Abstract
Recent events involving the global coronavirus pandemic have focused attention on vaccination strategies. Although tremendous advances have been made in subcutaneous and intramuscular vaccines during this time, one area that has lagged in implementation is mucosal immunization. Mucosal immunization provides several potential advantages over subcutaneous and intramuscular routes, including protection from localized infection at the site of entry, clearance of organisms on mucosal surfaces, induction of long-term immunity through establishment of central and tissue-resident memory cells, and the ability to shape regulatory responses. Despite these advantages, significant barriers remain to achieving effective mucosal immunization. The epithelium itself provides many obstacles to immunization, and the activation of immune recognition and effector pathways that leads to mucosal immunity has been difficult to achieve. This review will highlight the potential advantages of mucosal immunity, define the barriers to mucosal immunization, examine the immune mechanisms that need to be activated on mucosal surfaces, and finally address recent developments in methods for mucosal vaccination that have shown promise in generating immunity on mucosal surfaces in human trials.
Key words: Mucosal vaccines, sterile immunity, barriers to mucosal immunization, innate lymphoid cells, tissue resident memory cells, respiratory infections
Recent events involving the coronavirus pandemic have focused attention on new vaccination approaches.1 This has piqued interest in mucosal immunization, because it provides numerous potential advantages over subcutaneous and intramuscular vaccination routes.2 These major advantages include immune responses composed of secretory antibodies and tissue-resident effector- and long-lived memory T cells at mucosal surfaces.3 , 4 Because the mucosa is the port of entry for most major pathogens ranging from respiratory viruses to pathogens causing sexually transmitted and enteric diseases, mucosal immunity is a major asset in blocking establishment of initial infection and preventing transmission should infection be established.5 The importance of the latter has been underscored by the continued transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by infected vaccinated individuals.6
Other less well-defined benefits of mucosal vaccination include the induction of mucosal immunity at one site, producing immune crosstalk that provides protection at distal mucosal surfaces.7 In addition, the constant exposure of mucosal surfaces—particularly the respiratory and gastrointestinal tracts—to microbes and antigens from the environment makes these surfaces valuable for shaping tolerogenic responses in autoimmune and allergic disease.8 Finally, mucosal vaccines, particularly oral and intranasal vaccines, provide practical benefits including ease of administration and greater global accessibility for low-income countries.9
Unfortunately, although advances have recently been made in subcutaneous and intramuscular vaccines, mucosal vaccines have lagged in implementation. Mucosal vaccines have wide use and a long history of efficacy in veterinary applications.10 However, there are only 9 approved mucosal vaccines for use in humans.11 Although all of these are effective, they involve older technology using attenuated or inactivated pathogens. Eight are administered orally, and only 1 (Flumist) is given intranasally.11 Importantly, no new mucosal vaccine has been approved in the United States in the last 10 years.
The paucity of mucosal vaccines is the result of several significant hurdles facing effective mucosal immunization. The mucosa contains a unique immune cell milieu that functions to selectively identify pathogens within an environment that promotes tolerance toward commensal organisms. To overcome this requires different modes of immune activation that are not directly translatable from parenteral vaccines.12 This review will highlight the potential advantages of attaining mucosal immunity, define the barriers to mucosal immunization, and examine the unique immune processes that need to be engaged on mucosal surfaces for effective immunity. We will then address approaches that could improve the likelihood of achieving effective protective immunity on mucosal surfaces. In this last regard, given there are excellent recent articles that cover preclinical evaluations of these technologies,3 , 4 , 11 this review will focus on vaccine approaches that have reached human clinical trials.
Potential advantages of targeting mucosal-specific immunity
Mucosal surfaces are the entry point location of most pathogens infectious to humans.4 , 5 Addressing infections at their source has several advantages that cannot be achieved through traditional immunization (summarized in Table I ).
Table I.
Theoretical advantages of mucosal immunization
| Result of mucosal immunization | Specific immune mechanisms | Result of immune activation | Diseases where potentially valuable | Examples |
|---|---|---|---|---|
| Immune response homes to mucosal surfaces | Induction of immune cells with mucosal- targeting molecules | Focusing of the immune response to areas of need |
|
|
| Unique types of immunity produced on mucosal surfaces | IgA, IL-17, and CD8 immunity | Sterile immunity at mucosal surfaces, clearance of virally infected or transformed cells |
|
|
| Induction of long-term immunity | TRM cells | Long-term protection against recurrent disease |
|
|
| Regulatory immune responses | Tissue-resident regulatory T cells | Downregulation of immune inflammation and shifting to protective immunity |
|
Protection from the acute effects of both bacterial and viral infections could be enhanced through mucosal immunity
As demonstrated by SARS-CoV-2, early infection of mucosal surfaces can result in disruption of respiratory nasal and lung epithelium.13 This disruption and the secondary inflammatory response can lead to long-term damage even in individuals who recover from acute infection.14 It is not clear how the resulting chronic symptoms (“long COVID”), such as respiratory insufficiency and loss of smell and taste, are prevented in breakthrough infections with current intramuscular vaccines,15 but given the pathophysiology these might be ameliorated by mucosal vaccines. Besides pathogens that colonize the respiratory tract, there are pathogens that colonize the intestinal mucosa and induce acute morbidity and even death simply from disruption of epithelial function.16 This is demonstrated by bacteria such as Vibrio cholerae and enteropathogenic Escherichia coli, as well as rotavirus.17 , 18 In these situations, either local epithelial damage or toxin production that disrupts epithelial integrity leads to loss of fluids and local inflammation, which makes maintaining fluid and electrolyte balance difficult. A mucosal immune response composed of toxin-neutralizing IgA and IgG antibodies and neutrophils that clear organisms would be effective at reducing pathology and preventing systemic infection.19
Mucosal immunity reduces pathogen load on mucosal surfaces
Another advantage of mucosal immune responses is that it limits transmission and promotes pathogen clearance. Along with specific antibodies, the induction of TH17 immune responses on mucosal surfaces can markedly improve the clearance of pathogens.20 This can provide sterile immunity where individuals do not carry pathogens that can be transmitted to others. As now illustrated most clearly with coronavirus disease 2019 (COVID-19), individuals with personal protective immunity can still infect others through SARS-CoV-2 aerosol from their respiratory mucosa.21 Importantly, this transmission infects others while suppressing a vaccinated person’s symptoms, making it harder to identify infected individuals, which hinders infection control.22
Although respiratory transmission is commonly seen with viruses, such as influenza virus, SARS-CoV-2, and respiratory syncytial virus (RSV), it is also observed with bacterial infections. Pertussis can be spread through aerosols and has been increasing in prevalence because current Bordetella pertussis vaccines do not induce sterile immunity, resulting in several serious outbreaks.22 Mucosal immunization is being pursued to rid individuals of nasal carriage.23 Infections from organisms colonizing the gastrointestinal tract can be spread through fecal-oral transfer if not completely cleared from the intestines. This is observed with outbreaks of Clostridium difficile in health care facilities and enteropathogenic E coli in water parks.24 The causes of recurrent urinary tract infections are varied, but it is believed that chronic carriage of bacteria or ineffectual mucosal immunity is a significant factor.25 Although environmental control may limit these outbreaks, having an immune response that provides neutralizing mucosal immunity and neutralizes bacterial/toxins would lead to simpler and more effective control.
Mucosal immunization prevents reinfection through long-term immune memory
Long-term immunity is enhanced through the induction of antigen-specific, tissue- resident memory T (TRM) cells.4 , 26 These cells serve as sentinels in mucosal lymphoid tissue to provide memory responses on rechallenge with infectious agents. Although memory T cells can also be induced through other routes of immunization, having these persistent T-cell populations in the epithelial barrier tissue positions them for an immediate memory response before central memory T cells can arrive.4 , 26 This type of response along with the mucosal IgA it generates is crucial in response to an infection such as HIV in which the virus needs to be eradicated on the mucosal surface, because once it gets into systemic lymph nodes it quickly mutates to escape immune control.27
Mucosal immunization may modulate adverse immune responses
There has long been interest in using this approach as a means of suppressing autoimmunity, often applying autoantigens to mucosal surfaces to induce tissue-resident regulatory T cells. This would provide an antigen-specific suppression of autoimmunity. Trials using myelin basic protein to suppress immunity in patients with multiple sclerosis have yielded interesting findings but have yet to show therapeutic value.28 Some of the results have indicated that immune responses can be specifically modulated in patients. There have also been trials examining oral and nasal immunization with several antigens in patients with type 1 diabetes, although again, no positive clinical outcome has been observed.29
Mucosal immunization as an approach to modulate allergic disease
A new area of interest in mucosal immunization of interest to allergists is suppressing allergic disease. This involves antigen application to the mucosa to suppress type II immune responses or redirect these immune responses to produce protective immunity.30 Although the first application of mucosal immunization involved sublingual immunotherapy for treating respiratory allergic disease, including rhinoconjunctivitis, much of the current focus is on food allergy. Both oral and sublingual applications of food antigens have been shown to induce specific immune unresponsiveness to the food. However, this is short lived and ends rapidly after chronic ingestion is discontinued.30 The goal for mucosal immunization, potentially combined with immunomodulation therapeutics, would be to achieve long-term tolerance to the food.
Barriers to effective mucosal immunization
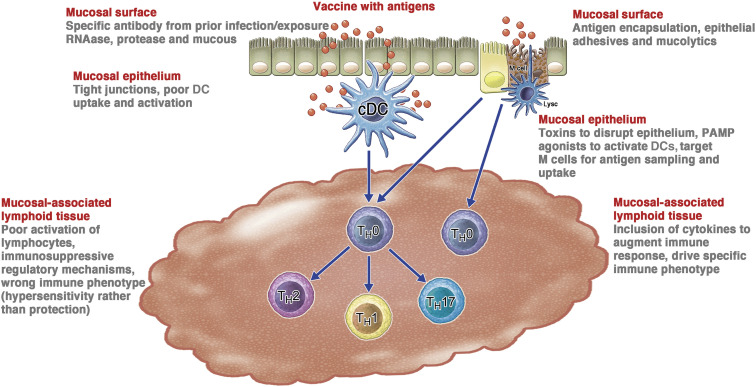
A major hurdle limiting effective mucosal immunization is the barrier that mucosal surfaces provide to prevent antigen delivery and immune stimulation (Fig 1 ). These barriers have evolved to protect the mucosa from infectious pathogens, toxins, and other noxious agents.31, 32, 33, 34 Therefore, it is no surprise that most effective mucosal vaccines have been adapted from infectious pathogens that have evolved to overcome these barriers.11 , 18 To better pursue rational mucosal vaccine design, one must first define epithelial barriers and understand potential ways that they can be overcome.
Fig 1.
Barriers to mucosal immunization. There are several layers of barriers that uniquely challenge mucosal immunization. These barriers can be conceptualized as associated with different components of the epithelial anatomy. The barriers at each level of the mucosa are enumerated on the left side of the figure, with potential approaches to overcome each impediment presented on the right side. cDC, Conventional DC.
Vaccine barriers on mucosal surfaces
Mucosal barriers include the mucus layer itself. This complex mix of glycoproteins, proteoglycans, lipids, DNA, and large protein polymers forms strands on surfaces that repel foreign proteins and particles.31 In addition, there are large amounts of proteases and nucleases on the surface that promote degradation of genetic material and proteins in vaccines.32 Ciliary function also helps to clear vaccine particles from mucosal surfaces, and in the gut, digestive enzymes and low pH and mucus also degrade vaccine formulations. Using mucoadhesive components32 such as positively charged polymers and lipids or using mucus-disruptive surfactants could provide enhanced antigen uptake. Also, including proteins or sugars, such as spike proteins or sialic acid residues, which mediate high avidity, multivalent virus binding to epithelial cells could provide the same type of interactions to optimize vaccine delivery and efficacy.
Epithelial tissue prevents the interaction of antigens and vaccines with the mucosal immune system
The epithelial barrier normally is very effective in preventing the penetration of antigens to mucosal immune tissue. Tight junctions between epithelial cells limit the access of antigens through the epithelium.33 These junctions prevent any material over 30 nm in diameter from directly penetrating the mucosal epithelium unless the surface is disrupted.34 One approach to overcome this barrier has been to use toxins or other approaches to disrupt epithelium integrity. In addition, although transcellular migration can occur, it is inefficient in most cells without specific binding and internalization as seen with viruses. In contrast, efficient transport of vaccine and antigen can be achieved through direct sampling of the lumen by dendritic cells (DCs) and M cells.35 However, as discussed later, studies suggest that using these specialized cells require either targeting molecules for M cells, or activation signals to engage DCs.
Avoiding vaccine neutralization by preexisting secreted IgA and cytotoxic T-cell–mediated immune responses is also critical to effective mucosal immunization because this can prevent a secondary response to the vaccine. This is especially important with live virus vaccines such as Flumist or adenovirus vector vaccines, where viral-mediated entry into antigen-presenting cells (APCs) and/or replication are necessary for the vaccine to work.36 , 37
Activating lymphoid tissues is a central challenge for mucosal vaccines
The mucosal immune system is constantly exposed to inhaled and ingested antigens, so it is biased toward tolerogenic immunity to maintain homeostasis and prevent hyperactive immune responses. Mucosal-administered antigens are therefore inherently less immunogenic, and mucosal vaccines must include danger signals such as pathogen-associated molecular patterns (PAMPs) and/or cytokines to overcome tolerogenic programming.26 , 38 The success of mucosal vaccines thus relies on adjuvants as part of novel delivery systems to provide these signals, and several vaccine approaches now use specific innate immune activation strategies to overcome these challenges.
Unique features of the induction of mucosal immunity
Inducing mucosal immunity involves activating the largest and most complex immune organ in the human body. Recent research has better defined the distinct organization of immune cells resident on the different epithelial surfaces (Fig 2 ). Each of these immune regions—mouth, nares, gut, rectum, urinary tract, and vagina—face unique challenges in maintaining homeostasis amidst the constant exposure of environmental antigens and commensal organisms, while blocking infection by pathogens.11 , 18 Engaging these networks to achieve useful immune responses while not disturbing its complex overall function is paramount to effective mucosal vaccines.
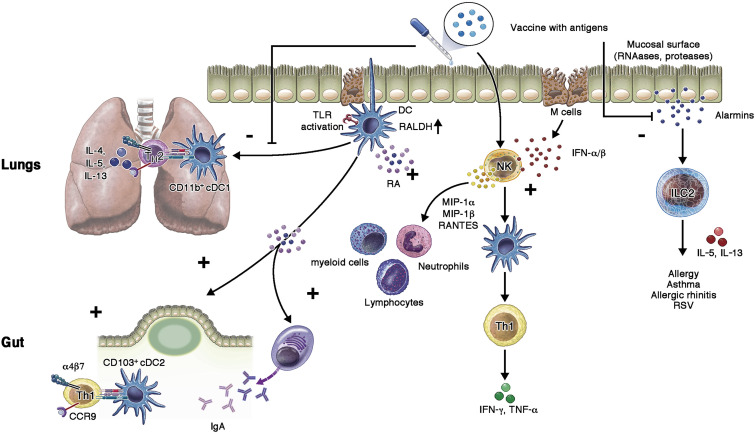
Fig 2.
The optimal approach to activating mucosal-specific immune responses by vaccines. When a vaccine is placed on the mucosal surface, immune activation occurs through DC sampling and activation. This can occur either through direct antigen sampling by DCs across the epithelium, DC sampling of infected or dying epithelial cells, or through sampling of antigens passed across the epithelium by M cells. After that first step, activating of PAMPs, along with retinoic acid pathways, can activate DCs to induce specific, effector immunity (plus signs) involving cellular cytotoxicity and antibodies. In contrast to enhancing pathways for protective immunity, those inducing hypersensitivity reactions (minus signs) including ILC2 and TH2 lymphocytes should be suppressed. α4β7, Alpha 4 beta 7 integrin; CCR9, chemokine CC receptor 9; NK, natural killer; RALDH, retinal dehydrogenase enzyme; TLR, Toll-like receptor.
Induction of immunity at different mucosal surfaces
Each mucosal surface consists of inductive and effector sites. Inductive sites, or mucosa-associated lymphoid tissue (eg, gut-associated lymphoid tissue and nasopharyngeal-associated lymphoid tissue), contain T and B cells along with APCs, macrophages, and DCs.18 , 39 , 40 The specialized peyer patches of the gut-associated lymphoid tissue additionally contain phagocytic M cells, which transcytose molecules across the epithelial barrier. Identification of non–self-microbial components as danger signals occurs within these inductive sites through recognition of PAMPs by the pattern recognition receptors of epithelial cells and tissue-resident APCs—mainly DCs and macrophages. These receptors include membrane-bound Toll-like receptors and C-type lectin receptors, along with cytoplasmic nucleotide-binding and oligomerization domain (NOD)-like receptors and retinoic acid-inducible gene-I (RIG-I)-like receptors.40 , 41
Although the PAMPs that are active on mucosal surfaces are not qualitatively different than those involved in activating pathways downstream of parenteral vaccines, the unique environment of the mucosal surfaces suggests a difference in the importance of these signals. Given the chronic exposure to bacterial PAMPs, such as endotoxin, mucosal surfaces may be less responsive to these signals.26 , 39 In contrast, given the many viruses that enter through the mucosa, receptors for unique genetic signatures and structures associated with RNA and DNA viruses would appear to be more important.42 Thus, although the receptors and ligands of PAMPs are the same as in nonmucosal structures, the environment alters the hierarchy of their significance.
Activation of these receptors produces cytokines that trigger lymphocyte maturation and induces migration to mucosal effector sites such as the lamina propria of the gastrointestinal tract and lymphoid tissue of the upper respiratory tract. Subsequently, TH cells, cytotoxic T cells, and antibody-producing plasma cells are engaged at these effector sites to induce specific immunity to the antigen. These cells recruit additional APCs to inflammatory sites to process antigens and prime effector T cells.11 , 26 This primary chain reaction—entry of pathogen recognition by APCs, communication with specific lymphocytes leading to mounting of protective immune responses—occurs locally in the subepithelium.
Despite these insights, mucosal vaccine development is challenging due to our limited understanding of the complex cellular interactions in the mucosal immune environment. In the following section, we will focus on what is known regarding these different immune cell types and their roles in mucosal immunity. These features of each immune cell type are potential targets that can be exploited for mucosal vaccine design.
Mucosal APCs
On initial pathogen encounter, epithelial cells process the antigens and pass them on to tissue-resident APCs, which further recruit additional APCs to inflammatory sites to sample the antigens and prime effector T-cell immunity. DCs in the mucosa particularly induce expression of specific chemokine receptors and integrins on T cells to direct their migration to the site of infection. Although mucosal conventional type 1 and 2 DCs are present throughout the mucosa and are similar to DCs seen elsewhere in the body,41 these APCs have unique functions in the mucosa.
Mucosal conventional type 1 DCs expressing CD103 provide cross-presentation to CD8+ T cells to mediate viral clearance; however, in the gut microenvironment, these cells actively metabolize vitamin A to produce retinoic acid.43 , 44 Retinoic acid is a crucial aspect of mucosal immune activation because retinoic acid produced by DCs imprints CCR9 and α4β7 on T cells, promoting T-cell homing to the epithelium in the gut and the activation of effector immune responses.45 In the lung, conventional DCs can induce TH2 immunity toward inhaled allergens, increasing lung inflammation and airway hypersensitivity (Fig 2).46 , 47 DC-activated T cells also migrate to mesenteric lymph nodes to drive differentiation of FoxP3+ regulatory T cells.48 , 49 The complexity of these networks is unique to the mucosa and makes orchestrating the optimal configuration of immune responses challenging for any mucosal vaccine.
In contrast to subcutaneous tissue, mucosal tissue-resident macrophages also phagocytose microbes to clear them from mucosal surfaces. However, these cells do not migrate to lymph nodes or directly activate T cells. Macrophages can transfer processed antigens to mucosal DCs, and this process appears unique to the mucosa and pivotal in inducing gut TH17 and IgA responses.26 , 48 , 49 Thus, delivering antigens to mucosal APCs and activating homing responses in DCs and macrophages is an important and unique aspect of mucosal immunization.
M cells
Given their unique functions, intestinal M cells and their equivalents in the nasal mucosa would seem to be an important target for antigen delivery.35 , 39 They act as portals to deliver antigen to underlying immune cells for antigen presentation, but this process has limitations. M cells do not sample regular antigens or DNA in vaccines effectively, and although targeting is possible through molecules such as lectins, bacterial toxins, and certain viral proteins, this usually requires construction of conjugates between the antigen and delivery molecules. Mouse studies suggest aging also degrades M-cell function, and M-cell antigen sampling under certain conditions can also induce tolerance.50 Therefore, targeting M cells may be no better than using other pathways to deliver antigens to mucosal DCs.
Innate lymphoid cells
Innate lymphoid cells (ILCs) are populations of immune cells unique to mucosa. ILCs are lymphocytes that lack antigen-specific receptors and instead respond to cytokines and danger signals in the microenvironment. ILCs are found in abundance in mucosal tissues and play crucial roles in inducing tolerance, as well as in fighting various pathogens. The ILCs are divided into type 1 ILCs (ILC1s), type 2 ILCs (ILC2s), and type 3 ILCs (ILC3s) on the basis of expression of transcription factors T-bet, GATA3, and ROR-γt. ILC1s mainly produce IFN-γ and TNF-α, ILC2s induce IL-5 and IL-13, while ILC3s make IL-17 and IL-22.51 Not surprisingly, ILC1s and ILC3s are important in mucosal protection, and ILC2s are associated with allergy and chronic inflammation. For example, during lung inflammation, ILC2s can secrete IL-13 to promote DC migration to regional lymph nodes to prime T cells to have a TH2 phenotype.26 , 51
Immune response polarization
Following innate pathway activation of APCs, naive T and B cells are primed to skew the specific cellular immune profile. The skewing of the cellular immune response can lead to enhanced pathogen clearance or pathogenicity. Optimal mucosal immunization correctly polarizes lymphocytic responses for protective immunity because preferential TH2 or Treg-cell responses could result in hypersensitivity or tolerance from a vaccine.11 , 18 , 42 Adjuvants capable of skewing toward a TH1 response are crucial particularly in preventing viral infections and may be helpful in ameliorating TH2-biased pathology such as that found in food allergy or RSV infection.
Vaccine administration routes and adjuvants affect ILC populations differently, and focusing on approaches that preferentially induce ILC1s and ILC3s would be important in designing a mucosal vaccine candidate.11 , 18 Adjuvants capable of skewing toward a TH1 response are crucial particularly in preventing viral infections and may be helpful in ameliorating TH2-biased pathology such as that found in food allergy or RSV infection. Several approaches have been shown to provide specific mucosal protection by eliciting TH1 and TH17 cellular responses as well as tissue-resident memory T-cell responses.26
Tissue-resident immune memory
As previously mentioned, TRM cells are uniquely important to mucosal immunity.52 TRM cells in local tissues can rapidly be converted into effector T cells when encountering a recalled infectious agent. CD4+ TRM cells support antibody production against bacterial pathogens, whereas CD8 TRM cells use cellular mechanisms to contain chronic and recurrent mucosal viruses. There is also evolving evidence that these cells are important in immune surveillance for mucosal cancers.
Although there is convincing evidence that these cells exist and are important in humans, no specific methods have been identified that can induce TRM cells. This capability would be highly desirable because it could aid sterile immunity due to the ability of TRM cells to respond quickly.4 Given the antigen/pathogen specificity, specific immune induction will be necessary, and the site of memory response and generation of specific memory response should be considered while developing a vaccine candidate. It does appear that presentation on specific mucosal sites is important to generating local TRM cells, and it is unclear that parenteral vaccination can induce these cells.53
Mucosal antibody responses
IgA produced by local plasma cells is the principal antibody found in mucosal secretions and acts as a primary defense in countering the pathogen. Parenteral immunization has not effectively induced secreted IgA responses, which are most optimally induced through mucosal immunization strategies. Recent advancements in mucosal vaccine design have led to the development of adjuvants capable of inducing pathogen-specific IgA production by engaging cells such as ILC3.51 , 54 Although IgG plays an important role in providing systemic protection against pathogens, the secretion of antigen-specific IgA at the mucosal surfaces could block pathogen entry and enhance clearance.
Platforms for the induction of mucosal immunity
There is no shortage of proposed approaches to overcome the barriers confronting mucosal immunization and provide effective immunity (summarized in Table II ). New mucosal vaccines fall into 2 general areas: live attenuated and genetically modified organisms or synthetic systems to deliver antigens, DNA, or genetic material. Although many approaches are in preclinical development, the utility of these for human vaccination is not clear. This is, in part, because animal models of mucosal immunization (other than primates) have not been predictive of success in humans, potentially related to differences in microbiota.55
Table II.
Potential approaches to human mucosal immunization
| Technology | Advantages | Limitations | Efficacy | Safety |
|---|---|---|---|---|
| New methods of producing live attenuated viral vectors | Genetic codon deoptimization can control expression, improve safety | Stability of expression control not tested in humans | Unknown. In human testing | Animal studies encouraging |
| Live attenuated viral vectors with transgenic expression | Proven technology in injectable vaccines. Genetic engineering well worked out | Prior immunity to virus can prevent immunization | Mixed data suggesting some immunogenicity in nasal applications in humans | Human phase I studies encouraging |
| Natural polymeric complexes (chitosan) | Chemistry used in injection vaccines. Shown to prevent nuclease and protease degradation | Not very immunogenic. Must be combined with immune system activation molecules to induce an immune response | Some failures as an injectable vaccine. No data yet from human studies | |
| Synthetic polymers complexes (PEG, PLGA) | Mucoadhesive. Proven to stabilize genetic material. Chemistry well defined. Proven utility in injectable vaccines | Disrupted by biological components in serum. Not very immunogenic | Pending in phase I trials | Appears good in early-stage human studies |
| Virus-like particles | Defined chemistry. Uniform structures readily conjugated to antigens | Not very immunogenic. Often must be conjugated with toxins to enhance immunogenicity | Pending in human mucosal applications. Proven in injectable applications | Excellent in human injection studies. Awaiting results from nasal immunization but no trial stopped for safety issues |
| Liposomes | Well-defined technology. Extensively studied and used for drug delivery in humans | Not mucoadhesive. Disrupted by biological components in serum. Not very immunogenic | Pending in phase I trials | Appears good in early-stage human studies |
| Emulsions | Easily produced, combine readily with proteins. Can be designed to be mucoadhesive and disruptive to epithelial cells | No data with genetic vaccines | Phase I data show some immune response | No evidence of toxicity in 2 phase I studies |
PEG, Polyethylene glycol; PLGA, poly(lactic-co-glycolic acid).
Live attenuated organisms
Since the classic work of Sabin on oral polio vaccine, mucosal immunization with live attenuated vaccines has shown promise.56 Attenuated organisms have provided the only viable oral and mucosal vaccines currently approved for human use.11 These organisms have evolved to overcome mucosal barriers, whereas the mucosal immune system has evolved to recognize and protect against these pathogens.3 , 9 , 12 , 26 These approaches inherently overcome the epithelial barriers because they have evolved to efficiently infect epithelial cells, which leads to DC and M-cell uptake and the production of PAMP agonists, which induce protective mucosal immune responses.42
Current approved vaccines uniformly seek to develop immunity to the organism used for the attenuated vaccine. A nasal vaccine is available based on attenuated organisms for influenza,57 and attenuated live virus vaccines for RSV,58 parainfluenza,59 , 60 and metapneumo viruses61 are in early-stage clinical trials and have shown acceptable safety and evidence of immunogenicity. It is likely that this approach will continue to be important as a means for new vaccine development.
Reactogenicity and safety have been the major concerns for attenuated live virus vaccines because they are essentially causing low-grade infections. Fever and malaise are common with these vaccines, and caution has been particularly high with viruses that cause severe disability and death. In particular, as background infections became less common, illness from vaccine strain organisms became a much larger issue.62
To resolve these issues, promising results have been shown with new methods of inactivation such as genetic codon deoptimizing63 that can potentially generate live attenuated viruses with higher safety profiles. A live attenuated SARS-CoV-2 vaccine (COVI-VAC) based on this approach demonstrated a good safety profile in animals and is currently in human trials, although the results are not yet available.64
Attenuated respiratory viruses as vaccine vectors
A new approach evolved from research stimulated by the COVID-19 pandemic involves using attenuated respiratory viruses as vectors to immunize against third-party antigens where the vector has been genetically programmed to express an exogenous antigen. This is similar to the concept of the parenteral COVID-19 vaccines based on adenovirus-derived vectors engineered to express the SARS-CoV-2 spike protein.1 These vaccines have shown good safety and efficacy, and because of the environmental stability of adenovirus, have less stringent storage requirements.
Attenuated viral vectors also are able to efficiently infect host respiratory cells, resulting in transduced expression of the exogenous antigen in the same cell types that are typically infected by the natural target pathogen. Accordingly, this has the potential to induce the types of immune responses more relevant to respiratory pathogens. However, much like injection applications of these technologies, preexisting immune response to the viral vector could significantly reduce the efficacy of this technology.2 , 9
Nine different nasal vaccines expressing SARS-CoV-2 spike protein based on recombinant virus technology are in clinical trials. These include vaccines based on engineered human65 and chimpanzee66 adenoviruses, parainfluenza67 and influenza viruses,68 Newcastle disease virus,69 and RSV.70 Although safety has been very good with these vaccines, several have been withdrawn from development because of poor immunogenicity. Therefore, the value of this approach as an effective human mucosal vaccine is yet to be proven.
Currently approved oral, live virus vaccines are being examined as platforms to deliver third-party antigens. Rotavirus vaccines have been a great advance in preventing infant mortality from infection-induced diarrhea in the developing world.71 Similarly, oral polio vaccine, which effectively targets M cells, has proven to be crucial to preventing paralysis.56 These oral vaccines are easily used because they do not require injection and do not need stringent storage conditions. They have had minimal toxicity other than rare cases of intussusception with rotavirus vaccine.71 Both viruses are now being examined as potential platforms for the development of other vaccines because they are also efficient at M-cell targeting and could be transmissible, expanding vaccination.72 , 73
Synthetic materials as a basis for recombinant protein and mRNA-based mucosal vaccines
Given the challenges of producing consistent virus-based vaccines, there is enthusiasm for using synthetic carriers to develop recombinant protein and mRNA-based mucosal vaccines. This type of approach has been remarkably successful for injectable vaccines. Virus-like particles made from recombinant protein subunits have created breakthroughs in prophylactic formulations for hepatitis B, human papilloma virus, herpes zoster, and COVID-19.74 However, there are significant challenges when adapting these approaches to mucosal immunization, and these formulations usually require some type of adjuvant system in injectable applications. There is a phase I clinical trial using a nasally applied COVID-19 protein: one using the spike receptor binding domain in conjunction with hepatitis B nucleocapsids, whereas the other used recombinant spike proteins.75 , 76 Both also tried intranasal/intramuscular administration, and although these approaches are well tolerated, conclusions on efficacy await larger trials.
Naturally occurring polymers
Natural polymers, such as chitosan, have been used complexed with protein and peptide antigens or nucleic acids for human injectable vaccines.77 Given the positive charge on this material, it binds well to phospholipids in mucus and cell membranes and can protect vaccines from protease and nuclease activity. The safety profile of injectable vaccines made with chitosan and similar polymers looks safe with mild reactions. Unfortunately, its ability to elicit effective immune responses has been varied in human studies as compared with animal models, and most believe it needs to be chemically modified or combined with pattern recognition receptor agonists to elicit effective immunity.77 , 78 The phase I trial involved intranasal Norwalk virus VLP-combined chitosan with MonoPhosphoryl Lipid (MPL) A and reported this material to be safe, immunogenic, and well tolerated.79
Liposomes
Liposomes are bicomponent oil and water mixtures extensively used as drug and gene delivery systems.80 They have well-defined lipid bilayers and are useful in solubilizing hydrophobic molecules because the interior of the structure is oil based. Liposomes can be manufactured in ways that could make them mucoadhesive, but, in most applications, the particles are too large for efficient DC uptake.81 New formulations may resolve this issue. A phase I trial using influenza virus antigens in a liposome formulation was conducted in 200 individuals in the early 2000s, but no results have been reported from that study.82
Emulsions
Emulsions involve simple lipid and water interfaces that are stabilized with surfactants. These are small particle-size droplet structures. These formulations are attractive because they can associate with and stabilize with either charged or hydrophobic materials. Emulsions are crucial to many of the vaccine formulations used for intramuscular immunization, but these materials have just recently been adapted to nasal administration. Formulations can be engineered to be strong adjuvants as well as provide mucus adhesion and enhanced uptake in DCs. Phase I clinical trials with whole-killed influenza virus83 and recombinant anthrax-protective antigen84 have shown some evidence of immunogenicity and excellent tolerability but are too preliminary to define efficacy.
Summary and future steps
As outlined above, vaccine administration at mucosal sites continues to be challenged by several complicating factors. Despite these hurdles, there remain many reasons to develop effective mucosal vaccines. New applications are being theorized that would increase the value of mucosal immunization. For example, mucosal immunization may be particularly effective in inducing immunity to epithelial cancers. Head and neck, lung, gastric, cervical, and bladder epithelia cancers are now major targets for immunotherapy.85 Combining immunization with immune checkpoint inhibitor therapies may improve outcomes of tumor immunotherapy.86 , 87 In particular, the concept of a combination of mucosal tumor vaccines, checkpoint inhibitor therapies, and alterations of the microbiome of the gut is an intriguing approach to broaden the efficacy of immunotherapy for mucosal cancers.88
Mucosal vaccine development will be aided by advances in understanding of the mechanisms required for immunity at mucosal surfaces and trafficking of tissue-resident cells between mucosal compartments. In recent years, fundamental advances in research on innate immunity facilitated the development of novel mucosal adjuvants. These include potentially nontoxic forms of bacterial enterotoxins (mCT and mLT).89, 90, 91 Despite these advances, untoward reactions still occur. For example, intranasal administration of an mLT LTK3 adjuvanted intranasal influenza vaccine caused transient peripheral facial nerve palsy (Bell’s palsy) in some vaccine recipients.92
The safety concerns with live attenuated vaccines and biologically derived adjuvants will continue to drive the development of less toxic, synthetic systems for vaccine delivery. Collaborations between bioengineering and immunology disciplines can mature platforms for mucosal antigen delivery, which include liposomes, emulsions, polymeric nanoparticles, and microneedle patches.81 , 93, 94, 95, 96, 97 Most of these approaches have demonstrated efficacy in preclinical models but are not yet in human trials.
Next-generation vaccines also need to focus on both the magnitude and the polarization of the induced immune response. This includes optimizing the combination of antibodies, cytokines, and T-cell types to prevent disease. Because adjuvants activate many innate signaling pathways, careful selection will be needed to avoid toxicity. Rational vaccine design is especially important in the development of vaccines for pathogens where traditional approaches have not been successful.98
Other factors offer promise for the development of successful mucosal vaccines. The combination of parenteral and mucosal prime-pull strategies is an interesting future direction for enhancing mucosal immune responses following injectable vaccines, as well as combining the specific cellular immune responses induced by parenteral and mucosal administration.99 Focus should also be directed at responses in specific populations (newborns and the aged, those with abnormal immunity, etc), which are key to addressing those individuals who respond poorly to current vaccines. Limits in our understanding of immune mechanisms of sterilizing immunity toward pathogens that enter at mucosal surfaces also remain a major gap in knowledge.
New mucosal vaccine development requires a complex approach that evaluates both immunogenicity and reactogenicity. Despite this complexity, these vaccines have the potential to improve current applications, provide new vaccines against pathogens currently without adequate protection, and extend the utility of vaccines to diseases such as cancer and allergies. The effort will require but also merits the type of investment that resulted in the parenteral COVID-19 vaccines.
Footnotes
Supported by the Michigan Nanotechnology Institute, the Mary H. Weiser Food Allergy Center, and grants N01 AI090031 to J.R.B. and R21 AI155944 to J.J.O.
Disclosure of potential conflict of interest: J. R. Baker is an inventor on patents for a nanoemulsion adjuvant and vaccines. This technology has been licensed to Blue Willow Biologics, and the University of Michigan has a financial interest in Blue Willow Biologics. This research is recognized in references 83 and 84. J. R. Baker is also on the Scientific Advisory Board for Aimmune and Moonlight Therapeutics, owns stock in Merck, and has received consulting fees from Takeda, GlaxoSmithKline, and HAL Allergy. P. T. Wong is an inventor on patents for a nanoemulsion adjuvant and vaccines. This technology has been licensed to Blue Willow Biologics, and the University of Michigan has a financial interest in Blue Willow Biologics. J. J. O’Konek is an inventor on patents for a nanoemulsion adjuvant for the suppression of allergic disease (PCT/US2015/054943 and PCT/US2021/065576). This technology has been licensed to Blue Willow Biologics, and the University of Michigan has a financial interest in Blue Willow Biologics. This research is recognized in references 83 and 84. J. J. O’Konek has received funding from Blue Willow as a subcontractor on a Small Business Innovation Research contract from the National Institutes of Health (grant no. 75N93019C00035). M. Farazuddin has no relevant conflicts of interest.
References
- 1.Mistry P., Barmania F., Mellet J., Peta K., Strydom A., Viljoen I.M., et al. SARS-CoV-2 variants, vaccines, and host immunity. Front Immunol. 2021;12:809244. doi: 10.3389/fimmu.2021.809244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alu A., Chen L., Lei H., Wei Y., Tian X., Wei X. Intranasal COVID-19 vaccines: from bench to bed. EBioMedicine. 2022;76:103841. doi: 10.1016/j.ebiom.2022.103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M., Wang Y., Sun Y., Cui H., Zhu S.J., Qiu H.J. Mucosal vaccines: strategies and challenges. Immunol Lett. 2020;217:116–125. doi: 10.1016/j.imlet.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Xu H., Cai L., Hufnagel S., Cui Z. Intranasal vaccine: factors to consider in research and development. Int J Pharm. 2021;609:121180. doi: 10.1016/j.ijpharm.2021.121180. [DOI] [PubMed] [Google Scholar]
- 5.van der Ley P.A., Zariri A., van Riet E., Oosterhoff D., Kruiswijk C.P. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front Immunol. 2021;12:781280. doi: 10.3389/fimmu.2021.781280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipsitch M., Krammer F., Regev-Yochay G., Lustig Y., Balicer R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol. 2022;22:57–65. doi: 10.1038/s41577-021-00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S.H., Jang Y.S. Recent insights into cellular crosstalk in respiratory and gastrointestinal mucosal immune systems. Immune Netw. 2020;20:e44. doi: 10.4110/in.2020.20.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinheiro-Rosa N., Torres L., de Almeida Oliveira M., Andrade-Oliveira M., Andrade de Freitas Guimaraes M., Coelho M., et al. Oral tolerance as antigen-specific immunotherapy. Immunother Adv. 2021;1:ltab017. doi: 10.1093/immadv/ltab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azegami T., Yuki Y., Kiyono H. Challenges in mucosal vaccines for the control of infectious diseases. Int Immunol. 2014;26:517–528. doi: 10.1093/intimm/dxu063. [DOI] [PubMed] [Google Scholar]
- 10.Gerdts V., Mutwiri G.K., Tikoo S.K., Babiuk L.A. Mucosal delivery of vaccines in domestic animals. Vet Res. 2006;37:487–510. doi: 10.1051/vetres:2006012. [DOI] [PubMed] [Google Scholar]
- 11.Miquel-Clopés A., Bentley E.G., Stewart J.P., Carding S.R. Mucosal vaccines and technology. Clin Exp Immunol. 2019;196:205–214. doi: 10.1111/cei.13285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf H., Kett V. Current prospects and future challenges for nasal vaccine delivery. Hum Vaccine Immunother. 2017;13:34–45. doi: 10.1080/21645515.2016.1239668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith N., Goncalves P., Charbit B., Grzelak L., Beretta M., Planchais C., et al. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat Immunol. 2021;22:1428–1439. doi: 10.1038/s41590-021-01028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S.Y., Yun S.G., Shin J.W., Lee B.Y., Son H.J., Lee S., et al. Persistent severe acute respiratory syndrome coronavirus 2 detection after resolution of coronavirus disease 2019-associated symptoms/signs. Korean J Intern Med. 2020;35:793–796. doi: 10.3904/kjim.2020.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med [published online ahead of print May 25, 2022]. doi: https://doi.org/10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed]
- 16.Navaneethan U., Giannella R.A. Mechanisms of infectious diarrhea. Nat Rev Pract Gastroenterol Hepatol. 2008;5:637–647. doi: 10.1038/ncpgasthep1264. [DOI] [PubMed] [Google Scholar]
- 17.Chen H.D., Frankel G. Enteropathogenic Escherichia coli: unravelling pathogenesis. FEMS Microbiol Rev. 2005;29:83–98. doi: 10.1016/j.femsre.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg H.B., Estes M.K. Rotaviruses: from pathogenesis to vaccination. Gastroenterology. 2009;136:1939–1951. doi: 10.1053/j.gastro.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mowat A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 20.Nemattalab M., Shenagari M., Taheri M., Mahjoob M., Nazari Chamaki F., Mojtahedi A., et al. Co-expression of interleukin-17A molecular adjuvant and prophylactic Helicobacter pylori genetic vaccine could cause sterile immunity in Treg suppressed mice. Cytokine. 2020;126:154866. doi: 10.1016/j.cyto.2019.154866. [DOI] [PubMed] [Google Scholar]
- 21.Brown C.M., Vostok J., Johnson H., Burns M., Gharpure R., Sami S., et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1059–1062. doi: 10.15585/mmwr.mm7031e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubois V., Locht C. Mucosal immunization against pertussis: lessons from the past and perspectives. Front Immunol. 2021;12:701285. doi: 10.3389/fimmu.2021.701285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warfel J.M., Zimmerman L.I., Merkel T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leffler D.A., Lamont J.T. Clostridium difficile infection. N Engl J Med. 2015;373:287–288. doi: 10.1056/NEJMc1506004. [DOI] [PubMed] [Google Scholar]
- 25.Abraham S.N., Miao Y. The nature of immune responses to urinary tract infections. Nat Rev Immunol. 2015;15:655–663. doi: 10.1038/nri3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavelle E.C., Ward R.W. Mucosal vaccines—fortifying the frontiers. Nat Rev Immunol. 2022;22:236–250. doi: 10.1038/s41577-021-00583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozlowski P.A., Aldovini A. Mucosal vaccine approaches for prevention of HIV and SIV transmission. Curr Immunol Rev. 2019;15:102–122. doi: 10.2174/1573395514666180605092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willekens B., Cools N. Beyond the magic bullet: current progress of therapeutic vaccination in multiple sclerosis. CNS Drugs. 2018;32:401–410. doi: 10.1007/s40263-018-0518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frumento D., Ben Nasr M., El Essawy B., D’Addio F., Zuccotti G.V., Fiorina P. Immunotherapy for type 1 diabetes. J Endocrinol Invest. 2017;40:803–814. doi: 10.1007/s40618-017-0641-y. [DOI] [PubMed] [Google Scholar]
- 30.Barshow S.M., Kulis M.D., Burks A.W., Kim E.H. Mechanisms of oral immunotherapy. Clin Exp Allergy. 2021;51:527–535. doi: 10.1111/cea.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillehoj E.R., Kim K.C. Airway mucus: its components and function. Arch Pharm Res. 2002;25:770–780. doi: 10.1007/BF02976990. [DOI] [PubMed] [Google Scholar]
- 32.Xing L., Zhou T., Fan Y., He Y., Pang T., Cho K., et al. Efficient mucosal immunization by mucoadhesive and pH-sensitive polymeric vaccine delivery system. Macromol Res. 2019;27:215–226. [Google Scholar]
- 33.Buckley A., Turner J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10:a029314. doi: 10.1101/cshperspect.a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith D.M., Simon J.K., Baker J.R. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraehenbuhl J.P., Neutra M.R. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301–332. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- 36.Monto A.S., Ohmit S.E., Petrie J.G., Johnson E., Truscon R., Teich E., et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 37.Chang J. Adenovirus vectors: excellent tools for vaccine development. Immune Netw. 2021;21:e6. doi: 10.4110/in.2021.21.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mabbott N.A., Donaldson D.S., Ohno H., Williams I.R., Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiyono H., Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tartey S., Takeuchi O. Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells. Int Rev Immunol. 2017;36:57–73. doi: 10.1080/08830185.2016.1261318. [DOI] [PubMed] [Google Scholar]
- 41.Merad M., Sathe P., Helft J., Miller J., Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou R., Liu L., Wang Y. Viral proteins recognized by different TLRs. J Med Virol. 2021;93:6116–6123. doi: 10.1002/jmv.27265. [DOI] [PubMed] [Google Scholar]
- 43.Helft J., Manicassamy B., Guermonprez P., Hashimoto D., Silvin A., Agudo J., et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sigmundsdottir H., Butcher E.C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mora J.R., Bono M.R., Manjunath N., Weninger W., Cavanagh L.L., Rosemblatt M., et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 46.Allard J.B., Rinaldi L., Wargo M.J., Allen G., Akira S., Uematsu S., et al. Th2 allergic immune response to inhaled fungal antigens is modulated by TLR-4-independent bacterial products. Eur J Immunol. 2009;39:776–788. doi: 10.1002/eji.200838932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar S., Jeong Y., Ashraf M.U., Bae Y.S. Dendritic cell-mediated Th2 immunity and immune disorders. Int J Mol Sci. 2019;20:2159. doi: 10.3390/ijms20092159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaensson E., Uronen-Hansson H., Pabst O., Eksteen B., Tian J., Coombes J.L., et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dillon A., Lo D.D. M cells: intelligent engineering of mucosal immune surveillance. Front Immunol. 2019;10:1499. doi: 10.3389/fimmu.2019.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Lange J., Rivera-Ballesteros O., Buggert M. Human mucosal tissue-resident memory T cells in health and disease. Mucosal Immunol. 2022;15:389–397. doi: 10.1038/s41385-021-00467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takamura S. Niches for the long-term maintenance of tissue-resident memory T cells. Front Immunol. 2018;9:1214. doi: 10.3389/fimmu.2018.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Domingues R.G., Hepworth M.R. Immunoregulatory sensory circuits in group 3 innate lymphoid cell (ILC3) function and tissue homeostasis. Front Immunol. 2020;11:116. doi: 10.3389/fimmu.2020.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hugenholtz F., de Vos W.M. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci. 2018;75:149–160. doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabin A.B. Properties and behavior of orally administered attenuated poliovirus vaccine. J Am Med Assoc. 1957;164:1216–1223. doi: 10.1001/jama.1957.62980110008008. [DOI] [PubMed] [Google Scholar]
- 57.Perego G., Vigezzi G.P., Cocciolo G., Chiappa F., Salvati S., Balzarini F., et al. Safety and efficacy of spray intranasal live attenuated influenza vaccine: systematic review and meta-analysis. Vaccines (Basel) 2021;9:998. doi: 10.3390/vaccines9090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rossey I., Saelens X. Vaccines against human respiratory syncytial virus in clinical trials, where are we now? Expert Rev Vaccines. 2019;18:1053–1067. doi: 10.1080/14760584.2019.1675520. [DOI] [PubMed] [Google Scholar]
- 59.Safety of a live attenuated human parainfluenza virus type 2 (HPIV2) vaccine for adults, children, and infants. https://ClinicalTrials.gov/show/NCT01139437 Available at: Accessed April 25, 2022.
- 60.Evaluating the safety and immunogenicity of a human parainfluenza type 3 (HPIV3) virus vaccine in infants and children. https://ClinicalTrials.gov/show/NCT01254175 Available at: Accessed April 25, 2022.
- 61.Karron R.A., San Mateo J., Wanionek K., Collins P.L., Buchholz U.J. Evaluation of a live attenuated human metapneumovirus vaccine in adults and children. J Pediatr Infect Dis Soc. 2018;7:86–89. doi: 10.1093/jpids/pix006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cáceres V.M., Sutter R.W. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin Infect Dis. 2001;33:531–541. doi: 10.1086/321905. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Yang C., Song Y., Coleman J.R., Stawowczyk M., Tafrova J., et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2102775118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Safety and immunogenicity of COVI-VAC, a live attenuated vaccine against COVID-19. https://ClinicalTrials.gov/show/NCT04619628 Available at: Accessed April 25, 2022.
- 65.A clinical trial of a recombinant adenovirus 5 vectored COVID-19 vaccine (Ad5-nCoV) with two doses in healthy adults. https://ClinicalTrials.gov/show/NCT04552366 Available at: Accessed April 25, 2022.
- 66.Phase I/II clinical trial of recombinant novel coronavirus (COVID-19) vaccine (adenovirus type 5 vector) for inhalation. https://ClinicalTrials.gov/show/NCT04840992 Available at: Accessed April 25, 2022.
- 67.Phase 1 study of intranasal PIV5-vectored COVID-19 vaccine expressing SARS-CoV-2 spike protein in healthy adults. https://ClinicalTrials.gov/show/NCT04954287 Available at: Accessed April 25, 2022.
- 68.An extended clinical trial of influenza virus vector COVID-19 vaccine for intranasal spray (DeINS1-2019-nCoV-RBD-OPT1) https://www.chictr.org.cn/showprojen.aspx?proj=55455 Available at: Accessed April 25, 2022.
- 69.Study of a live rNDV based vaccine against COVID-19. https://ClinicalTrials.gov/show/NCT04871737 Available at: Accessed April 25, 2022.
- 70.Safety and immunogenicity of an intranasal RSV vaccine expressing SARS-CoV-2 spike protein (COVID-19 vaccine) in adults. https://ClinicalTrials.gov/show/NCT04798001 Available at: Accessed April 25, 2022.
- 71.Crommelin D.J.A., Volkin D.B., Hoogendoorn K.H., Lubiniecki A.S., Jiskoot W. The science is there: key considerations for stabilizing viral vector-based Covid-19 vaccines. J Pharm Sci. 2021;110:627–634. doi: 10.1016/j.xphs.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samaranayake LP, Seneviratne CJ, Fakhruddin KS. Coronavirus disease 2019 (COVID-19) vaccines: a concise review [published online ahead of print May 15, 2021]. Oral Dis. https://doi.org/10.1111/odi.13916. [DOI] [PMC free article] [PubMed]
- 73.Kim S.H., Jang Y.S. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med. 2014;46:e85. doi: 10.1038/emm.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lua L.H., Connors N.K., Sainsbury F., Chuan Y.P., Wibowo N., Middelberg A.P. Bioengineering virus-like particles as vaccines. Biotechnol Bioeng. 2014;111:425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- 75.Shah S., Patel C., Patel D., Vadgama P., Patel M., Trivedi R. A review on modern use of intranasal vaccination in the treatment of SARS-COV-2. JDDT. 2021;11(4-S):263–270. [Google Scholar]
- 76.van der Lubben I.M., Verhoef J.C., Borchard G., Junginger H.E. Chitosan for mucosal vaccination. Adv Drug Deliv Rev. 2001;52:139–144. doi: 10.1016/s0169-409x(01)00197-1. [DOI] [PubMed] [Google Scholar]
- 77.Zhao J., Li J., Jiang Z., Tong R., Duan X., Bai L., et al. Chitosan, N,N,N-trimethyl chitosan (TMC) and 2-hydroxypropyltrimethyl ammonium chloride chitosan (HTCC): the potential immune adjuvants and nano carriers. Int J Biol Macromol. 2020;154:339–348. doi: 10.1016/j.ijbiomac.2020.03.065. [DOI] [PubMed] [Google Scholar]
- 78.Smith A., Perelman M., Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum Vaccin Immunother. 2014;10:797–807. doi: 10.4161/hv.27449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mateo R., Lindesmith L.C., Garg S.J., Gottlieb K., Lin K., Said S., et al. Production and clinical evaluation of Norwalk GI.1 virus Lot 001-09NV in norovirus vaccine development. J Infect Dis. 2020;221:919–926. doi: 10.1093/infdis/jiz540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Has C., Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J Liposome Res. 2020;30:336–365. doi: 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- 81.Bernasconi V., Norling K., Bally M., Höök F., Lycke N.Y. Mucosal vaccine development based on liposome technology. J Immunol Res. 2016;2016:5482087. doi: 10.1155/2016/5482087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liposomal based intranasal influenza vaccine: safety and efficacy. https://ClinicalTrials.gov/show/NCT00197301 Available at: Accessed April 25, 2022.
- 83.Stanberry L.R., Simon J.K., Johnson C., Robinson P.L., Morry J., Flack M.R., et al. Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W805EC combined with approved seasonal influenza antigens. Vaccine. 2012;30:307–316. doi: 10.1016/j.vaccine.2011.10.094. [DOI] [PubMed] [Google Scholar]
- 84.A safety and immunogenicity of intranasal nanoemulsion adjuvanted recombinant anthrax vaccine in healthy adults. https://ClinicalTrials.gov/show/NCT04148118 Available at: Accessed April 25, 2022.
- 85.Thallinger C., Füreder T., Preusser M., Heller G., Müllauer L., Höller C., et al. Review of cancer treatment with immune checkpoint inhibitors: current concepts, expectations, limitations and pitfalls. Wien Klin Wochenschr. 2018;130:85–91. doi: 10.1007/s00508-017-1285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webster R.M. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov. 2014;13:883–884. doi: 10.1038/nrd4476. [DOI] [PubMed] [Google Scholar]
- 87.Alex F., Alfredo A. Promising predictors of checkpoint inhibitor response in NSCLC. Expert Rev Anticancer Ther. 2020;20:931–937. doi: 10.1080/14737140.2020.1816173. [DOI] [PubMed] [Google Scholar]
- 88.Reens A.L., Cabral D.J., Liang X., Norton J.E., Therien A.G., Hazuda D.J., et al. Immunomodulation by the commensal microbiome during immune-targeted interventions: focus on cancer immune checkpoint inhibitor therapy and vaccination. Front Immunol. 2021;12:643255. doi: 10.3389/fimmu.2021.643255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lycke N., Lebrero-Fernández C. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr Opin Pharmacol. 2018;41:42–51. doi: 10.1016/j.coph.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 90.Quan F.S., Yoo D.G., Song J.M., Clements J.D., Compans R.W., Kang S.M. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J Virol. 2009;83:4489–4497. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qadri F., Akhtar M., Bhuiyan T.R., Chowdhury M.I., Ahmed T., Rafique T.A., et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2020;20:208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T., et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 93.Bernasconi V., Norling K., Gribonika I., Ong L.C., Burazerovic S., Parveen N., et al. A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection. Mucosal Immunol. 2021;14:523–536. doi: 10.1038/s41385-020-0334-2. [DOI] [PubMed] [Google Scholar]
- 94.Ball R.L., Bajaj P., Whitehead K.A. Oral delivery of siRNA lipid nanoparticles: fate in the GI tract. Sci Rep. 2018;8:2178. doi: 10.1038/s41598-018-20632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nelson S.A., Dileepan T., Rasley A., Jenkins M.K., Fischer N.O., Sant A.J. Intranasal nanoparticle vaccination elicits a persistent, polyfunctional CD4 T cell response in the murine lung specific for a highly conserved influenza virus antigen that is sufficient to mediate protection from influenza virus challenge. J Virol. 2021;95 doi: 10.1128/JVI.00841-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hajam I.A., Senevirathne A., Hewawaduge C., Kim J., Lee J.H. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet Res. 2020;51:37. doi: 10.1186/s13567-020-00762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rouphael N.G., Paine M., Mosley R., Henry S., McAllister D.V., Kalluri H., et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet. 2017;390:649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Hagan D.T., Friedland L.R., Hanon E., Didierlaurent A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr Opin Immunol. 2017;47:93–102. doi: 10.1016/j.coi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 99.Ozberk V., Reynolds S., Huo Y., Calcutt A., Eskandari S., Dooley J., et al. Prime-pull immunization with a bivalent M-protein and spy-CEP peptide vaccine adjuvanted with CAF®01 liposomes induces both mucosal and peripheral protection from covR/S mutant Streptococcus pyogenes. mBio. 2021;12 doi: 10.1128/mBio.03537-20. e03537-20. [DOI] [PMC free article] [PubMed] [Google Scholar]