Abstract
Rhodococcus sp. strain ECRD-1 was evaluated for its ability to desulfurize a 232 to 343°C middle-distillate (diesel range) fraction of Oregon basin (OB) crude oil. OB oil was provided as the sole source of sulfur in batch cultures, and the extent of desulfurization and the chemical fate of the residual sulfur in the oil after treatment were determined. Gas chromatography (GC), flame ionization detection, and GC sulfur chemiluminesce detection analysis were used to qualitatively evaluate the effect of Rhodococcus sp. strain ECRD-1 treatment on the hydrocarbon and sulfur content of the oil, respectively. Total sulfur was determined by combustion of samples and measurement of released sulfur dioxide by infrared absorption. Up to 30% of the total sulfur in the middle distillate cut was removed, and compounds across the entire boiling range of the oil were affected. Sulfur K-edge X-ray absorption-edge spectroscopy was used to examine the chemical state of the sulfur remaining in the treated OB oil. Approximately equal amounts of thiophenic and sulfidic sulfur compounds were removed by ECRD-1 treatment, and over 50% of the sulfur remaining after treatment was in an oxidized form. The presence of partially oxidized sulfur compounds indicates that these compounds were en route to desulfurization. Overall, more than two-thirds of the sulfur had been removed or oxidized by the microbial treatment.
The concentration of sulfur in crude oil is typically between 0.05 and 5.0% (by weight), although values as high as 13.95% have been reported (24). In general, the distribution of sulfur in crude oil is such that the proportion of sulfur increases along with the boiling point of the distillate fraction (24). As a result, the higher the boiling range of the fuel the higher the sulfur content will tend to be. For example, a middle-distillate-range fraction, e.g., diesel fuel, will typically have a higher sulfur content than the lower-boiling-range gasoline fraction. Upon combustion, the sulfur in fuels can contribute to air pollution in the form of particulate material and acidic gases, such as sulfur dioxide (26). To reduce sulfur-related air pollution, the level of sulfur in fuels is regulated, and to meet these regulations sulfur must be removed from fuels during the refining process.
Refineries remove organic sulfur from crude oil-derived fuels by hydrodesulfurization (HDS). HDS is a catalytic process that converts organic sulfur to hydrogen sulfide gas by reacting crude oil fractions with hydrogen at pressures between 150 and 3,000 lb/in2 and temperatures between 290 and 455°C, depending upon the feed and level of desulfurization required (26). Organic sulfur compounds in the lower-boiling fractions of petroleum, e.g., the gasoline range, are mainly thiols, sulfides, and thiophenes, which are readily removed by HDS. However, middle-distillate fractions, e.g., the diesel and fuel oil range, contain significant amounts of benzothiophenes and dibenzothiophenes (DBTs), which are considerably more difficult to remove by HDS. Among the most refractory of these compounds are DBTs with substitutions adjacent to the sulfur moiety (12). Compounds of this type are referred to as sterically hindered compounds because the substitutions are believed to sterically hinder access of the sulfur atom to the catalyst surface. Due to their resistance to HDS, sterically hindered compounds represent a significant barrier to reaching very low sulfur levels in middle- and heavy-distillate-range fuels. The high cost and inherent chemical limitations associated with HDS make alternatives to this technology of interest to the petroleum industry. Moreover, current trends toward stricter regulations on the content of sulfur in fuels provide incentive for the continued search for improved desulfurization processes.
Biodesulfurization has been studied as an alternative to HDS for the removal of organic sulfur from fuels. The use of hydrocarbon degradation pathways that attacked DBT were unsuccessful because these systems relied on the oxidation and mineralization of the carbon skeleton instead of on sulfur removal and therefore significantly reduced the fuel value of the desulfurized end product (9, 10, 14, 15, 16, 17, 18, 19, 20, 30). More recently, bacteria that desulfurize DBT and a variety of other organic sulfur compounds typically found in petroleum oils via a sulfur selective oxidative pathway that does not remove carbon (1, 7, 11, 13, 22, 27, 28) have been isolated. This pathway involves the sequential oxidation of the sulfur moiety followed by cleavage of the carbon sulfur bonds.
To be commercially useful, biodesulfurization must be able to remove the sulfur from fuels. Although considerable research on the desulfurization of model compounds via the sulfur selective oxidative pathway has been reported, little information on the desulfurization of fuel oils has been published. Rhodococcus sp. strain ECRD-1 was previously shown to be able to desulfurize sterically hindered DBTs by using the sulfur selective oxidative pathway (17). In this study, we evaluated the ability of ECRD-1 to desulfurize a middle-distillate fraction of crude and characterized the chemical fate of the remaining sulfur.
MATERIALS AND METHODS
Bacterial strain.
Biodesulfurization experiments using Rhodococcus sp. strain ECRD-1 (ATCC 55309), previously designated Arthrobacter sp. strain D-1 (ATCC 55309), were performed. This organism was isolated by enrichment culture from marine sediments based on its ability to selectively remove sulfur from the sterically hindered organic sulfur compound 4,6-DEDBT (17). ECRD-1 uses a sulfur selective oxidative pathway for sulfur removal, resulting in the formation of a hydroxylated sulfur-free end product (17). Frozen stocks were maintained at −80°C prior to use in desulfurization experiments.
Oil.
Oregon Basin (OB) crude oil, containing 2.1% sulfur, was obtained from the Exxon Company, Baytown, Tex. A 450-to-650°F (232-to-343°C) middle-distillate cut was prepared and used for desulfurization experiments. The oil was artificially weathered by evaporation under a stream of nitrogen to a constant weight to eliminate inconsistencies caused by evaporative loss of oil during culturing or extraction. Weathering of the OB oil resulted in a weight loss of less than 10%.
Biodesulfurization of oil.
Biodesulfurization of OB oil was performed by growing Rhodococcus sp. strain ECRD-1 in mineral salts sulfur-free medium (MSSF) using the OB oil as sulfur source. MSSF contained the following components per liter: 0.4 g of KH2PO4, 1.6 g of K2HPO4, 1.5 g of NH4Cl, 0.17 g of MgCl2 · 6H2O, 0.09 g of CaCl2 · 2H2O, 50 mg of Na2WO4 · 2H2O, 1 ml of vitamin solution, and 5 ml of mineral solution. The vitamin solution contained the following components per liter: 100 mg of thiamine, 50 mg of p-aminobenzoic acid, 50 mg of vitamin B12, and 10 mg of biotin. The mineral solution contained (per liter of deionized water) 1.5 g of nitrilotriacetic acid (dissolved in 500 ml of H2O and adjusted to pH 6.5 with 10 M KOH), 5.1 g of MgCl2 · 6H2O, 0.66 g of MnCl2 · 2H2O, 1.0 g of NaCl, 1.0 g of FeCl3 · 6H2O, 0.1 g of CaCl2 · 6H2O, 0.01 g of CuCl2 · 6H2O, 0.08 g of ZnCl2, 0.05 g of AlCl3, 0.01 g of H3BO3, and 0.04 g of Na2MoO4 · 2H2O. MSSF plates containing 130 mg of 4,6-DEDBT/liter (added as a 100× solution dissolved in ethanol) as the sole sulfur source were prepared from MSSF containing 0.8% SeaKem Gold agarose (FMC Corp.), a low-free-sulfate agarose.
Desulfurization of undiluted OB oil was performed with 1 ml of OB oil per liter of culture, which provided approximately 20 mg of organic sulfur (final concentration, 20 ppm). To minimize the degradation by ECRD-1 of alkanes present in the OB oil, desulfurization experiments were also performed with OB oil diluted in decane. Decane served as an abundant source of a readily assimilated alkane substrate for ECRD-1, minimizing the degradation of alkanes present in the OB oil. Two dilutions of OB oil were used to evaluate the effect of different sulfur concentrations on the extent of desulfurization. Ten milliliters of a 10-fold dilution of oil in decane per liter of culture was added to provide 20 mg of organic sulfur (final concentration, 20 ppm), and 10 ml of a 20-fold dilution of oil in decane per liter of culture was added to provide 10 mg of organic sulfur (final concentration, 10 ppm). Oil was autoclaved in sealed jars for 15 min at 121°C and 15 lb/in2, cooled, and then added at the indicated amounts prior to inoculation.
Inocula for the biodesulfurization experiments were prepared by inoculating 50 ml of sterile Luria-Bertani medium (25) with Rhodococcus sp. strain ECRD-1 grown on MSSF agar plates containing 4,6-DEDBT as the sole sulfur source. Cells were grown for 20 h until mid-log phase, and the entire culture was harvested by centrifugation at 3,000 × g at 4°C. The resulting cell pellet was washed twice with 50 ml of sterile 12 mM phosphate buffer (pH 7.0). Washed cell pellets were resuspended in 1/10 the original culture volume of chilled phosphate buffer and used immediately for inoculation. Two milliliters of a concentrated inoculum suspension was used to inoculate 1 liter of culture medium contained in 2-liter Erlenmeyer flasks. Biodesulfurization cultures were incubated for 7 days at 25°C with shaking at 200 rpm on a rotary shaker. The pH of the cultures was monitored at 1- to 2-day intervals with pH paper (J. T. Baker Inc.) by using 1-ml aliquots of the aqueous phase withdrawn with a sterile pipette. Care was taken to avoid removal of oil. If the pH deviated by more than 1.0 pH unit, it was adjusted to pH 7.0 with 1 M phosphoric acid. Sterile controls, which were not inoculated with ECRD-1, were prepared and treated in a manner identical to that for inoculated cultures.
Extraction of oil cultures.
Before extraction, cultures were brought to a pH of 2.0 with 1 N HCl. The entire content of each flask was extracted three times with 100 ml of methylene chloride, and the combined extracts were filtered through anhydrous sodium sulfate to remove water. The samples were evaporated to approximately 10 ml under a stream of nitrogen gas. Samples were subsequently filtered through a 0.22-μm-pore-size Teflon hydrophobic membrane syringe filter (13-mm diameter; Gelman Sciences) to remove turbidity (attributed to water condensate) appearing after volume reduction. The solutions were then concentrated by evaporation at room temperature under a stream of nitrogen gas to approximately 3.0 ml and used for analysis by gas chromatography (GC) and sulfur K-edge X-ray absorption-edge spectroscopy. For total sulfur analysis, a portion of the concentrated sample was evaporated under a stream of nitrogen gas to constant weight to remove residual methylene chloride and, if present, decane.
GC, FID, and SCD analyses.
GC analysis was performed in duplicate on 1 μl of sample extract by using a Perkin-Elmer GC Autosystem (split/splitless injector) and a Supelco SPB-1 column (30 m by 0.32 mm, 0.25-μm film thickness). The temperature zones for the GC were as follows: injector and detector temperature, 300°C; initial oven temperature, 40°C for 1 min, followed by a 4°C/min temperature ramp to 300°C for a final 10-min hold. The temperature zones for the GC were as follows: injector temperature, 275°C; detector temperature, 325°C; initial oven temperature, 50°C for 1 min followed by a 5°C/min temperature ramp to 300°C with a final 20-min hold. Hydrocarbon- and sulfur-containing compounds were detected by using a Perkin-Elmer flame ionization detection (FID) instrument and a Sievers Instruments model 355 sulfur chemiluminescence detection (SCD) instrument in tandem.
Total sulfur analysis.
Sulfur removal from OB oil was determined by the difference in sulfur content in sterile control oil and that in oil treated with ECRD-1. The total percentage of sulfur (by weight) was determined in triplicate for each sample by combustion of samples, and measurement of released sulfur dioxide was performed by infrared absorption using a model SC-432DR sulfur analyzer (LECO Corporation, St. Joseph, Mich.). Analysis was carried out in accordance with the procedures described in American Society for Testing Materials Method D-4239. The variation on the procedure included the use of Com-Aid (501-426) combustion aid (LECO).
Sulfur K-edge X-ray absorption-edge spectroscopy.
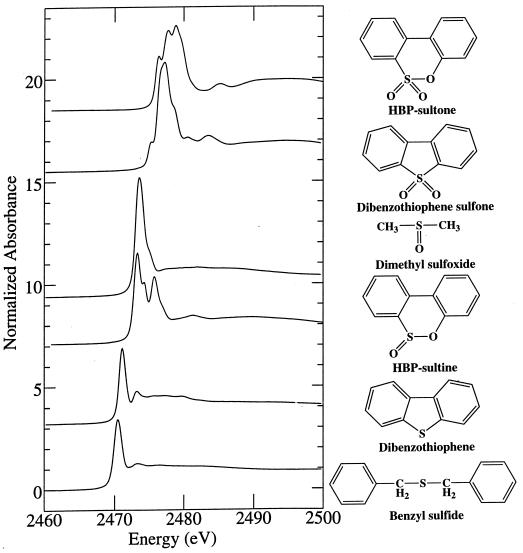
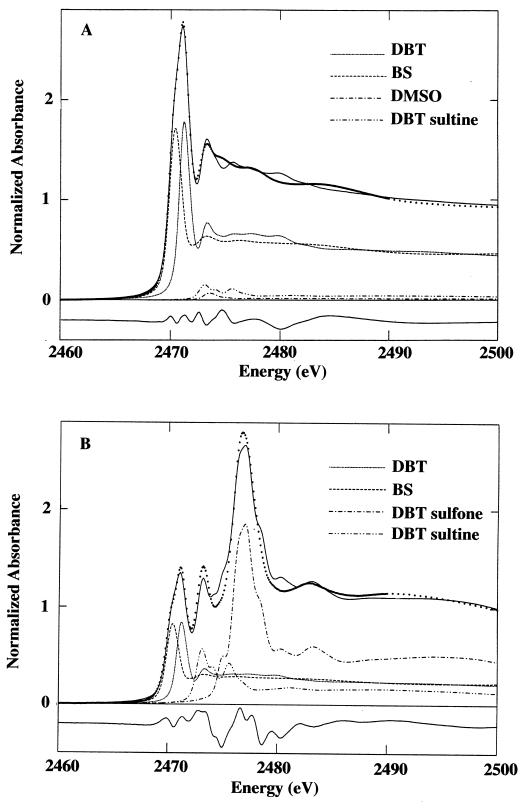
Sulfur K-edge X-ray absorption-edge spectroscopy was used to determine the effect of biodesulfurization on the residual sulfur content of the treated oils. This technique allows for the evaluation of the chemical state of sulfur compounds in a sample, e.g., sulfidic versus thiophenic, and the oxidation state, e.g., sulfoxide versus sulfone. Sulfur K-edge X-ray absorption-edge spectra were obtained on beamline 6-2 at the Stanford Synchrotron Radiation Laboratory. Where appropriate, model compounds were run as powder films, using electron yield detection, and liquid model compounds and the oils were run as dilute solutions in toluene, using fluorescence detection (4). In general, there is a trend toward higher absorbance energies in sulfidic, thiophenic, and oxidized species (in that order) (Fig. 1).
FIG. 1.
Sulfur K-edge X-ray absorption spectra of model compounds.
Crude oil and its distillate fractions contain a mixture of sulfur compounds. The sulfur K-edge X-ray absorption-edge spectra of samples with a mixture of sulfur compounds is equal to the sum of the spectra of the individual components. The contribution of each component sulfur compound to the total sulfur spectra is proportional to the amount of the total sulfur in the sample it represents. The relative amount of different sulfur types in biodesulfurized and control oils was determined from the combination and proportion of model compound spectra which gave the best fit to the spectra of the oil. Fits to the spectra were performed by using least-squares nonlinear optimization as previously described (5).
Although middle-distillate crude oil fractions contain a complex mixture of sulfur compounds, they can be represented by two general chemical types, aliphatic sulfides and thiophenes. These compounds, representing the substrates for biodesulfurization, were modeled by using DBT as a model thiophene and benzylsulfide (BS) as a model aliphatic sulfide. DBT sulfoxide and sulfone have been identified as intermediates of DBT desulfurization by Rhodococcus sp. strain ECRD-1 (17). The compounds 2-hydroxybiphenyl-2′-sulfinic acid and 2-hydroxybiphenyl-2′-sulfonic acid, cyclized during acid extraction to dibenz[c,e][1,2]-oxanthiin 6-oxide (HBP-sultine) and dibenz[c,e][1,2]-oxanthiin 6,6-dioxide (HBP-sultone), respectively, have been identified as intermediates of DBT desulfurization by Rhodococcus sp. strain IGTS8, which also uses a sulfur selective oxidative desulfurization pathway (6, 21). Potential intermediates of biodesulfurization were modeled by using dimethyl sulfoxide (DMSO), DBT sulfone, HBP-sultine, and HBP-sultone. Figure 1 shows the sulfur K-edge X-ray absorption-edge spectra of these compounds. DBT, BS, and DMSO were purchased from Aldrich. DBT sulfone was synthesized as previously described (17), and HBP-sultine and HBP-sultone were synthesized from 2-hydroxybiphenyl by the method of Hanson and Kemp (8). All compounds were greater than 98% pure as determined by GC analysis.
RESULTS
GC, SCD, FID, and total sulfur analyses.
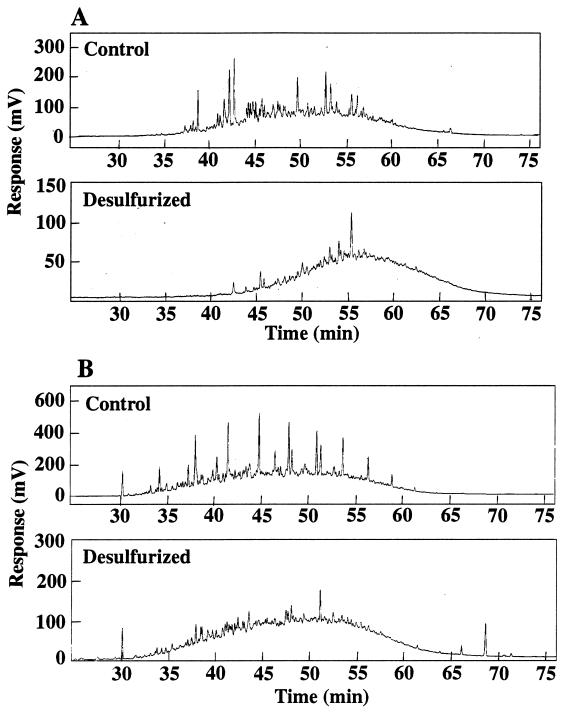
Desulfurization of OB oil by Rhodococcus sp. strain ECRD-1 was performed by using neat OB oil and OB oil diluted in decane. GC-SCD analysis of cultures grown on undiluted OB oil providing approximately 20 ppm of organic sulfur revealed extensive depletion of sulfur compounds across the entire boiling range of the oil (Fig. 2A). Comparison of the sulfur content of the treated versus sterile control oil showed that the treated oil had 8.1% less sulfur than the sterile control oil (Table 1). In addition to sulfur removal, the GC-FID chromatogram showed marked reduction of the resolvable peaks, largely linear alkanes (Fig. 2B). ECRD-1 is able to degrade alkanes but is unable to attack aromatic hydrocarbons (17). The loss of resolvable peaks in the treated OB oil chromatogram was attributed to alkane degradation.
FIG. 2.
GC-SCD (A) and GC-FID (B) chromatograms of sterile control and Rhodococcus sp. strain ECRD-1-desulfurized OB oil treated without decane.
TABLE 1.
Sulfur removal from OB oil by ECRD-1
| Culture | % Sulfur (± relative SD)
|
% Sulfur removed | |
|---|---|---|---|
| Sterile control | Biodesulfurized culture | ||
| Undiluted OB oil | 2.17 (0.44) | 1.99 (0.01) | 8.1 |
| OB oil diluted 10-fold in decane (20 ppm sulfur) | 2.05 (0.26) | 1.44 (0.30) | 29.8 |
| OB oil diluted 20-fold in decane (10 ppm sulfur) | 2.05 (0.26) | 1.45 (0.56) | 29.3 |
The loss of oil due to alkane degradation by ECRD-1 resulted in an underestimation of the extent of sulfur removal. To reduce the degradation of hydrocarbons in the OB oil, experiments using the oil diluted in decane were performed. The added decane provided a preferred alkane substrate that competed for alkane substrates present in the OB oil and was completely resolved from the hydrocarbons present in the OB oil by GC. This allowed for the evaluation of sulfur removal without significant hydrocarbon loss from the OB oil itself.
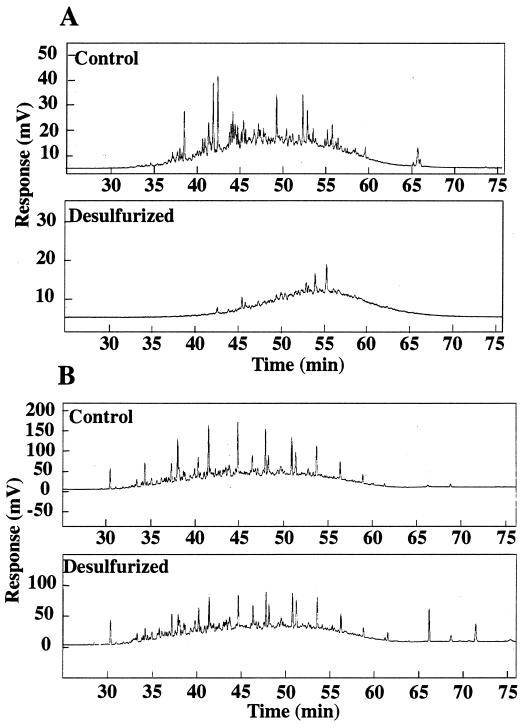
Equal volumes of OB oil diluted 10-fold and 20-fold in decane, providing a final sulfur concentration of approximately 20 and 10 ppm, respectively, were evaluated to assess the maximum amount of sulfur removal possible by ECRD-1. The GC-FID chromatogram of the OB oil sample from the culture receiving 10-fold-diluted OB oil (Fig. 3B) and the culture receiving 20-fold-diluted OB oil (data not shown) showed little change in the resolvable peaks, demonstrating that the decane effectively competed for other alkane substrates in the OB oil. The GC-SCD chromatogram of the OB oil samples from the culture receiving 10-fold-diluted OB oil (Fig. 3A) and the culture receiving 20-fold-diluted OB oil (data not shown) revealed effectively the same pattern of sulfur compound removal as observed without decane. Sulfur removal was calculated to be 30% for both cultures (Table 1). If sulfur removal in the culture receiving 10-fold-diluted OB oil was limited by the sulfur needs of ECRD-1, a greater sulfur reduction should have occurred in the culture receiving 20-fold-diluted OB oil. The fact that this did not occur indicates that 30% sulfur removal is the maximum obtainable by this organism for this oil.
FIG. 3.
GC-SCD (A) and GC-FID (B) chromatograms of sterile control and Rhodococcus sp. strain ECRD-1-desulfurized OB oil diluted in decane.
Sulfur K-edge X-ray absorption-edge spectroscopy.
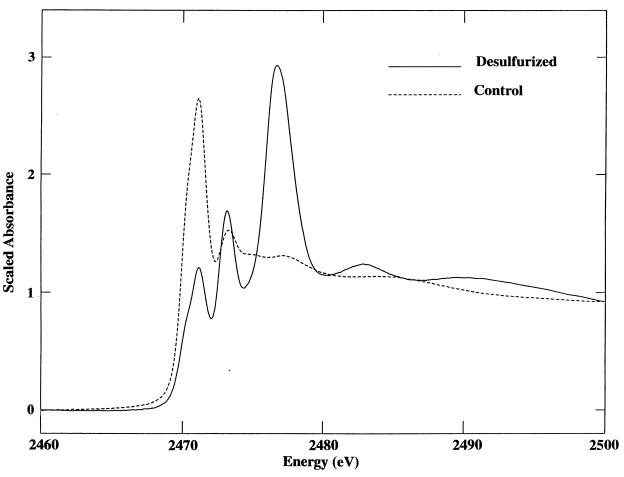
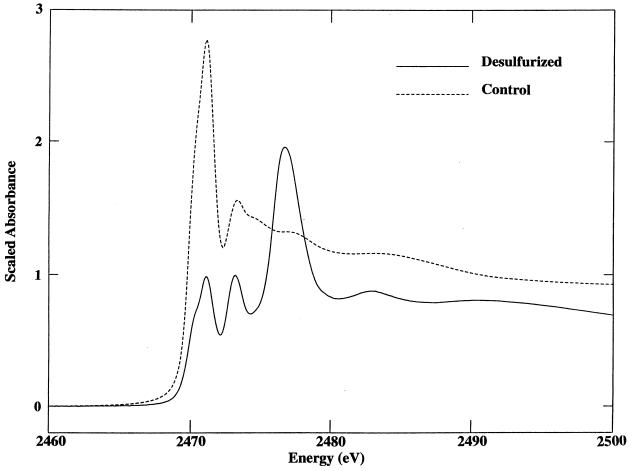
The effect of ECRD-1 biodesulfurization on the chemical state of the sulfur remaining in the OB oil after treatment was determined by analysis of the sulfur K-edge X-ray absorption-edge spectra of the recovered oils. The sulfur spectra of the sterile control and desulfurized OB oil without decane samples are shown in Fig. 4. The spectra of the original and the sterile control were virtually identical, indicating that there were no abiological effects on the sulfur composition due to the culture conditions used (data not shown). In contrast, the spectrum of the treated oil is markedly different from that of the sterile control, showing an increase in absorbance at approximately 2,473 and 2,477 eV that is characteristic of more highly oxidized sulfur species (Fig. 1). Table 2 shows the best-fit composition of sulfur compounds in the sterile control and in the treated oil. In the sterile control, the sulfur compounds are almost equally split between thiophenic and sulfidic forms with a small amount of oxidized sulfur present, modeled in this case by a 4% contribution of HBP-sultine. In marked contrast, the best fit to the treated OB oil sample spectra is obtained by including significant amounts of HBP-sultine (22%) and DBT sulfone (38%). Interestingly, thiophenic and sulfidic sulfur appeared to be affected to an equal extent by the microbial desulfurization. In this analysis and subsequent analyses of OB oil in decane cultures, the sultone could not be meaningfully fit to the data and was not considered to contribute to their spectra.
FIG. 4.
Sulfur K-edge X-ray absorption spectra of sterile control and Rhodococcus sp. strain ECRD-1-desulfurized OB oil treated without decane. Spectra are scaled relative to the levels of sulfur in the oils.
TABLE 2.
Chemical state of sulfur species: OB oil without decane
| Model compound | Relative % of total sulfur
|
|
|---|---|---|
| Sterile control | ECRD-1-desulfurized culture | |
| BS | 50 | 20 |
| DBT | 46 | 20 |
| DMSO | 0 | 0 |
| DBT sulfone | 0 | 38 |
| HBP-sultine | 4 | 22 |
The sulfur K-edge X-ray absorption-edge spectrum of the biodesulfurized OB oil diluted 10-fold in decane also showed a dramatic increase in oxidized sulfur species (Fig. 5). The fits to the spectra and the model spectra contributing to the fits are shown in Fig. 6A and B. The composition of oxidized species was similar to that of the OB oil desulfurized in the absence of decane, but less of the remaining sulfur species were present as HBP-sultine (14% versus 22%), and the total percentage of oxidized species was lower (Table 3). Overall, about 30% of the sulfur was removed, and 50% of the remaining sulfur was oxidized to a chemical state most similar to that of DBT sulfone and HBP-sultine, possibly representing compounds that had proceeded only partially along the desulfurization pathway. The sulfur remaining in the biodesulfurized sample from the culture receiving OB oil diluted 20-fold in decane was somewhat more oxidized than that from the culture receiving 10-fold-diluted OB oil, most noticeably with regard to species with spectra similar to HBP-sultine (data not shown).
FIG. 5.
Sulfur K-edge X-ray absorption spectra of sterile control and Rhodococcus sp. strain ECRD-1-desulfurized OB oil diluted in decane. Spectra are scaled relative to the levels of sulfur in the oils.
FIG. 6.
Linear fits (solid lines) of the sulfur K-edge X-ray absorption spectra of a combination of model compounds to the spectra of OB oil diluted in decane (dotted lines) from sterile control (A) and Rhodococcus sp. strain ECRD-1-desulfurized (B) cultures. The spectra of the individual model compounds contributing to the fit are shown below the oil spectra and fits. The difference between the fit and the experimental data is shown beneath each panel.
TABLE 3.
Chemical state of sulfur species: OB oil diluted 10-fold with decane
| Model compound | Relative % of total sulfur
|
|
|---|---|---|
| Sterile control | ECRD-1-desulfurized culture | |
| BS | 50 | 25 |
| DBT | 49 | 24 |
| DMSO | 1 | 0 |
| DBT sulfone | 0 | 37 |
| HBP-sultine | 0 | 14 |
DISCUSSION
The use of excess decane significantly reduced the extent of OB oil hydrocarbon degradation by Rhodococcus sp. strain ECRD-1 and allowed for an accurate measure of sulfur removal. ECRD-1 was able to remove a maximum of 30% of the total sulfur present in the middle-distillate OB oil. Reducing the amount of sulfur added for biodesulfurization from 20 ppm (OB oil diluted 10-fold in decane) to 10 ppm (OB oil diluted 20-fold in decane) did not increase sulfur removal but appeared to increase HBP-sultine formation. The fact that the final sulfur content of the OB oil was the same in both cases suggests that the substrate range of the desulfurization enzymes and/or bioavailability, not the sulfur requirements of the bacteria, limited further sulfur removal. Comparison of the GC-SCD chromatograms of the oils treated in the presence and absence of decane indicates that the pattern of sulfur compound removal is the same. This suggests that sulfur-containing compounds are not substrates for hydrocarbon degradation enzymes and that sulfur removal is strictly due to the activity of the desulfurization pathway.
Sulfur K-edge X-ray absorption-edge analysis revealed that sulfidic and thiophenic sulfur compounds were equivalently reduced, indicating that this pathway is active against a wide variety of sulfur compounds relevant to the fuel industry. Good fits to the biodesulfurized OB oil sample spectra were obtained with DBT sulfone and HBP-sultine as model oxidized sulfur compounds, consistent with the oxidative desulfurization pathway of Rhodococcus sp. strain IGTS8 described by Gray et al. (6). Gallagher et al. (3) proposed two desulfurization pathways for IGTS8 that are dependent upon the physiological state of the organism. During growth, the pathway followed the sequence DBT sulfoxide, DBT sulfone, HBP-sultone, and 2,2′-dihydroxybiphenyl. In contrast, when DBT was metabolized during stationary phase, 2-hydroxybiphenyl was identified as the end product, and HBP-sultine was observed as an intermediate, which is analogous to the results of Gray et al. (6). In our analysis, HBP-sultone could not be meaningfully fitted to the experimental data, suggesting that it is not a pathway intermediate or that under the conditions used here it is rapidly converted to other products.
In addition to removing 30% of the sulfur in the OB oil, biodesulfurization by ECRD-1 resulted in the conversion of greater than 50% of the remaining sulfur into oxidized forms. This suggests that the substrate range of the sulfur oxidation enzymes is broader than that of the enzymes involved in carbon-sulfur bond cleavage. If true, broadening the substrate range of the carbon-sulfur cleavage enzymes to include those oxidized compounds present in the treated oils should allow for considerably more desulfurization of middle-distillate crude oil fractions. The genes required for DBT desulfurization via the sulfur oxidative pathway have been cloned from strain IGTS8 (2, 23), and the enzymes and cofactors involved have recently been characterized (6). Not all of the oxidized species formed in the treated oils are necessarily due to the desulfurization activity. It is known that alkane degradation enzymes will oxidize sulfur compounds to the sulfoxides, although the production of sulfones and sultines has not been reported (29).
To be commercially useful, microbial desulfurization must be able to greatly reduce the sulfur content of fuel oils which contain a broad range of organic sulfur compounds. The results shown here demonstrate that significant biological sulfur removal can be achieved for a middle-distillate fraction of a crude oil. Further, hydrocarbon degradation of the desulfurized oil does not appear to be involved in desulfurization, indicating that hydrocarbon degradation and desulfurization are distinct and separable activities. Biodesulfurization using a strain lacking the ability to degrade hydrocarbons will clarify any ambiguities regarding the affect of hydrocarbon degradation on desulfurization.
Although significant, the degree of desulfurization shown here is not sufficient to meet the required sulfur levels for all fuels. Nonetheless, the ability to remove sterically hindered compounds not affected by HDS could prove valuable in and of itself.
ACKNOWLEDGMENTS
Stanford Synchrotron Radiation Laboratory is funded by the U.S. Department of Energy, Office of Basic Energy Sciences. The Biotechnology Program is supported by the National Institutes of Health, Biomedical Research Technology Program, Division of Research Resources. Further support is provided by the Department of Energy, Office of Health and Environmental Research.
REFERENCES
- 1.Campbell I M. An orphaned child makes good. The story of US DOE/PETC’s foray into fossil fuel biodesulphurization. American Chemical Society, Division of Petroleum Chemistry, Preprints. 1993;38:275–278. [Google Scholar]
- 2.Denome S A, Oldfield C, Nash L J, Young K D. Characterization of the desulfurization genes from Rhodococcus sp. strain IGTS8. J Bacteriol. 1994;176:6707–6716. doi: 10.1128/jb.176.21.6707-6716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher J R, Olson E S, Stanley D C. Microbial desulfurization of dibenzothiophene: a sulfur-specific pathway. FEMS Microbiol Lett. 1993;107:31–36. doi: 10.1016/0378-1097(93)90349-7. [DOI] [PubMed] [Google Scholar]
- 4.George G N, Gorbaty M L. Sulfur K-edge X-ray absorption spectroscopy of petroleum asphaltenes and model compounds. J Am Chem Soc. 1989;111:3182–3186. [Google Scholar]
- 5.George G N, Gorbaty M L, Keleman S R, Sansone M. Direct determination and quantification of sulfur forms in coals from the Argonne Premium Sample program. Energy Fuels. 1991;5:93–97. [Google Scholar]
- 6.Gray K A, Pogrebinsky O S, Mrachko G T, Lei Xi, Monticello D J, Squires C H. Nat. Biotechnol. 1996;14:1705–1709. doi: 10.1038/nbt1296-1705. [DOI] [PubMed] [Google Scholar]
- 7.Grossman M J. Microbial removal of organic sulfur from fuels: a review of past and present approaches. In: Occelli M L, Chianelli R, editors. Hydrotreating technology for pollution control: catalysts, catalysis, and processes. New York, N.Y: Marcel Dekker; 1996. pp. 345–359. [Google Scholar]
- 8.Hanson G, Kemp D S. Convenient routes to 4,4"-functionalized o-terphenyls and 2,2′-functionalized biphenyls. J Org Chem. 1981;46:5441–5443. [Google Scholar]
- 9.Hartdegen F J, Coburn J M, Roberts R L. The microbial desulfurization of petroleum. Chem Eng Prog. 1984;80:63–67. [Google Scholar]
- 10.Hou C T, Laskin A I. Microbial conversion of dibenzothiophene. Dev Ind Microbiol. 1976;17:351–362. [Google Scholar]
- 11.Izumi Y, Ohshiro T, Ogino H, Hine Y, Shimao M. Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Appl Environ Microbiol. 1994;60:223–226. doi: 10.1128/aem.60.1.223-226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabe T, Ishihara A, Tajima H. Hydrodesulfurization of sulfur-containing polyaromatic compounds in light oil. Ind Eng Chem Res. 1992;31:1577–1580. [Google Scholar]
- 13.Kilbane J J, Bielaga B A. Toward sulfur-free fuels. Chemtech. 1990;20:747–751. [Google Scholar]
- 14.Kodama K, Nakatani S, Umehara K, Shimizu K, Minoda Y, Yamada K. Microbial conversion of petrosulfur compounds. III. Isolation and identification of products from dibenzothiophene. Agric Biol Chem. 1970;34:1320–1324. [Google Scholar]
- 15.Kodama K, Umehara K, Shimizu K, Nakatani S, Minoda Y, Yamada K. Identification of microbial products from debinzothiophene and its proposed oxidation pathway. Agric Biol Chem. 1973;37:45–50. [Google Scholar]
- 16.Laborde A, Gibson D T. Metabolism of dibenzothiophene by Beijerinckia species. Appl Environ Microbiol. 1977;34:783–790. doi: 10.1128/aem.34.6.783-790.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M K, Senius J D, Grossman M J. Sulfur-specific microbial desulfurization of sterically hindered analogs of dibenzothiophene. Appl Environ Microbiol. 1995;61:4362–4366. doi: 10.1128/aem.61.12.4362-4366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik K A, Claus D. Microbial degradation of dibenzothiophene, abstr. 23.03. In: Dellweg H, editor. Abstracts of the Fifth International Fermentation Symposium. Berlin, Germany. 1976. p. 421. [Google Scholar]
- 19.Monticello D J, Bakker D, Finnerty W R. Plasmid-mediated degradation of dibenzothiophene by Pseudomonas species. Appl Environ Microbiol. 1985;49:756–760. doi: 10.1128/aem.49.4.756-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monticello D J, Finnerty W R. Microbial desulfurization of fossil fuels. Annu Rev Microbiol. 1985;39:371–389. doi: 10.1146/annurev.mi.39.100185.002103. [DOI] [PubMed] [Google Scholar]
- 21.Olson E S, Stanley D C, Gallagher J R. Characterization of intermediates in the microbial desulfurization of dibenzothiophene. Energy Fuels. 1993;7:159–164. [Google Scholar]
- 22.Omori T, Monna L, Saiki Y, Kodama T. Desulfurization of dibenzothiophene by Corynebacterium sp. strain SY1. Appl Environ Microbiol. 1992;58:911–915. doi: 10.1128/aem.58.3.911-915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piddington C S, Kovacevich B R, Rambosek J R. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1995;61:468–475. doi: 10.1128/aem.61.2.468-475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rall H T, Thompson C J, Coleman H J, Hopkins R L. Sulfur compounds in crude oil. Bulletin 659. Washington, D.C: U. S. Bureau of Mines; 1972. [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Speight J G. The desulfurization of heavy oils and residua. New York, N.Y: Marcel Dekker; 1981. [Google Scholar]
- 27.Van Afferden M, Schacht S, Klein J, Truper H G. Degradation of dibenzothiophene by Brevibacterium sp. DO. Arch Microbiol. 1990;153:324–328. [Google Scholar]
- 28.Wang P, Krawiec S. Desulfurization of dibenzothiophene to 2-hydroxybiphenyl by some newly isolated bacterial strains. Arch Microbiol. 1994;161:266–271. [Google Scholar]
- 29.Witholt B, De Smet M, Kingma J, Van Beilen J B, Kok M, Lageveen R G, Eggink G. Bioconversion of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. Trends Biotechnol. 1990;8:46–52. doi: 10.1016/0167-7799(90)90133-i. [DOI] [PubMed] [Google Scholar]
- 30.Yen K M, Gunsalus I C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci USA. 1982;79:874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]