twitch oxidative fibers and fewer hybrid fibers that confer intermediate contraction velocity and fatiguability on masseter muscle.
Despite bruxism was not a peripheral condition, these characteristics seem to provide favorable pathophysiological conditions that lead to its clinical expression. Moreover, compared with non-bruxers, bruxers had more atrial/neonatal type fibers, with higher percent occupancy. This particular fiber type has been shown to be commonly co-expressed with either type I or type IIA fibers, indicating that α–cardiac MyHC, which forms atrial fibers, is an intermediate isoform between the slow MyHC-I and the fast MyHC-IIA isoforms (Hämäläinen & Pette, 1997; Peuker et al., 1998). In addition, it has been speculated that fibers with different combinations of myosin composition increase the capacity of the jaw muscles to perform a wide variety of motor tasks, since these fibers have contractile properties that lie between those of pure fibers (Korfage et al., 2005b). The greater the composition of fibers, the more a continuum exists in contractile properties that could contribute to a precise modulation of mandibular position and force. In addition, all three common genotypes were associated with larger mean areas of type IIA fibers, although we did not find any difference in mean area of the type IIX fibers, indicating a tripartite relationship between bruxism, ACTN3 genotypes, and type IIA fiber enlargement. Both wild-type genotypes of rs1671064 (Q523R missense) and rs1815739 (R577X nonsense) also showed lower type I/II hybrid mean fiber area, while this parameter was increased for rs678397 (intronic SNP), suggesting a role of alpha-actinin-3 in bruxism, although the proportion of hybrid fibers changes when alpha-actinin-3 expression is lost. Moreover, multinomial logistic regression showed that the mutant ACTN3 genotypes protect against bruxism. Therefore, although muscle phenotype is strongly influenced by genetics, it is also subject to environmental influences (Isola et al., 2018). It is probable that, to some extent, the histomorphometric results are linked to fiber type transitions due to masseter muscle training or jaw disproportion, thus distorting the results of the statistical analysis (Nicot et al., 2020; Zebrick et al., 2014). This could explain why enlargement of type IIA fibers was not found to be a risk factor in the multinomial logistic regression.
On the other hand, this research supports the current paradigm switch, in which bruxism is considered to be an oral behavioral more than a pathology in most cases. Indeed, no association was found between bruxism and the different subtypes of TMD. Nevertheless, these results should be interpreted with caution given the small sample size. Moreover, all our results suggest the high adaptability of muscle pattern based on the possibility of fiber transition.
Our results are also particularly interesting from the perspective of the pathophysiology of botulinum toxin injections into masseter and temporal muscles for the treatment of bruxism. This approach produces a reduction in the muscular force and frequency of bruxism events and is therefore more and more used in the management of bruxism to improve the quality of life of patients (Ågren et al., 2020; Almukhtar & Fabi, 2019; Fernández-Núñez et al., 2019; Sendra et al., 2020; Villa et al., 2019). Indeed, injected masseters show a steep increase in the size of type IIX fibers, whereas fast fibers decreased by approximately 50% (Korfage et al., 2012). Therefore, botulinum toxin injections lead to a fiber type transition that could be unfavorable for the development of bruxism. These results are in accordance with those reported by Tsai et al., in which a reduction in muscle fiber size and transition of muscle fiber subtypes from type IIA to IIX or IIB was found to occur due to reduced masticatory function in a rat model treated with botulinum toxin injection (Gedrange et al., 2013; Tsai et al., 2012). Fiber transition after masticatory muscle botulinum toxin injections in humans remains to be investigated.
The initial objective of study was to investigate the musculoskeletal heritable influences on malocclusions. Therefore, the main limitation of this study concerns the diagnosis of bruxism, which was based on self-reporting and did not differentiate between the two patterns of the condition. Nevertheless, this study is, to date, the first to explore polymorphisms in the genes of interest and the histomorphometric patterns in the related muscles in human self-reported bruxism in patients with dentofacial deformity.
TABLE 5.
Muscle fiber percent occupancy depending on ACTN3 genotypes and alleles
| rs1815739 (R577X) |
p-value genotype/allele |
rs1671064 (Q523R) |
p-value genotype/allele |
rs678397 (intronic) |
p-value genotype/allele |
||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle fiber percent occupancy in %: my (SD) | |||||||||
| Type I | CC (N = 9) | 47.49 (13.09) | 0.011/0.318 | AA (8) | 48.31 (13.74) | 0.011/0.277 | CC (10) | 43.13 (18.51) | 0.011/0.857 |
| TC (33) | 38.93 (15.66) | GA (34) | 38.99 (15.43) | TC (15) | 37.83 (11.34) | ||||
| TT (12) | 49.00 (16.85) | GG (12) | 49.00 (16.84) | TT (25) | 49.40 (15.52) | ||||
| Type I/II hybrid | CC (9) | 20.27 (10.61) | 0.199/0.294 | AA (8) | 20.13 (11.33) | 0.199/0.316 | CC (10) | 18.47 (11.52) | 0.199/0.085 |
| TC (33) | 27.64 (19.16) | GA (34) | 27.46 (18.90) | TC (15) | 31.63 (17.73) | ||||
| TT (12) | 24.92 (15.47) | GG (12) | 24.92 (15.47) | TT (25) | 24.14 (16.91) | ||||
| Type IIA | CC (9) | 25.18 (10.71) | 0.203/0.160 | AA (8) | 25.17 (11.45) | 0.203/0.191 | CC (10) | 22.69 (12.80) | 0.203/0.173 |
| TC (33) | 18.49 (17.25) | GA (34) | 18.69 (17.03) | TC (15) | 16.47 (13.47) | ||||
| TT (12) | 14.53 (9.22) | GG (12) | 14.53 (9.22) | TT (25) | 16.79 (10.87) | ||||
| Type IIX | CC (9) | 1.82 (4.29) | 0.877/0.353 | AA (8) | 1.60 (4.53) | 0.877/0.363 | CC (10) | 1.64 (4.08) | 0.877/0.322 |
| TC (33) | 6.39 (14.91) | GA (34) | 6.31 (14.69) | TC (15) | 6.98 (15.69) | ||||
| TT (12) | 8.27 (19.43) | GG (12) | 8.27 (19.43) | TT (25) | 6.2 (17.57) | ||||
| Atrial type | CC (9) | 3.94 (4.65) | 0.589/0.867 | AA (8) | 3.39 (4.68) | 0.589/0.952 | CC (10) | 3.55 (4.55) | 0.589/0.939 |
| TC (33) | 4.30 (9.35) | GA (34) | 4.42 (9.23) | TC (15) | 5.48 (10.47) | ||||
| TT (12) | 1.15 (3.35) | GG (12) | 1.15 (3.35) | TT (25) | 0.92 (3.01) | ||||
| Neonatal type | CC (9) | 1.30 (2.45) | <0.0001/0.611 | AA (8) | 1.14 (2.59) | <0.0001/0.654 | CC (10) | 10.53 (29.27) | <0.0001/0.071 |
| TC (33) | 4.39 (16.55) | GA (34) | 4.27 (16.30) | TC (15) | 1.68 (3.24) | ||||
| TT (12) | 2.13 (5.09) | GG (12) | 2.13 (5.09) | TT (25) | 2.13 (4.73) | ||||
Abbreviations: my, mean; SD, standard deviation.
Bold values are statistically significant p-values.
ACKNOWLEDGEMENTS
This work is supported by the National Institute of Dental & Craniofacial research through a grant to Dr Sciote; Musculoskeletal Heritable Influences on Malocclusion - R21DE022427.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS COMMITTEE APPROVALS FOR THE STUDY
Temple University IRB: Certificate 13438. University of Lille CPP (Committee for Personal Protection): CPP12/44. University of Pittsburgh IRB: Certificate PRO12080373.
PATIENT CONSENT STATEMENT
Subjects were enrolled for study after participation was discussed and an informed consent was signed.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.14075.
DATA AVAILABILITY STATEMENT
All relevant data are within the paper and its Supporting Information files.
REFERENCES
- Ågren M, Sahin C, & Pettersson M (2020). The effect of botulinum toxin injections on bruxism: A systematic review. Journal of Oral Rehabilitation, 47, 395–402. [DOI] [PubMed] [Google Scholar]
- Ahmetov II, Druzhevskaya AM, Lyubaeva EV, Popov DV, Vinogradova OL, & Williams AG (2011). The dependence of preferred competitive racing distance on muscle fibre type composition and ACTN3 genotype in speed skaters. Experimental Physiology, 96, 1302–1310. [DOI] [PubMed] [Google Scholar]
- Almukhtar RM, & Fabi SG (2019). The masseter muscle and its role in facial contouring, aging, and quality of life: A literature review. Plastic and Reconstructive Surgery, 143, 39e–48e. [DOI] [PubMed] [Google Scholar]
- Baad-Hansen L, Thymi M, Lobbezoo F, & Svensson P (2019). To what extent is bruxism associated with musculoskeletal signs and symptoms? A systematic review. Journal of Oral Rehabilitation, 46, 845–861. [DOI] [PubMed] [Google Scholar]
- Broos S, Van Leemputte M, Deldicque L, & Thomis MA (2015). History-dependent force, angular velocity and muscular endurance in ACTN3 genotypes. European Journal of Applied Physiology, 115, 1637–1643. [DOI] [PubMed] [Google Scholar]
- Bruguiere F, Sciote JJ, Roland-Billecart T, Raoul G, Machuron F, Ferri J, & Nicot R (2019). Pre-operative parafunctional or dysfunctional oral habits are associated with the temporomandibular disorders after orthognathic surgery: An observational cohort study. Journal of Oral Rehabilitation, 46, 321–329. [DOI] [PubMed] [Google Scholar]
- Calvano Küchler E, Arid J, Palinkas M et al. (2020). Genetic polymorphisms in ACTN3 contribute to the etiology of bruxism in children. The Journal of Clinical Pediatric Dentistry, 44, 180–184. [DOI] [PubMed] [Google Scholar]
- de Oliveira RL, Ribeiro RA, Martins CC, & Devito KL (2019). Association between bruxism and temporomandibular disorders in children: A systematic review and meta-analysis. International Journal of Paediatric Dentistry, 29, 585–595. [DOI] [PubMed] [Google Scholar]
- Delaire J, Schendel SA, & Tulasne JF (1981). An architectural and structural craniofacial analysis: A new lateral cephalometric analysis. Oral Surgery, Oral Medicine, Oral Pathology, 52, 226–238. [DOI] [PubMed] [Google Scholar]
- Dworkin SF, & LeResche L (1992). Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. Journal of Craniomandibular Disorders Facial Oral Pain, 6, 301–355. [PubMed] [Google Scholar]
- Fernández-Núñez T, Amghar-Maach S, & Gay-Escoda C (2019). Efficacy of botulinum toxin in the treatment of bruxism: Systematic review. Medicina Oral, Patologia Oral Y Cirugia Bucal, 24, e416–e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N, & Olson EN (2002). Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. Journal of Biological Chemistry, 277, 13998–14004. [DOI] [PubMed] [Google Scholar]
- Gedrange T, Gredes T, Spassov A, Mai R, Kuhn DU, Dominiak M, & Kunert-Keil C (2013). Histological changes and changes in the myosin mRNA content of the porcine masticatory muscles after masseter treatment with botulinum toxin A. Clinical Oral Investigations, 17, 887–896. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N, & Pette D (1997). Expression of an alpha-cardiac like myosin heavy chain in diaphragm, chronically stimulated, and denervated fast-twitch muscles of rabbit. Journal of Muscle Research and Cell Motility, 18, 401–411. [DOI] [PubMed] [Google Scholar]
- Isola G, Anastasi GP, Matarese G, Williams RC, Cutroneo G, Bracco P, & Piancino MG (2018). Functional and molecular outcomes of the human masticatory muscles. Oral Diseases, 24, 1428–1441. [DOI] [PubMed] [Google Scholar]
- Jiménez-Silva A, Peña-Durán C, Tobar-Reyes J, & Frugone-Zambra R (2017). Sleep and awake bruxism in adults and its relationship with temporomandibular disorders: A systematic review from 2003 to 2014. Acta Odontologica Scandinavica, 75, 36–58. [DOI] [PubMed] [Google Scholar]
- Kawai N, Sano R, Korfage JAM, Nakamura S, Kinouchi N, Kawakami E, Tanne K, Langenbach GE, & Tanaka E (2010). Adaptation of rat jaw muscle fibers in postnatal development with a different food consistency: An immunohistochemical and electromyographic study. Journal of Anatomy, 216, 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi N, & Nakazato K (2015). Effective utilization of genetic information for athletes and coaches: Focus on ACTN3 R577X polymorphism. Journal of Exercise Nutrition & Biochemistry, 19, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiliaridis S, Engstrom C, & Thilander B (1988). Histochemical analysis of masticatory muscle in the growing rat after prolonged alteration in the consistency of the diet. Archives of Oral Biology, 33, 187–193. [DOI] [PubMed] [Google Scholar]
- Kiliaridis S, & Shyu BC (1988). Isometric muscle tension generated by masseter stimulation after prolonged alteration of the consistency of the diet fed to growing rats. Archives of Oral Biology, 33, 467–472. [DOI] [PubMed] [Google Scholar]
- Korfage J, Koolstra JH, Langenbach GEJ., & van Eijden T (2005a). Fiber-type composition of the human jaw muscles–(part 1) origin and functional significance of fiber-type diversity. Journal of Dental Research, 84, 774–783. 10.1177/154405910508400901 [DOI] [PubMed] [Google Scholar]
- Korfage J, Koolstra JH, Langenbach G, & van Eijden T (2005b). Fiber-type composition of the human jaw muscles–(part 2) role of hybrid fibers and factors responsible for inter-individual variation. Journal of Dental Research, 84, 784–793. 10.1177/154405910508400902 [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Wang J, Lie SHJTJ, & Langenbach GE (2012). Influence of botulinum toxin on rabbit jaw muscle activity and anatomy. Muscle and Nerve, 45, 684–691. [DOI] [PubMed] [Google Scholar]
- Lobbezoo F, Ahlberg J, Glaros AG, Glaros AG, Kato T, Koyano K, Lavigne GJ, De Leeuw R, Manfredini D, Svensson P, & Winocur E (2013). Bruxism defined and graded: An international consensus. Journal of Oral Rehabilitation, 40, 2–4. [DOI] [PubMed] [Google Scholar]
- Lobbezoo F, Ahlberg J, Manfredini D, & Winocur E (2012). Are bruxism and the bite causally related? Journal of Oral Rehabilitation, 39, 489–501. 10.1111/j.1365-2842.2012.02298.x [DOI] [PubMed] [Google Scholar]
- Lobbezoo F, Ahlberg J, Raphael KG, Wetselaar P, Glaros AG, Kato T, Santiago V, Winocur E, De Laat A, De Leeuw R, & Koyano K (2018). International consensus on the assessment of bruxism: Report of a work in progress. Journal of Oral Rehabilitation, 45, 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Yang Y, Li X,Zhou F, Gao C, Li M, & Gao L (2013). The association of sport performance with ACE and ACTN3 genetic polymorphisms: A systematic review and meta-analysis. PLoS One, 8, e54685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini D, Ahlberg J, & Lobbezoo F (2021). Bruxism definition: Past, present, and future - What should a prosthodontist know? Journal of Prosthetic Dentistry. 10.1016/j.prosdent.2021.01.026 [DOI] [PubMed] [Google Scholar]
- Manfredini D, Ahlberg J, Wetselaar P, Svensson P, & Lobbezoo F (2019). The bruxism construct: From cut-off points to a continuum spectrum. Journal of Oral Rehabilitation, 46, 991–997. [DOI] [PubMed] [Google Scholar]
- Miehe B, Fanghanel J, Kubein-Meesenburg D, Nagerl H, & Schwestka-Polly R (1999). Masticatory musculature under altered occlusal relationships–a model study with experimental animals. Annals of Anatomy-Anatomischer Anzeiger, 181, 37–40. [DOI] [PubMed] [Google Scholar]
- Moran CN, Yang N, Bailey MES, Tsiokanos A, Jamurtas A, MacArthur DG, North K, Pitsiladis YP, & Wilson RH (2007). Association analysis of the ACTN3 R577X polymorphism and complex quantitative body composition and performance phenotypes in adolescent Greeks. European Journal of Human Genetics, 15, 88–93. [DOI] [PubMed] [Google Scholar]
- Nicot R, Chung K, Vieira AR, Raoul G, Ferri J, & Sciote JJ (2020). Condyle modeling stability, craniofacial asymmetry and ACTN3 genotypes: Contribution to TMD prevalence in a cohort of dentofacial deformities. PLoS One, 15, e0236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North KN, & Beggs AH (1996). Deficiency of a skeletal muscle isoform of alpha-actinin (alpha-actinin-3) in merosin-positive congenital muscular dystrophy. Neuromuscular Disorders, 6, 229–235. [DOI] [PubMed] [Google Scholar]
- North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, & Beggs AH (1999). A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nature Genetics, 21, 353–354. [DOI] [PubMed] [Google Scholar]
- Peuker H, Conjard A, & Pette D (1998). Alpha-cardiac-like myosin heavy chain as an intermediate between MHCIIa and MHCI beta in transforming rabbit muscle. American Journal of Physiology, 274, C595–602. [DOI] [PubMed] [Google Scholar]
- Raoul G, Rowlerson A, Sciote J, Codaccioni E, Stevens L, Maurage CA, Duhamel A, & Ferri J (2011). Masseter myosin heavy chain composition varies with mandibular asymmetry. The Journal of Craniofacial Surgery, 22, 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter S, Goldsmith C, Emodi-Perlman A, Friedman-Rubin P, & Winocur E (2012). Masticatory muscle disorders diagnostic criteria: The American Academy of Orofacial Pain versus the research diagnostic criteria/temporomandibular disorders (RDC/TMD). Journal of Oral Rehabilitation, 39, 941–947. [DOI] [PubMed] [Google Scholar]
- Ringqvist M (1974). Fiber types in human masticatory muscles. Relation to function. Scandinavian Journal of Dental Research, 82, 333–355. [DOI] [PubMed] [Google Scholar]
- Rowlerson A, Raoul G, Daniel Y, Close J, Maurage CA, Ferri J, & Sciote JJ (2005). Fiber-type differences in masseter muscle associated with different facial morphologies. American Journal of Orthodontics and Dentofacial Orthopedics, 127, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Ohnuki Y, Yamane A, & Saeki Y (2002). Effects of diet consistency on the myosin heavy chain mRNAs of rat masseter muscle during postnatal development. Archives of Oral Biology, 47, 109–115. [DOI] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E et al. (2014). Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group†. Journal of Oral & Facial Pain and Headache, 28, 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciote JJ, Horton MJ, Rowlerson AM, Ferri J, Close JM, & Raoul G (2012). Human masseter muscle fiber type properties, skeletal malocclusions, and muscle growth factor expression. Journal of Oral and Maxillofacial Surgery, 70, 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciote JJ, Raoul G, Ferri J, Close J, Horton MJ, & Rowlerson A (2013). Masseter function and skeletal malocclusion. Revue De Stomatologie, De Chirurgie maxillo-faciale Et De Chirurgie Orale, 114, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciote JJ, Rowlerson AM, Hopper C, & Hunt NP (1994). Fibre type classification and myosin isoforms in the human masseter muscle. Journal of the Neurological Sciences, 126, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendra LA, Montez C, Vianna KC, & Barboza EP (2020). Clinical outcomes of botulinum toxin type A injections in the management of primary bruxism in adults: A systematic review. Journal of Prosthetic Dentistry, 126(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Seto JT, Quinlan KGR, Lek M, Zheng XF, Garton F, MacArthur DG, Hogarth MW, Houweling PJ, Gregorevic P, Turner N, & Cooney GJ (2013). ACTN3 genotype influences muscle performance through the regulation of calcineurin signaling. The Journal of Clinical Investigation, 123, 4255–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Hunter RB, Stevenson EJ, Felton HM, Kansagra NV, Lang JM, Esser KA, & Kandarian SC (2000). The calcineurin-NFAT pathway and muscle fiber-type gene expression. American Journal of Physiology Cell Physiology, 279, C915–C924. [DOI] [PubMed] [Google Scholar]
- Takasu H, Matsunaga T, Morita T, Hiraba K, Kuroki K, Saito K, Fujiwara T, & Goto S (2019). Changes in masseter muscle fibers by liquid diet rearing in rabbits and recovery by chewing of solid diet. Archives of Oral Biology, 108, 104548. [DOI] [PubMed] [Google Scholar]
- Tharabenjasin P, Pabalan N, & Jarjanazi H (2019). Association of the ACTN3 R577X (rs1815739) polymorphism with elite power sports: A meta-analysis. PLoS One, 14, e0217390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CY, Lin YC, Su B, Yang LY, & Chiu WC (2012). Masseter muscle fibre changes following reduction of masticatory function. International Journal of Oral and Maxillofacial Surgery, 41, 394–399. [DOI] [PubMed] [Google Scholar]
- Vieira AR, Scariot R, Gerber JT, Arid J, Küchler EC, Sebastiani AM, Palinkas M, Díaz-Serrano KV, Torres CP, Regalo SC, & Nelson-Filho P (2020). Bruxism throughout the lifespan and variants in MMP2, MMP9 and COMT. Journal of Personalized Medicine, 10, E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa S, Raoul G, Machuron F, Ferri J, & Nicot R (2019). Improvement in quality of life after botulinum toxin injection for temporomandibular disorder. Journal of Stomatology, Oral and Maxillofacial Surgery, 120, 2–6. [DOI] [PubMed] [Google Scholar]
- Vincent B, De Bock K, Ramaekers M, Van den Eede E, Van Leemputte M, Hespel P, & Thomis MA (2007). ACTN3 (R577X) genotype is associated with fiber type distribution. Physiological Genomics, 32, 58–63. [DOI] [PubMed] [Google Scholar]
- Walsh S, Liu D, Metter EJ, Ferrucci L, & Roth SM (2008). ACTN3 genotype is associated with muscle phenotypes in women across the adult age span. Journal of Applied Physiology, 1985(105), 1486–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Sugiyama G, & Mori Y (2020). Masticatory muscle function affects the pathological conditions of dentofacial deformities. Japanese Dental Science Review, 56, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, & North K (2003). ACTN3 genotype is associated with human elite athletic performance. American Journal of Human Genetics, 73, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrick B, Teeramongkolgul T, Nicot R, Horton MJ, Raoul G, Ferri J, Vieira AR, & Sciote JJ (2014). ACTN3 R577X genotypes associate with Class II and deepbite malocclusions. American Journal of Orthodontics and Dentofacial Orthopedics, 146, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchero TM, Cooper ME, Maher BS Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, & Natsume N (2004). Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. New England Journal of Medicine, 351, 769–780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.