Abstract
Mentha is an aromatic plant used since antiquity for its pharmaceutical virtues. The climate of Saudi Arabia favors the growth of aromatic plants including Mentha suaveolens L. The aim of this study is to analyze the volatile oils of different parts of fresh and dried Mentha suaveolens L. grown in Saudi Arabia (Aljouf area) using Gas Chromatography/Mass Spectrometry (GC/MS) and Gas Chromatography Flame Ionization Detector (GC/FID) techniques, to recognize the effect of drying on chemical composition, then to evaluate the antioxidant and antifungal activities of different extracts. In total, 118 compounds were identified via GC/MS and GC/FID, in which carvone is the main volatile constituent (stems, leaves, whole plant 45–64%). This investigation deduces that Mentha belonged to the carvone chemotype. Then, the analysis of non-volatile constituents of fresh and dried Mentha was performed by HPLC. The main phenolic compound of fresh and dried Mentha for different parts was rosmarinic acid (ranging from 28,002.5 to 6558 µg/g). The ethanolic extract of fresh stem showed the highest antifungal activity (53% inhibition) compared with miconazole (60% inhibition) but the ethanoic extract of dry stem showed no activity. Additionally, all ethanolic extracts, whether for fresh or dry Mentha, have antioxidant activity more than 90% while the antioxidant activity of whole plant volatile oil is equal to 53.33%. This research shows that M. suaveolens L. could be applied to manufacture natural antioxidants, antifungal, and flavoring agents.
Keywords: Mentha suaveolens L., carvone, rosmarinic acid, antioxidant, antifungal activity
1. Introduction
M. suaveolens L. is common plant in Saudi Arabia known for its strong antifungal, anti-aflatoxigenic, and antioxidant potential [1]. Mentha species have been well recognized for their aromatic and medicinal purposes since ancient times [2]. Recently, several Mentha species gained increasing interest for their potential use as natural food preservatives because of their strong antifungal, anti-aflatoxigenic, and antioxidant properties [1,3]. These biochemical properties are mainly due to the presence of several aromatic and phenolic compounds in different parts of the Mentha species. The chemical composition of Mentha species differs according to the growing season [4,5]. Additionally, the volatile oil composition of Mentha species from various populations and geographical regions indicated that the plants relate to either carvone or menthol or linalool chemotypes [6]. Several Mentha species were investigated in the Kingdom of Saudi Arabia. Burham et al. [7] analyzed the chemical composition of the volatile oils extracted from the leaves of M. longifolia harvested from the Albaha area in Saudi Arabia using GC/MS. As well as the compound categories identified, the content of oxygenated monoterpenes was the highest in comparison with other classes. A total of 46 compounds were detected, the main oxygenated monoterpene component was piperitone (30.77%). Caryophellene was the major sesquiterpene hydrocarbon identified (5.58%), while γ-terpinene was the main monoterpene hydrocarbon (1.36%) [7]. Abdel-Hameed et al. [8] examined the effect of extraction method on its yield and chemical composition for M. longifolia harvested from Taif, Saudi Arabia. The main chemical components identified were monoterpenes and sesquiterpenes; pulegone was the most prevalent [8]. The drying of aromatic plants is an essential step of the manufacture process to ensure the high quality of the end products [9]. Two Mentha varieties cultivated in Saudi Arabia, Medina, and Hasawi, were found to be high in volatile oils and phenolic content, especially in the soluble fractions. Regarding outside KSA, previous studies have shown that the major components of Moroccan M. suaveolens essential oils were piperitenone oxide (56.28%), piperitenone (11.64%), and pulegone (6.16%) [2]. Additionally, the study of the chemical composition in various regions of Morocco showed that the chemical composition changed and the main compounds were different: piperitenone oxide (56.00%), P-cymenol-8 (20.60%), caryophyllene (5.70%); piperitone oxide (26.00%), piperitenone oxide (25.00%), caryophyllene (9.80%); pulegone (50.00%), P-cymenol-8 (10.40%), and borneol (5.60%) [10]. The study of the Italian M. suaveolens showed that the piperitenone oxide was the main component [2]. Two different populations of M. suaveolens from Eastern Iberian Peninsula were studied and the results showed that the major components are different and correspond to two different chemotypes: piperitenone oxide (35.2–74.3%) and piperitone oxide (83.9–91.3%) [11]. A study of the chemical composition of Egyptian M. suaveolens showed that the major component was carvone (50.59%) followed by limonene (31.25%) [5]. Based on the results of these studies, this difference in chemical composition between the different geographic localities can be explained by many factors such as fertilization, drying effect, climatic change, and others.
M. suaveolens is an aromatic herb native to Southern and Western Europe. M. suaveolens is a perennial, herbaceous plant characterized by a sweet scent. The plant can grow up to 100 cm in height [12]. M. suaveolens cultivated in Egypt is rich in oxygenated compounds, with carvone and limonene being the predominant compounds, followed by hydrocarbons [4,5]. El-Kashoury et al. [4] identified two new triterpenes (3β-acetyl-22α-hydroxy ursa-12,20-diene and 2α, 3β-dihydroxy-olean-18-en-29-oic acid) in the ethanolic extracts of the aerial parts of M. suaveolens growing in Egypt. Moreover, seven known compounds were identified: β-sitosterol, β-sitosterol-3-O-β-D-glucoside, oleanolic acid, dihydrolimonene, 7-hydroxy-p-cymene, isoquercitrin, and rutin [4]. In Morocco, GC/MS analysis of the volatile constituents extracted from M. suaveolens L. revealed that the major components were piperitenone, pulegone, and piperitone [13]. In another study, the chemical composition of the volatile constituents extracted from 10 wild populations of M. suaveolens subsp. timija was analyzed using GC/MS analysis. Collectively, 44 compounds were identified in all samples with a percentage that reached at least 97.3% of the oil chemical composition. The major components identified were menthone, pulegone, cis-piperitone epoxide, piperitone, trans-piperitone epoxide, piperitenone, piperitenone oxide, (E)-caryophyllene, germacrene D, isomenthone, and borneol [14]. In a recent study, Benali et al. [15] investigated the chemical composition of the volatile constituents extracted from M. suaveolens in Morocco using GC/MS analysis. The main compounds identified were piperitenone oxide, germacrene D, β-trans-caryophyllene, piperitone oxide, 1-4-terpineol, and δ-terpinene, among a total of 17 compounds identified [15]. The major phenolic compounds identified by HPLC in different Mentha varieties are caffeoylquinic acid, salvianic acid, rosmarinic acid, luteolin, salvigenin, chrysoeriol, thymonin, carnosol [6]. Elansary et al. [16] investigated phenolic compounds of M. piperita and stated that rosmarinic acid, cryptochlorogenic acid, and chlorogenic acid are the major compounds. The non-volatile extract of Mentha plant extracts are rich sources of phenolic compounds and flavonoids [16]. The most important and frequently encountered phenolic acids in Mentha species are caffeic acid and its derivative compounds and other acids such as cinnamic acid, gentisic acid, protocatechuic acid, hydroxybenzoic acid, and vanillic acid [17]. Antioxidants are of major interest to scientists, however, many carcinogenic and toxic effects were reported for synthetic antioxidants, making natural antioxidants promising alternatives [18]. The volatile oils of M. suaveolens have several proven and beneficial biological activities, especially antioxidant activity [4]. Other studies also showed that the volatile oils extracted from M. suaveolens exhibited potent antifungal activity against Aspergillus niger and Candida albicans [5].
To our knowledge, there is no report on the chemical composition and biological activities of the volatile oils of M. suaveolens L. native to Saudi Arabia (Aljouf area). Therefore, the objective of this study was to extract non-volatile and volatile oils from M. suaveolens L. and to identify the chemical constituents using GC, GC-MS and high-performance liquid chromatography (HPLC). Moreover, biological activities (antioxidant and antifungal activities) are presented in this study.
2. Results
2.1. Analysis of Volatile Oils of Fresh and Dried M. suaveolens L. by GC/MS and GC/FID
The volatile constituents obtained from M. suaveolens L. under study were pale yellow with a pleasant and distinct odor. The total yield percentages of volatile constituents from M. suaveolens L. of fresh and dried whole plant extracts were 0.57 ± 0.03% and 0.225 ± 0.01%, respectively. The total yield percentages of fresh and dried leaf extracts were 0.235 ± 0.012% and 0.24 ± 0.016%, respectively. The total yields of fresh and dried stem extracts were 0.24 ± 0.016% and 0.9 ± 0.05%, respectively.
The analysis of volatile oils of fresh Mentha was performed by GC/MS and GC/FID; 125 volatile constituents of fresh Mentha were identified. The percentages of chemical identification of whole plant, leaf, and stem extracts were 99.14%, 97.35%, and 96.58%, respectively. The main volatile constituent of Mentha in whole plant, leaf, and stem extracts was carvone at 43.65%, 64.31%, and 58.8%, respectively. The other major volatile constituents in whole plant, leaf, and stem extracts were myrcenol (5.88%, 3.93%, and 3.85%, respectively), terpineol<1-> (4.29%, 5.61%, and 4.4%, respectively), pulegone (3.8%, 2.1%, and 2.2%, respectively), and limonen-10-ol (2.79%, 1.24%, and 0.15%, respectively). All components are listed in Table 1.
Table 1.
Chemical components of volatile oils from fresh and dried Mentha suaveolens L. analyzed by GC/FID and GC/MS.
| a Conc. % |
b Calculated KI |
c KI Data Average |
Name | d Type | Identification Methods | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Whole Plant | Leaves | Stems | ||||||||
| Fresh | Dried | Fresh | Dried | Fresh | Dried | |||||
| 0.01 | 3.22 | 0.03 | 0.18 | 0.01 | 0.08 | 978 | 977 | Octen-2-ol | LOC | KI and MS |
| 0.03 | 0.12 | 0.02 | 0.1 | 0.02 | 0 | 989 | 989 | Decan<1> | M | KI and MS and St. |
| 0.07 | 0.31 | 0.04 | 0.14 | 0.67 | 0.23 | 998 | 999 | Ethyl hexanoate | LOC | KI and MS |
| 0.04 | 0.05 | 0.02 | 0 | 0.34 | 0.22 | 1005 | 1007 | Hexenyl acetate(3Ƶ) | LOC | KI and MS |
| 0.07 | 0.06 | 0 | 0.02 | 0.03 | 0.02 | 1007 | 1007 | Hexenoic acid (2E) | LOC | KI and MS |
| 0.09 | 0.03 | 0.03 | 0 | 0.03 | 0.01 | 1008 | 1008 | Linalool oxide (dehydroxy-cis) | LOC | KI and MS |
| 0.1 | 0.06 | 0 | 0.04 | 0.01 | 0.01 | 1011 | 10,011 | δ-Carene (3) | M | KI and MS |
| 0.03 | 0.03 | 0 | 0.01 | 0.03 | 0.01 | 1013 | 1013 | Hexenyl acetate (2E) | LOC | KI and MS |
| 0.54 | 0.01 | 0.25 | 0 | 0.35 | 0 | 1014 | 1013 | 1,8 Cineole | LOC | KI and MS and St. |
| 0.39 | 0.03 | 0.16 | 0.02 | 0.24 | 0.86 | 1017 | 1017 | Terpinene(α) | M | KI and MS |
| 0.01 | 0 | 0 | 0 | 0.01 | 0.04 | 1024 | 1025 | p-Cymene | M | KI and MS |
| 0.12 | 0 | 0 | 0.08 | 0 | 0.08 | 1026 | 1026 | Menthene (1-p) | M | KI and MS |
| 0.04 | 0.01 | 0 | 0.01 | 0.03 | 0.09 | 1029 | 1029 | β-Phellandrene | M | KI and MS |
| 0.04 | 0.01 | 0.01 | 0.01 | 0.02 | 0 | 1037 | 1038 | Ocimene(Ƶ-β) | M | KI and MS |
| 0.62 | 0.15 | 0.33 | 0.31 | 0.1 | 0.18 | 1050 | 1048 | Ocimene(Ε-β) | M | KI and MS and St. |
| 0.09 | 0.03 | 0.04 | 0.01 | 0.03 | 0.01 | 1059 | 1060 | Terpinene(-γ-) | M | KI and MS |
| 0.13 | 0.03 | 0.03 | 0 | 0 | 0.1 | 1066 | 1065 | Octen-1-ol(2Ε) | LOC | KI and MS |
| 1.71 | 0.29 | 0.71 | 0.56 | 1.26 | 0.2 | 1076 | 1076 | Benzyl formate | LOC | KI and MS and St. |
| 0.81 | 0.25 | 0.49 | 0.48 | 0.34 | 0.25 | 1088 | 1086 | Terpinolene | M | KI and MS and St. |
| 1.54 | 0.46 | 1.13 | 0.9 | 1.18 | 0.18 | 1096 | 1097 | Linalool | LOC | KI and MS and St. |
| 0.03 | 0 | 0.01 | 0.01 | 0 | 0.05 | 1101 | 1102 | Hexyl propanoate | LOC | KI and MS |
| 0.05 | 0.03 | 0.04 | 0.01 | 0.03 | 0.01 | 1104 | 1103 | Methyl butyl isovalerate(2) | LOC | KI and MS |
| 0.13 | 0 | 0.08 | 0.05 | 0.02 | 0.05 | 1113 | 1115 | Camphenol(6-) | LOC | KI and MS |
| 0 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 1121 | 1122 | Menth-2-en-1-ol(cis-p) | LOC | KI and MS |
| 5.88 | 2.81 | 3.93 | 4.98 | 3.85 | 2.85 | 1122 | 1122 | Myrcenol | LOC | KI and MS and St. |
| 4.29 | 1.84 | 5.61 | 2.44 | 4.4 | 1.04 | 1133 | 1132 | Terpineol<1-> | LOC | KI and MS and St. |
| 0.22 | 0.05 | 0.17 | 0.09 | 0.1 | 0.04 | 1144 | 1143 | Ocimene(neo-allo) | M | KI and MS |
| 0.02 | 0.01 | 0.01 | 0 | 0.05 | 0.04 | 1153 | 1155 | Thujanol(neo-3) | LOC | KI and MS |
| 1.38 | 0.23 | 0.83 | 0.52 | 0.13 | 0.09 | 1159 | 1159 | Isopulegol(iso) | LOC | KI and MS |
| 0 | 0.01 | 0.03 | 0.03 | 0 | 0.08 | 1160 | 1159 | Isoborneol | LOC | KI and MS |
| 0.09 | 0.01 | 0.05 | 0.02 | 0.07 | 0.08 | 1164 | 1164 | Terpineol(cis dihydro) | LOC | KI and MS |
| 0.03 | 0.06 | 0.03 | 0.01 | 0.01 | 0.33 | 1165 | 1164 | Menthol(neo) | LOC | KI and MS |
| 0.3 | 0.07 | 0.17 | 0.11 | 0.07 | 0.15 | 1170 | 1170 | Pinocampheol | LOC | KI and MS |
| 0.26 | 0.03 | 0.13 | 0.06 | 0.07 | 0.06 | 1171 | 1171 | Iso pulegol (neoiso) | LOC | KI and MS |
| 0.16 | 0.05 | 0.09 | 0.04 | 0 | 0.09 | 1174 | 1174 | Linalool oxide (cis) | LOC | KI and MS |
| 0 | 0.03 | 0.01 | 0.03 | 0.02 | 0.05 | 1176 | 1176 | Linalool oxide (Trans) | LOC | KI and MS |
| 0.09 | 0.01 | 0.06 | 0 | 0.06 | 0.01 | 1177 | 1177 | Terpinen-4-ol | LOC | KI and MS and St. |
| 0.18 | 0.02 | 0.06 | 0.02 | 0.2 | 0.05 | 1186 | 1187 | Dillether | LOC | KI and MS |
| 0.15 | 0.07 | 0.08 | 0.04 | 0.05 | 0.01 | 1188 | 1188 | α-Terpineol | LOC | KI and MS and St. |
| 0.07 | 0.01 | 0.04 | 0.01 | 0.03 | 0 | 1189 | 1189 | Verbanol (neoiso) | LOC | KI and MS |
| 0.02 | 0.02 | 0.04 | 0.01 | 0.04 | 0.03 | 1192 | 1192 | Dihydro carveol | LOC | KI and MS |
| 0.05 | 0.01 | 0.01 | 0.01 | 0.05 | 0 | 1193 | 1194 | Dihydro carveol(neo) | LOC | KI and MS |
| 0.03 | 0.03 | 0.01 | 0 | 0.03 | 0.02 | 1196 | 1196 | Decanol(3-) | LOC | KI and MS |
| 0.27 | 0.15 | 0.45 | 0.23 | 0.11 | 0.06 | 1199 | 1199 | γ-Terpineol | LOC | KI and MS and St. |
| 0.91 | 0.09 | 0.44 | 0.15 | 0.08 | 0.08 | 1200 | 1201 | Dihydro carveol (Trans) | LOC | KI and MS |
| 0.15 | 0.01 | 0.11 | 0.03 | 0.72 | 0.03 | 1208 | 1208 | Piperitol | LOC | KI and MS |
| 0.01 | 0.03 | 0.02 | 0.05 | 0.9 | 0.69 | 1214 | 1213 | Pulegol (Trans) | LOC | KI and MS |
| 0.11 | 1.62 | 0 | 2.52 | 0.48 | 1.28 | 1215 | 1216 | Dihydro myrcenol acetate | LOC | KI and MS |
| 1.77 | 0.49 | 3.45 | 0.88 | 2.12 | 0.63 | 1216 | 1216 | Carveol (Trans) | LOC | KI and MS and St. |
| 0.05 | 0.01 | 0.1 | 0.02 | 0.05 | 0.01 | 1219 | 1220 | Cyclo citral | LOC | KI and MS |
| 0.05 | 0.07 | 0.27 | 0.13 | 0.01 | 0.11 | 1227 | 1227 | Prenyl cyclo pentanone | LOC | KI and MS |
| 0 | 1.84 | 2.24 | 2.65 | 2.5 | 0.02 | 1229 | 1229 | Carveol(cis) | LOC | KI and MS and St. |
| 0.54 | 0 | 0 | 0 | 0.03 | 1.69 | 1230 | 1232 | Mentha-1,8-dien-2-ol (cis-p) | LOC | KI and MS |
| 0.11 | 0 | 0 | 0.01 | 0 | 0.01 | 1234 | 1235 | linalool acetate (tetrahydro) | LOC | KI and MS |
| 43.65 | 45 | 64.31 | 53.45 | 58.8 | 49 | 1234 | 1234 | Carvone | LOC | KI and MS and St. |
| 3.8 | 4 | 2.1 | 0 | 2.2 | 0.01 | 1237 | 1237 | Pulegone | LOC | KI and MS and St. |
| 0.03 | 0.08 | 0.01 | 0 | 0.04 | 0.02 | 1242 | 1242 | Verbenyl acetate(Trans) | LOC | KI and MS |
| 0.12 | 0.07 | 0.06 | 0.02 | 0.2 | 0.06 | 1244 | 1244 | Isomenthene(2-ethyl) | S | KI and MS |
| 0 | 0.03 | 0.01 | 0 | 0.06 | 0.05 | 1247 | 1247 | Carvotan aceton | LOC | KI and MS |
| 0.01 | 0.02 | 0.15 | 0.01 | 0.05 | 0.01 | 1253 | 1254 | Myrtanal (cis) | LOC | KI and MS |
| 0.11 | 0.02 | 0 | 0.02 | 0.05 | 0.01 | 1254 | 1253 | Piperitone epoxide (cis) | LOC | KI and MS |
| 0.09 | 0.01 | 0 | 0.01 | 0.01 | 0.01 | 1255 | 1254 | Piperitone epoxide (trans) | LOC | KI and MS |
| 0.01 | 0.03 | 0.01 | 0.02 | 0.02 | 0.11 | 1256 | 1256 | Sabinene hydrate acetate | LOC | KI and MS |
| 0.34 | 0 | 0.13 | 0 | 0.32 | 0 | 1258 | 1257 | Carvenone | LOC | KI and MS |
| 0.11 | 0.02 | 0.03 | 0.01 | 0.17 | 1.26 | 1261 | 1262 | Myrtanol(Trans) | LOC | KI and MS |
| 0.05 | 0.01 | 0.02 | 0.02 | 0.07 | 0.04 | 1263 | 1263 | Carvonoxide(cis) | LOC | KI and MS |
| 0.05 | 0.06 | 0.05 | 0.01 | 0.06 | 0.06 | 1265 | 1265 | Cauaiacol acetate<o> | LOC | KI and MS |
| 0.03 | 0.01 | 0.15 | 0.01 | 0.05 | 0.34 | 1276 | 1267 | Thujanol acetate(neo-3) | LOC | KI and MS |
| 0.42 | 0 | 0 | 0 | 0.04 | 0 | 1277 | 1275 | Isopulegyl acetate | LOC | KI and MS |
| 0.33 | 0.46 | 0.19 | 0.85 | 0.22 | 0.04 | 1282 | 1282 | Verbenyl acetate(cis) | LOC | KI and MS |
| 0.22 | 0.08 | 0.07 | 0.11 | 0.08 | 0.01 | 1283 | 1282 | Thujanol acetate(neo iso-3) | LOC | KI and MS |
| 0 | 0.02 | 0.01 | 0 | 0.09 | 0.02 | 1285 | 1285 | Terpinen-7-al(α) | LOC | KI and MS |
| 0.09 | 0.34 | 0.4 | 0.02 | 0.27 | 0.01 | 1288 | 1288 | Fenchol(2-ethyl-endo) | LOC | KI and MS |
| 2.79 | 1.23 | 1.24 | 1.85 | 0.15 | 0.7 | 1289 | 1288 | Limonen-10-ol | LOC | KI and MS |
| 0.04 | 0.26 | 0.02 | 0 | 0.07 | 0.06 | 1290 | 1291 | Thymol | LOC | KI and MS |
| 2.66 | 2.32 | 1.09 | 2.14 | 0.65 | 1.2 | 1328 | 1329 | Silphiperfol-5-ene | S | KI and MS |
| 0.1 | 0.01 | 0 | 0.03 | 0.05 | 0.01 | 1333 | 1334 | cis-Carvyl acetate | LOC | KI and MS |
| 0.05 | 0.08 | 0.02 | 0.05 | 0.04 | 0.02 | 1336 | 1336 | Presilphiperfol-7-ene | S | KI and MS |
| 0.06 | 0.03 | 0.05 | 0.08 | 0.02 | 0.01 | 1338 | 1338 | Elemene(δ-) | S | KI and MS and St. |
| 1.69 | 1.29 | 0.68 | 1.31 | 0.44 | 0.7 | 1351 | 1351 | Cubebene(α) | S | KI and MS |
| 0.47 | 0.11 | 0 | 0.15 | 0.01 | 0.04 | 1352 | 1353 | Thymol acetate | LOC | KI and MS |
| 0.52 | 0.26 | 0.26 | 0.55 | 0.25 | 0.22 | 1353 | 1354 | Lengipinene(α) | S | KI and MS |
| 0.06 | 0 | 0.02 | 0.02 | 0.45 | 0.01 | 1354 | 1354 | Ethyl nerolate | LOC | KI and MS |
| 0.21 | 0.06 | 0.04 | 0.06 | 0.45 | 0.01 | 1359 | 1358 | Dihydro carveol acetate | LOC | KI and MS |
| 1.41 | 0.58 | 0.56 | 0.95 | 0.71 | 0.59 | 1371 | 1372 | Cyclo sativene | S | KI and MS |
| 0.17 | 0.02 | 0 | 0.06 | 0 | 0.08 | 1372 | 1373 | p-Menthane-1,2,3-triol | LOC | KI and MS |
| 1.37 | 0.6 | 0.53 | 0.8 | 0.13 | 0.57 | 1376 | 1375 | Copaene(α) | S | KI and MS and St. |
| 0.53 | 0.16 | 0.18 | 0.29 | 0.01 | 0.09 | 1380 | 1380 | cis-Jasmone | LOC | KI and MS |
| 0.04 | 0.02 | 0 | 0.01 | 0.02 | 0.04 | 1381 | 1381 | Patchoulene(β-) | S | KI and MS |
| 0.19 | 0.01 | 0.07 | 0.07 | 0.05 | 0 | 1382 | 1383 | Daucene | S | KI and MS |
| 0.57 | 0.21 | 0.23 | 0.3 | 0.06 | 0.24 | 1388 | 1388 | Cubebene(β-) | S | KI and MS |
| 0.25 | 0.1 | 0.07 | 0.1 | 0.08 | 0.13 | 1390 | 1391 | Longifolene(iso) | S | KI and MS |
| 0.14 | 0.06 | 0.07 | 0.04 | 0.09 | 0.07 | 1391 | 1392 | Elemene(β-) | S | KI and MS |
| 0.07 | 0.05 | 0.02 | 0.04 | 0.04 | 0.02 | 1392 | 1393 | Sativene | S | KI and MS |
| 0.32 | 0.17 | 0.16 | 0.08 | 0.27 | 0.1 | 1400 | 1400 | Longipinene(β-) | S | KI and MS |
| 0.83 | 0.04 | 0.35 | 0.36 | 0.54 | 0.14 | 1402 | 1403 | Funebrene(α) | S | KI and MS |
| 0.04 | 0.03 | 0.07 | 0.03 | 0.09 | 0.04 | 1408 | 1408 | Caryophyllene(Ƶ) | S | KI and MS |
| 0.19 | 0.12 | 0.1 | 0.05 | 0.1 | 0.11 | 1409 | 1409 | Gurjunene(α) | S | KI and MS |
| 0.07 | 0.03 | 0 | 0.03 | 0.04 | 0 | 1412 | 1412 | β-Caryophyllene | S | KI and MS |
| 0.56 | 0.16 | 0.29 | 0.2 | 0.13 | 14 | 1417 | 1416 | Santalene | S | KI and MS |
| 0 | 0.03 | 0.05 | 0.04 | 0.03 | 0.07 | 1419 | 1418 | Caryophyllene(Ε-) | S | KI and MS |
| 0.08 | 0.01 | 0.05 | 0.08 | 0.07 | 0.21 | 1423 | 1424 | Menth-1-on-9-ol acetate | LOC | KI and MS |
| 0.08 | 0.01 | 0.05 | 0.03 | 0.03 | 0.29 | 1430 | 1430 | Ionone(Ε-α) | LOC | KI and MS |
| 4 | 11 | 0.03 | 3.81 | 0.01 | 0.08 | 1431 | 1431 | Copaene(β) | S | KI and MS |
| 0.02 | 0.02 | 0.02 | 0.07 | 0.04 | 0.02 | 1436 | 1435 | Elemene(-γ) | S | KI and MS |
| 0.09 | 0.03 | 0.07 | 0.05 | 0.05 | 0.02 | 1441 | 1441 | Aromadendrene | S | KI and MS and St. |
| 0.03 | 0.04 | 0 | 0.1 | 0.03 | 0.08 | 1444 | 1444 | α-Humulene | S | KI and MS |
| 0.04 | 0.05 | 0 | 0.05 | 0.06 | 0.1 | 1446 | 1445 | γ-Muurolene | S | KI and MS |
| 0.13 | 0.04 | 0.1 | 0.03 | 0.01 | 0.02 | 1447 | 1447 | Cabreuva(A) | S | KI and MS |
| 0.14 | 0.07 | 0.07 | 0.05 | 0.18 | 0.13 | 1451 | 1451 | Himachalene(α) | S | KI and MS |
| 0.04 | 0.13 | 0.04 | 0.09 | 0.21 | 0.2 | 1454 | 1454 | Neryl propanoate | LOC | KI and MS |
| 0.02 | 0.05 | 0.03 | 0.1 | 0.07 | 0.01 | 1456 | 1457 | Carvyl propanoate(Trans) | HOC | KI and MS |
| 0.19 | 3.63 | 0.1 | 0.12 | 0.14 | 0.05 | 1460 | 1462 | Aromadendrene(allo) | S | KI and MS |
| 0.04 | 0.93 | 0.03 | 1.37 | 0.11 | 0.54 | 1466 | 1467 | Dodecanal | S | KI and MS |
| 0.1 | 0.43 | 0.07 | 0.21 | 0.07 | 0.05 | 1469 | 1469 | Ethyl-(2Ε,4Ƶ)-decadienoate | HOC | KI and MS |
| 0.02 | 0.72 | 0.01 | 1.08 | 0.07 | 0.01 | 1470 | 1471 | Pinchotene acetate | HOC | KI and MS |
| 0.02 | 0.8 | 0.05 | 0 | 0.02 | 0.04 | 1477 | 1478 | Geranyl propanoate | HOC | KI and MS |
| 0.06 | 0.06 | 0.02 | 2.43 | 0.09 | 2.81 | 1478 | 1478 | Allyl decanoate | HOC | KI and MS |
| 0.05 | 0.11 | 0.03 | 0 | 0.07 | 0.1 | 1480 | 1481 | Cabreuva oxide D | HOC | KI and MS |
| 0.01 | 0.03 | 0.02 | 0.08 | 0.06 | 0.06 | 1482 | 1482 | Menthyl lactate | HOC | KI and MS |
| 1.44 | 0.32 | 0.19 | 0.03 | 1.88 | 0.17 | 1515 | 1516 | Gernyl isobutanoate | HOC | KI and MS |
| 0.03 | 0.24 | 0.15 | 0.17 | 0.14 | 0.21 | 1517 | 1517 | Himachalene(α-dehydro-ar) | HOC | KI and MS |
| 0.13 | 2.68 | 0.1 | 2.23 | 0.13 | 1.91 | 1522 | 1522 | Isobornyl isovalerate | HOC | KI and MS |
| 0.13 | 2.34 | 0.18 | 2.86 | 0.26 | 7.06 | 1524 | 1524 | Isobornyl-2-methyl butanoate | HOC | KI and MS |
| 2.5 | 3.07 | 0.2 | 2.67 | 4.01 | 2.44 | 1640 | 1640 | Epi-α-Muurolol | HOC | KI and MS |
a concentration of area %, b KI (Kovat’s index), c reported Kovats index, [19,20,21,22,23,24,25,26,27,28]; www.webbook.nist.gov (accessed on 30 March 2022), d Sesquiterpenes (S), heavy oxygenated compounds (HOC), light oxygenated compounds (LOC), and monoterpenes (M). Standard compound (St). Notes: The analytical replicates were performed twice.
The analysis of dried Mentha volatile oils was completed by GC/MS and GC/FID. In total, 125 volatile constituents of dried Mentha were identified. The percentages of these chemical compounds of whole plant, leaves, and stems were 99.98%, 100%, and 99.28%, respectively. The major compound found in whole plant, leaf, and stem extracts was carvone at 45%, 53.45%, and 49% respectively. The other major volatile constituents in whole plant, leaves, and stems were copaene (β) (11%, 3.81%, and 0.08%, respectively), aromadendrene (allo) (3.63%, 0.12%, and 0.05%, respectively), octen-2-ol (3.22%, 0.18%, and 0.08%, respectively), epi-α-muurolol (3.07%, 2.67%, and 2.44%, respectively). All constituents are reported in Table 1.
2.2. Drying Effect on Volatile Oil Constituents of Mentha
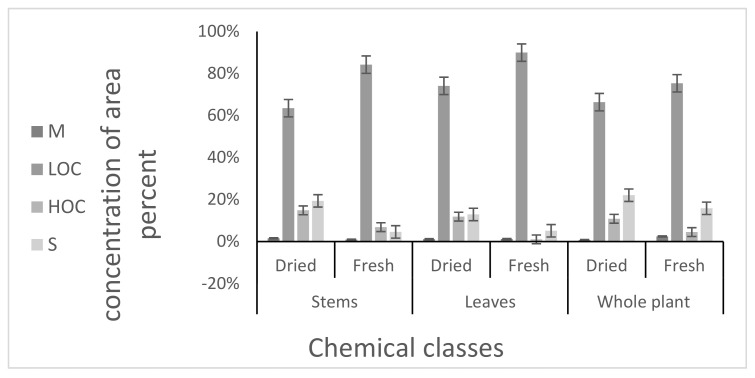
A variation in chemical composition concentration for some compounds was found due to the drying effect as shown in Figure 1. As shown in the figure, the LOC is the highest concentration in all plant extracts. While the S and the HOC concentration increases after the drying process in all plant extracts, no significant changes were found in the concentration of the M after the drying process.
Figure 1.
Drying effect on volatile oils constituents of M. suaveolens L. Sesquiterpenes (S), heavy oxygenated compounds (HOC), light oxygenated compounds (LOC), and monoterpenes (M).
2.3. Chemical Composition of Non-Volatile Constituents of Fresh and Dried Mentha by Using HPLC
The analysis of fresh Mentha non-volatile constituents was performed by HPLC. The major phenolic derivatives identified in the ethanolic extract from the fresh whole plant were rosmarinic acid (2223.3µg/g), rutin (676.7 µg/g), and ferulic acid (226.7 µg/g). Additionally, the main phenolic derivatives found in fresh leaves of Mentha were rosmarinic acid (28,002.5 µg/g), rutin (3383.8 µg/g), and ferulic acid (1520 µg/g). In fresh stems of Mentha, the main phenolic derivatives identified were rosmarinic acid (6558µg/g), catechin (1340.4 µg/g), and naringenin (371.8 µg/g). The other components are listed in Table 2. In addition, the analysis of dried Mentha non-volatile constituents was performed by HPLC. The major phenolic derivatives identified in the ethanolic extract from the dried whole plant of Mentha were rosmarinic acid (21,191.9 µg/g), rutin (252.16 µg/g), and quercetin (153.8 µg/g). The main phenolic derivatives found in dried leaves of Mentha were rosmarinic acid (15,165.1 µg/g), rutin (194.7 µg/g), and quercetin (156.98 µg/g). Moreover, the main phenolic derivatives found in dried stems of Mentha were rosmarinic acid (8378.4µg/g), naringenin (130.3 µg/g), and catechin (68.1 µg/g). The other components are recorded in Table 2.
Table 2.
The chemical composition of fresh and dried M. suaveolens L. analyzed by using HPLC.
| Phenolic Compounds | Concentration (µg/g) ± SD | |||||
|---|---|---|---|---|---|---|
| Whole Plant | LEAVES | Stems | ||||
| Fresh | Dried | Fresh | Dried | Fresh | Dried | |
| Protocatechuic acid | 5.5 ± 0.3 | n.d | 45.4 ± 3.21 | 3.5 ± 0.17 | 19.96 ± 1.56 | 3.6 ± 1.23 |
| p-hydroxybenzoic acid | n.d | 3.95 ± 0.23 | 526.9 ± 21.87 | 2.23 ± 0.15 | n.d | 2.62 ± 0.12 |
| Catechin | 198.3 ± 1.8 | 85.03 ± 0.98 | 462.3 ± 12.45 | 54.19 ± 3.14 | 1340.4 ± 13.76 | 68.1 ± 2.56 |
| Vanilic acid | n.d | n.d | 12.13 ± 1.12 | n.d | n.d | n.d |
| Cinnamic acid | 0.33 ± 0.01 | 10.51 ± 1.02 | 46.3 ± 3.05 | 15.35 ± 1.16 | 1.54 ± 0.13 | 1.84 ± 0.13 |
| Naringenin | 2.3 ± 0.15 | 53.22 ± 3.23 | 13 ± 1.23 | 7.2 ± 0.54 | 371.8 ± 3.89 | 130.3 ± 10.23 |
| Eugenol | n.d | n.d | n.d | n.d | 86.6 ± 7.34 | n.d |
| Caffeic Acid | n.d | 13.84 ± 1.08 | 1141.5 ± 13.35 | 1.79 ± 0.09 | n.d | 3.7 ± 023 |
| Coumaric acid | 0.05 ± 0.001 | n.d | 51 ± 3.67 | n.d | 5.8 ± 0.43 | n.d |
| Ferulic acid | 226.7 ± 3.8 | 3.89 ± 0.22 | 1520 ± 17.34 | 0.63 ± 0.0054 | 1.92 ± 0.12 | 1.95 ± 0.12 |
| Rutin | 676.7 ± 4.45 | 252.16 ± 12.92 | 3383.8 ± 15.45 | 194.7 ± 12.7 | 194.6 ± 12.43 | 41.6 ± 2.34 |
| Luteolin | 122.3 ± 1.17 | 78.65 ± 4.34 | 514.9 ± 12.34 | 83.7 ± 6.42 | 41.6 ± 3.52 | 38.6 ± 2.12 |
| Quercetin | 22.5 ± 1.08 | 153.8 ± 7.8 | 377.3 ± 24.20 | 156.98 ± 11.65 | n.d | 58.6 ± 6.23 |
| Rosmarinic acid | 2223.3 ± 9.8 | 21,191.9 ± 24.8 | 28,002.5 ± 32.6 | 15,165.1 ± 17.15 | 6558 ± 15.25 | 8378.4 ± 23.75 |
n.d: not detected. SD standard deviation. Notes: The HPLC analysis was carried out in duplicate
2.4. Biological Activities of Mentha suaveolens
2.4.1. Antioxidant Activity of Volatile Oils and Non-Volatile Extracts of Fresh and Dried Mentha suaveolens L.
Antioxidant activities of Mentha were evaluated using 1,1-diphenyl-2-picrylhydrazyl (DPPH) method. DPPH radical scavenging activity of the positive standard Butylated hydroxytoluene (BHT) and plant extracts were expressed by inhibition percentage (%). The ability of the tested extract to act as an electron donor in the conversion of DPPH• into DPPH-H was studied.
Antioxidant Activity of Volatile Oils from Mentha suaveolens L.
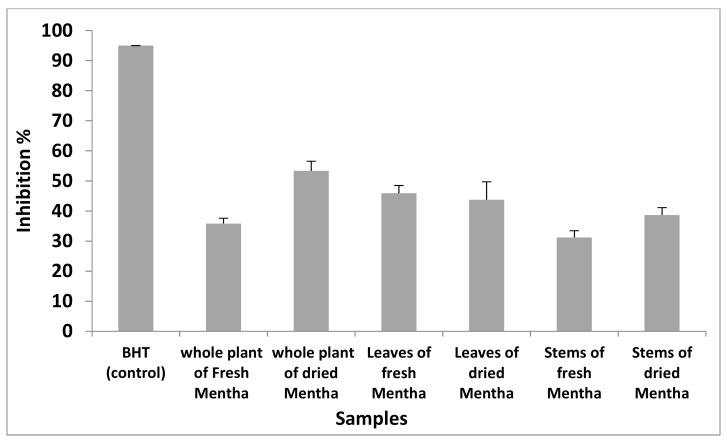
The percentages of inhibition were measured for all the samples presented in Figure 2. The results showed that the antioxidant activity of samples ranged from 31% to 53%.
Figure 2.
Antioxidant activity of volatile oils of M. suaveolens L.
Antioxidant Activity Non-Volatile Constituents from Mentha
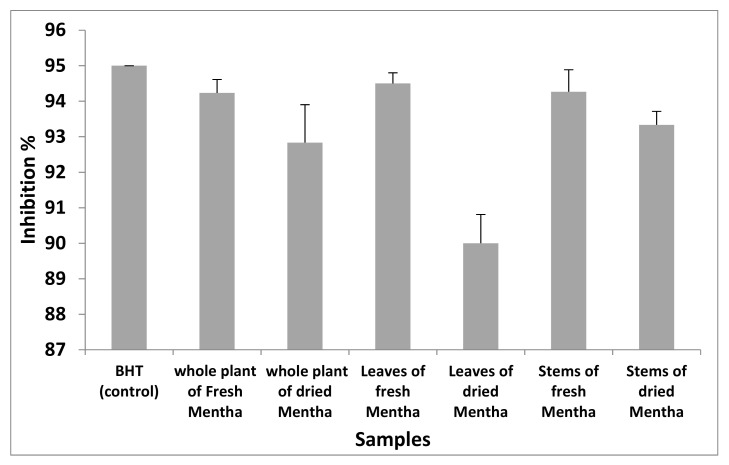
DPPH• (purple-colored) was reduced to DPPH-H (yellow colored) by using tested samples. The obtained results showed that all the ethanolic samples have good antioxidant activity with values exceeding 90%. The inhibition% measured for all samples is presented in Figure 3.
Figure 3.
Antioxidant activity of non-volatile constituents of M. suaveolens L.
2.4.2. Antifungal Activity of Fresh and Dried M. suaveolens L.
Isolated fungi of the studied white cheese samples show that a total of 13 fungal isolates were recovered on PDA media. The isolated fungi were identified as seven species belonging to four genera. The sequence of the most dominant species was closely related to Penicillium glabrum (Genbank accession number, MW534476.1) with 100% similarity https://www.ncbi.nlm.nih.gov/nuccore/MW534476.1 (2 February 2021).
Penicillium sp. was the most predominant genus encountered in 37.4% of the total fungi recovered on PDA media. Two species of Penicillia were most common, P. glabrum was the most prevalent (22.4% of the total fungi) and P. aurantiogriseum (14.9%). Three species of Aspergilla were identified, A. niger was the most prevalent (11.21% of the total fungi), followed by A. ustus (9.3%), and A. fumigatus (7.47%). Geotrichum was isolated from one sample only cultivated on PDA medium.
Antifungal Activity of Volatile Oils from M. suaveolens L.
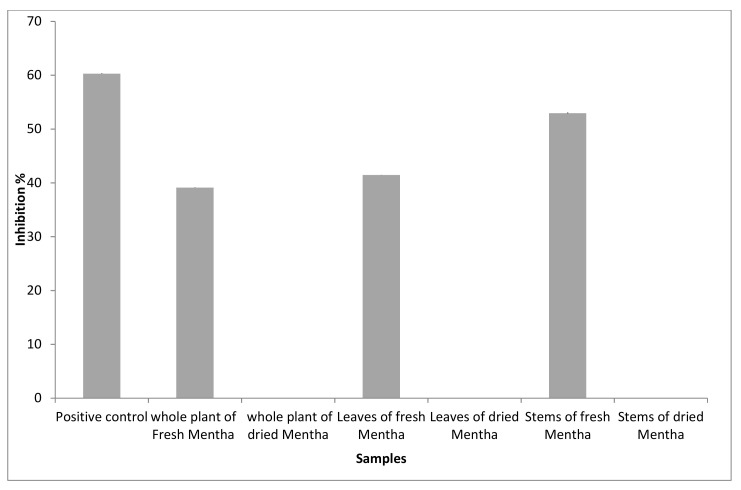
Antifungal activity was estimated by determining the capacity of inhibition of mycelial growth of the fungi species under study. Leaves of fresh Mentha presented the highest biological activity compared with the other extracts from Mentha, as shown in Figure 4. In total, 20 mg\ml of fresh and dried Mentha leaf extracts prevented the growth of the fungi by 51.18 ± 0.15 and 47.06 ± 0%, respectively. Additionally, the fungi can be inhibited by using fresh and dried stems of Mentha extracts, and the percentage is 48.53 ± 0.15% and 45.44 ± 0.23%, respectively, followed by inhibition by the whole plant, fresh and dried Mentha extracts, with the percentages of 41.53 ± 0.23% and 35.29 ± 0%, respectively.
Figure 4.
Antifungal activity of volatile oils of M. suaveolens L.
Antifungal Activity of Non-Volatile Constituents from Mentha
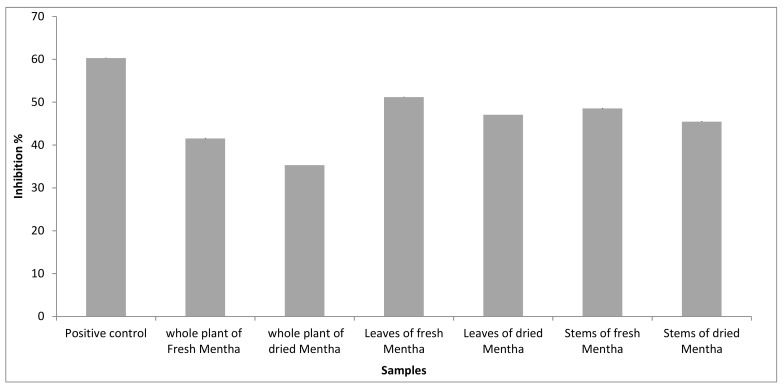
Antifungal activity was estimated by determining the capacity of inhibition of mycelial growth of the species under study. Fresh stem extracts of Mentha had the highest biological activity compared with the other extracts from Mentha. A concentration of 20 mg/mL of fresh stems, leaves, and whole plant extracts of Mentha can prevent the growth of the fungi by 52.94 ± 0.24%, 41.47 ± 0.05%, and 39.12 ± 0.096%, respectively, as shown in Figure 5. The other extracts, including dried (whole plant, leaves, and stems), of Mentha did not affect the growth and sporulation of the tested fungi.
Figure 5.
Antifungal activity of non-volatile constituents of M. suaveolens L.
Antifungal activity of volatile oils and non-volatile constituents of Mentha, examined using analysis of variance and presented in Table 3, shows that mean squares were highly significant for treatments.
Table 3.
Mean squares of analysis of variance for antifungal activity of volatile and non-volatile constituents of M. suaveolens L.
| Source of Variation | df | Volatile Oils | Ethanolic Non-Volatile Extracts |
|---|---|---|---|
| Between | 6 | 182.952 ** | 2160.532 ** |
| Within | 14 | 0.025 | 0.013 |
** indicates significance at 1% probability level.
3. Discussion
The volatile oils obtained from Mentha under study were pale yellow with a pleasant and distinct odor. The total yield percentages of volatile oils from different parts of fresh and dried Mentha varied from 0.225% to 0.9%. Our results were in close agreement with Petretto et al. [29] and Kasrati et al. [14], who reported that the volatile oil yields of the aerial parts of M. suaveolens ssp. insularis from Sardinia was 0.2%. The non-volatile constituents obtained from Mentha under study were yellow to deep green with a pleasant and distinct odor. The total yield percentages of non-volatile constituents from different parts of fresh and dried Mentha varied from 0.6% to 3.4%. Our results agree with Barchan et al. [30] who found that the content of non-volatile constituents of M. pulegium and M. piperita from Morocco to be 3.4% and 4%, respectively. In contrast, non-volatile constituents were found in high levels (5%) in the extracts of three Algerian mints [31]. Moreover, El-Ghorab [32] reported that non-volatile constituents of Egyptian Mentha were 4.5%.
The volatile constituents of fresh and dried Mentha from Sakaka belonged to the carvone chemotype. Our results agree with El-Kashoury et al. [4], who reported that the carvone was the main compound in Mentha in different seasons during the year (56% to 31%). Other studies are in agreement with our results where Mentha was classified by the carvone pathway [6,33]. Hussain et al. [34] and Boukhebti et al. [35] analyzed the chemical composition of Mentha from Pakistan and Algeria, in which the main compound in both studies was carvone (59.5% and 59.4%, respectively). Although the carvone percentage in leaves was higher (64.31%) than that reported by Hussain et al. [34], the percentage was lower in whole plant (43.65%) compared to Boukhebti et al. [35]. These differences in carvone concentration might be due to different environmental conditions. In another study of fresh Mentha in Morocco, pulegone was the major compound, accounting for 17.61% [13], while in our study, the percentage was 3.8% in the whole plant, 2.1% in leaves, and 2.2% in stems. Additionally, Sutour et al. [36] reported a higher level of pulegone in their samples (44.4%) compared to our results. In another study of dried Mentha in Morocco (Iguer region), pulegone was absent, agreeing with our results [14]. These changes in the concentration of pulegone might be due to the different habitats from which plants were collected. In our results, α-Terpineol concentration in fresh Mentha whole plant (0.15%) was lower than that reported by Salhi et al. (0.4%) [37]. In another study of M. suaveolens ssp. insularis in Sardinia, the percentage of the linalool was 1.37%, slightly lower than what was observed in this study for fresh whole plants (1.54%) [29]. In an recent study, α-Humulene was found in a high concentration (1.09%) in dried Mentha (Morocco) whole-plant extract compared to our results (0.04% in dried whole plant) [15]. In the same study, terpinene (γ) was among the major compounds accounting for 5.33% in dried whole-plant extract, which is a much higher percentage than the one we observed (0.03% in dried whole-plant extract).
The chemical compounds in fresh and dried Mentha analyzed by GC/MS can be categorized into four chemical classes: M, LOC, HOC, and S as shown in Table 2. The chemical classes were classified in the following decreasing order: high percentage of LOC due to its high content of carvone in all plant extracts, followed by S, HOC, and M in all different extracts of fresh and dried Mentha.
The above results (Table 2) were similar to those reported by Petretto et al. [29] during their work on fresh M. suaveolens ssp. insularis in Sardinia, especially high percentages were reported for oxygenated compounds (87.1%) in the whole plant extract. Moreover, the percentage of sesquiterpenes (14.2%) was similar to the percentage reported in the fresh whole plant extract (15.83%) in our study [29]. El-Kashoury et al. [4] analyzed the chemical composition of fresh M. suaveolens Ehrh. in Egypt. The highest percentage of oxygenated compounds was 62.9% in their whole plant extract, which was much lower than the percentages reported in our study. Our results were in close agreement with Hussain et al. [34], who identified oxygenated compounds in dried leaves of Mentha from Pakistan occurring at 81.5%. In the same study, sesquiterpenes were found at 6.1% in dried leaf extract compared to 12.90% in our analysis. However, the monoterpenes percentage was (1.15%) lower than reported in their study (9.09%) [34]. In another study of dried Mentha in Taif from KSA, the percentage of the oxygenated compounds was 91.9%, which was slightly higher than our results [8]. In contrast, Burham et al. [7] reported a much lower percentage of oxygenated compounds in dried leaves (30.4%) of Mentha from Albaha Area southern KSA. They also reported higher percentages of monoterpenes and sesquiterpenes (54.3% and 26.08%, respectively) compared to our observations in leaf extracts (1.15% and 12.90%, respectively) [7].
Based on the above results, significant change in the chemical composition of volatile oils might be due to different plant parts, environmental factors, seasons, geographical location, and plant age of the Mentha plant.
Previous investigation of the chemical composition of the volatile oils from Mentha in Senegal showed that the pulegone rate dropped in the oils of the dried plants [38]. Similarly, our results showed a sharp drop in pulegone concentration to very low or zero levels in dried M. suaveolens L. Kohari et al. [9] reported the drop in α-Terpineol percentage in the dried Japanese peppermint (0.27% in fresh to 0.18% in dried). This result was in close agreement with our results. The 1,8-cineole concentrations in Mentha from Senegal were lower in the dried plants, which was similar to our observations [38]. Moreover, in our results, the percentages of monoterpenes after drying the whole plant and leaves decreased. Similar results were previously reported [9,39]. In a study of Mentha from Senegal, monoterpene levels increased from 3.4% to 4.7% after drying [38]. Sesquiterpenes were also increased after drying M. suaveolens L. in our analysis, which also agrees with the studies of Diop et al. [38] and Ahmed et al. [39]. Different factors could contribute to the observed variation in volatile constituents. One of these factors is the chemical properties of volatile oils, mainly their structure and volatility. The type of plant is also a significant factor in this regard. There is also a chance for new compounds to form based on the chemical reactions involved, such as glycoside hydrolysis, oxidation, esterification, etc. [39]. Changes in volatile compound concentrations after drying have been reported and explained by the continuous biosynthesis after harvest [40].
Egyptian and Moroccan essential oils of M. suaveolens L. were studied and the results show that β-copaene is present in low amounts (less than 1%) [4,41]. Additionally, β-copaene had been detected in some Algerian species of Mentha with a concentration ranging from 0.82 to 0.9% [42]. Boukhebti et al. showed that the content of β-copaene in the essential oil of Mentha spicita was 0.347% [35]. Another study conducted in the USA showed that β-copaene was present in Peppermint EO (0.07%), Native Spearmint (0.25%), and Scotch Spearmint (0.18%) [43]. In this study, α and β copaene were detected in whole plant, leaves, and stems where the concentration of α copaene was 0.57%, 0.8%, and 1.37%, respectively and β copaene was 11%, 3.81%, and 0.08%, respectively.
Non-volatile components of fresh and dried Mentha were in close agreement with those reported by Elansary et al. [16] and Mišan et al. [44]. They investigated the phenolic compounds of Mentha species and reported, rosmarinic acid as a main compound. In comparison, Kulig et al. [45] reported that rutin was the primary phenolic acid in Mentha from the Slovak Republic, which was the case in our study for the fresh and dried whole plant and fresh and dried leaf extracts. Additionally, Farnad et al. [17] analyzed Mentha from West Azerbaijan and found that the main compounds were chlorogenic acid and rutin. Previous studies on Mentha species revealed some phenolic derivatives in the genus, such as rosmarinic acid, caffeic acid, ferulic acid, and catechin [31,43,44]. The highest antioxidant activity (53.33%) was observed in the whole-plant extract of dried Mentha, which can be explained by its high carvone content (45%), and which is known for its antioxidant activity. Indeed, Elmastaş et al. [46] reported that the antioxidant activity of carvone is equal to 95%. Additionally, it contains a high concentration of copaene (β) (11%), which has high antioxidant activity [47]. The mild antioxidant activity of the volatile oils of Mentha may be due to its weak phenolic compounds. Approximate results were obtained by Bardaweel et al. [48], who showed that volatile oils have weak antioxidant activity.
Good antioxidant activity of non-volatile constituents of Mentha was observed (94.23 ± 0.007% for the fresh whole plant, 94.50 ± 0.005% for fresh leaves, 94.27 ± 0.01% for fresh stems, 92.83 ± 0.02% for the whole dried plant, 90 ± 0.014% for dried leaves, and 93.33 ± 0.007% for dried stems). These results agree with Mata et al. [49], who reported that ethanol extracts have good antioxidant activity.
The high antioxidant activity of fresh whole plant can be explained by its high content of rosmarinic acid (2223.3 µg/g) [50,51], rutin (676.7 µg/g) [52], and ferulic acid (226.7 µg/g) [53], which are known for their high antioxidant activity. The large effect of fresh leaves was attributed to the high content of rosmarinic acid (28,002.5 µg/g) [50,51], rutin (3383.8 µg/g) [52], ferulic acid (1520 µg/g) [53], and caffeic acid (1141.5 µg/g) [53]. The phenolic compounds of fresh stems contain rosmarinic acid (6558 µg/g) [50,51], naringenin (371.8 µg/g) [54], and rutin (194.6 µg/g) [52], which also exhibited high antioxidant activity.
The good antioxidant activity for dried whole plant may be due to its high content of rosmarinic acid (21,191.9 µg/g) [50,51], rutin (252.16 µg/g) [52], and naringenin (53.22 µg/g) [54]. The high antioxidant activity of dried leaves was due to the high content of rosmarinic acid (15,165.1 µg/g) [50,51] and rutin (194.7 µg/g) [52]. The phenolic compounds of dried stems contain rosmarinic acid (8378.4 µg/g) [50,51], naringenin (130.3 µg/g) [54], and rutin (41.6 µg/g) [52], which have high antioxidant activity.
The high antifungal activity of volatile oils of Mentha can be explained by its high content of carvone, which showed high antifungal activity [55]. The volatile oils of fresh Mentha contain pulegone, which also exhibited high antifungal activity [56]. Additionally, it contains 1,8-cineole, which showed good antifungal activity but was lower than carvone [57].
The high antifungal activity was observed for the non-volatile constituents of fresh extracts of Mentha due to the high concentration of rosmarinic acid, catechin, and luteolin, known for their high antifungal activity [58,59,60]. Moreover, the fresh stem extracts of Mentha contained a high concentration of eugenol, which showed high antifungal activity [61].
4. Materials and Methods
4.1. Chemicals and Plant Material
All standard (authentic compounds of essential oils (Decan, 1,8 Cineole, Ocimene (Ε-β), Benzyl formate, Terpinolene, Linalool, Myrceno, Terpineol<1->, Terpinen-4-ol, α-Terpineol, γ-Terpineol, Carveol (Trans Carveol(cis), Carvone, Pulegone, Elemene (δ-), Copaene (α), Aromadendrene) and phenolic compounds (Protocatechuic acid, p-hydroxybenzoic acid, Catechin, Vanilic acid, Cinnamic acid, Naringenin, Eugenol, Caffeic Acid, Coumaric acid, Ferulic acid, Rutin, Luteolin, Quercetin and Rosmarinic acid)) and chemical reagents were supplied from Sigma Aldrich company (Burlington, MA, US and used without further purifications.
Fresh M. suaveolens L. plants were collected from a local farm in Sakaka, Aljouf, Saudi Arabia, in September 2020. Plant identification was performed by Hamdan A., Al-Jouf, KSA.) the herbarium (59-CPJU) voucher specimen was stored at College of Pharmacy, Jouf University (Sakaka, Saudi Arabia). Fresh M. suaveolens L. plants were dried under shade at room temperature in a fume hood.
4.2. Extraction Methods
4.2.1. Extraction of Volatile Oil of M. suaveolens L.
The volatile oils of different samples were extracted, 200 g of each sample, by hydro distillation using Clevenger apparatus (Clevenger Apparatus was supplied from Shiva Scientific Glass Private Limited, New Delhi, India.) during 5 h. Then, the volatile oils were extracted by dichloromethane (DCM) (3 × 50 mL) and dried by adding magnesium sulfate anhydrous. The DCM was filtered and removed in a rotary evaporator (Model: R-3001, Evaporating Flask: 500 mL, 1000 mL, Rotation Speed: 10–280 rpm, Temperature Range: Room temp +5 °C~95 °C. Evaporating Speed: 23.5 mL/min (Water) supplied from GWSI manufacture, Zhengzhou, China) and then stored at −4 °C in opaque containers [8].
4.2.2. Extraction of Non-Volatile Constituents of M. suaveolens L.
Each sample of Mentha (100 g) was extracted with 99.8% ethanol (200 mL for each part), for 72 h, in a dark place and with stirring. Then, the obtained extract was filtered and dried by adding anhydrous magnesium sulfate. The rotary evaporator was used for solvent removal from extract and then stored in dark containers at −4 °C [32].
4.3. Analysis of Volatile Oils of M. suaveolens L. by GC and GC/MS
4.3.1. GC Analysis
Volatile oils were analyzed using GC/MS and GC, instrumental details and condition according to the reported method [19]. See Supplementary Materials.
4.3.2. GC/MS Analysis
The quantitative determination and chemical composition of volatile oils were estimated using the adjusted reported method [14,15,16,17,18,19,20,21,22,23,24,62] (see Supplementary Materials).
4.4. HPLC Analysis of Non-Volatile Compounds
The non-volatile extracts were analyzed using HPLC, instrumental details and condition according to the reported method [63]. All phenolic standards were prepared in methanol within the range from 0.5 to 100 µg/mL and these standards were used for quantitative and qualitative identification of phenolic compounds in M. suaveolens L. extract chemical composition depending on the standard calibration curve for each standard and retention time, respectively (see Supplementary Materials).
4.5. Antioxidant Activity
The scavenging DPPH free radical ability was determined in vitro according to the procedure of Yue and Xu [64]. DPPH solution (0.1 mM, 1.8 mL) was mixed with 0.2 mL of each diluted Mentha extract stock solution (5 mg/mL) and incubated in the dark for 30 min at 25 °C. In this assay, the percentage of DPPH reduction by samples was compared to BHT. The experiment was repeated three times. The following formula was used to quantify radical scavenging activity:
| Inhibition (Scavenging effect) (%) = [A0 – A1)/A0] × 100 | (1) |
A0 is the absorbance of the control reaction (t = 0 min).
A1 is the absorbance of the tested extract solution (t = 30 min).
4.6. Antifungal Activity
Ten samples of locally fabricated cheese were gathered arbitrarily from farmer’s houses of Al-Jouf–Sakaka in KSA. Samples were collected in (sterile, clean, and dry) containers and shipped to test centre within 1–2 h of collection (at 4 + 2 °C).
4.6.1. Detection and Isolation of Fungi
The dilution-plate method [22] was used to isolate and identify fungi from the collected cheese samples. Samples were inoculated on potato dextrose agar (PDA) obtained from Oxoid [24]. Rose Bengal (30 ppm) was used as an antibacterial agent.
One gram of white cheese was suspended in 90 mL sterilized distilled H2O using a rotating shaker to homogenate the suspension. Then, 10 serial dilutions were prepared, and 1 mL of each dilution was inoculated into a petri dish. Then, melted medium was poured, mixed well, and left to solidify. After solidification, Petri dishes were incubated at 27 ± 2 °C for 5–7 days. Colonies were counted and isolated for purification and identification.
4.6.2. Identification of the Isolated Fungi
Morphological identification of the isolated fungi was carried out based on their macro and microscopic characteristics using the taxonomic methods [28,63]. Additionally, the most dominant species were identified using molecular analysis of the ITS1-5.8S rRNA–ITS2 region (animal health research institute, Dokki, Giza, Egypt).
4.6.3. Effect of Volatile Oils and Non-Volatile Extract on Mycelium Growth of Isolated Fungi
In total, 15 mL of PDA medium were placed in each Petri dish (9 cm diam.), and after solidification, a circular hole (2 cm, diam.) was formed. Then, 1 mL of each different concentration of the tested extract was added to each well. Each Petri dish was inoculated with four fungal species and incubated at 28 °C for seven days. PDA medium without additives was used as a negative control, whereas plates containing 20 mg/mL Miconazole Nitrate were used as a positive control. Each treatment was repeated three times and subjected to statistical analysis. The diameter of inhibition zones was measured for each well, and the results were statistically analyzed. In total, 1 mL of plant extract (20 mg/mL) was added to each well to determine the inhibition zone and inhibition percentage (%).
The diameter of inhibition zones was measured, and the percentage of antifungal activity was calculated according to Equation (2):
| (2) |
DC = the diameter of fungal mycelium growth in the control Petri dish.
DT = the diameter of fungal mycelium growth in the treated Petri dish, containing Mentha extracts.
4.7. Statistical Analysis
Separated completely randomized designs as one-way ANOVA were conducted for statistical analysis procedure of the obtained data. Both antioxidant and antifungal activities of volatile oils and non-volatile extracts of Mentha were carried out in triplicate experiments. Each experiment included seven treatments (whole plant of fresh Mentha, whole plant of dried Mentha, leaves of fresh Mentha, leaves of dried Mentha, stems of fresh Mentha and stems of dried Mentha as well as the control treatment with BHT). Duncan’s multiple range test [65] was used to estimate the performance of mean treatments depending on significance of mean of squares according to the ANOVA table at a significance level of p = 0.05, where a different superscript letter refers to a significant difference among those treatments. MSTAT- Cv.2.10 software program package processed all data [66].
5. Conclusions
M. suaveolens L. from the Sakaka region was investigated for its chemical components and biological activities for the first time. Regarding the main chemical components of volatile oils, carvone dominated in all samples. The volatile oil M. suaveolens L. was carvne chemo type. Additionally, the main phenolic compounds in ethanolic extract were rosmarinic acid, rutin, catechin, and naringenin which are responsible for the antioxidant and antifungal activities of Mentha. It was found that copaene had the highest concentration in M. suaveolens L. in comparison with the same species in another locality. Fungi (Penicillium glabrum) isolated from white cheese was inhibited by Mentha extracts for the first time as well as fungi (Penicillium glabrum) isolated from white cheese that was inhibited by Mentha extracts for the first time.
From the previous results, the M. suaveolens L. has high antioxidant and antifungal activity, confirming that it can be subject to application in pharmaceutical or food industries.
Acknowledgments
The authors would like to thank the Deanship of Graduate Studies at Jouf University for funding and supporting this research through the initiative of DGS, Graduate Students Research Support (GSR) at Jouf University, Saudi Arabia.
Supplementary Materials
The following supporting information can be downloaded at: The following are available online at https://www.mdpi.com/article/10.3390/molecules27092949/s1, Analysis of volatile oils of Mentha by GC and GC/MS and HPLC analysis of non-volatile compounds.
Author Contributions
Conceptualization, A.H.E.-G., H.B. and S.M.N.M.; methodology, B.A.; software, F.Q.A.; validation, H.M.A. and F.Q.A.; formal analysis, H.M.A., M.A.A. and M.H.; investigation, K.F.E.; writing—original draft preparation, B.A.; writing—review and editing, B.A., H.B., S.M.N.M. and A.H.E.-G.; supervision, A.H.E.-G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shahbazi Y. Application of Carboxymethyl Cellulose and Chitosan Coatings Containing Mentha spicata Essential Oil in Fresh Strawberries. Int. J. Biol. Macromol. 2018;112:264–272. doi: 10.1016/j.ijbiomac.2018.01.186. [DOI] [PubMed] [Google Scholar]
- 2.Božovic M., Pirolli A., Ragno R. Mentha suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules. 2015;20:8605–8633. doi: 10.3390/molecules20058605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dwivedy A.K., Prakash B., Chanotiya C.S., Bisht D., Dubey N.K. Chemically Characterized Mentha cardiaca L. Essential Oil as Plant Based Preservative in View of Efficacy against Biodeteriorating Fungi of Dry Fruits, Aflatoxin Secretion, Lipid Peroxidation and Safety Profile Assessment. Food Chem. Toxicol. 2017;106:175–184. doi: 10.1016/j.fct.2017.05.043. [DOI] [PubMed] [Google Scholar]
- 4.El-Kashoury E.S.A., El-Askary H.I., Kandil Z.A., Salem M.A., Sleem A.A. Chemical Composition and Biological Activities of the Essential Oil of Mentha suaveolens Ehrh. Z. Für Naturforsch. C. 2012;67:571–579. doi: 10.1515/znc-2012-11-1207. [DOI] [PubMed] [Google Scholar]
- 5.El-Sayeda A.E.-K., Hesham I.E.-A., Zeinab A.K., Shahira M.E., Mohamed A.S., Amany A.S. Chemical and Biological Study of Mentha suaveolens Ehrh. Cultivated in Egypt. J. Med. Plants Res. 2014;8:747–755. doi: 10.5897/JMPR2014.5324. [DOI] [Google Scholar]
- 6.Šarić-Kundalić B., Fialová S., Dobeš C., Ölzant S., Tekel’ová D., Grančai D., Reznicek G., Saukel J. Multivariate Numerical Taxonomy of Mentha Species, Hybrids, Varieties and Cultivars. Sci. Pharm. 2009;77:851–876. doi: 10.3797/scipharm.0905-10. [DOI] [Google Scholar]
- 7.Burham B.O., Osman O.A., Mohammed Nour A.A. Chemical Composition and Antibacterial Activity of Essential Oil of Mentha longifolia Leaf from Albaha Area Southern Saudi Arabia. Asian J. Biol. Life Sci. 2019;8:48–52. doi: 10.5530/ajbls.2019.8.9. [DOI] [Google Scholar]
- 8.Abdel-Hameed E.S.S., Salman M.S., Fadl M.A., Elkhateeb A., Hassan M.M. Chemical Composition and Biological Activity of Mentha longifolia L. Essential Oil Growing in Taif, KSA Extracted by Hydrodistillation, Solvent Free Microwave and Microwave Hydrodistillation. J. Essent. Oil-Bearing Plants. 2018;21:1–14. doi: 10.1080/0972060X.2018.1454343. [DOI] [Google Scholar]
- 9.Kohari Y., Yamashita S., Chiou T.Y., Shimotori Y., Ohtsu N., Nagata Y., Murata M. Hydrodistillation by Solvent-Free Microwave Extraction of Fresh Japanese Peppermint (Mentha arvensis L.) J. Essent. Oil-Bear. Plants. 2020;23:77–84. doi: 10.1080/0972060X.2020.1726825. [DOI] [Google Scholar]
- 10.El Hassani F.Z. Characterization, Activities, and Ethnobotanical Uses of Mentha Species in Morocco. Heliyon. 2020;6:e05480. doi: 10.1016/j.heliyon.2020.e05480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llorens-Molina J.A., Rivera Seclén C.F., Vacas Gonzalez S., Boira Tortajada H. Mentha suaveolens Ehrh. Chemotypes in Eastern Iberian Peninsula: Essential Oil Variation and Relation with Ecological Factors. Chem. Biodivers. 2017;14:e1700320. doi: 10.1002/cbdv.201700320. [DOI] [PubMed] [Google Scholar]
- 12.El-Kashoury E.S.A., El-Askary H.I., Kandil Z.A., Salem M.A. Botanical and Genetic Characterization of Mentha suaveolens Ehrh. Cultivated in Egypt. Pharmacogn. J. 2013;5:228–237. doi: 10.1016/j.phcgj.2013.10.002. [DOI] [Google Scholar]
- 13.Benayad N., Ebrahim W., Hakiki A., Mosaddak M. Chemical Characterization and Insecticidal Evaluation of the Essential Oil of Mentha suaveolens L. and Mentha pulegium L. Growing in Morocco. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2012;13:27–32. [Google Scholar]
- 14.Kasrati A., Alaoui Jamali C., Bekkouche K., Spooner-Hart R., Leach D., Abbad A. Chemical Characterization and Insecticidal Properties of Essential Oils from Different Wild Populations of Mentha suaveolens Subsp. Timija (Briq.) Harley from Morocco Properties. Chem. Biodivers. 2015;12:823–831. doi: 10.1002/cbdv.201400236. [DOI] [PubMed] [Google Scholar]
- 15.Benali T., Bouyahya A., Habbadi K., Zengin G., Khabbach A., Achbani E.H., Hammani K. Chemical Composition and Antibacterial Activity of the Essential Oil and Extracts of Cistus ladaniferus Subsp. Ladanifer and Mentha suaveolens against Phytopathogenic Bacteria and Their Ecofriendly Management of Phytopathogenic Bacteria. Biocatal. Agric. Biotechnol. 2020;28:101696. doi: 10.1016/j.bcab.2020.101696. [DOI] [Google Scholar]
- 16.Elansary H.O., Szopa A., Kubica P., Ekiert H., Klimek-Szczykutowicz M., El-Ansary D.O., Mahmoud E.A. Polyphenol Profile and Antimicrobial and Cytotoxic Activities of Natural Mentha × Piperita and Mentha longifolia Populations in Northern Saudi Arabia. Processes. 2020;8:479. doi: 10.3390/pr8040479. [DOI] [Google Scholar]
- 17.Farnad N., Heidari R., Aslanipour B. Phenolic Composition and Comparison of Antioxidant Activity of Alcoholic Extracts of Peppermint (Mentha piperita) J. Food Meas. Charact. 2014;8:113–121. doi: 10.1007/s11694-014-9171-x. [DOI] [Google Scholar]
- 18.Xu D.P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., Zhang J.J., Li H. Bin Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017;18:96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. pp. 804–806. [Google Scholar]
- 20.Alencar J.W., Craveiro A.A., Matos F.D.A. Kovats’ Indices as a Preselection Routine in Mass Spectra Library Searches of Volatiles. J. Nat. Prod. 1984;47:890–892. doi: 10.1021/np50035a028. [DOI] [Google Scholar]
- 21.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 22.Calva J., Castillo J.M., Bec N., Ramírez J., Andrade J.M., Larroque C., Armijos C. Chemical Composition, Enantiomeric Distribution and AChE-BChE Activities of the Essential Oil of Myrteola Phylicoides (Benth) Landrum from Ecuador. Rec. Nat. Prod. 2019;13:355–362. doi: 10.25135/rnp.112.18.09.893. [DOI] [Google Scholar]
- 23.Judzentiene A., Tomi F., Casanova J. Analysis of Essential Oils of Artemisia absinthium L. from Lithuania by CC, GC(RI), GC-MS and 13C NMR. 2010;1:1113–1118. [PubMed] [Google Scholar]
- 24.Mostafa E.M. Exploration of Aurora B and Cyclin-Dependent Kinase 4 Inhibitors Isolated from Scorzonera Tortuosissima Boiss. and Their Docking Studies. Pharmacogn. Mag. 2020;16:258. doi: 10.4103/pm.pm. [DOI] [Google Scholar]
- 25.Murbach Teles Andrade B.F., Nunes Barbosa L., Da Silva Probst I., Fernandes Júnior A. Antimicrobial Activity of Essential Oils. J. Essent. Oil Res. 2014;26:34–40. doi: 10.1080/10412905.2013.860409. [DOI] [Google Scholar]
- 26.Ribeiro P.H.S., Dos Santos M.L., Da Camara C.A.G., Born F.S., Fagg C.W. Seasonal Chemical Compositions of the Essential Oils of Two Eugenia Species and Their Acaricidal Properties. Quim. Nova. 2016;39:38–43. doi: 10.5935/0100-4042.20150161. [DOI] [Google Scholar]
- 27.Tian M., Zhao X., Wu X., Hong Y., Chen Q., Liu X., Zhou Y. Chemical Composition, Antibacterial and Cytotoxic Activities of the Essential Oil from Ficus Tikoua Bur. Rec. Nat. Prod. 2020;14:219–224. doi: 10.25135/rnp.161.19.10.1450. [DOI] [Google Scholar]
- 28.Yan J., Liu X.B., Zhu W.W., Zhong X., Sun Q., Liang Y.Z. Retention Indices for Identification of Aroma Compounds by GC: Development and Application of a Retention Index Database. Chromatographia. 2015;78:89–108. doi: 10.1007/s10337-014-2801-y. [DOI] [Google Scholar]
- 29.Petretto G.L., Fancello F., Zara S., Foddai M., Mangia N.P., Sanna M.L., Omer E.A., Menghini L., Chessa M., Pintore G. Antimicrobial Activity against Beneficial Microorganisms and Chemical Composition of Essential Oil of Mentha suaveolens ssp. Insularis Grown in Sardinia. J. Food Sci. 2014;79:369–377. doi: 10.1111/1750-3841.12343. [DOI] [PubMed] [Google Scholar]
- 30.Barchan A., Bakkali M., Arakrak A., Pagán R., Laglaoui A. The Effects of Solvents Polarity on the Phenolic Contents and Antioxidant Activity of Three Mentha Species Extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014;3:399–412. [Google Scholar]
- 31.Fatiha B., Didier H., Naima G., Khodir M., Martin K., Léocadie K., Caroline S., Mohamed C., Pierre D. Phenolic Composition, in Vitro Antioxidant Effects and Tyrosinase Inhibitory Activity of Three Algerian Mentha Species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae) Ind. Crops Prod. 2015;74:722–730. doi: 10.1016/j.indcrop.2015.04.038. [DOI] [Google Scholar]
- 32.El-Ghorab A.H. The Chemical Composition of the Mentha pulegium L. Essential Oil from Egypt and Its Antioxidant Activity. J. Essent. Oil-Bearing Plants. 2006;9:183–195. doi: 10.1080/0972060X.2006.10643491. [DOI] [Google Scholar]
- 33.Hendriks H., Van Os F.H.L. Essential Oil of Two Chemotypes of Mentha suaveolens during Ontogenesis. Phytochemistry. 1976;15:1127–1130. doi: 10.1016/0031-9422(76)85115-1. [DOI] [Google Scholar]
- 34.Hussain A.I., Anwar F., Nigam P.S., Ashraf M., Gilani A.H. Seasonal Variation in Content, Chemical Composition and Antimicrobial and Cytotoxic Activities of Essential Oils from Four Mentha Species. J. Sci. Food Agric. 2010;90:1827–1836. doi: 10.1002/jsfa.4021. [DOI] [PubMed] [Google Scholar]
- 35.Boukhebti H., Chaker A.N., Belhadj H., Sahli F., Ramdhani M., Laouer H., Harzallah D. Chemical Composition and Antibacterial Activity of Mentha pulegium L. and Mentha spicata L. Essential Oils. Der Pharm. Lett. 2011;3:267–275. [Google Scholar]
- 36.Sutour S., Bradesi P., de Rocca-Serra D., Casanova J., Tomi F. Chemical Composition and Antibacterial Activity of the Essential Oil from Mentha suaveolens Ssp. Insularis (Req.) Greuter. Flavour Fragr. J. 2008;23:107–114. doi: 10.1002/ffj.1863. [DOI] [Google Scholar]
- 37.Salhi A., Bouyanzer A., Chetouani A., El Ouariachi E., Zarrouk A., Hammouti B., Desjobert J.M., Costa J. Chemical Composition, Antioxidant and Anticorrosion Activities of Mentha suaveolens. J. Mater. Environ. Sci. 2017;8:1718–1728. [Google Scholar]
- 38.Diop S.M., Guèye M.T., Ndiaye I., Hadji E., Ndiaye B., Diop M.B., Heuskin S., Lognay G. Chemical Composition of Essential Oils and Floral Waters of Mentha longifolia (L.) Huds. from Senegal. Am. J. Essent. Oils Nat. Prod. 2016;4:46–49. [Google Scholar]
- 39.Ahmed A., Ayoub K., Chaima A.J., Hanaa L., Abdelaziz C. Effect of Drying Methods on Yield, Chemical Composition and Bioactivities of Essential Oil Obtained from Moroccan Mentha pulegium L. Biocatal. Agric. Biotechnol. 2018;16:638–643. doi: 10.1016/j.bcab.2018.10.016. [DOI] [Google Scholar]
- 40.Zrira S., Benjilali B. Effect of Drying on Leaf Oil Production of Moroccan Eucalyptus Camaldulensis. J. Essent. Oil Res. 1991;3:117–118. doi: 10.1080/10412905.1991.9697921. [DOI] [Google Scholar]
- 41.Hamdani I., Chikri M., Fethi F., Salhi A., Bouyanzer A., Zarrouk A., Hammouti B., Costa J., Desjobert J.M. Essential Oil Mentha suaveolens L: Chemical Composition, Anticorrosive Properties on Mild Steel in 0.5 M H2SO4 and Chemometric Approach. J. Mater. Environ. Sci. 2017;8:526–538. [Google Scholar]
- 42.Benabdallah A., Boumendjel M., Aissi O., Rahmoune C., Boussaid M., Messaoud C. Chemical Composition, Antioxidant Activity and Acetylcholinesterase Inhibitory of Wild Mentha Species from Northeastern Algeria. South African J. Bot. 2018;116:131–139. doi: 10.1016/j.sajb.2018.03.002. [DOI] [Google Scholar]
- 43.Wu Z., Tan B., Liu Y., Dunn J., Martorell Guerola P., Tortajada M., Cao Z., Ji P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules. 2019;24:2825. doi: 10.3390/molecules24152825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mišan A.Č., Mimica-Dukić N.M., Mandić A.I., Sakač M.B., Milovanović I.L., Sedej I.J. Development of a Rapid Resolution HPLC Method for the Separation and Determination of 17 Phenolic Compounds in Crude Plant Extracts. Cent. Eur. J. Chem. 2011;9:133–142. doi: 10.2478/s11532-010-0126-8. [DOI] [Google Scholar]
- 45.Kulig D., Matysiak M., Baldovská S., Štefániková J., Maruniaková N., Mňahončáková E., Árvay J., Galbavý D., Kolesárová A. Screening of Polyphenolic Compounds from Traditional Medicinal Herbs. J. Microbiol. Biotechnol. Food Sci. 2019;9:487–491. doi: 10.15414/jmbfs.2019.9.special.487-491. [DOI] [Google Scholar]
- 46.Elmastaş M., Dermirtas I., Isildak O., Aboul-Enein H.Y. Antioxidant Activity of S-Carvone Isolated from Spearmint (Mentha spicata L. Fam Lamiaceae). J. Liq. Chromatogr. Relat. Technol. 2006;29:1465–1475. doi: 10.1080/10826070600674893. [DOI] [Google Scholar]
- 47.Türkez H., Çelik K., Toğar B. Effects of Copaene, a Tricyclic Sesquiterpene, on Human Lymphocytes Cells in Vitro. Cytotechnology. 2014;66:597–603. doi: 10.1007/s10616-013-9611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bardaweel S.K., Bakchiche B., ALSalamat H.A., Rezzoug M., Gherib A., Flamini G. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of Essential Oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan Atlas. BMC Complement. Altern. Med. 2018;18:201. doi: 10.1186/s12906-018-2274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mata A.T., Proença C., Ferreira A.R., Serralheiro M.L.M., Nogueira J.M.F., Araújo M.E.M. Antioxidant and Antiacetylcholinesterase Activities of Five Plants Used as Portuguese Food Spices. Food Chem. 2007;103:778–786. doi: 10.1016/j.foodchem.2006.09.017. [DOI] [Google Scholar]
- 50.Shahidi F. Antioxidants in Food and Food Antioxidants. Nahrung Food. 2000;44:158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Erkan N., Ayranci G., Ayranci E. Antioxidant Activities of Rosemary (Rosmarinus officinalis L.) Extract, Blackseed (Nigella sativa L.) Essential Oil, Carnosic Acid, Rosmarinic Acid and Sesamol. Food Chem. 2008;110:76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 52.Yang J., Guo J., Yuan J. In Vitro Antioxidant Properties of Rutin. LWT Food Sci. Technol. 2008;41:1060–1066. doi: 10.1016/j.lwt.2007.06.010. [DOI] [Google Scholar]
- 53.Kikuzaki H., Hisamoto M., Hirose K., Akiyama K., Taniguchi H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 54.Mundlia J., Ahuja M., Kumar P., Pillay V. Improved Antioxidant, Antimicrobial and Anticancer Activity of Naringenin on Conjugation with Pectin. 3 Biotech. 2019;9:312. doi: 10.1007/s13205-019-1835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soković M.D., Vukojević J., Marin P.D., Brkić D.D., Vajs V., Van Griensven L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules. 2009;14:238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flamini G., Cioni P.L., Puleio R., Morelli I., Panizzi L. Antimicrobial Activity of the Essential Oil of Calamintha Nepeta and Its Constituent Pulegone against Bacteria and Fungi. Phyther. Res. 1999;13:349–351. doi: 10.1002/(SICI)1099-1573(199906)13:4<349::AID-PTR446>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 57.Griffin S.G., Markham J.L., Leach D.N. An Agar Dilution Method for the Determination of the Minimum Inhibitory Concentration of Essential Oils. J. Essent. Oil Res. 2000;12:249–255. doi: 10.1080/10412905.2000.9699509. [DOI] [Google Scholar]
- 58.Fialová S.B., Kello M., Čoma M., Slobodníková L., Drobná E., Holková I., Garajová M., Mrva M., Zachar V., Lukáč M. Derivatization of Rosmarinic Acid Enhances Its in Vitro Antitumor, Antimicrobial and Antiprotozoal Properties. Molecules. 2019;24:1078. doi: 10.3390/molecules24061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alves C.T., Ferreira I.C.F.R., Barros L., Silva S., Azeredo J., Henriques M. Antifungal Activity of Phenolic Compounds Identified in Flowers from North Eastern Portugal against Candida Species. Future Microbiol. 2014;9:139–146. doi: 10.2217/fmb.13.147. [DOI] [PubMed] [Google Scholar]
- 60.Rashed K., Ćirić A., Glamočlija J., Soković M. Antibacterial and Antifungal Activities of Methanol Extract and Phenolic Compounds from Diospyros virginiana L. Ind. Crops Prod. 2014;59:210–215. doi: 10.1016/j.indcrop.2014.05.021. [DOI] [Google Scholar]
- 61.Zabka M., Pavela R. Antifungal Efficacy of Some Natural Phenolic Compounds against Significant Pathogenic and Toxinogenic Filamentous Fungi. Chemosphere. 2013;93:1051–1056. doi: 10.1016/j.chemosphere.2013.05.076. [DOI] [PubMed] [Google Scholar]
- 62.Ramadan M., El-Ghorab A., Ghanem K. Volatile Compounds, Antioxidants, and Anticancer Activities of Cape Gooseberry Fruit (Physalis peruviana L.): An in-Vitro Study. J. Arab Soc. Med. Res. 2015;10:56. doi: 10.4103/1687-4293.175556. [DOI] [Google Scholar]
- 63.El-Massrry K.F., El-Ghorab A.H., Shaaban H.A., Shibamoto T. Chemical Compositions and Antioxidant/Antimicrobial Activities of Various Samples Prepared from Schinus Terebinthifolius Leaves Cultivated in Egypt. J. Agric. Food Chem. 2009;57:5265–5270. doi: 10.1021/jf900638c. [DOI] [PubMed] [Google Scholar]
- 64.Yue X., Xu Z. Changes of Anthocyanins, Anthocyanidins, and Antioxidant Activity in Bilberry Extract during Dry Heating. J. Food Sci. 2008;73:494–499. doi: 10.1111/j.1750-3841.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 65.Duncan D.B. Multiple Range and Multiple F Tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 66.Freed R., Einensmith S.P., Guets S., Reicosky D., Smail V.W., Wolberg P. User’s Guide to MSTAT-C Analysis of Agronomic Research Experiments. Michigan State University; East Lansing, MI, USA: 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.