Abstract
Although some data have linked proton pump inhibitor (PPI) use to risk of depression and anxiety, there are no studies investigating this safety issue in children. This study investigated the association between PPI use and risk of depression and anxiety in children. We conducted a nationwide register‐based cohort study in Sweden, July 1, 2007, to December 31, 2016. Following matching on age and propensity score, we included 29,320 pairs of PPI initiators and noninitiators among children aged 7–17 years old. The primary analysis examined the risk of incident depression and anxiety, a composite outcome defined as a diagnosis of depression, anxiety, or a prescription for an antidepressant. Children who initiated PPI use had higher hazards for risk of depression and anxiety compared with noninitiators (hazard ratios [HRs], 2.61; 95% confidence interval [CI], 2.32–2.94). In analyses of the timing of depression and anxiety onset after PPI initiation, the HRs were 3.71 (95% CI, 2.17–6.34) for 1–30 days, 3.47 (95% CI, 2.33–5.18) for 31–90 days, 2.71 (2.04–3.60) for 91–180 days, 2.52 (2.00–3.16) for 181–365 days, and 2.34 (1.94–2.82) for 366–730 days. Significant associations were observed across all age groups. The magnitude of the association increased with longer duration of PPI use (p for trend < 0.0001). The association was consistent through all sensitivity analyses, including high‐dimensional propensity score matching (HR, 2.31, 95% CI, 2.05–2.61). PPI use was associated with increased risk of depression and anxiety in children. Further investigation is warranted to confirm or refute this potential association.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Limited animal and human studies indicate a potential association between proton pump inhibitor (PPI) use and risk of depression and anxiety. However, there are no studies investigating whether PPI use is associated with increased risk of depression and anxiety in children.
WHAT QUESTION DID THIS STUDY ADDRESS?
We aimed to evaluate whether PPI use was associated with the risk of depression and anxiety in children.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
A total of 29,320 pairs of children were included in this cohort study after 1:1 propensity score matching. Initiation of PPI use compared to nonuse was significantly associated with approximately 2.6‐fold increased risk of depression and anxiety.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
This study indicates that PPI use may be associated with increased risk of depression and anxiety in children, adding new data to the limited evidence of PPIs. However, further investigation is warranted to confirm or refute this potential association.
INTRODUCTION
Proton pump inhibitors (PPIs), potent acid‐suppressive drugs, are core treatment for a variety of gastrointestinal disorders and increasingly prescribed to children in the last decade. 1 Although PPIs are empirically regarded as effective treatment for children, data on their pediatric safety profile are limited.
Recently, an increased risk of depression and anxiety with PPI use has emerged as a safety concern, as suggested by two analytical studies in adults. 2 , 3 Anxiety and depression are the most common psychiatric disorders in children and adolescents, affecting 2.6–6.5% of the pediatric population worldwide. 4 Moreover, anxiety and depression in childhood not only has been shown to predict a variety of psychiatric disorders in adult life, 5 but also to contribute to a considerable economic burden. 6
Growing data 7 , 8 , 9 , 10 have suggested that the human microbiome is a critical communicator between the gut and the brain through the microbiota‐gut‐brain axis. Alterations in the human microbiome could therefore contribute to the pathogenesis of anxiety and depression. PPIs are known to dysregulate the microbiome. However, whether PPI use is associated with risk of anxiety and depression in children is not known.
Aiming to investigate the association between PPI use among children and the risk of depression and anxiety, a Swedish nationwide register‐based cohort study with a propensity score matched new user design was conducted.
METHODS
Data sources
We conducted a cohort study (Figure S1) using several nationwide registers linked through commonly anonymous identifiers for individuals. The National Patient Register (NPR) contains complete records of disease diagnoses and surgical procedures from all inpatient and outpatient hospital and emergency department encounters in Sweden. The Prescribed Drug Register collects data on medications dispensed at all pharmacies, including drug name, dispensing date, and drug amount. The Total Population Register and Statistics Sweden provides details on demographic and socioeconomic characteristics. The study was approved by the Regional Ethics Committee in Stockholm, Sweden, which did not require informed consent because this was a registry‐based study.
Cohort
The source population consisted of all children in Sweden aged 7–17 years at some point from July 1, 2007, to June 30, 2016. From the source population, we identified all children who initiated PPI use, defined as patients filling their first prescription for a PPI during the study period and who had no PPI prescription in the previous 2 years. The PPI dispensing date served as the index date. Specific PPIs included omeprazole, esomeprazole, pantoprazole, lansoprazole, and rabeprazole (Table S1). The cohort was established through a two‐step matching process, which was aimed at selecting appropriate comparators (those who did not initiate use) from the source population, as described previously. 11 Patients with a history of any psychiatric diseases and use of psychiatric treatments before or on the index date were excluded, including depression, anxiety, alcohol and substance use disorders, schizophrenia and related psychoses, bipolar disorder, other mood disorders, attention deficit disorder with/without hyperactivity (ADHD), autism spectrum disorders, eating disorders, developmental disorders and sleep disorders, as well as use of antidepressants, antipsychotics, anxiolytics, hypnotics, ADHD treatments, and drugs against dependence. Other exclusion criteria were severe liver dysfunction, malignant tumors, brain injuries, and use of other anti‐acid drugs within 2 years before or on the index date (definitions in Table S1).
Outcome
The primary outcome was incident depression and anxiety, defined as any incident primary diagnosis of depression or anxiety, assigned in inpatient, urgent care, or outpatient settings, or any new dispensing record for antidepressants (definitions in Table S1). The first date that any of these criteria was met was assigned as the date of occurrence of the primary outcome. The validity of disease diagnoses in the NPR has been established in validation studies showing positive predictive values ranging from 81% to 96% for a range of psychiatric diseases, such as social anxiety disorder. 12 , 13 , 14 , 15 In addition, depression diagnosis in the NPR was validated in another study, showing high accordance with gold‐standard diagnoses based on multidisciplinary inpatient evaluations (κ of 0.32, 88% full agreement). 16
Propensity score
Propensity score matching was used to control for potential confounding. The propensity score was derived from fitting a multivariable logistic regression model based on all covariates listed in Table S1, including patient demographic and socioeconomic characteristics, medical history, comedications, and healthcare utilization within 2 years before or on the index date. Each child who initiated PPI use was matched to a child who did not based on propensity score and age group (in 1‐year age bands); the greedy nearest neighbor matching algorithm without replacement, with a caliper of 0.2 SDs of the logit of the propensity score, was used. The absolute mean standardized difference was used to assess covariate balance between the two groups; a covariate was considered well balanced if the standardized difference was less than 10%.
Statistical analysis
We followed up the matched study cohort from the day after the index date until a first event of primary outcome or censoring due to end of the study period (December 31, 2016), emigration, death, age 18 years, 2‐year follow‐up, occurrence of any other psychiatric diseases, and initiation of other psychiatric drugs, whichever occurred first. We computed incidence rates and absolute risk difference in incidence with 95% confidence interval (CIs) based on Poisson regression. Cox proportional hazards regression was performed to estimate HRs with 95% CIs, with days since the start of follow‐up as the underlying time scale. In subgroup analyses, proportional hazards regression models with an interaction term were applied to test whether the risk of primary outcome varied across baseline characteristics. In the analysis of cumulative duration, the linear trend of the risk of the primary outcome across different lengths of duration was assessed by the Cochran‐Armitage trend test. All analyses were performed in SAS Enterprise Guide, version 9.4 (SAS Institute Inc). A 95% CI that did not overlap 1.00 and two‐tailed p less than 0.05 were considered statistically significant.
Secondary analyses
Five secondary analyses were conducted. First, we repeated the analysis for the three conditions that comprised the primary outcome definition separately, including diagnosis‐based depression, diagnosis‐based anxiety, and use of antidepressants. Second, to investigate the potential latent period from PPI initiation to occurrence of primary outcome, we assessed the timing of primary outcome, categorizing the time since initiation of PPI use into five groups (1–30 days, 31–90 days, 91–180 days, 181–365 days, and 366–730 days). Third, we performed an analysis of individual PPIs, for which we created mutually exclusive matched subcohorts according to individual PPI at index date and drug‐specific propensity scores. Fourth, we investigated the risk of the primary outcome according to cumulative duration of PPI use. The duration of PPI use was calculated based on the drug amount from dispensed prescriptions; use was considered ongoing and added to duration for as long as prescriptions were refilled and overlapping. Accounting for irregularities and gaps in continuous treatment, refill gaps were permitted to 50% of the duration of the preceding dispensing. For each individual, we summed the durations during follow‐up in a time‐dependent manner, categorizing duration into four groups (1–30 days, 31–90 days, 91–180 days, and ≧181 days) and analyzed the risk of primary outcome for the full 2‐year follow‐up period for each category. Finally, to explore the pattern of the association in relation to ongoing treatment, we conducted an analysis based on an as‐treated approach. In this analysis, the follow‐up period was divided into five groups: ongoing PPI treatment and 1–90 days, 91–180 days, 181–365 days, and 366–730 days since PPI discontinuation.
Subgroup analyses and sensitivity analyses
In subgroup analyses, we examined the risk of the primary outcome stratified by age groups with 2‐year bands, infectious disease (yes/no), and use of systemic antibiotics (yes/no; Table S1) to assess whether these factors were potential effect modifiers. To evaluate the robustness of our primary findings, we conducted several sensitivity analyses. First, we used alternative narrower outcome definitions, including (1) any primary diagnosis of depression or anxiety from any hospital admission, urgent care visits or at least two outpatient visits within 3 months, or a prescription for an antidepressant; (2) any primary diagnosis of primary outcome from any hospital admission, urgent care visit or outpatient visit, or consecutive refill of at least two prescriptions for antidepressants; and (3) any primary diagnosis of depression or anxiety made in specialist psychiatric care. Second, to minimize the impact of potential residual confounding, we repeated the primary analysis within a high‐dimensional propensity score‐matched (HdPS) cohort (Table S3). 11 Third, we restricted the patients who received PPI monotherapy at the index date and did not have any prescription for antibiotics for Helicobacter pylori eradication (clarithromycin, amoxicillin, metronidazole, or tetracycline hydrochloride) within 30 days before or after the index date. Fourth, we restricted the patients without any hospitalization within 30 days before or on the index date to reduce potential confounding from general serious illness. Fifth, we repeated the primary analysis with a 6‐month age band matching to test the impact of more fine‐granular age‐adjustment. Sixth, we repeated the primary analysis adopting a lag‐time approach to reduce the potential influence of protopathic bias (i.e., the possibility that PPI initiation is related to early symptoms of undiagnosed depression and anxiety). Specifically, we introduced 90, 180, and 365‐day lag time periods immediately after the index date. Finally, we adopted an analysis of a negative control outcome by investigating the association between PPIs and a composite of several psychiatric conditions that have not been linked to PPI use previously, including developmental disorder and mental retardation (definitions in Table S1).
Role of the founding sources
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
RESULTS
Cohort
A total of 2,041,808 children aged 7–17 years, and who had continuously lived in Sweden at least 2 years during the study period were identified as the source population. Following exclusions, 29,648 children who initiated PPIs and 687,323 who did not were eligible for matching (Figure 1). The baseline characteristics of the two groups before and after one‐to‐one matching on propensity score and age are detailed in Table 1. Before matching, children initiating PPIs were somewhat older (mean [SD] age, 11.9 [2.7] vs. 11.4 [2.6]), were more often girls and had a higher prevalence of healthcare utilization (e.g., ≥2 hospital admissions, 3.3% vs. 0.5%), comorbidities (e.g., infection, 12.4% vs. 6.8%) and comedication use (e.g., systemic corticosteroids, 8.0% vs. 2.4%). After matching, the study cohort included 29,320 children who initiated PPI treatment and 29,320 matched children who did not, with a mean (SD) age of 11.9 (2.7) and a mean (SD) follow‐up time of 1.6 (0.6) years for both groups. All baseline characteristics between matched pairs were well‐balanced, with all standard differences were less than 5%.
FIGURE 1.
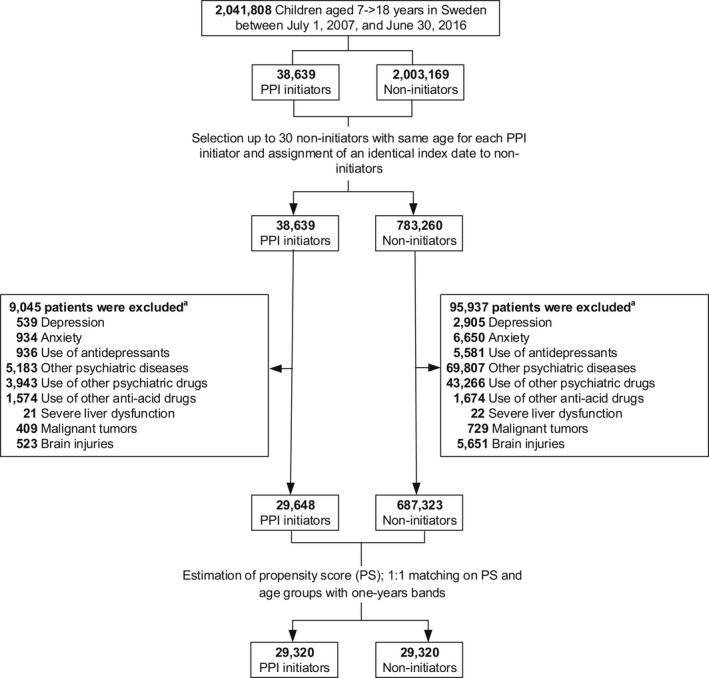
Flowchart for study cohort. PPI, proton pump inhibitor. aSome patients met multiple exclusion criteria
TABLE 1.
Baseline characteristics of PPI initiators and noninitiators before and after matching
| Before propensity score matching (%) | After propensity score matching (%) | |||||
|---|---|---|---|---|---|---|
| PPI initiators (n = 29,648) | Noninitiators (n = 687,323) | SDiff, % | PPI initiators (n = 29,320) | Noninitiators (n = 29,320) | SDiff, % | |
| Characteristics | ||||||
| Sex (male) | 11,915 (40.2) | 345,631 (50.3) | 20.4 | 11,805 (40.3) | 11,662 (39.8) | 1.0 |
| Age, mean ± SD, years | 11.9 ± 2.7 | 11.4 ± 2.6 | 21.1 | 11.9 ± 2.7 | 11.9 ± 2.7 | 0.3 |
| Age group, years | ||||||
| 7 | 2185 (7.4) | 69,875 (10.2) | 9.9 | 2164 (7.4) | 2164 (7.4) | 0 |
| 8 | 2740 (9.2) | 80,737 (11.7) | 8.2 | 2713 (9.3) | 2713 (9.3) | 0 |
| 9 | 3349 (11.3) | 89,981 (13.1) | 5.5 | 3297 (11.2) | 3297 (11.2) | 0 |
| 10 | 3664 (12.4) | 95,123 (13.8) | 4.4 | 3646 (12.4) | 3646 (12.4) | 0 |
| 11 | 3666 (12.4) | 87,742 (12.8) | 1.2 | 3614 (12.3) | 3614 (12.3) | 0 |
| 12 | 3201 (10.8) | 70,236 (10.2) | 1.9 | 3165 (10.8) | 3165 (10.8) | 0 |
| 13 | 3166 (10.7) | 63,051 (9.2) | 5.0 | 3133 (10.7) | 3133 (10.7) | 0 |
| 14 | 2982 (10.1) | 54,432 (7.9) | 7.5 | 2943 (10.0) | 2943 (10.0) | 0 |
| 15 | 2355 (7.9) | 39,394 (5.7) | 8.8 | 2327 (7.9) | 2327 (7.9) | 0 |
| 16 | 1686 (5.7) | 26,405 (3.8) | 8.7 | 1665 (5.7) | 1665 (5.7) | 0 |
| 17 | 654 (2.2) | 10,347 (1.5) | 5.2 | 653 (2.2) | 653 (2.2) | 0 |
| Calendar year | ||||||
| 2007 | 141 (0.5) | 2860 (0.4) | 0.9 | 135 (0.5) | 130 (0.4) | 0.3 |
| 2008 | 560 (1.9) | 9848 (1.4) | 3.6 | 550 (1.9) | 486 (1.7) | 1.7 |
| 2009 | 911 (3.1) | 16,315 (2.4) | 4.3 | 886 (3) | 849 (2.9) | 0.7 |
| 2010 | 1522 (5.1) | 29,496 (4.3) | 4.0 | 1495 (5.1) | 1510 (5.2) | 0.2 |
| 2011 | 2231 (7.5) | 46,842 (6.8) | 2.8 | 2199 (7.5) | 2082 (7.1) | 1.5 |
| 2012 | 3296 (11.1) | 69,969 (10.2) | 3.0 | 3262 (11.1) | 3265 (11.1) | <0.1 |
| 2013 | 4244 (14.3) | 96,511 (14.0) | 0.8 | 4184 (14.3) | 4225 (14.4) | 0.4 |
| 2014 | 5441 (18.4) | 127,484 (18.5) | 0.5 | 5394 (18.4) | 5365 (18.3) | 0.3 |
| 2015 | 6875 (23.2) | 169,744 (24.7) | 3.5 | 6815 (23.2) | 6877 (23.5) | 0.5 |
| 2016 | 4427 (14.9) | 118,254 (17.2) | 6.2 | 4400 (15.0) | 4531 (15.5) | 1.2 |
| Season | ||||||
| Spring (Mar–May) | 9158 (30.9) | 215,302 (31.3) | 0.9 | 9036 (30.8) | 9019 (30.8) | 0.1 |
| Summer (Jun–Aug) | 4734 (16.0) | 112,375 (16.3) | 1.0 | 4688 (16.0) | 4587 (15.6) | 0.9 |
| Autumn (Sep–Nov) | 7309 (24.7) | 163,359 (23.8) | 2.1 | 7234 (24.7) | 7184 (24.5) | 0.4 |
| Winter (Dec–Feb) | 8447 (28.5) | 196,287 (28.6) | 0.2 | 8362 (28.5) | 8530 (29.1) | 1.3 |
| Birth country | ||||||
| Scandinavia | 27,131 (91.5) | 616,533 (89.7) | 6.2 | 26,827 (91.5) | 27,063 (92.3) | 3.0 |
| Rest of Europe | 604 (2.0) | 20,251 (2.9) | 5.8 | 600 (2.0) | 524 (1.8) | 1.9 |
| Outside Europe | 1912 (6.4) | 50,471 (7.3) | 3.5 | 1892 (6.5) | 1733 (5.9) | 2.3 |
| Missing value | 1 (<0.1) | 68 (<0.1) | 0.8 | 1 (<0.1) | 0 (0) | 0.8 |
| Parental education, years a | ||||||
| ≤9 | 1426 (4.8) | 31,846 (4.6) | 0.8 | 1409 (4.8) | 1340 (4.6) | 1.1 |
| 10–12 | 11,851 (40.0) | 246,273 (35.8) | 8.5 | 11,696 (39.9) | 11,818 (40.3) | 0.9 |
| ≥13 | 16,296 (55.0) | 405,972 (59.1) | 8.3 | 16,140 (55.0) | 16,114 (55.0) | 0.2 |
| Missing value | 75 (0.3) | 3232 (0.5) | 3.6 | 75 (0.3) | 48 (0.2) | 2.0 |
| Parental income (SEK) by quartile, SEK a | ||||||
| 0–<257,374 | 7619 (25.7) | 171,136 (24.9) | 1.8 | 7505 (25.6) | 7389 (25.2) | 0.9 |
| 257,374–<321,184 | 8127 (27.4) | 170,807 (24.9) | 5.8 | 8044 (27.4) | 8109 (27.7) | 0.5 |
| 321,184–<408,943 | 7314 (24.7) | 171,621 (25) | 0.7 | 7249 (24.7) | 7285 (24.8) | 0.3 |
| 408,943≤ | 6560 (22.1) | 172,377 (25.1) | 7.0 | 6494 (22.1) | 6517 (22.2) | 0.2 |
| Missing value | 28 (0.1) | 1382 (0.2) | 2.8 | 28 (0.1) | 20 (0.1) | 1.0 |
| Healthcare utilization | ||||||
| Number of hospital admissions | ||||||
| 0 | 26,223 (88.4) | 662,013 (96.3) | 30.0 | 26,072 (88.9) | 26,187 (89.3) | 1.3 |
| 1 | 2450 (8.3) | 21,996 (3.2) | 21.9 | 2367 (8.1) | 2338 (8.0) | 0.4 |
| ≥2 | 975 (3.3) | 3314 (0.5) | 20.7 | 881 (3.0) | 795 (2.7) | 1.8 |
| Number of outpatient visits | ||||||
| 0 | 13,849 (46.7) | 490,225 (71.3) | 51.7 | 13,833 (47.2) | 13,917 (47.5) | 0.6 |
| 1–2 | 9050 (30.5) | 137,328 (20) | 24.5 | 8960 (30.6) | 9166 (31.3) | 1.5 |
| 3–4 | 3323 (11.2) | 36,866 (5.4) | 21.3 | 3257 (11.1) | 3102 (10.6) | 1.7 |
| ≥5 | 3426 (11.6) | 22,904 (3.3) | 31.7 | 3270 (11.2) | 3135 (10.7) | 1.5 |
| Number of emergency department visits | ||||||
| 0 | 17,496 (59) | 553,157 (80.5) | 48.1 | 17,434 (59.5) | 16,846 (57.5) | 4.1 |
| 1–2 | 9739 (32.8) | 123,017 (17.9) | 34.9 | 9595 (32.7) | 10,275 (35) | 4.9 |
| 3–4 | 1794 (6.1) | 9382 (1.4) | 25.0 | 1729 (5.9) | 1659 (5.7) | 1.0 |
| ≥5 | 619 (2.1) | 1767 (0.3) | 17.1 | 562 (1.9) | 540 (1.8) | 0.6 |
| Number of unique drugs | ||||||
| 0 | 6943 (23.4) | 331,222 (48.2) | 53.5 | 6938 (23.7) | 6702 (22.9) | 1.9 |
| 1–2 | 10,836 (36.5) | 234,086 (34.1) | 5.2 | 10,793 (36.8) | 11,290 (38.5) | 3.5 |
| 3–4 | 6001 (20.2) | 77,568 (11.3) | 24.8 | 5918 (20.2) | 5896 (20.1) | 0.2 |
| ≥5 | 5868 (19.8) | 44,447 (6.5) | 40.3 | 5671 (19.3) | 5432 (18.5) | 2.1 |
| Medical history | ||||||
| Cardiovascular disease | 168 (0.6) | 2248 (0.3) | 3.6 | 165 (0.6) | 174 (0.6) | 0.4 |
| Infection | 3672 (12.4) | 46,419 (6.8) | 19.2 | 3607 (12.3) | 3424 (11.7) | 1.9 |
| Epilepsy | 39 (0.1) | 629 (0.1) | 1.2 | 39 (0.1) | 35 (0.1) | 0.4 |
| Thyroid disease | 146 (0.5) | 1312 (0.2) | 5.2 | 141 (0.5) | 137 (0.5) | 0.2 |
| Kidney disease | 161 (0.5) | 353 (0.1) | 9.0 | 153 (0.5) | 155 (0.5) | 0.1 |
| Autoimmune diseases | 1682 (5.7) | 7618 (1.1) | 25.4 | 1562 (5.3) | 1384 (4.7) | 2.8 |
| Obesity | 410 (1.4) | 5418 (0.8) | 5.7 | 401 (1.4) | 383 (1.3) | 0.5 |
| Asthma | 1724 (5.8) | 21,608 (3.1) | 12.9 | 1702 (5.8) | 1625 (5.5) | 1.1 |
| Allergy | 2308 (7.8) | 26,697 (3.9) | 16.7 | 2255 (7.7) | 2040 (7.0) | 2.8 |
| Pain | 6608 (22.3) | 20,932 (3) | 60.4 | 6323 (21.6) | 6692 (22.8) | 3.0 |
| Headache | 1174 (4.0) | 8711 (1.3) | 16.9 | 1136 (3.9) | 1083 (3.7) | 1.0 |
| Nausea and vomiting | 884 (3.0) | 1383 (0.2) | 22.4 | 759 (2.6) | 680 (2.3) | 1.7 |
| Upper and lower endoscopy | 958 (3.2) | 956 (0.1) | 24.2 | 785 (2.7) | 604 (2.1) | 4.1 |
| Comedications | ||||||
| Asthma inhalants | 4138 (14.0) | 53,867 (7.8) | 19.7 | 4090 (13.9) | 4043 (13.8) | 0.5 |
| Opioids | 585 (2.0) | 4042 (0.6) | 12.3 | 555 (1.9) | 520 (1.8) | 0.9 |
| Antiepileptics | 67 (0.2) | 632 (0.1) | 3.4 | 64 (0.2) | 59 (0.2) | 0.4 |
| Systemic steroids | 2379 (8.0) | 16,814 (2.4) | 25.2 | 2254 (7.7) | 1934 (6.6) | 4.2 |
| NSAIDs | 2976 (10.0) | 14,152 (2.1) | 34.0 | 2877 (9.8) | 2800 (9.5) | 0.9 |
| Oral contraceptives | 1221 (4.1) | 6744 (1.0) | 20.0 | 1189 (4.1) | 1181 (4) | 0.1 |
| Antimigraine drugs | 174 (0.6) | 1190 (0.2) | 6.7 | 168 (0.6) | 138 (0.5) | 1.4 |
| Penicillin antibiotics | 9294 (31.3) | 147,285 (21.4) | 22.7 | 9166 (31.3) | 9020 (30.8) | 1.1 |
| Non‐penicillin antibiotics | 3952 (13.3) | 41,038 (6.0) | 25.1 | 3847 (13.1) | 3786 (12.9) | 0.6 |
| Systemic antihistamines | 4426 (14.9) | 65,633 (9.5) | 16.5 | 4368 (14.9) | 4145 (14.1) | 2.2 |
Abbreviations: NSAID, non‐steroidal anti‐inflammatory drugs; PPI, proton pump inhibitor; SDiff, standardized difference; SEK, Swedish krona.
Covariate based on the parent with the highest achieved education and income, respectively.
Main findings
The results of primary and secondary analyses are presented in Table 2. As the primary finding, there were 959 primary outcome events among PPI initiators (20.4 events per 1000 person‐years) and 373 primary outcome events among noninitiators (7.8 events per 1000 person‐years). PPI initiation was associated with an increased risk of the primary outcome (HR, 2.61, 95% CI, 2.32–2.94). In secondary analyses, the HR for diagnosis of depression or anxiety was 2.46 (95% CI, 2.15–2.82), whereas that for use of antidepressants was 3.15 (95% CI, 2.45–4.03). In analysis of timing of primary outcome onset after PPI initiation, HRs were 3.71 (95% CI, 2.17–6.34) for 1–30 days, 3.47 (95% CI, 2.33–5.18) for 31–90 days, 2.71 (95% CI, 2.04–3.60) for 91–180 days, 2.52 (95% CI, 2.00–3.16) for 181–365 days, and 2.34 (95% CI, 1.94–2.82) for 366–730 days after PPI initiation. In analysis of individual drugs, HRs were 2.66 (95% CI, 1.82–3.89) for esomeprazole and 2.65 (95% CI, 2.33–3.02) for omeprazole. The risk of the primary outcome increased with increasing cumulative duration of PPI use (Table 3). Compared to noninitiators, the HRs were 2.02 (95% CI, 1.76–2.33), 2.85 (95% CI, 2.46–3.29), 3.89 (95% CI, 3.21–4.72), and 3.81 (95% CI, 2.77–5.26) for cumulative duration of 1–30 days, 31–90 days, 91–180 days, and greater than or equal to 181 days, respectively (p < 0.0001 for linear trend). In the as‐treated analysis, HRs were 4.48 (95%, 3.43–5.86) for ongoing PPI treatment, and 3.28 (95%, 2.55–4.21) for 1–90 days, 2.85 (95%, 2.18–3.72) for 91–180 days, 2.24 (95%, 1.79–2.80) for 181–365 days, and 1.88 (95%, 1.55–2.29) for 366–730 days since PPI discontinuation (Table S2).
TABLE 2.
Main results of associations between PPI use and risk for depression and anxiety
| PPI initiators | Noninitiators | Absolute risk difference in incidence (95% CI) a | HR (95% CI) | |||
|---|---|---|---|---|---|---|
| No. of events | Incidence rate a | No. of events | Incidence rate a | |||
| Primary analysis | 959 | 20.4 | 373 | 7.8 | 12.61 (11.09–14.13) | 2.61 (2.32–2.94) |
| Secondary analyses | ||||||
| Definition of depression or anxiety | ||||||
| Diagnosis of depression or anxiety | 705 | 15.0 | 291 | 6.1 | 8.92 (7.61–10.23) | 2.46 (2.15–2.82) |
| Diagnosis of depression | 273 | 5.8 | 123 | 2.6 | 3.24 (2.41–4.06) | 2.25 (1.82–2.79) |
| Diagnosis of anxiety | 432 | 9.2 | 168 | 3.5 | 5.68 (4.66–6.70) | 2.61 (2.19–3.12) |
| Use of antidepressants | 254 | 5.4 | 82 | 1.7 | 3.69 (2.93–4.45) | 3.15 (2.45–4.03) |
| Timing of depression and anxiety onset (days after treatment initiation) | ||||||
| 1–30 days | 63 | 26.2 | 17 | 7.1 | 19.17 (11.87–26.47) | 3.71 (2.17–6.34) |
| 31–90 days | 107 | 22.5 | 31 | 6.5 | 16.03 (11.19–20.86) | 3.47 (2.33–5.18) |
| 91–180 days | 174 | 24.8 | 65 | 9.2 | 15.65 (11.34–19.95) | 2.71 (2.04–3.60) |
| 181–365 days | 255 | 19.4 | 103 | 7.7 | 11.72 (8.91–14.54) | 2.52 (2.00–3.16) |
| 366–730 days | 360 | 18.3 | 157 | 7.8 | 10.49 (8.24–12.74) | 2.34 (1.94–2.82) |
| Individual drugs b , c | ||||||
| Esomeprazole | 97 | 19.7 | 37 | 7.4 | 12.29 (7.71–16.88) | 2.66 (1.82–3.89) |
| Omeprazole | 843 | 20.9 | 323 | 7.9 | 13.00 (11.35–14.64) | 2.65 (2.33–3.02) |
Abbreviations: CI, confidence interval; HR, hazard ratio; PPI, proton pump inhibitor.
Events per 1000 person‐years.
Lansoprazole, pantoprazole, and rabeprazole were not analyzed due to the small sample size (n = 865 for lansoprazole, 209 for pantoprazole, and 0 for rabeprazole).
The numbers of matched pairs of each subcohort were for 3328 esomeprazole and 25,061 for omeprazole.
TABLE 3.
Associations between PPI use and risk for depression and anxiety, stratified by cumulative duration of PPI use
| Person‐years (% of total person‐year) | No. of events | Incidence rate a | Absolute risk difference in incidence a (95% CI) | HR (95% CI) | |
|---|---|---|---|---|---|
| Noninitiators | 47,697 (100) | 373 | 7.8 | Reference | Reference |
| ≤30 days | 24,935 (53) | 404 | 16.2 | 8.38 (6.61–10.15) | 2.02 (1.76–2.33) |
| 31–90 days | 16,013 (34) | 366 | 22.9 | 15.04 (12.56–17.51) | 2.85 (2.46–3.29) |
| 91–180 days | 4639 (10) | 147 | 31.7 | 23.87 (18.68–29.05) | 3.89 (3.21–4.72) |
| ≥181 days | 1356 (3) | 42 | 31.0 | 23.15 (13.75–32.55) | 3.81 (2.77–5.26) |
| p for trend | <0.0001 |
Abbreviations: CI, confidence interval; PPI, proton pump inhibitor.
Events per 1000 person‐years.
Subgroup analyses and sensitivity analyses
The results of subgroup analyses and sensitivity analyses are shown in Figure 2 and Table S4. In subgroup analyses, we observed that the association between PPI use and risk of primary outcome significantly varied across different age groups (p = 0.03 for interaction), with HRs ranging from 1.29 (95% CI, 1.77–1.71) to 3.41 (95% CI, 2.58–5.50). In contrast, neither history of infection nor earlier systemic antibiotic use were effect modifiers of the risk of primary outcome. In addition, our primary finding was consistent in all sensitivity analyses, including alternative definitions of primary outcome with narrower criteria and with adoption of HdPS matching (HR, 2.31, 95% CI, 2.05–2.61). Finally, we observed a null relationship between PPI use and risk of the negative control outcome (HR, 1.11, 95% CI, 0.82–1.50).
FIGURE 2.
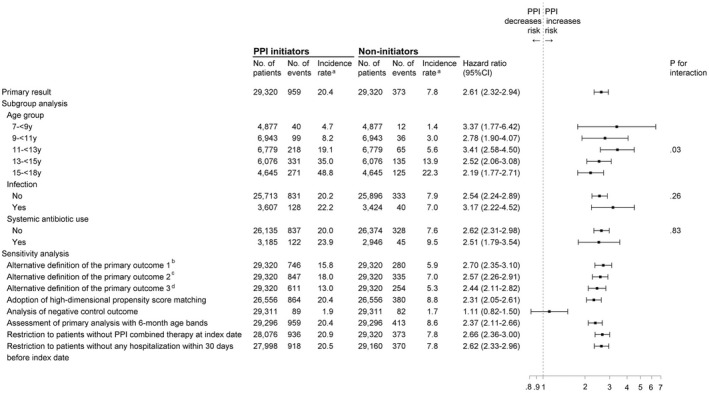
Subgroup and sensitivity analyses of association between proton pump inhibitor (PPI) use and risks for depression and anxiety. aEvents per 1000 person‐years. bDefined as a primary diagnosis of depression or anxiety from a hospital admission, an emergency department (ED) visit, or greater than or equal to two outpatient visits within 3 months, or any use of antidepressants. cDefined as a primary diagnosis of depression or anxiety from a hospital admission, an ED visit, or an outpatients visit, or consecutive refill of at least two prescriptions of antidepressants. dDefined as a primary diagnosis of depression or anxiety made in specialist psychiatric care
Discussion
In this nationwide cohort study, initiation of PPI use compared to nonuse was associated with a 2.6‐fold increased risk of depression and anxiety in children. PPI initiation was associated with both immediate and delayed risks of depression and anxiety. The magnitude of the association was stronger in the younger age groups, significantly increasing with longer duration of PPI use, but similar among individual PPIs. In addition, the risk was gradually attenuated but remained significant even 1 year after PPI discontinuation. A series of sensitivity analyses, including HdPS matching and an analysis of negative control outcome, all supported our main findings.
To our knowledge, only two analytical studies in adults 2 , 3 have so far explored this safety issue. The first study identified 2366 cases with depression and observed that risk of depression was associated with PPI treatment prescribed at cumulative daily doses of 121–365 (odds ratio [OR] 1.61, 95% CI, 1.41–1.84) and greater than 365 (OR 2.08, 95% CI, 1.61–2.68), as compared to less than or equal to 30 doses. 2 Similarly, the other study, a cross‐sectional analysis that included 344 elderly patients, reported an association between ongoing PPI use and depression (OR 2.38, 95% CI, 1.02–5.58). 3 Our findings included a treatment duration trend that is in line with the first study 2 and also adds comprehensive information on the association between PPI use and risk of depression and anxiety across the entire age range of children and adolescents.
There are some potential biologic mechanisms that might explain our findings. First, the human microbiome has been linked with brain function through immunologic, neuronal, and metabolic pathways. 7 , 8 , 9 , 10 PPI interference with the microbiome may hence contribute to the pathogenesis of depression and anxiety. Our study found a persistent association even 1 year after PPI discontinuation; this would be consistent with the long‐lasting impact of the microbiome on the central nervous system immune activity that affects development of brain function and mental health in the later life. 7 Furthermore, hypergastrinemia caused by PPI may strengthen cytokine expression in the brain to induce depressive and anxiety‐like conditions. 17 Similarly, in a recent experimental rat model, anxiety‐like behavior and a decline in neurotransmission of serotonin and dopamine in the brain was detected shortly after short‐term PPI administration (15 days). 18 Further studies are needed to understand potential mechanisms linking PPI to depression and anxiety.
Our study has several strengths. With up to 2 years of follow‐up, we included a nationwide population comprising 29,320 children who initiated PPI treatment and the same number of children with no PPI treatment who were matched on age and a propensity score including a large number of potential confounders. This enabled sufficient statistical power for examining the primary outcome and an extensive evaluation of different age groups, timing, duration, and individual PPIs.
Our study has some limitations. First, some outcome misclassification possibly existed because we were not able to capture mild depression or anxiety cases with non‐pharmacological support from primary care settings. This is unlikely to have been differential between the groups. Further, antidepressant use may be less specific for identifying depression and anxiety, as antidepressants may be prescribed for other diseases than depression and anxiety. However, the result for the antidepressant component was similar to that using diagnosed‐based outcome definitions only, albeit the magnitude of the association was somewhat larger. Second, we could not exclude the possibility of exposure misclassification because we lack information on medication adherence, use of over‐the‐counter medications, and medication use during hospitalization; however, assuming that misclassification was nondifferential, all these scenarios would bias results toward the null. Third, despite the inclusion of a broad range of covariates, an active comparator design was infeasible in our study, confounding by indication remains a possibility and overestimation of risks with PPI use cannot be ruled out. Furthermore, protopathic bias might explain the immediate risk increase of depression and anxiety observed within 30 days of PPI initiation. Gastrointestinal symptoms are occasionally prominent in depression and anxiety; early such manifestations in patients with undiagnosed depression or anxiety could have led to PPI treatment, appearing in the analysis as an association between PPIs and subsequently depression and anxiety. However, the observed association remained significant after 1 year since PPI initiation in the analysis according to time since PPI initiation and in the sensitivity analysis with a 1‐year lag, at which time point protopathic bias is less likely to influence. On the other hand, if any potential effects of PPIs on depression and anxiety wane over time, residual or unmeasured confounding might explain the significant 2.3‐fold increased risk after 1 year since PPI initiation. Still, our null findings in the analysis of a negative control outcome, which constituted other psychiatric conditions, such as mental retardation and developmental disorders, may be interpreted as pointing against underlying residual or unmeasured confounding driving an association between PPI initiation and psychiatric disease. In addition, we could not control for some unmeasured risk factors of depression and anxiety, including parental, genetic, lifestyle (e.g., smoking and obesity), psychosocial (e.g., war trauma), and other factors that may alter the microbiome (e.g., diet). Finally, whereas we implemented advanced propensity‐score matching techniques to address unmeasured confounding, as in any observational study, residual or unmeasured confounding cannot be ruled out. Still, confirmation of our findings is an important avenue for future research.
This study indicates that PPI use may be associated with increased risk of depression and anxiety in children, adding new data to the limited evidence on pediatric safety of PPIs. However, further investigation is warranted to confirm or refute this potential association.
CONFLICT OF INTEREST
Dr. Svanström has received consulting fees from Celgene and is employed by IQVIA, outside of the submitted work. Dr. Ludvigsson coordinates, on behalf of the Swedish IBD quality register (SWIBREG); a study that has received funding from Janssen Corporation. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
Y.H.W. wrote the manuscript. Y.H.W., V.W., and B.P. designed the research. Y.H.W., V.W., J.F.L., H.S., and B.P. performed the research. Y.H.W. analyzed the data.
Supporting information
Supplementary Material
Wang Y‐H, Wintzell V, Ludvigsson JF, Svanström H, Pasternak B. Proton pump inhibitor use and risk of depression and anxiety in children: nationwide cohort study. Clin Transl Sci. 2022;15:1112‐1122. doi: 10.1111/cts.13225
Funding information
Swedish Research Council and Frimurare Barnhuset Foundation. Dr Pasternak was supported by an investigator grant from the Strategic Research Area Epidemiology program at Karolinska Institutet
REFERENCES
- 1. Hales CM, Kit BK, Gu Q, Ogden CL. Trends in prescription medication use among children and adolescents‐United States, 1999–2014. JAMA. 2018;319(19):2009‐2020. 10.1001/jama.2018.5690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang W‐S, Bai Y‐M, Hsu J‐W, et al. Use of proton pump inhibitors and risk of major depressive disorder: a nationwide population‐based study. Psychother Psychosom. 2018;87(1):62‐64. [DOI] [PubMed] [Google Scholar]
- 3. Laudisio A, Antonelli Incalzi R, Gemma A, et al. Use of proton‐pump inhibitors is associated with depression: a population‐based study. Int Psychogeriatr. 2018;30(1):153‐159. [DOI] [PubMed] [Google Scholar]
- 4. Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: A meta‐analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345‐365. [DOI] [PubMed] [Google Scholar]
- 5. Copeland WE, Shanahan L, Costello EJ, Angold A. Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Arch Gen Psychiatry. 2009;66(7):764‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bui AL, Dieleman JL, Hamavid H, et al. Spending on children's personal health care in the United States, 1996–2013. JAMA Pediatr. 2017;171(2):181‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kennedy PJ, Murphy AB, Cryan JF, Ross PR, Dinan TG, Stanton C. Microbiome in brain function and mental health. Trends Food Sci Technol. 2016;57:289‐301. [Google Scholar]
- 8. Halverson T, Alagiakrishnan K. Gut microbes in neurocognitive and mental health disorders. Ann Med. 2020;52(8):423‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simpson CA, Diaz‐Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CS. The gut microbiota in anxiety and depression – A systematic review. Clin Psychol Rev. 2021;83:101943. [DOI] [PubMed] [Google Scholar]
- 10. Foster JA, McVey Neufeld KA. Gut‐brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305‐312. [DOI] [PubMed] [Google Scholar]
- 11. Wang YH, Wintzell V, Ludvigsson JF, Svanström H, Pasternak B. Association between proton pump inhibitor use and risk of fracture in children. JAMA Pediatr. 2020;174(6):543‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vilaplana‐Pérez A, Isung J, Krig S, et al. Validity and reliability of social anxiety disorder diagnoses in the Swedish National Patient Register. BMC Psychiatry. 2020;20(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sellgren C, Landén M, Lichtenstein P, Hultman CM, Långström N. Validity of bipolar disorder hospital discharge diagnoses: file review and multiple register linkage in Sweden. Acta Psychiatr Scand. 2011;124(6):447‐453. [DOI] [PubMed] [Google Scholar]
- 14. Idring S, Rai D, Dal H, et al. Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS One. 2012;7(7):e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fazel S, Wolf A, Chang Z, Larsson H, Goodwin GM, Lichtenstein P. Depression and violence: a Swedish population study. Lancet Psychiatry. 2015;2(3):224‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Polimeni G, Cutroneo P, Gallo A, Gallo S, Spina E, Caputi AP. Rabeprazole and psychiatric symptoms. Ann Pharmacother. 2007;41(7):1315‐1317. [DOI] [PubMed] [Google Scholar]
- 18. Ali SB, Mahmood K, Saeed R, Salman T, Choudhary MI, Haleem DJ. Elevated anxiety, hypoactivity, memory deficits, decreases of brain serotonin and 5‐HT‐1A receptors expression in rats treated with omeprazole. Toxicol Res. 2020;37(2):237‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


