Abstract
Screening assays for environmental mycotoxins in bulk samples currently use cytotoxicity in cell cultures, but their application to air particulate samples often lacks sensitivity and specificity for fungal spores. An assay based on inhibition of protein synthesis using translation of firefly luciferase in a rabbit reticulocyte system has been developed for the detection of trichothecene mycotoxins found in the spores of toxigenic fungi. Ethanol extracts of air particulates trapped on polycarbonate filters are ultrafiltered and applied at several dilutions to a translation reaction mixture. The activity of translated luciferase is measured directly in a luminometer, eliminating the need for radioisotopes and time-consuming sample processing. Parallel standard curves using a commercially available trichothecene provide for expression of the results in T-2 toxin equivalents per cubic meter of air. The assay can be completed in 2 h and is readily applicable to multiple samples. Comparison to the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cytotoxicity assay indicates a 400-fold increase in sensitivity of trichothecene detection in addition to a much higher specificity for these toxins. Initial field testing indicates a strong correlation between the measured level of toxicity and the presence of toxigenic fungi detected with microbiological methods. In conclusion, this luciferase translation assay offers a rapid and highly sensitive and specific method for quantitative detection of trichothecene mycotoxin activity in air particulate samples.
Because fungal viability may be short-lived compared to toxin stability, methods of detecting toxins or toxicity are much preferred over those requiring fungal culturing. Quantitative tests for airborne environmental fungi which are most widely used are based on culturing of air particulates collected on filters and determination of the number of viable spores. The making of public health decisions would be greatly facilitated by the development of rapid and affordable strategies which provide accurate quantitative assessment of possible environmental exposure to fungal toxins.
Existing methods of trichothecene toxin detection include costly, highly technical approaches, such as gas chromatography or mass spectroscopy (23). Immunodetection requires specific antibodies which are not readily available at the present time (8). Thin-layer chromatography has been used to detect mycotoxins, but its sensitivity is significantly lower than that of cytotoxicity measurements (25). Cell culture-based cytotoxicity assays (12, 14, 19) appear to work well with samples generated under controlled conditions, such as growing fungi on sterile substrates, but interpretation of the results becomes problematic when environmental bulk samples are studied. In addition to fungi, those samples are commonly heavily contaminated by bacteria, which raises the possibility of various synergistic effects of mycotoxins and other substances, such as endotoxin. Specificity may be less of a problem in the application of cytotoxicity assays to airborne particulates, but the sensitivity of these tests would preclude quantitative evaluation (22). In an attempt to use this approach with airborne particulates, we have tested swine kidney cells by using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (14). We have found that cytotoxicity assays, while yielding reproducible results, lack both the specificity and sensitivity needed to detect mycotoxins in fungal spores collected on filters in indoor air sampling using low (3 liters/min)-to-medium (22 liters/min)-flow pumps. For cytotoxicity assays, either high-flow pumps or prolonged collection periods are needed to sample the large quantities of air required.
Mycotoxin detection based on the inhibition of protein synthesis has been described by others (28). Toxin detection and methods used in studying the mechanism of action at the protein translation level (27) have relied on the use of radioisotopes and vary in sensitivity to 12,13-epoxytrichothecenes. Although they can have serious limitations, bioassays based on inhibition of protein synthesis demonstrate high specificity and sensitivity toward trichothecene mycotoxins. We have developed a nonradioactive assay based on translation of firefly luciferase in a rabbit reticulocyte system and have compared its sensitivity and specificity with those of the MTT assay by using both pure mycotoxins and air particulate samples collected from fungus-contaminated houses.
MATERIALS AND METHODS
Materials and reagents.
PK15 cells were obtained from the American Type Culture Collection, Manassas, Va. DON (deoxynivalenol) and T-2 toxin were purchased from Sigma, St. Louis, Mo. Satratoxin G was a generous gift from Bruce Jarvis, University of Maryland. The rabbit reticulocyte lysate, luciferase mRNA, amino acids, magnesium acetate, potassium chloride, recombinant RNase inhibitor, and luciferase assay reagent used were from Promega, Madison, Wis. RNase T-1 from Aspergillus oryzae was from Gibco BRL, Gaithersburg, Md.
Collection and processing of air samples.
Air samples from fungus-contaminated houses, as well as clean control rooms, were collected on polycarbonate filters (pore size, 0.8 μm; Poretics Corp., Livermore, Calif.) using low-flow (3 liters/min for 24 h) and medium-flow (18 and 22 liters/min for 8 h) pumps. Filters were extracted overnight in 10 ml of 95% ethanol and sonicated for 30 min. Another 5 ml of ethanol was added to the filter, and sonication was repeated for 30 min. Extracts were passed through 0.22-μm-pore-size GV Millex (Millipore Corp., Bedford, Mass.) filters to remove particulates and evaporated. Ethanol was the solvent of choice because of its compatibility with the filters used to remove endotoxin and RNase. Dried samples were reconstituted in small volumes of ethanol and appropriately diluted with buffer or culture medium for testing. For cytotoxicity studies, samples were filtered through Ultrasart D20 (Sartorius, Goettingen, Germany) to remove endotoxin. Extracts used in translation inhibition assays were passed through Millipore Ultrafree-MC 5000 NMWL filter units to remove proteins.
Bulk samples and isolated fungal spores.
Stachybotrys chartarum, originally isolated from a home in Cleveland, Ohio (JS5817; American Type Culture Collection catalog no. 201211), was grown in culture on rice. Rice (100 g) was suspended in 60 ml of distilled water and allowed to stand for 1 to 2 h before autoclaving. The rice was sterilized by autoclaving, inoculated with suspensions of 7-day-old conidia, and incubated at 28°C for 4 weeks. Additional water (5 ml) was added axenically after 48 h of incubation. Cultures were stored at 4°C until needed. Small portions of rice culture (volume not important) were shaken into a small plexiglass chamber (8 by 8 cm [internal dimensions]) provided with two openings. The chamber had previously been disinfected with 70% isopropanol. Incoming air was filtered through sterile glass wool in a 37-mm filter cassette, and the air-entrained spores were collected on an open-faced 37-mm cassette connected to an external vacuum source. The chamber was hand shaken to aerosolize the spores within the chamber, and the entire operation was performed in a chemical fume hood. When the collection filters were completely black, the vacuum was stopped, the chamber was disassembled, and the filters were transferred to sterile 50-ml centrifuge tubes for transport. The operation was repeated until it was no longer possible to collect spores from the rice. Samples from the filters were examined microscopically for the presence of hyphae and/or conidiophores and tested for fungal and bacterial contamination by streaking on malt extract agar and incubation in trypticase soy broth, respectively. Filters containing spores were rinsed with 1 to 2 ml of phosphate-buffered saline. Spores released from the filter were enumerated microscopically with a hemocytometer. Known numbers of spores were then pelleted by centrifugation and extracted with ethanol as described above. Bulk samples (fragments of wallpaper and drywall and samples of dust, carpet fibers, etc.) were weighed prior to the extraction procedure. Extraction was performed as described for filters.
Identification of fungal species in residences with water problems.
Bulk samples collected in residences (surface samples such as dust, wallpaper, etc.) were cultured under standard conditions on potato dextrose agar and Rose Bengal plates for 1 to 3 weeks at 30°C. Fungi were identified based on their morphology.
Cell culture.
PK15 porcine kidney cells were cultured at 37°C in Eagle’s minimum essential medium with 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, Earle’s balanced salt solution, and 5% newborn calf serum antibiotic free in an atmosphere of 5% CO2. For cytotoxicity measurements, cells were trypsinized (0.25% trypsin, 0.03% EDTA) and passaged onto 96-well plates at a density of 5 × 105/ml and a volume of 150 μl/well. After 24 h, confluent cells were exposed to toxins and extracts for 72 h prior to MTT assay.
Preparation and storage of trichothecene mycotoxins and air particulate extracts.
Concentrated stocks of T-2 toxin, satratoxin G, and DON (1 mg/ml) were prepared in ethanol to ensure their complete solubility and subsequently diluted in the culture medium used to grow porcine kidney cells as described above just prior to their addition to the cultures. The final concentration of ethanol did not exceed 1% in the culture medium and was kept under 0.05% in the translation reaction mixture. Storage of trichothecenes and filter extracts in aqueous solutions was avoided at all times to prevent the loss of toxin activity observed by others (28).
MTT assay.
MTT assays (20) were performed as described by Hanelt et al. (14) by using a Bio-Rad 3550 plate reader.
Translation of firefly luciferase mRNA.
The translation reaction was carried out with 33% rabbit reticulocyte lysate, 0.25 mM magnesium acetate, 110 mM potassium chloride, 8.3 ng of luciferase mRNA per μl, 0.33 U of rRNasin RNase inhibitor per μl, an 8.3 μM amino acid mixture, 4 mM dithiothreitol, and diluted toxins or extracts in a final volume of 1 to 20 μl. Using smaller volumes allows one to save reagents but is technically difficult when using manual pipetting. Following incubation at 30°C for 90 min, samples were rapidly frozen on dry ice. The luciferase translation assay has to be performed with great caution to avoid the introduction of RNase from the laboratory environment. We routinely use sterile techniques, sterile glassware and plasticware, and RNase-free water and reagents.
Luciferase activity assay.
The luciferase assay was performed following sample thawing and 20-fold dilution with 20 mM Tris/HCl (pH 7.8). Luciferase assay reagent (50 μl) containing 20 mM tricine, 1.07 mM (MgCO3)4Mg(OH)2 · 5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 33.3 mM dithiothreitol, 270 μM coenzyme A, 470 μM luciferin, and 530 μM ATP (pH 7.8) was mixed quickly with 5 μl of the diluted translation mixture and read in an Optocompt I photon-counting luminometer (MGM Instruments Inc.). The activity of all samples was expressed as percent control (water added in place of toxin or extract). A purified luciferase preparation was used to choose the range of light intensity (relative light units [RLU]) proportional to the amount of luciferase present in the sample. Control samples (no toxin added) consistently yielded about 60,000 RLU, corresponding to 2 μg of luciferase per liter.
Data analysis.
Dose-response curves were plotted and analyzed by using SigmaPlot and TableCurve programs (Jandel Scientific). Data from dose-response experiments were fitted into logistic dose-response equations, and 50% effective concentrations were calculated. Correlation coefficient (r2) values were used to assess the goodness of fit and ranged from 0.950 to 0.999.
RESULTS
Cytotoxicity assays.
Porcine kidney cells are highly susceptible to trichothecene mycotoxins, as demonstrated by Hanelt et al. (14) in a study comparing three different cell lines. We have found that swine kidney PK15 cells were more sensitive to trichothecenes and more resistant to solvents such as methanol and ethanol (no significant changes in MTT cleavage activity were detected at up to 5% ethanol) than the MRC-5 human lung fibroblast cells used by others for cytotoxicity measurements (19). Based on these findings, PK15 cells were chosen for further studies.
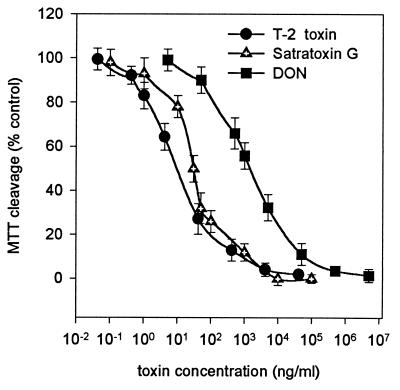
Three mycotoxins were tested for their cytotoxic effects on PK15 cells: two simple trichothecenes, T-2 toxin and DON, produced by Fusarium sp., which are commercially available, and a macrocyclic trichothecene, satratoxin G, isolated from S. chartarum (18). As shown in Fig. 1, T-2 toxin was found to inhibit MTT cleavage by 50% at a concentration of 9.14 ± 0.95 ng/ml. Satratoxin G produced the same effect at about three times the concentration (29.9 ± 2.6 ng/ml). DON was much less potent against PK15 cells, with 50% inhibition at 1.47 ± 0.12 μg/ml.
FIG. 1.
Effects of T-2 toxin, satratoxin G, and DON on the MTT cleavage activity of PK15 cells. The values are means ± SEM (n = 8). T-2 toxin yielded 50% inhibition at 9.14 ng/ml (r2 = 0.98), satratoxin G did so at 29.9 ng/ml (r2 = 0.98), and DON did so at 1,470 ng/ml (r2 = 0.98).
The cytotoxicity of air particulates collected in several homes with visible mold growth was evaluated by using material equivalent to 0.1 to 4.0 m3 of air. Under these conditions, the MTT test did not reveal any cytotoxic effects of air particulate extracts (data not shown). Based on the sensitivity of the MTT test (see Table 2), the cytotoxicity of those samples was lower than that of 1 ng of T-2 toxin/m3.
TABLE 2.
Comparison of trichothecene detection limits obtained by different methods
| Toxin and parameter | Luciferase translation assaya | MTT cytotoxicity assayb | MTT/LTb | Cytotoxicitycd | Thin-layer chromatographyc | Enzyme-linked immunosorbent assayef |
|---|---|---|---|---|---|---|
| T-2 toxin | ||||||
| Concn at 80% activity (pg/ml) | 20,000 | 1,000 | ||||
| Vol used (μl) | 0.25 (1) | 100 | ||||
| Amt detected (pg) | 5 (20) | 100 | 10–40,000 | 500,000 | 1–40 | |
| Ratio | 5–20 | |||||
| Satratoxin G | ||||||
| Concn at 80% activity (pg/ml) | 50,000 | 10,000 | ||||
| Vol used (μl) | 0.25 (1) | 100 | ||||
| Amt detected (pg) | 12.5 (50) | 1,000 | 630,000 | |||
| Ratio | 20–80 | |||||
| DON | ||||||
| Concn at 80% activity | 200,000 | 200,000 | ||||
| Vol used (μl) | 0.25 (1) | 100 | ||||
| Amt detected (pg) | 50 (200) | 20,000 | 100,000 | 500,000 | 250,000 | |
| Ratio | 100–400 |
The value in parentheses is the practical volume that can be accurately dispensed. This volume is four times larger than the amount needed for the luciferase assay and results in an increase in the detection limit (see text).
This report. LT, luciferase translation assay.
Robb and Norval (25).
Hanelt et al. (14).
Dietrich et al. (8).
Casale et al. (5).
Bulk samples such as dust, fragments of carpeting, plaster, or wallpaper collected in houses with moisture and fungus problems were demonstrated to contain very high levels of cytotoxicity. While the level of cytotoxicity was significantly decreased by using filters which exclude bacterial endotoxin, e.g., Ultrasart D20 (data not shown), other toxic agents from paint, glue, or dyes that are highly soluble in ethanol are likely to be present.
Translation inhibition assay.
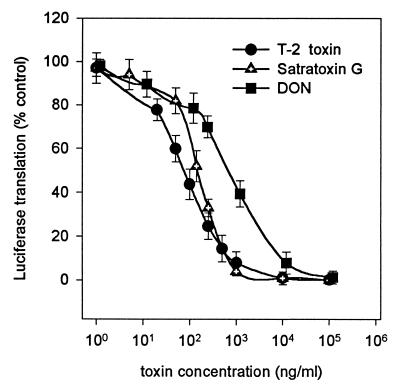
The in vitro luciferase translation system was used to study the effect of trichothecenes on protein translation in a cell-free rabbit reticulocyte system. The standard reaction conditions for rabbit reticulocyte lysate (24) have been modified as described in Materials and Methods to reach high translation reaction efficiency and, at the same time, limit the use of reagents. Figure 2 shows the concentration-dependent inhibition of translation of firefly luciferase mRNA by T-2 toxin, satratoxin G, and DON. Similar to the cell culture-based system, there is a significant difference in the effect of DON (50% inhibition at 757 ± 43 ng/ml), satratoxin G (50% inhibition at 148.5 ± 7.7 ng/ml), and T-2 toxin (50% inhibition at about 78.53 ± 13 ng/ml). Interestingly, T-2 toxin and satratoxin G are much less effective in this system than in PK15 cells.
FIG. 2.
Effects of T-2 toxin, satratoxin G, and DON on luciferase translation in rabbit reticulocyte lysate. The values shown are means ± SEM (n = 8). T-2 toxin yielded 50% inhibition at 78.5 ng/ml (r2 = 0.99), satratoxin G did so at 148.5 ng/ml (r2 = 0.99), and DON did so at 757 ng/ml (r2 = 0.99).
The air particulate extracts from fungus-contaminated houses were strongly inhibitory in the rabbit reticulocyte system (Table 1). Control sterile filters showed no inhibition of luciferase translation.
TABLE 1.
Toxicity of air particulates collected in residences with moisture problems
| House no., room(s) | Fungus cultured | Toxin equivalents (ng/m3)a
|
Vol of air containing particulates causing 50% translation inhibition (m3) | |
|---|---|---|---|---|
| T-2 | SG | |||
| 71, bedroom | Stachybotrys sp. | 1.09 | 2.06 | 1.441 |
| 71, kitchen | Stachybotrys sp. | 1.66 | 3.15 | 0.941 |
| 73, bedroom | Stachybotrys sp. | 1.75 | 3.33 | 0.899 |
| 74A, bedroom | Stachybotrys sp. | 0.98 | 1.86 | 1.600 |
| 74B, bedroom | ||||
| Test 1 | NAb | 0.50 | 0.94 | 3.152 |
| Test 2 | 0.47 | 0.89 | 3.333 | |
| Test 3 | 0.54 | 1.02 | 2.900 | |
| 75, baby’s room | Stachybotrys sp. | 1.10 | 2.09 | 1.421 |
| 75, toy room | Stachybotrys sp. | 1.12 | 2.12 | 1.395 |
| 78, baby’s room | Rhizopus sp.c | 1.91 | 3.64 | 0.816 |
| 78, basement | Rhizopus sp.c | 4.56 | 8.68 | 0.342 |
| 79A, bedroom | Stachybotrys sp. | 17.90 | 34.10 | 0.087 |
| 79B, bedroom | Stachybotrys sp. | 8.00 | 15.20 | 0.195 |
| 80, front room | Stachybotrys sp. | 14.10 | 27.00 | 0.110 |
| 80, baby’s room | Stachybotrys sp. | 17.30 | 33.00 | 0.090 |
| Control rooms (n = 3) | NA | 0d | 0d | NA |
| Control rooms (n = 2) | NA | 0.01d | 0.02d | NA |
| Control room | NA | 0.09d | 0.20d | NA |
r2 values for fitted dose-response curves ranged from 0.950 to 0.998. T-2, T-2 toxin; SG, satratoxin G.
NA, not available.
Heavy growth precluded identification of other fungi.
Single-dose measurement.
The content of extracts remained the main concern with this highly sensitive assay, especially with respect to RNase, which can be a serious problem in translation-based applications. The recombinant inhibitor that inhibits the RNases A, B, and C included in the reaction mixture is not effective against many bacterial and fungal RNases (3).
To determine whether the observed inhibition can be attributed to RNases or trichothecenes, the extracts were filtered through Millipore Ultrafree-MC 5000 NMWL centrifuge filter units with a molecular weight exclusion limit of 5,000. This procedure should remove proteins, most importantly, RNases and proteases. As shown in Fig. 3, such filtration of extracts of environmental air particulates leads to reduction of inhibitory activity. This suggests that these extracts contain significant levels of RNases or other high-molecular-weight compounds that interfere with the translation process or destroy the translation product (proteases). The concern about RNase interference was further investigated by incubating luciferase mRNA (1 μg) with air particulate extracts under conditions identical to those used for translation. Subsequent electrophoresis on 1% agarose (Fig. 4, lane 2) shows that RNA disappears completely following incubation with unfiltered extract. However, incubation with the same extract filtered through Millipore Ultrafree-MC 5000 NMWL units did not lead to detectable degradation of RNA.
FIG. 3.
Effect of filtration through Millipore Ultrafree-MC 5000 NMWL centrifuge filters on the protein translation inhibition activity of three different air particulate sample extracts. The values are means ± SEM (n = 3).
FIG. 4.
Luciferase mRNA (1 μg) incubated with air particulate sample extracts. Lanes 1, control (water added instead of extract); 2, extract showing a high degree of luciferase translation inhibition; 3, the same extract as in lane 1 filtered through a Millipore Ultrafree-MC 5000 NMWL centrifuge filter; 4, 1 μg of standard luciferase mRNA.
In order to quantitate the sensitivity of the translation assay to RNase, we used fungal RNase T1 from A. oryzae (molecular mass, 11 kDa). As shown in Fig. 5, subpicogram amounts of RNase affect the translation of luciferase. Filtration through Millipore Ultrafree-MC 5000 NMWL filters efficiently removes up to almost 2 ng of RNase. Increasing the concentration of RNase results in leaking of enzymatic activity through the filter. To be sure that the extracts were RNase free, they were filtered and assayed a second time by using the amount that reduced the translational activity by about 50% in the first assay. If the inhibitory effect on luciferase translation is reduced following the second filtration, it could be attributed to the presence of RNase in the extract. If observed changes remain within the limits of experimental error (several percent), we conclude that other inhibitors, most likely mycotoxins, were responsible for the inhibition of protein translation. To date, none of the environmental samples have shown reduced inhibition after the second filtration, demonstrating that the single filtration is sufficient to remove low levels of RNase present in the air particulate extracts.
FIG. 5.
Effect of T1 RNase on luciferase translation. The inhibitory effect of RNase was measured before and after filtration through Millipore Ultrafree-MC 5000 NMWL units. The values are means ± SEM (n = 3).
Control experiments performed with pure T-2 toxin indicate that there is no loss of trichothecenes in extracts due to the filtration. T-2 toxin solutions can be passed through Millipore Ultrafree-MC 5000 NMWL units up to three times without any significant change in toxicity as estimated by the luciferase translation assay.
Because the focus of the tests is on fungal spores, it was important to determine if the spores contain detectable amounts of RNase activity. The presence of RNase in fungal spores has been reported (16, 30). S. chartarum spores were extracted in accordance with our standard ethanol procedure and used in the luciferase translation assay. The dose-response curves (Fig. 6) generated with extracts obtained before and after filtration through Millipore Ultrafree-MC 5000 NMWL units were virtually superimposable, indicating the lack of RNase activity in spore ethanol extracts. This suggests that either S. chartarum spores do not contain RNase activity or none of the enzyme is extracted or active under the conditions of the experiment.
FIG. 6.
Effect of S. chartarum spore extracts on luciferase translation in the rabbit reticulocyte system. Comparison of extracts filtered through Millipore Ultrafree-MC 5000 NMWL units with unfiltered extracts. The values are means ± SEM (n = 3).
In addition to removing traces of RNases, ultrafiltration would remove other possible interfering agents, such as proteases or endotoxin. We have not been able to detect any effect of endotoxin on luciferase translation at concentrations of up to 1 μg/ml.
Testing and quantitating the toxicity of environmental samples.
The luciferase translation method was used to detect and quantitate the toxicity of air particulates collected in houses with known water and mold problems, where the presence of toxigenic mold has been confirmed by culturing of bulk samples. Dose-response curves were generated by using filtered (Millipore Ultrafree-MC 5000 NMWL filters) extracts of polycarbonate filters. Dose-response curves for T-2 toxin or satratoxin G were run in parallel with each experiment. The results are expressed as toxin equivalents per cubic meter of air determined by matching the 50% inhibition points of the experimental extract curves and the T-2 toxin and satratoxin G curves. The amounts of T-2 toxin and satratoxin G (nanograms) causing 50% inhibition were equated to the volume of extract (microliters) causing 50% inhibition of the luciferase translation. The volume of extract was then converted to the volume of air sampled (cubic meters), and toxin equivalents (nanograms per cubic meter) were obtained.
Table 1 shows the results of toxicity tests in several houses and rooms. The highest toxicity corresponds to about 17 ng of T-2 toxin or 34 ng of satratoxin G present in 1 m3 of air. Either control rooms (clean rooms with no evidence of mold) had no detectable toxicity, or their toxicity was no higher than 0.091 ng of T-2 toxin equivalents/m3 (5 to 200 times lower than that of contaminated rooms). Detecting toxicity of control rooms required using much larger amounts of extracts, corresponding to 5 to 10 m3 of sampled air. With the routine sampling of residences yielding a maximum of 10 m3 (8 h at 22 liters/min), only a single-point reading could be obtained.
To further validate the testing procedure, the reproducibility of multiple screening was assessed. Table 1 (house 74B) shows results obtained after collecting air samples in the same room three times for 8 h each time within a period of 72 h. The three separate samplings yielded toxin equivalent values of 0.502, 0.471, and 0.54 (mean, 0.505; standard error of the mean [SEM], 0.02) ng/m3.
DISCUSSION
The aim was to develop a rapid and inexpensive method to quantitatively assess exposure to trichothecenes as a biomarker for toxigenic fungi such as S. chartarum, which has recently been linked to an outbreak of pulmonary hemosiderosis in infants (see reference 10). Currently, airborne exposure to toxigenic fungi can only be estimated based on the results of culturing or spore counting of air particulate samples. Airborne concentrations of detected culturable spores are often falsely low (1, 11). Furthermore, because different isolates of the same fungal species can produce various amounts of mycotoxins, depending on the growth conditions (18, 21), the isolation of a toxigenic fungus from a building cannot be taken as an indication of the level of toxin exposure. Spores that have lost the ability to germinate still contain stable trichothecene mycotoxins. Toxicity tests may confirm both the presence and the toxic potential of fungal isolates in a particular home environment. Thus, measurement of total trichothecene toxicity rather than the number of viable spores is a more accurate approach.
Existing literature on cytotoxic effects of fungal spores collected on polycarbonate filters and pure mycotoxins suggests that cytotoxicity assays may be suitable for evaluation of inhalation exposure to toxigenic fungi (12, 14). Cytotoxicity has been used to measure toxic effects of fungal spores under controlled experimental conditions. Pasanen and coworkers (22) employed the fetal lung cell-based assay to demonstrate toxicity of airborne spores of S. chartarum growing in the laboratory on substrates, such as hay, grain, and wallpaper, that have been sterilized prior to fungal contamination. In a study of problem buildings using kidney cells and an MTT test, Gareis (12) demonstrated the cytotoxicity of fungus-contaminated samples of gypsum board. However, the specificity and quantitative aspect of those assays have not been tested in large practical building surveillance studies.
Cytotoxicity experiments employing the MTT assay described in this report demonstrate that porcine kidney (PK15) cells are highly susceptible to pure trichothecene toxins. Similar midpoint toxicity values for T-2 toxin of 2.8, 5.6, and 9.8 ng/ml for melanoma cells, keratinocytes, and hepatoma cells were reported by others using the neutral red assay (2). The MTT assay used with MDBK cells yielded 50% inhibition at 1 to 1.5 ng of T-2 toxin per ml (15). Cytotoxity experiments performed with numerous mycotoxins and a different line of swine kidney cells yielded 0.8 μg of DON per ml and 6.2 μg of satratoxin G per ml for 80% MTT cleavage activity (14).
By using extracts of polycarbonate filters which have been exposed to the air in houses with mold and moisture problems, we were unable to detect any cytotoxic effect on PK15 cells. In contrast, bulk samples collected in parallel with the air samples exhibited very high cytotoxicity. This was expected, since bulk samples, in general, contain much higher concentrations of microbes than can be found in air particulates (11). It seems that at least some of that toxicity could be attributed to the presence of bacterial endotoxin and other high-molecular-weight compounds. In most cases, filtration of ethanol extracts through Ultrasart D20 filters, which removes molecules larger than 20 kDa, led to reduction of the observed cytotoxicity. In summary, we find the MTT cleavage-based cytotoxicity assay to be suitable for screening of highly toxic bulk samples, especially after removal of endotoxin and other high-molecular-weight compounds, but not sufficiently sensitive or specific to detect and quantify the trichothecene toxicity of air particulates. High-flow pumps with impingers collecting much larger samples may help solve the sensitivity problem, but this does not appear to be practical for routine sampling, especially in residential buildings. The lack of specificity toward fungal toxins and the potential for synergistic effects do not appear to be readily resolvable in this system.
The primary mode of trichothecene action in living cells is inhibition of the protein translation process (6). Assays based on protein synthesis have been used to detect and compare mycotoxins, as well as to study their mechanism of action (27, 28). Translation inhibition-based tests performed with cultured cells appear to be highly sensitive to trichothecenes, with T-2 toxin 50% inhibition values of 10 to 15 ng/ml for CHO cells and 1 ng/ml for MDBK cells (15). T-2 toxin has been shown to be much less effective in cell-free translation systems, such as rabbit reticulocyte lysates requiring microgram-per-milliliter concentrations (27). The protein translation assays previously described all involve the use of radioactive amino acids and require several hours to several days to complete the tedious and labor-intensive processing of samples.
In recent years, the translation of firefly luciferase has been used in molecular biology as a nonradioactive alternative to detect and quantitate the expression of reporter genes and as a control for in vitro translation. The luminescence of in vitro-translated luciferase can be easily detected and quantified. Luciferase catalyzes ATP-dependent conversion of luciferin to oxyluciferin with concomitant release of light. The quantum yield of this reaction is the highest in efficiency of any known biological reaction (26). The light emitted from firefly luciferase is directly proportional to the number of luciferase enzyme molecules when the substrate is not in excess (7). Luciferase activity can be measured directly in the translation mixture within seconds. The entire testing procedure, including protein translation, can be completed in less than 2 h. The rabbit reticulocyte system has been extensively studied and optimized to yield functional, biologically active proteins (13) and is currently available from several commercial sources.
We have demonstrated that the trichothecenes T-2 toxin, satratoxin G, and DON readily inhibit the translation of firefly luciferase mRNA in a cell-free rabbit reticulocyte system. T-2 toxin and, to a lesser extent, satratoxin G are not as effective in the reticulocyte lysate as they are in PK15 cells. The greater potency in intact cells can be explained by toxicity independent of translational mechanisms such as effect on membranes or additional toxicity of toxin metabolites (4, 29). In the case of DON, only a small difference in potency between PK15 cells and the luciferase translation system was detected. DON contains different specific side groups than T-2 toxin and inhibits both the elongation and termination steps of the protein translation process, whereas T-2 toxin inhibits the initiation step (9, 27).
Although the effective toxin concentrations in the luciferase translation assays may be similar to (as in the case of DON) or even higher than (as in the cases of T-2 toxin and satratoxin G) those in the cytotoxicity assay, the practical sensitivity advantage of the luciferase translation assay results from the very small volume of extract that can be used. This is evident from Table 2, which contains a comparison of the detection limits of the luciferase translation method and the MTT cytotoxicity assay described in this report, as well as methods described by others, including cytotoxicity tests, thin-layer chromatographic analysis, and immunodetection. The luciferase translation test is a two-step procedure composed of a protein translation step and a luciferase assay step. Only 0.25 μl of translation mixture is required to obtain readings of about 60,000 RLU. Practically, one is not able to attain this limit in the first step by using regular pipetting, by which only 1 μl of translation mixture can be accurately dispensed. This increases the practical detection limit by a factor of four, hence, the difference between the real and the practical (in parentheses) volumes and amounts detected (Table 2). Use of robotic devices allowing accurate dispensing of nanoliter volumes should close the gap between the real and practical detection limits. The sensitivity of the assay remains comparable to the range of immunodetection. However, unlike immunodetection, the luciferase translation assay does not require an array of specific antibodies and measures combined toxicity rather than concentrations of individual toxins, thus providing a broader assessment of exposure. Toxicity analysis of problem houses finds toxin levels corresponding to nanogram amounts of T-2 toxin and satratoxin G, which is almost 1,000 times more than our practical detection limits. Furthermore, the procedure is conveniently standardized by parallel determination of T-2 toxin and satratoxin G dose-response curves. Although our use of rabbit reticulocytes yields very reproducible results, some batches of lysate occasionally demonstrate slightly different initial activity and altered sensitivity to trichothecenes, especially after prolonged storage. Therefore, using standard toxins provides an additional measure of interexperiment reproducibility and allows the expression of toxicity in terms of T-2 toxin and/or satratoxin G equivalents. The choice of standard toxins is somewhat arbitrary. Initially, T-2 toxin was selected because of its commercial availability and low cost and the relative abundance of available literature. Satratoxin G was subsequently included because it was detected in isolates of S. chartarum from water-damaged houses in Cleveland, Ohio, included in a study of pulmonary hemosiderosis in infants (18). However, this toxin is not commercially available, and the literature describing its action is rather scarce (17). Although possible effects of RNases and proteases, as well as other, unidentified substances interfering with protein translation, are potentially serious limitations of the assay, we have largely excluded these problems by the double filtration of extracts to remove enzymes and other large molecules. Exclusion of RNases and certain biomarkers, such as endotoxin, indicative of the presence of gram-negative bacteria allows one to focus on trichothecene mycotoxins as an indicator of exposure to toxigenic fungi.
In addition to its sensitivity, the luciferase translation assay yields highly reproducible results, as demonstrated by multiple screening of the same moisture problem house. As an activity assay, the luciferase translation test does not provide the toxin composition of environmental samples, which can only be investigated by using costly multimethod approaches, as described by Andersson et al. (1). Rather, the luciferase translation assay is a practical, rapid, and inexpensive means to detect and quantify the fungus-derived toxicity of air particulates from problem indoor environments. Inclusion of this assay in a battery of fungal tests to assess indoor environments is planned in order to demonstrate its projected utility.
ACKNOWLEDGMENT
Funding for this work was provided by NIEHS grant IR03ES08549-01.
REFERENCES
- 1.Andersson M A, Nikulin M, Koljalg U, Andersson M C, Rainey F, Reijula K, Hintikka E-L, Salkinija-Salonen M. Bacteria, molds and toxins in water-damaged building materials. Appl Environ Microbiol. 1997;63:387–393. doi: 10.1128/aem.63.2.387-393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babich H, Borenfreund E. Neutral red assay for toxicology in vitro. In: Watson R, editor. In vitro methods of toxicology. Boca Raton, Fla: CRC Press, Inc.; 1995. pp. 237–251. [Google Scholar]
- 3.Blackburn P, Moore S. Pancreatic ribonucleases. In: Boyer H W, editor. Enzymes. XV, part B. New York, N.Y: Academic Press, Inc.; 1982. pp. 317–433. [Google Scholar]
- 4.Bunner D L, Morris E R. Alteration of multiple cell functions in L-6 myoblasts by T-2 toxin: an important mechanism of action. Toxicol Appl Pharmacol. 1988;92:113–121. doi: 10.1016/0041-008x(88)90233-5. [DOI] [PubMed] [Google Scholar]
- 5.Casale W, Pestka J, Hart P. Enzyme linked immunosorbent assay employing monoclonal antibody specific for deoxynivalenol (vomitoxin) and several analogues. J Agric Food Chem. 1988;36:663–688. [Google Scholar]
- 6.Cundliffe E, Cannon M, Davies J. Mechanism of inhibition of eucaryotic protein synthesis by trichothecene fungal toxins. Proc Natl Acad Sci USA. 1974;71:30–34. doi: 10.1073/pnas.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLuca M, McElroy W D. Kinetics of the firefly luciferase catalyzed reactions. Biochemistry. 1974;13:921–925. doi: 10.1021/bi00702a015. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich R, Schneider E, Usleber E, Martlbauer E. Use of monoclonal antibodies for the analysis of mycotoxins. Nat Toxins. 1995;3:288–293. doi: 10.1002/nt.2620030423. [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich K C, Daigle K W. Protein synthesis inhibition by 8-oxo-12,13-epoxytrichothecenes. Biochim Biophys Acta. 1987;923:206–213. doi: 10.1016/0304-4165(87)90005-5. [DOI] [PubMed] [Google Scholar]
- 10.Etzel R, Montana E, Sorenson W, Kullman G, Allan T, Miller D, Jarvis B, Dearborn D. Acute hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch Pediatr Adolesc Med. 1998;152:757–762. doi: 10.1001/archpedi.152.8.757. [DOI] [PubMed] [Google Scholar]
- 11.Flannigan B. Biological particles in the air of indoor environments. In: Johanning E, Yang C S, editors. Fungi and bacteria in indoor air environments. Proceedings of the international conference. Latham, N.Y: Eastern New York Occupational Health Program; 1995. pp. 21–29. [Google Scholar]
- 12.Gareis M. Cytotoxicity testing of samples originating from problem buildings. In: Johanning E, Yang C S, editors. Fungi and bacteria in indoor air environments. Proceedings of the international conference. Latham, N.Y: Eastern New York Occupational Health Program; 1995. pp. 139–144. [Google Scholar]
- 13.Gould S J, Subramani S. Firefly luciferase as a tool in molecular biology. Anal Biochem. 1988;175:5–13. doi: 10.1016/0003-2697(88)90353-3. [DOI] [PubMed] [Google Scholar]
- 14.Hanelt M, Gareis M, Kollarczik B. Cytotoxicity of mycotoxin evaluated by the MTT cell-culture assay. Mycopathologia. 1994;128:167–174. doi: 10.1007/BF01138479. [DOI] [PubMed] [Google Scholar]
- 15.Holt P, DeLoach J. Cellular effects of T-2 mycotoxin on two different cell lines. Biochim Biophys Acta. 1988;971:1–8. doi: 10.1016/0167-4889(88)90155-3. [DOI] [PubMed] [Google Scholar]
- 16.Horikoshi K. Studies on the conidia of Aspergillus oryzae. Latent ribonuclease activity in the conidia of Aspergillus oryzae. Biochim Biophys Acta. 1979;240:532–540. doi: 10.1016/0005-2787(71)90710-6. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis B. Macrocyclic trichothecenes. In: Sharma R, Salunkhe D, editors. Mycotoxins and phytoalexins. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 361–421. [Google Scholar]
- 18.Jarvis B B, Sorenson W G, Hintikka E-L, Nikulin M, Zhou Y, Jiang J, Wang S, Hinkley S, Etzel R A, Dearborn D. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl Environ Microbiol. 1998;64:3620–3625. doi: 10.1128/aem.64.10.3620-3625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis C, Smith J, Anderson J, Murad T. The presence of mycotoxin-associated fungal spores isolated from the indoor air of the damp domestic environment and cytotoxic to human cell lines. Indoor Environ. 1994;3:323–330. [Google Scholar]
- 20.Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Nikulin M, Pasanen A-L, Berg S, Hintikka E-L. Stachybotrys atra growth and toxin production in some building materials and fodder under different relative humidities. Appl Environ Microbiol. 1994;60:3421–3424. doi: 10.1128/aem.60.9.3421-3424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasanen A, Nikulin M, Tuomainen M, Berg S, Parikka P, Hintikka E. Laboratory experiments on membrane filter sampling of airborne mycotoxins produced by Stachybotrys atra corda. Atmos Environ. 1993;27A:9–13. [Google Scholar]
- 23.Pathre S, Mirocha C. Assay methods for trichothecenes and review of their natural occurrence. In: Rodricks J, Hasseltine C, Mehlman M, editors. Mycotoxins in human and animal health. Park Forest South, Ill: Pathotox Publishers; 1977. pp. 229–253. [Google Scholar]
- 24.Promega. Technical bulletin no. 127. Promega, Madison, Wis.
- 25.Robb J, Norval M. Comparison of cytotoxicity and thin-layer chromatography methods for detection of mycotoxins. Appl Environ Microbiol. 1983;46:948–950. doi: 10.1128/aem.46.4.948-950.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seliger H H, McElroy W D. Spectral emission and quantum yield of firefly bioluminescence. Arch Biochem Biophys. 1960;88:136–141. doi: 10.1016/0003-9861(60)90208-3. [DOI] [PubMed] [Google Scholar]
- 27.Smith K E, Cannon M. Inhibition at the initiation level of eucaryotic protein synthesis by T-2 toxin. FEBS Lett. 1975;50:8–12. doi: 10.1016/0014-5793(75)81028-3. [DOI] [PubMed] [Google Scholar]
- 28.Thompson W, Wannemacher R. Detection and quantitation of T-2 mycotoxin with a simplified protein synthesis inhibition assay. Appl Environ Microbiol. 1984;48:1176–1180. doi: 10.1128/aem.48.6.1176-1180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno Y, Nakajima M, Sakai K, Ishii K, Sato N, Shimada N. Comparative toxicology of trichothecene mycotoxins: inhibition of protein synthesis in animal cells. J Biochem. 1973;74:283–296. [PubMed] [Google Scholar]
- 30.Van Etten J L, Dunkle L D, Knight R H. Nucleic acids and fungal spore germination. In: Weber D J, Hess W M, editors. The fungal spore. New York, N.Y: John Wiley & Sons, Inc.; 1974. pp. 243–300. [Google Scholar]