Abstract
BACKGROUND
Fluoxetine is one of the most widely prescribed anti-depressant drugs belonging to the category of selective serotonin reuptake inhibitors. Long-term fluoxetine treatment results in hepatotoxicity. Baicalin, a natural compound obtained from the Chinese herb Scutellaria baicalensis is known to have antioxidant, hepatoprotective and anti-inflammatory effects. However, the beneficial effects of baicalin against fluoxetine-induced hepatic damage have not previously been reported.
AIM
To evaluate the protective action of baicalin in fluoxetine-induced liver toxicity and inflammation.
METHODS
Male albino Wistar rats were divided into seven groups. Group 1 was the normal control. Oral fluoxetine was administered at 10 mg/kg body weight to groups 2, 3, 4 and 5. In addition, groups 3 and 4 were also co-administered oral baicalin (50 mg/kg and 100 mg/kg, respectively) while group 5 received silymarin (100 mg/kg), a standard hepatoprotective compound for comparison. Groups 6 and 7 were used as a positive control for baicalin (100 mg/kg) and silymarin (100 mg/kg), respectively. All treatments were carried out for 28 d. After sacrifice of the rats, biomarkers of oxidative stress [superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), glutathione-S-transferase (GST), advanced oxidation protein products (AOPP), malondialdehyde (MDA)], and liver injury [alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total protein, albumin, bilirubin] were studied in serum and tissue using standard protocols and diagnostic kits. Inflammatory markers [tumor necrosis factor (TNF-α), interleukin (IL)-6, IL-10 and interferon (IFN)-γ] in serum were evaluated using ELISA-based kits. The effect of baicalin on liver was also analyzed by histopathological examination of tissue sections.
RESULTS
Fluoxetine-treated rats showed elevated levels of the serum liver function markers (total bilirubin, ALT, AST, and ALP) and inflammatory markers (TNF-α, IL-6, IL-10 and IFN-γ), with a decline in total protein and albumin levels. Biochemical markers of oxidative stress such as SOD, CAT, GST, GSH, MDA and AOPP in the liver tissue homogenate were also altered indicating a surge in reactive oxygen species leading to oxidative damage. Histological examination of liver tissue also showed degeneration of hepatocytes. Concurrent administration of baicalin (50 and 100 mg/kg) restored the biomarkers of oxidative stress, inflammation and hepatic damage in serum as well as in liver tissues to near normal levels.
CONCLUSION
These findings suggested that long-term treatment with fluoxetine leads to oxidative stress via the formation of free radicals that consequently cause inflammation and liver damage. Concurrent treatment with baicalin alleviated fluoxetine-induced hepatotoxicity and liver injury by regulating oxidative stress and inflammation.
Keywords: Fluoxetine, Hepatotoxicity, Oxidative stress, Baicalin, Anti-inflammatory, Hepatoprotective and antioxidant
Core Tip: Prolonged treatment with the antidepressant drug fluoxetine causes severe hepatic damage. This study evaluated fluoxetine-induced liver damage in male albino Wistar rats. Oral fluoxetine was administered (10 mg/kg) for 28 d and caused significant alterations in serum and tissue biomarkers. Baicalin and silymarin were co-administered to facilitate the amelioration of oxidative stress-mediated hepatic damage and inflammation. The biochemical markers (total protein, albumin, total bilirubin, alanine transaminase, aspartate transaminase, alkaline phosphatase, superoxide dismutase, catalase, glutathione, glutathione-S-transferase, malondialdehyde and advanced oxidation protein products) and inflammatory markers [tumor necrosis factor-α, interleukin (IL)-6, IL-10 and interferon-γ] were markedly restored to near normal levels after treatment with the natural flavonoid compound baicalin. Histopathological examination of liver slices showing cellular degeneration and increased vacuolation in the fluoxetine-treated rats also corroborated the results obtained for biomarkers of liver function, oxidative stress and inflammation. The baicalin-treated rats demonstrated normal vacuolation and cellular pattern. Thus, baicalin acts as an antioxidant, anti-inflammatory and hepatoprotective agent in mitigating fluoxetine-induced toxicity. To the best of our knowledge, this is the first study to report the hepatoprotective efficacy of baicalin in fluoxetine-induced liver damage.
INTRODUCTION
Fluoxetine {N-methyl-3-phenyl-3-[4-(trifluoromethyl) phenoxy] propan-1-amine} is the most commonly prescribed drug for depression and other neuro-psychotic disorders. It belongs to the category of selective serotonin reuptake inhibitors, and is widely used due to its higher tolerability and fewer side effects[1,2]. Fluoxetine has a long half-life, is metabolized in the liver and excreted via urine. The active metabolite of fluoxetine is norfluoxetine. Fluoxetine acts by inhibiting cytochrome P450 (2D6) and its other isozymes, leading to potential drug interactions[3]. Although clinically approved, prolonged use of fluoxetine may cause various adverse effects such as anxiety, sleeplessness, nausea, diarrhea, metabolic disorders and sexual dysfunction[4,5]. The metabolism of fluoxetine results in excess production of free radicals that consequently causes liver damage. In addition, inflammation in the hepatic tissue is also due to over production of superoxide, hydroxyl, and some non-radical species like hydrogen peroxide (H2O2) along with surplus phagocyte formation which in turn can cause further tissue damage[6]. Liver diseases resulting from the excessive consumption of drugs are a leading cause of mortality worldwide. The mammalian system has evolved several enzymatic and non-enzymatic pathways that can counter the drug induced adverse effects arising from the action of free radicals[7-9]. In the past decade, several studies have reported the adverse effects caused by fluoxetine such as hyperglycemic effect, hepatic damage, bipolar disorders and even organ failure in extreme cases[10-13]. Further studies also showed a similar toxicity profile of fluoxetine mediated by oxidative stress and inflammation[14].
Baicalin (5,6,7-trihydroxyflavone 7-O-β-D-glucuronide) is a flavonoid primarily obtained from the Chinese herb Scutellaria baicalensis. It is known to possess several pharmacological activities including anti-diabetic, anti-inflammatory, hepatoprotective, neuroprotective, antioxidant and anticancer properties[15]. Structurally, baicalin possesses a di-ortho hydroxyl functional group on its aromatic rings (Figure 1). The divalent metal ion chelating and free radical scavenging actions of baicalin can be attributed to this structural feature[16]. Baicalin extracted from Scutellaria roots has exhibited antidepressant properties in mice and rats[17]. Several researchers have reported the neuroprotective effects of baicalin in rats with cerebral ischemia[18,19]. The antioxidant and hepatoprotective activities of baicalin in mice have also been reported[20]. Baicalin is also capable of inducing colon cancer cell apoptosis by inhibiting onco-microRNAs[21]. To date, no studies have assessed the hepatoprotective activity of baicalin against fluoxetine-induced toxicity. Moreover, prolonged treatment with fluoxetine in depression cases may result in liver dysfunction. Thus, the present study was undertaken to demonstrate the protective ability of baicalin against fluoxetine-induced hepatotoxicity, inflammation and oxidative stress in male albino Wistar rats.
Figure 1.
Structure of baicalin.
MATERIALS AND METHODS
Drugs and chemicals
Fluoxetine hydrochloride and silymarin were procured from Sigma-Aldrich. Baicalin, thiobarbituric acid (TBA), trichloroacetic acid (TCA), chloramines-T, 5-5’-Dithio-bis (2-nitrobenzoic acid) (DTNB), reduced glutathione (GSH), 1-chloro-2,4-dinitrobenzene (CDNB), pyrogallol, bovine serum albumin (BSA), hematoxylin, and eosin were purchased from TCI Chemicals, India. Diagnostics kits were obtained from Erba Diagnostics Mannheim, Germany. Inflammatory marker kits were supplied by Krishgen BioSystems, India.
Animals
Healthy male albino Wistar rats of similar age (weight: 200-250 g) were acclimatized at 23 ± 2 °C for one week prior to the experiment. The animals were given a standard pellet diet and water ad libitum. The in vivo experiments were carried out as per the norms of the Institutional Animal Ethics Committee, University of Allahabad, Allahabad [IAEC/AU/2017(1)/003].
Animal treatment
The rats were divided into seven groups (n = 6). Oral fluoxetine was administered for 28 d to induce toxicity. Group 1 rats, treated as the normal control, were fed with feed and water. Group 2 rats were administered fluoxetine (10 mg/kg) only. Group 3 (fluoxetine 10 mg/kg + baicalin 50 mg/kg), Group 4 (fluoxetine 10 mg/kg + baicalin 100 mg/kg) and group 5 (fluoxetine 10 mg/kg + silymarin 100 mg/kg) were the drug combination groups. Group 6 and group 7 were treated as positive controls and were only given baicalin (100 mg/kg) and silymarin (100 mg/kg), respectively. Silymarin was used as a standard hepato-protectant for comparison. After completion of 28 d treatment, the rats were sacrificed by cervical dislocation. Blood and liver tissue were collected for the evaluation of enzymatic and non-enzymatic biochemical markers.
Measurement of body weight
The body weight of rats was measured every day for 28 d until sacrifice.
Serum and plasma collection from rat blood
At the end of experiment, approximately 5 mL blood was drawn by heart puncture. About half the blood was left to clot for 20 min at approximately 25 ºC and serum was obtained at 5000 rpm for 10 min. The rest of the blood was collected in heparin containing vials. This was centrifuged in a cooling centrifuge at 4000 rpm for 20 min, and clear non-hemolyzed plasma was obtained. The plasma and serum samples thus separated were transferred to fresh microfuge tubes and preserved at -80 °C, until further assessment.
Evaluation of serum markers
The serum was used for assessment of the following enzymes: Alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and non-enzymatic parameters such as total bilirubin, albumin and total protein using Erba Diagnostics kits. The levels of serum inflammatory markers tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10 and interferon (IFN)-γ were evaluated using ELISA kits (Krishgen BioSystems).
Evaluation of hepatic and oxidative stress markers in liver homogenate
Tissue homogenization: 10% (w/v) liver tissue homogenate was prepared in 0.1 M phosphate buffer with 0.15 M KCl, at pH 7.4. The clear supernatant was separated after centrifugation at 4000 × g for 15 min at 4 °C and was used for further analysis.
Evaluation of malondialdehyde in liver homogenate: The amount of lipid peroxidation in the liver tissues was determined by the method of Niehaus and Samuelsson[22]. 100 mL tissue homogenate was mixed with 2 mL TBA reagent comprising TBA 0.37%, 15% TCA and 0.25 N HCl, and the tubes were placed in a hot water bath for 10 min and cooled at room temperature followed by centrifugation. The supernatant was used for spectrophotometric assessment at 532 nm against a reference blank. An extinction coefficient of 1.56 × 105 M−1cm−1 was used and the results were denoted as nmol malondialdehyde (MDA)/mg.
Evaluation of advanced oxidation protein products in liver homogenate: The levels of advanced oxidation protein products (AOPP) were determined by the method of Witko-Sarsat[23]. To 2 mL of liver homogenate [1:5 diluted in phosphate buffer solution (PBS)], 100 mL KI (1.16 M) was added followed by 200 mL glacial acetic acid after 2 min. The absorbance of the reaction mixture was read spectrophotometrically at 340 nm against a reference blank. The blank contained the same reaction mixture, except that the homogenate sample was replaced by 2 mL of PBS. The concentrations of AOPP were denoted as μmol/L of chloramine-T equivalents.
Evaluation of reduced GSH in liver homogenate: GSH content in liver homogenates was determined by the method of Ellman et al[24]. 250 mL of liver homogenate was added to the reaction mixture comprising 100 mL of 6 mmol/L DTNB, 300 mL of 0.2 M phosphate buffer (pH 8.0) and 50 mL 0.3M NaOH. Absorbance of the reaction mixture was determined at 412 nm. GSH was used as a standard and the results were represented as μg mg−1 protein.
Determination of the activity of antioxidant enzymes in liver homogenate: The activity of glutathione-S-transferase (GST) was measured by the method of Habig et al[25]. The activity assay was performed in a reaction mixture of 1 mL comprising 0.1 M phosphate buffer (pH 6.5), 1 mmol/L CDNB, 1 mmol/L GSH and 100 mL supernatant of liver homogenate. The change in absorbance on account of conjugate formation of GSH and CDNB was measured at 340 nm. GST activity was represented as μmol min−1mg−1 protein.
Superoxide dismutase (SOD) activity was evaluated by the method of Marklund and Marklund[26]. Formation of the colored complex takes place due to auto-oxidation of pyrogallol. This was measured for 3 min at an interval of 60 s at 412 nm in the presence or absence of the enzyme. One unit of enzyme activity was represented as 50% inhibition of auto-oxidation of pyrogallol per minute.
Catalase (CAT) activity was assessed by the method of Beers and Sizer[27]. The decreasing absorbance of H2O2 consumption was measured at 240 nm at an interval of 60 s for 3 min. One unit of CAT activity was expressed in μmol of H2O2 decomposed per minute with an extinction coefficient of H2O2 of 43.6 M−1cm−1.
Total protein assay
The total protein content in serum and tissue samples was estimated by the method of Lowry et al[28] with BSA as standard.
Histopathological study of liver tissue
Following sacrifice of the rats, the tissue samples were washed with cold saline and fixed in 10% formalin. The samples were then processed further for the preparation of paraffin wax blocks. Sectioning was done with a rotatory microtome and stained with hematoxylin and eosin[29]. The slides were then examined under the light microscope at 40 × magnification to observe the protective effect of baicalin against fluoxetine-mediated oxidative damage in liver tissue.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 5 software. An unpaired t test was used for statistical comparisons and the results were expressed as mean ± SD. A P value < 0.05 was considered significant.
RESULTS
Change in body weight
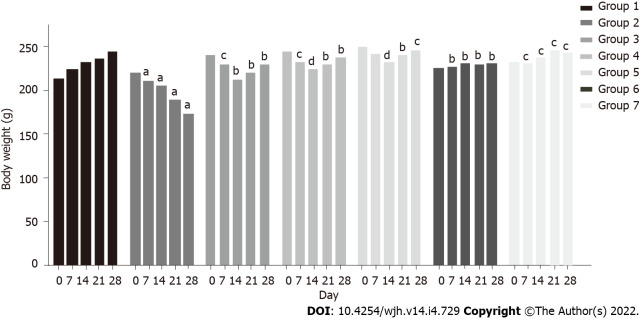
A marked decline in body weight was observed in group 2 rats (220 g to 173 g) within 28 d, while a constant increase was observed in group 1 rats (213 g to 245 g) (P < 0.05). In group 3 rats, body weight declined (240 g to 213 g) up to the second week, and increased thereafter (213 g to 229 g) (P < 0.05). A similar pattern was observed in group 4 (245 g to 224 g up to the second week and 224 g to 238 g thereafter) (P < 0.005) and group 5 (250 g to 231 g up to the second week and 231 g to 245 g thereafter) (P < 0.0005). Co-administration of baicalin and silymarin in groups 3, 4 and 5 helped body weight gain from day 14 onwards, after initial weight loss due to fluoxetine treatment. Groups 6 and 7 exhibited a normal pattern of body weight similar to the control group (Figure 2).
Figure 2.
Effect of baicalin on body weight of rats with fluoxetine-induced toxicity. Group 1: Control rats; group 2: Fluoxetine treated rats (10 mg/kg); group 3: Fluoxetine (10 mg/kg) + baicalin (50 mg/kg); group 4: Fluoxetine (10 mg/kg) + baicalin (100 mg/kg); group 5: Fluoxetine (10 mg/kg) + silymarin (100 mg/kg); group 6: Baicalin (100 mg/kg); group 7: Silymarin (100 mg/kg). Weight of rats is shown in grams. Data represent mean ± SD, n = 6. aRepresents a significant difference compared with group 1, P < 0.05; brepresents a significant difference compared with group 2, P < 0.05; crepresents a significant difference compared with group 2, P < 0.05; drepresents a significant difference compared with group 2, P < 0.0005.
Effect of baicalin on serum liver function enzymes
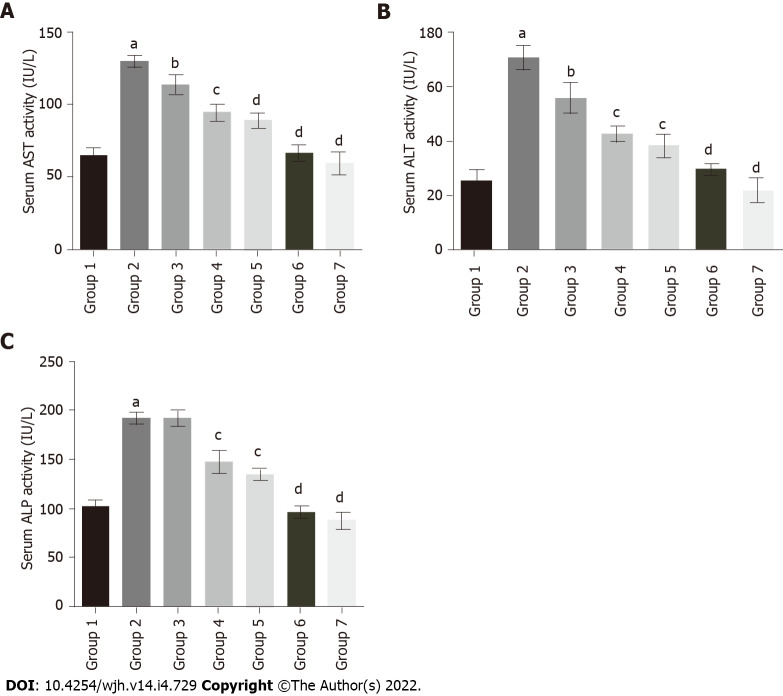
To evaluate the protective effect of baicalin against fluoxetine-induced hepatic injury, liver function marker enzymes were assessed in serum. Fluoxetine treatment for 28 d caused a significant increase in serum AST levels in group 2 rats (129.29 IU/L) in comparison to the control group (64.28 IU/L). Concurrent administration of baicalin significantly alleviated serum AST in group 3 (113.49 IU/L) and group 4 (94.51 IU/L) rats (Figure 3A). Group 5 rats treated with silymarin, a standard hepato-protectant, also showed a decline in AST levels to near normal (88.82 IU/L). ALT showed a similar pattern with a significant rise in group 2 (70.40 IU/L) as compared to the control group 1 (25.63 IU/L). Oral administration of baicalin decreased ALT levels in group 3 (55.84 IU/L) and group 4 (42.67 IU/L) rats (Figure 3B). Silymarin-treated group 5 rats also showed a reduction in ALT (38.22 IU/L). Similar to ALT and AST, group 2 rats also exhibited a marked increase (218.95 IU/L) in the level of serum ALP in comparison to group 1 (102.66 IU/L). Concurrent administration of baicalin produced a little decline in serum ALP levels in group 3 (191.26 IU/L), while a significant decline in group 4 (147.33 IU/L) and silymarin-treated group 5 (134.46 IU/L) was observed (Figure 3C). The greatest ameliorative potential in relation to serum enzymes was observed in group 5 rats treated with the standard hepato-protectant silymarin, while group 4 rats treated with baicalin (100 mg/kg) clearly exhibited greater restoration ability compared to group 3 rats treated with baicalin (50 mg/kg). Groups 6 and 7 served as positive controls and showed no significant changes compared to the control (group 1) for all the biomarkers.
Figure 3.
Effect of baicalin on serum enzymatic markers of liver function in fluoxetine-induced hepatotoxicity. A: Aspartate transaminase; B: Alanine transaminase; C: Alkaline phosphatase. Group 1: Control rats; group 2: Fluoxetine treated rats (10 mg/kg); group 3: Fluoxetine (10 mg/kg) + baicalin (50 mg/kg); group 4: Fluoxetine (10 mg/kg) + baicalin (100 mg/kg); group 5: Fluoxetine (10 mg/kg) + silymarin (100 mg/kg); group 6: Baicalin (100 mg/kg); group 7: Silymarin (100 mg/kg). Data represent mean ± SD, n = 6. aRepresents a significant difference compared with group 1, P < 0.05; brepresents a significant difference compared with group 2, P < 0.05; crepresents a significant difference compared with group 2, P < 0.005; drepresents a significant difference compared with group 2, P < 0.0001. AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphatase.
Effect of baicalin on serum bilirubin, total protein and albumin
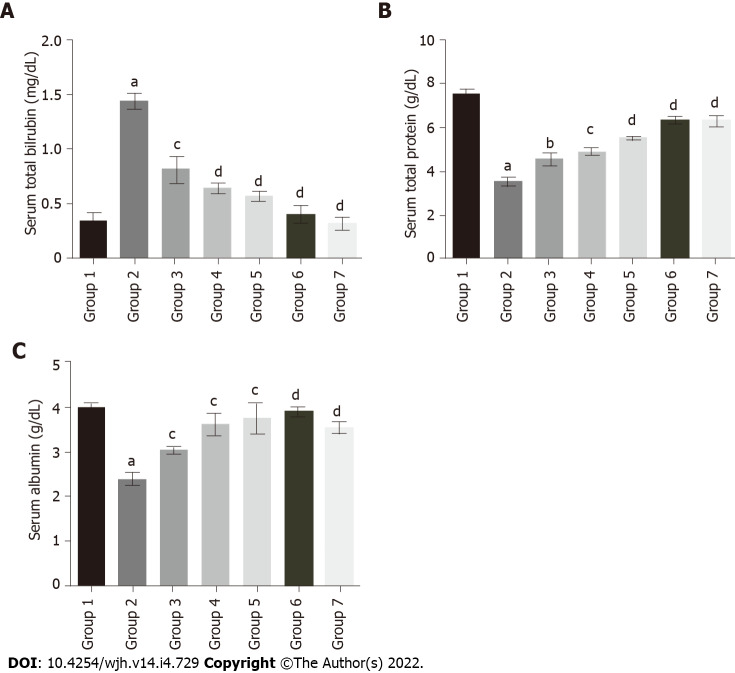
The serum total bilirubin levels in group 2 rats (1.44 mg/DL) were significantly increased compared to group 1 (0.35 mg/DL). Baicalin treatment along with fluoxetine led to a decline in bilirubin level in group 3 (0.81 mg/DL), and group 4 (0.64 mg/DL) (Figure 4A). Similarly, co-administration of silymarin with fluoxetine in group 5 caused a reduction in bilirubin (0.57 mg/DL). However, the level of total protein in group 2 (3.53 g/DL) was significantly decreased compared to group 1 rats (7.52 g/DL). Concurrent administration of baicalin resulted in a significant improvement in the total protein levels of group 3 (4.55 g/DL), group 4 (4.89 g/DL), and group 5 (5.52 g/DL) rats (Figure 4B). Similarly, the level of albumin was also decreased in group 2 (2.39 g/DL) compared to group 1 (3.97 g/DL), which increased with baicalin administration in group 3 (3.02 g/DL), group 4 (3.61 g/DL), and group 5 (3.75 g/DL) (Figure 4C).
Figure 4.
Effect of baicalin on serum bilirubin, total protein and albumin in fluoxetine-induced hepatotoxic rats. A: Serum bilirubin; B: Serum total protein; C: Serum albumin. Group 1: Control rats; group 2: Fluoxetine treated rats (10 mg/kg); group 3: Fluoxetine (10 mg/kg) + baicalin (50 mg/kg); group 4: Fluoxetine (10 mg/kg) + baicalin (100 mg/kg); group 5: Fluoxetine (10 mg/kg) + silymarin (100 mg/kg); group 6: Baicalin (100 mg/kg); group 7: Silymarin (100 mg/kg). Data represent mean ± SD, n = 6. aRepresents a significant difference from group 1, P < 0.05; brepresents a significant difference compared with group 2, P < 0.05; crepresents a significant difference compared with group 2, P < 0.005; drepresents a significant difference compared with group 2, P < 0.0001.
Effect of baicalin on hepatic antioxidant enzymes in liver homogenate
The enzymatic activities of SOD, CAT and GST were evaluated in liver tissue homogenates of rats treated with fluoxetine, baicalin and silymarin. The changes in activities in the different groups are shown in Table 1. A 55% reduction in the activity of SOD was observed in group 2 (6.357 U/mg) compared to group 1 (14.04 U/mg). Groups 3, 4, and 5 (7.73 U/mg, 11.11 U/mg and 10.37 U/mg, respectively) showed significant restoration in the presence of baicalin and silymarin. A similar pattern was observed in CAT activity with group 2 showing a decline (3.17 U/mg) as compared to control group 1 (7.26 U/mg). Co-administration of baicalin (50 and 100 mg/kg) and silymarin resulted in improved CAT activity in groups 3, 4 and 5 (3.74 U/mg, 4.94 U/mg and 5.37 U/mg, respectively). GST activity in group 2 rats (1.29 U/mg) showed decreased activity compared to group 1 (2.14 U/mg). In groups 3, 4 and 5, the activity of GST was increased towards normal values (1.32 U/mg, 1.41 U/mg, 1.54 U/mg, respectively).
Table 1.
The effect of baicalin and silymarin on the antioxidant status of liver tissue in fluoxetine-treated rats
|
Groups
|
SOD (U/mg)
|
CAT (U/mg)
|
GST (U/mg)
|
GSH (nM/mg)
|
MDA (nM/mg)
|
AOPP (nM/mg)
|
| 1 | 14.04 ± 0.58 | 7.26 ± 0.78 | 2.14 ± 0.22 | 115.76 ± 1.74 | 0.68 ± 0.04 | 0.48 ± 0.06 |
| 2 | 6.357 ± 0.50a | 3.17 ± 0.33a | 1.29 ± 0.15a | 52.12 ± 1.90a | 2.07 ± 0.18a | 1.12 ± 0.17a |
| 3 | 7.73 ± 0.65b | 3.74 ± 0.45 | 1.32 ± 0.17c | 78.87 ± 1.52c | 1.73 ± 0.16c | 0.83 ± 0.12c |
| 4 | 11.11 ± 0.4d | 4.94 ± 0.66c | 1.41 ± 0.23c | 97.02 ± 1.43c | 1.32 ± 0.11c | 0.64 ± 0.08c |
| 5 | 10.37 ± 0.46d | 5.37 ± 0.47c | 1.54 ± 0.11d | 104.91 ± 1.88c | 0.96 ± 0.07c | 0.56 ± 0.06d |
| 6 | 14.73 ± 0.31d | 7.27 ± 0.42d | 1.86 ± 0.24c | 110.24 ± 1.12c | 0.56 ± 0.13c | 0.49 ± 0.06c |
| 7 | 13.98 ± 0.3d | 6.66 ± 0.63d | 1.68 ± 0.10c | 106.58 ± 1.98c | 0.69 ± 0.08c | 0.50 ± 0.09c |
Represents a significant difference compared with group 1, P < 0.05.
Represents a significant difference compared with group 2, P < 0.05.
Represents a significant difference compared with group 2, P < 0.005.
Represents a significant difference compared with group 2, P < 0.0005.
The activities of biomarkers were assessed in tissue homogenate. Superoxide dismutase, catalase and glutathione-S-transferase are expressed as U/mg. Reduced glutathione, malondialdehyde and advanced oxidation protein products are expressed as nM/mg. Group 1: Control rats; group 2: Fluoxetine treated rats (10 mg/kg); group 3: Fluoxetine (10 mg/kg) + baicalin (50 mg/kg); group 4: Fluoxetine (10 mg/kg) + baicalin (100 mg/kg); group 5: Fluoxetine (10 mg/kg) + silymarin (100 mg/kg); group 6: Baicalin (100 mg/kg); group 7: Silymarin (100 mg/kg). Data represent mean ± SD, n = 6. SOD: Superoxide dismutase; CAT: Catalase; GST: Glutathione-S-transferase; GSH: Reduced glutathione; MDA: Malondialdehyde; AOPP: Advanced oxidation protein products.
Assessment of the non-enzymatic antioxidant markers in liver tissue
Treatment with fluoxetine caused increased levels of MDA and AOPP, along with a decline in GSH as compared with the control group (Table 1). Fluoxetine administration led to a decline in GSH in group 2 animals (52.12 Nm/mg) by more than 50% compared to group 1 (115.76 Nm/mg). The levels of GSH in group 3 (78.87 U/mg), group 4 (97.02 U/mg) and group 5 (104.91 U/mg) animals were subsequently elevated to near normal levels following administration of baicalin and silymarin. The level of MDA in group 2 exhibited an almost three-fold increase (2.07 Nm/mg) compared with control group 1 (0.68 Nm/mg). This was again restored partially in groups 3, 4 and 5 (1.73 Nm/mg, 1.32 Nm/mg and 0.96 Nm/mg, respectively). The AOPP levels also exhibited an identical pattern of an almost three-fold increase in group 2 animals (1.12 Nm/mg) compared with control group 1 (0.48 Nm/mg). Groups 3, 4 and 5 treated with baicalin and silymarin showed restoration of AOPP levels to near normal (0.83 Nm/mg, 0.64 Nm/mg and 0.56 Nm/mg, respectively) suggesting the appreciable antioxidant potential of baicalin. These results were found to be comparable to silymarin.
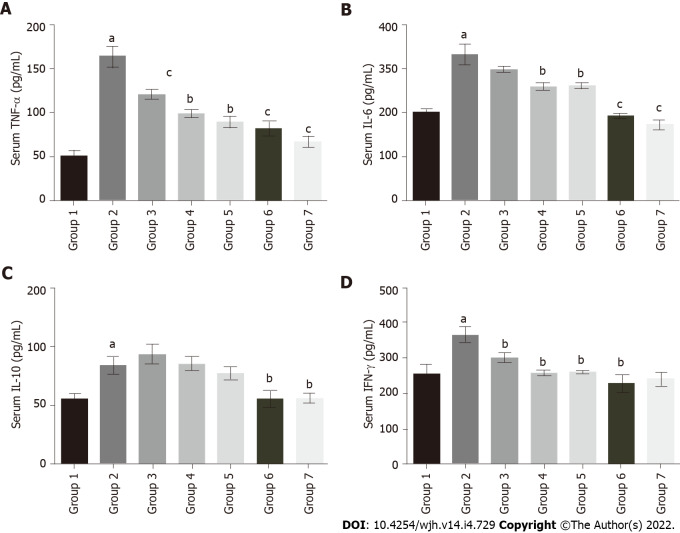
Assessment of inflammatory markers in serum
The serum TNF-α level in fluoxetine-treated group 2 (163.75 pg/mL) significantly increased as compared to group 1 (49.9 pg/mL). Baicalin and silymarin treatments showed improvement in groups 3 (120.36 pg/mL), 4 (98.67 pg/mL) and 5 (88.8 pg/mL) (Figure 5A). Similarly, serum IL-6 was elevated in group 2 (331.81 pg/mL) relative to group 1 (200.07 pg/mL), but there was a subsequent decline in groups 3 (298.18 pg/mL), 4 (258.24 pg/mL) and 5 (260.39 pg/mL) (Figure 5B). IL-10 levels slightly increased in group 2 (84.06 pg/mL) compared to group 1 (55.24 pg/mL), while the baicalin and silymarin treated groups 3 (93.7 pg/mL), 4 (85.36 pg/mL) and 5 (77.47 pg/mL) showed comparable results to group 2 (Figure 5C). The serum IFN-γ levels followed a similar pattern. There was a rise in fluoxetine-treated group 2 (365.14 pg/mL) compared to group 1 (256.73 pg/mL), whereas groups 3 (298.17 pg/mL), 4 (258.24 pg/mL) and 5 (260.39 pg/mL) showed considerable restoration to near normal levels (Figure 5D). In all treatment groups, baicalin at a dose of 100 mg/kg showed better efficacy as compared to baicalin at 50 mg/kg.
Figure 5.
Effect of baicalin on serum inflammatory markers in fluoxetine treated rats. A: Tumor necrosis factor-α; B: Interleukin (IL)-6; C: IL-10; D: Interferon-γ. The values are expressed in pg/mL. Group 1: Control rats; group 2: Fluoxetine treated rats (10 mg/kg); group 3: Fluoxetine (10 mg/kg) + baicalin (50 mg/kg); group 4: Fluoxetine (10 mg/kg) + baicalin (100 mg/kg); group 5: Fluoxetine (10 mg/kg) + silymarin (100 mg/kg); group 6: Baicalin (100 mg/kg); group 7: Silymarin (100 mg/kg). Data represent mean ± SD, n = 6. aRepresents a significant difference compared with group 1, P < 0.05; brepresents a significant difference compared with group 2, P < 0.05; crepresents a significant difference compared with group 2, P < 0.005; drepresents a significant difference compared with group 2, P < 0.0001. TNF: Tumor necrosis factor; IL: Interleukin; IFN: Interferon.
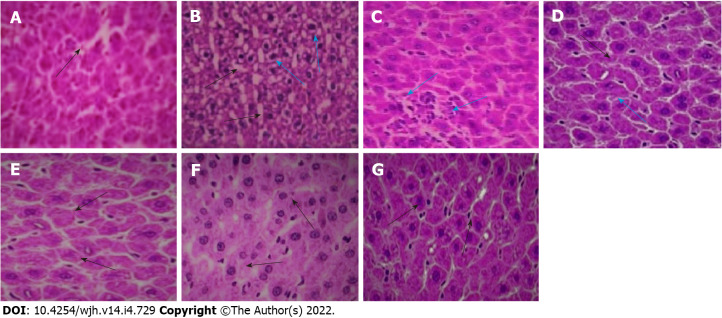
Histopathological studies
Histopathological examination of the control liver section revealed normal cellular architecture with mild vacuolation, presence of the central vein and sinusoidal spaces with intact hepatic cells (Figure 6A). The fluoxetine-treated liver slices showed an irregular pattern of hepatic cells, increased vacuolation, dilation of hepatic sinusoids, inflammatory cell infiltration, cellular disintegration and the initial stage of bridging necrosis that links the terminal veins to the portal tracts (Figure 6B). Co-administration of baicalin and silymarin prevented the disarrangement of hepatic cells, and normal sinusoidal spaces were observed without abnormal alterations (Figures 6C, 6D and 6E). The positive control groups 6 and 7 did not exhibit any alterations and showed a normal cellular pattern (Figures 6F and 6G).
Figure 6.
Histological sections of rat liver. A: Normal control (the black arrow shows normal intercellular spaces and negligible vacuolation); B: Fluoxetine (10 mg/kg) treated (the black arrows show increased vacuolation and cellular degeneration, blue arrows indicate presence of inflammatory cells); C: Fluoxetine + baicalin (50 mg/kg) treated (blue arrows indicate a patch of inflammatory cell infiltration); D: Fluoxetine + baicalin (100 mg/kg) treated (the black arrow depicts regular cellular pattern and normal sinusoidal space, blue arrow indicates fewer inflammatory cells); E: Fluoxetine + silymarin (100 mg/kg) treated (the black arrows show normal cells and intercellular spaces similar to normal control); F: Baicalin (100 mg/kg) treated (the black arrows show normal cellular structure); G: Silymarin (100 mg/kg) treated (the black arrows depict normal cells and intercellular spaces).
DISCUSSION
Liver is associated with the biotransformation of the entire xenobiotic load in the body. Drugs and various chemicals ingested are transformed with the help of cytochrome P450 in the liver. The active metabolites produced during biotransformation elevate the levels of free radicals and reactive oxygen species (ROS) thus disrupting the redox homeostasis[30,31]. Hepatic injury and toxicity can be produced by the use of numerous chemical agents or drugs, heavy metals and pesticides[32]. In the present study, the dose-dependent protective efficacy of baicalin was evaluated in fluoxetine-induced hepatotoxicity in male Wistar rats. Baicalin is well known in the treatment of several liver-related anomalies. It ameliorates the effects of estrogen-induced liver injury by up-regulating the expression of hepatic efflux transporters and down-regulating hepatic uptake transporters[33]. The neuroprotective and antidepressant properties of baicalin and its derivatives have also been widely studied. Baicalin facilitates stimulation of neurogenesis, the production of neurotrophic factors and modulation of the hypothalamic-pituitary-adrenal axis, which further counter oxidative stress, and inflammation. Similar to fluoxetine and its metabolite norfluoxetine, baicalin also elicits an anti-depressant effect by regulation of the gamma-aminobutyric acid (GABA) neurotransmitter system, and upregulating GABA receptors[17,34,35].
Long-term fluoxetine treatment in male albino Wistar rats resulted in reduced food intake over 28 d thereby resulting in weight loss (50 g in 28 d). Fluoxetine increases serotonin signaling in the brain, and higher serotonin levels help in activating the satiety neurons thus decreasing appetite. In addition, the active metabolite of fluoxetine, norfluoxetine is slow to metabolize and causes anorexia[36]. However, co-administration of baicalin prevented excess weight loss from the second week onwards. Baicalin has been reported to alleviate anorexia by inhibiting the over-expression of pro-inflammatory cytokines TNF-α and IL-6. The hypothalamic region in the brain that regulates food intake is stimulated by cytokine levels thus helping to increase appetite and body weight[37].
Fluoxetine caused a significant increase in the serum levels of ALT, AST and ALP in rats. This could be attributed to the fluoxetine-induced membrane alterations resulting from ROS action leading to cellular disintegration and necrosis of hepatic cells. As these enzymes reside in the cytoplasm, their elevated levels in the serum indicate breakdown of the hepatocellular membrane[31,38]. ALT is a liver injury biomarker while ALP is a marker of hepatic biliary injury and cholestasis. These results are further corroborated by earlier reports that showed enhanced levels of ALT, AST and ALP upon fluoxetine treatment[9,11]. Oral administration of baicalin led to a substantial decrease in serum ALT, AST and ALP levels in fluoxetine-treated rats, indicating its hepatoprotective action. Baicalin at 100 mg/kg was more effective in restoring enzyme levels to near normal than at 50 mg/kg. The efficacy of higher dose baicalin treatment was comparable to the effect of silymarin (100 mg/kg), a standard hepato-protectant used in experimental studies. Silymarin can bind to the receptors on the hepatocyte membrane to prevent the entry of toxic substances in the liver. The antioxidant properties of silymarin can reduce ROS, thus inhibiting cellular damage[39]. Fluoxetine treatment led to an elevation in serum bilirubin along with a reduction in total protein and albumin. Higher serum bilirubin is an indicator of lower hepatic clearance suggesting liver function abnormality that may cause jaundice and other hepatic symptoms[40]. Following treatment with baicalin, the serum bilirubin levels were considerably restored to near normal.
The decline in total protein and albumin levels also point to an anomaly in the protein synthesis machinery leading to alterations in cellular physiology and hepatocellular function in fluoxetine-treated rats. This subsequently causes a decline in cytochrome P450 activity. Baicalin treatment increased the total protein and albumin levels, and thereby improved cellular functions. Previous studies also demonstrated the potential of baicalin as a hepato-protectant that helps in restoring the total protein and albumin levels in mice[17]. Fluoxetine treatment caused elevated AOPP levels along with increased lipid peroxidation as indicated by higher MDA content in liver tissue suggesting the overproduction of ROS and free radicals. Moreover, decreased levels/activity of GSH, SOD, CAT and GST in liver tissue further supports enhanced oxidative stress in fluoxetine-treated rats.
GSH plays a key role in intracellular defense against free radical-induced oxidative stress. It is an intermediate in the pathways involving antioxidant enzymes such as GSH peroxidase and GSH reductase. The other important biological roles of GSH include regulation of signal transduction, transport of sulfate, modulation of cell growth and division, metabolite conjugation, protein and nucleic acid synthesis, xenobiotic detoxification, promoting metal ion chelation and enzymatic reactions[41]. Low GSH acts as an indicator of oxidative stress and tissue damage and adversely affects redox equilibrium with an increased oxidized state of the system[42,43]. GSH acts as a free radical scavenging and membrane stabilizing agent. It is capable of minimizing radical-linked membrane damage and prevents lipid peroxidation. Thus, reduced GSH content in fluoxetine-treated rats could be responsible for an increase in lipid peroxidation. Elevated levels of MDA, a product of lipid peroxidation, in the tissue also signify the oxidized state of the system that is beyond the control of the antioxidant defense system[44-48]. The higher levels of AOPP also point towards oxidative stress causing protein damage following prolonged fluoxetine treatment in rats. Proteins are often targeted by ROS that cause modification of amino acids which are measured quantitatively to determine the extent of oxidative damage. As fluoxetine is prescribed as an antidepressant in psychotic disorders, it is possible that its long-term intake causes oxidative damage to proteins in hepatocytes. Chloramines are oxidants that are produced in neutrophils by the enzyme myeloperoxidase. These oxidants result in the formation of advanced oxidation di-tyrosine cross-linked protein products. They are estimated quantitatively and act as biomarkers of protein oxidation[49]. The excessive production of AOPP is also suggestive of the onset of numerous diseases such as Alzheimer’s disease, rheumatoid arthritis, muscular dystrophy and respiratory diseases[50]. Hence, the decline in MDA and AOPP in rats treated with baicalin could be attributed to its antioxidant and oxidative stress lowering potential. SOD causes dismutation of the superoxide radicals generated in tissue into H2O2 and O2. CAT, in the peroxisomes further converts excess H2O2 produced by SOD action into water and O2[51,52]. During the present study, rats treated with baicalin and silymarin exhibited restoration of GSH, along with enhancement of SOD, CAT and GST activities. Nuclear respiratory factor 2 (Nrf2) is a major factor involved in maintaining cellular redox homeostasis. Activated Nrf2 helps to maintain the mitochondrial redox balance, increases the expression of antioxidant enzymes, and promotes mitochondrial biogenesis by increased transcription of Nrf1[53,54]. The rise in SOD and CAT activities could be due to increased activation of Nrf2 by baicalin and silymarin[55,56]. This further supports the role of baicalin treatment in improving and maintaining the redox balance in fluoxetine-treated rats.
GST is a cytosolic enzyme that helps to detoxify the toxic metabolites generated from cellular processes. It acts as a defense mechanism against oxidative stress, and regulates GSH homeostasis via mitogen activated protein kinase (MAPK) pathways that is involved in cellular response to stress[57]. The decline in GST activity is an indicator of oxidative stress. In the current study, fluoxetine treatment in rats led to a reduction in GST activity suggesting increased ROS production in the system. Thus, decreased GST activity further hinders the detoxification process[58]. The co-administration of baicalin (50 and 100 mg/kg) resulted in increased GST activity signifying a reduction in oxidative stress.
Baicalin also showed considerable efficacy against the fluoxetine-induced inflammatory response. The levels of serum inflammatory markers TNF-α, IL-6, IL-10 and IFN-γ were considerably elevated following fluoxetine treatment. Baicalin administration significantly restored the levels of TNF-α, IL-6, IL-10 and IFN-γ in fluoxetine-treated rats. The anti-inflammatory effects of baicalin and its derivative baicalein have been reported in several studies[59]. Baicalin helps to reduce the elevated levels of cytokines TNF-α and IL-6 by regulating the p38 MAPK signaling cascade[60]. Notably, IL-10 is an anti-inflammatory cytokine that binds to the IL receptor proteins and induces the STAT3 signaling cascade. In previous reports, fluoxetine administration elevated the level of IL-10 in depressive patients as it inhibits the synthesis of other pro-inflammatory cytokines such as IL-6 and IFN-γ[61]. However, in this study, the IL-10 level in fluoxetine-treated rats was slightly increased compared to the control group. In liver cells, IFN-γ is produced by natural killer cells and T lymphocytes. During hepatic injury and inflammation, the IFN-γ receptor expression is up-regulated which stimulates the secretion of IFN-γ. This in turn activates the macrophages, producing other cytokines such as TNF-α in abundance[62]. Thus, the elevated levels of IFN-γ in fluoxetine-treated rats indicate hepatic injury and oxidative stress. Baicalin treatment led to restoration of the IFN-γ levels to near normal. The efficacy of baicalin was better at the dose of 100 mg/kg than at 50 mg/kg. Histopathological examination of liver sections of rats treated with fluoxetine and baicalin showed signs of improvement as indicated by reduced vacuolation in cells, decreased cellular degeneration, less inflammatory cell infiltration and regular cellular architecture.
Thus, long-term intake of fluoxetine caused hepatotoxicity due to increased production of ROS during its biotransformation in liver. Baicalin, acting as an oxidative stress mitigator, led to improved structural and functional aspects of the liver as shown by biochemical and histopathological indices. In general, baicalin administration at 100 mg resulted in an appreciable reduction in fluoxetine-induced hepatic damage and inflammation in rats by restoring liver function markers and inflammatory cytokines to near normal levels. This was comparable to the effect of silymarin, a standard hepato-protectant at the same dose. In addition, it has been reported that baicalin possesses higher oral bioavailability than silymarin. Baicalin inhibits efflux transporters to increase the bioavailability of silymarin[63]. Therefore, baicalin can be used over silymarin as an alternative hepatoprotective compound to prevent fluoxetine-induced liver toxicity. Furthermore, it also improved the antioxidant status of the liver which consequently diminished ROS production and associated injury. Thus, co-treatment of baicalin with prolonged fluoxetine treatment proved beneficial for the liver and overall health status of the rats.
CONCLUSION
Fluoxetine is a commonly prescribed antidepressant drug used for long-term treatment. This study revealed that long-term fluoxetine treatment induced oxidative stress, hepatotoxicity and inflammation in rats. Baicalin administration prevented fluoxetine-induced liver damage and inflammation in rats by alleviating liver function biomarkers and inflammatory cytokines. Furthermore, it also prevented ROS-mediated damage by strengthening the antioxidant defense system at the enzymatic and non-enzymatic levels. Baicalin exhibited considerable hepatoprotective activity at a dose of 100 mg/kg and was found to be comparable to the standard compound silymarin at the same dose. Hence, to defend against oxidative stress and hepatotoxicity due to prolonged fluoxetine treatment, baicalin administration could be a drug of choice. However, further research is needed for a better understanding of the key pathways and mechanisms that could explain the protective effects of baicalin against fluoxetine-induced liver injury, oxidative damage and particularly the anti-inflammatory response.
ARTICLE HIGHLIGHTS
Research background
Fluoxetine is one of the most commonly prescribed drugs for depression and anxiety disorders. Prolonged use of fluoxetine results in hepatic toxicity. Baicalin is a natural compound obtained from the ancient Chinese herb Scutellaria baicalensis. Baicalin is known to possess several antioxidant, anti-inflammatory, anticancer, neuroprotective, cardioprotective and hepatoprotective effects.
Research motivation
The hepatotoxic effects of fluoxetine following prolonged treatment have been reported previously. As baicalin has anti-inflammatory and hepatoprotective properties, the aim of this study was to evaluate the hepatoprotective and anti-inflammatory properties of baicalin when co-administered with fluoxetine.
Research objectives
The objective of this study was to assess the protective action of baicalin in fluoxetine-induced liver toxicity and inflammation.
Research methods
Male albino Wistar rats were divided into seven groups. Group 1 was the normal control. Oral fluoxetine was administered at 10 mg/kg body weight to groups 2, 3, 4 and 5. In addition, groups 3 and 4 were also co-administered with oral baicalin (50 mg/kg and 100 mg/kg, respectively) while group 5 received silymarin (100 mg/kg). Groups 6 and 7 were used as positive controls for baicalin (100 mg/kg) and silymarin (100 mg/kg). All treatments were carried out for 28 d. Biomarkers of oxidative stress [superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), glutathione-S-transferase (GST), advanced oxidation protein products (AOPP), malondialdehyde (MDA)], and liver injury [alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total protein, albumin, bilirubin] were studied in serum and tissue using standard protocols and diagnostic kits. Inflammatory markers [tumor necrosis factor (TNF-α), interleukin (IL)-6, IL-10 and interferon (IFN)-γ] in serum were evaluated using ELISA kits. The effect of baicalin on the liver was also analyzed by histopathological examination of tissue sections.
Research results
Fluoxetine-treated rats showed elevated levels of serum liver function markers (total bilirubin, ALT, AST, and ALP) and inflammatory markers (TNF-α, IL-6, IL-10 and IFN-γ), with a decline in total protein and albumin levels. The biochemical markers of oxidative stress such as SOD, CAT, GST, GSH, MDA and AOPP in the liver tissue homogenate were also altered indicating a surge in reactive oxygen species leading to oxidative damage. Histological examination of liver tissue also showed degeneration of hepatocytes. Concurrent administration of baicalin (50 and 100 mg/kg) restored the biomarkers of oxidative stress, inflammation and hepatic damage in serum as well as in liver tissues to near normal levels.
Research conclusions
The results suggested that prolonged fluoxetine treatment leads to oxidative stress via the formation of free radicals that consequently cause inflammation and liver damage. Co-administration of baicalin alleviated fluoxetine-induced hepatotoxicity and liver injury by regulating oxidative stress and inflammation.
Research perspectives
Baicalin exhibited considerable hepatoprotective activity at a dose of 100 mg/kg and it was found to be comparable to the standard compound silymarin at the same dose. Therefore, baicalin can be used along with fluoxetine to prevent hepatic toxicity and inflammation. However, further research is needed for a better understanding of the key pathways and mechanisms that could explain the protective effects of baicalin against fluoxetine-induced liver injury, oxidative damage and particularly the anti-inflammatory response.
ACKNOWLEDGEMENTS
Ganguly R acknowledges financial support from University Grants Commission, New Delhi, India in the form of UGC-Junior Research Fellowship and Senior Research Fellowship. Kumar R acknowledges financial support from Council of Scientific & Industrial Research, New Delhi, India in the form of CSIR-Junior Research Fellowship and Senior Research Fellowship. All the authors also acknowledge DST-FIST and UGC-SAP facilities of the Department of Biochemistry, University of Allahabad, Prayagraj, India.
Footnotes
Institutional animal care and use committee statement: All animal experiments conformed to the internationally accepted principles for the care and use of laboratory animals, registered IAEC approval is No. IAEC/AU/2017(1)/003.
Conflict-of-interest statement: The authors declare no conflicts of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Peer-review started: July 23, 2021
First decision: September 5, 2021
Article in press: March 25, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nussler AK, Germany; Tunsophon S, Thailand S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
Contributor Information
Risha Ganguly, Department of Biochemistry, University of Allahabad, Prayagraj 211002, India.
Ramesh Kumar, Department of Biochemistry, University of Allahabad, Prayagraj 211002, India.
Abhay K Pandey, Department of Biochemistry, University of Allahabad, Prayagraj 211002, India. akpandey23@rediffmail.com.
Data sharing statement
No additional data are available.
References
- 1.Wernicke JF. Safety and side effect profile of fluoxetine. Expert Opin Drug Saf. 2004;3:495–504. doi: 10.1517/14740338.3.5.495. [DOI] [PubMed] [Google Scholar]
- 2.Tripathi A, Avasthi A, Desousa A, Bhagabati D, Shah N, Kallivayalil RA, Grover S, Trivedi JK, Shinfuku N. Prescription pattern of antidepressants in five tertiary care psychiatric centres of India. Indian J Med Res. 2016;143:507–513. doi: 10.4103/0971-5916.184289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preskorn SH, Shah R, Neff M, GolbeckAL , Choi J. The potential for clinically significant drug-drug interactions involving the CYP 2D6 system: effects with fluoxetine and paroxetine versus sertraline. J Psychiatr Pract. 2007;13:5–12. doi: 10.1097/00131746-200701000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Mielniczuk L, DeKemp RA, Dennie C, Yoshinaga K, Burwash IG, Bénard F, Haddad H, Beanlands DS, Beanlands RS. Images in cardiovascular medicine. Fluorine-18-labeled deoxyglucose positron emission tomography in the diagnosis and management of aortitis with pulmonary artery involvement. Circulation. 2005;111:e375–e376. doi: 10.1161/CIRCULATIONAHA.104.477703. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla P, Cipriani A, Hotopf M, Barbui C. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38:69–77. doi: 10.1055/s-2005-837806. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Kumar R, Ganguly R, Singh AK, Rana HK, Pandey AK. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol Rep. 2021;8:44–52. doi: 10.1016/j.toxrep.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma UK, Kumar R, Gupta A, Ganguly R, Singh AK, Ojha AK, Pandey AK. Ameliorating efficacy of eugenol against metanil yellow induced toxicity in albino Wistar rats. Food Chem Toxicol. 2019;126:34–40. doi: 10.1016/j.fct.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Mishra A, Sharma AK, Kumar S, Saxena AK, Pandey AK. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed Res Int. 2013;2013:915436. doi: 10.1155/2013/915436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diepenbroek C, Rijnsburger M, Eggels L, van Megen KM, Ackermans MT, Fliers E, Kalsbeek A, Serlie MJ, la Fleur SE. Infusion of fluoxetine, a serotonin reuptake inhibitor, in the shell region of the nucleus accumbens increases blood glucose concentrations in rats. Neurosci Lett. 2017;637:85–90. doi: 10.1016/j.neulet.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Inkielewicz-Stępniak I. Impact of fluoxetine on liver damage in rats. Pharmacol Rep. 2011;63:441–447. doi: 10.1016/s1734-1140(11)70510-2. [DOI] [PubMed] [Google Scholar]
- 12.Yılmaz A, Elbey B, Yazgan ÜC, Dönder A, Arslan N, Arslan S, Alabalık U, Aslanhan H. Protective Effects of Caffeic Acid Phenethyl Ester on Fluoxetine-Induced Hepatotoxicity: An Experimental Study. Biomed Res Int. 2016;2016:1247191. doi: 10.1155/2016/1247191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Özden H, Bildirici K, Üstüner D, Üstüner C, Cengiz BP, Tülay A, Yilmaz V. Histopathologic examination of rat liver after experimental application of fluoxetine. Türkiye Ekopatoloji Dergisi. 2005;11:9–15. [Google Scholar]
- 14.Elgebaly HA, Mosa NM, Allach M, El-Massry KF, El-Ghorab AH, Al Hroob AM, Mahmoud AM. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2018;98:446–453. doi: 10.1016/j.biopha.2017.12.101. [DOI] [PubMed] [Google Scholar]
- 15.Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018;8 doi: 10.3390/brainsci8060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol Res. 2015;100:296–308. doi: 10.1016/j.phrs.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhu W, Ma S, Qu R, Kang D, Liu Y. Antidepressant effect of baicalin extracted from the root of Scutellaria baicalensis in mice and rats. Pharmaceut Biol. 2006;44:503–510. [Google Scholar]
- 18.Tu L, Wu ZY, Yang XL, Zhang Q, Gu R, Wang Q, Tian T, Yao H, Qu X, Tian JY. Neuroprotective effect and mechanism of baicalin on Parkinson's disease model induced by 6-OHDA. Neuropsychiatr Dis Treat. 2019;15:3615–3625. doi: 10.2147/NDT.S165931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang W, Huang X, Chen W. The Effects of Baicalin and Baicalein on Cerebral Ischemia: A Review. Aging Dis. 2017;8:850–867. doi: 10.14336/AD.2017.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He P, Wu Y, Shun J, Liang Y, Cheng M, Wang Y. Baicalin Ameliorates Liver Injury Induced by Chronic plus Binge Ethanol Feeding by Modulating Oxidative Stress and Inflammation via CYP2E1 and NRF2 in Mice. Oxid Med Cell Longev. 2017;2017:4820414. doi: 10.1155/2017/4820414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao Y, Zhan S, Wang Y, Zhou G, Liang H, Chen X, Shen H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci Rep. 2018;8:14477. doi: 10.1038/s41598-018-32734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niehaus WG Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 23.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 26.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 27.Beers RF Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 28.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014:655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 30.Park JW, Reed JR, Brignac-Huber LM, Backes WL. Cytochrome P450 system proteins reside in different regions of the endoplasmic reticulum. Biochem J. 2014;464:241–249. doi: 10.1042/BJ20140787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Kumar R, Dwivedi A, Pandey AK. In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. Biomed Res Int. 2014;2014:459452. doi: 10.1155/2014/459452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A, Pandey AK. Aceclofenac-induced hepatotoxicity: An ameliorative effect of Terminalia bellirica fruit and ellagic acid. World J Hepatol. 2020;12:949–964. doi: 10.4254/wjh.v12.i11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Xiang D, He W, Liu Y, Lan L, Li G, Jiang C, Ren X, Liu D, Zhang C. Baicalin Protects Against 17α-Ethinylestradiol-Induced Cholestasis via the Sirtuin 1/Hepatic Nuclear Receptor-1α/Farnesoid X Receptor Pathway. Front Pharmacol. 2019;10:1685. doi: 10.3389/fphar.2019.01685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limanaqi F, Biagioni F, Busceti CL, Polzella M, Fabrizi C, Fornai F. Potential Antidepressant Effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang K, He M, Wang F, Zhang H, Li Y, Yang J, Wu C. Revealing Antidepressant Mechanisms of Baicalin in Hypothalamus Through Systems Approaches in Corticosterone- Induced Depressed Mice. Front Neurosci. 2019;13:834. doi: 10.3389/fnins.2019.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halford JC, Harrold JA, Lawton CL, Blundell JE. Serotonin (5-HT) drugs: effects on appetite expression and use for the treatment of obesity. Curr Drug Targets. 2005;6:201–213. doi: 10.2174/1389450053174550. [DOI] [PubMed] [Google Scholar]
- 37.Li B, Wan L, Li Y, Yu Q, Chen P, Gan R, Yang Q, Han Y, Guo C. Baicalin, a component of Scutellaria baicalensis, alleviates anorexia and inhibits skeletal muscle atrophy in experimental cancer cachexia. Tumour Biol. 2014;35:12415–12425. doi: 10.1007/s13277-014-2558-9. [DOI] [PubMed] [Google Scholar]
- 38.Sharma AK, Kumar S, Chashoo G, Saxena AK, Pandey AK. Cell cycle inhibitory activity of Piper longum against A549 cell line and its protective effect against metal-induced toxicity in rats. Indian J Biochem Biophys. 2014;51:358–364. [PubMed] [Google Scholar]
- 39.de Avelar CR, Pereira EM, de Farias Costa PR, de Jesus RP, de Oliveira LPM. Effect of silymarin on biochemical indicators in patients with liver disease: Systematic review with meta-analysis. World J Gastroenterol. 2017;23:5004–5017. doi: 10.3748/wjg.v23.i27.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Strader DB, Bernardo J, Venkataramanan R, Sterling TR ATS (American Thoracic Society) Hepatotoxicity of Antituberculosis Therapy Subcommittee. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 41.Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plantarum. 1997;100:241–254. [Google Scholar]
- 42.Rojas C, Cadenas S, Pérez-Campo R, López-Torres M, Barja G. Effect of vitamin C on antioxidants, lipid peroxidation, and GSH system in the normal guinea pig heart. J Nutr Sci Vitaminol (Tokyo) 1994;40:411–420. doi: 10.3177/jnsv.40.411. [DOI] [PubMed] [Google Scholar]
- 43.Gupta A, Singh AK, Kumar R, Jamieson S, Pandey AK, Bishayee A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv Nutr. 2021;12:1211–1238. doi: 10.1093/advances/nmab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Dwivedi A, Kumar R, Pandey AK. Preliminary evaluation of biological activities and phytochemical analysis of Syngonium podophyllum leaf. Natl Acad Sci Lett. 2015;38:143–146. [Google Scholar]
- 45.Sharma UK, Kumar R, Ganguly R, Gupta A, Sharma AK, Pandey AK. Cinnamaldehyde, an active component of cinnamon provides protection against food colour induced oxidative stress and hepatotoxicity in albino Wistar rats. Vegetos. 2018;31:123–129. [Google Scholar]
- 46.Gupta A, Kumar R, Pandey AK. Antioxidant and antidiabetic activities of Terminalia bellerica fruit in alloxan induced diabetic rats. S Afr J Bot. 2020;130:308–315. [Google Scholar]
- 47.Mishra A, Mishra A, Bhargava A, Pandey AK. Antimicrobial activity of essential oils from the leaves of Cinnamomum spp. Natl Acad Sci Lett. 2008;31:341–345. [Google Scholar]
- 48.Pandey AK, Mishra AK, Mishra A. Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamomum tamala. Cell Mol Biol (Noisy-le-grand) 2012;58:142–147. [PubMed] [Google Scholar]
- 49.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 50.Zhao Y, Zhang L, Ouyang X, Jiang Z, Xie Z, Fan L, Zhu D, Li L. Advanced oxidation protein products play critical roles in liver diseases. Eur J Clin Invest. 2019:e13098. doi: 10.1111/eci.13098. [DOI] [PubMed] [Google Scholar]
- 51.Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018;54:287–293. [Google Scholar]
- 52.Pandey AK, Kumar S, Pandey AK, Reis F. Editorial: Combating Redox Imbalance-Associated Complications With Natural Products. Front Pharmacol. 2021;12:802750. doi: 10.3389/fphar.2021.802750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A, Singh AK, Kumar R, Ganguly R, Rana HK, Pandey PK, Sethi G, Bishayee A, Pandey AK. Corilagin in Cancer: A Critical Evaluation of Anticancer Activities and Molecular Mechanisms. Molecules. 2019;24 doi: 10.3390/molecules24183399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi L, Hao Z, Zhang S, Wei M, Lu B, Wang Z, Ji L. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: The involvement of ERK1/2 and PKC. Biochem Pharmacol. 2018;150:9–23. doi: 10.1016/j.bcp.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 56.Vargas-Mendoza N, Morales-González Á, Morales-Martínez M, Soriano-Ursúa MA, Delgado-Olivares L, Sandoval-Gallegos EM, Madrigal-Bujaidar E, Álvarez-González I, Madrigal-Santillán E, Morales-Gonzalez JA. Flavolignans from Silymarin as Nrf2 Bioactivators and Their Therapeutic Applications. Biomedicines. 2020;8 doi: 10.3390/biomedicines8050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Röth E, Marczin N, Balatonyi B, Ghosh S, Kovács V, Alotti N, Borsiczky B, Gasz B. Effect of a glutathione S-transferase inhibitor on oxidative stress and ischemia-reperfusion-induced apoptotic signalling of cultured cardiomyocytes. Exp Clin Cardiol. 2011;16:92–96. [PMC free article] [PubMed] [Google Scholar]
- 58.Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, AlOlayan EM. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev. 2012;2012:194829. doi: 10.1155/2012/194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–271. doi: 10.1055/s-2002-23143. [DOI] [PubMed] [Google Scholar]
- 60.Farzaei MH, Singh AK, Kumar R, Croley CR, Pandey AK, Coy-Barrera E, Kumar Patra J, Das G, Kerry RG, Annunziata G, Tenore GC, Khan H, Micucci M, Budriesi R, Momtaz S, Nabavi SM, Bishayee A. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kostadinov I, Delev D, Petrova A, Stanimirova I, Draganova K, Kostadinova I, Murdjeva M. Study on anti-inflammatory and immunomodulatory effects of clomipramine in carrageenan- and lipopolysaccharide-induced rat models of inflammation. Biotechnol Biotechnol Equip. 2014;28:552–558. doi: 10.1080/13102818.2014.932136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horras CJ, Lamb CL, Mitchell KA. Regulation of hepatocyte fate by interferon-γ. Cytokine Growth Factor Rev. 2011;22:35–43. doi: 10.1016/j.cytogfr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu P, Zhou H, Li YZ, Yuan ZW, Liu CX, Liu L, Xie Y. Baicalein Enhances the Oral Bioavailability and Hepatoprotective Effects of Silybin Through the Inhibition of Efflux Transporters BCRP and MRP2. Front Pharmacol. 2018;9:1115. doi: 10.3389/fphar.2018.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.