Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), as the pathogen of coronavirus disease 2019 (COVID-19), has infected millions of people and took hundreds of thousands of lives. Unfortunately, there is deficiency of effective medicines to prevent or treat COVID-19. 3C like protease (3CLPro) of SARS-CoV-2 is essential to the viral replication and transcription, and is an attractive target to develop anti-SARS-CoV-2 agents. Targeting on the 3CLPro, we screened our protease inhibitor library and obtained compound 10a as hit to weakly inhibit the SARS-CoV-2 3CLPro, and determined the co-crystal structure of 10a and the protease. Based on the deep understanding on the protein-ligand complexes between the hit and SARS-CoV-2 3CLPro, we designed a series of peptidomimetic inhibitors, with outstanding inhibitory activity against SARS-CoV-2 3CLPro and excellent anti-viral potency against SARS-CoV-2. The protein-ligand complexes of the other key inhibitors with SARS-CoV-2 3CLPro were explicitly described by the X-ray co-crystal study. All such results suggest these peptidomimetic inhibitors could be further applied as encouraging drug candidates.
Keywords: SARS-CoV-2, 3C Like protease, Peptidomimetic inhibitors
Graphical abstract
1. Introduction
With the worldwide epidemic of Coronavirus Disease 2019 (COVID-19), the public health and economy has suffered a serious devastation [1]. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is the pathogen of COVID-19. In the early stage, the broad spectrum antiviral agents, such as Lopinavir, Ritonavir, interferon-α and Remdesivir, are used in clinical and preclinical experiment, the limit therapeutic effect of these medicines restrict the applications on the treatment of COVID-19 [2, 3]. Recently, PF-00835231, as a 3C-like protease inhibitor, was reported to demonstrate good efficacy against SARS-CoV-2 and possess high metabolic stability. However, this inhibitor cannot be taken orally, which can only be administered intravenously with relatively high effective dose [4,5]. In addition, in February of this year, China Food and Drug Administration approved the import registration of Pfizer's antiviral drug Paxlovid, used to treat adults with mild to moderate COVID-19 symptoms. Its main component PF-07321332, the first orally administered SARS-CoV-2 inhibitor, shows great enzymatic activity and cellular antiviral activity against SARS-CoV-2. More importantly, it demonstrates excellent off-target selectivity and in vivo safety profiles [6]. At present, the number of small molecule inhibitors used to treat COVID-19 is very few and most of them are administered in combination. As primary, it is still urgent to comprehensively decipher the interactive mechanism of peptidomimetics inhibitor with the target protein and more detailed investigation of the structure and activity relationship. This will be helpful to extend peptidomimetic inhibitor and provide a basement to develop more effective and safe antiviral drugs against COVID-19.
SARS-CoV-2 possess a single-stranded positive-sense RNA genome with two overlapping open reading frames (ORF1a and ORF1b), which encode two polyprotein precursors (pp1a and pp1ab). Subsequently, sixteen non-structural proteins are released from pp1a (nsp 1–11) and pp1ab (nsp 1–10, nsp 12–16) upon the proteolytic cleavage by two viral proteases, 3C-like protease (3CLPro, otherwise known as main protease) and papain-like protease (PLPro), which further participant in the life cycle of SARS-CoV-2 [7, 8]. As the main protease, 3CLPro is responsible for proteolytic processing of the majority of polyprotein cleavage sites and is essential for the viral replication and transcription. This enables 3CLPro to be a highly attractive target for antiviral drug development [[9], [10], [11]].
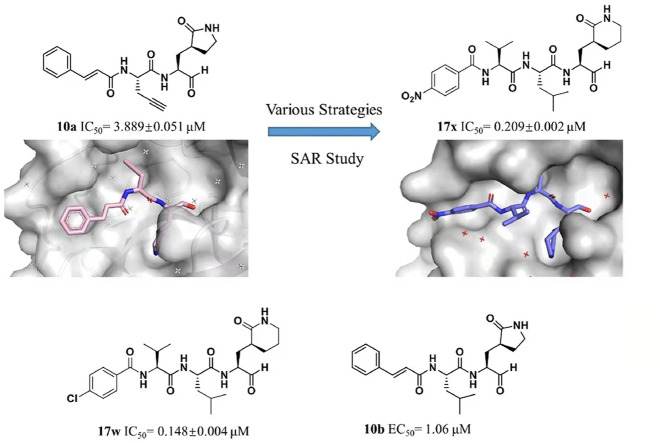
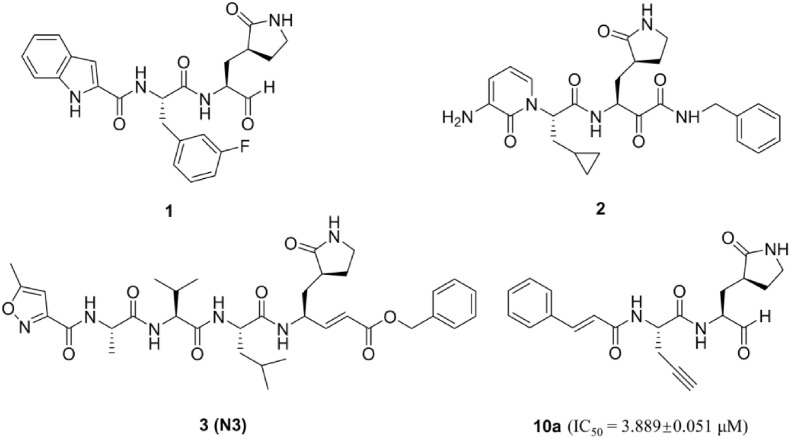
Active sites of coronavirus 3CLPro are well conserved [12]. SARS-CoV-2 3CLPro holds a dyad (His41-Cys145) and adopts a nucleophile manner to cleave the polyproteins as generally favored Leu-Gln↓Ser(Ala, Gly) sequence [[13], [14], [15]]. Additionally, a partial negative charge cluster (Arg-Tyr-Asp) and a conserved water to accelerate catalysis are exploited in other two highly pathogenic coronaviruses (MERS-CoV and SARS-CoV) 3CLPro [16]. Moreover, as 3CLPro exhibits genus-species specific, inhibitors that structurally mimic those proteolytic cleavage sites can specifically target the 3CLPro with little or no impact on host cellular proteases [17]. In retrospect, several series of peptidomimetic SARS-CoV-2 3CLPro inhibitors were successfully developed with different warhead moieties, such as aldehyde inhibitor 1, α-ketoamide inhibitor 2, Michael acceptor inhibitor 3 (N3) (Fig. 1 ) [18,19]. Despite a flood of SARS-CoV-2 research published every week, the clinically approved 3CLPro targeted agents against COVID-19 remain elusive. This inspires us to perform further investigations on the protein-ligand complexes of inhibitor with the 3CLPro and the structure-activity relationship (SAR) of the 3CLPro inhibitors. Because of 3CLPro's similarities to the picornaviral 3CPro, lots of picornaviral 3CPro inhibitors were reported to inhibit 3CLPro [20]. Our group previously developed a series of covalent and noncovalent inhibitors against Enterovirus 71 (a kind of picornavirus) 3CPro [[21], [22], [23], [24]]. Among those inhibitors, peptidomimetic aldehyde 10a, exhibited certain inhibitory activity against SARS-CoV-2 3CLPro (IC50 = 3.889 ± 0.051 μM), which was chosen as the hit compound for further research.
Fig. 1.
The structure of reported peptidomimetic inhibitors against SARS-CoV-2 3CLPro.
In the current study, we report accumulating co-crystal structures and compounds to gain the comprehensive insights on the SAR of the peptidomimetic inhibitors of SARS-CoV-2 3CLPro. With the structural optimization strategies, the inhibitor 17w (IC50 = 0.148 ± 0.004 μM) with excellent inhibitory potency against SARS-CoV-2 3CLPro and 10b with notable anti-viral activity against SARS-CoV-2 were obtained (EC50 = 1.06 μM), which provides a lead compound to develop clinical candidate. In general, these investigations might provide the solid basis for effective inhibitor development and accelerating development of SARS-CoV-2 3CLPro inhibitors into clinical candidates.
2. Results and discussion
2.1. Chemistry
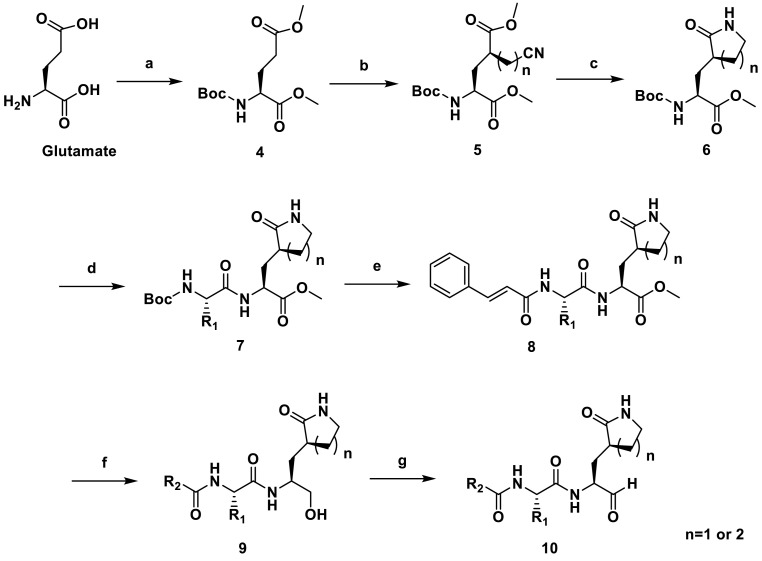
Synthetic route and chemical structures of the inhibitors were shown in Scheme 1, Scheme 2, Scheme 3 . After the esterification of l-glutamic acid, its amino group was protected by Boc group to form compound 4. The enol intermediate formed by the reaction of compound 4 with LiHDMS is then reacted with 2-bromoacetonitrile or 3-bromopropionitrile to obtain alkylation product 5. Lactam ring compound 6 was formed under the condition of sodium borohydride and hydrated cobalt chloride. Removal of the Boc group of 6 with TFA followed by the amide bond formation using EDCI as coupling reagent resulted in 7. The Boc group in 7 were removed and the resulting species were reacted with different acids to give 8. The primary alcohols 9 were derived from the reduction of the corresponding esters with NaBH4, which were oxidized to aldehydes 10 with Dess-Martin periodinane (DMP). The synthesis of 14 and 17 are similar to the above.
Scheme 1.
Synthetic Scheme of 10a-10l. Reagents and conditions: (a) (1) SOCl2, MeOH, reflux, 3 h, (2) Di-tert-butyl pyrocarbonate, TEA, THF, 25 °C, 12 h; yield: 98.4% for two steps; (b) (1) LiHMDS, anhydrous THF, −78 °C, 3 h, argon atmosphere; (2) 2-Bromoacetonitrile or 3-Bromopropionitrile (dissolved in anhydrous THF), −78 °C, 1.5 h; yield: 55.4%–59% for two steps; (c) CoCl2. 6H2O, NaBH4, MeOH, 0 °C, 48 h, yield: 46.3%; (d) (1) TFA, anhydrous DCM, 25 °C, 3 h, add TEA to adjust pH to 7.0; (2) various Boc-protected amino acid, EDCI, HOBt and TEA, anhydrous DCM, 25 °C, 12 h; 47%–65% for two steps; (e) (1) TFA, anhydrous DCM, RT, 3 h, add TEA to adjust pH to 7.0; (2) Cinnamic acid, EDCI, HOBt and TEA, anhydrous DCM, 25 °C, 12 h, 32–44%. (f) NaBH4, MeOH, 0 °C–25 °C, 3 h, 56–77%; (g) Dess-Martin Reagent, DCM, 25 °C, 2 h, 71–91%.
Scheme 2.
Synthetic Scheme of 14a-14d. Reagents and conditions: (a) (1) TFA, anhydrous DCM, RT, 3 h, add TEA to adjust pH to 7.0; (2) various Boc-l-amino acid, EDCI, HOBt and TEA, anhydrous DCM, 25 °C, 12 h, 41–49% for two steps; (b) (1) TFA, anhydrous DCM, RT, 3 h, add TEA to adjust pH to 7.0; (2) Cinnamic acid, EDCI, HOBt and TEA, anhydrous DCM, 25 °C, 12 h; 31–49%. (c) NaBH4, MeOH, 0 °C to RT, 25 °C; 41–57%; (d) Dess-Martin Reagent, DCM, 25 °C, 2 h; 68–81%.
Scheme 3.
Synthetic Scheme of 17a-17z. Reagents and conditions: (a) (1) TFA, anhydrous DCM, RT, 3 h, add TEA to adjust pH to 7.0; (2) various Boc-l-amino acid, EDCI, HOBt and TEA, anhydrous DCM, 25 °C, 12 h, 39–58% for two steps; (b) NaBH4, MeOH, 0 °C to RT, 25 °C; 40–52%; (d) Dess-Martin Reagent, DCM, 25 °C, 2 h; 50–71%.
2.2. Biological activity and SAR study
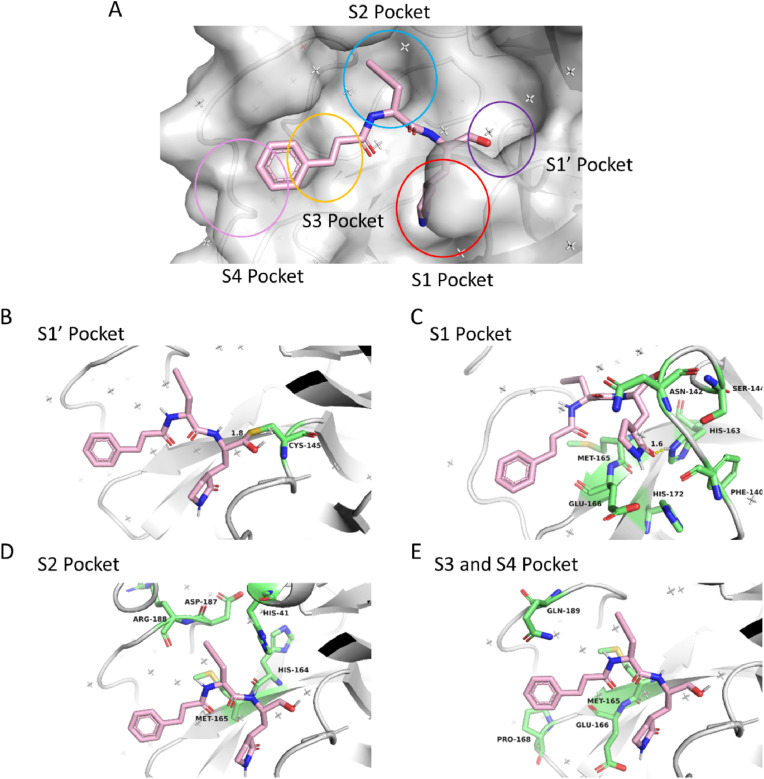
For rationally optimizing the peptidomimetic inhibitor, we determined the co-crystal structure of 10a with SARS-CoV-2 3CLPro (PDB: 7DHJ). According to thoroughly penetrating the structure, the aldehyde group of 10a forms a covalent bond with the catalytic residue Cys145, the γ-lactam ring group of 10a forms tight hydrogen bond with His163 of 3CLPro and suitably binds with the S1 pocket of 3CLPro, the rigid alkynyl group of 10a as the P2 site group inserts into the S2 pocket of 3CLPro enclosed by His41, His164, Met 165, Asp187 and Arg188, and the cinnamoyl group of 10a occupies the channel formed by Met165, Glu166, Pro168 and Gln189 (Fig. 2 A–E). With further investigation on the structure of the protease, due to the S2 pocket embed into the surface of the protease and exhibited hydrophobicity, we attempted to introduce the hydrophobic alkyl group with suitable sharp and volume to occupy the S2 pocket for optimizing candidate. Besides, owing to the imidazole side chain of His41 located at the S2 pocket, the group with π-electron is expected to be introduced into the P2 site of the inhibitor for forming π-π stacking interaction with His41 and improving the inhibitory activity of compounds (Fig. 3 ).
Fig. 2.
The protein-ligand complexes of SARS-CoV-2 3CLPro with aldehyde 10a. (A) the surface structure of SARS-CoV-2 3CLPro with aldehyde 10a. The detailed protein-ligand complexes of SARS-CoV-2 3CLPro with aldehyde 10a in S1′ pocket (B), S1 pocket (C), S2 pocket (D), S3 and pocket (E) respectively. In the structure, the protease is presented as a white cartoon. The significant residues and inhibitor are shown as sticks (the protease residues are in green, 10a is in pink).
Fig. 3.
Design of the peptidomimetic inhibitors.
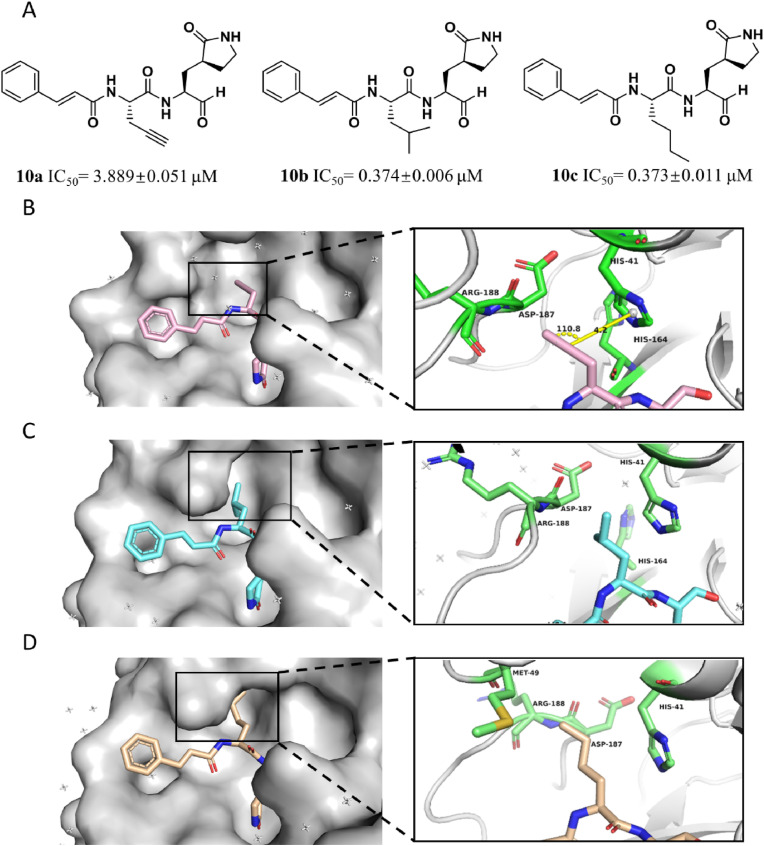
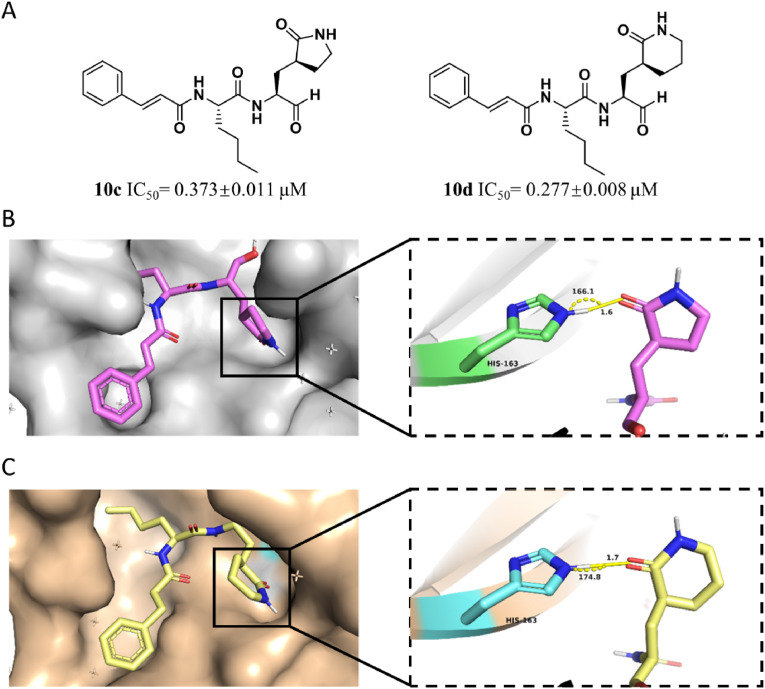
Based on the analysis toward S2 pocket, other two typical inhibitors with different alkyl group at P2 position were designed and synthesized. After evaluation of inhibitory potency, 10b (IC50 = 0.374 ± 0.006 μM) with branched isobutyl group at P2 site and 10c (IC50 = 0.373 ± 0.011 μM) with linear chain butyl group were noticed to commendably inhibit the proteolysis of SARS-CoV-2 3CLPro than 10a and the positive control (compound 1) (Table S1).
In order to clearly illustrate the contribution of the P2 site chemical group to the inhibitors, we further determined the crystal structure of 3CLPro in complex with the inhibitor 10b (PDB: 7DGB) or 10c (PDB: 7DGG). Combination with the complex structure of the protease with 10a, the aldehyde of the inhibitor (10a, 10b or 10c) forms a covalent bond with the catalytic residue Cys145 in each complex structure. These three inhibitors are all tripeptidomimetic inhibitors with the same P1 and P3 groups, but differ in the P2 site group. As expected, each inhibitor binds to the active site of 3CLPro in almost the same manner, except for the P2 group (Fig. 4 ). In 10a, we have expected the P2 alkynyl group to introduce π-electron interaction with the imidazole group of His41. However, the 3CLPro-10a complex structure showed that due to the lack of flexibility, the alkynyl group is 4.2 Å away from the His41 imidazole group and in an inappropriate angle of 110.8° (Fig. 4B), indicating that the alkynyl group of 10a is vain to improve the inhibitory activity of the inhibitor. On the contrary, the P2 site isobutyl group of 10b and n-butyl group of 10c, which are of higher flexibility, fit better in the S2 pocket (Fig. 4C and D). Especially N-butyl group of 10b approaches side chain of Met168, thereby improving their hydrophobic interaction. These structural observations are coincident with the IC50 values of the three inhibitors, that the 10b (IC50 = 0.374 ± 0.006 μM) showed lowest IC50 and 10c (IC50 = 0.373 ± 0.011 μM) showed almost the same IC50 to 10b, while the IC50 of 10a (IC50 = 3.889 ± 0.051 μM) was an order of magnitude higher than 10b and 10c. Consistent with the reported conclusion, these results proved that a hydrophobic alkyl group at the P2 site is more favored for the inhibitory activity. Meanwhile, compound 10c exhibited similar inhibitory activity with compound 10b against SARS-CoV2 3CLPro, which proved that the S2 pocket, as a high flexible pocket, interact with the peptidomimetic inhibitor in induced-fitting mode and the branched alkyl group is suitable to be substituted with long chain alkyl group.
Fig. 4.
The protein-ligand complexes of SARS-CoV-2 3CLPro with three aldehydes. (A) the structures and inhibitory activity of aldehyde 10a, 10b and 10c. The co-crystal structures of SARS-CoV-2 3CLPro in complex with aldehyde 10a (B), 10b (C), and 10c (D), respectively. In the structure, the protease is presented as a white cartoon. The significant residues and inhibitor are shown as sticks (the protease residues are in green, 10a is in pink, 10b is in cyan and 10c is in wheat).
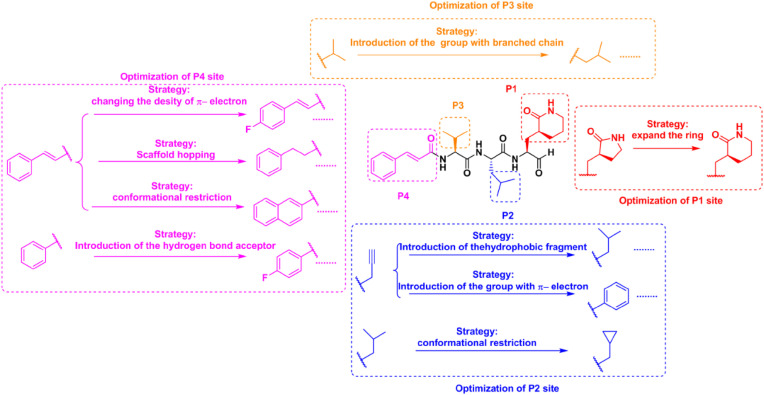
For further optimization of the inhibitory potency of the peptidomimetic inhibitor, the complex structure of 10c with the protease was penetrated in detail. In S1 pocket, the relative open lateral area with hydrophobicity made it reasonable to expand the (S)-γ-lactam ring of 10c into (S)-δ-lactam ring for preferable fitting the S1 pocket (Fig. 2A and C). As the result, the 10d (IC50 = 0.277 ± 0.008 μM) with (S)-δ-lactam ring was synthesized and exhibited more excellent inhibitory activity than 10c (Fig. 5 A).
Fig. 5.
The protein-ligand complexes between SARS-CoV-2 3CLPro and two inhibitors. (A) The structures of 10c and 10d. (B) Co-crystal structure of SARS-CoV-2 3CLPro in complex with 10c (C) The co-crystal structure of the protease with 10d. The significant residues and inhibitor are shown as sticks (The protease residues are in green and cyan. 10a is in violet and 10b is in yellow).
To clarify why the (S)-δ-lactam ring at the P1 site shows better inhibitory activity than the (S)-γ-lactam ring, we further determined the crystal structure of 3CLPro in complex with the inhibitor 10d (PDB: 7DGF) (Fig. 5B and C). The inhibitor binds to the active site of 3CLPro and forms a covalent bond with Cys145, as expected. Since the inhibitors 10c and 10d are the same except that they have (S)-δ-lactam ring and (S)-γ-lactam ring at the P1 site, respectively, so we focused on the P1 site and compared the structures of 3CLPro-10c complex and 3CLPro-10d complex. The (S)-γ-lactam ring of 10c and (S)-δ-lactam ring of 10d form hydrogen bonds with His163 respectively, with similar bond length and bond angle (Fig. 5B and C). Compared with the (S)-γ-lactam ring, the (S)-δ-lactam ring does not bring any additional polar interactions with 3CLPro. However, when measuring the contact area, we found that the contact area between the (S)-δ-lactam ring and 3CLPro is ∼135 Å2, while the (S)-γ-lactam ring is ∼125 Å2. The (S)-δ-lactam ring contributes ∼10 Å2 more contact area with 3CLPro than the (S)-γ-lactam ring. Therefore, inhibitor 10d exhibited better inhibitory activity than 10c.
Based on the insight on the above complex structures, the protein-ligand complexes of the typical inhibitors with the protease at P1 and P2 sites were summarized. For further check the validity of the model, a series of inhibitors with (S)-δ-lactam ring at the P1 site and various alkyl groups at P2 site were designed, synthesized and evaluated (Fig. 3). According to comparing the inhibitory activity of 10a with 10l, 10b with 10i, and 10c with 10d, it was firm to confirm (S)-δ-lactam ring with more contact area exhibited preferable inhibitory activity than (S)-γ-lactam ring. Following comparing the inhibitory activity of 10d-10l, long hydrophobic flexible chain alkyl group was suitable to occupy the S2 pocket of the protease (Table S1).
When further insight on the complex structure of 10d with the protease, a long narrow channel nearby the S1 and S2 pockets attracted our attention and thus the tripeptidomimetic inhibitors were designed to evolve into tetrapeptidomimetic inhibitors to occupy the channel as far as possible for improving the inhibitory activity of peptidomimetic inhibitors (Fig. 2, Fig. 3). In detail tetrapeptidomimetic inhibitors 14a-14d with branched alkyl groups at P3 position for preferably stucking the S3 pocket were synthesized and evaluated. As the results, the superior inhibitory activities were reflected by tetrapeptidomimetic inhibitors than tripeptidomimetic inhibitors (Fig. 4 and Tables S1 and S2), which illustrated the stereoscopic extension of the inhibitor is meaningful to improve the inhibitory potency of the inhibitor. Moreover, the superior inhibitory activity of 14c (IC50 = 0.281 ± 0.033 μM) made it reasonable to fix the branched isopropyl group at P3 site of tetrapeptidomimetic inhibitors to further design and optimize P4 site of tetrapeptidomimetic inhibitors (Fig. 3).
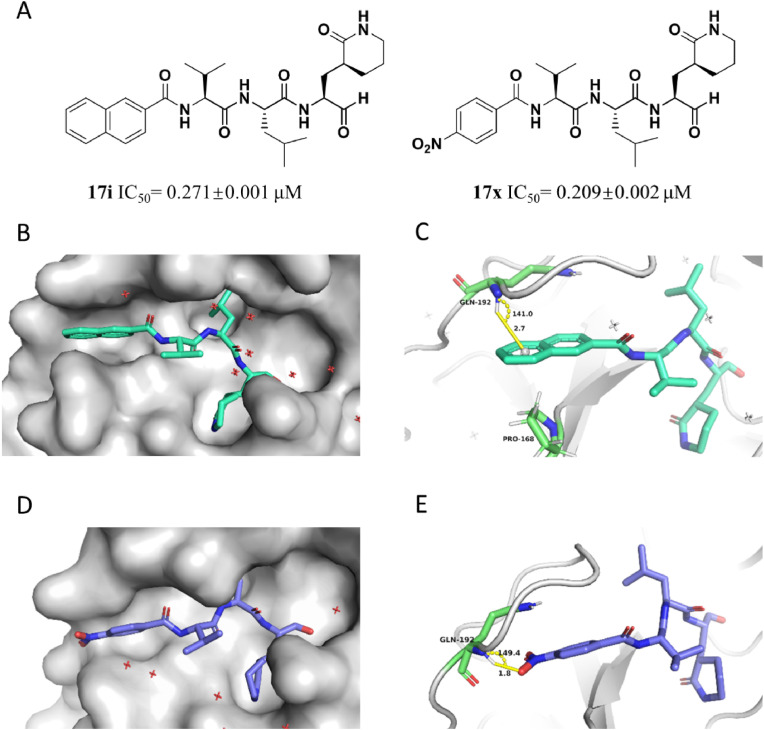
For the discovery of the suitable group at P4 site (Table S2), a series of aldehydes with various substituted cinnamic acids were obtained and evaluated. 17a (IC50 = 0.196 ± 0.004 μM) and 17g (IC50 = 0.173 ± 0.014 μM) exhibited excellent inhibitory activities against the 3CLPro. Subsequently, the inhibitor 14c with cinnamic acid fragment was evolved into 17i (IC50 = 0.271 ± 0.001 μM) with naphthyl group and 17k (IC50 = 0.221 ± 0.001 μM) with indole group via conformational restriction; 17l (IC50 = 0.199 ± 0.001 μM) with phenylethyl group and 17m (IC50 = 0.257 ± 0.024 μM) with phenyl group via scaffold hopping strategy; and 17q (IC50 = 0.173 ± 0.013 μM) with furanyl group and 17r (IC50 = 0.172 ± 0.018 μM) with pyrrolyl group via bioisostere strategy, respectively. All of these inhibitors displayed preferable inhibitory activity than that of 14c. Considering of the ligand efficiency, 14c was evolved into 17m for further research. Previously, we proposed a strategy to introduce a hydrogen bond acceptor into the P4 site of inhibitor for the construction of tight interaction with Q195 and enhancement of the potency of the MERS-CoV 3CLPro inhibitor. The high similarity of sequence and catalytic mechanism between SARS-CoV-2 3CLPro and MERS-CoV 3CLPro makes this rational strategy to optimize inhibitory activity against SARS-CoV-2 3CLPro. Following enzymatic assay, compared with 17m, the superior inhibitory potency reflected by 17s (IC50 = 0.153 ± 0.010 μM), 17v (IC50 = 0.197 ± 0.008 μM), 17w (IC50 = 0.148 ± 0.004 μM) and 17x (IC50 = 0.209 ± 0.002 μM) forcefully reiterated effectivity of the strategy. In addition, the inferior inhibitory potency of 17t (IC50 = 0.640 ± 0.009 μM) and 17u (IC50 = 0.565 ± 0.006 μM) than 17s denied the significance to introduce pyridine group at P4 site. 17y (IC50 = 1.426 ± 0.026 μM) exhibited notable reduced inhibitory activity than 17v, which suggested the importance of the location of the introduced hydrogen bond acceptor group. 17z (IC50 = 0.285 ± 0.006 μM) failed to exhibit comparable inhibitory activity with 17s, which proved the significance of aromatic ring at P4 position. Most of the tetrapeptidomimetic aldehydes (except 14g, 17o and 17y) exhibited preferable inhibitory activity than the positive control 1, and 17w especially achieved 4-fold increased inhibitory potency.
For tetrapeptidomimetic inhibitors, we managed to obtain the complex structures of 3CLPro-17i (PDB: 7DGH) and 3CLPro-17x (PDB: 7DGI). In each complex structure, the inhibitor is covalently linked to the catalytic residue Cys145 of 3CLPro via its aldehyde. The P4 group of each inhibitor fits in the S4 pocket of 3CLPro consequently increasing the contact area between the enzyme and the inhibitor. Therefore, the tetrapeptidomimetic inhibitors should exhibit better inhibitory activity than tripeptidomimetic inhibitors, such as 10d. However, although inhibitor 17x showed a lower IC50 than 10d, the tetrapeptidomimetic inhibitor 17i showed only a similar IC50 to 10d, indicating that there is selectivity between the S4 pocket and the P4 group (Fig. 6 A). To elucidate the selectivity, we compared the complex structures of 3CLPro-17i and 3CLPro-17x. As shown in Fig. 6B-E, the P4 naphthyl group of inhibitor 17i is sterically larger than the nitrophenyl group of 17x. The carbonyl group of 3CLPro Gln192 is flipped in the 3CLPro-17i complex structure due to steric effects, but not in the 3CLPro-17x complex structure. As a result, the backbone of Gln192 adopts an unfavorable conformation in 3CLPro-17i complex structure. Such energy consuming change in 3CLPro may cancel out the benefits of the interaction between the P4 group and the S4 pocket. Therefore, the inhibitor 17i cannot significantly promote its inhibitory activity. From the IC50 data we noticed that a smaller aromatic group at the P4 site was prone to better improve the inhibitor's inhibitory activity. This phenomenon is consistent with our structural observations.
Fig. 6.
The protein-ligand complexes of SARS-CoV-2 3CLPro with 17i and 17x. (A) The structures and inhibitory activity of 17i and 17x. (B) The overall structure of 17i binding to the active pocket of the protease. (C) The protein-ligand complexes of the naphthyl group of 17i with the S4 pocket of SARS-CoV-2 3CLPro in detail. (D) The overall structure of 17x binding to the active pocket of the protease. (E) The protein-ligand complexes of the naphthyl group of 17x with the S4 pocket of SARS-CoV-2 3CLPro in detail. In the structure, the protease is presented as a white cartoon, and the significant residues and inhibitor are shown as sticks (the protease residues are in green and the inhibitor are in lime green and violet).
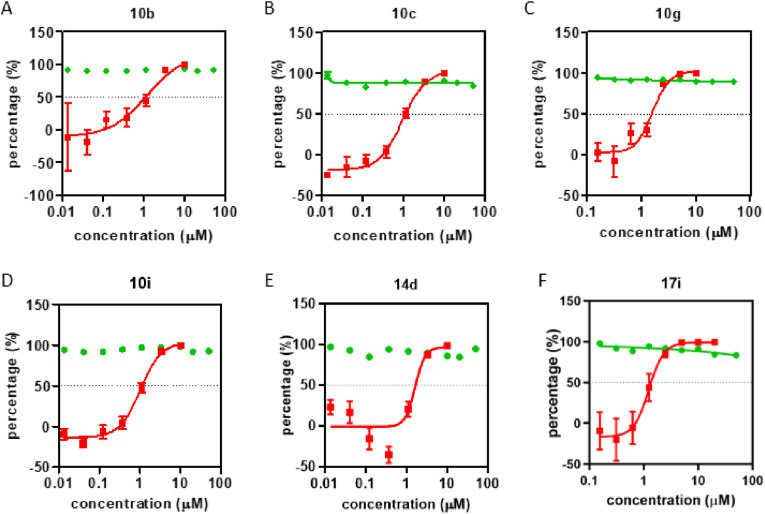
Encouraged by the excellent 3CLPro inhibitory activities of the peptidomimetic inhibitors, we further explored the anti-SARS-CoV-2 activity of those inhibitors in vitro. First, we indicated that all of inhibitors did not manifest the obvious cytotoxicity at A549 cell lines, which was used for simulation of the lung environment (Table S1 and Table S2). These inhibitors were then screened at concentration of 10 μM in VeroE6 cells for their anti-SARS-CoV-2 activity. As the tripeptidomimetic aldehydes, 10b, 10c, 10g, and 10h reduced the viral RNA more than 2logs (Fig. 7 ). Then, the EC50 and CC50 of 10b, 10c, 10g, and 10i were then tested (Fig. 7). While none of these compounds showed significant cytotoxity on VeroE6 cells, all compounds showed a dose-dependent inhibition effect on SARS-CoV-2 virus proliferation. Among the tested tripeptidomimetic inhibitors, 10b with (S)-γ-lactam ring at P1 site, isobutyl group at P2 site and cinnamic acid fragment at P3 site had the most potential anti-SARS-CoV-2 activity with EC50 of 1.06 μM, which makes it become an attractive potential clinic candidate medicine.
Fig. 7.
The anti-SARS-CoV-2 activity and cytotoxicity of the peptidomimetic inhibitors 10b (A), 10c (B), 10g (C), 10i (D), 14d (E), 17i (F). As green line, the peptidomimetic inhibitors were screened for viral inhibitory activity in Vero E6 cells. Vero E6 cells were infected with SARS-CoV-2 at 0.01 multiplicity of infection (MOI) in the presence of different compounds, and viral RNA in the supernatant was quantified by qRT-PCR at 24 hpi. As red line, the dose-dependent anti-SARS-CoV-2 activity of selected compounds were determined by serial dilution. Vero E6 cells were treated with series diluted compounds, and infected with SARS-CoV-2 virus at a MOI of 0.01. Viral RNA in supernatant was quantified by qRT-PCR and the inhibition rate was calculated by comparing with the viral RNA level in the DMSO control group. The cell viability was tested by MTT assay after compounds treatment. The cell viability was calculated as the percentage of the absorption value of the compounds treated cells to that of the DMSO treated cells. The cell viability of DMSO treated cells was set as 100%, and higher value of cell viability indicates less cytotoxicity.
Though most of the tetrapeptidomimetic inhibitors have preferable inhibitory activity on 3CLpro than the tripeptidomimetic inhibitors, the tetrapeptidomimetic inhibitors did not show superior antiviral activity. Among the tetrapeptidomimetic aldehydes, only compounds 14d and 17i inhibited SARS-CoV-2 viral RNA more than 2logs at concentration of 10 μM (Fig. 7). The EC50 and CC50 of 14j and 17i were determined (Fig. 7). None of the tested compounds influencs the cell viability, while all compounds dose-dependently inhibit SARS-CoV-2 viral propagation. Though 14c with isopropyl group at P3 site and cinnamic acid fragment at P4 had no antiviral activity, 17i which changed cinnamic acid fragment to naphthyl group at P4 site could inhibit SARS-CoV-2 and the EC50 was calculated as 1.36 μM, indicated the possibility to adjust P4 site group and enhance the anti-viral activity of the tetrapeptidomimetic inhibitors.
3. Discussion and conclusion
Novel small-molecule drugs might not be developed fast enough against COVID-19. However, as the pandemic threaten remains a long-term problem, antiviral molecules will play an important role of defense. Based on the structure of the hit compound 10a, various co-crystal structures and a series of strategies were applied to optimize the inhibitory potency of the peptidomimetic inhibitors against SARS-CoV-2 3CLPro. The results showed that most of the inhibitors exhibit excellent inhibitory activity against SARS-CoV-2 3CLPro, which were systematically summarized to the discussion on the SAR of peptidomimetic aldehyde inhibitor of SARS-CoV-2 3CLPro. For the discussion on the anti-viral activity of the designed compounds, the anti-viral activities of these compounds fail to present a perfect correlation with the enzymatic inhibitory activity. In detail, the tetrapeptidomimetic inhibitors generally exhibit inferior activity to reduce the viral titer than tripeptidomimetic inhibitors, which is considered the poor membrane permeability of tetrapeptidomimetic inhibitors. Among all of inhibitors, the tripeptidomimetic inhibitor 10b exhibited the striking anti-viral activity against SARS-CoV-2 3CLPro and might be further developed into effective candidate.
The coronavirus, as the single strand positive RNA virus, is accessible to variation due to getting rid of double stranded shackles. In the last 20 years, there are three outbreaks of coronavirus related diseases (SARS, MERS and COVID-19). However, 3CLPro remains the conserved key sites and catalytic mechanism during the virus evolution. This study comprehensively reveals the protein-ligand complexes of SARS-CoV-2 3CLPro with peptidomimetic inhibitors, including the SAR of the peptidomimetic inhibitor and co-crystal structure of SARS-CoV-2 3CLPro with inhibitors. The dimer co-crystal structure may afford a potential allosteric site for SARS-CoV-2 3CLPro inhibitor design. We believe this work could provide bedrock to promote the development of antiviral agents against SARS-CoV-2 and even novel coronavirus in the future.
4. Materials and methods
General methods: All reagents were purchased from commercial suppliers and used as received. NMR spectra were recorded on a Bruker Ascend 400 in the indicated solvent. (400 MHz for 1H and 101 MHz for 13C) (Bruker, Karlsruhe, Germany) NMR spectrometer. Molecular mass was determined on a mass spectrometry (Shimadzu (China) Co., Ltd.). All tested compounds exhibited purities of >95% as analyzed by HPLC (Dionex UltiMate 3000, Germany).
4.1. Preparation of 10a-10l
The synthesis procedure of (S)-2-cinnamamido-N-((S)-1-oxo-3-((S)-2-oxopyrrolidin- 3-yl)propan-2-yl)pent-4-ynamide 10a was exhibited as an example to illustrate the general procedure to process the peptidomimetic inhibitors 10a-10l.
The SOCl2 (54.23 mL, 747.64 mmol) was added in drop-wise to a suspension of l-glutamic acid (50.00 g, 339.84 mmol) in anhydrous MeOH (500 mL) at 0 °C. After stirring for 30 min in the ice-bath, the reaction was heated to reflux status for 3 h. After cooling, the mixture was evaporated to remove the solvent and the redundant SOCl2. Then, the anhydrous THF was added to suspend the residue (1.0 L) and other reagents (ditertbutyl dicarbonate (111.25 g, 509.75 mmol) and triethylamine (51.58 g, 509.75 mmol)) were subsequently added into the reaction at ice-bath. Then, the reaction mixture was allowed to stir overnight at room temperature. Following removing the solvent of the mixture, the residue was dissolved in DCM (800 mL) and washed with H2O (400 mL × 2), saturated citric acid solution (400 mL × 2), saturated NaHCO3 solution (400 mL × 2) and brine (400 mL × 2). After the organic phase was concentrated, column chromatography (EtOAc: petroleum ether, 1: 5 v/v) was used to give the pure product as colorless oil dimethyl (tert-butoxycarbonyl)-L-glutamate compound 4 (92.06 g, 334.40 mmol, 98.40%). 1H NMR (400 MHz, CDCl3) δ: 5.44 (d, J = 8.1 Hz, 1H), 4.33 (dd, J = 12.6, 7.5 Hz, 1H), 3.75 (s, 3H), 3.68 (s, 3H), 2.51–2.34 (m, 2H), 2.24–2.13 (m, 1H), 1.97 (td, J = 14.7, 8.2 Hz, 1H), 1.44 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 173.02, 172.58, 155.32, 79.64, 52.73, 52.18, 51.56, 29.92, 28.11, 27.42.
To a solution of 4 (20.0 g, 72.65 mmol) dissolved in anhydrous THF (500 mL), lithium hexamethyldisilazide/THF (159.83 mL, 1 mol/L, 159.83 mmol) was added in drop-wise under argon atmosphere at −78 °C. After a further 2 h of stirring at −78 °C, bromoacetonitrile (10.71 g, 79.91 mmol) was dissolved in anhydrous THF and the solution was added in drop-wise to the reaction mixture over a period of 2 h at −78 °C under argon atmosphere. Following an additional 2 h at −78 °C under the argon atmosphere, 20 mL pre-cooled methanol and 10 mL pre-cooled acetic acid were added to the reaction for quenching the reaction. After a further 10 min of stirring at −78 °C, the reaction was allowed to stir at 25 °C overnight. After removing the insoluble salt by filtration, the filtrate was evaporated and the obtained residue was purified by column chromatography (EtOAc: Petroleum ether, 1: 5 v/v) to give the pure product as yellow oil dimethyl (2S,4R)-2-((tert-butoxycarbonyl)amino)-4-(cyanomethyl) pentanedioate 5a (13.50g, 42.86 mmol, 59.12%). 1H NMR (400 MHz, CDCl3) δ: 5.34 (d, J = 8.8 Hz, 1H), 4.27 (dd, J = 14.2, 7.8 Hz, 1H), 3.70–3.58 (m, 6H), 2.83–2.72 (m, 1H), 2.70–2.57 (m, 2H), 2.11–1.99 (m, 2H), 1.33 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 172.49, 172.01, 155.55, 117.24, 80.19, 52.55, 52.52, 50.98, 38.16, 33.43, 28.11, 18.83.
The similar procedure with compound 5a was executed to obtain compound yellow oil dimethyl (2S,4S)-2-((tert-butoxycarbonyl)amino)-4-(2-cyanoethyl)pentanedioate 5b via the replacement of 3-bromopropionitrile into 3-Bromopropionitrile. 1H NMR (400 MHz, CDCl3) δ: 5.11 (d, J = 8.0 Hz, 1H), 4.48–4.31 (m, 1H), 3.74 (s, 3H), 3.72 (s, 3H), 2.70–2.58 (m, 1H), 2.48–2.35 (m, 2H), 2.13–1.92 (m, 4H), 1.45 (s, 9H). 13C NMR (100 MHz, CDCl3) δ: 174.39, 172.34, 155.38, 118.71, 80.28, 52.54, 52.16, 51.56, 40.79, 34.37, 28.24, 27.30, 15.12. yield 57.50%.
To a solution of 5a (10 g, 31.81 mmol) dissolved into anhydrous MeOH (400 mL), CoCl2·6H2O (4.54 g, 19.09 mmol) was added at −10 °C. Subsequently, NaBH4 (7.22 g, 31.81 mmol) was carefully added portion-wise at 0 °C. Following additional 48 h of stirring at 0 °C, saturated ammonium chloride solution (50 mL) was added to the reaction for quenching the reaction. Then, the mixture was filtered to remove insoluble substances. After removing the solvent, the residue was extracted with DCM (100 mL × 3) and further purified by column chromatography (EtOAc: petroleum ether, 2.5:1 v/v) to give the pure yellow oil methyl (S)-2-((tert-butoxycarbonyl)amino)-3-((S)-2-oxopyrrolidin-3-yl)propanoate 6a (4.22 g, 14.73 mmol, 46.30%) 1H NMR (400 MHz, CDCl3) δ: 7.56 (s, 1H), 5.99 (d, J = 8.1 Hz, 1H),4.37–4.24 (m, 1H), 3.74 (s, 3H), 3.42–3.25 (m, 2H), 2.56–2.38 (m, 2H), 2.22–2.07 (m, 1H), 1.93–1.75 (m, 2H), 1.44 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 180.04, 173.01, 155.79, 79.72, 52.32, 52.25, 40.46, 38.28, 33.91, 28.24, 27.96.
The similar operation was applied to obtain methyl (S)-2-((tert-butoxycarbonyl)amino)-3- ((S)-2-oxopiperidin-3-yl)propanoate 6b as yellow oil. 1H NMR (400 MHz, CDCl3) δ: 7.24 (s, 1H), 5.94 (d, J = 8.1 Hz, 1H), 4.29 (t, J = 7.8 Hz, 1H), 3.72 (s, 3H), 3.37–3.16 (m, 2H), 2.44–2.22 (m, 2H), 2.18–1.99 (m, 1H), 1.96–1.79 (m, 2H), 1.78–1.63 (m, 1H), 1.61–1.50 (m, 1H), 1.44 (s, 9H). 13C NMR (100 MHz, CDCl3) δ174.67, 173.23, 155.91, 79.51, 52.12, 51.68, 42.05, 37.85, 34.01, 28.21, 26.42, 21.43.
To a solution of 6a (1.0 g, 3.49 mmol) dissolved in anhydrous DCM (50 mL), CF3COOH (2.5 mL) was added slowly at 0 °C. Subsequently, the reaction mixture was allowed to continuously stir at room temperature for 3 h and concentrated to remove the redundant trifluoroacetic acid. Then, the triethylamine was added to the solution of the residue dissolved in DCM (60 mL) to adjust the pH value of the solution to 7.0. Subsequently, the L-2-((tert-butoxycarbonyl)amino)pent-4-ynoic acid (743.7 mg, 3.49 mmol), EDCI (803.4 mg, 4.19 mmol) and HOBt (566.3 mg, 4.19 mmol) were sequentially added. Following TEA (1.94 mL, 13.97 mmol) was added in drop-wise, the reaction mixture was stirred at ambient temperature overnight. Followed by washing with H2O (50 mL × 2), saturated citric acid solution (50 mL × 2), saturated NaHCO3 solution (50 mL × 2) and saturated brine (50 mL × 2), the organic phase was purified by column chromatography (DCM: MeOH, 100: 1 to 60: 1v/v) to afford the pure product methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)pent-4-ynamido)-3-((S)-2-oxopyrrolidin-3-yl)propanoate 7a (850.0 mg, 2.13 mmol, 61%) as light yellow foam. 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 7.9 Hz, 1H), 7.02 (s, 1H), 5.63 (d, J = 8.4 Hz, 1H), 4.65–4.51 (m, 1H), 4.43 (d, J = 8.0 Hz, 1H), 3.73 (s, 3H), 3.39–3.26 (m, 2H), 2.82–2.62 (m, 2H), 2.55–2.34 (m, 2H), 2.25 (d, J = 13.2 Hz, 1H), 2.09 (s, 1H), 1.91–1.77 (m, 2H), 1.46 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 179.89, 172.04, 170.68, 155.24, 79.67, 79.46, 71.45, 52.78, 52.37, 50.92, 40.43, 38.17, 33.47, 28.24, 27.91, 22.91.
The process to synthesize 7b-7l was similar to the 7a.
Compound 7b (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)-4-methylpentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.94 (d, J = 6.7 Hz, 1H), 7.30 (s, 1H), 5.41 (d, J = 8.3 Hz, 1H), 4.60–4.42 (m, 1H), 4.36–4.21 (m, 1H), 3.72 (s, 3H), 3.46–3.23 (m, 2H), 2.58–2.31 (m, 2H), 2.30–2.14 (m, 1H), 1.92–1.78 (m, 2H), 1.77–1.69 (m, 1H), 1.68–1.59 (m, 1H), 1.54–1.48 (m, 1H), 1.42 (s, 9H), 1.06–0.82 (m, 6H); 13C NMR (100 MHz, CDCl3) δ: 179.91, 173.40, 172.25, 155.61, 79.54, 52.82, 52.24, 50.89, 42.06, 40.44, 38.26, 33.05, 28.23, 27.90, 24.55, 22.81, 22.07.
Compound 7c (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)hexanamido)-3-((S)-2- oxopyrrolidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.95 (d, J = 7.8 Hz, 1H), 7.07 (s, 1H), 5.59 (d, J = 8.8 Hz, 1H), 4.61–4.52 (m, 1H), 4.45 (d, J = 8.1 Hz, 1H), 3.73 (s, 3H), 3.51–3.36 (m, 2H), 2.56–2.39 (m, 1H), 2.45–2.39 (s, 1H), 2.16–2.07 (m, 1H), 1.94–1.81 (m, 2H), 1.80–1.72 (m, 1H), 1.69–1.60 (m, 1H), 1.43 (s, 9H), 1.38–1.29 (d, J = 4.7 Hz, 4H), 0.89 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 178.31, 172.85, 172.41, 155.68, 79.69, 54.31, 52.26, 50.44, 42.53, 38.90, 33.61, 33.46, 28.38, 27.21, 26.58, 22.16, 13.98.
Compound 7d (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)hexanamido)-3-((S)-2- oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, J = 7.1 Hz, 1H), 6.69 (s, 1H), 5.27 (d, J = 8.3 Hz, 1H), 4.57–4.50 (d, J = 7.2 Hz, 1H), 4.25 (dd, J = 13.5, 7.3 Hz, 1H), 3.71 (s, 3H), 3.37–3.24 (m, 2H), 2.45–2.34 (m, 1H), 2.33–2.27 (s, 1H), 2.12–2.01 (m, 1H), 1.91–1.83 (m, 2H), 1.83–1.74 (m, 1H), 1.74–1.67 (m, 1H), 1.67–1.57 (m, 1H), 1.57–1.48 (m, 1H), 1.43 (s, 9H), 1.38–1.29 (d, J = 4.7 Hz, 4H), 0.89 (t, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 174.62, 172.90, 172.42, 155.55, 79.57, 54.26, 52.24, 50.40, 42.18, 37.90, 33.26, 33.09, 28.32, 27.37, 26.53, 22.46, 21.59, 13.96.
Compound 7e (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)butanamido)-3-((S)-2- oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.93 (d, J = 6.8 Hz, 1H), 6.82 (s, 1H), 5.41 (d, J = 7.2 Hz, 1H), 4.63–4.48 (m, 1H), 4.38–4.27 (m, 1H), 3.71 (s, 3H), 3.37–3.24 (m, 2H), 2.47–2.27 (m, 2H), 2.13–2.02 (m, 1H), 1.96–1.82 (m, 2H), 1.79–1.65 (m, 1H), 1.59–1.50 (m, 1H), 1.43 (s, 9H), 1.38 (d, J = 7.0 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 174.65, 173.49, 172.48, 155.30, 79.57, 52.28, 50.29, 49.85, 42.18, 37.83, 33.41, 28.32, 26.42, 21.49, 19.22.
Compound 7f (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)-3-methylbutanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.99 (d, J = 6.7 Hz, 1H), 6.86 (s, 1H), 5.32 (d, J = 8.0 Hz, 1H), 4.51 (m, 1H), 4.14 (dd, J = 8.5, 6.2 Hz, 1H), 3.70 (s, 3H), 3.33–3.26 (m, 2H), 2.40 (ddd, J = 13.8, 11.9, 4.8 Hz, 1H), 2.31 (s, 1H), 2.08 (ddd, J = 15.9, 10.9, 4.9 Hz, 2H), 1.93–1.81 (m, 2H), 1.76–1.64 (m, 1H), 1.60–1.49 (m, 1H), 1.43 (s, 9H), 0.99 (d, J = 6.7 Hz, 3H), 0.94 (d, J = 6.7 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 174.66, 172.43, 172.21, 155.86, 79.43, 59.26, 52.16, 50.56, 42.11, 37.95, 33.11, 31.75, 28.31, 26.55, 21.57, 19.03, 17.73.
Compound 7g (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.90 (d, J = 7.2 Hz, 1H), 6.85 (s, 1H), 5.21 (d, J = 8.6 Hz, 1H), 4.54 (t, J = 7.3 Hz, 1H), 4.31 (dd, J = 14.2, 8.5 Hz, 1H), 3.71 (s, 3H), 3.32–3.22 (m, 2H), 2.47–2.26 (m, 2H), 2.11–2.01 (m, 1H), 1.90–1.82 (m, 2H), 1.79–1.68 (m, 2H), 1.67–1.60 (m, 1H), 1.57–1.48 (m, 2H), 1.43 (s, 9H), 0.95 (dd, J = 6.3, 3.9 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ: 174.54, 173.38, 172.50, 155.57, 79.60, 52.82, 52.24, 50.20, 42.25, 42.11, 37.77, 33.19, 28.31, 26.33, 24.61, 22.87, 22.17, 21.59.
Compound 7h (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)-3,3-dimethylbutanamido)- 3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.13 (d, J = 6.6 Hz, 1H), 7.34 (d, J = 3.8 Hz, 1H), 5.41 (d, J = 9.8 Hz, 1H), 4.51–4.40 (m, 1H), 4.19 (d, J = 9.8 Hz, 1H), 3.71 (s, 3H), 3.28 (m, 2H), 2.47 (td, J = 14.0, 4.2 Hz, 1H), 2.25 (dd, J = 9.1, 4.7 Hz, 1H), 2.08–1.97 (m, 1H), 1.93–1.76 (m, 2H), 1.66 (dd, J = 23.9, 12.9 Hz, 1H), 1.42 (s, 9H), 1.03 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 174.49, 172.50, 171.61, 155.91, 79.29, 61.46, 52.03, 50.38, 41.86, 37.77, 34.82, 32.57, 28.27, 26.41, 26.19, 21.75.
Compound 7i (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)-3- cyclopropylpropanamido) -3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.91 (d, J = 7.3 Hz, 1H), 7.11 (s, 1H), 5.45 (d, J = 7.9 Hz, 1H), 4.61–4.38 (m, 1H), 4.32–4.05 (m, 1H), 3.59 (s, 3H), 3.31–3.07 (m, 2H), 2.39–2.16 (m, 2H), 2.00–1.92 (m, 1H), 1.85–1.69 (m, 2H), 1.66–1.55 (m, 2H), 1.53–1.39 (m, 2H), 1.33 (s, 9H), 0.79–0.56 (m, 1H), 0.36 (d, J = 7.6 Hz, 2H), 0.05–0.04 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 174.59, 172.75, 172.46, 155.41, 79.32, 54.64, 52.13, 50.08, 42.02, 37.99, 37.62, 33.34, 28.28, 26.22, 21.43, 7.09, 4.30, 4.17.
Compound 7j (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)-2-phenylacetamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.15 (d, J = 6.7 Hz, 1H), 7.42 (d, J = 6.9 Hz, 2H), 7.33–7.25 (m, 3H), 7.06 (s, 1H), 6.04 (d, J = 7.3 Hz, 1H), 5.42 (d, J = 7.6 Hz, 1H), 4.50–4.50 (m, 1H), 3.53 (s, 3H), 3.37–3.09 (m, 2H), 2.47–2.29 (m, 2H), 2.08–1.99 (m, 1H), 1.91–1.76 (m, 2H), 1.72–1.60 (m, 1H), 1.55–1.46 (m, 1H), 1.41 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 174.61, 172.12, 170.89, 155.17, 138.27, 128.54 (double peak height), 127.89, 127.09, 126.93, 79.69, 57.77, 53.52, 50.51, 42.07, 37.73, 33.27, 28.30, 26.30, 21.35.
Compound 7k (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)pent-4-enamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.97 (d, J = 6.9 Hz, 1H), 6.90 (s, 1H), 5.95–5.71 (m, 1H), 5.36 (d, J = 7.9 Hz, 1H), 5.20–5.07 (m, 2H), 4.65–4.51 (m, 1H), 4.35 (dd, J = 7.8, 3.8 Hz, 1H), 3.71 (s, 3H), 3.43–3.21 (m, 2H), 2.61–2.52 (m, 1H), 2.51–2.41 (m, 1H), 2.41–2.26 (m, 2H), 2.14–2.02 (m, 1H), 1.94–1.82 (m, 2H), 1.77–1.65 (m, 1H), 1.61–1.49 (m, 1H), 1.43 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 174.64, 172.40, 172.07, 155.41, 133.10, 118.59, 79.64, 53.56, 52.25, 50.25, 42.16, 37.77, 37.50, 33.43, 28.29, 26.35, 21.45.
Compound 7l (methyl (S)-2-((S)-2-((tert-butoxycarbonyl)amino)pent-4-ynamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.83 (d, J = 6.9 Hz, 1H), 6.66 (s, 1H), 5.52 (d, J = 7.8 Hz, 1H), 4.60–4.49 (m, 1H), 4.39 (dd, J = 8.2, 4.9 Hz, 1H), 3.64 (s, 3H), 3.26–3.17 (m, 2H), 2.75–2.55 (m, 2H), 2.41–2.27 (m, 2H), 2.07–1.99 (m, 2H), 1.86–1.77 (m, 2H), 1.69–1.61 (m, 1H), 1.50–1.42 (m, 1H), 1.39 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 174.54, 172.25, 170.68, 155.23, 80.15, 79.49, 71.42, 52.72, 52.34, 50.24, 42.26, 37.75, 33.74, 28.29, 26.37, 23.11, 21.50.
The synthesis of 8a-8l is referred to the procedure of preparation of 7a-7l.
Compound 8a (methyl (S)-2-((S)-2-cinnamamidopent-4-ynamido)-3-((S)-2-oxopyrrolidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 7.3 Hz, 1H), 7.63 (d, J = 15.7 Hz, 1H), 7.50–7.44 (m, 2H), 7.37–7.30 (m, 3H), 7.20 (d, J = 8.4 Hz, 1H), 6.88 (s, 1H), 6.55 (d, J = 15.7 Hz, 1H), 5.04–4.98 (m, 1H), 4.57–4.50 (m, 1H), 3.71 (s, 3H), 3.39–3.24 (m, 2H), 2.89–2.74 (m, 2H), 2.52–2.43 (m, 1H), 2.40–2.31 (m, 1H), 2.30–2.20 (m, 1H), 2.10 (s, 1H), 1.94–1.73 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 179.88, 171.99, 170.62, 165.88, 141.74, 134.64, 129.84, 128.82, 127.91, 120.28, 79.53, 71.42, 52.46, 51.55, 51.41, 40.56, 38.43, 33.00, 28.14, 23.02.
Compound 8b (methyl (S)-2-((S)-2-cinnamamido-4-methylpentanamido)-3-((S)-2-oxopyrrolidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.43 (d, J = 6.8 Hz, 1H), 7.58 (d, J = 15.6 Hz, 1H), 7.46 (dd, J = 6.4, 2.6 Hz, 2H), 7.33 (dd, J = 8.9, 5.2 Hz, 4H), 7.19 (d, J = 9.1 Hz, 1H), 6.53 (d, J = 15.6 Hz, 1H), 5.02 (td, J = 8.9, 5.0 Hz, 1H), 4.46–4.37 (m, 1H), 3.70 (s, 3H), 3.43–3.24 (m, 2H), 2.45–2.25 (m, 3H), 1.89–1.70 (m, 4H), 1.70–1.58 (m, 1H), 0.98 (d, J = 4.0 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ: 179.47, 173.56, 172.15, 165.84, 141.26, 134.81, 129.69, 128.78, 127.85, 120.71, 52.31, 51.36, 51.34, 42.61, 40.55, 38.42, 32.74, 27.94, 24.72, 22.90, 22.20.
Compound 8c (methyl (S)-2-((S)-2-cinnamamidohexanamido)-3-((S)-2-oxopyrrolidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ 8.52 (d, J = 6.8 Hz, 1H), 7.78 (d, J = 8.4 Hz, 1H), 7.66–7.55 (m, 2H), 7.50–7.43 (m, 2H), 7.34–7.25 (m, 3H), 6.65 (d, J = 15.7 Hz, 1H), 5.02–4.94 (m, 1H), 4.51–4.43 (m, 1H), 3.68 (s, 3H), 3.38–3.21 (m, 2H), 2.51–2.24 (m, 3H), 1.97–1.68 (m, 4H), 1.48–1.30 (m, 4H), 0.89 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 179.59, 173.43, 172.15, 165.98, 140.95, 134.91, 129.57, 128.73, 127.84, 120.97, 52.92, 52.24, 51.29, 40.55, 38.40, 33.26, 32.75, 27.85, 27.59, 22.46, 13.98.
Compound 8d (methyl (S)-2-((S)-2-cinnamamidohexanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.41 (d, J = 7.0 Hz, 1H), 7.59 (d, J = 15.6 Hz, 1H), 7.51–7.43 (m, 2H), 7.33 (dd, J = 5.0, 1.8 Hz, 3H), 7.06 (d, J = 8.5 Hz, 1H), 7.01 (s, 1H), 6.53 (d, J = 15.6 Hz, 1H), 4.87 (dd, J = 14.1, 7.6 Hz, 1H), 4.55–4.46 (m, 1H), 3.70 (s, 3H), 3.35–3.22 (m, 2H), 2.53–2.41 (m, 1H), 2.39–2.27 (m, 1H), 2.07–1.98 (m, 1H), 1.96–1.79 (m, 3H), 1.78–1.64 (m, 2H), 1.57–1.44 (m, 1H), 1.44–1.30 (m, 4H), 0.89 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 174.49, 172.91, 172.40, 165.64, 141.08, 134.84, 129.64, 128.78, 127.83, 120.78, 52.92, 52.25, 50.65, 42.19, 37.88, 33.25, 33.12, 27.38, 26.47, 22.49, 21.53, 13.98.
Compound 8e (methyl (S)-2-((S)-2-cinnamamidopropanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.43 (d, J = 7.2 Hz, 1H), 7.58 (d, J = 15.7 Hz, 1H), 7.53–7.40 (m, 3H), 7.37–7.26 (m, 3H), 7.23 (s, 1H), 6.56 (d, J = 15.7 Hz, 1H), 4.91 (d, J = 7.0 Hz, 1H), 4.54 (ddd, J = 11.4, 7.2, 3.9 Hz, 1H), 3.68 (s, 3H), 3.31–3.11 (m, 2H), 2.50–2.33 (m, 2H), 2.04–1.96 (m, 1H), 1.96–1.86 (m, 1H), 1.85–1.77 (m, 1H), 1.73–1.60 (m, 1H), 1.54–1.51 (m, 1H), 1.49 (d, J = 8.4 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ: 174.64, 173.68, 172.46, 165.59, 140.87, 134.89, 129.55, 128.73, 127.80, 120.88, 52.23, 50.50, 48.74, 42.10, 37.77, 33.18, 26.24, 21.40, 19.10.
Compound 8f (methyl (S)-2-((S)-2-cinnamamido-3-methylbutanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate):1H NMR (400 MHz, CDCl3) δ: 8.47 (d, J = 6.7 Hz, 1H), 7.60 (d, J = 15.6 Hz, 1H), 7.48 (dd, J = 6.5, 3.0 Hz, 2H), 7.33 (dd, J = 5.0, 1.8 Hz, 3H), 7.11 (s, 1H), 7.05 (d, J = 9.1 Hz, 1H), 6.57 (d, J = 15.6 Hz, 1H), 4.76 (dd, J = 9.1, 6.7 Hz, 1H), 4.49 (ddd, J = 10.7, 6.7, 3.6 Hz, 1H), 3.70 (s, 3H), 3.28 (dd, J = 14.7, 5.0 Hz, 2H), 2.54–2.42 (m, 1H), 2.36–2.26 (m, 1H), 2.19 (m, 1H), 2.08–1.95 (m, 1H), 1.85 (ddd, J = 14.3, 9.0, 4.5 Hz, 2H), 1.74–1.60 (m, 1H), 1.54–1.42 (m, 1H), 1.05 (d, J = 6.7 Hz, 3H), 1.01 (d, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 174.49, 172.45, 172.24, 165.89, 141.09, 134.89, 129.61, 128.77, 127.82, 120.90, 57.99, 52.17, 50.78, 42.15, 37.91, 33.05, 32.16, 26.49, 21.52, 19.08, 18.21.
Compound 8g (methyl (S)-2-((S)-2-cinnamamido-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.46 (d, J = 6.8 Hz, 1H), 7.58 (d, J = 15.6 Hz, 1H), 7.51–7.43 (m, 2H), 7.39–7.30 (m, 3H), 7.19 (s, 1H), 7.10 (d, J = 8.8 Hz, 1H), 6.53 (d, J = 15.6 Hz, 1H), 5.01 (td, J = 8.8, 5.1 Hz, 1H), 4.53–4.44 (m, 1H), 3.69 (s, 3H), 3.34–3.25 (m, 2H), 2.60–2.48 (m, 1H), 2.38–2.25 (m, 1H), 2.05–1.96 (m, 1H), 1.89–1.79 (m, 2H), 1.79–1.71 (m, 2H), 1.71–1.57 (m, 2H), 1.53–1.39 (m, 1H), 0.97 (d, J = 3.7 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ: 174.18, 173.47, 172.48, 165.70, 141.13, 134.87, 129.62, 128.76, 127.84, 120.79, 52.25, 51.37, 50.49, 42.66, 42.15, 37.74, 32.94, 26.30, 24.71, 22.90, 22.26, 21.63.
Compound 8h (methyl (S)-2-((S)-2-cinnamamido-3,3-dimethylbutanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.52 (d, J = 6.3 Hz, 1H), 7.60 (d, J = 15.6 Hz, 1H), 7.48 (dd, J = 6.5, 2.8 Hz, 2H), 7.37–7.29 (m, 4H), 6.85 (d, J = 9.7 Hz, 1H), 6.55 (d, J = 15.6 Hz, 1H), 4.86 (d, J = 9.7 Hz, 1H), 4.43 (ddd, J = 12.1, 6.1, 3.2 Hz, 1H), 3.73 (s, 3H), 3.35–3.24 (m, 2H), 2.52 (m, 1H), 2.34–2.21 (m, 1H), 2.04–1.96 (m, 1H), 1.90–1.77 (m, 2H), 1.75–1.58 (m, 1H), 1.46 (m, 1H), 1.09 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 174.27, 172.56, 171.43, 165.73, 141.18, 134.80, 129.66, 128.79, 127.80, 120.90, 59.96, 52.10, 50.81, 42.12, 37.87, 35.47, 32.95, 26.62, 26.47, 21.65.
Compound 8i (methyl (S)-2-((S)-2-cinnamamido-3-cyclopropylpropanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.28 (d, J = 7.0 Hz, 1H), 7.46 (d, J = 15.7 Hz, 1H), 7.41–7.28 (m, 2H), 7.24–7.15 (m, 3H), 7.04 (d, J = 8.4 Hz, 1H), 6.86 (s, 1H), 6.41 (d, J = 15.7 Hz, 1H), 4.82 (dd, J = 14.8, 6.7 Hz, 1H), 4.44–4.32 (m, 1H), 3.56 (s, 3H), 3.33–3.05 (m, 2H), 2.37–2.28 (m, 1H), 2.27–2.17 (m, 1H), 1.97–1.83 (m, 1H), 1.82–1.63 (m, 3H), 1.63–1.50 (m, 2H), 1.44–1.27 (m, 1H), 0.78–0.67 (m, 1H), 0.34 (d, J = 8.0 Hz, 2H), 0.06 to −0.06 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 174.49, 172.72, 172.43, 165.58, 141.03, 134.86, 129.62, 128.77, 127.83, 120.82, 53.47, 52.26, 50.68, 42.21, 38.23, 37.90, 33.28, 26.52, 21.49, 7.26, 4.48, 4.30.
Compound 8j (methyl (S)-2-((S)-2-cinnamamido-2-phenylacetamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.61 (d, J = 7.1 Hz, 1H), 7.77 (d, J = 8.0 Hz, 1H), 7.56 (dd, J = 14.0, 1H), 7.48 (d, J = 7.5 Hz, 2H), 7.44–7.37 (m, 2H), 7.32–7.18 (m, 8H), 6.56 (d, J = 15.7 Hz, 1H), 6.06 (d, J = 8.0 Hz, 1H), 4.63–4.50 (m, 1H), 3.51 (s, 3H), 3.27–3.05 (m, 2H), 2.52–2.33 (m, 2H), 2.09–1.95 (m, 1H), 1.90–1.75 (m, 2H), 1.69–1.58 (m, 1H), 1.50–1.39 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 174.47, 172.14, 171.09, 165.40, 141.35, 138.04, 134.86, 129.64, 128.77, 128.60, 127.89, 127.29, 127.08, 120.64, 56.40, 52.14, 50.78, 42.13, 37.79, 33.27, 26.27, 21.35.
Compound 8k (methyl (S)-2-((S)-2-cinnamamidopent-4-enamido)-3-((S)-2oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.43 (d, J = 7.0 Hz, 1H), 7.59 (d, J = 15.7 Hz, 1H), 7.48–7.44 (m, 2H), 7.38–7.30 (m, 3H), 7.12–6.94 (m, 2H), 6.52 (d, J = 15.7 Hz, 1H), 5.90–5.79 (m, 1H), 5.13 (t, J = 13.9 Hz, 2H), 4.96 (dd, J = 13.6, 7.0 Hz, 1H), 4.58–4.47 (m, 1H), 3.70 (s, 3H), 3.38–3.18 (m, 2H), 2.73–2.64 (m, 1H), 2.61–2.53 (m, 1H), 2.51–2.40 (m, 1H), 2.39–2.31 (m, 1H), 2.06–1.98 (m, 1H), 1.95–1.80 (m, 2H), 1.77–1.62 (m, 1H), 1.59–1.44 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 174.54, 172.37, 172.04, 165.62, 141.18, 134.79, 133.07, 129.66, 128.77, 127.84, 120.65, 118.65, 52.34, 52.28, 50.65, 42.21, 37.92, 37.65, 33.23, 26.44, 21.48.
Compound 8l (methyl (S)-2-((S)-2-cinnamamidopent-4-ynamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.41 (d, J = 7.2 Hz, 1H), 7.63 (d, J = 15.6 Hz, 1H), 7.49–7.45 (d, J = 4.2 Hz, 2H), 7.36–7.31 (m, 3H), 7.12 (d, J = 8.2 Hz, 1H), 6.76 (s, 1H), 6.54 (d, J = 15.6 Hz, 1H), 5.01 (dd, J = 13.1, 5.9 Hz, 1H), 4.57 (dd, J = 11.1, 7.1 Hz, 1H), 3.70 (s, 3H), 3.37–3.19 (m, 2H), 2.94–2.74 (m, 2H), 2.46–2.33 (m, 2H), 2.15–2.00 (m, 2H), 1.96–1.80 (m, 2H), 1.78–1.62 (m, 1H), 1.58–1.48 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 174.65, 172.23, 170.57, 165.75, 141.68, 134.67, 129.80, 128.80, 127.90, 120.30, 79.61, 71.35, 52.38, 51.35, 50.82, 42.29, 38.02, 33.47, 26.58, 23.14, 21.45.
To a solution of 8a (370 mg, 0.90 mmol) dissolved into anhydrous MeOH (30.0 mL), NaBH4 (0.51 g, 13.53 mmol) was added at 0 °C. Then, the reaction mixture was stirred at ambient temperature for 2 h. Saturated NH4Cl solution (20 mL) was added to the mixture for quench the reaction. Following evaporating the solvent, EA (60 mL × 2) was added to extract the aqueous components. The organic phase was washed with H2O (30 mL × 2), saturated brine (30 mL × 2), and concentrated. Finally, the residue was purified by column chromatography (DCM: MeOH, 25:1 v/v) to afford the pure product as white solid (S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)pent-4-ynamide 9a (276 mg, yield 80%).Compound 9a: 1H NMR (400 MHz, MeOD) δ 7.60–7.51 (m, 3H), 7.42–7.34 (m, 3H), 6.73 (d, J = 15.8 Hz, 1H), 4.62 (t, J = 6.7 Hz, 1H), 4.06–3.97 (m, 1H), 3.63–3.47 (m, 2H), 3.32–3.23 (m, 2H), 2.75–2.71 (m, 2H), 2.63–2.49 (m, 1H), 2.43 (t, J = 2.6 Hz, 1H), 2.39–2.30 (m, 1H), 2.03–1.94 (m, 1H), 1.82–1.71 (m, 1H), 1.59–1.51 (m, 1H).13C NMR (101 MHz, MeOD) δ 181.33, 171.23, 167.13, 141.11, 134.80, 129.61, 128.60, 127.55, 120.03, 79.09, 71.15, 64.09, 52.83, 49.52, 40.15, 38.19, 32.03, 27.57, 21.45.
The procedure to obtain 9b-9l is similar to the procedure of preparation of 9a.
Compound 9b ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.95 (d, J = 6.8 Hz, 1H), 7.49 (d, J = 15.6 Hz, 1H), 7.35 (s, 2H), 7.30–7.18 (m, 4H), 6.88 (s, 1H), 6.47 (d, J = 15.6 Hz, 1H), 4.75–4.57 (m, 1H), 4.23–4.11 (m, 1H), 3.96 (dd, J = 13.3, 12.9 Hz, 1H), 3.25–3.04 (m, 2H), 3.05–2.88 (m, 1H), 2.43–2.28 (m, 1H), 2.28–2.14 (m, 1H), 2.08–1.86 (m, 1H), 1.78–1.53 (m, 4H), 1.53–1.41 (m, 1H), 0.87 (s, 6H); 13C NMR (101 MHz, MeOD) δ: 181.03, 173.62, 166.17, 141.22, 134.74, 129.72, 128.80, 127.83, 120.67, 65.47, 52.26, 50.41, 42.16, 40.64, 38.31, 32.22, 28.32, 24.92, 23.06, 22.07.
Compound 9c ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)hexanamide): 1H NMR (400 MHz, MeOD) δ 7.59–7.46 (m, 3H), 7.43–7.30 (m, 3H), 6.74 (d, J = 15.8 Hz, 1H), 4.47–4.41 (m, 1H), 4.13–3.95 (m, 1H), 3.63–3.49 (m, 2H), 3.35–3.20 (m, 2H), 2.62–2.53 (m, 1H), 2.38–2.28 (m, 1H), 2.06–1.97 (m, 1H), 1.93–1.82 (m, 1H), 1.81–1.68 (m, 2H), 1.61–1.52 (m, 1H), 1.49–1.32 (m, 4H), 0.94 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, MeOD) δ 181.31, 173.55, 173.47, 167.10, 140.67, 134.86, 129.53, 128.60, 127.55, 120.27, 64.19, 54.12, 49.31, 40.17, 38.18, 32.13, 31.75, 27.90, 27.60, 22.14, 13.03.
Compound 9d ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)hexanamide): 1H NMR (400 MHz, MeOD) δ: 7.58–7.51 (m, 3H), 7.39–7.34 (m, 3H), 6.73 (d, J = 15.8 Hz, 1H), 4.43 (dd, J = 8.5, 5.7 Hz, 1H), 4.10–4.02 (m, 1H), 3.59–3.47 (m, 2H), 3.24–3.19 (m, 2H), 2.45–2.33 (m, 1H), 2.19–2.09 (m, 1H), 2.09–1.99 (m, 1H), 1.91–1.81 (m, 1H), 1.80–1.59 (m, 4H), 1.52–1.45 (m, 1H), 1.44–1.33 (m, 4H), 0.93 (t, J = 6.9 Hz, 3H).; 13C NMR (101 MHz, MeOD) δ: 176.00, 173.47, 167.07, 140.69, 134.90, 129.52, 128.60, 127.52, 120.33, 64.30, 54.09, 48.50, 41.62, 37.28, 32.51, 31.71, 27.86, 25.71, 22.13, 20.70, 13.01.
Compound 9e (N-((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-1-oxopropan-2-yl)cinnamamide): 1H NMR (400 MHz, MeOD) δ: 7.62–7.50 (m, 3H), 7.44–7.31 (m, 3H), 6.69 (d, J = 15.8 Hz, 1H), 4.44 (q, J = 7.1 Hz, 1H), 4.06–3.97 (m, 1H), 3.55–3.46 (m, 2H), 3.27–3.17 (m, 2H), 2.44–2.31 (m, 1H), 2.16–2.00 (m, 2H), 1.85–1.77 (m, 1H), 1.75–1.66 (m, 1H), 1.66–1.57 (m, 1H), 1.53–1.45 (m, 1H), 1.42 (d, J = 7.1 Hz, 3H).; 13C NMR (101 MHz, MeOD) δ: 176.05, 174.00, 166.90, 140.68, 134.88, 129.51, 128.57, 127.48, 120.23, 64.22, 49.52, 48.40, 41.62, 37.26, 32.50, 25.71, 20.66, 16.88.
Compound 9f ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-3-methylbutanamide): 1H NMR (400 MHz, MeOD) δ: 7.63–7.48 (m, 3H), 7.44–7.29 (m, 3H), 6.77 (d, J = 15.8 Hz, 1H), 4.27 (d, J = 7.4 Hz, 1H), 4.10–3.99 (m, 1H), 3.58–3.44 (m, 2H), 3.27–3.15 (m, 2H), 2.44–2.32 (m, 1H), 2.22–2.06 (m, 2H), 2.06–1.98 (m, 1H), 1.87–1.73 (m, 1H), 1.73–1.58 (m, 2H), 1.54–1.40 (m, 1H), 1.01 (d, J = 6.5 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 176.01, 172.49, 167.21, 140.78, 134.93, 129.48, 128.57, 127.48, 120.29, 64.27, 59.56, 48.40, 41.58, 37.30, 32.32, 30.29, 25.66, 20.70, 18.42, 17.45.
Compound 9g ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 8.01 (d, J = 8.7 Hz, 1H), 7.61–7.49 (m, 3H), 7.44–7.32 (m, 3H), 6.70 (d, J = 15.8 Hz, 1H), 4.49 (t, J = 7.4 Hz, 1H), 4.10–3.95 (m, 1H), 3.56–3.44 (m, 2H), 3.27–3.16 (m, 2H), 2.44–2.30 (m, 1H), 2.18–1.96 (m, 2H), 1.86–1.76 (m, 1H), 1.75–1.58 (m, 5H), 1.52–1.43 (m, 1H), 0.97 (dd, J = 11.7, 6.4 Hz, 6H).; 13C NMR (101 MHz, MeOD) δ: 176.05, 173.83, 167.12, 140.72, 134.88, 129.51, 128.58, 127.49, 120.20, 64.23, 52.40, 48.45, 41.60, 40.75, 37.25, 32.43, 25.68, 24.64, 22.07, 20.67, 20.65.
Compound 9h ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-3,3-dimethylbutanamide): 1H NMR (400 MHz, MeOD) δ: 7.61–7.49 (m, 3H), 7.42–7.31 (m, 3H), 6.86 (d, J = 15.8 Hz, 1H), 4.38 (s, 1H), 4.11–3.98 (m, 1H), 3.60–3.46 (m, 2H), 3.23–3.18 (m, 2H), 2.42–2.34 (m, 1H), 2.17–2.05 (m, 1H), 2.04–1.96 (m, 1H), 1.84–1.71 (m, 1H), 1.70–1.59 (m, 2H), 1.52–1.39 (m, 1H), 1.08 (s, 9H); 13C NMR (101 MHz, MeOD) δ: 176.00, 171.56, 171.47, 166.97, 140.80, 134.99, 129.46, 128.58, 127.52, 120.44, 64.29, 61.71, 61.66, 41.59, 37.30, 33.62, 32.33, 25.98, 25.70, 20.71.
Compound 9i (N-((S)-3-cyclopropyl-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-1-oxopropan-2-yl)cinnamamide): 1H NMR (400 MHz, MeOD) δ: 7.47–7.33 (m, 3H), 7.29–7.14 (m, 3H), 6.55 (d, J = 15.8 Hz, 1H), 4.33 (dd, J = 7.8, 6.4 Hz, 1H), 3.91–3.83 (m, 1H), 3.34 (ddd, J = 16.9, 10.9, 5.5 Hz, 2H), 3.09–2.99 (m, 2H), 2.31–2.18 (m, 1H), 2.04–1.81 (m, 2H), 1.71–1.41 (m, 5H), 1.38–1.23 (m, 1H), 0.75–0.58 (m, 1H), 0.39–0.24 (m, 2H), 0.07–0.08 (m, 2H); 13C NMR (101 MHz, MeOD) δ: 173.66, 170.78, 164.59, 138.27, 132.47, 127.08, 126.15, 125.07, 117.87, 61.75, 52.14, 45.96, 39.19, 34.82, 34.40, 30.05, 23.28, 18.25, 4.87, 1.42, 1.08.
Compound 9j (N-((S)-2-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-2-oxo-1-phenylethyl)cinnamamide): 1H NMR (400 MHz, MeOD) δ 7.62–7.49 (m, 5H), 7.43–7.35 (m, 6H), 6.81 (d, J = 15.8 Hz, 1H), 5.56 (s, 1H), 4.05 (d, J = 5.6 Hz, 1H), 3.50 (dd, J = 10.9, 5.0 Hz, 1H), 3.39 (dd, J = 10.8, 6.3 Hz, 1H), 3.27 (dd, J = 10.4, 6.6 Hz, 2H), 2.45–2.38 (m, 1H), 2.18–2.07 (m, 2H), 1.83 (s, 1H), 1.75–1.65 (m, 2H), 1.57–1.49 (m, 1H). 13C NMR (101 MHz, MeOD) δ 176.09, 171.45, 166.80, 140.97, 137.29, 134.90, 129.51, 128.54, 128.44, 127.97, 127.52, 127.44, 120.11, 64.06, 58.15, 48.65, 41.61, 37.33, 32.51, 25.72, 20.64.
Compound 9k ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pent-4-enamide): 1H NMR (400 MHz, MeOD) δ: 7.69–7.48 (m, 3H), 7.48–7.31 (m, 3H), 6.71 (d, J = 15.8 Hz, 1H), 5.84 (ddt, J = 17.1, 10.1, 7.0 Hz, 1H), 5.24–5.07 (m, 2H), 4.49 (dd, J = 8.0, 5.9 Hz, 1H), 4.08–3.97 (m, 1H), 3.60–3.41 (m, 2H), 3.28–3.15 (m, 2H), 2.66–2.42 (m, 2H), 2.42–2.29 (m, 1H), 2.16–1.98 (m, 2H), 1.87–1.75 (m, 1H), 1.74–1.58 (m, 2H), 1.55–1.40 (m, 1H); 13C NMR (101 MHz, MeOD) δ: 176.03, 172.43, 167.05, 140.81, 134.86, 133.33, 129.53, 128.58, 127.49, 120.17, 117.29, 64.23, 53.60, 48.46, 41.61, 37.25, 36.16, 32.45, 25.68, 20.66.
Compound 9l ((S)-2-cinnamamido-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pent-4-ynamide): 1H NMR (400 MHz, MeOD) δ: 7.66–7.51 (m, 3H), 7.38 (d, J = 5.8 Hz, 3H), 6.74 (d, J = 15.8 Hz, 1H), 4.62 (t, J = 6.4 Hz, 1H), 4.13–3.97 (m, 1H), 3.53 (ddd, J = 16.8, 10.9, 5.5 Hz, 2H), 3.29–3.13 (m, 2H), 2.73 (d, J = 6.3 Hz, 2H), 2.48–2.30 (m, 2H), 2.21–1.96 (m, 2H), 1.85–1.76 (m, 1H), 1.74–1.56 (m, 2H), 1.56–1.45 (m, 1H); 13C NMR (101 MHz, MeOD) δ: 176.05, 171.22, 167.10, 141.07, 134.81, 129.62, 128.62, 127.56, 120.05, 79.09, 71.14, 64.19, 52.81, 48.65, 41.62, 37.24, 32.47, 25.69, 21.40, 20.66.
To a solution of 9a (184 mg, 0.48 mmol) in anhydrous DCM (30.0 mL), Dess-Martin reagent (306.2 mg, 0.72 mmol) was added at ice-bath. Then, the reaction mixture was allowed to stir at ambient temperature for 2 h. A solution of NaHCO3 and solid Na2S2O3 were added to quench the reaction. After 40 min of stirring, the reaction mixture was extracted by DCM (30.0 mL × 2). The organic phase was washed with brine (30 mL × 2), dried over Na2SO4, and concentrated, and the residue was purified by column chromatography (DCM: MeOH, 50:1 to 35:1 v/v) to afford the product as a white solid (S)-2-cinnamamido-N-((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl) propan-2-yl)pent-4-ynamide 10a (157 mg, yield 86%). Owing the existence of MeOH, the aldehyde prefers to form diastereoisomers hemiacetals. For obtaining the purity aldehyde as far as possible, abundant CCl4 and hexane were added into the eluent to form azeotropes. Then, the eluent was concentrated at 44 °C and give the residue which mainly contain aldehyde. Following the abundant hexane was added into the solution of residue dissolved into chloroform, the precipitation was filtered and gave the purity aldehyde. Compound 10a: 1H NMR (400 MHz, CDCl3) δ 9.55 (d, J = 14.3 Hz, 1H), 8.24 (d, J = 7.6 Hz, 1H), 7.63 (d, J = 15.6 Hz, 1H), 7.54–7.44 (m, 2H), 7.38–7.30 (m, 3H), 7.16 (t, J = 9.3 Hz, 1H), 6.71 (s, 1H), 6.57 (d, J = 15.7 Hz, 1H), 5.01–4.93 (m, 1H), 4.62–4.39 (m, 1H), 3.00–2.70 (m, 2H), 3.34–3.24 (m, 2H), 2.45–2.35 (m, 1H), 2.24–2.17 (m, 1H), 2.16–2.09 (m, 1H), 2.02–1.97 (d, J = 9.8 Hz, 1H), 1.93–1.79 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 199.92, 180.88, 171.18, 165.94, 141.85, 134.60, 130.97, 128.84, 127.92, 120.19, 79.38, 71.73, 57.89, 54.97, 41.27, 38.06, 29.68, 28.42, 23.85. HRMS (m/z): 382.1690 (M + H) +.
The similar procedure with the preparation of 10a were applied to obtain compound 10b-10l.
Compound 10b ((S)-2-cinnamamido-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl) propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.43 (s, 1H), 8.60 (d, J = 6.1 Hz, 1H), 7.51 (d, J = 15.6 Hz, 1H), 7.38 (dd, J = 6.2, 2.6 Hz, 2H), 7.32–7.21 (m, 3H), 7.03 (s, 1H), 6.97 (d, J = 8.8 Hz, 1H), 6.44 (d, J = 15.6 Hz, 1H), 4.95–4.82 (m, 1H), 4.28–4.17 (m, 1H), 3.35–3.14 (m, 2H), 2.49–2.32 (m, 1H), 2.32–2.17 (m, 2H), 2.12–1.97 (m, 1H), 1.81–1.71 (m, 1H), 1.70–1.64 (m, 2H), 1.63–1.54 (m, 1H), 0.91 (d, J = 5.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.73, 179.85, 174.02, 165.94, 141.46, 134.70, 129.79, 128.82, 127.85, 120.46, 57.65, 51.55, 42.57, 40.63, 38.14, 29.70, 28.30, 24.92, 22.94, 22.12. HRMS (m/z): 400.2160 (M + H) +
Compound 10c ((S)-2-cinnamamido-N-((S)-1-oxo-3-((S)-2-oxopyrrolidin-3-yl)propan-2-yl)hexanamide): 1H NMR (400 MHz, CDCl3) δ 9.52 (s, 1H), 8.61 (d, J = 6.2 Hz, 1H), 7.61 (s, 1H), 7.52–7.41 (m, 2H), 7.32 (d, J = 4.4 Hz, 3H), 7.06 (d, J = 8.7 Hz, 1H), 6.99 (s, 1H), 6.53 (d, J = 15.5 Hz, 1H), 4.87 (q, J = 7.5 Hz, 1H), 4.34 (p, J = 4.5 Hz, 1H), 3.32 (dq, J = 17.4, 9.3 Hz, 2H), 2.46 (p, J = 8.1 Hz, 1H), 2.33 (dt, J = 15.7, 7.8 Hz, 1H), 2.17–2.02 (m, 1H), 1.91 (td, J = 15.3, 14.8, 7.3 Hz, 2H), 1.76 (dq, J = 15.8, 8.4, 7.6 Hz, 2H), 1.37 (dp, J = 19.1, 6.8, 6.1 Hz, 4H), 0.88 (q, J = 9.2, 8.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 199.65, 179.90, 173.53, 165.88, 141.37, 134.71, 129.78, 128.82, 127.85, 120.53, 57.68, 53.09, 40.62, 38.14, 33.21, 29.70, 28.35, 27.67, 22.45, 13.94. HRMS (m/z): 400.2159 (M + H) +
Compound 10d ((S)-2-cinnamamido-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)hexanamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.59 (d, J = 6.6 Hz, 1H), 7.62–7.55 (m, 1H), 7.46 (dd, J = 6.4, 2.8 Hz, 2H), 7.37–7.29 (m, 3H), 7.04 (d, J = 8.2 Hz, 1H), 6.89 (s, 1H), 6.52 (d, J = 15.6 Hz, 1H), 4.81 (dd, J = 14.0, 7.6 Hz, 1H), 4.46–4.36 (m, 1H), 3.39–3.16 (m, 2H), 2.44–2.30 (m, 1H), 2.30–2.17 (m, 1H), 2.06–1.89 (m, 2H), 1.89–1.79 (m, 2H), 1.79–1.64 (m, 2H), 1.56–1.44 (m, 1H), 1.43–1.30 (m, 4H), 0.89 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 199.86, 174.92, 173.35, 165.83, 141.29, 134.74, 129.74, 128.81, 127.84, 120.55, 57.13, 53.20, 42.23, 37.29, 33.09, 30.70, 27.65, 27.11, 22.47, 21.31, 13.95. HRMS (m/z): 414.2317 (M + H) +.
Compound 10e (N-((S)-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino) propan-2-yl)cinnamamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.53 (d, J = 6.4 Hz, 1H), 7.61 (d, J = 15.7 Hz, 1H), 7.48 (dd, J = 6.5, 2.9 Hz, 2H), 7.39–7.30 (m, 3H), 6.85 (d, J = 7.5 Hz, 1H), 6.56–6.39 (m, 2H), 4.96–4.70 (m, 1H), 4.48–4.25 (m, 1H), 3.34–3.19 (m, 2H), 2.46–2.30 (m, 1H), 2.28–2.06 (m, 2H), 2.06–1.95 (m, 1H), 1.94–1.78 (m, 2H), 1.76–1.65 (m, 1H), 1.52 (d, J = 6.9 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 199.89, 174.99, 173.75, 165.54, 141.34, 134.72, 129.78, 128.83, 127.85, 120.45, 57.32, 48.92, 42.32, 37.60, 30.80, 27.42, 21.33, 19.40. HRMS (m/z): 372.1847 (M + H) +.
Compound 10f ((S)-2-cinnamamido-3-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl) propan-2-yl)butanamide): 1H NMR (400 MHz, CDCl3) δ: 9.43 (s, 1H), 8.40 (d, J = 5.9 Hz, 1H), 7.55 (d, J = 15.6 Hz, 1H), 7.43 (d, J = 4.5 Hz, 2H), 7.29 (d, J = 4.6 Hz, 3H), 6.52–6.37 (m, 2H), 6.15 (s, 1H), 4.64–4.51 (m, 1H), 4.38–4.23 (m, 1H), 3.31–3.08 (m, 2H), 2.36–2.22 (m, 1H), 2.19–2.04 (m, 2H), 2.00–1.87 (m, 1H), 1.86–1.74 (m, 2H), 1.67–1.60 (m, 1H), 1.55–1.43 (m, 1H), 0.96 (dd, J = 14.2, 6.8 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.70, 174.94, 172.35, 165.90, 141.43, 134.75, 129.76, 128.83, 127.84, 120.56, 58.19, 57.55, 42.33, 37.64, 31.81, 30.74, 27.49, 21.35, 19.26, 18.11. HRMS (m/z): 400.2158 (M + H) +.
Compound 10g ((S)-2-cinnamamido-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl) propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.64 (d, J = 6.2 Hz, 1H), 7.58 (d, J = 15.6 Hz, 1H), 7.45 (d, J = 3.7 Hz, 2H), 7.32 (s, 3H), 7.06 (d, J = 8.8 Hz, 2H), 6.51 (d, J = 15.6 Hz, 1H), 5.04–4.86 (m, 1H), 4.44–4.28 (m, 1H), 3.37–3.15 (m, 2H), 2.44–2.19 (m, 2H), 2.05–1.90 (m, 1H), 1.90–1.78 (m, 2H), 1.77–1.60 (m, 4H), 1.52–1.38 (m, 1H), 0.98 (d, J = 5.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.96, 174.70, 173.88, 165.85, 141.29, 134.77, 129.71, 128.79, 127.84, 120.57, 57.01, 51.64, 42.48, 42.21, 37.17, 30.59, 27.01, 24.93, 22.94, 22.18, 21.43. HRMS (m/z): 414.2317 (M + H) +.
Compound 10h ((S)-2-cinnamamido-3,3-dimethyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl) propan-2-yl)butanamide): 1H NMR (400 MHz, CDCl3) δ: 9.53 (s, 1H), 8.57 (d, J = 5.5 Hz, 1H), 7.61 (d, J = 15.6 Hz, 1H), 7.49 (d, J = 3.8 Hz, 2H), 7.35 (d, J = 3.5 Hz, 3H), 6.87 (d, J = 11.2 Hz, 1H), 6.65 (d, J = 9.2 Hz, 1H), 6.52 (d, J = 15.6 Hz, 1H), 4.75 (d, J = 9.4 Hz, 1H), 4.42–4.30 (m, 1H), 3.39–3.21 (m, 2H), 2.40–2.22 (m, 2H), 2.04–1.94 (m, 1H), 1.91–1.77 (m, 2H), 1.76–1.62 (m, 1H), 1.57–1.44 (m, 1H), 1.09 (s, 9H); 13C NMR (101 MHz, CDCl3) δ: 199.64, 174.73, 171.62, 165.77, 141.50, 134.72, 129.79, 128.84, 127.84, 120.63, 60.31, 57.50, 42.27, 37.46, 35.01, 30.57, 27.24, 26.74, 21.45. HRMS (m/z): 414.2319 (M + H) +.
Compound 10i (N-((S)-3-cyclopropyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl) propan-2-yl)amino)propan-2-yl)cinnamamide): 1H NMR (400 MHz, CDCl3) δ: 9.37 (s, 1H), 8.45 (d, J = 6.5 Hz, 1H), 7.45 (d, J = 15.6 Hz, 1H), 7.33 (dd, J = 6.5, 2.8 Hz, 2H), 7.24–7.15 (m, 3H), 6.90 (d, J = 8.2 Hz, 1H), 6.68 (s, 1H), 6.37 (d, J = 15.6 Hz, 1H), 4.76 (dd, J = 14.6, 6.8 Hz, 1H), 4.33–4.19 (m, 1H), 3.23–3.03 (m, 2H), 2.23 (dt, J = 15.9, 6.2 Hz, 1H), 2.18–2.03 (m, 1H), 1.92–1.79 (m, 1H), 1.77–1.66 (m, 2H), 1.63 (t, J = 6.8 Hz, 2H), 1.60–1.50 (m, 1H), 1.44–1.29 (m, 1H), 0.78–0.60 (m, 1H), 0.40–0.26 (m, 2H), 0.09 to −0.08 (m, 2H); 13C NMR (101 MHz, CDCl3) δ: 199.82, 174.92, 173.11, 165.68, 141.28, 134.75, 129.74, 128.81, 127.85, 120.57, 57.22, 53.69, 42.26, 38.07, 37.31, 30.80, 27.21, 21.34, 7.38, 4.63, 4.36. HRMS (m/z): 412.2158 (M + H) +.
Compound 10j (N-((S)-2-oxo-2-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-1-phenylethyl)cinnamamide): 1H NMR (400 MHz, CDCl3) δ: 9.31 (s, 1H), 8.64 (d, J = 5.6 Hz, 1H), 7.56 (d, J = 15.5 Hz, 1H), 7.46 (dd, J = 16.9, 5.4 Hz, 4H), 7.38–7.25 (m, 6H), 7.11 (d, J = 7.0 Hz, 1H), 6.45 (d, J = 15.6 Hz, 1H), 6.37 (s, 1H), 5.83 (d, J = 7.2 Hz, 1H), 4.43–4.29 (m, 1H), 3.37–3.19 (m, 2H), 2.50–2.27 (m, 1H), 2.26–2.10 (m, 1H), 2.08–1.94 (m, 1H), 1.92–1.82 (m, 1H), 1.78–1.66 (m, 1H), 1.61–1.46 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 199.88, 174.91, 171.35, 165.18, 141.67, 138.13, 134.69, 129.78, 129.01, 128.80, 128.37, 127.86, 127.26, 120.21, 57.54, 56.96, 42.34, 37.74, 30.89, 27.49, 21.26. HRMS (m/z): 434.2006 (M + H) +
Compound 10k ((S)-2-cinnamamido-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl) pent-4-enamide): 1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.61 (d, J = 6.4 Hz, 1H), 7.59 (d, J = 15.6 Hz, 1H), 7.46 (dd, J = 6.4, 2.7 Hz, 2H), 7.40–7.29 (m, 3H), 6.97 (d, J = 8.0 Hz, 1H), 6.86 (s, 1H), 6.51 (d, J = 15.6 Hz, 1H), 5.90–5.74 (m, 1H), 5.15 (t, J = 13.4 Hz, 2H), 4.93 (dd, J = 13.9, 6.6 Hz, 1H), 4.47–4.31 (m, 1H), 3.37–3.16 (m, 2H), 2.73–2.54 (m, 2H), 2.43–2.30 (m, 1H), 2.30–2.17 (m, 1H), 2.05–1.92 (m, 1H), 1.91–1.78 (m, 2H), 1.74–1.60 (m, 1H), 1.57–1.43 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 199.96, 174.91, 172.35, 165.74, 141.43, 134.69, 132.90, 129.79, 128.82, 127.86, 120.42, 118.91, 57.21, 52.51, 42.25, 37.64, 37.36, 30.77, 27.15, 21.33. HRMS (m/z):398.2003 (M + H) +.
Compound 10l ((S)-2-cinnamamido-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl) pent-4-ynamide): 1H NMR (400 MHz, CDCl3) δ: 9.53 (s, 1H), 8.73 (d, J = 5.1 Hz, 1H), 7.64 (d, J = 15.6 Hz, 1H), 7.49 (s, 2H), 7.35 (s, 3H), 6.87 (d, J = 7.3 Hz, 1H), 6.52 (d, J = 15.9 Hz, 1H), 6.38 (s, 1H), 5.02–4.84 (m, 1H), 4.45–4.32 (m, 1H), 3.33 (d, J = 23.2 Hz, 2H), 2.97–2.71 (m, 2H), 2.46–2.32 (m, 1H), 2.26–2.16 (m, 1H), 2.10 (s, 1H), 2.07–1.96 (m, 1H), 1.93–1.80 (m, 2H), 1.78–1.65 (m, 1H), 1.63–1.49 (m, 1H); 13C NMR (101 MHz, CDCl3) δ: 200.18, 175.09, 170.88, 165.70, 141.91, 134.60, 129.92, 128.86, 127.94, 120.07, 79.33, 71.72, 57.70, 51.50, 42.39, 37.80, 30.88, 27.64, 23.17, 21.37. HRMS (m/z): 396.1846 (M + H) +.
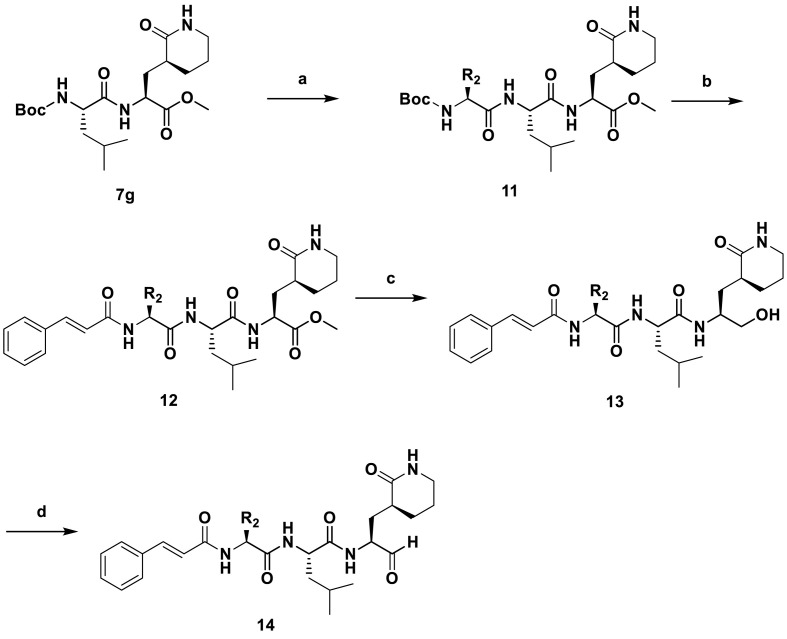
4.2. Preparation of 14a-14d
The compound 14a-14d were prepared by following Scheme 2, and the detailed synthetic procedure was similar with the synthesis of compound 10a-10l. the relevant NMR data were exhibited below:
Compound 11a (methyl (6S,9S,12S)-9-isobutyl-2,2-dimethyl-4,7,10-trioxo-12-(((S)-2- oxopiperidin-3-yl)methyl)-6-propyl-3-oxa-5,8,11-triazatridecan-13-oate): 1H NMR (400 MHz, CDCl3) δ: 7.95 (d, J = 6.2 Hz, 1H), 7.44 (d, J = 7.4 Hz, 1H), 7.19 (s, 1H), 5.18 (d, J = 7.4 Hz, 1H), 4.80–4.63 (m, 1H), 4.64–4.49 (m, 1H), 4.19–4.00 (m, 1H), 3.70 (s, 3H), 3.38–3.17 (m, 2H), 2.28–2.01 (m, 2H), 1.90–1.80 (m, 1H), 1.78–1.62 (m, 4H), 1.59–1.46 (m, 3H), 1.44–1.34 (m, 13H), 0.98–0.84 (m, 9H); 13C NMR (101 MHz, CDCl3) δ: 174.39, 172.71, 172.40, 172.05, 155.68, 79.86, 54.45, 52.19, 51.58, 49.79, 42.25, 42.12, 37.58, 34.45, 33.40, 28.25, 26.12, 24.55, 22.95, 21.95, 21.53, 18.79, 13.75.
Compound 11b (methyl (6S,9S,12S)-6-((S)-sec-butyl)-9-isobutyl-2,2-dimethyl-4,7,10- trioxo-12-(((S)-2-oxopiperidin-3-yl)methyl)-3-oxa-5,8,11-triazatridecan-13-oate): 1H NMR (400 MHz, CDCl3) δ: 7.87 (d, J = 7.1 Hz, 1H), 7.59 (d, J = 7.6 Hz, 1H), 7.31 (s, 1H), 5.17 (d, J = 8.6 Hz, 1H), 4.81–4.54 (m, 2H), 3.99–3.78 (m, 1H), 3.69 (s, 3H), 3.32–3.26 (m, 2H), 2.45–2.24 (m, 2H), 2.15–2.08 (m, 1H), 1.92–1.78 (m, 3H), 1.74–1.58 (m, 4H), 1.52–1.26 (m, 11H), 1.20–1.10 (m, 1H), 0.93–0.85 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 174.40, 172.66, 172.31, 171.53, 155.94, 79.86, 59.27, 52.19, 51.62, 49.47, 42.41, 42.11, 37.49, 36.71, 33.47, 28.24, 25.92, 24.84, 24.62, 22.94, 21.95, 21.50, 15.41, 10.95.
Compound 11c (methyl (6S,9S,12S)-9-isobutyl-6-isopropyl-2,2-dimethyl-4,7,10-trioxo-12-(((S)-2-oxopiperidin-3-yl)methyl)-3-oxa-5,8,11-triazatridecan-13-oate): 1H NMR (400 MHz, CDCl3) δ: 7.94 (d, J = 7.2 Hz, 1H), 7.22 (d, J = 7.8 Hz, 1H), 6.90 (d, J = 27.0 Hz, 1H), 5.15 (d, J = 9.1 Hz, 1H), 4.66 (td, J = 8.9, 4.9 Hz, 1H), 4.56 (s, 1H), 3.88 (t, J = 7.9 Hz, 1H), 3.70 (s, 3H), 3.36–3.16 (m, 2H), 2.46–2.24 (m, 3H), 2.14–1.99 (m, 2H), 1.92–1.79 (m, 2H), 1.73–1.62 (m, 3H), 1.54–1.48 (m, 1H), 1.43 (s, 9H), 0.99–0.89 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 174.57, 172.63, 172.29, 171.54, 79.88, 60.18, 52.24, 51.64, 50.09, 42.18, 40.59, 37.74, 33.50, 30.80, 28.26, 26.31, 24.61, 22.89, 22.00, 21.44, 19.20, 18.03.
Compound 11d (methyl (6S,9S,12S)-6-(cyclohexylmethyl)-9-isobutyl-2,2-dimethyl-4,7,10-trioxo-12-(((S)-2-oxopiperidin-3-yl)methyl)-3-oxa-5,8,11-triazatridecan-13-oate): 1H NMR (400 MHz, CDCl3) δ: 7.92 (d, J = 7.3 Hz, 1H), 7.45 (d, J = 4.5 Hz, 1H), 7.11 (s, 1H), 5.45 (s, 1H), 4.64 (d, J = 4.2 Hz, 1H), 4.44–4.21 (m, 2H), 3.59 (s, 3H), 3.35–3.27 (m, 2H), 2.48–2.23 (m, 2H), 2.30 (d, J = 4.5 Hz, 1H), 2.19–1.32 (m, 16H), 1.24–1.15 (m, 4H), 0.92 (dd, J = 12.2, 4.8 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 174.58, 172.61, 172.55, 172.17, 155.05, 79.88, 52.31, 52.05, 51.67, 49.94, 42.32, 42.43, 39.75, 37.45, 33.64, 33.49, 32.53, 28.12, 26.18, 26.11, 26.05, 26.00, 24.52, 23.09, 22.08, 21.49.
Compound 12a (methyl (S)-2-((S)-2-((S)-2-cinnamamidopentanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.56 (t, J = 11.1 Hz, 3H), 7.38 (d, J = 6.1 Hz, 3H), 6.65 (d, J = 15.8 Hz, 1H), 4.61–4.50 (m, 2H), 4.48–4.40 (m, 1H), 3.74 (s, 3H), 3.32–3.19 (m, 2H), 2.45–2.24 (m, 2H), 2.11–1.98 (m, 1H), 1.97–1.79 (m, 3H), 1.77–1.51 (m, 6H), 1.49–1.33 (m, 2H), 1.03–0.88 (m, 9H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.06, 173.55, 172.86, 172.52, 167.13, 141.21, 134.77, 129.66, 128.67, 127.69, 120.15, 53.32, 51.97, 51.93, 50.02, 41.81, 40.74, 37.37, 34.19, 33.18, 25.77, 24.50, 22.40, 21.36, 20.86, 18.82, 13.28.
Compound 12b (methyl (S)-2-((S)-2-((2S,3S)-2-cinnamamido-3-methylpentanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.64–7.49 (m, 3H), 7.38 (t, J = 5.4 Hz, 3H), 6.66 (d, J = 15.7 Hz, 1H), 4.55 (ddd, J = 11.5, 7.7, 3.8 Hz, 1H), 4.48–4.35 (m, 2H), 3.73 (s, 3H), 3.32–3.23 (m, 2H), 2.44–2.23 (m, 2H), 2.08–1.98 (m, 1H), 1.96–1.82 (m, 3H), 1.79–1.49 (m, 6H), 1.29–1.16 (m, 1H), 1.02–0.84 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 174.49, 172.77, 172.20, 171.17, 165.78, 58.97, 52.19, 51.82, 51.47, 42.86, 42.38, 37.85, 36.79, 33.42, 25.99, 24.82, 24.41, 23.09, 22.15, 21.66, 15.91, 11.55.
Compound 12c (methyl (S)-2-((S)-2-((S)-2-cinnamamido-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:3) δ: 7.58 (dd, J = 16.8, 5.8 Hz, 3H), 7.45–7.29 (dt, J = 10.5, 4.0 Hz, 3H), 6.68 (d, J = 15.7 Hz, 1H), 4.60–4.50 (m, 1H), 4.49–4.39 (m, 1H), 4.40–4.30 (m, 1H), 3.73 (s, 3H), 3.33–3.24 (m, 2H), 2.44–2.24 (m, 2H), 2.18–1.99 (m, 2H), 1.95–1.82 (m, 2H), 1.78–1.50 (m, 5H), 1.05–0.87 (m, 12H).; 13C NMR (101 MHz, MeOD:CDCl3 = 1:3) δ: 178.59, 175.10, 172.48, 172.07, 167.04, 141.35, 134.74, 129.70, 128.70, 127.72, 120.12, 58.73, 52.03, 49.90, 41.87, 40.73, 37.34, 33.20, 31.01, 25.79, 24.47, 22.39, 21.48, 20.84, 18.93, 17.87.
Compound 12d (methyl (S)-2-((S)-2-((S)-2-cinnamamido-3-cyclohexylpropanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 7.98 (d, J = 7.3 Hz, 1H), 7.71 (d, J = 7.9 Hz, 1H), 7.60 (d, J = 15.6 Hz, 1H), 7.47 (d, J = 3.7 Hz, 2H), 7.39–7.23 (m, 3H), 7.15–6.87 (m, 2H), 6.52 (d, J = 15.6 Hz, 1H), 4.72 (dd, J = 14.1, 7.2 Hz, 1H), 4.67–4.46 (m, 2H), 3.68 (s, 3H), 3.46–3.17 (m, 2H), 2.49–2.25 (m, 2H), 2.19–2.01 (m, 1H), 2.00–1.46 (m, 14H), 1.46–1.32 (m, 1H), 1.30–1.04 (m, 4H), 0.88 (dd, J = 12.4, 4.9 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 174.57, 172.62, 172.35, 165.96, 141.38, 134.80, 129.68, 128.78, 127.89, 120.48, 52.18, 52.04, 51.33, 50.06, 42.34, 41.80, 39.85, 37.79, 34.01, 33.82, 33.58, 32.80, 26.44, 26.35, 26.12, 26.01, 24.73, 22.88, 22.10, 21.40.
Compound 13a ((S)-2-((S)-2-cinnamamidopentanamido)-N-((S)-1-hydroxy-3-((S)-2- oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.65–7.48 (m, 3H), 7.38 (d, J = 6.4 Hz, 3H), 6.71 (d, J = 15.8 Hz, 1H), 4.49–4.32 (m, 2H), 4.11–3.93 (m, 1H), 3.51 (qd, J = 10.9, 5.6 Hz, 2H), 3.27–3.11 (m, 2H), 2.39–2.23 (m, 1H), 2.19–1.98 (m, 2H), 1.88–1.76 (m, 2H), 1.76–1.56 (m, 6H), 1.56–1.34 (m, 3H), 1.03–0.81 (m, 9H); 13C NMR (101 MHz, MeOD) δ: 175.91, 173.57, 173.15,167.41, 140.93, 134.89, 129.51, 128.55, 127.54, 120.05, 64.22, 53.74, 52.21, 48.45, 41.61, 40.37, 37.26, 33.87, 32.76, 25.70, 24.53, 22.05, 20.70, 20.60, 18.80, 12.76.
Compound 13b ((2S,3S)-2-cinnamamido-N-((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)-3-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.67–7.48 (m, 3H), 7.44–7.31 (m, 3H), 6.75 (d, J = 15.8 Hz, 1H), 4.44–4.30 (m, 2H), 4.09–3.96 (m, 1H), 3.51 (ddd, J = 17.0, 11.0, 5.6 Hz, 2H), 3.27–3.15 (m, 2H), 2.40–2.26 (m, 1H), 2.15–1.98 (m, 2H), 1.94–1.75 (m, 2H), 1.75–1.54 (m, 6H), 1.53–1.45 (m, 1H), 1.32–1.20 (m, 1H), 1.03–0.86 (m, 12H); 13C NMR (101 MHz, MeOD) δ: 175.92, 173.50, 172.42, 167.46, 140.96, 134.93, 129.50, 128.56, 127.55, 120.18, 64.27, 58.37, 52.35, 48.41, 41.61, 40.52, 37.23, 36.85, 32.74, 25.73, 24.73, 24.49, 22.02, 20.73, 20.64, 14.68, 10.08.
Compound 13c ((S)-2-((S)-2-cinnamamido-3-methylbutanamido)-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.56 (dd, J = 8.5, 7.3 Hz, 3H), 7.45–7.33 (m, 3H), 6.77 (d, J = 15.8 Hz, 1H), 4.45–4.27 (m, 2H), 4.01 (d, J = 3.0 Hz, 1H), 3.57–3.42 (m, 2H), 3.28–3.15 (m, 2H), 2.40–2.26 (m, 1H), 2.20–1.96 (m, 3H), 1.88–1.75 (m, 1H), 1.75–1.47 (m, 6H), 1.05–0.88 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.90, 173.50, 172.28, 167.39, 140.95, 134.93, 129.50, 128.56, 127.56, 120.16, 64.28, 59.22, 52.23, 48.36, 41.61, 40.50, 37.22, 32.71, 30.67, 27.34, 25.71, 24.48, 22.02, 20.69, 18.47, 17.49.
Compound 13d ((S)-2-((S)-2-cinnamamido-3-cyclohexylpropanamido)-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.62 (d, J = 14.7 Hz, 1H), 7.29 (dd, J = 8.4, 7.0 Hz, 2H), 7.22–7.13 (m, 3H), 6.56 (d, J = 15.6 Hz, 1H), 4.38–4.28 (m, 1H), 4.22–4.14 (m, 1H), 4.01–3.98 (m, 1H), 3.36 (dd, J = 12.1, 5.8 Hz, 2H), 3.11–3.05 (m, 1H), 2.56–2.44 (m, 2H), 2.16–2.08 (m, 1H), 1.98–1.88 (m, 2H), 1.67–1.31 (m, 14H), 1.27–1.05 (dd, J = 23.7, 11.2 Hz, 3H), 0.97 (dd, J = 12.2, 4.2 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 174.84, 172.65, 172.42, 166.53, 140.27, 134.03, 128.39, 127.44, 126.41, 119.04, 64.87, 52.31, 51.31, 49.72, 42.28, 41.88, 39.33, 36.93, 35.04, 32.58, 32.21, 31.49, 24.86, 24.73, 24.77, 24.61, 23.47, 20.92, 19.34, 18.94.
Compound 14a ((S)-2-((S)-2-cinnamamidopentanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2- oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, DMSO) δ: 9.40 (s, 1H), 8.45 (d, J = 7.8 Hz, 1H), 8.21 (dd, J = 24.3, 7.9 Hz, 2H), 7.64–7.32 (m, 7H), 6.80 (d, J = 15.8 Hz, 1H), 4.43 (dd, J = 13.6, 8.0 Hz, 1H), 4.38–4.21 (m, 2H), 3.18–3.03 (m, 2H), 2.23–2.02 (m, 2H), 1.94–1.84 (m, 1H), 1.79–1.58 (m, 4H), 1.58–1.41 (m, 4H), 1.39–1.25 (m, 3H), 1.00–0.78 (m, 9H); 13C NMR (101 MHz, DMSO) δ: 201.50, 173.14, 173.07, 172.14, 165.27, 139.32, 135.38, 129.42, 128.67, 127.98, 122.57, 55.80, 52.77, 51.56, 41.69, 41.12, 37.10, 34.99, 30.09, 26.08, 24.66, 23.32, 22.24, 21.85, 18.98, 14.22. HRMS (m/z): 513.3000 (M + H) +.
Compound 14b ((2S,3S)-2-cinnamamido-3-methyl-N-((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.32 (d, J = 6.8 Hz, 1H), 7.63 (d, J = 15.6 Hz, 1H), 7.54–7.44 (m, 2H), 7.38–7.27 (m, 3H), 7.19 (d, J = 6.5 Hz, 1H), 6.87 (d, J = 8.3 Hz, 1H), 6.67–6.43 (m, 2H), 4.74–4.59 (m, 1H), 4.59–4.36 (m, 2H), 3.37–3.14 (m, 2H), 2.41–2.12 (m, 2H), 2.12–1.80 (m, 4H), 1.74–1.50 (m, 6H), 1.24–1.06 (m, 1H), 1.02–0.85 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 201.49, 173.14, 172.97, 171.47, 165.29, 139.32, 135.44, 129.41, 128.64, 127.97, 122.70, 57.15, 55.71, 51.62, 41.68, 41.15, 37.53, 37.04, 30.09, 26.04, 24.84, 24.63, 23.27, 22.29, 21.79, 15.72, 11.46. HRMS (m/z): 527.3158 (M + H) +.
Compound 14c ((S)-2-((S)-2-cinnamamido-3-methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.32 (s, 1H), 7.64 (d, J = 15.5 Hz, 1H), 7.50 (d, J = 3.6 Hz, 1H), 7.38–7.31 (m, 2H), 7.27 (s, 3H), 7.16 (dd, J = 26.3, 7.4 Hz, 1H), 6.67 (d, J = 8.4 Hz, 1H), 6.52 (d, J = 15.5 Hz, 1H), 6.31 (s, 1H), 4.70–4.55 (m, 1H), 4.50 (dd, J = 16.3, 8.5 Hz, 1H), 4.44–4.34 (m, 1H), 3.40–3.19 (m, 2H), 2.43–2.27 (m, 1H), 2.25–2.11 (m, 2H), 2.05–1.96 (m, 1H), 1.93–1.81 (m, 2H), 1.74–1.50 (m, 5H), 1.01–0.85 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.80, 174.93, 173.62, 171.99, 166.02, 141.67, 134.70, 129.84, 128.86, 127.89, 121.01, 58.74, 57.27, 52.00, 42.39, 41.56, 37.08, 31.60, 31.36, 30.98, 24.85, 22.86, 22.06, 21.32, 19.31, 18.34. HRMS (m/z): 513.3001 (M + H) +.
Compound 14d ((S)-2-((S)-2-cinnamamido-3-cyclohexylpropanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.49 (s, 1H), 8.31 (d, J = 6.7 Hz, 1H), 7.60 (t, J = 10.2 Hz, 2H), 7.48 (d, J = 3.1 Hz, 2H), 7.32 (d, J = 2.6 Hz, 3H), 6.90 (d, J = 8.0 Hz, 1H), 6.82–6.67 (m, 1H), 6.52 (dd, J = 15.6, 6.9 Hz, 1H), 4.71 (dd, J = 13.9, 8.0 Hz, 1H), 4.65–4.53 (m, 1H), 4.53–4.35 (m, 1H), 3.33–3.15 (m, 2H), 2.47–2.12 (m, 2H), 2.10–1.96 (m, 1H), 1.95–1.45 (m, 13H), 1.45–1.09 (m, 4H), 1.09–0.71 (m, 8H); 13C NMR (101 MHz, CDCl3) δ: 199.83, 174.83, 173.14, 172.47, 166.09, 141.55, 134.73, 129.78, 128.82, 127.89, 120.36, 56.96, 52.17, 51.51, 42.32, 41.52, 39.73, 37.39, 34.11, 33.62, 32.79, 31.00, 27.14, 26.38, 26.18, 26.04, 24.87, 22.91, 22.04, 21.29. HRMS (m/z): 567.3469 (M + H) +.
4.3. Preparation of 17a-17z
The compound 17a-17e were obtained by via Scheme 3, and the detailed synthetic procedure was similar with the synthesis of compound 10a-10m. the relevant NMR data were exhibited below:
Compound 15a (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-((E)-3-(p-tolyl)acrylamido) butanamido)pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.56 (d, J = 15.7 Hz, 1H), 7.43 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 8.0 Hz, 2H), 6.60 (d, J = 15.7 Hz, 1H), 4.61–4.54 (m, 1H), 4.51–4.43 (m, 1H), 4.38 (dd, J = 11.1, 5.1 Hz, 1H), 3.34–3.21 (m, 2H), 2.42–2.32 (m, 4H), 2.33–2.25 (m, 1H), 2.19–2.09 (m, 1H), 2.08–1.97 (m, 1H), 1.96–1.82 (m, 2H), 1.78–1.63 (m, 3H), 1.63–1.48 (m, 2H), 1.02–0.86 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.06, 173.30, 172.48, 172.08, 167.18, 141.37, 140.06, 132.00, 129.43, 127.74, 119.18, 58.79, 52.07, 52.00, 50.07, 41.92, 40.89, 37.40, 33.30, 31.14, 25.87, 24.50, 22.44, 21.62, 20.97, 20.88, 18.98, 17.98.
Compound 15b (methyl (S)-2-((S)-2-((S)-2-((E)-3-(4-isopropylphenyl)acrylamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.56 (d, J = 16.1 Hz, 1H), 7.48 (d, J = 7.5 Hz, 2H), 7.25 (d, J = 7.5 Hz, 2H), 6.63 (d, J = 15.7 Hz, 1H), 4.55 (t, J = 7.5 Hz, 1H), 4.45 (dd, J = 14.1, 7.4 Hz, 1H), 4.37 (t, J = 7.8 Hz, 1H), 3.73 (s, 3H), 3.33–3.20 (m, 2H), 2.99–2.84 (m, 1H), 2.47–2.22 (m, 2H), 2.20–2.08 (m, 1H), 2.08–1.97 (m, 1H), 1.97–1.80 (m, 2H), 1.81–1.49 (m, 5H), 1.26 (d, J = 7.2 Hz, 6H), 1.04–0.88 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.10, 173.39, 172.50, 172.14, 167.32, 151.01, 141.36, 132.38, 127.86, 126.80, 119.19, 58.84, 52.02, 52.00, 50.02, 41.88, 40.80, 37.36, 33.97, 33.25, 31.05, 25.81, 24.48, 23.40 (double peak height), 22.39, 21.51, 20.86, 18.93, 17.89.
Compound 15c (methyl (S)-2-((S)-2-((S)-2-((E)-3-([1,1′-biphenyl]-4-yl)acrylamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.67–7.53 (m, 7H), 7.43 (t, J = 7.5 Hz, 2H), 7.35 (t, J = 7.3 Hz, 1H), 6.74 (d, J = 15.7 Hz, 1H), 4.57 (ddd, J = 11.5, 7.8, 3.9 Hz, 1H), 4.49 (dd, J = 14.1, 8.0 Hz, 1H), 4.42 (dd, J = 10.9, 5.2 Hz, 1H), 3.72 (s, 3H), 3.33–3.19 (m, 2H), 2.45–2.25 (m, 2H), 2.20–2.08 (m, 1H), 2.09–1.97 (m, 1H), 1.97–1.79 (m, 2H), 1.79–1.47 (m, 5H), 1.05–0.84 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.02, 173.41, 172.52, 172.14, 167.09, 142.45, 140.80, 140.11, 133.82, 128.71, 128.26, 127.57, 127.24, 126.74, 120.23, 58.90, 52.00, 51.95, 50.02, 41.84, 40.86, 37.38, 33.21, 31.10, 25.80, 24.50, 22.36, 21.55, 20.91, 18.93, 17.96.
Compound 15d (methyl (S)-2-((S)-2-((S)-2-((E)-3-(4-methoxyphenyl)acrylamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.57–7.47 (m, 3H), 6.92 (d, J = 8.7 Hz, 2H), 6.54 (d, J = 15.7 Hz, 1H), 4.55 (ddd, J = 11.5, 7.8, 3.9 Hz, 1H), 4.51–4.40 (m, 1H), 4.37 (t, J = 6.7 Hz, 1H), 3.84 (s, 3H), 3.72 (s, 3H), 3.32–3.20 (m, 2H), 2.44–2.25 (m, 2H), 2.19–2.07 (m, 1H), 2.07–1.97 (m, 1H), 1.97–1.82 (m, 2H), 1.80–1.49 (m, 5H), 1.01–0.91 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.06, 173.40, 172.50, 172.17, 167.43, 161.06, 141.02, 129.32, 127.49, 117.69, 114.12, 58.77, 54.94, 52.00, 51.95, 50.02, 41.84, 40.80, 37.37, 33.21, 30.99, 25.81, 24.47, 22.34, 21.46, 20.87, 18.90, 17.86.
Compound 15e (methyl (S)-2-((S)-2-((S)-2-((E)-3-(4-(dimethylamino)phenyl)acrylamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.51 (d, J = 15.6 Hz, 1H), 7.42 (d, J = 8.8 Hz, 2H), 6.68 (d, J = 8.8 Hz, 2H), 6.44 (d, J = 15.6 Hz, 1H), 4.56 (ddd, J = 11.5, 7.9, 3.9 Hz, 1H), 4.51–4.44 (m, 1H), 4.42–4.36 (m, 1H), 3.72 (s, 3H), 3.34–3.19 (m, 2H), 3.00 (s, 6H), 2.44–2.25 (m, 2H), 2.21–2.07 (m, 1H), 2.07–1.97 (m, 1H), 1.97–1.80 (m, 2H), 1.78–1.47 (m, 5H), 1.03–0.90 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.00, 173.38, 172.52, 172.30, 167.99, 151.60, 141.88, 129.30, 122.58, 114.73, 111.88, 58.74, 53.42, 51.99, 49.99, 41.86, 40.87, 39.73, 37.36, 33.20, 31.14, 25.80, 24.48, 22.39, 21.60, 20.92, 18.96, 17.98.
Compound 15f (methyl (S)-2-((S)-2-((S)-2-((E)-3-(4-fluorophenyl)acrylamido)-3- methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.56 (dd, J = 14.7, 8.9 Hz, 3H), 7.09 (t, J = 8.6 Hz, 2H), 6.64 (d, J = 15.7 Hz, 1H), 4.56 (ddd, J = 11.6, 7.8, 3.8 Hz, 1H), 4.51–4.41 (m, 1H), 4.37 (t, J = 6.7 Hz, 1H), 3.31–3.21 (m, 2H), 2.45–2.25 (m, 2H), 2.20–2.07 (m, 1H), 2.07–1.97 (m, 1H), 1.97–1.79 (m, 2H), 1.79–1.47 (m, 5H), 1.01–0.91 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.03, 173.47, 172.50, 172.13, 166.96, 163.59 (d, J = 249.7 Hz), 139.90, 131.22 (d, J = 3.1 Hz), 129.62 (d, J = 8.4 Hz), 120.07 (d, J = 2.1 Hz), 115.60 (d, J = 22.0 Hz), 58.81, 51.82, 49.95, 49.84, 41.77, 40.70, 37.33, 33.14, 30.94, 25.73, 24.45, 22.25, 21.36, 20.89, 18.81, 17.79.
Compound 15g (methyl (S)-2-((S)-2-((S)-2-((E)-3-(2-fluorophenyl)acrylamido)-3- methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.71 (d, J = 15.9 Hz, 1H), 7.64–7.55 (m, 1H), 7.41–7.29 (m, 1H), 7.23–7.06 (m, 2H), 6.79 (d, J = 15.9 Hz, 1H), 4.59–4.52 (m, 1H), 4.52–4.43 (m, 1H), 4.39 (dd, J = 11.0, 5.3 Hz, 1H), 3.34–3.22 (m, 2H), 2.44–2.23 (m, 2H), 2.19–2.07 (m, 1H), 2.07–1.96 (m, 1H), 1.97–1.81 (m, 2H), 1.79–1.49 (m, 5H), 0.97 (ddd, J = 20.3, 11.5, 5.6 Hz, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.04, 173.36, 172.50, 172.03, 166.79, 161.24 (d, J = 252.5 Hz), 133.88 (d, J = 2.4 Hz), 131.14 (d, J = 8.6 Hz), 128.98 (d, J = 2.9 Hz), 124.37 (d, J = 3.4 Hz), 122.95 (d, J = 6.3 Hz), 122.72 (d, J = 11.7 Hz), 115.87 (d, J = 22.0 Hz), 58.91, 52.00(double peak height), 50.03, 41.87, 40.87, 37.38, 33.25, 31.08, 25.83, 24.49, 22.38, 21.57, 20.89, 18.94, 17.95.
Compound 15h (methyl (S)-2-((S)-2-((S)-2-((E)-3-(3-fluorophenyl)acrylamido)-3- methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.55 (d, J = 15.7 Hz, 1H), 7.40–7.23 (m, 3H), 7.07 (t, J = 8.4 Hz, 1H), 6.66 (d, J = 15.7 Hz, 1H), 4.55 (ddd, J = 11.5, 7.8, 3.8 Hz, 1H), 4.44–4.41 (m, 1H), 4.37 (dd, J = 11.0, 5.3 Hz, 1H), 3.33–3.23 (m, 2H), 2.41–2.23 (m, 2H), 2.18–1.98 (m, 2H), 1.97–1.83 (m, 2H), 1.81–1.50 (m, 5H), 1.03–0.87 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.13, 173.27, 172.47, 171.95, 166.53, 163.01 (d, J = 245.9 Hz), 140.01, 137.16 (d, J = 7.8 Hz), 130.33 (d, J = 8.3 Hz), 123.89, 121.72, 116.43 (d, J = 21.6 Hz), 113.85 (d, J = 21.9 Hz), 58.86, 52.12, 52.02, 50.10, 41.94, 40.89, 37.41, 33.36, 31.13, 25.89, 24.51, 22.46, 21.62, 20.84, 18.97, 17.96.
Compound 15i (methyl (S)-2-((S)-2-((S)-2-(2-naphthamido)-3-methylbutanamido)-4- methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1)) δ: 8.88–8.71 (m, 1H), 8.63–8.39 (m, 3H), 8.06–7.77 (m, 4H), 7.65–7.44 (m, 2H), 4.75–4.43 (m, 3H), 3.73 (s, 3H), 3.42–3.15 (m, 2H), 2.67–2.45 (m, 1H), 2.38–2.11 (m, 3H), 1.90–1.51 (m, 6H), 1.50–1.41 (m, 1H)1.07 (s, 6H), 1.00–0.85 (m, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 180.21, 173.55, 172.57, 172.24, 168.80, 134.90, 132.57, 131.23, 128.69, 127.93, 127.71, 127.50, 127.39, 126.46, 123.74, 59.62, 52.07, 51.50, 50.41, 41.59, 40.54, 38.10, 32.48, 30.90, 26.28, 24.38, 21.88, 21.14, 18.65, 18.14.
Compound 15j (methyl (S)-2-((S)-2-((S)-2-(1H-indole-5-carboxamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.66 (d, J = 7.9 Hz, 1H), 7.46 (d, J = 8.3 Hz, 1H), 7.27 (t, J = 7.5 Hz, 1H), 7.19–7.07 (m, 2H), 4.67–4.55 (m, 2H), 4.55–4.46 (m, 1H), 3.74 (s, 3H), 3.31–3.20 (m, 2H), 2.44–2.27 (m, 2H), 2.24–2.09 (m, 1H), 2.09–1.98 (m, 1H), 1.98–1.79 (m, 2H), 1.77–1.62 (m, 3H), 1.62–1.47 (m, 2H), 1.02 (d, J = 6.3 Hz, 6H), 0.90 (dd, J = 15.0, 4.3 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.12, 173.27, 172.47, 171.95, 162.16, 136.90, 130.09, 127.42, 124.33, 121.84, 120.28, 112.07, 104.01, 58.65, 53.39, 52.24, 50.06, 41.98, 40.81, 37.43, 33.25, 31.44, 25.86, 24.57, 22.46, 21.67, 20.79, 19.04, 18.32.
Compound 15k (methyl (S)-2-((S)-2-((S)-2-(1H-indole-2-carboxamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.65 (dd, J = 8.5, 4.6 Hz, 2H), 7.45 (d, J = 8.5 Hz, 1H), 7.30 (d, J = 2.6 Hz, 1H), 6.58 (d, J = 2.3 Hz, 1H), 4.60–4.41 (m, 3H), 3.72 (s, 3H), 3.31–3.16 (m, 2H), 2.44–2.35 (m, 1H), 2.33–2.25 (m, 1H), 2.24–2.12 (m, 1H), 2.07–1.96 (m, 1H), 1.94–1.79 (m, 2H), 1.78–1.47 (m, 5H), 1.03 (d, J = 6.6 Hz, 6H), 0.94 (dd, J = 17.9, 6.2 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.09, 173.41, 172.53, 172.47, 169.91, 138.15, 127.56, 126.14, 124.73, 120.37, 120.26, 111.08, 102.50, 59.03, 52.11, 52.01, 50.04, 41.85, 40.73, 37.36, 33.21, 31.41, 25.81, 24.49, 22.36, 21.50, 20.85, 19.02, 18.13.
Compound 15l (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-(3-phenylpropanamido) butanamido)pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD) δ: 7.27–7.12 (m, 5H), 4.54 (dd, J = 11.8, 4.1 Hz, 1H), 4.45–4.38 (m, 1H), 4.20–4.13 (dt, J = 7.5, 3.8 Hz, 1H), 3.69 (s, 3H), 3.28–3.16 (m, 2H), 2.90 (t, J = 7.7 Hz, 2H), 2.64–2.48 (m, 2H), 2.45–2.25 (m, 2H), 2.04–1.92 (m, 2H), 1.92–1.77 (m, 2H), 1.75–1.64 (m, 2H), 1.64–1.54 (m, 2H), 1.53–1.41 (m, 1H), 0.95 (dd, J = 20.3, 6.5 Hz, 6H), 0.85 (dd, J = 17.3, 6.8 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 174.94, 173.71, 173.45, 172.44, 172.19, 140.77, 128.09, 128.03, 125.80, 58.62, 51.78, 51.40, 49.57, 41.56, 40.55, 37.23, 37.20, 32.82, 31.45, 30.58, 25.49, 24.35, 21.92, 21.04, 20.93, 18.41, 17.47.
Compound 15m (methyl (S)-2-((S)-2-((S)-2-benzamido-3-methylbutanamido)-4- methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.04 (d, J = 7.6 Hz, 1H), 7.79 (d, J = 7.6 Hz, 2H), 7.70 (d, J = 8.2 Hz, 1H), 7.55–7.41 (m, 3H), 7.17 (d, J = 8.4 Hz, 1H), 7.02 (s, 1H), 4.69–4.61 (m, 2H), 4.53 (t, J = 7.2 Hz, 1H), 3.72 (s, 3H), 3.28 (s, 2H), 2.41–2.37 (m, 1H), 2.35–2.27 (m, 1H), 2.26–2.15 (m, 1H), 2.12–2.05 (m, 1H), 1.94–1.81 (m, 2H), 1.79–1.62 (m, 3H), 1.57–1.48 (m, 2H), 0.97 (dd, J = 6.4, 1.6 Hz, 6H), 0.88 (dd, J = 11.4, 6.4 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 174.09, 172.53, 172.26, 171.19, 167.54, 134.20, 131.53, 128.54, 127.26, 59.01, 52.11, 51.99, 50.04, 42.24, 41.97, 37.85, 33.64, 31.49, 26.22, 24.75, 22.63, 22.02, 21.67, 19.38, 18.59.
Compound 15n (methyl (S)-2-((S)-2-((S)-2-([1,1′-biphenyl]-4-carboxamido)-3- methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.93 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 7.3 Hz, 2H), 7.47 (t, J = 7.5 Hz, 2H), 4.63–4.54 (m, 1H), 4.52–4.40 (m, 2H), 3.34–3.21 (m, 2H), 2.44–2.24 (m, 2H), 2.25–2.11 (m, 1H), 2.11–1.98 (m, 1H), 1.96–1.81 (m, 2H), 1.79–1.50 (m, 5H), 1.03 (dd, J = 6.7, 3.0 Hz, 6H), 0.95 (dd, J = 17.4, 6.3 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.11, 173.30, 172.48, 172.11, 168.08, 144.60, 139.84, 132.48, 128.82, 127.95, 127.71, 127.05, 127.03, 59.05, 52.10 (double peak height), 50.07, 41.93, 40.83, 37.40, 33.32, 31.40, 25.90, 24.52, 22.44, 21.61, 20.87, 19.05, 18.19.
Compound 15o (methyl (S)-2-((S)-2-((S)-2-(cyclohexanecarboxamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 4.54 (ddd, J = 11.7, 7.9, 3.9 Hz, 1H), 4.43 (ddd, J = 8.0, 6.7, 4.0 Hz, 1H), 4.21 (dd, J = 11.3, 5.3 Hz, 1H), 3.34–3.23 (m, 2H), 2.43–2.17 (m, 3H), 2.10–1.96 (m, 2H), 1.95–1.76 (m, 6H), 1.76–1.65 (m, 3H), 1.65–1.50 (m, 3H), 1.49–1.37 (m, 2H), 1.36–1.18 (m, 4H), 1.01–0.86 (m, 12H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 177.48, 175.09, 173.34, 172.46, 172.18, 58.19, 52.00, 51.92, 50.02, 45.20, 41.88, 40.89, 37.38, 33.28, 30.92, 29.77, 29.14, 25.86 (double peak height), 25.57, 25.45, 24.47, 22.40, 21.46, 20.87, 18.90, 17.79.
Compound 15p (methyl (S)-2-((S)-2-((S)-2-(furan-2-carboxamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.46 (s, 1H), 7.14 (d, J = 3.4 Hz, 1H), 6.49 (dd, J = 3.4, 1.7 Hz, 1H), 4.76–4.65 (m, 1H), 4.59 (ddd, J = 11.7, 7.7, 3.7 Hz, 1H), 4.52 (dd, J = 8.8, 7.2 Hz, 1H), 3.70 (s, 3H), 3.39–3.18 (m, 2H), 2.50–2.37 (m, 1H), 2.37–2.25 (m, 1H), 2.25–2.10 (m, 1H), 2.08–1.98 (m, 1H), 1.96–1.78 (m, 2H), 1.75–1.59 (m, 3H), 1.59–1.45 (m, 2H), 0.97 (dd, J = 6.7, 3.3 Hz, 6H), 0.89 (t, J = 6.6 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 174.43, 172.73, 172.30, 170.84, 158.18, 147.52, 144.27, 114.69, 112.14, 58.04, 52.21, 51.82, 50.04, 42.23, 41.91, 37.74, 33.44, 31.67, 26.22, 24.68, 22.74, 22.11, 21.53, 19.24, 18.27.
Compound 15q (methyl (S)-2-((S)-2-((S)-2-(furan-3-carboxamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.39 (s, 1H), 7.24 (s, 1H), 6.77 (s, 1H), 4.76–4.61 (m, 2H), 4.56 (t, J = 8.5 Hz, 1H), 3.69 (s, 3H), 3.39–3.22 (m, 2H), 2.53–2.24 (m, 2H), 2.19–2.00 (m, 2H), 1.97–1.79 (m, 2H), 1.76–1.58 (m, 3H), 1.58–1.40 (m, 2H), 0.95 (d, J = 6.5 Hz, 6H), 0.84 (t, J = 5.5 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 174.40, 172.70, 172.50, 171.65, 162.63, 145.44, 143.43, 122.26, 108.75, 58.74, 52.17, 51.86, 49.83, 42.17, 41.78, 37.65, 33.54, 31.14, 26.10, 24.62, 22.64, 22.23, 21.47, 19.26, 18.82.
Compound 15r (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-(1H-pyrrole-2-carboxamido) butanamido)pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 6.94 (s, 1H), 6.86 (d, J = 3.2 Hz, 1H), 6.25–6.16 (m, 1H), 4.62–4.44 (m, 3H), 3.73 (s, 3H), 3.33–3.18 (m, 2H), 2.45–2.29 (m, 2H), 2.20–2.06 (m, 1H), 2.06–1.97 (m, 1H), 1.96–1.80 (m, 2H), 1.76–1.60 (m, 3H), 1.60–1.47 (m, 2H), 1.07–0.94 (m, 6H), 0.95–0.85 (m, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.06, 173.52, 172.55, 172.51, 161.81, 125.05, 122.21, 111.04, 109.33, 58.52, 51.99, 51.96, 49.98, 41.84, 40.71, 37.35, 33.13, 31.31, 25.73, 24.50, 22.30, 21.50, 20.86, 18.91, 18.22.
Compound 15s (methyl (S)-2-((S)-2-((S)-2-(isonicotinamido)-3-methylbutanamido)-4- methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 8.65 (dd, J = 4.4, 1.4 Hz, 2H), 7.66 (dd, J = 4.4, 1.6 Hz, 2H), 4.44–4.35 (m, 1H), 4.34–4.26 (m, 1H), 4.03–3.95 (m, 1H), 3.75 (s, 3H), 3.24–3.12 (m, 2H), 2.37–1.96 (m, 4H), 1.82–1.51 (m, 6H), 1.46–1.37 (m, 1H), 1.01 (d, J = 6.6 Hz, 6H), 0.95 (dd, J = 17.9, 6.7 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.45, 173.03, 172.92, 171.55, 166.25, 147.38, 141.04, 122.15, 57.47, 53.39, 52.17, 51.44, 42.12, 40.28, 37.14, 32.47, 30.39, 25.76, 24.37, 22.07, 20.49, 20.75, 18.74, 17.95.
Compound 15t (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-(nicotinamido)butanamido) pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 9.05 (dd, J = 2.1, 0.6 Hz, 1H), 8.70 (dd, J = 4.9, 1.6 Hz, 1H), 8.34–8.22 (m, 1H), 7.51 (ddd, J = 8.0, 5.0, 0.6 Hz, 1H), 4.58 (dd, J = 11.7, 4.0 Hz, 1H), 4.50 (dd, J = 8.7, 4.3 Hz, 2H), 3.73 (s, 3H), 3.32–3.20 (m, 2H), 2.47–2.27 (m, 2H), 2.27–2.11 (m, 1H), 2.10–1.97 (m, 1H), 1.97–1.82 (m, 2H), 1.79–1.48 (m, 5H), 1.02 (dd, J = 6.7, 1.1 Hz, 6H), 0.98–0.89 (m, 6H); 13C NMR (101 MHz, CDCl3) δ: 174.94, 173.26, 172.51, 171.86, 166.13, 151.51, 148.14, 135.94, 130.25, 123.75, 59.31, 51.99, 51.90, 49.79, 41.83, 40.78, 37.32, 33.16, 30.98, 25.75, 24.49, 22.35, 21.58, 20.94, 18.97, 18.35.
Compound 15u (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-(picolinamido)butanamido) pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ 8.56 (d, J = 4.7 Hz, 1H), 8.19 (d, J = 7.8 Hz, 1H), 7.86 (td, J = 7.7, 1.8 Hz, 1H), 7.44 (ddd, J = 7.6, 4.7, 1.2 Hz, 1H), 4.76 (td, J = 8.4, 5.2 Hz, 1H), 4.57 (td, J = 10.2, 9.5, 5.2 Hz, 2H), 3.71 (s, 3H), 3.33 (d, J = 5.6 Hz, 2H), 2.51 (ddd, J = 14.0, 12.1, 4.2 Hz, 1H), 2.29 (ddt, J = 20.4, 13.6, 5.8 Hz, 2H), 2.08–1.98 (m, 1H), 1.87 (ddd, J = 13.6, 9.9, 3.6 Hz, 2H), 1.75–1.60 (m, 3H), 1.58–1.40 (m, 2H), 1.00 (dd, J = 6.7, 2.3 Hz, 6H), 0.88 (dd, J = 8.6, 6.0 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 174.37, 172.86, 172.40, 170.85, 164.32, 149.34, 148.27, 137.33, 126.35, 122.37, 58.60, 52.21, 51.61, 49.85, 42.12, 41.97, 37.55, 33.10, 31.52, 25.94, 24.62, 22.69, 22.21, 21.58, 19.30, 18.25.
Compound 15v (methyl (S)-2-((S)-2-((S)-2-(4-fluorobenzamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.84 (dd, J = 8.6, 5.4 Hz, 2H), 7.08 (t, J = 8.6 Hz, 2H), 6.89 (s, 1H), 4.67–4.50 (m, 3H), 3.70 (s, 3H), 3.36–3.24 (m, 2H), 2.41–2.27 (m, 2H), 2.25–2.13 (m, 1H), 2.10–2.00 (m, 1H), 1.92–1.81 (m, 2H), 1.72–1.64 (m, 3H), 1.57–1.49 (m, 2H), 0.98 (d, J = 6.7 Hz, 6H), 0.86 (dd, J = 10.7, 6.0 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 174.63, 172.60, 172.30, 171.28, 166.42, 164.82 (d, J = 252.2 Hz), 130.26 (d, J = 2.6 Hz), 129.62 (d, J = 8.9 Hz), 115.49 (d, J = 21.8 Hz), 59.06, 52.24, 51.97, 50.23, 42.28, 41.74, 37.82, 33.67, 31.46, 26.38, 24.69, 22.71, 22.10, 21.36, 19.25, 18.52.
Compound 15w (methyl (S)-2-((S)-2-((S)-2-(4-chlorobenzamido)-3-methylbutanamido)-4-methylpentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, CDCl3) δ: 8.09 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.48 (d, J = 8.4 Hz, 1H), 7.39 (d, J = 8.5 Hz, 2H), 7.18 (d, J = 8.6 Hz, 1H), 6.75 (s, 1H), 4.73–4.44 (m, 3H), 3.69 (s, 1H), 3.38–3.21 (m, 2H), 2.44–2.25 (m, 2H), 2.18 (td, J = 13.5, 6.7 Hz, 1H), 2.12–1.99 (m, 1H), 1.94–1.81 (m, 2H), 1.76–1.59 (m, 3H), 1.59–1.45 (m, 2H), 0.97 (d, J = 6.4 Hz, 6H), 0.87 (dd, J = 10.2, 6.1 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 174.54, 172.51, 172.29, 171.02, 166.43, 137.90, 132.46, 128.76, 128.70, 59.06, 52.30, 51.92, 50.30, 42.34, 41.89, 37.93, 33.64, 31.49, 26.52, 24.70, 22.75, 22.13, 21.48, 19.26, 18.53.
Compound 15x (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-(4-nitrobenzamido)butanamido)pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 8.32 (d, J = 8.9 Hz, 2H), 8.05 (d, J = 8.9 Hz, 2H), 4.57–4.51 (m, 1H), 4.49–4.38 (m, 2H), 3.73 (s, 3H), 3.34–3.21 (m, 2H), 2.43–2.34 (m, 1H), 2.32–2.12 (m, 2H), 2.10–1.98 (m, 1H), 1.98–1.83 (m, 2H), 1.77–1.54 (m, 5H), 1.02 (dd, J = 6.7, 3.8 Hz, 6H), 0.95 (dd, J = 18.5, 6.3 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.14, 173.31, 172.47, 171.80, 166.31, 149.62, 139.66, 128.60, 123.54, 59.37, 52.14, 52.11, 50.06, 41.94, 40.80, 37.39, 33.35, 31.16, 25.89, 24.52, 22.44, 21.57, 20.83, 19.01, 18.25.
Compound 15y (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-(4-(trifluoromethyl) benzamido)butanamido)pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD:CDCl3 = 1:1) δ: 7.99 (d, J = 8.1 Hz, 2H), 7.73 (d, J = 8.2 Hz, 2H), 4.56–4.52 (m, 1H), 4.50–4.42 (m, 2H), 3.73 (s, 3H), 3.33–3.19 (m, 2H), 2.43–2.24 (m, 2H), 2.22–2.11 (m, 1H), 2.08–1.98 (m, 1H), 1.94–1.82 (m, 2H), 1.77–1.68 (m, 2H), 1.67–1.52 (m, 3H), 1.02 (dd, J = 6.7, 3.6 Hz, 6H), 0.95 (dd, J = 18.0, 6.3 Hz, 6H); 13C NMR (101 MHz, MeOD:CDCl3 = 1:1) δ: 175.09, 173.31, 172.47, 171.93, 167.13, 137.39, 133.34, 133.01, 127.82, 125.34 (dd, J = 7.3, 3.6 Hz, 1H), 125.04, 122.34, 59.26, 52.10, 52.01, 50.07, 41.88, 40.81, 37.40, 33.30, 31.15, 25.88, 24.51, 22.36, 21.51, 20.86, 18.96, 18.17.
Compound 15z (methyl (S)-2-((S)-4-methyl-2-((S)-3-methyl-2-(tetrahydro-2H-pyran-4- carboxamido)butanamido)pentanamido)-3-((S)-2-oxopiperidin-3-yl)propanoate): 1H NMR (400 MHz, MeOD) δ: 4.59–4.49 (m, 1H), 4.42 (dd, J = 9.1, 5.8 Hz, 1H), 4.25–4.15 (m, 1H), 4.05–3.96 (m, 2H), 3.73 (s, 3H), 3.47 (tdd, J = 11.6, 5.6, 2.5 Hz, 2H), 3.32–3.23 (m, 2H), 2.61–2.46 (m, 1H), 2.42–2.20 (m, 2H), 2.10–1.98 (m, 2H), 1.95–1.84 (m, 2H), 1.84–1.65 (m, 6H), 1.65–1.50 (m, 3H), 0.95 (ddd, J = 10.2, 8.8, 5.5 Hz, 12H); 13C NMR (101 MHz, MeOD) δ: 175.61, 175.09, 173.28, 172.45, 172.03, 67.09, 58.34, 52.00, 51.78, 49.86, 41.86, 41.62, 40.80, 37.35, 33.22, 30.86, 29.29, 28.68, 25.80, 24.46, 22.38, 21.43, 20.85, 18.88, 17.79.
Compound 16a ((S)–N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methyl-2-((S)-3-methyl-2-((E)-3-(p-tolyl)acrylamido)butanamido)pentanamide): 1H NMR (400 MHz, MeOD) δ: 7.56–7.42 (m, 3H), 7.23 (d, J = 8.0 Hz, 2H), 6.69 (d, J = 15.4 Hz, 1H), 4.61–4.55 (m, 1H), 4.53–4.47 (m, 1H), 4.18–4.07 (m, 1H), 3.38–3.25 (m, 2H), 2.44–2.26 (m, 5H), 2.22–1.84 (m, 4H), 1.76–1.51 (m, 5H), 1.00–0.89 (m, 12H); 13C NMR (101 MHz, MeOD) δ: 175.36, 173.16, 172.53, 167.68, 140.87, 140.16, 132.30, 129.63, 127.69, 120.01, 64.29, 58.99, 52.37, 49.07, 42.16, 41.25, 37.49, 33.38, 31.22, 25.91, 24.63, 22.51, 21.66, 20.99, 20.85, 19.39, 18.27.
Compound 16b ((S)–N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-2-((S)-2-((E)-3-(4-isopropylphenyl)acrylamido)-3-methylbutanamido)-4-methylpentanamide): 1H NMR (400 MHz, CDCl3: MeOD = 1:1) δ: 7.56 (d, J = 15.7 Hz, 1H), 7.49 (d, J = 7.9 Hz, 2H), 7.25 (d, J = 7.9 Hz, 2H), 6.67 (d, J = 15.7 Hz, 1H), 4.46–4.36 (m, 1H), 4.31 (d, J = 7.1 Hz, 1H), 4.08–3.95 (m, 1H), 3.54 (ddd, J = 26.3, 11.1, 5.3 Hz, 2H), 3.32–3.16 (m, 2H), 2.99–2.86 (m, 1H), 2.34–2.23 (m, 1H), 2.21–2.10 (m, 1H), 2.10–1.96 (m, 2H), 1.90–1.76 (m, 1H), 1.76–1.65 (m, 2H), 1.65–1.58 (m, 3H), 1.57–1.45 (m, 1H), 1.26 (d, J = 6.9 Hz, 6H), 1.01 (dd, J = 6.4, 3.6 Hz, 6H), 0.94 (dd, J = 15.2, 6.0 Hz, 6H); 13C NMR (101 MHz, CDCl3: MeOD = 1:1) δ: 176.04, 173.41, 172.28, 167.70, 150.98, 141.38, 132.44, 127.86, 126.75, 119.08, 64.48, 59.36, 52.26, 48.86, 41.86, 40.65, 37.34, 33.96, 32.97, 30.69, 25.94, 24.61, 23.30 (double peak height), 22.47, 21.10, 20.68, 18.84, 17.85.
Compound 16c ((S)-2-((S)-2-((E)-3-([1,1′-biphenyl]-4-yl)acrylamido)-3-methylbutanamido)-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, CDCl3) δ: 7.68–7.59 (m, 7H), 7.47 (dd, J = 14.9, 7.5 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 6.70 (d, J = 15.7 Hz, 1H), 4.41 (dd, J = 9.1, 5.4 Hz, 1H), 4.32 (d, J = 7.1 Hz, 1H), 4.04–3.94 (m, 1H), 3.55 (qd, J = 11.3, 5.2 Hz, 2H), 3.33–3.19 (m, 2H), 2.33–2.22 (m, 1H), 2.21–2.10 (m, 1H), 2.10–1.99 (m, 2H), 1.90–1.78 (m, 1H), 1.77–1.59 (m, 5H), 1.59–1.48 (m, 1H), 1.01 (dd, J = 6.7, 4.1 Hz, 6H), 0.94 (dd, J = 14.0, 5.8 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 176.11, 173.25, 172.14, 167.31, 142.55, 141.06, 140.14, 133.74, 128.77, 128.31, 127.65, 127.34, 126.83, 119.96, 64.64, 59.29, 52.29, 52.25, 42.02, 40.74, 37.46, 33.01, 30.82, 26.12, 24.67, 22.65, 21.30, 20.69, 18.99, 17.98.
Compound 16d ((S)–N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-2-((S)-2-((E)-3-(4-methoxyphenyl)acrylamido)-3-methylbutanamido)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.89 (d, J = 8.9 Hz, 1H), 7.51 (dd, J = 12.2, 3.4 Hz, 2H), 6.94 (s, 1H), 6.92 (s, 1H), 6.62 (d, J = 15.8 Hz, 1H), 4.39 (dt, J = 8.5, 5.5 Hz, 1H), 4.31 (dt, J = 7.6, 3.9 Hz, 1H), 4.01 (dd, J = 10.0, 7.0 Hz, 1H), 3.81 (s, 3H), 3.58–3.42 (m, 2H), 3.28–3.16 (m, 2H), 2.42–2.25 (m, 1H), 2.17–1.98 (m, 3H), 1.87–1.75 (m, 1H), 1.75–1.56 (m, 5H), 1.54–1.44 (m, 1H), 1.02–0.88 (m, 12H); 13C NMR (101 MHz, MeOD) δ: 175.90, 173.50, 172.44, 167.88, 167.80, 161.25, 140.76, 129.18, 127.51, 117.65, 117.59, 113.95, 64.29, 59.34, 59.25, 54.45, 52.33, 41.62, 40.59, 37.26, 32.73, 30.66, 27.35, 25.74, 24.50, 22.02, 20.71, 18.49, 17.51.
Compound 16e ((S)-2-((S)-2-((E)-3-(4-(dimethylamino)phenyl)acrylamido)-3- methylbutanamido)-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.47 (dd, J = 27.0, 12.2 Hz, 2H), 7.08 (d, J = 8.6 Hz, 1H), 6.71 (t, J = 8.5 Hz, 2H), 6.46 (d, J = 15.6 Hz, 1H), 4.44–4.34 (m, 1H), 4.30–4.22 (m, 1H), 4.06–3.93 (m, 1H), 3.63–3.43 (m, 2H), 3.31–3.14 (m, 2H), 3.01 (s, 3H), 2.88 (s, 3H), 2.34–2.20 (m, 1H), 2.20–1.95 (m, 3H), 1.90–1.76 (m, 1H), 1.73–1.57 (m, 5H), 1.51 (dd, J = 9.8, 3.4 Hz, 1H), 1.05–0.83 (m, 12H); 13C NMR (101 MHz, MeOD) δ: 176.01, 174.40, 172.45, 168.49, 151.69, 142.02, 131.22, 129.29, 128.70, 122.60, 114.45, 111.86, 64.46, 59.34, 52.22, 48.68, 41.86, 40.64, 39.57, 37.36, 32.93, 30.67, 30.43, 25.97, 24.61, 22.46, 21.08, 20.69, 18.84, 17.83.
Compound 16f ((S)-2-((S)-2-((E)-3-(4-fluorophenyl)acrylamido)-3-methylbutanamido)-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.64–7.50 (m, 3H), 7.11 (t, J = 8.7 Hz, 2H), 6.71 (d, J = 15.8 Hz, 1H), 4.40 (dd, J = 8.6, 6.4 Hz, 1H), 4.34 (d, J = 7.3 Hz, 1H), 4.10–3.96 (m, 1H), 3.52 (ddd, J = 25.2, 11.0, 5.6 Hz, 2H), 3.29–3.15 (m, 2H), 2.41–2.25 (m, 1H), 2.21–1.99 (m, 3H), 1.87–1.75 (m, 1H), 1.75–1.57 (m, 5H), 1.52–1.40 (m, 1H), 1.00 (dd, J = 6.6, 4.0 Hz, 6H), 0.93 (dd, J = 16.0, 6.4 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.89, 173.46, 172.25, 167.20, 163.61 (d, J = 248.8 Hz), 139.65, 131.40 (d, J = 3.3 Hz), 129.62 (d, J = 8.5 Hz), 120.13, 115.43 (d, J = 22.1 Hz), 64.31, 59.20, 52.24, 48.41, 41.63, 40.57, 37.26, 32.72, 30.72, 25.74, 24.51, 22.03, 20.76 (double peak height), 18.50, 17.54.
Compound 16g ((S)-2-((S)-2-((E)-3-(2-fluorophenyl)acrylamido)-3-methylbutanamido)-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.75–7.59 (m, 2H), 7.39 (dd, J = 13.8, 7.0 Hz, 1H), 7.17 (dt, J = 19.3, 8.0 Hz, 2H), 6.87 (d, J = 15.9 Hz, 1H), 4.40 (dd, J = 8.8, 6.3 Hz, 1H), 4.33 (d, J = 7.3 Hz, 1H), 4.08–3.96 (m, 1H), 3.51 (ddd, J = 25.1, 11.0, 5.6 Hz, 2H), 3.28–3.15 (m, 2H), 2.43–2.27 (m, 1H), 2.22–1.99 (m, 3H), 1.86–1.76 (m, 1H), 1.75–1.55 (m, 5H), 1.53–1.40 (m, 1H), 1.00 (dd, J = 6.6, 3.9 Hz, 6H), 0.93 (dd, J = 16.2, 6.4 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.91, 173.38, 172.18, 167.05, 161.20 (d, J = 251.4 Hz), 133.19 (d, J = 3.0 Hz), 131.15 (d, J = 8.7 Hz), 128.68 (d, J = 2.7 Hz), 124.37 (d, J = 3.5 Hz), 122.88 (d, J = 6.0 Hz), 122.69 (d, J = 11.7 Hz), 115.60 (d, J = 22.1 Hz), 64.27, 59.26, 52.20, 48.35, 41.62, 40.59, 37.27, 32.73, 30.65, 25.76, 24.50, 22.01, 20.74 (double peak height), 18.48, 17.50.
Compound 16h ((S)-2-((S)-2-((E)-3-(3-fluorophenyl)acrylamido)-3-methylbutanamido)-N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methylpentanamide): 1H NMR (400 MHz, MeOD) δ: 7.54 (d, J = 15.8 Hz, 1H), 7.44–7.28 (m, 3H), 7.09 (dd, J = 10.3, 5.7 Hz, 1H), 6.79 (d, J = 15.8 Hz, 1H), 4.41 (dd, J = 8.6, 6.5 Hz, 1H), 4.35 (d, J = 7.2 Hz, 1H), 4.10–3.98 (m, 1H), 3.52 (ddd, J = 24.8, 11.0, 5.6 Hz, 2H), 3.29–3.16 (m, 2H), 2.40–2.27 (m, 1H), 2.21–2.00 (m, 3H), 1.88–1.76 (m, 1H), 1.76–1.55 (m, 5H), 1.54–1.41 (m, 1H), 1.01 (dd, J = 6.6, 3.7 Hz, 6H), 0.93 (dd, J = 15.8, 6.4 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.91, 173.38, 172.20, 166.90, 163.10 (d, J = 245.0 Hz), 139.53 (d, J = 2.4 Hz), 137.46 (d, J = 7.8 Hz), 130.35 (d, J = 8.4 Hz), 123.77 (d, J = 2.6 Hz), 121.79, 116.05 (d, J = 21.6 Hz), 113.53 (d, J = 22.2 Hz), 64.29, 59.23, 52.21, 48.33, 41.63, 40.57, 37.27, 32.69, 30.73, 25.74, 24.51, 22.02, 20.76 (double peak height), 18.50, 17.54.
Compound 16i (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)-2-naphthamide): 1H NMR (400 MHz, MeOD) δ: 8.42 (s, 1H), 8.02–7.86 (m, 4H), 7.61–7.51 (m, 2H), 4.53 (t, J = 6.8 Hz, 1H), 4.45 (dd, J = 8.9, 6.2 Hz, 1H), 4.12–3.98 (m, 1H), 3.53 (ddd, J = 25.3, 11.0, 5.6 Hz, 2H), 3.27–3.14 (m, 2H), 2.42–2.32 (m, 1H), 2.31–2.19 (m, 1H), 2.18–2.08 (m, 1H), 2.08–1.99 (m, 1H), 1.83–1.71 (m, 2H), 1.71–1.58 (m, 4H), 1.51–1.38 (m, 1H), 1.08 (dd, J = 6.7, 3.6 Hz, 6H), 0.93 (dd, J = 14.5, 6.5 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.89, 173.33, 172.40, 168.88, 134.93, 132.61, 131.19, 128.69, 127.95, 127.68, 127.51, 127.39, 126.47, 123.68, 64.32, 59.73, 52.29, 48.30, 41.62, 40.67, 37.27, 32.64, 30.78, 25.76, 24.51, 21.99, 20.88, 20.79, 18.65, 18.08.
Compound 16j (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)-1H-indole-5-carboxamide): 1H NMR (400 MHz, CDCl3: MeOD = 1:2) δ: 7.83 (d, J = 8.8 Hz, 1H), 7.68–7.62 (m, 1H), 7.50 (d, J = 8.3 Hz, 1H), 7.31–7.22 (m, 1H), 7.09 (t, J = 7.5 Hz, 1H), 4.46–4.37 (m, 2H), 4.08–3.98 (m, 1H), 3.55 (ddd, J = 17.1, 11.1, 5.5 Hz, 2H), 3.29–3.15 (m, 2H), 2.36–2.26 (m, 1H), 2.25–2.14 (m, 2H), 2.04–1.97 (m, 1H), 1.86–1.75 (m, 1H), 1.72–1.57 (m, 5H), 1.55–1.44 (m, 1H), 1.06 (dd, J = 6.7, 2.2 Hz, 6H), 0.91 (dd, J = 12.6, 6.0 Hz, 6H); 13C NMR (101 MHz, CDCl3: MeOD = 1:2) δ: 176.07, 173.44, 172.40, 162.89, 137.09, 130.06, 127.42, 124.19, 121.68, 120.11, 112.08, 104.27, 64.52, 59.57, 53.37, 52.39, 41.86, 40.39, 37.34, 32.69, 30.81, 25.82, 24.65, 22.43, 21.02, 20.65, 18.81, 18.24.
Compound 16k (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl) amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)-1H-indole-2-carboxamide): 1H NMR (400 MHz, MeOD) δ: 8.16 (s, 1H), 7.64 (dd, J = 8.5, 1.4 Hz, 1H), 7.43 (d, J = 8.6 Hz, 1H), 7.32 (d, J = 3.1 Hz, 1H), 6.56 (d, J = 3.1 Hz, 1H), 4.43 (ddd, J = 15.1, 8.4, 4.1 Hz, 2H), 4.06–3.98 (m, 1H), 3.50 (ddd, J = 23.9, 11.0, 5.6 Hz, 2H), 3.26–3.09 (m, 2H), 2.42–2.29 (m, 1H), 2.27–2.15 (m, 1H), 2.15–2.05 (m, 1H), 2.05–1.94 (m, 1H), 1.81–1.68 (m, 2H), 1.66–1.51 (m, 4H), 1.49–1.33 (m, 1H), 1.04 (dd, J = 6.7, 2.3 Hz, 6H), 0.92 (dd, J = 16.0, 6.4 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.92, 173.38, 172.73, 170.28, 138.23, 127.62, 125.96, 124.64, 120.33, 120.22, 110.71, 102.24, 64.30, 59.56, 52.28, 48.24, 41.61, 40.58, 37.22, 32.61, 30.84, 25.71, 24.48, 21.99, 20.81, 20.76, 18.65, 18.01.
Compound 16l ((S)–N-((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)-4-methyl-2-((S)-3-methyl-2-(3-phenylpropanamido)butanamido)pentanamide): 1H NMR (400 MHz, MeOD) δ: 7.32–7.10 (m, 5H), 4.44–4.33 (m, 1H), 4.14 (dt, J = 7.6, 3.9 Hz, 1H), 4.08–3.97 (m, 1H), 3.52 (ddd, J = 24.7, 11.0, 5.6 Hz, 2H), 3.30–3.18 (m, 2H), 2.93 (t, J = 7.7 Hz, 2H), 2.60 (t, J = 7.7 Hz, 2H), 2.38–2.23 (m, 1H), 2.17–1.93 (m, 3H), 1.89–1.77 (m, 1H), 1.74–1.65 (m, 2H), 1.64–1.57 (m, 3H), 1.55–1.41 (m, 1H), 0.96 (dd, J = 17.9, 6.5 Hz, 6H), 0.89 (dd, J = 15.2, 6.8 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.95, 174.15, 174.06, 173.44, 140.78, 128.07, 128.03, 125.79, 64.29, 59.03, 52.14, 48.44, 41.62, 40.57, 37.23, 37.18, 32.80, 31.42, 30.41, 25.72, 24.48, 22.04, 20.72(double peak height), 18.40, 17.44.
Compound 16m (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)benzamide): 1H NMR (400 MHz, MeOD) δ: 7.89–7.79 (m, 2H), 7.58–7.50 (m, 1H), 7.46 (t, J = 7.5 Hz, 2H), 4.45–4.34 (m, 2H), 4.07–3.96 (m, 1H), 3.49 (ddd, J = 24.6, 11.0, 5.6 Hz, 2H), 3.28–3.16 (m, 2H), 2.41–2.29 (m, 1H), 2.24–1.99 (m, 3H), 1.85–1.76 (m, 1H), 1.75–1.53 (m, 5H), 1.51–1.40 (m, 1H), 1.02 (d, J = 6.7 Hz, 6H), 0.93 (dd, J = 18.2, 6.5 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.94, 173.44, 172.37, 168.93, 133.98, 131.46, 128.19, 127.12, 64.28, 59.57, 52.30, 48.28, 41.62, 40.55, 37.21, 32.65, 30.67, 25.72, 24.47, 21.97, 20.79, 20.75, 18.56, 17.94.
Compound 16n (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)-[1,1′-biphenyl]-4-carboxamide): 1H NMR (400 MHz, CDCl3: MeOD = 1:1) δ: 7.94 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 8.5 Hz, 2H), 7.67–7.59 (m, 2H), 7.46 (dd, J = 10.3, 4.7 Hz, 2H), 7.40–7.34 (m, 1H), 4.52–4.38 (m, 2H), 4.10–3.95 (m, 1H), 3.62–3.46 (m, 2H), 3.32–3.19 (m, 2H), 2.39–2.27 (m, 1H), 2.27–2.15 (m, 1H), 2.15–1.98 (m, 2H), 1.88–1.78 (m, 1H), 1.77–1.57 (m, 5H), 1.55–1.47 (m, 1H), 1.08–0.90 (m, 12H); 13C NMR (101 MHz, CDCl3: MeOD = 1:1) δ: 176.03, 173.29, 172.27, 168.43, 144.56, 139.82, 132.45, 128.74, 127.86, 127.78, 126.91, 126.89, 64.43, 59.46, 52.32, 48.51, 41.84, 40.73, 37.33, 32.80, 30.98, 25.94, 24.60, 22.36, 21.20, 20.78, 18.90, 18.19.
Compound 16o (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)cyclohexanecarboxamide): 1H NMR (400 MHz, CDCl3: MeOD = 1:1) δ: 4.37 (t, J = 7.3 Hz, 1H), 4.18 (d, J = 7.4 Hz, 1H), 4.05–3.91 (m, 1H), 3.52 (ddd, J = 27.9, 11.1, 5.1 Hz, 2H), 3.33–3.21 (m, 2H), 2.35–2.17 (m, 2H), 2.12–1.99 (m, 3H), 1.93–1.75 (m, 5H), 1.75–1.55 (m, 6H), 1.54–1.35 (m, 3H), 1.34–1.21 (m, 3H), 0.99–0.85 (m, 12H); 13C NMR (101 MHz, CDCl3: MeOD = 1:1) δ: 177.67, 176.11, 173.25, 172.21, 64.51, 58.41, 52.16, 48.70, 45.09, 41.95, 40.85, 37.42, 32.88, 30.74, 29.82, 29.06, 26.06, 25.59, 25.56, 25.44, 24.60, 22.52, 21.24, 20.78, 18.91, 17.83.
Compound 16p (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)furan-2-carboxamide): 1H NMR (400 MHz, MeOD) δ: 7.69 (dd, J = 1.6, 0.6 Hz, 1H), 7.19 (dd, J = 3.5, 0.7 Hz, 1H), 6.60 (dd, J = 3.5, 1.8 Hz, 1H), 4.48–4.35 (m, 2H), 4.11–3.97 (m, 1H), 3.52 (ddd, J = 17.0, 11.0, 5.6 Hz, 2H), 3.31–3.18 (m, 2H), 2.43–2.30 (m, 1H), 2.23–2.00 (m, 3H), 1.89–1.77 (m, 1H), 1.76–1.56 (m, 5H), 1.55–1.40 (m, 1H), 1.05–0.89 (m, 12H); 13C NMR (101 MHz, MeOD) δ: 175.92, 173.38, 172.06, 159.10, 147.22, 145.16, 114.51, 111.74, 64.34, 58.57, 53.47, 52.41, 41.64, 40.64, 40.59, 37.25, 32.63, 30.95, 25.76, 24.49, 22.00, 20.83, 18.54, 17.73.
Compound 16q (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)furan-3-carboxamide): 1H NMR (400 MHz, MeOD) δ: 8.15 (s, 1H), 7.56 (t, J = 1.7 Hz, 1H), 6.86 (d, J = 1.2 Hz, 1H), 4.45–4.32 (m, 2H), 4.12–3.98 (m, 1H), 3.50 (ddd, J = 25.4, 11.0, 5.6 Hz, 2H), 3.29–3.16 (m, 2H), 2.40–2.28 (m, 1H), 2.18–1.99 (m, 3H), 1.88–1.76 (m, 1H), 1.75–1.55 (m, 5H), 1.52–1.39 (m, 1H), 0.99 (d, J = 6.7 Hz, 6H), 0.92 (dd, J = 16.5, 6.5 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.90, 173.39, 172.34, 163.73, 145.54, 143.81, 121.93, 108.40, 64.32, 59.09, 52.27, 48.33, 41.64, 40.61, 37.26, 32.67, 30.55, 25.75, 24.48, 21.99, 20.82 (double peak height), 18.53, 18.01.
Compound 16r (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)-1H-pyrrole-2-carboxamide): 1H NMR (400 MHz, MeOD) δ: 6.93 (dd, J = 14.3, 2.4 Hz, 2H), 6.23–6.15 (m, 1H), 4.41 (t, J = 6.5 Hz, 2H), 4.14–3.95 (m, 1H), 3.52 (ddd, J = 17.1, 10.9, 5.7 Hz, 2H), 3.30–3.17 (m, 2H), 2.42–2.29 (m, 1H), 2.28–2.10 (m, 2H), 2.10–1.99 (m, 1H), 1.92–1.77 (m, 1H), 1.76–1.56 (m, 5H), 1.56–1.41 (m, 1H), 1.02 (d, J = 6.7 Hz, 6H), 0.91 (dd, J = 12.4, 6.4 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.96, 173.43, 172.74, 162.23, 124.98, 122.05, 111.14, 108.92, 64.32, 59.01, 52.25, 48.27, 41.63, 40.39, 37.25, 32.49, 30.75, 25.65, 24.50, 21.97, 20.74, 20.69, 18.49, 17.90.
Compound 16s (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)isonicotinamide): 1H NMR (400 MHz, MeOD) δ: 8.69 (dd, J = 4.6, 1.5 Hz, 2H), 7.81 (dd, J = 4.6, 1.6 Hz, 2H), 4.39 (dd, J = 8.7, 5.5 Hz, 2H), 4.11–3.96 (m, 1H), 3.49 (ddd, J = 24.6, 10.9, 5.7 Hz, 2H), 3.28–3.16 (m, 2H), 2.41–2.28 (m, 1H), 2.24–1.99 (m, 3H), 1.87–1.76 (m, 1H), 1.76–1.54 (m, 5H), 1.53–1.39 (m, 1H), 1.02 (d, J = 6.7 Hz, 6H), 0.93 (dd, J = 18.1, 6.5 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.91, 173.31, 171.95, 166.62, 149.54, 142.23, 121.77, 64.28, 59.80, 52.27, 49.23, 48.27, 41.62, 40.55, 37.21, 32.64, 30.57, 25.73, 24.48, 21.97, 20.80, 20.78, 18.51, 17.98.
Compound 16t (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)nicotinamide): 1H NMR (400 MHz, MeOD) δ: 9.08–8.97 (m, 1H), 8.69 (dd, J = 4.9, 1.6 Hz, 1H), 8.34–8.23 (m, 1H), 7.99 (d, J = 8.9 Hz, 1H), 7.54 (ddd, J = 8.0, 5.0, 0.6 Hz, 1H), 4.47–4.33 (m, 2H), 4.09–3.95 (m, 1H), 3.50 (ddd, J = 25.3, 10.9, 5.6 Hz, 2H), 3.28–3.17 (m, 2H), 2.40–2.27 (m, 1H), 2.25–2.15 (m, 1H), 2.14–1.99 (m, 2H), 1.88–1.77 (m, 1H), 1.76–1.56 (m, 5H), 1.51–1.39 (m, 1H), 1.03 (dd, J = 6.7, 1.8 Hz, 6H), 0.93 (dd, J = 17.6, 6.5 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.88, 173.39, 172.09, 166.63, 151.37, 147.99, 135.92, 130.42, 123.71, 64.31, 59.76, 52.29, 48.31, 41.63, 40.61, 37.24, 32.67, 30.56, 25.75, 24.49, 22.00, 20.82, 18.56, 18.03.
Compound 16u (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)picolinamide): 1H NMR (400 MHz, CDCl3) δ 8.57 (d, J = 4.6 Hz, 1H), 8.20–8.15 (m, 1H), 7.86 (td, J = 7.7, 1.8 Hz, 1H), 7.44 (ddd, J = 7.6, 4.8, 1.2 Hz, 1H), 4.75–4.55 (m, 2H), 4.19–4.01 (m, 2H), 3.74–3.63 (m, 1H), 3.55(ddd, J = 11.7, 7.6, 5.2 Hz, 1H), 3.32 (d, J = 5.0 Hz, 2H), 2.26 (dq, J = 13.3, 6.4, 5.8 Hz, 3H), 2.10–1.99 (m, 2H), 1.86 (dt, J = 13.7, 4.5 Hz, 1H), 1.76–1.60 (m, 4H), 1.54 (dtd, J = 14.7, 11.1, 10.7, 5.5 Hz, 2H), 1.00 (d, J = 6.8 Hz, 6H), 0.89 (t, J = 5.6 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 175.58, 173.12, 170.97, 164.70, 149.26, 148.30, 137.42, 126.45, 122.38, 65.68, 58.68, 52.00, 49.52, 42.28, 41.90, 37.83, 32.54, 31.58, 26.54, 24.87, 22.83, 22.08, 21.51, 19.30, 18.33.
Compound 16v (4-fluoro-N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl) propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)benzamide): 1H NMR (400 MHz, MeOD) δ: 7.84–7.79 (m, 2H), 7.09 (t, J = 8.7 Hz, 2H), 4.36–4.24 (m, 2H), 4.01–3.87 (m, 1H), 3.40 (ddd, J = 25.6, 11.0, 5.6 Hz, 2H), 3.18–3.07 (m, 2H), 2.31–2.18 (m, 1H), 2.13–1.90 (m, 3H), 1.76–1.66 (m, 1H), 1.66–1.47 (m, 5H), 1.44–1.29 (m, 1H), 0.92 (d, J = 6.7 Hz, 6H), 0.82 (dd, J = 17.6, 6.4 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.93, 173.41, 172.32, 167.75, 164.89 (d, J = 250.5 Hz), 130.32 (d, J = 2.9 Hz), 129.79 (d, J = 9.0 Hz), 114.99 (d, J = 22.2 Hz), 64.29, 59.69, 52.31, 48.27, 41.63, 40.59, 37.25, 32.69, 30.62, 25.76, 24.48, 21.97, 20.77(double peak height), 18.55, 17.95.
Compound 16w (4-chloro-N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl) propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)benzamide): 1H NMR (400 MHz, MeOD) δ: 7.86 (d, J = 7.8 Hz, 2H), 7.49 (d, J = 8.0 Hz, 2H), 4.49–4.32 (m, 2H), 4.11–3.97 (m, 1H), 3.59–3.43 (m, 2H), 3.30–3.14 (m, 2H), 2.43–2.30 (m, 1H), 2.25–1.99 (m, 3H), 1.91–1.78 (m, 1H), 1.68 (ddd, J = 33.8, 16.7, 9.8 Hz, 5H), 1.56–1.40 (m, 1H), 1.04 (d, J = 6.2 Hz, 6H), 0.95 (dd, J = 17.9, 5.9 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.92, 173.33, 172.25, 167.78, 137.49, 132.62, 128.89, 128.33, 64.27, 59.73, 52.26, 48.25, 41.62, 40.56, 37.24, 32.65, 30.60, 25.75, 24.48, 21.96, 20.79, 20.75, 18.53, 17.95.
Compound 16x (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)-4-nitrobenzamide): 1H NMR (400 MHz, MeOD) δ: 8.22 (d, J = 8.8 Hz, 2H), 7.95 (d, J = 8.8 Hz, 2H), 4.33–4.23 (m, 2H), 3.99–3.85 (m, 1H), 3.39 (ddd, J = 24.5, 11.0, 5.7 Hz, 2H), 3.18–3.08 (m, 2H), 2.30–2.19 (m, 1H), 2.17–2.03 (m, 1H), 2.03–1.90 (m, 2H), 1.77–1.67 (m, 1H), 1.67–1.55 (m, 2H), 1.55–1.44 (m, 3H), 1.42–1.29 (m, 1H), 0.94 (d, J = 6.7 Hz, 6H), 0.84 (dd, J = 18.8, 6.5 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 175.93, 173.42, 172.06, 167.06, 149.68, 139.76, 128.60, 123.19, 64.27, 59.92, 52.32, 48.29, 41.62, 40.50, 37.21, 32.69, 30.56, 25.73, 24.47, 21.97, 20.78, 20.70, 18.49, 17.95.
Compound 16y (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)-4-(trifluoromethyl)benzamide): 1H NMR (400 MHz, MeOD) δ: 8.00 (d, J = 8.1 Hz, 2H), 7.76 (t, J = 9.4 Hz, 2H), 4.41 (dd, J = 15.3, 7.6 Hz, 2H), 4.10–3.92 (m, 1H), 3.52 (ddd, J = 30.1, 11.1, 5.4 Hz, 2H), 3.32–3.17 (m, 2H), 2.38–2.25 (m, 1H), 2.25–2.12 (m, 1H), 2.12–2.00 (m, 2H), 1.92–1.79 (m, 1H), 1.77–1.59 (m, 5H), 1.58–1.44 (m, 1H), 1.03 (d, J = 6.6 Hz, 6H), 0.94 (dd, J = 16.7, 6.2 Hz, 6H); 13C NMR (101 MHz, MeOD) δ: 176.09, 173.33, 171.98, 167.34, 137.36, 133.32, 133.00, 127.88, 125.34 (dd, J = 7.3, 3.6 Hz, 1H)., 124.48, 122.36, 64.47, 59.58, 52.38, 48.78, 41.90, 40.75, 37.38, 32.90, 30.92, 26.02, 24.62, 22.41, 21.23, 20.76, 18.91, 18.19.
Compound 16z (N-((S)-1-(((S)-1-(((S)-1-hydroxy-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)-4-methyl-1-oxopentan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)tetrahydro-2H-pyran-4-carboxamide): 1H NMR (400 MHz, MeOD) δ: 4.36 (dd, J = 8.5, 6.6 Hz, 1H), 4.15 (d, J = 7.6 Hz, 1H), 4.08–3.96 (m, 1H), 3.58–3.39 (m, 4H), 3.29–3.20 (m, 2H), 2.64–2.48 (m, 1H), 2.37–2.24 (m, 1H), 2.13–1.97 (m, 3H), 1.90–1.64 (m, 7H), 1.63–1.55 (m, 3H), 1.54–1.43 (m, 1H), 1.00–0.87 (m, 12H); 13C NMR (101 MHz, MeOD) δ: 176.07, 176.01, 173.33, 172.24, 66.96, 64.31, 58.70, 52.15, 52.10, 41.74, 41.38, 40.67, 37.25, 32.81, 30.54, 29.36, 28.59, 25.81, 24.50, 22.25, 20.93, 20.74, 18.66, 17.62.
Compound 17a ((S)-4-methyl-2-((S)-3-methyl-2-((E)-3-(p-tolyl)acrylamido)butanamido)-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.39 (d, J = 6.9 Hz, 1H), 7.62 (t, J = 11.2 Hz, 2H), 7.38 (d, J = 8.0 Hz, 2H), 7.12 (d, J = 8.0 Hz, 2H), 6.90 (d, J = 8.2 Hz, 1H), 6.67 (s, 1H), 6.52 (d, J = 15.6 Hz, 1H), 4.65 (dd, J = 14.3, 8.1 Hz, 1H), 4.56 (t, J = 8.1 Hz, 1H), 4.51–4.40 (m, 1H), 3.37–3.19 (m, 2H), 2.34 (s, 3H), 2.30–2.08 (m, 3H), 2.07–1.94 (m, 1H), 1.94–1.79 (m, 2H), 1.73–1.56 (m, 4H), 1.54–1.42 (m, 1H), 1.00–0.87 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.81, 174.78, 173.12, 171.56, 166.25, 141.51, 140.02, 132.01, 129.54, 127.87, 119.45, 58.66, 56.95, 51.99, 42.32, 41.53, 37.27, 31.54, 30.89, 27.03, 24.85, 22.78, 22.20, 21.42, 21.32, 19.28, 18.50. HRMS (m/z): 527.3156 (M + H) +.
Compound 17b ((S)-2-((S)-2-((E)-3-(4-isopropylphenyl)acrylamido)-3-methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.49 (s, 1H), 8.32 (d, J = 6.9 Hz, 1H), 7.61 (d, J = 15.6 Hz, 1H), 7.41 (t, J = 10.4 Hz, 2H), 7.26 (s, 1H), 7.18 (d, J = 8.1 Hz, 2H), 6.75 (d, J = 8.7 Hz, 1H), 6.51 (dd, J = 24.2, 16.8 Hz, 2H), 4.62 (dd, J = 14.0, 8.2 Hz, 1H), 4.55–4.48 (m, 1H), 4.48–4.36 (m, 1H), 3.35–3.22 (m, 2H), 2.89 (dt, J = 13.8, 6.9 Hz, 1H), 2.44–2.28 (m, 1H), 2.26–2.09 (m, 2H), 2.05–2.00 (m, 1H), 1.94–1.80 (m, 2H), 1.77–1.63 (m, 3H), 1.63–1.49 (m, 2H), 1.23 (d, J = 6.9 Hz, 6H), 0.93 (ddd, J = 12.9, 11.5, 6.4 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.72, 174.82, 173.04, 171.48, 166.27, 150.97, 141.56, 132.38, 127.97, 126.92, 119.46, 58.74, 57.06, 52.04, 42.33, 41.55, 37.38, 34.01, 31.39, 30.94, 28.30, 27.19, 24.86, 23.77, 22.81, 22.13, 21.32, 19.28, 18.43. HRMS (m/z): 555.3471 (M + H) +.
Compound 17c ((S)-2-((S)-2-((E)-3-([1,1′-biphenyl]-4-yl)acrylamido)-3-methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.51 (s, 1H), 8.40 (d, J = 6.6 Hz, 1H), 7.69 (d, J = 15.6 Hz, 1H), 7.55 (t, J = 6.0 Hz, 6H), 7.37 (dt, J = 25.1, 7.2 Hz, 3H), 7.26 (s, 1H), 6.97 (s, 1H), 6.62 (d, J = 15.6 Hz, 1H), 6.50 (s, 1H), 4.73–4.53 (m, 2H), 4.45 (t, J = 10.3 Hz, 1H), 3.42–3.19 (m, 2H), 2.43–2.29 (m, 1H), 2.26–2.10 (m, 2H), 2.02–1.94 (m, 1H), 1.94–1.80 (m, 2H), 1.77–1.43 (m, 5H), 0.95 (ddd, J = 16.8, 8.7, 4.7 Hz, 12H).; 13C NMR (101 MHz, CDCl3) δ: 199.76, 174.80, 173.09, 171.56, 166.08, 142.41, 141.05, 140.15, 133.77, 128.85, 128.40, 127.70, 127.43, 126.94, 120.47, 58.73, 57.08, 52.04, 42.34, 41.54, 37.39, 31.59, 30.93, 27.13, 24.89, 22.78, 22.25, 21.32, 19.29, 18.55. HRMS (m/z): 589.3313 (M + H) +.
Compound 17d ((S)-2-((S)-2-((E)-3-(4-methoxyphenyl)acrylamido)-3-methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.49 (s, 1H), 8.32 (d, J = 6.7 Hz, 1H), 7.59 (d, J = 15.5 Hz, 1H), 7.44 (d, J = 8.7 Hz, 2H), 7.27 (s, 1H), 6.85 (d, J = 8.7 Hz, 2H), 6.73 (s, 1H), 6.54 (s, 1H), 6.41 (d, J = 15.6 Hz, 1H), 4.62 (dd, J = 13.9, 8.3 Hz, 1H), 4.51 (t, J = 8.0 Hz, 1H), 4.48–4.36 (m, 1H), 3.81 (s, 3H), 3.38–3.23 (m, 2H), 2.43–2.28 (m, 1H), 2.28–2.08 (m, 2H), 2.04–1.97 (m, 1H), 1.92–1.81 (m, 2H), 1.77–1.50 (m, 5H), 0.93 (ddd, J = 12.6, 10.3, 6.4 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.75, 174.87, 173.04, 171.55, 166.42, 160.97, 141.23, 129.47, 127.47, 118.02, 114.26, 58.72, 57.07, 55.33, 52.04, 42.33, 41.50, 37.37, 31.40, 30.93, 27.19, 24.86, 22.82, 22.12, 21.31, 19.30, 18.42. HRMS (m/z): 543.3109 (M + Na) +.
Compound 17e ((S)-2-((S)-2-((E)-3-(4-(dimethylamino)phenyl)acrylamido)-3- methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.30 (d, J = 6.9 Hz, 1H), 7.55 (dd, J = 12.1, 7.0 Hz, 1H), 7.38 (d, J = 8.7 Hz, 2H), 7.26 (s, 1H), 6.76–6.44 (m, 4H), 6.30 (d, J = 15.4 Hz, 1H), 4.65–4.54 (m, 1H), 4.52–4.32 (m, 2H), 3.27 (s, 2H), 2.99 (s, 6H), 2.44–2.27 (m, 1H), 2.25–2.10 (m, 2H), 2.08–1.95 (m, 1H), 1.93–1.79 (m, 2H), 1.74–1.47 (m, 5H), 1.01–0.83 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.88, 174.93, 173.07, 171.73, 167.08, 151.45, 142.06, 131.45, 129.46, 128.86, 122.56, 114.99, 111.89, 58.79, 57.10, 52.07, 42.32, 41.36, 40.15, 37.39, 31.24, 30.96, 27.19, 24.86, 22.86, 22.05, 21.24, 19.34, 18.41. HRMS (m/z): 556.3422 (M + H) +
Compound 17f ((S)-2-((S)-2-((E)-3-(4-fluorophenyl)acrylamido)-3- methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.38 (d, J = 6.2 Hz, 1H), 7.62 (t, J = 15.5 Hz, 2H), 7.53–7.39 (m, 2H), 7.08–6.85 (m, 3H), 6.60 (s, 1H), 6.49 (d, J = 15.6 Hz, 1H), 4.71–4.60 (m, 1H), 4.56 (t, J = 7.7 Hz, 1H), 4.51–4.39 (m, 1H), 3.40–3.19 (m, 2H), 2.43–2.27 (m, 1H), 2.24–2.09 (m, 2H), 2.05–1.94 (m, 1H), 1.94–1.80 (m, 2H), 1.78–1.63 (m, 3H), 1.63–1.49 (m, 2H), 1.01–0.85 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.71, 174.79, 173.08, 171.49, 165.89, 163.56 (d, J = 250.2 Hz, 1C), 140.29, 131.04 (d, J = 3.0 Hz, 1C), 129.69 (d, J = 8.4 Hz, 1C), 120.28, 115.92 (d, J = 21.8 Hz, 1C), 58.66, 57.01, 52.02, 42.33, 41.59, 37.32, 31.57, 30.90, 27.11, 24.86, 22.77, 22.20, 21.34, 19.26, 18.48. HRMS (m/z): 531.2905 (M + H) +.
Compound 17g ((S)-2-((S)-2-((E)-3-(2-fluorophenyl)acrylamido)-3- methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.49 (s, 1H), 8.32 (d, J = 6.8 Hz, 1H), 7.71 (d, J = 15.8 Hz, 1H), 7.49 (t, J = 7.3 Hz, 1H), 7.30 (dd, J = 13.9, 7.3 Hz, 1H), 7.26 (s, 1H), 7.16–7.01 (m, 2H), 6.78 (d, J = 8.7 Hz, 1H), 6.66 (d, J = 15.8 Hz, 1H), 6.41 (s, 1H), 4.61 (dd, J = 13.9, 8.2 Hz, 1H), 4.51 (t, J = 7.9 Hz, 1H), 4.43 (dd, J = 12.8, 8.1 Hz, 1H), 3.40–3.20 (m, 2H), 2.35 (td, J = 12.9, 6.6 Hz, 1H), 2.26–2.10 (m, 2H), 2.06–1.97 (m, 1H), 1.94–1.85 (m, 2H), 1.78–1.46 (m, 5H), 1.05–0.81 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.71, 174.85, 172.97, 171.29, 165.90, 161.35 (d, J = 253.4 Hz, 1C), 134.58, 131.08 (d, J = 8.7 Hz, 1C), 129.63 (d, J = 2.7 Hz, 1C), 124.41 (d, J = 3.3 Hz, 1C), 123.27 (d, J = 7.0 Hz, 1C), 122.78 (d, J = 11.8 Hz, 1C), 116.11 (d, J = 22.0 Hz, 1C), 58.78, 57.20, 52.06, 42.38, 41.58, 37.49, 31.40, 30.98, 27.35, 24.86, 22.82, 22.09, 21.32, 19.28, 18.37. HRMS (m/z): 531.2907 (M + H) +.
Compound 17h ((S)-2-((S)-2-((E)-3-(3-fluorophenyl)acrylamido)-3- methylbutanamido)-4-methyl-N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.49 (s, 1H), 8.34 (d, J = 6.8 Hz, 1H), 7.59 (d, J = 15.6 Hz, 1H), 7.35–7.26 (m, 3H), 7.26 (s, 2H), 7.24–7.16 (m, 1H), 7.06–6.99 (m, 1H), 6.82 (s, 1H), 6.54 (d, J = 15.5 Hz, 1H), 6.34 (s, 1H), 4.66–4.57 (m, 1H), 4.52 (t, J = 8.0 Hz, 1H), 4.42 (d, J = 10.9 Hz, 1H), 3.30 (d, J = 8.3 Hz, 2H), 2.43–2.30 (m, 1H), 2.24–2.09 (m, 2H), 2.07–1.96 (m, 1H), 1.92–1.82 (m, 2H), 1.77–1.51 (m, 5H), 0.97 (d, J = 6.7 Hz, 6H), 0.93–0.83 (m, 6H). 13C NMR (101 MHz, CDCl3) δ: 199.62, 174.84, 172.91, 171.25, 165.58, 162.94 (d, J = 257.2 Hz, 1C), 140.31 (d, J = 2.6 Hz, 1C), 137.73 (d, J = 2.2 Hz, 1C) 130.35 (d, J = 8.4 Hz, 1C), 123.88 (d, J = 2.6 Hz, 1C), 122.21 (d, J = 2.0 Hz, 1C), 116.60 (d, J = 21.9 Hz, 1C), 114.08 (d, J = 21.5 Hz, 1C), 58.72, 57.29, 52.07, 42.39, 41.60, 37.56, 31.48, 30.99, 27.42, 24.87, 22.82, 22.13, 21.33, 19.27, 18.37. HRMS (m/z):531.2905 (M + H) +.
Compound 17i (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)-2-naphthamide): 1H NMR (400 MHz, CDCl3) δ: 9.51 (s, 1H), 8.38 (d, J = 7.0 Hz, 1H), 8.31 (s, 1H), 7.95 (d, J = 8.1 Hz, 1H), 7.91–7.77 (m, 4H), 7.50 (dt, J = 14.7, 6.9 Hz, 2H), 7.41 (d, J = 8.6 Hz, 1H), 6.93 (s, 1H), 4.80–4.63 (m, 2H), 4.53–4.38 (m, 1H), 3.40–3.18 (m, 2H), 2.42–2.15 (m, 3H), 1.99–1.76 (m, 3H), 1.74–1.56 (m, 4H), 1.51–1.34 (m, 1H), 1.02 (t, J = 7.0 Hz, 6H), 0.86 (dd, J = 8.0, 6.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.72, 174.77, 173.21, 171.56, 167.64, 134.80, 132.56, 131.35, 128.98, 128.35, 127.78, 127.68, 126.71, 123.80, 59.07, 56.81, 52.06, 42.22, 41.60, 37.09, 31.59, 30.78, 26.83, 24.87, 22.72, 22.17, 21.31, 19.34, 18.66. HRMS (m/z): 537.3000 (M + H) +.
Compound 17j (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)-1H-indole-5-carboxamide): 1H NMR (400 MHz, CDCl3) δ: 11.36 (s, 1H), 9.61 (s, 1H), 8.82 (s, 1H), 7.64 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.32–7.20 (m, 2H), 7.11 (t, J = 7.6 Hz, 2H), 7.03 (s, 1H), 6.88 (s, 1H), 5.06 (t, J = 8.3 Hz, 1H), 4.96 (dd, J = 15.7, 7.6 Hz, 1H), 4.50 (d, J = 10.0 Hz, 1H), 3.39–3.22 (m, 2H), 2.46–2.30 (m, 2H), 2.23–2.09 (m, 1H), 2.02–1.94 (m, 2H), 1.89–1.80 (m, 1H), 1.72–1.57 (m, 3H), 1.53–1.44 (m, 2H), 1.03 (t, J = 7.0 Hz, 6H), 0.80 (dd, J = 30.1, 6.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.90, 174.64, 173.81, 171.26, 161.80, 137.24, 130.15, 127.43, 124.46, 121.73, 120.46, 112.57, 102.80, 58.29, 57.18, 51.41, 42.25, 41.88, 37.42, 32.37, 30.56, 26.77, 24.99, 22.55, 22.44, 21.50, 19.13, 18.92. HRMS (m/z): 526.2953 (M + H) +
Compound 17k (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)-1H-indole-2-carboxamide):1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.70 (s, 1H), 8.30 (d, J = 6.7 Hz, 1H), 8.11 (d, J = 5.9 Hz, 1H), 7.64 (dd, J = 8.5, 1.5 Hz, 1H), 7.47–7.33 (m, 1H), 7.30–7.27 (m, 1H), 7.16 (d, J = 8.0 Hz, 1H), 6.92 (d, J = 8.3 Hz, 1H), 6.61 (s, 1H), 6.35 (s, 1H), 4.65–4.50 (m, 2H), 4.44–4.33 (m, 1H), 3.30–3.16 (m, 2H), 2.35–2.23 (m, 2H), 2.23–2.10 (m, 1H), 2.05–1.94 (m, 1H), 1.88–1.79 (m, 2H), 1.75–1.57 (m, 4H), 1.55–1.42 (m, 1H), 1.03 (d, J = 6.6 Hz, 6H), 0.89 (dd, J = 8.4, 6.1 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.94, 174.89, 173.06, 171.66, 168.81, 137.73, 130.10, 127.55, 125.77, 121.13, 120.51, 111.09, 103.66, 59.20, 57.32, 52.12, 42.32, 41.40, 37.53, 30.96, 27.48, 24.86, 22.89, 21.90, 21.26, 19.48, 18.38. HRMS (m/z):526.2956 (M + H) +
Compound 17l ((S)-4-methyl-2-((S)-3-methyl-2-(3-phenylpropanamido)butanamido)- N-((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)pentanamide): 1H NMR (400 MHz, CDCl3) δ: 9.47 (s, 1H), 8.29 (d, J = 6.9 Hz, 1H), 7.41–7.31 (m, 1H), 7.31–7.23 (m, 3H), 7.19 (d, J = 5.9 Hz, 2H), 6.60 (dd, J = 30.2, 16.6 Hz, 1H), 6.49–6.32 (m, 1H), 4.66–4.50 (m, 1H), 4.47–4.35 (m, 1H), 4.35–4.23 (m, 1H), 3.35–3.19 (m, 2H), 3.01–2.83 (m, 2H), 2.61–2.48 (m, 2H), 2.38–2.27 (m, 1H), 2.22–2.08 (m, 2H), 2.05–1.93 (m, 2H), 1.94–1.79 (m, 2H), 1.73–1.49 (m, 4H), 0.93 (dd, J = 10.9, 6.1 Hz, 6H), 0.82 (dd, J = 14.7, 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.66, 174.85, 173.09, 172.36, 171.30, 140.65, 128.53, 128.30, 126.26, 58.39, 56.91, 51.92, 42.32, 41.68, 38.13, 37.23, 31.61, 31.16, 30.89, 27.03, 24.86, 22.82, 22.14, 21.30, 19.14, 18.19. HRMS (m/z): 515.3156 (M + H) +.
Compound 17m (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)benzamide):1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.32 (d, J = 6.4 Hz, 1H), 7.83 (d, J = 7.6 Hz, 3H), 7.47 (t, J = 6.8 Hz, 1H), 7.40 (t, J = 7.5 Hz, 2H), 7.22 (d, J = 8.3 Hz, 1H), 6.96 (s, 1H), 4.81–4.53 (m, 2H), 4.51–4.34 (m, 1H), 3.27 (s, 2H), 2.38–2.12 (m, 3H), 2.06–1.76 (m, 3H), 1.74–1.45 (m, 5H), 0.92 (ddd, J = 15.9, 8.1, 5.0 Hz, 12H); 13C NMR (101 MHz, CDCl3) δ: 200.28, 175.48, 173.95, 171.94, 167.88, 134.68, 132.40, 129.60, 126.77, 59.88, 55.88, 51.03, 42.91, 41.17, 36.76, 31.79, 30.52, 27.09, 25.11, 23.09, 21.85, 21.19, 19.55, 18.39. HRMS (m/z): 487.2843 (M + H) +.
Compound 17n (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)-[1,1′-biphenyl]-4-carboxamide):1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.36 (d, J = 7.0 Hz, 1H), 7.88 (d, J = 8.1 Hz, 2H), 7.73 (d, J = 8.2 Hz, 1H), 7.60 (dd, J = 16.9, 7.8 Hz, 3H), 7.41 (dt, J = 14.2, 7.3 Hz, 3H), 7.34–7.16 (m, 2H), 6.82 (s, 1H), 4.72–4.54 (m, 2H), 4.46 (dd, J = 14.5, 6.2 Hz, 1H), 3.32–3.16 (m, 2H), 2.41–2.10 (m, 3H), 2.07–1.93 (m, 1H), 1.92–1.81 (m, 1H), 1.74–1.56 (m, 4H), 1.49 (dd, J = 20.8, 10.4 Hz, 1H), 1.43 (s, 1H), 1.04–0.82 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.72, 174.84, 173.14, 171.45, 167.25, 144.51, 139.92, 132.69, 128.93, 128.42, 128.04, 127.76, 127.18, 58.99, 56.90, 52.09, 42.28, 41.58, 37.20, 31.52, 30.84, 27.03, 24.88, 22.78, 22.12, 21.33, 19.35, 18.54. HRMS (m/z): 563.3158 (M + H) +.
Compound 17o (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)cyclohexanecarboxamide): 1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.25 (d, J = 7.0 Hz, 1H), 7.38 (d, J = 8.3 Hz, 1H), 6.77 (s, 1H), 6.29 (d, J = 8.8 Hz, 1H), 4.68–4.58 (m, 1H), 4.44 (td, J = 9.7, 7.0 Hz, 1H), 4.35–4.25 (m, 1H), 3.40–3.23 (m, 2H), 2.42–2.27 (m, 1H), 2.25–2.10 (m, 2H), 2.10–1.98 (m, 2H), 1.95–1.75 (m, 6H), 1.73–1.60 (m, 4H), 1.60–1.49 (m, 2H), 1.49–1.37 (m, 2H), 1.33–1.18 (m, 3H), 1.00–0.84 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.69, 176.28, 174.81, 173.14, 171.46, 58.05, 56.80, 51.88, 45.40, 42.31, 41.76, 37.21, 31.10, 30.85, 29.96, 29.36, 27.01, 25.72, 25.59, 24.84, 22.86, 22.06, 21.35, 19.28, 18.22. HRMS (m/z): 493.3316 (M + H) +
Compound 17p (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)furan-2-carboxamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.30 (d, J = 6.9 Hz, 1H), 7.76 (d, J = 8.3 Hz, 1H), 7.46 (s, 1H), 7.16 (d, J = 3.2 Hz, 1H), 7.08 (d, J = 8.9 Hz, 1H), 6.99 (s, 1H), 6.50 (d, J = 1.5 Hz, 1H), 4.76–4.64 (m, 1H), 4.62–4.52 (m, 1H), 4.46 (dd, J = 12.6, 7.8 Hz, 1H), 3.40–3.20 (m, 2H), 2.40–2.11 (m, 3H), 2.04–1.94 (m, 1H), 1.93–1.79 (m, 2H), 1.79–1.62 (m, 3H), 1.62–1.43 (m, 2H), 0.96 (d, J = 6.7 Hz, 6H), 0.89 (dd, J = 15.1, 8.4 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.67, 174.80, 173.21, 171.04, 158.25, 147.52, 144.29, 114.75, 112.20, 57.91, 56.80, 51.94, 42.22, 41.66, 37.03, 31.69, 30.70, 26.92, 24.84, 22.74, 22.11, 21.42, 19.25, 18.24. HRMS (m/z): 477.2636 (M + H) +.
Compound 17q (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)furan-3-carboxamide): 1H NMR (400 MHz, CDCl3) δ: 9.50 (s, 1H), 8.35 (d, J = 7.1 Hz, 1H), 8.13 (d, J = 8.0 Hz, 1H), 8.08 (s, 1H), 7.40 (d, J = 1.5 Hz, 1H), 7.34 (d, J = 8.7 Hz, 1H), 7.09 (s, 1H), 6.76 (d, J = 1.2 Hz, 1H), 4.72–4.63 (m, 1H), 4.60 (t, J = 8.4 Hz, 1H), 4.49 (dd, J = 13.1, 8.0 Hz, 1H), 3.39–3.18 (m, 2H), 2.41–2.29 (m, 1H), 2.28–2.17 (m, 1H), 2.17–2.05 (m, 1H), 2.05–1.79 (m, 3H), 1.77–1.44 (m, 5H), 0.94 (dd, J = 6.3, 4.4 Hz, 6H), 0.86 (t, J = 6.2 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.72, 174.77, 173.24, 171.84, 162.70, 145.45, 143.55, 122.23, 108.70, 58.60, 56.78, 52.00, 42.20, 41.48, 37.08, 31.26, 30.80, 26.80, 24.80, 22.67, 22.18, 21.29, 19.26, 18.74. HRMS (m/z): 477.2636 (M + H) +.
Compound 17r (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)-1H-pyrrole-2-carboxamide):1H NMR (400 MHz, CDCl3) δ: 11.08 (s, 1H), 9.52 (s, 1H), 8.43 (d, J = 6.7 Hz, 1H), 7.75 (s, 1H), 6.97 (s, 1H), 6.70 (s, 1H), 6.48 (d, J = 7.3 Hz, 1H), 6.26 (d, J = 26.0 Hz, 2H), 4.79–4.68 (m, 1H), 4.68–4.57 (m, 1H), 4.42 (qt, J = 7.7, 3.9 Hz, 1H), 3.37–3.23 (m, 2H), 2.45–2.12 (m, 3H), 2.05–1.80 (m, 3H), 1.73–1.62 (m, 2H), 1.62–1.40 (m, 3H), 1.00 (t, J = 6.1 Hz, 6H), 0.93–0.81 (m, 6H); 13C NMR (101 MHz, CDCl3) δ: 200.33, 174.84, 173.47, 171.23, 161.67, 125.00, 123.00, 110.05, 109.63, 58.85, 56.81, 51.76, 42.37, 41.20, 37.50, 31.34, 30.50, 26.64, 24.98, 22.78, 21.90, 21.12, 19.29, 18.41. HRMS (m/z): 476.2798 (M + H) +.
Compound 17s (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)isonicotinamide): 1H NMR (400 MHz, CDCl3) δ: 9.49 (s, 1H), 8.73 (s, 2H), 8.38 (d, J = 6.6 Hz, 1H), 7.68 (d, J = 4.5 Hz, 3H), 7.57 (d, J = 8.4 Hz, 1H), 6.77 (s, 1H), 4.63 (dt, J = 16.2, 7.5 Hz, 2H), 4.44 (t, J = 9.9 Hz, 1H), 3.39–3.21 (m, 2H), 2.41–2.27 (m, 1H), 2.26–2.12 (m, 2H), 2.07–1.92 (m, 1H), 1.94–1.80 (m, 2H), 1.76–1.63 (m, 3H), 1.60–1.45 (m, 2H), 0.97 (t, J = 5.2 Hz, 6H), 0.89 (dd, J = 10.2, 5.5 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.49, 174.86, 173.03, 171.08, 165.66, 150.52, 141.17, 121.18, 59.08, 57.08, 52.04, 42.30, 41.64, 37.31, 31.52, 30.87, 27.12, 24.85, 22.74, 22.15, 21.28, 19.25, 18.54. HRMS (m/z): 488.2797 (M + H) +.
Compound 17t (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)nicotinamide): 1H NMR (400 MHz, CDCl3) δ: 9.51 (s, 1H), 9.13 (s, 1H), 8.72 (d, J = 4.5 Hz, 1H), 8.29–8.10 (m, 1H), 7.39 (dd, J = 7.8, 4.8 Hz, 1H), 4.88–4.42 (m, 3H), 3.31–3.19 (m, 2H), 2.41–2.13 (m, 3H), 2.07–1.79 (m, 3H), 1.70–1.47 (m, 5H), 1.06–0.94 (m, 6H), 0.91–0.81 (m, 6H).; 13C NMR (101 MHz, CDCl3) δ: 199.56, 174.83, 173.10, 171.33, 165.80, 152.14, 148.50, 135.69, 129.93, 123.53, 59.24, 57.01, 52.03, 42.24, 41.57, 37.11, 31.33, 29.70, 27.03, 24.82, 22.76, 22.11, 21.31, 19.34, 18.58. HRMS (m/z): 488.2796 (M + H) +.
Compound 17u (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)picolinamide): 1H NMR (400 MHz, CDCl3) δ 9.52 (s, 1H), 8.56 (d, J = 5.0 Hz, 1H), 8.20 (d, J = 7.9 Hz, 1H), 7.86 (ddd, J = 7.8, 6.4, 1.8 Hz, 1H), 7.48–7.42 (m, 1H), 4.71 (td, J = 8.4, 5.7 Hz, 1H), 4.58 (dd, J = 9.1, 6.7 Hz, 1H), 4.51–4.43 (m, 1H), 3.31 (d, J = 3.1 Hz, 2H), 2.41–2.33 (m, 1H), 2.27 (dtd, J = 14.0, 8.6, 7.8, 3.3 Hz, 2H), 2.04–1.95 (m, 1H), 1.88 (dq, J = 16.2, 4.0, 3.5 Hz, 2H), 1.69 (dq, J = 15.3, 6.6, 6.2 Hz, 3H), 1.60–1.48 (m, 2H), 1.00 (dd, J = 6.8, 1.5 Hz, 6H), 0.92–0.85 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 199.88, 174.87, 173.31, 171.09, 164.52, 149.33, 148.28, 137.39, 126.41, 122.39, 58.57, 56.68, 51.87, 42.15, 41.61, 36.90, 31.43, 30.57, 26.74, 24.83, 22.75, 22.12, 21.41, 19.35, 18.17. HRMS (m/z): 488.2796 (M + H) +.
Compound 17v (4-fluoro-N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)benzamide): 1H NMR (400 MHz, CDCl3) δ: 9.48 (d, J = 4.2 Hz, 1H), 8.32 (s, 1H), 7.92–7.74 (m, 2H), 7.56 (d, J = 4.7 Hz, 1H), 7.21–6.99 (m, 3H), 6.69 (s, 1H), 4.69–4.59 (m, 1H), 4.56 (td, J = 8.0, 3.6 Hz, 1H), 4.50–4.37 (m, 1H), 3.42–3.23 (m, 2H), 2.41–2.28 (m, 1H), 2.27–2.15 (m, 2H), 2.07–1.95 (m, 1H), 1.94–1.81 (m, 2H), 1.76–1.46 (m, 5H), 0.97 (s, 6H), 0.93–0.81 (m, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.61, 174.84, 173.08, 171.36, 166.46, 164.85 (d, J = 252.4 Hz, 1C), 130.20 (d, J = 3.0 Hz, 1C), 129.58 (d, J = 8.9 Hz, 1C), 115.58 (d, J = 21.8 Hz, 1C), 58.96, 57.01, 52.04, 42.32, 41.62, 37.31, 31.48, 30.89, 27.13, 24.85, 22.75, 22.08, 21.30, 19.28, 18.47.HRMS (m/z): 505.2751 (M + H) +
Compound 17w (4-chloro-N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)benzamide): 1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.33 (d, J = 5.7 Hz, 1H), 7.76 (d, J = 7.6 Hz, 2H), 7.48 (d, J = 7.4 Hz, 1H), 7.39 (d, J = 7.5 Hz, 2H), 7.14 (d, J = 7.6 Hz, 1H), 6.60 (s, 1H), 4.61 (ddd, J = 12.7, 9.2, 3.3 Hz, 1H), 4.55 (t, J = 7.1 Hz, 1H), 4.48–4.35 (m, 1H), 3.35–3.21 (m, 2H), 2.43–2.29 (m, 1H), 2.28–2.11 (m, 3H), 2.00 (dt, J = 20.0, 8.1 Hz, 1H), 1.91–1.84 (m, 1H), 1.77–1.47 (m, 5H), 0.98 (d, J = 5.5 Hz, 6H), 0.93–0.80 (m, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.61, 173.04, 171.29, 166.50, 137.98, 132.42, 128.80, 128.68, 58.99, 57.09, 52.06, 42.33, 41.61, 37.35, 31.48, 30.90, 27.19, 24.85, 22.76, 22.08, 21.28, 19.28, 18.46. HRMS (m/z): 521.2454 (M + H) +.
Compound 17x (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)-4-nitrobenzamide): 1H NMR (400 MHz, CDCl3) δ: 9.48 (s, 1H), 8.38 (d, J = 6.7 Hz, 1H), 8.27 (d, J = 8.7 Hz, 2H), 8.00 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 8.1 Hz, 1H), 7.50 (d, J = 8.6 Hz, 1H), 6.70 (s, 1H), 4.74–4.56 (m, 2H), 4.56–4.39 (m, 1H), 3.40–3.19 (m, 2H), 2.42–2.28 (m, 1H), 2.28–2.10 (m, 2H), 2.08–1.95 (m, 1H), 1.95–1.82 (m, 2H), 1.79–1.47 (m, 5H), 0.98 (t, J = 6.4 Hz, 6H), 0.90 (dd, J = 11.6, 5.9 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.42, 174.84, 172.99, 171.04, 165.56, 149.67, 139.62, 128.55, 123.74, 59.16, 57.16, 52.03, 42.34, 41.74, 37.34, 31.61, 30.89, 27.23, 24.87, 22.73, 22.20, 21.33, 19.25, 18.55.. HRMS (m/z):532.2694 (M + H) +.
Compound 17y (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)-4-(trifluoromethyl)benzamide): 1H NMR (400 MHz, CDCl3) δ: 9.49 (s, 1H), 8.39 (d, J = 6.8 Hz, 1H), 7.92 (d, J = 8.1 Hz, 2H), 7.66 (d, J = 8.2 Hz, 3H), 7.36 (d, J = 8.7 Hz, 1H), 6.70 (s, 1H), 4.71–4.55 (m, 2H), 4.45 (ddd, J = 14.2, 11.5, 8.5 Hz, 1H), 3.38–3.21 (m, 2H), 2.41–2.32 (m, 1H), 2.26–2.12 (m, 2H), 2.04–1.95 (m, 1H), 1.93–1.80 (m, 2H), 1.76–1.45 (m, 5H), 0.98 (dd, J = 6.5, 4.4 Hz, 6H), 0.88 (dd, J = 10.3, 6.0 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 199.53, 174.86, 173.08, 171.21, 166.28, 137.35, 133.52, 133.19, 127.75, 125.55, 124.98, 122.27, 59.06, 57.07, 52.03, 42.31, 41.67, 37.30, 31.54, 30.88, 27.12, 24.86, 22.71, 22.12, 21.27, 19.24, 18.52. HRMS (m/z): 555.2718 (M + H) +.
Compound 17z (N-((S)-3-methyl-1-(((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((S)-2-oxopiperidin-3-yl)propan-2-yl)amino)pentan-2-yl)amino)-1-oxobutan-2-yl)tetrahydro-2H-pyran-4-carboxamide): 1H NMR (400 MHz, CDCl3) δ: 9.47 (s, 1H), 8.29 (d, J = 6.4 Hz, 1H), 7.23 (d, J = 7.6 Hz, 1H), 6.59 (s, 1H), 6.42 (d, J = 6.8 Hz, 1H), 4.60 (dd, J = 13.3, 8.0 Hz, 1H), 4.48–4.37 (m, 1H), 4.32 (dd, J = 16.4, 8.6 Hz, 1H), 4.05–3.93 (m, 2H), 3.47–3.35 (m, 2H), 3.32 (d, J = 4.4 Hz, 2H), 2.51–2.37 (m, 1H), 2.37–2.26 (m, 1H), 2.24–2.13 (m, 1H), 2.13–1.99 (m, 2H), 1.96–1.42 (m, 11H), 0.98–0.78 (m, 12H); 13C NMR (101 MHz, CDCl3) δ: 199.59, 174.88, 174.50, 173.01, 171.35, 67.20, 58.10, 57.11, 51.93, 42.35, 42.14, 41.78, 37.41, 31.24, 30.88, 29.49, 28.95, 27.29, 24.85, 22.84, 22.09, 21.36, 19.26, 18.21. HRMS (m/z): 495.3107 (M + H) +.
4.4. Enzyme expression and purification
The SARS-CoV-2 3CLPro gene was synthesized by Genewiz, Inc and subsequently constructed into pGEX-6P vector (Novagen). Then, the verified plasmid was transformed into Escherichia coli BL21 (DE3) cells (TransGen Biotech, Beijing, China) and the Escherichia coli BL21 (DE3) cells was induced by 0.25 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 16 °C for 18 h. The harvested cells were resuspended into the buffer contain 20 mM Tris-HCl (pH 8), 150 mM NaCl, 5% glycerol, and homogenized by ultrasonic cell disintegration at 4 °C. After removal of the cell debris by centrifugation at 12000 rpm for 40 min at 4 °C, the supernatant was loaded onto the Ni-nitrilotriacetic acid (Ni-NTA) column (GE Healthcare). Following washing the resin with the washing buffer contain 20 mM imidazole (pH 8), PPase was added to generate the mature SARS-CoV-2 3CLPro. Crude protein was purified by Superdex 200 gel filtration chromatography (GE Healthcare), and verified by SDS-PAGE analysis. Finally, the target protein was concentrated into 50 mg/mL and stored at −80 °C.
4.5. Crystallization, data collection, structure determination, and refinement
Purified SARS-CoV-2 3CLPro was pre-incubated with the inhibitor in 5:1 stoichiometric ratio at 4 °C overnight and incubated with the diverse crystallization conditions. Following iterative rounds of optimization of the crystallization conditions, the crystals of SARS-CoV-2 3CLPro was suitable to grow in 0.1 M MES, pH 6.0, 10% PEG 6000 buffer condition and in hanging-drop, vapor diffusion method at 16 °C. For collection of X-Ray diffraction data, the crystals were flash-cooled in liquid nitrogen followed by dragging the crystals through the crystallization solution supplemented with 20% glycerol. The X-ray diffraction data were collected at the SSRF Beamline BL19U1. The data were processed by the HKL3000 package. The crystal structure of SARS-CoV-2 3CLPro (PDB code: 6LZE) was used as the initial searching model to determine the complex structure. Subsequently, the manual model was refined by performing COOT and PHENIX software through rigid-body refinement, energy minimization and individual b-factor refinement. Finally, the quality of the final refined model was verified by the program PHENIX validation module and the statistics information were summarized in Tables S3–S8.
4.6. Enzymatic inhibitory activity assay
The FRET-based peptide MCA-TSAVLQSGFRK(DNP)M was synthesized as a substrate via a solid-phase method. When the peptide was cleaved by 3CLPro at the Gln-Ser bond, the fluorescence was released. In detailed, a 2.0 μM solution of SARS-CoV-2 3CLPro dissolved in 25 μL assay buffer (pH = 8.0, 20 mM Tris-HCl, 150 mM NaCl) was incubated with 25 μL seven different concentrations of the inhibitor (2-fold dilution), respectively, and DMSO only as blank control at 37 °C for 30 min. Subsequently, a 50 μL solution contain 30 μM substrate was added to the mixture and the reaction was initiated. After 2h incubation, the change of relative fluorescence units was recorded by a microplate reader (Thermo Varioskan Flash, USA) at λex of 340 nm and λem of 440 nm. Finally, following inhibitory curve fitting by GraphPad Prism 7.0, the IC50 value of the inhibitor was obtained.
4.7. Cell viability assay
For matching the similar environment with the SARS-CoV-2 infection, the lung tumor cell line A549 was chosen to test the toxicity of peptidomimetic inhibitor. In brief, the cell (5000 per well) was incubated with the 25 μL seven different concentrations (2-fold dilution) of the peptidomimetic inhibitor, respectively, at 37 °C for 24h. Subsequently, the solution contained MTT was added to the plate and incubated with the cell for 4 h. Then, the super supernatant was discarded and the cell was washed by PBS buffer at 3 times. 200 μL DMSO was added and the absorbance at 490 nm was recorded by a microplate reader (Thermo Varioskan Flash, USA). Finally, the cellular viability rate was calculated and the CC50 value of the peptidomimetic was obtained by the survival curve fitting.
4.8. Anti-viral activity assay
SARS-CoV-2 (WIV04) was passaged in Vero E6 cells and titered by a plaque assay. Vero E6 cells were treated with compounds at the indicated concentrations and infected with SARS-CoV-2 virus at an MOI of 0.01, and maintained in DMEM with 2% FBS. The supernatants were collected at 24 h post infection (hpi). Magnetic Bead Virus RNA Extraction Kits (Shanghai Fine Gene Biotech, FG438) were used for viral RNA extraction and the viral RNA was quantified by real-time RT-PCR with TaqMan probes targeting the RBD region of S gene. Vero E6 cells were treated with different compounds at the indicated concentrations for 48 h and cell viability were tested by Cell Counting Kit-8 (GK10001, GLPBIO).
4.9. HPLC chromatography of all tested compounds
HPLC analysis was performed using the general method: equipment = Agilent 1260 HPLC; column = Phenomenex Luna C18 5micron column (250 mm × 4.60 mm, 5 μm); tested temporary = 35 °C; solvent = MeCN/0.1% TFA dissolved in H2O; Gradient = 10–90% MeCN in 0.1% TFA solution over 15 min at 1 mL/min flow rate; detector = The UV detection at 254 nm and 270 nm.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the staff of SSRF BL19U1 for their support in collecting the diffraction data. This work was supported by the National Key Research and Development Program of China (grant no. 2018YFA0507204), the National Natural Science Foundation of China (grant nos. 22177055), the Fundamental Research Funds for the Central Universities, Nankai University (63201229), the Zhejiang University special scientific research fund for COVID-19 prevention and control (2020XGZX087), the Advanced Customer Cultivation Project of Wuhan National Biosafety Laboratory (2021ACCP-MS10), the Natural Science Foundation of Tianjin (grant nos. 19JCZDJC33300).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmech.2022.114458.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Detailed synthetic procedures, compound characterization, and biology assays. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X., Peiris M., Yen H.-L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antivir. Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halford B. 2020. Pfizer's Novel COVID-19 Antiviral Heads to Clinical Trials.https://cen.acs.org/pharmaceuticals/drug-discovery/Pfizersnovel-COVID-19-antiviral/98/web/2020/09 [Google Scholar]
- 5.Hoffman R.L., Kania R.S., Brothers M.A., Davies J.F., Ferre R.A., Gajiwala K.S., He M., Hogan R.J., Kozminski K., Li L.Y., Lockner J.W., Lou J., Marra M.T., Mitchell L.J., Murray B.W., Nieman J.A., Noell S., Planken S.P., Rowe T., Ryan K., Smith G.J., Solowiej J.E., Steppan C.M., Taggart B. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J. Med. Chem. 2020;63:12725–12747. doi: 10.1021/acs.jmedchem.0c01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen D.R., Allerton C.M.N., Anderson A.S., Aschenbrenner L., Avery M., Berritt S., Boras B., Cardin R.D., Carlo A., Coffman K.J., Dantonio A., Di L., Eng H., Ferre R., Gajiwala K.S., Gibson S.A., Greasley S.E., Hurst B.L., Kadar E.P., Kalgutkar A.S., Lee J.C., Lee J., Liu W., Mason S.W., Noell S., Novak J.J., Obach R.S., Ogilvie K., Patel N.C., Pettersson M., Rai D.K., Reese M.R., Sammons M.F., Sathish, Singh J.G., P R.S., Steppan C.M., Stewart A.E., Tuttle J.B., Updyke L., Verhoest P.R., Wei L., Yang Q., Zhu Y. An oral SARS-CoV-2 mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 7.Kim D., Lee J.-Y., Yang J.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020;30 doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. Chembiochem: a European journal of chemical biology. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuong W., Khan M.B., Fischer C., Arutyunova E., Lamer T., Shields J., Saffran H.A., McKay R.T., Belkum M.J., Joyce M.A., Young H.S., Tyrrell D.L., Vederas J.C., Lemieux M.J. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2020;11:4282. doi: 10.1038/s41467-020-18096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coronaviridae V. Study Group of the International Committee on Taxonomy of, the species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 14.Hsu M.-F., Kuo C.-J., Chang K.-T., Chang H.-C., Chou C.-C., Ko T.-P., Shr H.-L., Chang G.-G., Wang A.H.J., Liang P.-H. Mechanism of the maturation process of SARS-CoV 3CL protease. J. Biol. Chem. 2005;280:31257–31266. doi: 10.1074/jbc.M502577200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pillaiyar T., Manickam M., Namasivayam V., Hayashi Y., Jung S.-H. An overview of severe acute respiratory syndrome–coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy. J. Med. Chem. 2016;59:6595–6628. doi: 10.1021/acs.jmedchem.5b01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., He S., Deng W., Zhang Y., Li G., Sun J., Zhao W., Guo Y., Yin Z., Li D., Shang L. Comprehensive insights into the catalytic mechanism of Middle East respiratory syndrome 3C-like protease and severe acute respiratory syndrome 3C-like protease. ACS Catal. 2020;10:5871–5890. doi: 10.1021/acscatal.0c00110. [DOI] [PubMed] [Google Scholar]
- 17.Vuong W., Fischer C., Khan M.B., van-Belkum M.J., Lamer T., Willoughby K.D., Lu J., Arutyunova E., Joyce M.A., Saffran H.A., Shields J.A., Young H.S., Nieman J.A., Tyrrell D.L., Lemieux M.J., Vederas J.C. Improved SARS-CoV-2 Mpro inhibitors based on feline antiviral drug GC376: structural enhancements, increased solubility, and micellar studies. Eur. J. Med. Chem. 2021;222 doi: 10.1016/j.ejmech.2021.113584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai W., Zhang B., Jiang X.-M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.-K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H.-M., Liang P.-H. Picornaviral 3C protease inhibitors and the dual 3C protease/coronaviral 3C-like protease inhibitors. Expert Opin. Ther. Pat. 2010;20:59–71. doi: 10.1517/13543770903460323. [DOI] [PubMed] [Google Scholar]
- 21.Zhai Y., Zhao X., Cui Z., Wang M., Wang Y., Li L., Sun Q., Yang X., Zeng D., Liu Y., Sun Y., Lou Z., Shang L., Yin Z. Cyanohydrin as an anchoring group for potent and selective inhibitors of enterovirus 71 3C protease. J. Med. Chem. 2015;58:9414–9420. doi: 10.1021/acs.jmedchem.5b01013. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y., Shang C., Yang P., Li L., Zhai Y., Yin Z., Wang B., Shang L. 4-Iminooxazolidin-2-one as a bioisostere of the cyanohydrin moiety: inhibitors of enterovirus 71 3C protease. J. Med. Chem. 2018;61:10333–10339. doi: 10.1021/acs.jmedchem.8b01335. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y., Li L., He S., Shang C., Sun Y., Liu N., Meek T.D., Wang Y., Shang L. Application of dually activated Michael acceptor to the rational design of reversible covalent inhibitor for enterovirus 71 3C protease. J. Med. Chem. 2019;62:6146–6162. doi: 10.1021/acs.jmedchem.9b00387. [DOI] [PubMed] [Google Scholar]
- 24.Zhai Y., Ma Y., Ma F., Nie Q., Ren X., Wang Y., Shang L., Yin Z. Structure–activity relationship study of peptidomimetic aldehydes as enterovirus 71 3C protease inhibitors. Eur. J. Med. Chem. 2016;124:559–573. doi: 10.1016/j.ejmech.2016.08.064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.