Abstract
Background
Crohn's disease (CD) is an inflammatory bowel disease that causes inflammation and stricture, of any part of the mucosa and the gut wall. It forms skip lesions, sparing the areas in between the affected parts of the gastrointestinal tract. Crohn's disease could have one of three complications; fistula, intestinal obstruction due to stricture, or gastrointestinal inflammation presenting as severe diarrhoea.
Stem cell therapy (SCT) is an innovative treatment that has been recently used in CD. The exact role of SCT in CD is still unclear. Stem cells modify the immunity of the patients or act as a “reset tool” for the immune system as in the case of systemically‐injected stem cells, or regenerate the affected area of necrotic and inflammatory tissue as in the case of local injection into the lesion. Stem cells are a wide variety of cells including pluripotent stem cells or differentiated stem cells. The hazards range from rejection to symptomatic manifestations as fever or increase infection.
Objectives
The objective of this Cochrane systematic review is to assess the effects of stem cell transplantation compared to standard of care alone or with placebo on efficacy and safety outcomes in patients with refractory CD.
Search methods
We searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and clinical trial registries (Clinicaltrials.gov, World Health Organization‐International Clinical Trials Registry Platform WHO ICTRP) from inception to 19 March 2021, without any language, publication year, or publication status restrictions. In addition, we searched references of included studies and review articles for further references. An update of the published studies was done during the writing of the review.
Selection criteria
We included only randomised controlled trials (RCTs) that assessed the effectiveness and safety of SCT in refractory CD versus standard care alone (control) or with placebo.
Data collection and analysis
Two review authors (SEN and SFA) independently screened the studies retrieved from the search results for inclusion, extracted data and assessed the risk of bias. Any disagreement was resolved through a consensus between the authors. We used standard methodological procedures expected by Cochrane.
Main results
We conducted our search on 19 March 2021 and identified 639 records. We added two records by a manual search of the published reviews on the topic to a total of 641 records. The Covidence program removed 125 duplicates making a total of 516 reports. Two review authors (SEN and SFA) screened titles and abstracts and excluded 451 records with the remaining 65 for full‐text records screened independently by the two authors; only 18 studies were considered for inclusion.
We included seven RCTs with a total of 442 participants for the meta‐analysis. The intervention group included 234 patients, and the control group included 208 patients. Nine trials are ongoing and, two abstracts are awaiting classification.
All patients in the control and intervention groups received the standard therapy for CD. Only three studies used blinding methods for the control group in the form of a placebo, with one study of the three stated that the blinding method was inefficient. The patients and personnel were aware of the intervention in the rest of the four studies as they were open‐label trials. However, the effect of unblinding was balanced by the low risk of detection bias in five of the included studies.
The evidence is uncertain about the effect of SCT on achieving clinical remission as compared to control/placebo (risk ratio (RR) 1.88, 95% Confidence Interval (CI) 0.80 to 4.41; 3 studies; low‐certainty evidence).
The evidence is very uncertain about the effect of SCT on achieving Crohn’s Disease Activity Index (CDAI) <150 at 24 weeks compared to control (RR1.02 95% CI 0.67 to 1.56; 4 studies; very‐low certainty evidence).
SCT is likely to achieve fistula closure as compared to the control/placebo both in the short term (RR 1.48, 95% CI 1.12 to 1.96); low‐certainty evidence) and in the long term (RR 1.42, 95% CI 1.09 to 1.87; 4 studies; low‐certainty evidence) follow‐up.
The evidence is very uncertain about the effect of SCT to cause no difference in the number of total adverse events as compared to the control/placebo (RR 0.99, 95% CI [0.88 to 1.13); 4 studies; very‐low‐certainty evidence). However, SCT is likely to increase the number of serious adverse events as compared to the control/placebo (RR 1.22, 95% CI 0.88 to 1.67; 7 studies; low‐certainty evidence).
The evidence is very uncertain about the effect of SCT to decrease the withdrawal due to adverse events as compared to the control/placebo (RR 0.78, 95% CI 0.32 to 1.89; 3 studies; very‐low certainty evidence).
Funding by pharmaceutical companies was found in three studies, with one including more than 50% of our studied population.
Authors' conclusions
SCT shows an uncertain effect on clinical remission with low certainty of evidence. SCT shows an uncertain effect on CDAI score to reach <150 after 24 weeks of treatment, with very low certainty evidence. SCT shows beneficial effects on fistula‐closure during short and long‐term follow‐up with low‐certainty evidence in both outcomes. There was no change in the total number of adverse events with SCT as compared to control, with very low certainty evidence. While there was a moderate effect on increasing the number of serious adverse events in the SCT group, as compared to the control with low‐certainty evidence. Withdrawal due to adverse events was slightly higher in the control group with very low certainty evidence.
All the participants were refractory to standard medical treatment, but the number of participants was small, this may limit the generalizability of the results. Further research is needed for validation. More objective outcomes are needed in the assessment of stem cell effectiveness in the treatment of Crohn's disease, especially the intestinal CD subtype; with standardization of the dose, methods of stem cell preparation, route of administration, and inclusion criteria to the studies to achieve clear results.
Keywords: Humans; Constriction, Pathologic; Crohn Disease; Crohn Disease/drug therapy; Hematopoietic Stem Cell Transplantation; Inflammation; Remission Induction
Plain language summary
Stem cells for treating patients not responding to Crohn's disease treatment
Question
Are stem cells (SCs) an effective and safe option in patients with Crohn's disease (CD) when they do not respond to their standard medical treatment?
Key messages
Stem cells when combined with standard medical treatment could be better than the medical treatment alone or with a placebo (a dummy treatment) in the healing of the opening in the perianal region connected to the bowels caused by CD (perianal fistula).
Stem cells when combined with standard medical treatment could be safe when compared to the medical treatment alone or with a placebo in treating the bowel inflammation associated with Crohn's disease (total and serious adverse events).
What are stem cells? Stem cells are the cells responsible for forming new cells and renewing the surrounding tissue. They are also responsible for modifying the immune system. There are various types of SCs, self‐stem cells extracted from the patient's own body (autologous) and non‐self stem cells extracted from other individuals (allogeneic). They could be found in the bone marrow, fat tissue, placenta, umbilical vein, etc.
What is Crohn's disease? It is an autoimmune (fighting the patient's own body) disease‐causing inflammation and stenosis of the bowel, or causing a bowel opening in the skin (fistula). CD usually follows an "on and off" pattern, it also affects the mortality, morbidity, and quality of life of the patients. Standard treatment of CD includes drugs that suppress the immunity of the patient including anti‐inflammatory, immunosuppressive, and biological drugs. However, one‐third of patients do not respond to medical or surgical treatment.
Why was this Cochrane Review conducted? To assess if SCs are effective and safe in patients with CD who do not respond to medical treatment (i.e. refractory CD).
What did we do? We assessed randomised controlled trials on the topic.
How up to date is this evidence?
This evidence is up‐to‐date to March 2021.
What did we find?
We found seven trials on the topic, including 442 patients (234 in the stem cell group and 208 in the placebo or control groups). The follow‐up duration in the studies varied from one to four years. We included seven RCTs with a total of 442 participants for the meta‐analysis. The intervention group included 234 patients, and the control group included 208 patients. We assessed the effect of both the systemic and local administration of SCs. The intervention group included 127 males (55.95%) and 100 females (44.05%), while the control/placebo group included 114 males (56.44%) and 88 females (43.56%). Studies were conducted in the UK, China, Spain, the Netherlands, and the USA.
We found data on clinical remission in three studies, data on achieving Crohn's Disease Activity Index (CDAI) <150 after 24 weeks in three studies, data on fistula closure short and long term in four studies, data on the total number of adverse events in four studies, data on serious adverse events in seven studies, and data on withdrawal due to adverse events in three studies.
What are our main results?
In patients who did not respond to standard medical treatment for CD, we found that: when using SCs combined with medical treatment compared to medical treatment alone or with placebo, it is unclear whether they cause an achievement of improvement in the clinical remission, or in the clinical score CDAI to <150 after 24 weeks. SCs combined with medical treatment, when compared to the standard medical treatment, are likely to lead to improvement in the rate of fistula closure in both the short and long term. SCs combined with medical treatment, when compared to the standard medical treatment, are less likely to change the number of total adverse events. SCs combined with medical treatment, when compared to the standard medical treatment, are more probable to increase the number of occurrences of serious adverse events, but are less likely to decrease the number of patients who withdrew due to adverse events.
What are the limitations of the evidence?
Three of the included trials were funded by pharmaceutical companies.
Only a small number of studies addressed the topic with small numbers of patients. Moreover, most of the studied population (> 60%) was in those funded three studies, with one study including >50% of the studied population.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Stem Cell compared to Placebo or Control for Induction of Remission in Medically Refractory Crohn?s Disease.
| Stem Cell compared to Placebo or Control for Induction of Remission in Medically Refractory Crohn's Disease | ||||||
| Patient or population: Induction of Remission in Medically Refractory Crohn's Disease Setting: Specialised centres Intervention: Stem Cell Comparison: Placebo or Control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Placebo or Control | Risk with Stem Cell | |||||
| Clinical remission | 315 per 1000 | 592 per 1000 (252 to 1000) | RR 1.88 (0.80 to 4.41) | 301 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | The three studies that included data about clinical remission were Hawkey 2015, Melmet 2015, and Panes 2016. Each had a different definition of clinical remission. |
| CDAI <150 at 24 weeks | 506 per 1000 | 516 per 1000 (339 to 789) | RR 1.02 (0.67 to 1.56) | 352 (4 RCTs) | ⊕⊝⊝⊝ Very lowc,d,e | The data regarding the CDAI at 24 weeks was not the primary outcome of any of the included studies. They also used different cut‐offs and different ranges. Here we included local and systemic stem cell therapy. Zhang et al 2018 had zero weight, as the number of events was 0 in both intervention and control arms (the authors stated that no patients achieved CDAI <150 at 24 weeks). The baseline CDAI was already low in the studies examining fistula management. |
| Fistula Closure short‐term assessed with: Clinically or MRI | 349 per 1000 | 516 per 1000 (391 to 684) | RR 1.48 (1.12 to 1.96) | 269 (4 RCTs) | ⊕⊕⊝⊝ Lowf,g | Fistula closure was assessed in four studies. Garcia‐Olmo et al 2009 assessed the outcome at 8 weeks, while Molendijk et al 2015, Panes et al 2016, and Zhou et al 2020 assessed the outcome at 24 weeks. It was assessed both clinically and with MRI |
| Fistula closure in long‐term Follow up of original studies assessed with: Clinicaaly or MRI | 390 per 1000 | 554 per 1000 (425 to 729) | RR 1.42 (1.09 to 1.87) | 250 (4 RCTs) | ⊕⊕⊝⊝ Lowh,i | The data on the long‐term effects were gathered from published papers after a long‐term follow‐up of the original studies. Except Zhou 2020, which reported their long‐term 1‐year follow‐up. |
| Total Adverse Events assessed with: Clinically | 730 per 1000 | 723 per 1000 (643 to 825) | RR 0.99 (0.88 to 1.13) | 293 (4 RCTs) | ⊕⊝⊝⊝ Very lowj,k,l | The range of total adverse effects stated was very wide across studies, from minimal abdominal pain or low‐grade fever to sepsis and the need for surgical operation. We collected the data on total adverse events without stating the level, or severity of the adverse events. |
| Serious Adverse Events assessed with: Clinically | 112 per 1000 | 137 per 1000 (99 to 187) | RR 1.22 (0.88 to 1.67) | 433 (7 RCTs) | ⊕⊕⊝⊝ Lowm,n | All 7 studies stated the number of patients suffering from serious adverse effects, mostly because these trials are addressing the safety issues of stem cell administration. The different definitions of serious adverse events among the studies make the outcome assessment heterogeneous and inconsistent, some studies did not define clearly what a serious adverse event stands for. |
| Withdrawal due to adverse events | 74 per 1000 | 58 per 1000 (24 to 140) | RR 0.78 (0.32 to 1.89) | 272 (3 RCTs) | ⊕⊝⊝⊝ Very lowo,p | Both Panes 2016 and Zhou 2020 stated clearly that the withdrawal was due to adverse events. But in Hawkey 2015, it was stated that one patient from the control group withdrew directly after randomization, and one patient in the active group withdrew after 26 weeks of mobilization for accelerated transplantation. In Zhou 2020, the patients withdrew due to adverse effects to receive a subsequent reoperation. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_423896885310176737. | ||||||
a The risk of bias is downgraded to serious, as two of the three studies are open‐label, thus the participants are not blinded including Panes 2016 which contains most of the studied population, and this outcome is a subjective outcome. b The inconsistency was downgraded by one level as the heterogeneity in the included studies was high in the form of the variability of the results due to different definitions of the outcome. c Inconsistency downgraded by one level to serious as the level of CDAI at the start of the studies varied according to the route of intervention, CDAI was low or normal at baseline in local intervention, while high at baseline in systemic intervention. d The imprecision was downgraded by one level to serious as the RR is 1.08 with CI 0.94‐1.24, which makes the intervention equally capable of doing harm or benefit. e Publication bias strongly suspected and downgraded by one level as there were two abstracts of unpublished complete data (Arturo 2017 and Lichtiger 2012). f The outcome was downgraded by one point to serious as the risk of allocation concealment was unclear in three studies (Garcia‐Olmo 2009 and Zhou 2020), and randomization was unclear in (Zhou 2020). Although the risk of bias for blinding of participants is high in (Zou 2020, Panes 2016, Garcia‐Olmo 2009), and unclear in (Molendijk 2015); three of these studies had a low risk for detection bias, while only Zhou 2020 had high risk. Also, the outcome is an objective outcome that doesn't change by the participant or the personnel being unblinded. g Imprecision was downgraded by one level to serious as the number of the studied population was small. h The risk of bias is downgraded to serious: as there are a lot of patients lost to long‐term follow‐up (missing data), thus high attrition. i The imprecision was downgraded by one level to serious as the number of patients and number of events were low. Also, the confidence interval was wide. j The risk of Bias was downgraded by one level to serious as three of the four studies had a high risk of performance bias and one unclear risk. Considering that the outcome is reporting about the adverse events, which are mostly subjective in the case of mild and moderate adverse events, we downgraded by one level. k Inconsistency is downgraded by one level to serious as the studies reported the category (All adverse events differently) l Impression was downgraded by one level as the number of participants was low m The risk of bias was downgraded by one level, as 5 trials had high‐performance bias (open‐label trials) but it is not an objective outcome, and allocation concealment is unclear in 4 trials and randomization is unclear in 2 trials. n Imperceision is downgraded by one level to serious because the CI was very wide (0.89‐1.93), so we are not certain if the intervention causes benefit or harm. o Indirectness was downgraded by one level to seriuos as the causes of withdrawal in (Hawkey 2015) were not stated as due to adverse events. p Imprecision was downgraded by two levels to very serious due to the low number of participants and wide CI (0.33‐1.91), so we are not confident if the intervention causes benefit or harm.
Background
Crohn's disease (CD) is one of the inflammatory diseases affecting the gut. The autoimmune origins are apparent in the pathogenic mechanism of the disease. Its clinical manifestation includes diarrhoea, intestinal fistulas, and strictures. The disease follows a relapsing and remitting pattern in most cases. Refractory CD was previously defined as a persistently symptomatic acute CD or actively chronic CD, not responding to medical anti‐inflammatory treatment, and not reaching remission (Tremaine 1997). The recent definition states that refractory CD is the failure of response to all licensed medical therapeutic approaches, while refractory perianal CD fistula means failure of at least one surgical therapeutic approach and anti‐tumour necrosis factor‐alpha (Raine 2021). Refractory disease to the current medical treatment is present in a considerable number of cases (Ng 2017; Ha 2015; Carvello 2019).
Stem Cells are an innovative tool to induce immunomodulatory response and reset the immune system. They could also act as a regenerative tool to induce the healing of tissues. Stem cells can be used as a local injection on‐site of the lesion or as a systemic infusion(Ruiz 2018).
Description of the condition
Crohn's disease is a chronic inflammatory disease of the gastrointestinal tract that typically affects young adults between 15 and 35 years of age. The prevalence of Crohn's disease is nearly 320 per 100,000, with the highest prevalence in Europe and North America. The prevalence of Crohn's disease in developing countries might be underestimated due to a lack of rigorous population screening studies (Molodecky 2012; Ng 2017).
Crohn's disease presents mainly with abdominal pain, diarrhoea, fever, malabsorption, and weight loss (Abraham 2009; Ruiz 2015). Crohn's disease causes both mucosal and transmural inflammation that can affect any part of the gastrointestinal tract, but mostly the small bowel (Wiarda 2012). There are three Crohn's disease behaviours (Montreal classification) that can occur at any time during the disease course. These are non‐stricturing, non‐penetrating, stricturing and penetrating disease (Satsangi 2006). Common complications of Crohn's disease include perianal fistulae and abscesses. Some patients may have immune‐mediated extraintestinal manifestations (i.e. arthritis, eye, skin, and liver) (Isene 2015; Peyrin‐Biroulet 2017). Crohn's disease follows a relapsing and remitting course (Nikfar 2013). The therapeutic goal of treatment is to induce and maintain clinical remission. Different interventions have been investigated for inducing remission in active Crohn's disease (Dassopoulos 2013).
Description of the intervention
Stem cell therapy includes haematopoietic stem cells (HSCs) and mesenchymal stem (stromal) cells (MSCs). Stem cell therapy, whether HSCs or Mesenchymal stem cells (MSCs), can be subdivided into autologous donation (isolated from the patient) or allogenic donation (isolated from a donor, ideally human leukocyte antigen matched) (Dalal 2012; Duran 2016).
HSCs can be administered by an intra‐arterial or intravenous approach (Duran 2016). HSCs are characterised by their differentiation abilities into multi lineage cell types, and their migration to the affected tissues under the control of chemokines (Rossi 2011). MSCs can be successfully isolated for clinical application from bone marrow, umbilical cord blood, or adipose tissue. MSCs can be administered by an intra‐arterial or intravenous route or by local injection (Duran 2016). Darvadstrocel is the first MSC to be approved by European guidelines for use in CD. It was approved after the results of the ADMIRE‐CD trial which showed a positive healing effect on the CD‐associated fistula. Darvadstocel is recommended for use only in refractory CD, after the failure of one or more of the standard therapies in adult patients (Scott 2018).
Comparator intervention (standard of care)
These interventions include systemic corticosteroids such as hydrocortisone or prednisolone (Benchimol 2008), locally‐acting corticosteroids such as budesonide (Rezaie 2015), sulphasalazine (Lim 2016), tumour necrosis factor‐alpha (TNF‐α) antagonists such as infliximab (Kawalec 2013), azathioprine (Chande 2016), interleukin inhibitors e.g. ustekinumab (MacDonald 2016), methotrexate (McDonald 2014) and alpha‐4 integrin monoclonal antibodies such as vedolizumab (Sandborn 2013). Unfortunately, the only therapy that showed a positive effect on fistula healing after one year of follow‐up is infliximab with a success rate approaching 23% (in the Sands' clinical trial) (Guadalajara 2020). Also, retreatment with anti‐TNF showed a lower response in both induction and maintenance of remission (Pockley 2018).
Immunosuppressive drugs are the standard treatment for CD. For those who do not respond or lose response to this therapy, treatment solutions become a challenge (Cooper 2017). Further, endoscopic recurrence the following surgery may occur in up to 70% of cases (Day 2013; Lawrance 2014).
How the intervention might work
The goal in treating CD is to achieve remission and halt any ongoing disease progression (Gomollón 2017). Stem cells have immunoregulatory potential. Therefore, stem cell therapy, either haematopoietic or mesenchymal, may induce remission in refractory CD (Dalal 2012; Dave 2015; Duran 2016; Ricart 2013).
Haematopoietic stem cells (HSCs) extend immune modulation and suppression by incrementing immune suppression to the point of immune ablation(Duran 2016). Thus, HSCs can induce remission of the refractory CD through different mechanisms; either ablation of the bone marrow cells in the conditioning phase, which causes the destruction of the bone marrow cells, or later when the bone marrow restores its function and resetting of the cells occurs. Thymic reactivation is the key for cellular restoration in this phase for the T, B, plasma, and natural killer cells progeny (Brierley 2018).
The role of HSCs in treating inflammatory bowel disease was originally supported by clinical remissions observed in patients undergoing stem cell transplant for haematological disorders. These observations led to trials of HSCs in patients with refractory CD (Burt 2003; Burt 2010; Cassinotti 2008; Clerici 2011; Craig 2003; Kreisel 2003; Oyama 2005). The largest multicentre, randomised clinical trial of autologous HSCs in refractory CD was conducted from 2007 to 2011, with follow‐up through 2013 (Hawkey 2015). The infusion of either autologous or allogeneic HSCs is associated with adverse events, with cardiovascular and pulmonary adverse events being common (Vidula 2015).
Mesenchymal stem (stromal); cells (MSCs) are multipotent cells that have immunomodulating capabilities to down‐regulate mucosal immune reactivity and promote tissue healing. MSCs can induce apoptosis (programmed cell death) of lymphocytes, thus, decreasing their proliferation in vitro. There are only a few studies reporting on the use of autologous (Duijvestein 2010), or allogeneic (Forbes 2014), bone marrow‐derived MSCs for luminal CD. In fistulising CD, local injection of MSCs may be beneficial for healing of the fistula (Ciccocioppo 2011; de la Portilla 2013; Garcia‐Olmo 2005; Garcia‐Olmo 2009; García‐Arranz 2016; Lee 2013).
The effect of stem cells on the treatment of CD might be due to their regenerative effect on local healing of fistulas and colitis. Mesenchymal stem cells are the commonest type of stem cells used in CD treatment, with the possibility of commercial availability and easier non‐hazardous preparation; with proper lab preparation and "Good manufacturing practice" (GMP). Limitations that might face this treatment are; the absence of dose standardisation, indeterminate concomitant medication "wash‐out" period, insufficient data about the effect of using allogenic versus autologous cells, high cost, and the ethical issues related to some sources of MSCs as the placenta and the umbilical cord (Lightner 2019a; Lightner 2019b).
There is a concern about the safety of hematopoietic stem cells use to "reset the immune system" as the hazards during the conditioning phase could overshadow the benefits (Jauregui‐Amezaga 2016).
Why it is important to do this review
Patients with refractory CD suffer high morbidity and mortality. Controversy regarding the potential benefits and harms of stem cell transplant for patients with refractory CD still exists (Duran 2016; Gomollón 2017). This systematic review summarises the current evidence regarding the efficacy and safety of stem cell transplantation in refractory CD.
Objectives
The objective of this review is to assess the effects of stem cell transplantation compared to standard of care alone or with placebo on efficacy and safety outcomes in patients with refractory Crohn's disease (CD).
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished, and ongoing randomised controlled trials (RCTs) that assessed the efficacy and safety of stem cell transplantation compared to standard of care alone or with placebo used for refractory Crohn's disease. We included studies with parallel comparisons, either two or more groups comparing separate doses of the intervention, only if the control group was presented separately as one of the parallel groups. In the case of multi‐arm trials, we combined the intervention arms as one and compared it with the control arm (section 23.3.2 Handbook Higgins 2021). This was done to avoid the repeated counting of the participants or unreasoned omission of relevant groups with the resulting over or underestimation of precision (Melmed 2015; Molendijk 2015).
We also included cross‐over trials (as indicated in the protocol) (Hawkey 2015), only if they had data available before the cross‐over phase. Two ongoing trials (NCT04519671; NCT04519697) stated that they are cross‐over trials; we will include them in future analysis if the data before the cross‐over is presented in their final data. RCTs that contained patients complaining of different diseases causing perianal fistula (mixed population)(Garcia‐Olmo 2009), but stated clearly and separately their data concerning the Crohn's disease‐associated fistula patients were also included. We did not include non‐randomised or quasi‐randomised trials.
Types of participants
We included participants with refractory Crohn's disease (Patients who received previously one or more failed standard treatments) as defined by conventional clinical, radiological or endoscopic criteria. We did not restrict inclusion by age or gender.
Types of interventions
Interventions that involved the administration of different types of stem cells were considered for inclusion.
We included the following comparisons.
Haematopoietic stem cells (HSCs) transplantation as compared with placebo or control receiving the standard of care.
Mesenchymal stem cells (MSCs) transplantation as compared with placebo or control receiving the standard of care.
Local MSCs injection as compared with placebo or control receiving the standard of care.
Types of outcome measures
We extracted primary and secondary outcomes.
Primary outcomes
The primary outcomes included the following.
Clinical remission, as defined by the original studies.
Crohn's Disease Activity Index (CDAI) of < 150),or a Pediatric Crohn’s Disease Activity Index (PCDAI) of < 15 at weeks four to six (early), weeks 10 to 12 (middle), and weeks 15 or later (late) following initiation of therapy. But no paediatric population was present in all the included studies.
Complete closure of the fistula as defined by original studies (e.g. complete closure of the fistula tract including internal and external openings without drainage or any sign of inflammation either detected; assessed clinically or by magnetic resonance imaging (MRI) or Perianal Disease Activity Index score (PDAI score)).
Secondary outcomes
Secondary outcomes included the following.
Clinical improvement, as defined by the original studies.
Endoscopic remission, as defined by the original studies e.g. Crohn's disease endoscopic index of severity (CDEIS), simple endoscopic score for Crohn's disease(SES‐CD), Rutgeerts' postoperative endoscopic index.
Endoscopic improvement, as defined by the original studies.
Adverse events (e.g. perianal abscess, bacterial gastroenteritis).
Serious adverse events (e.g. sepsis, graft versus host disease).
Withdrawals due to adverse events.
All‐cause mortality.
Quality of life as defined by the original studies e.g. Inflammatory Bowel Disease Questionnaire (IBDQ) or Short Form Health Survey(SF‐36), IBD‐Control 8, Crohn's Ulcerative Colitis Questionnaire‐8 (CUCQ‐8), or IMPACT III for pediatric patients.
Search methods for identification of studies
We searched electronic databases and trial registries. We also searched published reviews and meta‐analyses dealing with the topic in question for study inclusion.
Electronic searches
To identify relevant studies, we searched the following databases from inception to 19 March 2021 without imposing any language, publication year, or publication status restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL) (from inception, via Ovid Evidence‐Based Medicine Reviews Database (EBMR)) (Appendix 1).
-
MEDLINE (from 1946, via Ovid) (Appendix 2):
all from 1946 to 2021 March 19
-
Embase (from 1974, via Ovid) (Appendix 3):
Embase Classic+Embase 1947 to 2021 March 19
Embase 1974 to 2021 March 19
Embase 1974 to 2021 Week 11
Embase 1980 to 2021 March 19
Embase 1980 to 2021 Week 11
Embase Classic 1947 to 1973
The Cochrane Gut Group Specialised Register, as a part of CENTRAL.
The performed new search strategy was developed by a Cochrane Information Specialist in March 2021.
Searching other resources
We searched the following databases for ongoing trials.
United States (US) National Institutes of Health Trials Registry (clinicaltrials.gov) (Appendix 4).
The World Health Organization (WHO) Clinical Trials Registry Platform (apps.who.int/trialsearch/default.aspx) (Appendix 5).
Checking reference lists
We handsearched reference lists of all included primary studies and relevant review articles for additional studies.
Data collection and analysis
We used the standard methodological procedures as stated in the Cochrane Handbook in conducting and reporting this systematic review (Higgins 2016; Higgins 2021).
An expert Statistician (MEN) conducted the conversions done in data extraction and also helped in conducting the data analysis, both according to the Cochrane Handbook. The methods are mentioned in detail in (Appendix 6).
Selection of studies
We conducted a previous search in 2018, another separate updated search was conducted on 19 March 2021. Two review authors (SEN and SFA) independently reviewed the titles and abstracts of the studies identified from the literature search. The full texts were assessed by (SEN and SFA) for the final inclusion of studies. The screening of the titles, abstracts, and full texts was conducted by SEN and SFA using the COVIDENCE Program (Babineau 2014) in the updated search of 19 March 2021.
In the current 19 March 2021 search, two review authors (SEN and SFA) screened the results of the database search of 639 records with two records added from manual search to the total number of 641 records. The two records added by the manual search were (Melmed 2015 and Knyazev 2020). Then 125 duplicates were removed. Both SEN and SFA screened the titles and abstracts of the remaining 516 reports with excluding 451 reports. Furthermore, the two review authors screened the remaining 65 full‐text reports for final inclusion, and a total number of 18 studies were included (9 ongoing trials, seven included RCTs, two abstracts of studies were added to studies awaiting classification (Figure 1). Any discrepancies during the inclusion phase were resolved through consensus between the two authors (SEN and SFA). Then both authors added the updated extracted data in a data word sheet formed previously. SEN conducted the data analysis using RevMan Web (RevMan Web 2020) and formulated the summary of findings (SoF) table using (GRADEpro GDT) through the RevaMan Web‐GRADEPro integration. We created a study flow diagram using the RevMan web software (RevMan Web 2020); to map out the number of records identified, included, awaiting classification, ongoing, and excluded according to PRISMA guidelines, as shown in (Figure 1).
1.
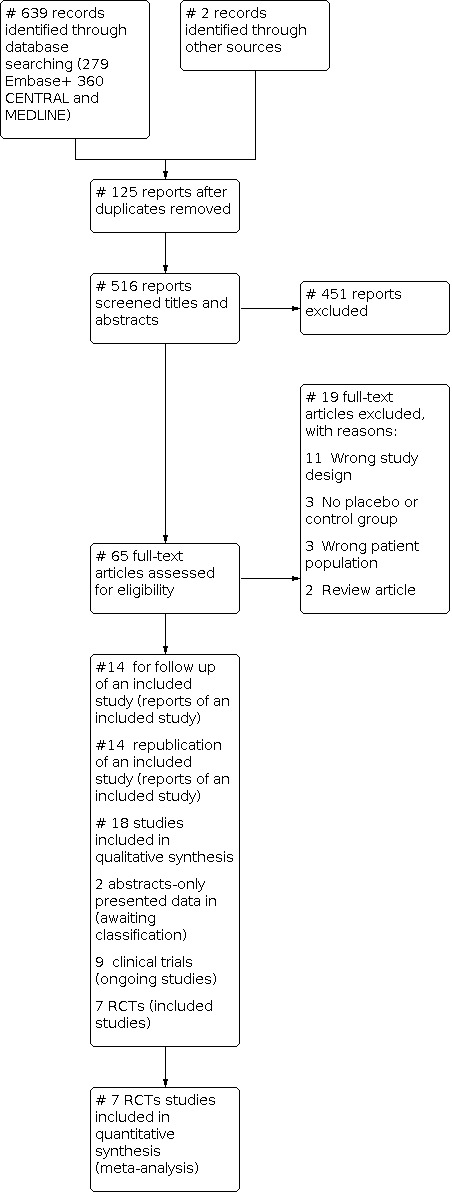
The flow diagram of the new search strategy (updated in 20 March 2021) Using COVIDENCE for screening (COVIDENCE Program)
We planned to include trials presented as abstracts if the full data was present after contacting the authors, or if the primary outcome is present otherwise the study was excluded.
We previously tried unsuccessfully to contact the authors of the two studies awaiting classification (Arturo 2017; Lichtiger 2012) and again contacted them in October 2021, and we are waiting for their reply. However, we found the data in the abstracts insufficient to include in quantitative or qualitative analysis, hence they were moved the two studies to the (Studies awaiting classification) section.
Data extraction and management
We designed a data extraction sheet and extracted data in our previous 2018 search.
In our current 19 March 2021 search, review authors SEN and SFA independently extracted the data from the included studies to our previously designed data extraction form. Any discrepancies were resolved by a consensus of the two authors (SEN and SFA). Data are presented in(Characteristics of included studies).
We entered data into Review Manager software (RevMan 2014). After October 2020 we used RevMan Web (RevMan Web 2020).
Extracted data included the following items: (as presented in Characteristics of included studies and additional Table 2).
1. Data Extracted from the included studies:
| Study name | Garcia‐Olmo 2009 | Molendijk 2015 | Melmed 2015 | Panes 2016 | Zhang 2018 | Hawkey 2015 | Zhou 2020 | |
| CCC of patients | Age | In ALL study 43.33, SD 9.9 I: mean 42.64, SD 10.93 P: 43.99, SD 8.97 |
Mean age: 38 Group 1: Mean: 40.4 (27‐54) Group 2: Mean: 40.8 (37‐47) Group 3: Mean: 33.4 (21‐48) Placebo: Mean: 37.3 (27‐49) | 1 unit: mean 35.3 SD 14 4 units: mean 36.2 SD 11.6 Placebo: mean 36.5 SD7.3 |
I: mean 39.0 (13.1) P: mean 37.6 (13.1) |
>18y‐70 years I:34.3 (21‐44) P: 32.7(20‐41) |
I: Median: 34.1 IQR: (26.1‐41.2) P: Median: 30.6 IQR:(24.0‐37.6) | Range from; 12‐51 28.86 ± 10.13 I: 24.4 ± 5.0 C: 24.9 ± 5.4 |
| sex | In all study 24/25 M/F I: 10/14 P: 14/11 |
Male in 4 groups: 4/5 4/5 1/5 3/6 M/F: 12/9 |
1 unit:53.3% M/F:8/7 4 units:33.3 % 5/10 placebo:43.8% 7/9 |
I: 60/47 P: 56/49 |
I: 24/17 P: 26/15 |
Women: I: 13/23 P: 11/22 |
1 female 21 male (M/F) Intervention: 11/0 Control: 10/1 |
|
| Disease duration in years | Not mentioned | In years in 4 groups I: 7.6 (5‐11) II: 16.8 (5‐28) III: 13.2 (2‐23) Placebo: 6.8 (1‐20) |
Mean I unit:18.5 yrs SD 13.8 4 units:10.4 yrs SD 10.7 placebo: 16.2 yrs SD9.4 |
I: 12.1 (10.0) P: 11.3 (8.9) years |
I: 7 (2‐15) C: 8 (3‐14) | I: 14.9 y P: 11.2 y |
Not mentioned | |
| Disease location | Rectovaginal fistula = 8/49 I= 4, P= 4 Suprasphincteric fistulous tract= 30/49 I= 14, P= 16 | Perianal Group 1: L1= 1, L2 =3, L3=1 Group 2: L1= 1, L2=2,L3= 2 Group 3: L1=2,L2=1,L3=2 Placebo: L1=1,L2=2,L3=2, L3+L4=1 |
Perianal High inter‐sphincteric, trans‐sphincteric, extra‐sphincteric or supra‐sphincteric. (?) |
I/C Ileal: 14/17 Colonic:18/14 Ileocolonic:9/10 Isolated upper:6/9 |
Intervention:
Ileum (L1) 1 (9.1%) Colon (L2) 4 (36.4%) Ileocolon (L3) 6 (54.6%) Upper GI (L4) 0 (0.0%) Control: Ileum (L1): 1 (9.1%) Colon (L2): 3 (27.3%) Ileocolon (L3): 7 (63.6%) Upper GI (L4) 0 (0.0%) |
|||
| Previous and concomitant medications | at least one complete course of antibiotics with a seton placement or conventional surgery (advancement flap or fistulectomy), at least one complete induction course of infliximab, unless anti‐TNF‐α treatment was contraindicated Concomitant: any except infliximab, tacrolimus, or cyclosporine |
Mesalamine, steroids, anti TNF, immunosuppressives | Aminosalicylates‐corticosteroids‐immunomodulators‐biologics (at least 3m before study) | concomitant but refractory to immunomodulatory and anti‐TNF and antibiotics at randomisation | Steroids for the last 6 months and as background treatment Anticoagulation prior to treatment (2,500 IU of low‐molecular‐weight heparin ) |
I/P Prior drugs: Azathioprine/6MP:22/22 Methotrexate:19/18 Anti TNF:23/22 Other:10/9 |
During the study, all patients received aminosalicylic acid (Mesalazine) and probiotic treatment. One patient in the observation group and three patients in the control group received immunomodulator treatment. One patient in each group was given antibiotics. |
|
| Previous bowel surgeries | Yes (adv flap or fistulectomy) Previous fistula surgery: 39/49 I= 17, P= 22 |
None | None | None | I:12 P:9 |
I: 2/23 P: 2/22 Ileostomy: I: 4/23 P: 4/22 |
Not mentioned | |
| Funding | This clinical trial has been sponsored by Cellerix S.L. | This work was supported by the DigestScience Foundation. | the study is funded by Celularity Incorporated | The study was funded by TiGenix | none mentioned | This study was sponsored by the European Group for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party and the European Crohn and Colitis Organisation (ECCO). | This work was funded by Key Medical Science and Technology Development | |
Data here are not mentioned in the characteristics of the studies.
Characteristics of patients: age, sex, disease duration, disease location, type of Crohn’s disease activity index used.
The total number of patients in each study and in each group.
Previous and concomitant medications used.
Outcomes: clinical remission, quality of life, mortality, adverse effects.
Type of intervention: HSCs or MSCs, etc.
Type of stem cells used: autologous or allogeneic.
Route of administration: systemic or local.
Mode and source of collection of the cells: direct marrow biopsy, cell mobilisation from the marrow, somatic cells reprogrammed, umbilical cord, adipose tissue.
Type of reconditioning used in cell collection if present.
Disease behaviour (inflammatory, fibro stenosing, penetrating).
We added the following to the previous items.
The dosage of the injected cells either local or systemic injection.
The selected endpoint of the primary study.
The number of centres in the study.
Primary country conducting the study.
The comparative intervention used (placebo or other intervention).
We added additional data extraction form for the included trials retrieved from the search strategy to assess their status and further characteristics(Characteristics of ongoing studies).
Whether published or not.
The type of stem cell therapy (SCT) they use.
The phase of the trial.
Status of the trial (ongoing, finished, or withdrawn).
Arms of the study.
Local or systemic injection.
Start and end dates of the trial.
Assessment of risk of bias in included studies
Two review authors (SEN and SFA) independently assessed the risk of bias for each study using the Cochrane risk of bias tool (Higgins 2011). Detailed methods for the risk of bias assessment are shown in Appendix 7.
We assessed the following items.
Random sequence generation (checking for possible selection bias).
Allocation concealment (checking for possible selection bias).
Blinding of participants and personnel (checking for possible performance bias).
Blinding of outcome assessment (checking for possible detection bias).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data).
Selective reporting (checking for reporting bias).
Other biases (checking for bias due to problems not covered by items above): e.g. funding issues, baseline characteristics of the patients across groups, type of stem cells used (single versus multiple donors), etc.
For each item, we made explicit judgments about the high, low, or unclear risk of bias. Overall, we made explicit judgments about whether studies were at (high, low, or some concerns) risk of bias. Two review authors (SEN and SFA) independently assessed the risk of bias using the ROB2 domains assessment format with guidance from version 6.2 of the Cochrane Handbook(Higgins 2021). For each item, we asked signalling questions to reach the judgment. Reasons for each judgment are written in detail in the Characteristics of included studies. When disagreement of the judgment occurred, it was resolved by a consensus between the two authors.
With reference to (1) to (7) above, we assessed the likely magnitude and direction of the bias and whether we consider it was likely to impact the findings. We planned to explore the impact of the level of bias by conducting sensitivity analysis (see Sensitivity analysis).
Measures of treatment effect
All data were analysed on an intention‐to‐treat (ITT) basis using Review Manager Web (RevMan Web 2020). We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI. In some analyses with differences in the presentation of the same outcome, we used standardised mean difference (SMD).
Unit of analysis issues
When studies reported multiple observations for the same outcome, we combined outcomes for fixed intervals of follow‐up (e.g. clinical remission at eight weeks).
We planned to include cross‐over trials if data were available from the first phase of the study (i.e. before cross‐over). Only one study (Hawkey 2015) was a cross‐over trial, where participants in the control group underwent the intervention after one year. We only included the results before the cross‐over as stated in the protocol.
In Garcia‐Olmo 2009, the studied population was heterogeneous, but the Crohn's disease group was documented separately in the results and methods section, so we could extract the data of the Crohn's disease‐only population.
Separate comparisons were planned to be conducted for stem cell therapy versus standard therapy alone versus standard therapy with placebo but the number of studies using placebo was small. If studies allocated participants to more than one stem cell treatment arm, these studies were pooled for the primary analysis as (Melmed 2015; Molendijk 2015).
Dealing with missing data
The analysis of the two primary outcomes was carried out using intention‐to‐treat (ITT) analyses. We planned to contact the trialists to request missing data, or to ascertain the reason for data loss, but we did not need to because most of the included studies provided online supplementary detailed data which we used. We only tried to contact the trialists with the unclear methodology of their trials, but got no response and a consensus was formed to exclude those studies due to unclear methodology (Kagramanova 2016; Knyazev 2015; Lazebnik 2010) whether randomised trials or cohort studies.
Although attrition was low in all studies, all the studies with missing data had supplementary material online in addition to the published manuscripts (Hawkey 2015, Molendijk 2015, and Panes 2016) providing a detailed explanation and how missing data were dealt with.
Otherwise, missing dichotomous data were planned to be assumed as treatment failure. The impact of this assumption on the effect estimate was planned to be assessed by performing sensitivity analyses where appropriate, but the number of studies was too low to conduct such analysis. We planned to conduct an available case analysis for continuous outcomes with missing data.
Assessment of heterogeneity
We assessed statistical heterogeneity for each pooled analysis using the Tau2, I2 and Chi2 statistics. We regarded heterogeneity as substantial if an I2 was greater than 50% and either the Tau2 was greater than 1, or the P value for the Chi2 test was statistically significant (i.e. less than 0.10).
We assessed heterogeneity using the Chi²test (P < 0.10, significant heterogeneity) and I² statistic (> 50%, substantial heterogeneity) using a random‐effects model along with visual inspection of forest plots. Following the guidance of the Cochrane Handbook (Higgins 2021), we defined I² = 0% to 30% as not important heterogeneity, 31% to 50% as moderate heterogeneity, 51% to 90% as substantial heterogeneity, and 91% to 00% as considerable heterogeneity. When substantial or considerable heterogeneity was found, possible explanations were investigated by subgroup and sensitivity analyses to test the robustness of the overall results.
Assessment of reporting biases
We aimed to include all the eligible studies either published or unpublished. In the case of unpublished trials and abstract‐only published studies, we contacted the authors for further data and classified them as studies "awaiting classification" until full data were available for quantitative and qualitative assessment. In case of unfinished trials, we classified them as "ongoing trials". We planned to investigate the reporting bias in the form of publication bias of all the reported studies (published and unpublished) by drawing a funnel plot and visualising any asymmetry, but as the number of the relevant studies was small (only seven published and two unpublished studies as compared to the minimum required number for assessment i.e. 10 studies) we could not assess the reporting publication bias.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014) and RevManweb (RevMan Web 2020) after 2020. We combined data from individual trials for meta‐analysis when the interventions, patient groups, and outcomes were sufficiently similar (as determined by consensus).
When we suspected a high degree of clinical or baseline heterogeneity supported by a high degree of statistical heterogeneity as detected by (I2 ≥ 75%), we planned not to pool data for meta‐analysis, and we did that with two outcomes where their I2 was >75%; endoscopy scores and CDAI after 24 weeks.
As for the mortality outcome, we found that only two patients died from two studies, and due to the high number of zeros in the other studies, we performed Peto Odds ratio (OR) as recommended by Dr. Burch.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses if we identified substantial heterogeneity (I2 statistic ≥ 50% or P for heterogeneity < 0.1) was detected.
Autologous versus allogeneic stem cells.
High‐dose versus low‐dose stem cells.
Paediatric versus adult participants.
Male versus female participants.
Treatment after recurrence versus treatment‐naive participants.
However, with only seven studies included in the review, there were insufficient data to perform these analyses. Consequently, results of included studies were reported only as narrative results as shown in Included studies.
Sensitivity analysis
We conducted the following sensitivity analyses for the primary outcomes.
Repeated analyses using a random‐effects model where we identified substantial heterogeneity.
Restricted analyses to trials with a low risk of bias.
Summary of findings and assessment of the certainty of the evidence
GRADE and summary of findings table
We used the GRADE approach (Schünemann 2009), to create a summary of findings table for the following main outcomes (Table 1).
Clinical remission.
Clinical improvement in the form of CDAI <150 achievement after 24 weeks.
Fistula closure (short and long term)
Total adverse events.
Serious adverse events.
Withdrawals due to adverse events.
We used GRADEpro GDT to import data from Review Manager Web (RevMan Web 2020) through "integration" in order to create the summary of findings table. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. Evidence from randomised trials starts as 'high certainty', the evidence is downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on the assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates and potential publication bias.
Results
Description of studies
For a full description of the included studies kindly see Characteristics of included studies, for secluded studies Characteristics of excluded studies, and for ongoing trials full description please see Characteristics of ongoing studies.
Results of the search
We conducted the first search in 2018 and conducted the updated search on 19 March 2021. We retrieved 639 records; 360 records from (CENTRAL Appendix 1 and MEDLINE Appendix 2), and 279 records from (Embase Appendix 3). We added two records through our manual search of published studies on the topic (Melmed 2015 and Knyazev 2020). The total number of studies retrieved was 641 records.
Two review authors (SEN and SFA) screened the results of the database search, 125 duplicates were removed by COVIDENCE program (Babineau 2014). Both SEN and SFA screened the titles and abstracts of the remaining 516 reports. We excluded 451 reports. The two review authors screened the remaining 65 full‐text reports for final inclusion, and 19 reports were excluded for the reasons mentioned in (Figure 1). We added a specific detailed description and reasons for the exclusion of the excluded studies in the Characteristics of excluded studies section.
Duplicate reports of the included studies and their follow‐up were 28 publications. We included the 28 reports of the seven included RCTs (14 follow‐up reports and 14 duplicate publications), they were in the form of, republication of the same data, trial registries of the same study in different databases, protocol publication, or follow‐up data publication. So we screened the reports for any relevant new data and added them to the datasheet of the originally included studies (the 7 RCTs). This included the long‐term follow‐up and mortality data from those reports to our final analysis and quality of evidence assessment. The follow‐up report for Panes 2016 was Panés 2018, the follow‐up report for Garcia‐Olmo 2009 was Guadalajara 2012, and the follow‐up report for Molendijk 2015 was Barnhoorn 2020.
A total number of 18 studies were finally included for qualitative analysis (two abstracts, nine ongoing trials, and seven RCTs). Any discrepancies were resolved through a consensus between the authors. The identified 18 studies for inclusion in the review were nine studies of ongoing trials(EUCTR2017‐000725‐12‐CZ; ISRCTN17160440; NCT00482092; NCT04010526; NCT04519671; NCT04519684; NCT04519697; NCT04548583; NCT04612465), and seven RCTs Garcia‐Olmo 2009; Hawkey 2015; Melmed 2015; Molendijk 2015; Panes 2016; Zhang 2018; Zhou 2020) for the final quantitative meta‐analysis. We found two studies (Arturo 2017 and Lichtiger 2012) presented as abstract‐only data which we moved to Studies awaiting classification.
Included studies
We ultimately included seven RCTs for meta‐analysis Garcia‐Olmo 2009; Hawkey 2015; Melmed 2015; Molendijk 2015; Panes 2016; Zhang 2018; Zhou 2020) for the final qualitative and quantitative analysis with their secondary reports and online supplements assessed for further data. All data are shown in detail in the Characteristics of included studies section. We found multiple reports of the same study for (Garcia‐Olmo 2009; Hawkey 2015; Molendijk 2015; Panes 2016). We included the long‐term follow‐up data from these records and mentioned the relevant references in the results.
Two of the included studies are awaiting classification (Arturo 2017; Lichtiger 2012), both were presented with an abstract‐only publication, without enough data to assess in the current review. (Lichtiger 2012) is a double‐blinded RCT with a subsequent compassionate open‐label trial on six patients (in the intervention group) with ileocolitis and intestinal CD. The patients were given four doses of remestemcel‐L (a commercial type of mesenchymal stem cell prepared from healthy young adults' bone marrow aspirate). The infused doses ranged from 0 to 400 million cells per dose, four subsequent infusions were given over the duration of several months. The study reported five of the six patients having a clinical response, with four of the six patients having improved CDAI >100 points and no reported adverse events. (Arturo 2017) is a single‐centre phase II open‐label RCT using autologous expanded bone marrow‐derived mesenchymal stem cells (axBM‐MSC), conducted on 26 patients. The routes of injection were the mesenteric arteries (superior and inferior), through colonic endovascular catheterization. They reported symptomatic improvement and lowering of the CDAI score in the intervention group as compared to the control group.
1‐Study design, setting, and duration
1.1 Phases of the included trials
Two of the trials were phase III studies (Hawkey 2015; and Panes 2016) while five were phase I‐II studies (Garcia‐Olmo 2009; Melmed 2015; Molendijk 2015; Zhang 2018; Zhou 2020).
Two studies were dose‐escalation studies with a placebo arm; (Molendijk 2015) included three arms; one placebo and three intervention groups with different stem cell doses, and (Melmed 2015) included two arms of intervention and one in placebo. The other five studies (Garcia‐Olmo 2009; Hawkey 2015; Panes 2016; Zhang 2018 ; Zhou 2020) consisted of two arms only one intervention and one control or placebo.
1.2 Study duration and follow‐up period
The study duration ranged from one year to four years in (Hawkey 2015) with a follow‐up duration period ranging from one to two years.
1.3 The countries and the number of centres included in the trials
Four of the studies were multicentre trials (Garcia‐Olmo 2009; Hawkey 2015; Melmed 2015; Panes 2016), while three (Molendijk 2015; Zhang 2018; Zhou 2020) were uni‐centre trials one in the Netherlands and two in China, respectively. The multicentre trials were also multinational trials in (Hawkey 2015; Panes 2016). Panes contained the largest number of centres with 49. Most of the studies were carried out in Europe with one in the USA (Melmed 2015) and one in China (Zhang 2018), also (Panes 2016) included Israel in their multinational European trial.
2‐ Participants
We included seven RCTs. The total number of participants was 442 with 234 in the stem cell group and 208 in the placebo or control groups, with around 50% of the participants included in one multicentre RCT (Panes 2016). Studies were conducted in the UK, China, Spain, the Netherlands, and the USA.
2.1 Treatment after recurrence versus treatment‐naive participants
All participants were refractory to the primary medical treatment and no treatment‐naive patients were recruited. The inclusion criteria ranged from moderate to severe cases of Crohn's disease either luminal or fistulising disease, no patients with mild disease, or already on remission in both categories were included.
All participants (both intervention and control groups) received concomitant therapy according to the study protocols as stated in details in (additional Table 2).
2.2 The age of the participants:
All the studies excluded pregnant women. Only one study included children (Zhou 2020), where the age of the included participants ranged from 12 to 51 years old. In the rest of the studies; the minimum age for inclusion was 18 years old and was stated clearly in the studies. The (Interquartile range (IQR) and median) or (mean and standard deviation SD) of individual studies are shown in (Additional Table 2). Mean and median ranged from (30 to 44) years old i.e. around 31 years old. Only three of the studies set a limit on the maximum age for the inclusion of the participants; in (Melmed 2015), it was 75 years old and in (Hawkey 2015), it was 50 years old.
2.3 The gender of the participants
From (Garcia‐Olmo 2009), we included only the subgroup of 14 people with Crohn's disease; their gender distribution was not reported separately (total numbers 24 males and 25 females). Also, in (Melmed 2015) there was an open Phase Ib non‐randomised one arm trial on four participants and Phase IIa trial on 46 participants; we only included the latter group. The rest of the studies with data on the number of male/female participants included a total number of 428 patients (Hawkey 2015; Molendijk 2015; Panes 2016; Zhang 2018; Zhou 2020) with 240 males (56.07%) and 188 females (43.93%).
There was no difference between the control and intervention groups regarding gender. The intervention group included 127 males (55.95%) and 100 females (44.05%), while the control/placebo group included 114 males (56.44%) and 88 females (43.56%).
3‐ Interventions
3.1 Type of control used in the trials
The placebo in the local injection was saline only as in (Panes 2016) or a combination of saline and albumin infusion without any cells in (Molendijk 2015). Unfortunately in (Panes 2016), masking of the treatment was not achieved because the consistency of the stem cell suspension was apparently different from the saline solution.
The fibrin‐glue in (Garcia‐Olmo 2009) was used as a background treatment for both the intervention and the control groups.
As for systemic infusion, the placebo group received a "vehicle control without any cells" in (Melmed 2015).
While (Hawkey 2015; Zhang 2018; Zhou 2020) did not state any placebo taken with the standard background treatment. Participants in the control group of Hawkey 2015 received SCT with a delay of one year as compared to the intervention group (cross‐over trial).
3.2 Autologous versus allogenic
The intervention was autologous stem cells in three trials; in the study of (Hawkey 2015); it was extracted from bone marrow stem cells and in both Garcia‐Olmo 2009 and Zhou 2020, the cells were extracted from adipose tissue through liposuction. The stem cells used were allogeneic in four trials (Melmed 2015; Molendijk 2015; Panes 2016; Zhang 2018). The used cells were commercially available (cenplacel‐L)‐ Human placenta‐derived cells (PDA‐001) in (Melmed 2015 ) from placental tissues, in (Panes 2016) Allogeneic, expanded, adipose‐derived stem cells (Cx601) cells‐ commercial cells through Human lipo aspirate from donor liposuction. While prepared allogeneic were from the umbilical cord of a newborn (Expanded Umbilical Cord Mesenchymal Stem Cells (UC‐MSCs)) in (Zhang 2018), and from five different donors of bone marrow aspirate in(Molendijk 2015) where each patient received their SCs doses from a single donor.
We identified no direct comparisons of autologous versus allogeneic stem cells and had insufficient data to explore this via subgroup analyses.
3.3 The route of administration
The route of administration for three studies was systemic infusion (Hawkey 2015; Melmed 2015; Zhang 2018), while four received a local injection into the fistula (Garcia‐Olmo 2009; Molendijk 2015; Panes 2016; Zhou 2020).
3.4 The doses of the stem cells
The doses varied among studies; (Garcia‐Olmo 2009) started with 2 million cells if no healing at eight weeks another dose of 4 million cells was given.
In (Molendijk 2015); Group I; 10 million, Group II; 30 million, and Group III; 90 million. The response according to the dose varied. The change in the PDAI score decreased from 4.4 to 1.8 in the first group after 24 weeks, while the decline in the second group was most apparent on week 12. Oddly enough there was no decline in the highest dose group of 90 million, which may indicate that a moderate dose of stem cells could be the most appropriate for local injection, but the number of participants was very small (five in each of the three intervention arms).
On the other hand, (Melmed 2015) had two arms of intervention in comparison to the placebo arm. Group I received 150 million cells (1 unit) and Group II received 600 million cells (4 units) and the dose was repeated after one week. The clinical response at four and six weeks; defined as the drop of CDAI by > or = 100 points and/or 25% decline. This was achieved in both arms of the intervention group regardless of the dose used in (10/28) patients ie 36% as compared to the placebo group where 0% achieved clinical remission P value = 0.026, but clinical remission after four and six weeks was achieved in (4/28) patients i.e. 14% in the intervention group versus 0% in the placebo group withP value = 0.3.
In (Panes 2016), the patients received 120 million cells in a single injection, while in (Zhang 2018) the patients received 1.6 million cells/ kg body weight once weekly with a total of four doses. In (Hawkey 2015) a minimum dose of 3 million cells/kg on day 7.
In (Zhou 2020) each fistula was injected with a different dose according to its diameter and length (< 1 cm injected with 1 mL, 1‐2 cm injected with 2 mL). The suspension contained 5 million cells/ml of Adipose‐derived stem cell (ADSC). Multiple injections were performed in all quadrants of the fistula.
3.5 The type of stem cells
Different studies used different types of stem cells; adipose tissue‐derived stem cells (a type of mesenchymal stem cells) in (Garcia‐Olmo 2009 and Zhou 2020), mesenchymal stem cells in (Molendijk 2015; Panes 2016; Zhang 2018), placental‐derived mesenchymal‐like stem cells (PDA‐001) in (Melmed 2015), and haematopoietic stem cells CD34+ in (Hawkey 2015).
3.6 Methods of preparation
Methods varied across the studies due to the different types of cells used. Conditioning was only used with Hawkey 2015.
4‐ Results of Sensitivity Analysis
We carried out sensitivity analysis for the all the primary outcomes as shown in (Appendix 8); clinical remission, CDAI < 24 weeks, fistula closure short term and long term, but we found no differences when using random‐effects versus fixed‐effect models. In addition, our conclusions remained unchanged when excluding high and unclear risk studies. This could be attributed to the weight of the study (Panes 2016)which has the largest population studied, with more than 50% of the patients, and also has a low risk of bias.
First, for clinical remission we found that there was a positive effect of the intervention as opposed to the control; risk ratio (RR) was 1.88, 95% confidence interval (CI) 0.80 to 4.41 with a random‐effects model, RR was 1.47, 95% CI [1.10 to 1.95] with a fixed‐effect model, and RR was 1.41 95% CI [1.06 to 1.88] after removing the high risk of bias study Melmed 2015 (for high risk of bias for randomisation and allocation).
Second, for CDAI <1 50, we found that there was no difference between the intervention and control groups after conducting sensitivity analysis, RR was 1.02, 95% CI [0.67 to 1.56] with random‐effects model, RR was 1.08, 95% CI [0.94, 1 to 24] with fixed‐effect model, and RR was 1.02, 95% CI [0.67 to 1.56] after removing the high risk of bias study Melmed 2015 (for unclear allocation and high risk of bias performance and detection blinding).
Third, for the outcome fistula closure, short term we found a positive effect of the intervention than the control after conducting sensitivity analysis, RR was 1.48, 95% CI [1.12 to 1.96] random‐effects model, RR was 1.53, 95% CI [1.15 to 2.03] fixed‐effect model, and RR was 1.47, 95% CI [1.07 to 2.01] after removal of high‐risk studies Garcia‐Olmo 2009 and Zhou 2020 (for unclear allocation and high risk of bias for blinding and detection).
Fourth, for the outcome fistula closure, long term we found a positive effect of the intervention after conducting sensitivity analysis RR was 1.42, 95% CI [1.09 to 1.87] for random‐effects model, RR was 1.47, 95% CI [1.12 to 1.94] for a fixed‐effect model, and RR was 1.48, 95% CI [1.10 to 1.99] after removal of high risk of bias studies Garcia‐Olmo 2009 and Zhou 2020 (for unclear allocation and high risk of bias for blinding and detection).
Excluded studies
We excluded three studies due to the absence of a placebo or control group (Cho 2013; Park 2014; Onken 2008; Dige 2019; Serrero 2019). In addition, three non‐randomised studies (Lazebnik 2010; Kagramanova 2016; Knyazev 2015) were excluded. Other reports of the same included studies were excluded as a primary study, but data were included in their primary studies as follow‐up data. One trial was withdrawn as the authors found that the protocol was not reflective of the clinical situation in question (FATT‐2 trial), thus was added to the excluded studies. For reasons of exclusion see (Figure 1).
Ongoing studies
We found nine ongoing studies (EUCTR2017‐000725‐12‐CZ; ISRCTN17160440; NCT00482092; NCT04010526; NCT04519671; NCT04519684; NCT04519697; NCT04548583; NCT04612465). For full characteristics of ongoing trials kindly see Characteristics of ongoing studies
Risk of bias in included studies
The graphical presentation of the risk of bias is presented in Figure 2 and Figure 3. Further details are presented in the Characteristics of included studies.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The random sequence generation method was clearly reported in five studies (Garcia‐Olmo 2009; Hawkey 2015; Molendijk 2015; Panes 2016; Zhang 2018) and was unclear in two (Melmed 2015 and Zhou 2020).
It was mentioned that centralised randomisation was carried out in (Garcia‐Olmo 2009; Hawkey 2015, and Panes 2016), but the method of allocation was not mentioned in ( Garcia‐Olmo 2009) so it was judged as unclear. It was computer‐generated in Zhang 2018, while in Molendijk 2015 randomisation was done in the immunology department with the researcher having no contact or knowledge of the patients entering the study.
Three trials clearly reported the allocation concealment method through having centralised randomisation Panes 2016 and Hawkey 2015), and one study (Molendijk 2015) mentioned that the researcher responsible for randomisation is not in contact with, or has knowledge of, the patients. Four studies had an unclear method of allocation concealment (Garcia‐Olmo 2009; Melmed 2015; Zhang 2018; Zhou 2020).
Blinding
Four of the studies had a high risk of performance bias as they were open trials with un‐blinding of the participants (Garcia‐Olmo 2009; Hawkey 2015; Zhang 2018; Zhou 2020). Three trials using placebo as a method of blinding but only two trials had a low risk of performance blinding (Melmed 2015; Molendijk 2015), while in the third trial (Panes 2016) the risk was high as the authors stated that the saline used was apparent to contain no cells which caused the unblinding of the participants and personnel.
On the other hand, five trials (Garcia‐Olmo 2009; Hawkey 2015; Melmed 2015; Molendijk 2015; Panes 2016) stated clearly that they had a low risk of detection bias, while in two trials (Zhang 2018 and Zhou 2020) the risk of detection bias was high, as the trials were open‐label, and no specific measure for blinding of the assessors was mentioned.
Incomplete outcome data
All the seven trials had a low risk of attrition bias (Garcia‐Olmo 2009; Hawkey 2015; Melmed 2015; Molendijk 2015; Panes 2016; Zhang 2018; Zhou 2020).
All the seven studies mentioned that they performed an ITT analysis (Garcia‐Olmo 2009; Hawkey 2015; Melmed 2015; Molendijk 2015; Panes 2016; Zhang 2018; Zhou 2020).
In (Hawkey 2015); eight patients withdrew from the placebo group before completing the one‐year follow‐up period due to the flare‐up of their disease and the need for either surgical intervention or early transplantation, and one patient withdrew after the randomisation. In the intervention group; one patient withdrew, and one patient died 20 days after the intervention. Although this created unbalance in the two groups, our primary outcomes measured (CDAI at 24 weeks and clinical remission) were not affected as the data on both were present in the primary study. The withdrawal of patients affected the outcome CDAI after one year and the authors had undergone implementation assuming the worst‐case scenario.
In the case of long‐term fistula follow‐up >1 year, we considered the attrition as high when assessing the certainty of evidence in the Table 1. This is because all four studies have a high percentage of patients lost in the long‐term follow‐up (Panes 2016; Molendijk 2015; Garcia‐Olmo 2009; Zhou 2020).
Selective reporting
We examined the trial registries against the published manuscript of the included studies to observe any differences in the following; the type of study, the collection sample, and the assigned primary outcomes. The trial registry of each of the included studies is presented in the Characteristics of included studies.
Five studies (Hawkey 2015; Melmed 2015; Molendijk 2015; Panes 2016; Zhang 2018) had a low risk of selective reporting. Garcia‐Olmo 2009h had an unclear risk of selective reporting. Zhou 2020 had a high risk of selective reporting as the protocol of the study stated that the study is a case‐control trial with 22 participants in each arm. The published study included only 11 participants in each arm without mentioning the cause of the decline in the sample size. Also, the published paper stated that it was a randomised controlled open‐label trial.
Other potential sources of bias
The overall judgment was a high risk of bias in(Garcia‐Olmo 2009). The randomization included 50 participants with a range of diseases and this review relied on data from a subgroup of the 14 participants with Crohn's disease, this caused a judgment of unclear risk of bias. Also, there is a high risk of funding bias in this study due to sponsoring by (Cellerix) company where the Primary investigator holds a chair in the company and on the advisory board, he also holds two patents of the Cx401 cellular composition.
In (Melmed 2015) there was a high risk of bias attributed to the suspension of the study before reaching the statistical power due to safety events, with the trial sponsored by a pharmaceutical company (Celularity Incorporated). In Panes 2016)there was a high risk of bias due to funding conflict, where the funder (TiGenix) had a role in the study design, data collection, analysis, interpretation, and writing of the final report of the study.
In addition, in Zhang 2018 there was a low risk of bias as the stem cells were obtained from a single donor, so the variation of results due to variation of donors was expectedly low. In other included RCTs using allogeneic stem cells; this risk of bias was unclear as in (Melmed 2015) it was placental tissue, and in (Panes 2016) it was commercially available mesenchymal cells, so we do not know if the cells came from single or multiple donors. In Molendijk 2015 there is a high risk of bias as the mesenchymal stem cells were prepared from five separate donors.
However, in the other three studies (Hawkey 2015; Molendijk 2015) no risk of other forms of bias was detected. (Zhou 2020) included mainly male participants (21 males versus 1 female).
Effects of interventions
See: Table 1
The main comparisons of stem cells versus placebo for medically refractory Crohn's disease‐Primary outcomes, Summary, and long‐term effects of the intervention are shown in Table 1and additional Table 2.
1‐Primary Outcomes
1.1 Clinical remission as defined by the primary studies
Clinical remission as defined by the primary studies is presented in Figure 4; Analysis 1.1. More people achieved clinical remission with stem cell therapy (SCT) than with placebo/no SCT, (risk ratio (RR) 1.88, 95% confidence interval (CI) 0.80 to 4.41), studies = 3; participants = 301; low certainty of evidence; Analysis 1.1).
4.

1.1. Analysis.

Comparison 1: Stem cells versus Control, Outcome 1: Stem cell therapy versus placebo or control, Outcome: Clinical remission
The definition was variable; in Hawkey 2015 it was defined as “free of active disease", the number of patients in each group was identical to Crohn’s Disease Activity Index (CDAI) < 150. In (Panes 2016) it was defined as clinical remission at 24 weeks with “closure of all treated external openings that were draining at baseline despite gentle finger compression”. In Melmed 2015, clinical remission induction was considered when CDAI ≤150 at four and six weeks.
1.2 Clinical improvement by CDAI < 150
The time frame assessment was different including 12 and 24 weeks. CDAI <150 at 24 weeks was presented Analysis 1.2 in four studies (Hawkey 2015; Molendijk 2015; Panes 2016; Zhang 2018) with (RR‐1.02 95% CI [0.67 to 1.56]; studies = 4; participants = 352; very‐low certainty of evidence; Analysis 1.2), and I2 68%; the impact of SCT is uncertain as the CIs are wide and cross the line of no effect.
1.2. Analysis.

Comparison 1: Stem cells versus Control, Outcome 2: CDAI <150 at 24 weeks
Only two studies had patients with intestinal Crohn’s disease (CD) (Hawkey 2015; Zhang 2018). (Hawkey 2015) showed improved CDAI after stem cells as compared to the control with (RR‐3.83 95% CI [0.91 to 16.07]; participants = 45), but the size of the study population was small with only 7.5% weight. While the RR could not be calculated in Zhang 2018 as none of the patients reached CDAI < 150 in the two groups (intervention and control). Molendijk 2015 and Panes 2016 both aimed at the treatment of CD fistula, not the intestinal CD, thus, the baseline CDAI in both studies was low, with no significant change after the intervention.
1.3 Fistula closure
Fistula closure was assessed in the studies dealing with perianal CD (Garcia‐Olmo 2009; Molendijk 2015; Panes 2016; Zhou 2020) with long‐term data available for all four studies.
The short‐term outcome presented in [Analysis 1.3, Figure 5] was assessed at eight weeks in (Garcia‐Olmo 2009)and 24 weeks in (Molendijk 2015; Panes 2016; Zhou 2020). More people had fistula closure with SCT than with placebo/no SCT, (RR 1.48, 95% CI [1.12 to 1.96], studies = 4; participants = 269; low certainty of evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Stem cells versus Control, Outcome 3: Stem cell therapy versus placebo or control, Outcome: Fistula Closure short‐term
5.

The result for longer‐term fistula closure (one to four years) presented in the primary study in Zhou 2020 or on follow‐up reports of the primary studies by the same authors in (Guadalajara 2012; Panés 2018; Barnhoorn 2020) is presented in[Analysis 1.4, Figure 6]. More people had fistula closure in the longer term with SCT than with placebo/no SCT, (RR 1.42, 95% CI [1.09 to 1.87], studies = 4; participants = 250; low certainty of evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Stem cells versus Control, Outcome 4: Stem cell therapy versus placebo or control, Outcome: Fistula closure in long‐term Follow up of original studies
6.

2‐Secondary Outcomes
2.1 Clinical improvement
Regarding the Perianal Disease Activity Index (PDAI) score; there was a change in the score from the baseline presented at 12 and 24 weeks in three studies (Molendijk 2015; Panes 2016; Zhou 2020), where PDAI at 12 weeks I2 = 82%. Therefore, a forest plot was done for PDAI after 12 weeks with no pooling of the results as stated in the protocol for any I2 more than 75%, for more detail on sensitivity analysis (please see Analysis 1.5). PDAI at 24 weeks was associated with a mild decrease of (RR ‐0.35, 95% CI ‐1.57 to 0.86), studies = 3; participants = 247; Analysis 1.6). I2 = 39% at 24 weeks.
Regarding the Crohn’s Disease Activity Index (CDAI) score ; we found data on the change in the score from baseline at 24 weeks of CDAI presented in the studies (Hawkey 2015; Molendijk 2015; Panes 2016; Zhang 2018. There was a decrease in the score after 24 weeks. I2 = 96% was high due to heterogeneity among studies, so a forest plot was done without pooling of the results as stated in the protocol for any I2 more than 75% [Analysis 1.7]. In (Melmed 2015) the outcome was not analysed quantitatively, the clinical response was defined as a decline > 100 points in the CDAI score and was achieved in 10 patients out of 28 in the intervention group versus none in the placebo group.
Data on change of Harvey‐Bradshaw Index (HBI) after 12 months was extracted from two studies (Hawkey 2015; Zhang 2018). There was a decline with intervention by (RR‐2.59, 95% CI ‐4.04, to1.14, studies = 2; participants = 124; Analysis 1.8), and I2 of 40%. The total number of participants was 62 in the stem cell group and 62 in the placebo group.
1.5. Analysis.

Comparison 1: Stem cells versus Control, Outcome 5: PDAI Change at week 12
1.6. Analysis.

Comparison 1: Stem cells versus Control, Outcome 6: PDAI change at week 24
1.7. Analysis.

Comparison 1: Stem cells versus Control, Outcome 7: CDAI change at 24 weeks
1.8. Analysis.

Comparison 1: Stem cells versus Control, Outcome 8: Harvey Bradshow Index (HBI) change after 12 months
2.2 Endoscopic scores
We found data for different scores used in different studies. Only three studies used endoscopic scores for patients' assessment. In Hawkey 2015; the Simple endoscopic score for Crohn's disease (SES‐CD) was used while in Molendijk 2015, and Zhang 2018 the Crohn's Disease Endoscopic Index of Severity (CDEIS) score was used. The change in the score was measured after 12 weeks. There was no change in the score after treatment. Data were presented with forest‐plot only and no pooling was done as the I2 was 92% (I2 > 75%was not conducted according to the protocol methodology) as shown in [Analysis 1.9].
1.9. Analysis.

Comparison 1: Stem cells versus Control, Outcome 9: Endoscopic score change after 12 weeks
2.3 Adverse events
We assessed total and serious adverse events as defined by the original studies.
2.3.1 Data on total adverse events
This was mentioned in four studies (Hawkey 2015; Molendijk 2015; Panes 2016; Zhou 2020) with (RR 0.99, 95% CI [0.88 to 1.13], studies = 4; participants = 293; very low certainty of evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Stem cells versus Control, Outcome 10: Total Adverse Events
We presented adverse events as the number of patients suffering from one or more adverse events as dichotomous outcomes only. In (Hawkey 2015) "the count data" of the total number of events per patient (events/patients) was presented in the study, so we converted it to a dichotomous outcome (number of patients suffering from adverse events versus the number of patients not suffering from adverse events). In (Molendijk 2015; Panes 2016) the data were originally presented as the number of patients suffering from adverse events, thus no conversion was performed.
The reported adverse events varied across studies; they included; headache, pyrexia, local reactions at the site of injection, non‐cardiac chest pain, upper respiratory tract infection, anaemia, leukopenia, thrombocytopenia, phlebitis, infections (viral, bacterial), nausea, and vomiting, abdominal pain, diarrhoea, and fistula.
In Hawkey 2015), the adverse events were more frequent (76 in 19 patients) in the intervention group as compared to the placebo group (38 in 15 patients).
In Panes 2016, it was reported that 53 patients in the intervention group developed antibodies to human leukocyte antigen (HLA) class‐I while none in the placebo group developed those donor‐specific antibodies, but no immune reaction occurred and also there was no relation between the treatment response and the antibody response.
In (Zhou 2020) total adverse events were found in 7/11 patients in the intervention group versus 11/11 patients in the control group.
2.3.2 Data on serious adverse events
This was found in all the seven studies with (RR 1.22, 95% CI 0.88 to 1.67, studies = 7; participants = 433; low certainty of evidence; Analysis 1.11).
1.11. Analysis.

Comparison 1: Stem cells versus Control, Outcome 11: Serious Adverse Events
Serious adverse events reported included; systemic hypersensitivity to the intervention, gastric ulcer perforation, Crohn’s disease flare, pneumonia, anal or perianal abscess, proctalgia, liver abscess.
Only (Zhang 2018 and Zhou 2020) reported the absence of serious adverse events in both the intervention and the control groups (zero events).
It has to be noted that the trial of Melmed 2015 stopped before enrolling the last two participants due to safety issues. As explained by the authors of Melmed 2015, the safety issues were reported in another study on rheumatoid arthritis (RA) patients, and those who received the intervention suffered from the following: (one patient had retinal artery spasm and one patient had an attack of acute myocardial infection), which led to the suspension of the RA trial. Other serious adverse events reported in (Melmed 2015); included anal (after 8.5 months) and colon cancer (after 74 days) on the long‐term follow‐up. The authors suspected that the cancers were related to the original disease, not the intervention, and argued that the duration of CD in those two patients was 46.8 years and 33.4 years, respectively.
In Molendijk 2015, one patient developed adenocarcinoma of the cecum with peritoneal carcinomatosis after 15 months from intervention and with a family history of colon cancer.
In Garcia‐Olmo 2009, one patient in the placebo (fibrin glue injection) group reported a flare of Crohn’s disease with intra‐abdominal abscess and recovered on medical treatment.
2.4 Withdrawal due to adverse events
Withdrawal due to adverse events was only reported in three studies with a total number of participants of 250; 124 in the placebo group and 126 in the intervention group. In the first study Hawkey 2015, one patient in the active and one patient in the placebo group. The second study was (Panes 2016), there were five patients from the active group and six patients in the placebo group who withdrew due to adverse events. In Zhou 2020, two patients in the intervention group and three patients in the control group withdrew due to adverse events and went on to receive a re‐operation, (RR0.78, 95% CI 0.32 to 1.89, studies = 3; participants = 272; very low certainty of evidence; Analysis 1.12).
1.12. Analysis.

Comparison 1: Stem cells versus Control, Outcome 12: Withdrawal due to adverse events
In Hawkey 2015, the authors stated that in the control group eight patients withdrew due to treatment failure, and underwent subsequent surgery or early transplantation, one patient died after 20 days from administration of the intervention.
2.5 All‐Cause mortality
We found data for all‐cause mortality in all seven studies with a total number of participants of 440 patients; 234 in the intervention group and 206 in the control/placebo group. No patients died in the control/placebo group, while two patients died in the intervention group. One died in Hawkey 2015, while the other case was in Molendijk 2015 where one patient died from a cancer rectum in the long follow‐up study (Barnhoorn 2020).
In Hawkey 2015), one patient died after 20 days from the intervention by sinusoidal obstructive syndrome diagnosed post‐mortem. He was suspected to have intraperitoneal sepsis and laparotomy was done but was negative, then he developed acute liver failure with no apparent history or previous biochemical data to support a definitive diagnosis.
The effect estimate as calculated by (Peto odds ratio (OR) 5.51, 95% CI 5.51 [0.30, 101.02], studies = 7; participants = 440; Analysis 1.13).
1.13. Analysis.

Comparison 1: Stem cells versus Control, Outcome 13: All cause mortality
2.6 Quality of life scores‐ change after 12 weeks in inflammatory bowel disease Questionnaire (IBDQ) score
Quality of life was reported in four studies (Hawkey 2015; Molendijk 2015; Panes 2016; Zhou 2020) with a total number of participants of 292; 152 in the intervention group and 140 in the placebo/control group. Different scores were used, but we found enough data for meta‐analysis of the IBDQ score, it was assessed both at baseline and at 12 weeks in (Hawkey 2015) and at 24 weeks in (Molendijk 2015; Panes 2016; Zhou 2020). The change in the score was calculated using the standardised mean difference (SMD); with (SMD 0.25, 95% CI 0.25 [‐0.18, 0.68], studies = 4; participants = 292; Analysis 1.14). The heterogeneity was I2 = 50%.
1.14. Analysis.

Comparison 1: Stem cells versus Control, Outcome 14: Quality of life score (change from base line) IBDQ
2.7 Additional outcomes found but not mentioned in the protocol methodology (C‐reactive protein (CRP), cytokines, and fecal calprotectin)
In Molendijk 2015, the mean levels of cytokines did not differ from baseline to after intervention event with clinical response.
In Melmed 2015, it was noted that only fecal calprotectin was decreased with the clinical response but CRP and cytokines showed no change.
This laboratory outcome is important in the daily clinical practice for patients' assessment at baseline and follow‐up.
Discussion
All of the randomised controlled trials (RCTs) included patients with Crohn's disease (CD) refractory to one or more standard medical treatment, with variability from moderate to severe activity, either colitis or fistulising disease. As the main concept is not to expose treatment‐naive and responding‐patients to unknown risks. So in naive patients to treatment data are lacking. This is in accordance with the latest European Union guidelines in which Darvadstrocel (a suspension drug of expanded allogeneic adipose‐derived stem cells (eASCs)) was approved after the results of the Adipose‐Derived Mesenchymal Stem Cells for Induction of Remission in Perianal Fistulizing Crohn's Disease (ADMIRE‐CD) trial (Panes 2016), with subsequent approval in the UK, Switzerland, and Israel. The mesenchymal stem cells (MSCs) are indicated for treatment in case of the refractory disease to at least one standard or biological therapy for CD with mild or non‐active disease status, and prior conditioning of the fistula has been performed (Takeda 2021, Scott 2018). A step‐wise algorithm was proposed in (Guadalajara 2020) for using the approved Darvastrocel. Also, a protocol was outlined for standardisation of MSC dosing in perianal disease associated with CD (Molendijk 2018).
Summary of main results
We included for the final qualitative and quantitative analysis seven RCTs; the total number of participants was 442, with 234 in the stem cell group and 208 in the placebo or control groups. It has to be noted that about 50% of the participants were from one RCT (Panes 2016).
Three RCTs assessed the main outcome of clinical remission (Hawkey 2015, Melmed 2015, Panes 2016). Clinical remission was achieved in the intervention group more than the control group with low certainty of evidence. But the definition of clinical remission was variable across studies, where two of the included studies (Hawkey 2015; Panes 2016) were open‐labelled with most of the studied population in (Panes 2016). Clinical remission and response are followed for a variable duration among studies (4, 6,12, 24 etc. weeks). Our results show that clinical remission may be achieved with the stem cell therapy (SCT) more than placebo or control, and there was no difference after conducting the sensitivity analysis for random‐effects versus fixed‐effect models or omitting high‐risk studies.
Four RCTs assessed the achievement ofCrohn’s Disease Activity Index ( CDAI) <150 at 24 weeks (Hawkey 2015; Molendijk 2015; Panes 2016; Zhang 2018), although the CDAI decrease was not the primary outcome in most of the studies. There was no apparent CDAI change after 24 weeks of the intervention. (Hawkey 2015) was the only study showing improved CDAI to < 150 after the 24 weeks from the administration of the SCT as compared to the control, however, the study weight was only 7.5% due to the small size of the studied population. While (Zhang 2018) stated that none of the patients reached the target CDAI at 24 weeks, so no risk ratio (RR) could be calculated for this study. In addition, two of the four included studies presented with normal baseline CDAI as they were examining the fistulising, not the inflammatory intestinal, a subcategory of CD fixed‐effect( Molendijk 2015 and Panes 2016). The level of certainty of the evidence was very low. Our results show that CDAI < 150 at 24 weeks may show no difference with the SCT as compared to the placebo or control, and there was no difference after conducting the sensitivity analysis for random‐effects versus fixed‐effect models or omitting high‐risk studies.
Four RCTs assessed the early fistula closure (Garcia‐Olmo 2009; Molendijk 2015; Panes 2016; Zhou 2020), although the time of determining the outcome was different, where (Garcia‐Olmo 2009) was at eight weeks, while (Molendijk 2015; Panes 2016; Zhou 2020)were at 24 weeks. It was assessed both clinically and with magnetic resonance imaging (MRI) with low certainty of evidence. Our results show that fistula closure in the short term may be achieved with the stem cell therapy more than placebo or control, and there was no difference after conducting the sensitivity analysis for random versus fixed‐effect models or omitting high‐risk studies.
Four RCTs assessed the long‐term follow‐up of fistula closure after one to several years (Zhou 2020; Panés 2018; Barnhoorn 2020; Guadalajara 2012) are presented. The number of patients was 250 with low certainty of evidence. The data were collected from published papers with long‐term follow‐up of the original studies (Zhou 2020; Panes 2016; Molendijk 2015; Garcia‐Olmo 2009), respectively. In Molendijk 2015, long‐term follow‐up study (after four years) (Barnhoorn 2020), three of the placebo group received SCT after two years from the original study. One study (Zhou 2020) stated both the short‐ and long‐term follow‐up after one year results of fistula healing. It was noticed that attrition was high in all studies in long‐term follow‐up of fistula healing, which resulted in a downgrading of the certainty of evidence to low. Our results show that fistula closure long term may be achieved with the SCT more than placebo or control, and there was no difference after conducting the sensitivity analysis for random‐effects versus fixed‐effect models or omitting high‐risk studies.
There were only two dose‐escalation studies (Melmed 2015; Molendijk 2015) where the effect on fistula closure was decreased by increasing the dose of stem cells in (Molendijk 2015), or no effect to increasing the dose in (Melmed 2015). Further analysis is needed to determine the exact effect of increasing the dose of stem cells on fistula healing.
The total number of adverse events was assessed in four studies (Hawkey 2015; Molendijk 2015; Panes 2016; Zhou 2020). There was no difference between the intervention and the control groups regarding the number of adverse events, with very low certainty of evidence.
All seven RCTs assessed the number of patients with serious adverse events (Garcia‐Olmo 2009; Hawkey 2015; Melmed 2015; Molendijk 2015; Panes 2016; Zhang 2018; Zhou 2020), although the definition was different across studies. This was expected as most of the studies are phase II dealing mainly with safety issues as the primary outcome. The outcome had a low certainty of evidence. There were different definitions of what constitutes a serious adverse effect. Autologous stem‐cell transplantation in treatment‐refractory Crohn's disease (ASCTIC) trial authors (Hawkey 2015) concluded that haematopoietic stem cells (HSCs) did not achieve clinical remission and were associated with increased harm due to adverse events, thus there are no data to support the continuation of the trial on a large scale. In haematopoietic stem cell transplantation (HSCT), infection was the most dangerous and common adverse event. This is related to pancytopenia resulting from conditioning chemotherapeutic regimen, which as the authors of the original study (Hawkey 2015) declared, could be the cause of death of the patient who died from sinusoidal obstruction syndrome. It is mentioned in Melmed 2015 that the study was stopped due to safety issues in a concomitant study using PDA‐100 stem cells, but we did not find any publication on the topic stated by the authors "Two SAEs were seen in a study of rheumatoid arthritis and led to the suspension of enrollment in this study". Thus safety issues concerning PDA‐100 have to be addressed further. Only one patient died during the conduct of the study in all the six studies, the patient was in the intervention arm of (Hawkey 2015). While only in one of the included studies (Molendijk 2015) one patient died from cancer cecum on long‐term follow‐up study by (Barnhoorn 2020).
Only three studies reported on the patients' withdrawal caused by adverse events (Hawkey 2015; Panes 2016; Zhou 2020). We found that the withdrawal of patients due to adverse events was higher in the control group compared to the intervention group with very low certainty of evidence.
Overall completeness and applicability of evidence
There is an apparent heterogeneity among the studies regarding; the doses and types of stem cells (SCs) used, route of administration of the intervention, outcome measures, and follow‐up duration, adverse events reported, concomitant medications given to participants, and definition of refractory disease for study inclusion.
There is an age restriction for children and also pregnant women due to safety and ethical issues related to SCs. In addition, the elderly were restricted in (Hawkey 2015) (age < 50 years) and in(Melmed 2015 ) (< 75 years). No gender restriction was observed among the trials, but there was inequality in the female/male ratio in (Zhou 2020 ) where only one female was included.
The effect of using multiple donors versus a single donor as a source ofSCs, and autologous versus allogeneic stem cells on the immune system is still an unresolved issue.
The local injection into the fistula could be more beneficial, and with fewer side effects when compared to systemic infusion, especially when using mesenchymal stem cells (MSCs).
Crohn’s Disease Activity Index (CDAI) is a subjective outcome (symptom dependent) through questionnaires, and most of the studies lack performance blinding which makes the certainty of evidence very weak. A proposal of other objective outcomes is more proper to use in clinical trials using stem cells in refractory CD. On the other hand; fistula closure is assessed clinically or by MRI which is more objective.
Laboratory cytokines and inflammatory markers are not changed in relation to the clinical response in early follow‐up, but it could more relevant if used in the long term.
Quality of the evidence
We used the GRADE approach (GRADEpro GDT) to assess the certainty of the evidence for the comparison between stem cell therapy and placebo for the induction of remission of medically refractory CD. We used the GRADEPro integration in RevMan Web 2020.
There were significant problems with risk of bias lead to downgrading risk of bias; most of the studies had a high or unclear risk of bias of randomisation (two unclear Melmed 2015 and Zhou 2020), allocation concealment (four unclear Garcia‐Olmo 2009Melmed 2015; Zhang 2018; Zhou 2020), and blinding (five high risk of bias Garcia‐Olmo 2009; Hawkey 2015; Panes 2016; Zhang 2018; Zhou 2020). However, most of the unblinded studies had a low risk of detection bias except for two studies (Zhang 2018; Zhou 2020). This leads to downgrading the risk of bias by one point only. Blinding of the intervention was hard to accomplish due to the structural appearance of the stem cells solution which is different from saline, the studies which tried blinding used albumin (Molendijk 2015) or used opaque bags as (Melmed 2015), or the conditioning procedure used for the preparation of stem cells from the patient (Hawkey 2015).
Moreover, concerns about the small number of the studied population lead to downgrading imprecision. No other issues regarding indirectness as all the outcomes in the summary of findings table were clinically relevant to the patients. Furthermore, publication bias was downgraded in one outcome (CDAI < 150 at 24 weeks) as two studies were presented as abstract only (Arturo 2017; Lichtiger 2012) and no published complete manuscript was found, also we contacted the authors for further data and are waiting for their reply.
Potential biases in the review process
We did a thorough screening of the meta‐analyses, systematic reviews, reviews, editorials, conference proceedings, and grey literature for the inclusion of possible studies related to our topic. We found four Russian studies published in English (Kagramanova 2016; Knyazev 2015; Knyazev 2018; Knyazev 2020) that compared the standard medical intervention versus stem cells combined with medical intervention in CD, but all those studies were reported as case‐control with no mention of randomisation, allocation concealment, or blinding in their published data. Also, no clinical trial registry was found in the published manuscripts. We tried contacting the authors for further information, but they did not respond, and through a consensus between the review authors we removed them as non‐RCT studies and presented them in the (Characteristics of excluded studies).
The adverse effects varied immensely in their reporting across the studies, and we tried to find common ground for extraction and analysis through discussion with (AFN). We divided the adverse effects into (total adverse events) including all the adverse events that occur to the patients regardless of their severity, and (serious adverse events) that are life‐threatening or requiring hospitalisation or surgical intervention. Mortality was recorded as a separate outcome and discussed narratively.
There was a limitation on the published data, where the authors of two conducted studies were published only as abstracts (Arturo 2017 and Lichtiger 2012) with foreseeable full manuscript publication. We tried to contact the authors, but we did not receive any response, so these studies were moved to (Studies awaiting classification). Both studies used the systematic injection of SCs. We re‐contacted the authors on October 2021 and are awaiting their response.
We did not explore the Chinese and Korean databases with limitations to the English databases containing the abstracts of the studies, although we did not limit the search to the English‐written manuscripts; we found none in the English databases.
There was an apparent issue regarding funding, as most of the included studies had either funding for the cellular component or support for the authors to publish as shown in (Characteristics of included studies). The cost of stem cell preparation or purchase is high, thus it is difficult for the researchers to obtain the stem cells without funding. This could be resolved by supplying this area of research through non‐profit clinical organisations as ECCO, which funded Hawkey 2015, or through applying strict regulations prohibiting pharmaceutical companies from controlling the data collection and subsequent publication, where this right remains in the hands of an independent group of investigators.
Agreements and disagreements with other studies or reviews
We assessed the systematic reviews published in the same topic area that shed the light on some data, but we found some shortcomings.
We noted multiple publications of different records for the same study in the following included studies (Garcia‐Olmo 2009; Hawkey 2015; Molendijk 2015; Panes 2016). This caused confusion in most of the published meta‐analyses and systematic reviews, where the authors included the population and the events twice, which caused a fault in the calculation of the results. Consequently, when a study (e.g. Panes 2016), which included over half of the studied population, this duplicate calculation will increase the weight of the study, the effect estimate of the intervention, and its confidence interval. This duplicate calculation was noticed in the following meta‐analyses (Ciccocioppo 2019; Castro‐Poceiro 2018; Cao 2021; Cheng 2019).
We noticed that some meta‐analyses did not include the Melmed 2015 study in their final results, which may have been caused by it not being apparent by the keywords used in their search, which also occurred in our study (we found the study through manual search of references in the review articles). This occurred in (Qiu 2017; Cao 2021).
Also, most of the meta‐analyses included one‐arm trials, dose‐escalation trials with no control group, case‐control, retrospective, and case series studies, which weakened the quality of evidence in their results and overestimated the effect of SCs. This happened in ; Cao 2021; Ciccocioppo 2019; Castro‐Poceiro 2018; Cheng 2019; Lightner 2017; Qiu 2017; Turse 2018).
Finally, some meta‐analyses used a non Crohn's disease population such as the Herreros 2012 study in their final results. This happened in Cao 2021; Castro‐Poceiro 2018; Ciccocioppo 2019.
The British Society of Gastroenterology (Lamb 2019) commented on the use of autologous HSCs used in (Hawkey 2015), as a hazardous intervention, and that the authors did not achieve their intended outcome but recommended further research in that refractory CD population. Moreover, they found Panes 2016 results encouraging, regarding the effect of treatment by allogeneic MSCs, and noted that 34% of the control group achieved remission due to removal of affected tissue and fibrin glue interventions only. In addition, National Institute for Health and Care Excellence (NICE) guidelines mentioned the ADMIRE‐CD trial in their (Technology appraisal guidance) as a promising new intervention for refractory CD patients with perianal fistula (NICE 2019). American College of Gastroenterology (ACG)(Lichtenstein 2018) did not mention the SCT, but mentioned the seton placement alone as a plausible intervention with moderate quality of evidence. As for American Gastroenterological Association (AGA) they did not mention stem cell therapy (SCT) in their most updated guidelines. (Feuerstein 2021), however, the European Crohn’s and Colitis Organisation (ECCO) (Adamina 2020) mentioned adipose tissue‐derived mesenchymal stem cells, either allogeneic or autologous, as a safe and effective treatment in patients with complex perianal fistula, in their most updated guidelines version.
Authors' conclusions
Implications for practice.
Stem cell therapy, when injected systemically, is uncertain to produce benefit or harm on achieving improvement in clinical remission( the data are uncertain) or CDAI score <150 at 24 weeks (the data are very uncertain), for the treatment of refractory CD. Stem cell therapy could have a beneficial effect when injected locally, on the achievement of fistula closure in the short and long terms (the data are uncertain). The stem cells may cause no effect on the number of total adverse events (the data are very uncertain). Moreover, SCs may increase the number of serious adverse events (the data are uncertain). The withdrawal due to adverse events may be decreased by stem cell therapy (the data are very uncertain).
Implications for research.
The effect on modifying and "resetting" the immune system didn't have enough clinical and laboratory data to support it, and further studies are needed to confirm or refute its benefit. We need a more clear and unified definition of clinical remission for future clinical trials. CDAI score as the primary outcome could be misleading due to its subjectivity and a more objective score could be more consistent.
Large‐scale randomized trials are needed to validate the efficacy and effectiveness of stem cells in the treatment of refractory Crohn's disease. Standardization of the doses, outcome measures, and patients' selection criteria will lead to more consistent and clear results.
The effect of SCT on other forms of IBD mainly UC needs to be further evaluated.
What's new
| Date | Event | Description |
|---|---|---|
| 24 May 2022 | Amended | Amended to make small correction in abstract. |
History
Protocol first published: Issue 7, 2018 Review first published: Issue 5, 2022
Acknowledgements
Cochrane Gut supported the authors in the development of this systematic review.
The following people conducted the editorial process for this article:
Co‐ordinating Editor/ Sign‐off Editor (final editorial decision): Professor Morris Gordon, Cochrane Gut ‐ UK, University of Central Lancashire.
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Ghazaleh Aali, Cochrane Gut Group ‐ UK, University of Central Lancashire.
Copy Editor (copy‐editing and production): Heather Maxwell, Wiley Copy Editor team, UK
Peer‐reviewers (provided comments and recommended an editorial decision): Ms. Sarah Rhodes, Centre for Biostatistics, University of Manchester (statistical review), Dr. Sami Hoque, Barts health NHS trust, Whipps cross university hospital /Queen Mary, University of London (clinical review), Dr. Shahida Din, Consultant Gastroenterologist, NHS Lothian (clinical review), Dr. Kelly Bracewell, University of Central Lancashire (Consumer review), Dr. Farhad Shokraneh, University College London (search review).
The authors would also like to acknowledge:
Dr. Jane Burch,peer‐review as a methodology expert before editorial submission. Mohamed El‐Nakeep (statistician), providing both statistical and mathematical expert opinion during data extraction and analysis phases. Dr. Tran Nguyen, for modifying the search strategy and conducting the search at the early stage of the review. The search strategies in the review were further revised and conducted by Dr. Yuhong Yuan (Information Specialist at the Cochrane Gut Group).
Appendices
Appendix 1. Cochrane CENTRAL search strategy
Crohn Disease/
Inflammatory Bowel Diseases/
(Crohn* or ileitis or regional enteritis or ileocolitis or granulomatous colitis or granulomatous enteritis).tw,kw.
(Inflammatory bowel disease* or IBD).tw,kw.
or/1‐4
exp Stem Cell Transplantation/
Stem cell*.tw,kw.
SCT.tw,kw.
exp Stem Cells/
exp Hematopoietic Stem Cells/
((autologous or autotransfusion or auto‐transfus* or autograft* or allogenic) adj3 (hematopoietic or haematopoietic)).tw,kw.
exp Bone Marrow Transplantation/
(bone marrow adj3 (transplant* or graft* or transfus*)).tw,kw.
BMT.tw,kw.
((autologous or autotransfusion or auto‐transfus* or autograft* or allogenic) adj3 bone marrow).tw,kw.
or/6‐15
5 and 16
Appendix 2. MEDLINE search strategy
Crohn Disease/
Inflammatory Bowel Diseases/
(Crohn* or ileitis or regional enteritis or ileocolitis or granulomatous colitis or granulomatous enteritis).tw,kw.
(Inflammatory bowel disease* or IBD).tw,kw.
or/1‐4
exp Stem Cell Transplantation/
Stem cell*.tw,kw.
SCT.tw,kw.
exp Stem Cells/
exp Hematopoietic Stem Cells/
((autologous or autotransfusion or auto‐transfus* or autograft* or allogenic) adj3 (hematopoietic or haematopoietic)).tw,kw.
exp Bone Marrow Transplantation/
(bone marrow adj3 (transplant* or graft* or transfus*)).tw,kw.
BMT.tw,kw.
((autologous or autotransfusion or auto‐transfus* or autograft* or allogenic) adj3 bone marrow).tw,kw.
or/6‐15
5 and 16
randomized controlled trial.pt.
controlled clinical trial.pt.
randomi?ed.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/18‐25
exp animals/ not humans/
26 not 27
17 and 28
Note: lines 18‐28. RCT filter: “Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format”.
Appendix 3. Embase search strategy
Crohn disease/
inflammatory bowel disease/
(Crohn* or ileitis or regional enteritis or ileocolitis or granulomatous colitis or granulomatous enteritis).tw,kw.
(Inflammatory bowel disease* or IBD).tw,kw.
or/1‐4
exp stem cell transplantation/
Stem cell*.tw,kw.
SCT.tw,kw.
exp stem cell/
((autologous or autotransfusion or auto‐transfus* or autograft* or allogenic) adj3 (hematopoietic or haematopoietic)).tw,kw.
exp bone marrow transplantation/
(bone marrow adj3 (transplant* or graft* or transfus*)).tw,kw.
BMT.tw,kw.
((autologous or autotransfusion or auto‐transfus* or autograft* or allogenic) adj3 bone marrow).tw,kw.
or/6‐14
5 and 15
random:.tw.
placebo:.mp.
double‐blind:.tw.
or/17‐19
exp animal/ not human/
20 not 21
16 and 22
Note: Lines 17‐20. RCT filter. Hedge Best balance of sensitivity and specificity filter for identifying randomized trials in Embase. https://hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx
Appendix 4. ClinicalTrials.gov search strategy
Advanced search:
Disease or condition: Crohn or crohn's or crohns
Intervention/treatment: stem cell*
Study type: Interventional studies (clinical trials)
Appendix 5. WHO ICTRP search strategy
Advanced search:
Conditon: Crohn*
Intervention: Stem cell*
Recruitment status: All
Appendix 6. Mathematical conversions done for extracted data according to the Cochrane Handbook
IQR was converted to SD by the equation from Handbook (chapter 7 section 7.7.3.5) (IQR range/1.35) assuming normality and using the median as the mean, in Hawkey 2015 study to calculate SD for all extracted data (CDAI change, HBI, SES‐CD score, and IBDQ).
Measuring from the graph (using GIMP 2.10 program on Mac OS) to get the mean and SD of PDAI change of Molendijk 2015 (figure no 3), the change was calculated using the combining of the data of the three intervention groups (dose escalation) versus placebo group in the study, while in Panes 2016 the data was present in the supplement.
In all the data extracted from Molendijk 2015, we converted SEM to SD (chapter 7 in the handbook section 7.7.3.2) , and combined all the 3 intervention groups' means and SDs using the equations in the Handbook (chapter 7 table 7.7a).
In Molendijk 2015 the changes (at 12 or 24 weeks) from the baseline in CDAI, IBDQ, and PDAI scores were calculated by subtracting individual group mean before combining the three groups change in means, then using the SD of a similar study in the patients characteristic and score ( we mentioned in the figures of the forest plots footnotes). " the appropriateness of using a standard deviation from another study relies on whether the studies used the same measurement scale, had the same degree of measurement error and had the same time periods (between baseline and final value measurement)." Handbook chapter 16 section 16.1.3.2
In Molendijk 2015, we combined the outcome (fistula closure) in the 3 intervention groups to one group versus placebo arm. According to the criteria of combing different study arms of the intervention versus the placebo chapter 16 section 16.5.4 of the Handbook.
We split the patients according to cut‐off value pre‐specified by the protocol for the primary outcome <150 CDAI (assuming a normal distribution) in the studies of Molendijk 2015 and Panes 2016, we used the mean and SD of each group (intervention versus control groups) of the studies were CDAI at 24 weeks was present to calculate the number of patients who will have CDAI <150), we used the online calculator http://www.onlinestatbook.com/2/calculators/normal_dist.html).
In the case of CDEIS outcome in Molendijk 2015 and Zhang 2018, we calculated the SD of change through calculating the correlation coefficient (chapter 16 section 16.1.3 in the Handbook) of the experimental and the control groups from the study (Hawkey 2015) which used SES‐CD) reported in considerable detail the SD and mean of the outcome (the baseline, the final, and the change). The CorrE was used to calculate the SD of the experimental groups while the CorrC was used to calculate the control groups, then used the SMD between the studies as they used different scales and time frames for the outcome. I2 was >92% so this result was excluded from our discussion.
Appendix 7. Risk of bias assessment
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess random sequence generation as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess allocation concealment as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess blinding of participants and personnel as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(4) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(5) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will include missing data in the analyses which we undertake.
We will assess incomplete outcome data as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(6) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where a protocol exists and it is clear that all pre‐specified outcomes are reported and where a protocol doesn't exist and all expected outcomes have been reported);
high risk of bias (where a protocol exists and not all of the pre‐specified outcomes have been reported; where a protocol doesn't exist and an expected outcome is reported incompletely or the study fails to report key outcomes that would have been expected to have been reported); or
unclear risk of bias.
(7) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias; or
unclear whether there is risk of other bias.
Appendix 8. Senstivity analysis for all the primary outcomes:
| Outcome | Random effect | Fixed effect | With high risk | Without high risk |
| Clinical remission | 1.88 [0.80, 4.41] | 1.47 [1.10, 1.95] | 1.41 [1.06, 1.88] Melmet 2015 (high randon and allocation) |
|
| CDAI <150 | 1.02 [0.67, 1.56] | 1.08 [0.94, 1.24] | 1.02 [0.67, 1.56] Zhang 2018 high blinding and detection unclear allocation |
|
| Fistula closure short | 1.48 [1.12, 1.96] | 1.53 [1.15, 2.03] | 1.47 [1.07, 2.01] Garcia 2009 and Zhou 2020 unclear allocation and high blinding and detection |
|
| Fistula closure long | 1.42 [1.09, 1.87] | 1.47 [1.12, 1.94] | 1.48 [1.10, 1.99] removing Garcia 2009 and Zhou 2020 |
Data and analyses
Comparison 1. Stem cells versus Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Stem cell therapy versus placebo or control, Outcome: Clinical remission | 3 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.80, 4.41] |
| 1.2 CDAI <150 at 24 weeks | 4 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.67, 1.56] |
| 1.3 Stem cell therapy versus placebo or control, Outcome: Fistula Closure short‐term | 4 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.12, 1.96] |
| 1.4 Stem cell therapy versus placebo or control, Outcome: Fistula closure in long‐term Follow up of original studies | 4 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.09, 1.87] |
| 1.5 PDAI Change at week 12 | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 PDAI change at week 24 | 3 | 247 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.57, 0.86] |
| 1.7 CDAI change at 24 weeks | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8 Harvey Bradshow Index (HBI) change after 12 months | 2 | 124 | Mean Difference (IV, Random, 95% CI) | ‐2.59 [‐4.04, ‐1.14] |
| 1.9 Endoscopic score change after 12 weeks | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10 Total Adverse Events | 4 | 293 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.88, 1.13] |
| 1.11 Serious Adverse Events | 7 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.88, 1.67] |
| 1.12 Withdrawal due to adverse events | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.32, 1.89] |
| 1.13 All cause mortality | 7 | 440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.51 [0.30, 101.02] |
| 1.14 Quality of life score (change from base line) IBDQ | 4 | 292 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.18, 0.68] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Garcia‐Olmo 2009.
| Study characteristics | ||
| Methods | Phase II multicentre (three centres in Spain) randomised controlled trial. Patients were recruited consecutively and enrolled in the study from October 2004 through March 2005. Follow‐up period: 1 year (after 8 weeks then every 3 months until 12 months) |
|
| Participants | Patients were eligible for the study if they were aged 18 years or older and had a complex perianal fistula (either of cryptoglandular origin or associated with Crohn’s disease) with a visible external opening. 50 patients with complex perianal fistulas (cryptoglandular origin, n = 35; associated with Crohn’s disease, n = 14). |
|
| Interventions | Intervention group: fibrin glue plus autologous adipose‐derived stem cells (ASCs) locally. The ASCs were extracted from Liposuction from the patients (AT). Dosage: 2 million cells, if no healing at 8 weeks another dose of 4 million is given. Control group: patients were randomly assigned to intra‐lesional treatment with fibrin glue locally. |
|
| Outcomes | The primary end point for efficacy was defined as the proportion of patients whose fistula had healed at eight weeks after the last treatment received. | |
| Notes | A fistula was considered complex; if at least one of the following conditions was fulfilled: 1) fistula tract under the perianal skin unidentifiable in the physical examination; 2) fistula tract parallel to the rectum when examined with a probe; 3) associated fecal incontinence; 4) at least one previous operation performed because of fistulous disease (fistulectomy or advancement flap); 5) supra‐sphincteric tracts; 6) the presence of Crohn’s disease; or 7) recto‐vaginal fistula. Trial start date: October 2004 Trial ending date: March 2005 Trial registry number: NCT00115466 Funding Source: this clinical trial has been sponsored by Cellerix S.L. Conflict of interest: Damian García‐Olmo is a holder of the UAM–Cellerix Chair of Cell Therapy and Regenerative Medicine to which Cellerix contributes 40,000€ per year. UAM and Cellerix S.A. share patent rights to Cx401. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A centralized randomisation procedure was set up by Logitest (Madrid, Spain), the external contract research organization responsible for monitoring the entire study." |
| Allocation concealment (selection bias) | Unclear risk | The authors onlu=y stated "Central randomization" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "open‐label, add‐on clinical trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Although patients and investigators were aware of the treatment allocation, healing was assessed by a blinded evaluation committee consisting of three independent surgeons, all of whom were experts in colorectal surgery." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was done. “One patient in the experimental treatment group did not receive the designated treatment because, for unknown reasons, the cells did not expand sufficiently and the protocol did not allow for a second liposuction.” |
| Selective reporting (reporting bias) | Unclear risk | Protocol linked to abstract on PubMed under ID NCT01803347 does not match some study aspects |
| Other bias | Low risk | Baseline Characteristics show no difference between groups |
Hawkey 2015.
| Study characteristics | ||
| Methods | Parallel‐group randomised clinical trial conducted in 11 European transplant units (6 European countries) from July 2007 to September 2011, with follow‐up through March 2013. | |
| Participants | 45 Patients aged 18 to 50 years with impaired quality of life from refractory Crohn's disease not amenable to surgery despite treatment with 3 or more immunosuppressive or biologic agents and corticosteroids. | |
| Interventions | 45 patients underwent stem cell mobilisation before 1:1 randomisation. Intervention group: immuno‐ablation and autologous hematopoietic stem cell transplantation (HSCT) (n = 23) injected in through route. HSCT was extracted from the bone marrow of the patients. Dosage: Minimum 3x106 CD34+ cells/kg on day 7 Control group: (HSCT deferred for 1 year [n = 22]). All were given standard Crohn's disease treatment as needed. |
|
| Outcomes |
|
|
| Notes |
Trial start date: from July 2007 to September 2011 Trial ending date: with follow‐up through March 2013 Trial registry number: NCT00297193 (ASTIC trial) Funding Source: Sponsor: European Group for Blood and Marrow Transplantation. Collaborator: The Broad Foundation. Conflict of interest: all authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Hawkey reported receiving a National Institute for Health Research Senior Investigator Award and receiving funding from the University of Nottingham Medical School Dean’s Fund and the Nottingham University Hospitals NHS Trust Research and Development Fund. No other authors reported disclosures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was centralized and used balanced non‐stratified (1:1) electronically generated random number tables in permuted blocks of 4 patients prepared by the Nottingham Clinical Trials Unit." |
| Allocation concealment (selection bias) | Low risk | "all parties, including the trial coordinator, were unaware of the randomization group until allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the intervention, patients, clinicians, investigators, and coordinators were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "an adjudication committee that reviewed all radiology and endoscopy reports to determine the presence and activity of Crohn disease within the GI tract were blinded to time of assessment and treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was done. All participants reported the primary outcome (CDAI at 24 weeks and clinical remission). Eight patients in the placebo group withdrew due to disease flare which required either surgical intervention or early transplant. and one patient after the randomization, thus the long‐term effect on CDAI couldn't be assessed and was measured by the "Worst‐case scenario" implementation. |
| Selective reporting (reporting bias) | Low risk | Primary outcome in protocol matches the one in the study. Note: Some secondary outcomes mentioned in protocol were not assessed in the study |
| Other bias | Low risk | No difference in baseline characteristics |
Melmed 2015.
| Study characteristics | ||
| Methods | A multi‐centre (13 centres in the USA), adaptive, phase 1b/2a dose‐ranging placebo‐controlled study was performed to evaluate the safety and efficacy of human placenta‐derived cells (PDA‐001) inparticipants with moderate‐to‐severe Crohn’s disease between August 2010 and November 2011. Follow‐up period: 2 years (wk 1,2,4,6,12,24 then every 6 months till 24 months). |
|
| Participants | Moderate to severe Chron’s CDAI score (220‐450 ) Active inflammation on colonoscopy or elevated fecal calprotectin and inadequate response to conventional therapy at enrolment and visual evidence of mucosal inflammation within 3 months of enrollment by colonoscopy. An elevated fecal calprotectin (>162.9 mg/g) could also serve as evidence of mucosal inflammation, but no patients were enrolled based on this criterion. Fifty participants were enrolled (safety analysis, 50 participants; efficacy analysis, 48 participants). Four subjectsparticipants received 8 units of PDA‐001 (phase 1b study); 46 participants were subsequently randomised to 1 or 4 units of PDA‐001 or placebo (phase 2a study). The age of the participants was ≥18‐75 years old. |
|
| Interventions | Participants received 8 units of PDA‐001 (cenplacel‐L) (1.5X108 cells per unit) in the phase 1b open‐label study. (not included in the analysis as it is non‐randomised). Intervention group: 1 unit, or 4 units of allogenic PDA‐001 (2 infusions 1 week apart) systemic infusion. The cells were extracted from placental tissue. Doses: Group I: 1.5x108, Group II: 6x108 Control group: patients in the placebo group received "vehicle control without any cells"(Infusion was done twice on 0 and 7 days) "Concomitant therapy with stable doses of immunomodulators and/or biologics was permitted." |
|
| Outcomes | The primary endpoint was induction of clinical response (> or =100 points and/or 25% decrease in CDAI) at 4 and 6 weeks. | |
| Notes | This manuscript included 2 phases: phase 1b not included in the analysis as it is non‐randomised, phase 2a included in the analysis as it is randomised. “The study was suspended before the last 2 enrolled subjects were randomized because of safety events” Trial start date: August 2010 Trial ending date: November 2011 Trial registry number: NCT01155362 Funding Source: sponsor: Celularity Incorporated. Collaborator: Celgene Corporation. Conflict of interest: G. Y. Melmed has provided consulting services for AbbVie, Amgen, Celgene, Given Imaging, Janssen, Luitpold Pharmaceuticals, and UCB and has received research funding from Pfizer, Prometheus Laboratories, and Shire Pharmaceuticals. W. M. Pandak has received research grants from Bayer, Novartis, MannKind, Bristol‐Myers Squibb, Ocera, Salix, GlobeImmune, Scynexix, Genzyme, Intermune, Hoffman Laroche, SciClone, Wyeth, Merck, UCB, Celgene, Centocor, Millenium, Osiris, Exilixis, AtheroNova, Pfizer, and GlaxoSmithKine. J. Valentine has received research funding from Pfizer, Celgene, Abbott, Bristol‐Myers Squibb, Takeda, Genentech, and the National Institutes of Health and has provided speaking services for AbbVie. D. Schwartz has provided consulting services for AbbVie, UCB, Janssen, Takeda, Celgene, and Tigenix and has received research funding from AbbVie and UCB. S. Lichtiger has provided consulting services for Jansen, AbbVie, Prometheus Laboratories, and Shire and has received research funding from Jansen, Pfizer, Osiris, and Millenium. B. Sands has provided consulting services for AbbVie, Amgen, AstraZeneca, Avaxia Biologics, Bristol‐Myers Squibb, Janssen Biotech, Luitpold Pharmaceuticals, MedImmune, Pfizer, Puretech Ventures, Salix, Shire, Takeda, Topivert Pharma, and Vedanta Biosciences; has received research funding from AbbVie, Amgen, Celgene, Janssen R&D, Millennium Pharmaceuticals, Pfizer, and Prometheus Laboratories; has received honoraria for lecturing in a CME program from IMEDEX, Strategic Consultants International, Focus Medical Communications, Curatio CME Medical Institute/Huntsworth Health NA, Creative Educational Concepts, and Scripps; has received honoraria as an associate editor from the American Gastroenterological Association Institute; and holds stock in Avaxia Biologics, a non‐publicly traded company. R. Richards has provided speaking services to AbbVie. K. Johnson, R. Hariri, and S. Fischkoff are full‐time employees of Celgene Corporation with stock and stock options. The remaining authors have no conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subsequent subjects were enrolled into the phase 2a study and were randomly assigned to receive placebo, 1 unit (1.5X108 cells), or 4 units (6X108 cells) of PDA‐001 in a double‐blinded fashion" The method of randomisation is not stated clearly. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Phase IIa: It was mentioned that it was a "double blinded study". also; "IP (investigational product was covered with an opaque bag to maintain blinding and administered peripherally through a volumetric pump over 2 hours." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mostly blinded due to blinding of personnel and patients. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was done. "one subject withdrew consent before the wk 4 efficacy endpoint, and a second subject did not provide the efficacy assessment at wk 4” |
| Selective reporting (reporting bias) | Low risk | Both primary and secondary outcomes match the protocol. |
| Other bias | Low risk | No differences in baseline characteristics. |
Molendijk 2015.
| Study characteristics | ||
| Methods | Single centre (Leiden University Medical Center, the Netherlands), phase I‐II dose‐escalation study, randomised controlled trial conducted from June 2012 through July 2014. | |
| Participants | Twenty‐one patients with refractory perianal fistulising Crohn’s disease were randomly assigned to three active groups and one placebo group. Eligible patients were men and women of at least 18 years of age with actively draining perianal fistulising Crohn’s disease refractory to conventional therapies. Eligible patients had to have 1‐2 internal openings and 1‐3 fistula tracts. |
|
| Interventions | The patients were randomly assigned to intervention or placebo. Intervention group: different doses of local injections of 1X107 (n = 5, group 1), 3X107 (n = 5, group 2), or 9X107 (n = 5, group 3) of allogenic mesenchymal stem cells (MSCs) into the wall of curettaged fistula, around the trimmed and closed internal opening. MSCs were extracted from 5 different donors from 50‐100 Bone marrow (BM) aspirates (1 donor /1 patient in each group) demineralised bone matrix (DMB) aspirate. Control group: placebo (n = 6) injected into the wall of curettaged fistula, around the trimmed and closed internal opening. Placebo (0.9% saline +human albumin with no cells). The placebo group received 0.9% NaCl/5% human albumin solution with no cells. |
|
| Outcomes | The primary outcome, fistula healing, was determined by physical examination 6, 12, and 24 weeks later; healing was defined as the absence of discharge and <2 cm of the fluid collection—the latter determined by MRI at week 12. 1ry safety endpoint: serious adverse events at 12 weeks. Efficacy: fistula healing and reduction of number at 12 weeks MRI at 12 weeks. |
|
| Notes | Additional criteria for inclusion were diagnosis of Crohn’s disease at least 3 months before enrollment, CDAI score of <250 at screening and baseline, a stable dose of current drugs (mesalamine and steroids 4 weeks; immunosuppressive drugs 8 weeks; anti‐TNF agents 8 weeks), which were continued during the entire study period. Trial start date: June 2012. Trial ending date: July 2014. Trial registry number: NCT01144962 Funding Source: Sponsors and collaborators: Leiden University Medical Center and DigestScience Foundation. Conflict of interest: the authors disclose no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed at the Immunohematology and Blood Transfusion Department by a researcher who did not have any contact with or any knowledge about the included patients." |
| Allocation concealment (selection bias) | Low risk | The method of allocation was not stated: "Randomization was performed at the Immunohematology and Blood Transfusion Department by a researcher who did not have any contact with or any knowledge about the included patients." "Two weeks before the intervention was planned, the patient was randomized to receive either MSCs or placebo" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo group received 0.9% NaCl/5% human albumin solution with no cells. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Safety was assessed blindly by a physician by monitoring for (serious) adverse events and changes in vital signs at the time of surgical intervention with MSC or placebo injection at the day of treatment and at all follow‐up visits." "Routine laboratory measurements were performed and complications after surgery (e.g, bleeding, wound infection, and perianal abscesses) were assessed blindly at weeks 6, 12, and 24 by a surgeon other than the surgeon who performed the surgical intervention with MSC or placebo injection." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was done. No missing data. |
| Selective reporting (reporting bias) | Low risk | Both primary and secondary outcomes match the protocol. |
| Other bias | Low risk | No differences between baseline charcteristics |
Panes 2016.
| Study characteristics | ||
| Methods | Multicentre phase III, placebo‐control randomised trial of two parallel groups (conducted in 49 hospitals in seven European countries and Israel). | |
| Participants | 212 participants were included from July 6, 2012 to July 27, 2015. Inclusion of adult participants (≥18 years) with Crohn's disease and treatment refractory draining complex perianal fistula. Inclusion criteria:
|
|
| Interventions | 212 Participants were randomly assigned (1:1) using a pre‐established randomisation list. Intervention group: 107 participants were assigned to allogeneic, expanded, adipose‐derived stem cells (Cx601) single intralesional injection of 120 million (5million cells/ml) single injection Cx601 cells. Cells were provided from Human lipoaspirates from donor liposuction (AT) Control group: 105 participants were assigned to placebo 24 mL saline solution. |
|
| Outcomes | The primary endpoint was combined remission at week 24 (i.e. clinical assessment of closure of all treated external openings that were draining at baseline, and absence of collections >2 cm of the treated perianal fistulas confirmed by masked central MRI. There were later published data of the follow‐up of patients for longer periods of time. |
|
| Notes |
Trial start date: July 6, 2012 Trial ending date: July 27, 2015 Trial registry number: NCT01541579 Funding Sourse: Sponsor: Tigenix S.A.U. Information provided by: Takeda ( Tigenix S.A.U. ) Conflict of interest: JP has received personal fees from TiGenix, AbbVie, Boehringer Ingelheim, Galapagos, Pfi zer, Janssen, and Takeda. DG‐O has received personal fees from TiGenix, and has a patent “Identifi cation and isolation of multipotentcells from non‐osteochondral mesenchymal tissue” (10157355957US), pending to TiGenix, and a patent “Use of adipose tissue‐derived stromal stem cells in treating fi stula” (US11/167061), pending to TiGenix. GVA has received personal fees from TiGenix, MSD, Janssen, and Takeda; and grants and personal fees from AbbVie. JFC has received grants and personal fees from AbbVie, Janssen, and Takeda; personal fees from Amgen, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Ferring, Genentech, Medimmune, Merck, Pfizer, Protagonist, Second Genome, Seres, Shire, Theradiag, and PPM Services; and stock options from Genfit and Intestinal Biotech Development. WR has received personal fees from TiGenix; has served as a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Centocor, Celltrion, Danone Austria, Elan, Falk Pharma, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, Schering‐Plough, Shire, Takeda, Therakos, Vifor, and Yakult; has served as a consultant for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia, BioClinica, Biogen IDEC, Boehringer Ingelheim, Bristol‐Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Covance, Danone Austria, Elan, Falk Pharma, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millennium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestlé, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Schering‐Plough, Second Genome, Setpointmedical, Takeda, Therakos, TiGenix, UCB, Vifor, Zyngenia, and 4SC; has served as an advisory board member for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas,AstraZeneca, Avaxia, Biogen IDEC, Boehringer Ingelheim, Bristol‐Myers Squibb, Cellerix, Chemocentryx, Celgene, Centocor, Celltrion, Danone Austria, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millennium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestlé, Novartis, Ocera, Otsuka, PDL, Pharmacosmos, Pfizer, Procter & Gamble, Prometheus, Schering‐Plough, Second Genome, Setpointmedical, Takeda, Therakos, TiGenix, UCB, Zyngenia, and 4SC; and has received research funding from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma, Immundiagnsotik, and MSD. DCB reports unrestricted research grants from Shire and Hitachi; personal fees and non‐financial support from AbbVie, Merck (MSD), Takeda, Ferring, Recordati, Genentech (Roche Group), Janssen, and Dr Falk; personal fees from Biogen, Foreward Pharma, and Tigenix; and non‐financial support from Nestlé. All of his activities and contracts conform with the “FSA‐Kodex Fachkreise” (voluntary selfmonitoring code for expert consultants to the pharmaceutical industry), have been checked by the legal department of Charité Universitätsmedizin Berlin, and have been approved by the directorate of the Faculty of Medicine Charité Universitätsmedizin Berlin. AD has received grants and non‐financial support from TiGenix; and personal fees and non‐financial support from AbbVie, Dr Falk, Ferring, MSD, Takeda, Pharmacosmos, Mundipharma, Vifor, Hospira, Hexal, Allergosan, Janssen, Otsuka, and TiGenix. MN has received personal fees and non‐financial support from AbbVie, MSD, and Takeda; and personal fees from Boehringer Ingelheim. MF has received non‐financial support from TiGenix; grants, personal fees, and non‐financial support from Takeda; and personal fees and non‐financial support from MSD, Janssen, AbbVie, Chiesi, Tillotts, Ferring, Falk, Mitsubishi, Zeria, and Boehringer Ingelheim. LK‐S has received non‐financial support from and been a principal investigator for a study sponsored by TiGenix; has been a principal investigator for a study sponsored by SigmaTau and Sanofi ; has received personal fees from MSD, AbbVie, Ferring, MerckSerono/Dr Falk, Chiesi, Novartis, Roche, Abbott, and Phadia Austria/Thermo Fisher Scientifi c; and has received non‐financial support from Mylan, Abbott, MSD, Gilead, MerckSerono/Dr Falk, and Novartis. MPR and AL have received personal fees from TiGenix. SD has received personal fees from AbbVie, MSD, Takeda, Janssen, Mundipharma, Hospira, and Pfizer. JCG, FdlP, and EG declare no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned (1:1) by a centrally located computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Low risk | "centrally located computer‐generated randomisation list" "Treatments were assigned using a pre‐established randomisation list generated by the Department of Biostatistics, Linical (Madrid, Spain)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Masking of treatments was not possible because the cell suspension was clearly different to saline solution (i.e., placebo)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a masked gastroenterologist and radiologist both assessing the therapeutic effect." "radiologists who centrally read MRI scans were provided with figures to identify the treated fistulas, but were masked to patient data, order of examinations, and treatment received. Surgeons were not permitted to share information about the treatment used in the surgical procedure with the gastroenterologist and were not allowed to participate in any clinical assessment of the fistula during the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was done Reasons for withdrawal in both groups were clearly mentioned |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes match those in protocol. |
| Other bias | Low risk | No differenecs in baseline characteristics |
Zhang 2018.
| Study characteristics | ||
| Methods | Phase I randomised, controlled, open‐label, single‐centre (Shaanxi Provincial People’s Hospital in China) clinical trial. | |
| Participants | 82 participants (age >18‐70 years old) were included from June 2012 to June 2015. Inclusion criteria: patients were above 18 years of age, with moderate to severe CD (Crohn’s disease activity index [CDAI] between 220 and 450). All patients had received steroid maintenance therapy for more than 6 months before enrolment. Concomitant immunosuppressive agents (including azathioprine, 6‐mercaptopurine or methotrexate) were allowed but the dosage was maintained unless steroid was discontinued. Anti‐tumour necrosis factor (anti‐TNF) therapy was not allowed within 3 months prior to the selection. |
|
| Interventions | Intervention group: 41 patients were randomly selected to receive a total of four peripheral intravenous infusions of 1×106 Expanded Umbilical Cord Mesenchymal Stem Cells UC‐MSCs/kg, with one infusion per week. UC‐MSCs were allogenic extracted from one donor (Umbilical cord of a newborn). Control group: received the associated immunosuppression only. |
|
| Outcomes | Primary endpoint: patients were followed up for 12 months. CDAI, Harvey‐Bradshaw index (HBI), and corticosteroid dosage were assessed. | |
| Notes | For preoperative prophylaxis of thrombosis, 500 IU of low‐molecular‐weight heparin were administered by subcutaneous injection once a day, for a total of 3 days. Trial start date: June 2012 Trial ending date: June 2015 Trial registry number: NCT02445547 Funding Source: Sponsor: Fuzhou General Hospital. Collaborator: Shaanxi Provincial People's Hospital Conflict of interest: No potential conflict of interest relevant to this article was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated block randomization was used to assign each participant to one of the study groups." |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was done. Reasons for withdrawal were clearly stated, “Four patients dropped out in the UC‐MSC group and three dropped out in the control group both due to non‐adherence.” |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes match those in protocol |
| Other bias | Low risk | No differences in baseline characteristics |
Zhou 2020.
| Study characteristics | ||
| Methods | Open‐label randomised‐ single centre in China (Nanjing Hospital of Chinese Medicine) | |
| Participants | Patients with refractory Crohn's disease in the form of complicated anal fistula. Age from 12‐51 years (children included). Inclusion criteria: 1‐ Diagnosis of complex Crohn’s fistula‐in‐ano. 2‐ Patients with Crohn’s disease should control their disease in remission or mild active phase, that is, simplified CDAI is less than 6 points. 3‐ There is no evidence of cancer or precancerous lesions in enteroscopy 1 year before admission. 4‐ There are no other cardio‐cerebrovascular diseases. Exclusion criteria: 1‐ acute infection stage of anal fistula (immature fistula). 2‐ Patients with Crohn’s disease’s simplified CDAI > 6. 3‐ An autoimmune disease other than Crohn’s disease. 4‐ Patients with infectious diseases. 5‐ Patients who were allergic to anaesthetics. 6‐ Patients who cannot tolerate liposuction. 7‐ Patients who were pregnant or were trying to become pregnant. |
|
| Interventions | Intervention group: autologous adipose‐derived stem cell (ADSC). Fistula preparation: after admission, patients received fistula preparation more than 2 weeks before ADSC injection, which included fistula exploration, curettage, and drainage with seton. T5 × 106cells/mL per injection. Plus 1 × 106 cells/mL serum suspension perfused into the fistula. The dosage of ADSCs is based on the diameter and length of the fistula measured before injection, and mainly according to the results of preoperative MRI and clinical evaluation at fistula preparation. The diameter of the fistula was less than 1 cm, and 1 mL ADSCs/cm was injected into the fistula. And 2 mL ADSCs/cm was injected into the fistula in the patients with the fistula diameter ranging from 1 and 2 cm. The cells are prepared from Liposuction (AT) Control group: traditional incision thread‐drawing procedure. |
|
| Outcomes | Primary endpoint: Healing and closure of fistulas at months 3, 6, and 12. A minimum follow‐up of 24 weeks to evaluate the efficacy and safety of the ADSC. |
|
| Notes |
Trial start date: Trial ending date: Trial registry number: ChiCTR1800014599 Funding Source: This study was funded by Key Medical Science and Technology Development Projects of Nanjing Commission of Health, No. ZKX17034. Conflict of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is not stated in the study. |
| Allocation concealment (selection bias) | Unclear risk | It is not stated in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data up to 6 months ie. 24 weeks are present for all the patients: "All patients completed the 3‐month and 6‐month follow‐up but only 17 patients completed 12‐month follow‐up because another five received reoperation due to the recurrence and no healing of fistulas." |
| Selective reporting (reporting bias) | High risk | There are many differences between the protocol and the published manuscript. The authors stated in the protocol that is a case‐control study, yet they stated in their published paper that this is a randomized open‐label trial. The authors restricted the age of the studied patients in the protocol to >18 years of age, yet in the published manuscript they stated that the age of their patients ranged from 12‐51 years old. The authors stated that the sample size is 20 in control and 20 in intervention, yet in the published manuscript the total number of the studied population was 22. |
| Other bias | Unclear risk | The study included mainly male participants (21 males versus 1 female). |
ADSC: adipose‐derived stem cell; ASCs: adipose‐derived stem cells; anti‐TNF: anti‐tumor necrosis factor; CDAI: Crohn’s Disease Activity Index; (Cx601) cells: allogeneic, expanded, adipose‐derived stem cells; GI: gastrointestinal; HSCT: Hematopoietic stem cell; transplantation; IU: international unit; MRI: Magnetic resonance imaging; MSCs: mesenchymal stem cells; PDA‐001: human placenta‐derived cells; UC‐MSCs: expanded umbilical cord mesenchymal stem cells; NaCl: sodium chloride
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avivar‐Valderas 2019 | This is a sub‐analysis of the ADMIRE‐CD study. Wrong population. |
| Burt | This is a case series study with no comparative control group. Wrong study design. |
| Cho 2013 | There is no placebo group. |
| Dige 2019 | Single‐arm prospective study, wrong study design. |
| Dozois 2019 | Population is not Crohn's patients. "Given the success seen in patients with Crohn’s disease, we hypothesized that patients with cryptoglandular fistulas may also benefit from this approach." |
| FATT‐2 trial | FATT‐2 study is excluded as it was terminated without any published data. The authors declared that the termination of the study was because the protocol was not reflective of the current clinical situation. Wrong study design. |
| FATT‐I (Fistula Advanced Therapy Trial I) | No Crohn's disease patients were included. Wrong population. |
| Garcia‐Olmo 2008 | This is a review article. |
| Hommes 2011 | This is a case study that inlcuded only three patients who received the intervention. Wrong study design. |
| Kagramanova 2016 | It is not an RCT. We contacted the authors for further details, but got not response. |
| Knyazev 2015 | It is not an RCT. We contacted the authors for further details but got no response. |
| Knyazev 2018 | It is not an RCT. We contacted the authors for further details, but got not response. |
| Knyazev 2020 | It is not an RCT. Found by manual search. |
| Lazebnik 2010 | it is not an RCT |
| López‐García 2017 | Wrong study design. This is a case series of 35 patients who received the intervention. |
| Onken 2008 | No placebo group |
| Park 2014 | No placebo group |
| Serrero 2019 | Single‐arm study. Wrong study design. |
| Snowden 2018 | This is a review article. |
Characteristics of studies awaiting classification [ordered by study ID]
Arturo 2017.
| Methods | single‐centre, randomised, open‐label, controlled trial |
| Participants | 26 patients with resistance and not response to the current treatments including anti TNF‐blockers, treated by the multidisciplinary surgical medical team |
| Interventions | Autologous bone marrow mesenchymal stem cells (axBM‐MSC) expanded and cultured in vitro during 1 month and subsequently supraselective infusion in the total colonic area by endovascular catheterism in superior and inferior mesenteric artery to accurate the arrival of the cells to the colonic tissue. |
| Outcomes | After one month of the treatment several changes in the patients was documented like decrease in the number of diarrhea episodes, bloodiness, pain and CDAI score. Absence of ulcerations and lesions of colitis activity in the colonoscopy and the pathological study. Improved laboratory systemic cytokines after treatment. |
| Notes | After our protcol publishing we could not find any contact to the author. But as contact information is now available, we contacted the author and waiting for response (October 2021). |
Lichtiger 2012.
| Methods | Double‐blind, placebo‐controlled, randomised trial After the randomised trial was complete, they were entered into the compassionate, open‐label trial at the request of the open‐label investigator. |
| Participants | Six patients with refractory Crohn’s disease (not durably responsive to steroids and immunomodulators and anti‐TNF agents) |
| Interventions | The patients received placebo or remestemcel‐L (Osiris Therapeutics, Columbia MD), comprising MSCs isolated and expanded from bone marrow aspiration of young healthy adults. Dose varied between 0 and 400 million cells at each infusion. Therapy consisted of 4 infusions in 2 weeks and up to four additional infusions over several months. |
| Outcomes | CDAI score decrease >100 after 28 days. Failure of therapy. Adverse effects. |
| Notes | Funding: Simon Lichtiger ‐ grant and research support Linda custer‐director, clinical trials, Osiris therapeutics. This research was supported by an industry grant from Osiris therapeutics. After our protcol publishing we tried to contact the authors unsucessfully. But as contact information is now available, we contacted the first author and waiting for response (October 2021). |
anti‐TNF: anti‐tumor necrosis factor; CDAI: Crohn’s Disease Activity Index.
Characteristics of ongoing studies [ordered by study ID]
EUCTR2017‐000725‐12‐CZ.
| Study name | A phase III, randomised, double‐blind, parallel group, placebo‐controlled, international, multicentre study to assess efficacy and safety of Cx601, adult allogeneic expanded adipose‐derived stem cells (eASC), for the treatment of complex perianal fistula(s) in patients with Crohn’s disease over a period of 24 weeks and a follow‐up period up to 52 weeks. ADMIRE‐CD II study. |
| Methods | A phase III, randomised, double‐blind, parallel group, placebo‐controlled,international, multicentre study. |
| Participants | Targeted sample: 554 Patients of either gender = 18 years and =75 years of age Patients with Crohn’s disease diagnosed at least 6 months prior to screening visit in accordance with accepted clinical, endoscopic, histological and/or radiological criteria with complex perianal fistula(s) |
| Interventions | Cx601, adult allogeneic expanded adipose‐derived stem cells (eASC) 5 million cells/ml suspension for injection CX601 |
| Outcomes | Primary end point(s): proportion of participants who achieve combined remission at week 24 after IMP administration, where combined remission is defined as: ‐ The closure of all treated external openings that were draining at baseline despite gentle finger compression AND ‐ Absence of collection(s) >2 cm (in at least 2 dimensions) of the treated perianal fistula(s) confirmed by blinded central MRI assessment. |
| Starting date | Date of first enrolment:08/08/2017 |
| Contact information | C/ Marconi 1. Parque Tecnológico de Madrid 28760 Madrid Spain +3491804 92 64 inmaculada.gilaberte@takeda.com |
| Notes | Authorised‐recruitment may be ongoing or finished Source(s) of Monetary Support: TiGenix S.A.U. other ID registries: NCT03279081 |
ISRCTN17160440.
| Study name | A randomised controlled trial to assess the safety and effectiveness of stem cell transplantation using a reduced intensity regimen in patients with treatment resistant Crohn’s disease (ASTIClite) |
| Methods | Randomised controlled trial, phase III |
| Participants | Patients aged 18‐60years who have refractory Crohn's disease |
| Interventions | Stem cell mobilisation with low dose cyclophosphamide 1g/m2 and G‐CSF followed by autologous transplantation with a reduced intensity (‘HSCTlite’) conditioning regimen (fludarabine 125mg/m2, cyclophosphamide 120mg/kg and rabbit‐ATG 7.5mg/kg) is safe and effective in inducing regression of ileo‐colonic ulceration in patients with refractory CD compared with standard care (control group). |
| Outcomes | Primary outcome: regression of mucosal ulceration is assessed using the SES‐CD ulcer sub score on colonoscopy at week 48. |
| Starting date | date applied 23/10/2017 |
| Contact information |
Miss Lizzie Swaby: United Kingdom +44 114 222 4023 e.a.swaby@sheffield.ac.uk Prof James Lindsay: United Kingdom +44 20 3954 3300 James.lindsay@bartshealth.nhs.uk |
| Notes | Sponsor information Organisation: Barts Health NHS Trust Sponsor details: The Royal London Hospital, Whitechapel, London, E1 1BB, United Kingdom |
NCT00482092.
| Study name | Evaluation of PROCHYMAL® Adult Human Stem Cells for Treatment‐resistant Moderate‐to‐severe Crohn's Disease Title: A Phase III, Multicenter, Placebo‐controlled, Randomized, Double‐blind Study to Evaluate the Safety and Efficacy of PROCHYMAL® (ex Vivo Cultured Adult Human Mesenchymal Stem Cells) Intravenous Infusion for the Induction of Remission in Subjects Experiencing Treatment‐refractory Moderate‐to‐severe Crohn's Disease |
| Methods | Randomised controlled Phase III trial. |
| Participants | Estimated 330 participants Enrolling subjects with moderate‐to‐severe Crohn's disease who are intolerant to, or have previously failed therapy with, at least one steroid and at least one immunosuppressant and a biologic monoclonal anti‐body to tumor necrosis factor alpha. subjects of 18 Years to 70 Years |
| Interventions | The protocol investigates the safety and efficacy of using PROCHYMAL® adult human stem cells to induce remission. PROCHYMAL is delivered through a vein in the arm four times over two weeks, for approximately an hour each time. Compartor arms: Arm 1: Low dose (600 million cells total over four infusions in two weeks) Arm 2: High dose (1200 million cells delivered in four infusions over two weeks) Control: Placebo |
| Outcomes | Primary outcome: Disease remission (CDAI at or below 150) [ Time Frame: 28 days ] |
| Starting date | May 2007 |
| Contact information | Study Director: Pushpam Bharathi Mesoblast International Sarl |
| Notes | estimated study completion time: July 2020 Sponsors and Collaborators: Mesoblast International Sàrl |
NCT04010526.
| Study name | Double‐blind Randomised Placebo Controlled Study Evaluating Local Co‐administration of Autologous ADIpose Derived Stromal Vascular Fraction With Microfat for Refractory Perianal CROHN's Fistulas. |
| Methods | Phase 2 RCT‐ double blinded Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Parallel arms |
| Participants | 84 participants randomised to receive either intervention or placebo CD patients with complex refractory perianal fistula refractory to conventional medical and surgical therapy |
| Interventions | Intervention arm: local co‐administration of autologous ADIpose derived stromal vascular fraction and microfat for refractory perianal CROHN's fistulas Each batch of the final product is composed of one 5 mL syringes containing 25,9 +/‐ 10,7 millions viable cells. Each syringe will be obstructed with a sterile stopper and packaged in an external packaging. Placebo arm: The study placebo will consist of a saline solution for intralesional administration and will follow the same administration schema described for the SFV |
| Outcomes | Primary outcomes: 1‐ clinically evaluated [ Time Frame: 24 weeks (w) ] number of fistula closure 2‐ MRI at 24 and 52 weeks for the confirmation of absence of collections > 2 cm of the treated perianal fistulas. |
| Starting date | March 2020 |
| Contact information | JEAN CHARLES GRIMAUD, MD+33491368739 mailto:Jean‐charles.GRIMAUD%40ap‐hm.fr?subject=NCT04010526, RCAPHM18_0013, Evaluation of Local Co‐administration of Autologous ADIpose Derived Stromal Vascular Fraction With Microfat for Refractory Perianal CROHN's Fistulas. |
| Notes | Sponsor: Assistance Publique Hopitaux De Marseille |
NCT04519671.
| Study name | A Phase IB/IIA Study of Adult Allogeneic Bone Marrow Derived Mesenchymal Stem Cells for the Treatment of Perianal Fistulizing Crohn's Disease |
| Methods | Phase IB/IIA Study, randomised, Crossover Assignment Masking: Single (Participant) |
| Participants | 40 participants randomised Patients with medically refractory perianal fistulizing Crohn's disease. 18‐75 years old |
| Interventions | Intervention arm: Direct injection of adult allogeneic bone marrow derived mesenchymal stem cell product, at a dose of 75 million cells into perianal fistula(s) Direct injection of adult allogeneic bone marrow derived mesenchymal stem cells, at a dose of 75 million cells into perianal fistula(s) at baseline with a possible repeat injection at 3 months if not completely healed from the first injection. Placebo arm: Normal saline Direct injection of normal saline. If not completely healed after 6 months, participants will then cross over to the treatment group to receive a direct injection of adult allogeneic bone marrow derived mesenchymal stem cells, at a dose of 75 million cells into perianal fistula(s) |
| Outcomes | Primary outcome: Treatment related adverse events [ Time Frame: Month 6 ] |
| Starting date | November 19, 2020 |
| Contact information | Contact: Kavita Elliott, BS216‐403‐3573 NCT04519671, CCF‐Stem Cells IBD‐001, Mesenchymal Stem Cells for the Treatment of Perianal Fistulizing Crohn's Disease" type="EXTERNAL">ibdstemcelltherapy@ccf.org Contact: Caroline Matyas, BS216‐212‐0746 mailto:ibdstemcelltherapy%40ccf.org?subject=NCT04519671, CCF‐Stem Cells IBD‐001, Mesenchymal Stem Cells for the Treatment of Perianal Fistulizing Crohn's Disease |
| Notes | Locations and recruiting team: Cleveland, Ohio, United States, 44195 Contact: Kavita Elliott Contact: Caroline Matyas |
NCT04519684.
| Study name | A Phase IB/IIA Study of Allogeneic Bone Marrow Derived Mesenchymal Stem Cells for the Treatment of Ileal Anal Anastomosis and Ileal Pouch Fistulas in the Setting of Crohn's Disease of the Pouch |
| Methods | Phase IB/IIA Study, RCT, |
| Participants | 40 participants 18 Years to 75 Years Patients with medically refractory peri‐pouch fistulizing disease in the setting of Crohn's disease of the pouch. |
| Interventions | Experimental: Mesenchymal stem cells Direct injection of allogeneic bone marrow derived mesenchymal stem cells at a dose of 75 million cells into the ileal pouch fistula(s) at baseline with a possible repeat injection at 3 months if not completely healed from the first injection. Placebo Comparator: Placebo: Normal saline Direct injection of normal saline. If not completely healed after 6 months, participants will then cross over to the treatment group to receive a direct injection of allogeneic bone marrow derived mesenchymal stem cells at a dose of 75 million cells into ileal pouch fistula(s). |
| Outcomes | Primary outcome: Treatment related adverse events [ Time Frame: Month 6 ] |
| Starting date | October 28, 2020 |
| Contact information | Contact: Kavita Elliott, BS216‐403‐3573 NCT04519684, CCF‐Stem Cells IBD‐002, Study of Mesenchymal Stem Cells for the Treatment of Ileal Pouch Fistula's in Participants With Crohn's Disease" type="EXTERNAL">ibdstemcelltherapy@ccf.org Contact: Caroline Matyas, BS216‐212‐0746 mailto:ibdstemcelltherapy%40ccf.org?subject=NCT04519684, CCF‐Stem Cells IBD‐002, Study of Mesenchymal Stem Cells for the Treatment of Ileal Pouch Fistula's in Participants With Crohn's Disease |
| Notes | Recruiting team: Cleveland, Ohio, United States, 44195, Contact: Kavita Elliott Principal Investigator:Amy Lightner, MDThe Cleveland Clinic Estimated completion date: October 2022 |
NCT04519697.
| Study name | A Phase IB/IIA Study of Adult Allogeneic Bone Marrow Derived Mesenchymal Stem Cells for the Treatment of Rectovaginal Fistulas in the Setting of Crohn's Disease. |
| Methods | Phase IB/IIA Study, RCT, Crossover Assignment Masking: Single (Participant) |
| Participants | 40 participants, 18 Years to 75 Years, Females Patients with rectovaginal fistulas in the setting of Crohn's disease. |
| Interventions | Experimental: Mesenchymal Stem Cells Direct injection of adult allogeneic bone marrow derived mesenchymal stem cells at a dose of 75 million cells into rectovaginal fistula at baseline with a possible repeat injection at 3 months if not completely healed from the first injection. Placebo Comparator: Placebo Direct injection of normal saline with a possible repeat injection at 3 months if not completely healed from the first injection. If not completely healed after 6 months, participants will then cross over to the treatment group to receive a direct injection of adult allogeneic bone marrow derived mesenchymal stem cells at a dose of 75 million cells into rectovaginal fistula. |
| Outcomes | Primary outcome: Treatment related adverse events [ Time Frame: Month 6 ] |
| Starting date | October 28, 2020 |
| Contact information | Contact: Caroline Matyas, BSPH216‐212‐0746NCT04519697, CCF‐Stem Cells IBD‐003, Mesenchymal Stem Cells for the Treatment of Rectovaginal Fistulas in Participants With Crohn's Disease" type="EXTERNAL">ibdstemcelltherapy@ccf.org Contact: Kavita Elliott, BS216‐403‐3573mailto:ibdstemcelltherapy%40ccf.org?subject=NCT04519697, CCF‐Stem Cells IBD‐003, Mesenchymal Stem Cells for the Treatment of Rectovaginal Fistulas in Participants With Crohn's Disease |
| Notes | Estimated completion date: October 2022 Location and recruiting: Cleveland, Ohio, United States, 44195 Contact: Kavita Elliott Contact: Caroline Matyas Principal Investigator:Amy Lightner, MDThe Cleveland Clinic |
NCT04548583.
| Study name | Study of Mesenchymal Stem Cells for the Treatment of Medically Refractory Crohn's Colitis Official title: A Phase IB/IIA Study of Remestemcel‐L, an Ex‐vivo Culture‐expanded Adult Allogeneic Bone Marrow Derived Mesenchymal Stem Cell Product for the Treatment of Medically Refractory Crohn's Colitis |
| Methods | Phase I‐II randomized control trial. |
| Participants | 24 participants are expected to be recruited. Patients with medically refractory Crohn's colitis: Crohn's colitis of at least 6 months duration with medically refractory symptoms who has failed one anti‐TNF therapy, with a next step of subtotal colectomy or escalation in medical management. Included participants' age is from 18 Years to 75 Years |
| Interventions |
Intervention arms will receive: adult allogeneic bone marrow derived mesenchymal stem cell product .
Placebo Comparator: Placebo (saline). Direct injection of normal saline into the submucosal layer of the colon wall. If not completely healed after 3 months, participants will then cross over to the treatment group to receive a direct injection of remestemcel‐L, at a dose of 150 or 300 million cells into the submucosal layer of the colon wall. |
| Outcomes |
Primary Outcome Measures: Treatment‐related adverse events [ Time Frame: 3 Month] To determine the safety and feasibility of endoscopic injection of remestemcel‐L, an ex vivo expanded allogeneic bone marrow‐derived mesenchymal stem cell product. Secondary Outcome measures:
|
| Starting date | November 4, 2020 |
| Contact information | Contact: Kavita Elliott, BS216‐403‐3573NCT04548583, CCF‐Stem Cells IBD‐004, Study of Mesenchymal Stem Cells for the Treatment of Medically Refractory Crohn's Colitis" type="EXTERNAL">ibdstemcelltherapy@ccf.org Contact: Caroline Matyas, BSPH216‐212‐0746mailto:ibdstemcelltherapy%40ccf.org?subject=NCT04548583, CCF‐Stem Cells IBD‐004, Study of Mesenchymal Stem Cells for the Treatment of Medically Refractory Crohn's Colitis |
| Notes | Sponsor: The Cleveland Clinic. Collaborator: Mesoblast, Inc. Principal Investigator: Amy Lightner, MD. The Cleveland Clinic. |
NCT04612465.
| Study name | Phase 3 Clinical Study to Evaluate Efficacy and Safety of ASC(Autologous Adipose‐derived Stem Cells) and Fibringlue or Fibringlue in Patients With Crohn's Fistula.: A Randomized Study |
| Methods | Phase III RCT, Open Label, Parallel Assignment |
| Participants | 36 Patient who has one or more Crohn's fistulas. 18 Years and older |
| Interventions | Experimental: ASC (Autologous Adipose‐derived Mesenchymal Stem Cells) The ASC injection dose is about 1x10^7 cells of ASC per 1cm^2 of the surface area of the fistula, and the additional injection dose is 1.5 times the initial injection dose. and up to 30% of the ASC injection dose is administered in combination with Fibringlu. Standard Comparator (control): Fibringlu |
| Outcomes | Primary outcome: Proportion of subjects who are completely blocked fistula [ Time Frame: In the 8th week after 1st injection ] ; complete blockage |
| Starting date | January 9, 2020 |
| Contact information | Contact: KyuJoo Park, MD. Ph D+82‐02‐2072‐2901 mailto:kjparkmd%40plaza.snu.ac.kr?subject=NCT04612465, ANTG‐ASC‐301, Clinical Study to Evaluate Efficacy and Safety of ASC and Fibringlue or Fibringlue in Patients With Crohn's Fistula |
| Notes | estimated study completion date: December 31, 2021 Locations for recruitments: 1‐ Seoul Natinoal Univetsity Hospital 2‐ Asan Medical Center 3‐ Samsung Medical Center Sponsors: Anterogen Co., Ltd. |
ATG: anti‐thymocyte globulin; CD: Crohn'sdisease; IMP: Investigational Medicinal Product; G‐CSF: granulocyte‐colony stimulating factor; MRI: magnetic resonance imaging
Differences between protocol and review
The search strategy was modified by Dr. Tran Nguyen to include more studies. The modified search strategy was used to retrieve studies in 2018. A second search strategy was modified for the 19th March 2021 update by the Information Specialist Dr. Yuan. The second one is the one used in the current review (appendices 1, 2, and 3).
Contributions of authors
SEN: writing of the review, screening of studies, data extraction, risk of bias assessment, data analysis, read and approved the final version of the manuscript.
AS: revision of the review and providing an expert clinical and gastrointestinal opinion, read and approved the final version of the manuscript.
SFA: screening of studies, data extraction, risk of bias assessment, read and approved the final version of the manuscript.
OAL: revision of the review and providing an expert immunological opinion, read and approved the final version of the manuscript.
Sources of support
Internal sources
-
Egyptian Center of Evidence Based Medicine, Egypt
Providing author training
External sources
-
No sources of support, Other
No sources of support
Declarations of interest
SEN: has declared that she has no conflicts of interest.
AS: has declared that she has no conflicts of interest.
SFA: has declared that she has no conflicts of interest.
OAL: has declared that she has no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Garcia‐Olmo 2009 {published data only}
- Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, et al.Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Diseases of the Colon and Rectum 2009;52(1):79-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hawkey 2015 {published data only}
- Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, Ricart E, et al.Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA 2015;314(23):2524-34. [PMID: ] [DOI] [PubMed] [Google Scholar]
Melmed 2015 {published data only}
- Melmed GY, Pandak WM, Casey K, Abraham B, Valentine J, Schwartz D, et al.Human placenta-derived Cells (PDA-001) for the treatment of moderate-to-severe Crohn's disease: a phase 1b/2a study. Inflammatory Bowel Diseases 2015;21(8):1809-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Molendijk 2015 {published data only}
- Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, et al.Allogeneic bone marrow-derived mesenchymal stromalcCells promote healing of refractory perianal fistulas in patients with Crohn's disease. Gastroenterology 2015;149(4):918-27.e6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Panes 2016 {published data only}
- Panes J, Garcia-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, et al.Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet (London, England) 2016;388(10051):1281-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang J, Lv S, Liu X, Song B, Shi L.Umbilical cord mesenchymal stem cell treatment for Crohn's disease: a randomized controlled clinical trial. Gut and Liver 2018;12(1):73-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2020 {published data only}
- Zhou C, Li M, Zhang Y, Ni M, Wang Y, Xu D, et al.Autologous adipose-derived stem cells for the treatment of Crohn’s fistula-in-ano: an open-label, controlled trial [Z]. Stem Cell Research & Therapy 2020;11(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Avivar‐Valderas 2019 {published data only}
- Avivar-Valderas Alvaro, Martín-Martín Cristina, Ramírez Cristina, Del Río Borja, Menta Ramón, Mancheño-Corvo Pablo, Ortiz-Virumbrales Maitane, Herrero-Méndez Ángel, Panés Julián, García-Olmo Damián, Castañer José Luís, Palacios Itziar, Lombardo Eleuterio, Dalemans Wilfried, DelaRosa Olga.Dissecting Allo-Sensitization After Local Administration of Human Allogeneic Adipose Mesenchymal Stem Cells in Perianal Fistulas of Crohn's Disease Patients. Front. Immunol. 2019;10:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burt {published data only}
- Burt RK, Craig RM, Milanetti F, Quigley K, Gozdziak P, Bucha J, et al.Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood 2010;116(26):6123-6132. [DOI] [PubMed] [Google Scholar]
Cho 2013 {published data only}
- Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS.Autologous adipose tissue-derived stem cells for the treatment of Crohn's fistula: a phase I clinical study. Cell transplantation 2013;22(2):279-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dige 2019 {published data only}
- Dige A, Hougaard HT, Agnholt J, Pedersen BG, Tencerova M, Kassem M, et al.Efficacy of injection of freshly collected autologous adipose tissue Into perianal fistulas in patients with Crohn's disease. Gastroenterology 2019;156(8):2208-2216.e1. [DOI] [PubMed] [Google Scholar]
Dozois 2019 {published data only}
- Dozois EJ, Lightner AL, Mathis KL, Chua HK, Kelley SR, Fletcher JG, et al.Early Results of a Phase I Trial Using an Adipose-Derived Mesenchymal Stem Cell-Coated Fistula Plug for the Treatment of Transsphincteric Cryptoglandular Fistulas. Diseases of the Colon & Rectum 2019;62(5):615-622. [DOI] [PubMed] [Google Scholar]
FATT‐2 trial {published data only}
- WHO trial.Randomized, single-blind, placebo controlled multicenter phase III study to assess the efficacy and safety of expanded autologous adipose-derived stem cells (eASCs) (CX-401), for treatment of complex perianal fistulas in perianal Crohn's disease. FATT II: fistula Advanced Therapy Trial (II) - FATT II. EUCTR2008-004286-25-NL 2008.
FATT‐I (Fistula Advanced Therapy Trial I) {published data only}
- A Phase III multicenter, single-blind, randomized, comparative, add-on clinical trial, in three parallel groups, to evaluate the efficacy and safety of a new therapy with adipose-derived autologous stem cells for the treatment of complex perianal fistula. FATT I: Fistula Advanced Therapy Trial (I) - FATT 1. International Clinical Trials Registry Platform (ICTRP) 2007.
Garcia‐Olmo 2008 {published data only}
- Garcia-Olmo D, Garcia-Arranz M, Herreros D.Expanded adipose-derived stem cells for the treatment of complex perianal fistula including Crohn's disease. Expert Opinion on Biological Therapy 2008;8(9):1417-1423. [DOI] [PubMed] [Google Scholar]
Hommes 2011 {published data only}
- Hommes DW, Duijvestein M, Zelinkova Z, Stokkers PCF, Ley MH-de, Stoker J, et al.Long-term follow-up of autologous hematopoietic stem cell transplantation for severe refractory Crohn's disease. Journal of Crohn's and Colitis 2011;5(6):543-549. [DOI] [PubMed] [Google Scholar]
Kagramanova 2016 {published data only}
- Kagramanova A, Knyazev, O, Konoplyannikov A.P-068 The combined of mesenchymal stem cells and anticytokine therapy reduces the recurrence rate of Crohn's disease. Inflammatory Bowel Diseases 2016;22(suppl-1):S31-S31. [Google Scholar]
Knyazev 2015 {published data only}
- Knyazev OV, Ivanovich PA, Georgievich KA, Kagramanova KA.Safety of mesenchymal stem cells therapy in patients with inflammatorybowel diseases a 5 year follow-up. Journal of Biotechnology & Biomaterials 2015;5(3):1-5. [Google Scholar]
Knyazev 2018 {published data only}
- Knyazev OV, Kagramanova AV, Fadeeva NA, Lishchinskaya AA, Boldyreva ON, Noskova KK, et, al.Mesenchymal stromal cells of bone marrow and azathioprine in Crohn's disease therapy. Terapevticheskii arkhiv 2018;90(2):47-52. [DOI] [PubMed] [Google Scholar]
Knyazev 2020 {published data only}
- Knyazev O, Kagramanova A, Lischinskaya A, Korneeva I, Zvyaglova M, Babayan A, et al.Stem cell therapy for perianal Crohn’s disease. Proceedings of the Latvian Academy of Sciences (Section B) 2020;74(2 (725)):68–74. [Google Scholar]
Lazebnik 2010 {published data only}
- Lazebnik L, Knyazev O, Konoplyannikov A, Parfenov A, Ruchkina I, Rogozina V, et al.T1751 Transplantation of allogenic mesenchimal stem cells is a new method of biological therapy of Crohn's disease. Gastroenterology 2010;138(5):S-571. [Google Scholar]
López‐García 2017 {published data only}
- López-GA, Rovira M, Jauregui-A, Marín P, Barastegui R, Salas A, et al.Autologous Haematopoietic Stem Cell Transplantation for Refractory Crohn’s Disease: Efficacy in a Single-Centre Cohort. Journal of Crohn's and Colitis 2017;11(10):1161-1168. [DOI] [PubMed] [Google Scholar]
Onken 2008 {published data only}
- Onken J, Jaffe T, Custer L.W1237 Long-term safety of prochymal adult mesenchymal stem cells in Crohn's disease. Gastroenterology 2008;134(4):A-661. [Google Scholar]
Park 2014 {published data only}
- Park KJ, Kim JS, Kim W, Kim HJ, Lee KY, Baik S H, et al.507 Allogeneic adipose-derived stem cells for the treatment of Crohn's perianal fistula: a Phase I/IIa clinical study. Gastroenterology 2014;146(5):S-1016. [Google Scholar]
Serrero 2019 {published data only}
- Serrero M, Grimaud F, Philandrianos C, Visée C, Sabatier F, Grimaud JC.Long-term safety and efficacy of local microinjection combining autologous microfat and adipose-derived stromal vascular fraction for the treatment of refractory perianal fistula in Crohn's disease. Gastroenterology 2019;156(8):2335-2337.e2. [DOI] [PubMed] [Google Scholar]
Snowden 2018 {published data only}
- Snowden JA, Panés J, Alexander T, Allez M, Ardizzone S, Dierickx D, and European Crohn’s and Colitis Organisation (ECCO), European Society for Blood and Marrow Transplantation (EBMT), Autoimmune Diseases Working Party (ADWP), Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT) and EBMT (JACIE).Autologous Haematopoietic Stem Cell Transplantation (AHSCT) in Severe Crohn’s Disease: A Review on Behalf of ECCO and EBMT. Journal of Crohn's and Colitis 2018;12(4):476-488. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Arturo 2017 {published data only}
- Arturo J, Lucena C, Perez C, Esteban C, Sandoval vivas AL, Bastidas Y et al.Efficacy of autologous expanded bone marrow derived mesenchymal stem cells (axBM-MSC) in the treatment of patients with chron's disease who have failed to standard therapy: an open-label, randomized controlled clinical trial phase II. Cytotherapy 2017;19(5):e23. [Google Scholar]
Lichtiger 2012 {published data only}
- Lichtiger, S.Remestemcel-L therapy is effective treatment in patients with refractory Crohn's disease. American Journal of Gastroenterology 2012;1:S689. [Google Scholar]
References to ongoing studies
EUCTR2017‐000725‐12‐CZ {published data only}
- A clinical research study of an investigational new drug to treat perianal fistulas in patients with Crohn’s disease (CD). EU Clinical Trials Register.
- Wexner S, Sandborn W, Panes J, Gilaberte I, Gulati P, Zhang B, et al.Design of a phase III, double-blind, randomized trial to evaluate the safety and efficacy of allogeneic stem cells (Darvadstrocel) for the treatment of complex perianal fistula(S) in patients with Crohn's disease (ADMIRE-CD II trial).. Diseases of the Colon and Rectum 2020;63(6):e300. [Google Scholar]
ISRCTN17160440 {published data only}
- A randomised controlled trial to assess the safety and effectiveness of stem cell transplantation using a reduced intensity regimen in patients with treatment resistant Crohn’s disease. ISRCTN registry.
- Snowden JA, Hawkey C, Hind D, Swaby L, Mellor K, Emsley R et al, Autologous Stem Cell Transplantation In Refractory CD - Low Intensity Therapy Evaluation Study Investigators, European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP).Autologous stem cell transplantation in refractory Crohn's disease - low intensity therapy evaluation (ASTIClite): study protocols for a multicentre, randomised controlled trial and observational follow up study. BMC Gastroenterology 2019;19(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00482092 {published data only}
- Evaluation of PROCHYMAL® adult human stem cells for treatment-resistant moderate-to-severe Crohn's disease. ClinicalTrials.gov.
NCT04010526 {published data only}
- Evaluation of local co-administration of autologous ADIpose derived stromal vascular fraction with microfat for refractory perianal CROHN's fistulas (ADICROHN2). NCT04010526 2019.
NCT04519671 {published data only}
- Mesenchymal stem cells for the treatment of Perianal Fistulizing Crohn's Disease (PFCD). NCT04519671 2020. [DOI] [PubMed]
NCT04519684 {published data only}
- Study of mesenchymal Stem cells for the treatment of Ileal Pouch Fistula's in participants with Crohn's disease (IPAAF). NCT04519684 2020.
NCT04519697 {published data only}
- Mesenchymal stem cells for the treatment of Rectovaginal Fistulas in participants with Crohn's disease (RVF). NCT04519697 2020.
NCT04548583 {published data only}
- Study of Mesenchymal Stem Cells for the Treatment of Medically Refractory Crohn's ColitisOfficial title: A Phase IB/IIA Study of Remestemcel-L, an Ex-vivo Culture-expanded Adult Allogeneic Bone Marrow Derived Mesenchymal Stem Cell Product for the Treatment of Medically Refractory Crohn's Colitis. Ongoing study. November 4, 2020. Contact author for more information.
NCT04612465 {published data only}
- Clinical study to evaluate efficacy and safety of ASC and fibringlue or fibringlue in patients with Crohn's fistula. NCT04612465 2020.
Additional references
Abraham 2009
- Abraham C, Cho JH.Inflammatory bowel disease. New England Journal of Medicine 2009;361(21):2066-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adamina 2020
- Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, etal, and European Crohn’s and Colitis Organisation [ECCO].ECCO Guidelines on Therapeutics in Crohn’s Disease: Surgical Treatment. Journal of Crohn's and Colitis 2020;14(2):155-168. [DOI] [PubMed] [Google Scholar]
Babineau 2014
- Babineau J.Product Review: Covidence (Systematic Review Software). Journal of the Canadian Health Libraries Association / Journal De l’Association Des bibliothèques De La Santé Du Canada 2014;35(2):68-71. [DOI: ] [Google Scholar]
Barnhoorn 2020
- Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars WJ, Molendijk I, Bonsing BA, et al.Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn's disease perianal fistulas. Journal of Crohn's & Colitis 2020;14(1):64-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benchimol 2008
- Benchimol EI, Seow CH, Steinhart AH, Griffiths AM.Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD006792. [DOI: 10.1002/14651858.CD006792.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brierley 2018
- Brierley CK, Castilla-Llorente C, Labopin M, Badoglio M, Rovira M, Ricart E, et al, and European Society for Blood and Marrow Transplantation [EBMT] Autoimmune Diseases Working Party [ADWP].Autologous haematopoietic stem cell transplantation for Crohn’s disease: a retrospective survey of long-term outcomes from the European Society for Blood and Marrow Transplantation. Journal of Crohn's and Colitis 2018;12(9):1097-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burt 2003
- Burt RK, Traynor A, Oyama Y, Craig R.High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory Crohn disease. Blood 2003;101(5):2064-6. [DOI] [PubMed] [Google Scholar]
Burt 2010
- Burt RK, Craig RM, Milanetti F, Quigley K, Gozdziak P, Bucha J, et al.Autologous nonmyeloablative hematopoietic stem cell transplantation in patients with severe anti-TNF refractory Crohn disease: long-term follow-up. Blood 2010;116(26):6123-32. [DOI] [PubMed] [Google Scholar]
Cao 2021
- Cao Y, Su Q, Zhang B, Shen F, Li S.Efficacy of stem cells therapy for Crohn’s fistula: a meta-analysis and systematic review. Stem Cell Research & Therapy 2021;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carvello 2019
- Carvello M, Lightner A, Yamamoto T, Kotze PG, Spinelli A.Mesenchymal stem cells for perianal Crohn's disease. Cells 2019;8(7):764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassinotti 2008
- Cassinotti A, Annaloro C, Ardizzone S, Onida F, Della Volpe A, Clerici M, et al.Autologous haematopoietic stem cell transplantation without CD34+ cell selection in refractory Crohn's disease. Gut 2008;57(2):211-7. [DOI] [PubMed] [Google Scholar]
Castro‐Poceiro 2018
- Castro-Poceiro J, Fernández-Clotet A, Panés J.Mesenchymal stromal cells in the treatment of perianal fistulas in Crohn's disease. Immunotherapy 2018;10(14):1203-17. [DOI] [PubMed] [Google Scholar]
Chande 2016
- Chande N, Townsend CM, Parker CE, MacDonald JK.Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2016, Issue 10. Art. No: CD000545. [DOI: 10.1002/14651858.CD000545.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2019
- Cheng F, Huang Z, Li Z.Mesenchymal stem-cell therapy for perianal fistulas in Crohn’s disease: a systematic review and meta-analysis. Techniques in Coloproctology 2019;23(7):613-23. [DOI] [PubMed] [Google Scholar]
Ciccocioppo 2011
- Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al.Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut 2011;60(6):788-98. [DOI] [PubMed] [Google Scholar]
Ciccocioppo 2019
- Ciccocioppo R, Klersy C, Leffler DA, Rogers R, Bennett D, Corazza GR.Systematic review with meta-analysis: Safety and efficacy of local injections of mesenchymal stem cells in perianal fistulas. JGH Open 2019;3(3):249-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clerici 2011
- Clerici M, Cassinotti A, Onida F, Trabattoni D, Annaloro C, Della Volpe A, et al.Immunomodulatory effects of unselected hematopoietic stem cells autotransplantation in refractory Crohn's disease. Digestive and Liver Disease 2011;43(12):946-52. [DOI] [PubMed] [Google Scholar]
Cooper 2017
- Cooper J, Blake I, Lindsay JO, Hawkey CJ.Living with Crohn's disease: an exploratory cross-sectional qualitative study into decision-making and expectations in relation to autologous haematopoietic stem cell treatment (the DECIDES study). BMJ Open 2017;7(9):e015201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Craig 2003
- Craig RM, Traynor A, Oyama Y, Burt RK.Hematopoietic stem cell transplantation for severe Crohn's disease. Bone Marrow Transplant 2003;32:S57-9. [DOI] [PubMed] [Google Scholar]
Dalal 2012
- Dalal J, Gandy K, Domen J.Role of mesenchymal stem cell therapy in Crohn's disease. Pediatric Research 2012;71(4 Pt 2):445-51. [DOI] [PubMed] [Google Scholar]
Dassopoulos 2013
- Dassopoulos T, Sultan S, Falck–Ytter YT, Inadomi JM, Hanauer SB.American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti–TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology 2013;145(6):1464-78. [DOI] [PubMed] [Google Scholar]
Dave 2015
- Dave M, Mehta K, Luther J, Baruah A, Dietz AB, Faubion WA Jr.Mesenchymal stem cell Therapy for inflammatory bowel disease: a systematic review and meta-analysis. Inflammatory Bowel Diseases 2015;21(11):2696-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Day 2013
- Day AS, Burgess L.Exclusive enteral nutrition and induction of remission of active Crohn's disease in children. Expert Review of Clinical Immunology 2013;9(4):375-83. [DOI] [PubMed] [Google Scholar]
de la Portilla 2013
- la Portilla F, Alba F, Garcia-Olmo D, Herrerias JM, Gonzalez FX, Galindo A.Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn's disease: results from a multicenter phase I/IIa clinical trial. International Journal of Colorectal Disease 2013;28(3):313-23. [DOI] [PubMed] [Google Scholar]
Duijvestein 2010
- Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, et al.Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut 2010;59(12):1662-9. [DOI] [PubMed] [Google Scholar]
Duran 2016
- Duran NE, Hommes DW.Stem cell-based therapies in inflammatory bowel disease: promises and pitfalls. Therapeutic Advances in Gastroenterology 2016;9(4):533-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feuerstein 2021
- Feuerstein JD, Ho EY, Shmidt E, Singh H, Falck-YY, Sultan S, et al.AGA Clinical Practice Guidelines on the medical management of moderate to severe luminal and perianal fistulizing Crohn’s disease. Gastroenterology 2021;160(7):496-2508. [DOI] [PubMed] [Google Scholar]
Forbes 2014
- Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, et al.A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clinical Gastroenterology and Hepatology 2014;12(1):64-71. [DOI] [PubMed] [Google Scholar]
García‐Arranz 2016
- García-Arranz M, Herreros MD, González-Gómez C, la Quintana P, Guadalajara H, Georgiev-Hristov T, et al.Treatment of Crohn's-related rectovaginal fistula with allogeneic expanded-adipose derived stem cells: a phase I-IIa clinical trial. Stem Cells Translational Medicine 2016;5(11):1441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Olmo 2005
- Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA.A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Diseases of the Colon and Rectum 2005;48(7):1416-23. [DOI] [PubMed] [Google Scholar]
Garcia‐Olmo 2009
- Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, et al.Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Diseases of the Colon and Rectum 2009;52(1):79-86. [DOI] [PubMed] [Google Scholar]
Gomollón 2017
- Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al.3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and Medical Management. Journal of Crohn's and Colitis 2017;11(1):3-25. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT.Version accessed 3 July 2018. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Guadalajara 2012
- Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D.Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. International Journal of Colorectal Disease 2012;27(5):595-600. [DOI] [PubMed] [Google Scholar]
Guadalajara 2020
- Guadalajara H, García-Arranz M, Herreros MD, Borycka-Kiciak K, Lightner AL, García-Olmo D.Mesenchymal stem cells in perianal Crohn’s disease. Techniques in Coloproctology 2020;24(8):883-9. [DOI] [PubMed] [Google Scholar]
Ha 2015
- Ha F Khalil H.Crohn's disease: a clinical update. Therapeutic Advances in Gastroenterology 2015;8(6):352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkey 2015
- Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, Ricart E, et al.Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA 2015;314(23):2524-34. [DOI] [PubMed] [Google Scholar]
Herreros 2012
- Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D, FATT Collaborative Group.Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Diseases of the Colon and Rectum 2012;55(7):762-72. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors).Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
Higgins 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R.Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version 1.02, 2016.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors).Cochrane. Available from www.training.cochrane.org/handbook.. Chichester (UK): John Wiley & Sons, 2021. [Google Scholar]
Isene 2015
- Isene R, Bernklev T, Hoie O, Munkholm P, Tsianos E, Stockbrugger R, et al.Extraintestinal manifestations in Crohn's disease and ulcerative colitis: results from a prospective, population-based European inception cohort. Scandinavian Journal of Gastroenterology 2015;50(3):300-5. [DOI] [PubMed] [Google Scholar]
Jauregui‐Amezaga 2016
- Jauregui-Amezaga A, Rovira M, Marín P, Salas A, Pinó-Donnay S, Feu F, et al.Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn's disease. Gut 2016;65(9):1456-62. [DOI] [PubMed] [Google Scholar]
Kawalec 2013
- Kawalec P, Mikrut A, Wiśniewska N, Pilc A.Tumor necrosis factor-α antibodies (infliximab, adalimumab and certolizumab) in Crohn's disease: systematic review and meta-analysis. Archives of Medical Science 2013;9(5):765-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreisel 2003
- Kreisel W, Potthoff K, Bertz H, Schmitt-Graeff A, Ruf G, Rasenack J, et al.Complete remission of Crohn's disease after high-dose cyclophosphamide and autologous stem cell transplantation. Bone Marrow Transplant 2003;32(3):337-40. [DOI] [PubMed] [Google Scholar]
Lamb 2019
- Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al.British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68(Suppl 3):s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrance 2014
- Lawrance IC.What is left when anti-tumour necrosis factor therapy in inflammatory bowel diseases fails? World Journal of Gastroenterology 2014;20(5):1248-58. [DOI] [PMC free article] [PubMed]
Lee 2013
- Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim DS, et al.Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn's fistula. Stem Cells (Dayton, Ohio) 2013;31(11):2575-81. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lichtenstein 2018
- Lichtenstein GR, Loftus E V, Isaacs KL, Regueiro MD, Gerson LB, Sands BE.ACG Clinical Guideline: management of Crohn's disease in adults. Official journal of the American College of Gastroenterology (ACG) 2018;113(4):481-517. [DOI] [PubMed] [Google Scholar]
Lightner 2017
- Lightner AL, Faubion WA.Mesenchymal stem cell injections for the treatment of perianal Crohn's disease: what we have accomplished and what we still need to do. Journal of Crohn's & Colitis 2017;11(10):1267-76. [DOI] [PubMed] [Google Scholar]
Lightner 2019a
- Lightner AL.The present state and future Direction of regenerative medicine for perianal Crohn's disease. Gastroenterology 2019;156(8):2128-2130. e4. [DOI] [PubMed] [Google Scholar]
Lightner 2019b
- Lightner AL, Du Z, Peterson TE, Shi A, Li M, Romero AS, Behfar A.Commonly used immunosuppressives affect mesenchymal stem cell viability and function: should we rethinking clinical trial inclusion and exclusion criteria? Crohn's Colitis 360 2019;1(3):1-8. [Google Scholar]
Lim 2016
- Lim W-C, Wang Y, MacDonald JK, Hanauer S.Aminosalicylates for induction of remission or response in Crohn's disease. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD008870. [DOI: 10.1002/14651858.CD008870.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 2016
- MacDonald JK, Nguyen TM, Khanna R, Timmer A.Anti-IL-12/23p40 antibodies for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD007572. [DOI: 10.1002/14651858.CD007572.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2014
- McDonald JW, Wang Y, Tsoulis DJ, MacDonald JK, Feagan BG.Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database of Systematic Reviews 2014, Issue 8. Art. No: CD003459. [DOI: 10.1002/14651858.CD003459.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molendijk 2018
- Molendijk I, Meulen-de Jong AE, Verspaget HW, Veenendaal RA, Hommes DW, Bonsing BA, et al.Standardization of mesenchymal stromal cell therapy for perianal fistulizing Crohn’s disease. European Journal of Gastroenterology & Hepatology 2018;30(10):1148-54. [DOI] [PubMed] [Google Scholar]
Molodecky 2012
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al.Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46-54. [DOI] [PubMed] [Google Scholar]
Ng 2017
- Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al.Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390(10114):2769-78. [DOI] [PubMed] [Google Scholar]
NICE 2019
- NICE.Darvadstrocel for treating complex perianal fistulas in Crohn’s disease. National Institute for Health and Care Excellence (NICE)Guidelines, Technology Appraisal Guidance 9 January 2019;[TA556]:1-24. [Google Scholar]
Nikfar 2013
- Nikfar S, Ehteshami-Afshar S, Abdollahi M.Is Certolizumab pegol safe and effective in the treatment of patients with moderate to severe Crohn's disease? A meta-analysis of controlled clinical trials. Iranian Red Crescent Medical Journal 2013;15(8):668-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oyama 2005
- Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, et al.Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology 2005;128(3):552-63. [DOI] [PubMed] [Google Scholar]
Panés 2018
- Panés J, García-Olmo D, Van AG, Colombel JF, Reinisch W, Baumgart DC, et al.Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn's disease. Gastroenterology 2018;154(5):1334-1342.e4. [DOI] [PubMed] [Google Scholar]
Peyrin‐Biroulet 2017
- Peyrin-Biroulet L, Van AG, Gomez-Ulloa D, Garcia-Alvarez L, Lara N, Black CM, et al.Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clinical Gastroenterology and Hepatology 2017;15(1):25-36.e27. [DOI] [PubMed] [Google Scholar]
Pockley 2018
- Pockley AG, Lindsay JO, Foulds GA, Rutella S, Gribben JG, Alexander T, et al.Immune reconstitution after autologous hematopoietic stem cell transplantation in Crohn's disease: current status and future directions. A review onbBehalf of the EBMT Autoimmune Diseases Working Party and the Autologous Stem Cell Transplantation In Refractory CD-Low Intensity Therapy Evaluation Study Investigators. Frontiers In Immunology 2018;9:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiu 2017
- Qiu X, Feng J-R, Chen L-P, Liu S, Zhang M, Zhou Z, et al.Efficacy and safety of autologous hematopoietic stem cell therapy for refractory Crohn's disease: a systematic review and meta-analysis. Medicine 2017;96(26):e7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raine 2021
- Tim R, Bram V, Uri K, Konstantinos K, Rimma G, Raja A, et al.ECCO topical review: refractory inflammatory bowel disease. Journal of Crohns and Colitis 2021;15(10):1605-20. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan).Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2020 [Computer program]
- RevMan Manager Web (RevMan Web).Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Rezaie 2015
- Rezaie A, Kuenzig ME, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH, et al.Budesonide for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD000296. [DOI: 10.1002/14651858.CD000296.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ricart 2013
- Ricart E, Jauregui-Amezaga A, Ordas I, Pino S, Ramirez AM, Panes J.Cell therapies for IBD: what works? Current Drug Targets 2013;14(12):1453-9. [DOI] [PubMed] [Google Scholar]
Rossi 2011
- Rossi L, Challen GA, Sirin O, Lin KK-Y, Goodell MA.Hematopoietic stem cell characterization and isolation. Methods in Molecular Biology (Clifton, N.J.) 2011;750:47-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz 2015
- Ruiz MA, Kaiser JRL, Gouvea FMA, Quadros LG.Remission of refractory Crohn's disease after autologous hematopoietic stem cell transplantation. Revista Brasileira de Hematologia e Hemoterapia 2015;37(2):136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz 2018
- Ruiz MA, Kaiser JRL, Piron-Ruiz L, Peña-Arciniegas T, Saran PS, De Quadros LG.Hematopoietic stem cell transplantation for Crohn's disease: Gaps, doubts and perspectives. World Journal of Stem Cells 2018;10(10):134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandborn 2013
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al.Vedolizumab as induction and maintenance therapy for Crohn's disease. New England Journal of Medicine 2013;369(8):711-21. [DOI] [PubMed] [Google Scholar]
Satsangi 2006
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF.The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55(6):749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ.GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391-400. [DOI] [PubMed] [Google Scholar]
Scott 2018
- Scott Lesley J.Darvadstrocel: a review in treatment-refractory complex perianal fistulas in Crohn’s disease. BioDrugs 2018;32(6):627-34. [DOI] [PubMed] [Google Scholar]
Takeda 2021
- Takeda Pharmaceutical Company Limited.Takeda submits new drug application to manufacture and market Darvadstrocel in Japan for treatment of complex perianal fistulas in adult patients with Crohn’s disease. Takeda Pharmaceutical Company Limited (TSE:4502/NYSE:TAK) news release 2021;Online:Online. [NEWS RELEASE: https://www.takeda.com/newsroom/newsreleases/2021/takeda-submits-new-drug-application-to-manufacture-and-market-darvadstrocel--in-japan-for-treatment-of-complex-perianal-fistulas-in-adult-patients-with-crohns-disease/] [Google Scholar]
Tremaine 1997
- Tremaine W J.Refractory IBD: medical management. Netherlands Journal of Medicine 1997;50(2):S12-S14. [DOI] [PubMed] [Google Scholar]
Turse 2018
- Turse EP, Dailey FE, Naseer M, PartykaEK, Bragg JD, Tahan V.Stem cells for luminal, fistulizing, and perianal inflammatory bowel disease: a comprehensive updated review of the literature. Stem Cells and Cloning : Advances And Applications 2018;11:95-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vidula 2015
- Vidula N, Villa M, Helenowski IB, Merchant M, Jovanovic BD, Meagher R, et al.Adverse events during hematopoietic stem cell Infusion: analysis of the infusion product. Clinical Lymphoma, Myeloma & Leukemia 2015;15(11):e157-62. [DOI] [PubMed] [Google Scholar]
Wiarda 2012
- Wiarda BM, Mensink PB, Heine DG, Stolk M, Dees J, Hazenberg H, et al.Small bowel Crohn's disease: MR enteroclysis and capsule endoscopy compared to balloon-assisted enteroscopy. Abdominal Imaging 2012;37(3):397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
El‐Nakeep 2018/07/09
- El‐Nakeep S, Abdel Latif O, Shawky A, Nabhan AF.Stem cell transplantation for induction of remission in medically refractory Crohn’s disease. The Cochrane Database of Systematic Reviews 2018;2018(7):CD013070. [DOI] [PMC free article] [PubMed] [Google Scholar]


