Abstract
Familial hypocalciuric hypercalcemia (FHH) is a mostly benign condition of elevated calcium and PTH levels based on a hyposensitive calcium sensing receptor (CaSR) in FHH 1 or its downstream regulatory pathway in FHH2 and FHH3. In children, adolescents and young adults with FHH the main challenge is to distinguish the condition from primary hyperparathyroidism and thereby to avoid unnecessary treatments including parathyroidectomy. However, inheritance of FHH may result in neonatal hyperparathyroidism (NHPT) or neonatal severe hyperparathyroidism (NSHPT), conditions with high morbidity, and in the latter even high mortality. This review focuses on the genetic and pathophysiological framework that leads to the severe neonatal form, gives recommendations for counselling and summarizes treatment options.
Keywords: familial hypocalciuric hypercalcemia (FHH), calcium sensing receptor (CaSR), neonatal hyperparathyroidism (NHPT), neonatal severe hyperparathyroidism (NSHPT), cinacalcet
1. Introduction
Loss-of-function (LOF) mutations in the calcium-sensing pathway may cause various calcium-hyposensitivity-diseases which are characterized by elevated serum calcium levels. Pathophysiologically, this is based on a shift of the ‘set point’ of the calcium concentration at which PTH secretion is half-maximal [1]. The resulting disorders share a constellation of inappropriately high PTH concentrations despite elevated serum calcium levels and are categorized into three groups based on genetic origin, time of onset and severity of clinical symptoms, namely familial hypocalciuric hypercalcemia (FHH), neonatal severe primary hyperparathyroidism (NSHPT), and neonatal hyperparathyroidism (NHPT) [2,3,4].
FHH comprises a genetically heterogeneous group [5]: FHH1 [OMIM #145980] is usually caused by heterozygous inactivating mutations in the calcium sensing receptor gene (CaSR) and accounts for about 65% of all FHH cases [3]. Mutations in other genes encoding for proteins with a role in calcium signaling (GNA11 and AP2S1) cause FHH2 and 3 [6,7,8].
The aims of this review are to present the pathophysiology of calcium-sensing receptor signaling disorders with a focus on intra-uterine calcium homeostasis and the differing clinical outcomes in neonates.
2. Epidemiology
FHH1 is considered a rare disease. However, the prevalence of FHH is difficult to estimate since many of those affected remain asymptomatic and remain undetected. In 2002, Hinnie et al. estimated a minimal prevalence for FHH of 1 in 78,000 (1.3 cases in 100,000) [9].
However, in a recent report, Dershem et al., presented results from whole exome analyses in 51,289 probands of the DiscovEHR cohort in northern USA and detected a prevalence of CaSR mutations (giving rise to FHH1) of 74.1 per 100,000. The authors state that these results indicate a prevalence of FHH comparable to that of primary hyperparathyroidism (PHPT). If these findings can be confirmed, FHH cannot be considered a rare disease anymore [10].
In Germany, with an estimated livebirths of 780,000 per year, this prevalence would give rise to 580 neonates with an inherited form of FHH. Of these, about 50% will be affected by paternal inheritance and in addition, sporadic cases will occur. However, given the fact that NHPT is reported so rarely in the literature, these calculations are difficult to perform. [11].
3. Pathophysiology
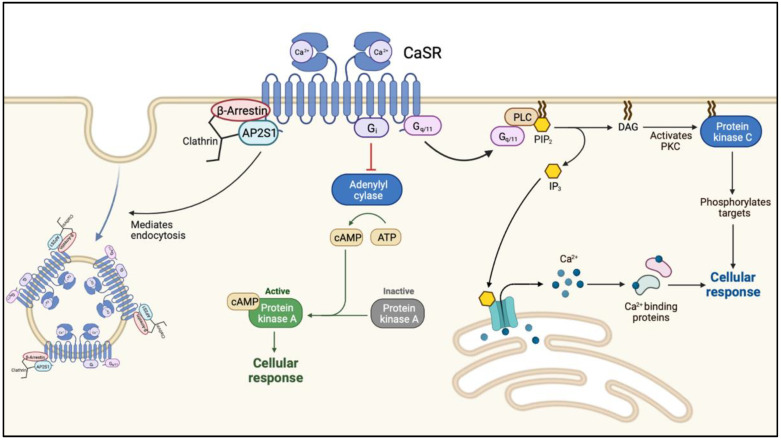
FHH1 (OMIM: 145980) is caused loss-of-function (LOF) mutations in CaSR on chromosome 3q21.1. This is the most common form of FHH with more than 300 mutations in CaSR reported to date. The calcium-sensing receptor (CaSR) is a G protein-coupled transmembrane receptor. When activated, the receptor couples to heterotrimeric G proteins (Ga11) leading to an increased phospholipase C activity with a subsequent elevation of inositol 1,4,5-trisphosphate (IP3) and a rise in cytoplasmic ionized calcium [12,13] (Figure 1).
Figure 1.
The intracellular cascade following ligand (calcium-) binding to the calcium-sensing receptor. Abbreviations: Ca2+, calcium; CaSR, calcium sensing receptor; AP2S1, adaptor-related protein complex 2 subunit 2; Gi, inhibitory G-protein; cAMP, cyclic adenosine monophosphate; PLC, phospholipase C; PIP3, phosphatidylinositol-3,4,5-triphosphate; DAG, diacylglycerol; PKC, protein kinase C.
The level of plasma membrane expression is regulated by the balance of agonist-driven insertional signaling (ADIS) and the activity of adaptor-related protein complex 2 (AP2). ADIS provide trafficking of CaSR from the intracellular reservoir to the plasma membrane after sustained receptor activation [14]. AP2 together with β-arrestin represent the key regulators of CaSR internalization and facilitate clathrin-mediated endocytosis (Figure 1) [15].
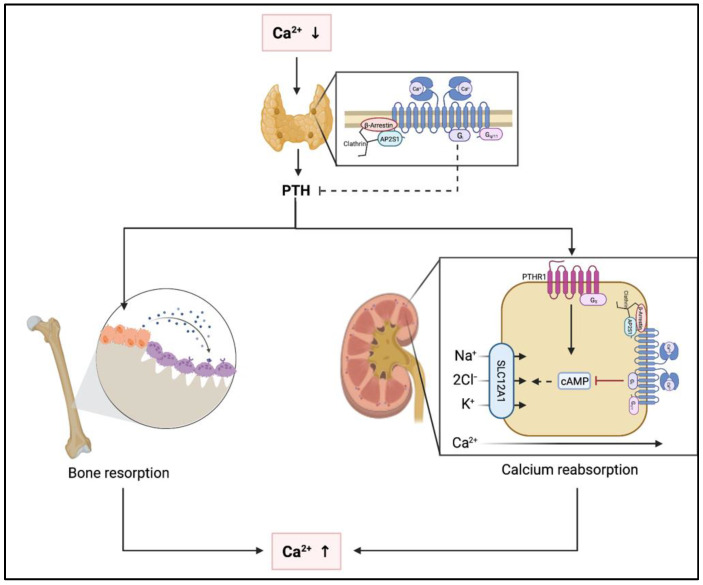
Under physiological conditions, several organ systems are affected by activation of the CaSR due to hypercalcemia (Figure 2). In the parathyroid glands, activation of the CaSR results in suppression of PTH secretion [16,17]. In the kidneys, the CaSR is expressed throughout most of the nephron and stimulation of CaSR by Ca2+ leads to reduced urinary concentration capacity [18,19,20]. In addition, CaSR is expressed in both osteoblasts and osteoclasts. Stimulation of the CaSR by hypercalcemia leads to inhibition of osteoclastic resorption and increased osteoblastic activity [21,22,23]. However, these effects do not result in increased bone mass as indirectly concluded by the lack of BMD changes with calcium only supplementation or calcimimetic administration or in prolonged hypercalcemic states. Relevance of CaSR expression in other skeletal cells such as osteocytes or chondrocytes remains to be determined [24].
Figure 2.
Pathophysiological effects of hypo- and hypercalcemia at the parathyroid glands, the kidneys and the skeleton. Abbreviations: Ca2+, calcium; PTH, parathyroid hormone; PTHR1, parathyroid hormone receptor 1; cAMP, cyclic adenosine monophosphate; SLC1A1, solute carrier 1A1; Na+, sodium; Cl−, chloride; K+, potassium.
FHH2 (OMIM: 145981) is caused by inactivating mutations of the gene GNA11 located on chromosome 19p, encoding the Gα11 protein [25,26]. LOF mutations in GNA11 lead to disruption of the G-protein activation that impairs CaSR signal transduction [27]. FHH3 (OMIM: 600740) is caused by mutations of the gene AP2S1 located on chromosome 19q13.3. AP2S1 encodes the sigma subunit of the adaptor-related protein-2 (AP2σ), which is involved in clathrin-mediated CaSR endocytosis. The mutant AP2σ leads to enhanced CaSR cell-membrane expression but also impaired CaSR signaling [6,28].
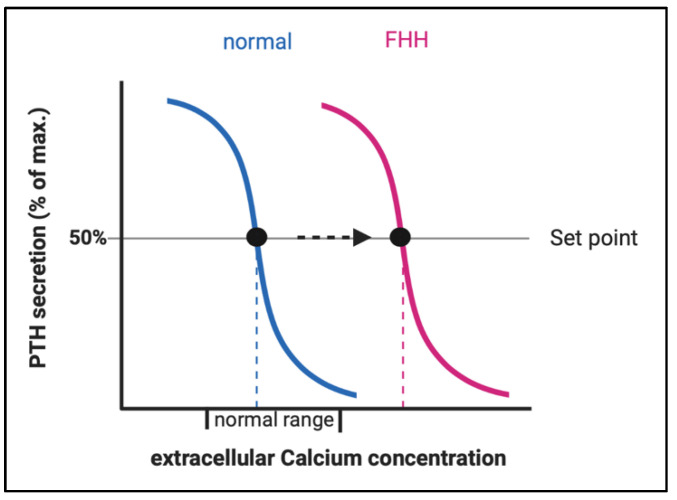
LOF of CaSR (in FHH1) or its downstream partner signaling proteins (in FHH2 and FHH3) result in an elevation of the body’s calcium set point, defined as the serum calcium concentration at which PTH secretion is half-maximal. Patients with FHH display higher levels of plasma PTH and it takes a higher level of plasma calcium to suppress PTH secretion (Figure 3). The elevated PTH stimulates bone resorption resulting in elevated serum calcium levels. The resulting hypercalcemia would normally inhibit osteoclast activity, however, due to the LOF in the CaSR-pathway in osteoclasts, this mechanism does not work properly.
Figure 3.
Change of the set point of the calcium concentration for half maximal PTH secretion in FHH. Abbreviations: FHH, familial hypocalciuric hypercalcemia.
Further, CaSR is expressed in the nephron of the kidneys and in the LOF situation, the renal threshold for calcium is increased and results in low renal calcium excretion compared to the enhanced filtered load of calcium.
4. Diagnosis, Screening and Prevention
4.1. Clinical Signs and Symptoms
Clinically, FHH is a benign, typically asymptomatic disease and usually presents with the biochemical triad: life-long, non-progressive hypercalcemia, normal or slightly increased serum PTH levels and hypocalciuria [2]. Serum phosphate levels are often reduced, circulating levels of 25OHD are reported as normal in FHH [2]. However, as discussed below, vitamin D status may influence the phenotype of FHH [29]. 1,25-(OH)2D levels are normal or elevated [2,30] and mild hypermagnesemia may be present [5,25,31].
Accordingly, the majority of patients with FHH do not require medical or surgical treatment [5,32].
Although typically asymptomatic, some patients with moderate-to-severe hypercalcemia may present with classical hypercalcemic symptoms (i.e., polyuria- polydipsia, fatigue, acute and chronic pancreatitis, gallstones and chondrocalcinosis, acute pancreatitis, chondrocalcinosis, and nephrolithiasis) [5,33,34,35,36,37]. Although, PTH is elevated in FHH, patients generally have BMDs and Z-scores comparable to normal controls [2,38,39].
The phenotypes of the different subtypes of FHH are generally similar [5,26], aside from some reports of more symptomatic disease in FHH3 with more severe hypercalcemia, hypermagnesemia as well as more pronounced hypocalciuria. Low bone mineral density (BMD) and cognitive impairment have been reported in FHH3, aswell [15,40].
4.2. Neonatal Diseases
In contrast to the clinically benign course of FHH, neonates with de-novo or paternally derived mutations in CaSR may present with neonatal severe primary hyperparathyroidism (NSHPT). NSHPT [OMIM #239200] is a clinically severe, ultra-rare disease associated with a high mortality usually caused by homozygous or compound heterozygous inactivating mutations in CaSR [2,41]. Infants with NSHPT develop significant and symptomatic hypercalcemia with muscular weakness, respiratory distress, fractures, and failure to thrive in the early days of life [17,42,43]. Swift and rigorous interventions to decrease calcium levels and suppress PTH secretion are necessary to decrease mortality in this condition. Treatment is discussed below.
Milder phenotypes are termed neonatal hyperparathyroidism (NHPT). These conditions are characterized by elevated serum PTH levels and subsequent bone disease, however only moderate hypercalcemia. Infants with NHPT are typically symptomatic as hypercalcemia leads to poor feeding with resulting dehydration, and lethargy, while PTH excess causes skeletal demineralization. These patients usually carry heterozygous, LOF- mutations in CaSR [4].
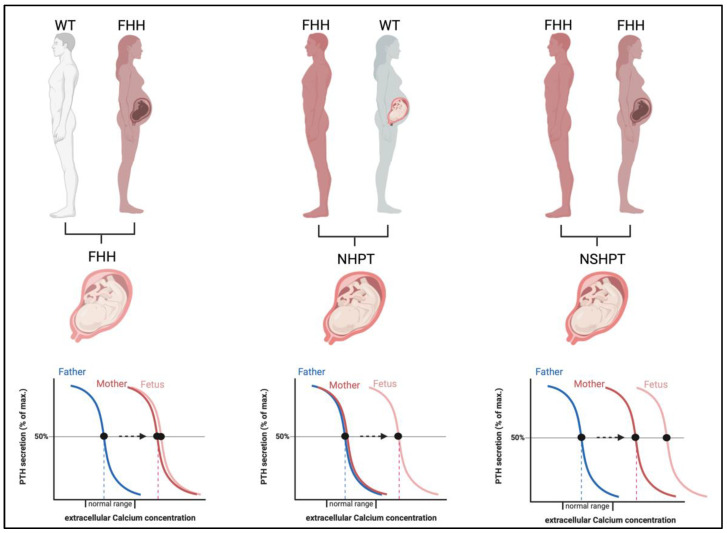
The severity of the neonatal disease in infants with heterozygous LOF-CaSR mutations is not only defined by the underlying genetic mutation but is significantly modified by the parental origin of the mutation (Figure 4) [17,44].
Figure 4.
Impact of the in-utero environment on fetal calcium homeostasis in calcium-hyposensitivity disorders. If the fetus is affected by heterozygous CaSR mutations, fetal and maternal calcium needs are concordant (left panel). If the maternal environment is normocalcemic (middle panel) but the fetus affected by a paternally derived or de-novo mutation in CaSR, the maternal environment is perceived as hypocalcemic by the fetus and PTH stimulation arises resulting in NHPT. If both parents are affected by FHH (right panel), the fetus may inherit both mutations and a most severe stimulation of PTH and hypercalcemia arises, a NSHPT. Abbreviations: FHH, familial hypocalciuric hypercalcemia; WT, wild type; NHPT, neonatal hyperparathyroidism; NSHPT, neonatal severe primary hyperparathyroidism.
When the mutation is maternally derived, both the mother and fetus share a need for increased calcium levels and coincide in the regulation of calcium homeostasis [11]. Thus, these pregnancies are likely uneventful. In contrast, when the mutation is paternally derived or sporadic (de novo), this result in differing calcium setpoints in mother and fetus with a different calcium need and thus conflicting regulation of calcium levels. In these pregnancies, the normocalcemic maternal environment is sensed as hypocalcemic by the fetus and thus induces fetal hyperparathyroidism to support increased fetal calcium levels at the expense of skeletal mineralization. This may result in bone fragility and pre- and perinatal fractures [11,44,45].
Accordingly, the majority of the reported cases of NHPT/NSHPT based on heterozygous mutations in CaSR, are neonates born to normocalcemic mothers with a paternally inherited or a de-novo mutation in CaSR [41,46,47,48]. The maternal vitamin D status during pregnancy may be a modifying factor in the phenotypic variability of heterozygous CaSR mutations [29,41,49]. Despite the severity of the phenotype, spontaneous recoveries are common. Wilkonson et al. reported on spontaneous clinical improvement in an infant with NHPT due to a paternally inherited heterozygous CaSR mutation [50]. In fact, spontaneous recovery should be expected in NHPT since after birth, the counter-regulating maternal environment is no longer influencing the fetal organism [45].
4.3. Establishing the Diagnosis
As the biochemical hallmarks of FHH are elevated calcium and PTH levels, the main differential diagnosis for FHH is primary hyperparathyroidism (PHPT), a condition that is ultra-rare in pediatric patients and has never been reported in neonates [51]. As such, in the neonatal setting the diagnostic challenge is to distinguish forms of NSHPT based on homozygous or compound heterozygous mutations in CaSR, which require aggressive and definite calcium and PTH suppressive measures, from milder forms such as NHPT which will resolve within the first months of life. Ultimately, the severity of the disease dictates the extent of treatment, and the differential diagnosis will be made based on molecular genetic findings [11].
In the clinical practice for older children (often adolescents) and adults, the differential diagnosis between primary hyperparathyroidism (PHPT) and FHH is essential, as parathyroidectomy may be indicated in PHPT and in neonates with NSHPT but usually not in older patients with FHH [32,52,53].
Recommendations on the practical management of parathyroid disorders is provided in the recently published European expert consensus paper [53].
In cases of chronic hypercalcemia, it is of utmost importance to measure calcium excretion in the urine. Neither the calcium level nor the PTH level allows a differentiation of FHH and PHPT [53]. However, more than 80% of FHH-affected individuals present with hypocalciuria (Ca creatinine clearance ratio [CCCR] < 0.01) in the presence of hypercalcemia, whereas less than 20% of patients with PHPT present with a CCCR < 0.01 [54,55]. In general, a CCCR less than 0.01 is considered to proof FHH [56,57]. However, a CCCR between 0.01 and 0.02 has been reported in some patients with FHH and Christensen et al. found a diagnostic sensitivity for a threshold of 0.02 [58].
In 2018, Bertocchio et al. introduced the pro-FHH (probability of having FHH) score with a specificity for FHH and PHPT of 100%. Pro-FHH takes plasma calcium, PTH, and serum osteocalcin concentrations, and CCCR calculated from 24-h urine collection into account [59]. However, the pro-FHH score has only been evaluated in adults and interpretation has been shown to be difficult in patients with elevated PTH levels [56].
Vargas-Poussou et al. compared a large cohort of FHH and PHPT patients and found considerable overlap in the phenotypes, making the distinction based on laboratory data alone difficult [60]. Thus, Christensen et al. suggest a two-step diagnostic procedure, where the first step is based on the CCCR with a cut-off at <0.020, and the second step is CaSR gene analysis. The clinical presentation in FHH1 and FHH2 is usually similar. Mutation analysis of AP2S1 gene for FHH3 should be performed in FHH patients with marked hypermagnesemia, cognitive impairment and low bone mineral density [15].
5. Management
A specific treatment is not necessary in the majority of patients. In contrast, morbidity may result from inappropriate surgical intervention [5]. The prognosis in FHH is good, and the life expectancy is probably normal [2].
However, in rare instances when calcium levels are very high or the patients become symptomatic from hypercalcemia, it becomes necessary to treat FHH [11,61].
Calcimimetic drugs, such as cinacalcet, are allosteric agonists at the CaSR [61]. They enhance the effect of extracellular calcium at the CaSR in the parathyroid cell, thereby decreasing PTH secretion and consequently serum calcium levels. In FHH, cinacalcet results in a significant reduction of serum calcium levels and improvement of calcium clearance after initiation of cinacalcet therapy [17,61,62,63,64,65,66,67].
Treatment with cinacalcet has also been reported in FHH2 [7] and FHH3 [68], with successful normalization of serum calcium concentrations. In addition, infants with NHPT and NSHPT have also been treated successfully with cinacalcet [11,17,42]. However, in NHPT it remains unclear, whether the use of cinacalcet is necessary or whether surveillance alone is sufficient to confirm a spontaneous improvement over time. In NSHPT, total parathyroidectomy is generally considered the definitive treatment. Subtotal parathyroidectomy is ineffective and therefore not recommended [69,70]. Prior to surgery, serum calcium levels need to be lowered. For treatment of hypercalcemia bisphosphonates are well established and their use has been reported in neonates with NSHPT and NHPT [67].
6. Genetic Counselling and Neonatal Management
Based on the fact that most patients with FHH do not require any form of treatment and that patients are at risk of unnecessary (partial) parathyroidectomies the focus is based on (genetic) counselling [53]. Perinatal complications in pregnancies affected by FHH should also be brought to the attention of affected patients of reproductive age and to the clinicians potentially involved in their care, e.g., human geneticists, obstetricians, (pediatric) endocrinologists, and neonatologists. The fact that the paternally derived mutations cause a greater risk for neonates to suffer from hyperparathyroid complications emphasizes the need to specifically instruct male patients with FHH on these potential complications.
Therefore, we agree with the recommendation of Ghaznavi et al., that it is advisable to offer genetic counseling to all pregnant women with confirmed FHH or a partner with FHH given the range of possible neonatal outcomes [52], including NSHPT in neonates with homozygous or compound heterozygous inactivating mutations [32].
7. Summary
While FHH in general is a benign condition, neonates with inactivating mutations in CaSR are at risk to develop NHPT or even NSHPT. This risk is highest if the mother is not affected by FHH or if homozygous or compound heterozygous mutations affect the neonate. In NHPT clinical signs are often temporary and resolve within weeks or months once the neonate has established calcium levels according to her/his individual threshold. At that point, the excess PTH will drop to the individual threshold and the skeletal system may mineralize appropriately in the further course. However, in NSHPT (due to homozygous or compound heterozygous mutations in CaSR), therapeutic interventions must aim to lower calcium and PTH levels until a diagnosis is established and a definite (surgical) treatment can be pursued. Adult patients with FHH of reproductive age should be counselled about the potential peripartal complications in this condition.
Author Contributions
All authors have contributed to review of the papers. J.H. and C.G. have designed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hannan F., Kallay E., Chang W., Brandi M.L., Thakker R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018;15:33–51. doi: 10.1038/s41574-018-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christensen S.E., Nissen P.H., Vestergaard P., Mosekilde L. Familial hypocalciuric hypercalcaemia. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:359–370. doi: 10.1097/MED.0b013e32834c3c7c. [DOI] [PubMed] [Google Scholar]
- 3.Vannucci L., Brandi M.L. Familial Hypocalciuric Hypercalcemia and Neonatal Severe Hyperparathyroidism. Front. Horm. Res. 2018;51:52–62. doi: 10.1159/000491038. [DOI] [PubMed] [Google Scholar]
- 4.Brown E.M. Clinical lessons from the calcium-sensing receptor. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:122–133. doi: 10.1038/ncpendmet0388. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.Y., Shoback D.M. Familial hypocalciuric hypercalcemia and related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32:609–619. doi: 10.1016/j.beem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nesbit M.A., Hannan F.M., Howles S.A., Reed A.A.C., Cranston T., Thakker C.E., Gregory L., Rimmer A.J., Rust N., Graham U., et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat. Genet. 2013;45:93–97. doi: 10.1038/ng.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorvin C.M., Hannan F.M., Cranston T., Valta H., Makitie O., Schalin-Jantti C., Thakker R.V. Cinacalcet Rectifies Hypercalcemia in a Patient with Familial Hypocalciuric Hypercalcemia Type 2 (FHH2) Caused by a Germline Loss-of-Function Gα11 Mutation. J. Bone Miner. Res. 2018;33:32–41. doi: 10.1002/jbmr.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannan F.M., Babinsky V.N., Thakker R.V. Disorders of the calcium-sensing receptor and partner proteins: Insights into the molecular basis of calcium homeostasis. J. Mol. Endocrinol. 2016;57:R127–R142. doi: 10.1530/JME-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinnie J., Bell E., McKillop E., Gallacher S. The Prevalence of Familial Hypocalciuric Hypercalcemia. Calcif. Tissue Res. 2001;68:216–218. doi: 10.1007/s002230001201. [DOI] [PubMed] [Google Scholar]
- 10.Dershem R., Gorvin C.M., Metpally R.P., Krishnamurthy S., Smelser D.T., Hannan F., Carey D.J., Thakker R.V., Breitwieser G.E. Familial Hypocalciuric Hypercalcemia Type 1 and Autosomal-Dominant Hypocalcemia Type 1: Prevalence in a Large Healthcare Population. Am. J. Hum. Genet. 2020;106:734–747. doi: 10.1016/j.ajhg.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Höppner J., Lais S., Roll C., Wegener-Panzer A., Wieczorek D., Högler W., Grasemann C. Case Report: Severe Neonatal Course in Paternally Derived Familial Hypocalciuric Hypercalcemia. Front. Endocrinol. 2021;12:1–7. doi: 10.3389/fendo.2021.700612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofer A.M., Brown E.M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 13.Breitwieser G., Gama L. Calcium-sensing receptor activation induces intracellular calcium oscillations. Am. J. Physiol. Physiol. 2001;280:C1412–C1421. doi: 10.1152/ajpcell.2001.280.6.C1412. [DOI] [PubMed] [Google Scholar]
- 14.Grant M.P., Stepanchick A., Cavanaugh A., Breitwieser G.E. Agonist-Driven Maturation and Plasma Membrane Insertion of Calcium-Sensing Receptors Dynamically Control Signal Amplitude. Sci. Signal. 2011;4:ra78. doi: 10.1126/scisignal.2002208. [DOI] [PubMed] [Google Scholar]
- 15.Tőke J., Czirják G., Enyedi P., Tóth M. Rare diseases caused by abnormal calcium sensing and signaling. Endocrine. 2021;71:611–617. doi: 10.1007/s12020-021-02620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown E.M., MacLeod R.J. Extracellular Calcium Sensing and Extracellular Calcium. Physiol. Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 17.Reh C.M.S., Hendy G.N., Cole D.E.C., Jeandron D.D. Neonatal Hyperparathyroidism with a Heterozygous Calcium-Sensing Receptor (CASR) R185Q Mutation: Clinical Benefit from Cinacalcet. J. Clin. Endocrinol. Metab. 2011;96:707–712. doi: 10.1210/jc.2010-1306. [DOI] [PubMed] [Google Scholar]
- 18.Sands J.M., Naruse M., Baum M., Jo I., Hebert S.C., Brown E.M., Harris H.W. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J. Clin. Investig. 1997;99:1399–1405. doi: 10.1172/JCI119299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riccardi D., Valenti G. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 2016;12:414–425. doi: 10.1038/nrneph.2016.59. [DOI] [PubMed] [Google Scholar]
- 20.Riccardi D., Hall A.E., Chattopadhyay N., Xu J.Z., Brown E.M., Hebert S.C. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am. J. Physiol. Physiol. 1998;274:611–622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 21.Kanatani M., Sugimoto T., Kanzawa M., Yano S., Chihara K. High Extracellular Calcium Inhibits Osteoclast-like Cell Formation by Directly Acting on the Calcium-Sensing Receptor Existing in Osteoclast Precursor Cells. Biochem. Biophys. Res. Commun. 1999;261:144–148. doi: 10.1006/bbrc.1999.0932. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi T., Kifor O., Chattopadhyay N., Brown E.M. Expression of Extracellular Calcium (Ca(o)2+)-Sensing Receptor in the Clonal Osteoblast-like Cell Lines, UMR-106 and SAOS-2. Biochem. Biophys. Res. Commun. 1998;243:753–757. doi: 10.1006/bbrc.1998.8178. [DOI] [PubMed] [Google Scholar]
- 23.Lorget F., Kamel S., Mentaverri R., Wattel A., Naassila M., Maamer M., Brazier M. High Extracellular Calcium Concentrations Directly Stimulate Osteoclast Apoptosis. Biochem. Biophys. Res. Commun. 2000;268:899–903. doi: 10.1006/bbrc.2000.2229. [DOI] [PubMed] [Google Scholar]
- 24.Chang W., Tu C., Chen T.-H., Komuves L., Oda Y., Pratt S.A., Miller S., Shoback D. Expression and Signal Transduction of Calcium-Sensing Receptors in Cartilage and Bone. Endocrinology. 1999;140:5883–5893. doi: 10.1210/endo.140.12.7190. [DOI] [PubMed] [Google Scholar]
- 25.Nesbit M.A., Hannan F.M., Howles S.A., Babinsky V.N., Head R.A., Cranston T., Rust N., Hobbs M.R., Heath H., Thakker R.V. Mutations Affecting G-Protein Subunit α11 in Hypercalcemia and Hypocalcemia. N. Engl. J. Med. 2013;368:2476–2486. doi: 10.1056/NEJMoa1300253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorvin C., Cranston T., Hannan F., Rust N., Qureshi A., Nesbit M.A., Thakker R.V. A G-protein Subunit-α11 Loss-of-Function Mutation, Thr54Met, Causes Familial Hypocalciuric Hypercalcemia Type 2 (FHH2) J. Bone Miner. Res. 2016;31:1200–1206. doi: 10.1002/jbmr.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorvin C.M., Cranston T., Hannan F.M., Valta H., Makitie O., Schalin-Jantti C., Thakker R.V. G [alpha] 11-Phe220Ser loss-of-function mutation causes familial hypocalciuric hypercalcemia type-2 (FHH2) by disrupting a hydrophobic cluster critical for G-protein signaling. Bioscientifica. 2017;49 doi: 10.1530/endoabs.49.OC10.5. [DOI] [Google Scholar]
- 28.Gorvin C.M. Insights into calcium-sensing receptor trafficking and biased signalling by studies of calcium homeostasis. J. Mol. Endocrinol. 2018;61:R1–R12. doi: 10.1530/JME-18-0049. [DOI] [PubMed] [Google Scholar]
- 29.Zajickova K., Vrbikova J., Canaff L., Pawelek P., Goltzman D., Hendy G.N. Identification and Functional Characterization of a Novel Mutation in the Calcium-Sensing Receptor Gene in Familial Hypocalciuric Hypercalcemia: Modulation of Clinical Severity by Vitamin D Status. J. Clin. Endocrinol. Metab. 2007;92:2616–2623. doi: 10.1210/jc.2007-0123. [DOI] [PubMed] [Google Scholar]
- 30.Christensen S.E., Nissen P.H., Vestergaard P., Heickendorff L., Rejnmark L., Brixen K., Mosekilde L. Plasma 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and parathyroid hormone in familial hypocalciuric hypercalcemia and primary hyperparathyroidism. Eur. J. Endocrinol. 2008;159:719–727. doi: 10.1530/EJE-08-0440. [DOI] [PubMed] [Google Scholar]
- 31.Hendy G.N., Canaff L., Newfield R.S., Tripto-Shkolnik L., Wong B.Y.L., Lee B.S.P., Cole D.E.C. Codon Arg15 mutations of the AP2S1 gene: Common occurrence in familial hypocalciuric hypercalcemia cases negative for calcium-sensing receptor (CASR) mutations. J. Clin. Endocrinol. Metab. 2014;99:1311–1315. doi: 10.1210/jc.2014-1120. [DOI] [PubMed] [Google Scholar]
- 32.Jones A.R., Hare M.J., Brown J., Yang J., Meyer C., Milat F., Allan C.A. Familial Hypocalciuric Hypercalcemia in Pregnancy: Diagnostic Pitfalls. JBMR Plus. 2020;4:e10362. doi: 10.1002/jbm4.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law W.M. Familial Benign Hypercalcemia (Hypocalciuric Hypercalcemia): Clinical and pathogenetic studies in 21 families. Annals of Internal Medicine. Ann. Intern. Med. 1985;102:511–519. doi: 10.7326/0003-4819-102-4-511. [DOI] [PubMed] [Google Scholar]
- 34.Anderson R.M., Funnell M.M. Patient empowerment: Myths and misconceptions. Patient Educ. Couns. 2010;79:277–282. doi: 10.1016/j.pec.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volpe A., Guerriero A., Marchetta A., Caramaschi P., Furlani L. Familial hypocalciuric hypercalcemia revealed by chondrocalcinosis. Jt. Bone Spine. 2009;76:708–710. doi: 10.1016/j.jbspin.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Stuckey B.G.A., Gutteridge D.H., Kent G.N., Reed W.D. Familial hypocalciuric hypercalcaemia and pancreatitis: No causal link proven. Aust. N. Z. J. Med. 1990;20:718–719. doi: 10.1111/j.1445-5994.1990.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 37.Ritter A., Vargas-Poussou R., Mohebbi N., Seeger H. Recurrent Nephrolithiasis in a Patient with Hypercalcemia and Normal to Mildly Elevated Parathyroid Hormone. Am. J. Kidney Dis. 2021;77:A13–A15. doi: 10.1053/j.ajkd.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Nissen P.H., Vestergaard P., Heickendorff L., Rejnmark L., Brixen K., Mosekilde L., Christensen S.E. Skeletal consequences of familial hypocalciuric hypercalcaemia vs. primary hyperparathyroidism. Clin. Endocrinol. 2009;71:798–807. doi: 10.1111/j.1365-2265.2009.03557.x. [DOI] [PubMed] [Google Scholar]
- 39.Jakobsen N.F.B., Rolighed L., Moser E., Nissen P.H., Mosekilde L., Rejnmark L. Increased Trabecular Volumetric Bone Mass Density in Familial Hypocalciuric Hypercalcemia (FHH) Type 1: A Cross-Sectional Study. Calcif. Tissue Int. 2014;95:141–152. doi: 10.1007/s00223-014-9877-0. [DOI] [PubMed] [Google Scholar]
- 40.Hannan F., Howles S., Rogers A., Cranston T., Gorvin C., Babinsky V.N., Reed A.A., Thakker C.E., Bockenhauer D., Brown R.S., et al. Adaptor protein-2 sigma subunit mutations causing familial hypocalciuric hypercalcaemia type 3 (FHH3) demonstrate genotype-phenotype correlations, codon bias and dominant-negative effects. Hum. Mol. Genet. 2015;24:5079–5092. doi: 10.1093/hmg/ddv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marx S.J., Sinaii N. Neonatal Severe Hyperparathyroidism: Novel Insights from Calcium, PTH, and the CASR Gene. J. Clin. Endocrinol. Metab. 2019;105:1061–1078. doi: 10.1210/clinem/dgz233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulcan-Kersin S., Kirkgoz T., Eltan M., Rzayev T., Ata P., Bilgen H., Ozek E., Bereket A., Turan S. Cinacalcet as a First-Line Treatment in Neonatal Severe Hyperparathyroidism Secondary to Calcium Sensing Receptor (CaSR) Mutation. Horm. Res. Paediatr. 2020;93:313–321. doi: 10.1159/000510623. [DOI] [PubMed] [Google Scholar]
- 43.Marx S.J., Attie M.F., Spiegel A.M., Levine M.A., Lasker R.D., Fox M. An Association between Neonatal Severe Primary Hyperparathyreoidism and Familial Hypocalciuric Hypercalcemia in three kindreds. N. Engl. J. Med. 1982;306:257–264. doi: 10.1056/NEJM198202043060502. [DOI] [PubMed] [Google Scholar]
- 44.Obermannova B., Banghova K., Sumnik Z., Dvorakova H.M., Betka J., Fencl F., Kolouskova S., Cinek O., Lebl J. Unusually severe phenotype of neonatal primary hyperparathyroidism due to a heterozygous inactivating mutation in the CASR gene. Eur. J. Pediatr. 2009;168:569–573. doi: 10.1007/s00431-008-0794-y. [DOI] [PubMed] [Google Scholar]
- 45.Fox L., Sadowsky J., Pringle K.P., Kidd A., Murdoch J., Cole D.E., Wiltshire E. Neonatal Hyperparathyroidism and Pamidronate Therapy in an Extremely Premature Infant. Pediatrics. 2007;120:e1350–e1354. doi: 10.1542/peds.2006-3209. [DOI] [PubMed] [Google Scholar]
- 46.Bai M., Pearce S., Kifor O., Trivedi S., Stauffer U.G., Thakker R., Brown E.M., Steinmann B. In vivo and in vitro characterization of neonatal hyperparathyroidism resulting from a de novo, heterozygous mutation in the Ca2+—sensing receptor gene: Normal maternal calcium homeostasis as a cause of secondary hyperparathyroidism in familial benign hypocalciuric hypercalcemia. J. Clin. Investig. 1997;99:88–96. doi: 10.1172/jci119137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waller S., Kurzawinski T., Spitz L., Thakker R., Cranston T., Pearce S., Cheetham T., Hoff W.G.V. Neonatal severe hyperparathyroidism: Genotype/phenotype correlation and the use of pamidronate as rescue therapy. Eur. J. Pediatr. 2004;163:589–594. doi: 10.1007/s00431-004-1491-0. [DOI] [PubMed] [Google Scholar]
- 48.Appelman-Dijkstra N.M., Ertl D.-A., Zillikens M.C., Rjenmark L., Winter E.M. Hypercalcemia during pregnancy: Management and outcomes for mother and child. Endocrine. 2021;71:604–610. doi: 10.1007/s12020-021-02615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarz P., Larsen N.E., Friis I.M.L., Lillquist K., Brown E.M., Gammeltoft S. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism associated with mutations in the human Ca2+—sensing receptor gene in three Danish families. Scand. J. Clin. Lab. Investig. 2000;60:221–228. doi: 10.1080/003655100750044875. [DOI] [PubMed] [Google Scholar]
- 50.Wilkinson H., James J. Self limiting neonatal primary hyperparathyroidism associated with familial hypocalciuric hypercalcaemia. Arch. Dis. Child. 1993;69:319–321. doi: 10.1136/adc.69.3_Spec_No.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodd C., Goodyer P. Hypercalcemia of the newborn: Etiology, evaluation, and management. Pediatr. Nephrol. 1999;13:542–547. doi: 10.1007/s004670050654. [DOI] [PubMed] [Google Scholar]
- 52.Ghaznavi S.A., Saad N.M.A., Donovan L.E. The Biochemical Profile of Familial Hypocalciuric Hypercalcemia and Primary Hyperparathyroidism during Pregnancy and Lactation: Two Case Reports and Review of the Literature. Case Rep. Endocrinol. 2016;2016:1–6. doi: 10.1155/2016/2725486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bollerslev J., Rejnmark L., Zahn A., Heck A., Appelman-Dijkstra N.M., Cardoso L., Hannan F.M., Cetani F., Sikjaer T., Formenti A.M., et al. European expert consensus on practical management of specific aspects of parathyroid disorders in adults and in pregnancy: Recommendations of the ESE Educational Program of Parathyroid Disorders (PARAT 2021) Eur. J. Endocrinol. 2022;186:R33–R63. doi: 10.1530/EJE-21-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eastell R., Brandi M.L., Costa A.G., D’Amour P., Shoback D.M., Thakker R. Diagnosis of Asymptomatic Primary Hyperparathyroidism: Proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab. 2014;99:3570–3579. doi: 10.1210/jc.2014-1414. [DOI] [PubMed] [Google Scholar]
- 55.Moore E.C., Berber E., Jin J., Krishnamurthy V., Shin J., Siperstein A. Calcium-Creatinine Clearance Ratio is not Helpful in Differentiating Primary Hyperparathyroidism from Familial Hypercalcemic Hypocalciuria: A Study of 1000 Patients. Endocr. Pract. 2018;24:988–994. doi: 10.4158/EP-2018-0350. [DOI] [PubMed] [Google Scholar]
- 56.Quarde A., Leonhard J., Fan C. Performance of the Pro-FHH Score in a Delayed Diagnosis of Familial Hypocalciuric Hypercalcemia Type-1. SSRN Electron. J. 2021 doi: 10.2139/ssrn.3843235. [DOI] [Google Scholar]
- 57.Vahe C., Benomar K., Espiard S., Coppin L., Jannin A., Odou M.F., Vantyghem M.C. Diseases associated with calcium-sensing receptor. Orphanet J. Rare Dis. 2017;12:1–9. doi: 10.1186/s13023-017-0570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christensen S.E., Nissen P.H., Vestergaard P., Heickendorff L., Brixen K., Mosekilde L. Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: A follow-up study on methods. Clin. Endocrinol. 2008;69:713–720. doi: 10.1111/j.1365-2265.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 59.Bertocchio J.-P., Tafflet M., Koumakis E., Maruani G., Vargas-Poussou R., Silve C., Nissen P.H., Baron S., Prot-Bertoye C., Courbebaisse M., et al. Pro-FHH: A Risk Equation to Facilitate the Diagnosis of Parathyroid-Related Hypercalcemia. J. Clin. Endocrinol. Metab. 2018;103:2534–2542. doi: 10.1210/jc.2017-02773. [DOI] [PubMed] [Google Scholar]
- 60.Vargas-Poussou R., Mansour-Hendili L., Baron S., Bertocchio J.-P., Travers C., Simian C., Treard C., Baudouin V., Beltran S., Broux F., et al. Familial Hypocalciuric Hypercalcemia Types 1 and 3 and Primary Hyperparathyroidism: Similarities and Differences. J. Clin. Endocrinol. Metab. 2016;101:2185–2195. doi: 10.1210/jc.2015-3442. [DOI] [PubMed] [Google Scholar]
- 61.Khadilkar K.S., Jagtap V., Lila A., Bandgar T., Shah N.S. Utility of cinacalcet in familial hypocalciuric hypercalcemia. Indian Journal of Endocrinology and Metabolism. Indian J. Endocrinol. Metab. 2017;21:362–363. doi: 10.4103/2230-8210.202031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timmers H.J.L.M., Karperien M., Hamdy N.A.T., De Boer H., Hermus A.R.M.M. Normalization of serum calcium by cinacalcet in a patient with hypercalcaemia due to a de novo inactivating mutation of the calcium-sensing receptor. J. Intern. Med. 2006;260:177–182. doi: 10.1111/j.1365-2796.2006.01684.x. [DOI] [PubMed] [Google Scholar]
- 63.Alon U.S., Vandevoorde R.G. Beneficial effect of cinacalcet in a child with familial hypocalciuric hypercalcemia. Pediatr. Nephrol. 2010;25:1747–1750. doi: 10.1007/s00467-010-1547-5. [DOI] [PubMed] [Google Scholar]
- 64.Rasmussen A.Q., Jørgensen N.R., Schwarz P. Clinical and biochemical outcomes of cinacalcet treatment of familial hypocalciuric hypercalcemia: A case series. J. Med. Case Rep. 2011;5:564. doi: 10.1186/1752-1947-5-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forman T., Niemi A.-K., Prahalad P., Shi R.Z., Nally L.M. Cinacalcet therapy in an infant with an R185Q calcium-sensing receptor mutation causing hyperparathyroidism: A case report and review of the literature. J. Pediatr. Endocrinol. Metab. 2019;32:305–310. doi: 10.1515/jpem-2018-0307. [DOI] [PubMed] [Google Scholar]
- 66.Giraldi F.P., Fatti L.M., Bertola G., Balza G., Lavezzi E., Pesce S., Scacchi M., Cavagnini F. Correction of hypercalcaemia by cinacalcet in familial hypocalciuric hypercalcaemia. Clin. Endocrinol. 2007;68:324–325. doi: 10.1111/j.1365-2265.2007.03024.x. [DOI] [PubMed] [Google Scholar]
- 67.Mayr B.M., Schnabel D., Dörr H.-G., Schöfl C. Gain and loss of function mutations of the calcium-sensing receptor and associated proteins: Current treatment concepts. Eur. J. Endocrinol. 2016;174:R189–R208. doi: 10.1530/EJE-15-1028. [DOI] [PubMed] [Google Scholar]
- 68.Howles S., Hannan F., Babinsky V.N., Rogers A., Gorvin C., Rust N., Richardson T., McKenna M.J., Nesbit M.A., Thakker R.V. Cinacalcet for Symptomatic Hypercalcemia Caused by AP2S1 Mutations. N. Engl. J. Med. 2016;374:1396–1398. doi: 10.1056/NEJMc1511646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marx S.J. Calcimimetic Use in Familial Hypocalciuric Hypercalcemia—A Perspective in Endocrinology. J. Clin. Endocrinol. Metab. 2017;102:3933–3936. doi: 10.1210/jc.2017-01606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sperlongano P., Sperlongano S., Foroni F., De Lucia F.P., Pezzulo C., Manfredi C., Esposito E., Sperlongano R. Postoperative hypocalcemia: Assessment timing. Int. J. Surg. 2014;12:S95–S97. doi: 10.1016/j.ijsu.2014.05.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.