Abstract
Nanotechnology sculptures the current scenario of science and technology. The word nano refers ‘small’ which ranges from 10 to 100 nm in size. Silver and gold nanoparticles can be synthesized at nanoscale and have unique biological properties like antibacterial, antifungal, antiviral, antiparasitic, antiplatelet, anti-inflammatory, and anti-tumor activity. In this mini review, we shall discuss the various applications of silver and gold nanoparticles (AuNPs) in the field of therapy, imaging, biomedical devices and in cancer diagnosis. The usage of silver nanoparticles(AgNPs) in dentistry and dental implants, therapeutic abilities like wound dressings, silver impregnated catheters, ventricular drainage catheters, combating orthopedic infections, and osteointegration will be elaborated. Gold nanoparticles in recent years have garnered large importance in bio medical applications. They are being used in diagnosis and have recently seen a surge in therapeutics. In this mini review, we shall see about the various applications of AuNP and AgNP, and highlight their evolution in theranostics.
Keywords: Silver nanoparticle, Gold nanoparticle, Nanotechology, Biomedical application, Evolution in theranostics
Introduction
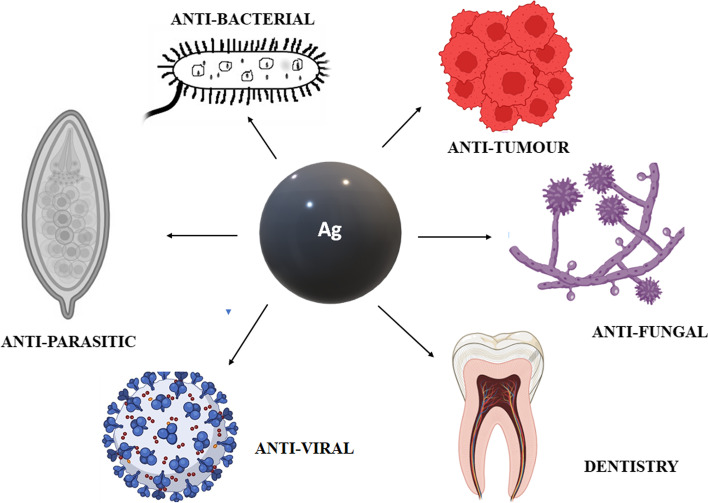
Nanotechnology is known as multifaceted field of science which deals with the particles at nanosized ranges from 10 to 100 nm. There are various types of nanomaterials used in fields of science and technology such as medicine, construction, agriculture, energy production, and food industry [1–5]. The characterizations of nanoparticles (NPs) are based on their shape (rod, triangular, polyhedral, octagonal, round) and among the various NPs, metal NPs has gained more attention in recent years due to their distinctive property. Their use is more primitive in comparison to other nanostructures and gold particles were used as medicine in India and China for ayurvedhic medicine preparation [6, 7]. Among metal NPs, gold and silver nanoparticles (AgNPs) have various potential application and uses due to their application in allied disciplines and biomedical properties. AgNP is used in cleansing up the environmental pollution, medical imaging techniques and also exhibit more in the biomedical field because of large surface area to volume ratio [8–10]. AgNP is a powerful disinfectant and antibacterial agent and is used as a topical silver sulfadiazine cream as an antibiotic for treating burn wounds. It also shows antibacterial activity with garlic, cinnamon extract and is reported to have anticancer, anti-inflamatory activity etc., as shown in Fig. 1 [11–15]. The recent advancements and developments of gold nanoparticle (AuNP) in medicine and in theranostics have also gained attention. They are being used in diagnosis and have recently seen a surge in therapeutics. AuNP has a deep connection with chemistry, and in the Roman era(735BC) they were used in art decorations [16]. It has been identified that gold nanoparticles are used in various field of sciences such as diagnosis, cancer treatment, antibacterial and anti viral agent [17]. Noble metal are those which are defiance to oxidation at higher temperatures and corrosion. Some of the metals used are palladium, osmium, ruthenium, rhodium, iridium, platinum, copper, silver, gold, etc. These metal nanoparticles play a prominent role in the development of nanobiosensors in point of care testing, gene deivery, gene detection, intracellular trafficking targeting, cancer treatment, preventing tuberculosis, HIV, keratitis, imaging and therapeutics [18]. Metal nanoparticles can produce excessive reactive oxygen species, protein damage, inflammation which leads to toxicity, and it can also produce hyperthermia locally. The fundamendal principle is that it exhibits photocatalytic and photothermal effects by which under the light the metal nanoparticles gets excited at different wavelength [19]. In this review, we will discuss about the various applications of AgNP and AuNP in the field of theranostics and biomedical science.
Fig. 1.
Applications of silver nanoparticles
Biological Properties of Silver Nanoparticle
Antibacterial Agent
Multi-drug–resistant bacteria have become very prominent these days which has limited the resources for controlling infections using conventional therapies, such as, antibiotics, radiation etc. AgNPs is used as a superior antibacterial agent and has been found to be effective against a broad spectrum of vancomycin resistant strains in Gram-positive and Gram-negative bacteria [20–24]. Silver ions released by the AgNPs attaches to the thiol (SH) group of the sulfur and hydrogen present in the bacterial proteins and inhibits the bacterial growth [25–27]. AgNPs are potent against both anaerobic and aerobic bacteria and it functions by obstructing the bacterial electron transport chain system and precipitating the bacterial cellular proteins [28–30]. As AgNPs have larger surface area to volume ratio, it shows better efficiency [22, 31] and the mechanism of action are as follows,
The silver ions in AgNPs inhibits the function by reacting with phosphorous present in the DNA and sulfur containing proteins present in the bacterial membranes [32].
A nanometer scale silver provides firm attachment to the cell membrane and penetrates the bacterial cell wall [33, 34].
Ag+ ions in AgNPs causes cell death by bombarding the electron transport chain in bacterial mitochondria [35].
Continuous detachment of silver ions in the bacterial cell from AgNPs with an environment of lower pH produces free radicals, induces oxidative stress, and enhances the antibacterial activity [27, 36].
AgNPs attaches to the bacteria and it penetrates inside the cell wall, further leading to death [31].
The shape, size, and concentration of the AgNPs determines the antibacterial efficacy. Studies state that enlarging the surface area of AgNPs can enhance the antibacterial activity [37]. Researchers have demonstrated that AgNPs possessed 50% inhibition activity against multidrug resistant bacteria (MDR)- Staphylococcus aureus and E. coli at a very low concentration of 20 μg/ml. At a further high concentration of about 40 μg /mL, it exhibited an efficient inhibition of both microorganisms. In another study, biosynthesized AgNP using marine macroalgae Padina species observed a good bacteriostatic activity against pathogenic Gram-positive organism like Bacillus subtilis, Staphylococcus aureus and Gram-negative bacteria like Pseudomonas aeruginosa, E. coli, Salmonella typhi. AgNPs with the concentration of 1mg/ml exhibited a higher sensitivity against Pseudomonas aeruginosa with diameter of zone of inhibition of 13.33±0.76 mm and Staphylococcus aureus of 15.17±0.58 mm respectively, where as the negative control possesed 0.00 mm. [38]. In another study, a comparative analysis was done with small AgNPs and PEGylated AgNPs of different molecular weight against a prime pathogen Staphylococcus aureus. In this study, 12 different sizes of AgNPs have been synthesized with a size range of 29.7 nm ± 0.02 to 35.5 ± 0.02 nm in three different pH of 10,11 and 12. Excellent bactericidal activity was brought in by the PEGylated AgNPs with the zone of inhibition of about 29 mm at a pH 10. This was because PEG possessed a high hydrophilic property that eliminated more water and terminated the microbes [39]. Veerasamy et al have observed antimicrobial activity in the Green synthesized AgNP using Malia Azedarach at the concentrations of 25, 50, 75, 100 μg/ml in which highest zone of inhibition was observed at 100 μg/ml as 21 mm and 12 mm for Pseudomonas aeruginosa and Bacillus subtilis respectively [40].
Antifungal Agent
Prolonged use of antifungal drugs leads to multidrug resistance especially for Candida species [41]. AgNPs coated reverse osmosis membrane exerted better antifungal activity against Candida albicans, Candida glabrata, Candida krusei, Candida tropicalis [24]. AgNP exerts antifungal property by interrupting the cell membrane and inhibits the normal asexual reproduction process by destroying the membrane integrity [42]. Mallmann et al., reported that AgNPs prepared by bio synthesis using SDS as stabilizer and reducing agent as ribose exhibited increased antifungal activity against C. albicans and C. tropicalis [43]. Additionally, AgNPs suppresses the growth of Aspergillus niger by 70% and Cladosporium cladosporoides by 90 %. Increasing the concentration of nanosilver minimizes the fungal growth in a dose dependent manner. AgNPs coated in cotton has shown antifungal activity against Aspergillus sp and the zone of inhibition was observed at 14.33±3.51 mm [44]. A study was done with biosynthesized AgNPs using Malva parviflora and leaf extract of Malva parviflora (LEMP) to monitor the antifungal activity against Alternaria alternata, Fusarium oxysporum Fusarium solani and Helminthosporium rostratum. With an average diameter of 50.6 nm, the AgNP mitigate the mycelia growth of H. rostratum at 88.6%, A.alternata at 83.0%, F.solani at 81.1% and F.oxyspoum at 80.7% where as the LEMP showed growth inhibition at 65.3% for F.solani, 54.7% F.oxyspoum, 53.6% H.rostratum and 45.6% for A.alternata [45]. Another study discussed about the pathogenic fungus which affects the quality of crops. To overcome this, mycosynthesized AgNPs using A. niger fungal isolate to invade against plant pathogenic fungi was utilized. At a concentration of about 10 μg/ml, AgNPs showed inhibition of 91.0% for Fusarium oxysporum, 97.3% for Aspergillus flavvus, and 93.75% for Penicillin digitatum. This is because on treatment with Ag+, the DNA ruins its ability to replicate, thereby resulting in dormant expression of ribosomal subunit proteins and the enzymes needed for ATP production. [46]. A synergistic antifungal activity of Epoxiconazole and Lingustrum-lucidum leaf extract against Setosphaeria turcica showed the inhibitory percentage of 50 % against the organism at 170.20 μg/ml concentration. At a ratio of 8:2 and 9:1 synergistic antifungal activity was seen in combination with epoxiconazole. This method gave a new perspective for the integrative control of plant pathogen [47].
Antiviral Agent
Metal NPs like gold or silver exhibit antiviral activity against broad spectrum of virus by direct interaction with nanomaterial and viral surface proteins and reduce the infectivity of the viral cultured cells. Recent studies have shown that metal NPs are effective antiviral agents against HIV 1, Respiratory syncytial virus, HSV type1, monkey pox virus, Tacaribe virus and influenza virus [48–52]. AgNP coated with poly (N-vinyl-2-pyrrolidone) showed antiviral property against HIV1 by interacting with gp 120 [53]. AgNPs and polysaccharide coated AgNPs ranging from10-80 nm size was potent against monkey pox virus of Poxviridae family by blocking the virus – host cell binding and penetration [52]. AgNP at non-toxic concentration are used effectively to inhibit tacaribe virus (TCRV) of Arenaviridae family by inactivating the viral particles at their initial entry [54]. Saadh et al., observed that AgNP integrated with epigallocatechingallate (EGCG) and doped with zinc sulphate showed a potent antiviral activity against Avian influenza A virus H9N2. At a concentration of 50 μM EGCG, it reduced the titre of AI H9N2 (logEID50/ml was 4.2). By increasing the concentration of EGCG >50 μM, it did not show any enhanced antiviral effect. Effective result was obtained by combining EGCG with zinc sulphate showing logEID50/ml (1.5±0.6) [55]. Another study was on synthesizing AgNPs using Lamprathus coccineus and Malephora lutea. This study possessed that the hexane nanoextract of L.coccineus showed 50% inhibition against HAV-10 virus, HSV-1virus and CoxB4 virus with concentration 11.71 ng/ml, 36.36 μg/ml and 12.74 μg/ml respectively [56].
Antiparasitic Agent
Saad and colleagues synthesized silver and copper NPs and studied the antiparasitic activity which showed remarkable decrease in the oocyte availability in Cryptosporidium parvum. They have also studied that AgNPs are effective against parasitic infection of Entamoeba histolytica and Cryptosporidium parvum. AgNPs expresses larvicidal activities against Aedes aegypti and Culex quinquefasciatus [57]. Allahverdiyev et al. demonstrated a study to assess the effects of AgNPs over the biological parameters of Leishmania tropica. AgNPs attack the parasites by impairing the lipophosphoglycan and glycoprotein 63 present in the surface of the parasite which is responsible for the infection. The study revealed that AgNPs have the potential to inhibit the promastigote’s proliferation activity. And further, the AgNPs in the presence of increased UV light hinders the endurance of amastigotes in host cell [58]. A study on biosynthesis of AgNPs using Corn cob nanoxylan as a reducing and stabilizing agent observed a minimum inhibitory concentration (IC50) of 25 μg/mL against Leishmania amazonensis promastigotes [59]. In an in vitro study of AgNPs against Entamoeba histolytica trophozoite, the result showed a mortality percentage of 46.2 %, 42.4 % for 75 μg/ml and 46.2 %, 46.7 % for 100 μg/ml of concentration after 24 h and 48 h incubation respectively [60].
Anti-inflammatory Agent
Silver-based NPs is powerful in preventing the bacterial infections and inflammation [61]. Intra-peritoneal injections of AgNPs in animal models showed decrease in the degree of post operative fibrous adhesions. Hebeish et al., have done the in vivo efficacy of albino rats’ and grouped the 24 albino rats into 4 groups. Group 1 was the negative control and given 1 ml of saline. Group 2 & 3 rats were administered with two different AgNPs concentration of 250 ppm and 124 ppm respectively. Group 4 was the reference drug administered with indomethacin at concentration of 20 mg/kg rat. After one hour, all animals were given a sub planter injection of 1% carrageenan solution in 0.1ml of saline over the right hind paw and 0.1 ml saline in left hind paw. After four hours of administration, rat’s both the paws were excised and weighed separately. The oedema percentage showed that the synthesized AgNP could significantly decrease in degree of rat oedema. The oedema percent of 250 ppm concentration of AgNPs gave the same effect as the standard drug indomethacin. Thus, it was indicated that AgNPs could reduce inflammation significantly [62]. Shensha et al., made a study on Nigella sativa oil mediated AgNPs to assess the anti-inflammatory activity in male Wister rats which showed inhibitory concentration at 54.40% (1 h) and 60.30% (5 h) with the dose of 0.3 mg/kg bodyweight [63]. Researchers have biosynthesized AgNPs using Selaginella myosurus aqueous extract which showed significant inhibition of paw edema of Wistar rats of 44.30% (1h), 57.60% (5 h) and 60.50% (5 h) for the concentration 0.1, 0.2 and 0.4 mg/Kg (body weight) respectively [64].
Antiplatelet Agent
Thrombotic disorders have become the remarkable problem in the medical field. Anticoagulant and thrombolytic therapy can lead to many bleeding complications. Recently Dakshayan et al. demonstrated the role of Selaginella bryopetris (Sanjeevini) plant extract supported AgNPs (SPE @AgNP) in platelets. Platelet aggregation assay was performed in platelet rich plasma with ADP and Epinephrin as agonists. SPE @AgNP inhibited ADP only and not the Epinephrin. Platelet exhibits its major role in arresting the bleeding in addition to the coagulation factors and they tend to aggregate at the injury site forming a platelet plug and it is vulnerable to the collagen present in endothelium [65]. Thrombin, ADP, epinephrine, thromboxane, thrombin, platelet activating factors etc. are the agonist that activates the platelets [66]. Formation of thrombus in arteries and vein occurs when platelets get hyperactivated. Thus, SPE @AgNP was used in the treatment of thrombotic disorders as a superior antiplatelet agent along with its anticoagulant activity [67]. Additionally, Shrivastava et al., demonstrated that AgNPs inhibited the integrin mediated platelet functional responses like aggregation, adhesion, secretion to immobilized fibrinogen or collagen [26].
Therapeutic Application of Silver Nanoparticle
Wound Dressing
AgNPs are used in wound dressing, in case of toxic epidermal and necrolysis, severe burns etc. AgNPs takes about 3.35 days on an average to heal the wound and the bacterial load decreases in wound area with no further effects [68]. AgNPs works more effectively when compared with 1 % silver sulfadiazine for superficial burns. In case of deep burns, AgNPs and 1 % silver sulfadiazine does the same work and AgNPs promotes the restoration of intact epidermal barrier but do not form any new tissue [69]. The application of biopolymer based biomaterial increases because of biocompatibility, biodegradability, non-immunogenicity and nontoxicity. The biopolymers used are gelatine, collagen, keratin, natural rubber proteins, polysaccharide. Collagen is a natural biopolymer and the usage of biopolymers in wound healing showed a better positive output in the clinical trials. Collagen sponges and glycosaminoglycans act as a double layered artificial skin and heals the wound. These act as a good carrier for AgNP to release the drug in a sustained manner [70]. Hasari et al., reported that silk based novel bilayer wound dressing material with gelatine is less toxic to skin cell than other wound dressing available and it also promotes the wound healing by increasing the collagen production [71]. Studies have shown that chitosan and keratin have wound healing effects and antimicrobial activity. Chitosan is effective for the treatment of chronic periodontitis [72]. Scientists have emerged with worlds first AgNP based commercially available wound dressing material (ACTICOAT: Smith and Nephew, UK) that covers the huge area of burns and increases the wound healing activity [73]. A study was done with konjac glucomann silver nanoparticle (KGM/AgNP) a composite sponge which possessed a better wound healing capacity and also exhibited good cytocompatibility. By animal experiments they have confirmed the activity of wound healing, and on the day 14, KGM/Ag3 wound healing capacity was increased to 99 % [74].
Silver Impregnated Catheters
The prevalence of central venous catheter (CVC)–related bloodstream infections were about 80,000 cases annually. However, the use of CVC in hospitals of the USA was around 5 million for 1 year [75]. Patients with tumour, intracerebral haemorrhage, subarachnoid haemorrhage were implanted with external ventricular drain (EVD) catheters usually for the therapy of acute hydrocephalus. The EVP catheters were significantly used for monitoring the draining CSF and intracranial pressure. Previously catheters were impregnated with antibiotics that decreased the colonization rates [76], which led to bacterial resistance. Therefore, a new trend of silver coated catheters was used in clinical field, in which the silver ions bind with an inert ceramic zeolite by the help of inorganic silver powder. Recent studies described that, there was a remarkable reduction in the colonization rate in silver impregnated CVC [77]. In a previous study, A.baumanni was made to form a biofilm to mimic the in vivo infection conditions and the CVC was coated with polydopamine and tested for the bactericidal activity. By surface characterization with field emission scanning electron microscopy, water contact angle (CA), Raman spectroscopy the results showed that dopamine coated AgNPs had a CA value of 49.1 ± 0.3 [78]. Fichtner et al. conducted retrospective clinical analysis to see the contrasting efficacy between silver coated EVD catheters and standard non coated catheters. There was a significant reduction in the positive culture of CRI. Bacterial colonization was likely to be 4 times in EVD catheters in comparison to the standard non coated catheters. Another study showed that the growth of Staphylococcus aureus reduced in the silver coated EVD catheters [79].
Silver in Orthopaedics
Benchmark treatment for the arthritic disease is the artificial joint replacement. The use of bone cements like poly methyl methacrylate (PMMA) resulted in high rate of infection when integrated in the bones. Nanotechnology has evolved in the field of orthopaedics and trauma. Hence, nano silver coated bone cement resulted in outrageous antibacterial activity against broad range of bacteria including methicillin resistant Staphylococcus aureus (MRSA). Taken further, the nanoparticle did not show any cytotoxicity. Ultra high molecular weight polyethylene (UHMWPE) was used for the artificial joint replacement but the only disadvantage was with the wear and tear and associated debris generation, which led to inflammation and failure of joints in the body. This major problem was overcome by integration of AgNPs with bone cement. This protocol consequently reduced the polymer debris formation [20, 80]. The rate of infection was reduced by adding the AgNPs to the outer layer of the implants. A group of scientist demonstrated that there was an effective resistance to Escherichia coli by exposing it to modified titanium film. It also had a role in reducing the pin tract associated infections [69, 81]. Orthopaedic infections resulted in high morbidity when osteoblast like cells, and bone marrow mesenchymal stem cells were exposed to AgNPs and showed maximum inhibitory concentration of 25 μg/ml [82].
Silver Surgical Meshes
Prosthetic mesh is not commonly used implanted devices for pelvic reconstructive surgery and hernia repair. The incidence of mesh associated infections ranges between 0.6% and 8.0% [83, 84]. In accordance to the one million herniorrhaphies there were around 30,000–50,000 prosthetic mesh infection in the USA [85]. Multiple antibacterial coatings were used on the medical devices such as urinary catheters, central venous catheters and surgical meshes to reduce the infection rate [86]. To reduce the occurrence of prosthetic mesh infections in the post pelvic and hernia surgery, nanocrystalline silver particles with polypropylene was used [87]. The antimicrobial activity of silver nanoparticle relies in the electrical state of the ions. Silver is biologically active in its soluble form as Ag+. Commonly used topicals like silver sulfadiazine, silver nitrate, has silver in the form of Ag +. Topical silver formulations were used two to twelve times a day in burn areas because of the rapid inactivation of silver ions with chloride or organic ions present in the wounds. In comparison to Ag+, Ag0 does not form complex resulting in halt in rapid inactivation of microbes [88]. Nanocrystalline silver has decreased one million nosocomial infections in a year with the patients implanted with prosthetic material [89]. Cohen et al., reported that polypropylene integrated with nanocrystalline silver particle (NCSP) showed that silver particles circulated inside the mesh and produced a zone of inhibition and higher inhibition efficacy against Staphylococcus aureus. The zone of inhibition increased in a dose dependent manner with increase in the concentration of silver [90]. NCSP also holds the property of anti-inflammatory agent. Secondary mechanism of NCSP is to repress tumour necrosis factor α and interleukin (IL)-12 and initiate inflammatory cell apoptosis [91].
Dentistry
AgNPs pops up as a promising agent used in dentistry. This property is because of their integration of antimicrobial property in dental biomaterials [92]. The major mechanism is by liberating cationic silver and its oxidative potential [93]. Using AgNPs in dentistry, is effective against multidrug resistant bacteria and for its prophylactic action. AgNPs are used in different streams like preventive dentistry, orthodontics, endodontics, periodontics and in oral dentistry. AgNPs inhibit the growth of Staphylococcus aureus, Streptococcus mitis, Streptococcus gordoinii biofilms. Additionally recent study states that AgNPs exert its antibacterial activity against Streptococcus sobrinus, Lactobacillus acidophilus, Lactobacillus casei, Streptococcus sanguinis, Enterococcus faecalis and Actinomyces actinomycetemcomitans [94]. Incorporation of AgNPs into polymers used as denture base and tissue conditioners in stomatitis have showed superior antimicrobial activity and capacity to fight against oral infections. Another study stated that modified denature base acrylic combined with AgNPs at 20.0 wt.% showed antifungal property [95]. AgNPs with smaller diameter size exerts good biofilm inhibition when compared with larger particles. Biological synthesis of AgNPs using neem, onion, and tomato with size measuring 26.2 to 33.3 nm showed antimicrobial activity against Staphylococcus aureus because of the high concentration of flavonoids and terpenoids in it [96]. Pérez-Díaz et al., reported that AgNPs inhibited the growth of planktonic Streptococcus mutans and killed the Streptococcus mutans biofilms. Thus, AgNPs play a significant role in dentistry and prevents dental caries [97]. Decrease in adhesion of biofilm and production of lactate by microorganism is seen in AgNPs treated with titanium disc -based composites [98]. A nano bacteriostatic agent silver Nano fluoride had inhibited the growth of Streptococcus mutans and it could be used once a year, easy to use, and had a better cost benefit ratio. This was a superior replacement for sodium fluorine [99]. Titanium micro implants with biopolymer coated AgNPs (Ti-BP-AgNp) showed largest zone of inhibition of 50.58 ± 4.88 mm2 for Streptococcus mutans. The zone of inhibition for S. sanguinis was 27 ± 3.01 mm2 and for A. actinomycetemcometans showed a smaller zone of inhibition of about 25 ± 3.06 mm2 with the control showing no zone of inhibition [100].
Cancer Theranostics
Cancer is a global menace and is caused by environmental effects and mutations in genes which activates the sequence of events in molecular level and leads to tumour formation. [101]. There are two major causes for cancer, they are external and internal factors. The external factors include radiation, viruses, chemical exposures, environmental conditions etc. [102]. Internal factors comprise the mutations, hormones and immune conditions which trigger the process of carcinogenesis [103]. Chronic low dose exposure to oxidative stress is also known to be a contributor for cancer onset by circumventing apotosis [104]. Many studies showed that AgNPs get localized inside perinuclear space of cytoplasm and endo lysosomal compartment cells by entering cells through endocytosis [105, 106]. AgNPs affect the respiration of cells and produce reactive oxygen species (ROS). As AgNPs are harmful to cells and proceeds to oxidative stress, damage of DNA, trigger the apoptosis and damage of mitochondria to cancer cells [107–112]. Studies showed that AgNPs can affect the activity of vascular endothelial growth factor which are involved in angiogenesis [113]. Theranostics is the mixture of diagnostics and therapy. Biosynthesized AgNP possess theranostic applications as it holds the anti-cancer property and used in targeted drug delivery and bioimaging vehicle [114]. It is a cost effective, safe, simple and eco-friendly approach [115]; the different applications of AgNPs in anti-cancer therspy is summarised in Table 1.
Table 1.
Antitumor effects of AgNPs
| S.No. | Types of Silver nanoparticles Used | Model used | Outcome | References |
|---|---|---|---|---|
| 1. | Green synthesis of AgNPs using Ganoderma neo-japonicum Imazeki | Breast cancer cell lines | AgNP increased the production of hydroxyl radical and reactive oxygen species by inhibiting the cell viability. They play a prime role in apoptosis by activation of caspase 9 and DNA nuclear fragmentation. | Gurunathan et al., [116] |
| 2. | Green synthesis of AgNPs using Escherichia fergusoni | Breast cancer MCF 7 cell lines | Cytotoxicity effects of bio synthesized AgNP reduced the activation of LDH, increases the ROS production and results in apoptosis. | Gurunathan et al., [117] |
| 3. | Green synthesis of AgNPs using sucrose | Malignant skin melanoma (HT144 cell line) and squamous cell lung carcinoma (H157 cell line) | Observed a prominent antitumor activity against vincristine and methotrexate. | Nazir et al.,[118] |
| 4. | Green synthesis of AgNPs using Taraxacum officinale | Liver hepatocellular carcinoma in HepG2 cell line | Possess an enhanced activity against the commercial AgNP and in increased cytotoxic effects against HepG2 cell line | Saratale et al.,[119] |
| 5. | Chemical biosynthesis of AgNPs using phycocyanin extracted from Nostoc linckia as reducing agent | Human breast adenocarcinoma | Observed a significant cytotoxic activity against MCF-7 cell line with the inhibitory concentration (IC50) of about 27.79±2.3 μg/ml | Naggar et al.,[120] |
| 6. | Green synthesis of AgNPs using Sargassum vulgare | Human myeloblastic leukaemia in HL60 and HeLa cells | Prevents carcinogenesis related with irradiation by inhibiting lipid peroxidation – mediated reactive oxygen species generation which leads to apoptosis. | Govindaraju et al., [121] |
| 7. | Green synthesis of AgNPs using Piper longum leaf | Hep 2 cell line | Observed effective cytotoxic effect of 94.02% at 500 μg/ml due to formation of ROS. | Jacob et al., [122] |
| 8. | Green synthesis of AgNPs using Inonotus obliquus extract | Human lung cancer in A549 cell line and breast cancer in MCF -7 cell line. | Cell lines shows significant cytotoxic effects. | Nagajyothi et al., [123] |
| 9. | Green synthesis of AgNPs using Commelina nudiflora | HCT-116 Colon cancer cell line. | AgNP showed less toxicity when compared with AuNP and inhibitory concentration (IC50) was 100 μg/ml. | Kuppusamy et al., [124] |
| 10. | Green synthesis of AgNPs using Melia dubia leaf | Human breast cancer cell line (KB) | The inhibitory concentration (IC50) was 31.2 μg/ml. | Kathiravan et al., [125] |
| 11. | Green synthesis of AgNPs using Dimocarpus longan Lour | H1299 lung cancer cell line. | Possess inhibitory effect with (IC50) value of 5.33±0.37 μg/ml and suppress the growth of H1299 tumours in SCID mice. | He et al., [126] |
| 12. | Cisplatin (cis-diamminedichloroplantium II) bound bio AgNPs using Penicillium, Fusarium and Aspergillus. | Prostate cancer cell line (PC -3). | Decreases the toxic effects and increases the efficacy against human prostate cancer | El-Sheikh et al., [127] |
| 13. | Green synthesis of AgNPs using root extract of Erythrina indica | Breast and lung cancer cell line (MCF-7 and HEP G2). | Viability of the cells decreased with increase in concentration of AgNP. At 25μg/ml, the viability percentage is 23.89±0.39 for MCF-7 cell line and 13.86±0.95 for HEP G2 cell line. This root mediated synthesis plays a role in cancer chemotherapy and chemoprevention. | Sre et al., [128] |
| 14. | Green synthesis of AgNPs using Acalypha indica leaves extract. | Human breast cancer MDA-MB-231 cells | Four different concentrations were used, such as, 1, 10, 50, 100 µg/ml, in which 100 µg/ml AgNP exposed toxicity to some extent. | Krishnaraj.C et al., [129] |
| 15. | Green synthesis of AgNPs using leaf extract of mistletoe Dendrophthoe falcata (L.f) Ettingsh | Human breast carcinoma (MCF-7 cells) | At merest dosage of 5 μg/ml, of fabricated AgNP they observed the enhanced cytotoxic effect. This concentration is the IC50 value. | Sathishkumar et al., [130] |
| 16. | Green synthesis of AgNPs using Datura inoxia leaves. | Human breast cancer (MCF-7 cell line) | The inhibitory concentration at 50% (IC50) was 20 μg/ml. It also seizes the cell cycle phase, suppresses the growth, finally induces apoptosis and exhibits the antiproliferative activity against MCF -7 cell line. | Gajendran et al., [131] |
| 17. | Green Synthesis of AgNPs using Clinacanthus Nutans leaves extract. | Oral squamous cell carcinoma cell line (HSC-4) | Observed a prominent cytotoxic effect at the concentration of 1.61 ±0.14 μg/ml by repressing the release of Bcl-2 protein. | Yakop et al., [132] |
| 18. | Green synthesis of AgNPs using Pimpinella anisum seeds | Human neonatal skin stromal cells (hSSCs) and colon cancer cells (HT115) | Observed lower cytotoxicity for bio-synthesized AgNP of about 51.39% in comparison with chemically synthesized one that showed 85.45%. And it is useful in pharmacological applications for producing nanodrugs. | AlSalhi et al., [133] |
| 19. | Biosynthesized AgNPs using aqueous fruit extract of Chaenomeles sinensis (CS) | Human breast cancer cell line (MCF-7) | At concentration of 0.01 μg/ml, the viability percentage of cells was remarkably reduced. | Keun Hyun Oh et al., [134] |
| 20. | Green synthesis of AgNPs using Saccharomyces boulardii | Human breast cancer cell line (MCF-7) | IC50 of bio-synthesized AgNP was about less than 10 μg/ml. This indicated that AgNP with low concentration exhibited almost 80% of inhibition of the cancer cells. They observed no significant changes in the higher concentration (10-100 μg/ml) | Kaler et al., [135] |
| 21. | Green synthesis of AgNPs using Indigofera tinctoria leaf extract | Lung cancer cell line (A549) | IC50 value of AgNP-tinctoria was71.92 ±0.76 μg/ml. This is because of the NPs induced ROS. | Vijayan et al., [136] |
| 22. | PVP coated AgNPs | Human lung cancer cell line (Alveolar cell line A549) |
1. Ag+ with 0-10 μg/ml and AgNP with 0-20 μg/ml concentration exhibited similar toxic effects and a decrease in mitochondrial function. 2. Study showed that oxidative stress was induced by both AgNP and Ag+ by correlating with geno and cytotoxicity. |
Foldbjerg et al., [137] |
| 23. | Green synthesis of AgNPs using aqueous extract of Phyllanthus emblica (PE) fruit. | Laryngeal carcinoma cells (Hep2 cell line) | Observed a potent cytotoxic effect, the IC50 value of PE alone was 30 μg/ml and PE-AgNP was 20 μg/ml. | Rosarin et al., [138] |
| 24. | Green synthesis of AgNPs using Padina tetrastromatica seaweed extract | Human breast cancer cell line (MCF-7) | With increased concentration, the percentage of inhibition increased. The bio-AgNP showed a value of IC50 as 86.7 μg/ml and AgNP value was 200 μg/ml. The observed cytotoxicity effect of AgNP was because of caspase 3 mediated apoptosis. | Selvi et al., [139] |
| 25. | Green synthesis of AgNPs using leaves of Vitex negundo | Human colon cancer (HCT15 cell line) | Proliferation of HCT-15 was inhibited with a concentration of 20 μg/ml (IC50) at 48h incubation. They exhibited a antiproliferative effect by seizing the G0/G1- phase and induced programmed apoptosis. | Prabhu et al., [140] |
| 26. | Green synthesis of AgNPs using Artemisia turcomanica leaf extract | Gastric cancer (AGS cell line) | The IC50 value of bio-synthesized AgNP was 4.88 μg/ml and commercial AgNP showed a value of 6.37 μg/ml. This study inferred that least concentration of bio-AgNP was sufficient to inhibit the cell growth when compared with commercial AgNP. | Mousavi et al., [141] |
| 27. | Green synthesis of AgNPs using Bacillus licheniformis in tumour bearing mice. | Dalton’s lymphoma ascites (DLA cell line) | Observed a prominent decrease in tumour volume from 7.3ml to 2.6ml in the group of mice treated with AgNP with the concentration of 500 μg/ml in about 15 days. | Sriram et al., [142] |
| 28. | Chemical synthesis of AgNPs. | Glioma (U251 glioblastoma cells) | AgNP exhibited better inhibition over U251 glioma cells in comparison to AuNP. The IC50 of AgNP was 75.9 μg/ml. | Liu et al., [143] |
| 29. | Metal silver and PVP coated AgNPs on tumor bearing mice | Lymphoma | 70% and 60% of mice survived at the day 35 with the metal silver and PVP AgNP administered at day 0. | Lara-Gonzalez et al., [144] |
| 30. | Poly vinyl pyrrolidone -coated nano silver (PVP-AgNP) and bare nano silver (AgNP) | Human hepatoma cell line (HepG2 cell line) and mice. |
1. AgNP caused more DNA damage to HepG2 cells than PVP-AgNP, whereas PVP-AgNP possessed more chromosomal aberration in comparison to AgNP. 2. At the highest dose of 250 mg/mL, they observed no inhibitory effects in the bone marrow cells of mice. |
Wang et al., [145] |
| 31. | Green synthesis of AgNPs using curcumin derivative (ST06) | Cervical cancer in HeLa cell line and EAC (Ehrlich Ascites carcinoma) tumour bearing mice. |
1. At a concentration of 1 μg/ml of ST06 and 1 μg/ml of ST06-AgNP, 50% of the cells were killed in a HeLa cell line. 2. Inferred that at the concentration of 5 μg/ml (ST06-AgNP) intraperitoneally inhibited the tumour growth in a tumour bearing mice which did not affect the body weight. |
Murugesan et al., [146] |
| 32. | Green synthesis of AgNPs using Spinacia oleracea leaves. | Myoblast cancer (mouse C2 C12 cell and in zebra fish) |
1. AgNP showed 100% inhibition of growth at low concentration of about 20 μg/ml and AgNP, at a concentration of 100 μg/ml exhibited 20% viability of cells, whereas the plant extract did not possess any significant cytotoxic effects against C2 C12 cell. 2. In zebrafish embryo, AgNP was more toxic and exhibited 100% mortality at concentration of 3 μg/ml and AuNP showed the 100% mortality only at higher concentration of 300 mg/ml. Moreover, plant extract did not cause any mortality. |
Ramachandran et al., [147] |
| 33. | Green synthesis of AgNPs using poisonous plant Cleistanthus collinus extract. | Lung cancer cell line (A549 cell line) and mice. |
1. Poisonous plant at a finite dosage was used as an anticancer agent. The inhibitory concentration (IC50) was found to be 30 μg/ml. 2. In vivo histopathological findings in mice treated with bio-synthesized AgNP did not show any edema or inflammation in the organs. Thus, it could be used for diagnostic and therapeutic purposes. |
Kanipandian et al., [148] |
| 34. | Green synthesis of AgNPs using Teucrium polium leaf extract | Human gastric cancer (MNK45 cell line) | The IC50 value of T. polium-AgNP is 68.2 μg/ml after 48h exposure. | Hashemi et al., [149] |
| 35. | Green synthesis of AgNPs using Albizia adianthifolia leaf extract. | Lung cancer (A549 cell line) | Observed significant cytotoxic effect and the cell viability percentage was 79% and 27% in the concentration of 10 μg/ml and 50 μg/ml, respectively. | Gengen et al.,[150] |
| 36. | Green synthesis of AgNPs using aqueous extract of Punica granatum | Lung cancer (A549 cell line) | Possessed cytotoxic effect to cancer cells but not to the normal cells. Potent cytotoxicity i.e.,50% growth inhibition was observed after 48h at a concentration of 5 μg/ml. | Annu et al., [151] |
| 37. | Green synthesis of AgNPs using walnut fruits (Juglans regia) | Breast cancer (MCF-7) | Observed a significant cytotoxic effect at the concentration of 60 μg/ml as 70% and 42% for AgNP and extract, respectively. | Khorrami et al,[152] |
| 38. | Green synthesis of AgNPs using Aspergillus niger | Human colon cancer (HT29 cell line) | Highest cell viability percentage was at 10 μg/ml and lowest at 160 μg/ml with exposure time of 24-72h by exhibiting ROS mediated apoptosis. | Chengzheng et al., [153] |
| 39. | Green synthesis of AgNPs using alcoholic extract of Argemone Mexicana leaves. | Cervical cancer (SiHa human cervical cancer cell line) | Observed a decrease in percentage of cell viability (70-80%) with the concentration of 100μg/ml. | Jha et al., [154] |
| 40. | Green synthesis of AgNPs using Pseudomonas aeruginosa | Thyroid cancer (TCL1 cell line) | IC50 value was observed as 48.5 μg/ml by increasing the lipid peroxidation, decreasing the mitochondrial membrane potential, reducing antioxidants and finally cell condensation took place. | Yang et al., [155] |
| 41. | Green synthesis of AgNPs using the extract of red sea weed Pterocladiella capillacea | Human hepatocellular carcinoma (HepG2 cell line) | Infers that the level of cytotoxicity increased with higher concentration. Untreated cells were used as negative control. The 50% of cell inhibition (IC50) occurred at 3.7 μg/ml of concentration. | El Kassas et al.,[156] |
Silver Nanoparticle in Imaging
Silver Nanoparticle as Photoacoustic Imaging
In the field of biomedical applications, research has increased in the area of designing and delivery of NPs to the specific organ. To know whether the nanoparticle has delivered to the diseased tissue and to also know about the intended function of NPs, a new combination of photoacoustic imaging modality and custom designed nano system has emerged [157, 158]. The main principle behind this is, first, the object will absorb light, then absorbed light energy will develop as heat and finally because of thermoelastic expansion, acoustic waves will be release out [159]. Photoacoustic imaging was first proposrd by Oraevsky, for the use of biomedical applications [160]. AgNPs possess strong optical absorbance and scattering properties and are used as contrast agents for imaging therapies. With the light of wavelength 800 nm and radiation, the NP’s injected 1cm deep in ex vivo pancreatic tissue could be detected using ultrasound imaging and photoacoustics. First mode is, AgNPs are engineered to target the tumour site specifically to the leaky blood vessels of the tumor and the reduced the rate of clearance due to lack of functional lymphatic vessels, and will retain the AgNPs [161]. The second one is, at the site of tumour, the AgNPs gets conjugated with antibodies and bind to the antigens present. By localizing the AgNPs at the tumour site, it provides photoacoustic contrast with normal tissues thereby useful in in vivo examination of tumour [16].
Gold Nanoparticles and Their Applications
Over in the last half century, AuNPs have been developed in many ways. Synthesizing AuNPs is now more reliable and are high in yields. These AuNP have distinct traits, namely size and shape dependent electrical and optical features, surfaces that can be changed with ligands containing functional groups. With the help of these functional groups to hold the ligands, and other biomolecules such as antibodies, proteins , etc. the newly formed gold nanoconjugates possess a wider range of research in therapeutics [16]. AuNP have provided useful materials for various biomedical applications, such as material crystallizations, programmed assembly, conversion of NPs into dimers and trimers and then to DNA templates, detection methods and bioelectronics. In diagnosis of a disease, the process of binding between the analytes and AuNPs, the physicochemical properties of AuNPs can be altered. We shall see about the various applications of AuNP and highlight their evolution in theranostics [162] in the sections mentioned below.
Medical Uses of Gold
Gold has been used in medicinal practices for many decades including Chinese and Indian medicines. They were mainly used for the treatment of arthritic diseases. But it was later found that nephrotoxicity was caused by gold due to the prolonged exposure. Hence, they were not used in medical practices until researchers developed a new compound called auranofins and aurothioglucose [163]. Yao et al, made a comparitive study of Gold clusters using Bovine serum albumin as template, Gold clusters using glutathione and Au nanoparticles with large particle diameter in both in vitro and in vivo. Gold nanoparticles acts by suppressing the proinflammatory mediators which is produced by lipopolyschharide. In comparing the above three, Au clusters with glutathione as template produced a better antiinflammatory effects thus it is one of potent nanodrug for treating Rheumatoid arthritis [164]. Osteoarthritis is kind of the arthritis which is attributed by swelling, stiffness and joint pain. Researchers studied the use of AuNPs for improvising the delivery of chondroitin sulfate. Chrondroitin sulfate is one of the drug for treating osteoarthritis. The combination of Chondroitin sulfate and AuNP augment the production of extracellular matrix and proliferation of chrondrocytes [165]. Since they are non-toxic, they are vividly used in biomedical applications as well as in gene and drug delivery. The optical intensity of AuNP has been subjugated for polynucleotide detections. Gold consists of a dielectric core and can be used suitably to make nanoshells, which are very useful in Surface enhanced Raman spectroscopy (SERS). AuNP has the ability to aggregate upon themselves when they interact with proteins. One of the basis for the quantitative method of colorimetric determination of proteins were the sudden colour change in the solution. This process helped in a better accurate result for many analytical methods including ELISA. AuNP is used in photothermal therapy. This is used in tumor therapy and infectious diseases. AuNP tend to become hot when they reach their maximum absorption in visible or near infrared region at the corresponding light wavelength. This kills the cells that are located around or inside the target cells. Today controlled and direct damaging of the tumor tissues is possible in cancer thermotherapy using laser radiation. But there are a lot of questions regarding its biodistribution, blood stream circulation, pharmacokinetics and toxicity. There are numerous research groups which use AuNP in their projects. There is a vast difference in the experiments, functionalizing methods, and dosages. Due to this, there has been major inconsistency with the data and the kinetics of biodistribution for toxicity estimation. Due to the development of efficient medical tools, AuNP has provided technologies for the functionalization of molecules providing stabilization in vivo. AuNP is now widely being used as problem solvers in bioimaging. [166]. AuNP have a great future in the medicine field, but one important concern is their safety level towards humans and environment. The AuNP toxicity depends on their physical dimensions and surface chemistry. Studies based on cytotoxicity of AuNP in human cells was done and the research showed that AuNP are nontoxic upto 250 mM, while ionic gold showed cytotoxicity at 25 mM. Lot of similar results were produced using AuNP in therapies [167].
Gold Nanoparticles for Cancer Theranostics
Cancer therapy has grown rapidly in the past few years. But surgery with chemoradiotherapy still remains the go to procedure for fighting malignant cancer. Nanotechnology has recently been in the main topic in medical research, and many NPs have been studied for cancer therapy. The color of AuNP changes when there is increase in their size. Because of this unique property AuNP can be used in detecting various biomolecule and easily identify the tumor targets that are accumulated in vitro and in vivo. The most commonly used metal in cancer theranostics are gold and iron oxide because of their structure stability, variability of the size, controlled release and low toxicity during cancer theranostics [168] . Table 2 shows the different anti tumor effect of AuNPs.
Table 2.
Antitumor effects of AuNPs
| S.No. | Type of Gold Nanoparticles used | Model used | Outcome | References |
|---|---|---|---|---|
| 1. | Green synthesis of AuNPs using Commelina nudiflora | HCT-116 Colon cancer cell line. | AuNP at high concentration of 400 μg/ml showed 90% cell death in HCT-116 cell line and the inhibitory concentration (IC50) was 200 μg/ml. | Kuppusamy et al., [124] |
| 2. | Green synthesis of AuNPs using Acalypha indica leaves extract. | Human breast cancer MDA-MB-231 cells | Four different concentrations were used - 1, 10, 50, 100 μg/ml, higher toxic effects were showed by AuNP at 100 μg/ml posessing 40 % of cell toxicity. This exhibited AuNPs as a novel anticancer agent which could be used for the human breast cancer. | Krishnaraj.C et al., [129] |
| 3. | Biosynthesized AuNPs using aqueous fruit extract of Chaenomeles sinensis (CS) | Human breast cancer cell line (MCF-7) | Observed a significant cytotoxicity at >25 μg/ml. | Keun Hyun Oh et al., [134] |
| 4. | Green synthesis of AuNPs using Indigofera tinctoria leaf extract | Lung cancer cell line (A549) | IC50 value of AuNP- tinctoria was 56.62±0.86 μg/ml. This was because of the NPs induced ROS. | Vijayan et al., [136] |
| 5. | Chemical synthesis of AuNPs | Glioma (U251 glioblastoma cells) | The IC50 of AuNP was 116.3 μg/ml which was less potent than AgNP. | Liu et al., [143] |
| 6. | Gold nanopartcles with hibiscus and curcumin extracts | Human colorectal cancer (HCT 116) and Michagen Cancer Foundation-7 (MCF-7) | The IC50 of AuNP- Hibiscus against HCT-116 and MCF-7 was found to be 5.80±0.91 μg/ml and 3.67±0.75 μg/ml and for AuNP-Curcumin was 4.94±0.84 μg/ml and 3.91±0.65 μg/ml respectively. | Akhtar et al, 2022 [169] |
| 7. | Gold nano bioconjugates with Elephantopus Scaber (linn,) hydro methalic extract. | Michagen Cancer Foundation-7 (MCF-7), A-549 cells, Squamous cell carcinoma (SCC-40) and Human colon cancer cell lines (COLO- 205) | The 50 % cell growth inhibition (GI 50) for AuNP was found to be < 10 μg/ml against MCF-7 cell line where as in A-549, SCC-40, COLO-205 was 33.0, 28.3 and 24.7 μg/ml respectively. Thus AuNP was one of the good antiproliferative agent. | Shinde et al, 2022 [170] |
AuNPs are used for both cell imaging and CT imaging in vivo. They are used mainly because they act as an alternative to X-ray based CT machines. They are used because of their better absorbent coefficient, easy attachment to the moieties and better body tolerance. AuNP has a great X-ray attenuation because of their high concentration and smaller size. Due to this it becomes easier to diagnose cancer with imaging [171]. The tissue absorption is very weak for light of wavelength >650 and <2,000 nm and that is the reason the NIR light is used for deep tumor imaging. AuNPs are the NIR-active probe for imaging the cancer cells in our body which is used for whole body scans. AuNPs are conjugated with anti-EGFR antibodies and used as a contrast agent in tumor imaging [172, 173].
Gold Nanoparticles for Cancer Therapy
Photothermal Therapy (PTT)
Gold has shown promising results in various cancer treatment such as photodynamic and photothermal therapies. This photothermal therapy is done through converting absorbed light into heat by nonradiative process. There are two main process:
The heat which is absorbed from energy conversion is given to the surrounding environment through the phonon relaxation within 100 ps.
A meticulous process of heating the electron and being cooled by the surrounding medium takes place, when the rate of heating is higher than the rate of cooling.
To use the photothermal therapy, a continuous wave laser is overlapped maximally with the AuNP resonance band. To achieve PTT using gold nanospheres the resonance of Continuous-Wave visible lasers should be in the visible region. This method can be used in treatment of shallow tumors. Recently, the development of antibody targeted gold nanospheres were done to primarily target EGFR on squalor carcinoma cells [172]. Researchers have developed new type of nanomatryoshkas which consists of PEG stabilizing ligands and concentric gold silica layers. In comparison to the existing gold-silica nanoshells which is approximately 150 nm, these nanomatryoshkas could facilitate higher concentration in tumors due to their ability to infiltrate the smaller AuNP in to the tissue. The survival rate of TNBC model was lesser than 1000 mm3 which significantly decreased the size of the tumors by gold nanomatryoshkas and this was superior than the conventional silica-gold nanoshells. The results were taken under irradiation with a CW laser emitting 3W/cm2 at a wavelength of 808 nm [174].
Photodynamic Therapy (PDT)
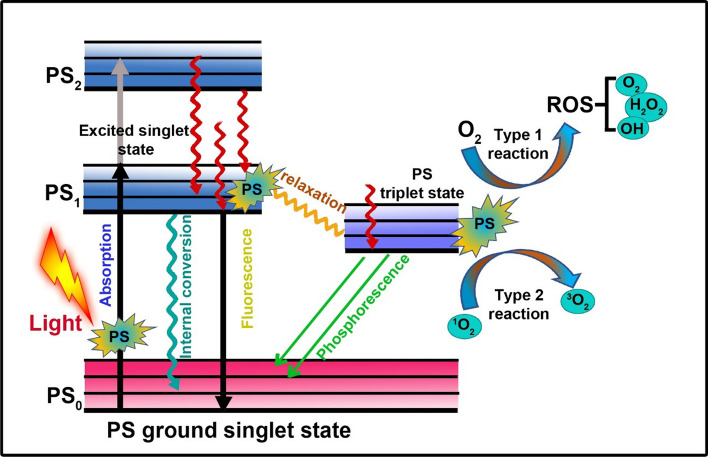
Photo synthesizers tend to convert the surrounding oxygen into a toxic reactive oxygen species when they are stimulated under a specific wavelength that might destroy the malignant cells in the surrounding proximity, which is now known as cancer PDT [175, 176]. To treat deeply buried tumors, AuNP exert PDT over NIR light activation. In addition, the incorporation of various photosensitizers with NIR active property into the AuNP can also be done with low dosages of organic photosensitizers and lasers with short exposure irradiation for PDT. When tested under an 808 nm laser, CS-AuNR-ICG NSs at the same time produced reactive oxygen species and hyperthermia, which attained complete inhibition of tumor growth in xenografted mice. In comaprison to PTT or PDT the combined therapy showed a drastically better therapeutic effectiveness [177]. The principle of photodynamic therapy is explained in Figure 2. Nanobiosensors has emerged as great tool in diagnosing cancer. Figure 3 describes the principle of nanobiosensor. Functionalized gold nanoparticles are immbolized on a template which can detect the analytes (proteins, toxins, antibodies, disease amrkers, cells etc.) with high specificity and sensitivity. The combination of analyte and recognition element which is attached to the gold nanoparticles gives a signal (heat, light, fluorescence, electrochemical change-current, potential and conduction, sound) which is converetd into measurable elctrical or optical signal. The amplifiers then amplify those signals to make them measurable using electronics [178].
Fig. 2.
Mechanism of Photodynamic therapy. PDT takes place through type I and type II reactions that can create ROS for killing the cancer cells
Fig. 3.
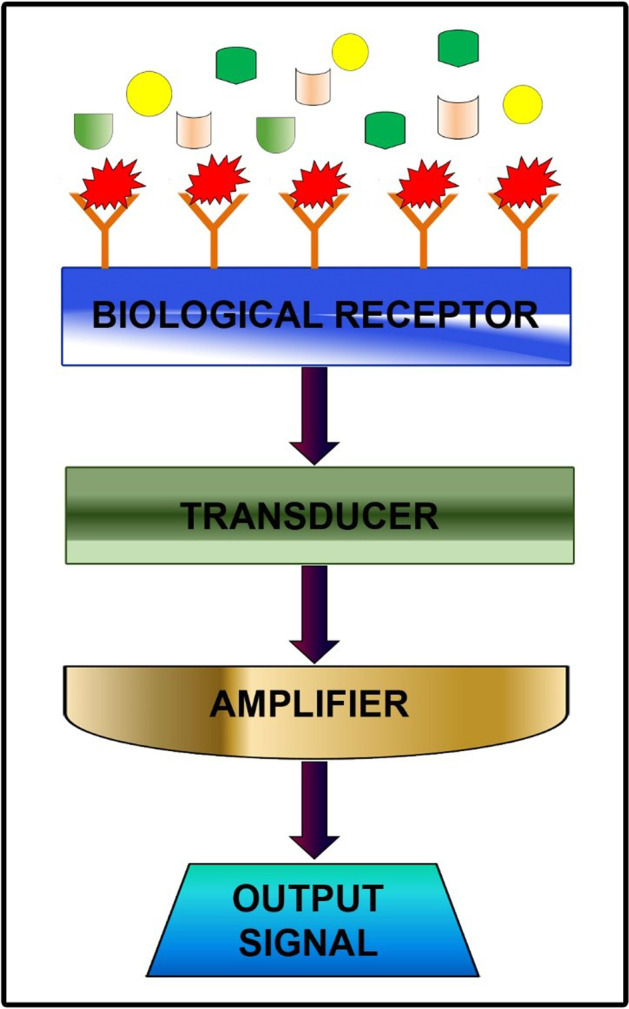
Mechanism of Nanobiosensor. The biological receptor contains the recognition element that can specifically bind with the target molecule and covert them into measurable signal through the transducer. The signal gets amplified and recorded as output
Use of Gold Nanoparticles in SARS-cov-2 Virus
COVID Test
AuNP can be used as a test kit to find out whether a person is infected by the virus or not. As we know the existing test kits require atleast 12 h to find whether the person is infected or not. In these critical 12 h the person might come in contact with another infected person and acquire the virus. With the help of AuNP we can reduce both the amount of time required for testing a person and the amount needed to spend for testing. These AuNP based assay test is done in an molecular level i.e. it checks the RNA of the person. This helps in accurate result for the identification of the virus. If this is the case, then it might prove positive for everyone because it'll be helpful in finding out who is infected and can treat the person well before it gets worsened. Because the test kits available in the market doesn't give the results at the earliest time, this methododlogy can be helpful. [179].
Covid Vaccines
The endurance of the human for the past 3 years become an dispute due to the outbreak of Covid 19. Severe Acute respiratory syndrome-Cov-2 (SARS-CoV-2) affects both humans and animals. Many researchers were in demand of developing Covid vaccines by collaborating with pharma companies. WHO reported on 9th June 2020 that among 136 vaccine candidates, 16 where nano based vaccines which are under clinical trials [180]. S proteins are the chief goal for the Corona virus vaccine production as it plays a role in its pathogenisis. Chen et al made a study using synthetic viral like particles as an effective vaccination tool in an avian model of Corona virus infection. Synthetic virus like particles were prepared by incubating the 100 nm gold nanoparticles in the optimal viral protein concentration solution. This enhanced the antibody titer, increased the lymphatic antigenic delivery, splenic T-cell response and decreased the infection symptoms. In comparison with the whole inactivated virus, synthetic viral like particles produced a better antiviral protection [181]. NPs in general are most preferred when it comes to destroy a deadly virus. This is mainly because of their nano size and the flexibility to alter a nanoparticle according to the virus. Covid 19 virus, an enveloped shape virus, which ranges from 50nm - 150nm in diameter has undergone many mutations. The exterior of this virus is covered by proteins in form of spikes which infects our cells. The genetic material of this virus is said to be ssRNA. This is one of the main reason for using a nanoparticle in killing this virus. Nanoparticle has the tendency to mimic a virus. This a good thing because it gives us the ability to directly attack the virus without affecting the nearby cells. The main path of these virus is to attack our respiratory system by binding with the cells in the respiratory system and affecting them with the help of protein spikes. There many ways to stop this from happening. As of now there are no confirmed therapeutic ways to destroy these viruses with the help of NPs. All of these are still in clinical trials. But it is well known that NPs is the way to destroy the virus efficiently [182].
Current Limitations
As we have discussed above, AuNP gives us the possibility to be used in cancer diagnostics and therapeutics. But it is foolish to not consider the other side of the coin. These NPs might have side effects on health of the human beings. There are many studies in the cytotoxicity of AuNP, toxic effects of size, efficacy, response of NPs, biodistribution. But these researches give out contradicting results leaving us in a challenging situation. Absence of solid information on the effects of NPs could have serious effects and a negative impact on human health. Chaves et al., studied toxicity of gold nanoparticles by in vitro using HT- 29 and HepG2 cell lines and in vivo using Wister rats. For this study, reserchers used 10 ppm of 10, 30 or 60 nm gold nanoparticles for the experiments. AuNP increased the production of ROS in cells at 16 h and at the 32 h the overproduction was normalised. As a result of in vivo studies, AuNP produced an increase in protein carbonyl groups formation and lipid peroxidation, which was measured by Thiobarbituric acid reactive substance (TBARS). Gold content got accumulated in liver, intestine, spleen, faeces,urine and kidney of rats [183]. Li et al., proposed that AuNP obstructs the proliferation of cells by dysregulating the cell cycle genes and it also affects the genomic stability and DNA repair [184]. This findings shows that there is a side of bane for gold nanoparticles and it is very important to evaluate the toxicity of any metal and metaloxide nanoparticles [185].
Conclusion
There are various metal NPs that as a predominant application in various fields of science. In which AuNP and AgNP has a vast range of application such as antimicrobial, antiinflamatory, diagnosis, anticancer agent and also has therapeutic activity such as in dentistry, orthopedics, cancer therapy, etc.,. which are mentioned above. Most of the researches are still in clinical trials. Some of them were already applied for treatment and diagnosis. Future research is necessary to conclude the safety aspects of nano silver and gold.
Acknowledgements
The authors are grateful to Chettinad Academy of Research and Education, Kelambakkam, Tamilnadu, India, for providing the infrastructural support.
Author Contribution
Sakthi Devi R and Siddharth M have collected the significant articles from Google Scholar and PubMed with appropriate keywords and written the first draft. Agnishwar Girigoswami and Koyeli Girigoswami have given the concept, and prepared the final manuscript.
Funding
The study was funded by Council of Scientific and Industrial Research (CSIR), INDIA, Scheme No. 01(2868)/17/EMR-II.
Data Availability
All the authors declare that all data and materials support their published claims and comply with field standards.
Code Availability
Not Applicable
Declarations
Ethics Approval
• The manuscript is not be submitted to more than one journal for simultaneous consideration.
• The submitted work is original and should not have been published elsewhere in any form or language (partially or in full).
• A single study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time (i.e. ‘salami-slicing/publishing’).
• Results are presented clearly, honestly, and without fabrication, falsification or inappropriate data manipulation (including image based manipulation). Authors have adhered to discipline-specific rules for acquiring, selecting and processing data.
• No data, text, or theories by others are presented as if they were the author’s own (‘plagiarism’). Proper acknowledgements to other works have been given.
Informed Consent
Not applicable
Consent to Participate
Yes
Consent for Publication
Yes
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh H, Du J, Singh P, Yi TH. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46:1163–1170. doi: 10.1080/21691401.2017.1362417. [DOI] [PubMed] [Google Scholar]
- 2.Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK, Rather IA. Application of nanotechnology in food science: perception and overview. Frontiers in Microbiology. 2017;8:1501. doi: 10.3389/fmicb.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta N, Ranjan S, Ramalingam C. Applications of nanotechnology in agriculture and water quality management. Environmental Chemistry Letters. 2017;15:591–605. doi: 10.1007/s10311-017-0648-9. [DOI] [Google Scholar]
- 4.Girigoswami K, Viswanathan M, Murugesan R, Girigoswami A. Studies on Polymer-Coated Zinc Oxide Nanoparticles: UV-blocking Efficacy and in vivo Toxicity. Materials Science and Engineering: C. 2015;56:501–510. doi: 10.1016/j.msec.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Girigoswami A, Girigoswami K. Nanotechnology in Detection of Food Toxins–Focus on the Dairy Products. Biointerface Research in Applied Chemistry. 2021;11:14155–14172. doi: 10.33263/BRIAC116.1415514172. [DOI] [Google Scholar]
- 6.Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. Journal of Drug Delivery Science and Technology. 2019;53:101174. doi: 10.1016/j.jddst.2019.101174. [DOI] [Google Scholar]
- 7.Ghosh D, Girigoswami A, Chattopadhyay N. Superquenching of coumarin 153 by gold nanoparticles. Journal of Photochemistry and Photobiology A. 2012;242:44–50. doi: 10.1016/j.jphotochem.2012.05.027. [DOI] [Google Scholar]
- 8.Ge L, Li Q, Wang M, Ouyang J, Li X, Xing MM. Nanosilver particles in medical applications: synthesis, performance, and toxicity. International Journal of Nanomedicine. 2014;9:2399. doi: 10.2147/IJN.S55015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthu MS, Wilson B. Multifunctional radionanomedicine: a novel nanoplatform for cancer imaging and therapy. Nanomedicine. 2010;5(2):169–171. doi: 10.2217/nnm.09.107. [DOI] [PubMed] [Google Scholar]
- 10.Haribabu V, Girigoswami K, Girigoswami A. Magneto-silver core–shell nanohybrids for theragnosis. Nano-Struct.Nano-Objects. 2021;25:100636. doi: 10.1016/j.nanoso.2020.100636. [DOI] [Google Scholar]
- 11.Liu X, Gao P, Du J, Zhao X, Wong KK. Long-term anti-inflammatory efficacy in intestinal anastomosis in mice using silver nanoparticle-coated suture. Journal of Pediatric Surgery. 2017;52(12):2083–2087. doi: 10.1016/j.jpedsurg.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Keshvadi M, Karimi F, Valizadeh S, Valizadeh A. Comparative study of antibacterial inhibitory effect of silver nanoparticles and garlic oil nanoemulsion with their combination. Biointerface Research in Applied Chemistry. 2019;9:4560–4566. doi: 10.33263/BRIAC96.560566. [DOI] [Google Scholar]
- 13.Ali G, Abd El-Moez S, Abdel-Fattah W. Synthesis and characterization of nontoxic silver nano-particles with preferential bactericidal activity. Biointerface Research in Applied Chemistry. 2019;9:4617–4623. doi: 10.33263/BRIAC96.617623. [DOI] [Google Scholar]
- 14.Kavya J, Amsaveni G, Nagalakshmi M, Girigoswami K, Murugesan R, Girigoswami A. Silver Nanoparticles Induced Lowering of BCl2/Bax Causes Dalton's Lymphoma Tumour Cell Death in Mice. Journal of Bionanoscience. 2013;7(3):276–281. doi: 10.1166/jbns.2013.1135. [DOI] [Google Scholar]
- 15.Girigoswami A, Wafic Y, Sharmiladevi P, Haribabu V, Girigoswami K. Camouflaged Nanosilver with Excitation Wavelength Dependent High Quantum Yield for Targeted Theranostic. Scientific Reports. 2018;8:16459. doi: 10.1038/s41598-018-34843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angewandte Chemie, International Edition. 2010;49(19):3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maleki M, Pourhassan-Moghaddam M, Karimi A, Akbarzadeh A, Zarghami N, Mohammadi S. Synthesis, characterisation, and application of chamomile gold nanoparticles in molecular diagnostics: a new component for PCR kits. Biointerface Research in Applied Chemistry. 2019;9(6):4635–4641. doi: 10.33263/BRIAC96.635641. [DOI] [Google Scholar]
- 18.Azharuddin M, Zhu GH, Das D, Ozgur E, Uzun L, Turner AP, Patra HK. A repertoire of biomedical applications of noble metal nanoparticles. Chemical Communications. 2019;55(49):6964–6996. doi: 10.1039/c9cc01741k. [DOI] [PubMed] [Google Scholar]
- 19.Ye L, Cao Z, Liu X, Cui Z, Li Z, Liang Y, Zhu S, Wu S. Noble Metal-Based Nanomaterials as Antibacterial Agents. Journal of Alloys and Compounds. 2022;2022:164091. doi: 10.1016/j.jallcom.2022.164091. [DOI] [Google Scholar]
- 20.Alt V, Bechert T, Steinrücke P, Wagener M, Seidel P, Dingeldein E, et al. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25(18):4383–4391. doi: 10.1016/j.biomaterials.2003.10.078. [DOI] [PubMed] [Google Scholar]
- 21.Panáček A, Kvitek L, Prucek R, Kolář M, Večeřová R, Pizúrová N, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. The Journal of Physical Chemistry. 2006;110(33):16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 22.Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, et al. Silver nanoparticles: partial oxidation and antibacterial activities. Journal of Biological Inorganic Chemistry. 2007;12(4):527–534. doi: 10.1007/s00775-007-0208-z. [DOI] [PubMed] [Google Scholar]
- 23.Ovington LG. The truth about silver. Ostomy/Wound Management. 2004;50(9A Suppl):1S–10S. [PubMed] [Google Scholar]
- 24.Manjumeena R, Duraibabu D, Sudha J, Kalaichelvan P. Biogenic nanosilver incorporated reverse osmosis membrane for antibacterial and antifungal activities against selected pathogenic strains: an enhanced eco-friendly water disinfection approach. Journal of Environmental Science and Health, Part A. 2014;49(10):1125–1133. doi: 10.1080/10934529.2014.897149. [DOI] [PubMed] [Google Scholar]
- 25.Sanpui P, Murugadoss A, Prasad PD, Ghosh SS, Chattopadhyay A. The antibacterial properties of a novel chitosan–Ag-nanoparticle composite. International Journal of Food Microbiology. 2008;124(2):142–146. doi: 10.1016/j.ijfoodmicro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 2009;3(6):1357–1364. doi: 10.1021/nn900277t. [DOI] [PubMed] [Google Scholar]
- 27.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 28.Leaper DJ. Silver dressings: their role in wound management. International Wound Journal. 2006;3(4):282–294. doi: 10.1111/j.1742-481X.2006.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barreiro E, Casas JS, Couce MD, Sánchez A, Seoane R, Sord OJ, et al. Synthesis and antimicrobial activities of silver (i) sulfanylcarboxylates. Structural isomers with identically or unequally coordinated Ag centers in an Ag 4 S 4 ring. Dalton Transactions. 2007;28:3074–3085. doi: 10.1039/b702936e. [DOI] [PubMed] [Google Scholar]
- 30.Thomas V, Yallapu MM, Sreedhar B, Bajpai SA. versatile strategy to fabricate hydrogel–silver nanocomposites and investigation of their antimicrobial activity. Journal Colloid and Interface Science. 2007;315(1):389–395. doi: 10.1016/j.jcis.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 31.Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. Journal of Proteome Research. 2006;5(4):916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 32.Thomas JG, Chenoweth CE, Sullivan SE. Iatrogenic Creutzfeldt-Jakob disease via surgical instruments. Journal of Clinical Neuroscience. 2013;20(9):1207–1212. doi: 10.1016/j.jocn.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Feng QL, Wu J, Chen GQ, Cui F, Kim T, Kim JA. Mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Materials Research. 2000;52(4):662–668. doi: 10.1002/1097-4636(20001215)52:4<662::AID-JBM10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 35.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. Journal Colloid and Interface Science. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Song H, Ko K, Oh L, Lee B. Fabrication of silver nanoparticles and their antimicrobial mechanisms. European Cells & Materials. 2006;11(Suppl 1):58. [Google Scholar]
- 37.Ghodake G, Kim M, Sung JS, Shinde S, Yang J, Hwang K, et al. Extracellular synthesis and characterization of silver nanoparticles—Antibacterial activity against multidrug-resistant bacterial strains. Nanomaterials. 2020;10(2):360. doi: 10.3390/nano10020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhuyar P, Rahim MHA, Sundararaju S, Ramaraj R, Maniam GP, Govindan N. Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef University Journal of Basic and Applied Sciences. 2020;9(1):1–15. doi: 10.1186/s43088-019-0031-y. [DOI] [Google Scholar]
- 39.Khan B, Nawaz M, Hussain R, Price GJ, Warsi MF, Waseem M. Enhanced antibacterial activity of size-controlled silver and polyethylene glycol functionalized silver nanoparticles. Chemical Papers. 2021;75(2):743–752. doi: 10.1007/s11696-020-01335-7. [DOI] [Google Scholar]
- 40.Thiruvengadam V, Bansod AV. Green Synthesis of Silver Nanoparticles Using Melia Azedarach and its Characterization, Corrosion and Antibacterial Properties. Biointerface Research in Applied Chemistry. 2020;11(1):8577–8586. doi: 10.33263/BRIAC111.85778586. [DOI] [Google Scholar]
- 41.Pulit J, Banach M, Szczygłowska R, Bryk M. Nanosilver against fungi. Silver nanoparticles as an effective biocidal factor. Acta Biochimica Polonica. 2013;60(4):795–798. doi: 10.18388/abp.2013_2060. [DOI] [PubMed] [Google Scholar]
- 42.Thirumalai Arasu V, Prabhu D, Soniya M. Stable silver nanoparticle synthesizing methods and its applications. Research Journal of Biological Sciences. 2010;1:259–270. [Google Scholar]
- 43.Mallmann EJJ, Cunha FA, Castro BN, Maciel AM, Menezes EA, Fechine PBA. Antifungal activity of silver nanoparticles obtained by green synthesis. Revista do Instituto de Medicina Tropical de São Paulo. 2015;57:165–167. doi: 10.1590/S0036-46652015000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fajar M, Endarko E, Rubiyanto A, Malek N, Hadibarata T, Syafiuddin A. A green deposition method of silver nanoparticles on textiles and their antifungal activity. Biointerface Research in Applied Chemistry. 2019;10:4902–4907. doi: 10.33263/BRIAC101.902907. [DOI] [Google Scholar]
- 45.Al-Otibi F, Perveen K, Al-Saif NA, Alharbi RI, Bokhari NA, Albasher G, et al. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi Journal of Biological Sciences. 2021;28(4):2229–2235. doi: 10.1016/j.sjbs.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Zubaidi S, Al-Ayafi A, Abdelkader H. Biosynthesis, characterization and antifungal activity of silver nanoparticles by Aspergillus niger isolate. Journal Nanobiotechnology. 2019;1(1):23–36. doi: 10.26502/jnr.2688-8521002. [DOI] [Google Scholar]
- 47.Huang W, Yan M, Duan H, Bi Y, Cheng X, Yu H. Synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against Setosphaeria turcica. Journal of Nanomaterials. 2020;2020:9535432. doi: 10.1155/2020/9535432. [DOI] [Google Scholar]
- 48.Lara HH, Ixtepan-Turrent L, Garza-Treviño EN, Rodriguez-Padilla C. PVP-coated silver nanoparticles block the transmission of cell-free and cell-associated HIV-1 in human cervical culture. Journal Nanobiotechnology. 2010;8(1):1–11. doi: 10.1186/1477-3155-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L, Singh AK, Vig K, Pillai SR, Singh SR. Silver nanoparticles inhibit replication of respiratory syncytial virus. Journal of Biomedical Nanotechnology. 2008;4(2):149–158. doi: 10.1166/jbn.2008.012. [DOI] [Google Scholar]
- 50.Baram-Pinto D, Shukla S, Gedanken A, Sarid R. Inhibition of HSV-1 attachment, entry, and cell-to-cell spread by functionalized multivalent gold nanoparticles. Small. 2010;6(9):1044–1050. doi: 10.1002/smll.200902384. [DOI] [PubMed] [Google Scholar]
- 51.Papp I, Sieben C, Ludwig K, Roskamp M, Böttcher C, Schlecht S, et al. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small. 2010;6(24):2900–2906. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]
- 52.Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM. A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Research Letters. 2008;3(4):129–133. doi: 10.1007/s11671-008-9128-2. [DOI] [Google Scholar]
- 53.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. Journal Nanobiotechnology. 2010;8(1):1–10. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speshock JL, Murdock RC, Braydich-Stolle LK, Schrand AM, Hussain SM. Interaction of silver nanoparticles with Tacaribe virus. Journal Nanobiotechnology. 2010;8(1):1–9. doi: 10.1186/1477-3155-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saadh MJ, Aggag MM, Alboghdadly A, Kharshid AM, Aldalaen SM, Abdelrazek MA. Silver nanoparticles with epigallocatechingallate and zinc sulphate significantly inhibits avian influenza A virus H9N2. Microbial Pathogenesis. 2021;158:105071. doi: 10.1016/j.micpath.2021.105071. [DOI] [PubMed] [Google Scholar]
- 56.Haggag EG, Elshamy AM, Rabeh MA, Gabr NM, Salem M, Youssif KA, et al. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Biomolecules. 2019;14:6217. doi: 10.2147/IJN.S214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saad AHA, Soliman MI, Azzam AM, Mostafa AB. Antiparasitic activity of silver and copper oxide nanoparticles against Entamoeba histolytica and Cryptosporidium parvum cysts. Journal of the Egyptian Society of Parasitology. 2015;45(3):593–602. doi: 10.12816/0017920. [DOI] [PubMed] [Google Scholar]
- 58.Allahverdiyev AM, Abamor ES, Bagirova M, Ustundag CB, Kaya C, Kaya F, et al. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. International Journal of Nanomedicine. 2011;6:2705. doi: 10.2147/IJN.S23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva Viana RL, Pereira Fidelis G, Jane Campos Medeiros M, Antonio Morgano M, Chagas Faustino Alves MG, Domingues Passero LF, et al. Green synthesis of antileishmanial and antifungal silver nanoparticles using corn cob xylan as a reducing and stabilizing agent. Biomolecules. 2020;10(9):1235. doi: 10.3390/biom10091235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zahra’a AA, Mustafa TA, Ardalan NM, Idan EM. In vitro toxicity evaluation of silver nanoparticles on Entamoeba histolytica trophozoite. Baghdad Science Journal. 2017;14(3):509–515. doi: 10.21123/bsj.14.3.509-515. [DOI] [Google Scholar]
- 61.Boateng J, Catanzano O. Silver and silver nanoparticle-based antimicrobial dressings. Therapeutic dressings and wound healing applications. 2020;2020:157–184. doi: 10.1002/9781119433316.ch8. [DOI] [Google Scholar]
- 62.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proceedings of the Society for Experimental Biology and Medicine. 1962;111(3):544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 63.Shehensha S, Jyothi MV. Anti-inflammatory Activity of Nigella sativa oil Mediated Silver Nanoparticles. Pharmacognosy Magazine. 2020;12(5):1086–1092. doi: 10.5530/pj.2020.12.153. [DOI] [Google Scholar]
- 64.Kedi PBE, Meva FEA, Kotsedi L, Nguemfo EL, Zangueu CB, Ntoumba AA, et al. Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. International Journal of Nanomedicine. 2018;13:8537. doi: 10.2147/IJN.S174530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devaraja S, Nagaraju S, Mahadeswaraswamy Y, Girish K, Kemparaju K. A low molecular weight serine protease: Purification and characterization from Hippasa agelenoides (funnel web) spider venom gland extract. Toxicon. 2008;52(1):130–138. doi: 10.1016/j.toxicon.2008.04.168. [DOI] [PubMed] [Google Scholar]
- 66.Poon M, d'Oiron R. Recombinant activated factor VII (NovoSeven®) treatment of platelet-related bleeding disorders. Blood Coagulation & Fibrinolysis. 2000;11:S55–S68. doi: 10.1097/00001721-200004001-00013. [DOI] [PubMed] [Google Scholar]
- 67.Kenawy HI, Boral I, Bevington A. Complement-coagulation cross-talk: a potential mediator of the physiological activation of complement by low pH. Frontiers in Immunology. 2015;6:215. doi: 10.3389/fimmu.2015.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y, Li X, Liao Z, Zhang G, Liu Q, Tang J, et al. A randomized comparative trial between Acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysis. Burns. 2007;33(2):161–166. doi: 10.1016/j.burns.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 69.Chen J, Han C, Lin X, Tang Z, Su S. Effect of silver nanoparticle dressing on second degree burn wound. Chinese Journal of Surgery. 2006;44(1):50–52. [PubMed] [Google Scholar]
- 70.Chandika P, Ko SC, Jung WK. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. International Journal of Biological Macromolecules. 2015;77:24–35. doi: 10.1016/j.ijbiomac.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 71.Hasatsri S, Yamdech R, Chanvorachote P, Aramwit P. Physical and biological assessments of the innovative bilayered wound dressing made of silk and gelatin for clinical applications. Journal of Biomaterials Applications. 2015;29(9):1304–1313. doi: 10.1177/0885328214559138. [DOI] [PubMed] [Google Scholar]
- 72.Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Review of Anti-Infective Therapy. 2011;9(7):857–879. doi: 10.1586/eri.11.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burgess R. Understanding nanomedicine: an introductory textbook. CRC Press; 2012. [Google Scholar]
- 74.Chen H, Lan G, Ran L, Xiao Y, Yu K, Lu B, et al. A novel wound dressing based on a Konjac glucomannan/silver nanoparticle composite sponge effectively kills bacteria and accelerates wound healing. Carbohydrate Polymers. 2018;183:70–80. doi: 10.1016/j.carbpol.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 75.Mermel LA. Prevention of intravascular catheter–related infections. Annals of Internal Medicine. 2000;132(5):391–402. doi: 10.7326/0003-4819-132-5-200003070-00009. [DOI] [PubMed] [Google Scholar]
- 76.Sheng WH, Ko WJ, Wang JT, Chang SC, Hsueh PR, Luh KT. Evaluation of antiseptic-impregnated central venous catheters for prevention of catheter-related infection in intensive care unit patients. Diagnostic Microbiology and Infectious Disease. 2000;38(1):1–5. doi: 10.1016/s0732-8893(00)00166-8. [DOI] [PubMed] [Google Scholar]
- 77.Khare MD, Bukhari SS, Swann A, Spiers P, McLaren I, Myers J. Reduction of catheter-related colonisation by the use of a silver zeolite-impregnated central vascular catheter in adult critical care. The Journal of Infection. 2007;54(2):146–150. doi: 10.1016/j.jinf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Neethu S, Midhun SJ, Radhakrishnan E, Jyothis M. Surface functionalization of central venous catheter with mycofabricated silver nanoparticles and its antibiofilm activity on multidrug resistant Acinetobacter baumannii. Microbial Pathogenesis. 2020;138:103832. doi: 10.1016/j.micpath.2019.103832. [DOI] [PubMed] [Google Scholar]
- 79.Fichtner J, Güresir E, Seifert V, Raabe A. Efficacy of silver-bearing external ventricular drainage catheters: a retrospective analysis. Journal of Neurosurgery. 2010;112(4):840–846. doi: 10.3171/2009.8.JNS091297. [DOI] [PubMed] [Google Scholar]
- 80.Morley K, Webb P, Tokareva N, Krasnov A, Popov V, Zhang J, et al. Synthesis and characterisation of advanced UHMWPE/silver nanocomposites for biomedical applications. European Polymer Journal. 2007;43(2):307–314. doi: 10.1016/j.eurpolymj.2006.10.011. [DOI] [Google Scholar]
- 81.Zhao Y, Xing Q, Janjanam J, He K, Long F, Low KB, et al. Facile electrochemical synthesis of antimicrobial TiO2 nanotube arrays. International Journal of Nanomedicine. 2014;9:5177. doi: 10.2147/IJN.S65386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castiglioni S, Cazzaniga A, Locatelli L, Maier JA. Silver nanoparticles in orthopedic applications: New insights on their effects on osteogenic cells. Nanomaterials. 2017;7(6):124. doi: 10.3390/nano7060124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cobb WS, Harris JB, Lokey JS, McGill ES, Klove KL. Incisional herniorrhaphy with intraperitoneal composite mesh: a report of 95 cases. The American Surgeon. 2003;69(9):784–787. [PubMed] [Google Scholar]
- 84.Read RC. Milestones in the history of hernia surgery: prosthetic repair. Hernia. 2004;8(1):8–14. doi: 10.1007/s10029-003-0169-2. [DOI] [PubMed] [Google Scholar]
- 85.Beard JH, Ohene-Yeboah M, de Vries CR, Schecter W. Hernia and hydrocele. In: Debas HT, Donkor P, Gawande A, Jamison DT, Kruk ME, Mock CN, editors. Essential Surgery: Disease Control Priorities. 3rd Eds. The International Bank for Reconstruction and Development / The World Bank; 2015. pp. 151–171. [Google Scholar]
- 86.Samuel U, Guggenbichler J. Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. International Journal of Antimicrobial Agents. 2004;23:75–78. doi: 10.1016/j.ijantimicag.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 87.Klasen H. Historical review of the use of silver in the treatment of burns. I. Early uses. Burns. 2000;26(2):117–130. doi: 10.1016/s0305-4179(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 88.Dunn K, Edwards-Jones V. The role of Acticoat™ with nanocrystalline silver in the management of burns. Burns. 2004;30:S1–S9. doi: 10.1016/s0305-4179(04)90000-9. [DOI] [PubMed] [Google Scholar]
- 89.Darouiche RO. Treatment of infections associated with surgical implants. The New England Journal of Medicine. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 90.Cohen MS, Stern JM, Vanni AJ, Kelley RS, Baumgart E, Field D, et al. In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surgical Infections. 2007;8(3):397–404. doi: 10.1089/sur.2006.032. [DOI] [PubMed] [Google Scholar]
- 91.Bhol K, Schechter P. Topical nanocrystalline silver cream suppresses inflammatory cytokines and induces apoptosis of inflammatory cells in a murine model of allergic contact dermatitis. The British Journal of Dermatology. 2005;152(6):1235–1242. doi: 10.1111/j.1365-2133.2005.06575.x. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, Zheng Y, Li Y, Wang L, Bai Y, Zhao Q, et al. Tantalum nitride-decorated titanium with enhanced resistance to microbiologically induced corrosion and mechanical property for dental application. PLoS One. 2015;10(6):e0130774. doi: 10.1371/journal.pone.0130774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Porenczuk A, Grzeczkowicz A, Maciejewska I, Gołaś M, Piskorska K, Kolenda A, et al. An initial evaluation of cytotoxicity, genotoxicity and antibacterial effectiveness of a disinfection liquid containing silver nanoparticles alone and combined with a glass-ionomer cement and dentin bonding systems. Advances in Clinical and Experimental Medicine. 2019;28(1):75–83. doi: 10.17219/acem/76160. [DOI] [PubMed] [Google Scholar]
- 94.Fernandez CC, Sokolonski AR, Fonseca MS, Stanisic D, Araújo DB, Azevedo V, et al. Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation. International Journal of Molecular Sciences. 2021;22(5):2485. doi: 10.3390/ijms22052485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Monteiro DR, Gorup LF, Takamiya AS, de Camargo ER, Filho ACR, Barbosa DB. Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles. Journal of Prosthodontics. 2012;21(1):7–15. doi: 10.1111/j.1532-849X.2011.00772.x. [DOI] [PubMed] [Google Scholar]
- 96.Chand K, Abro MI, Aftab U, Shah AH, Lakhan MN, Cao D, et al. Green synthesis characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Advances. 2019;9(30):17002–17015. doi: 10.1039/C9RA01407A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pérez-Díaz MA, Boegli L, James G, Velasquillo C, Sanchez-Sanchez R, Martinez-Martinez RE, et al. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Materials Science and Engineering: C. 2015;55:360–366. doi: 10.1016/j.msec.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 98.Besinis A, Hadi SD, Le H, Tredwin C, Handy R. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology. 2017;11(3):327–338. doi: 10.1080/17435390.2017.1299890. [DOI] [PubMed] [Google Scholar]
- 99.Nozari A, Ajami S, Rafiei A, Niazi E. Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: an in vitro study. Journal of Clinical and Diagnostic. 2017;11(9):ZC97. doi: 10.7860/JCDR/2017/30108.10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Venugopal A, Muthuchamy N, Tejani H, Gopalan AI, Lee KP, Lee HJ, et al. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. The Korean Journal of Orthodontics. 2017;47(1):3–10. doi: 10.4041/kjod.2017.47.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hollstein M, Alexandrov L, Wild C, Ardin M, Zavadil J. Base changes in tumour DNA have the power to reveal the causes and evolution of cancer. Oncogene. 2017;36(2):158–167. doi: 10.1038/onc.2016.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manzoor M, Khan AHA, Ullah R, Khan MZ, Ahmad I. Environmental epidemiology of cancer in South Asian population: risk assessment against exposure to polycyclic aromatic hydrocarbons and volatile organic compounds. Arabian Journal for Science and Engineering. 2016;41(6):2031–2043. doi: 10.1007/s13369-016-2139-x. [DOI] [Google Scholar]
- 103.Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm.Res. 2008;25(9):2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ghosh R, Girigoswami K. NADH dehydrogenase subunits are overexpressed in cells exposed repeatedly to H2O2. Mutation Research, Fundamental and Molecular Mechanisms of Mutagenesis. 2008;638(1-2):210–215. doi: 10.1016/j.mrfmmm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Asharani P, Hande MP, Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biology. 2009;10(1):1–14. doi: 10.1186/1471-2121-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Greulich C, Diendorf J, Simon T, Eggeler G, Epple M, Köller M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomaterialia. 2011;7(1):347–354. doi: 10.1016/j.actbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicology In Vitro. 2009;23(6):1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Rosas-Hernández H, Jiménez-Badillo S, Martínez-Cuevas PP, Gracia-Espino E, Terrones H, Terrones M, et al. Effects of 45-nm silver nanoparticles on coronary endothelial cells and isolated rat aortic rings. Toxicology Letters. 2009;191(2-3):305–313. doi: 10.1016/j.toxlet.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 109.Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicology Letters. 2008;179(3):130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 110.Sanpui P, Chattopadhyay A, Ghosh SS. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Applied Materials & Interfaces. 2011;3(2):218–228. doi: 10.1021/am100840c. [DOI] [PubMed] [Google Scholar]
- 111.Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicology and Applied Pharmacology. 2008;33(3):404–410. doi: 10.1016/j.taap.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 112.Sukirtha R, Priyanka KM, Antony JJ, Kamalakkannan S, Thangam R, Gunasekaran P, et al. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochemistry. 2012;47(2):273–279. doi: 10.1016/j.procbio.2011.11.003. [DOI] [Google Scholar]
- 113.Kalishwaralal K, Banumathi E, Pandian SRK, Deepak V, Muniyandi J, Eom SH, et al. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids and Surfaces, B: Biointerfaces. 2009;73(1):51–57. doi: 10.1016/j.colsurfb.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 114.Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system) Theranostics. 2014;4(3):316. doi: 10.7150/thno.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yesilot S, Aydin C. Silver nanoparticles; a new hope in cancer therapy? Eastern Journal of Medicine. 2019;24(1):111–116. doi: 10.5505/ejm.2019.66487. [DOI] [Google Scholar]
- 116.Gurunathan S, Raman, J. Abd Malek, S.N.; John, P.A.; Vikineswary, S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells Int. Journal Nanomedicine. 2013;8:4399. doi: 10.2147/IJN.S51881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gurunathan S, Han JW, Dayem AA, Eppakayala V, Park JH, Cho SG, et al. Green synthesis of anisotropic silver nanoparticles and its potential cytotoxicity in human breast cancer cells (MCF-7) Journal of Industrial and Engineering Chemistry. 2013;19(5):1600–1605. doi: 10.3390/ijms17101603. [DOI] [Google Scholar]
- 118.Nazir S, Hussain T, de Iqbal MMK, Muazzam A, Ismail JM. Novel and cost-effective green synthesis of silver nano particles and their in vivo antitumor properties against human cancer cell lines. BioScience Technologies. 2011;2(6):425–430. [Google Scholar]
- 119.Saratale RG, Benelli G, Kumar G, Kim DS, Saratale GD. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environmental Science and Pollution Research. 2018;25(11):10392–10406. doi: 10.1007/s11356-017-9581-5. [DOI] [PubMed] [Google Scholar]
- 120.El-Naggar NEA, Hussein MH, El-Sawah AA. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Scientific Reports. 2017;7(1):1–20. doi: 10.1038/s41598-017-11121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Govindaraju K, Krishnamoorthy K, Alsagaby SA, Singaravelu G, Premanathan M. Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnology. 2015;9(6):325–330. doi: 10.1049/iet-nbt.2015.0001. [DOI] [PubMed] [Google Scholar]
- 122.Jacob SJP, Finub J, Narayanan A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids and Surfaces, B: Biointerfaces. 2012;91:212–214. doi: 10.1016/j.colsurfb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 123.Nagajyothi P, Sreekanth T, Lee JI, Lee KD. Mycosynthesis: antibacterial, antioxidant and antiproliferative activities of silver nanoparticles synthesized from Inonotus obliquus (Chaga mushroom) extract. Journal of Photochemistry and Photobiology A. 2014;130:299–304. doi: 10.1016/j.jphotobiol.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 124.Kuppusamy P, Ichwan SJ, Al-Zikri PNH, Suriyah WH, Soundharrajan I, Govindan N, et al. In vitro anticancer activity of Au, Ag nanoparticles synthesized using Commelina nudiflora L. aqueous extract against HCT-116 colon cancer cells. Biological Trace Element Research. 2016;173(2):297–305. doi: 10.1007/s12011-016-0666-7. [DOI] [PubMed] [Google Scholar]
- 125.Kathiravan V, Ravi S, Ashokkumar S. Synthesis of silver nanoparticles from Melia dubia leaf extract and their in vitro anticancer activity. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 2014;130:116–121. doi: 10.1016/j.saa.2014.03.10. [DOI] [PubMed] [Google Scholar]
- 126.He Y, Du Z, Ma S, Liu Y, Li D, Huang H, et al. Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. International Journal of Nanomedicine. 2016;11:1879. doi: 10.2147/IJN.S103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.El-Sheikh, S. M., Edrees, N., Hend, E. S., Khamis, T., Arisha, A. H., Metwally, M. M., et al. (2021, 1-9). Could Cisplatin Loading on Biosynthesized Silver Nanoparticles Improve Its Therapeutic Efficacy on Human Prostate Cancer Cell Line and Reduce Its In Vivo Nephrotoxic Effects? Biological Trace Element Research. 10.1007/s12011-021-02677-3 [DOI] [PubMed]
- 128.Sre PR, Reka M, Poovazhagi R, Kumar MA, Murugesan K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy. 2015;135:1137–1144. doi: 10.1016/j.saa.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 129.Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran M, Kalaichelvan P. Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnology Reports. 2014;4:42–49. doi: 10.1016/j.btre.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sathishkumar G, Gobinath C, Wilson A, Sivaramakrishnan S. Dendrophthoe falcata (Lf) Ettingsh (Neem mistletoe): A potent bioresource to fabricate silver nanoparticles for anticancer effect against human breast cancer cells (MCF-7) Spectrochimica Acta Part A: Molecular and Biomolecular. 2014;128:285–290. doi: 10.1016/j.saa.2014.02.096. [DOI] [PubMed] [Google Scholar]
- 131.Gajendran B, Chinnasamy A, Durai P, Raman J, Ramar M. Biosynthesis and characterization of silver nanoparticles from Datura inoxia and its apoptotic effect on human breast cancer cell line MCF7. Materials Letters. 2014;122:98–102. doi: 10.1016/j.matlet.2014.02.003. [DOI] [Google Scholar]
- 132.Yakop F, Abd Ghafar SA, Yong YK, Saiful Yazan L, Mohamad HR, Lim V, et al. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46(sup2):131–139. doi: 10.1080/21691401.2018.1452750. [DOI] [PubMed] [Google Scholar]
- 133.AlSalhi MS, Devanesan S, Alfuraydi AA, Vishnubalaji R, Munusamy MA, Murugan K, et al. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. International Journal of Nanomedicine. 2016;11:4439. doi: 10.2147/IJN.S113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oh KH, Soshnikova V, Markus J, Kim YJ, Lee SC, Singh P, et al. Biosynthesized gold and silver nanoparticles by aqueous fruit extract of Chaenomeles sinensis and screening of their biomedical activities. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46(3):599–606. doi: 10.1080/21691401.2017.1332636. [DOI] [PubMed] [Google Scholar]
- 135.Kaler A, Jain S, Banerjee UC. Green and rapid synthesis of anticancerous silver nanoparticles by Saccharomyces boulardii and insight into mechanism of nanoparticle synthesis. BioMed Research International. 2013;2013:872940. doi: 10.1155/2013/872940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vijayan R, Joseph S, Mathew B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46(4):861–871. doi: 10.1080/21691401.2017.1345930. [DOI] [PubMed] [Google Scholar]
- 137.Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Archives of Toxicology. 2011;85(7):743–750. doi: 10.1007/s00204-010-0545-5. [DOI] [PubMed] [Google Scholar]
- 138.Rosarin FS, Arulmozhi V, Nagarajan S, Mirunalini S. Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pacific Journal of Tropical Medicine. 2013;6(1):1–10. doi: 10.1016/S1995-7645(12)60193-X. [DOI] [PubMed] [Google Scholar]
- 139.Selvi BCG, Madhavan J, Santhanam A. Cytotoxic effect of silver nanoparticles synthesized from Padina tetrastromatica on breast cancer cell line. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2016;7(3):035015. doi: 10.1088/2043-6262/7/3/035015. [DOI] [Google Scholar]
- 140.Prabhu D, Arulvasu C, Babu G, Manikandan R, Srinivasan P. Biologically synthesized green silver nanoparticles from leaf extract of Vitex negundo L. induce growth-inhibitory effect on human colon cancer cell line HCT15. Process Biochemistry. 2013;48(2):317–324. doi: 10.1016/j.procbio.2012.12.013. [DOI] [Google Scholar]
- 141.Mousavi B, Tafvizi F, Zaker BS. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS) Artificial Cells, Nanomedicine and Biotechnology. 2018;46(sup1):499–510. doi: 10.1080/21691401.2018.1430697. [DOI] [PubMed] [Google Scholar]
- 142.Sriram MI, Kanth SBM, Kalishwaralal K, Gurunathan S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. International Journal of Nanomedicine. 2010;5:753. doi: 10.2147/IJN.S11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu P, Jin H, Guo Z, Ma J, Zhao J, Li D, et al. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. International Journal of Nanomedicine. 2016;11:5003. doi: 10.2147/IJN.S115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lara-González, J. H., Gomez-Flores, R., Tamez-Guerra, P., Monreal-Cuevas, E., Tamez-Guerra, R., & Rodríguez-Padilla, C. (2013). In vivo antitumor activity of metal silver and silver nanoparticles in the L5178Y-R murine lymphoma model. Journal of Advances in Medicine, 1308–1316. 10.9734/BJMMR/2013/3108
- 145.Wang X, Li T, Su X, Li J, Li W, Gan J, et al. Genotoxic effects of silver nanoparticles with/without coating in human liver HepG2 cells and in mice. Journal of Applied Toxicology. 2019;39(6):908–918. doi: 10.1002/jat.3779. [DOI] [PubMed] [Google Scholar]
- 146.Murugesan K, Koroth J, Srinivasan PP, Singh A, Mukundan S, Karki SS, et al. Effects of green synthesised silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models. International Journal of Nanomedicine. 2019;14:5257. doi: 10.2147/IJN.S202404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147.Ramachandran R, Krishnaraj C, Sivakumar AS, Prasannakumar P, Kumar VA, Shim KS, et al. Anticancer activity of biologically synthesized silver and gold nanoparticles on mouse myoblast cancer cells and their toxicity against embryonic zebrafish. Materials Science and Engineering: C. 2017;73:674–683. doi: 10.1016/j.msec.2016.12.110. [DOI] [PubMed] [Google Scholar]
- 148.Kanipandian N, Kannan S, Ramesh R, Subramanian P, Thirumurugan R. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Materials Research Bulletin. 2014;49:494–502. doi: 10.1016/j.materresbull.2013.09.016. [DOI] [Google Scholar]
- 149.Hashemi SF, Tasharrofi N, Saber MM. Green synthesis of silver nanoparticles using Teucrium polium leaf extract and assessment of their antitumor effects against MNK45 human gastric cancer cell line. Journal of Molecular Structure. 2020;1208:127889. doi: 10.1016/j.molstruc.2020.127889. [DOI] [Google Scholar]
- 150.Gengan R, Anand K, Phulukdaree A, Chuturgoon A. A549 lung cell line activity of biosynthesized silver nanoparticles using Albizia adianthifolia leaf. Colloids and Surfaces. B, Biointerfaces. 2013;105:87–91. doi: 10.1016/j.colsurfb.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 151.Annu M, Ahmed S, Kaur G, Sharma P, Singh S, Ikram S. Evaluation of the antioxidant, antibacterial and anticancer (lung cancer cell line A549) activity of Punica granatum mediated silver nanoparticles. Toxicology Research. 2018;7(5):923–930. doi: 10.1039/c8tx00103k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khorrami S, Zarrabi A, Khaleghi M, Danaei M, Mozafari M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. International Journal of Nanomedicine. 2018;13:8013. doi: 10.2147/IJN.S189295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chengzheng W, Jiazhi W, Shuangjiang C, Swamy MK, Sinniah UR, Akhtar M, et al. Biogenic synthesis, characterization and evaluation of silver nanoparticles from Aspergillus niger JX556221 against human colon cancer cell line HT-29. Journal of Nanoscience and Nanotechnology. 2018;18(5):3673–3681. doi: 10.1166/jnn.2018.15364. [DOI] [PubMed] [Google Scholar]
- 154.Jha AK, Prasad K. Green synthesis of silver nanoparticles and its activity on SiHa cervical cancer cell line. Advanced Materials Letters. 2014;5(12):501–505. doi: 10.5185/amlett.2014.4563. [DOI] [Google Scholar]
- 155.Yang J, Wang Q, Wang C, Yang R, Ahmed M, Kumaran S, et al. Pseudomonas aeruginosa synthesized silver nanoparticles inhibit cell proliferation and induce ROS mediated apoptosis in thyroid cancer cell line (TPC1) Artificial Cells, Nanomedicine, and Biotechnology. 2020;48(1):800–809. doi: 10.1080/21691401.2019.1687495. [DOI] [PubMed] [Google Scholar]
- 156.El Kassas HY, Attia AA. Bactericidal application and cytotoxic activity of biosynthesized silver nanoparticles with an extract of the red seaweed Pterocladiella capillacea on the HepG 2 cell line. Asian Pacific Journal of Cancer Prevention. 2014;15(3):1299–13306. doi: 10.7314/apjcp.2014.15.3.1299. [DOI] [PubMed] [Google Scholar]
- 157.Chen H, Roco MC, Li X, Lin Y. Trends in nanotechnology patents. Nature Nanotechnology. 2008;3(3):123–125. doi: 10.1038/nnano.2008.51. [DOI] [PubMed] [Google Scholar]
- 158.Roco MC. Nanotechnology: convergence with modern biology and medicine. Current Opinion in Biotechnology. 2003;14(3):337–346. doi: 10.1016/s0958-1669(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 159.Xia J, Yao J, Wang LV. Photoacoustic tomography: principles and advances. Electromagn Waves (Camb). 2014;147:1–22. doi: 10.2528/pier14032303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Homan KA, Shah J, Gomez S, Gensler H, Karpiouk AB, Brannon-Peppas L, et al. Silver nanosystems for photoacoustic imaging and image-guided therapy. Journal of Biomedical Optics. 2010;15(2):021316. doi: 10.1117/1.3365937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu Y, Miyoshi H, Nakamura M. Nanomedicine for drug delivery and imaging: a promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. International Journal of Cancer. 2007;120(12):2527–2537. doi: 10.1002/ijc.22709. [DOI] [PubMed] [Google Scholar]
- 162.Yeh YC, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4(6):1871–1880. doi: 10.1039/c1nr11188d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yamashita M. Auranofin: Past to Present and repurposing. International Immunopharmacology. 2021;101(pt B):108272. doi: 10.1016/j.intimp.2021.108272. [DOI] [PubMed] [Google Scholar]
- 164.Zhao Y, He Z, Wang R, Cai P, Zhang X, Yuan Q, Zhang J, Gao F, Gao X. Comparison of the therapeutic effects of gold nanoclusters and gold nanoparticles on rheumatoid arthritis. Journal of Biomedical Nanotechnology. 2019;15(11):2281–2290. doi: 10.1166/jbn.2019.2848. [DOI] [PubMed] [Google Scholar]
- 165.Dwivedi P, Nayak V, Kowshik M. Role of gold nanoparticles as drug delivery vehicles for chondroitin sulfate in the treatment of osteoarthritis. Biotechnology Progress. 2015;31(5):1416–1422. doi: 10.1002/btpr.2147. [DOI] [PubMed] [Google Scholar]
- 166.Dykman L, Khlebtsov N. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae. 2011;3(2):34–55. doi: 10.32607/20758251-2011-3-2-34-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vecchio G, Galeone A, Brunetti V, Maiorano G, Rizzello L, Sabella S, et al. Mutagenic effects of gold nanoparticles induce aberrant phenotypes in Drosophila melanogaster. Nanomedicine: Nanotechnology, Biology and Medicine. 2012;8(1):1–7. doi: 10.1016/j.nano.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 168.Tan E, Yin P, Lang X, Wang X, You T, Guo L. Functionalized gold nanoparticles as nanosensor for sensitive and selective detection of silver ions and silver nanoparticles by surface-enhanced Raman scattering. The Analyst. 2012;137(17):3925–3928. doi: 10.1039/c2an35670. [DOI] [PubMed] [Google Scholar]
- 169.Akhtar S, Asiri SM, Khan FA, Gunday ST, Iqbal A, Alrushaid N, Labib OA, Deen GR, Henari FZ. Formulation of gold nanoparticles with hibiscus and curcumin extracts induced anti-cancer activity. Arabian Journal of Chemistry. 2022;15(2):103594. doi: 10.1016/j.arabjc.2021.103594. [DOI] [Google Scholar]
- 170.Shinde, A. S., & Mendhulkar, V. D. (2022). Anticancer activity of gold nanobioconjugates synthesized from Elephantopus scaber (linn.) leaf extract. Journal Canadian Research. 10.4103/jcrt.JCRT_1043_20 [DOI] [PubMed]
- 171.Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, Zhai ZB, Meng AM, et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes Int. Journal of Nanomedicine. 2010;5:771. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guo J, Rahme K, He Y, Li LL, Holmes JD, O’Driscoll CM. Gold nanoparticles enlighten the future of cancer theranostics. International Journal of Nanomedicine. 2017;12:6131. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Research. 2003;63(9):1999–2004. [PubMed] [Google Scholar]
- 174.Link S, El-Sayed MA. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. International Reviews in Physical Chemistry. 2000;19(3):409–453. doi: 10.1080/01442350050034180. [DOI] [Google Scholar]
- 175.Ayala-Orozco C, Urban C, Bishno S, Urban A, Charron H, Mitchell T, et al. Sub-100 nm gold nanomatryoshkas improve photo-thermal therapy efficacy in large and highly aggressive triple negative breast tumors. Journal of Controlled Release. 2014;191:90–97. doi: 10.1016/j.jconrel.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vimaladevi M, Divya KC, Girigoswami A. Liposomal nanoformulations of rhodamine for targeted photodynamic inactivation of multidrug resistant gram negative bacteria in sewage treatment plant. Journal of Photochemistry and Photobiology B: Biology. 2016;162:146–152. doi: 10.1016/j.jphotobiol.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 177.Allison RR, Moghissi K. Photodynamic therapy (PDT): PDT mechanisms. Clinical Endoscopy. 2013;46(1):24. doi: 10.5946/ce.2013.46.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Metkar SK, Girigoswami K. Diagnostic biosensors in medicine–a review. Biocatalysis and Agricultural Biotechnology. 2019;17:271–283. doi: 10.1016/j.bcab.2018.11.029. [DOI] [Google Scholar]
- 179.Wang J, Drelich AJ, Hopkins CM, Mecozzi S, Li L, Kwon G, et al. Gold nanoparticles in virus detection: Recent advances and potential considerations for SARS-CoV-2 testing development. Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology. 2021;2021:e1754. doi: 10.1002/wnan.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.WHO. (2020b). Draft landscape of COVID-19 candidate vaccines. World Health Organization. R & D Blue Prints. https://www.who.int/who-documents-detail-redirect/draft-landscape-of-covid-19-candidate-vaccines. Accessed July 2020.
- 181.Chen HW, Huang CY, Lin SY, Fang ZS, Hsu CH, Lin JC, Chen YI, Yao BY, Hu CM. Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials. 2016;106:111–118. doi: 10.1016/j.biomaterials.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alphandéry E. The potential of various nanotechnologies for coronavirus diagnosis/treatment highlighted through a literature analysis. Bioconjugate Chemistry. 2020;31(8):1873–1882. doi: 10.1021/acs.bioconjchem.0c00287. [DOI] [PubMed] [Google Scholar]
- 183.Lopez-Chaves C, Soto-Alvaredo J, Montes-Bayon M, Bettmer J, Llopis J, Sanchez-Gonzalez C. Gold nanoparticles: distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed.:Nanotechnol.Biol.Med. 2008;14(1):1–2. doi: 10.1016/j.nano.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 184.Li JJ, Zou LI, Hartono D, Ong CN, Bay BH, Lanry Yung LY. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Advanced Materials. 2008;20(1):138–142. doi: 10.1002/adma.200701853. [DOI] [Google Scholar]
- 185.Girigoswami K. Toxicity of Metal Oxide Nanoparticles. Advances in Experimental Medicine and Biology. 2018;1048:99–122. doi: 10.1007/978-3-319-72041-8_7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the authors declare that all data and materials support their published claims and comply with field standards.
Not Applicable