Abstract
Fifty-two isolates of Fusarium species were obtained from soybean seeds from various parts of Korea and identified as Fusarium oxysporum, F. moniliforme, F. semitectum, F. solani, F. graminearum, or F. lateritium. These isolates were grown on autoclaved wheat grains and examined for toxicity in a rat-feeding test. Nine cultures were toxic to rats. One of these, a culture of Fusarium sp. strain KCTC 16677, produced apicidin, an antiprotozoal agent that caused toxic effects in rats (including body weight loss; hemorrhage in the stomach, intestines, and bladder; and finally death) when rats were fed diets supplemented with 0.05 and 0.1% apicidin. The toxin was toxic to brine shrimp (the 50% lethal concentration was 40 μg/ml) and was weakly cytotoxic to human and mouse tumor cell lines.
Fusarium species are known to occur throughout the world in a variety of climates and on many plant species as epiphytes, parasites, or pathogens. There is a long history of toxicosis associated with the consumption of Fusarium-infected cereals by people and domestic animals (16). Intensive studies of some of these outbreaks of toxicosis have led to the identification of a series of mycotoxins, including trichothecenes, zearalenone (ZEA), moniliformin (MON), and fumonisins (4, 5, 8, 23).
Soybean, Glycine max (L.) Merril, has been cultivated in eastern Asia for several thousand years and is grown to some extent in most of the world for both vegetable oil and protein. Fusarium-induced diseases of soybeans have been attributed to different species; fusarium blight or wilt and root rot are caused by F. oxysporum (Schlect.) emend. Snyd. & Hans., pod and collar rot is caused by F. semitectum Berk. & Rav., and sudden death syndrome is caused by F. solani (Mart.) Appel & Wr. emend. Snyd. & Hans. (21). In addition, F. equiseti (Corda) Sacc., F. graminearum Schwabe, and F. moniliforme Sheldon have also been reported to be pathogenic to soybeans. These Fusarium species are seed borne and are frequently found in soybean seed lots (17).
Despite the widespread occurrence of Fusarium species in soybean seeds, there has been limited study of the ability of Fusarium isolates to produce mycotoxins in soybeans. Richardson et al. (19) reported that Fusarium isolates were able to produce ZEA or T-2 toxin on soybeans and soybean meal. On the other hand, only trace levels of fumonisins and fusarin C were detected in soybeans which were inoculated with F. moniliforme isolates (3, 12).
The mycotoxins responsible for hemorrhage in cases of mycotoxicoses in farm animals have not all been identified, although trichothecenes, such as T-2 and diacetoxyscirpenol (DAS), may be responsible for some of the occurrences of the hemorrhagic disease syndrome observed under field conditions (1). We have been seeking the mycotoxins produced by Fusarium species, other than the trichothecenes, that can account for hemorrhage in the stomachs and intestines of farm animals. In a previous study by members of our group (13), sambutoxin was isolated as a hemorrhagic factor from cultures of F. oxysporum. Recently, production of apicidin was reported for liquid cultures of F. pallidoroseum (= F. semitectum), which was obtained from Acacia species (22). Apicidin is known to be an antiprotozoal agent that inhibits parasite histone deacetylase (9), but little information on its toxicity is available.
We obtained Fusarium isolates from soybean seeds and tested them for both toxicity and production of mycotoxins. During chemical analyses of culture extracts of the toxic isolates, we found that one isolate of Fusarium sp. strain KCTC 16677 produced a substantial amount of apicidin as a hemorrhagic factor. The objectives of this study were to purify and identify apicidin from wheat cultures of Fusarium sp. strain KCTC 16677 and to establish a cause-and-effect relationship between apicidin and hemorrhage in a rat-feeding test. Toxicity of apicidin to brine shrimp and several tumor cell lines is also reported.
MATERIALS AND METHODS
Soybean samples.
Twenty samples of soybeans, approximately 500 g each, were collected from 15 different farmers’ stocks in six provinces of Korea during November 1995.
Isolation and culture of Fusarium species.
For each sample, 100 seeds were soaked in 2% NaOCl for 1 min, rinsed in sterile distilled water, transferred to potato dextrose agar (Bacto Potato Dextrose Agar; Difco Laboratories, Detroit, Mich.), and incubated at 25°C for 4 to 7 days. Fusarium isolates were transferred from the seeds to noncommercial potato dextrose agar, carnation leaf agar (11), or both; incubated under fluorescent lamps (cool white type, 5,000 lx) at 25°C; and identified to the species level as described by Nelson et al. (18). A total of 52 isolates were obtained from soybean seeds. The isolates were stored in sterilized soil (15) and recovered on potato dextrose agar as needed. Later, the toxigenic isolates were deposited with the Korean Collection for Type Cultures at the Genetic Resources Center, Korea Research Institute of Bioscience and Biotechnology, Taejon, Korea.
Erlenmeyer flasks (1 liter), each containing 200 g of wheat and 120 ml of distilled water, were autoclaved for 1 h at 121°C twice with a 24-h interval. The wheat was inoculated with mycelial plugs from a 5-day-old potato dextrose agar plate containing the fungus. The flasks were incubated for 2 weeks at 25°C and 2 weeks for 10°C. The mycelial mass and substrate were dispersed onto a screen-bottom tray and allowed to air dry for 5 days at room temperature in a ventilated hood (airflow, 0.6 m/s). When dry, this inoculated substrate was ground to the consistency of flour and stored at −15°C until used.
Rat-feeding test.
Female Sprague-Dawley rats, 21 days old and weighing approximately 50 g each, were obtained from the Experimental Animal Center, Seoul National University. The rats were housed in individual cages and fed a 1:1 mixture of ground moldy wheat and complete rat diet. The rats were observed for 10 days, and major symptoms and death were recorded. Surviving rats were sacrificed by cervical dislocation and examined for pathological changes in the tissues.
Mycotoxin standards.
T-2 toxin, HT-2, neosolaniol, T-2 tetraol, DAS, MON, fusarochromanone, and wortmannin were supplied by C. J. Mirocha, Department of Plant Pathology, University of Minnesota. Ketotrichothecenes, including deoxynivalenol (DON), nivalenol (NIV), 15-acetyl-DON, 3-acetyl-DON, 4-acetyl-NIV, and ZEA were purchased from Sigma Chemical Co. (St. Louis, Mo.). Isoverrucarol (14), sambutoxin (13), fumonisin B1 (FB1), fumonisin B2 (FB2), and fumonisin B3 (FB3) were prepared in our laboratory.
Detection of known mycotoxins in Fusarium extracts.
Trichothecenes, MON, fumonisins, sambutoxin, fusarochromanone, and wortmannin were extracted by methods described by Tanaka et al. (26), Scott and Lawrence (20), Sydenham et al. (24), Kim and Lee (13), Lee et al. (15), and Abbas and Mirocha (1), respectively. Mycotoxins were identified by cochromatography with authentic standards in at least two solvent systems and by identical color reactions of the spots after treatment with p-anisaldehyde, 20% sulfuric acid in methanol, or the reagents of Takitani et al. (25). The last system is more specific for trichothecenes because the reagents detect the epoxide group present in this class of toxins.
Extraction and purification of apicidin. (i) Extraction.
Two kilograms of wheat culture of Fusarium sp. strain KCTC 16677 was extracted successively with n-hexane (15 liters) and ethyl acetate (15 liters) in a reciprocating shaker (120 rpm) at room temperature. The extracts were filtered through Whatman no. 2 filter paper and concentrated to dryness in vacuo. The two extracts were bioassayed in a rat-feeding test.
(ii) Florisil column chromatography.
The ethyl acetate extract was dissolved in a minimal volume of chloroform and loaded onto a Florisil column (5 cm [inside diameter] by 60 cm) containing 500 g of Florisil (60 to 100 mesh; Fisher Scientific Co., Pittsburgh, Pa.). The column was eluted with a solvent system using a step gradient of chloroform to chloroform-methanol (3:1, vol/vol). The eluate was collected in 10-ml fractions with a fraction collector. The fractions were monitored with thin-layer chromatography (TLC) plates (Kiesel gel 60, 20 by 20 cm, 0.25 mm thick; E. Merck, Darmstadt, Germany); fractions containing apicidin (fraction 1) and its related compound (fraction 2) were retained. The fractions were bioassayed in a rat-feeding test.
(iii) Purification by Chromatotron.
Further purification of apicidin was accomplished with preparative TLC (Chromatotron, model 7924T; Harrison Research, Palo Alto, Calif.). The toxic fraction (1.2 g) was dissolved in 10 ml of chloroform and applied to a preparative TLC circular glass plate with silica gel (2 mm thick; particle size, 2 to 25 μm, with gypsum binder and fluorescent indicator; Aldrich Chemical Company, Inc., Milwaukee, Wis.), which was eluted with chloroform-methanol (7:3, vol/vol) at a flow rate of 8 ml/min under nitrogen gas. During the separation, the chemical was visualized under UV light (254 nm). Apicidin was purified as an amorphous white powder.
(iv) Bulk purification of apicidin.
Approximately 1.6 kg of culture material was extracted with ethyl acetate and concentrated to dryness. The residue was fractionated as described above. After the active fraction from Chromatotron was concentrated in vacuo, 800 mg of apicidin was obtained.
Determination of structure.
The structure of the toxin was determined by UV spectroscopy, infrared spectroscopy, melting-point measurement, and mass spectrometry as previously described (13). A Carlo Erba model 1106 apparatus was used for the analysis. Mass spectra were recorded on a double-focusing high-resolution mass spectrometer (JMS-AX 505; JEOL Ltd., Tokyo, Japan). 1H, 13C, and 15N nuclear magnetic resonance spectra were obtained on a JEOL 600 ECP (600 MHz) spectrometer at 600, 150.8, and 60.7 MHz, respectively, in CDCl3. The configurations of isoleucine and N-methoxytryptophan of apicidin were determined by acid hydrolysis and amino acid oxidase tests as described by Singh et al. (22) and Closse and Huguenin (7), respectively.
Toxicity test of apicidin. (i) Rat-feeding test.
Apicidin was incorporated into complete rat diets at concentrations of 0.05 and 0.1% and fed to 21-day-old female Sprague-Dawley rats. Each treatment group consisted of three rats. After a 2-week feeding period, all surviving rats were sacrificed and examined for pathological changes in tissues.
(ii) Brine shrimp toxicity.
We tested brine shrimp larvae (Artemia salina L.) for sensitivity to apicidin by the procedure of Visconti et al. (27). Bioassays were performed on 24-well cell culture plates (Nunclon; Delat, Roskilde, Denmark) containing 60 to 80 larvae in 1 ml of seawater and 1% methanolic test solutions of apicidin. Each treatment consisted of three replicates. The number of dead shrimp was determined microscopically after incubation at 27°C for 36 h. The total number of shrimp per well was counted after the remaining shrimp were killed by freezing at −20°C for 12 h.
(iii) In vitro cytotoxicity test.
Apicidin was dissolved in 50% ethanol (1 mg/ml) and serially diluted with RPMI 1640 (GIBCO, Grand Island, N.Y.) containing 10% fetal bovine serum immediately before use. Two human tumor cell lines and one mouse tumor cell lines were used: K562, a human leukemia cell line; MOF-7, a human breast carcinoma cell line; and P388, a mouse leukemia cell line. All the cell lines were maintained in tissue culture flasks (Costar 3055) at 37°C in a humidified atmosphere supplemented with 5% CO2. The medium used was RPMI 1640 supplemented with 10% fetal bovine serum, penicillin G (100 U/ml), and streptomycin (100 μg/ml). The in vitro cytotoxicity test was performed with a tetrazolium-based semiautomated colorimetric assay described by Carmichael et al. (6).
Production of apicidin by the toxic isolates.
The presence of apicidin was investigated with the three toxic cultures of F. semitectum and a Fusarium isolate not identified to the species level. A 20-g portion of culture was extracted with 100 ml of ethyl acetate for 30 min in a wrist action shaker. After filtration through Whatman no. 2 filter paper, the filtrate was concentrated to dryness. The residue was dissolved in 2 ml of chloroform and applied to a Florisil column (2 cm [inside diameter] by 20 cm). The column was packed with 10 g of Florisil (60 to 100 mesh) topped with 5 g of anhydrous sodium sulfate. After being washed with 100 ml of n-hexane, the column was eluted with chloroform-methanol (3:1, vol/vol). The eluate was concentrated to dryness and redissolved in 2 ml of methanol. The extract was analyzed by TLC and high-performance liquid chromatography (HPLC). For the HPLC analysis, the following equipment and conditions were used: instrument, TSP Spectra system (Thermo Separation Products Inc., San Jose, Calif.); Bondclone 10 C18 column (4.9 mm [inside diameter] by 300 mm; particle size, 10 μm; Phenomenex Co., Torrance, Calif.); mobile phase, acetonitrile-water (60:40, vol/vol); flow rate, 1 ml/min; UV detector wavelength, 292 nm. The retention time of apicidin was 7.4 min.
RESULTS
Toxicity of Fusarium isolates.
Fifty-two isolates of Fusarium were obtained from 20 samples of soybeans collected at 15 sites. Representatives of 7 species were identified: F. oxysporum (13 isolates), F. moniliforme (14 isolates), F. graminearum (9 isolates), F. solani (4 isolates), F. sporotrichioides (3 isolates), F. semitectum (3 isolates), and F. lateritium (1 isolate). Five isolates could not be identified to the species level. Of these 52 isolates, 9 caused death (Table 1), 10 caused a loss in body weight of 1 to 20 g, and 33 caused a gain in body weight of 4 to 19 g. Cultures of F. sporotrichioides, F. lateritium, and an unknown Fusarium sp. caused hemorrhage and the production of excess mucus in the stomach, intestines, or bladder or in all of these organs.
TABLE 1.
Toxicities of Fusarium isolates from soybean seeds and lethalities to rats
| Organism | Strain | No. of rats which dieda | Toxic sign(s)b | Mycotoxins (concn, μg/g)c |
|---|---|---|---|---|
| None (control) | 0 | — | ||
| F. graminearum | KCTC 16671 | 1 | IC | NIV (650), 4-acetyl-NIV (50), ZEA (10) |
| F. semitectum | KCTC 16672 | 1 | IM, IC | Unknown metabolites |
| F. sporotrichioides | KCTC 16673 | 3 | IH, IM, SD | T-2 (100), HT-2 (30), neosolaniol (40), T-2 tetraol (5) |
| KCTC 16674 | 3 | SH, IM | T-2 (75), HT-2 (20), neosolaniol (140), T-2 tetraol (5) | |
| F. lateritium | KCTC 16675 | 3 | BH, ID, IM | Isoverrucarol (320), unidentified trichothecenes |
| F. moniliforme | KCTC 16678 | 1 | IM | FB1 (800), FB2 (180), FB3 (50) |
| KCTC 16679 | 2 | IM, ID | FB1 (1,500), FB2 (450), FB3 (100) | |
| Fusarium sp. | KCTC 16676 | 1 | IH, IM | Unknown metabolites |
| KCTC 16677 | 3 | BH, IH, IM | Unknown metabolites |
Experimental rats were fed ground moldy wheat-complete diet (1:1), and control rats were fed an autoclaved wheat-complete diet (1:1). Three rats were used for each treatment.
—, no detectable toxic effect. Abbreviations indicate toxic effects (congestion [C], excess mucus production [M], hemorrhage [H], and degeneration [D]) in various organs (intestine [I], stomach [S], and bladder [B]).
Toxins were quantified by TLC.
The levels of trichothecenes and fumonisins in cultures of the toxic Fusarium isolates could account for their toxicities (Table 1). None of the F. semitectum isolates or isolates of unknown Fusarium species produced trichothecenes, fumonisins, ZEA, MON, fusarochromanone, wortmannin, or sambutoxin.
Isolation of apicidin.
The toxicities of culture material of Fusarium sp. strain KCTC 16677 and culture extracts to rats are shown in Table 2. Rats fed a diet containing a 1:1 mixture of crude culture and complete rat diet died of hemorrhaging in the stomach, intestines, and bladder within 5 days after treatment. No toxicity was observed in the control group fed complete rat diet with 50 ml of acetone. After extraction, most of the toxicity was recovered in the ethyl acetate extract. After Florisil column chromatography, most of the toxicity was recovered in the retained fraction, although a portion of the toxicity was found in another fraction (Table 2). During the large-scale extraction, 800 mg of purified apicidin was obtained from 1.6 kg of crude culture.
TABLE 2.
Toxicity to rats of Fusarium sp. strain KCTC 16677 culture material and fractions obtained from the extraction and Florisil column fractionation steps
| Fractiona | No. of rats which diedb | Mean wt change (g)c |
|---|---|---|
| Control | 0 | 24 |
| Culture material | 3 | NDd |
| n-Hexane extract | 0 | 30 |
| Ethyl acetate extract | 3 | ND |
| Fraction 1 | 3 | ND |
| Fraction 2 | 1 | −18 |
The control was prepared by mixing 50 ml of acetone alone with 200 g of complete rat diet and drying the mixture at 40°C overnight. The extract obtained from 2 kg of culture material was dissolved in 500 ml of acetone, and 50 ml of the solution was mixed with 200 g of complete rat diet and dried at 40°C overnight. The fractions from the ethyl acetate extraction through the Florisil column were dissolved in acetone, and a portion of the solution equivalent to 200 g of fungal culture was mixed with 200 g of complete rat diet and dried at 40°C overnight.
Three rats were used for each treatment.
Mean weight change of surviving rats (minus sign signifies weight loss).
ND, not determined because rats died.
Characterization of apicidin.
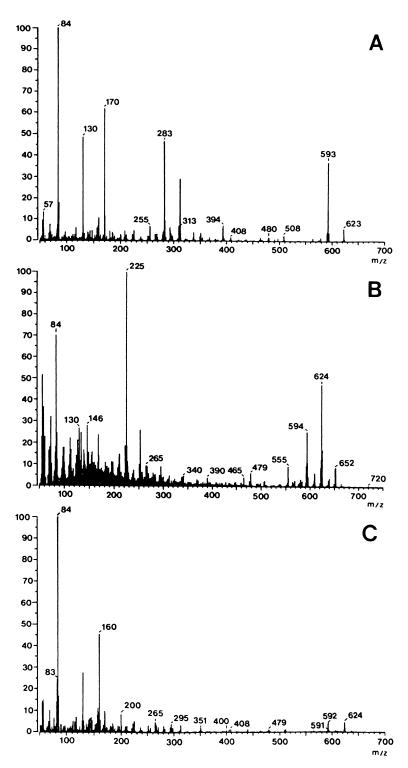
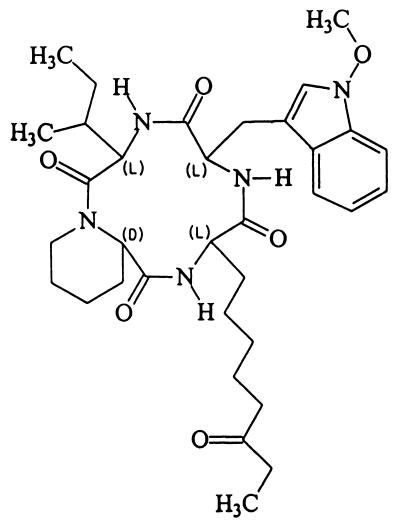
The low-resolution (LR)-electron impact mass spectrum of the toxin displayed a strong molecular ion at m/z 623 and fragment ions at m/z 593, 314, 283, 170, 130, and 84 (Fig. 1A). The LR-chemical ionization mass spectrum had strong [M+1]+, [M+15]+, and [M+29]+ ion peaks at m/z 624, 638, and 652, respectively (Fig. 1B). The fast atom bombardment mass spectrum displayed a protonated molecular ion at m/z 624 (Fig. 1C). High-resolution mass spectrometry and elemental analyses gave the molecular formula C34H49N5O6. The interpretation of the nuclear magnetic resonance data and amino oxidase experiments suggests that the toxin is identical to apicidin (Fig. 2), which is cyclo-{l-(2-amino-8-oxodecanoyl)–l-(N-methoxytryptophan)–l-isoleucyl–d-pipecolinyl} (22).
FIG. 1.
LR mass spectrum of apicidin obtained by electron impact at 70 eV (the molecular ion is m/z 623) (A), by chemical ionization at 200 eV ([M+1]+ peak is at m/z 624) (B), and by fast atom bombardment ([M+1]+ peak is at m/z 624) (C).
FIG. 2.
Chemical structure of apicidin.
Toxicity of apicidin.
In the rat-feeding test, a 0.05% level of apicidin in rat diet caused death 10 to 14 days after treatment and a 0.1% level caused death within 7 days of the initial treatment. Rats that died following either treatment had hemorrhaging in the stomach, intestines, and bladder accompanied by tissue degeneration. Apicidin was toxic in brine shrimp bioassay, and the 50% lethal dose was 40 μg/ml. Apicidin had weak in vitro cytotoxic activity; the 50% inhibitory concentrations were 2.1 μg/ml for P388 cells, 16 μg/ml for K562 cells, and 25 μg/ml for MOF-7 cells.
Production of apicidin by the toxic isolates.
Apicidin production by two toxic Fusarium isolates of unknown species and one F. semitectum isolate was measured. Apicidin was produced by the two unknown isolates but not by the F. semitectum isolate. The average concentrations of apicidin in wheat cultures of Fusarium sp. strain KCTC 16676 and Fusarium sp. strain KCTC 16677 were 340 and 680 μg/g, respectively.
The two apicidin-producing isolates were submitted to W. F. O. Marasas for identification. He determined that the two isolates are conspecific but that the species to which the cultures belong is uncertain. These isolates resemble F. sambucinum Fuckel according to the criteria of Nelson et al. (18) because of the curved, snout-like apical cells of the macroconidia. However, some other features of the cultures, such as the rapid growth rate at 30°C, are not typical of F. sambucinum. In addition, neither of the isolates produced DAS, which is a common toxic metabolite of F. sambucinum.
DISCUSSION
The predominant Fusarium species isolated from soybean seeds was F. oxysporum, followed by F. moniliforme and F. graminearum. Yum and Park (28) reported F. equiseti in addition to these species to be the dominant species in soybean seed lots in Korea.
The toxic isolates of F. graminearum, F. sporotrichioides, F. lateritium, and F. moniliforme produced known trichothecenes, ZEA, or fumonisins, suggesting that these toxins may occur naturally in moldy soybeans in Korea. On the other hand, the toxic isolates of Fusarium (two isolates) and F. semitectum (one isolate) produced no known trichothecenes or other known mycotoxins. Fusarium sp. strain KCTC 16677 produced apicidin and caused severe hemorrhaging in internal organs. Apicidin also is produced by F. pallidoroseum (= F. semitectum [22]) and is known to be an apicomplexan histone deacetylase inhibitor and to have activity against a broad spectrum of apicomplexan parasites in vitro. Recently, Darkin-Rattray et al. (9) also reported that apicidin has in vivo activity against Plasmodium berghei malaria.
When the rats were fed complete diets supplemented with 0.05 or 0.1% apicidin, apicidin caused loss of body weight; hemorrhage in the stomach, intestines, and bladder; and death. The acute lethal toxicity of apicidin at the 0.05% level corresponds approximately to the toxicity of the 1:1 mixture of crude culture and control diet. These results suggest that apicidin does not account for all of the toxicity associated with Fusarium sp. strain KCTC 16677. The fraction containing unpurified toxins other than apicidin also caused death accompanied by hemorrhage in the stomach, intestines, and bladder in the feeding test (Table 2). When fraction 2 was examined by TLC, one compound other than apicidin turned purple with p-anisaldehyde and 20% sulfuric acid, suggesting that this component is structurally related to apicidin. This component, together with apicidin in the crude culture, may be responsible for the hemorrhage and death in the feeding test. Apicidin was toxic to brine shrimp. For brine shrimp (10), the 50% lethal concentration of apicidin is higher than those of trichothecenes and lower than that of chlamydosporol (2). In addition, apicidin was weakly cytotoxic to several human and mouse tumor cells.
Although apicidin caused hemorrhage and death at high doses in this study, more toxicological data are needed to account for a portion of the cases of hemorrhagic disease syndrome found in the stomachs and intestines of farm animals. Surveys on the natural occurrence in agricultural products including soybeans are expected to provide valuable information on risk assessment of apicidin. Also, the possibility that apicidin is produced by Fusarium species, including F. semitectum and F. sambucinum, from other sources requires further investigation.
ACKNOWLEDGMENTS
This work was supported by the Korea Science and Engineering Foundation through the Research Center for New Biomaterials in Agriculture at Seoul National University.
We thank W. F. O. Marasas of PROMEC, Medical Research Council, Tygerberg, South Africa, for identification of the apicidin-producing isolates.
REFERENCES
- 1.Abbas H K, Mirocha C J. Isolation and purification of a hemorrhagic factor (wortmannin) from Fusarium oxysporum (N17B) Appl Environ Microbiol. 1988;54:1268–1274. doi: 10.1128/aem.54.5.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas H K, Mirocha C J, Shier W T. Isolation, identification and biological activity of chamydosporol from Fusarium culmorum HN-8. Mycopathologia. 1992;118:115–123. doi: 10.1007/BF00442540. [DOI] [PubMed] [Google Scholar]
- 3.Bacon C W, Marijanovic D R, Norred W P, Hinton D M. Production of fusarin C on cereal and soybean by Fusarium moniliforme. Appl Environ Microbiol. 1989;55:2745–2748. doi: 10.1128/aem.55.11.2745-2748.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamburg J R, Strong F M. 12,13-Epoxytrichothecenes. In: Kadis S, Ciegler A, Ajl S, editors. Microbial toxins. Vol. 7. New York, N.Y: Academic Press, Inc.; 1971. pp. 207–292. [Google Scholar]
- 5.Bezuidenhout S C, Gelderblom W C A, Gorst-Allman C P, Horak R M, Marasas W F O, Spiteller G, Vleggaar R. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme. J Chem Soc Chem Commun. 1988;1988:743–745. [Google Scholar]
- 6.Carmichael J, Degraff W G, Gazdar A F, Minna J D, Mitchell J B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 7.Closse A, Huguenin R. Isolation and structure elucidation of chladomycin. Helv Chim Acta. 1974;57:533–545. doi: 10.1002/hlca.19740570306. [DOI] [PubMed] [Google Scholar]
- 8.Cole R J, Kirksey J W, Cutler H G, Doupnik B L, Peckham J C. Toxin from Fusarium moniliforme: effects on plants and animals. Science. 1973;179:1324–1326. doi: 10.1126/science.179.4080.1324. [DOI] [PubMed] [Google Scholar]
- 9.Darkin-Rattray S J, Gurnett A M, Myers R W, Dulski P M, Crumley T M, Allocco J J, Cannova C, Meinke P T, Colletti S L, Bednarek M A, Singh S B, Goetz M A, Dombrowski A W, Polishook J D, Schmatz D M. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc Natl Acad Sci USA. 1996;93:13143–13147. doi: 10.1073/pnas.93.23.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eppley R M. Sensitivity of brine shrimp (Artemia salina) to trichothecenes. J Assoc Off Anal Chem. 1974;57:618–620. [PubMed] [Google Scholar]
- 11.Fisher N L, Burgess L W, Tousson T A, Nelson P E. Carnation leaves as a substrate and for pressing cultures of Fusarium species. Phytopathology. 1982;72:151–153. [Google Scholar]
- 12.Holcomb M, Sutherland J B, Chiarelli M P, Korfmacher W A, Thompson H C, Jr, Lay J O, Jr, Hankins L J, Cerniglia C E. HPLC and FAB mass spectrometry analysis of fumonisin B1 and B2 produced by Fusarium moniliforme on food substrates. J Agric Food Chem. 1993;41:357–360. [Google Scholar]
- 13.Kim J-C, Lee Y-W. Sambutoxin, a new mycotoxin produced by toxic Fusarium isolates obtained from rotted potato tubers. Appl Environ Microbiol. 1994;60:4380–4386. doi: 10.1128/aem.60.12.4380-4386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K-H, Lee Y-W, Mirocha C J, Pawlosky R J. Isoverrucarol production by Fusarium oxysporum CJS-12 isolated from corn. Appl Environ Microbiol. 1990;56:260–263. doi: 10.1128/aem.56.1.260-263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y-W, Mirocha C J, Shroeder D J, Walser M M. TDP-1, a toxic component causing tibial dyschondroplasia in broiler chickens, and trichothecenes from Fusarium roseum ‘Graminearum.’. Appl Environ Microbiol. 1985;50:102–107. doi: 10.1128/aem.50.1.102-107.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marasas W F O, Nelson P E, Tousson T A. Toxigenic Fusarium species—identity and mycotoxicology. University Park: Pennsylvania State University Press; 1984. [Google Scholar]
- 17.Neergaard P. Seed pathology. I and II. London, England: The Macmillan Press Ltd.; 1977. [Google Scholar]
- 18.Nelson P E, Tousson T A, Marasas W F O. Fusarium species—an illustrated manual for identification. University Park: Pennsylvania State University Press; 1983. [Google Scholar]
- 19.Richardson K E, Hagler W M, Jr, Haney C A, Hamilton R B. Zearalenone and trichothecene production in soybeans by toxigenic Fusarium. J Food Prot. 1985;48:240–243. doi: 10.4315/0362-028X-48.3.240. [DOI] [PubMed] [Google Scholar]
- 20.Scott P M, Lawrence G A. Liquid chromatographic determination and stability of the Fusarium mycotoxin moniliformin in cereal grains. J Assoc Off Anal Chem. 1987;70:850–853. [PubMed] [Google Scholar]
- 21.Sinclair J B, Blackman P A, editors. Compendium of soybean diseases. St. Paul, Minn: APS Press; 1989. [Google Scholar]
- 22.Singh S B, Zink D L, Polishook J D, Dombrososki A W, Darkin-Rattray S J, Schmatz D M, Goetz M A. Apicidins: novel cyclic tetrapeptides as coccidiostats and antimalarial agents from Fusarium pallidoroseum. Tetrahedron Lett. 1996;37:8077–8080. [Google Scholar]
- 23.Stob J, Baldwin R S, Tuite J, Andrews F N, Gillette K G. Isolation of an anabolic, uterotrophic compound from corn infected with Gibberella zeae. Nature. 1962;196:1318. doi: 10.1038/1961318a0. [DOI] [PubMed] [Google Scholar]
- 24.Sydenham E W, Shephard G S, Thiel P G. Liquid chromatographic determination of fumonisin B1, B2, and B3 in foods and feeds. J Assoc Off Anal Chem. 1992;75:313–318. [Google Scholar]
- 25.Takitani S, Asabe Y, Kato T, Suzuki M, Ueno Y. Spectrodensitometric determination of trichothecene mycotoxins with 4-(p-nitrobenzyl)pyridine on silica gel thin-layer chromatograms. J Chromatogr. 1979;172:335–339. doi: 10.1016/s0021-9673(00)90970-1. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Hasegawa A, Matsuki Y, Ishii K, Ueno Y. Improved methodology for the simultaneous detection of the trichothecene mycotoxins deoxynivalenol and nivalenol in cereals. Food Addit Contam. 1985;2:125–137. doi: 10.1080/02652038509373534. [DOI] [PubMed] [Google Scholar]
- 27.Visconti A, Mirocha C J, Logrieco A, Bottalico A, Solfrizzo M. Mycotoxins produced by Fusarium acuminatum. Isolation and characterization of acuminatin: a new trichothecene. J Agric Food Chem. 1989;37:1348–1351. [Google Scholar]
- 28.Yum K-J, Park E-W. Occurrence and distribution of soybean seed-borne fungi in Korea. Korean J Plant Pathol. 1989;5:287–293. [Google Scholar]