Abstract
Autoimmune pancreatitis (AIP) has attracted much attention in the last two decades, and due to the diagnostic value of immunoglobulin G4 (IgG4), the number of cases diagnosed in clinical practice has markedly increased. However, in contrast to prototypic IgG4-related type 1 AIP, a minor subtype of AIP, referred to as type 2 AIP, is less widely known and has thus not yet been characterized in detail. Type 2 AIP is unrelated to IgG4 and is a completely distinct entity from type 1 AIP. One confusing factor is that the two types of AIP share patterns of clinical presentation (e.g., acute pancreatitis and painless jaundice) and imaging abnormalities (e.g., diffuse or segmental enlargement). Since there are currently no established serum markers, the diagnosis of type 2 AIP is highly challenging and requires the tissue confirmation of neutrophilic injury to the pancreatic ducts, a finding designated as a granulocytic epithelial lesion. Approximately one-third of cases are associated with inflammatory bowel disease, particularly ulcerative colitis; however, the pathological relationship between these two conditions has not yet been clarified. Unanswered questions relate to its pathophysiology, the potential development of a similar granulocytic injury in other organs, and the characteristics of pediatric cases. This review summarizes consensus and controversies surrounding type 2 AIP, with the aim of increasing awareness and highlighting the unmet needs of this underrecognized condition.
Keywords: Idiopathic duct-centric pancreatitis; Autoimmune pancreatitis; Immunoglobulin G; Granulocytic epithelial lesion; Colitis, ulcerative
INTRODUCTION
A distinct form of pancreatitis, which is currently regarded as type 2 autoimmune pancreatitis (AIP), was initially identified in 2003 in two pathological studies that histologically reviewed cases of suspected AIP.1,2 Descriptive diagnostic terms, such as idiopathic duct-centric pancreatitis and granulocytic epithelial lesion (GEL)-positive pancreatitis, were originally used.1,2 After the distinct clinical features of these cases were confirmed, an international expert group proposed the diagnostic term “type 2 AIP” in 2010 in order to distinguish it from the prototypic form of AIP (currently referred to as type 1 AIP or immunoglobulin G4 [IgG4]-related AIP).3 Since only limited advances have been made in the past 10 years, a more detailed understanding of this condition is needed. Although the incidence of type 2 AIP is expected to be similar among different ethnicities,4 the incidence of its diagnosis in real clinical practice significantly varies among countries or even centers in the same country, indicating the challenges associated with establishing a diagnosis.
Consensus and controversies surrounding type 2 AIP are reviewed herein, with the aim of summarizing what has been discovered and also what remains to be investigated in this condition.
CONSENSUS
1. Consensus 1: type 2 AIP is distinct from type 1 AIP
Although type 2 AIP shares some features with type 1 AIP, these two conditions are distinct without any overlaps.5 Table 1 summarizes the features of two types of AIP. In contrast to type 1 AIP, which is a pancreatic manifestation of IgG4-related disease, serum IgG4 concentrations are normal or only mildly elevated in type 2 AIP.5 Serum IgG4 elevations >300 mg/dL (or >2-fold higher than the normal range) are highly suggestive of type 1 AIP.6-8 Since the ratio of serum IgG4/IgG is typical >10% in type 1 AIP and ≤10% in other conditions, this calculation is useful for cases with only a mild IgG4 elevation.9 Although specific serological markers are not currently available for type 2 AIP, anti-neutrophil cytoplasmic antibodies can be detected in patients with type 2 AIP.10
Table 1.
Comparison between Type 1 and Type 2 AIP
| Variable | Type 1 AIP | Type 2 AIP |
|---|---|---|
| Mean age, yr | 60 (typically >40) |
30 (including children) |
| Male sex, % | 80 | 50 |
| Serum IgG4 elevation, % | 80–90 | 10 |
| Other organ involvement, %* | 50 | 0 |
| Inflammatory bowel disease, % | <5 | 40 |
| Presentation, % | ||
| Pain/acute pancreatitis | 10 | 60 |
| Painless jaundice | 80 | 30 |
| Others | 10 | 10 |
| Histology | ||
| Pattern of inflammation | Lobule centric | Duct centric |
| Lymphoplasmacytic infiltration | ++ | ++ |
| Storiform fibrosis | ++ | + |
| Obliterative phlebitis | ++ | + |
| Granulocytic epithelial lesion | - | ++ |
| Granulocytic acinar injury | - | ++ |
| IgG4+ cells | ++ | – or + |
| Steroid responsiveness, % | ~100 | ~100 |
| Relapse, % | 30–50 | <10 |
AIP, autoimmune pancreatitis; IgG4, immunoglobulin G4.
*Including upstream sclerosing cholangitis, sialadenitis, dacryoadenitis, tubulointerstitial nephritis, retroperitoneal fibrosis, and periaortitis.
The assessment of extrapancreatic diseases is useful for the typing of AIP. The involvement of other organs in type 1 AIP has been demonstrated in various organs (e.g., the salivary glands, lacrimal glands, retroperitoneum, abdominal aorta, kidneys, and lungs).11,12 Extrapancreatic bile duct involvement is not expected in type 2 AIP, and the radiological confirmation of diffuse cholangiopathy is highly suggestive of type 1 AIP.6,7,13
In contrast, inflammatory bowel disease (IBD) is a single potential extrapancreatic disease in patients with type 2 AIP, with approximately 40% of cases being diagnosed with IBD (75%, ulcerative colitis; 15%, Crohn’s disease; 10%, unclassified).6,7 Since the relationship between type 1 AIP and IBD is very weak, AIP that develops in patients with known IBD is most likely type 2. In a French and Belgian multicenter study on IBD patients, 91 were found to have AIP; 89 (98%) had type 2 AIP and only two (2%) had type 1 AIP.14 IBD is typically diagnosed before or at the same time as an episode of type 2 AIP (80%), but may also develop after the diagnosis of type 2 AIP (20%).14 Although IBD increases the risk of type 2 AIP and primary sclerosing cholangitis (PSC), cases with both type 2 AIP and PSC have not yet been described in the literature.
2. Consensus 2: type 2 AIP is less common than type 1 AIP
In an international multicenter study, 1,064 cases of AIP were identified. The proportion of type 2 AIP was 14% in the United States, 13% in Europe, and 4% in Asia.4 However, a recent Korean study reported a higher proportion of type 2 AIP (11%).7 This difference in the incidence of type 2 AIP in Asia may be due to an increased awareness of this condition, the use of international diagnostic criteria,15 and the increased availability and quality of endoscopic ultrasound-guided pancreatic biopsy. Therefore, the incidence of type 2 AIP does not significantly differ among ethnic groups. Based on a nationwide study in Japan, the overall prevalence of AIP is 4.6/100,000 population,16 suggesting that the prevalence of type 2 AIP is approximately 0.5/100,000.
3. Consensus 3: histology is the gold standard for diagnosing type 2 AIP
Since type 2 AIP is a condition discovered by microscopic examination, it is defined by histological features. Serological markers that are highly specific for type 2 AIP have not yet been identified.13
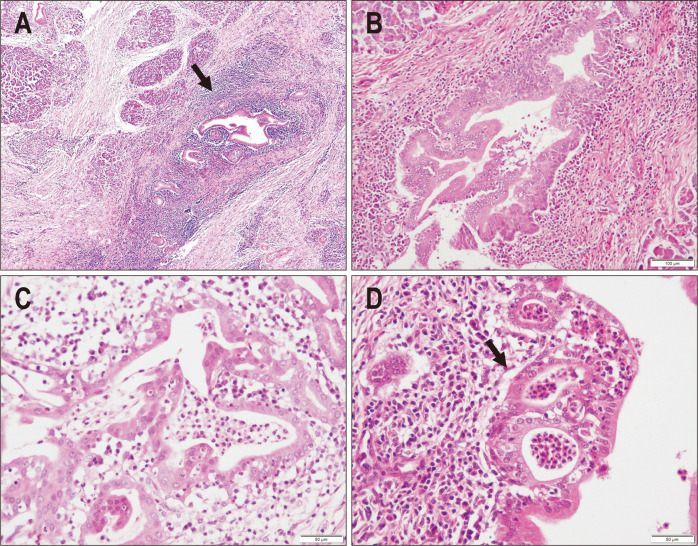
Similar to type 1 AIP, type 2 is characterized by a massive inflammatory infiltrate including many lymphocytes and plasma cells.17 In contrast, the pattern and cellular components of inflammation differ between the two types (Table 1).3,18 One significant difference is the distribution of inflammation. In type 2 AIP, periductal inflammation is more pronounced than lobular inflammation (Fig. 1A and B), while type 1 shows a lobule-centered pattern of injury. This difference may be one of the reasons why type 2 AIP less commonly shows a capsule-like rim on imaging than type 1 because this radiological finding represents inflammatory extension from the lobules to pancreatic adipose tissue.19 Obliterative phlebitis and storiform fibrosis, two characteristic findings of IgG4-related disease, are detected in type 2 AIP, but are less frequent and conspicuous.3,18
Fig. 1.
Microscopic features of type 2 autoimmune pancreatitis (AIP). (A) The pancreatic duct and lobules are both involved in the fibroinflammatory process, with the inflammatory infiltrate being more pronounced in the periductal area (arrow) (H&E, ×20). (B) The pancreatic duct is heavily infiltrated by inflammatory cells, including lymphocytes and plasma cells, and the architecture of the lining epithelium is irregular (H&E, ×40). (C) The pancreatic duct is severely damaged with many infiltrating neutrophils, which is consistent with granulocytic epithelial lesions (H&E, ×100). (D) Neutrophilic aggregates in the duct lumen resemble crypt abscesses in inflammatory bowel disease (H&E, ×100).
The neutrophil-rich nature of inflammation discriminates type 2 from type 1 AIP.1,2,17 Characteristically, many neutrophils infiltrate the epithelial layer of the pancreatic ducts, a finding designated as GEL (Fig. 1C).2 Intraductal clusters of neutrophils resembling crypt abscesses in IBD often co-exist (Fig. 1D). Neutrophilic infiltration is also present in acini or around small ductules, but is less specific for the diagnosis of type 2 AIP.3,18 In contrast, type 1 AIP and IgG4-related disease in extrapancreatic organs typically lack neutrophilic infiltration or abscess formation.
In contrast to type 1 AIP, which shows diffuse infiltration of IgG4-positive plasma cells, type 2 AIP typically lacks the marked increase in IgG4-positive plasma cells; however, isolated pockets with numerous amounts of these cells are not uncommon.17,20,21 The number of IgG4-positive cells is typically <10 per high-power field (HPF) of biopsy material and <50 per HPF of resected samples of type 2 AIP.22 Similarly, the IgG4/IgG-positive plasma cell ratio does not exceed 40% in type 2 AIP.22
4. Consensus 4: imaging studies are useful for the diagnosis, but not typing, of AIP
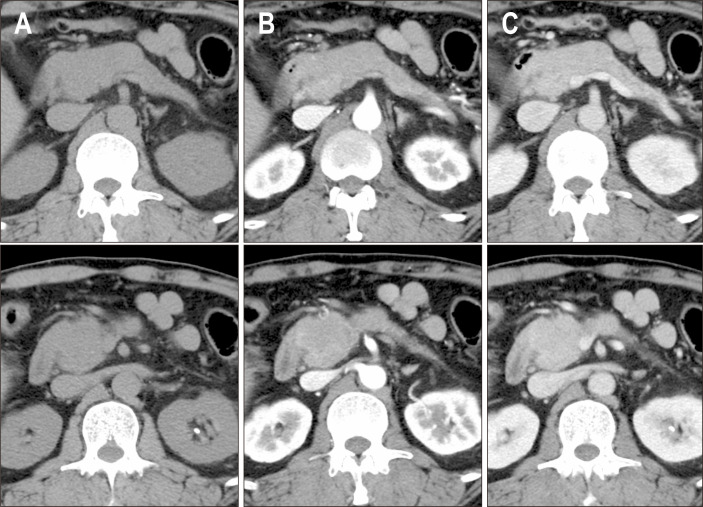
Imaging abnormalities of the pancreas are almost indistinguishable between the two types of AIP, with diffuse sausage-like and segmental enlargements being the two most common manifestations (Fig. 2).19,23-25 This is one of the reasons why the same term of AIP is still used for these totally distinct entities. Multifocal hypoattenuation areas may also occur in either type.
Fig. 2.
Imaging findings of type 2 autoimmune pancreatitis (AIP). The pancreatic head and body show global enlargement with gradual enhancement. The diagnosis of type 2 AIP was confirmed by the presence of a granulocytic epithelial lesion in the pancreatic biopsy. (A) Non-contrast, (B) early phase enhancement, and (C) late phase enhancement.
Dynamic studies reveal decreased enhancement in the pancreatic phase, homogeneous delayed enhancement in the portal venous phase, and contrast retention in the delayed phase.26,27 On magnetic resonance imaging, the affected pancreatic parenchyma typically appeared as hypointense on T1-weighted images and slightly hyperintense on T2-weighted images.23 A systematic comparison of imaging findings between the two types failed to demonstrate a significant difference, except for the involvement of other organs; however, in our experience, a capsule-like rim and thickening and enhancement of the intrapancreatic bile duct are less common in type 2 AIP. It is important to note that peripancreatic fluid may be mistaken for a capsule-like rim.19 Peripancreatic fluid often has an irregular border, T2 hyperintensity, and no enhancement.19
5. Consensus 5: broad differential diagnosis of type 2 AIP
Due to the tumefactive nature of either type of AIP, the diagnosis of AIP requires the careful exclusion of pancreatic malignancy by imaging studies, tumor markers, and tissue or cytological examinations. Since abdominal pain and the diffuse enlargement of the pancreas are common findings in type 2 AIP, acute interstitial pancreatitis is another differential diagnosis, particularly in young patients.
Type 2 AIP is a potential cause of acute pancreatitis in patients with known IBD.14 However, a Korean study on 1,106 patients with ulcerative colitis indicated that the estimated prevalence of AIP (including both types) was 0.54%;28 therefore, alternative etiologies, such as medication (e.g., mesalamine and azathioprine) and gallstones, need to be considered. in the first instance.6 Likewise, in a consecutive, non-selective cohort of acute pancreatitis, only a small proportion of cases were attributable to AIP.29 Drug-induced pancreatitis often radiologically mimics type 2 AIP, and a clinical evaluation of the chronological relationship between drug use and the onset of pancreatitis is useful for a differential diagnosis.
The histological diagnosis of type 2 AIP in surgically resected specimens is relatively straightforward, while a biopsy diagnosis remains challenging. Type 2 AIP needs to be discriminated from type 1 AIP by morphological features and IgG4 immunostaining. Correlations with clinical features, particularly serum IgG4 concentrations and other organ involvement, facilitate the diagnosis of cases with non-conclusive biopsy findings. Acute interstitial pancreatitis may be mistaken for type 2 AIP because it is often associated with neutrophilic infiltration into small tubules. The presence or absence of dense lymphocytic infiltration in the background assists in discrimination. Another caveat is that neutrophilic infiltration in the duct epithelium or lobules can be focally detected in peritumoral pancreatitis.
6. Consensus 6: the diagnosis requires a multi-disciplinary approach
A diagnosis of type 2 AIP needs to be established using a combination of imaging abnormalities, biopsy findings, a history of IBD, and responses to steroids. According to the international diagnostic criteria,15 an imaging abnormality consistent with AIP is an essential element. Typical radiological findings listed in the criteria include diffuse enlargement with delayed enhancement and long or multiple strictures of the pancreatic ducts without marked upstream dilatation. Findings that are less specific for, but consistent with AIP are focal or segmental enlargement with delayed enhancement and focal or segmental narrowing without marked upstream duct dilatation.
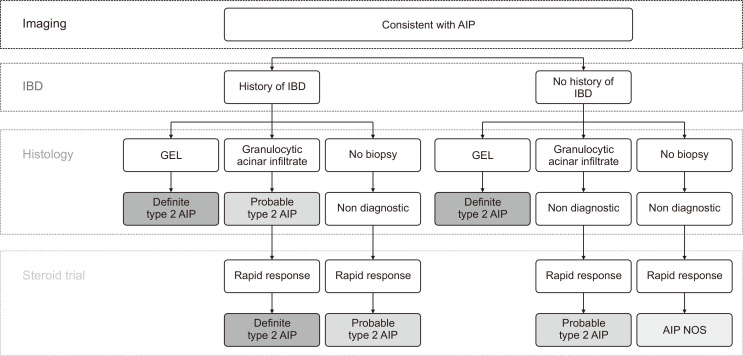
When imaging criteria are met, type 2 AIP needs to be diagnosed in a step-by-step manner, as summarized in Fig. 3. In contrast to type 1 AIP, the diagnosis of which may be established without biopsy, type 2 AIP generally requires the tissue confirmation of neutrophilic infiltration into the ducts or acini.15 Without histology, type 2 AIP cannot be diagnosed in patients without history of IBD, even if they show typical imaging features and good response to corticosteroids. AIP not-otherwise-specified is the proposed diagnostic term for these cases.15,30 Scarce IgG4-positive plasma cells (<10 cells/HPF) also need to be confirmed by immunostaining.
Fig. 3.
Diagnostic algorithm based on international diagnostic criteria.
AIP, autoimmune pancreatitis; IBD, inflammatory bowel disease; GEL, granulocytic epithelial lesion; NOS, not-otherwise-specified.
A steroid trial using 0.6 to 1.0 mg/kg prednisolone followed by a reassessment of imaging findings and carbohydrate antigen 19-9 (CA19-9) levels after 2 weeks of treatment can be used as part of the diagnostic process.15 Definite improvements in pancreatic enlargement and a decrease in CA19-9 levels, if elevated before treatment, are expected. However, a steroid trial needs to be considered with caution because various conditions, including peritumoral pancreatitis, potentially respond to immunosuppression. Pancreatic biopsy needs to be considered before a steroid trial, which veils pathognomonic histological changes.
7. Consensus 7: immunosuppression with corticosteroids is the treatment of choice
Type 2 AIP is a steroid-responsive disorder, and rapid improvements in symptoms and imaging abnormalities are expected within 2 or 3 weeks of the commencement of corticosteroids.6,7 Spontaneous regression without immunosuppression has also been documented. In contrast to type 1 AIP, in which relapse is relatively common (30% to 50%), disease relapse is uncommon in type 2 AIP (10%);6,7 therefore, maintenance therapy is unnecessary in most patients.
CONTROVERSY
1. Controversy 1: diversity of clinical presentation
The majority of patients with type 2 AIP present with features of acute pancreatitis (60%) or painless jaundice (30%).6,7 Episodes of acute pancreatitis in patients with type 2 AIP are clinically mild without the need for intensive care unit admission, the development of organ failure, or peripancreatic fluid collection.6 Other less common patterns of presentation include liver dysfunction, non-specific abdominal symptoms, and the incidental detection of a pancreatic mass on images taken for other purposes.
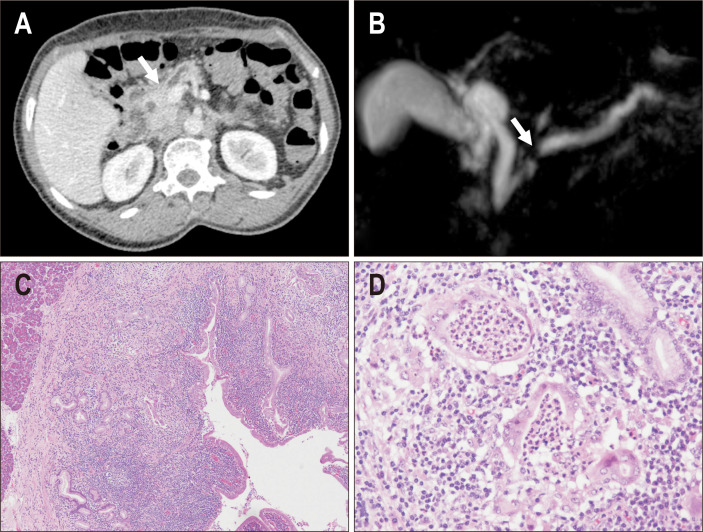
We recently encountered two patients with type 2 AIP who presented with pancreatic duct obstruction, but no mass or parenchymal abnormalities (Fig. 4). They underwent pancreatectomy for a suspected small neoplasm obstructing the main pancreatic duct. Histologically, a lymphoplasmacytic infiltrate with GEL was observed along the ductal system, a finding that is consistent with type 2 AIP. However, the inflammatory process was mostly restricted to the periductal areas, and the parenchyma lacked significant inflammation. These cases suggest that type 2 AIP presents with only duct abnormalities. Due to the lack of characteristic imaging findings, these cases do not meet the current diagnostic criteria of type 2 AIP.
Fig. 4.
A case of type 2 autoimmune pancreatitis (AIP) that presented with pancreatic duct obstruction. (A) Computed tomography showed duct obstruction in the pancreatic head (arrow); however, a mass lesion was not confirmed. (B) Magnetic resonance cholangiopancreatography showing obstruction of the main duct at the pancreatic head (arrow) and dilatation of the upstream duct. (C) In the resected specimen, a dense inflammatory infiltrate was observed along the duct system, and pancreatic parenchyma was not inflamed (left upper area) (H&E, ×20). (D) A granulocytic epithelial lesion was confirmed in the damaged pancreatic duct (H&E, ×100).
A broader awareness of this condition among gastroenterologists and pathologists may lead to the identification of unusual manifestations of type 2 AIP. Without these studies, the diversity of the clinical and pathological manifestations of type 2 AIP will remain unclear.
2. Controversy 2: is AIP in children always type 2?
This is a debatable topic. Some autoimmune diseases show different characteristics between adult and pediatric patients. In autoimmune hepatitis, type 2 disease characterized by a positive liver kidney microsomal type 1 antibody generally develops in children.31 Pediatric cases of PSC often have positive autoantibodies and significant inflammatory activity resembling autoimmune hepatitis, and, thus, it is sometimes referred to as autoimmune sclerosing cholangitis.32 Serum IgG4 concentrations are elevated in 10% to 15% of otherwise typical adult PSC,33,34 and this ratio increases up to 40% in pediatric PSC.
A limited number of reports of pediatric AIP are available in the literature and the use of inconsistent diagnostic criteria preclude a systematic comparison between adult and pediatric patients.35-39 In 2014, we performed a literature review of pediatric AIP to clarify which type of AIP is dominant in the pediatric population by applying the international diagnostic criteria to reported cases.10 The 18 cases identified (including two own cases) were classified as definite type 2 AIP (n=10), probable type 2 AIP (n=1), AIP not-otherwise-specified (n=5), and probable type 1 AIP (n=2).10 The diagnosis of probable type 1 AIP was based on a mildly elevated serum IgG4 level (224 mg/dL) or a borderline increase in IgG4-positive plasma cells (10 cells/HPF).37
In 2017, an international multicenter study successfully identified 18 pediatric patients with AIP from the registry database of acute recurrent and chronic pancreatitis, and examined the features of these cases along with reported cases in the literature (n=48 in total).40 The majority of patients presented with abdominal pain (91%) with or without obstructive jaundice (42%). Serum IgG4 levels were elevated in 22% of patients, and antinuclear antibodies were detected in 29%.40 The involvement of other organs suggestive of IgG4-related disease was observed in 4% of patients, and IBD was confirmed in 20%. Histology findings were available in 26 children, and most had lymphoplasmacytic infiltration (92%) and GEL (69%).40 IgG4-positive plasma cell infiltration (>10/HPF) was confirmed in a single case (4%). These findings suggested that most cases of pediatric AIP are type 2. However, it currently remains unclear whether type 1 AIP still, although rarely, occurs in children, if pediatric patients with type 2 AIP have any unique features, or whether the international diagnostic criteria of AIP are applicable to children.41
3. Controversy 3: is type 2 AIP a pancreas-restricted condition?
Although type 2 AIP is generally recognized as a pancreas-oriented condition, a few studies suggested that a similar GEL-positive duct injury may occur in other organs. We reported a 13-year-old boy with IBD and steroid-responsive cholangitis.42 He presented with abdominal pain, bloody diarrhea, abnormal liver function tests, and weight loss. IBD was diagnosed by barium follow-through, endoscopy, and biopsy. Endoscopic retrograde cholangiopancreatography revealed a normal pancreatogram, but intrahepatic cholangiopathy. Liver biopsy showed portal tract changes that were consistent with sclerosing cholangitis (e.g., periductal fibrosis); bile duct injury was associated with the infiltration of many neutrophils in the epithelial layer, resembling GEL of type 2 AIP.42 The serum concentration of IgG4 was within the normal range and IgG4 immunostaining was normal. Based on the diagnosis of sclerosing cholangitis with IBD, the patient was treated with sulfasalazine and prednisolone. Sclerosing cholangitis showed an excellent response to steroids, and no relapse was observed in a follow-up period of 24 years.42
Following the report of the index case, we reviewed the liver biopsies of 103 children and 142 adults with PSC, and detected GEL in four children and one adult.43 One patient had concomitant pancreatitis. All patients went into remission with prednisolone and/or ursodeoxycholic acid, and their liver function tests remained completely normal without relapse over a follow-up period of 6 to 16 years.43 These benign clinical courses indicated that sclerosing cholangitis with GEL is distinct from PSC and may be a biliary counterpart of type 2 AIP.
Another recent study described GEL-positive sialadenitis in a patient with histology-proven type 2 AIP.44 A few other reports also reported cholangiopathy and sialadenitis in patients with type 2 AIP.45-47 GEL-positive duct injuries in the pancreas, salivary glands and biliary tree may be analogical conditions. According to the international diagnostic criteria, type 2 AIP associated with intrahepatic cholangiopathy or sialadenitis is potentially misclassified as type 1 AIP without the histological confirmation of GEL.
4. Controversy 4: pathophysiology
Although the pathogenesis or immunological features of type 2 AIP remain largely unknown, the relationship with IBD is a potential clue.5,17
We previously examined the expression of various cytokines in pancreatic tissue samples of type 2 AIP.48 In type 2 AIP, the expression of interleukin (IL)-4, IL-10, and tumor necrosis factor α was almost undetectable, while that of interferon-γ was similar between types 1 and 2 AIP. The expression of IL-8 mRNA was markedly stronger in type 2 AIP (13-fold) than in type 1 AIP).48 Immunostaining revealed that IL-8 was expressed in neutrophils, T lymphocytes, and the damaged duct epithelium.48 IL-8-positive cells were scarce in type 1 AIP and peritumoral pancreatitis. Based on strong chemotactic effects for neutrophils, the aberrant expression of IL-8 in the duct epithelium may be an immunological event underlying the development of GEL. Similarly, IL-8-expressing T cells may also contribute to the recruitment of neutrophils to the pancreas. Another interesting finding obtained in that study was that the similar expression of IL-8 in the epithelium was confirmed in colonic biopsies of ulcerative colitis.48 IL-8 appeared to be positive in the epithelium involving crypt abscesses, suggesting that type 2 AIP and ulcerative colitis share immunological features with neutrophilic epithelial injury, thereby linking the two conditions.
We recently observed an elevated serum IL-8 level of 8.3 pg/mL (normal range <2.0) in a patient with histology-proven type 2 AIP, indicating the diagnostic value of serum IL-8 levels for type 2 AIP. Since serum IL-8 levels are elevated in some patients with pancreatic cancer, this cytokine may not be useful for discriminating between type 2 AIP and pancreatic cancers.49 However, we still suspect that serum IL-8 in combination with IgG4 may assist in the typing of AIP in patients with suspected AIP. A systematic investigation using a large cohort is needed to establish whether IL-8 may serve as a serum marker for type 2 AIP.
Another pathological study revealed the aberrant expression of programmed death-ligand 1 (PD-L1) in the pancreatic duct epithelium in type 2 AIP.50 Positive immunoreactivity was identified in 70% of cases of type 2 AIP, but was almost entirely negative in cases of type 1 AIP, other forms of pancreatitis, and the background pancreas around pancreatic cancer (99% specificity).50 PD-L1 was expressed in the ducts involved in GEL as well as in those without neutrophilic injury. Despite its diagnostic value, the biological significance of this finding remains unclear. The expression of PD-L1 did not correlate with the number of immune cells positive for programmed death 1 (PD-1) or CD8.50
These studies investigated selected immunological markers. A large, non-biased, comprehensive analysis of various immunological aspects is awaited. An immunological comparison between type 2 AIP and IBD will clarify whether these two conditions have a pathophysiological link.
5. Controversy 5: long-term outcomes
Type 2 AIP is considered to have a benign clinical course with a low risk of relapse. However, only a small number of clinical studies from selected institutes are currently available;6,7 the median follow-up period was 3 years, which was not long enough to characterize long-term outcomes, particularly those of inflammatory conditions.
CONCLUSION
The typical clinical and pathological features of type 2 AIP have been characterized. However, unusual manifestations, pathophysiology, and long-term outcomes warrant further research. Large-scale studies are needed to address these aspects, and improvements in diagnostic processes will be a crucial element for the further development of this field.
ACKNOWLEDGEMENTS
This study received a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (grant number: 18H02630).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119–1127. doi: 10.1097/00000478-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Zamboni G, Lüttges J, Capelli P, et al. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552–563. doi: 10.1007/s00428-004-1140-z. [DOI] [PubMed] [Google Scholar]
- 3.Chari ST, Kloeppel G, Zhang L, et al. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549–554. doi: 10.1097/MPA.0b013e3181e4d9e5. [DOI] [PubMed] [Google Scholar]
- 4.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut. 2013;62:1771–1776. doi: 10.1136/gutjnl-2012-303617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149:39–51. doi: 10.1053/j.gastro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Hart PA, Levy MJ, Smyrk TC, et al. Clinical profiles and outcomes in idiopathic duct-centric chronic pancreatitis (type 2 autoimmune pancreatitis): the Mayo Clinic experience. Gut. 2016;65:1702–1709. doi: 10.1136/gutjnl-2015-309275. [DOI] [PubMed] [Google Scholar]
- 7.Oh D, Song TJ, Moon SH, et al. Type 2 autoimmune pancreatitis (idiopathic duct-centric pancreatitis) highlighting patients presenting as clinical acute pancreatitis: a single-center experience. Gut Liver. 2019;13:461–470. doi: 10.5009/gnl18429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 9.Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 2015;94:e680. doi: 10.1097/MD.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zen Y, Grammatikopoulos T, Hadzic N. Autoimmune pancreatitis in children: insights into the diagnostic challenge. J Pediatr Gastroenterol Nutr. 2014;59:e42–e45. doi: 10.1097/MPG.0b013e3182994559. [DOI] [PubMed] [Google Scholar]
- 11.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 12.Kamisawa T, Zen Y, Nakazawa T, Okazaki K. Advances in IgG4-related pancreatobiliary diseases. Lancet Gastroenterol Hepatol. 2018;3:575–585. doi: 10.1016/S2468-1253(18)30121-3. [DOI] [PubMed] [Google Scholar]
- 13.de Pretis N, Frulloni L. Autoimmune pancreatitis type 2. Curr Opin Gastroenterol. 2020;36:417–420. doi: 10.1097/MOG.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo D, Maire F, Stefanescu C, et al. Features of autoimmune pancreatitis associated with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:59–67. doi: 10.1016/j.cgh.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 16.Kanno A, Masamune A, Okazaki K, et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2011. Pancreas. 2015;44:535–539. doi: 10.1097/MPA.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 17.Zen Y, Deshpande V. Tumefactive inflammatory diseases of the pancreas. Am J Pathol. 2019;189:82–93. doi: 10.1016/j.ajpath.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Chari S, Smyrk TC, et al. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172–1179. doi: 10.1097/MPA.0b013e318233bec5. [DOI] [PubMed] [Google Scholar]
- 19.Khandelwal A, Inoue D, Takahashi N. Autoimmune pancreatitis: an update. Abdom Radiol (NY) 2020;45:1359–1370. doi: 10.1007/s00261-019-02275-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Notohara K, Levy MJ, Chari ST, Smyrk TC. IgG4-positive plasma cell infiltration in the diagnosis of autoimmune pancreatitis. Mod Pathol. 2007;20:23–28. doi: 10.1038/modpathol.3800689. [DOI] [PubMed] [Google Scholar]
- 21.Zen Y, Harada K, Sasaki M, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193–1203. doi: 10.1097/01.pas.0000136449.37936.6c. [DOI] [PubMed] [Google Scholar]
- 22.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 23.Negrelli R, Boninsegna E, Avesani G, et al. Type 1 and type 2 autoimmune pancreatitis: distinctive clinical and pathological features, but are there any differences at magnetic resonance? Experience from a referral center. Pancreas. 2018;47:1115–1122. doi: 10.1097/MPA.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 24.Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, Pozzi Mucelli R. Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology. 2011;260:428–436. doi: 10.1148/radiol.11101729. [DOI] [PubMed] [Google Scholar]
- 25.Negrelli R, Manfredi R, Pedrinolla B, et al. Pancreatic duct abnormalities in focal autoimmune pancreatitis: MR/MRCP imaging findings. Eur Radiol. 2015;25:359–367. doi: 10.1007/s00330-014-3371-y. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N, Fletcher JG, Fidler JL, Hough DM, Kawashima A, Chari ST. Dual-phase CT of autoimmune pancreatitis: a multireader study. AJR Am J Roentgenol. 2008;190:280–286. doi: 10.2214/AJR.07.2309. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Fletcher JG, Hough DM, et al. Autoimmune pancreatitis: differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. AJR Am J Roentgenol. 2009;193:479–484. doi: 10.2214/AJR.08.1883. [DOI] [PubMed] [Google Scholar]
- 28.Park SH, Kim D, Ye BD, et al. The characteristics of ulcerative colitis associated with autoimmune pancreatitis. J Clin Gastroenterol. 2013;47:520–525. doi: 10.1097/MCG.0b013e31827fd4a2. [DOI] [PubMed] [Google Scholar]
- 29.Sah RP, Chari ST, Pannala R, et al. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140–148. doi: 10.1053/j.gastro.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 30.de Pretis N, Vieceli F, Brandolese A, Brozzi L, Amodio A, Frulloni L. Autoimmune pancreatitis not otherwise specified (NOS): clinical features and outcomes of the forgotten type. Hepatobiliary Pancreat Dis Int. 2019;18:576–579. doi: 10.1016/j.hbpd.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Sokollik C, McLin VA, Vergani D, Terziroli Beretta-Piccoli B, Mieli-Vergani G. Juvenile autoimmune hepatitis: a comprehensive review. J Autoimmun. 2018;95:69–76. doi: 10.1016/j.jaut.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Terziroli Beretta-Piccoli B, Vergani D, Mieli-Vergani G. Autoimmune sclerosing cholangitis: Evidence and open questions. J Autoimmun. 2018;95:15–25. doi: 10.1016/j.jaut.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Alswat K, Al-Harthy N, Mazrani W, Alshumrani G, Jhaveri K, Hirschfield GM. The spectrum of sclerosing cholangitis and the relevance of IgG4 elevations in routine practice. Am J Gastroenterol. 2012;107:56–63. doi: 10.1038/ajg.2011.375. [DOI] [PubMed] [Google Scholar]
- 34.Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070–2075. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 35.Bartholomew SV, Zigman A, Sheppard B. Lymphoplasmacytic sclerosing pancreatitis presenting as a pancreatic head mass in a child: case report and management recommendations. J Pediatr Surg. 2006;41:e23–e25. doi: 10.1016/j.jpedsurg.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 36.Blejter J, Weller S, Pace R, Cusumano H, Giambini D. Autoimmune pancreatitis: an adolescent case and review of literature. J Pediatr Surg. 2008;43:1368–1372. doi: 10.1016/j.jpedsurg.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 37.Friedlander J, Quiros JA, Morgan T, et al. Diagnosis of autoimmune pancreatitis vs neoplasms in children with pancreatic mass and biliary obstruction. Clin Gastroenterol Hepatol. 2012;10:1051–1055. doi: 10.1016/j.cgh.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Gargouri L, Ponsot P, Viala J, et al. Recurrent autoimmune pancreatitis in a 10-year-old boy. J Pediatr Gastroenterol Nutr. 2009;48:374–377. doi: 10.1097/MPG.0b013e3181826dca. [DOI] [PubMed] [Google Scholar]
- 39.Refaat R, Harth M, Proschek P, Lindemayr S, Vogl TJ. Autoimmune pancreatitis in an 11-year-old boy. Pediatr Radiol. 2009;39:389–392. doi: 10.1007/s00247-008-1132-2. [DOI] [PubMed] [Google Scholar]
- 40.Scheers I, Palermo JJ, Freedman S, et al. Autoimmune pancreatitis in children: characteristic features, diagnosis, and management. Am J Gastroenterol. 2017;112:1604–1611. doi: 10.1038/ajg.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hart PA, Chari ST. Editorial: autoimmune pancreatitis in children: is this a new subtype of disease or early-onset idiopathic duct-centric chronic pancreatitis? Am J Gastroenterol. 2017;112:1613–1614. doi: 10.1038/ajg.2017.236. [DOI] [PubMed] [Google Scholar]
- 42.Grammatikopoulos T, Zen Y, Portmann B, et al. Steroid-responsive autoimmune sclerosing cholangitis with liver granulocytic epithelial lesions. J Pediatr Gastroenterol Nutr. 2013;56:e3–e4. doi: 10.1097/MPG.0b013e3182487173. [DOI] [PubMed] [Google Scholar]
- 43.Zen Y, Grammatikopoulos T, Heneghan MA, Vergani D, Mieli-Vergani G, Portmann BC. Sclerosing cholangitis with granulocytic epithelial lesion: a benign form of sclerosing cholangiopathy. Am J Surg Pathol. 2012;36:1555–1561. doi: 10.1097/PAS.0b013e31825faae0. [DOI] [PubMed] [Google Scholar]
- 44.Detlefsen S, Olesen SS. Sialadenitis in a patient with ulcerative colitis and autoimmune pancreatitis type 2. Pathol Res Pract. 2020;216:153072. doi: 10.1016/j.prp.2020.153072. [DOI] [PubMed] [Google Scholar]
- 45.Ollo D, Terraz S, Arnoux G, Puppa G, Frossard JL, Bichard P. Biliary involvement in type 2 autoimmune pancreatitis. Case Rep Gastroenterol. 2019;13:200–206. doi: 10.1159/000499422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Detlefsen S, Zamboni G, Frulloni L, et al. Clinical features and relapse rates after surgery in type 1 autoimmune pancreatitis differ from type 2: a study of 114 surgically treated European patients. Pancreatology. 2012;12:276–283. doi: 10.1016/j.pan.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 47.Maire F, Le Baleur Y, Rebours V, et al. Outcome of patients with type 1 or 2 autoimmune pancreatitis. Am J Gastroenterol. 2011;106:151–156. doi: 10.1038/ajg.2010.314. [DOI] [PubMed] [Google Scholar]
- 48.Ku Y, Hong SM, Fujikura K, et al. IL-8 expression in granulocytic epithelial lesions of idiopathic duct-centric pancreatitis (type 2 autoimmune pancreatitis) Am J Surg Pathol. 2017;41:1129–1138. doi: 10.1097/PAS.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Shi M, Yu GZ, et al. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol. 2012;18:1123–1129. doi: 10.3748/wjg.v18.i10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta R, Neyaz A, Chougule A, et al. Autoimmune pancreatitis type 2: diagnostic utility of PD-L1 immunohistochemistry. Am J Surg Pathol. 2019;43:898–906. doi: 10.1097/PAS.0000000000001282. [DOI] [PubMed] [Google Scholar]