Abstract
Background
There was considerable debate regarding the effect of mean blood glucose (MBG) and glycemic variability (GV) on the mortality of septic patients. This retrospective cohort study aimed to assess the association between MBG and GV with ICU mortality of sepsis patients and to explore the optimal MBG range.
Methods
Sepsis patients were enrolled from the Medical Information Mart for Intensive Care IV database (MIMIC-IV). MBG and glycemic coefficient of variation (GluCV) were, respectively, calculated to represent the overall glycemic status and GV during ICU stay. The associations between MBG, GluCV, and ICU mortality of the septic patients were assessed by using multivariate logistic regression in different subgroups and the severity of sepsis. Restricted cubic splines evaluated the optimal MBG target.
Results
A total of 7,104 adult sepsis patients were included. The multivariate logistic regression results showed that increased MBG and GluCV were significantly correlated with ICU mortality. The adjusted odds ratios were 1.14 (95% CI 1.09–1.20) and 1.05 (95% CI 1.00–1.12). However, there was no association between hyperglycemia and ICU mortality among diabetes, liver disease, immunosuppression, and hypoglycemia patients. And the impact of high GluCV on ICU mortality was not observed in those with diabetes, immunosuppression, liver disease, and non-septic shock. The ICU mortality risk of severe hyperglycemia (≧200 mg/dl) and high GluCV (>31.429%), respectively, elevated 2.30, 3.15, 3.06, and 2.37, 2.79, 3.14-folds in mild (SOFA ≦ 3), middle (SOFA 3–7), and severe group (SOFA ≧ 7). The MBG level was associated with the lowest risk of ICU mortality and hypoglycemia between 120 and 140 mg/dl in the subgroup without diabetes. For the diabetic subset, the incidence of hypoglycemia was significantly reduced when the MBG was 140–190 mg/dl, but a glycemic control target effectively reducing ICU mortality was not observed.
Conclusion
MBG and GluCV during the ICU stay were associated with all-cause ICU mortality in sepsis patients; however, their harms are not apparent in some particular subgroups. The impact of hyperglycemia and high GV on death increased with the severity of sepsis. The risk of ICU mortality and hypoglycemia in those with no pre-existing diabetes was lower when maintaining the MBG in the range of 120–140 mg/dl.
Keywords: sepsis, glucose metabolism disorders, mortality, restricted cubic splines regression, glycemic control
Introduction
Sepsis, defined as organ dysfunction caused by a dysregulated host response to infection by the 2021 Surviving Sepsis Campaign (SSC) Guideline, is associated with high mortality and rapidly became a significant global health burden (1, 2). The glycometabolism disorder is highly prevalent in critically ill patients, especially those with sepsis (3). The activation of stress induces this disturbance, typically manifested as hyperglycemia and increased glycemic variability (GV) (4). Specifically, under the attack of infections, the overwhelming release of pro-inflammatory mediums results in excessive hepatic gluconeogenesis and peripheral insulin resistance during sepsis (5). Catecholamines and cortisol, released by the adrenal cortex through the activated hypothalamic-pituitary-adrenocortical axis, also play significant roles (6).
The unified blood glucose (BG) management protocols for sepsis patients has not been established, even though much research has been conducted to clarify the specific mechanisms of the glycometabolism disorder. At present, the controversy on glycemic management in patients with sepsis mainly focuses on two aspects. First, the influence of elevated BG has not been fully elucidated. Previous literature has examined the impact of hyperglycemia on poor prognosis in different critically ill patients, such as those with myocardial infarction (7), acute pancreatitis (8), and stroke (9). However, these connections are not consistent across sepsis patients. Many trials have reported that hyperglycemia is associated with increased short-term mortality of sepsis patients (10–12), but neutral even lower mortality risks have also been found (13–16). These seemingly opposite phenomena suggested complex non-linear relationships between the hyperglycemic effect and the prognosis in sepsis patients (17). Although diabetic condition may be associated with the apparent inconsistencies, it was unreasonable to consider it as a specific interpretation. This was because the influence of hyperglycemia also differs in sepsis patients combined with diabetes (11, 13), and it suggested that other disease states likely also play a role. Second, the interaction between overall BG and GV levels was not clear. Although Magee et al. and Chao et al. have respectively demonstrated that early fluctuation disorder in BG increased 30-day mortality and all-cause hospital mortality in sepsis patients (18, 19), the majority of sepsis patients experienced a relapse of the disease. Thus, the overall GV levels during ICU hospitalization seem more relevant to septic prognosis than early BG fluctuation. Third, the optimal BG target is not yet confirmed. Several multicenter studies have disproved the protective effect of traditional intensive glucose control in sepsis patients, such as VISEP and NICE-SUGAR (20, 21). Furthermore, the 2021 SSC Guidelines recommend initiating insulin therapy when the glucose level ≧180 mg/dl and maintenance ranges from 144 to 180 mg/dl (1). Nevertheless, this recommendation draws on the American Diabetes Association Standards of Medical Care in Diabetes Guideline, specific to the entire critically ill population (22). Few studies have focused on the optimal target of BG control, and thus further investigations are necessary considering the heterogeneity of septic patients.
Therefore, we performed a retrospective cohort study based on an extensive, publicly available database called Medical Information Mart for Intensive Care IV (MIMIC-IV). Our primary aim was to examine the association of overall BG and GV levels during ICU admission with all-cause ICU mortality in sepsis patients. The secondary aim of this study was to investigate the optimal range of BG in patients with sepsis and each subgroup. We hypothesized that the influence of BG and GV in different subgroups of sepsis patients on ICU mortality might differ, and the ideal glucose range might also be different across sepsis subgroups.
Methods
Study Population and Data Extraction
The Massachusetts Institute of Technology established the MIMIC-IV (1.0 version) database, which contained the medical records of 382,278 in-patients who received care at the Beth Israel Deaconess Medical Center between 2008 and 2019 (23). The latter is one of the preeminent academic medical and referral centers in the Boston area, in which 77 critical care beds are contained. Users can screen demographic characteristics, vital signs, laboratory test results, imaging examinations of each patient by using a unique code given during admission. Lu has completed the Collaborative Institutional Training Initiative program course (Certification number 36763801). Because the MIMIC-IV database is a publicly available anonymized database, approval from the ethical committee was not necessary.
In the present study, we extracted patients' parameters, including (1) demographic features (age, gender), type of care unit, body mass index (BMI); (2) neutrophil to lymphocyte ratio (NLR), white blood cell count (WBC), Sequential Organ Failure Assessment (SOFA) score, Acute Physiology Score III (APS III), Charlson Comorbidity Index within the first 24 h after ICU admission; (3) anamnesis (diabetes, immunosuppression, myocardial infarct, congestive heart failure, peripheral vascular disease, liver disease, renal disease, cerebrovascular disease, and chronic obstructive pulmonary disease), infection site; (4) mean BG and glucose variability during ICU stay; (5) the use of mechanical ventilation (MV), renal replacement therapy (RRT), norepinephrine and insulin during ICU stay; (6) incidence of septic shock and hypoglycemia during ICU stay; (7) the length of ICU stay, 7-day mortality, 28-day mortality, ICU mortality of all patients. Immunosuppression was defined as having any of the following major immune diseases: lymphoma, acquired immune deficiency syndrome, solid metastatic tumor, malignant tumor, or autoimmune diseases. All related diseases were identified by the International Classification of Diseases, Ninth Revision (ICD-9), combined with Tenth Revision (ICD-10) diagnosis codes when the patient is discharged.
All adult sepsis patients (≧18 years) were screened for analysis. We excluded patients who stayed <48 h in the ICU to avoid inaccurate valuation of the condition of glycemic fluctuations. Furthermore, patients were also excluded if they had missing daily BG records. In this study, the diagnosis of sepsis was based on the criteria of the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), which define sepsis as SOFA ≧2 and the presence of infection or suspected infection (24). Suspected infection refers to antibiotics administered within 3 days or before 24 h of culture collection. It is difficult to implement the procedure which is strictly based on the Sepsis-3 standard to screen septic shock patients in the MIMIC-IV database, and thus we draw on previous experience in this study (25). Septic shock was defined as sepsis with hypotension, and the hypotension was assumed for sepsis patients when any vasopressor was administered during the ICU stay, including norepinephrine, epinephrine, phenylephrine, vasopressin, and dopamine dobutamine or milrinone. For patients with multiple ICU and hospital admissions, we only included data from the first hospital admission and first ICU stay. The flowchart is shown in Supplementary Figure 1.
Glucose Measurement and Glycemic Variability Definition
For each included patient, we have calculated the mean BG (MBG) during ICU stay using all biochemical glucose records. MBG were stratified as follows: no hyperglycemia (≦140 mg/dl), mild hyperglycemia (140–200 mg/dl), and severe hyperglycemia (≧200 mg/dl) based on previous work (26). We defined hypoglycemia as at least one glucose record <70 mg/dl during ICU stay. Here, we considered glucose≦140 mg/dl as a reference value to which each category is compared. In this analysis, the overall GV was evaluated by calculating the coefficient of variation (GluCV), which is the ratio of the standard deviation (GluCD) to the glycemic average. Due to the lack of universally accepted clinical criteria for grading the GluCV status of critically ill patients, we grouped GluCV into three categories according to the percentiles (low: <25th; mild: 25–75th; high: >75th).
Restricted Cubic Splines
Linear regression was often used to identify the relationship between independent and dependent variables in clinical trials, but this linear relationship was not always easy to meet and particularly likely to occur when the independent variable was continuous. We usually transformed continuous variables into categorical variables based on some special cutoff points to explore the unknown non-linear relationship. However, this approach may change the shape of the dose-response relationship and induce inevitable information loss. Restricted cubic splines (RCS) analysis as a smoothness function is well-fit to non-linear relationships and retains independent local structure. Recently, RCS was widely used to assess the dose-response relationship between continuous variables and dependent variables (27, 28). RCS can be seen as a piecewise polynomial, it requires a continuous second-order derivative existing in each segmented spot (29). The main operation of RCS is that the setting of the knots count and position is required before its use and it may have an influence on the overall structure. With the reference from the previous study (28), we used RCS with five knots, corresponding to the 5, 35, 50, 65, and 95th percentiles, to explore the relationship between MBG with all-cause ICU mortality in sepsis patients. The reference was set at 140 mg/dl.
Statistical Analysis
The present retrospective study of the collected observational data set was stratified according to MBG and GluCV. We performed a normality test (Agostino tests), followed by a descriptive analysis of the data. Continuous variables were expressed as mean (standard deviation) while non-parametric variables were expressed as the median (interquartile ranges, IQR) and were compared using the one-way ANOVA test or non-parametric Kruskal-Wallis test. The categorical variables are expressed as a frequency (percentage) and were compared using the X2 or rank-sum tests. The random forests function handled missing values. However, the variable was deleted when >30% of the values were lacking. Outlier expressions were defined as values that are greater than the 99th or lower than the 1st percentile. Variables with outliers were winsorized using the winsor2 command in STATA software.
Multivariate logistic regression was performed to determine the connection between MBG, GluCV, and ICU mortality of sepsis patients separately. MBG was modeled as both continuous and categorical scale; while the MBG category set cutoffs on 140 and 200 mg/dl, and the GluCV set on the first and third quartile. The potential confounders were adjusted gradually in three models. Initially, we adjusted for age and gender (Model 1). Subsequently, related comorbidities, such as diabetes and immunosuppression, have been adjusted (Model 2). Finally, we adjusted for NLR- related early disease severity scores (APS III, SOFA, and Charlson Comorbidity Index), MBG/GluCV, occurrence or not of septic shock and hypoglycemia, and related interventions including the use of MV, RRT, and insulin except norepinephrine during ICU stay (Model 3).
In the subgroup analyses, we stratified the study population by age (≧65, <65 years), gender (male, female), diabetes, immunosuppression, liver disease, hypoglycemia, and septic shock. The interaction of the levels of MBG and GluCV with the above covariates for stratification of ICU mortality was examined by including two-factor interaction terms in the multivariate logistic regression model. Meanwhile, the interactions were visualized by the slopes of the regression lines.
To evaluate the performance of MBG and GluCV in predicting ICU mortality in sepsis patients, we conducted receiver operating characteristic (ROC) curves. We also conducted the dose-response association using the RCS model with five knots located at the 5, 35, 50, 65, and 95th percentiles of the overall distribution for MBG levels based on the multivariate logistic regression model. The exact number and location of knots from the overall population splines were also applied in the splines for each subgroup to allow direct comparison of the overall and stratified analyses. All statistical analyses were performed using STATA 15.1 (College Station, Texas) and R 3.6.2 (Chicago, Illinois) software. The p-values with < 0.05 were taken as statistically significant (two-sided).
Results
Characteristics of Included Sepsis Participants
In the MIMIC-IV database, a total of 12,274 patients were diagnosed with sepsis at their first ICU admission according to the definition of sepsis 3.0; ultimately, 7,104 patients were included in the analysis; 2,661 patients lacked the height data. Thus, all pre-defined features were included except the BMI index. During the whole ICU stay, the minimum and maximum values of MBG were 81.33 and 294.78 mg/dl, respectively; in addition, the minimum GluCV was 4.22 %, and the maximum GluCV was 84.76%. The incidence of septic shock was 40.36% (2,867/7,104), insulin treatment was 54.43% (3,867/7,104), hypoglycemia was 14.26% (1,013/7,104), diabetes was 31.53% (2,240/7,104), and liver disease was 18.65% (1,325/7,104). Among the included septic patients, 841 (11.84%) died during the ICU stay. The MBG of patients who died was significantly elevated compared with survivors [128 (112–155) vs. 142 (119–173); p < 0.001]. The dead group also showed significantly increased GluCV [21.5 (14.8–30.5) vs. 26.4 (18.4–37.1); p < 0.001]. The distribution of MBG, GluCV within the two cohorts is shown in Supplementary Figure 2.
The clinical characteristics based on MBG and GluCV categories can be found in Table 1. An upward trend was observed at higher MBG levels for initial NLR value, APS III scoring, and the prevalence of diabetes, myocardial infarct, congestive heart failure, peripheral vascular disease, cerebrovascular disease, and renal disease. Similarly, as MBG levels increased, elevated risk of urinary tract infection and septicemia was also presented; but higher MBG levels were not positively correlated with poor prognosis in sepsis patients. For different GluCV categories, the initial inflammatory markers (WBC, NLR) were higher in individuals with higher GluCV, and the incidence of diabetes, septic shock, myocardial infarct, congestive heart failure, renal disease, and related treatments (RRT, MV, norepinephrine, and insulin infusion) was also elevated. Unlike the MBG levels, there was a positive association between GluCV and the risk of poor outcomes.
Table 1.
Baseline demographic and clinical characteristics by mean glucose level and glycemic variability in patients with sepsis.
| Features | Mean blood glucose level during ICU hospitalization | p-value | Glycemic variability during ICU hospitalization | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Glucose <= 140 | Glucose 140 to <200 | Glucose > = 200 | GlUcv < 15.174 | GLUcv 15.174 to <= 31.429 | GlUcv > 31.429 | |||
| N = 4,407 | N = 2,029 | N = 668 | N = 1,776 | N = 3,552 | N = 1,776 | |||
| Demographic features | ||||||||
| Age (years) | 68.0 (54.9, 80.5) | 69.8 (58.8, 79.8) | 68.8 (59.6, 78.1) | 0.001 | 68.5 (55.5, 80.7) | 68.5 (56.4, 80.1) | 68.8 (57.4, 79.6) | 0.9 |
| Gender | 0.289 | <0.001 | ||||||
| Female | 2,010 (45.6%) | 920 (45.3%) | 283 (42.4%) | 720 (40.5%) | 1,670 (47.0%) | 823 (46.3%) | ||
| Male | 2,397 (54.4%) | 1,109 (54.7%) | 385 (57.6%) | 1,056 (59.5%) | 1,882 (53.0%) | 953 (53.7%) | ||
| Care unit type | <0.001 | <0.001 | ||||||
| Cardiac vascular intensive care unit | 568 (12.9%) | 171 (8.43%) | 13 (1.95%) | 267 (15.0%) | 339 (9.54%) | 146 (8.22%) | ||
| Coronary care unit | 267 (6.06%) | 184 (9.07%) | 72 (10.8%) | 114 (6.42%) | 254 (7.15%) | 155 (8.73%) | ||
| Medical intensive care unit | 1,403 (31.8%) | 647 (31.9%) | 267 (40.0%) | 507 (28.5%) | 1,180 (33.2%) | 630 (35.5%) | ||
| Medical/surgical intensive care unit | 964 (21.9%) | 411 (20.3%) | 166 (24.9%) | 364 (20.5%) | 765 (21.5%) | 412 (23.2%) | ||
| Neuro intermediate | 112 (2.54%) | 38 (1.87%) | 11 (1.65%) | 55 (3.10%) | 81 (2.28%) | 25 (1.41%) | ||
| Neuro stepdown | 60 (1.36%) | 7 (0.34%) | 5 (0.75%) | 23 (1.30%) | 43 (1.21%) | 6 (0.34%) | ||
| Neuro surgical intensive care unit | 70 (1.59%) | 42 (2.07%) | 11 (1.65%) | 41 (2.31%) | 64 (1.80%) | 18 (1.01%) | ||
| Surgical intensive care unit | 559 (12.7%) | 316 (15.6%) | 78 (11.7%) | 228 (12.8%) | 482 (13.6%) | 243 (13.7%) | ||
| Trauma SICU | 404 (9.17%) | 213 (10.5%) | 45 (6.74%) | 177 (9.97%) | 344 (9.68%) | 141 (7.94%) | ||
| Inflammatory indicators # | ||||||||
| WBC_max (X 103/uL) | 13.6 (9.50, 19.0) | 15.1 (10.7, 20.6) | 15.0 (10.9, 20.3) | <0.001 | 13.4 (9.60, 18.3) | 14.2 (9.80, 19.6) | 15.1 (10.6, 21.1) | <0.001 |
| NLR | 8.73 (5.80, 14.4) | 9.46 (6.33, 15.9) | 9.71 (6.90, 16.4) | <0.001 | 8.20 (5.55, 12.9) | 9.13 (6.13, 15.3) | 9.77 (6.61, 16.7) | <0.001 |
| Severe scoring | ||||||||
| SOFA | 3.00 (2.00, 5.00) | 3.00 (2.00, 5.00) | 3.00 (2.00, 4.00) | 0.231 | 3.00 (2.00, 4.00) | 3.00 (2.00, 5.00) | 3.00 (2.00, 5.00) | <0.001 |
| APSIII | 54.0 (39.0, 73.0) | 61.0 (47.0, 82.0) | 65.0 (52.0, 83.0) | <0.001 | 49.0 (36.0, 63.0) | 58.0 (43.0, 77.0) | 67.0 (50.0, 87.0) | <0.001 |
| Charlson comorbidity index | 6.00 (4.00, 8.00) | 6.00 (5.00, 8.00) | 7.00 (5.00, 9.00) | <0.001 | 5.00 (4.00, 7.25) | 6.00 (4.00, 8.00) | 6.00 (5.00, 8.00) | <0.001 |
| Comorbidity | ||||||||
| Diabetes | 708 (16.1%) | 996 (49.1%) | 536 (80.2%) | <0.001 | 324 (18.2%) | 992 (27.9%) | 924 (52.0%) | <0.001 |
| Septic shock | 1,761 (40.0%) | 868 (42.8%) | 238 (35.6%) | 0.003 | 624 (35.1%) | 1,465 (41.2%) | 778 (43.8%) | <0.001 |
| Immunosuppression | 839 (19.0%) | 373 (18.4%) | 124 (18.6%) | 0.811 | 323 (18.2%) | 707 (19.9%) | 306 (17.2%) | 0.046 |
| Myocardial infarct | 598 (13.6%) | 374 (18.4%) | 174 (26.0%) | <0.001 | 234 (13.2%) | 554 (15.6%) | 358 (20.2%) | <0.001 |
| Congestive heart failure | 1,279 (29.0%) | 728 (35.9%) | 263 (39.4%) | <0.001 | 503 (28.3%) | 1,139 (32.1%) | 628 (35.4%) | <0.001 |
| Peripheral vascular disease | 455 (10.3%) | 214 (10.5%) | 84 (12.6%) | 0.211 | 167 (9.40%) | 362 (10.2%) | 224 (12.6%) | 0.004 |
| Cerebrovascular disease | 612 (13.9%) | 330 (16.3%) | 122 (18.3%) | 0.002 | 299 (16.8%) | 517 (14.6%) | 248 (14.0%) | 0.034 |
| COPD | 1,215 (27.6%) | 576 (28.4%) | 187 (28.0%) | 0.79 | 444 (25.0%) | 1,052 (29.6%) | 482 (27.1%) | 0.001 |
| Renal disease | 899 (20.4%) | 568 (28.0%) | 224 (33.5%) | <0.001 | 353 (19.9%) | 794 (22.4%) | 544 (30.6%) | <0.001 |
| Liver disease | 808 (18.3%) | 398 (19.6%) | 119 (17.8%) | 0.398 | 281 (15.8%) | 686 (19.3%) | 358 (20.2%) | 0.001 |
| Infection site | ||||||||
| Skin subcutaneous tissue | 334 (7.58%) | 173 (8.53%) | 63 (9.43%) | 0.16 | 137 (7.71%) | 286 (8.05%) | 147 (8.28%) | 0.823 |
| Catheter related | 88 (2.00%) | 56 (2.76%) | 16 (2.40%) | 0.154 | 32 (1.80%) | 84 (2.36%) | 44 (2.48%) | 0.325 |
| Urinary tract | 874 (19.8%) | 359 (17.7%) | 150 (22.5%) | 0.016 | 331 (18.6%) | 707 (19.9%) | 345 (19.4%) | 0.545 |
| Intestinal infection | 248 (5.63%) | 110 (5.42%) | 32 (4.79%) | 0.667 | 75 (4.22%) | 214 (6.02%) | 101 (5.69%) | 0.023 |
| Septicemia | 1,584 (35.9%) | 762 (37.6%) | 286 (42.8%) | 0.002 | 516 (29.1%) | 1,370 (38.6%) | 746 (42.0%) | <0.001 |
| Pulmonary infection | 1,468 (33.3%) | 706 (34.8%) | 210 (31.4%) | 0.239 | 530 (29.8%) | 1,282 (36.1%) | 572 (32.2%) | <0.001 |
| Curing | ||||||||
| RRT | 419 (9.51%) | 271 (13.4%) | 79 (11.8%) | <0.001 | 106 (5.97%) | 388 (10.9%) | 275 (15.5%) | <0.001 |
| MV | 2,408 (54.6%) | 1,273 (62.7%) | 389 (58.2%) | <0.001 | 814 (45.8%) | 2,144 (60.4%) | 1,112 (62.6%) | <0.001 |
| MV duration (h) | 6.50 (0.00, 45.0) | 19.0 (0.00, 80.0) | 14.3 (0.00, 61.0) | <0.001 | 0.00 (0.00, 22.4) | 14.0 (0.00, 66.3) | 20.0 (0.00, 75.4) | <0.001 |
| Norepinephrine | 1,156 (26.2%) | 614 (30.3%) | 188 (28.1%) | 0.003 | 343 (19.3%) | 1,028 (28.9%) | 587 (33.1%) | <0.001 |
| Insulin | 1,627 (36.9%) | 1,597 (78.7%) | 643 (96.3%) | <0.001 | 667 (37.6%) | 1,884 (53.0%) | 1,316 (74.1%) | <0.001 |
| Outcomes | ||||||||
| Length of ICU stay | 3.92 (2.76, 6.64) | 4.52 (2.91, 8.65) | 3.89 (2.74, 6.87) | <0.001 | 3.28 (2.55, 5.05) | 4.48 (2.93, 8.01) | 4.37 (2.86, 7.93) | <0.001 |
| Mortality_hospital | 666 (15.1%) | 481 (23.7%) | 152 (22.8%) | <0.001 | 223 (12.6%) | 652 (18.4%) | 424 (23.9%) | <0.001 |
| Mortality_ICU | 407 (9.24%) | 330 (16.3%) | 104 (15.6%) | <0.001 | 124 (6.98%) | 416 (11.7%) | 301 (16.9%) | <0.001 |
| Mortality_ICU_7day | 262 (5.95%) | 205 (10.1%) | 68 (10.2%) | <0.001 | 105 (5.91%) | 242 (6.81%) | 188 (10.6%) | <0.001 |
| Mortality_ICU_28day | 607 (13.8%) | 434 (21.4%) | 142 (21.3%) | <0.001 | 209 (11.8%) | 587 (16.5%) | 387 (21.8%) | <0.001 |
| Hypoglycemia | 759 (17.2%) | 198 (9.76%) | 56 (8.38%) | <0.001 | 43 (2.42%) | 460 (13.0%) | 510 (28.7%) | <0.001 |
Continuous data are presented as the median (interquartile range) and categorical data as n (%).
WBC, white blood cell; NLR, neutrophil to lymphocyte ratio; SOFA, Sequential Organ Failure Assessment; APS III, Acute Physiology Score III; COPD, chronic obstructive pulmonary disease; RRT, renal replacement therapy; MV, mechanical ventilation.
Inflammatory indicators use the maximum value in the first 24 h after ICU admission.
Association Between MBG, GluCV, and ICU Mortality
On a continuous scale, the results of multivariable logistic regression showed that every 20 mg/dl or 10% rise in MBG and GluCV was, respectively, associated with 1.14-fold (95% CI 1.09–1.20) and 1.05-fold (95% CI 1.00–1.12) increase in the risk of ICU mortality (Table 2, Model 3). As described previously, we divided the patients into three tertiles according to their MBG levels. Compared with MBG levels ≦140 mg/dl, septic patients with MBG levels between 140 and 200 mg/dl and ≧200 mg/dl had an increased risk of ICU mortality, the aORs were 1.97 (95% CI 1.61–2.41) and 2.23 (95% CI 1.64–3.03), respectively (Table 2, Model 3). Similarly, the 25 and 75th percentiles of GluCV were used as the cutoff values to subdivide patients with sepsis into three risk categories. Mortality among patients in the lowest category of GluCV was 6.98%, increasing to 11.7 and 16.9% in the median and highest category (Table 1). The patients with GluCV ≧ 31.429% had a 0.36 (95% CI 0.04–0.77) higher risk of ICU mortality than those with GluCV < 15.174% (Table 2, Model 3).
Table 2.
Odds ratio for death in ICU according to the mean glucose levels and glycemic variability on a continuous scale or in tertile groups.
| ICU mortality | N | Event | Crude OR (95% CI) | P a | a.OR (95% CI) | P b | a.OR (95% CI) | P c | a.OR (95% CI) | P d | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||||
| MBG | Glucose (per 20 mg/dl) | 7,104 | 841 | 1.12 (1.08, 1.15) | <0.001 | 1.12 (1.08, 1.15) | <0.001 | 1.17 (1.13, 1.21) | <0.001 | 1.14 (1.09, 1.20) | <0.001 |
| Glucose (mg/dl) | |||||||||||
| Glucose < =140 | 4,407 | 407 | Ref | Ref | Ref | Ref | |||||
| Glucose >140 and <200 | 2,029 | 330 | 1.91 (1.63,2.23) | <0.001 | 1.88 (1.61, 2.19) | <0.001 | 2.16 (1.82, 2.55) | <0.001 | 1.97 (1.61, 2.41) | <0.001 | |
| Glucose >=200 | 668 | 104 | 1.81 (1.43, 2.28) | <0.001 | 1.80 (1.42, 2.16) | <0.001 | 2.39 (1.83, 3.10) | <0.001 | 2.23 (1.64, 3.03) | <0.001 | |
| GLUcv | GLUcv (per 10%) | 7,104 | 841 | 1.18 (1.13, 1.23) | <0.001 | 1.18 (1.13, 1.23) | <0.001 | 1.21 (1.16, 1.26) | <0.001 | 1.05 (1.00, 1.12) | 0.05 |
| GLUcv %; (quartile: 25%, 75%) | |||||||||||
| GLUcv <15.174 | 1,776 | 124 | Ref | Ref | Ref | Ref | |||||
| GLUcv >=15.174 and < = 31.429 | 3,552 | 416 | 1.77 (1.44, 2.19) | <0.001 | 1.76 (1.43, 2.18) | <0.001 | 1.76 (1.43, 2.19) | <0.001 | 1.02 (0.81, 1.29) | 0.86 | |
| GLUcv >= 31.429 | 1,776 | 301 | 2.72 (2.19, 3.40) | <0.001 | 2.71 (2.18, 3.39) | <0.001 | 2.98 (2.37, 3.76) | <0.001 | 1.36 (1.04, 1.77) | 0.03 | |
Model 1: adjusted for age and gender.
Model 2: adjusted for age, gender and comorbidity.
Model 3: adjusted for age, gender, comorbidity, SOFA, APS III, Charlson Comorbidity Index, MBG/GluCV, septic shock or not, mechanical ventilation, renal replacement therapy, insulin infusion, NLR, and hypoglycemic event.
aOR and corresponding 95% CIs are presented in bold when the 95% CI cross 1.
P for crude analysis.
P for analysis based on Model 1.
P for analysis based on Model 2.
P for analysis based on Model 3.
A subgroup analyses indicated that the effect of hyperglycemia on ICU mortality is more pronounced in non-diabetic, non-immunosuppression, non-liver disease, non-hypoglycemia, and septic shock patients. Interestingly, different levels of hyperglycemia did not seem to have obvious adverse impacts on the risk of ICU mortality in patients with diabetes or liver disease. Additionally, a significant interaction effect was found between diabetes (p < 0.001), hypoglycemia (p = 0.001), liver disease (p = 0.002), and MBG levels (Table 3). Supplementary Figures 3–5 visually depicted these interactions, respectively. We observed that the ICU mortality risk among non-diabetics was consistently higher than among people with diabetes at the same level as hyperglycemia (Supplementary Figure 3). Meanwhile, increased MBG had a weak impact on ICU mortality risk for patients who experienced at least one episode of hypoglycemia (Supplementary Figure 4). One of the possible reasons is that the influence of hypoglycemia may mask the effect of hyperglycemia on death. And this phenomenon also occurred in the liver disease cohort (Supplementary Figure 5). The impact of GluCV on different subgroups varied greatly. Despite the ICU mortality risk appearing incremental with increasing GluCV, the difference was only significant in non-elderly, males, non-diabetics, non-immunosuppression, non-hypoglycemia, non-liver disease, septic shock, and patients not treated with insulin (Table 4). Furthermore, a significant interaction between age and GluCV was observed (p = 0.02) (Supplementary Figure 6).
Table 3.
Results of subgroup analyses of mean blood glucose level and ICU mortality according to clinical characteristics.
| Subgroup | N | Event | a.OR (95%CI) | Subgroup | N | Event | a.OR (95%CI) | P for interaction |
|---|---|---|---|---|---|---|---|---|
| Age < 65 | Age > = 65 | 0.98 | ||||||
| Glucose < = 140 | 1,923 | 157 | 1.00 (1.00, 1.00) | Glucose < = 140 | 2,484 | 250 | 1.00 (1.00, 1.00) | |
| 140 < Glucose <200 | 763 | 111 | 1.99 (1.42, 2.81) | 140 < Glucose <200 | 1,266 | 219 | 1.98 (1.54, 2.55) | |
| Glucose >=200 | 259 | 33 | 2.32 (1.35, 3.93) | Glucose >=200 | 409 | 71 | 2.22 (1.52, 3.24) | |
| Male | Female | 0.88 | ||||||
| Glucose < = 140 | 2,397 | 221 | 1.00 (1.00, 1.00) | Glucose < = 140 | 2,010 | 186 | 1.00 (1.00, 1.00) | |
| 140 < Glucose <200 | 1,109 | 158 | 1.83 (1.37, 2.44) | 140 < Glucose <200 | 920 | 172 | 2.16 (1.63, 2.86) | |
| Glucose >=200 | 385 | 65 | 2.77 (1.83, 4.19) | Glucose >=200 | 283 | 39 | 1.67 (1.04, 2.66) | |
| Diabetes | Non-Diabetes | <0.001 | ||||||
| Glucose < = 140 | 708 | 66 | 1.00 (1.00, 1.00) | Glucose < = 140 | 3,699 | 341 | 1.00 (1.00, 1.00) | |
| 140 < Glucose <200 | 996 | 114 | 1.04 (0.70, 1.55) | 140 < Glucose <200 | 1,033 | 216 | 2.44 (1.94, 3.06) | |
| Glucose >=200 | 536 | 66 | 1.21 (0.77, 1.93) | Glucose >=200 | 132 | 38 | 3.52 (2.18, 5.60) | |
| Immunosuppression | Non-Immunosuppression | 0.83 | ||||||
| Glucose < = 140 | 839 | 114 | 1.00 (1.00, 1.00) | Glucose < = 140 | 3,568 | 293 | 1.00 (1.00, 1.00) | |
| 140 < Glucose <200 | 373 | 79 | 1.73 (1.13, 2.63) | 140 < Glucose <200 | 1,656 | 251 | 2.15 (1.71, 2.71) | |
| Glucose >=200 | 124 | 27 | 1.83 (0.97, 3.42) | Glucose >=200 | 544 | 77 | 2.64 (1.85, 3.76) | |
| Liver disease | Non-Liver disease | 0.002 | ||||||
| Glucose < = 140 | 808 | 137 | 1.00 (1.00, 1.00) | Glucose < = 140 | 3,599 | 270 | 1.00 (1.00, 1.00) | |
| 140 < Glucose <200 | 398 | 70 | 1.15 (0.74, 1.79) | 140 < Glucose <200 | 1,631 | 260 | 2.36 (1.88, 2.96) | |
| Glucose >=200 | 119 | 17 | 1.15 (0.56, 2.32) | Glucose >=200 | 549 | 87 | 2.85 (2.01, 4.01) | |
| Hypoglycemia | Non-Hypoglycemia | 0.001 | ||||||
| Glucose < = 140 | 759 | 141 | 1.00 (1.00, 1.00) | Glucose < = 140 | 3,648 | 266 | 1.00 (1.00, 1.00) | |
| 140 < Glucose <200 | 198 | 48 | 1.33 (0.81, 2.18) | 140 < Glucose <200 | 1,831 | 282 | 2.22 (1.78, 2.77) | |
| Glucose >=200 | 56 | 11 | 0.91 (0.39, 2.04) | Glucose >=200 | 612 | 93 | 2.78 (1.98, 3.88) | |
| Septic-shock | Non-Septic shock | 0.25 | ||||||
| Glucose < = 140 | 1,761 | 186 | 1.00 (1.00, 1.00) | Glucose < = 140 | 2,646 | 221 | 1.00 (1.00, 1.00) | |
| 140 < Glucose <200 | 868 | 176 | 2.31 (1.73, 3.09) | 140 < Glucose <200 | 1,161 | 154 | 1.69 (1.27, 2.23) | |
| Glucose >=200 | 238 | 50 | 2.79 (1.77, 4.37) | Glucose >=200 | 430 | 54 | 1.84 (1.20, 2.79) |
Adjustment factors are the same as those in Model 3.
Table 4.
Results of subgroup analyses of GluCV level and ICU mortality according to clinical characteristics.
| Subgroup | N | Event | a.OR (95%CI) | Subgroup | N | Event | a.OR (95%CI) | P for interaction |
|---|---|---|---|---|---|---|---|---|
| Age < 65 | Age> = 65 | 0.02 | ||||||
| GluCV <15.174 | 741 | 34 | 1.00 (1.00, 1.00) | GluCV <15.174 | 1,035 | 90 | 1.00 (1.00, 1.00) | |
| 15.174 < = GluCV < =31.429 | 1,480 | 152 | 1.40 (0.93, 2.16) | 15.174 < = GluCV < =31.429 | 2,072 | 264 | 0.85 (0.64, 1.13) | |
| GluCV >31.429 | 724 | 115 | 2.21 (1.39, 3.57) | GluCV >31.429 | 1,052 | 186 | 1.06 (0.76, 1.47) | |
| Male | Female | 0.95 | ||||||
| GluCV <15.174 | 1,056 | 79 | 1.00 (1.00, 1.00) | GluCV <15.174 | 720 | 45 | 1.00 (1.00, 1.00) | |
| 15.174 < = GluCV < =31.429 | 1,882 | 201 | 0.92 (0.68, 1.26) | 15.174 < = GluCV < =31.429 | 1,670 | 215 | 1.22 (0.85, 1.78) | |
| GluCV >31.429 | 953 | 164 | 1.45 (1.02, 2.06) | GluCV >31.429 | 823 | 137 | 1.35 (0.89, 2.05) | |
| Diabetes | Non-Diabetes | 0.4 | ||||||
| GluCV <15.174 | 324 | 23 | 1.00 (1.00, 1.00) | GluCV <15.174 | 1,452 | 101 | 1.00 (1.00, 1.00) | |
| 15.174 < = GluCV < =31.429 | 992 | 103 | 0.92 (0.55, 1.58) | 15.174 < = GluCV < =31.429 | 2,560 | 313 | 1.03 (0.80, 1.35) | |
| GluCV >31.429 | 924 | 120 | 0.78 (0.46, 1.39) | GluCV >31.429 | 852 | 181 | 1.75 (1.29, 2.39) | |
| Immunosuppression | Non-Immunosuppression | 0.97 | ||||||
| GluCV <15.174 | 323 | 32 | 1.00 (1.00, 1.00) | GluCV <15.174 | 1,453 | 92 | 1.00 (1.00, 1.00) | |
| 15.174 < = GluCV < =31.429 | 707 | 119 | 1.17 (0.74, 1.90) | 15.174 < = GluCV < =31.429 | 2,845 | 297 | 0.99 (0.76, 1.30) | |
| GluCV >31.429 | 306 | 69 | 1.23 (0.70, 2.18) | GluCV >31.429 | 1470 | 232 | 1.42 (1.05, 1.93) | |
| Liver disease | Non-Liver disease | 0.74 | ||||||
| GluCV <15.174 | 281 | 25 | 1.00 (1.00, 1.00) | GluCV <15.174 | 1,495 | 99 | 1.00 (1.00, 1.00) | |
| 15.174 < = GluCV < =31.429 | 686 | 118 | 1.03 (0.60, 1.80) | 15.174 < = GluCV < =31.429 | 2,866 | 298 | 1.02 (0.79, 1.33) | |
| GluCV >31.429 | 358 | 81 | 1.12 (0.61, 2.10) | GluCV >31.429 | 1,418 | 220 | 1.43 (1.06, 1.93) | |
| Hypoglycemia | Non-Hypoglycemia | 0.66 | ||||||
| GluCV <15.174 | 43 | 5 | 1.00 (1.00, 1.00) | GluCV <15.174 | 1,733 | 119 | 1.00 (1.00, 1.00) | |
| 15.174 < = GluCV < =31.429 | 460 | 92 | 1.29 (0.46, 4.33) | 15.174 < = GluCV < =31.429 | 3,092 | 324 | 0.97 (0.77, 1.25) | |
| GluCV >31.429 | 510 | 103 | 1.29 (0.45, 4.37) | GluCV >31.429 | 1,266 | 198 | 1.42 (1.07, 1.88) | |
| Septic-shock | Non-Septic shock | 0.28 | ||||||
| GluCV <15.174 | 624 | 45 | 1.00 (1.00, 1.00) | GluCV <15.174 | 1,152 | 79 | 1.00 (1.00, 1.00) | |
| 15.174 < = GluCV < =31.429 | 1,465 | 204 | 1.22 (0.84, 1.79) | 15.174 < = GluCV < =31.429 | 2,087 | 212 | 0.92 (0.68, 1.25) | |
| GluCV >31.429 | 778 | 163 | 1.58 (1.05, 2.41) | GluCV >31.429 | 998 | 138 | 1.24 (0.87, 1.77) |
Adjustment factors are the same as those in Model 3.
In this study, we also subdivided the severity of sepsis according to the initial SOFA score. Those with SOFA scores ≦ 3 (25th) and ≧ 7 (75th) were correspondingly assigned to the mild group and severe group, while the 3–7 were defined as the middle group. The results demonstrated that the impact of MBG on death increased with the severity of sepsis; besides, hyperglycemia was independently associated with increased ICU mortality in each group (Supplementary Figure 7A). In addition, the same trends were also found for the relationship between septic severity and GluCV (Supplementary Figure 7B).
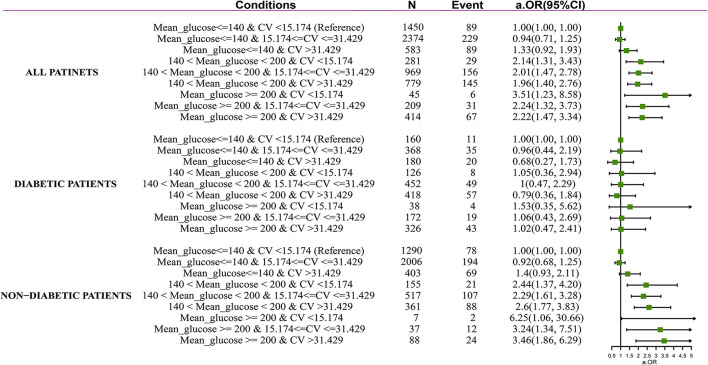
After adjustment for confounders contained in Model 3, among the subjects with non-hyperglycemia, increased GluCV did not associate with an increased risk of ICU mortality (mild GluCV: aOR 0.94, 95% CI 0.71–1.25; high GluCV: aOR 1.33, 95% CI 0.92–1.93). Among the patients with hyperglycemia, the risk of ICU mortality significantly increases regardless of higher GluCV. Notably, adjusted odds of death were markedly higher in patients with MBG above 200 mg/dl and lower GluCV values (aOR 3.51, 95% CI 1.23–8.58). In the patients without pre-existing diabetes, mild and severe hyperglycemia were also associated with increased mortality when in combination with various levels of GluCV, that is, mild hyperglycemia plus low GluCV level (aOR 2.44, 95% CI 1.37–4.20), mild hyperglycemia plus mild GluCV level (aOR 2.29, 95% CI 1.61–3.28), mild hyperglycemia plus high GluCV level (OR 2.6, 95% CI 1.77–3.83), high hyperglycemia plus low GluCV level (aOR 6.25, 95% CI 1.06–30.66), high hyperglycemia plus mild GluCV level (aOR 3.24, 95% CI 1.34–7.51), and high hyperglycemia plus high GluCV level (aOR 3.46, 95% CI 1.86–6.29). By contrast, in combination with any GluCV levels, hyperglycemia was not associated with increased mortality in diabetes patients (Figure 1).
Figure 1.
Forest plot depicting ICU mortality risk in septic patients with and without diabetes. Adjustment factors are the same as those in Model 3.
The area under the curve (AUC) of MBG, GluCV, and the combination of two indicators for predicting ICU mortality of all sepsis patients were 0.59, 0.61, and, 0.62, respectively. Three indicators significantly improved risk discrimination in non-diabetics with the AUC increasing from 0.54 to 0.64, 0.55 to 0.64, and 0.56 to 0.66 for the MBG, GluCV, and combination, respectively, compared with those in the people with diabetes. Nevertheless, the overall predictive performance was only moderate (Supplementary Figure 8, Supplementary Table 1).
Mean Glucose With the Lowest Risk of ICU Mortality
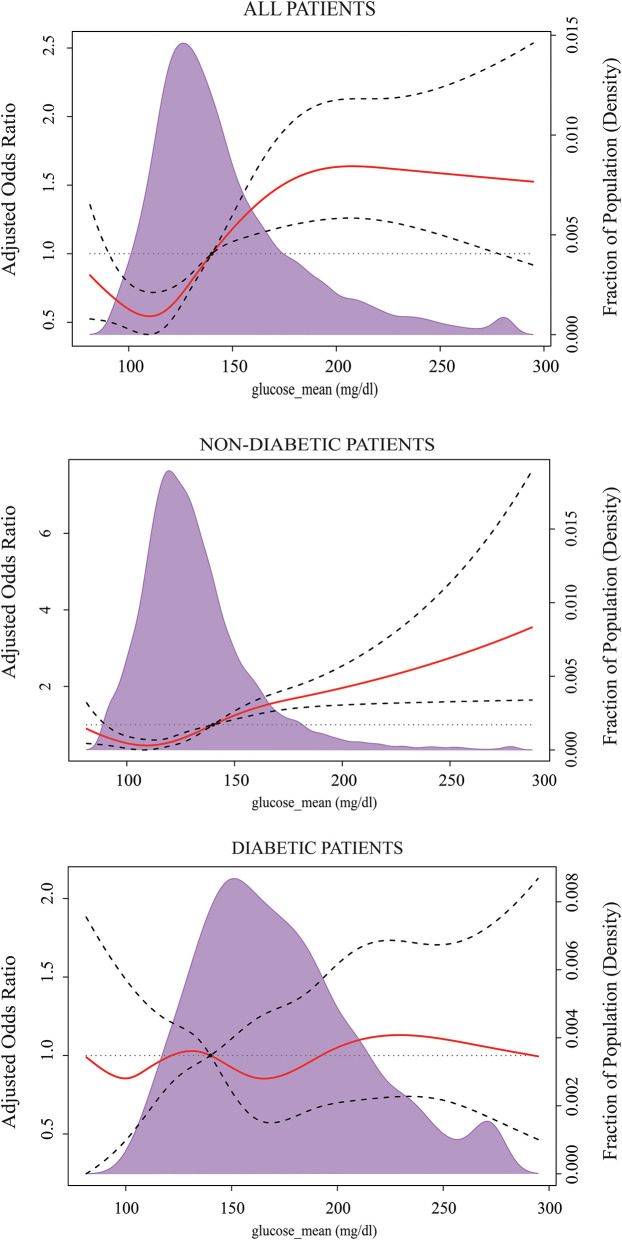
The results of RCS after multivariable adjustment presented a non-linear dose-response relationship between the levels of MBG on a continuous scale and the risk of ICU mortality. The concentration of MBG associated with the lowest risk of ICU mortality was ~120 mg/dl in the overall population. The value of aOR has an initial steep increase when MBG is lower than 120 mg/dl or ranges from 120–200 mg/dl, then plateaued. Similarly, the risk reached a minimum when the concentrations of MBG were around 120 mg/dl in non-diabetes patients, and up perpetually with MBG increasing. There was a trend for decreasing the risk of ICU mortality when MBG was between 140 and 190 mg/dl for people with diabetes, but it did not reach statistical significance (Figure 2).
Figure 2.
Multivariable-adjusted odds ratios for ICU mortality according to the levels of the mean blood glucose (MBG) on a continuous scale. Solid red lines are multivariable-adjusted odds ratios, with dashed bold lines showing 95% confidence intervals derived from restricted cubic spline regressions with five knots. Reference lines for no association are indicated by the black dashed lines at a hazard ratio of 1.0, and the reference knot set at 140 mg/dl. Purple regions indicate the fraction of the population with different levels of MBG. Adjustment factors are the same as those in Model 3 of Table 2.
Considering the risk of hypoglycemia, we further compared the incidence of hypoglycemia and death in sepsis patients when containing MBG below 120 mg/dl and in the range of 120–140 mg/dl. The results showed that the hazard of hypoglycemia for patients who maintained MBG lower than 120 mg/dl was significantly greater than the rate for those who maintained MBG between 120 and 140 mg/dl (22.8 vs. 9.35%, p < 0.001), while there was no significant difference in ICU mortality (8.57 vs. 10.2%, p = 0.08). Within non-diabetics, the risk of hypoglycemia was significantly reduced when MBG was between 120 and 140 mg/dl compared to the MBG level below 120 mg/dl (22.8 vs. 9.35%, p < 0.001). And there was no difference in ICU mortality (8.5 vs. 10.3%, p = 0.069). For diabetics, patients with 140–190 mg/dl of MBG had a lower hypoglycemic event rate than those who maintained MBG below 140 mg/dl (26 vs. 13%, p < 0.001). In addition, no statistical difference was observed between the two divided groups in terms of ICU mortality (9.25 vs. 10.2%, p = 0.589).
Discussion
This study demonstrated the association between MBG, GluCV during ICU stay, and the increased ICU mortality of septic patients. For the entire cohort, the MBG levels of 140–200 mg/dl, ≧ 200 mg/dl induced a 1.97- and 2.23-fold higher risk of ICU mortality, respectively; and GluCV of ≦ 31.429% connected with 1.36-fold higher risk. Nevertheless, we found that the effect of MBG and GluCV on ICU mortality differed among different subgroups. The unfavorable influence of hyperglycemia was more pronounced in non-diabetic, non-immunosuppression, non-liver disease, and non-hypoglycemia patients. And the impact of high GluCV was more significant in non-elderly, males, non-diabetic, non-immunosuppression, non-liver disease, non-hypoglycemia, and septic shock patients. Furthermore, the impact of hyperglycemia and high GluCV on death increased with the severity of sepsis. Our results also indicated that the optimal MBG target of sepsis patients without diabetes during ICU stay was 120–140 mg/dl. In diabetic patients, the incidence of hypoglycemia was significantly reduced when the MBG level was set between 140 and 190 mg/dl. A trend of decreased ICU mortality was observed in this BG range, but statistical differences were not reached.
High GV during ICU stay has a solid and consistent relation with adverse prognosis in critically ill patients (30–32). However, there was no consensus regarding the effect of GV on mortality in septic patients. In this study, we calculated the GluCV using all available biochemical BG records, reflecting the overall intervention status. The results identified that death presented a higher GluCV than the surviving patients, and a high GluCV level (>31.43%) was independently associated with an increased risk of ICU mortality among septic patients. In line with our findings, a recent study demonstrated that the rise of the mean amplitude of glycemic excursions and GluCV within the 1st day of ICU admission was related to increased risk of 30-day mortality in septic patients; in contrast, these relations do not exist in those with diabetes (33). In addition, Ali et al. also reported that GV was an important factor connected with hospital mortality using all biochemical and capillary glucose values for the entire hospitalization (19). Unfortunately, they did not further probe whether GV may vary across different populations.
Of course, the divergent results among such trials may not just depend on the presence of diabetes (30–33). Our study also found that the influence of high GluCV on ICU mortality was attenuated in the elderly, females, or patients with immunosuppression and hypoglycemia. Although the mechanisms underlying these phenomena are unclear, two reasons could explain this discrepancy. First, a higher incidence of cardiovascular disease, diabetes, and the use of related medications increased as the individuals aged, which changed the natural process of GV and obscured their adverse effects. Second, the risk of hypoglycemia induced by increased GV masked the association between GV and mortality of septic patients. Septic patients are especially vulnerable to hypoglycemia, and the occurrence risk is proportional to the viscera injury severity (34). Previous trials have proved the interaction between hypoglycemia and GV in intensive and non-intensive patients (35–37). The present study similarly showed that the probability of hypoglycemic occurrence rises with increases in GluCV.
The debate surrounding the effect of hyperglycemia on septic patients has been ongoing for more than 10 years. In some studies, hyperglycemia has been argued as an adaptive response under a stress state and plays a protective role in reducing the mortality of septic patients (15, 16). Due to the small number of samples included in these two trials, the stability of this conclusion may be questioned. In contrast, a large multicenter cohort study that contained 7,754 emergency department patients with sepsis demonstrated that high initial BG (>200 mg/dl) was significantly related to increased mortality in non-diabetic patients, but not in those with diabetes (13). In addition, Zohar et al. reported that BG over 200 mg/dl at admission resulted in a 1.48-fold increase in in-hospital mortality, 1.8-fold increase in 30-day mortality, and 1.68-fold increase in 90-day mortality of septic patients (10). However, they claimed that the harm of hyperglycemia was more robust in diabetic patients than in those without diabetes. Although diabetic patients have a greater chance of suffering chronic hyperglycemia, most published papers support that increased BG may not be harmful in septic patients with diabetes (13, 15, 38). Similarly, in this study, we did not observe any relevance between hyperglycemia and the ICU mortality risk of diabetic patients after adjustment in demographic characteristics, other comorbidities, and illness severity. Furthermore, the interaction test also proved that patients without diabetes had a higher risk of mortality in the same MBG range than diabetic patients. Interestingly, our results found that other comorbidities and pathological states, such as immunosuppression, hypoglycemia, liver disease, and septic shock, maybe also affecting the effect of hyperglycemia on the outcomes in septic patients (Table 2). However, no existing study targeted this particular population.
Different diseases may require a different optimal range of BG levels to achieve a better prognosis, and it will impact subsequent medical strategy and interventions (39, 40). However, in patients with sepsis, a firm consensus on optimal BG level is not available. The latest 2021 SSC Guideline recommended that BG should be kept in the range of 144–180 mg/dl for sepsis patients (1), and this recommendation was based on the results of a multicenter RCT (NICE-SUGAR) (21). The NICE-SUGAR study randomized 6,104 critically ill patients to either an intensive glycemic control group with BG of 81–108 mg/dl or a conventional glycemic control group in which insulin was administered if the BG level exceeded 180 mg/dl, and then maintained BG in the range of 144–180 mg/dl. The results presented that the patients in the intensive glycemic control group had lower 90-day mortality (27.5 vs. 24.9%, P = 0.02). Nevertheless, it is important to note that there was no statistically significant difference in the all-cause mortality between the two groups in the severe sepsis subgroup (OR 1.13, 95% CI 0.89–1.44). Furthermore, the definition of sepsis has undergone a dramatic change in the past 10 years. Thus, these differences limited the application of this recommendation in clinical practice.
The management protocol of BG in septic patients was usually developed according to the local conditions and experiences of the physicians. The optimum glycemic management needs to consider both the survival benefit and the risk of hypoglycemia. This study found that overall, the patients achieved relatively low mortality and hypoglycemic risk when keeping MBG in the range of 120–140 mg/dl; this range was equally applied to those without diabetes. For the diabetic subset, this study did not find an effective MBG interval that could significantly decrease ICU mortality. Nevertheless, we suggest that diabetes patients maintain MBG between 140 and 190 mg/dl to avoid hypoglycemia. A few published papers have explored the optimal level of BG control in septic patients. In 2019, Wang et al. found that the MBG at admission between 145 and 155 mg/dl was associated with the lowest hospital mortality both in the sepsis patients with and without diabetes based on a dose-response meta-analysis (17). The discrepancies between the two studies were on account of different BG measurements. Considering the effect of subsequent interventions, the MBG during the ICU stay was usually lower than MBG at admission.
Strengths and Limitations
The strengths of our study were that it was a large cohort that assessed the relationship between MBG, GluCV, and ICU mortality in sepsis patients and each subgroup. In addition, we used RCS to explore the optimal MBG range of sepsis patients in ICU stay. Although residual confounding cannot be completely removed, detailed adjustment for potential confounders about patients themselves and subsequent therapies limited the degree of confounding as far as possible.
Despite these strengths, this study has several limitations. First, we were unable to quantify the timing of each BG measurement, such as fasting or non-fasting in this real-world observational study. Thus, each BG record in this study should be regarded as a random BG. Second, the recent BG control of included patients cannot be accurately reflected due to the lack of complete HbA1c records in the MIMIC-IV database. However, chronic hyperglycemia is strongly associated with the risk of death in critically ill patients (41). Therefore, there could be bias affecting the influence of hyperglycemia in diabetic patients, especially in those with better glycemic control. Third, numerous medications used in the ICU patients and the routes of nutrition are associated with blood glucose metabolism. Nevertheless, this study aimed to determine whether there was a difference in the association of the overall BG and GluCV levels with the prognosis of sepsis patients in the context of the above measures and the reasons for the discrepancy. Furthermore, these related interventions recorded in the MIMIC-IV database were reasonable and recognized. Fourth, this study was a single-center, retrospective cohort study. Our findings need to be validated by an external population.
Clinical Implications and Future Perspectives
The most salient finding of this study is the evidence for differences in the effects of BG and GV in various septic subgroups, and the reason for this discrepancy is not simply due to the diabetes states. Age, gender, immunosuppression, liver disease, septic shock, and the hypoglycemic event also play an essential role in associating overall BG and GV with ICU mortality in sepsis patients. The current investigation findings have important implications for the development of a reasonable medical strategy and individualized treatment. On the other hand, our results suggest that the glycemic management of septic patients during the acute phase should be assessed individually rather than a “one size fits all” approach.
Moreover, this study questions the plausibility of the latest published 2021 SSC Guideline, which recommends a glycemic target range of 140–180 mg/dl for septic or septic shock patients (1). Given the risk of mortality and hypoglycemia, the optimal range of BG should be different between diabetic and non-diabetic patients. The occurrence of hyperglycemia (>140 mg/dl) should be avoided as much as possible for those without diabetes. In contrast, for diabetic patients, the BG should be maintained at a relatively high level to reduce the risk of hypoglycemia.
Although the mechanisms behind these phenomena are currently unknown, this research has provided further explorations some enlightenment. The enlightenment were listed below, as presented in 1.,2.,3.,4:
1. It is necessary to consider BG and GV levels together when implementing glycemic management in septic patients.
2. Future studies should focus on investigating the glycometabolism disorder among specific subgroups rather than all the septic patients.
3. The optimal glycemic target range of septic patients and related subsets is still controversial. Hence, further studies are warranted to resolve it.
4. Despite some new biomarkers and technologies such as capnography and continuous glucose monitoring systems showing a positive effect on clinical glucose management (42, 43), they did not seem to be widely available in sepsis patients. Further studies and consensus are necessary to standardize blood sample collection frequency and time points during the BG monitoring and management of sepsis patients.
Conclusion
This study demonstrated that MBG and Glucv during the ICU stay were associated with all-cause ICU mortality in sepsis patients. However, the harm of hyperglycemia and high GV was not apparent in some particular subgroups, such as those with diabetes, immunosuppression, liver disease, and documented hypoglycemia. Furthermore, the results presented that the impact of hyperglycemia and high GV on death increased with the severity of sepsis based on the initial SOFA scores. We also found that patients with severe hyperglycemia (≥200 mg/dl) and low GV (<15.174%) during ICU hospitalization always had the highest all-cause ICU mortality of any subsets regardless of having diabetes or not, indicating that persistent hyperglycemia states were a significant risk factor for ICU deaths of sepsis patients. Although the AUC of MBG combined with Glucv was superior to either of them alone for predicting ICU mortality in sepsis patients, the overall predictive performance was moderate. Finally, the results of the RCS analysis showed that the risk of ICU mortality and hypoglycemia of those with no pre-existing diabetes were lower when maintaining the MBG in the range of 120–140 mg/dl, whereas in sepsis patients with diabetes, the incidence of hypoglycemia significantly reduced when the MBG level was set between 140 and 190 mg/dl, but a glycemic control target effectively reducing the ICU mortality was not observed.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZL, MY, and HZ designed the study. XS, MJ, and YZ extracted the data. ZL, YL, ML, and TH conducted data quality management and statistical analysis and drafted the manuscript. JZ and WX participated in the literature search. GT, MY, HZ, and TH critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a research grant from the National Natural Science Foundation of China (No. 82072134) and the National Natural Science Foundation Youth Science Foundation (No. 81601661).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all participants in the Second Affiliated Hospital of Anhui Medical University and Anhui University.
Glossary
Abbreviations
- SSC
Surviving Sepsis Campaign
- GV
glycemic variability
- MBG
mean blood glucose
- MIMIC-IV
Medical Information Mart for Intensive Care IV
- NLR
neutrophil to lymphocyte ratio
- WBC
white blood cell count
- SOFA
Sequential Organ Failure Assessment
- APS III
Acute Physiology Score III
- MV
mechanical ventilation
- RRT
renal replacement therapy
- ICD-9, International Classification of Diseases
Ninth Revision
- ICD-10, International Classification of Diseases
Tenth Revision
- RCS
Restricted0cubic0splines
- ROC
receiver operating characteristic.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.857368/full#supplementary-material
The flowchart of this present study.
The distribution of mean blood glucose (MBG) (A,B), glycemic coefficient of variation (GluCV) (A,B) within survivors and deaths during ICU stay. The orange and green solid lines present the density curve.
The interaction between mean blood glucose (MBG) and diabetes. The abscissa and ordinate, respectively, represent the MBG values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The interaction between mean blood glucose (MBG) and hypoglycemia. The abscissa and ordinate, respectively, represent the MBG values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The interaction between mean blood glucose (MBG) and liver disease. The abscissa and ordinate respectively represent the MBG values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The interaction between the glycemic coefficient of variation (GluCV) and age. The abscissa and ordinate, respectively, represent the GluCV values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The associations between MBG and GluCV with the ICU mortality of sepsis patients in different severity degrees according to the initial SOFA score.
The ROC curve of the mean blood glucose (MBG), glycemic coefficient of variation (GluCV), and MBG + GluCV in all septic patients (A), diabetic patients (B), and non-diabetic patients (C).
Comparison of performance of MBG, GluCV, and MBG combined with GluCV in predicting the ICU mortality of septic patients.
References
- 1.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med. (2020) 46:1552–62. 10.1007/s00134-020-06151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamler R, LeRoith D, Roth J. Intensive insulin therapy in the medical ICU. N Engl J Med. (2006) 354:2069–71. 10.1056/NEJMc060566 [DOI] [PubMed] [Google Scholar]
- 4.Vanhorebeek I, Gunst J, Van den Berghe G. Critical care management of stress-induced hyperglycemia. Curr Diab Rep. (2018) 18:17. 10.1007/s11892-018-0988-2 [DOI] [PubMed] [Google Scholar]
- 5.Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care Med. (2013) 41:e93–4. 10.1097/CCM.0b013e318283d124 [DOI] [PubMed] [Google Scholar]
- 6.Finfer S. Clinical controversies in the management of critically ill patients with severe sepsis: resuscitation fluids and glucose control. Virulence. (2014) 5:200–5. 10.4161/viru.25855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira JA, Baptista RM, Monteiro SR, Gonçalves FM, Monteiro PF, Gonçalves LM, et al. Admission hyperglycemia and all-cause mortality in diabetic and non-diabetic patients with acute myocardial infarction: a tertiary center analysis. Intern Emerg Med. (2021) 16:2109–19. 10.1007/s11739-021-02693-0 [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Zhang R, Jin T, Zhu P, Yao L, Li L, et al. Stress hyperglycemia is independently associated with persistent organ failure in acute pancreatitis. Dig Dis Sci. (2021) 10:8. 10.1007/s10620-021-06982-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou D, Zhong P, Ye X, Wu D. Persistent hyperglycemia is a useful glycemic pattern to predict stroke mortality: a systematic review and meta-analysis. BMC Neurol. (2021) 21:487. 10.1186/s12883-021-02512-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zohar Y, Zilberman Itskovich S, Koren S, Zaidenstein R, Marchaim D, Koren R. The association of diabetes and hyperglycemia with sepsis outcomes: a population-based cohort analysis. Intern Emerg Med. (2021) 16:719–28. 10.1007/s11739-020-02507-9 [DOI] [PubMed] [Google Scholar]
- 11.van Vught LA, Wiewel MA, Klein Klouwenberg PM, Hoogendijk AJ, Scicluna BPY, Ong DS, et al. Admission hyperglycemia in critically ill sepsis patients: association with outcome and host response. Crit Care Med. (2016) 44:1338–46. 10.1097/CCM.0000000000001650 [DOI] [PubMed] [Google Scholar]
- 12.Fabbri A, Marchesini G, Benazzi B, Morelli A, Montesi D, Bini C, et al. Stress hyperglycemia and mortality in subjects with diabetes and sepsis. Crit Care Explor. (2020) 2:e0152. 10.1097/CCE.0000000000000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuetz P, Jones AE, Howell MD, Trzeciak S, Ngo L, Younger JG, et al. Diabetes is not associated with increased mortality in emergency department patients with sepsis. Ann Emerg Med. (2011) 58:438–44. 10.1016/j.annemergmed.2011.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan MC, Tseng JS, Hsu KH, Shih SJ Yi CY, Wu CL, et al. A minimum blood glucose value less than or equal to 120 mg/dL under glycemic control is associated with increased 14-day mortality in nondiabetic intensive care unit patients with sepsis and stress hyperglycemia. J Crit Care. (2016) 34:69–73. 10.1016/j.jcrc.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 15.Tiruvoipati R, Chiezey B, Lewis D, Ong K, Villanueva E, Haji K, et al. Stress hyperglycemia may not be harmful in critically ill patients with sepsis. J Crit Care. (2012) 27:153–8. 10.1016/j.jcrc.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 16.Wernly B, Lichtenauer M, Franz M, Kabisch B, Muessig J, Masyuk M, et al. Differential impact of hyperglycemia in critically ill patients: significance in acute myocardial infarction but not in sepsis? Int J Mol Sci. (2016) 17:1586. 10.3390/ijms17091586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Chen W, Liu Y, Li L, Li S, Tan J, et al. Blood glucose levels and mortality in patients with sepsis: dose-response analysis of observational studies. J Intensive Care Med. (2021) 36:182–90. 10.1177/0885066619889322 [DOI] [PubMed] [Google Scholar]
- 18.Magee F, Bailey M, Pilcher DV, Mårtensson J, Bellomo R. Early glycemia and mortality in critically ill septic patients: interaction with insulin-treated diabetes. J Crit Care. (2018) 45:170–7. 10.1016/j.jcrc.2018.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Ali NA, O'Brien JM Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. (2008) 36:2316–21. 10.1097/CCM.0b013e3181810378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. (2008) 358:125–39. 10.1056/NEJMoa070716 [DOI] [PubMed] [Google Scholar]
- 21.NICE-SUGAR Study Investigators, Finfer S, Chittock DR, Su SY, Blair D, Foster D, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. (2009) 360:1283–97. 10.1056/NEJMoa0810625 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association . Diabetes care in the hospital: standards of medical care in diabetes-2018. Diabetes Care. (2018) 41(Suppl.1):S144–51. 10.2337/dc18-S014 [DOI] [PubMed] [Google Scholar]
- 23.Johnson A, Bulgarelli L, Pollard T, Horng S, Celi LA, Mark R. MIMIC-IV (version 1.0) PhysioNet. (2021). Available online at: https://physionet.org/content/mimiciv/1.0/ (accessed May 07, 2021).
- 24.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). J Am Med Assoc. (2016) 315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Wang X, Gao Y, Yu S, Zhao L, Zhang Z, et al. Efficacy and safety of corticosteroids for septic shock in immunocompromised patients: a cohort study from MIMIC. Am J Emerg Med. (2021) 42:121–6. 10.1016/j.ajem.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 26.van Vught LA, Holman R, de Jonge E, de Keizer NF, van der Poll T. Diabetes is not associated with increased 90-day mortality risk in critically ill patients with sepsis. Crit Care Med. (2017) 45:e1026–35. 10.1097/CCM.0000000000002590 [DOI] [PubMed] [Google Scholar]
- 27.Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. (2021) 372:n422. 10.1136/bmj.n422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Y, Zhou JH, Zhang ZW, Tan QY, Zhang MY, Li J, et al. Application of restricted cube spline in cox regression model. Chin J Prev Med. (2020) 54:1169–73. 10.3760/cma.j.cn112150-20200804-01092 [DOI] [PubMed] [Google Scholar]
- 29.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC. Predicted lean body mass, fat mass, and all cause and cause specific mortality in men: prospective US cohort study. BMJ. (2018) 362:k2575. 10.1136/bmj.k2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. (2008) 36:3008–13. 10.1097/CCM.0b013e31818b38d2 [DOI] [PubMed] [Google Scholar]
- 31.Krinsley JS, Egi M, Kiss A, Devendra AN, Schuetz P, Maurer PM, et al. Diabetic status and the relation of the three domains of glycemic control to mortality in critically ill patients: an international multicenter cohort study. Crit Care. (2013) 17:R37. 10.1186/cc12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermanides J, Vriesendorp TM, Bosman RJ, Zandstra DF, Hoekstra JB, Devries JH. Glucose variability is associated with intensive care unit mortality. Crit Care Med. (2010) 38:838–42. 10.1097/CCM.0b013e3181cc4be9 [DOI] [PubMed] [Google Scholar]
- 33.Chao WC, Tseng CH, Wu CL, Shih SJ, Yi CY, Chan MC. Higher glycemic variability within the first day of ICU admission is associated with increased 30-day mortality in ICU patients with sepsis. Ann Intensive Care. (2020) 10:17. 10.1186/s13613-020-0635-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmoodpoor A, Hamishehkar H, Beigmohammadi M, Sanaie S, Shadvar K, Soleimanpour H, et al. Predisposing factors for hypoglycemia and its relation with mortality in critically ill patients undergoing insulin therapy in an intensive care unit. Anesth Pain Med. (2016) 6:e33849. 10.5812/aapm.33849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruginski D, Précoma DB, Sabbag A, Olandowski M, et al. Impact of glycemic variability and hypoglycemia on the mortality and length of hospital stay among elderly patients in Brazil. Curr Diabetes Rev. (2020) 16:171–80. 10.2174/1573399815999190619141622 [DOI] [PubMed] [Google Scholar]
- 36.Uemura F, Okada Y, Torimoto K, Tanaka Y. Relation between hypoglycemia and glycemic variability in type 2 diabetes patients with insulin therapy: a study based on continuous glucose monitoring. Diabetes Technol Ther. (2018) 20:140–6. 10.1089/dia.2017.0306 [DOI] [PubMed] [Google Scholar]
- 37.Arnold P, Paxton RA, McNorton K, Szpunar S, Edwin SB. The effect of a hypoglycemia treatment protocol on glycemic variability in critically ill patients. J Intensive Care Med. (2015) 30:156–60. 10.1177/0885066613511048 [DOI] [PubMed] [Google Scholar]
- 38.Stegenga ME, Vincent JL, Vail GM, Xie J, Haney DJ, Williams MD, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med. (2010) 38:539–45. 10.1097/CCM.0b013e3181c02726 [DOI] [PubMed] [Google Scholar]
- 39.Chiang HY, Lin KR, Hsiao YL, Huang HC, Chang SN, Hung CH, et al. Association between preoperative blood glucose level and hospital length of stay for patients undergoing appendectomy or laparoscopic cholecystectomy. Diabetes Care. (2021) 44:107–15. 10.2337/dc19-0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadegari H, Bozorgmanesh M, Hadaegh F, Azizi F. Non-linear contribution of glucose measures to cardiovascular diseases and mortality: reclassifying the Framingham's risk categories: a decade follow-up from the Tehran lipid and glucose study. Int J Cardiol. (2013) 167:1486–94. 10.1016/j.ijcard.2012.04.053 [DOI] [PubMed] [Google Scholar]
- 41.Plummer MP, Bellomo R, Cousins CE, Annink CE, Sundararajan K, Reddi BA, et al. Dysglycaemia in the critically ill and the interaction of chronic and acute glycaemia with mortality. Intensive Care Med. (2014) 40:973–80. 10.1007/s00134-014-3287-7 [DOI] [PubMed] [Google Scholar]
- 42.Soleimanpour H, Taghizadieh A, Niafar M, Rahmani F, Golzari SE, Esfanjani RM. Predictive value of capnography for suspected diabetic ketoacidosis in the emergency department. West J Emerg Med. (2013) 14:590–4. 10.5811/westjem.2013.4.14296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moser O, Sternad C, Eckstein ML, Szadkowska A, Michalak A, Mader JK, et al. Performance of intermittently scanned continuous glucose monitoring systems in people with type 1 diabetes: a pooled analysis. Diabetes Obes Metab. (2022) 24:522–9. 10.1111/dom.14609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The flowchart of this present study.
The distribution of mean blood glucose (MBG) (A,B), glycemic coefficient of variation (GluCV) (A,B) within survivors and deaths during ICU stay. The orange and green solid lines present the density curve.
The interaction between mean blood glucose (MBG) and diabetes. The abscissa and ordinate, respectively, represent the MBG values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The interaction between mean blood glucose (MBG) and hypoglycemia. The abscissa and ordinate, respectively, represent the MBG values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The interaction between mean blood glucose (MBG) and liver disease. The abscissa and ordinate respectively represent the MBG values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The interaction between the glycemic coefficient of variation (GluCV) and age. The abscissa and ordinate, respectively, represent the GluCV values and predictive risk probability of ICU mortality by multivariable logistic regression analysis. Gray solid dots indicate the distribution of each included patient. Solid blue lines are multivariable regression lines, with gray regions showing 95% confidence intervals. Adjustment factors are the same as those in Model 3 of Table 2.
The associations between MBG and GluCV with the ICU mortality of sepsis patients in different severity degrees according to the initial SOFA score.
The ROC curve of the mean blood glucose (MBG), glycemic coefficient of variation (GluCV), and MBG + GluCV in all septic patients (A), diabetic patients (B), and non-diabetic patients (C).
Comparison of performance of MBG, GluCV, and MBG combined with GluCV in predicting the ICU mortality of septic patients.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.