Abstract
Introduction
Pain is a common postoperative complication. The ideal postoperative analgesia is awake, safe, mobile, and without side effects. The objective of this study is to provide new ideas for postoperative analgesia by observing the safety and analgesic effect of different analgesic methods in patients undergoing laparotomy after surgery.
Methods
Patients, who underwent laparotomy between September 2019 and December 2020, were randomly divided into three groups: group S received sufentanil, group N received nalbuphine, group T + N received postoperative bilateral transversus abdominis plane block (TAPB) and nalbuphine. The primary outcomes included visual analog scale (VAS) score and the use of postoperative analgesic pump. Secondary outcomes included quality of life recovery (QoR-15) scale score and incidence of postoperative adverse reactions.
Results
Compared with group S and N, there were significant differences in the resting VAS score within 48 h after surgery, dynamic VAS score within 12 h after surgery, the first compression time, and cumulative use of patient-controlled intravenous analgesia (PCIA) drugs at 24 h in group T + N (P < 0.05). The QoR-15 score within 48 h after surgery in group T + N was significantly higher than group N (P < 0.05). The first exhaust time and the incidence of nausea and vomiting in group T + N were significantly lower than those in group N (P < 0.05).
Conclusions
Sufentanil PCIA and nalbuphine PCIA have equivalent analgesic effects, while TAPB combined with nalbuphine PCIA can ensure a good analgesic effect, thereby reducing the incidence of adverse reactions.
Keywords: Transversus abdominis plane block, Nalbuphine, Intravenous patient-controlled analgesia, Postoperative analgesia
Key Summary Points
| Why carry out this study? |
| Postoperative pain is a common postoperative complication. Multimodal analgesia refers to the combination of different drugs or analgesic methods, which has nearly reached the ideal postoperative analgesia. |
| Transversus abdominis plane block (TAPB) is an anesthetic technique that can be used for postoperative analgesia in a variety of abdominal surgeries. Nalbuphine has a unique analgesia for visceral pain and its analgesia effect lasts for a long time. |
| We hypothesized that TAPB combined with nalbuphine patient-controlled intravenous analgesia (PCIA) could provide good analgesia for patients undergoing laparotomy and reduce the occurrence of adverse reactions. |
| What was learned from the study? |
| TAPB combined with nalbuphine PCIA can effectively relieve postoperative pain of patients undergoing laparotomy, reduce the dosage of nalbuphine in a PCIA pump and the incidence of adverse reactions and improve patient satisfaction. |
| Nalbuphine can replace sufentanil equivalently using postoperative analgesia after laparotomy. |
| TAPB combined with nalbuphine PCIA is a safe and effective mode of postoperative analgesia that can be used in patients with different types of laparotomy. |
Introduction
Comfortable medicine and enhanced recovery after surgery (ERAS) are becoming a main goal for doctors [1]. Postoperative analgesia is a vital part of comfortable medicine. Intense pain after laparotomy is a common symptom, with significantly higher postoperative pain scores than other surgeries, increasing the perioperative risk [2–5]. Effective postoperative analgesia can minimize the unpleasant emotional experience caused by pain, thereby improving patient satisfaction; moreover, it can reduce the occurrence of postoperative complications, accelerating the rehabilitation and recovery of patients [6].
Patient-controlled epidural analgesia (PCEA) and patient-controlled intravenous analgesia (PCIA) are the main analgesic methods after laparotomy in clinical practice, combined with oral or intramuscular analgesic drugs. These analgesic methods could meet the basic analgesic needs of patients, although there are obvious limitations [7]. Therefore, “multimodal analgesia” or “balanced analgesia” is thought to be the most important concept for present postoperative pain [8]. Multimodal analgesia (MMA) is a relatively new analgesic mode combining different kinds of analgesic drugs or analgesic methods, to reduce the central and peripheral pain sensitivity of patients through multi-target action [9]. MMA could reduce the dosage of certain analgesic drugs and related adverse reactions under the premise of ensuring satisfactory analgesic effect, which is a better analgesic mode recommended by ERAS at present [10].
In the 2016 US Clinical Guidelines for Postoperative Pain Management, opioids are considered an important component of postoperative MMA (strong recommendation, medium quality) [11]. Sufentanil is widely used in clinical practice, because it not only has a strong analgesic effect but also limited accumulation in the body [12]. However, increasing the dosage of sufentanil could increase adverse reactions, such as excessive sedation, respiratory depression, nausea, and vomiting. Nalbuphine hydrochloride (referred to as “nalbuphine”) is a centrally acting opioid, which can activate κ receptors to produce central analgesic and sedative effects; it also partially antagonizes μ receptors, thereby reducing the side effects associated with activating μ receptors, such as respiratory depression, nausea and vomiting, and skin itching [13]. Several studies have shown that nalbuphine has the advantages of good analgesic effect, rapid onset, long duration of action, and less incidence of adverse reactions [14]. Both nalbuphine and equivalent doses of sufentanil can provide good intraoperative and postoperative analgesia [15]. It is worth noting that nalbuphine is significantly more effective than sufentanil in inhibiting visceral pain [16].
Transversus abdominis plane block (TAPB) is an anesthetic technique that can provide analgesia in the anterior and external abdominal wall [17–19] that is widely used for postoperative analgesia in various abdominal surgeries [20]. In recent years, with the introduction of ultrasound, the success rate of TAPB has greatly improved. TAPB is mainly aimed at somatic-related pain. Nevertheless, visceral pain resulting from laparotomy remains an important cause of postoperative pain as well; consequently TAPB is often used in combination with other analgesic methods in clinical practice [21].
Sufentanil PCIA was used as a positive control group in this study to evaluate the safety and analgesic effect of nalbuphine PCIA alone and TAPB combined with nalbuphine PCIA in postoperative analgesia in patients undergoing laparotomy, in order to provide new ideas for postoperative analgesia of patients undergoing laparotomy in clinical practice.
Methods
Study Participants
Ethical approval for this study was granted by the Medical Research Ethics Committee of Xianyang Hospital of Yan’an University, Shaanxi province, China, on August 28, 2019 (YDXY-KY-2019-004). The clinical research was conducted in accordance with the Declaration of Helsinki, and all patients signed the written informed consent form. We recruited 197 patients to participate in this prospective, randomized, clinical trial, who underwent laparotomy (mainly including cholecystectomy or cholangioenterostomy, partial gastrectomy, hemicolectomy, pancreaticoduodenectomy, and hysterectomy) between September 2019 and December 2020 without any robot-assisted and minimally invasive procedures. Finally, 180 patients completed the study. Inclusion criteria were the following: patients with ASA I–II scheduled for laparotomy under total intravenous general anesthesia; patients with clear consciousness, normal communication ability, voluntary acceptance of PCIA after surgery, and correct use of PCIA through learning; platelet and coagulation profile normal; no skin damage and infection at the TABP puncture site; patients who did not participate in other clinical trials within 3 months before the start of the study; patients and their families signed informed consent. Exclusion criteria were the following: use of narcotic analgesics within 48 h preoperative or history of opioid dependence; serious postoperative status leading to unstable vital signs and even death; history of allergy to anesthetic; pregnant and lactating women; cognitive dysfunction, or severe neuropsychiatric disorders, or inability to understand the scoring criteria and communication disorders with the physician.
Randomization and Blinding
The patients were randomly divided into three groups according to different postoperative analgesia methods (n = 60 each) by using a computer-generated random number list: patient-controlled intravenous analgesia with sufentanil alone (group S), patient-controlled intravenous analgesia with nalbuphine alone (group N), and ultrasound-guided transversus abdominis plane block combined with patient-controlled intravenous analgesia with nalbuphine (group T + N). Group S was used as a positive control. Postoperative follow-up of the patients was performed by an assistant researcher who was not involved in any previous process.
Intervention Protocols
Prior to surgery, all participants were given an information leaflet on the visual analog scale (VAS), quality of life recovery scale (QoR-15), and PCIA, which included scoring rules on the VAS and QoR-15 and the method of using the PCIA pump. No information related to the intervention was given to participants other than what was included in the manual.
All patients were anesthetized by intravenous general anesthesia, and anesthesia induction was performed using propofol 1.5–2 mg/kg, sufentanil 0.4–0.6 μg/kg, cisatracurium 0.2 mg/kg, midazolam 0.05 mg/kg. After the consciousness of patients disappeared, mechanical ventilation with endotracheal intubation was performed to maintain the end-tidal carbon dioxide concentration (EtCO2) at 35–45 mmHg. Standard monitoring was established before anesthesia induction, including electrocardiogram, noninvasive blood pressure (NBP) (necessary invasive blood pressure), pulse saturation, and bispectral index (BIS) to monitor the depth of anesthesia. During intraoperative maintenance of anesthesia, continuous infusion of propofol 4–10 mg/(kg·h), remifentanil 0.05–0.2 μg/(kg·h), intermittent additional cisatracurium as needed to maintain muscle relaxation. Dosage of propofol and remifentanil was adjusted according to hemodynamic changes and BIS value, which was maintained between 40 and 60. When hypotension (mean arterial pressure < 60 mmHg) or bradycardia (heart rate < 45 beats/min) exceeded 5 min, 6 mg ephedrine or 0.5 mg atropine was administered after intraoperative fluid resuscitation. After surgery, every patient received a PCIA pump. The background dose of the analgesic pump was 2 mL/h, the bolus dose was 1 mL, and the lockout time was 10 min. The patients returned to the surgical ward after extubation.
Group S was given sufentanil 2 mg/kg in a PCIA pump. Group N was given nalbuphine 2 mg/kg in a PCIA pump. Group T + N received TAPB block + nalbuphine 1.4 mg/kg in a PCIA pump after the operation, in which 20 ml 0.4% ropivacaine hydrochloride was used for TAPB block. In the three groups, 10 mg tropisetron was added to the analgesic pump and diluted to 100 ml with 0.9% sodium chloride injection.
In order to reduce the errors caused by the selection of operators and approaches, the implementation of TAPB in group N + T was performed by the same skilled anesthetist using the same approach under the guidance of visual ultrasound. The operation method of TAPB block was as follows: the supine position was taken immediately after the end of laparotomy. After skin disinfection, an ultrasound linear probe and 0.9 mm × 80 mm 22G sterile puncture needle were used. The probe was moved perpendicular to the level of anterior axillary line between the iliac crest and costal arch to find a satisfactory image. The skin, subcutaneous fat, external oblique muscle, internal oblique muscle, transverse abdominal muscle, and abdominal cavity could be displayed under ultrasound. The needle was inserted along the direction parallel to the long axis of the ultrasound probe, and the puncture needle was inserted into a vectorial plane about 3–4 cm medial to the ultrasound probe guiding the needle tip to the neuro-fascial plane between the internal oblique and transverse abdominal muscles. After the correct position of the needle tip and without blood and gas was determined, 1 ml normal saline was injected to open the plane between the two muscles, followed by 20 ml 0.4% ropivacaine hydrochloride injection, blocked the other side in the same way, and no catheter was placed, which was a one-time injection (the choice of this dosage was determined by the relevant guidelines in China and literature and that has also been proved to be feasible in preliminary experiments).
Outcome Measurements
The primary outcomes included VAS score, the first compression time of the PCIA pump, and the cumulative use of PCIA drugs at 24 h after the operation. The VAS scores of the three groups were recorded 2, 6, 12, 24, and 48 h after surgery. The resting VAS score measured the intensity of pain at rest (when the patient is placed in a comfortable position, lying still or sitting still). The dynamic VAS score measured the pain intensity during postoperative functional exercises (limb elevation exercises, ambulation with weight bearing, etc.).
Secondary outcomes included quality of life recovery (QoR-15) scale score and incidence of postoperative adverse reactions. The QoR-15 scores were recorded 24 h before surgery and 24 h and 48 h after surgery. The QoR-15 scale is divided into A and B volumes, with a total of 15 items, each of which is 0–10 points. The sum is the final evaluation criteria, and the score is the patient’s quality of life recovery. Simultaneously, the first exhaust time and postoperative adverse reactions, such as nausea and vomiting, skin itching, dizziness, respiratory depression, or puncture site hematoma, were recorded in the three groups. The first exhaust time was defined as the time of the first flatus. Additional secondary outcomes including mean arterial pressure (MAP), heart rate (HR), and pulse oxygen saturation (SpO2) to evaluate the safety of the analgesic method in each group, which were recorded at 2, 6, 12, 24, and 48 h after the operation.
Statistical Analysis
Before the start of this study, we conducted a preliminary in which 15 patients were selected in each group. The VAS scores of groups S, N, and T + N at 24 h after surgery were 1.50 ± 0.33, 1.56 ± 0.30, and 1.44 ± 0.35, respectively. Using an sample size calculation online tool (https://www.cnstat.org/samplesize/), we decided to recruit 60 patients to each group, allowing for a 20% dropout rate.
All data were processed using Statistical Package for Social Sciences (SPSS) software, version 26.0 (IBM SPSS, Armonk, NY, USA). Continuous data were expressed as mean ± standard deviation, followed by test of normality. If the variance was homogeneous, t test and analysis of variance (ANOVA) test were used; non-parametric test and Welch’s ANOVA test were used to determine the heterogeneity of variance. Enumeration data were expressed as frequency, and chi-square test and multiple independent sample non-parametric tests were used for comparison.
Results
Comparison of General Data Among Three Groups
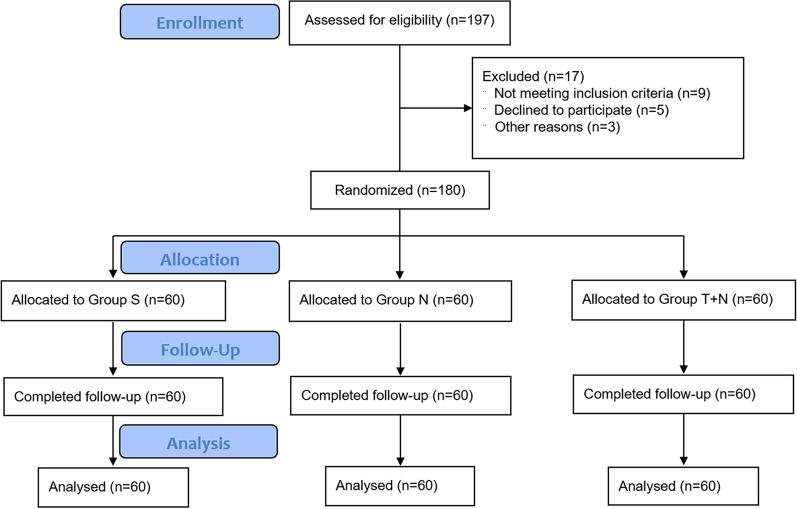
We enrolled and assessed a total of 197 patients for eligibility from September 2019 to December 2020, and excluded 17 patients, of whom nine did not meet inclusion criteria, five declined to participate, and three withdrew for other reasons. Finally, 180 patients were randomly assigned to three groups of 60 patients each (Fig. 1). Basic characteristics of the patients are listed in Table 1. There was no significant difference in age, gender, BMI (body mass index), type of operation, hypertension, diabetes, preoperative TP, preoperative TG, preoperative MAP, HR, and SpO2 among the three groups (P > 0.05). There were significant differences in ALB and ALT among the three groups (P < 0.05), which may be caused by different types of surgery. We therefore consider that there was no clinical significance in the statistical differences.
Fig. 1.
CONSORT diagram of patient flow
Table 1.
Basic characteristics of patients
| Group S | Group N | Group T + N | P | |
|---|---|---|---|---|
| Gender (male/female) | 40/20 | 42/18 | 42/18 | 0.902 |
| Age (year) | 45.78 ± 17.13 | 44.52 ± 17.71 | 46.28 ± 13.18 | 0.827 |
| BMI (kg/m2) | 26.67 ± 3.23 | 27.37 ± 3.44 | 27.85 ± 3.01 | 0.135 |
| Surgery type | 0.994 | |||
| Cholecystectomy or cholangioenterostomy | 28 (45.00%) | 27 (45.00) | 25 (41.67) | |
| Partial gastrectomy | 3 (6.67%) | 4 (6.67) | 4 (6.67) | |
| Hemicolectomy | 8 (16.67%) | 10 (16.67) | 11 (18.33) | |
| Pancreaticoduodenectomy | 2 (5.00%) | 3 (5.00) | 2 (3.33) | |
| Hysterectomy | 19 (26.67%) | 16 (26.67) | 18 (30.00) | |
| Preoperative hypertension | 2 (5%) | 3 (5) | 3 (5) | 0.877 |
| Preoperative diabetes | 1 (3.33%) | 2 (3.33) | 2 (3.33) | 0.814 |
| Preoperative ALB (g/l) | 39.67 ± 3.55 | 39.79 ± 3.68 | 38.31 ± 3.29 | < 0.05 |
| Preoperative ALT (µ/l) | 35.89 ± 4.65 | 34.73 ± 3.45 | 33.20 ± 5.36 | < 0.05 |
| Preoperative TP (g/l) | 67.88 ± 5.13 | 68.36 ± 5.34 | 68.35 ± 6.04 | 0.862 |
| Preoperative TG (mmol/l) | 1.03 ± 0.26 | 0.97 ± 0.19 | 1.04 ± 0.30 | 0.267 |
| MAP (mmHg) | 88.34 ± 5.65 | 87.95 ± 5.26 | 88.38 ± 6.32 | 0.903 |
| HR (times/min) | 72.24 ± 8.21 | 72.17 ± 9.48 | 72.61 ± 10.42 | 0.963 |
| SpO2 (%) | 98.78 ± 1.25 | 98.97 ± 1.41 | 98.94 ± 1.15 | 0.681 |
Data are presented as mean ± SD and number (percentage)
BMI body mass index, ALB albumin, ALT alanine aminotransferase, TP total protein, TG triglyceride, MAP mean arterial pressure, HR heart rate, SpO2 pulse oxygen saturation
Safety Evaluation
There were significant differences in MAP, HR, and SpO2 within 6 h after surgery (P < 0.05). MAP and HR at 2 h and 6 h after the operation in group T + N were significantly lower than those in group S and group N, and SpO2 at 2 h after the operation was significantly higher than that in group S and group N (P < 0.001). However, there was no significant difference in MAP and HR at 12, 24, and 48 h after the operation and SpO2 at 6, 12, 24, and 48 h after the operation among the three groups (P > 0.05) (Table 2).
Table 2.
Comparison of MAP, HR, and SpO2 among the three groups at different time points
| Observational period | Group S | Group N | Group T + N | P | |
|---|---|---|---|---|---|
| MAP | Postoperative 2 h | 84.67 ± 5.03 | 85.63 ± 4.55 | 80.57 ± 4.30 | < 0.001 |
| Postoperative 6 h | 86.42 ± 4.21 | 87.37 ± 4.14 | 81.67 ± 5.36 | < 0.001 | |
| Postoperative 12 h | 87.64 ± 6.03 | 88.78 ± 4.98 | 89.40 ± 5.32 | 0.204 | |
| Postoperative 24 h | 88.54 ± 4.35 | 89.52 ± 4.61 | 88.78 ± 4.32 | 0.452 | |
| Postoperative 48 h | 87.34 ± 3.65 | 86.95 ± 5.23 | 87.28 ± 2.32 | 0.842 | |
| HR | Postoperative 2 h | 78.39 ± 5.36 | 78.58 ± 4.48 | 72.72 ± 4.06 | < 0.001 |
| Postoperative 6 h | 75.97 ± 4.02 | 76.54 ± 3.95 | 72.58 ± 4.26 | < 0.001 | |
| Postoperative 12 h | 77.34 ± 5.31 | 76.40 ± 4.53 | 77.82 ± 4.24 | 0.247 | |
| Postoperative 24 h | 78.31 ± 6.32 | 78.72 ± 4.28 | 78.60 ± 4.63 | 0.905 | |
| Postoperative 48 h | 79.21 ± 5.35 | 79.43 ± 5.67 | 79.54 ± 6.01 | 0.949 | |
| SpO2 | Postoperative 2 h | 97.35 ± 1.15 | 97.57 ± 1.04 | 98.49 ± 0.73 | < 0.001 |
| Postoperative 6 h | 97.23 ± 2.12 | 97.53 ± 2.22 | 97.83 ± 1.12 | 0.233 | |
| Postoperative 12 h | 98.23 ± 0.35 | 98.34 ± 0.82 | 98.45 ± 0.75 | 0.204 | |
| Postoperative 24 h | 98.43 ± 0.54 | 98.36 ± 0.73 | 98.41 ± 0.69 | 0.834 | |
| Postoperative 48 h | 98.54 ± 0.51 | 98.47 ± 0.69 | 98.71 ± 0.76 | 0.127 |
Data are presented as mean ± SD
MAP mean arterial pressure, HR heart rate, SpO2 pulse oxygen saturation
Primary Outcome: Evaluation of Postoperative Analgesic Effect
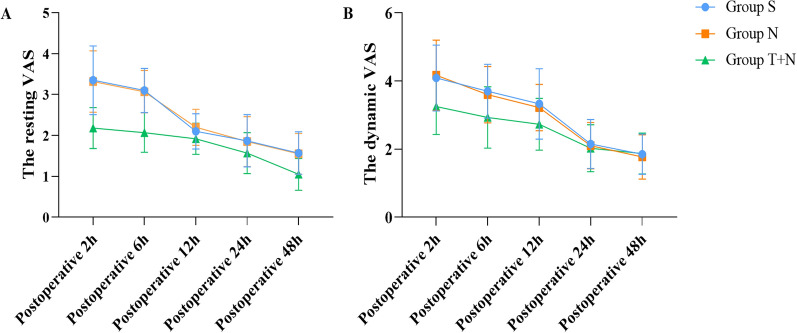
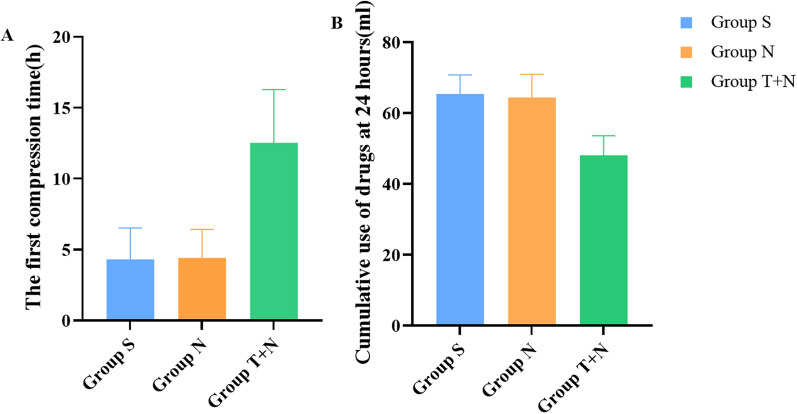
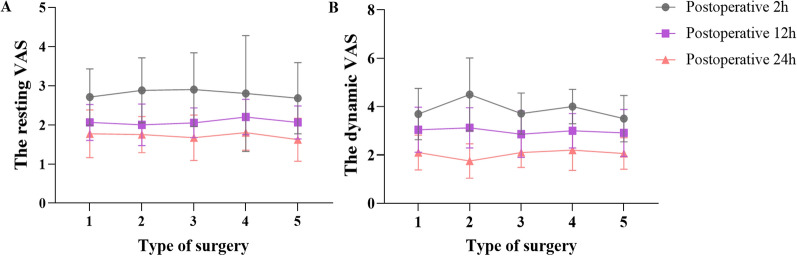
We first compared group S and group N and found that there was no significant difference in the resting and dynamic VAS score, the first compression time, and the cumulative use of PCIA drugs at 24 h between the two groups (P > 0.05). After that, compared with group N, there were significant differences in the resting VAS score within 48 h after surgery, dynamic VAS score within 12 h after surgery in group T + N (P < 0.05), but there was no significant difference in dynamic VAS score at 24 h and 48 h after surgery (P > 0.05). Among them, the resting VAS score at 2, 6, 12, and 48 h after surgery and the dynamic VAS score at 2 and 6 h after surgery in the T + N group were significantly lower than those in the N group (P < 0.001) (Fig. 2). In addition, the first compression time of group N + T was significantly later than that of group N, while the cumulative use of PCIA drugs at 24 h was also significantly less than that of group N (P < 0.05) (Fig. 3). Meanwhile, we also compared the VAS scores of patients with different types of laparotomy and found that there were no significant differences in the resting and dynamic VAS scores of cholecystectomy or cholangioenterostomy, partial gastrectomy, hemicolectomy, pancreaticoduodenectomy, and hysterectomy at 2, 12, and 24 h after surgery (P > 0.05) (Fig. 4).
Fig. 2.
Comparison of VAS score at different time points. Mean VAS score for pain intensity at different times postoperatively. Data are presented as mean and SD; whiskers represent SD. a Compared with group S and N, the resting VAS of group T + N was significantly lower (P < 0.05) at 2, 6, 12, 24, and 48 h postoperatively. b Compared with group S and N, the dynamic VAS of group T + N was significantly lower (P < 0.05) at 2, 6, and 12 h postoperatively
Fig. 3.
Comparison of use of PCIA pump between group N and group T + N. Data are presented as mean and SD. a Compared with group S and N, the first compression time of group T + N was significantly later (P < 0.05). b Compared with group S and N, the cumulative use of drugs at 24 h of group T + N was significantly lower (P < 0.05)
Fig. 4.
Comparison of VAS scores of different types of surgery at different time points. Mean VAS score for pain intensity at different times postoperatively. Data are presented as mean and SD; whiskers represent SD. 1 cholecystectomy or cholangioenterostomy; 2 partial gastrectomy; 3 hemicolectomy; 4 pancreaticoduodenectomy; 5 hysterectomy. There was no significant difference (P > 0.05) in a resting VAS scores or b dynamic VAS scores among cholecystectomy or cholangioenterostomy, partial gastrectomy, hemicolectomy, pancreaticoduodenectomy, and hysterectomy at 2 h, 12 h, and 24 h postoperatively
Secondary Outcomes: Recovery Status and Incidence of Postoperative Adverse Reactions
In the same way, we first compared the QoR-15 score, the postoperative initial exhaust time, and the incidence rate of adverse reactions in group S and group N, and found that the difference was not statistically significant (P > 0.05). Compared with group N, there were significant differences in the QoR-15 score at 24 h and 48 h after surgery in group T + N (P < 0.05) (Fig. 5). The first exhaust time and the incidence of nausea and vomiting in group T + N were significantly lower than those in group N, and the differences were statistically significant (P < 0.05), while the incidences of skin itching, dizziness, respiratory depression, and puncture site hematoma were not significantly different (P > 0.05) (Tables 3, 4).
Fig. 5.
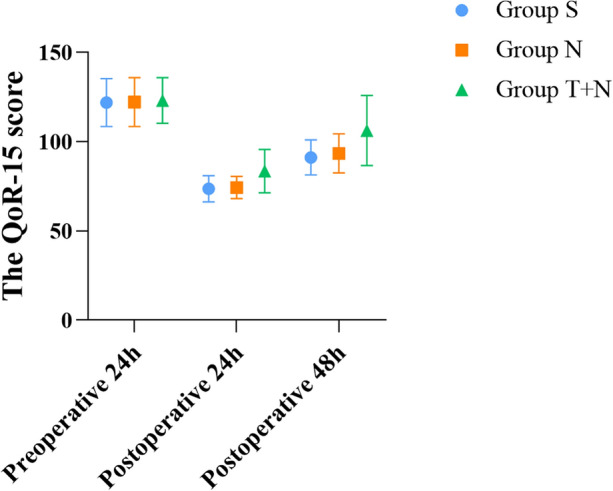
Comparison of QoR-15 score. Mean QoR score for quality of recovery in patients at different times postoperatively. Data are presented as mean and SD. Compared with group S and N, the QoR score of group T + N was significantly higher (P < 0.05) at 24 h and 48 h postoperatively
Table 3.
Comparison of postoperative adverse reaction between group S and group N
| Group S | Group N | P | |
|---|---|---|---|
| First exhaust time (h) | 6.01 ± 0.49 | 5.90 ± 0.50 | 0.226 |
| Nausea and vomiting | 9 (15.00) | 8 (13.33) | 0.793 |
| Skin itching | 3 (5.00) | 2 (3.33) | 1 |
| Dizziness | 1 (1.67) | 0 | 1 |
| Respiratory depression | 3(5.00) | 2 (3.33) | 1 |
| Puncture site hematoma | 0 | 0 | 1 |
Data are presented as mean ± SD and number (percentage)
Table 4.
Comparison of postoperative adverse reaction between group N and group T + N
| Group N | Group T + N | P | |
|---|---|---|---|
| First exhaust time (h) | 5.90 ± 0.50 | 4.35 ± 0.42 | < 0.001 |
| Nausea and vomiting | 8 (13.33) | 1 (1.67) | < 0.05 |
| Skin itching | 2 (3.33) | 0 | 0.496 |
| Dizziness | 0 | 0 | 1 |
| Respiratory depression | 2 (3.33) | 1 (1.67) | 0.560 |
| Puncture site hematoma | 0 | 0 | 1 |
Data are presented as mean ± SD and number (percentage)
Discussion
Pain is an inevitable complication after surgery, which will bring great suffering to patients, cause changes in endocrine and metabolic functions of patients, delay postoperative recovery, and increase medical costs [14]. Therefore, how to relieve postoperative pain has always been a hot issue for anesthetists [22]. Among the previous analgesic methods, PCEA and PCIA are two main forms [23]. PCEA has a definite analgesic effect, but patients can experience some adverse events such as lower limb numbness, weakness, and urinary retention. In addition, a change in patient position may cause the posterior catheter patch to fall off the catheter, increasing the risk of puncture site infection [24]. PCIA is more widely used in clinical practice owing to its relatively simple postoperative care and low risk of infection. Despite PCIA having an acceptable analgesic effect on resting pain, it is not effective for wound pain caused by abdominal wall tension traction during postoperative turning, patting the back, and coughing [25]. Currently, sufentanil is the preferred analgesic drug for PCIA after laparotomy, but with the increase of sufentanil concentration in the PCIA pump, the adverse reactions would increase. This study is mainly dedicated to the study of postoperative pain treatment in patients undergoing laparotomy in an effort to find a superior method of analgesia.
MMA is an analgesic concept, which is mainly a method to exert combined analgesic effect through multi-target action using different kinds of analgesic drugs [26]. Pain after laparotomy mainly includes visceral pain and somatic pain. According to the mechanism of postoperative pain, satisfactory analgesic effect can only be achieved by combining two or more drugs and methods, which is the primary reason that MMA has become the analgesic mode recommended by ERAS. Regional nerve block technique is one of the important components of MMA, with definite analgesic effect, but little effect on respiration, circulation, postoperative movement of patients, and functional exercise [27]. TAPB is a regional block anesthesia technique that can effectively reduce abdominal incision pain [28, 29]. Studies showed that TAPB combined with an opioid-sparing analgesia in the setting of laparoscopic colorectal surgery is feasible and effective in postoperative analgesia [30, 31]. TAPB is mainly for somatic pain, whereas pain after laparotomy is complex. Compared with equivalent doses of sufentanil, nalbuphine provides good analgesic effect, the incidence of adverse reactions is lower [15, 32, 33], and it has a good visceral analgesic effect [34]. Therefore, our study explored a better postoperative analgesic mode by comparing the analgesic effect, safety, and reliability of sufentanil PCIA, nalbuphine PCIA, and TAPB combined with nalbuphine intravenous PCIA.
This study found that the MAP, HR, and SpO2 of group S, group N and group T + N at five different time points (2, 6, 12, 24, and 48 h) after surgery were within the clinically normal range, indicating that the three analgesic methods can be safely used for postoperative analgesia. MAP and HR in the T + N group were significantly lower than in the group S and N at 2 and 6 h after surgery, suggesting that the onset of action of TAPB peaked at this time and minimized the stimulation of surgical pain. It also indicated that multimodal analgesia with TAPB combined with nalbuphine PCIA was more beneficial to maintain hemodynamic stability than sufentanil PCIA and nalbuphine PCIA, which was consistent with the results of previous studies [35].
The VAS score is a simple and practical evaluation scale that is commonly used in clinical practice to evaluate the degree of pain [36, 37]. At the same time, the time of the first postoperative PCIA pump compression and the cumulative use of postoperative PCIA drugs can also indirectly imply the patient’s postoperative pain. Therefore, the VAS score and the use of postoperative analgesic pump were used as the primary outcome measures in this study. Firstly, we observed that there was no significant difference in resting and dynamic VAS score within 48 h after surgery between group S and N, and the resting and dynamic VAS scores within 12 h after surgery in group T + N were significantly lower than in group S and N within 48 h after surgery, which suggested that the analgesic effects of group N and T + N were good, and TAPB combined with nalbuphine PCIA could improve the quality of analgesia within 48 h after surgery in the resting state and within 12 h after surgery in the exercise state. Secondly, we found that the difference of resting VAS score after 6 h after surgery in group T + N was reduced compared with the other two groups, while the dynamic VAS score after 12 h after surgery was not significantly different compared with the other two groups, which may be related to the decrease of TAPB drug concentration. Next, considering whether the degree of pain in different types of surgery would have an impact on the whole study, we compared the resting and dynamic VAS scores at 2 h, 12 h, and 24 h after different laparotomies and found no significant difference. Meanwhile, other results showed that the first compression time of group T + N was significantly later and the cumulative use of drugs at 24 h was significantly less than in group S and N, which again indicated that the analgesic effect could be significantly improved after TAPB combined with nalbuphine PCIA, and the dosage of opioids could also be reduced.
Postoperative recovery is, as a key evaluation indicator in postoperative analgesia study, a complex multidimensional concept. The QoR-15 scale is a postoperative assessment tool proposed, which has a total score of 150 points and includes several aspects such as psychological support, emotional state, physical comfort, independence, and pain [38, 39], which has been proved to be effective, reliable, clinically acceptable, and feasible. In this study, the QoR-15 score of group N + T was significantly higher than in group S and N at 24 h and 48 h after surgery, suggesting that compared with nalbuphine PCIA and sufentanil PCIA alone, the mode of TAPB combined with nalbuphine PCIA can increase patient comfort. In addition, several researchers have found that the TAPB block can not only reduce opioid consumption and postoperative pain but also improve quality of recovery in patients undergoing abdominal surgery [40–43]. The aforementioned studies were consistent with the results of our study, which showed that TAPB combined with nalbuphine PCIA can provide better postoperative analgesia and quality of recovery for patients.
Acute postoperative pain can cause gastrointestinal disorders and slowed gastrointestinal motility, and opioids may also cause flatulence and decreased intestinal motility. Zafar et al. found that patients who received TAPB significantly improved postoperative gastrointestinal function recovery time and shortened anus exhaust time [44]. In our study, the first exhaust time and the incidence of postoperative nausea and vomiting in group N + T were significantly less than those in group S and N. We considered that this may be because TAPB effectively inhibited sympathetic stimulation, or the dose of opioids in the PCIA pump was reduced after combined use of TAPB, or nalbuphine reduced side effects including gastrointestinal reactions by antagonizing μ receptors. It is worth noting that there were occasional cases of pruritus, dizziness, and respiratory depression in each group, but there was no statistically significant difference.
This study has the following limitations: this study is a single-center study and lacks multicenter study results; TAPB requires advanced color Doppler machines and skilled physicians for operation, which may have some associated limitations in clinical application; the VAS score of pain assessment index is subjective, related to the cognitive level and emotional stability of patients. For more in-depth studies in the future, we should conduct a multicenter study and find an easy to operate and more objective postoperative analgesia scoring tool. In addition, at present, we have no specific method to differentiate the effects of TABP combined with nalbuphine on human somatic pain and visceral pain, but in our further study, we intend to build different animal models of pain to explore the effect of this analgesic pattern in somatic pain and visceral pain.
Conclusions
In the study, we found that sufentanil or nalbuphine PCIA alone and TAPB combined with nalbuphine PCIA can safely and effectively relieve postoperative pain in patients undergoing laparotomy. Sufentanil PCIA and nalbuphine PCIA have the same analgesic effect, while TAPB combined with nalbuphine PCIA can not only ensure a good analgesic effect but can also reduce the dosage of nalbuphine in the PCIA pump, reduce the incidence of adverse reactions, and improve patient satisfaction.
Acknowledgements
Thanks to our colleagues for their help in this study, and also to the participants in this study.
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s Rapid Service Fee of the study was funded by the researchers themselves.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written by Kunyu Han and Yuhe Zhang, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Kunyu Han, Yuhe Zhang, Ruiping Bai, Rui An, Simei Zhang, Mengwen Xue and Xin Shen declare that they have no conflict of interest.
Compliance with Ethics Guidelines
Ethical approval for this study was granted by the Medical Research Ethics Committee of Xianyang Hospital of Yan’an University, Shaanxi province, China, on August 28, 2019 (YDXY-KY-2019–004). The clinical research was conducted from September 2019 to December 2021 in accordance with the Declaration of Helsinki. It is a pity that the trial was not registered in the Clinical Trial Registry before our study, but the study was fully compliant with Good Clinical Practice (GCP), passed rigorous ethical review, and all patients signed the informed consent form. This study, as a graduation topic for inservice graduate student, did not consider there will be a worthy and promising result for clinical analgesia at the beginning. Besides, we worried that it may affect the progress of graduation and therefore missed the timing of clinical registration. We sincerely hope to show our study to the researchers according to the journal. Next, our team plan to carry out relative multicenter RCT.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Kunyu Han and Yuhe Zhang contributed equally to this work.
Contributor Information
Mengwen Xue, Email: xuemengwen@xjtu.edu.cn.
Xin Shen, Email: shenxin6125@mail.xjtu.edu.cn.
References
- 1.Kehlet H. Enhanced postoperative recovery: good from afar, but far from good? Anaesthesia. 2020;75(Suppl 1):e54–e61. doi: 10.1111/anae.14860. [DOI] [PubMed] [Google Scholar]
- 2.Small C, Laycock H. Acute postoperative pain management. Br J Surg. 2020;107(2):e70–e80. doi: 10.1002/bjs.11477. [DOI] [PubMed] [Google Scholar]
- 3.Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4(5):451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 4.Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi: 10.1016/S0140-6736(19)30352-6. [DOI] [PubMed] [Google Scholar]
- 5.Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264:73–80. doi: 10.1097/SLA.0000000000001691. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yang D, Zhao S, et al. Postoperative pain management in Chinese hospitals: a national survey. Br J Anaesth. 2021;127(6):e200–e202. doi: 10.1016/j.bja.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Mann C, Pouzeratte J, Boccara G, et al. Comparison of intravenous or epidural patient-controlled analgesia in the elderly after major abdominal surgery. Anesthesiology. 2000;92(2):433–441. doi: 10.1097/00000542-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Chen YK, Boden KA, Schreiber KL. The role of regional anaesthesia and multimodal analgesia in the prevention of chronic postoperative pain: a narrative review. Anaesthesia. 2021;76(Suppl 1):8–17. doi: 10.1111/anae.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691–697. doi: 10.1001/jamasurg.2017.0898. [DOI] [PubMed] [Google Scholar]
- 11.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Chen Y, Huang S, Sun X. Interaction of analgesic effects of dezocine and sufentanil for relief of postoperative pain: a pilot study. Drug Des Dev Ther. 2020;3(14):4717–4724. doi: 10.2147/DDDT.S270478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MP, Fernandez C, Regel S, McPherson ML. Does nalbuphine have a niche in managing pain? J Opioid Manag. 2018;14(2):143–151. doi: 10.5055/jom.2018.0441. [DOI] [PubMed] [Google Scholar]
- 14.Fang P, Qian J, Ding J, et al. Comparison of analgesic effects between nalbuphine and sufentanil in first-trimester surgical abortion: a randomized, double-blind, controlled trial. Pain Ther. 2022;11(1):121–132. doi: 10.1007/s40122-021-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Zhou Y, Cai Y, et al. The ED95 of nalbuphine in outpatient-induced abortion compared to equivalent sufentanil. Basic Clin Pharmacol Toxicol. 2018;123(2):202–206. doi: 10.1111/bcpt.13022. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Hu J, Hu X, et al. Preemptive intravenous nalbuphine for the treatment of post-operative visceral pain: a multicenter, double-blind, placebo-controlled, randomized clinical trial. Pain Ther. 2021;10(2):1155–69. [DOI] [PMC free article] [PubMed]
- 17.Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56(10):1024–1026. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 18.McDonnell JG, O’Donnell BD, Tuite D, et al. The regional abdominal field infiltration (R.A.F.I.) technique computerised tomographic and anatomical identification of a novel approach to the transversus abdominis neuro-vascular fascial plain. In: Proceedings of the American Society of Anesthesiologists annual meeting, 2004, p A-899.
- 19.Turan A, Cohen B, Elsharkawy H, et al. Transversus abdominis plane block with liposomal bupivacaine versus continuous epidural analgesia for major abdominal surgery: the EXPLANE randomized trial. J Clin Anesth. 2022;77:110640. doi: 10.1016/j.jclinane.2021.110640. [DOI] [PubMed] [Google Scholar]
- 20.Kamel AAF, Amin OAI, Ibrahem MAM. Bilateral ultrasound-guided erector spinae plane block versus transversus abdominis plane block on postoperative analgesia after total abdominal hysterectomy. Pain Physician. 2020;23(4):375–382. doi: 10.36076/ppj.2020/23/375. [DOI] [PubMed] [Google Scholar]
- 21.Jankovic Z, Ahmad N, Ravishankar N, et al. Transversus abdominis plane block: how safe is it? Anesth Analg. 2016;107(5):1758–1759. doi: 10.1213/ane.0b013e3181853619. [DOI] [PubMed] [Google Scholar]
- 22.Kehlet H. Postoperative pain, analgesia, and recovery-bedfellows that cannot be ignored. Pain. 2018;159(Suppl 1):S11–S16. doi: 10.1097/j.pain.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 23.Klotz R, Larmann J, Klose C, et al. Gastrointestinal complications after pancreatoduodenectomy with epidural vs patient-controlled intravenous analgesia: a randomized clinical trial. JAMA Surg. 2020;155(7):e200794. doi: 10.1001/jamasurg.2020.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Dong H, Tan S, Qian Y, Jin W. Effects of thoracic epidural anesthesia/analgesia on the stress response, pain relief, hospital stay, and treatment costs of patients with esophageal carcinoma undergoing thoracic surgery: a single-center, randomized controlled trial. Medicine (Baltimore) 2019;98(7):e14362. doi: 10.1097/MD.0000000000014362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian P, Fu X, Li ZJ, Ma XL. Comparison of patient-controlled epidural analgesia and patient-controlled intravenous analgesia after spinal fusion surgery: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2015;15(16):388. doi: 10.1186/s12891-015-0849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladha KS, Patorno E, Huybrechts KF, et al. Variations in the use of perioperative multimodal analgesic therapy. Anesthesiology. 2016;124:837–845. doi: 10.1097/ALN.0000000000001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kehlet H. Modification of responses to surgery and anesthesia by neural blockade: clinical implications. In: Cousins MJ, Bridenbaugh PO, editors. Neural blockade in clinical anesthesia and management of pain. Philadelphia: Lippincott; 1987. pp. 145–188. [Google Scholar]
- 28.Owen DJ, Harrod I, Ford J, et al. The surgical transversus abdominis plane block—a novel approach for performing an established technique. BJOG. 2010;118(1):24–27. doi: 10.1111/j.1471-0528.2010.02779.x. [DOI] [PubMed] [Google Scholar]
- 29.Desai N, El-Boghdadly K, Albrecht E. Epidural vs. transversus abdominis plane block for abdominal surgery—a systematic review, meta-analysis and trial sequential analysis. Anaesthesia. 2021;76(1):101–117. doi: 10.1111/anae.15068. [DOI] [PubMed] [Google Scholar]
- 30.Ismail S, Ahmed A, Hoda MQ, et al. Mid-axillary transversus abdominis plane block and stress response after abdominal hysterectomy: a randomised controlled placebo trial. Eur J Anaesthesiol. 2021;38(7):768–776. doi: 10.1097/EJA.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 31.Yang P, Luo Y, Lin L, et al. The efficacy of transversus abdominis plane block with or without dexmedetomidine for postoperative analgesia in renal transplantation. A randomized controlled trial. Int J Surg. 2020;79:196–201. doi: 10.1016/j.ijsu.2020.05.073. [DOI] [PubMed] [Google Scholar]
- 32.Sun S, Guo Y, Wang T, Huang S. Analgesic effect comparison between nalbuphine and sufentanil for patient-controlled intravenous analgesia after cesarean section. Front Pharmacol. 2020;11:574493. doi: 10.3389/fphar.2020.574493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F, Wang CY, Zhang J, et al. Comparison of postoperative analgesic effects between nalbuphine and fentanyl in children undergoing adenotonsillectomy: a prospective, randomized, double-blind, multicenter study. Front Pharmacol. 2020;11(5):97550. doi: 10.3389/fphar.2020.597550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Z, Zhu Z, Yang G, Zheng H. The 95% effective dose of nalbuphine in patient-controlled intravenous analgesia for patients undergoing laparoscopic total hysterectomy compared to equivalent sufentanil. Medicine (Baltimore) 2020;99(22):e20424. doi: 10.1097/MD.0000000000020424. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Bacal V, Rana U, McIsaac DI, Chen I. Transversus abdominis plane block for post hysterectomy pain: a systematic review and meta-analysis. J Minim Invas Gynecol. 2019;26(1):40–52. doi: 10.1016/j.jmig.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Heller GZ, Manuguerra M, Chow R. How to analyze the visual analogue scale: myths, truths and clinical relevance. Scand J Pain. 2016;13:67–75. doi: 10.1016/j.sjpain.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Shafshak TS, Elnemr R. The visual analogue scale versus numerical rating scale in measuring pain severity and predicting disability in low back pain. J Clin Rheumatol. 2021;27(7):282–285. doi: 10.1097/RHU.0000000000001320. [DOI] [PubMed] [Google Scholar]
- 38.Kleif J, Waage J, Christensen KB, Gogenur I. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth. 2018;120:28–36. doi: 10.1016/j.bja.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Campfort M, Cayla C, Lasocki S, et al. Early quality of recovery according to QoR-15 score is associated with one-month postoperative complications after elective surgery. J Clin Anesth. 2022;78:110638. doi: 10.1016/j.jclinane.2021.110638. [DOI] [PubMed] [Google Scholar]
- 40.Karaman T, Ozsoy AZ, Karaman S, et al. The effects of transversus abdominis plane block on analgesic and anesthetic consumption during total abdominal hysterectomy: a randomized controlled study. Braz J Anesthesiol. 2018;68(3):285–291. doi: 10.1016/j.bjan.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon S, Song GY, Lee J, et al. Ultrasound-guided bilateral subcostal transversus abdominis plane block in gastric cancer patients undergoing laparoscopic gastrectomy: a randomised-controlled double-blinded study. Surg Endosc. 2022;36(2):1044–1052. doi: 10.1007/s00464-021-08370-9. [DOI] [PubMed] [Google Scholar]
- 42.Vindal A, Sarda H, Lal P. Laparoscopically guided transversus abdominis plane block offers better pain relief after laparoscopic cholecystectomy: results of a triple blind randomized controlled trial. Surg Endosc. 2021;35(4):1713–1721. doi: 10.1007/s00464-020-07558-9. [DOI] [PubMed] [Google Scholar]
- 43.Shim JW, Ko J, Lee CS, et al. Better timing of ultrasound-guided transversus abdominis plane block for early recovery after open inguinal herniorrhaphy: a prospective randomised controlled study. Asian J Surg. 2021;44(1):254–261. doi: 10.1016/j.asjsur.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Zafar N, Davies R, Greenslade GL, Dixon AR. The evolution of analgesia in an 'accelerated' recovery programme for resectional laparoscopic colorectal surgery with anastomosis. Colorectal Dis. 2010;12(2):119–124. doi: 10.1111/j.1463-1318.2009.01768.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.