Abstract
Cancer is an immunosuppressive disorder with characteristic features of unchecked cell growth, invasion, and sometimes thromboembolism leading to multiple systemic sequelae, including infective endocarditis. This article has compiled some of the crucial mechanisms by which infective endocarditis occurs in cancer patients, its risk factors, and the existing treatment interventions. It has focused on the necessity of being aware that these multiple pathogeneses are involved in the development of infective endocarditis (IE) in cancer patients, which would help delineate the risk factors associated with the condition and help physicians screen better for specific red flags. Identifying these risk factors and patient-oriented therapy, targeting the necessary elements such as causative organism, patient immune status, type of cancer, choosing evidence-based treatment modalities, and to improve the outcome of the disease in an already exasperating condition called cancer.
Keywords: colorectal cancer and endocarditis, malignancy and endocarditis, streptococcus bovis endocarditis, s. anginosus endocarditis, marantic endocarditis, non-bacterial thrombotic endocarditis, infective endocarditis
Introduction and background
Cancer is the uncontrolled growth of a cell with the subsequent development of metastatic features. Malignancy is when the unchecked cell growth develops into a metastatic state by downregulation of cell adhesion receptors and up-regulation of receptors for increased cell motility for its spread far from its origin [1]. Cancer patients with infective endocarditis (IE) might be active or previously diagnosed. IE can also be a marker for the suspicion of an underlying malignancy [2,3]. One of the first associations between IE and malignancy was described decades ago in patients with colorectal cancer (CRC) [3]. The occurrence of IE in cancer patients is surprisingly uncommon and was found to be 18% [4]. The most common cancers in the United States and Europe are colorectal, rectal, breast, and prostate, with a collective incidence of 800,000 and 1,370,000, respectively [5,6]. A recent study identified that IE occurred more commonly in patients with lung, prostate, and colorectal cancers than in patients without a cancer diagnosis [3]. Other extensive databases have observed that some digestive, respiratory, and hematologic malignancies are related to IE [7]. IE cancer patients belonged to the elderly population and were most often males [4,8,9].
Cancer patients undergo a substantial amount of invasive procedures during diagnosis and treatment, which puts them at risk of developing IE [10]. The thrombotic nature of most malignancies is one of the most well-discussed mechanisms of IE in patients with cancer [11]. In recent times, technological advancement has shed some light on the relatively less known mechanism by which malignancies serve as a threat to the development of IE, such as acting as a port of entry (POE) via the skin, oral, biliary, urinary, female genital tract [12] or by the associated immune suppression. Certain bacteria can adhere and invade tissues as another crucial etiology of IE in cancer patients [7]. Hematologic malignancies and specific chemotherapeutic treatments are prone to cause immunosuppression, which acts as a segue for IE development [13]. Compared to classic IE in non-cancer patients, IE in cancer patients present atypically with significantly less fever and a new heart murmur [14]. The complications of cancer with subsequent IE have a slightly different and increased preference for acute renal failure, followed by emboli events and congestive heart failure (CHF) [14].
Positron emission tomography with 2-deoxy-2-fluorine-18 fluoro- D-glucose integrated with computed tomography ((18F-FDG) PET/CT) has a significant diagnostic value in cancer and non-cancer patients. However, it has some drawbacks, such as the inability to differentiate between metastatic lesions, inflammatory foci, and emboli of IE. Although surgical indications are similar to non-cancer patients, cardiac surgeries are not often performed. The cancer patients with IE were denied surgery mainly because of their high-risk features, and delaying the surgery may lead to death before performing the procedure itself [14].
There remain unfamiliar grounds for the disease characteristics and risk factors of IE that could occur in patients with an underlying malignancy, which would be helpful to understand the disease course better and provide appropriate treatment [15]. This article aims to discuss the pathophysiology of IE in patients diagnosed with malignancy and its risk factors, making the patient susceptible to the simultaneous diagnosis, and highlighting the treatment modalities available for IE in cancer patients.
Review
Pathogenesis
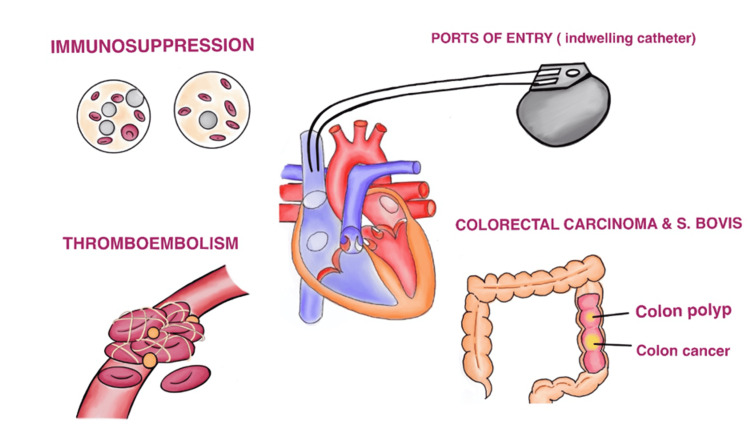
As discussed earlier, IE can originate from various sources in cancer patients, as depicted in Figure 1.
Figure 1. Pathogenesis of infective endocarditis in cancer patients.
Image credits - Lakshmi Sree Pugalenthi
S. bovis - Streptococcus bovis
Immunosuppression
Immune suppression plays a vital role in the pathophysiology of endocarditis in cancer patients. Immune suppression has various etiologies in such patients, such as chemotherapy, alcoholism, metastatic disease, and old age [1,3,12,16,17]. Patients with hematologic malignancies have neutropenia associated with poor phagocytosis, abnormal B & T cells, POE from venous catheter routes, splenectomy, and chemotherapy-related immune suppression. These are some of the suggested conditions responsible for the development of endocarditis in cancer patients [7].
Cancer patients are prone to be neutropenic, making them susceptible to IE. Cristina et al. conducted a study over 10 years, from 2005 to 2015, in Santa Cruz de Tenerife. The study population included 208 IE patients, of which 32 people were also diagnosed with cancer. The study concluded that neutropenia was detected during the time of the diagnosis of IE in only 15.6% of the patients [18]. Chemotherapeutic agents inhibit the synthesis of DNA and RNA, which results in the suppressed production of immune cells [19]. Cancer patients have a poor nutritional status which further impairs the B & T cells' immuno-protective effects [19].
S. bovis endocarditis and CRC
The association between CRC and Streptococcal endocarditis was discussed in 1951 [3]. The strain, Streptococcus bovis, seemed to have an undeniable relationship with colon cancer [20]. S. bovis-associated endocarditis has an unusual and slightly less understood relationship with colorectal carcinoma. S. bovis is a commensal bacteria inhabiting the lumens of our gastrointestinal system and is responsible for 10-15% of all IE cases [21,22]. Any colonic lesion may serve as a niche for the colonization of S. bovis/gallolyticus. Hence premalignant colonic lesions serve as a site of colonization for S. bovis. The ability of the bacteria to survive for a more extended period is increased due to the conducive environment provided by the tumor microenvironment [23]. Once intestinal micro-perforations occur in CRC, these colonized organisms find their way into the blood circulation [21,22].
The diagnosis of advanced adenoma or cancer with S. bovis colonization is made before the development of endocarditis has been found in many cases [24]. S. bovis has characteristic traits such as adhesion to intestinal cells by pili expression due to its interaction with mucin and collagen [25,26] and the ability to grow in bile compared to other alpha-hemolytic streptococci. Due to these properties, S. bovis can bypass the hepatic-reticuloendothelial system and enter the systemic circulation directly [27,28].
S. bovis has also been known to play a role in the development of CRC actively, explained by an increased interleukin 1 (IL-1), cyclooxygenase-2 (COX-2), and interleukin-8 (IL-8). These cytokines stage an inflammatory sequence for tumor development and progression [29]. The permeability of the intestinal wall is further altered by the production of cytokines by the bacteria itself, promoting its entry from the intestinal lumen into the blood, resulting in a myriad of infectious complications [30].
Port of entry
As the name suggests, POE serves as a port for bacterial organisms to cause bacteremia and subsequently IE. Bacterial characteristics such as pili [31,32], biofilm formation for adhesion to epithelial cells [33-37], the ability to attach to the extracellular matrix of the heart valves [33,34,36,37], and its capacity to evade phagocytosis [35] and thereby aiding in bacterial virulence. The most common POEs are the skin, oral cavity, and digestive system [38]. Identifying possible POE is crucial in reducing the incidence of a new episode of IE. The various POE and the associated risk factors are discussed in Table 1 [38].
Table 1. Ports of entry and their risk factors .
POE - Port of Entry
IV - Intravenous
BPH - Benign Prostatic Hyperplasia
ENT - Ear, nose & throat
| POE | RISK FACTORS |
| Cutaneous | Healthcare-associated Vascular access, infection of cardiac implantable electronic device, infection of the operation site, community-acquired {domestic wound, ulcers (diabetic foot ulcer, venous ulcer, pressure ulcer), insect bite}, IV drug use, inoculation disease (louse bite, tick bite, cat-scratch disease). |
| Oral/ dental infective foci | Somatologic examination, dental infectious focus, and endodontic & periodontal disease. |
| Colonic Lesions | Those who underwent colonoscopy for polyps, diverticulosis, diffuse angiodysplasia, and adenocarcinoma. |
| Urinary Lesions | Who underwent urinary examinations: Prostate cancer, BPH with urine retention, urethral stenosis, pyelonephritis, cystinuria with repetitive renal lithiasis, post-radiotherapy bladder, and extrinsic urethral compression by colon cancer. |
| ENT lesions | Sinusitis, otomastoiditis, etc. |
Delahaye et al. conducted a study in France over six years in a sample population of 318 hospitalized patients, excluding 82 patients who died during the hospitalization. They found that 78% of the patients were identified with POE. Cutaneous POE (40%) was the most commonly identified source, followed by 29% and 24% in dental/oral POE and gastrointestinal POE, respectively. The study concluded that POE is directly associated with a diagnosis of IE and that patients with such possible POE must be examined regularly [38].
Invasive procedures pose a risk for the development of IE. Kim et al. conducted a study from 2011 to 2015 on 170 patients, of which 30 patients had active cancer. It was found that non-dental procedures can also lead to IE, such as intravenous catheter insertion and endoscopic or genitourinary invasive procedures [18].
Non-bacterial thrombotic endocarditis
Non-bacterial thrombotic endocarditis (NBTE) is common among those between 40 to 80 years of age, with no preference for a specific gender. Adenocarcinoma is a malignancy that is most commonly associated with NBTE, especially in the lungs, pancreas, and gastric adenocarcinomas [39].
Many studies have estimated that 15% of cancer patients have developed a thromboembolic event throughout their disease course and 50% have a documented venous thromboembolic event [40,41]. NBTE is characterized by the presence of vegetations on the heart valves, which are sterile and non-infective [42]. Cancers are associated with the increased secretion of cytokines such as tumor necrosis factor (TNF) or IL-1, which causes local tissue damage; and subsequently, vegetation formation [43]. NBTE is based on the mechanism of endothelial cell injury with a coexisting hypercoagulable state. NBTE is found to have increased levels of circulating tissue factors. The monocytes on valvular lesions express more tissue factor messenger RNA (mRNA), which contributes to the mechanism of coagulation cascade initiation [44]. This injury leads to thrombi formed by platelet accumulation and inflammatory mononuclear cells entangled with fibrin and immune complexes [45]. Cardiac lesions in such a setting are always devoid of microorganisms making NBTE distinct from IE. They break apart more easily and can seed out into the systemic circulation. NBTE vegetative lesions can equally affect damaged and undamaged valves, chordae tendineae, or the endocardium [45]. Histologically, these vegetations seem to show some evidence of abnormal collagen and elastic fibers. Valvular leaflets with areas of high flow seem to influence the development of valvular lesions associated with NBTE [43]. The interaction between macrophages and malignant cells leads to the release of cytokines such as TNF, IL-1, and IL-6, promoting endothelium damage, platelet deposition, and the formation of friable thrombi. Such interactions also amplify the coagulation cascade, resulting in a worsened hypercoagulable state [46]. These patients become clinically symptomatic only when the thrombus has embolized to the brain or any other vital organ [38]. Recent studies have found a link between oncogene and activation and hemostasis, whereby the mesenchymal-epithelial transition factor (MET) oncogene causes transcriptional response and upregulation of plasminogen activator inhibitor type 1 (PAI-1) and cyclooxygenase 2 (COX-2), ultimately resulting in increased coagulation [47].
Treatment modalities and outcomes
Mistiaen and Gebruers conducted a literature search from 2010 to 2018 focusing on IE and malignancy. It was found that the short-term outcome of IE was not altered significantly by malignancy, and no particular pattern of management strategy was observed [7].
Del Castillo-Payá conducted a retrospective observational study from 2005 to 2015 in 208 IE patients, of which 32 patients were also diagnosed with cancer. They concluded that the Charlson comorbidity index remained unchanged irrespective of whether the patient was diagnosed with cancer or not. Surgery was not performed in 18.7% of the patients even though there was an indication [48]. The in-hospital mortality and survival rate is comparatively higher in cancer patients with IE than in non-cancer patients with IE (Table 2); and hence, a worse outcome.
Table 2. Outcomes of patients with IE and cancer .
IE - Infective Endocarditis
| REFERENCE | TIMELINE OF STUDY | STUDY TYPE | POPULATION | CONCLUSION |
| Cullen Grable, et al. (2021) [49]. | 2001-2006 and 2015-2018 | Retrospective | 56 patients with the diagnosis of cancer and IE | Cancer IE patients had a poorer survival rate than those in remission (HR 2.497; 95% CI 1.062 to 5.868; p=0.0358). |
| Bernard Cosyns, et al. (2020) [50]. | 2016-2018 | Prospective cohort study | 3085 IE patients (359 cancer IE patients, 2726 cancer free IE patients) from 40 countries | Higher in-hospital mortality in cancer IE patients (23.4 vs. 16.1%, P = 0.006, and 18.0 vs. 10.2%; P < 0.001, respectively.) |
| Del Castillo et al. (2018) [48] | 2005 to 2015 | Retrospective observational study | 208 IE patients, of which 32 patients also had cancer | In-hospital mortality was 45.5%, and the probability of survival was 40% |
| Kyu Kim, et al. (2017) [18] | 2011 to 2015 | Retrospective cohort study | 170 patients with IE | In-hospital mortality was higher in patients with an additional diagnosis (34.4% vs. 12.4%, P<0.001). |
| Ana Fernández-Cruz, et al. (2017) [8]. | 2008 to 2014 | Prospectively included | 161 patients diagnosed with IE in 30 hospitals | Cancer patients with IE had higher In-hospital mortality (34.8% vs. 25.8%, P = .012) and 1-year mortality (47.8% vs. 30.9%, P |
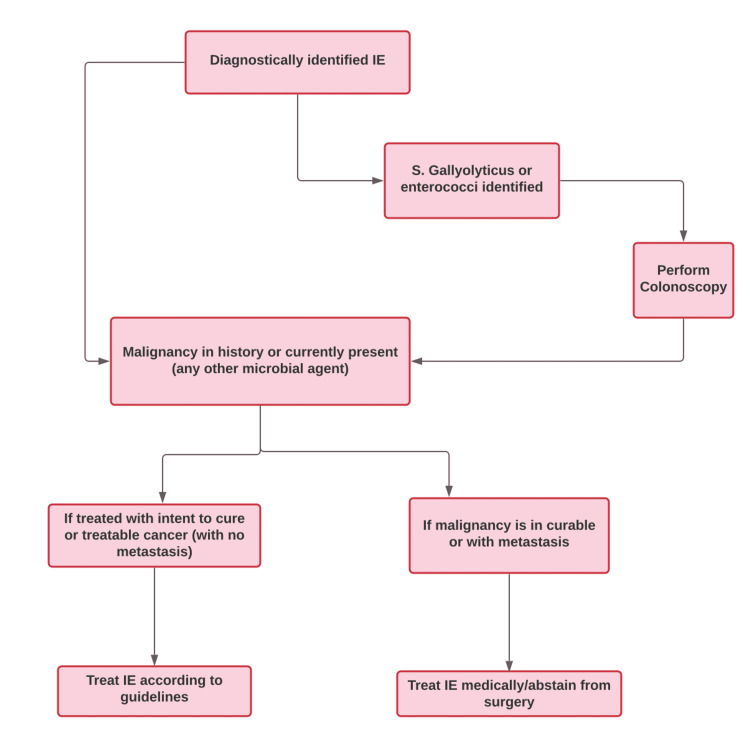
The goal of treating IE in cancer patients should be to (1) prevent bacteremia, (2) consider fever as a warning sign, and (3) rule out an alternate diagnosis to endocarditis [48]. It is suggested that patients with a treatable malignancy and concomitant IE should be prioritized and be treated for IE according to available guidelines [7]. A proposed treatment guideline for IE in cancer patients is depicted in Figure 2.
Figure 2. Proposed treatment guidelines for infective endocarditis in cancer patients.
Image credits - Lakshmi Sree Pugalenthi
IE - Infective Endocarditis
S. gallolyticus - Streptococcus gallolyticus
Medical intervention
Empiric antimicrobial treatment depends on various factors such as microorganisms suspected and the location of the cardiac lesion [51,52]. Staphylococcus aureus must always be a suspected pathogen in right-sided native valve endocarditis (NVE). Antibiotic therapy must be targeted with S. aureus coverage, such as penicillinase-resistant penicillin or vancomycin, based on the prevalence of methicillin-resistant S. aureus (MRSA) in the locality [53,54].
Trends are slightly starting to change in recent years with the empiric treatment. Cosyns et al. conducted a study across 40 countries from 2016 to 2018 with 3085 patients diagnosed with IE. They reported that IE-cancer patients were more frequently treated with amoxicillin and daptomycin than with vancomycin [49].
Although invasive procedures serve as POE in cancer patients, the effects of antibiotic prophylaxis on such patients are still being studied. Since patients with cancer are not typically considered at risk of developing IE, antibiotic prophylaxis is not currently a recommendation [18]. Although still under evaluation, antibiotic prophylaxis has not shown significant results in preventing increased infection rates associated with invasive procedures in critical patients (Table 3).
Table 3. Outcomes of antibiotic prophylaxis as per included studies.
CRI - Catheter-Related Infections
CVAP - Central Venous Access Ports
| Reference | Design | Cases | Conclusion |
| Evan Johnson et al. (2016) [55] | Meta-analysis | 2154 | No significant difference in infection rates. |
| Anne M. Covey et al. (2012) [56] | Retrospective chart review | 1183 | The infection rate without antibiotic prophylaxis is < 1%. |
| Courtney L Scaife et al. (2010) [57] | Retrospective review | 459 | All 9 (2%) CRIs occurred in the non-prophylactic antibiotic group (P = .218), with 5 infections resulting in port removal. Single-dose perioperative antibiotics may decrease CVAP infection rates and should be studied further in a prospective randomized trial. |
Patients with malignancy-associated NBTE were found to have a poor long-term outcome. Still, such patients were treated with anticoagulant therapies with significant palliative effects [43]. Treatment in NBTE aims to control underlying cancer and its instigating factor, thromboembolism, with or without associated disseminated intravascular coagulation (DIC) [58]. The American Association of Colleges of Pharmacy (AACP) guidelines have recommended that patients with NBTE and systemic/pulmonary emboli be given full-dose intravenous unfractionated heparin or subcutaneous low molecular weight heparin, and patients with disseminated cancer with aseptic vegetations should be treated with full-dose intravenous unfractionated heparin or subcutaneous low molecular weight heparin [59].
Surgical management
The main goal of surgical intervention in IE is to remove the infected tissue, reduce embolic events and morbidity, and reduce mortality in appropriate clinical scenarios [60-64]. The indications for surgical interventions are explained in Table 4 [65]. Cosyns et al. conducted a study across 40 countries from 2016 to 2018 with 3085 adult patients diagnosed with IE. Three hundred fifty-nine IE-cancer patients were compared with 2726 IE patients without cancer. It was found that the difference in the theoretical indication for cardiac surgery was minimum between the two groups. The study also found that cancer-IE patients were more frequently treated with bio-prosthetic valves than non-cancer IE patients (aortic bio-prosthesis - 76.3% vs. 56.2%, respectively; mitral bio-prosthesis 41.5% v.s 37.2%, respectively). Bio-prosthetic valve replacement was also preferred over mechanical valve replacement in cancer IE patients (aortic bio-prosthesis: 76.3% vs. mechanical 14.0%; mitral bio-prosthesis: 41.5% vs. mechanical 18.5%) [14].
Table 4. Echocardiographic features suggesting surgical intervention.
| ECHOCARDIOGRAPHIC FEATURES SUGGESTING SURGICAL INTERVENTION | |
| Vegetation | Persistent vegetation after systemic embolization.: Anterior mitral leaflet vegetation with size >10mm, ≥1 embolic event during the first two weeks of antimicrobial therapy, or ≥ two embolic events during or after antimicrobial treatment. |
| Increase in vegetation size after four weeks of antimicrobial therapy | |
| Valvular dysfunction | Mitral valve insufficiency with signs of ventricular failure or aortic valve insufficiency |
| Heart failure in patients who are unresponsive to medical therapy. | |
| Ruptured or perforated wall | |
| Perivalvular extension | Valvular dehiscence, rupture, or fistula |
| New heart block | |
| Abscess or its extension despite appropriate antimicrobial therapy. | |
Although inconclusive, surgery in cancer patients for endocarditis seems to have an excellent prognostic effect compared to those who don't undergo surgery. Patients who were not surgically treated also had to discontinue their antitumor therapy due to the IE, worsening their life span [4]. Vivian et al. conducted a study that included patients with definite IE (International Collaboration on Endocarditis-PLUS, ICE-PLUS) from 2008 to 2012. The study concluded that patients with an indication for surgical intervention, who did undergo surgery were associated with a higher six-month survival than those who didn't receive surgery. It also found that those with higher and lower operative risks had similar outcomes when undergoing surgery. Still, those with a higher risk and who didn't undergo surgery had poorer outcomes [66]. Even though cancer patients have similar indications, only 50% of the patients undergo surgery. Infection is the most common indication for surgery in cancer-IE patients. But the most common reasons for denying surgery are the surgical risk itself and not cancer, death before surgery, or simply patient refusal [14]. The outcomes of surgery on cancer patients conducted by some studies are presented in Table 5.
Table 5. Outcomes of surgery as per included studies.
IE - Infective Endocarditis
| REFERENCE | TIMELINE | TYPE OF STUDY | POPULATION | CONCLUSION |
| Cullen Grable, et al. (2021) [50]. | 2001-2006 and 2015-2018 | Retrospective | 56 patients with cancer and IE | Not associated with significant increase in death (HR 0.671; 95% CI 0.086 to 5.242; p=0.7036). |
| Ana Fernández-Cruz, et al. (2017) [8]. | 2008 to 2014 | Prospectively included | 161 patients diagnosed with IE | Both groups had similar outcomes. |
| Kyu Kim, et al. (2017) [18] | 2011 to 2015 | Retrospective cohort study | 170 patients with IE | One-fifth of the IE patients with cancer underwent surgery, and the in-hospital mortality rate was 53.3%. |
In the case of NBTE, indications for surgical interventions have not yet been established. Still, surgery can be considered in patients with benign or curable cancers or in those patients who have severe valvular dysfunction or recurrent embolic events beyond sufficient anticoagulation therapy [67].
Limitations
IE has multimodal pathophysiology in cancer patients owing to the plethora of malignancies and because of their specific characteristics makes them susceptible to the disease. This article has only broadly discussed the most common etiologies among all cancers. The intricate mechanisms in each type of cancer and their targeted therapies need to be discussed in detail with much more data to follow the evidence-based medical practice.
Conclusions
Infective endocarditis and malignancies, both being different entities in their own categories, have an entangled relationship. This article clears out some of the lesser-known pathogenesis of IE in cancer patients, such as immunosuppression, S. bovis colonization, POE, and hypercoagulability. Cancer patients need to undergo various invasive procedures, especially during the diagnostic phase, which places them at risk of being exposed to various pathogens in their already immune-compromised state. The inherent nature of many malignancies is to facilitate the formation of thromboembolism, which should direct us to be wary of those specific cancers and keep IE in the back of our minds. Although surgical indications are similar in both cancer-IE and non-cancer IE, they are less often considered. However, they do have some proven benefits over those not undergoing surgical treatment. Various medical interventions are still being studied intensely, and existing protocol suggested treatment options depend upon the type of causative microorganism and treating the underlying cancer. Antibiotic prophylaxis is still debated if there is substantial value in preventing IE in cancer patients; hence, they are not yet recommended. The clinical significance of this review helps doctors deal with such patients in understanding how to be cautious and choose the appropriate mode of intervention based on the evidence available. Each cancer patient is different and can be classified according to their relevant risk factors and be screened for possible IE. We feel that there are still more unknowns in both diagnostic and treatment protocols for these conditions, which once determined could pave the way for better prognostic outcomes in such patients.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Cancer development, progression, and therapy: an epigenetic overview. Sarkar S, Horn G, Moulton K, Oza A, Byler S, Kokolus S, Longacre M. Int J Mol Sci. 2013;14:21087–21113. doi: 10.3390/ijms141021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endocarditis and risk of cancer: a Danish nationwide cohort study. Thomsen RW, Farkas DK, Friis S, Sværke C, Ording AG, Nørgaard M, Sørensen HT. Am J Med. 2013;126:58–67. doi: 10.1016/j.amjmed.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Infective endocarditis and cancer in the elderly. García-Albéniz X, Hsu J, Lipsitch M, Logan RW, Hernández-Díaz S, Hernán MA. Eur J Epidemiol. 2016;31:41–49. doi: 10.1007/s10654-015-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Infective endocarditis in cancer patients―causative organisms, predisposing procedures, and prognosis differ from infective endocarditis in non-cancer patients. Kim K, Kim D, Lee SE, Cho IJ, Shim CY, Hong GR, Ha JW. Circ J. 2019;83:452–460. doi: 10.1253/circj.CJ-18-0609. [DOI] [PubMed] [Google Scholar]
- 5.Colorectal cancer statistics, 2014. Siegel R, Desantis C, Jemal A. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 6.Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 7.How to manage patients in whom malignancy and infective endocarditis are associated: a review. Mistiaen WP, Gebruers N. Scand Cardiovasc J. 2020;54:70–76. doi: 10.1080/14017431.2019.1698762. [DOI] [PubMed] [Google Scholar]
- 8.Infective endocarditis in patients with cancer: a consequence of invasive procedures or a harbinger of neoplasm?: A prospective, multicenter cohort. Fernández-Cruz A, Muñoz P, Sandoval C, et al. Medicine (Baltimore) 2017;96:0. doi: 10.1097/MD.0000000000007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Habib G, Erba PA, Iung B, et al. Eur Heart J. 2019;40:3222–3232. doi: 10.1093/eurheartj/ehz620. [DOI] [PubMed] [Google Scholar]
- 10.Invasive procedures associated with the development of infective endocarditis. Janszky I, Gémes K, Ahnve S, Asgeirsson H, Möller J. J Am Coll Cardiol. 2018;71:2744–2752. doi: 10.1016/j.jacc.2018.03.532. [DOI] [PubMed] [Google Scholar]
- 11.Infective endocarditis, and cancer risk: a population-based cohort study. Sun LM, Wu JN, Lin CL, Day JD, Liang JA, Liou LR, Kao CH. Medicine (Baltimore) 2016;95:0. doi: 10.1097/MD.0000000000003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streptococcus gallolyticus bacteraemia in hepatobiliary-pancreatic and colonic pathologies. Zammit SC, Azzopardi N, Ellul P. QJM. 2014;107:355–361. doi: 10.1093/qjmed/hct261. [DOI] [PubMed] [Google Scholar]
- 13.A special topic in onco-cardiology: how to deal with a patient with endocarditis and malignancy. Mistiaen WP. Future Cardiol. 2020;16:61–63. doi: 10.2217/fca-2019-0062. [DOI] [PubMed] [Google Scholar]
- 14.Cancer and infective endocarditis: characteristics and prognostic impact. Cosyns B, Roosens B, Lancellotti P, et al. Front Cardiovasc Med. 2021;8:766996. doi: 10.3389/fcvm.2021.766996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culture-positive and culture-negative endocarditis in patients with cancer: a retrospective observational study, 1994-2004. Yusuf SW, Ali SS, Swafford J, et al. Medicine (Baltimore) 2006;85:86–94. doi: 10.1097/01.md.0000208503.06288.7b. [DOI] [PubMed] [Google Scholar]
- 16.Age adjusted Charlson Co-morbidity Index is an independent predictor of mortality over long-term follow-up in infective endocarditis. Lu KJ, Kearney LG, Ord M, Jones E, Burrell LM, Srivastava PM. Int J Cardiol. 2013;168:5243–5248. doi: 10.1016/j.ijcard.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Is there a need for bacterial endocarditis prophylaxis in patients undergoing gastrointestinal endoscopy? Patanè S. J Cardiovasc Transl Res. 2014;7:372–374. doi: 10.1007/s12265-014-9553-9. [DOI] [PubMed] [Google Scholar]
- 18.Infective endocarditis in patients with cancer: incidence, provoking factors, and the potential need for preprocedural antibiotic prophylaxis. Kim K, Kim D, Lee SE, Cho IJ, Shim CY, Hong GR, Ha JW. https://www.ahajournals.org/doi/10.1161/circ.136.suppl_1.17824 Circulation. 2017;136:0. [Google Scholar]
- 19.Endocarditis in the immunocompromised. Caldwell DA, Lovasik D. Am J Nurs. 2002;Suppl:32–36. doi: 10.1097/00000446-200205001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Association of Streptococcus bovis with carcinoma of the colon. Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. N Engl J Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- 21.The itinerary of Streptococcus gallolyticus infection in patients with colonic malignant disease. Boleij A, Tjalsma H. Lancet. 2013;13:719–724. doi: 10.1016/S1473-3099(13)70107-5. [DOI] [PubMed] [Google Scholar]
- 22.The relationship between the new taxonomy of Streptococcus bovis and its clonality to colon cancer, endocarditis, and biliary disease. Lazarovitch T, Shango M, Levine M, et al. Infection. 2013;41:329–337. doi: 10.1007/s15010-012-0314-x. [DOI] [PubMed] [Google Scholar]
- 23.Increased exposure to bacterial antigen RpL7/L12 in early stage colorectal cancer patients. Boleij A, Roelofs R, Schaeps RM, et al. Cancer. 2010;116:4014–4022. doi: 10.1002/cncr.25212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colon cancer with Streptococcus gallolyticus aortic valve endocarditis: a missing link? Chime C, Patel H, Kumar K, Elwan A, Bhandari M, Ihimoyan A. Case Rep Gastrointest Med. 2019;2019:4205603. doi: 10.1155/2019/4205603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Pil3 pilus of Streptococcus gallolyticus binds to intestinal mucins and to fibrinogen. Martins M, Porrini C, du Merle L, Danne C, Robbe-Masselot C, Trieu-Cuot P, Dramsi S. Gut Microbes. 2016;7:526–532. doi: 10.1080/19490976.2016.1239677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molecular characterization of a Streptococcus gallolyticus genomic island encoding a pilus involved in endocarditis. Danne C, Entenza JM, Mallet A, et al. J Infect Dis. 2011;204:1960–1970. doi: 10.1093/infdis/jir666. [DOI] [PubMed] [Google Scholar]
- 27.Biliary tract infection due to bile-soluble bacteria: an intriguing paradox. Luk WK, Liu CL, Yuen KY, Wong SS, Woo PC, Fan ST. Clin Infect Dis. 1998;26:1010–1012. doi: 10.1086/517638. [DOI] [PubMed] [Google Scholar]
- 28.Streptococcus bovis bacteraemia: identification within organism complex and association with endocarditis and colonic malignancy. Vaska VL, Faoagali JL. Pathology. 2009;41:183–186. doi: 10.1080/00313020802436816. [DOI] [PubMed] [Google Scholar]
- 29.Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S.bovis) Biarc J, Nguyen IS, Pini A, et al. Carcinogenesis. 2004;25:1477–1484. doi: 10.1093/carcin/bgh091. [DOI] [PubMed] [Google Scholar]
- 30.Streptococcus bovis endocarditis and colon cancer: myth or reality? A case report and literature review. Galdy S, Nastasi G. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enterococcal endocarditis in the beginning of the 21st century: analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Chirouze C, Athan E, Alla F, et al. Clin Microbiol Infect. 2013;19:1140–1147. doi: 10.1111/1469-0691.12166. [DOI] [PubMed] [Google Scholar]
- 32.Construction of isogenic mutants in Streptococcus gallolyticus based on the development of new mobilizable vectors. Danne C, Guérillot R, Glaser P, Trieu-Cuot P, Dramsi S. Res Microbiol. 2013;164:973–978. doi: 10.1016/j.resmic.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Genomics, evolution, and molecular epidemiology of the Streptococcus bovis/Streptococcus equinus complex (SBSEC) Jans C, Meile L, Lacroix C, Stevens MJ. Infect Genet Evol. 2015;33:419–436. doi: 10.1016/j.meegid.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Streptococcus gallolyticus Pil3 pilus is required for adhesion to colonic mucus and for colonization of mouse distal colon. Martins M, Aymeric L, du Merle L, et al. J Infect Dis. 2015;212:1646–1655. doi: 10.1093/infdis/jiv307. [DOI] [PubMed] [Google Scholar]
- 35.Pathogenesis of streptococcus infantarius subspecies coli isolated from sea otters with infective endocarditis. Counihan KL, Gill VA, Miller MA, Burek-Huntington KA, LeFebvre RB, Byrne BA. Comp Immunol Microbiol Infect Dis. 2015;40:7–17. doi: 10.1016/j.cimid.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Clinical Importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and meta-analysis. Boleij A, van Gelder MM, Swinkels DW, Tjalsma H. Clin Infect Dis. 2011;53:870–878. doi: 10.1093/cid/cir609. [DOI] [PubMed] [Google Scholar]
- 37.Bacteremia with the bovis group streptococci: species identification and association with infective endocarditis and with gastrointestinal disease. Marmolin ES, Hartmeyer GN, Christensen JJ, et al. Diagn Microbiol Infect Dis. 2016;85:239–242. doi: 10.1016/j.diagmicrobio.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Systematic search for present and potential portals of entry for infective endocarditis. Delahaye F, M'Hammedi A, Guerpillon B, et al. J Am Coll Cardiol. 2016;67:151–158. doi: 10.1016/j.jacc.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 39.Nonbacterial thrombotic endocarditis: a review. Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Am Heart J. 1987;113:773–784. doi: 10.1016/0002-8703(87)90719-8. [DOI] [PubMed] [Google Scholar]
- 40.Cancer and thrombosis: mechanisms and treatment. Deitcher SR. J Thromb Thrombolysis. 2003;16:21–31. doi: 10.1023/B:THRO.0000014589.17314.24. [DOI] [PubMed] [Google Scholar]
- 41.Nuttall GA. Anesthesia & Analgesia. Vol. 104. Philadelphia: Lippincott Williams & Wilkins; 2007. Hemostasis and thrombosis: basic principles and clinical practice; p. 1317. [Google Scholar]
- 42.Nonbacterial thrombotic endocarditis related to adenocarcinoma of the uterine cervix. Kijpaisalratana N, Chutinet A, Travanichakul S, Kitjawijit T, Yokumporn P, Duangjino K, Suwanwela NC. Case Rep Neurol. 2020;12:183–188. doi: 10.1159/000507277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. el-Shami K, Griffiths E, Streiff M. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 44.Tissue factor is associated with the nonbacterial thrombotic endocarditis induced by a hypobaric hypoxic environment in rats. Nakanishi K, Tajima F, Nakata Y, et al. Virchows Arch. 1998;433:375–379. doi: 10.1007/s004280050262. [DOI] [PubMed] [Google Scholar]
- 45.Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985-2000. Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ. Mayo Clin Proc. 2001;76:1204–1212. doi: 10.4065/76.12.1204. [DOI] [PubMed] [Google Scholar]
- 46.Marantic endocarditis and disseminated intravascular coagulation with systemic emboli in presentation of pancreatic cancer. Smeglin A, Ansari M, Skali H, Oo TH, Maysky M. J Clin Oncol. 2008;26:1383–1385. doi: 10.1200/JCO.2007.12.9148. [DOI] [PubMed] [Google Scholar]
- 47.The MET oncogene drives a genetic programme linking cancer to haemostasis. Boccaccio C, Sabatino G, Medico E, et al. Nature. 2005;434:396–400. doi: 10.1038/nature03357. [DOI] [PubMed] [Google Scholar]
- 48.Infective endocarditis in patients with oncological diseases. Mesa Del Castillo-Payá C, Rodríguez-Esteban M, Quijada-Fumero A, Carballo-Arzola L, Farrais-Villalba M, Afonso R, Trugeda-Padilla A. Enferm Infecc Microbiol Clin (Engl Ed) 2018;36:72–77. doi: 10.1016/j.eimc.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Characteristics of infective endocarditis in a cancer population. Grable C, Yusuf SW, Song J, et al. Open Heart. 2021;8 doi: 10.1136/openhrt-2021-001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cancer and infective endocarditis: diagnosis and prognostic impact. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Cosyns B, Roosens B, Lancellotti P, et al. Eur Heart J. 2020;41:946–2014. [Google Scholar]
- 51.The medical complications of drug addiction and the medical assessment of the intravenous drug user: 25 years later. Cherubin CE, Sapira JD. Ann Intern Med. 1993;119:1017–1028. doi: 10.7326/0003-4819-119-10-199311150-00009. [DOI] [PubMed] [Google Scholar]
- 52.From the Alcohol, Drug Abuse, and Mental Health Administration. Serious infections other than human immunodeficiency virus among intravenous drug abusers. Haverkos HW, Lange WR. J Infect Dis. 1990;161:894–902. doi: 10.1093/infdis/161.5.894. [DOI] [PubMed] [Google Scholar]
- 53.Bacteremia in narcotic addicts at the Detroit Medical Center. I. Microbiology, epidemiology, risk factors, and empiric therapy. Crane LR, Levine DP, Zervos MJ, Cummings G. Rev Infect Dis. 1986;8:364–373. doi: 10.1093/clinids/8.3.364. [DOI] [PubMed] [Google Scholar]
- 54.Bacteremia in narcotic addicts at the Detroit Medical Center. II. Infectious endocarditis: a prospective comparative study. Levine DP, Crane LR, Zervos MJ. Rev Infect Dis. 1986;8:374–396. doi: 10.1093/clinids/8.3.374. [DOI] [PubMed] [Google Scholar]
- 55.Routine antibiotic prophylaxis for totally implantable venous access device placement: meta-analysis of 2,154 patients. Johnson E, Babb J, Sridhar D. J Vasc Interv Radiol. 2016;27:339–343. doi: 10.1016/j.jvir.2015.11.051. [DOI] [PubMed] [Google Scholar]
- 56.Totally implantable venous access device placement by interventional radiologists: are prophylactic antibiotics necessary? Covey AM, Toro-Pape FW, Thornton RH, et al. J Vasc Interv Radiol. 2012;23:358–362. doi: 10.1016/j.jvir.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antibiotic prophylaxis in the placement of totally implanted central venous access ports. Scaife CL, Gross ME, Mone MC, et al. Am J Surg. 2010;200:719–722. doi: 10.1016/j.amjsurg.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 58.Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Mazokopakis EE, Syros PK, Starakis IK. Cardiovasc Hematol Disord Drug Targets. 2010;10:84–86. doi: 10.2174/187152910791292484. [DOI] [PubMed] [Google Scholar]
- 59.Valvular and structural heart disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Salem DN, O'Gara PT, Madias C, Pauker SG. Chest. 2008;133:593–629. doi: 10.1378/chest.08-0724. [DOI] [PubMed] [Google Scholar]
- 60.Early surgery versus conventional treatment for infective endocarditis. Kang DH, Kim YJ, Kim SH, et al. N Engl J Med. 2012;366:2466–2473. doi: 10.1056/NEJMoa1112843. [DOI] [PubMed] [Google Scholar]
- 61.Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Lalani T, Cabell CH, Benjamin DK, et al. Circulation. 2010;121:1005–1013. doi: 10.1161/CIRCULATIONAHA.109.864488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Impact of valve surgery on 6-month mortality in adults with complicated, left-sided native valve endocarditis: a propensity analysis. Vikram HR, Buenconsejo J, Hasbun R, Quagliarello VJ. JAMA. 2003;290:3207–3214. doi: 10.1001/jama.290.24.3207. [DOI] [PubMed] [Google Scholar]
- 63.The use and effect of surgical therapy for prosthetic valve infective endocarditis: a propensity analysis of a multicenter, international cohort. Wang A, Pappas P, Anstrom KJ, et al. Am Heart J. 2005;150:1086–1091. doi: 10.1016/j.ahj.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 64.The impact of valve surgery on short- and long-term mortality in left-sided infective endocarditis: do differences in methodological approaches explain previous conflicting results? Bannay A, Hoen B, Duval X, et al. Eur Heart J. 2011;32:2003–2015. doi: 10.1093/eurheartj/ehp008. [DOI] [PubMed] [Google Scholar]
- 65.Diagnosis and management of infective endocarditis and its complications. Bayer AS, Bolger AF, Taubert KA, et al. Circulation. 1998;98:2936–2948. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 66.Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Chu VH, Park LP, Athan E, et al. Circulation. 2015;131:131–140. doi: 10.1161/CIRCULATIONAHA.114.012461. [DOI] [PubMed] [Google Scholar]
- 67.Nonbacterial thrombotic endocarditis with recurrent embolic events as manifestation of ovarian neoplasm. Aryana A, Esterbrooks DJ, Morris PC. J Gen Intern Med. 2006;21:0–5. doi: 10.1111/j.1525-1497.2006.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]