Abstract
The respiratory and photosynthetic quinones of microbial mats which occurred in Japanese sulfide-containing neutral-pH hot springs at different temperatures were analyzed by spectrochromatography and mass spectrometry. All of the microbial mats that developed at high temperatures (temperatures above 68°C) were so-called sulfur-turf bacterial mats and produced methionaquinones (MTKs) as the major quinones. A 78°C hot spring sediment had a similar quinone profile. Chloroflexus-mixed mats occurred at temperatures of 61 to 65°C and contained menaquinone 10 (MK-10) as the major component together with significant amounts of either MTKs or plastoquinone 9 (PQ-9). The sunlight-exposed biomats growing at temperatures of 45 to 56°C were all cyanobacterial mats, in which the photosynthetic quinones (PQ-9 and phylloquinone) predominated and MK-10 was the next most abundant component in most cases. Ubiquinones (UQs) were not found or were detected in only small amounts in the biomats growing at temperatures of 50°C and above, whereas the majority of the quinones of a purple photosynthetic mat growing at 34°C were UQs. A numerical analysis of the quinone profiles was performed by using the following three parameters: dissimilarity index (D), microbial divergence index (MDq), and bioenergetic divergence index (BDq). A D matrix tree analysis showed that the hot spring mats consisting of the sulfur-turf bacteria, Chloroflexus spp., cyanobacteria, and purple phototrophic bacteria formed distinct clusters. Analyses of MDq and BDq values indicated that the microbial diversity of hot spring mats decreased as the temperature of the environment increased. The changes in quinone profiles and physiological types of microbial mats in hot springs with thermal gradients are discussed from evolutionary viewpoints.
Geothermal hot spring streams provide favorable conditions for the development of microbial mats, which contain physiologically and phylogenetically different groups of procaryotes, such as chemotrophic sulfur bacteria, cyanobacteria, and anoxygenic phototrophic bacteria, depending on the temperature, pH, sulfide concentration, and some other environmental conditions (for reviews see references 4, 8–10, and 37). The different types of hot spring microbial mats have been studied extensively as simple and stable systems in order to understand the ecology and evolution of microbial communities. The major constituents of hot spring microbial mats are not cultured in many cases. Therefore, non-culture-dependent approaches are essential to study the microbial communities of hot spring environments.
One of the most promising culture-independent approaches in this research area is an rRNA approach which involves selective cloning of naturally occurring 16S rRNA as cRNA or PCR cloning of environmental 16S rRNA genes (2, 3, 24, 40, 41, 44). In a previous study, we used the 16S ribosomal DNA (rDNA) clone library method to perform a community analysis of the so-called sulfur-turf bacterial mats and found that novel 16S rDNA phylotypes that represent a deeply branching clade within the Aquifex-Hydrogenobacter complex (Aquificales) predominated in the sulfur-turf mats (46).
Another culture-independent approach useful for hot spring community analysis is a chemical biomarker approach which involves direct extraction and identification of cell envelope lipid constituents (36). Quinone profiling, which is a chemical method for detecting various structural types of respiratory and photosynthetic quinones in microbial cells, not only has been useful in microbial chemotaxonomy (11, 12) but also has potential for estimating microbial redox states (16) and community structures (17–23, 27) in the environment. The fact that representative species of thermophilic and hyperthermophilic bacteria contain specific quinone homologs provides a basis for using the quinone profile method for analyzing the communities of hot spring bacterial mats. For example, Hydrogenobacter species, which are the hot spring-inhabiting chemolithotrophs of the earliest branching eubacterial lineage, the Aquificales (5, 39), contain novel sulfur-containing naphthoquinones called methionaquinones (MTKs) (26, 28, 43). Chloroflexus species, which are widespread in thermophilic photosynthetic bacterial mats (8–10, 38), contain menaquinones (MKs) with 10 isoprene units in the side chain as the main components (13–15). Cyanobacteria are common photosynthetic procaryotes in hot spring microbial mats as well (6–10) and contain plastoquinones (PQs) and phylloquinone (K1) (11). Moreover, purple phototrophic bacteria, which occasionally exhibit massive red growth in hot spring streams (4, 30, 31, 33), contain ubiquinones (UQs) with eight isoprene units in most cases, and some of these organisms also contain MKs (25).
In this study, the quinone profile method was used to characterize biomat communities in sulfide-containing neutral-pH hot springs which are growing at different temperatures in geographically different areas of Japan. Relationships between microbial quinone profiles and thermal gradients of hot springs are discussed below from evolutionary and ecological viewpoints. To our knowledge, this report is the first description of the quinone profiles of thermal microbial communities.
MATERIALS AND METHODS
Mat samples.
Microbial mats were collected from the following four hot springs in Japan: Ganiba (39°47′N, 140°48′E) in Akita Prefecture; Nakanoyu (36°12′N, 137°36′E) in Nagano Prefecture; Yumata (36°24′N, 137°41′E) in Nagano Prefecture; and Nikko-Yumoto (36°48′N, 139°25′E) in Tochigi Prefecture. A sediment sample was also collected from a hot spring in Yufuin (33°15′N, 131°20′E) in Oita Prefecture. For comparison, a sewage mat sample was collected from a sewage ditch in Toyohashi in Aichi Prefecture. Detailed information concerning these samples is shown in Table 1. Temperature and pH were measured in situ with a portable thermometer and pH meter. Surface material was collected from microbial mats and sediments in sterile polyethylene bottles and was transported in an insulated cooler to the laboratory. Biomass and suspended solids were harvested by centrifugation, washed once with 50 mM phosphate buffer (pH 7.0), and stored at −20°C until they were analyzed.
TABLE 1.
Microbial mats and sediment samples studied
| Sample | Sampling site (prefecture) | Date of sampling | Water temp (°C) | pH | Color | Appearance |
|---|---|---|---|---|---|---|
| GANI | Ganiba spa (Akita) | 16 May 1995 | 50 | 8.0 | White | Sulfur-turf mat |
| YUM-1 | Yumata spa (Nagano) | 8 August 1996 | 73 | 6.5 | Black | Sulfur-turf-like mat |
| YUM-2 | Yumata spa (Nagano) | 8 August 1996 | 68 | 5.9 | White | Sulfur-turf mat |
| YUM-3 | Yumata spa (Nagano) | 8 August 1996 | 65 | 6.2 | Yellow | Chloroflexus-mixed mat |
| YUM-4 | Yumata spa (Nagano) | 8 August 1996 | 50 | 6.4 | Green | Cyanobacterial mat |
| NAK-1 | Nakanoyu spa (Nagano) | 25 May 1996 | 75 | 6.6 | White-yellow | Sulfur-turf mat |
| NAK-2 | Nakanoyu spa (Nagano) | 25 May 1996 | 61 | 6.5 | Orange-green | Chloroflexus-mixed mat |
| NAK-3 | Nakanoyu spa (Nagano) | 25 May 1996 | 53 | 5.7 | Green | Cyanobacterial mat |
| NAK-4 | Nakanoyu spa (Nagano) | 25 May 1996 | 52 | 5.8 | Green | Cyanobacterial mat |
| NIK-1 | Nikko-Yumoto spa (Tochigi) | 10 October 1995 | 56 | 7.5 | Green | Cyanobacterial mat |
| NIK-2 | Nikko-Yumoto spa (Tochigi) | 10 October 1995 | 45 | 7.4 | Green | Cyanobacterial mat |
| NIK-3 | Nikko-Yumoto spa (Tochigi) | 10 October 1995 | 34 | 6.8 | Red | Purple photosynthetic mat |
| YUF | Yuhin hot spring (Oita) | 3 May 1996 | 78 | 8.0 | Hot spring sediment | |
| SEW | Toyohashi (Aichi) | 9 June 1996 | 22 | 7.0 | Gray | Sewage ditch mat |
Microscopic and spectroscopic studies.
Portions of washed mat samples were appropriately diluted with 50 mM phosphate buffer (pH 7.0) and observed with an Olympus model BX-50 phase-contrast microscope. Pigments were extracted from washed biomass with an acetone-methanol mixture (7:2, vol/vol), and absorption spectra were measured with a Shimadzu model BioSpec-1600 spectrophotometer.
Quinone extraction and fractionation.
Washed samples (ca. 1 to 5 g [wet weight] for mats; 40 g [wet weight] for sediments) were resuspended in 50 mM phosphate buffer (pH 7.0). Each suspension was mixed with 2.5 volumes of a chloroform-methanol mixture (2:1, vol/vol), sonicated for 1 min on ice (20 kHz; output power, 100 W), and centrifuged at 5,000 × g for 10 min. The resulting upper aqueous layer was discarded, and the lipid layer was collected with a pipette. The residue was extracted once with acetone and then twice (30 min each) with the chloroform-methanol mixture. All extracts were combined, evaporated under a vacuum, and reextracted three times with n-hexane–1% saline (1:1, vol/vol). The hexane extract was concentrated and then applied to a chromatography column with Sep-Pak Vac cartridges (Waters Corp., Milford, Mass.) to separate the MK and UQ fractions. Detailed information concerning the procedures used has been given elsewhere (22, 27).
Analysis of quinones.
Quinone components were separated and identified by spectrochromatography with a Beckman model 110B liquid chromatograph or a Shimadzu model LC-10 liquid chromatograph equipped with a diode array detector. Mass spectrometry was used to confirm the chemical structures of quinones separated by high-performance liquid chromatography (HPLC). Details of the analytical methods used have been described previously (22). For quantification of quinones, detector response factors were determined on the basis of HPLC data obtained by using a known concentration of quinones which had been determined by the reduced-minus-oxidized difference spectrum method (29). Since the extinction coefficients of MTKs are not known, MTKs were quantified by assuming that they have the same extinction coefficients at 263 nm as their analogs, the MKs.
Abbreviations of quinones and standard quinones.
UQs, PQs, MKs, and MTKs with n isoprene units in their side chains were abbreviated Q-n, PQ-n, MK-n, and MTK-n, respectively. Partially hydrogenated MKs and MTKs were designated MK-n(Hx) and MTK-n(Hx), where x indicates the number of hydrogen atoms saturating the side chain. Standard quinones were prepared from raw sewage and activated sludge as described previously (22). In addition, MK-10 and MTK-7(H4) were purified from Chloroflexus aurantiacus J-10-fl and Hydrogenobacter thermophilus JCM 7687, respectively, and were used as the standard quinones.
Numerical analysis.
Differences in the community structures of the microbial mats were estimated simply by visually interpreting quinone profile data. To enhance the objectivity of the information and to make quantitative estimates of population shifts over time and space, however, it was necessary to process data by performing an appropriate numerical cluster analysis (20, 27). Three parameters, the microbial divergence index (MDq), the bioenergetic divergence index (BDq), and the dissimilarity index (D) (27), were used in the numerical analysis of quinone profiles. MDq is given by:
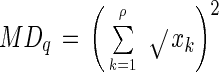 |
where xk ≥ 0.001 and xk indicates the molar ratio of the content of the quinone homolog k to the total quinone content (defined as 1). Since MDq values indicate the divergence of the quinone structural types detected, this parameter indicates the extent of the diversity of microbial taxa (23, 27). BDq is given by:
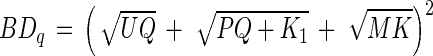 |
where UQ, (PQ + K1), MK ≥ 0.001, and UQ, PQ, K1, and MK indicate the molar fractions of UQs, PQs, K1, and MKs (plus their derivatives), respectively, compared to the total quinone content. Therefore, BDq is a reflection of the divergence of bioenergetic modes of microbes (i.e., the balance of UQ-mediated aerobic respiration, oxygenic photosynthesis, and anaerobic and/or MK-mediated aerobic respiration) (27). D is given by:
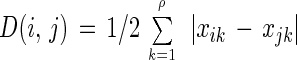 |
where xik, xjk ≥ 0.01, ∑ xjk = ∑ xjk = 100, and xjk and xjk indicate the levels (expressed as moles percent) of the quinone homolog k in samples i and j, respectively. The D values can be used as indicators of differences in community structure among samples (20). The numerical analysis was performed with a personal computer program, BioCLUST, written by one of us (A.H.) for use with an IBM personal computer and other compatible computers (27). The algorithm of the neighbor-joining (NJ) method (42) was used to construct a dendrogram based on D matrix data. A dendrogram was drawn by using the TreeView program (35).
RESULTS
Microscopic and spectroscopic observations.
On the basis of in situ observations and microscopic and spectroscopic studies, the hot spring microbial mats from high-temperature environments (temperatures, >45°C) were classified into the following three major types: sulfur-turf mats, Chloroflexus-mixed mats, and cyanobacterial mats. In addition to these types, a purple photosynthetic mat (NIK-3) was found in the Nikko-Yumoto spa (temperature, 34°C (Table 1 and Fig. 1).
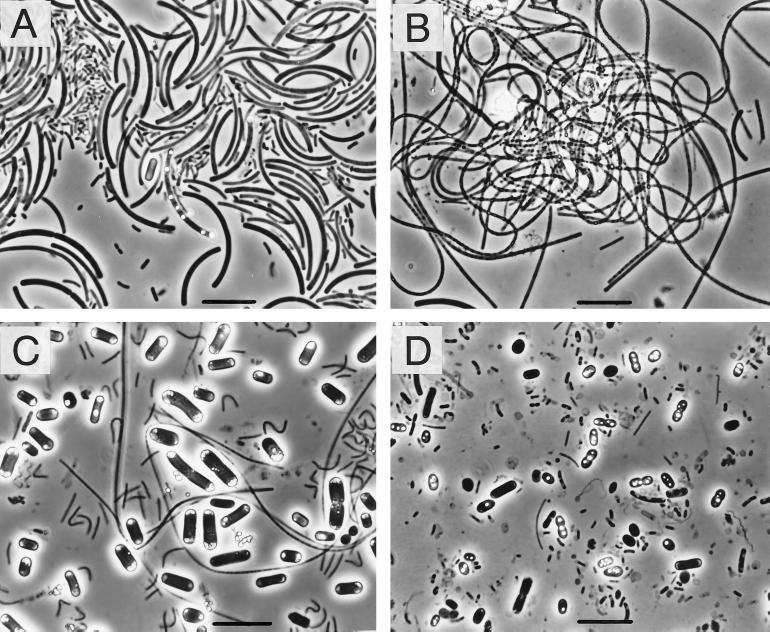
FIG. 1.
Phase-contrast micrographs showing typical cell morphologies of the predominant bacteria in hot spring microbial mats. (A) Sulfur-turf mat (YUM-2). (B) Chloroflexus-mixed mat (NAK-2). (C) Cyanobacterial mat (NAK-3). (D) Purple photosynthetic mat (NIK-3). Bars = 10 μm.
All of the sulfur-turf mats studied contained great numbers of relatively large sausage-shaped bacteria (Fig. 1A); this morphotype has been described previously as the typical morphotype of the sulfur-turf bacteria (32, 46). The cells of some of these sausage-shaped bacteria were accompanied by elemental sulfur granules. In the Chloroflexus-mixed mats, flexible filamentous bacteria, possibly Chloroflexus species, were abundant (Fig. 1B), and the sausage-shaped sulfur-turf bacteria were present in smaller numbers. The cyanobacterial mats contained large rod-shaped cyanobacteria that occurred singly and occasionally in chains (Fig. 1C). In the purple photosynthetic mat, elemental sulfur-containing large cells, possibly Chromatium cells or cells of related purple sulfur bacteria, occurred in large numbers together with much smaller rods and cocci (Fig. 1D).
Spectrophotometric measurements showed that all of the Chloroflexus-mixed mats and cyanobacterial mats studied contained a photosynthetic pigment having an absorption maximum at 660 nm in the acetone-methanol mixture, indicating that bacteriochlorophyll c and/or chlorophyll a was present (data not shown).
HPLC elution patterns.
HPLC experiments revealed that most hot spring samples contained quinones consisting of both UQ and MK fractions; the only exception was sediment sample YUF, which contained no UQs. In the UQ fractions, it was easy to identify each component by spectrochromatography alone, because all of the samples produced a simple elution pattern, such as that found in wastewater sludges, which in general produce three major components, Q-8, Q-9, and Q-10 (17–22). On the other hand, the MK fractions from all of the hot spring samples produced simple but characteristic HPLC patterns which have never been found in wastewater environments.
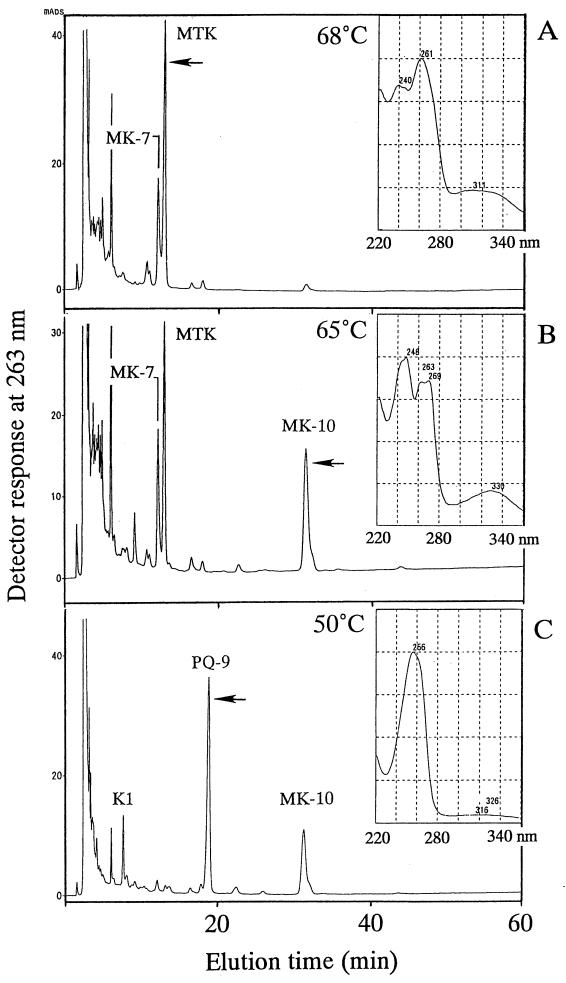
The MK fractions of the microbial mat samples from the high-temperature environments (temperatures, >45°C) produced three major HPLC patterns depending on the type of microbial mats and the temperature at which they occurred in situ. Examples of the three patterns which were obtained from the Yumata spa samples (samples YUM-2, YUM-3, and YUM-4) are shown in Fig. 2. Sample YUM-2, which was a typical sulfur-turf mat sample obtained from a 68°C stream, produced a major component having an HPLC retention time of 13.0 min (Fig. 2A) and absorption maxima at 240, 261 (maximum), and 311 nm (Fig. 2A, inset). This spectral pattern was the same as that of MTK-7(H4) purified from H. thermophilus. However, the HPLC elution time of the main component of the YUM-2 sample differed from the HPLC elution time of MTK-7(H4), which eluted at 17.9 min (data not shown). This suggested that the main YUM-2 component was an MTK homolog that differed from MTK-7(H4) in the structure of the multiprenyl side chain. MK-7 eluted at 12.2 min as the second most abundant component in sample YUM-2. Sample YUM-3 was from a Chloroflexus-mixed mat developing at 65°C and produced a major MK component that eluted at 27 min in addition to the MTK component noted above (Fig. 2B). This MK component was identified as MK-10 by comparing its HPLC elution time and UV absorption spectrum with the HPLC elution time and UV absorption spectrum of the MK standards. In sample YUM-4, which was from a cyanobacterial mat growing at 50°C, PQ-9 was detected as the predominant component together with significant amounts of MK-10 and K1 (Fig. 2C).
FIG. 2.
HPLC elution profiles of the MK fractions of three microbial mats from the Yumata hot spring stream growing at different temperatures. The UV absorption spectra of the predominant components (indicated by arrows) are shown in insets. (A) Components of sulfur-turf mat growing at 68°C (YUM-2). (B) Components of Chloroflexus-mixed mat growing at 65°C (YUM-3). (C) Components of cyanobacterial mat growing at 50°C (YUM-4).
Mass spectrometry.
The chemical structures of all of the quinone components detected by HPLC were confirmed by mass spectroscopy. For example, a mass spectroscopy analysis of the MK fraction of YUM-2 showed that the major MK component that eluted at 12.2 min had a molecular ion peak (M+1) at m/z 649.6, thereby confirming that it was MK-7. The predominant MTK component that appeared at 13.0 min had a molecular ion peak (M+1) at m/z 681.6, a value which is 4 mass units lower than that of MTK-7(H4) (26). Thus, on the basis of the results of both spectrochromatography and mass spectrometry, the major MTK component detected in this study was identified tentatively as fully unsaturated MTK-7. A detailed structural determination analysis of this MTK component will be described elsewhere. The minor component that eluted at 17.9 min had a molecular ion peak (M+1) at m/z 685.6 and was identified as MTK-7(H4).
Quinone compositions.
The quinone contents of all test samples, as determined by HPLC and mass spectrometry, are summarized in Table 2. There were marked differences in quinone patterns, as well as in the total quinone contents, among the samples examined. MTKs (mostly MTK-7) predominated (>68%) in the sulfur-turf (GANI, YUM-1, YUM-2, and NAK-1) and sediment (YUF) samples. In most of the sulfur-turf mats, considerable amounts of MK-7 also occurred, but other quinone homologs were not detected or were present at only low levels. In the Chloroflexus-mixed mats (YUM-3 and NAK-2), MK-10 was a major component (37 to 55%) comparable to MTKs. All of the cyanobacterial mat samples (YUM-4, NAK-3, NAK-4, NIK-1, and NIK-2) produced the photosynthetic quinones (PQ-9 and K1) as the primary components (34 to 79%). In these green mats at temperatures of ≥50°C, MK-10 also constituted a significant proportion of the total content (17 to 27%), suggesting that Chloroflexus spp. were present in the cyanobacterial mats. In all samples from mats at temperatures of ≥50°C, UQs constituted small fractions of the total content (<10%), whereas in the 34°C purple photosynthetic mat (NIK-2), UQs (with Q-8 predominating) accounted for the majority of the total quinone content.
TABLE 2.
Quinone compositions of microbial mat and sediment samples
| Homolog | Quinone composition (mol%)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GANI (50°C) | YUM-1 (75°C) | YUM-2 (68°C) | YUM-3 (65°C) | YUM-4 (50°C) | NAK-1 (73°C) | NAK-2 (61°C) | NAK-3 (53°C) | NAK-4 (52°C) | NIK-1 (56°C) | NIK-2 (45°C) | NIK-3 (34°C) | YUF (78°C) | SEW (22°C) | |
| Q-7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.02 | 0 | 0.62 |
| Q-8 | 0.48 | 1.85 | 1.81 | 2.91 | 1.70 | 0.45 | 2.23 | 1.14 | 6.70 | 4.52 | 8.46 | 38.25 | 0 | 30.65 |
| Q-9 | 0 | 0 | 0.51 | 0 | 0.40 | 0.19 | 0 | 0.44 | 0.26 | 0.18 | 1.21 | 6.12 | 0 | 2.98 |
| Q-10 | 0 | 1.56 | 1.29 | 0.71 | 0.66 | 0.17 | 1.08 | 1.85 | 1.10 | 1.06 | 4.55 | 6.12 | 0 | 8.46 |
| Q-11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.33 |
| PQ-9 | 0 | 0 | 0 | 0 | 73.17 | 0 | 9.03 | 20.09 | 32.49 | 35.87 | 58.66 | 7.69 | 0 | 19.62 |
| K1 | 0 | 0 | 0 | 0 | 5.59 | 0 | 1.12 | 14.88 | 21.00 | 12.65 | 10.01 | 1.22 | 0 | 1.89 |
| MK-6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.92 | 1.30 | 1.55 | 1.03 | 2.13 | 0 | 15.56 |
| MK-7 | 4.36 | 13.02 | 24.49 | 18.39 | 0 | 14.35 | 18.20 | 6.54 | 5.05 | 7.21 | 7.89 | 17.20 | 12.98 | 10.32 |
| MK-8 | 0 | 0 | 0 | 0 | 0 | 0 | 0.80 | 9.72 | 2.79 | 2.45 | 4.57 | 23.50 | 1.65 | 1.56 |
| MK-9 | 0 | 0 | 0 | 1.02 | 1.31 | 0.20 | 1.44 | 7.94 | 1.89 | 2.23 | 0.33 | 1.32 | 0.06 | 0.88 |
| MK-10 | 0 | 0 | 2.09 | 38.36 | 17.16 | 0 | 49.82 | 27.32 | 19.02 | 31.05 | 3.02 | 0.12 | 0.10 | 1.56 |
| MK-11 | 0 | 0 | 0 | 0.45 | 0 | 0 | 1.55 | 8.16 | 3.52 | 1.23 | 0.12 | 0 | 0 | 0.24 |
| MK-12 | 0 | 0 | 0 | 0 | 0 | 0 | 0.09 | 0 | 0 | 0 | 0 | 0 | 0 | 0.10 |
| MK-8(H4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.12 | 0 | 1.72 |
| MK-8(H6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.06 |
| MK-9(H2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.41 |
| MK-9(H4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.16 | 0 | 2.06 |
| MK-9(H8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.04 | 0 | 0.98 |
| MTK-7 | 95.20 | 65.54 | 67.98 | 36.73 | 0 | 81.95 | 14.64 | 0 | 4.88 | 0 | 0 | 0 | 45.13 | 0 |
| MTK-7(H4) | 0 | 18.03 | 1.83 | 0.93 | 0 | 2.69 | 0 | 0 | 0 | 0 | 0 | 0 | 40.08 | 0 |
The total quinone contents of the GANI, YUM-1, YUM-2, YUM-3, YUM-4, NAK-1, NAK-2, NAK-3, NAK-4, NIK-1, NIK-2, NIK-3, YUF, and SEW samples were 11.3, 9.3, 35.9, 42.4, 47.8, 50.1, 101, 129, 135, 162, 110, 105, 5.6, and 156 nmol/g (wet weight), respectively.
Numerical analysis.
Differences in quinone profiles among the mat samples were quantitatively estimated by using D (Table 3). Also, the quinone profiles obtained were evaluated by using MDq and BDq, which were considered reflections of the diversity of the microbial taxa and the diversity of their bioenergetic modes, respectively. The MDq values for microbial mats seemed to increase with decreasing in situ temperature, as the MDq values were 1.57 to 3.59 for the sulfur-turf mats (temperatures of most mates, ≥68°C; GANI temperature, 50°C), 3.44 to 6.44 for the Chloroflexus-mixed mats (61 to 65°C), 4.75 to 8.59 for the cyanobacterial mats (45 to 55°C), and 7.65 for the purple photosynthetic mat (34°C). This was also the case for the BDq values.
TABLE 3.
D value matrix data and MDq and BDq values for microbial mat and sediment samples
| Sample |
D value with:
|
MDq | BDq | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GANI | YUM-1 | YUM-2 | YUM-3 | YUM-4 | NAK-1 | NAK-2 | NAK-3 | NAK-4 | NIK-1 | NIK-2 | NIK-3 | YUF | |||
| GANI | 1.57 | 1.14 | |||||||||||||
| YUM-1 | 29.6 | 3.44 | 1.36 | ||||||||||||
| YUM-2 | 27.2 | 16.5 | 3.68 | 1.37 | |||||||||||
| YUM-3 | 58.2 | 46.5 | 39.1 | 4.75 | 1.37 | ||||||||||
| YUM-4 | 99.5 | 97.6 | 95.1 | 79.2 | 3.59 | 2.20 | |||||||||
| NAK-1 | 13.3 | 18.1 | 15.0 | 46.9 | 99.0 | 2.71 | 1.18 | ||||||||
| NAK-2 | 80.5 | 69.4 | 62.2 | 24.1 | 69.0 | 70.2 | 6.44 | 2.05 | |||||||
| NAK-3 | 95.2 | 90.8 | 88.5 | 62.6 | 53.6 | 92.5 | 50.0 | 8.64 | 2.45 | ||||||
| NAK-4 | 90.3 | 87.1 | 84.8 | 65.7 | 40.8 | 89.1 | 53.8 | 29.0 | 8.59 | 2.67 | |||||
| NIK-1 | 95.2 | 89.9 | 87.7 | 56.4 | 37.5 | 91.8 | 44.8 | 23.6 | 18.2 | 7.18 | 2.60 | ||||
| NIK-2 | 95.1 | 88.6 | 86.3 | 84.7 | 29.6 | 91.0 | 74.3 | 50.8 | 37.0 | 33.9 | 6.81 | 2.62 | |||
| NIK-3 | 97.7 | 86.1 | 81.6 | 80.3 | 89.4 | 87.2 | 71.0 | 70.6 | 75.0 | 72.7 | 65.4 | 7.65 | 2.72 | ||
| YUF | 50.5 | 23.9 | 40.0 | 49.0 | 99.8 | 39.1 | 71.4 | 91.7 | 88.3 | 91.0 | 90.2 | 87.7 | 3.33 | 1.00 | |
| SEW | 95.2 | 86.3 | 84.5 | 83.1 | 73.3 | 88.7 | 72.7 | 62.4 | 59.8 | 59.7 | 51.7 | 37.9 | 88.0 | 10.97 | 2.94 |
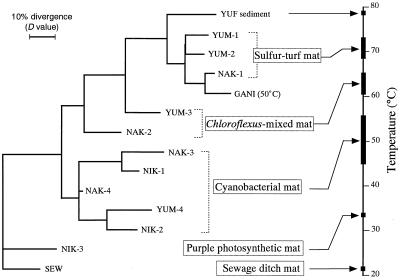
On the basis of the D matrix data shown in Table 3, we constructed an NJ dendrogram which grouped the hot spring microbial mats from environments having different temperatures (Fig. 3). The four physiologically different types of microbial mats (i.e., the sulfur-turf bacterial, Chloroflexus, cyanobacterial, and purple phototrophic bacterial mats) occurred in distinct clusters on the dendrogram. The branching order of the clusters was correlated with temperatures from which the samples were collected.
FIG. 3.
NJ dendrogram showing relationships among community structures of different microbial mats based on D value matrix data. The ranges of temperatures from which the mat samples were collected are shown. Sample SEW was used as an outgroup to root the tree.
Relationship between quinone profiles and temperature.
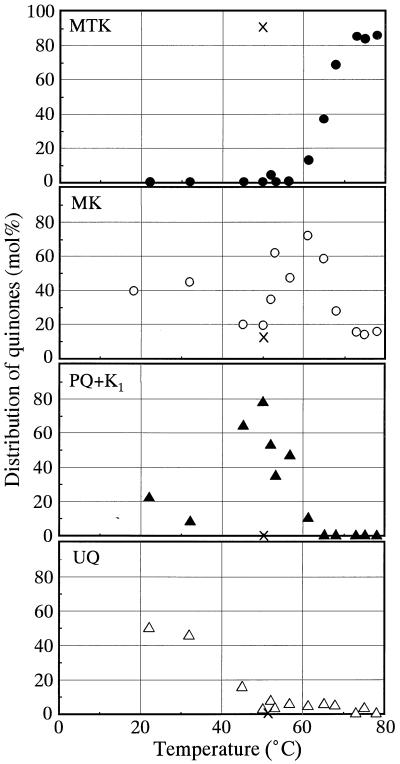
To clarify the relationship between quinone profiles and temperature in hot spring environments, the contents of the major quinone ring structural types in the hot spring environments were plotted as a function of in situ temperature (Fig. 4). It was clear that MTKs predominated only in high-temperature environments with temperatures above 65°C, although we found that one 50°C sample (GANI) contained a high proportion of MTKs. The maximum proportion of MKs was found at temperatures around 60°C. PQs and K1 were the most frequent structural types at temperatures between 50 and 60°C. UQs were abundant only in the environments where the temperature was less than 40°C.
FIG. 4.
Changes in the proportions of major quinone ring structural types detected in hot spring mats as a function of temperature. ×, data for the 50°C sulfur-turf mat sample (sample GANI).
DISCUSSION
We found that quinone profiling of the hot spring bacterial mats from environments having different temperatures resulted in some interesting observations. One of our most striking findings is that MTKs predominated in all of the sulfur-turf bacterial mats studied, despite the fact that samples were collected from geographically different areas in Japan. Previously, MTKs have been found to be the sole quinones in the thermophilic chemolithotrophic bacteria Hydrogenobacter spp., and MTK-7(H4) is the major homolog in all of these bacteria (26, 28, 43). On the other hand, this study showed that all of the major MTK types of the sulfur-turf bacteria were MTK-7 types.
A previous molecular approach showed that a new 16S rDNA phylotype that branches deeply from the Aquificales lineage predominates in sulfur-turf mats (46). A concurrent in situ hybridization assay performed with a 16S rRNA-targeted oligonucleotide probe demonstrated that the sausage-shaped large bacteria that commonly predominate in sulfur-turf mats (32) correspond to this phylotype (46). Our quinone profile findings strongly suggest that the sulfur-turf bacteria having the phylotype and morphotype mentioned above contain MTK-7 as their major quinone component, and this chemotaxonomic feature is consistent with the phylogenetic positions of these organisms as members of the Aquificales. Although there is no information available concerning the quinone systems of members of the Aquificales other than Hydrogenobacter spp. and the sulfur-turf bacteria, it has been suggested that MTKs are essential components of the respiratory chains in this (hyper-)thermophilic phylogenetic group. Recently, MTKs have also been found in an aerobic hyperthermophilic archaeon (34).
Another interesting observation is that the quinone profiles and the physiological types of microbial mats were dramatically different depending on the temperature at which the mats developed in situ and that these differences occurred independent of the geographical locations of the mats sampled. The predominant structural types of quinones detected changed with decreasing temperature of the hot springs, as follows: >68°C, MTK-7; 61 to 65°C, MK-10; 45 to 56°C, PQ-9 plus K1; <40°C, Q-8. These differences in the main structural types of quinones in response to temperature were a reflection of the development of physiologically and phylogenetically different bacterial communities; namely, specific detection of MTK-7, MK-10, PQ-9, and Q-8 as the major quinone types at the different temperatures indicated that the sulfur-turf bacteria, Chloroflexus spp., thermophilic cyanobacteria, and purple phototrophic bacteria, respectively, were the predominant organisms. An exception was the 50°C GANI sample, which contained a high proportion of MTKs. Since the GANI sampling site was in a thermal stream pipe not exposed to sunlight, it did not provide favorable conditions for photosynthetic growth. This explains why the MTK-producing bacteria predominated at such a low temperature.
MK homologs other than MK-10, such as MK-7, were found to be widespread in the hot spring mats independent of temperature. Detection of these quinone homologs may have been due to the presence of phylogenetically diverse thermophilic chemotrophs. In fact, microscopic studies revealed that all of the microbial mats studied contained various morphological types of unknown bacteria in addition to the typical sulfur-turf bacteria (sausage-shaped bacteria), Chloroflexus spp., cyanobacteria, and purple phototrophic bacteria. Also, at this time, we cannot exclude the possibility that archaeal species, which produce MKs in general, coexist in hot spring microbial mats.
The numerical analysis based on the D value matrix data revealed that the microbial mats containing the sulfur-turf bacteria, Chloroflexus spp., cyanobacteria, and purple phototrophic bacteria form distinct clusters. These four physiologically different types of mats could also be characterized by using MDq and BDq values. Interestingly, the microbial diversity of hot spring biomats shown by MDq and BDq values was related to the temperatures of the environments from which the biomats were taken. The extent of hot spring microbial diversity with respect to both species composition and energy-yielding modes of the inhabitants decreased as the in situ temperature increased.
In conclusion, quinone profiling of hot spring environments is useful not only for characterization of microbial community structures but also for evaluation of the energy-yielding modes of the microbes present, both of which change sharply in response to the temperature of the environment. The changes occur with decreasing temperature in the following order: MTK-mediated respiration by members of the Aquificales→MK-involved anoxygenic photosynthesis by Chloroflexus spp.→PQ-involved photosynthesis by cyanobacteria→UQ-involved anoxygenic photosynthesis by purple bacteria. The phylogenetic tree of modern organisms provides circumstantial evidence that life on Earth arose from hyperthermophilic ancestors (1, 5, 45) and that the four phylogenetic groups described above branch more deeply in the following order: Aquificales←Chloroflexus←cyanobacteria←purple bacteria. Thus, the succession of different quinone-containing bacterial populations found in extant hot spring environments with a thermal gradient may have some evolutionary implications.
ACKNOWLEDGMENTS
We thank S. Higuchi, Nagano Research Institute for Health and Pollution, for his help with sampling and S. Ishii, Department of Biotechnology, The University of Tokyo, for making his unpublished data on Hydrogenobacter quinones available to us. We especially thank K. Matsuura, Department of Biology, Tokyo Metropolitan University, for his interest and stimulating discussions.
This study was supported in part by the Decoding the Earth Evolution Program, Intensified Study Area Program of the Ministry of Culture, Science, Sports and Education, Japan (grant 259, 1955–1997).
REFERENCES
- 1.Achenbach-Richter L, Gupta R, Stetter K O, Woese C R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- 2.Barns S M, Fundyga R E, Jeffries M W, Pace N R. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc Natl Acad Sci USA. 1994;91:1609–1613. doi: 10.1073/pnas.91.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barns S M, Delwiche C F, Palmer J D, Pace N R. Perspective on archaeal diversity, thermophily and monophyly from environmental rRNA sequences. Proc Natl Acad Sci USA. 1996;93:9188–9193. doi: 10.1073/pnas.93.17.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock T D. Thermophilic microorganisms and life at high temperatures. New York, N.Y: Springer-Verlag; 1978. [Google Scholar]
- 5.Burggraf S, Olsen G J, Woese C R. A phylogenetic analysis of Aquifex pyrophilus. Syst Appl Microbiol. 1992;15:352–356. doi: 10.1016/S0723-2020(11)80207-9. [DOI] [PubMed] [Google Scholar]
- 6.Castenholz R W. The effect of sulfide on the blue-green algae in hot springs. I. New Zealand and Iceland. J Phycol. 1976;12:54–68. [Google Scholar]
- 7.Castenholz R W. The effect of sulfide on the blue-green algae in hot springs. II. Yellowstone National Park. Microb Ecol. 1977;3:79–105. doi: 10.1007/BF02010399. [DOI] [PubMed] [Google Scholar]
- 8.Castenholz R W. Composition of hot spring microbial mats: a summary. In: Cohen Y, Castenholtz R W, Halvorson H O, editors. Microbial mats: stromatolites. New York, N.Y: Alan R. Liss, Inc.; 1984. pp. 101–119. [Google Scholar]
- 9.Castenholz R W. The green sulfur and nonsulfur bacteria of hot springs. In: Olson J M, Ormerod J G, Amez J, Stackebrandt E, Trüper H G, editors. Green photosynthetic bacteria. New York, N.Y: Plenum Press; 1988. pp. 237–245. [Google Scholar]
- 10.Castenholtz R W, Pierson B K. Ecology of thermophilic anoxygenic phototrophs. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 87–103. [Google Scholar]
- 11.Collins M D. Analysis of isoprenoid quinones. Methods Microbiol. 1985;18:329–366. [Google Scholar]
- 12.Collins M D, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol Rev. 1981;45:316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hale M B, Blankenship R E, Fuller R C. Menaquinone is the sole quinone in the facultatively aerobic green photosynthetic bacterium Chloroflexus aurantiacus. Biochim Biophys Acta. 1983;723:376–382. [Google Scholar]
- 14.Hanada S, Hiraishi A, Shimada K, Matsuura K. Isolation of Chloroflexus aurantiacus and related thermophilic phototrophic bacteria from Japanese hot springs using an improved isolation procedure. J Gen Appl Microbiol. 1995;41:119–120. [Google Scholar]
- 15.Hanada S, Hiraishi A, Shimada K, Matsuura K. Chloroflexus aggregans sp. nov., a filamentous phototrophic bacterium which forms dense cell aggregates by active gliding movement. Int J Syst Bacteriol. 1995;45:676–681. doi: 10.1099/00207713-45-4-676. [DOI] [PubMed] [Google Scholar]
- 16.Hedrick D B, White D C. Microbial respiratory quinones in the environment. I. A sensitive liquid chromatographic method. J Microbiol Methods. 1986;5:243–254. [Google Scholar]
- 17.Hiraishi A. Respiratory quinone profiles as tools for identifying different bacterial populations in activated sludge. J Gen Appl Microbiol. 1988;34:39–56. [Google Scholar]
- 18.Hiraishi A. Isoprenoid quinone profiles for identifying and classifying microorganisms in the environment. In: Hattori T, Ishida Y, Maruyama Y, Morita R Y, Uchida A, editors. Recent advances in microbial ecology. Tokyo, Japan: Japan Scientific Societies Press; 1989. pp. 663–668. [Google Scholar]
- 19.Hiraishi A, Masamune K, Kitamura H. Characterization of the bacterial population structure in an anaerobic-aerobic activated sludge system on the basis of respiratory quinone profiles. Appl Environ Microbiol. 1989;55:897–901. doi: 10.1128/aem.55.4.897-901.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraishi A, Morishima Y, Takeuchi J. Numerical analysis of lipoquinone patterns in monitoring bacterial community dynamics in wastewater treatment systems. J Gen Appl Microbiol. 1991;37:57–70. [Google Scholar]
- 21.Hiraishi A, Ueda Y, Ishihara J. Quinone profiling of bacterial communities in natural and synthetic sewage activated sludge for enhanced phosphate removal. Appl Environ Microbiol. 1998;64:992–998. doi: 10.1128/aem.64.3.992-998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraishi A, Ueda Y, Ishihara J, Mori T. Comparative lipoquinone analysis of influent sewage and activated sludge by high-performance liquid chromatography and photodiode array detection. J Gen Appl Microbiol. 1996;42:457–469. [Google Scholar]
- 23.Hu H-I. Kinetic and ecological studies on aerobic submerged biofilter for wastewater treatment. Ph.D. thesis. Yokohama, Japan: Yokohama National University; 1993. . (In Japanese.) [Google Scholar]
- 24.Hugenholtz P, Pitulle C, Hershberger K L, Pace N R. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–376. doi: 10.1128/jb.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imhoff J F. Quinones of phototrophic purple bacteria. FEMS Microbiol Lett. 1984;25:85–89. [Google Scholar]
- 26.Ishii M, Kawasumi T, Igarashi Y, Kodama T, Minoda Y. 2-Methylthio-1,4-naphthoquinone, a unique sulfur-containing quinone from a thermophilic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. J Bacteriol. 1987;169:2380–2384. doi: 10.1128/jb.169.6.2380-2384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki M, Hiraishi A. A new approach to numerical analyses of microbial quinone profiles in the environment. Microbes Environ. 1998;13:67–76. [Google Scholar]
- 28.Kawasumi T, Igarashi Y, Kodama T, Minoda Y. Hydrogenobacter thermophilus gen. nov., sp. nov., an extremely thermophilic, aerobic, hydrogen-oxidizing bacterium. Int J Syst Bacteriol. 1984;34:5–10. [Google Scholar]
- 29.Kröger A. Determination of contents and redox states of ubiquinone and menaquinone. Methods Enzymol. 1978;53:579–591. doi: 10.1016/s0076-6879(78)53059-0. [DOI] [PubMed] [Google Scholar]
- 30.Madigan M T. A novel photosynthetic purple bacterium isolated from a Yellowstone hot spring. Science. 1984;225:313–315. doi: 10.1126/science.225.4659.313. [DOI] [PubMed] [Google Scholar]
- 31.Madigan M T. Chromatium tepidum sp. nov., a thermophilic photosynthetic bacterium of the family Chromatiaceae. Int J Syst Bacteriol. 1986;36:222–227. [Google Scholar]
- 32.Maki Y. Study of the sulfur-turf: a community of colorless sulfur bacteria growing in hot spring effluent. Bull Jpn Soc Microb Ecol. 1991;6:33–43. [Google Scholar]
- 33.Miyoshi M. Studien über die Schwefelrasenbildung und die Schwefelbakterien der Thermen von Yumoto bei Nikko. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt 2. 1897;3:526–527. [Google Scholar]
- 34.Nishijima, M. Personal communication.
- 35.Page R D M. TreeView, version 1.5. Glasgow, United Kingdom: University of Glasgow; 1997. [Google Scholar]
- 36.Parkes R J. Analysis of microbial communities within sediments using biomarkers. In: Fletcher M, Gray T R G, Jones J G, editors. Ecology of microbial communities. Cambridge, United Kingdom: Cambridge University Press; 1987. pp. 141–177. [Google Scholar]
- 37.Pierson B K. Modern mat-building microbial communities: a key to the interpretation of proterozoic stromatolitic communities—introduction. In: Schoph J W, Klein C, editors. The proterozoic biosphere. A multidisciplinary study. London, United Kingdom: Cambridge University Press; 1994. pp. 247–251. [Google Scholar]
- 38.Pierson B K, Castenholz R W. The family Chloroflexaceae. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. Berlin, Germany: Springer-Verlag; 1991. pp. 3754–3774. [Google Scholar]
- 39.Pitulle C, Yang Y, Marchiani M, Moore E R B, Siefert J L, Arano M, Jurtshuk P, Jr, Fox G E. Phylogenetic position of the genus Hydrogenobacter. Int J Syst Bacteriol. 1994;44:620–626. doi: 10.1099/00207713-44-4-620. [DOI] [PubMed] [Google Scholar]
- 40.Reysenbach A-L, Wickham G S, Pace N R. Phylogenetic analysis of the hyperthermophilic pink filament community in Octopus Spring, Yellowstone National Park. Appl Environ Microbiol. 1994;60:2113–2119. doi: 10.1128/aem.60.6.2113-2119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruff-Roberts A L, Kuenen G J, Ward D M. Distribution of cultivated and uncultivated cyanobacteria and Chloroflexus-like bacteria in hot spring microbial mats. Appl Environ Microbiol. 1994;60:697–704. doi: 10.1128/aem.60.2.697-704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 43.Shima S, Suzuki K. Hydrogenobacter acidophilus sp. nov., a thermoacidophilic, aerobic, hydrogen-oxidizing bacterium requiring elemental sulfur for growth. Int J Syst Bacteriol. 1993;43:703–708. [Google Scholar]
- 44.Ward D M, Weller R W, Bateson M M. 16S rRNA sequences reveal uncultured inhabitants of a well-studied thermal community. FEMS Microbiol Rev. 1990;75:105–116. doi: 10.1111/j.1574-6968.1990.tb04088.x. [DOI] [PubMed] [Google Scholar]
- 45.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto H, Hiraishi A, Kato K, Chiura H X, Maki Y, Shimizu A. Phylogenetic evidence for the existence of novel thermophilic bacteria in hot spring sulfur-turf microbial mats in Japan. Appl Environ Microbiol. 1998;64:1680–1687. doi: 10.1128/aem.64.5.1680-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]