Abstract
Simple Summary
Natural formulations and phytotherapies have shown promising antitumor activities. This review assesses the antitumor effects of curcumin on breast cancer. In particular, we discuss the effects of curcumin on the proliferation, viability, and apoptosis of breast cancer cell lineages and tumor volume. Studies have shown that curcumin administered at different concentrations inhibited proliferation, decreased viability, and induced apoptosis in human and animal breast cancer cells. Nanoparticle formulations of curcumin administered orally, via implant, or intraperitoneally reduced the tumor volume of human and murine mammary cells in vivo. Moreover, curcumin nanoformulations facilitate tumor growth inhibition in animal models of breast cancer. Randomized clinical trials are warranted to assess the efficacy and safety of curcumin formulations for clinical use.
Abstract
Breast cancer is one of the most common neoplasms among women. Anticancer strategies using natural formulations and phytotherapies are promising antitumor treatment alternatives. This review assesses the antitumor effects of curcumin on breast cancer reported in preclinical in vitro and in vivo animal models. We used five databases to search for preclinical studies published up to May 2021. The assessments included the effects of curcumin on the proliferation, viability, and apoptosis of breast cancer cell lineages and on tumor volume. In total, 60 articles met the inclusion criteria. Curcumin administered at different concentrations and via different routes of administration inhibited proliferation, decreased viability, and induced apoptosis in human and animal breast cancer cells. Nanoparticle formulations of curcumin administered orally, via implant, and intraperitoneally reduced the tumor volume of human and murine mammary cells in vivo. Moreover, curcumin nanoformulations exert positive effects on tumor growth inhibition in animal models of breast cancer. Further randomized clinical trials are warranted to assess the efficacy and safety of curcumin formulations for clinical use.
Keywords: turmeric, anticancer, breast tumor, in vitro, in vivo, nanoparticles
1. Introduction
Breast cancer is one of the most common neoplasms in women and an important public health problem worldwide [1]. Breast cancer has surpassed lung neoplasm as the most frequently diagnosed cancer, with approximately 2.3 million new cases reported (11.7%) in 2020, according to the Global Cancer Observatory [2].
Conventional treatment for breast cancer includes surgical resection, radiotherapy, and chemotherapy [3]. In addition, promising alternative approaches, such as targeted therapy, immunotherapy, and hormone therapy, are currently under investigation [4,5]. These therapies vary in their mechanisms of action. The appropriate treatment regime is determined based on the type of tumor, disease stage, and clinical condition of patients [4,5].
Although chemotherapy remains the gold standard for treating several types of cancer, severe adverse reactions and tumor resistance to treatment and hormone therapy are considered negative aspects of paramount importance [3,6]. Therefore, alternative anticancer therapeutic strategies, such as the use of low-toxicity natural subproducts and extracts, are promising modalities [6,7].
Previous studies have reported that curcumin, a turmeric-derived phytochemical, exhibits beneficial biological activities, including antibacterial, antiviral, anticancer, anti-inflammatory, and antioxidant properties, and was found to exert preventive and therapeutic effects in various cancers, including breast cancer [7,8,9,10,11,12]. However, the therapeutic applicability of curcumin remains limited owing to its low water solubility and bioavailability [7,13]. Only two systematic reviews on the effects of curcumin on breast cancer have been reported to date [13,14]. Gianfredi et al. [14] investigated the bioactive effects of a curcumin-containing diet on human breast cancer cell lines [14]. Meanwhile, Ombredane et al. [13] reported the in vivo efficacy and toxicity of curcumin nanoparticles (CUR-NPs) as a treatment strategy against breast cancer. Therefore, there remain gaps in the literature regarding the effects of curcumin on tumors. In this systematic review, we collated data from preclinical in vitro and in vivo studies conducted on animal models to investigate the effects of curcumin on the proliferation, viability, and apoptosis of breast cancer cells and tumor volume, focusing on dose and administration route. We systematically reviewed the antitumor effects of curcumin on breast cancer previously reported. To our knowledge, this is the first systematic review of in vitro preclinical studies on the effect of curcumin on breast cancer cell lineages and animal models of breast cancer.
2. Materials and Methods
2.1. Protocol and Registration
The study design was based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [15] and the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) [16]. A protocol was published in the International Prospective Register of Systematic Reviews: Review of animal data from experimental studies databases (CRD42021256605). We included data from preclinical in vitro and in vivo studies conducted using animal models.
2.2. Search Strategy and Eligibility Criteria
The PubMed, Embase, Scopus, Web of Science, and SciELO databases were used for data retrieval. The research period was limited to 23 May 2021. Google Scholar and the reference lists of primary studies were consulted to search for additional studies. The following uniterms were used: “Curcumin”; “Curcuma longa”; “Turmeric”; “Natural yellow 3”; “Turmeric yellow”; “Indian saffron”; “Kacha haldi”; “Curcumin Nanoparticles”; “Breast Cancer”; “Breast Neoplasms”; “Triple Negative”; “Breast Neoplasms”; “Breast Tumor”; “Inflammatory Breast Neoplasms”; “Carcinoma, Ductal, Breast”; “Carcinoma Lobular”; “Her-2 Positive”; “Breast Cancer”; “In Vitro”; “Mouse”; “Animal”. The search strategy adopted for each database is listed in (Table S1).
The participants, intervention, comparator, and outcomes (PICO) framework was used to determine the eligibility criteria for the systematic review of preclinical animal studies, as follows:
Patient: laboratory animals with induced breast cancer (all species).
Intervention(s): curcumin
Comparator(s): control group or comparison with no treatment, treatment with other drugs, and/or traditional radiotherapy or chemotherapy regimens.
Outcomes: antitumor activity (reduction in tumor volume and dimensions) in in vitro studies.
The inclusion criteria were as follows: (1) in vitro and animal experimental model investigations on the effects of curcumin on human and animal breast cancer cells of different lineages, (2) peer-reviewed original research articles, (3) no language restrictions, and (4) no publication year restriction. The exclusion criteria were as follows: (1) doctoral and master’s theses, (2) case studies, (3) editorials, (4) letters to editor, (5) duplicate studies found in more than one database and in silico studies, (6) epidemiological studies, (7) clinical assays and articles that requested permission from authors without response, (8) studies irrelevant to the antitumor effect of curcumin on breast cancer, (9) trials performed in non-oncological clinical conditions, (10) studies involving a sole treatment protocol based on the association between curcumin and other treatment modalities, and (11) trials involving immunodeficient animal models.
Definitions
Cell proliferation: increase in cell count owing to cell division [17]. Cell proliferation was strictly controlled without any alterations. In contrast, neoplastic cells exhibited massive and uncontrolled proliferation [18].
Cell viability: quantification of viable cells for estimating cytotoxicity [19] and investigating cell activity and integrity [19,20].
Apoptosis: programmed cell death under physiological and pathological conditions [21,22]. In cancer, disparity between cell replication and death causes malignancy [22].
2.3. Review Process
Two authors (K.A.B. and C.R.M.) performed a peer review of the titles and abstracts of the articles using Rayyan software. The selected articles were assessed by the authors and critically evaluated based on the known antitumor effects of curcumin on breast cancer. Next, the selected articles were assessed and the inclusion/exclusion criteria were applied. Doubts and disagreements regarding article selection were discussed with the research team. If some published studies were associated with the same project or were retrieved from the same database, the most complete study was selected [23,24].
2.4. Training of Reviewers
The authors participating in eligibility assessments completely understood each step of the review process, primarily the inclusion/exclusion criteria, and practiced eligibility assessments on 50 test abstracts prior to coding articles. The authors also used risk-of-bias instruments and performed quality assessments and data extraction on five articles that were not included in the review [23].
2.5. Evidence Synthesis
The following data were extracted: authorship, year of publication, country, cell lineage, concentration, exposure time, animal experimental model, follow-up, sample, dosing, route of administration, and main outcomes. The outcomes included antitumor activity, including cell proliferation, viability, apoptosis, and/or cessation of the cell cycle in in vitro studies and changes in tumor volume and magnitude in animal models.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool, adapted for in vitro study designs [25], was used to assess quality, since methods specifically for this purpose are lacking. In vitro trials were ranked as “high,” “moderate,” and “low” in terms of quality [25] based on the analysis of each study.
The SYRCLE RoB Toll tool was used to assess the quality of animal model studies [26]. Selection, performance, detection, attrition, reporting, and other biases were investigated.
There was substantial heterogeneity among the studies, which was detrimental to meta-analysis. Therefore, narrative synthesis was performed without statistical or sensitivity analysis, assessing publication bias using the funnel plot and Egger’s and Begg’s tests.
3. Results
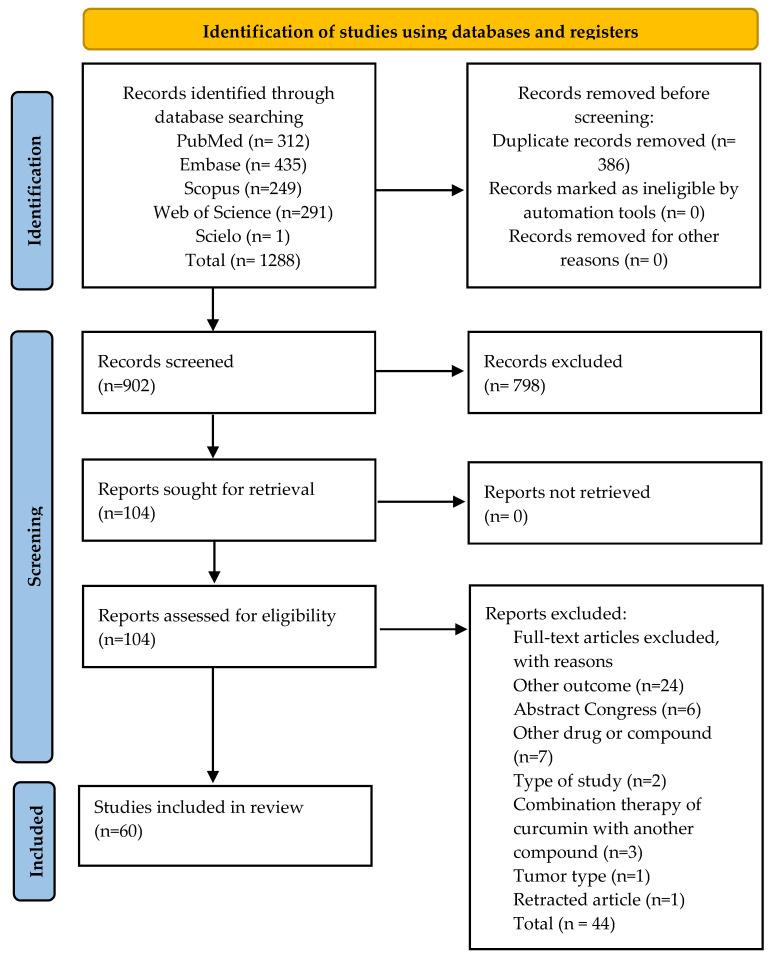
The bibliographic survey yielded 1288 articles. After titles and abstracts from the records were screened, 104 potentially eligible articles were identified and selected for complete reading. Following a review of all texts, 44 articles were excluded. Details of the search strategies are provided in (Table S1). The reasons for exclusion are provided in (Table S2). The flowchart of the study is shown in Figure 1.
Figure 1.
Flowchart for study selection (PRISMA Flow Diagram 2020).
3.1. General Characteristics of the Studies
Sixty studies on the effect of curcumin on breast cancer [3,7,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] were included in this investigation, with 23 in vitro trials [27,28,31,33,34,35,36,37,44,45,48,51,52,54,59,64,65,70,73,74,76,80,84], 20 studies on animal models [29,30,32,40,41,46,57,60,62,66,68,69,71,72,75,77,79,82,83], and 17 studies with both in vitro and in vivo experimental designs [3,7,39,42,43,47,49,50,53,55,56,58,61,63,67,78,81]. The oldest and most recent articles were published in 1997 [64] and 2021 [39,81], respectively. The general characteristics of the selected articles are presented in Table 1 and Table 2.
Table 1.
Characteristics of the in vitro studies included in the systematic review on curcumin and breast cancer.
| Author/Year/Country | Type of Cell/Model | Intervention | Outcomes | Conflict of Interest | |||
|---|---|---|---|---|---|---|---|
| Antitumor Activity | |||||||
| Concentration (Component) | Treatment Duration | Cell Proliferation In Vitro | Cell Viability | Apoptosis and/or Cell Cycle Interruption | |||
| Abbaspour and Afshar, 2018 [27] Iran |
MCF-7 Human |
Curcumin at 10, 20 and 30 μg/mL |
24, 48, and 72 h | MTT assay ↓ cell proliferation owing to downregulation of ODC1 and ADA gene expression. |
MTT assay ↓ viability of cells in a time- and dose-dependent manner. |
Not reported | None |
| Abuelba et al., 2015 [28] Romania |
MDA-MB-231 Human |
Curcumin at 15–19 μM |
24, 48, and 72 h | MTT assay ↓ cell proliferation upon treatment with 15 μM curcumin. |
MTT assay ↓ cell viability by up to 25% upon treatment with 15 μM curcumin. |
MTT assay Pro-apoptotic effects on MDA-MB-231 cells cultured in a single layer, without photoactivation. |
None |
| Bimonte et al., 2015 [7] Italy |
MDA.MB231 Human |
Curcumin at 10 and 50 μM |
48 h | MTT assay Inhibition of breast cancer cell migration in 48 h. ↓ cell proliferation (p < 0.05). |
Not reported | Flow cytometry Curcumin (10 μM) ↑ apoptosis (p < 0.0001). |
None |
| Calaf et al., 2018 [31] Chile |
MCF7 MDA-MB-231 Human |
Curcumin at 30 µM |
48 h | Not reported | Not reported | Flow cytometry Apoptosis MDA-MB-231: 14.2% MCF7: 4.6% |
None |
| Chiu and Su, 2009 [33] China |
MDA-MB-231 Human |
Curcumin at 10, 20, and 30 μg/mL |
48 h | MTT Assay ↓ proliferation of MDA-MB-231 cells via p21 expression. |
Not reported | Flow cytometry Curcumin induced apoptosis via positive regulation of the Bax:Bcl-2 ratio. |
None |
| Choudhuri et al., 2002 [34] India |
MCF-7 Human |
Curcumin at 10 and 25 μM |
24 h | Quantitative image analysis Cessation of cell growth followed by significant cell death. Optimal inhibition was obtained upon treatment with 25 μM curcumin. |
Not reported | Quantitative image analysis techniques Curcumin induced apoptosis. |
None |
| Coker-Gurkan et al., 2019 [35] Turkey |
T47D Human |
Curcumin at 30 µM | 24 and 48 h | Not reported | MTT assay ↓ cell viability by 48% and 60% upon treatment with 20 µM curcumin (p < 0.0024). |
Double staining with Annexin-V/PI Curcumin induced apoptosis in 10.9% and 5.2% of the cell populations. |
None |
| Coker-Gurkan et al., 2018 [36] Turkey |
MCF-7 MDA-MB-231 Human |
Curcumin at 30 µM | 24 and 48 h | Not reported | MTT assay ↓ cell viability MCF-7 cells by 49% and of MDA-MB-453 cells by 48% upon treatment for 24 h with 20 µM curcumin |
MTT assay Curcumin induced apoptotic cell death. |
None |
| Fan et al., 2016 [37] China |
MDA-MB-231 Human |
Curcumin at 50 μg/mL | 24 h | Not reported | MTT assay ↓ cell viability (% NR) (P:NR) |
MTT assay Curcumin induced apoptosis. |
None |
| Ghosh et al., 2021 [39] India |
MDA-MB 231 Human |
Curcumin at 50 μg/mL Nanostructured platform Nanoparticles, MSN-Curcumin (MSN-C), and MSN-Hyaluronic acid-Curcumin (MSN-HA-C) |
48 h | MTT Assay MSN-HA-C blocked cell proliferation, in contrast to free curcumin. The treatment agent exhibited anticancer properties at 20 μg/mL. |
Not reported | MTT assay Cell death MSN-HA-C: 58% MSN-C: 34% (with equivalent dose of 12 μg/mL curcumin). MDA-MB-231 cycle arrest ↓ G1-phase cells: 32.5% Control: 54.6% G2/M phase cells: 37.8% Controls: 11.4%. |
None |
| Hashemzehi et al., 2018 [42] Iran |
MCF-7 Human |
Curcumin at 1 mM Nanostructured platform Nano-curcumin—phytosomalcurcumin |
24 h | Transwell assay ↓ cell invasion MTT assay ↓ cell growth in a dose-dependent manner. |
Not reported | Not reported | None |
| He et al., 2019 [43] China |
4T1 Mouse |
Curcumin at 50 μg/mL Nanostructured platform Polymeric micellar NPs [amphiphilic diblock copolymer—mPEG-b-PLG (Se) -TP] |
48 h | Not reported | MTT assay ↓ of cell viability upon treatment with CUR-NP and Free CUR: 15% |
Not reported | None |
| Hu et al., 2018 [44] China |
T47D, MCF7 Human |
Curcumin at 10 or 30 µM |
72 h | MTT assay ↓ cell proliferation |
Not reported | Flow cytometry Apoptosis T47D cells: 13.87% and 30.09%. MCF7 cells: 15.14% and 35.04%. |
None |
| Hua et al., 2010 [45] China |
MDA-MB-435 Human |
Curcumin at 10, 25, 50, and 75 μM | 12, 24, or 48 h. | MTT assay ↓ cell proliferation, inducing arrest in the G1 phase. |
Not reported | Not reported | NR |
| Ji et al., 2020 [47] China |
MDA-MB-231 Human |
Curcumin at 50 μg/mL |
24 h | Not reported | Not reported | Flow cytometry Apoptosis HA@CUR-NCs 80%. |
None |
| Jiang et al., 2013 [48] China |
MCF-7/LCC2 and LCC9 Human |
Curcumin at 10 and 30 μM |
24, 48, 72, and 96 h | Colony formation assay ↓ colony formation Complete suppression of colony formation upon treatment with 30 μM curcumin. |
Not reported | Annexin-V/PI staining and flow cytometry 30 μM curcumin caused a significant increase (28.72% in MCF-7 cells, 31.36% in MCF-7/LCC2 cells, and 34.70% in MCF-7/LCC9 cells) in the percentage of late apoptotic cells. |
None |
| Jin et al., 2017 [49] China and USA |
MCF-7 Human |
Curcumin at 10 µg/mL Nanostructured platform CUR-NP; GE11-CUR-NP; Free CUR |
24 h | Not reported | Nanostructured platform CUR-NP, GE11-CUR-NP, and Free CUR |
Flow cytometry Apoptosis CUR-NP: 14.9%; GE11-CUR-NP: 18.9%; Free CUR 11.0%. |
None |
| Jung et al., 2018 [50] South Korea |
MDA-MB-468 Human |
Curcumin at 5 and 10 μM |
72 and 96 h | Colony formation assay ↓ number of colonies over 2 weeks to 36.9 ± 7.7% upon treatment with 5 µM curcumin. |
Unclear method ↓ significantly decreased cell viability (41.5 ± 2.8% of basal level) upon treatment with 10 µM curcumin |
Not reported | None |
| Kim et al., 2012 [51] Coreia do Sul |
MCF-7 Human |
Curcumin at 1, 5, 10, 30, and 50 μM |
24 h | Not reported | MTT assay Curcumin exerted no effect on the viability of MCF-7 cells |
Not reported | None |
| Kumari et al., 2017 [52] India |
MDA-MB-231 Human |
Curcumin at 50 and 100 μg/mL Nanostructured platform free CUR and CUR-mPEG-PLA-Ch micelles |
24 h | Not reported | MTT assay CUR: 55.26 ± 3.7% Free CUR: 66.84 ± 2.4% (p = 0.079) |
Not reported | None |
| Kumari et al., 2020 [53] India |
MDA-MB-231 Human 4T1 Mouse |
Curcumin at 50 μg/mL Nanostructured platform CUR treatment (Free CUR group—24 μg/mL) and CUR-HSA-DOPE NPs treatment (CUR-HSA-DOPE group) |
6 and 24 h | Not reported | MTT Assay MDA-MB-231 Cur-HSA-DOPE NPs 24.34 ± 6.1% and 33.99 ± 4.5% free CUR 34.87 ± 4.9% and 43.12 ± 2.4% 50 μg/mL curcumin 4T1 CUR-HSA-DOPE NPs 25.2 ± 5.8% and 11.9 ± 8.6% free CUR 34.5 ± 6.6% and 48.3 ± 7.2% 50 μg of curcumin |
Immunofluorescence TUNEL assay ↑ Apotosis CUR-HSA-DOPE NPs |
None |
| Kumari et al., 2016 [54] India |
MDA-MB-231 Human |
Curcumin at 50 μg/mL Curcumin and curcumin-loaded nanoparticles (curcumin in mPEG-PLA micelles) (CUR-HSA-DOPE NPs) |
24 h | Not reported | MTT Assay CUR-mPEG-PLA231 35.1 ± 8.5 free CUR 65.7 ± 1.0% 50 μg/mL |
Not reported | None |
| Laha et al., 2018 [55] India and USA |
MDA-MB-468 Human |
Curcumin at 20, 40, 60, 80, 100, and 120 mM |
12 and 24 h | Not reported | Not reported | Annexin V-FITC staining Apoptotic cells: 25% and 91%. |
None |
| Lai et al., 2012 [56] China |
MCF-7, BT-474, MDA-MB-231, and normal breast cells Human |
Curcumin at 10 μg/mL |
72 h | Colorimetric analysis of sulforhodamine B ↓ cell proliferation (MCF-7, BT-474, and MDA-MB-231 cells). |
Not reported | Not reported | None |
| Li et al., 2018 [3] China |
MDA-MB-231 Human |
Curcumin at 10 g/mL Nanostructured platform curcumin and curcumin nanoparticle MSN/IR780-PEI-FA 160 mg/kg |
24 and 48 h | Not reported | Not reported | Flow cytometry CUR and free MSN/CUR induced the G2/M phase of the cell cycle. |
|
| Liu et al., 2013 [58] China |
4T1 Mouse |
Curcumin at 100 μg/mL Nanostructured platform Nanoparticle with self-assembled polymeric micelles (CUR-M) loaded with curcumin (CUR) |
48 h | Not reported | MTT assay Both CUR-M and Free CUR drastically inhibited cell growth in a dose-dependent manner. |
TUNEL assay by immunofluorescence staining Apoptotic index CUR-M: 15.77 ± 2.74%, Free CUR: 9.42 ± 2.13% p < 0.001) |
None |
| Liu et al., 2009 [58] China |
MDA-MB-231 Human |
Curcumin at 1, 1.25, 2.5, 5, 10, and 20 mg/mL |
24 and 48 h | Method (NR) Inhibition of cell growth by 60–70% with 1.25 mg/mL curcumin. Inhibition of cell growth by 50–60% with 2.5 mg/mL curcumin. |
Not reported | Not reported | NR |
| Lv et al., 2014 [61] China |
MDA-MB-231 Human |
Curcumin at 1–100 μL |
24 and 48 h | Not reported | MTT assay ↓ significant reduction in the number of viable cells in a time- and dose-dependent manner. |
Flow cytometry of fixed nuclei ↑ in the number of apoptotic cells in a dose-dependent manner. |
None |
| Masuelli et al., 2013 [63] Italy |
MDA-MB-231, MDA -MB-435, MDA-MB-453, MDA-MB-468, T-47D, MCF7, BT-474, SK-BR-3 Human Mammary cancer cells (H-2”) (TUBO) Humanized mouse Mammary cancer cells (H-2”) (TUBO) Mouse |
Curcumin 6 to 50 pM |
24 and 48 h | Not reported | Not reported | Pro-apoptotic Bax and anti-apoptotic Bcl-2 expression CUR induced apoptosis in all investigated cell types. |
None |
| Mehta et al., 1997 [64] USA |
MCF7 Human |
Curcumin 1 to 3 μg/mL |
72 h | [3H]thymidine incorporation and flow cytometry. Cell growth inhibition in a time- and dose-dependent manner, correlated with the inhibition of ornithine decarboxylase activity. |
Not reported | Flow cytometry Curcumin-induced cell death was not due to apoptosis or any significant change in the expression of apoptosis-related genes, including the Bcl-2, p53, cyclin B, and transglutaminase genes. |
NR |
| Montazeri et al., 2017 [65] Iran |
MCF7 Human |
Curcumin at 23, 17, and 14 µM Dendrosomal curcumin (DNC) for 48 h (28–35 μM) and 72 h (23–25 μM) |
24, 48, and 72 h | Not reported | Not reported | Flow cytometry Total apoptosis by DNC: 24 h: 30.34 ± 0.011% 48 h: 33.83 ± 0.005% 72 h: 61.83 ± 0.009% |
None |
| Mukhopadhyay et al., 2020 [67] India |
MDA-MB-231 Human |
5 mg of curcumin Nanostructured platform Polymeric NPs PLGA/PVA with or without folate (F) |
24 h | Not reported | Not reported | Flow cytometry Apoptosis CUR-NP-F: 29% Free CUR: 20% |
|
| Sarighieh et al., 2020 [70] Irã |
MCF7 Human |
Curcumin 5, 10, 20, 40, 80, and 160 μM |
24 h | Not reported | MTT assay Curcumin decreased the cell viability of MCF-7 cells. |
Flow cytometry Apoptosis 24.6% |
None |
| Sun Shih-Han et al., 2012 [73] Taiwan |
MDA-MB-231/Her2 Human |
Curcumin at 30 and 50 mM |
24 h | Not reported | Not reported | Flow cytometry Apoptosis occurred at a higher dosage (50 mM). |
None |
| Sun Xiao-Dong et al., 2012 [74] China |
MDA-MB-231 Human |
Curcumin at 10, 20, and 30 μmol/mL |
48 h | MTT assay The inhibitory effect on MDA-MB-231 cell proliferation peaked upon treatment with 30 μmol/mL curcumin (p < 0.01). |
Not reported | Flow cytometry Apoptosis control 2.76% and Curcumin 26.34%, 30 μmol/mL (p < 0.01). |
None |
| Wang Xet al., 2017 [76] China |
MCF-7 Human |
Curcumin [0 (with DMSO vehicle), 0.5, 1.0, 2.0, 5.0, and 10.0 µM] | 24, 48, and 72 h | MTT assay ↓ cell growth (treatment with 0, 0.5, 1.0, 2.0, 5.0, and 10.0 µM curcumin). |
Not reported | Flow cytometry Apoptotic cell death within 48 h upon treatment with 2 µM (p = 0.0021) and 5 µM (p = 0.0004) curcumin. |
None |
| Yang et al., 2017 a [76] China | MCF-7 Human |
Curcumin at 50 μm Nanostructured platform Micelle NPs (PPBV triblock copolymer) |
24 h | Not reported | Not reported | Flow cytometry Apoptotic cell death |
|
| Younesian et al., 2017 [80] Irã |
SKBR3 Human |
Curcumin at 2.5, 10, 15, 20, 25, and 30 μM | 24, 48, and 72 h | Not reported | Not reported | Flow cytometry Apoptosis: 4.37% with 0 μM, 27.46% with 5 μM, 64.98% with 10 μM, 75.90% with 15 μM, and 76.92% with 20 μM curcumin. |
None |
| Yu et al., 2021 [81] China |
4T1 Mouse |
Curcumin at 5, 10, and 15 μM |
24 h | Not reported | MTT assay ↓ of cell viability by 16% using 15 μg/mL curcumin |
Not reported | None |
| Zong et al., 2012 [84] China |
MCF-7 Human |
Curcumin at 10, 20, 50, and 100 μM | 48 h | MTT assay ↓ cell growth by 37%, 54%, and 73% using 20, 50, and 100 μM curcumin, respectively. |
Not reported | Not reported | None |
MTT assay, MTT Assay Protocol for Cell Viability and Proliferation, ↓: inhibition, ↑: activation.
Table 2.
Characteristics of the studies conducted on experimental animal models included in the systematic review on curcumin and breast cancer.
| Author/Year/Country | Experimental Animal Model * | Intervention | Outcome | Conflicts of Interest | Ethical Approval | |
|---|---|---|---|---|---|---|
| Treatment Follow-Up |
Dose (mg/kg)/Administration Route | Anti-Tumor Activity (Size or Volume of the Tumor) | ||||
| Abd-Ellatef et al., 2020 [38] Italy and Egypt |
Balb/c/n = 8/JC/mouse/(1 × 107 cells)/mammary fat pad | VT: 50 mm3; three times (on days 1, 7, and 14); vehicle-free CUR: 10% DMSO suspension v/v Follow-up: 18 days Nanostructured platform Solid lipid nanoparticles (SLNs) with or without chitosan (CS) coating (cholesterol; trilaurin, butyl lactate, Epikuron® 200, Cremophor® RH60, sodium taurocholate, Pluronic® F68) |
5 mg/kg; Intravenous administration | CURC-CS-SLN and CURC ↓ VT (35%); Free CUR: no VT ↓; p < 0.01 |
None | Yes |
| Alizadeh et al., 2015 [29] Iran |
Balb/c/n = 8/Transplantation of spontaneous mouse mammary tumor/pieces < 0.3 cm3/subcutaneous administration in the left flank | 14 days after tumor induction; daily for 24 days Follow-up: 35 days Nanostructured platform Micelles/polymersomes NPs (PNP) [monomethoxyPEG (mPEG 2000), oleic acid (OA)] |
Dose: (NR); Intraperitoneal administration | CUR-NP ↓ VT (80%); p < 0.05 | None | Yes |
| Bansal et al., 2014 [30] USA |
Female ACI mice/ 5 to 6 weeks old/mammary tumorigenesis mediated by 17β-estradiol (E2)/9 mg of E2/back |
4 days after tumor induction/ Curcumin implants (n = 6) Curcumin diet (n = 6) Follow-up: 6 months |
Curcumin 1000 ppm via diet Two 2 cm implants, 200 mg/implant, 20% p/p drug load 10.9 mg of curcumin for 25 days subcutaneous administration |
Curcumin implant: ↓ VT (35%) Curcumin administration via diet: ineffective |
None | Yes |
| Bimonte et al., 2015 [7] Italy |
Foxn1 nu/nu female mice/n = 16, 6-to-8-week-old/human breast cancer cell line MDA.MB231/2.5 × 106 cells/right flank | After reaching 30–60 mm3, normal diet (n = 8) and diet containing 0.6% curcumin were administered (n = 8). Follow-up: 6 weeks |
0.6% Curcumin administration via diet |
↓ VT (% NR) (p = 0.0195) | None | NR |
| Chen et al., 2017 [32] China |
Balb/c/n = 5/BT-549/human (2 × 106 cells)/subcutaneous administration in the right upper thigh | 200 mm3 VT 35 mg/kg; Fourteen days, every 2 days Intratumoral—Vehicle Free CUR: NR Follow-up: 30 days Nanostructured platform Micelle NPs [POCA4C6 (phosphorylated calixarene) micelles—PM] |
5 mg/kg; Intratumoral administration |
CUR-NP ↓ VT (60%); Free CUR: ↓ VT (34%); p < 0.05 |
None | Yes |
| Ghosh et al., 2021 [39] India |
Swiss albino mice/3 groups (n = 5)/MCF-7 and MDA-MB 231 cells (human)/vein | Alternating days after tumor induction Follow-up: 2 weeks Nanostructured platform Nanoparticles: MSN-Curcumin (MSN-C) and MSN-Hyaluronic acid-Curcumin (MSN-HA-C) |
10 mg/kg; intravenous administration |
MSN-HA-C ↓ VT (% NR); p < 0.05 |
None | Yes |
| Greish et al., 2018 [40] Bahrain | Balb/c/n = 5/4T1/mouse/(1 × 106 cells)/bilaterally on flanks | VT: 100 mm3; frequency of treatment: unclear; Treatment: 10 days Follow-up: 9 days Nanostructured platform Micelles (curcumin-metal complex and SMA) |
10 and 20 mg/kg; Intravenous administration | CUR-NP-10 mg/kg ↓ VT (61%); CUR-NP-20 mg/kg ↓ VT (92%); p < 0.05 |
None | NR |
| Grill et al., 2018 [41] Estados Unidos |
Balb-neuT mice/n = NR/HER-2-positive breast cancer cells/ten breast pads |
At 2, 4, 7, or 12 weeks of age, and once a month thereafter Follow-up: 24 weeks Nanostructured platform Curcumin-loaded microparticles Curcumin (20 mg) and PLGA (20 mg) |
140 mg of microparticles, corresponding to 58.2 mg of curcumin/administered via subcutaneous injection | Curcumin MP ↓ VT (60%); p < 0.05 | None | Yes |
| Hashemzehi et al., 2018 [42] Iran |
Balb mice/n = 4/MCF-7 cells (human)/flanks | VT: 100 mm3; 7 days after tumor induction Follow-up: 22 days Nanostructured platform Nanocurcumin—phytosomal curcumin |
Dose: (NR); NR | Curcumin ↓ VT (22.2%) Curcumin + 5-FU ↓ VT (53.3%) |
None | Yes |
| He et al., 2019 [43] China |
Balb/c/n = 6/4T1/mouse/(1 × 106 cells)/subcutaneous administration in right back | VT: 100 mm3 Every 4 days for 4 times Free CUR: (NM) Follow-up: 21 days Nanostructured platform Polymeric micellar NPs [amphiphilic diblock copolymer—mPEG-b-PLG (Se)-TP] |
5 mg/kg; Intravenous administration |
CUR-NP ↓ VT (62.9%); Free CUR: ↓ VT (55%); p < 0.05 |
None | Yes |
| Huang et al., 2020 [46] China | Balb/c/n = 5/4T1/mouse/NR/Flank mice | VT: 40–50 mm3/every 2 days for 5 times Follow-up: 16 days Nanostructured platform |
50 mg/kg; Intravenous |
CUR-NP ↓ VT (38%); p < 0.05 | None | Yes |
| Ji et al., 2020 [47] China |
Balb/c/n = 5/4T1/mouse/(1 × 106 cells)/subcutaneous administration in the right flank | Polymeric NPs (HA-CHEMS); pH-sensitive First day of treatment: NR; Every 2 days Vehicle-free CUR: (NM) Follow-up: 10 days Nanostructured platform Nanocrystal NPs with or without HA |
5 mg/kg; Intravenous | HA@CUR-NCs ↓ VT (86%); CUR-NP ↓ VT (39%); Free CUR: ↓ VT (21%); p < 0.05 | None | Yes |
| Jin et al., 2017 [49] China and USA |
Balb/c nude rats/n = 5/MCF-7/human/(1 × 107 cells)/subcutaneous administration in the dorsal flank | 7 days after tumor induction; every 24 h for 20 times Free CUR: NR Follow-up: 3 weeks Nanostructured platform Polymeric NPs with or without EGFR-targeting peptides (GE11) (PLGA-PEG); |
5 mg/kg; Intravenous administration | CUR-NP-GE11 and CUR-NP ↓ VT (80%); Free CUR: sem VT ↓; p < 0.05 |
None | Yes |
| Jung et al., 2018 [50] República da Coréia | Balb/c nude rats/n = 4/MDA-MB-468 cells/human/(5 × 106 cells)/right shoulder |
VT: 50 mm3; three times a week; eight injections in all Follow-up: NR Nanostructured platform CUR-NP e EGF-CUR-NP |
10 mg/kg; Intraperitoneal administration | CUR-NP-EGFR ↓ VT (59.1%); CUR-NP no ↓ VT; p < 0.05 |
None | Yes |
| Kumari et al., 2020 [52] India |
Balb/c mice/n = 18/Mouse (4T1)/50 μL, 1 × 106 cells/subcutaneous administration in left flank |
VT: 50 mm3; Follow-up: 21 days Nanostructured platform CUR treatment (Free CUR group (0–24 μg/mL)) and CUR-HSA-DOPE NPs treatment (CUR-HSA-DOPE group) |
25 mg/kg; Intravenous administration |
CUR-HSA-DOPE ↑ VT (80.41%); Free CUR ↑ VT (86.30%) |
None | Yes |
| Laha et al., 2018 [55] India and USA |
Balb/c/n = 6/4T1/mouse/NR/mammary fat pad | 10 days after tumor induction; every 5 days for four times Follow-up: 20 days Nanostructured platform Metal organic frameworks NPs (IRMOF-3) with or without folic acid (FA) [(Zn(NO3)2; NH2-H2 BDC] |
2 mg/kg (* unclear); Route of administration: (NM) |
CUR-NP-FA ↓ VT (61%); CUR-NP ↓ VT (44%); p < 0.05 | None | Yes |
| Lai et al., 2012 [56] Taiwan |
Nude mice/n = 16/BT-474 cells overexpressing HER-2 (1 × 107)/right flank subcutaneous route of administration | 21–28 days after xenograft inoculation. VT:50–100 mm3 Follow-up: after 4 weeks |
45 mg/kg curcumin injected intra-peritoneally |
Herceptin and curcumin VT 34.1 ± 25.0 mm3 Curcumin VT 63.6 ± 25.7 mm3 p = 0.079 |
||
| Li et al., 2018 [3] China |
Balb/c/n = 4/MDA-MB-231/human/(1 × 107 cells)/subcutaneous administration | Tumor diameter: 4 mm; every 3 days for six times in all Free CUR: NR Follow-up: 18 days Nanostructured platform Mesoporous silica nanoparticles with hyaluronan (MSN-HA) or polyethyleneimine-folic acid (MSN-PEI-FA). |
8 mg/kg; Intravenous administration | CUR-NP-PEI-HA ↓ VT (50%); Free CUR: no VT ↓; p < 0.01 |
None | Yes |
| Lin et al., 2016 [57] China | Balb/c nude mice/n = 6/MCF-7/ human/(NM)/Subcutaneous administration in the right axilla | First day of treatment: NR once every 3 days for 15 days Vehicle-Free CUR: (NM) Follow-up: 15 days Nanostructured platform Lipid-based NPs (NLC) with or without folate coating (FA) (PEG-DSPE, soy lecithin, castor oil, Tween 80, and Precirol ATO-5) |
Dose: NR; Intravenous administration |
CUR-NP-FA ↓ VT (~83%); CUR-NP ↓ VT (~66%); Free CUR: ↓ VT (31%) | None | NR |
| Liu et al., 2013 [58] China |
Balb/c mice n = 12; 6 per group/4T1/5 × 105 cells/right flank/subcutaneous administration | From day 4, palpable tumors were daily injected with the treatment agent intravenously for 10 days Follow-up: 25 days Nanostructured platform Self-assembled polymeric micelles (CUR-M) loaded with curcumin (CUR) |
CUR-M (30 mg/kg body weight) Free CUR (30 mg/kg body weight) |
CUR-M ↓ VT (68%); p < 0.01 Free CUR: sem ↓ VT (35%) |
None | Yes |
| Lv et al., 2014 [61] China |
Balb/c nude mice/n = 8 per group /MCF-7 and MDA-MB-231/2 × 106 cells/subcutaneous administration in the back | After reaching 60 mm3/treatment days alternating Follow-up: 4 weeks |
Curcumin 50 µg/kg, 200 µg/kg Intraperitoneal injections |
Cur 50 µg/kg ↓ VT (54%); p < 0.05 Cur 200 µg/kg ↓ (73%); p < 0.05 VT |
None | Yes |
| Lv et al., 2015 [60] China |
Kunming mice/n = 6/EMT6/mouse/(1.0 × 107 cells/mL)/Subcutaneous administration | VT: 300 mm3; daily for 9 days Vehicle-free CUR: cremophor EL:dehydrated alcohol (1:1, v/v) and diluted with saline solution Follow-up: 14 days Nanostructured platform Polymeric NPs (PEG-PCDA) with or without biotin |
10 mg/kg; Intravenous administration | CUR-NP ↓ VT (69%); CUR-NP-biotin ↓ VT (79%); Free CUR: ↓ TV (32%); p < 0.05 |
NR | Yes |
| Mahalunkar et al., 2019 [62] India, Germany and Norway |
Balb/c/n = 6/4T1/mouse/(1 × 105 cells)/Mammary fat pad | First day of treatment: (NM) Twice a week for 2 weeks Vehicle-free CUR: (NM) Follow-up: 21 days Nanostructured platform Metallic gold NPs (CurAu-PVP) with folic acid (FA) (HAuCl4 and PVP polymer) |
10 mg/kg; Intratumoral administration | CUR-NP-FA ↓ VT (51%); Free CUR: no ↓ VT; p < 0.006 |
None | Yes |
| Masuelli et al., 2013 [63] Italy |
Transgenic BALB-neuT mouse/n = 5 per group /NR | After the diameter reached 15 mm, CUR (2 mg in 50 |.il oil with), with oil (50 |.il) or water (50 |.il) was administered three times a week. Follow-up: 30 weeks |
Curcumin 6–50 µM Oral administration |
No mice treated with CUR exhibited tumor growth at week 22, (p < 0.01). Cur ↓ VT (52%) (p < 0.05) |
None | NR |
| Mukerjee et al., 2016 [66] USA | Balb/c nude rats/n = 8/MCF10CA1a/human/(3 × 106 cells)/flank | VT: 70 mm3; Three times a week for 30 days Follow-up: 32 days Nanostructured platform Polymeric NPs [PLGA/PVA with or without antibody targeting (AnxA2)] |
20 mg/kg; Intravenous administration | CUR-NP-AnxA2 ↓ VT (44.0%); CUR-NP ↓ VT (33.5%); p < 0.05 CUR-NP-AnxA2 ↓ PT (53.0%); CUR-NP ↓ PT (30%); p < 0.05 |
NR | NR |
| Mukhopadhyay et al., 2020 [67] India |
Balb/c nude rat/n = 5/MDA-MB-231/human/(5 × 106 cells)/Right flank | 8 days after induction; three times a week Follow-up: 29 days Nanostructured platform Polymeric NPs [PLGA/PVA with or without folate (F)] |
20 mg/kg Route of administration: unclear |
CUR-NP-F ↓ VT (90%); CUR-NP ↓ VT (75%); p < 0.05 | NR | Yes |
| Pal et al., 2019 [68] India |
Balb/c mice/n = 5 per group /human MCF-7, MDA-MB-231, MDA-MB-468, and murine 4T1/100 L/abdominal skin |
Treatment for 20 days at 3-day intervals after 10 days of tumor implantation Follow-up: 30 days Nanostructured platform Synthesis of curcumin-loaded microsphere (10% by weight polymer) PLGA@CCM@FA |
2000 µg/kg Route of administration: unclear |
PLGA—VT 0.092 mm3 ↓ VT (25%) PLGA @ CCM—VT 0.064 mm3 ↓ VT (48%) PLGA @ CCM @FA-VT—0.031 mm3 ↓ VT (75%) |
NR | NR |
| Sahne et al., 2019 [69] Irã | Balb/c/n = 4/4T1/mouse/NR/ssubcutaneous administration in the flank | VT: 50–100 mm3; daily follow-up: 3 weeks Nanostructured platform Graphene oxide NPs (GO NPs with CMC, PVP, PEG, and FA) |
4 mg/kg; Intravenous administration | CUR-NP-FA ↓ VT (86%); p < 0.05 | None | Yes |
| Shiri et al., 2015 [71] Irã |
Balb/c/n = 9/4T1/mouse/(1 × 106 cells)/left flank | Third day after tumor induction Follow-up: 35 days Nanostructured platform Dendrosome NPs (DNC) [composition: not mentioned (patent number: 71753)]. |
40 or 80 mg/kg Route of administration: NR |
NP-40 mg/kg ↓ VT (72%); NP-80 mg/kg ↓ VT (76%); p < 0.05 NP-40 mg/kg ↓ VT (61%); NP-80 mg/kg ↓ VT (64%); p < 0.05 |
NR | Yes |
| Shukla et al., 2017 [72] India |
Balb/c mice/n = 3/(1 × 106 cells)/subcutaneous administration in hind skin | 10 days from tumor inoculation; daily administration for 28 days: gum acacia (1%, w/v). Follow-up: 42 days Nanostructured platform Lipid-based CPC-SNEDDS NPs (Phospholipid, castor oil, Tween 80, and PEG 400) |
100 mg/kg; oral | 1) CUR-NP ↓ VT (58.9%); Free CUR ↓ VT (29.5%); p < 0.001 |
None | Yes |
| Vakilinezhad et al., 2019 [75] Irã |
Sprague–Dawley rats/n = 6/Chemically-induced mammary tumors (MNU) | 4 months after tumor induction; Once a week for 4 weeks Free curing vehicle: aqueous suspension Follow-up: 20 weeks Nanostructured platform Polymeric NPs (PLGA-PVA) |
2.5 mg; Intravenous |
CUR-NP ↓ VT (20%); Free CUR: ↓ VT (16%); p < 0.05 | None | Yes |
| Wang et al., 2018 [77] China | Nude mice/n = (NM)/MDA-MB-231/human/(1.5 × 106 cells)/subcutaneous | 2 months after tumor induction; daily Free CUR: (NM) Follow-up: 2 weeks Nanostructured platform Polymeric NPs (MPEG-PCL) |
1 × 10−3 M; Intravenous administration |
CUR-NP ↓ VT (82%); Free CUR: ↓ VT (49%); p < 0.01 | None | Yes |
| Yang et al., 2017 a [78] China | Balb/c nude mice/n = 5 MCF-7/human/(1 × 107 cells)/subcutaneous administration in the flank | VT: 200 mm3 Every other day, five times; total duration: 20 days Free CUR vehicle: NR Follow-up: 20 days Nanostructured platform Hybrid NPs [PLGA NPs coated with a modified hyaluronic acid (HA hybrid)] |
15 mg/kg; Intravenous | HA-Hybrid NPs/CUR ↓ VT (43.8%, day 12); ↓ VT (24%, day 20); p < 0.05 | NR | Yes |
| Yang et al., 2017 b [79] China | Balb/c nude mice/n = 5 MCF-7/human/(1 × 107 cells)/subcutaneous administration in the flank | VT: 200 mm3 Every other day, five times; total duration: 20 days Free CUR vehicle: NR Follow-up: 20 days Nanostructured platform Micelle NPs (PPBV triblock copolymer) |
10 mg/kg; Intravenous | PPBV micelles/CUR ↓ VT (58.5%, day 12); ↓ VT (28.9%, day 20); p < 0.05 |
NR | Yes |
| Yu et al., 2014 [82] China | Balb/c nude mice/n = 5/MCF-7/human/(3 × 106 cells)/subcutaneous administration in the right flank | VT: 100–400 mm3; Every other day for 5 times for 24 days in all Follow-up: 25 days Nanostructured platform Micelle NPs (MPEG-PLA with or without PAE) |
40 mg/kg; Intravenous administration |
CUR-NP-PAE ↓ VT (65.6%); CUR-NP ↓ VT (47.1%); p < 0.05 | NR | Yes |
| Yu et al., 2021 [81] China |
Balb/c mice/ murine 4T1/NR/intradermal administration in the back of the neck | VT: 150–200 mm3, administration via tail vein every 3 days; 14 days in all Follow-up: 16 days Nanostructured platform curcumin (CUR), zeolitic imidazolate framework-8 nanoparticles (ZIF-8), and hyaluronic acid (HA) |
CUR@ZIF-8 19.6 mg of CUR@ZIF-8@ HA 20.9 mg Intravenous administration |
CUR@ZIF-8 ↓ VT (12.5%); CUR@ZIF-8@HA ↓ VT (62.5%); |
None | Yes |
| Yuan et al., 2018 [83] China | Balb/c nude mice/n = 6/MCF-7/human/(3 × 106 cells)/right flank | VT: 100 mm3; every other day, four times Follow-up: 18 days Nanostructured platform Polymeric NPs (mPEG-PLGA-Pglu) |
2.5 mg/kg; intravenous administration | CUR-NP ↓ VT (28.0%); p < 0.05 CUR-NP ↓ PT (22.5%); p < 0.05 |
None | Yes |
* Animal type/sample size/injected cell type/source/cell concentration/cell insertion site; NR: not reported; VT, tumor volume; ↓: inhibition; ↑: activation.
3.2. Summary of the Results
3.2.1. In Vitro Studies
Forty studies were conducted using in vitro design and assessment (Table 1) [3,7,27,28,31,33,34,35,36,37,39,42,43,44,45,47,48,49,50,51,52,53,54,55,56,58,59,61,63,64,65,67,70,73,74,76,79,80,81,84].
The human breast cancer cell lineages used in the studies were as follows: MCF-7 [27,31,34,36,42,44,48,49,51,56,64,65,70,76,78,79], MDA-MB-435 [45,63], T47D [35,44], MCF-7/LCC2 [48], LCC9 [48], MDA-MB-468 [50,63], and BT-474 [63].
Moreover, studies conducted using the triple-negative breast cancer cell line MDA-MB-231 [3,28,31,33,36,37,39,47,52,53,54,56,59,61,63,67,73,74] and human breast cancer cell lineage expressing the Her2 SK-BR-3 gene [63,80] were also assessed. In animal models, murine mammary carcinoma 4T1 [43,53,58,81] and H-2” (TUBO) [63] cell lineages were investigated.
3.2.2. In Vitro Cell Proliferation
The in vitro proliferation of breast cancer cell lineages was assessed using a quantitative image assessment technique [34], Transwell assay [42], colony formation assay [48,50], sulforhodamine B, colorimetric analysis [56], method (NR) for determining inhibition of cell growth [59], thymidine incorporation assay [3H], flow cytometry tests [64], and MTT assay, as described in most studies (Table 1).
The effect of curcumin on cell proliferation was investigated only in human cell lines. Curcumin administered at concentrations of 1, 3, 10, 20, 30, and 50 μg/mL for 24 h inhibited the proliferation of MCF-7 cells, with growth recurrence in the subsequent 72 h [27,34,42,44,56,64,76]. Optimal inhibition was achieved upon treatment with a single dose of 25 μM curcumin for 24 h [34]. A substantial reduction in growth was observed in malignant MCF-7 cell lines, with 37%, 54%, and 73% reduction upon treatment with 20, 50, and 100 μM curcumin, respectively [84].
Proliferation in MDA-MB-435 cell lineages was inhibited following treatment with 0, 10, 25, 50, and 75 μM curcumin [45,63]. The formation of colonies from MCF-7/LCC2 cells was inhibited following treatment with 30 μM curcumin [48]. The number of colonies in MDA-MB-468 cell cultures reduced over two weeks upon treatment with 5 µM curcumin [50]. Likewise, the proliferation of BT-474 cell cultures was inhibited upon treatment with 10 μg/mL curcumin [56]. In studies on triple-negative MDA-MB-231 cell lineages, cell proliferation was inhibited upon treatment with 0, 1, 25, 2,5, 5, 10, 15, 20, 30, and 50 μM curcumin for 24 and 48 h [7,28,33,59,73]. Furthermore, MSN-curcumin nanoparticles exhibited anticancer properties at 20 μg/mL [39].
3.2.3. Cell Viability
Cytotoxicity in breast cancer cell lineages was assessed using the MTT assay. Curcumin significantly decreased the viability of MCF-7 malignant cells in a time and dose-dependent manner [27,49,70]. In another trial, a decrease in the viability of MCF-7 cells by 49% and of MDA-MB-453 cell cultures by 48% following treatment with 20 µM curcumin for 24 h was observed [36], while another study reported that curcumin did not affect the viability of MCF-7 cell cultures [51]. Cells were treated with 1, 5, 10, 30, and 50 μM curcumin for 24 h at 37 °C.
There was a significant decrease in the viability of MDA-MB-468 cells upon treatment with 10 µM curcumin [50]. The viability of triple-negative MDA-MB-231 cell cultures reduced by up to 25% upon treatment with 15–100 μM curcumin for 24 h [28,37,53,54,61]. There was a 55.2% reduction in the viability of MDA-MB-23 colonies upon treatment with 50 μg/mL curcumin [52]. In T47D cell lineages, viability reduced by 48% and 60% upon treatment with 20 µM curcumin [35].
Mouse 4T1 cultures showed a significant reduction in cell viability upon treatment with pure 6–50 pM curcumin [43,53,81]. Curcumin CUR-M and free CUR nanoparticles also inhibited cell growth in a dose-dependent manner [58].
3.2.4. Apoptosis and/or Interruption of Cell Cycle
In most studies, apoptosis and/or interruption of the cell cycle were assessed using the MTT assay [28,36,37,39], quantitative image analysis [34], Annexin-V/PI double staining [35,48], immunofluorescence TUNEL assay [53,58], Annexin V-FITC staining [55], pro-apoptotic Bax and anti-apoptotic Bcl-2 expression evaluation [63], and flow cytometry.
In the breast cancer MCF-7 cell lineage, apoptosis occurred in 4.6% of the cells upon treatment with 25 μM curcumin [34], and in 28.7% and 49% of the cells upon treatment with 30 μM curcumin [31,36,48]. Other studies reported 14.9% apoptosis in MCF-7 colonies treated with 10 µg/mL curcumin delivered via nanoparticles [49]. There was 24.6% apoptosis in MCF-7 cells incubated under normoxic and hypoxic conditions for 24 h and treated with curcumin at different concentrations (5, 10, 20, 40, 80, and 160 μM) [70]. Wang et al. also reported apoptosis following treatment with 2 and 5 µM curcumin for 48 h [76].
In the triple-negative MDA MB-468 cell lineage, the apoptosis frequency was 25% and 91% after treatment for 12 and 24 h, respectively [55]. In addition, 30 μM curcumin induced apoptosis in 31.36% of MCF-7/LCC2 cells [48] and 34.70% of LCC9 cells [48]. Other studies also reported apoptosis in triple-negative MDA-MB-231 cells treated with 10, 12, 20, 30, and 50 μM curcumin for 24 and 48 h [3,7,28,31,33,37,39,47,53,61,67,74], and in colonies of SK-BR-3 cells treated with 5, 10, 15, and 20 μM curcumin [63,80]. In T47D cells, 30 µM curcumin induced 10.9% apoptosis in 24 h, 5.2% apoptosis in 48 h [35], and 30.09% apoptosis in 72 h [44].
Mouse 4T1 cell lines showed increased apoptosis in response to treatment with 6 to 50 pM curcumin [43,53,63]. Moreover, CUR-NPs at 0–100 μg/mL also induced apoptosis in a dose-responsive manner [58].
3.2.5. Animal Studies
Thirty-seven studies on animal models met the inclusion criteria [3,7,29,30,32,38,39,40,41,42,43,46,47,49,50,53,55,56,57,58,60,61,62,63,66,67,68,69,71,72,75,77,78,79,81,82,83]. Curcumin was delivered via diet in two studies [7,63], diet and implant in one study [30], intraperitoneal injection in two studies [56,61], and different modes using nanoparticles in 32 studies. The results of these studies are listed in Table 2, with the animal species, sampling size, type of cells injected, cell concentration, cell insertion site, treatment, follow-up, dose, and route of administration specified. The studies were heterogeneous with respect to the animal model, follow-up, curcumin dose, and route of administration.
A curcumin-encapsulated polymer micelle formulation was developed showing antitumor and anti-metastatic activities in breast cancer cells [58]. Micelles loaded with curcumin inhibited tumor activity and induced minimal collateral effects in vivo compared to a free curcumin formulation (free-CUR) [43]. Reduction in tumor volume increased significantly following treatment with CUR-NPs (20–92%) [29,32,38,40,46,57,58,60,67,72,75,76,83] rather than with free CUR (0–55%) [32,38,40,43,47,49,60,62,72,75,77,83].
Other curcumin delivery methods and their corresponding tumor magnitude percentile reductions were as follows: HA@CUR-NCs (86%) [47], CUR-NP-biotin (79%) [60], curcumin + 5-FU (53.3%) [42], CUR-NP- AnxA2 (44.0%) [66], CUR-NP-PEI-HA (50%) [3], HA-Hybrid NPs/CUR (43.8%) [78], PPBV micelles/CUR (58.5%) [79], CUR-NP-FA (51–86%) [57,62,69], CUR-NP-PAE (65.6%) [82], CUR@ZIF-8@HA (62.5%) [81], and CUR-NP-EGFR (59.1%) [55]. Furthermore, the synthesized nano-hybrid MSN-HA-C increased anticancer efficacy when compared to Free CUR [39].
Intracellularly degradable, self-assembled amphiphilic biotin-poly (ethylene glycol)-b-poly (curcumin–dithiodipropionic acid) nanoparticles exhibited excellent anticancer activity in vivo due to their high tumor-targeted accumulation and stimuli-triggered intracellular drug release [60]. Moreover, these nanoparticles could be loaded with other anticancer drugs, which could promote synergistic oncologic effects in vivo [60].
In another trial, compared to control PLGA microparticles, curcumin-loaded microparticles retarded oncogenesis in a Balb-neuT transgenic mouse model. PLGA microparticles accelerated oncogenesis compared to a saline control. This unanticipated collateral effect of PLGA microparticles may be related to the high dose of microparticles for optimal in vivo concentration of curcumin [41].
3.3. Conflict of Interest and Ethics Committee Approval
Only six studies were approved by their respective ethics committees on animal use [7,40,57,63,66,68], while there was no mention of potential conflicts of interest in eight studies [60,66,67,68,71,78,79,82]. The authors of the remaining articles declared no conflicts of interest.
3.4. Overall Quality of Evidence
Thirty-nine studies were rated as moderate with respect to quality of evidence using the GRADE approach [25], as shown in (Table S3). These studies were not representative of the results of all assessed outcomes.
The evaluation of the risk of bias based on the SYRCLE RoB Toll guidelines for animal model studies is shown in Table 3. Most studies did not clearly state information on assignment, randomization, and blinding, which are critical aspects for assessing the quality of evidence.
Table 3.
Risk of bias according to the SYRCLE’s RoB Toll criteria for animal models.
| Authors | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Biases | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Abd-Ellatef et al., 2020 [38] |

|

|

|

|

|

|

|

|

|

|
| Alizadeh et al., 2015 [29] |

|

|

|

|

|

|

|

|

|

|
| Bansal et al., 2014 [30] |

|

|

|

|

|

|

|

|

|

|
| Bimonte et al., 2015 [7] |

|

|

|

|

|

|

|

|

|

|
| Chen et al., 2017 [32] |

|

|

|

|

|

|

|

|

|

|
| Ghosh et al., 2021 [39] |

|

|

|

|

|

|

|

|

|

|
| Greish et al., 2018 [40] |

|

|

|

|

|

|

|

|

|

|
| Grill et al., 2018 [41] |

|

|

|

|

|

|

|

|

|

|
| Hashemzehi et al., 2018 [42] |

|

|

|

|

|

|

|

|

|

|
| He et al., 2019 [43] |

|

|

|

|

|

|

|

|

|

|
| Huang et al., 2020 [46] China |

|

|

|

|

|

|

|

|

|

|
| Ji et al., 2020 [47] |

|

|

|

|

|

|

|

|

|

|
| Jin et al., 2017 [49] |

|

|

|

|

|

|

|

|

|

|
| Jung et al., 2018 [50] |

|

|

|

|

|

|

|

|

|

|
| Kumari et al., 2020 [52] |

|

|

|

|

|

|

|

|

|

|
| Laha et al., 2018 [55] |

|

|

|

|

|

|

|

|

|

|
| Lai et al., 2012 [56] |

|

|

|

|

|

|

|

|

|

|
| Li et al., 2018 [3] |

|

|

|

|

|

|

|

|

|

|
| Lin et al., 2016 [57] |

|

|

|

|

|

|

|

|

|

|
| Liu et al., 2013 [58] |

|

|

|

|

|

|

|

|

|

|
| Lv et al., 2014 [61] |

|

|

|

|

|

|

|

|

|

|
| Lv et al., 2015 [60] |

|

|

|

|

|

|

|

|

|

|
| Mahalunkar et al., 2019 [62] |

|

|

|

|

|

|

|

|

|

|
| Masuelli et al., 2013 [63] |

|

|

|

|

|

|

|

|

|

|
| Mukerjee et al., 2016 [66] USA |

|

|

|

|

|

|

|

|

|

|
| Mukhopadhyay et al., 2020 [67] |

|

|

|

|

|

|

|

|

|

|
| Pal et al., 2019 [68] |

|

|

|

|

|

|

|

|

|

|
| Sahne et al., 2019 [69] |

|

|

|

|

|

|

|

|

|

|
| Shiri et al., 2015 [71] |

|

|

|

|

|

|

|

|

|

|
| Shukla et al., 2017 [72] |

|

|

|

|

|

|

|

|

|

|
| Vakilinezhad et al., 2019 [75] |

|

|

|

|

|

|

|

|

|

|
| Wang et al., 2018 [77] |

|

|

|

|

|

|

|

|

|

|
| Yang et al., 2017 a [78] |

|

|

|

|

|

|

|

|

|

|
| Yang et al., 2017 b [79] |

|

|

|

|

|

|

|

|

|

|
| Yu et al., 2014 [82] |

|

|

|

|

|

|

|

|

|

|
| Yu et al., 2021 [81] |

|

|

|

|

|

|

|

|

|

|
| Yuan et al., 2018 [83] |

|

|

|

|

|

|

|

|

|

|
YES  NO
NO  UNCLEAR
UNCLEAR  . YES indicates low risk of bias; NO indicates high risk of bias; UNCLEAR indicates inability of bias assignment. The ten items assessed included: 1 Was the sequence of assignment generated and applied properly? 2 Were the groups similar at baseline, or were they adjusted for confounders in the analysis? 3 Was the allocation to the different groups adequately concealed? 4 Were the animals randomly housed during the experiment? 5 Were caregivers and/or investigators blinded to the intervention each animal received during the experiment? 6 Were the animals randomly selected for the evaluation of results? 7 Was the outcome assessor blinded? 8 Were data of incomplete results handled appropriately? 9 Are study reports exempt from selective result reporting? 10 Was the study apparently free from other problems that could cause a high risk of bias?
. YES indicates low risk of bias; NO indicates high risk of bias; UNCLEAR indicates inability of bias assignment. The ten items assessed included: 1 Was the sequence of assignment generated and applied properly? 2 Were the groups similar at baseline, or were they adjusted for confounders in the analysis? 3 Was the allocation to the different groups adequately concealed? 4 Were the animals randomly housed during the experiment? 5 Were caregivers and/or investigators blinded to the intervention each animal received during the experiment? 6 Were the animals randomly selected for the evaluation of results? 7 Was the outcome assessor blinded? 8 Were data of incomplete results handled appropriately? 9 Are study reports exempt from selective result reporting? 10 Was the study apparently free from other problems that could cause a high risk of bias?
4. Discussion
This systematic review highlighted some of the promising antitumor activities of curcumin reported in in vitro studies, as well as its potential for tumor volume reduction in animal models. At different concentrations, curcumin inhibited cell proliferation, reduced cell viability, and induced apoptosis in several human and animal breast cancer cell subtypes. In vivo data showed that curcumin reduced tumor volume in human and murine mammary cells when administered either orally, via implants, or via intraperitoneal injection, or when delivered via different curcumin nanoparticle formulations.
In vitro studies showed inhibitory activity of curcumin on cell proliferation, induction of cell viability, and apoptosis at different concentrations. The anti-proliferative effect of curcumin was attributed to its regulatory effects on protein kinases, the cell cycle, and transcription factors, including NF-κB [85]. Curcumin significantly inhibited the growth of MDA-MB-231 and MCF-7 human breast cancer cells by inducing apoptosis in a gradual, dose-dependent method, which was related the increase in the Bax/Bcl-2 ratio [34,61].
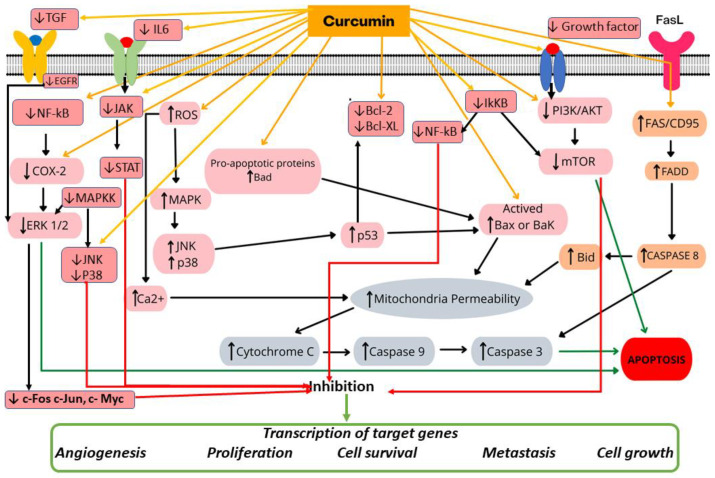
The cell cycle is divided into four phases: G1, S, G2, and M [85]. Dendrosomal curcumin increases the number of cells in the SubG1 phase and reduces the number of cells in the G1, S, and G2/M phases [65]. Early-stage apoptosis showed the inhibition of cell growth through the early phase. Real-time PCR revealed a gradual increase in the mRNA levels of BAX, NOXA, and p21, with a decrease in Bcl-2 expression [65]. The magnitude of anticancer effects and induction of apoptosis are essential for investigating antineoplastic therapy. Apoptosis occurred via intrinsic or mitochondrial pathways [85]. Apoptotic pathways were modulated via NF-κB and Bax [39,67]. Curcumin was also shown to downregulate the expression of cyclin D1, PECAM-1, and p65, which are regulated by NF-κB [7,35]. Figure 2 shows different mechanisms of action of curcumin in breast cancer, including cell proliferation, cell viability, and apoptosis.
Figure 2.
Cellular and molecular mechanisms of action of curcumin in breast cancer. Curcumin exerts its anticancer effect by modulating cell proliferation, inducing apoptosis and inhibiting cancer spread. JAK: janus kinase, STAT: signal transducer and activator of transcription, IL-6: interleukin-6, IκKB: inhibitor of kappa B kinase, TGF: transforming growth factor, EGFR: epidermal growth factor receptor, MAPK: mitogen-activated protein kinase, MAPKK: MAPK kinase, JNK: c-Jun N-terminal kinases, Bcl-2: B-cell lymphoma 2, Bak: Bcl-2 homologous antagonist/killer, Bad: BCL2 associated agonist of cell death, Bid: BH3 interacting-domain death agonist, Bax: Bcl-2 associated X protein, Bcl-xL: ROS: reactive oxygen species, NF-κB: nuclear factor-κ-gene binding, COX-2: Cyclooxygenase 2, ERK1/2: extracellular regulated protein kinase 1 and 2, PI3K: phosphatidylinositol 3-kinase, Akt: protein kinase B, mTOR: mammalian target of rapamycin, JNK: Jun N-terminal kinase, FADD: Fas-associated protein with death domain, p38: mitogen-activated protein kinases, FAZ/CD95: type-II transmembrane protein that belongs to the tumor necrosis fator, Caspases: cysteine-dependent aspartate-specific protease, p53: tumor-suppressor protein, ↓: inhibition, ↑: activation.
The PLGA@CCM@FA nanoparticle formulation triggered apoptosis in human triple-negative breast cancer cells by positively regulating cleaved caspase-3 and downregulating p-AKT expression [68]. Curcumin also induced caspase-mediated apoptosis by activating the expression of polyamine catabolic enzymes, with the subsequent generation of toxic molecules such as H2O2 in MCF-7, MDA-MB-453, and MDA-MB-231 GH+ breast cancer cells [35]. Curcumin-encapsulated polymeric micelles should be considered for breast cancer treatment, as they reduced the proliferation of breast cancer cells [58]. Curcumin-loaded micelles also showed significant tumor-inhibiting properties as well as minimal in vivo collateral effects compared to free-CUR formulations [43]. A study revealed that alginate–chitosan hydrogel loaded with curcumin significantly reduced the viability and induced the apoptosis of malignant cells. Therefore, this system presents promising anticancer drug delivery properties [86].
Conversely, one of the pharmacological limitations of orally administered curcumin is its low bioavailability owing to its low solubility in water and rapid metabolism, which may hinder its clinical application [72,87]. In a randomized clinical study, water-soluble injection formulations of curcumin for parenteral/intravenous administration showed up to 100% bioavailability, demonstrating its potential clinical application [87]. Moreover, a liquid droplet nanomicellar formulation containing Gelucire® and polysorbate 20 (BioCurc®) showed optimal bioavailability, with more than 400-fold greater absorption than non-formulated curcumin [88].
This study highlights different curcumin nanoparticle formulations with optimal bioavailability, causing substantial mammary tumor-reducing effects. Recent advances in micro-and nanoformulations of curcumin with enhanced absorption yield helped improve the serum levels of the active components. These formulations have a wide range of potential applications and properties, including tissue protection [89].
The results discussed in this review support randomized clinical investigations of the antitumor properties of curcumin in patients with breast cancer. Considering the diversity and heterogeneity of breast cancer subtypes, further studies will provide deeper insights into the effects of curcumin on specific types of mammary neoplasms to determine the effects on tumor markers, metastasis, and patient outcomes. Moreover, the efficiency and safety of curcumin in combination with other chemotherapeutic drugs should be established. In future clinical trials, tumor characteristics should be considered to support clinical decision-making. Both human patients and animals showing mammary neoplasms may benefit from curcumin-based therapies in the near future, as indicated by evidence from studies on animal models.
Although eight ongoing clinical assays on the effects of curcumin on breast cancer have been registered on clinicaltrials.gov to date, to the best of our knowledge, only one randomized controlled double-blinded clinical trial has been published [87]. In the said study, 150 women with advanced metastatic breast cancer were randomly assigned to receive paclitaxel chemotherapy (80 mg/m2) plus placebo or paclitaxel with curcumin (CUC-1® solution, 300 mg, administered intravenously once a week) for 12 weeks, with three months of follow-up. The paclitaxel–curcumin combination provided a superior objective response and physical performance after two weeks of treatment. Intravenous curcumin was safe, did not negatively affect the patients’ quality of life, and decreased fatigue [87].
Currently, the data available only pertain to a trial at an advanced stage; therefore, studies focusing on early stages and, in particular, net-adjuvant chemotherapy are lacking. Addressing this knowledge gap remains essential. There are good prospects for the use of curcumin in cancer management, although its clinical development is limited due to its low bioavailability and aqueous solubility [90]; however, efforts have been made to improve the solubility, stability, and bioavailability of curcumin. For example, one strategy employed to obtain curcumin derivatives is chemical modification or synthesis of their analogues. Furthermore, curcumin encapsulated in protein nanoparticles demonstrated improved anticancer activity in MCF-7 cells and increased oral bioavailability in rats [90].
A systematic review [91] indicated that curcumin reduces the side effects of chemotherapy or radiotherapy, thereby improving the quality of life for patients. Furthermore, the authors reported that curcumin increases patient survival and decreases the level of tumour markers through several molecular pathways including hypoxic stress, angiogenesis, adhesion molecules, and extracellular matrix degradation [91].
Another review highlighted curcumin’s ability to interrupt important stages of tumorigenesis, including proliferation, survival, angiogenesis, and metastasis, in hormone-independent breast cancer, via the modulation of multiple signaling paths. The anticancer activity of curcumin in breast cancer was associated with the PI3K/Akt/mTOR, JAK/STAT, MAPK, NF-ĸB, p53, and Wnt/β-catenin pathways, as well as the apoptosis and cell cycle paths [9].
This systematic review provided a thorough overview of evidence from in vitro and animal model studies on the antitumor effects of curcumin in breast cancer. Our investigation was based on analysis of the five most important databases, with no restrictions imposed on the year of publication and language in the inclusion criteria. We included studies conducted in several countries, including China, India, Turkey, Iran, Italy, USA, Taiwan, Egypt, Bahrain, Romania, and Chile, which helped provide a broad perspective of the topic. However, this review had certain limitations. First, a meta-analysis could not be performed because of the high heterogeneity in the presentation of outcome measures, including different dosing and modes of delivery of curcumin, animal models, and methods of follow-up in the different studies. Furthermore, the adverse effects of curcumin formulations are yet to be investigated thoroughly. As the review did not focus on this aspect, we emphasize the importance of further studies investigating the adverse effects, toxicity, safety, tumor markers, and therapeutic responses in experimental trials and studies conducted on human patients. We believe that the results of ongoing clinical assays will provide a deeper understanding of the therapeutic potential of curcumin as an efficient alternative or adjuvant treatment.
5. Conclusions
This systematic review highlighted the beneficial effects of curcumin against human and animal breast cancer cells with respect to the inhibition of cell proliferation, reduction of malignant cell viability, and induction of apoptosis, and discussed the efficacy of curcumin in tumor growth reduction in experimental breast cancer models. These results were obtained from studies based on the delivery of curcumin via oral administration, implantation, intraperitoneal injection, and nanoparticle formulations. The information presented herein supports randomized clinical trials on the adjuvant properties of curcumin in the treatment of breast mammary neoplasms.
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de Goiás/ Fundação de Apoio à Pesquisa/ Universidade Federal de Goiás (FAPEG/ FUNAPE/UFG), Universidade de Rio Verde (UniRV) and Instituto Federal Goiano (IFG) for partially supporting this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14092165/s1, Table S1: Search strategies for use in the databases. Table S2: Articles excluded and reasons for exclusion. Table S3: Quality of evidence in the preclinical studies.
Author Contributions
Conceptualization: K.A.B., C.R.M., M.N., A.F.B., C.R.D.F. and M.A.M.S.; Methodology: K.A.B., C.R.M., M.N., A.F.B., C.R.D.F. and M.A.M.S.; Formal Analysis: K.A.B. and C.R.M.; Investigation: K.A.B., C.R.M., M.N., A.F.B., C.R.D.F. and M.A.M.S.; Resources: K.A.B., C.R.M., M.N., A.F.B., C.R.D.F. and M.A.M.S.; Data Curation: K.A.B.; and M.A.M.S.; Writing—Original Draft Preparation: K.A.B., C.R.M. and M.A.M.S.; Writing—Review and Editing: K.A.B., C.R.M., M.N., A.F.B., C.R.D.F. and M.A.M.S.; Visualization: K.A.B., C.R.M., M.N., A.F.B., C.R.D.F. and M.A.M.S.; Supervision: A.F.B. and M.A.M.S.; Project Administration: K.A.B.; Funding Acquisition: M.N., A.F.B. and M.A.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Momenimovahed Z., Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med. Press) 2019;11:151–164. doi: 10.2147/BCTT.S176070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Li N., Wang Z., Zhang Y., Zhang K., Xie J., Liu Y., Li W., Feng N. Curcumin-loaded redox-responsive mesoporous silica nanoparticles for targeted breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018;46:921–935. doi: 10.1080/21691401.2018.1473412. [DOI] [PubMed] [Google Scholar]
- 4.Inotai A., Ágh T., Maris R., Erdősi D., Kovács S., Kaló Z., Senkus E. Systematic review of real-world studies evaluating the impact of medication non-adherence to endocrine therapies on hard clinical endpoints in patients with non-metastatic breast cancer. Cancer Treat. Rev. 2021;100:102264. doi: 10.1016/j.ctrv.2021.102264. [DOI] [PubMed] [Google Scholar]
- 5.Terret C., Russo C. Pharmacotherapeutic Management of Breast Cancer in Elderly Patients: The Promise of Novel Agents. Drugs Aging. 2018;35:93–115. doi: 10.1007/s40266-018-0519-5. [DOI] [PubMed] [Google Scholar]
- 6.Sen G.S., Mohanty S., Hossain D.M.S., Bhattacharyya S., Banerjee S., Chakraborty J., Saha S., Ray P., Bhattacharjee P., Mandal D., et al. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of p53–p300 in breast cancer. J. Biol. Chem. 2011;286:42232–42247. doi: 10.1074/jbc.M111.262295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bimonte S., Barbieri A., Palma G., Rea D., Luciano A., D’Aiuto M., Arra C., Izzo F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. BioMed Res. Int. 2015;2015:878134. doi: 10.1155/2015/878134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witika B.A., Makoni P.A., Matafwali S.K., Mweetwa L.L., Shandele G.C., Walker R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules. 2021;26:4244. doi: 10.3390/molecules26144244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farghadani R., Naidu R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers. 2021;13:3427. doi: 10.3390/cancers13143427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farghadani R., Naidu R. Curcumin as an Enhancer of Therapeutic Efficiency of Chemotherapy Drugs in Breast Cancer. Int. J. Mol. Sci. 2022;23:2144. doi: 10.3390/ijms23042144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha D., Biswas J., Sung B., Aggarwal B.B., Bishayee A. Chemopreventive and chemotherapeutic potential of curcumin in breast cancer. Curr. Drug Targets. 2012;13:1799–1819. doi: 10.2174/138945012804545632. [DOI] [PubMed] [Google Scholar]
- 12.Song X., Zhang M., Dai E., Luo Y. Molecular targets of curcumin in breast cancer (Review) Mol. Med. Rep. 2019;19:23–29. doi: 10.3892/mmr.2018.9665. [DOI] [PubMed] [Google Scholar]
- 13.Ombredane A.S., Silva V.R.P., Andrade L.R., Pinheiro W.O., Simonelly M., Oliveira J.V., Pinheiro A.C., Gonçalves G.F., Felice G.J., Garcia M.P., et al. In Vivo Efficacy and Toxicity of Curcumin Nanoparticles in Breast Cancer Treatment: A Systematic Review. Front. Oncol. 2021;11:612903. doi: 10.3389/fonc.2021.612903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianfredi V., Nucci D., Vannini S., Villarini M., Moretti M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer. 2017;69:969–978. doi: 10.1080/01635581.2017.1359322. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Vries R.B.M., Hooijmans C.R., Langendam M.W., van Luijk J., Leenaars M., Ritskes-Hoitinga M., Wever K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evid. Based Preclin. Med. 2015;2:e00007. doi: 10.1002/ebm2.7. [DOI] [Google Scholar]
- 17.Yang N., Ray S.D., Krafts K. Cell Proliferation. In: Wexler P., editor. Encyclopedia of Toxicology. 3rd ed. Academic Press; Oxford, UK: 2014. [Google Scholar]
- 18.Peng Y., Li J., Zhu L. Chapter 8—Cancer and non-coding RNAs. In: Ferguson B.S., editor. Nutritional Epigenomics. Volume 14. Academic Press; Oxford, UK: 2019. pp. 119–132. [Google Scholar]
- 19.Fang I.J., Trewyn B.G. Application of mesoporous silica nanoparticles in intracellular delivery of molecules and proteins. Methods Enzymol. 2012;508:41–59. doi: 10.1016/B978-0-12-391860-4.00003-3. [DOI] [PubMed] [Google Scholar]
- 20.Khan Y. Characterizing the Properties of Tissue Constructs for Regenerative Engineering. Elsevier; Amsterdam, The Netherlands: 2019. [DOI] [Google Scholar]
- 21.Brown D.A., Yang N., Ray S.D. Apoptosis. In: Wexler P., editor. Encyclopedia of Toxicology. 3rd ed. Academic Press; Oxford, UK: 2014. [Google Scholar]
- 22.Wong R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noll M., Wedderkopp N., Mendonça C.R., Kjaer P. Motor performance and back pain in children and adolescents: A systematic review and meta-analysis protocol. Syst. Rev. 2020;9:212. doi: 10.1186/s13643-020-01468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noll M., Kjaer P., Mendonça C.R., Wedderkopp N. Motor performance and back pain in children and adolescents: A systematic review. Eur. J. Pain. 2022;26:77–102. doi: 10.1002/ejp.1850. [DOI] [PubMed] [Google Scholar]
- 25.Pavan L.M.C., Rêgo D.F., Elias S.T., De Luca Canto G., Guerra E.N.S. In vitro Anti-Tumor Effects of Statins on Head and Neck Squamous Cell Carcinoma: A Systematic Review. PLoS ONE. 2015;10:e0130476. doi: 10.1371/journal.pone.0130476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei D., Tang K., Wang Q., Estill J., Yao L., Wang X., Chen Y., Yang K. The use of GRADE approach in systematic reviews of animal studies. J. Evid.-Based Med. 2016;9:98–104. doi: 10.1111/jebm.12198. [DOI] [PubMed] [Google Scholar]
- 27.Abbaspour H., Safipour Afshar A. Curcumin inhibits the expression of ornithine decarboxylase and adenosine deaminase genes in MCF-7 human breast cancer cells. Arch. Biol. Sci. 2018;70:639–645. doi: 10.2298/ABS180209025A. [DOI] [Google Scholar]
- 28.Abuelba H., Cotrutz C.E., Stoica B.A., Stoica L., Olinici D., Petreuş T. In vitro evaluation of curcumin effects on breast adenocarcinoma 2D and 3D cell cultures. Rom. J. Morphol. Embryol. = Rev. Roum. De Morphol. Et Embryol. 2015;56:71–76. [PubMed] [Google Scholar]
- 29.Alizadeh A.M., Sadeghizadeh M., Najafi F., Ardestani S.K., Erfani-Moghadam V., Khaniki M., Rezaei A., Zamani M., Khodayari S., Khodayari H., et al. Encapsulation of Curcumin in Diblock Copolymer Micelles for Cancer Therapy. BioMed Res. Int. 2015;2015:824746. doi: 10.1155/2015/824746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bansal S.S., Kausar H., Vadhanam M.V., Ravoori S., Pan J., Rai S.N., Gupta R.C. Curcumin implants, not curcumin diet, inhibit estrogen-induced mammary carcinogenesis in ACI rats. Cancer Prev. Res. 2014;7:456–465. doi: 10.1158/1940-6207.CAPR-13-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calaf G.M., Ponce-Cusi R., Carrión F. Curcumin and paclitaxel induce cell death in breast cancer cell lines. Oncol. Rep. 2018;40:2381–2388. doi: 10.3892/or.2018.6603. [DOI] [PubMed] [Google Scholar]
- 32.Chen W., Li L., Zhang X., Liang Y.-k., Pu Z., Wang L., Mo J.J.D.D. Curcumin: A calixarene derivative micelle potentiates anti-breast cancer stem cells effects in xenografted, triple-negative breast cancer mouse models. Drug Deliv. 2017;24:1470–1481. doi: 10.1080/10717544.2017.1381198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu T.L., Su C.C. Curcumin inhibits proliferation and migration by increasing the Bax to Bcl-2 ratio and decreasing NF-kappaBp65 expression in breast cancer MDA-MB-231 cells. Int. J. Mol. Med. 2009;23:469–475. doi: 10.3892/ijmm_00000153. [DOI] [PubMed] [Google Scholar]
- 34.Choudhuri T., Pal S., Agwarwal M.L., Das T., Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002;512:334–340. doi: 10.1016/S0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 35.Coker-Gurkan A., Bulut D., Genc R., Arisan E.D., Obakan-Yerlikaya P., Palavan-Unsal N. Curcumin prevented human autocrine growth hormone (GH) signaling mediated NF-κB activation and miR-183-96-182 cluster stimulated epithelial mesenchymal transition in T47D breast cancer cells. Mol. Biol. Rep. 2019;46:355–369. doi: 10.1007/s11033-018-4479-y. [DOI] [PubMed] [Google Scholar]
- 36.Coker-Gurkan A., Celik M., Ugur M., Arisan E.D., Obakan-Yerlikaya P., Durdu Z.B., Palavan-Unsal N. Curcumin inhibits autocrine growth hormone-mediated invasion and metastasis by targeting NF-κB signaling and polyamine metabolism in breast cancer cells. Amino Acids. 2018;50:1045–1069. doi: 10.1007/s00726-018-2581-z. [DOI] [PubMed] [Google Scholar]
- 37.Fan H., Liang Y., Jiang B., Li X., Xun H., Sun J., He W., Lau H.T., Ma X. Curcumin inhibits intracellular fatty acid synthase and induces apoptosis in human breast cancer MDA-MB-231 cells. Oncol. Rep. 2016;35:2651–2656. doi: 10.3892/or.2016.4682. [DOI] [PubMed] [Google Scholar]
- 38.Fathy Abd-Ellatef G.E., Gazzano E., Chirio D., Hamed A.R., Belisario D.C., Zuddas C., Peira E., Rolando B., Kopecka J., Assem Said Marie M., et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics. 2020;12:96. doi: 10.3390/pharmaceutics12020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh S., Dutta S., Sarkar A., Kundu M., Sil P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B Biointerfaces. 2021;197:111404. doi: 10.1016/j.colsurfb.2020.111404. [DOI] [PubMed] [Google Scholar]
- 40.Greish K., Pittalà V., Taurin S., Taha S., Bahman F., Mathur A., Jasim A., Mohammed F., El-Deeb I.M., Fredericks S., et al. Curcumin-Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer. Nanomaterials. 2018;8:884. doi: 10.3390/nano8110884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grill A.E., Shahani K., Koniar B., Panyam J. Chemopreventive efficacy of curcumin-loaded PLGA microparticles in a transgenic mouse model of HER-2-positive breast cancer. Drug Deliv. Transl. Res. 2018;8:329–341. doi: 10.1007/s13346-017-0377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashemzehi M., Behnam-Rassouli R., Hassanian S.M., Moradi-Binabaj M., Moradi-Marjaneh R., Rahmani F., Fiuji H., Jamili M., Mirahmadi M., Boromand N., et al. Phytosomal-curcumin antagonizes cell growth and migration, induced by thrombin through AMP-Kinase in breast cancer. J. Cell Biochem. 2018;119:5996–6007. doi: 10.1002/jcb.26796. [DOI] [PubMed] [Google Scholar]
- 43.He H., Zhuang W., Ma B., Su X., Yu T., Hu J., Chen L., Peng R., Li G., Wang Y. Oxidation-Responsive and Aggregation-Induced Emission Polymeric Micelles with Two-Photon Excitation for Cancer Therapy and Bioimaging. ACS Biomater. Sci. Eng. 2019;5:2577–2586. doi: 10.1021/acsbiomaterials.9b00212. [DOI] [PubMed] [Google Scholar]
- 44.Hu S., Xu Y., Meng L., Huang L., Sun H. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp. Ther. Med. 2018;16:1266–1272. doi: 10.3892/etm.2018.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hua W.F., Fu Y.S., Liao Y.J., Xia W.J., Chen Y.C., Zeng Y.X., Kung H.F., Xie D. Curcumin induces down-regulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells. Eur. J. Pharmacol. 2010;637:16–21. doi: 10.1016/j.ejphar.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 46.Huang C., Chen F., Zhang L., Yang Y., Yang X., Pan W. (99 m)Tc Radiolabeled HA/TPGS-Based Curcumin-Loaded Nanoparticle for Breast Cancer Synergistic Theranostics: Design, in vitro and in vivo Evaluation. Int. J. Nanomed. 2020;15:2987–2998. doi: 10.2147/IJN.S242490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ji P., Wang L., Chen Y., Wang S., Wu Z., Qi X. Hyaluronic acid hydrophilic surface rehabilitating curcumin nanocrystals for targeted breast cancer treatment with prolonged biodistribution. Biomater. Sci. 2020;8:462–472. doi: 10.1039/C9BM01605H. [DOI] [PubMed] [Google Scholar]
- 48.Jiang M., Huang O., Zhang X., Xie Z., Shen A., Liu H., Geng M., Shen K. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules. 2013;18:701–720. doi: 10.3390/molecules18010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin H., Pi J., Zhao Y., Jiang J., Li T., Zeng X., Yang P., Evans C.E., Cai J. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale. 2017;9:16365–16374. doi: 10.1039/C7NR06898K. [DOI] [PubMed] [Google Scholar]
- 50.Jung K.H., Lee J.H., Park J.W., Kim D.H., Moon S.H., Cho Y.S., Lee K.H. Targeted therapy of triple negative MDA-MB-468 breast cancer with curcumin delivered by epidermal growth factor-conjugated phospholipid nanoparticles. Oncol. Lett. 2018;15:9093–9100. doi: 10.3892/ol.2018.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J.M., Noh E.M., Kwon K.B., Kim J.S., You Y.O., Hwang J.K., Hwang B.M., Kim B.S., Lee S.H., Lee S.J., et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKCα-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine. 2012;19:1085–1092. doi: 10.1016/j.phymed.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Kumari P., Muddineti O.S., Rompicharla S.V., Ghanta P., Karthik B.B.N.A., Ghosh B., Biswas S. Cholesterol-conjugated poly (D, L-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017;24:209–223. doi: 10.1080/10717544.2016.1245365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumari P., Paul M., Bobde Y., Soniya K., Kiran Rompicharla S.V., Ghosh B., Biswas S. Albumin-based lipoprotein nanoparticles for improved delivery and anticancer activity of curcumin for cancer treatment. Nanomedicine. 2020;15:2851–2869. doi: 10.2217/nnm-2020-0232. [DOI] [PubMed] [Google Scholar]
- 54.Kumari P., Swami M.O., Nadipalli S.K., Myneni S., Ghosh B., Biswas S. Curcumin Delivery by Poly(Lactide)-Based Co-Polymeric Micelles: An In Vitro Anticancer Study. Pharm. Res. 2016;33:826–841. doi: 10.1007/s11095-015-1830-z. [DOI] [PubMed] [Google Scholar]
- 55.Laha D., Pal K., Ray Chowdhuri A., Parida P., Sahu S., Jana K., Karmakar P. Fabrication of curcumin loaded folic acid tagged metal organic framework for triple negative breast cancer therapy in in vitro and in vivo system. New J. Chem. 2018;43:217–229. doi: 10.1039/C8NJ03350A. [DOI] [Google Scholar]
- 56.Lai H.-W., Chien S.-Y., Kuo S.-J., Tseng L.-M., Lin H.-Y., Chi C.-W., Chen D.-R. The Potential Utility of Curcumin in the Treatment of HER-2-Overexpressed Breast Cancer: An In Vitro and In Vivo Comparison Study with Herceptin. Evid. Based Complement. Altern. Med. 2012;2012:486568. doi: 10.1155/2012/486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin M., Teng L., Wang Y., Zhang J., Sun X. Curcumin-guided nanotherapy: A lipid-based nanomedicine for targeted drug delivery in breast cancer therapy. Drug Deliv. 2016;23:1420–1425. doi: 10.3109/10717544.2015.1066902. [DOI] [PubMed] [Google Scholar]
- 58.Liu L., Sun L., Wu Q., Guo W., Li L., Chen Y., Li Y., Gong C., Qian Z., Wei Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013;443:175–182. doi: 10.1016/j.ijpharm.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 59.Liu Q., Loo W.T., Sze S.C., Tong Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine. 2009;16:916–922. doi: 10.1016/j.phymed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Lv L., Guo Y., Shen Y., Liu J., Zhang W., Zhou D., Guo S. Intracellularly Degradable, Self-Assembled Amphiphilic Block Copolycurcumin Nanoparticles for Efficient In Vivo Cancer Chemotherapy. Adv. Healthc. Mater. 2015;4:1496–1501. doi: 10.1002/adhm.201500075. [DOI] [PubMed] [Google Scholar]
- 61.Lv Z.D., Liu X.P., Zhao W.J., Dong Q., Li F.N., Wang H.B., Kong B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014;7:2818–2824. [PMC free article] [PubMed] [Google Scholar]
- 62.Mahalunkar S., Yadav A.S., Gorain M., Pawar V., Braathen R., Weiss S., Bogen B., Gosavi S.W., Kundu G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019;14:8285–8302. doi: 10.2147/IJN.S215142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masuelli L., Benvenuto M., Fantini M., Marzocchella L., Sacchetti P., Di Stefano E., Tresoldi I., Izzi V., Bernardini R., Palumbo C., et al. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J. Biol. Regul. Homeost. Agents. 2013;27:105–119. [PubMed] [Google Scholar]
- 64.Mehta K., Pantazis P., McQueen T., Aggarwal B.B. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anti-Cancer Drugs. 1997;8:470–481. doi: 10.1097/00001813-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 65.Montazeri M., Pilehvar-Soltanahmadi Y., Mohaghegh M., Panahi A., Khodi S., Zarghami N., Sadeghizadeh M. Antiproliferative and Apoptotic Effect of Dendrosomal Curcumin Nanoformulation in P53 Mutant and Wide-Type Cancer Cell Lines. Anticancer Agents Med. Chem. 2017;17:662–673. doi: 10.2174/1871520616666160815124537. [DOI] [PubMed] [Google Scholar]
- 66.Mukerjee A., Ranjan A.P., Vishwanatha J.K. Targeted Nanocurcumin Therapy Using Annexin A2 Anitbody Improves Tumor Accumulation and Therapeutic Efficacy Against Highly Metastatic Breast Cancer. J. Biomed. Nanotechnol. 2016;12:1374–1392. doi: 10.1166/jbn.2016.2240. [DOI] [PubMed] [Google Scholar]
- 67.Mukhopadhyay R., Sen R., Paul B., Kazi J., Ganguly S., Debnath M.C. Gemcitabine Co-Encapsulated with Curcumin in Folate Decorated PLGA Nanoparticles; a Novel Approach to Treat Breast Adenocarcinoma. Pharm. Res. 2020;37:56. doi: 10.1007/s11095-020-2758-5. [DOI] [PubMed] [Google Scholar]
- 68.Pal K., Laha D., Parida P.K., Roy S., Bardhan S., Dutta A., Jana K., Karmakar P. An In Vivo Study for Targeted Delivery of Curcumin in Human Triple Negative Breast Carcinoma Cells Using Biocompatible PLGA Microspheres Conjugated with Folic Acid. J. Nanosci. Nanotechnol. 2019;19:3720–3733. doi: 10.1166/jnn.2019.16292. [DOI] [PubMed] [Google Scholar]
- 69.Sahne F., Mohammadi M., Najafpour G.D. Single-Layer Assembly of Multifunctional Carboxymethylcellulose on Graphene Oxide Nanoparticles for Improving in Vivo Curcumin Delivery into Tumor Cells. ACS Biomater. Sci. Eng. 2019;5:2595–2609. doi: 10.1021/acsbiomaterials.8b01628. [DOI] [PubMed] [Google Scholar]
- 70.Sarighieh M.A., Montazeri V., Shadboorestan A., Ghahremani M.H., Ostad S.N. The Inhibitory Effect of Curcumin on Hypoxia Inducer Factors (Hifs) as a Regulatory Factor in the Growth of Tumor Cells in Breast Cancer Stem-Like Cells. Drug Res. 2020;70:512–518. doi: 10.1055/a-1201-2602. [DOI] [PubMed] [Google Scholar]
- 71.Shiri S., Alizadeh A.M., Baradaran B., Farhanghi B., Shanehbandi D., Khodayari S., Khodayari H., Tavassoli A. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac. J. Cancer Prev. 2015;16:3917–3922. doi: 10.7314/APJCP.2015.16.9.3917. [DOI] [PubMed] [Google Scholar]
- 72.Shukla M., Jaiswal S., Sharma A., Srivastava P.K., Arya A., Dwivedi A.K., Lal J. A combination of complexation and self-nanoemulsifying drug delivery system for enhancing oral bioavailability and anticancer efficacy of curcumin. Drug Dev. Ind. Pharm. 2017;43:847–861. doi: 10.1080/03639045.2016.1239732. [DOI] [PubMed] [Google Scholar]
- 73.Sun S.H., Huang H.C., Huang C., Lin J.K. Cycle arrest and apoptosis in MDA-MB-231/Her2 cells induced by curcumin. Eur. J. Pharmacol. 2012;690:22–30. doi: 10.1016/j.ejphar.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 74.Sun X.D., Liu X.E., Huang D.S. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol. Med. Rep. 2012;6:1267–1270. doi: 10.3892/mmr.2012.1103. [DOI] [PubMed] [Google Scholar]
- 75.Vakilinezhad M.A., Amini A., Dara T., Alipour S. Methotrexate and Curcumin co-encapsulated PLGA nanoparticles as a potential breast cancer therapeutic system: In vitro and in vivo evaluation. Colloids Surfaces. B Biointerfaces. 2019;184:110515. doi: 10.1016/j.colsurfb.2019.110515. [DOI] [PubMed] [Google Scholar]
- 76.Wang X., Hang Y., Liu J., Hou Y., Wang N., Wang M. Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncol. Lett. 2017;13:4825–4831. doi: 10.3892/ol.2017.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y., Luo Z., Wang Z., You M., Xie S., Peng Y., Yang H. Effect of curcumin-loaded nanoparticles on mitochondrial dysfunctions of breast cancer cells. J. Nanopart. Res. 2018;20:1–11. doi: 10.1007/s11051-018-4382-4. [DOI] [Google Scholar]
- 78.Yang Z., Sun N., Cheng R., Zhao C., Liu J., Tian Z. Hybrid nanoparticles coated with hyaluronic acid lipoid for targeted co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. J. Mater. Chem. B. 2017;5:6762–6775. doi: 10.1039/C7TB01510K. [DOI] [PubMed] [Google Scholar]
- 79.Yang Z., Sun N., Cheng R., Zhao C., Liu Z., Li X., Liu J., Tian Z. pH multistage responsive micellar system with charge-switch and PEG layer detachment for co-delivery of paclitaxel and curcumin to synergistically eliminate breast cancer stem cells. Biomaterials. 2017;147:53–67. doi: 10.1016/j.biomaterials.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 80.Younesian O., Kazerouni F., Dehghan-Nayeri N., Omrani D., Rahimipour A., Shanaki M., Rezapour Kalkhoran M., Cheshmi F. Effect of Curcumin on Fatty Acid Synthase Expression and Enzyme Activity in Breast Cancer Cell Line SKBR3. Int. J. Cancer Manag. 2017;10:e8173. doi: 10.5812/ijcm.8173. [DOI] [Google Scholar]
- 81.Yu S., Wang S., Xie Z., Yu S., Li L., Xiao H., Song Y. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: An in vitro and in vivo study. Colloids Surf. B Biointerfaces. 2021;203:111759. doi: 10.1016/j.colsurfb.2021.111759. [DOI] [PubMed] [Google Scholar]
- 82.Yu Y., Zhang X., Qiu L. The anti-tumor efficacy of curcumin when delivered by size/charge-changing multistage polymeric micelles based on amphiphilic poly(β-amino ester) derivates. Biomaterials. 2014;35:3467–3479. doi: 10.1016/j.biomaterials.2013.12.096. [DOI] [PubMed] [Google Scholar]
- 83.Yuan J.D., ZhuGe D.L., Tong M.Q., Lin M.T., Xu X.F., Tang X., Zhao Y.Z., Xu H.L. pH-sensitive polymeric nanoparticles of mPEG-PLGA-PGlu with hybrid core for simultaneous encapsulation of curcumin and doxorubicin to kill the heterogeneous tumour cells in breast cancer. Artif. Cells Nanomed. 2018;46:302–313. doi: 10.1080/21691401.2017.1423495. [DOI] [PubMed] [Google Scholar]
- 84.Zong H., Wang F., Fan Q.X., Wang L.X. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol. Biol. Rep. 2012;39:4803–4808. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- 85.Liu H.-T., Ho Y.-S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness. 2018;7:134–137. doi: 10.1016/j.fshw.2018.06.001. [DOI] [Google Scholar]
- 86.Abbasalizadeh F., Alizadeh E., Bagher Fazljou S.M., Torbati M., Akbarzadeh A. Anticancer Effect of Alginate-Chitosan Hydrogel Loaded with Curcumin and Chrysin on Lung and Breast Cancer Cell Lines. Curr. Drug Deliv. 2021 doi: 10.2174/1567201818666210813142007. [DOI] [PubMed] [Google Scholar]
- 87.Saghatelyan T., Tananyan A., Janoyan N., Tadevosyan A., Petrosyan H., Hovhannisyan A., Hayrapetyan L., Arustamyan M., Arnhold J., Rotmann A.R., et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2020;70:153218. doi: 10.1016/j.phymed.2020.153218. [DOI] [PubMed] [Google Scholar]
- 88.Stohs S.J., Ji J., Bucci L.R., Preuss H.G. A Comparative Pharmacokinetic Assessment of a Novel Highly Bioavailable Curcumin Formulation with 95% Curcumin: A Randomized, Double-Blind, Crossover Study. J. Am. Coll. Nutr. 2018;37:51–598. doi: 10.1080/07315724.2017.1358118. [DOI] [PubMed] [Google Scholar]
- 89.Stohs S.J., Chen O., Ray S.D., Ji J., Bucci L.R., Preuss H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules. 2020;25:1397. doi: 10.3390/molecules25061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giordano A., Tommonaro G. Curcumin and Cancer. Nutrients. 2019;5:2376. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mansouri K., Rasoulpoor S., Daneshkhah A., Abolfathi S., Salari N., Mohammadi M., Rasoulpoor S., Shabani S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer. 2020;24:791. doi: 10.1186/s12885-020-07256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.