Abstract
The presence or absence of molecular oxygen has been shown to play a crucial role in the degradability of haloaromatic compounds. In the present study, it was shown that anaerobic phototrophic 3-chlorobenzoate (3CBA) metabolism by Rhodopseudomonas palustris DCP3 is oxygen tolerant up to a concentration of 3 μM O2. Simultaneous oxidation of an additional carbon source permitted light-dependent anaerobic 3CBA degradation at oxygen input levels which, in the absence of such an additional compound, would result in inhibition of light-dependent dehalogenation. Experiments under the same experimental conditions with strain DCP3 in coculture with an aerobic 3CBA-utilizing heterotroph, Alcaligenes sp. strain L6, revealed that light-dependent dehalogenation of 3CBA did not occur. Under both oxygen limitation (O2 < 0.1 μM) and low oxygen concentrations (3 μM O2), all the 3CBA was metabolized by the aerobic heterotroph. These data suggest that biodegradation of (halo)aromatics by photoheterotrophic bacteria such as R. palustris DCP3 may be restricted to anoxic photic environments.
Herbicides and products of aerobic transformations of halogenated alkyl benzenes and polychlorinated biphenyls are important sources of halogenated aromatic compounds, including chlorinated benzoates, that occur in the environment (1, 19, 34). In addition to the wide range of man-made chemicals, a great variety of halogenated aromatic compounds are produced naturally in soils and aquatic environments (17, 39). Whether or not (halogenated) organic compounds are biodegraded depends on the chemical structure of the compound, the prevailing physicochemical conditions, and, obviously, the presence of microorganisms with the appropriate catabolic capacities. Of these factors, molecular oxygen in particular plays a crucial role in determining the fate of aromatic compounds. This is due to its key role in establishing the redox state of a given environment, thereby determining the metabolic options available to the microbial community. In addition, oxygen is often used as a cosubstrate in aerobic degradation processes of (halo)aromatic substrates. Dioxygenases, which are involved in ring fission processes, incorporate molecular oxygen into the aromatic ring. However, the activity and synthesis of such dioxygenases under oxic conditions is considerably reduced with decreasing oxygen concentrations (37, 42). As a result, reduced oxygen tensions may lead to the accumulation of toxic intermediates such as chlorocatechols (11, 18). Oxic-anoxic interfaces in soils, as well as top layers of aquatic sediments and freshwater and marine ecosystems, are habitats where such redox conditions often prevail. Bacteria using metabolic routes which are less affected by decreased pO2 may play a crucial role in the decomposition of haloaromatics at such interfaces. So far, only a few aerobic bacteria that are able to degrade (halo)aromatic compounds at appreciable rates under reduced partial pressures of oxygen have been described (26, 28, 29, 33). At aquatic sediment surfaces, in microbial mats, and in shallow ponds and lakes, oxic-anoxic interfaces also experience gradients of light, subject to a diel cycle. Anoxygenic photoheterotrophs are known to abound in such interface environments, and many are known to degrade (halo)aromatic compounds (22). However, the biodegradation of haloaromatic compounds at low pO2 by these facultative anaerobic bacteria has received little attention. Many Rhodopseudomonas species are known to be capable of growth on methyl benzenes, aminobenzenes, and phenolics under anoxygenic phototrophic conditions. In addition, some Rhodopseudomonas species have been shown to degrade (aromatic) xenobiotics, such as halocarboxylic acids, chlorobenzoates, polychlorinated biphenyls, and dinitrophenols (6, 21, 23, 25, 30, 31). Therefore, such organisms may, in principle, play a significant role in the decomposition of xenobiotic compounds at oxic-anoxic interfaces. These bacteria degrade such aromatic substrates via a reductive ring fission pathway, as has also been found for denitrifying sulfate-reducing, methanogenic, and fermentative bacteria (4, 24, 36, 43). The recently described anoxygenic photoheterotroph Rhodopseudomonas palustris DCP3, isolated by Van der Woude et al. (41), is the first example of an R. palustris strain that can use 3-chlorobenzoate (3CBA) as sole source of carbon under anoxic conditions in the presence of light. A unique property of this bacterium is that it does not need a cosubstrate for growth on the chlorinated compound, which is in contrast to previously described anaerobic phototrophic bacteria (6, 23).
To investigate the role of ecology in the biodegradation of xenobiotics by these anoxygenic phototrophs in oxic-anoxic interfaces, the abundance of these bacteria in micro-oxic environments must be demonstrated and their actual involvement in situ in dehalogenation reactions of haloaromatics must be shown. However, first of all, the fundamental issue of the degree of oxygen sensitivity of the reductive degradation pathways in these bacteria should be resolved. For this reason, the following three questions were asked: (i) how do low-oxygen conditions (≤10% air saturation) affect light-dependent growth at the expense of 3CBA, (ii) is it possible to readily switch between anoxic phototrophic growth on 3CBA and aerobic metabolism upon temporarily increased levels of oxygen, and (iii) do these phototrophs effectively compete for aromatic compounds with aerobic bacteria at low oxygen concentrations? To answer these questions, we used R. palustris DCP3 as an example of dechlorinating photoheterotrophs with 3CBA as a model substrate for growth at various oxygen concentrations in batch and continuous cultures. Furthermore, the competitiveness of this phototroph for growth on 3CBA at very low oxygen concentrations was investigated in chemostats with mixed cultures of R. palustris DCP3 and Alcaligenes sp. strain L6, an aerobic heterotroph previously shown to be well adapted to growth at low oxygen partial pressures (26) and to be able to grow at the expense of 3CBA as the sole carbon and energy source under both oxic and hypoxic conditions but unable to grow on succinate under either condition.
MATERIALS AND METHODS
Organisms.
R. palustris DCP3 was isolated from a mixture of samples taken from several freshwater ditches and a polluted marsh sediment (41). This anoxygenic phototroph is able to grow anaerobically on 3CBA as the sole source of carbon and electrons in the presence of light. Alcaligenes sp. strain L6 is able to grow aerobically on 3CBA as the sole carbon and energy source; it was isolated from polluted marsh sediment under a reduced partial pressure of oxygen (26).
Media and growth conditions.
Low-chloride minimal medium (13) in 100-ml serum bottles with butyl rubber stoppers was used to cultivate both R. palustris DCP3 and Alcaligenes sp. strain L6. Filter-sterilized vitamins (1 ml/liter) were added after the medium was autoclaved (41). Anoxic phototrophic batch cultures were routinely incubated under saturating light intensities at 30°C under a nitrogen atmosphere. 3CBA was added from separately autoclaved stock solutions to a final concentration of 2 mM. Sterile air was added to obtain final aqueous oxygen concentrations of either 3, 6, or 12 μM. The oxygen concentration in the liquid phase was kept in equilibrium with the gas phase by incubation in a rotary incubator at 150 rpm. Oxygen concentrations in the gas phase were measured with a gas chromatograph (Pye Unicam 104, equipped with a katharometer and a Poropack Q [Water Associates Inc.] 100 to 120 mesh column), both in advance and after finishing batch experiments. The ratio between the gas phase volume and the volume of the liquid phase was 6.5, which ensured that consumption of oxygen by aerobic metabolism did not significantly affect the oxygen concentration. Continuous cultivation was performed in chemostat vessels (working volume, 500 ml) with low-chloride minimal medium which contained 0.5 mM chlorobenzoate plus 10 mM succinate as substrates. The temperature was set to 30°C, and the chemostat was illuminated by four 40-W light bulbs (20,000 lux). A flow of nitrogen gas or a mixture of nitrogen and air was passed over the culture at a final rate of 60 ml/h. The oxygen concentration was automatically regulated by coupling the stirring rate to continuous oxygen readings from a polarographic electrode (Ingold, Urdorf, Switzerland). The detection limit of these electrodes is approximately 0.1 μM. During prolonged continuous operation at low oxygen concentrations above this detection limit, the zero reading of the electrode was readjusted to correct for drifting of the readings at zero oxygen. The pH was regulated at pH 7 by automatic titrations with either KOH (1 M) or H3PO4 (1 M).
Analytical and microbiological procedures.
The purity of cultures was routinely checked by streaking on nutrient broth agar plates which were incubated under both oxic and anoxic conditions in the presence of light. Chloride concentrations in the medium were determined colorimetrically by the method of Bergman and Sanik (5), with NaCl as a standard. 3CBA was measured by gas chromatography as described previously (13), with benzoate as an internal standard. Succinate was also measured by gas chromatography after methylation with methanol and extraction with chloroform (32). Cell densities were quantified by measuring the optical densities at 660 nm.
Whole-cell hybridization with 16S rRNA oligonucleotide probes.
Enumeration and identification of R. palustris DCP3 and Alcaligenes sp. strain L6 in mixed cultures were done with 16S rRNA targeted oligonucleotide probes. Samples were taken from the chemostat, centrifuged (10 min at 10,000 × g, and 4°C), washed twice with phosphate-buffered saline (PBS; 130 mM sodium chloride, 10 mM sodium phosphate buffer [pH 7.2]), and resuspended in PBS. Prior to hybridization by standard procedures (3), the cells were fixed with 3% paraformaldehyde and stored in 50% PBS–50% (vol/vol) ethanol at 4°C. Fixed cells were dehydrated and placed at 50°C in hybridization buffer containing 0.9 M NaCl, 0.1% sodium dodecyl sulfate, 20 mM Tris-HCl (pH 7.2), 15% formamide, and 5 ng of oligonucleotide probe per μl. For total-cell counts in mixed cultures, samples were counted with an epifluorescence microscope (Axioskop; Zeiss Nederland BV) equipped with an Hg arc lamp and filter set Blue 450–490 (excitation wavelength) for detection of fluorescein after hybridization with a fluorescein-labelled eubacterial 16S rRNA oligonucleotide probe EUB338 probe, 5′-GCTGCCTCCCGTAGGAGT-3′ (3). Specific counts (Alcaligenes sp. strain L6) in these samples from mixed cultures were counted by epifluorescence microscopy with filter set Green 546 (excitation wavelength) for detection of rhodamine after hybridization with a rhodamine-labelled 16S rRNA oligonucleotide probe specific for Alcaligenes sp. strain L6. This L6 probe, 5′-GCCGGCGCCGTTTCTTCCCT-3′, targets the 16S rRNA of strain L6 (EMBL accession no. X92415) starting at position 444 (Escherichia coli numbering). Subtracting the number of Alcaligenes sp strain L6 cells from the total count yielded the number of R. palustris DCP3 cells in the mixed cultures.
Resting-cell experiments.
The rates of 3CBA and succinate metabolism by mixed resting-cell suspensions of R. palustris DCP3 and Alcaligenes sp. strain L6 were determined. Aliquots of the chemostat culture were obtained, centrifuged (10 min at 4°C and 11,000 × g), and washed twice in LMM buffer (pH 7.0) consisting of 25 mM K(NH4)PO4, 0.1 g of MgSO4 · 7H2O per liter, and 0.05 g of Ca(NO3)2 · 4H2O per liter. Cell pellets were resuspended in LMM buffer, and chloroamphenicol (30 mg/liter) was added to prevent protein synthesis. Both 3CBA (1 mM) and succinate (3 mM) were added to these suspensions at t = 0 h, and disappearance of the substrates was monitored in samples taken every hour from duplicate incubations which were incubated for a total of 6 h in a rotary incubator (150 rpm) under (i) anoxic phototrophic conditions at 30°C or (ii) phototrophic conditions at 30°C in the presence of 3 μM O2.
RESULTS
Light-dependent 3CBA degradation by R. palustris DCP3 in anoxic and micro-oxic batch cultures.
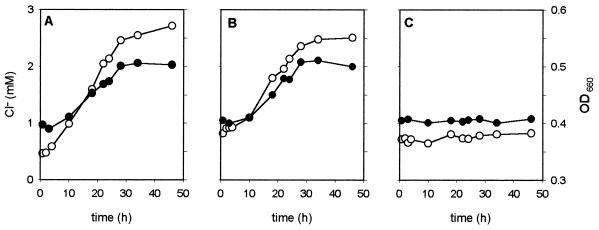
To investigate the oxygen sensitivity of anoxygenic photoheterotrophic 3CBA metabolism by R. palustris DCP3, cells were exposed to various concentrations of oxygen during exponential phototrophic growth. To this end, R. palustris DCP3 was initially grown on 3CBA under fully anoxic conditions in the light. After mid-log phase (optical density at 660 nm, 0.4 to 0.5) had been reached, aliquots of these cultures were transferred to fresh medium in the presence of 0, 3, or 6 μM oxygen. In the presence of light and the absence of oxygen, exponential growth on 3CBA continued at the same rate as observed in the initial culture (0.027 h−1) (data not shown), as determined by a comparable increase in cell density and release of chloride to the medium (Fig. 1A). During the course of incubation, samples taken from the cultures were subjected to gas chromatography for quantification of residual 3CBA. This revealed that the decrease in the 3CBA concentration was equal to the amount of chloride produced. In the presence of 3 μM oxygen, consumption of 3CBA and growth also continued at similar rates to those observed under anoxic conditions (Fig. 1B). However, 6 μM oxygen resulted in no 3CBA disappearance and total inhibition of growth (Fig. 1C). In the dark, none of these cultures showed any degradation of 3CBA, release of chloride, or growth (data not shown).
FIG. 1.
Release of chloride (•) and change in cell density (○) in batch cultures of R. palustris DCP3 grown on 3CBA anaerobically (A), in the presence of 3 μM O2 (B), and in the presence of 6 μM O2 (C). The data shown are mean values of duplicate experiments. Cells were pregrown phototrophically in anoxic batch culture, and aliquots were taken to inoculate all the cultures at time zero. OD, optical density.
3CBA metabolism by R. palustris DCP3 in continuous culture and its response to oxygen.
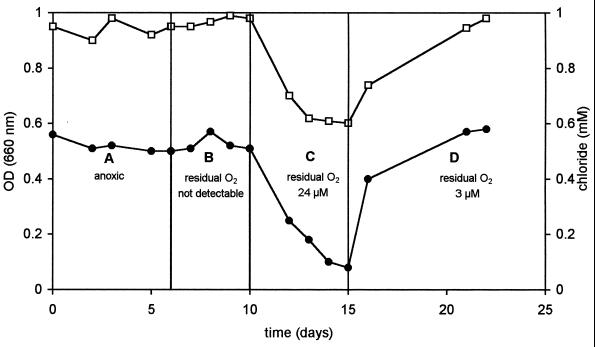
One might expect that in natural habitats containing numerous sources of additional carbon at low concentrations, growth on 3CBA as the sole source of carbon would be unlikely. The O2 tolerance of R. palustris DCP3 during 3CBA metabolism may be different in the presence of additional carbon sources from when 3CBA was the sole source of carbon. If one or more of the additional carbon sources could be metabolized aerobically, the oxygen concentration would be reduced in the direct environment of the organism. Under these conditions, it is possible that the phototroph would be able to simultaneously utilize 3CBA at O2 concentrations much higher than 3 μM. Since succinate was shown to be a good carbon source for both aerobic and anaerobic growth of strain DCP3, this was used as an additional carbon substrate in 3CBA-grown chemostat cultures of strain DCP3 in the presence and absence of oxygen. During initial anoxic growth in the light, a steady state was obtained at a dilution rate of 0.021 h−1, with complete consumption of succinate (data not shown) and 3CBA, as reflected by chloride release (Fig. 2A). Subsequently, the oxygen input level was increased to determine the influence of oxygen on 3CBA metabolism by R. palustris DCP3. At a low oxygen input level that was sufficient for aerobic metabolism of only a portion of the succinate supplied, there was no residual oxygen. Despite this limited oxygen concentration, all the succinate was degraded, apparently through anaerobic metabolism of the residual succinate, which was not degraded aerobically (data not shown). Complete degradation of 3CBA was observed, as indicated by the stoichiometric release of chloride (Fig. 2B). High cell densities, comparable to those obtained under fully anoxic conditions, were attained. Residual oxygen concentrations of 24 and 3 μM were obtained by adjusting the oxygen supply above the total amount needed for complete aerobic degradation of succinate. When the residual oxygen concentration was 24 μM, R. palustris DCP3 was no longer able to degrade 3CBA, as indicated by a sharp decrease in the amount of chloride released and a parallel drop in culture density (Fig. 2C). During this time, the cells turned from red to white, indicating that the synthesis of photopigments had been repressed by oxygen. This suggests that succinate was completely metabolized aerobically and not phototrophically. The degradation of 3CBA resumed at a high rate when the oxygen input level was reduced, so that there was a much lower residual level of O2 (3 μM O2 in the culture liquid) (Fig. 2D). This coincided with the renewed synthesis of photopigments, as observed by a switch from white to red cells. These data show that the phototroph was able to metabolize 3CBA when there were high oxygen input levels, as long as there was a second substrate present that could be aerobically metabolized and the residual oxygen concentration was 3 μM or less.
FIG. 2.
Optical density (OD) (□) and release of chloride (•) in a pure culture of R. palustris DCP3 grown in continuous culture (D = 0.021 h−1) on a mixture of 3CBA (0.5 mM) and succinate (10 mM) in constant light. (A) Fully anoxic conditions. (B) Oxygen-limiting conditions (<0.1 μM O2). (C) Low-oxygen conditions (24 μM O2). (D) Very low (micro-oxic) conditions (3 μM O2).
Competition for 3CBA between Alcaligenes sp. strain L6 and R. palustris DCP3.
For photoheterotrophs to play a significant role in degradation of xenobiotic compounds in low-oxygen photic habitats, they must not only be aerotolerant but also able to successfully compete with other xenobiotic-degrading bacteria. The competitiveness of R. palustris DCP3 for growth on 3CBA was investigated in mixed continuous cultures with Alcaligenes sp. strain L6 under anoxic and hypoxic conditions. Succinate was used as additional carbon source to support growth of the phototroph during oxic conditions and hence to avoid possible washout of R. palustris DCP3. The metabolic characteristics for growth on 3CBA and succinate of both strains are summarized in Table 1.
TABLE 1.
Main metabolic characteristics for succinate and 3CBA of R. palustris DCP3 and Alcaligenes sp. strain L6
| Organism | Growth conditions | Carbon source | Energy source |
|---|---|---|---|
| R. palustris DCP3 | Anoxic | Succinate | Light |
| 3CBA | Light | ||
| Micro-oxic | Succinate | Light and succinate | |
| 3CBA | Light | ||
| Oxic | Succinate | Succinate | |
| Alcaligenes sp. strain L6 | Anoxic | None | None |
| Micro-oxic | 3CBA | 3CBA | |
| Oxic | 3CBA | 3CBA |
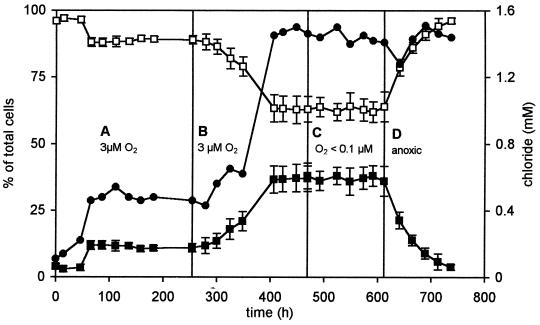
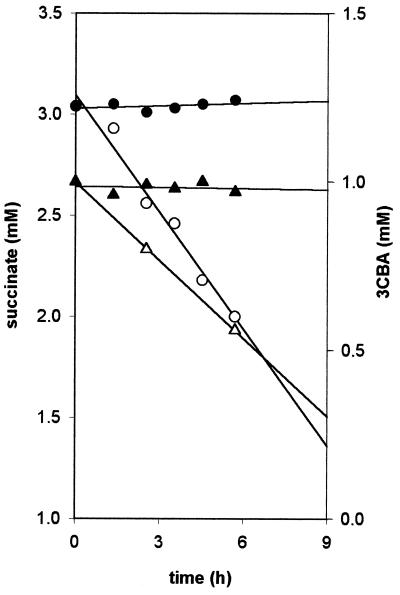
The competition experiments were started by inoculating the chemostat with 3CBA-grown cells of R. palustris DCP3 and Alcaligenes sp. strain L6, which were pregrown in batch cultures. Both 3CBA and succinate were present in the feed, and hypoxic conditions (3 μM residual O2) were applied in the presence of light. It was observed that Alcaligenes sp. strain L6 used part of the 3CBA in the presence of 3 μM oxygen, since the percentage of cells of this aerobe was maintained in the mixed culture (Fig. 3A). Succinate was degraded completely (data not shown), resulting in large numbers of DCP3 cells. Stoichiometric chloride was produced due to complete degradation of 3CBA. The percentage of strain L6 cells increased when the 3CBA concentration in the feed was increased from 0.5 to 1.5 mM. There was a concomitant increase in chloride concentration (Fig. 3B). Washed cell suspensions of this culture (sampled at 450 h) that were incubated in the presence of light and chloramphenicol did not degrade 3CBA under anaerobic conditions. In contrast, 3CBA was rapidly degraded under aerobic conditions. These data indicate that in the chemostat at this time, the 3CBA was aerobically metabolized entirely by Alcaligenes sp. strain L6 and that the enzymes required for 3CBA metabolism by R. palustris DCP3 were not induced (Fig. 4).
FIG. 3.
Composition of a mixed continuous culture of R. palustris DCP3 (□) and Alcaligenes sp. strain L6 (■) during growth on a mixture of succinate (10 mM) and 3CBA (0.5 to 1.5 mM) in the feed at a constant dilution rate of 0.021 h−1 and in constant light. The release of chloride (•) was monitored to reflect the use of 3CBA. The chemostat inoculum consisted of 50 and 5 ml of R. palustris DCP3 and Alcaligenes sp. strain L6, resulting in initial relative population sizes of 95 and 5% based on cell numbers of the two species. (A) Low oxygen concentrations (3 μM O2) and 3CBA plus succinate limitation. (B) Low oxygen concentrations (3 μM O2) with increased 3CBA (1.5 mM) added to the feed at t = 250 h. (C) Oxygen-limiting conditions (<0.1 μM O2) and the same feed as in panel B. (D) Fully anoxic conditions and the same feed as in panel B.
FIG. 4.
Consumption rates of succinate (circles) and 3CBA (triangles) in the absence of oxygen (solid symbols) and in the presence of low oxygen (3 μM) concentrations (open symbols) in phototrophically incubated resting-cell suspensions from aliquots taken from the chemostat at t = 450 h (Fig. 3) in the presence of chloramphenicol. The data shown are mean values of duplicates.
The relative proportion of the two strains remained constant when the oxygen supply was further reduced to impose oxygen-limiting conditions (below the detection limit of the oxygen electrode, i.e., <0.1 μM O2) (Fig. 3C). This suggests that even under oxygen limitation, 3CBA was still exclusively metabolized by strain L6. If a significant fraction had been metabolized phototrophically by R. palustris DCP3, the relative proportion of the strain L6 population would have decreased. When fully anoxic conditions were imposed, Alcaligenes sp. strain L6 washed out from the chemostat and R. palustris DCP3 represented 100% of the culture and fully degraded 3CBA, as indicated by the stoichiometric release of chloride (Fig. 3D). These data indicate that R. palustris DCP3 was not able to compete effectively with the aerobic heterotroph Alcaligenes sp. strain L6 during growth on 3CBA in the presence of low oxygen concentrations (≤3 μM O2) and in the light. This was apparently due to a relative low affinity for 3CBA.
DISCUSSION
The ecological relevance of the different types of bacterial degradation processes contributing to the decomposition of halogenated aromatic compounds in low-oxygen environments has received relatively little attention. Facultatively anaerobic bacteria able to degrade haloaromatic compounds are also expected to be active at oxic-anoxic interfaces. Therefore, they may be significant competitors for aerobes that specialize in metabolism of such haloaromatic compounds at low pO2. The 3CBA-degrading facultatively anaerobic bacterium R. palustris DCP3 was isolated from a highly polluted freshwater sediment surface. It was postulated that this organism would be well adapted to cope with and probably make use of low concentrations of oxygen during anaerobic 3CBA metabolism. Results from pure batch cultures support this postulate. However, the outcome of competition experiments in mixed chemostate cultures with a 3CBA-degrading aerobic heterotroph, Alcaligenes sp. strain L6, has clearly demonstrated that even at very low oxygen concentrations (<0.1 μM O2), the aerobe used up all the 3CBA with oxygen as electron acceptor.
Data obtained in batch cultures in which 3CBA was the sole substrate revealed that 3CBA could indeed be degraded phototrophically by R. palustris DCP3 in the presence of low levels of oxygen (≤3 μM O2). However, the inability of R. palustris to degrade 3CBA in the dark under anoxic, micro-oxic, or fully oxic conditions implies that R. palustris DCP3 was not able to use 3CBA as an oxidizable energy source. This strictly light-dependent degradation of 3CBA also indicates that energy, in the form of light, is probably needed for the initial metabolic steps in the 3CBA degradation pathway. Thus, although the enzymes involved in 3CBA metabolism were present in cells which were initially cultured under anoxic phototrophic conditions, dechlorination did not occur. Activation of the primary substrate, 3CBA, into a coenzyme A ester may be needed, as has been shown for many anaerobic degradation pathways of benzenoid compounds (10, 12). In addition, this inability of R. palustris DCP3 to use 3CBA as the sole energy source in the presence of (low) oxygen concentrations excludes the existence of an additional 3CBA degradation pathway, distinct from the anaerobic pathway, as shown for the metabolism of some nonhalogenated aromatic compounds in several R. palustris strains (15, 21, 41).
Chemostat cultivation was used to study the influence of an additional carbon source (succinate) on the use of 3CBA at low and accurately controlled oxygen concentrations. The results showed that 3CBA was indeed degraded by R. palustris DCP3 in the presence of 3 μM O2, confirming the data obtained in batch cultures. In addition, it was demonstrated that oxygen inputs leaving residual oxygen concentrations exceeding the oxygen tolerance for 3CBA metabolism (>3 μM O2) in the absence of additional carbon sources could be scavenged by the organism’s own aerobic metabolism of the additional substrate succinate. Evidently, these cultures were able (i) to carry out simultaneous aerobic respiratory and anaerobic phototrophic metabolism either within all individual cells or distributed over anaerobically and aerobically growing cells within the culture and (ii) to create conditions with a pO2 low enough to permit light-dependent dechlorination. The abilities to switch from anaerobic to aerobic metabolism and to perform both types of metabolism at the same time at low oxygen concentrations were also shown for other facultatively anaerobic bacteria grown under a dual limitation of carbon substrate and oxygen (14, 16, 20). Moreover, simultaneous phototrophic and oxygen-dependent chemotrophic growth was also observed in the anoxygenic phototroph Thiocapsa roseopersicina with thiosulfate as the source of electrons for energy generation. This purple sulfur bacterium showed significantly lower cell yields under oxic conditions than under anoxic phototrophic conditions, since during chemotrophic growth part of the substrate was respired instead of being used as a carbon source (35). Similarly, the much lower cell densities reached after switching over to growth of R. palustris DCP3 on succinate and 3CBA in the presence of 24 μM O2 (compared with phototrophic growth) is probably not caused solely by the inability of this strain to use 3CBA under these conditions, but may also be due to the lower cell yields obtained during aerobic respiration of succinate. Besides losing the capacity to metabolize 3CBA at oxygen concentrations exceeding 3 μM, R. palustris DCP3 stops synthesizing photopigments. It has long been known that photopigment synthesis in anoxygenic phototrophs is indeed generally dependent on anoxic conditions (8), although some Rhodopseudomonas species begin to repress the synthesis of photosynthetic pigments only at oxygen tensions well above strictly anoxic conditions (2, 7). For R. palustris DCP3, it is probably the combination of the absence of pigments needed for energy supply and the oxygen sensitivity of the reductive pathway which caused the inhibition of 3CBA degradation at these levels of molecular oxygen.
The experiments investigating the competition between the “low-oxygen specialist” Alcaligenes sp. strain L6 and the facultative anaerobe R. palustris DCP3 for growth on 3CBA revealed that at low oxygen concentrations (3 μM O2) and under oxygen-limiting conditions (<0.1 μM O2), Alcaligenes sp. strain L6 outcompeted the phototroph. Only under fully anoxic conditions did the phototroph become dominant, obviously as a result of the inability of Alcaligenes sp. strain L6 to grow under anoxic conditions. Siefert et al. (38) also demonstrated that anoxygenic phototrophic bacteria which were incubated in activated and digestor sludge under different environmental conditions could compete successfully only with other bacteria under anoxic conditions in the light. The phototrophic bacteria were not capable of competing with chemotrophic bacteria under other conditions because of either the limited availability of light or the presence of too much oxygen. However, in our particular case, during competition between R. palustris DCP3 and Alcaligenes sp. strain L6, the high affinity of the latter for the substrate 3CBA most probably explains the dominance of the Alcaligenes sp., since studies in pure cultures clearly demonstrated that R. palustris is able to degrade 3CBA under micro-oxic conditions. In previous studies, it has been shown that the high-substrate affinity for 3CBA indeed determines the dominance of strain L6 during competition for 3CBA (27).
The outcome of our experiments raises the question whether the contribution of R. palustris DCP3-type phototrophs to the decomposition of haloaromatics at low oxygen concentrations is important. However, an important feature determining the actual competitive strength of bacteria relative to other organisms which has not been considered in these experiments is the capacity of the various inhabitants to adapt to alternating conditions, such as light intensity due to day-night rhythms and oxygen production and consumption by other members of the community, and the rate at which they do so. It is to be expected that the competitive advantage of phototrophs will vary strongly during day-night rhythms, which often are parallelled by changes in oxygen concentrations (9, 40). To further investigate the ecological relevance of these phototrophs, experiments are now being undertaken to determine the abundance of chlorinated aromatic-degrading phototrophs and to demonstrate their haloaromatic-degrading activities relative to aerobic and anaerobic heterotrophs in low oxygen environments in situ.
ACKNOWLEDGMENTS
We are very grateful to Rudolf A. Prins for helpful and valuable discussions during this research. Much to our sorrow, he passed away on 26 February 1997.
This research received financial support from the National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands.
REFERENCES
- 1.Abramowitz D A. Aerobic and anaerobic biodegradation of PCBs: a review. Crit Rev Biotechnol. 1990;10:241–251. [Google Scholar]
- 2.Albers H, Gottschalk G. Acetate metabolism in Rhodopseudomonas gelatinosa and several other Rhodospirillaceae. Arch Microbiol. 1976;111:45–49. doi: 10.1007/BF00446548. [DOI] [PubMed] [Google Scholar]
- 3.Amann R, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balba M T, Evans W C. The methanogenic fermentation of aromatic substrates. Biochem Soc Trans. 1977;5:302–304. doi: 10.1042/bst0050302. [DOI] [PubMed] [Google Scholar]
- 5.Bergman J G, Sanik J. Determination of trace amounts of chlorine in naphtha. Anal Chem. 1957;29:241–243. [Google Scholar]
- 6.Blasco R, Castillo F. Light-dependent degradation of nitrophenols by the phototrophic bacterium Rhodobacter capsulatus E1F1. Appl Environ Microbiol. 1992;58:690–695. doi: 10.1128/aem.58.2.690-695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butow B, Bergstein-Ben Dan T. Effects of growth on acetate utilization by Rhodopseudomonas palustris isolated from a freshwater lake. Microb Ecol. 1991;22:317–328. doi: 10.1007/BF02540233. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Bazire G, Sistrom W R, Stanier R W. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol. 1957;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 9.de Wit R. Interactions between phototrophic bacteria in marine sediments. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1989. [Google Scholar]
- 10.Elder D J E, Kelly D J. The bacterial degradation of benzoic acid and benzenoid compounds under anaerobic conditions: unifying trends and new perspectives. FEMS Microbiol Rev. 1994;13:441–468. doi: 10.1111/j.1574-6976.1994.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 11.Fava F, Di Gioia D, Romagnoli C, Marchetti L, Mares D. Biosynthesis and cytoplasmic accumulation of a chlorinated catechol pigment during 3-chlorobenzoate aerobic co-metabolism in Pseudomonas fluorescens. Arch Microbiol. 1993;160:350–357. doi: 10.1007/BF00252220. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs G, Mohamed M E S, Altenschmidt U, Koch J, Lack A, Brackmann R, Lochmeyer C, Oswald B. Biochemistry of anaerobic biodegradation of aromatic compounds. In: Ratledge C, editor. Biochemistry of microbial degradation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 513–553. [Google Scholar]
- 13.Gerritse J, Gottschal J C. Mineralization of the herbicide 2,3,6-trichlorobenzoic acid by a co-culture of anaerobic and aerobic bacteria. FEMS Microbiol Ecol. 1992;101:89–98. [Google Scholar]
- 14.Gerritse J, Gottschal J C. Oxic and anoxic growth of a new Citrobacter species on amino acids. Arch Microbiol. 1993;160:51–61. [Google Scholar]
- 15.Gibson J, Dispensa M, Harwood C S. 4-Hydroxybenzoyl coenzyme A reductase (dehydroxylating) is required for anaerobic degradation of 4-hydroxybenzoate by Rhodopseudomonas palustris and shares features with molybdenum-containing hydroxylases. J Bacteriol. 1997;179:634–642. doi: 10.1128/jb.179.3.634-642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschal J C, Szewzyk R. Growth of a facultative anaerobe under oxygen-limiting conditions in pure culture and in co-culture with a sulphate reducing bacterium. FEMS Microbiol Ecol. 1985;31:159–170. [Google Scholar]
- 17.Gribble G W. The natural production of chlorinated compounds. Environ Sci Technol. 1994;28:310–319. doi: 10.1021/es00056a712. [DOI] [PubMed] [Google Scholar]
- 18.Haller H D, Finn R K. Biodegradation of 3-chlorobenzoate and formation of black colour in the presence and absence of benzoate. Eur J Appl Microbiol Biotechnol. 1979;8:191–205. [Google Scholar]
- 19.Harkness M R, McDermott J B, Abramowitz D A, Salvo J J, Flanagan W P, Stephens M L, Mondello F J, May R J, Lobos J H, Caroll K M, Brennan M J, Bracco A A, Fish K M, Warner G L, Wilson P R, Dietrich D K, Lin D T, Morgan C B, Gately W L. In situ stimulation of aerobic PCB biodegradation in Hudson river sediments. Science. 1993;259:503–507. doi: 10.1126/science.8424172. [DOI] [PubMed] [Google Scholar]
- 20.Harrison D E F, Loveless J E. The effect of growth conditions on respiratory activity and growth efficiency in facultative anaerobes in chemostat culture. J Gen Microbiol. 1971;68:35–43. doi: 10.1099/00221287-68-1-35. [DOI] [PubMed] [Google Scholar]
- 21.Harwood C S, Gibson J. Anaerobic and aerobic metabolism of diverse aromatic compounds by the photosynthetic bacterium Rhodopseudomonas palustris. Appl Environ Microbiol. 1988;54:712–717. doi: 10.1128/aem.54.3.712-717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imhoff J F. Taxonomy and physiology of phototrophic purple bacteria and green sulfur bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1–15. [Google Scholar]
- 23.Kamal V S, Wyndham R C. Anaerobic phototrophic metabolism of 3-chlorobenzoate by Rhodopseudomonas palustris WS17. Appl Environ Microbiol. 1990;56:3871–3873. doi: 10.1128/aem.56.12.3871-3873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keith C L, Bridges R L, Fina L R, Iverson K L, Cloran J A. The anaerobic decomposition of benzoic acid during methane formation. IV. Dearomazation of the ring and volatile fatty acids formed on ring rupture. Arch Microbiol. 1978;118:173–176. doi: 10.1007/BF00415726. [DOI] [PubMed] [Google Scholar]
- 25.Khanna P, Rajkumar B, Jothikumar N. Anoxygenic degradation of aromatic substances by Rhodopseudomonas palustris. Curr Microbiol. 1992;26:1–9. doi: 10.1007/BF01570961. [DOI] [PubMed] [Google Scholar]
- 26.Krooneman J, Wieringa E B A, Moore E R B, Gerritse J, Prins R A, Gottschal J C. Isolation of Alcaligenes sp strain L6 at low oxygen concentrations and degradation of 3-chlorobenzoate via a pathway not involving (chloro)catechols. Appl Environ Microbiol. 1996;62:2427–2434. doi: 10.1128/aem.62.7.2427-2434.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krooneman J, Moore E R B, van Velzen J C L, Prins R A, Forney L J, Gottschal J C. Competition for oxygen and 3-chlorobenzoate between two aerobic bacteria using different degradation pathways. FEMS Microbiol Ecol. 1998;26:171–179. [Google Scholar]
- 28.Kukor J J, Olsen R H. Catechol 2,3-dioxygenases functional in oxygen-limited (hypoxic) environments. Appl Environ Microbiol. 1996;62:1728–1740. doi: 10.1128/aem.62.5.1728-1740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leahy J G, Olsen R H. Kinetics of toluene degradation by toluene-oxidizing bacteria as a function of oxygen concentration, and the effect of nitrate. FEMS Microbiol Ecol. 1997;23:23–30. [Google Scholar]
- 30.McGrath J E, Harfoot C G. Reductive dehalogenation of halocarboxylic acids by phototrophic genera Rhodospirillum and Rhodopseudomonas. Appl Environ Microbiol. 1997;63:3333–3335. doi: 10.1128/aem.63.8.3333-3335.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery L, Vogel T M. Dechlorination of 2,3,5,6-tetrachlorobiphenyl by a phototrophic enrichment culture. FEMS Microbiol Lett. 1992;94:247–250. doi: 10.1016/0378-1097(92)90638-5. [DOI] [PubMed] [Google Scholar]
- 32.Nanninga H J, Gottschal J C. Amino acid fermentation and hydrogen transfer in mixed cultures. FEMS Microbiol Ecol. 1985;31:261–269. [Google Scholar]
- 33.Olsen R H, Mikesell M D, Kukor J J. Enumeration and characterisation of BTEX-degrading bacteria from hypoxic environments functional with mixed electron acceptors. Res Microbiol. 1994;145:47–49. doi: 10.1016/0923-2508(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 34.Reineke W. Microbial degradation of halogenated aromatic compounds. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 319–360. [Google Scholar]
- 35.Schaub B E M, Van Gemerden H. Simultaneous phototrophic and chemotrophic growth in the purple sulfur bacterium Tiocapsa roseopersicina M1. FEMS Microbiol Ecol. 1994;13:185–196. [Google Scholar]
- 36.Schennen U, Braun K, Knackmuss H J. Anaerobic degradation of 2-fluorobenzoate by benzoate-degrading denitrifying bacteria. J Bacteriol. 1985;161:321–325. doi: 10.1128/jb.161.1.321-325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaler T A, Klečka G M. Effects of dissolved oxygen concentration on biodegradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1986;51:950–955. doi: 10.1128/aem.51.5.950-955.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siefert E, Irgens R L, Pfennig N. Phototrophic purple and green bacteria in a sewage treatment plant. Appl Environ Microbiol. 1978;35:38–44. doi: 10.1128/aem.35.1.38-44.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swarts H J, Verhagen F J M, Field J A, Wijnberg J B P A. Novel chlorometabolites produced by Bjerkandera species. Phytochemistry (Oxford) 1996;42:1699–1701. [Google Scholar]
- 40.van de Ende F P. Microbial ecology of oxygen-sulfide interfaces in marine benthic ecosystems. Ph.D. thesis. Groningen, The Netherlands: University of Groningen; 1997. [Google Scholar]
- 41.Van der Woude B J, De Boer M, Van der Put N M J, Van der Geld F M, Prins R A, Gottschal J C. Anaerobic degradation of halogenated benzoic acids by photoheterotrophic bacteria. FEMS Microbiol Lett. 1994;119:199–208. doi: 10.1111/j.1574-6968.1994.tb06889.x. [DOI] [PubMed] [Google Scholar]
- 42.Viliesid F, Lilly M D. Influence of dissolved oxygen tension on the synthesis of catechol 1,2-dioxygenase by Pseudomonas putida. Enzyme Microb Technol. 1992;14:561–565. [Google Scholar]
- 43.Williams R J, Evans W C. The metabolism of benzoate by Moraxella species through anaerobic nitrate respiration. Biochem J. 1975;148:1–10. doi: 10.1042/bj1480001a. [DOI] [PMC free article] [PubMed] [Google Scholar]