Abstract
Introduction
Recent studies have revealed that inflammation is a key factor in the causation of opioid analgesic tolerance. Opioids can induce a massive release of inflammatory cytokines and disruption of intestinal barrier function by activating Toll-like receptors 2/4 (TLR2/4), eventually resulting to sustained bacterial transmission and persistent systemic inflammation. However, most of the relevant analyses available were conducted at the level of animal experiments. It is necessary to explore the potential association between opioid tolerance and inflammatory cytokines and gut microbiota in patients with cancer pain.
Methods
We retrospectively analyzed cytokines, lymphocyte subsets and blood cells in 186 cancer patients to examine the effect of oral opioids on inflammatory cytokines in patients with moderate to severe cancer pain. The control group constituted tumor patients without cancer pain, while patients with moderate to severe cancer pain taking oral opioids made up the observation group. Fecal samples collected from 25 cancer patients were also analyzed for the composition and diversity of gut microbiota using 16S rRNA sequencing to explore the association between oral opioids and dynamic changes in gut microbiota.
Results
Patients with moderate to severe cancer pain taking oxycodone had significantly higher levels of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ than those in the control group (p < 0.001). The difference in the relative abundance of Lactobacillus (p = 0.025), Anaerostipes (p = 0.034), Megamonas (p = 0.0080), Monoglobus (p = 0.0080), and the Rikenellaceae_RC9_gut_group (p = 0.022) between the opioid and control group was significant.
Conclusion
Oral oxycodone can cause abnormal changes in cytokine levels and gut microbiota of patients with moderate to severe cancer pain, prompting chronic systemic inflammation. Analgesic tolerance induced by long-term oxycodone use could be closely related to the consistent upregulation of IL-6 and TNF-α levels.
Keywords: Opioids, Oxycodone, Opioid tolerance, Inflammatory cytokines, Gut microbiota, 16S rRNA
Key Summary Points
| Why carry out this study? |
| Recent studies have revealed that inflammation is a key factor in the causation of opioid analgesic tolerance. Opioids can induce a massive release of inflammatory cytokines and disruption of intestinal barrier function by activating TLR2/4, eventually resulting to sustained bacterial transmission and persistent systemic inflammation. |
| Most of the relevant analyses available were conducted at the level of animal experiments. It is necessary to explore the potential association between opioid tolerance and inflammatory cytokines and gut microbiota in patients with cancer pain. |
| What was learned from the study? |
| Oral oxycodone can cause abnormal alterations in body inflammatory cytokine levels and gut microbiota of patients with moderate to severe cancer pain, promoting chronic systemic inflammation. |
| Analgesic tolerance induced by long-term oxycodone use may be closely related to the consistent upregulation of IL-6 and TNF-α levels. |
Introduction
Cancer is a malignant disease with a severe impact on human health and survival. Cancer pain is one of the most common symptoms observed in patients with malignant tumors: more than 70% of patients report cancer-related pain [1], which severely affects their quality of life. Although treatment options are available, up to 50% of patients fail to relieve their cancer pain effectively using these approaches [1].
Opioids are an important option in the treatment of moderate to severe cancer pain. Oxycodone and morphine are used as first-line oral opioids for cancer pain relief [2]. Yet, long-term use can give rise to a tolerance state, which requires using higher doses to maintain the analgesic effect [3]. However, dose escalation can increase the risk of adverse effects, including addiction, withdrawal symptoms, and respiratory depression [4] and, in the end, still be insufficient to overcome tolerance and restore analgesic efficacy. In reality, opioid tolerance is a significant barrier to adequate pain relief in approximately 60% of cancer patients [5].
Currently, the main mechanisms involved in opioid tolerance are opioid-induced hyperalgesia, genetic polymorphism, opioid receptor desensitization and downregulation, internalization, and heterodimerization with other receptors [6–8]. Recent studies have revealed that inflammation is a key factor in the causation of opioid analgesic tolerance [9, 10]. Investigations have shown that opioids can induce a massive release of proinflammatory cytokines by activating TLR2/4, which, in turn, disrupts intestinal barrier function to trigger intestinal bacterial translocation and the activation of peripheral immune responses, eventually resulting in sustained bacterial transmission and persistent systemic inflammation [9, 11]. However, most of the relevant analyses available were conducted at the level of animal experiments, and few clinical studies are available. Additionally, the effects of oral opioid on inflammatory factors and gut microbiota in patients with chronic cancer pain are yet to be well understood.
In this study, we retrospectively investigated the effects of oral opioid on cytokines and the immune system of patients with cancer pain. We recruited tumor patients without cancer pain as control and those with moderate to severe cancer pain taking oral opioids as the observation group. We also analyzed the composition and diversity of gut microbiota using the 16S rRNA gene sequencing technology to elucidate the association between oral opioids and dynamic changes in gut microbiota.
Methods
Research Subjects
All cases in this study were from the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology (Wuhan, Hubei Province, China). The research was approved by the hospital’s ethics committee. We selected a total of 186 tumor patients receiving treatment in our hospital from 1 May 2020 to 1 May 2021, including 86 patients with moderate to severe cancer pain taking opioids (opioid group, see Table 1 for opioid use) and 100 patients without cancer pain (control group).
Table 1.
General characteristics of all enrolled cancer patients (n = 186)
| Opioid group (n = 86) | Control (n = 100) | p value | |
|---|---|---|---|
| Age, years | 55.74 ± 9.91 | 56.86 ± 8.35 | 0.41 |
| Sex | – | – | 0.64 |
| Female | 24(28%) | 31(31%) | – |
| Male | 62(72%) | 69(69%) | – |
| Cancer type | |||
| Lung cancer | 39(45%) | 52(52%) | 0.37 |
| Liver cancer | 12(14%) | 7(7%) | 0.12 |
| Gastric cancer | 11(13%) | 12(12%) | 0.87 |
| Colorectal cancer | 9(10%) | 12(12%) | 0.74 |
| Pancreatic cancer | 7(8%) | 8(8%) | 0.97 |
| Esophageal cancer | 4(5%) | 5(5%) | 0.91 |
| Other | 4(5%) | 4(4%) | 0.83 |
| Cancer staging | |||
| I | 1(1%) | 1(1%) | 0.91 |
| II | 2(2%) | 3(3%) | 0.78 |
| III | 17(20%) | 25(25%) | 0.39 |
| IV | 66(77%) | 71(71%) | 0.38 |
| ECOG | |||
| 0 | 12(14%) | 20(20%) | 0.28 |
| 1 | 65(76%) | 76(76%) | 0.95 |
| 2 | 9(10%) | 4(4%) | 0.08 |
| Body mass index (BMI, kg/m2) | 21.42 ± 3.06 | 22.22 ± 2.64 | 0.06 |
| Total protein (g/l) | 66.05 ± 7.61 | 68.02 ± 6.57 | 0.06 |
| Pain management | |||
| Oxycodone | 86(100%) | NA | NA |
| Dose (mg/day) | 28.95 ± 22.96 | NA | NA |
| Usage days | 27.64 ± 30.02 | NA | NA |
There were no significant differences in age, gender, tumor type and stage, ECOG score, BMI, and total protein between the two tumor groups. Data are presented as the mean ± SD or n (%)
NA not applicable
p values are for comparisons between the opioid and control group
Inclusion criteria: (1) the diagnosis of cancer with confirmed results of clinical manifestations (symptoms, signs), auxiliary examinations (serological indicators, such as tumor markers), and pathological testing (tissue biopsy); (2) age between 18 and 70 years old; (3) complete clinical data.
Exclusion criteria: (1) the use or combinational use of non-steroidal anti-inflammatory drugs; (2) patients with poor general conditions (e.g., ECOG score > 2); (3) patients treated with antibiotics, glucocorticoids, immunosuppressants, and microecological agents within the past 3 months; (4) patients who received radiotherapy within the past month; (5) patients with gastrointestinal perforation, acute pancreatitis, acute intestinal obstruction, acute cerebral infarction, acute fever, and acute and chronic infectious diseases; (6) patients with hepatic and renal insufficiency and congenital heart disease; (7) patients with a previous history of psychiatric disorders; (8) pregnant and lactating women; (9) patients with a previous history of drug abuse or addiction; (10) patients with opioid allergy.
Data Collection
The basic information and results of tests on cytokines, lymphocyte subsets, and blood routine examination, as well as serum biochemical test results, were collected from all patients recruited for this study. Two researchers also independently reviewed the data collection forms to double-check the data collected.
Fecal Sample Collection, DNA Extraction, and 16S rRNA Gene Sequencing
Fecal sampling was performed 2 h after breakfast. About 2 g of samples was collected in a sterile container and immediately stored at − 80 °C until further processing. Total genome DNA from samples was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, USA) per the manufacturer’s protocols. The adequacy of the amount of extracted DNA from the samples was verified with fluorometric quantitation (Thermo Fisher Scientific, USA); 20–30 ng DNA was used to generate amplicons. Primers 5' ACACTCTTTCCCTACACGACGCTCTTCCGATCT 3' and 5' CGGTCTCGGCATTCCTGCTGAACCGCTCTTCCGATCT 3′ were employed in the amplification of the V3–V4 hypervariable regions of bacteria and Archaea 16S rDNA. A linker with an index was added to the end of the PCR product of 16S rDNA via PCR for NGS sequencing. The library was then purified with magnetic beads, after which the concentration was determined using a microplate reader and the fragment size established with agarose gel electrophoresis before quantification of the library to 10 nM and paired-end sequencing of PE250/FE300 following instructions in the Illumina MiSeq/Novaseq (Illumina, San Diego, CA, USA) instrument manual. The MiSeq Control Software (MCS)/Novaseq Control Software (NCS) was used to read sequence information.
Bioinformatics Analysis
We employed the Quantitative Insights Into Microbial Ecology pipeline to process the sequencing data (QIIME 1.9.0, http://qiime.org/) and performed Operational Taxonomic Unit (OTU) clustering on the resulting sequences using VSEARCH 1.9.6 (sequence similarity was set to 97%). The 16 s rRNA reference database for comparison was Silva 138.1 (http://www.arb-silva.de/). We conducted taxonomic analysis on the representative sequences of OTU utilizing the RDP Classifier Bayesian algorithm and counted the community composition of each sample at different species classification levels. Alpha-diversity indexes were compared in QIIME with a nonparametric two-sample t-test, whereas Adonis tests were used for beta-diversity comparisons. Metastats difference analysis, STAMP analysis, and Wilcoxon rank-sum test were applied in the comparison of microbiological differences between groups.
Statistical Analysis
Data analysis was performed using the SPSS 23.0 statistical software. Data are expressed as the mean ± SD or n (%). GraphPad version 8.0.1 software was utilized for plotting. Differences between groups were evaluated using the unpaired two-sided Student’s t-test for continuous measurement data and the χ2 test for count data. Differences of P < 0.05 were considered statistically significant.
Compliance with Ethics Guidelines
The research was approved by the Medical Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (consent no. 2020.0265).
Results
Basic Information About the Selected Tumor Patients
As shown in Table 1, there were 62 males and 24 females, aged 55.74 ± 9.91 years old, in the opioid group and 69 males and 31 females, aged 56.86 ± 8.35 years old, in the control group. No significant difference was registered in age between the two groups. Tumor types included lung, liver, stomach, colorectal, pancreatic, and esophageal cancers, and there were no significant differences between the two groups. Similarly, there were no significant differences in tumor stages (AJCC 8th edition), including stages I, II, III, and IV, between the two groups of tumor patients. In addition, the differences in the physical status and nutritional status between the two groups of tumor patients were not significant. These results indicate that the basic information of the two groups of patients was similar and comparable.
Effect of Opioids on Cytokines in Patients with Moderate to Severe Cancer Pain
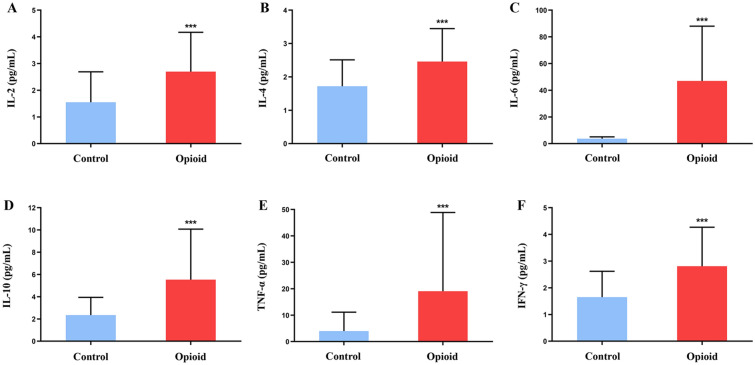
As shown in Fig. 1A–F, patients with moderate to severe cancer pain taking opioids had significantly higher levels of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ than those in the control group.
Fig. 1.
Effect of oxycodone on inflammatory cytokines in patients with moderate to severe cancer pain. A–F: IL-2 (A), IL-4 (B), IL-6 (C), IL-10 (D), TNF-α (E), and IFN-γ (F) levels in the two groups of cancer patients. Control: the control group (n = 100). Opioid: the opioid group (n = 86). Data are expressed as the mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group
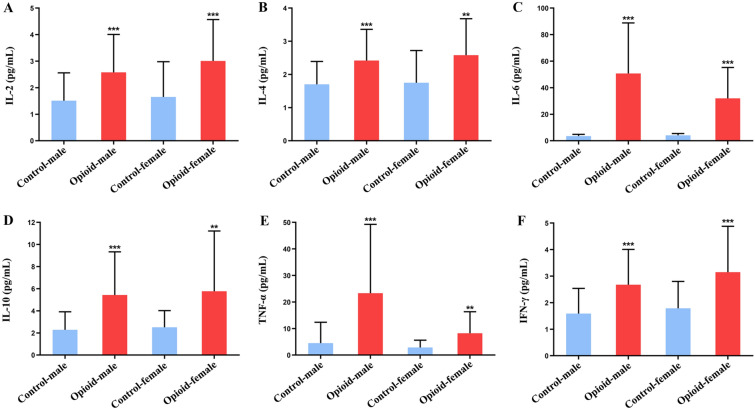
To further verify whether there is gender difference in the effects of opioids on cytokines in patients with moderate to severe cancer pain, we conducted a stratified analysis. As shown in Fig. 2A–F, the levels of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ were significantly higher in the male and female patients with moderate and severe cancer pain treated with opioids than in the control group. There were no significant differences in inflammatory cytokine levels between males and females in the control group or in the opioid group.
Fig. 2.
Effect of oxycodone on inflammatory cytokines in male and female cancer patients. A–F IL-2 (A), IL-4 (B), IL-6 (C), IL-10 (D), TNF-α (E), and IFN-γ (F) levels in patients of different genders in the control and opioid groups. Control-male group: n = 69. Control-female group: n = 31. Opioid-male group: n = 62. Opioid-female group: n = 24. Data are expressed as the mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 opioid-male group vs. control-male group and opioid-female group vs. control-female group
Effects of Opioids on Lymphocyte Subsets and Blood Routine in patIents with Moderate to Severe Cancer Pain
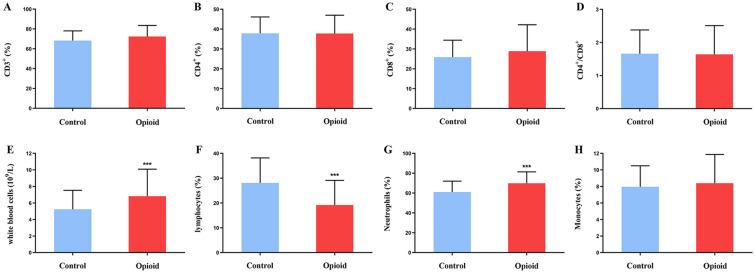
As shown in Fig. 3A–D, there were no significant differences in CD3+ percentage, CD4+ percentage, CD8+ percentage, and CD4+/CD8+ ratio between patients of the opioid and control group.
Fig. 3.
Effect of oxycodone on lymphocyte subpopulation and complete blood count in patients with moderate to severe cancer pain. A–H: CD3+ % (A), CD4+ % (B), CD8+ % (C), CD4+/CD8+ (D), white blood cells (E), lymphocytes % (F), neutrophils % (G), and monocytes % (H) levels in the two groups of cancer patients. Control: the control group (n = 100). Opioid: the opioid group (n = 86). Data are expressed as the mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 vs. the control group
Per Fig. 3E, patients in the opioid group had considerably higher leukocyte levels than those in the control group. In Fig. 3F, lymphocyte% was substantially lower in patients in the opioid group than in those in the control group. However, neutrophil % was pointedly higher in the opioid group patients than in their control group counterparts (Fig. 3G). There was no remarkable difference in monocytes % between patients of both groups (Fig. 3H).
Effects of Opioids on Gut Microbiota in Patients with Moderate to Severe Cancer Pain
To further investigate the effect of opioids on the gut microbiota of patients with moderate to severe cancer pain, fecal samples were collected from the enrolled patients. However, because this was a retrospective study, only 25 patients’ fecal samples have been preserved in the laboratory, including 7 in the control group and 18 in the opioid group. The fecal samples of other patients have been cleaned regularly or no laboratory examination of feces has been done. For the opioid group, a stratified analysis was performed for further grouping according to the duration and dose of opioids. Those who had been taking opioids on time for at least 1 week with a total daily dose of at least 30 mg of oral oxycodone were placed in the opioid-tolerant group (Opioid-T, n = 8), while those who did not meet the above requirements for duration and dose of continuous analgesia were classed in the opioid-sensitive group (Opioid-S, n = 10). No statistically significant differences in age, gender, tumor type and stage, ECOG score, BMI, and total protein (Table 2) were noted among the three groups (Opioid-T, Opioid-S, and control group).
Table 2.
General information of the 25 cancer patients whose fecal samples were analyzed
| Opioid-S (n = 10) | Opioid-T (n = 8) | Control (n = 7) | |
|---|---|---|---|
| Age, years | 61.20 ± 6.68 | 54.75 ± 11.60 | 59.71 ± 7.85 |
| Sex | |||
| Female | 0(0) | 3(37%) | 2(29%) |
| Male | 10(100%) | 5(63%) | 5(71%) |
| Cancer type | |||
| Lung cancer | 9(90%) | 8(100%) | 6(86%) |
| Esophageal cancer | 1(10%) | 0(0) | 1(14%) |
| Cancer staging | |||
| I | 0(0) | 0(0) | 1(14%) |
| II | 0(0) | 0(0) | 1(14%) |
| III | 2(20%) | 2(25%) | 1(14%) |
| IV | 8(80%) | 6(75%) | 4(57%) |
| ECOG | |||
| 0 | 3(30%) | 2(25%) | 3(43%) |
| 1 | 6(60%) | 4(50%) | 4(57%) |
| 2 | 1(10%) | 2(25%) | 0(0) |
| Body mass index (BMI, kg/m2) | 23.54 ± 3.41 | 21.25 ± 3.83 | 20.59 ± 1.91 |
| Total protein (g/l) | 66.41 ± 4.09 | 67.49 ± 4.94 | 71.09 ± 4.71 |
| Pain management | |||
| Oxycodone | 10(100%) | 8(100%) | NA |
| Dose (mg/day) | 21.00 ± 3.16 | 57.59 ± 19.23 | NA |
| Usage days | 14.00 ± 9.25 | 14.88 ± 7.49 | NA |
There were no significant differences in age, gender, tumor type and stage, ECOG score, BMI, and total protein in patients with moderate to severe cancer pain on opioids compared to the control group. Data are presented as the mean ± SD or n (%)
NA not applicable
Opioid-S: Opioid-sensitive group, regular administration of opioid for < 1 week or a daily total dose of < 30 mg oral oxycodone; Opioid-T: Opioid-tolerant group, took opioid medication regularly for at least 1 week, with a daily total dose of at least 30 mg of oral oxycodone; Control: Cancer patients without cancer pain
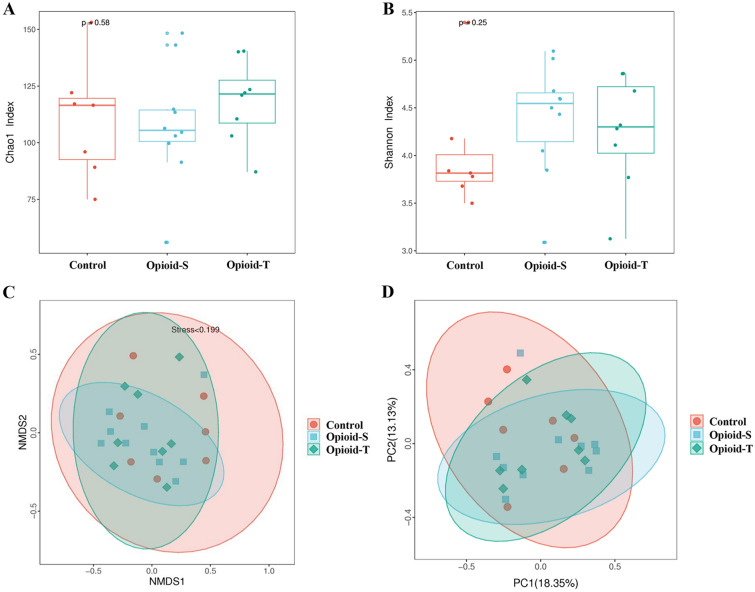
Analysis of alpha diversity in the groups was predicted using Chao 1 and Shannon indexes. There were no significant differences in the Chao 1 diversity indexes among the Control, Opioid-S, and Opioid-T groups (p = 0.58, Fig. 4A). Similarly, there were no significant differences in the Shannon indexes among the Control, Opioid-S, and Opioid-T groups (p = 0.25, Fig. 4B). Beta diversity was assessed using unweighted UniFrac analysis, non-metric multidimensional scaling (NMDS), and principal coordinates analysis (PCoA). The results of NMDS (Fig. 4C) and PCoA (Fig. 4D) showed no significant difference in gut microbiota diversity among the Control, Opioid-S, and Opioid-T groups.
Fig. 4.
Comparison of alpha diversity and beta diversity of gut microbial communities between patients with moderate to severe cancer pain on opioids (opioid groups) and patients without cancer pain (control group). The analysis of alpha diversity in groups was carried out using Chao 1 index (A) and Shannon index (B). The beta diversity of samples was assessed with non-metric multidimensional scaling (NMDS, C) and principal coordinates analysis (PCoA, D) based on the unweighted-UniFrac distances. Orange for the Control, blue for the Opioid-S, and green for the Opioid-T. (Opioid-S (n = 10): Opioid-sensitive group, regular administration of opioid for < 1 week or a daily total dose of < 30 mg oral oxycodone; Opioid-T (n = 8): Opioid-tolerant group, took opioid medication regularly for at least 1 week, with a daily total dose of at least 30 mg of oral oxycodone; Control (n = 7): Cancer patients without cancer pain)
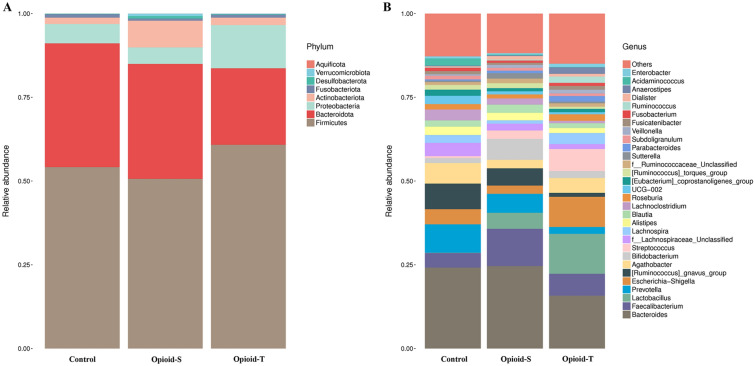
The abundance and distribution of gut microbiota in the three groups of tumor patients were analyzed. The non-metric multidimensional scale revealed differences in the bacterial spectra of the three groups of tumor patients (Fig. 5). Specifically, as shown in Fig. 5A, at the phylum level, there were no significant differences in the relative abundance of Firmicutes, Bacteroidota, Proteobacteria, Actinobacteriota, Fusobacteriota, Desulfobacterota, Verrucomicrobiota, and Aquificota among the three groups (p > 0.05). Figure 5B shows the relative abundance of each of the top 30 species at the genus level, including Bacteroides, Faecalibacterium, Lactobacillus, Prevotella, Escherichia-Shigella, Ruminococcus_gnavus_group, Agathobacter, Bifidobacterium, Streptococcus, f_Lachnospiraceae_Unclassified, Lachnospira, Alistipes, Blautia, Lachnoclostridium, Roseburia, UCG-002, Eubacterium_coprostanoligenes_group, Ruminococcus_torques_group, f_Ruminococcaceae_Unclassified, Sutterella, Parabacteroides, Subdoligranulum, Veillonella, Fusicatenibacter, Fusobacterium, Ruminococcus, Dialister, Anaerostipes, Acidaminococcus, Enterobacter, and others. While differences existed in the relative abundance of bacteria, such as Faecalibacterium, Lactobacillus, Prevotella, and Streptococcus, among the three groups, the difference was not statistically significant (p > 0.05).
Fig. 5.
Histogram of the top 30 community distributions of gut microbiota in patients with moderate to severe cancer pain on opioids and patients without cancer pain using different classification methods. Phylum-level (A) and genus-level (B) taxonomic distribution of gut microbiota in samples. The pillars with different colors represent different species, and the lengths of the pillars denote the proportion of the species. (Opioid-S (n = 10): Opioid-sensitive group, regular administration of opioid for < 1 week or a daily total dose of < 30 mg oral oxycodone; Opioid-T (n = 8): Opioid-tolerant group, took opioid medication regularly for at least 1 week with a daily total dose of at least 30 mg oral oxycodone; Control (n = 7): Cancer patients without cancer pain)
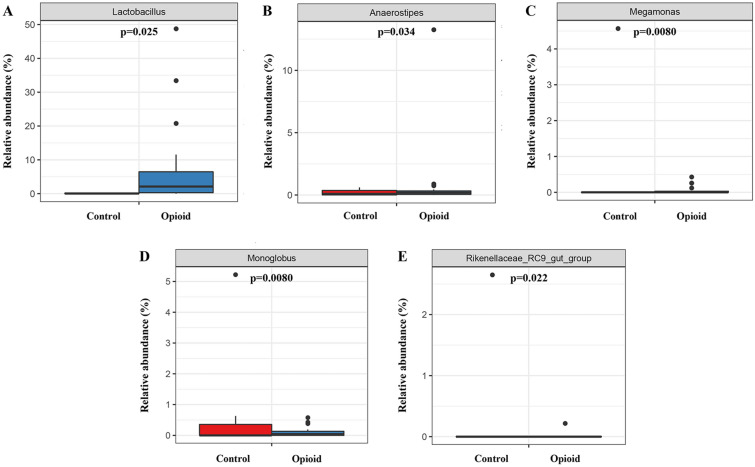
Because the stratification analysis performed on the opioid group showed no remarkable difference in the composition of gut microbiota between the opioid-sensitive and opioid-tolerant groups, the subsequent Metastat abundance difference analysis was performed without stratification. Per the findings, the changes in the abundance of Lactobacillus (p = 0.025), Anaerostipes (p = 0.034), Megamonas (p = 0.0080), Monoglobus (p = 0.0080), and Rikenellaceae_ RC9_gut_group (p = 0.022) between the opioid group and the control group were significant (Fig. 6).
Fig. 6.
Comparison of the relative abundance of species in the gut microbiota at the genus level between patients with moderate to severe cancer pain on opioids and patients without cancer pain. The relative abundances of Lactobacillus (A), Anaerostipes (B), Megamonas (C), Monoglobus (D), and Rikenellaceae_RC9_gut_group (E) were significantly different between the two groups based on Metastat abundance difference analysis (p < 0.05). Control: control group (n = 7); Opioid: opioid group (n = 18)
Discussion
According to the three-step analgesic principle established by the World Health Organization (WHO), oral opioids are the first choice for the treatment of moderate to severe cancer pain. Oxycodone hydrochloride sustained-release tablets are prepared by improved AcroContin sustained-release technology with oxycodone as the active ingredient. Oxycodone, as a full agonist of opioid receptors with the main sites of action being opioid μ and κ receptors, can effectively control different types of pain such as visceral and neuropathic pain [12]. It is widely used in clinical practice for the treatment of patients with moderate to severe cancer pain, but long-term use can lead to analgesic tolerance. Recent studies have shown that inflammation is a key factor in causing analgesic tolerance to opioids [9, 10]. Results from numerous animal experiments have demonstrated dysregulated gut microbiota, massive release of inflammatory cytokines, impaired intestinal mucosal barrier function, and bacterial translocation in opioid-tolerant mice that trigger persistent chronic systemic inflammation [9, 11, 13]. The current study was the first retrospective cohort study to analyze the effects of first-line oral opioid oxycodone on inflammatory cytokines and gut microbiota in patients with chronic cancer pain. It aimed to elucidate the association between oral opioids and changes in inflammatory cytokines and gut microbiota dynamics as well as provide insights into cancer pain treatment in the future.
In clinical observations, opioid users have been found to exhibit abnormal cytokine changes characterized by a disruption of the balance between plasma proinflammatory cytokines (IL-1β, IL-2, IL-6, TNF-α, and IFN-γ) and anti-inflammatory cytokines (IL-4, IL-10, and IL-13) [14–17]. IL-6, TNF-α, and IFN-γ are essential for the activation of the acute or chronic phases of inflammation [14, 18]. In this study, we showed that serum levels of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ, particularly IL-6 and TNF-α levels, increased significantly (p < 0.001) in patients with moderate to severe cancer pain on oral oxycodone. IL-6 is an exceptionally critical inflammatory factor involved in inflammation and immune response. It can upregulate STAT3-mediated retinoid orphan receptor γt (RORγt), which promotes the differentiation of CD4+ naive T cells into Th17 cells and suppresses regulatory T cell (Treg) function. Th17/Treg imbalance can disrupt the immune tolerance of the body [19], playing a major role in the development of chronic inflammation. IL-6 has also been shown to be associated with opioid tolerance, with high levels of IL-6 detected in the blood-brain barrier of morphine-tolerant rats [20].
An almost ubiquitous feature of opioid-induced neuroinflammation is the production of TNF-α. The release of the MOR-PKCε-Akt-ERK1/2 signaling pathway mediated by TNF-α is linked to morphine tolerance. Findings from animal experiments have shown that inhibiting TNF-α signaling could hinder the development of morphine tolerance [21, 22]. Our results here that oral oxycodone causes abnormal changes in cytokine levels and analgesic tolerance induced by long-term oxycodone use is closely associated with the persistent upregulation of IL-6 and TNF-α levels are in line with these findings. Moreover, we also observed a significant increase in leukocytes (p < 0.001) and neutrophils % (p < 0.001) and a substantial decrease in lymphocytes % (p < 0.001) in the opioid group patients in this study, further supporting the point that patients with moderate to severe cancer pain who medicate with oxycodone endure persistent systemic inflammation.
To further investigate the potential mechanism of how oral oxycodone causes abnormal alteration in inflammatory cytokine levels in patients with moderate to severe cancer pain, fecal samples from 25 of these tumor patients were analyzed for the composition and diversity of the gut microbiota using the 16S rRNA gene sequencing technology. The intestine is not only one of the tissues in closest contact with the external environment, but it is also the largest immune organ of the body. The amount of symbiotic microbiota in the intestine that play essential roles in physiological functions, such as immunity and neurology, as well as in the development of many diseases, is massive [23, 24]. Recent studies have shown that opioids can cause alterations in the composition and function of the gut microbiota and that dysregulated gut microbiota could initiate the activation of TLR2/4, prompting a massive release of proinflammatory cytokines (TNF-α, IL-1β, and IL-6), which would trigger local intestinal inflammation and drive morphine tolerance through the microbial-gut-brain axis [9, 11, 13]. Restoring gut microbial homeostasis by microbiota transplantation or probiotic interventions is seen as a possibly vital therapeutic measure to improve opioid tolerance.
Notably, the number of Lactobacillus (p = 0.025) and Anaerostipes (p = 0.034) changed considerably among the Top 30 gut microbiotas of patients in the opioid group in this study. This result suggests that the oral administration of oxycodone could disrupt the balance and stability of gut microbiota and get in the way of the intestinal micro-ecological environment in patients with cancer pain. Regrettably, however, the sample size of this study was small, and a follow-up study with a larger scale must be conducted for corroboration.
Activated glia (i.e., microglia and astrocytes) have also been shown to promote pain transmission and counteract morphine analgesia [25–27]. Opioids activate glial cells by binding to the glycoprotein myeloid differentiation factor-2 (MD-2) on the innate immune receptor TLR4 [28], prompting increased production and release of proinflammatory cytokines (including TNF-α, IL-1β, and IL-6) and neuroexcitotoxic free radicals (NO, NOS, iNOS), and these signaling factors are associated with opioid tolerance [29–31]. The binding of IL-1β and TNF-α to their target receptors on astrocytes and microglia leads to the further release of proinflammatory cytokines (IL-1β, IL-6, TNF-α), creating a vicious cycle of neuroinflammation [32, 33]. Cancer pain is typically caused by neuroinflammation or neurological dysfunction, as tumor expansion can lead to tissue damage and the release of various inflammatory mediators [34]. The massive release of inflammatory cytokines observed in this study could be the synergistic result of opioids and neuroinflammation in cancer pain.
Limitations
Despite its promising revelations, there are some limitations to the current study. The sample size was small, with 186 cases in the retrospective study and only 25 fecal samples available for analysis: it must, therefore, be expanded for a larger-scale study. This study was limited by a retrospective study and could not include moderate to severe cancer pain patients who did not take opioids and could not rule out the effect of cancer pain on inflammatory cytokines in cancer patients. We will conduct further prospective studies in the future. In addition, this research was a single-center clinical investigation limited by the variety of oral opioids in our hospital. Only the effect of oxycodone on cytokines and gut microbiota in cancer pain patients was analyzed. More patients on other oral opioids must be assessed in a multicenter clinical study to explore the possibility of specific changes in inflammatory cytokines and gut microbiota in their bodies.
Conclusions
Overall, we have established here that oral oxycodone can cause abnormal alterations in body cytokine levels and gut microbiota, promoting chronic systemic inflammation. We have also revealed that analgesic tolerance induced by long-term oxycodone use may be closely linked to the consistent upregulation of IL-6 and TNF-α levels. By using a highly homogeneous clinical biological sample, we have provided direct evidence that oral opioids can cause abnormal alterations in cytokine levels in patients with chronic cancer pain, laying the foundation for future in-depth investigations into the potential mechanisms of opioid tolerance.
Acknowledgements
The authors thank all study participants, as well as the Cancer Center workers who supported our research.
Funding
This study was funed by the China International Medical Foundation (Z-2018-35-2004) and Hubei Provincial Health Commission (WJ2021Q060). The Rapid Service Fee was funded by the China International Medical Foundation (Z-2018-35-2004) and Hubei Provincial Health Commission (WJ2021Q060).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Hanxiang Wang and Chen Shi conceived and designed the research. Hanxiang Wang, Juan Luo, Xu Chen and Huiping Hu performed the research. Yu Zhang and Shijun Li analyzed the data. Hanxiang Wang and Chen Shi wrote the paper. All authors reviewed the manuscript.
Disclosures
Hanxiang Wang, Juan Luo, Xu Chen, Huiping Hu, Shijun Li, Yu Zhang and Chen Shi have nothing to disclose.
Compliance with Ethics Guidelines
The research was approved by the Medical Ethics Committee of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (consent No. 2020.0265).
Data Availability
All data generated or analyzed during this study are included in this published article.
Contributor Information
Yu Zhang, Email: whxhzy@163.com.
Chen Shi, Email: whxhchen@163.com.
References
- 1.Neufeld NJ, Elnahal SM, Alvarez RH. Cancer pain: a review of epidemiology, clinical quality and value impact. Future Oncol. 2017;13(9):833–841. doi: 10.2217/fon-2016-0423. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS. Oxycodone for cancer-related pain. Cochrane Database Syst Rev. 2017;8(8):CD003870. doi: 10.1002/14651858.CD003870.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Phys. 2008;11(2 Suppl):S181–200. doi: 10.36076/ppj.2008/11/S181. [DOI] [PubMed] [Google Scholar]
- 4.Trescot AM, Boswell MV, Atluri SL, et al. Opioid guidelines in the management of chronic non-cancer pain. Pain Phys. 2006;9(1):1–39. [PubMed] [Google Scholar]
- 5.Gulur P, Williams L, Chaudhary S, Koury K, Jaff M. Opioid tolerance–a predictor of increased length of stay and higher readmission rates. Pain Phys. 2014;17(4):503–507. doi: 10.36076/ppj.2014/17/E503. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 7.Compton P, Halabicky OM, Aryal S, Badiola I. Opioid taper is associated with improved experimental pain tolerance in patients with chronic pain: an observational study. Pain Ther. 2022;11(1):303–313. doi: 10.1007/s40122-021-00348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi C, Liu J, Hu J, et al. Genetic and clinical factors associated with opioid response in Chinese han patients with cancer pain: an exploratory cross-sectional study. Pain Ther. 2022;11(1):269–288. doi: 10.1007/s40122-022-00353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Meng J, Ban Y, et al. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci. 2019;116(27):13523–13532. doi: 10.1073/pnas.1901182116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Gao H, Gao C, Liu W, Xing D. Bindarit attenuates pain and cancer-related inflammation by influencing myeloid cells in a model of bone cancer. Arch Immunol Ther Exp (Warsz) 2018;66(3):221–229. doi: 10.1007/s00005-017-0497-z. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Sindberg G, Wang F, et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 2016;9(6):1418–1428. doi: 10.1038/mi.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Wang Y, Jiang G. Oxycodone versus morphine for cancer pain titration: a systematic review and pharmacoeconomic evaluation. PLoS ONE. 2020;15(4):e0231763. doi: 10.1371/journal.pone.0231763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep. 2018;8(1):3596. doi: 10.1038/s41598-018-21915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SH, Chen SS, Wang YP, Chen LK. Effects of systemic and neuraxial morphine on the immune system. Medicine. 2019;98(19):e15375. doi: 10.1097/MD.0000000000015375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijerink H, Indrati A, Utami F, et al. Heroin use is associated with suppressed pro-inflammatory cytokine response after LPS exposure in HIV-infected individuals. PLoS ONE. 2015;10(4):e0122822. doi: 10.1371/journal.pone.0122822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. Toll-like receptor 4 (TLR4)/opioid receptor pathway crosstalk and impact on opioid analgesia, immune function, and gastrointestinal motility. Front Immunol. 2020;11:1455. doi: 10.3389/fimmu.2020.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SL, Lee SY, Tao PL, et al. Dextromethorphan attenuated inflammation and combined opioid use in humans undergoing methadone maintenance treatment. J Neuroimmune Pharmacol. 2012;7(4):1025–1033. doi: 10.1007/s11481-012-9400-1. [DOI] [PubMed] [Google Scholar]
- 18.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Lin MY, Wang D, Zhang Y, Hong Y. Involvement of adrenomedullin in spinal glial activation following chronic administration of morphine in rats. Eur J Pain. 2014;18(9):1323–1332. doi: 10.1002/j.1532-2149.2014.493.x. [DOI] [PubMed] [Google Scholar]
- 21.Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A, Borea PA. Morphine mediates a proinflammatory phenotype via μ-opioid receptor-PKCε-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol. 2013;86(4):487–496. doi: 10.1016/j.bcp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Tu H, Chu H, Guan S, et al. The role of the M1/M2 microglia in the process from cancer pain to morphine tolerance. Tissue Cell. 2021;68:101438. doi: 10.1016/j.tice.2020.101438. [DOI] [PubMed] [Google Scholar]
- 23.Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eidson LN, Murphy AZ. Inflammatory mediators of opioid tolerance: Implications for dependency and addiction. Peptides. 2019;115:51–58. doi: 10.1016/j.peptides.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24(1):83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain. 2003;104(3):655–664. doi: 10.1016/S0304-3959(03)00138-6. [DOI] [PubMed] [Google Scholar]
- 28.Lewis SS, Hutchinson MR, Rezvani N, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165(2):569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10(1):40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Loram LC, Ramos K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci. 2012;109(16):6325–6330. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487–498. doi: 10.1007/s40122-020-00200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30(11):581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28(12):661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Dai Z, Chu H, Ma J, Yan Y, Zhang X, Liang Y. The regulatory mechanisms and therapeutic potential of microRNAs: from chronic pain to morphine tolerance. Front Mol Neurosci. 2018;11:80. doi: 10.3389/fnmol.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.