Abstract
In nature, lectins are widely dispersed proteins that selectively recognize and bind to carbohydrates and glycoconjugates via reversible bonds at specific binding sites. Many viral diseases have been treated with lectins due to their wide range of structures, specificity for carbohydrates, and ability to bind carbohydrates. Through hemagglutination assays, these proteins can be detected interacting with various carbohydrates on the surface of cells and viral envelopes. This review discusses the most robust lectins and their rationally engineered versions, such as lectibodies, as antiviral proteins. Fusion of lectin and antibody’s crystallizable fragment (Fc) of immunoglobulin G (IgG) produces a molecule called a “lectibody” that can act as a carbohydrate-targeting antibody. Lectibodies can not only bind to the surface glycoproteins via their lectins and neutralize and clear viruses or infected cells by viruses but also perform Fc-mediated antibody effector functions. These functions include complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and antibody-dependent cell-mediated phagocytosis (ADCP). In addition to entering host cells, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein S1 binds to angiotensin-converting enzyme 2 (ACE2) and downregulates it and type I interferons in a way that may lead to lung disease. The SARS-CoV-2 spike protein S1 and human immunodeficiency virus (HIV) envelope are heavily glycosylated, which could make them a major target for developing vaccines, diagnostic tests, and therapeutic drugs. Lectibodies can lead to neutralization and clearance of viruses and cells infected by viruses by binding to glycans located on the envelope surface (e.g., the heavily glycosylated SARS-CoV-2 spike protein).
Keywords: Lectins, Lectibody, Carbohydrates, Virus envelope, SARS-CoV-2, HIV, EBV, HCV
Introduction
As proteins without any enzymatic activity, lectins are ubiquitous among various kinds of living things. These proteins act as adhesion molecules in the colonization processes of bacteria, archaea, protists, and fungi. Also, they play an essential role in the defense mechanisms and nodulation of Plantae. Interestingly, they are involved in various functions of Animalia, such as cell migration and adhesion, opsonization, immune responses and phagocytosis, and glycoprotein production. These proteins act as cell-agglutinating and carbohydrate-specific proteins and recognition molecules in cell–molecule and cell–cell interactions. Regarding these functions of lectins, they play an indispensable role in many biological processes and mediate biological recognition events. Therefore, they can be a suitable tool for investigating carbohydrate indicators on cell surfaces, mainly the modifications occurring in malignancies and isolation and characterization of glycoproteins.
Infection with enveloped viruses (e.g., coronaviruses and HIV) is one of the leading causes of mortality and morbidity globally, even with the recent development of highly effective direct-acting antivirals. These viruses have envelope glycoproteins that are heavily glycosylated with a high proportion of high-mannose-type glycans (HMGs), protecting them from antibody neutralization and enabling them to interact with cell entry receptors. However, there is no approved therapeutic targeting this potentially druggable biomarker.
HMGs are highly rare in eukaryotic cell-surface glycoproteins because of the processing steps conducted by the Golgi apparatus that make them hybrid and complex glycoforms. Interestingly, despite eukaryotic cells, HMGs are plentiful at the surface glycoproteins of various viruses, shielding them against attacks from the host immune system. Therefore, glycosylation is pivotal for a broad range of functions in viruses: entrance into host cells; protein expression and assembly, and evading the immune system, for example, are the significant contributions of glycosylation of virus surface proteins. Hence, lectins as agents that identify and neutralize virus-associated glycans, particularly HMGs, are valuable tools in antiviral medicine as potent antiviral microbiocides. The antiviral lectins that have been used against human viral infections are from two origins, endogeneous or exogeneous (endo- and exolectins, respectively), the former being animal lectins expressed in a variety of cell types and carrying out a diverse range of activities such as the first-line defense in innate immune system, mediation of cellular adhesion, regulation of glycoprotein synthesis, and signal transduction, while the latter (the focus of this article) are lectins originating from other species. All the exolectins assessed herein have the typical, protein nature, except pradimicin A (PRM-A), which is an antibiotic with a nonprotein origin [1–4].
Although lectin’s attachment to monosaccharides is weak, in the case of more complex ligands, they employ subsite and subunit multivalency to improve the affinity and specificity of their interactions. Bivalent or multivalent lectins reversibly and selectively interconnect with their ligands (carbohydrates or glycoproteins) in solution or on the cell surface. Therefore, this class of proteins is detectable through agglutination assays. In this regard, plant lectins, because of their high stability even in unfadable pH/temperature and exposure to insect/animal proteases, are widely used in clinical and experimental biology and medicine, given their abundant availability; For example, serology arrays were the first field of medicine to use lectins, to distinguish human blood types based on their carbohydrate indicators [1–3, 5].
Accordingly, the diversity of lectins and their capacity to bind to carbohydrates have led to their widespread application in several fields of sciences, including biochemistry, cellular and molecular biology, immunology, pharmacology, medicine, and clinical analysis. Hence, in addition to their antiviral effect, which is the main subject of discussion herein, they can uniquely cause mitogenic stimulation and enforce quiescent lymphocytes to grow and replicate. Cancer detection and treatment is another field of application of lectins [6–8]. A detailed investigation into the field of lectins would provide valuable information for future antiviral investigations. This review aims to present an in-depth analysis of the most potent antiviral lectins and to shed light on the drug selection path for the clinical use of lectins and drug research and development.
Structure and classification of lectins
The primary structure of lectins contains carbohydrate-recognition domains (CRDs) with the highest variability, located in their internal repeat sequences. The CRDs are not rigidly fixed in position, such that their structural elements orient them in space, leading to variations in the specific avidity for the three-dimensional (3D) structures of carbohydrates (Fig. 1a–d). In this respect, the sequence similarity of lectins, primarily found in the central region, varies from 10% to 100%. The tertiary structure of different lectins shows great variety, along with the identical function of specific recognition and tight bindings to their ligands. Moreover, some structural elements, namely disulfide bonds and oligomerization, exist in a few lectins but are not prerequisite components. For example, disulfide bonds are located as inter or intradomains of cyanovirin (CV-N) and actinohivin (AH) lectins [9]. In addition, scytovirin (SVN) is a strictly monomeric lectin, consisting of two internal duplicated domains (SD1 and SD2) with 90% identity. These domains are separated by a short linker that is dominantly composed of proline residues. Ten cysteine residues in the SVN constitute five disulfide bridges [10–12]. An example of an intra-disulfide bind is Serpula vermicularis lectin (SVL). SVL is a Ca2+-independent homotetrameric marine invertebrate lectin (Mw = 12,700), comprising two similar domains connected via disulfide bridges [13, 14].
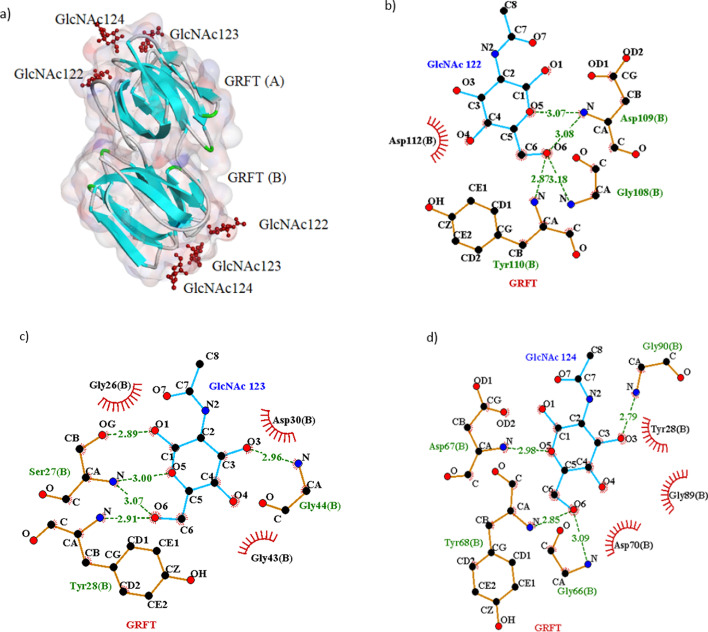
Fig. 1.
Interaction between lectin griffithsin (GRFT) (PDB ID: 2NU5) that was homodimer (A and B chain) with N-acetylglucosamine (GlcNAc) as ligand. a The interacting domains of the lectin with GlcNAc, depicted by BIOVIA Discovery Studio Visualizer, are shown. In the figure, the lectin is shown schematically in blue, while ligands are shown in scaled ball-and-stick style in red. b–d Residues and atoms participating in the interaction between B chain of the lectin and three residues of ligand (GlcNAc 122, 123 and 124), depicted by using the LigPlot+ v.2.2 program [15]. The bonds shown by dashed lines in olive-green color represent hydrogenic bonds, while the bonds shown by radius lines in brick-red color represent hydrophobic bonds. The numbers on the hydrogen bond show bond distances. Note: Two carbohydrates of N-acetylglucosamine and mannose are contained in SARS-CoV-2 surface glycan, and the residues are exposed to the innate immune system [16]. So, the crystal structure of complexes of antiviral lectin GRFT with glucose and N-acetylglucosamine were solved and refined at high resolution. In both complexes, all six monosaccharide-binding sites of GRFT were occupied, and the mode of binding was similar to that of mannose [17]. Therefore, as an example of multiple lectins, the interaction pattern of GRFT with GlcNAc is selected to be shown in the figure
The classification of lectins is based on the CRD specificity for carbohydrate ligands such as glucose, mannose, N-acetylglucosamine, N-acetylgalactosamine, and other glycans. Some important antiviral lectins are presented alongside their detailed properties, including origins, structure, and glycan specificity, in Table 1. As mentioned above, the spatial orientation of CRDs in the tertiary structure of lectins enhances their affinity for complex carbohydrates. In line with this finding, several antiviral lectins merely couple with high-mannose oligosaccharides, whereas others possess chitobiose units and high-mannose lateral branches. Duplication of binding domains is another mechanism leading to increased avidity for branched-chain carbohydrates. Besides, binding with linear oligomannose leads to less affinity than the branched structures. Therefore, the spatial position, orientation, and distance of the carbohydrate ligand are essential for the specificity and classification of lectins [9].
Table 1.
General features of antiviral lectins
| Source | Kingdom | Lectin | Mw (× 1000) per monomer | Residue per monomer | Oligomeric status | CRDs | Structure class | Glycan specificity | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Actinomycete Longisporum albida | Bacteria | AH | 12.5 | 114 | Monomeric | 3 | β-Trefoil | α(1,2)-Mannose | [153–156] |
| Cyanobacterium Nostoc ellipsosporum | Bacteria | CV-N | 11 | 101 | Monomeric–dimeric | 2 | Cyanovirin-like | α(1,2)-Mannose | [41, 46] |
| Cyanobacterium Microcystis aeruginosa | Bacteria | MVN | 14.2 | 108 | Monomeric | 1 | Cyanovirin-like | α(1,2)-Mannose | [47–49] |
| Cyanobacterium Microcystis viridis | Bacteria | MVL | 13 | 113 | Homodimeric | 4 | Cyanovirin-like | Man3GlcNAc2, Man6GlcNAc2 | [176, 177] |
| Cyanobacterium Scytonema varium | Bacteria | SVN | 9.7 | 95 | Monomeric | 2 | Cyanovirin-like | Man-α(1–2) Man-α(1–6) Man-α(1–6) Man | [10–12] |
| Cyanobacterium Oscillatoria agardhii | Bacteria | OAA | 13.9 | 133 | Monomeric | 2 | OAAH | Man-α(1–6)Man, Man-8/9 | [52–55] |
| Actinomadura hibisca | Bacteria | PRM-A | 8.5 | – | Dimeric | 4 | – | α(1,2)-Mannose | [58–61] |
| Griffithsia sp. | Protista | GRFT | 12.7 | 121 | Homodimeric | 6 | β-Prism type 1 | α(1,2), α(1,6), mannotetrose, man5-9 | [103–107, 178] |
| Boodlea coacta | Protista | BCA | 13.8 | 118 | Monomeric | 3 | β-Prism type 1 | α(1,2)-Mannose | [64] |
| Musa acuminata cultivars | Plantae | BanLec | 15 | 141 | Homotetrameric | 8 | β-Prism type 1 | α-1,6 mannotetrose α-D manno/glycosyl, α-1,3 mannosyl1/β-1,3-glycosyl | [166–170] |
| Galanthus nivalis | Plantae | GNA | 12.5 | 157 | Homotetrameric | 12 | β-Prism type 2 | α1-3 or α1-6 linked mannose | [125–129] |
| Hippeastrum hybrid | Plantae | HHA | 12.5 | 157 | Homotetrameric | 12 | β-Prism type 2 | α1-3 or α1-6 linked mannose | [125–129] |
| Polygonatum cyrtonema Hau | Plantae | PCL | 12 | 110 | Dimeric | 6 | β-Prism type 2 | α(1,3)-Dimannoside | [179–181] |
| Urtica dioicia | Plantae | UDA | 8.7 | 89 | Monomeric | 2 | Hevein-like | (N-acetyl-d-glucosamine)3 | [182–184] |
| Nicotiana tabacum var. Samsun NN | Plantae | NICTABA | 19 | 165 | Homodimeric | 2 | Unk | GlcNAc2Man3 | [185–187] |
| Phaseolus vulgaris | Plantae | PHA | ~ 30 | – | Homo/heterotetrameric | 4 | β-Sandwich | Galβ-(1–4)GlcNAcβ-(1–2)Man | [135–138] |
| Lens culinaris | Plantae | LCA | ~ 25 | – | Homodimeric | 2 | β-Sandwich | FucMan3GlcNAc2, Man5-9, GlcNAc | [140–144] |
| Serpula vermicularis | Animalia | SVL | ~ 12.7 | – | Homotetrameric | – | Unk | N-acetyl-d-glucosamine | [13] |
| Crenomytilus grayanus | Animalia | CGL | 18 | 150 | Homodimeric | 6 | β-Trefoil | GalNAc/Gal | [65–73] |
AH actinohivin, CV-N cyanovirin, MVN microvirin, MVL Microcystis viridis lectin, SVN scytovirin, OAA Oscillatoria agardhii agglutinin, PRM-A pradimicin A, GRFT griffithsin, BCA Boodlea coacta agglutinin, BanLec banana lectin, GNA Galanthus nivalis agglutinin, HHA Hippeastrum hybrid agglutinin, PCL Polygonatum cyrtonema lectin, UDA Urtica dioicia agglutinin, NICTABA Nicotiana tabacum agglutinin, PHA phytohemagglutinin, LCA Lens culinaris agglutinin, SVL Serpula vermicularis lectin, CGL Crenomytilus grayanus lectin
Mechanisms of antiviral lectins
Recognition of glycosylated envelope proteins by cell-surface receptors is the usual mechanism for virus recognition and entry. In this respect, lectins coevolved in parallel to impede virus entry and activate host defense mechanisms to neutralize invading viruses. Virus-neutralizing lectins mainly recognize carbohydrate moieties on enveloped viruses in mono- or oligomeric states. They interact with these configurations endowed via CRDs, which are usually repeated in the sequence of lectins, thereby inhibiting viral entry into host cells, on the one hand, and helping the host defense system to find alien viruses on the other hand. Interestingly, duplication of CRDs and multivalent binding features in some lectins are helpful tools by which they can bind to branched sugar moieties on the envelope of viruses more strongly. All of the antiviral lectins characterized and mentioned herein aim to neutralize different viruses by wrapping envelope glycosylated proteins, thereby creating a barrier between these essential recognition tools and their counterpart receptors on the host cell surface [18–22].
Lectin action on the surface of enveloped viruses
Lectins and HIV envelope interactions
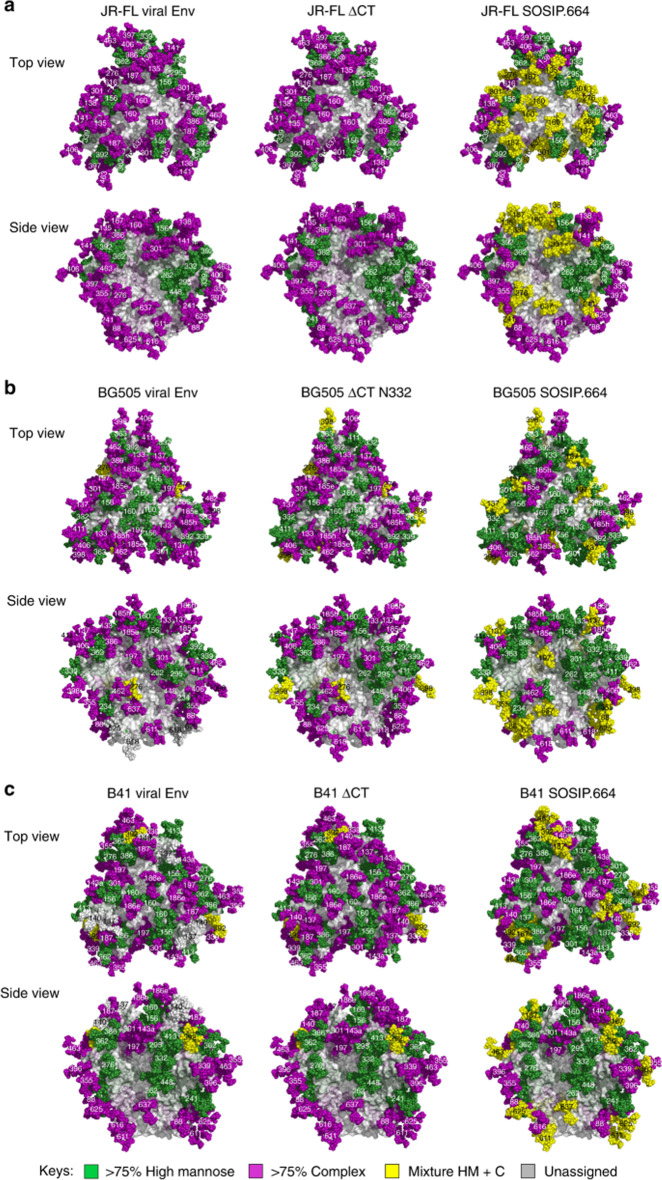
As an example of an enveloped virus, the human immunodeficiency virus (HIV) surface is covered with gp120 and gp41. These heavily glycosylated proteins consist of almost 50% glycans. Sugar-binding proteins (such as lectins) specifically and strongly interact with the glycans on the viral surface and disturb the interactions of such invasive viruses with their target receptors on host cells [19, 23, 24]. Mannose oligomers are the central part of HIV carbohydrates that are directed against plant lectins. A map of site-specific N-glycan processing onto the structures of the HIV-1 envelope is shown in Fig. 2a–c.
Fig. 2.
Map of site-specific N-glycan processing onto the structure of HIV-1 envelope: a JR-FL strain, b BG505 strain, and c B41 strain. The fully glycosylated models were created with JR-FL ΔCT (PDB: 5FUU), BG505 SOSIP (PDB: 5FYK), and B41 SOSIP. The surfaces of the trimers are represented in grey, and the glycans are represented as spheres colored by the proportion of oligomannose content at that site. The glycans are shown in ball-and-stick representation: > 75% high mannose (green), > 75% complex type glycosylation (purple), mixture of high mannose and complex type glycosylation (25% < high-mannose glycosylation < 75%) (yellow), and the glycosites that were not detected (gray) [25]
Monocotyledonous families are the best to exemplify HIV-inhibitory plant lectins. Also, reverse transcriptase (RT), the critical enzyme in the life cycle of HIV, is another target of plant lectin. Pholiota adipose mushroom, extralong autumn purple bean, and black soybean are the primary sources of plant lectins averting RT activity [26–33]. Cyanovirin-N (CV-N), a lectin that has its origin in blue–green algae, controls a wide range of antiviral properties. Envelope glycoprotein gp120 on the HIV surface is one suggested target for CV-N. CV-N consists of 101 amino acids (Mw = 11,000) with two sequence repeats, exhibiting even specialty toward α-(1–2)-mannose moieties but different affinities for their carbohydrate binding. In this regard, domain B has higher affinity (Kd = 14 nM) compared with domain A with a lower affinity (Kd = 1.5 µM) [34–36]. It has been demonstrated that each domain individually does not have sufficient avidity toward gp120 and dissociation constants in the micrometric range, resulting in loss of the virucide function [37–39]. The lectin exists in monomeric and domain-swapped dimer forms, with the latter being unstable at physiological temperatures. However, structure-guided design of CV-N aided the production of a stable domain-swapped dimer form (in a head-to-tail manner), which exhibited elevated anti-HIV activity compared with wild-type CV-N [40].
Multivalency is a common feature of lectins to bind with higher affinity to carbohydrates. In the case of CV-N, the antiviral activity is not enhanced due to geometric constraints when increasing the number of CRDs. Interestingly, Woodrum et al. (2016) reported an increased anti-HIV-virus action (IC50 value of 7.5 nM compared with 0.5 nM for WT CV-N) by engineering a flexible dimer of CV-N (called “nested CV-N”), lacking rigidity and spatial constraints [41].
The specific viral targets of CV-N are not limited to HIV and retroviruses. Generally, due to its stable binding, CV-N can inhibit the cytopathic effects of different viruses. For example, CV-N inhibits human influenza A/B strains and the Ebola virus, respectively, by binding to hemagglutinin surface glycoprotein and surface envelope glycoproteins [42–45].
O’Keefe et al. (2003) reported the neutralizing functions of CV-N against viruses at three levels. At level 1, there was no antiviral activity against enteric viruses and rhinoviruses in the first studied category. At level 2, moderate antiviral activity was shown for the second category encompassing herpesviruses and flaviviruses. Finally, at level 3 (which consisted of influenza A and B strains), viruses were susceptible to CV-N except for NWS/33 (H1N1) and A/PR/8/34 (H1N1), which were resistant to even high concentration of CV-N. The results of this study indicate that CV-N hinders viral infection through direct binding and inactivating the invasive virus [43].
Additionally, Maier et al. (2021) designed various domain-swapped dimers differing in the number of B and A domains, then tested their binding affinities against the envelopes of HIV-1, influenza, and Ebola. For the first two, the 2B + 1A form showed higher Kd values than the other forms including 1B + 1A, 2A, and 1H, suggesting a significant role for multivalency in these bindings. The Kd values for all the forms against the Ebola envelope protein GP1,2 were similar (Kd = 26–72 nM) [46].
Another bacterial-derived lectin with high similarity to CVN is a 108-amino-acid lectin with molecular weight of 14,200, specific for Manα-(1–2) Man configurations, named microvirin (MVN). This lectin shows a monodisperse monomer in solution with only one carbohydrate recognition site, very similar to its counterpart in domain A of CV-N [47]. The lectin has slight antiviral potency compared with CV-N, but a narrower antiviral profile (50-fold lower cytotoxicity), which may make it a higher priority for rational engineering to boost its antiviral potency [48, 49]. It can be concluded that the avidity between lectins and mono- and oligosaccharides can be augmented by increasing the number of binding units, thus Yuan-Qin Min et al. (2017) produced oligomer types of MVN, then investigated their binding activities against hepatitis C virus (HCV). All of the oligomers showed higher binding activities compared with the natural monomer type (tetra > tri > di > monomers), while long peptide linkers displayed better anti-HIV binding activity [50]. Munazza Shahid and coworkers (2020) recently engineered an MWN lectin, named LUMS1, by duplicating domain A, which, as a result, is predicted to have higher activity (2CRDs). However, the engineered lectin exhibited less potential against HIV-1 and HCV compared with the unmanipulated lectin (EC50 values for HIV-1 and HCV of 32.8–41.6 and 6.6–9.4 nM versus 26.7–63.9 and 31.6–39.5 nM, respectively), apparently indicating the need for further optimization and structures suitable for interaction with carbohydrate moieties [51].
Oscillatoria agardhii agglutinin (OAA) is a single polypeptide (133 residues, MW = 13,900) composed of one domain and two CRDs with β-barrel-like topology. The lectin has no affinity for monosaccharides, and its recognition of a pentasaccharide [Man-α-(1–3) Man-α-(1–6) Man-β-(1–4) GlcNAc-β-(1–4) GlcNAc] is pivotal for its binding to Man-α-(1–6) Man disaccharide units [52, 53].
In comparison with all other antiviral lectins, recognizing Man-9 by OAA is unprecedented, while this lectin also has a unique amino acid composition with roughly 20% glycine residues in its amino acid sequence [54, 55]. OAA is a potent anti-HIV-1 lectin with reported EC50 values of 30–45 nM [56, 57].
Pradimicin A (PRM-A), an antifungal nonpeptidic benzonaphthacenequinone antibiotic (Mw = 8500), is the first archetype of this class containing d-alanine, d-xylose, 4,6-dideoxy-4-methylamino-d-galactose, and a substituted 5,6-dihydrobenzo[a]naphtacenequinone.
PRM-A acts like a c-type “artificial lectin” and predominantly binds to α-(1–2)-mannose configurations. Two molecules of PRM-A cooperate in the carbohydrate-binding processes. First, the binary complex [PRM2/Ca2+] forms due to the Ca2+ bridging effect (i.e., assembly of two molecules of PRM). Next, the ternary complex [PRM2/Ca2+/Man2] organizes by incorporating two molecules of Man with high affinity. Ultimately, the final complex [PRM2/Ca2+/Man4] forms by binding two more Man molecules with low affinity. Thus, PRM-A makes water-insoluble aggregates by cross-linking glycans at the surface of virus particles [58, 59].
PRM-A would be a promising lead for treating various viral infections, particularly HIV. The main advantages of PRM-A include its small size, lack of steric hindrance, that it is unaffected by proteases, chemical stability, neither cytotoxicity nor mitogenicity, quick large-scale production, long-term storage, easy modification, and high genetic barrier [60, 61]. There are numerous binding sites on the gp120 envelope of HIV for PRM-A, following the deletion of some glycans by the virus, i.e., insensitivity to lectin binding. Therefore, not only would the antiviral potential of the lectin not decline but this also makes the virus vulnerable to immune system recognition and elimination. PRM-A inhibits HIV1/2 and simian immunodeficiency viruses (SIVs) with EC50 values of 1.6–10 and 5 µM, respectively [62, 63].
Boodlea coacta agglutinin (BCA) is a monomeric protein composed of 118 amino acids (Mw = 13,800). Its single domain is separated into three subdomains, whose primary targets are oligosaccharides of α-(1–2)-mannose residues at the non-reducing end (the D1 arm of gp120) with no affinity for internal ones. Studies have shown that influenza H3N2 is the strain that is most affected by BCA (with EC50 values of 18.8–74.2 nM). This lectin has also been shown to potently inhibit HIV-1 infection (EC50 of 8.2 nM) by binding directly to the gp120 subunit of the virus [64].
Two animal-derived lectins, SVL and CGL, were recently evaluated for their anti-HIV infection properties. Serpula vermicularis lectin (SVL) is a Ca2+-independent homotetrameric marine invertebrate lectin (Mw = 12,700) comprising two similar domains connected via disulfide bridges. SVL is specific for N-acetyl-d-glucosamine monosaccharides and inhibits HIV-1 infection through an unclear mechanism (with an EC50 value of 2.8 µM and no cytotoxicity up to > 16 µM) [13, 14]. Crenomytilus grayanus lectin (CGL) is a Ca2+ independent, dumbbell-shaped, homodimeric protein (Mw = 18,000) whose subunits contain three similar (64–73% identity) tandem subdomains (150 residues per monomer), folded into a β-trefoil configuration. Since the lectin lacks cysteine amino acid, its oligomeric state, presumably, results from hydrophobic interactions [65–70]. CGL has some similarities, such as glycan preferences, with galectins, some of the common animal lectins, but its amino acid sequence is entirely unique. The lectin recognizes O-glycans containing GalNAc/Gal of the nonreducing ends such as mucin-type glycoproteins [71–73]. Regarding recent studies, CGL inhibits the HIV-1 virus (EC50 value of 2.5 µM) and has not shown any cytotoxicity (up to a CC50 value of 14.6 µM) [14].
Lectins as potential molecules in coronavirus treatment/recognition
Like the mentioned enveloped viruses, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has a glycoprotein envelope around the virion particle. Also, SARS-CoV-2 has glycoproteins on its envelope, suggesting opportunities to use lectins as a treatment strategy [74, 75]. The host cell provides a two-layer envelope for the virus during budding, making its compounds dependent on the cell membrane of origin [76]. Host enzymes glycosylate some of the proteins in the layers of the SARS-CoV-2 envelope. These glycoproteins participate in the adhesion, invasion, and entry of the virus and the formation/modulation of immune system responses. The spike and membrane proteins, called S-protein and M-protein, respectively, are examples of glycoproteins on the envelope of SARS-CoV-2 with important roles in its pathogenesis (Fig. 3) [77–81].
Fig. 3.
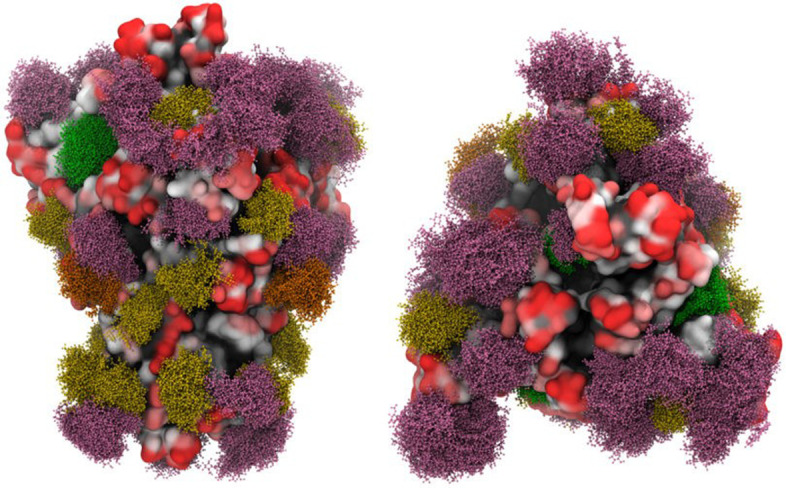
Overlay of snapshots from molecular dynamics (MD) simulation of SARS-CoV-2 S glycoprotein with site-specific glycosylation. The glycans are shown in ball-and-stick representation: high mannose (green), paucimannose (dark yellow), hybrid (orange), and biantennary complex (purple) [82]
The S-protein arranges as trimers and mediates adhesion between SARS-CoV-2 and the host cell through an interaction with angiotensin-converting enzyme 2 (ACE2) [24, 83–87]. The number of potential glycosylation sites localized in subunits S1 and S2 is 3 for O-glycosylation and 22 for N-glycosylation. In this regard, the S1 glycoprotein of SARS-CoV-2 exhibits ligands for several innate immune receptors, particularly C-type lectin receptors (CLRs), which are known to bind specific glycans mainly in a manner dependent on C-type lectin [88]. CLRs, such as macrophage mannose receptor (MMR), macrophage galactose-type lectin (MGL), dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), lymph node-specific intercellular adhesion molecule-3-grabbing integrin (L-SIGN), and Dectin-2, are widely expressed by immune system cells such as macrophages, dendritic cells (DCs), and monocytes, which can exert their role as the first line of defense against viruses and pathogens, including SARS-CoV-2 [89].
There is a D614G substitution in the spike protein of all SARS-CoV-2 variants classified as variants of concern (B.1.1.7/alpha, P.1/gamma, B1.351/beta, B.1.617.2/delta, and the newly emerged omicron/B.1.1.529) and variants of Interest (B.1.427/epsilon, B.1.429/epsilon, B.1.525/eta, B.1.526/iota, B.1.617.1/kappa, B.1.617.3, and P2/zeta) called S-D614G, that results in an altered conformation that enhances ACE2 binding and increases transmission and infectivity.
Data show that half of the N-glycosylation sequences changed their distribution of glycans in the S-614G variant. The S-D614G variant shows a reduction in the relative level of complex-type glycans (up to 45%) and an increase in oligomannose glycans (up to 33%) in all altered sequences [90]. Han and coworkers [91] also showed different degrees of binding affinity caused by different S glycoprotein mutations, thus indicating that the 501Y.V1 variant yielded the highest enhancements in binding affinity (increased by 36.8%), followed by the N439K variant (increased by 29.5%) and the 501Y.V2 variant (increased by 19.6%). Moreover, N165A and N234A mutations led to glycan deletions at the respective sites, reducing binding of the mutated virus to the ACE2 receptor. This observation suggests that these glycans are actually important for the conformational plasticity of the spike protein receptor binding domain (RBD) of SARS-CoV-2 and hence its ACE2 interaction [92]. Another study investigated the effect of five common SARS-CoV-2 RBD mutations (K417N, K417T, N501Y, E484K, and S477N) on the RBD–ACE2 interaction. They demonstrated that S477N and E484K mutations enhanced transmission primarily by enhancing binding of RBD and ACE2, while K417N and K417T mutations decreased this affinity. It was also indicated that N501Y, E484K, K417N and K417T mutations facilitated immune escape [93]. So, mutations in spike protein S1 of SAR-CoV-2 improve its affinity with ACE2 and, consequently, increase its infectivity and pathogenicity.
On the other hand, through the Janus-activated kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway, interferon-stimulated genes (ISGs) are activated by binding interferins to their receptors on the cell surface, leading to immune responses [94]. In this regard, ACE2 is one of the ISGs, and its expression levels correlate with type I interferons. Reduced levels of ACE2 in the lung are beneficial for the host in controlling viral replication and transmission. Nevertheless, if there is insufficient ACE2 for a prolonged period of time, angiotensin II would not be properly converted into ang1-7 by the function of ACE2. The resulting accumulation of angiotensin II will have a negative impact on immune activation and may eventually cause lung disease. After binding of SAR-CoV-2 spike protein S1 to ACE2, it downregulates ACE2 and type I interferons, which may directly contribute to SARS-CoV-2-associated lung disease [95]. It has also been indicated that induction of ACE2 and type I interferons by poly I:C, an interferon inducer, is suppressed by S1 protein in primary cells of lung bronchoalveolar lavage (BAL) from naive rhesus macaques. Thus, the S1 and S2 glycoproteins are golden targets for the host immune system and drug and vaccine design, including the lectibodies discussed in the next section. In this regard, glycosylated nonstructural proteins (e.g., the 3a protein, which plays a pivotal role in SARS-CoV-2 virulence) are also suitable targets for lectibodies [78, 96–102].
In this regard, griffithsin (GRFT) is a potent antiviral lectin (121 amino acids, Mw = 12,700) with domain-swapped dimer folding. High-resolution crystallographic experiments have revealed three identical carbohydrate-binding sites per monomer, resembling a β-prism-1 motif. Each CRD recognizes one terminal mannose monosaccharide on N-linked Man5-9GlcNAc2 configurations. Indeed, GRFT shows antiviral activity against a broad range of viral infections, including HIV (EC50 values of 0.03–1.3 nM), HCV (6.7–13.9 nM), and SARS-CoV (48 nM) [103–107].
This property is attributed to its favorable preclinical features such as lack of toxicity and mitogenicity, synergistic effect with various other lectins and drugs, and inexpensive bulk production [108, 109]. In this regard, GRFT has been the subject of two phase I clinical studies investigating its toxicity in healthy populations. One part of a two-part study looked at the safety of GRFT injected intravaginally for a single dose, followed by 14 consecutive days of use in healthy women. The second part of the study involved 30 subjects receiving placebo and 30 subjects receiving GRFT gel. The results of cell-based assays and cervical explants showed that GRFT was safe for vaginal use up to 14 days with potent anti-HIV activity [110, 111].
Another phase I clinical study on GRFT (PREVENT, pre-exposure prevention of viral entry) has been conducted since 2014. GRFT was studied for the purpose of providing a comprehensive dataset that would facilitate an informed decision on whether the topical microbicide should proceed. Study students who were HIV-1 seronegative and engaged in unprotected receptive anal interactions (URAI) in this double-blind, randomized, phase 1 study were given GRFT enema rectally [112].
However, a recent preformulation study on GRFT by Kramzer et al. (2021) revealed that slow oxidation of GRFT occurs following long-term storage (at methionine 78, in particular). Hence, more studies are required to protect GRFT from oxidation [113–123]. GRFT also shows synergy when used together with other antivirals. Cai et al. (2021) reported that the combination of EK1 (which binds to the HR1 in the S2 subunit of SARS-CoV-2) and GRFT (which binds to the RBD in the S1 subunit of the same virus) potently inhibits the SARS-CoV-2 virus. In this respect, the EC50 values of GRFT and EK1 alone were 511 and 2459 nM, respectively, while the EC50 for GRFT-L25-EK1 (where L25 refers to the 25-mer linker) was 20 nM, demonstrating the efficacy of the synergism [124].
Galanthus nivalis agglutinin (GNA) and Hippeastrum hybrid agglutinin (HHA) are two lectins that highly resemble each other, in terms of their monomeric molecular weight (12,500), oligomeric status (homotetrameric, resulting from noncovalent interactions among four monomers), number of sugar-binding sites per monomer (three CRDs), and secondary structure (β-prism-type 2). Nevertheless, they show distinct potential binding targets; while GNA exclusively binds to α-(1,3)-mannose ends, HHA CBAs can corecognize internal and external α-(1,3)-or α-(1,6)-linked mannose moieties. In addition to binding to mannose termini, GNA also recognizes some other carbohydrates, including lactosamine structures found on N- and/or O-glycans [125–127]. These lectins have dual antiviral activity: First, by their numerous carbohydrate-recognition sites, they bind to several target sugars, then force the target virus to eliminate some glycans on its envelope. Second, now that mutant virus strains expose their protein motifs to the immune system, Nab can now simply trigger immune responses against the invading virus [128, 129]. Both GNA and HHA have demonstrated potent antiviral activities against SARS-CoV (6.2 and 3.2 µg/ml, respectively) [130–134].
Moreover, phytohemagglutinin (PHA) shows affinity toward N-glycans of complex type, but not monosaccharides. PHA is considered to be a “complex-type” specific lectin, recognizing the Gal-β-(1–4)GlcNAc-β-(1–2)Man structure [135]. There are two types of subunits, named E (because of binding to erythrocytes and then agglutinin activity) and L (owing to its association with leukocytes and then mitogenic activity), that assemble into five different types of tetramers (Mw = ~ 120,000), i.e., E4, E3L1, E2L2, E1L3, and L4 [136, 137]. Despite the 70% sequence similarity between the E and L subunits, they have different glycan-binding preferences. Indeed, while PHA-E prefers terminal Gal and GlcNAc glycans, PHA-L recognizes Man residues through interaction with both α1–3 and α1–6 branches [138].
Wang et al. (2021) recently investigated the antiviral potency of PHA-E and L against SARS-CoV-2 pseudovirus and reported no cytotoxicity with EC50 values of 141–200.1 and 184.9–217.9 nM, respectively [139].
Lens culinaris agglutinin (LCA) is a homodimeric lectin in solution, where each monomer is composed of two distinct polypeptides, viz. α (the light chain with 52 amino acids) and β (the heavy chain with 180 residues), which assemble to an α2β2 composition (with molecular mass of 49,000) with a β-sandwich structure [140–142]. Binding to one metal ion (usually Mn2+) and one Ca2+ per subunit is obligatory for sugar binding (one CRD per monomer) [143, 144]. Lentil lectin needs a FucMan3GlcNAc2 core for its binding; it can bind to N-glycan oligosaccharides of Man-5 to Man-9 with the highest affinity, as well as GlcNAc residues at the nonreducing terminals with lower affinity. LCA has been shown to increase HIV infection and transmission to CD4+ cells through an unknown mechanism, likely due to the high expression level of fucosylated glycans on the surface of eukaryotic cells [145]. More recently, Wang and colleagues (2021) showed for the first time that LCA has potent antiviral activity against SARS-CoV-2 pseudovirus (with EC50 values of 152.3–186.6 nM) with no cytotoxicity effect [139]. A list of antiviral lectins with their cytotoxicity and mitogenicity properties and effective concentrations used to treat enveloped viruses is presented in Table 2.
Table 2.
Toxicity and antiviral activity of lectins
| Lectin | Cytotoxicity | Mitogenicity | Activity (nM unless otherwise noted) | Refs. |
|---|---|---|---|---|
| AH | No | No | HIV-1 (2–110), HIV-2 (3–14), | [148, 157–164] |
| CV-N | Yes | Yes | HIV (0.1–33.7), HCV (1.6), Ebo (100) | [34–40] |
| MVN | No | No | HIV-1 (2–167), HCV (31–39) | [50, 51] |
| MVL | Yes | Unk | HIV-1 (30–37), HCV (14–34) | [188, 189] |
| SVN | No | Unk | HIV-1 (0.3–22), EBOV (41) | [190–194] |
| OAA | Yes | Unk | HIV-1 (30–45) | [56, 57] |
| PRM-A | No | No | HIV-1/2 (1.6-10 µM), SIV (5 µM) | [62, 63] |
| GRFT | No | No | HIV (0.03–1.3), HCV (6.7–13.9), SARS-Cov (48), JEV (20), HSV-2(230), HPV (0.4–1.39 µM), NiV (20–60), ANDV (180–230) | [104–109, 113–124, 178, 195, 196] |
| BCA | No | Unk | HIV-1 (8.2), influenza H3N2 (18.8–74.2) | [64] |
| BanLec | Yes | Yes | HIV-1 (0.8–14), HIV-2 (3.7) | [171–174] |
| GNA | No | Unk | HCV (11.1–25.5), influenza A H1N1 (0.1–268), influenza A H3N2 (0.4–6.4), influenza B (0.016–0.89), HIV-1 (0.3–4.7 μg/ml), HIV-2 (0.1–0.2 μg/ml), SIV (2.7 μg/ml), FIV (0.09 μg/ml), SARS-COV (6.2 μg/ml), FIPV (3.9 μg/ml) | [130–134] |
| HHA | No | Unk | Influenza A H1N1 (0.05–121), influenza A H3N2 (0.10–3), influenza B (0.015–1.8), HIV-1 (0.3–3.2 μg/ml), HIV-2 (0.1–0.2 μg/ml), SIV (0.6 μg/ml), FIV (0.1 μg/ml), SARS-COV (3.2 μg/ml), FIPV (2.6 μg/ml) | [130–134] |
| PCL | No | Unk | HIV-1 (0.05–0.08 μg/ml), HIV-2 (0.08–0.1 μg/ml) | [179, 197] |
| UDA | Yes | Yes | HIV-1(100–180), HIV-2 (240–420), HSV-1(9.6- > 11 µM), HSV-2 (1.1–1.3 µM), SIV (130–190), MSV (> 20 µg/ml), SARS-CoV (0.9–2.6 µg/ml), influenza A H1N1 (5–435), influenza A H3N2 (5.8–83), and influenza B (0.64–14) | [182–187, 198–203] |
| NICTABA | No | Unk | HSV-1 (171–263), HSV-2 (41–64), influenza A H1N1 (21–43), influenza A H3N2 (13–23), influenza B (11), RSV (105), and DENV type 2 (323–729) | [107, 176–178] |
| PHA | No | Yes | SARS-CoV-2 (141–217.9), HIV-1 (50 µg/ml) | [116, 122] |
| LCA | No | Unk | SARS-CoV-2 (152.3–186.6) | [116] |
| SVL | No | Unk | HIV-1 (2.8 µM) | [14] |
| CGL | No | Yes | HIV-1 (2.5 µM) | [14] |
HIV human immunodeficiency virus, HCV hepatitis C virus, EBOV Ebola virus, SIV simian immunodeficiency virus, SARS-CoV severe acute respiratory syndrome coronavirus, JEV Japanese encephalitis virus, HSV herpes simplex virus, HPV human papillomavirus, NiV Nipah virus, ANDV Andes orthohantavirus, FIV feline immunodeficiency virus, FIPV feline infectious peritonitis virus, MSV maize streak virus, RSV respiratory syncytial virus, DENV dengue virus
New generation of lectins: lectibodies
Besides their size limitations, short stability in the body environment, vulnerability to proteolytic lysis, and challenges regarding bulk production, the cytotoxicity, mitogenicity, and proinflammatory properties of lectins raise several questions regarding their valuable usage as antiviral agents. In this regard, several protein engineering techniques have been applied to produce modified lectins, such as lectibodies, to overcome these issues [146, 147].
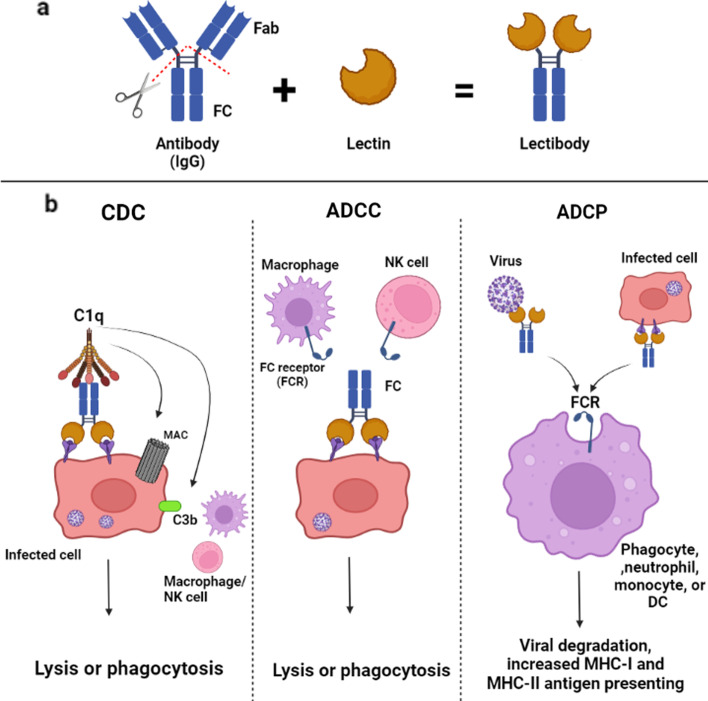
Fusion of lectin and antibody’s crystallizable fragment (Fc) of immunoglobulin G (IgG) produces a molecule called a “lectibody” that can act as a carbohydrate-targeting antibody (Fig. 4a). Lectibodies can bind to surface glycoproteins via their lectins, neutralize viruses or cells infected by viruses, and perform Fc-mediated antibody effector functions that include complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and antibody-dependent cell-mediated phagocytosis (ADCP) (Fig. 4b) [148].
Fig. 4.
a Fusion of lectin and antibody’s crystallizable fragment (Fc) of immunoglobulin G (IgG) produces a molecule called a “lectibody.” b Lectibodies can bind to surface glycoproteins via their lectins, neutralize viruses or cells infected by viruses, and help the innate and adaptive immune systems function against pathogens through functions including complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and antibody-dependent cell-mediated phagocytosis (ADCP)
The CDC mechanism is activated by binding of C1q to Fc-bound virus-infected cells, initiating the cascade. By releasing C3 and C5 molecules, immune effector cells are recruited and activated, while C3b binds to pathogens and infected cells to initiate immune complex clearance and phagocytosis. Following assembly of a membrane attack complex, infected cells undergo lysis.
The ADCC response is triggered by the binding of Fc gamma receptor (FcγR) on natural killer (NK) cells to Fc domain on antibodies bound to viral antigens on infected cells [149]. Upon release of cytotoxic granules, infected cells are killed. Liu et al. demonstrated that anti-Ebola monoclonal antibodies have predominantly NK cell ADCC activity [150].
During ADCP, phagocytic cells ingest virus–antibody or antibody–infected cell complexes. The antigen is then processed and presented on major histocompatibility complex (MHC) molecules on cell surfaces, or transferred to lysosomes to be degraded. ADCP has been shown to reduce SARS-CoV infection when anti-SARS-CoV antibodies were administered to mice [151, 152].
CVN-Fc, as a lectibody, has an intense inhibiting activity on free enveloped viruses. CVN-Fc prevents virus attachment and entrance into the target infection cell and attracts host defense cells to the virus war zone. HCV, HIV, Ebola, and influenza are among the list of CVN-Fc’s target viruses. In addition, actinovirin (AH), an actinomycete-derived lectin, is a single polypeptide with 114 residues (Mw = 12,500) that folds into a β-trefoil structure. This structure has three highly conserved tandem repeats (HMG-binding pocket) specific toward α-1,2-mannose oligomers (D1) of high-mannose-type glycans (HMTGs) on the HIV envelope [153–156]. Chiba et al. (2004) reported the inhibitory activity of AH against T- and M-tropic HIV-1 strains (with IC50 values of 2–110 nM and 38 nM, respectively) and HIV-2 (with an IC50 value of 3–14 nM). They also found that, although three CRDs in AH recognize gp120 on the env-expressing cells specifically and cooperatively, they have no significant affinity toward chemokine receptor-expressing cells such as CD4+ [157]. Besides the antiviral properties of AH, it is highly hydrophobic and prone to aggregate. Therefore, Hamorsky et al. (2019) produced a soluble AH variant in Nicotiana, with more efficient biochemical and pharmaceutical properties, named avaren (AV). They also fused AV to the fragment Fc fragment of IgG1 to create the avaren-Fc (AvFc) lectibody. Avaren-Fc (AvFc) is a binding neutralizing lectibody for HIV and simian immunodeficiency virus (SIV) strains, without affecting normal human blood cells, which attaches to the HMGs on the gp120 of HIV envelope. AvFc exhibited an extended serum half-life in rats and macaques, whereas repeated systemic administration in mice did not result in any noticeable toxicity. Moreover, surface plasmon resonance (SPR) analysis revealed that AvFc has tenfold higher affinity to the gp120 of HIV relative to AH, indicating the high selectivity and specificity of the lectibodies. Currently, no pharmaceutical agent chooses HMG selectively as a target. Therefore, developing AvFc and/or lectibodies in general may be unprecedented for selectively rendering HIV and SIV ineffective subtypes [158].
HCV is another enveloped virus that is densely glycosylated with HMGs. In addition to facilitating cell entry through surface receptors, these HMGs act as an armor-like shield in front of neutralizing antibodies. Previous studies have shown the affinity of AvFc to a recombinant HCV E2 envelope glycoprotein and its ability to prevent viral infection of Huh-7-hepatocyte-derived cellular carcinoma cell by cell culture-derived HCV (HCVcc).
Although the antiviral function of AH is not the strongest when compared with other lectins, it does not show any cytotoxicity and mitogenicity, which are common side effects among lectins. Takahashi et al. (2011) demonstrated that, via dimerizing AH proteins, its activities against various HIV strains can be increased due to the “cluster effect” of lectin (with IC50 values of 12–290 nM) [159–161]. Hydrophobic nature and high aggregation rate are the major drawbacks of AH. Hence, developing a more efficient recombinant product is necessary to make lectin a potent anti-HIV drug. Its soluble variant Avaren was obtained by structure-guided mutations and fused to Fc of human IgG1. As a result, the “lectibody” AvFc was created, which is more active against HIV infection (with IC50 values of 5.6 and 0.3 nM for HIV-1 and 2, respectively) compared with AH (with IC50 values of 60.4 and 156.3 nM against HIV-1 and 2, respectively). It was also reported that AvFc might inhibit SIV (with IC50 values of 3.8–15.3 nM) and HCV (with IC50 values of 1.69–2.85 nM) [148, 158, 162–164]. These results suggest that lectibodies can act as inhibitors of viral entry into host cells and represent a potential solution to reduce the mortality rate of hepatitis C [165].
Banana lectin (BanLec) is a 141-residue plant-derived lectin (Mw = 15,000). Native mass spectrometry (MS) demonstrated that BanLec assembles as a homotetramer in solution, with each subunit folding into a β-prism-1 topology, characteristic of Jacalin-related lectins (JLRs). There are two sugar-binding sites in each subunit (octavalent for native tetrameric BanLec), recognizing 1,3-sugar moieties, particularly α/β-d-mannosyl/glycosyl configurations at the reducing ends, plus internal α-1,3-linked glucosyl residues. Concomitantly, the lectin recognizes numerous N-glycans on different HIV trimers, making the virus particles cross-link and aggregate [166–170].
The major drawback of BanLec is its mitogenic effect on T-cells. However, it has been shown previously that an engineered type of this lectin (BanLec H84T) avoids any mitogenicity while retaining the broad-spectrum antiviral functions. The rationally engineered lectin inhibits HIV-1 and HIV-2 with EC50 values of 0.4–4.1 nM and 0.3 nM, whereas the EC50 values for the wild type are 0.8–14 nM and 3.7 nM, respectively [171–173]. Moreover, a fluorescently labeled BanLec (BanLec-eGFP) was produced recently by Lopandic et al. (2021) and exploited as a versatile detection tool to investigate tissues and pathological processes structurally and functionally [174].
In recent years, IgG monoclonal antibodies have played an essential role in producing therapeutic drugs (> 70 antibody drugs) for many diseases, such as cancer, autoimmune illnesses, and viral infections. The Fc domain in IgG is constituted from two constant domains CH2 and CH3, which are responsible for the ADCC and complement activation produced by IgG. Previously, lectibodies were produced by fusing a lectin and Fc domain genetically. However, such production of full-length antibodies in large quantities in bacterial systems is challenging because disulfide bonds needed for proper folding cannot be created. Moreover, large-scale production of antibodies in mammalian expression systems is expensive and slow, and also needs more time for optimization, in addition to the problem of heterogeneous expression of foreign proteins from different organisms. More recently, Jaakkonen et al. (2020) exploited an off-the-shelf approach as a solution for the production of Fc fusions, where each domain of a bivalent lectibody preproduced in their ideal host organism and expression system is then ligated to produce engineered bispecific antibodies. This approach avoids the time-consuming optimization of the expression and purification steps, while only the ligation step needs to be optimized. Indeed, the heavy-chain antibodies in camelids, which bear only two heavy chains, inspired this novel approach. They used in vitro protein trans-splicing (PTS) to replace the antigen-binding domains of IgG with the SVN lectin to produce a lectin fusion protein, i.e., lectibody, that can specifically attach to the HMGs on the surface of diverse virus particles such as HIV, SARS coronavirus, and Ebola virus, on the one hand, and call the immune system to wipe out these viruses out, on the other. They also documented that the size of the Fc domain and its separate valency could, respectively, enhance the stability and binding capacity of the small protein SVN [175].
Conclusions and perspectives
Lectins are potent antiviral agents with a promising future for the treatment/recognition of viral infections. Unlike most antiviral compounds that inhibit virus replication, antiviral lectins target the entry of viruses into cells, leading to lower toxicity in topical use. However, since various obstacles prevent their clinical use, future investigations should focus on these. The risks or limitations to the significant use of antiviral lectins are their size, short stability in the body environment, cytotoxicity and mitogenicity (for some lectins), the potential for awakening the immune system (thereby resulting in deleterious responses), vulnerability to proteolytic lysis, and challenges regarding affordable bulk production. Fortunately, computational tools or gene manipulation can produce more effective derivatives of these valuable proteins, such as lectibodies, to improve their stability and robustness and remove their mitogenic potential. In this regard, lectibodies can be considered to represent a new and promising approach, with high specificity and cost-effectiveness, to treat viral infections by identifying and neutralizing carbohydrates on the surface of the viral envelope. Lectibodies can not only neutralize the virus and inhibit its entry into the host cell through specific cell surface receptors but also, with the assistance of immune system mechanisms such as CDC, ADCC, and ADCP, induce the clearance of the virus or infected cells. Virus envelope glycans may be able to exert their role as a shield to reduce the normalizing function of antibodies against the virus. However, lectibodies may be superior in this respect, especially in terms of the performance of vaccines against newer variants of a virus that is consistently developing new mutations. However, because lectibodies have recently provided new insight into viruses, particularly SARS-CoV-2 and HIV, further studies and accurate clinical trials are needed to investigate their tissue distribution and effect on tissue metabolism and confirm their specificity and engagement of cell receptors, to prevent their possible binding to unwanted glycosylated targets or undesirable local immune responses.
Acknowledgements
Not applicable.
Abbreviations
- HMGs
High proportion of high-mannose-type glycans
- PRM-A
Pradimicin A
- CRDs
Carbohydrate recognition domains
- CV-N
Cyanovirin
- AH
Actinohivin
- SVN
Scytovirin
- SVL
Vermicularis
- GRFT
Griffithsin
- GlcNAc
N-acetylglucosamine
- HIV
Human immunodeficiency virus
- RT
Reverse transcriptase
- MVN
Microvirin
- HCV
Hepatitis C virus
- OAA
Oscillatoria agardhii agglutinin
- SIV
Simian immunodeficiency virus
- BCA
Boodlea coacta agglutinin
- CGL
Crenomytilus grayanus lectin
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ACE2
Angiotensin-converting enzyme 2
- CLRs
C-type lectin receptors
- MMR
Macrophage mannose receptor
- MGL
Macrophage galactose-type lectin
- DC-SIGN
Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin
- L-SIGN
Lymph node-specific intercellular adhesion molecule-3-grabbing integrin
- DCs
Dendritic cells
- GNA
Galanthus nivalis agglutinin
- HHA
Hippeastrum hybrid agglutinin
- PHA
Phytohemagglutinin
- LCA
Lens culinaris agglutinin
- Fc
Crystallizable fragment
- IgG
Immunoglobulin G
- CDC
Complement-dependent cytotoxicity
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- ADCP
Antibody-dependent cell-mediated phagocytosis
- FcγR
Fc gamma receptor
- NK
Natural killer
- MHC
Major histocompatibility complex
- SPR
Surface plasmon resonance
- HCVcc
Cell culture-derived HCV
- BanLec
Banana lectin
- JLRs
Jacalin-related lectins
- PTS
Protein trans-splicing
- BanLec
Banana lectin
- CRDs
Carbohydrate recognition domains
Author contributions
MN-A: conceptualization, writing-review and editing, investigation, visualization. MH: writing-review and editing. HZ: writing-review, editing, and figure drawing. IA: software. AS: writing-original draft. PS: writing-original draft. SA: supervision, writing-review and editing. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohsen Nabi-Afjadi and Morteza Heydari contributed equally to this work as first authors
References
- 1.Santos AF, Da Silva M, Napoleão T, Paiva P, Correia MdS, Coelho L. Lectins: function, structure, biological properties and potential applications. 2014.
- 2.Elgavish S, Shaanan B. Lectin-carbohydrate interactions: different folds, common recognition principles. Trends Biochem Sci. 1997;22:462–467. doi: 10.1016/S0968-0004(97)01146-8. [DOI] [PubMed] [Google Scholar]
- 3.Bah CSF, Fang EF, Ng TB. Medicinal applications of plant lectins. Antitumor Potential and other Emerging Medicinal Properties of Natural Compounds. 2013:55–74.
- 4.Tsaneva M, Van Damme EJ. 130 years of plant lectin research. Glycoconj J. 2020:1–19.
- 5.Singh RS, Walia AK. Lectins from red algae and their biomedical potential. J Appl Phycol. 2018;30:1833–1858. doi: 10.1007/s10811-017-1338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilpatrick DC. Mechanisms and assessment of lectin-mediated mitogenesis. Mol Biotechnol. 1999;11:55. doi: 10.1007/BF02789176. [DOI] [PubMed] [Google Scholar]
- 7.Patel S, Goyal A. Recent developments in mushrooms as anti-cancer therapeutics: a review. 3 Biotech. 2012;2:1–15. doi: 10.1007/s13205-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keyaerts E, Vijgen L, Pannecouque C, Van Damme E, Peumans W, Egberink H, et al. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell CA, Ramessar K, O'Keefe BR. Antiviral lectins: selective inhibitors of viral entry. Antiviral Res. 2017;142:37–54. doi: 10.1016/j.antiviral.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong C, O’Keefe BR, Byrd RA, McMahon JB. Potent anti-HIV activity of scytovirin domain 1 peptide. Peptides. 2006;27:1668–1675. doi: 10.1016/j.peptides.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 11.McFeeters RL, Xiong C, O’Keefe BR, Bokesch HR, McMahon JB, Ratner DM, et al. The novel fold of scytovirin reveals a new twist for antiviral entry inhibitors. J Mol Biol. 2007;369:451–461. doi: 10.1016/j.jmb.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moulaei T, Botos I, Ziółkowska NE, Bokesch HR, Krumpe LR, McKee TC, et al. Atomic-resolution crystal structure of the antiviral lectin scytovirin. Protein Sci. 2007;16:2756–2760. doi: 10.1110/ps.073157507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molchanova V, Chikalovets I, Chernikov O, Belogortseva N, Li W, Wang J-H, et al. A new lectin from the sea worm Serpula vermicularis: isolation, characterization and anti-HIV activity. Comp Biochem Physiol C: Toxicol Pharmacol. 2007;145:184–193. doi: 10.1016/j.cbpc.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Lukyanov P, Chernikov O, Kobelev S, Chikalovets I, Molchanova V, Li W. Carbohydrate-binding proteins of marine invertebrates. Russ J Bioorg Chem. 2007;33:161–169. doi: 10.1134/S1068162007010190. [DOI] [Google Scholar]
- 15.Laskowski RA, Swindells MB. LigPlot+: multiple ligand–protein interaction diagrams for drug discovery. ACS Publications; 2011.
- 16.Trbojević-Akmačić I, Petrović T, Lauc G. SARS-CoV-2 S glycoprotein binding to multiple host receptors enables cell entry and infection. Glycoconj J. 2021;38:611–623. doi: 10.1007/s10719-021-10021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziółkowska NE, Shenoy SR, O'Keefe BR, Wlodawer A. Crystallographic studies of the complexes of antiviral protein griffithsin with glucose and N-acetylglucosamine. Protein Sci. 2007;16:1485–1489. doi: 10.1110/ps.072889407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloor A, Zhang J, Gashash EA, Parameswaran A, Chrzanowski M, Ma C, et al. Site-specific N-glycosylation on the AAV8 capsid protein. Viruses. 2018;10:644. doi: 10.3390/v10110644. [DOI] [Google Scholar]
- 19.Balzarini J. Inhibition of HIV entry by carbohydrate-binding proteins. Antiviral Res. 2006;71:237–247. doi: 10.1016/j.antiviral.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Dimitrov DS. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Microbiol. 2004;2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolchinsky P, Kiprilov E, Sodroski J. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J Virol. 2001;75:2041–2050. doi: 10.1128/JVI.75.5.2041-2050.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zalpoor H, Akbari A, Samei A, Forghaniesfidvajani R, Kamali M, Afzalnia A, et al. The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: inflammasome and JaK inhibitors as potential promising therapies. Cell Mol Biol Lett. 2022;27:1–21. doi: 10.1186/s11658-022-00311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letafati A, Najafi S, Mottahedi M, Karimzadeh M, Shahini A, Garousi S, et al. MicroRNA let-7 and viral infections: focus on mechanisms of action. Cell Mol Biol Lett. 2022;27:1–47. doi: 10.1186/s11658-022-00317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao L, Pauthner M, Andrabi R, Rantalainen K, Berndsen Z, Diedrich JK, et al. Differential processing of HIV envelope glycans on the virus and soluble recombinant trimer. Nat Commun. 2018;9:1–14. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazalovska M, Kouokam JC. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. BioMed Res Int. 2018;2018.
- 27.Miller NL, Clark T, Raman R, Sasisekharan R. Glycans in virus-host interactions: a structural perspective. Front Mol Biosci. 2021;8:505. doi: 10.3389/fmolb.2021.666756. [DOI] [Google Scholar]
- 28.Jan M, Upadhyay C, Hioe CE. HIV-1 envelope glycan composition as a key determinant of efficient virus transmission via DC-SIGN and resistance to inhibitory lectins. Iscience. 2019;21:413–427. doi: 10.1016/j.isci.2019.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jan M, Upadhyay C, Alcami Pertejo J, Hioe CE, Arora SK. Heterogeneity in glycan composition on the surface of HIV-1 envelope determines virus sensitivity to lectins. PLoS ONE. 2018;13:e0194498. doi: 10.1371/journal.pone.0194498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang G, Sun J, Wang H, Ng T. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim Pol. 2009;56.
- 31.Lin P, Ye X, Ng T. Purification of melibiose-binding lectins from two cultivars of Chinese black soybeans. Acta Biochim Biophys Sin. 2008;40:1029–1038. doi: 10.1111/j.1745-7270.2008.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fei Fang E, Ho Wong J, Lin P, Bun NT. Biochemical and functional properties of a lectin purified from korean large black soybeans—a cultivar of Glycine max. Protein Pept Lett. 2010;17:690–698. doi: 10.2174/092986610791190309. [DOI] [PubMed] [Google Scholar]
- 33.Cheung AH, Wong JH, Ng T. Musa acuminata (Del Monte banana) lectin is a fructose-binding lectin with cytokine-inducing activity. Phytomedicine. 2009;16:594–600. doi: 10.1016/j.phymed.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Barrientos LG, Gronenborn AM. The highly specific carbohydrate-binding protein cyanovirin-N: structure, anti-HIV/Ebola activity and possibilities for therapy. Mini Rev Med Chem. 2005;5:21–31. doi: 10.2174/1389557053402783. [DOI] [PubMed] [Google Scholar]
- 35.Barrientos LG, Matei E, Lasala F, Delgado R, Gronenborn AM. Dissecting carbohydrate–Cyanovirin-N binding by structure-guided mutagenesis: functional implications for viral entry inhibition. Protein Eng Des Sel. 2006;19:525–535. doi: 10.1093/protein/gzl040. [DOI] [PubMed] [Google Scholar]
- 36.Helle F, Wychowski C, Vu-Dac N, Gustafson KR, Voisset C, Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J Biol Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 37.Fromme R, Katiliene Z, Giomarelli B, Bogani F, Mc Mahon J, Mori T, et al. A monovalent mutant of cyanovirin-N provides insight into the role of multiple interactions with gp120 for antiviral activity. Biochemistry. 2007;46:9199–9207. doi: 10.1021/bi700666m. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Carroll JR, Holt LA, McMahon J, Giomarelli B, Ghirlanda G. Multivalent interactions with gp120 are required for the anti-HIV activity of Cyanovirin. Pept Sci. 2009;92:194–200. doi: 10.1002/bip.21173. [DOI] [Google Scholar]
- 39.Keeffe JR, Gnanapragasam PN, Gillespie SK, Yong J, Bjorkman PJ, Mayo SL. Designed oligomers of cyanovirin-N show enhanced HIV neutralization. Proc Natl Acad Sci. 2011;108:14079–14084. doi: 10.1073/pnas.1108777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandre KB, Gray ES, Mufhandu H, McMahon JB, Chakauya E, O'Keefe BR, et al. The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4+ cells. Virology. 2012;423:175–186. doi: 10.1016/j.virol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodrum BW, Maxwell J, Allen DM, Wilson J, Krumpe LR, Bobkov AA, et al. A designed “nested” dimer of cyanovirin-N increases antiviral activity. Viruses. 2016;8:158. doi: 10.3390/v8060158. [DOI] [Google Scholar]
- 42.Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O'Keefe BR, Mori T, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/AAC.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T, et al. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrientos LG, Lasala F, Otero JR, Sanchez A, Delgado R. In vitro evaluation of cyanovirin-N antiviral activity, by use of lentiviral vectors pseudotyped with filovirus envelope glycoproteins. J Infect Dis. 2004;189:1440–1443. doi: 10.1086/382658. [DOI] [PubMed] [Google Scholar]
- 45.Ziółkowska NE, Wlodawer A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim Pol. 2006;53:617–626. doi: 10.18388/abp.2006_3290. [DOI] [PubMed] [Google Scholar]
- 46.Maier I, Schiestl RH, Kontaxis G. Cyanovirin-N binds viral envelope proteins at the low-affinity carbohydrate binding site without direct virus neutralization ability. Molecules. 2021;26:3621. doi: 10.3390/molecules26123621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huskens D, Férir G, Vermeire K, Kehr J-C, Balzarini J, Dittmann E, et al. Microvirin, a novel α (1, 2)-mannose-specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J Biol Chem. 2010;285:24845–24854. doi: 10.1074/jbc.M110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahzad-ul-Hussan S, Gustchina E, Ghirlando R, Clore GM, Bewley CA. Solution structure of the monovalent lectin microvirin in complex with Manα (1–2) Man provides a basis for anti-HIV activity with low toxicity. J Biol Chem. 2011;286:20788–20796. doi: 10.1074/jbc.M111.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Souza RC, de Medeiros MG, Siqueira AS, de Melo LA, da Silva AP, Gonçalves EC, et al. Investigating the effects of point mutations on the affinity between the cyanobacterial lectin microvirin and high mannose-type glycans present on the HIV envelope glycoprotein. J Mol Model. 2016;22:1–9. doi: 10.1007/s00894-016-2961-9. [DOI] [PubMed] [Google Scholar]
- 50.Min Y-Q, Duan X-C, Zhou Y-D, Kulinich A, Meng W, Cai Z-P, et al. Effects of microvirin monomers and oligomers on hepatitis C virus. Biosci Rep. 2017;37:BSR20170015. doi: 10.1042/BSR20170015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahid M, Qadir A, Yang J, Ahmad I, Zahid H, Mirza S, et al. An engineered microvirin variant with identical structural domains potently inhibits human immunodeficiency virus and hepatitis C virus cellular entry. Viruses. 2020;12:199. doi: 10.3390/v12020199. [DOI] [Google Scholar]
- 52.Sato Y, Okuyama S, Hori K. Primary structure and carbohydrate binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium Oscillatoria agardhii. J Biol Chem. 2007;282:11021–11029. doi: 10.1074/jbc.M701252200. [DOI] [PubMed] [Google Scholar]
- 53.Koharudin LM, Furey W, Gronenborn AM. Novel fold and carbohydrate specificity of the potent anti-HIV cyanobacterial lectin from Oscillatoria agardhii. J Biol Chem. 2011;286:1588–1597. doi: 10.1074/jbc.M110.173278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koharudin LM, Gronenborn AM. Structural basis of the anti-HIV activity of the cyanobacterial Oscillatoria agardhii agglutinin. Structure. 2011;19:1170–1181. doi: 10.1016/j.str.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koharudin LM, Kollipara S, Aiken C, Gronenborn AM. Structural insights into the anti-HIV activity of the Oscillatoria agardhii agglutinin homolog lectin family. J Biol Chem. 2012;287:33796–33811. doi: 10.1074/jbc.M112.388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitley MJ, Furey W, Kollipara S, Gronenborn AM. Burkholderia oklahomensis agglutinin is a canonical two-domain OAA-family lectin: structures, carbohydrate binding and anti-HIV activity. FEBS J. 2013;280:2056–2067. doi: 10.1111/febs.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Férir G, Huskens D, Noppen S, Koharudin LM, Gronenborn AM, Schols D. Broad anti-HIV activity of the Oscillatoria agardhii agglutinin homologue lectin family. J Antimicrob Chemother. 2014;69:2746–2758. doi: 10.1093/jac/dku220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Meer F, de Haan C, Schuurman N, Haijema B, Verheije M, Bosch B, et al. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J Antimicrob Chemother. 2007;60:741–749. doi: 10.1093/jac/dkm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balzarini J, Van Laethem K, Daelemans D, Hatse S, Bugatti A, Rusnati M, et al. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human immunodeficiency virus. J Virol. 2007;81:362–373. doi: 10.1128/JVI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balzarini J, François KO, Van Laethem K, Hoorelbeke B, Renders M, Auwerx J, et al. Pradimicin S, a highly soluble nonpeptidic small-size carbohydrate-binding antibiotic, is an anti-HIV drug lead for both microbicidal and systemic use. Antimicrob Agents Chemother. 2010;54:1425–1435. doi: 10.1128/AAC.01347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakagawa Y, Doi T, Masuda Y, Takegoshi K, Igarashi Y, Ito Y. Mapping of the primary mannose binding site of pradimicin A. J Am Chem Soc. 2011;133:17485–17493. doi: 10.1021/ja207816h. [DOI] [PubMed] [Google Scholar]
- 62.Nakagawa Y, Doi T, Taketani T, Takegoshi K, Igarashi Y, Ito Y. Mannose-binding geometry of pradimicin A. Chemistry. 2013;19:10516–10525. doi: 10.1002/chem.201301368. [DOI] [PubMed] [Google Scholar]
- 63.Nakagawa Y. Paving the way for practical use of sugar-binding natural products as lectin mimics in glycobiological research. ChemBioChem. 2020;21:1567–1572. doi: 10.1002/cbic.201900781. [DOI] [PubMed] [Google Scholar]
- 64.Sato Y, Hirayama M, Morimoto K, Yamamoto N, Okuyama S, Hori K. High mannose-binding lectin with preference for the cluster of α1–2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J Biol Chem. 2011;286:19446–19458. doi: 10.1074/jbc.M110.216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ovodova RG, Glazkova VE, Mikheyskaya LV, Molchanova VI, Isakov VV, Ovodov YS, et al. The structure of mytilan, a bioglycan-immunomodulator isolated from the mussel Crenomytilus grayanus. Carbohyd Res. 1992;223:221–226. doi: 10.1016/0008-6215(92)80018-V. [DOI] [Google Scholar]
- 66.Belogortseva NI, Molchanova VI, Kurika AV, Skobun AS, Glazkova VE. Isolation and characterization of new GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol. 1998;119:45–50. doi: 10.1016/s0742-8413(97)00180-1. [DOI] [PubMed] [Google Scholar]
- 67.Kovalchuk SN, Chikalovets IV, Chernikov OV, Molchanova VI, Li W, Rasskazov VA, et al. cDNA cloning and structural characterization of a lectin from the mussel Crenomytilus grayanus with a unique amino acid sequence and antibacterial activity. Fish Shellfish Immunol. 2013;35:1320–1324. doi: 10.1016/j.fsi.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Jakób M, Lubkowski J, O'Keefe BR, Wlodawer A. Structure of a lectin from the sea mussel Crenomytilus grayanus (CGL) Acta Crystallogr Sect F: Struct Biol Commun. 2015;71:1429–1436. doi: 10.1107/S2053230X15019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kovalchuk SN, Golotin VA, Balabanova LA, Buinovskaya NS, Likhatskaya GN, Rasskazov VA. Carbohydrate-binding motifs in a novel type lectin from the sea mussel Crenomytilus grayanus: homology modeling study and site-specific mutagenesis. Fish Shellfish Immunol. 2015;47:565–571. doi: 10.1016/j.fsi.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 70.Chernikov OV, Wong W-T, Li L-H, Chikalovets IV, Molchanova VI, Wu S-H, et al. A GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus modulates immune response in macrophages and in mice. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-06647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chernikov O, Kuzmich A, Chikalovets I, Molchanova V, Hua K-F. Lectin CGL from the sea mussel Crenomytilus grayanus induces Burkitt’s lymphoma cells death via interaction with surface glycan. Int J Biol Macromol. 2017;104:508–514. doi: 10.1016/j.ijbiomac.2017.06.074. [DOI] [PubMed] [Google Scholar]
- 72.Kovalchuk SN, Buinovskaya NS, Likhatskaya GN, Rasskazov VA, Son OM, Tekutyeva LA, et al. Mutagenesis studies and structure-function relationships for GalNAc/Gal-specific lectin from the sea mussel Crenomytilus grayanus. Mar Drugs. 2018;16:471. doi: 10.3390/md16120471. [DOI] [Google Scholar]
- 73.Chikalovets I, Filshtein A, Molchanova V, Mizgina T, Lukyanov P, Nedashkovskaya O, et al. Activity dependence of a novel lectin family on structure and carbohydrate-binding properties. Molecules. 2020;25:150. doi: 10.3390/molecules25010150. [DOI] [Google Scholar]
- 74.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bianchi M, Benvenuto D, Giovanetti M, Angeletti S, Ciccozzi M, Pascarella S. Sars-CoV-2 envelope and membrane proteins: structural differences linked to virus characteristics? BioMed Res Int. 2020;2020.
- 76.Cosset F-L, Lavillette D. Cell entry of enveloped viruses. Adv Genet. 2011;73:121–183. doi: 10.1016/B978-0-12-380860-8.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verma J, Subbarao N, Rajala MS. Envelope proteins as antiviral drug target. J Drug Target. 2020;28:1046–1052. doi: 10.1080/1061186X.2020.1792916. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [Google Scholar]
- 79.Zalpoor H, Bakhtiyari M, Liaghat M, Nabi‐Afjadi M, Ganjalikhani‐Hakemi M. Quercetin potential effects against SARS‐CoV‐2 infection and COVID‐19‐associated cancer progression by inhibiting mTOR and hypoxia‐inducible factor‐1α (HIF‐1α). Phytotherapy Research. 2022.
- 80.Sedighimehr N, Fathi J, Hadi N, Rezaeian ZS. Rehabilitation, a necessity in hospitalized and discharged people infected with COVID-19: a narrative review. Phys Therapy Rev. 2021;26:202–210. doi: 10.1080/10833196.2021.1899472. [DOI] [Google Scholar]
- 81.Khesht AMS, Karpisheh V, Saeed BQ, Zekiy AO, Yapanto LM, Afjadi MN, et al. Different T cell related immunological profiles in COVID-19 patients compared to healthy controls. Int Immunopharmacol. 2021;97:107828. doi: 10.1016/j.intimp.2021.107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grant OC, Montgomery D, Ito K, Woods RJ. 3D Models of glycosylated SARS-CoV-2 spike protein suggest challenges and opportunities for vaccine development. BioRxiv. 2020.
- 83.Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili S-M, Bahreini E. A comprehensive review of COVID-19 characteristics. Biol Proced online. 2020;22:1–10. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khomari F, Nabi-Afjadi M, Yarahmadi S, Eskandari H, Bahreini E. Effects of cell proteostasis network on the survival of SARS-CoV-2. Biol Proced Online. 2021;23:1–10. doi: 10.1186/s12575-021-00145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Payandeh Z, Mohammadkhani N, Nabi Afjadi M, Khalili S, Rajabibazl M, Houjaghani Z, et al. The immunology of SARS-CoV-2 infection, the potential antibody based treatments and vaccination strategies. Expert Rev Anti Infect Ther. 2021;19:899–910. doi: 10.1080/14787210.2020.1863144. [DOI] [PubMed] [Google Scholar]
- 86.Khezri MR, Varzandeh R, Ghasemnejad-Berenji M. The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS-CoV-2 induced coagulopathy. Cell Mol Biol Lett. 2022;27:1–10. doi: 10.1186/s11658-022-00308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zalpoor H, Akbari A, Nabi-Afjadi M. Ephrin (Eph) receptor and downstream signaling pathways: a promising potential targeted therapy for COVID‑19 and associated cancers and diseases. Human Cell. 2022:1–3.
- 88.Amraei R, Yin W, Napoleon MA, Suder EL, Berrigan J, Zhao Q, et al. CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2.
- 89.Gupta A, Gupta G. Status of mannose-binding lectin (MBL) and complement system in COVID-19 patients and therapeutic applications of antiviral plant MBLs. Mol Cell Biochem. 2021:1–26.
- 90.Wang D, Zhou B, Keppel TR, Solano M, Baudys J, Goldstein J, et al. N-glycosylation profiles of the SARS-CoV-2 spike D614G mutant and its ancestral protein characterized by advanced mass spectrometry. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han Y, Wang Z, Wei Z, Schapiro I, Li J. Binding affinity and mechanisms of SARS-CoV-2 variants. Comput Struct Biotechnol J. 2021;19:4184–4191. doi: 10.1016/j.csbj.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao X, Chen H, Wang H. Glycans of SARS-CoV-2 spike protein in virus infection and antibody production. Front Mol Biosci. 2021;8:53. [Google Scholar]
- 93.Barton MI, MacGowan SA, Kutuzov MA, Dushek O, Barton GJ, van der Merwe PA. Effects of common mutations in the SARS-CoV-2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. eLife. 2021;10:e70658. doi: 10.7554/eLife.70658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nabi-Afjadi M, Karami H, Goudarzi K, Alipourfard I, Bahreini E. The effect of vitamin D, magnesium and zinc supplements on interferon signaling pathways and their relationship to control SARS-CoV-2 infection. Clin Mol Allergy. 2021;19:1–10. doi: 10.1186/s12948-021-00161-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sui Y, Li J, Venzon DJ, Berzofsky JA. SARS-CoV-2 spike protein suppresses ACE2 and type I interferon expression in primary cells from macaque lung bronchoalveolar lavage. Front Immunol. 2021:2061.
- 96.Issa E, Merhi G, Panossian B, Salloum T, Tokajian S. SARS-CoV-2 and ORF3a: nonsynonymous mutations, functional domains, and viral pathogenesis. Msystems. 2020;5:e00266–e320. doi: 10.1128/mSystems.00266-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1–12. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(281–92):e6. [Google Scholar]
- 99.Almehdi AM, Khoder G, Alchakee AS, Alsayyid AT, Sarg NH, Soliman SS. SARS-CoV-2 spike protein: pathogenesis, vaccines, and potential therapies. Infection. 2021;49:855–876. doi: 10.1007/s15010-021-01677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alavi M, Asare-Addo K, Nokhodchi A. Lectin protein as a promising component to functionalize micelles, liposomes and lipid NPs against coronavirus. Biomedicines. 2020;8:580. doi: 10.3390/biomedicines8120580. [DOI] [Google Scholar]
- 101.Barre A, Van Damme EJ, Simplicien M, Le Poder S, Klonjkowski B, Benoist H, et al. Man-specific lectins from plants, fungi, algae and cyanobacteria, as potential blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) coronaviruses: biomedical perspectives. Cells. 2021;10:1619. doi: 10.3390/cells10071619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watanabe Y, Berndsen ZT, Raghwani J, Seabright GE, Allen JD, Pybus OG, et al. Vulnerabilities in coronavirus glycan shields despite extensive glycosylation. Nat Commun. 2020;11:1–10. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mori T, O'Keefe BR, Sowder RC, Bringans S, Gardella R, Berg S, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 104.Meuleman P, Albecka A, Belouzard S, Vercauteren K, Verhoye L, Wychowski C, et al. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother. 2011;55:5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xue J, Gao Y, Hoorelbeke B, Kagiampakis I, Zhao B, Demeler B, et al. The role of individual carbohydrate-binding sites in the function of the potent anti-HIV lectin griffithsin. Mol Pharm. 2012;9:2613–2625. doi: 10.1021/mp300194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barton C, Kouokam JC, Lasnik AB, Foreman O, Cambon A, Brock G, et al. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob Agents Chemother. 2014;58:120–127. doi: 10.1128/AAC.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xue J, Hoorelbeke B, Kagiampakis I, Demeler B, Balzarini J, LiWang PJ. The griffithsin dimer is required for high-potency inhibition of HIV-1: evidence for manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob Agents Chemother. 2013;57:3976–3989. doi: 10.1128/AAC.00332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lusvarghi S, Bewley CA. Griffithsin: an antiviral lectin with outstanding therapeutic potential. Viruses. 2016;8:296. doi: 10.3390/v8100296. [DOI] [Google Scholar]
- 109.Kouokam JC, Lasnik AB, Palmer KE. Studies in a murine model confirm the safety of griffithsin and advocate its further development as a microbicide targeting HIV-1 and other enveloped viruses. Viruses. 2016;8:311. doi: 10.3390/v8110311. [DOI] [Google Scholar]
- 110.Developing and Testing a Griffithsin (non-ARV) Microbicide. Available online: https://www.popcouncil.org/research/developing-and-testing-a-griffithsin-non-arv-microbicide (accessed on 5 October 2019).
- 111.Study to Evaluate the Safety of Griffithsin in a Carrageenan Gel in Healthy Women. Available online: https://clinicaltrialsgov/ct2/show/study/NCT02875119 (accessed on 5 October 2019).
- 112.Palmer K. Griffithsin-based rectal microbicides for prevention of viral entry (PREVENT). 2014.
- 113.Millet JK, Séron K, Labitt RN, Danneels A, Palmer KE, Whittaker GR, et al. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lo MK, Spengler JR, Krumpe LR, Welch SR, Chattopadhyay A, Harmon JR, et al. Griffithsin inhibits Nipah virus entry and fusion and can protect Syrian golden Hamsters from lethal Nipah virus challenge. J Infect Dis. 2020;221:S480–S492. doi: 10.1093/infdis/jiz630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Habibi P, Soccol CR, O’Keefe BR, Krumpe LR, Wilson J, de Macedo LLP, et al. Gene-silencing suppressors for high-level production of the HIV-1 entry inhibitor griffithsin in Nicotiana benthamiana. Process Biochem. 2018;70:45–54. doi: 10.1016/j.procbio.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoelscher M, Tiller N, Teh AY-H, Wu G-Z, Ma JK, Bock R. High-level expression of the HIV entry inhibitor griffithsin from the plastid genome and retention of biological activity in dried tobacco leaves. Plant Mol Biol. 2018;97:357–370. doi: 10.1007/s11103-018-0744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee C. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: from discovery to clinical application. Mar Drugs. 2019;17:567. doi: 10.3390/md17100567. [DOI] [Google Scholar]
- 118.Cai Y, Xu W, Gu C, Cai X, Qu D, Lu L, et al. Griffithsin with a broad-spectrum antiviral activity by binding glycans in viral glycoprotein exhibits strong synergistic effect in combination with a pan-coronavirus fusion inhibitor targeting SARS-CoV-2 spike S2 subunit. Virol Sin. 2020;35:857–860. doi: 10.1007/s12250-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shrivastava-Ranjan P, Lo MK, Chatterjee P, Flint M, Nichol ST, Montgomery JM, et al. Hantavirus infection is inhibited by griffithsin in cell culture. Front Cell Infect Microbiol. 2020;10:619. doi: 10.3389/fcimb.2020.561502. [DOI] [Google Scholar]
- 120.Alexandre K, Malatji K, Mulaudzi T. Comparison of the antiviral activity of the microbicide candidate griffithsin and its tandemers derivatives against different modes of HIV-1 transmission. Virology. 2020;544:12–20. doi: 10.1016/j.virol.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 121.Jiang Y-P, Zhao X-X, Lv H-Q, Wen C-P. Drug screening and development from the affinity of S protein of new coronavirus with ACE2. Eur J Clin Microbiol Infect Dis. 2021;40:715–723. doi: 10.1007/s10096-020-04048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cai Y, Xu W, Tang J, Cao N, Lan Q, Lu L, et al. A bivalent protein targeting glycans and HR1 domain in spike protein potently inhibited infection of SARS-CoV-2 and other human coronaviruses. Cell Biosci. 2021;11:1–14. doi: 10.1186/s13578-021-00638-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kramzer LF, Hamorsky KT, Graebing PW, Wang L, Fuqua JL, Matoba N, et al. Preformulation characterization of griffithsin, a biopharmaceutical candidate for HIV prevention. AAPS PharmSciTech. 2021;22:1–13. doi: 10.1208/s12249-021-01931-0. [DOI] [Google Scholar]
- 124.Ishag HZ, Li C, Wang F, Mao X. Griffithsin binds to the glycosylated proteins (E and prM) of Japanese encephalitis virus and inhibit its infection. Virus Res. 2016;215:50–54. doi: 10.1016/j.virusres.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu L, Bao J-K. Anti-tumor and anti-viral activities of Galanthus nivalis agglutinin (GNA)-related lectins. Glycoconjugate J. 2013;30:269–279. doi: 10.1007/s10719-012-9440-z. [DOI] [Google Scholar]
- 126.Barre A, Bourne Y, Van Damme EJ, Rougé P. Overview of the structure–function relationships of mannose-specific lectins from plants, algae and fungi. Int J Mol Sci. 2019;20:254. doi: 10.3390/ijms20020254. [DOI] [Google Scholar]
- 127.Kong CK, Low LE, Wei Sheng S, Yap WH, Goh PH, Ming LC, et al. Biological activities of snowdrop (Galanthus spp., Family Amaryllidaceae) Front Pharmacol. 2020;11:2394. [Google Scholar]
- 128.Balzarini J, Hatse S, Vermeire K, Princen K, Aquaro S, Perno C-F, et al. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother. 2004;48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mishra A, Behura A, Mawatwal S, Kumar A, Naik L, Mohanty SS, et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem Toxicol. 2019;134:110827. doi: 10.1016/j.fct.2019.110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vanderlinden E, Van Winkel N, Naesens L, Van Damme EJ, Persoons L, Schols D. In vitro characterization of the carbohydrate-binding agents HHA, GNA, and UDA as inhibitors of influenza A and B virus replication. Antimicrob Agents Chemother. 2020;65:e01732–e1820. [Google Scholar]
- 131.Saïdi H, Nasreddine N, Jenabian M-A, Lecerf M, Schols D, Krief C, et al. Differential in vitro inhibitory activity against HIV-1 of alpha-(1–3)-and alpha-(1–6)-D-mannose specific plant lectins: implication for microbicide development. J Transl Med. 2007;5:1–9. doi: 10.1186/1479-5876-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jenabian M-A, Saïdi H, Charpentier C, Van Herrewege Y, Son JC, Schols D, et al. In vitro synergistic activity against CCR5-tropic HIV-1 with combinations of potential candidate microbicide molecules HHA, KRV2110 and enfuvirtide (T20) J Antimicrob Chemother. 2009;64:1192–1195. doi: 10.1093/jac/dkp380. [DOI] [PubMed] [Google Scholar]
- 133.Mathys L, Balzarini J. Exposure of HIV-1 to a combination of two carbohydrate-binding agents markedly delays drug resistance development and selects for virus strains with compromised fitness. J Antimicrob Chemother. 2014;69:582–593. doi: 10.1093/jac/dkt414. [DOI] [PubMed] [Google Scholar]
- 134.Khabbazi SD, Bakhsh A, Sancak C, Özcan S. Molecular characterization of snowdrop lectin (GNA) and its comparison with reported lectin sequences of Amaryllidaceae. Czech J Genetics Plant Breeding. 2016;52:94–100. doi: 10.17221/58/2016-CJGPB. [DOI] [Google Scholar]
- 135.Nagae M, Soga K, Morita-Matsumoto K, Hanashima S, Ikeda A, Yamamoto K, et al. Phytohemagglutinin from Phaseolus vulgaris (PHA-E) displays a novel glycan recognition mode using a common legume lectin fold. Glycobiology. 2014;24:368–378. doi: 10.1093/glycob/cwu004. [DOI] [PubMed] [Google Scholar]
- 136.He S, Simpson BK, Sun H, Ngadi MO, Ma Y, Huang T. Phaseolus vulgaris lectins: a systematic review of characteristics and health implications. Crit Rev Food Sci Nutr. 2018;58:70–83. doi: 10.1080/10408398.2015.1096234. [DOI] [PubMed] [Google Scholar]
- 137.Nath AB, Sivaramakrishna A, Marimuthu K, Saraswathy R. A comparative study of phytohaemagglutinin and extract of Phaseolus vulgaris seeds by characterization and cytogenetics. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;134:143–147. doi: 10.1016/j.saa.2014.05.086. [DOI] [Google Scholar]
- 138.Ekowati H, Arai J, Putri ASD, Nainu F, Shiratsuchi A, Nakanishi Y. Protective effects of Phaseolus vulgaris lectin against viral infection in Drosophila. Drug Discov Ther. 2017;11:329–335. doi: 10.5582/ddt.2017.01071. [DOI] [PubMed] [Google Scholar]
- 139.Wang W, Li Q, Wu J, Hu Y, Wu G, Yu C, et al. Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg Microbes Infect. 2021;10:1519–1529. doi: 10.1080/22221751.2021.1957720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schwarz FP, Puri KD, Bhat R, Surolia A. Thermodynamics of monosaccharide binding to concanavalin A, pea (Pisum sativum) lectin, and lentil (Lens culinaris) lectin. J Biol Chem. 1993;268:7668–7677. doi: 10.1016/S0021-9258(18)53009-X. [DOI] [PubMed] [Google Scholar]
- 141.Loris R, Steyaert J, Maes D, Lisgarten J, Pickersgill R, Wyns L. Crystal structure determination and refinement at 2.3-. ANG. resolution of the lentil lectin. Biochemistry. 1993;32:8772–8781. doi: 10.1021/bi00085a007. [DOI] [PubMed] [Google Scholar]
- 142.Loris R, Casset F, Bouckaert J, Pletinckx J, Dao-Thi M-H, Poortmans F, et al. The monosaccharide binding site of lentil lectin: an X-ray and molecular modelling study. Glycoconj J. 1994;11:507–517. doi: 10.1007/BF00731301. [DOI] [PubMed] [Google Scholar]
- 143.Marcos MJ, Villar E, Gavilanes F, Zhadan GG, Shnyrov VL. Compact residual structure in lentil lectin at pH 2. Eur J Biochem. 2000;267:2127–2132. doi: 10.1046/j.1432-1327.2000.01233.x. [DOI] [PubMed] [Google Scholar]
- 144.Naseem F, Khan RH. Fluoroalcohol-induced stabilization of the α-helical intermediates of lentil lectin: implication for non-hierarchical lectin folding. Arch Biochem Biophys. 2004;431:215–223. doi: 10.1016/j.abb.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 145.Jan M, Tomer S, Arora SK. α (1–6)-fucosylated complex glycan-binding lentil lectin enhances in vitro HIV-1 infection and DC-SIGN-mediated viral capture and transmission to CD4 cells. AIDS Res Hum Retroviruses. 2018;34:641–644. doi: 10.1089/aid.2018.0045. [DOI] [PubMed] [Google Scholar]
- 146.Carvalho EV, Oliveira WF, Coelho LC, Correia MT. Lectins as mitosis stimulating factors: briefly reviewed. Life Sci. 2018;207:152–157. doi: 10.1016/j.lfs.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 147.Nascimento da Silva LC, Mendonça JSP, de Oliveira WF, Batista KLR, Zagmignan A, Viana IFT, et al. Exploring lectin–glycan interactions to combat COVID-19: lessons acquired from other enveloped viruses. Glycobiology. 2021;31:358–371. doi: 10.1093/glycob/cwaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dent M, Hamorsky K, Vausselin T, Dubuisson J, Miyata Y, Morikawa Y, et al. Safety and efficacy of avaren-Fc lectibody targeting HCV high-mannose glycans in a human liver chimeric mouse model. Cell Mol Gastroenterol Hepatol. 2021;11:185–198. doi: 10.1016/j.jcmgh.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Van Erp EA, Luytjes W, Ferwerda G, Van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019;10:548. doi: 10.3389/fimmu.2019.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Q, Fan C, Li Q, Zhou S, Huang W, Wang L, et al. Antibody-dependent-cellular-cytotoxicity-inducing antibodies significantly affect the post-exposure treatment of Ebola virus infection. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yasui F, Kohara M, Kitabatake M, Nishiwaki T, Fujii H, Tateno C, et al. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology. 2014;454:157–168. doi: 10.1016/j.virol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tay M, Wiehe K, Pollara J. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front Immunol. 2019;10:332. doi: 10.3389/fimmu.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hoorelbeke B, Huskens D, Férir G, François KO, Takahashi A, Van Laethem K, et al. Actinohivin, a broadly neutralizing prokaryotic lectin, inhibits HIV-1 infection by specifically targeting high-mannose-type glycans on the gp120 envelope. Antimicrob Agents Chemother. 2010;54:3287–3301. doi: 10.1128/AAC.00254-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang F, Hoque MM, Jiang J, Suzuki K, Tsunoda M, Takeda Y, et al. The characteristic structure of anti-hiv actinohivin in complex with three HMTG D1 chains of HIV-gp120. ChemBioChem. 2014;15:2766–2773. doi: 10.1002/cbic.201402352. [DOI] [PubMed] [Google Scholar]
- 155.Hoque MM, Suzuki K, Tsunoda M, Jiang J, Zhang F, Takahashi A, et al. Structural insights into the specific anti-HIV property of actinohivin: structure of its complex with the α (1–2) mannobiose moiety of gp120. Acta Crystallogr D Biol Crystallogr. 2012;68:1671–1679. doi: 10.1107/S0907444912040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suzuki K, Ohbayashi N, Jiang J, Zhang X, Hoque M, Tsunoda M, et al. Crystallographic study of the interaction of the anti-HIV lectin actinohivin with the α (1–2) mannobiose moiety of gp120 HMTG. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68:1060–1063. doi: 10.1107/S1744309112031077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiba H, Inokoshi J, Nakashima H, Ōmura S, Tanaka H. Actinohivin, a novel anti-human immunodeficiency virus protein from an actinomycete, inhibits viral entry to cells by binding high-mannose type sugar chains of gp120. Biochem Biophys Res Commun. 2004;316:203–210. doi: 10.1016/j.bbrc.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 158.Hamorsky KT, Kouokam JC, Dent MW, Grooms TN, Husk AS, Hume SD, et al. Engineering of a lectibody targeting high-mannose-type glycans of the HIV envelope. Mol Ther. 2019;27:2038–2052. doi: 10.1016/j.ymthe.2019.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Takahashi A, Inokoshi J, Tsunoda M, Suzuki K, Takenaka A, Sekiguchi T, et al. Actinohivin: specific amino acid residues essential for anti-HIV activity. J Antibiot. 2010;63:661–665. doi: 10.1038/ja.2010.106. [DOI] [Google Scholar]
- 160.Takahashi A, Inokoshi J, Hachiya A, Oka S, Omura S, Tanaka H. The high mannose-type glycan binding lectin actinohivin: dimerization greatly improves anti-HIV activity. J Antibiot. 2011;64:551–557. doi: 10.1038/ja.2011.51. [DOI] [Google Scholar]
- 161.Matoba N, Husk AS, Barnett BW, Pickel MM, Arntzen CJ, Montefiori DC, et al. HIV-1 neutralization profile and plant-based recombinant expression of actinohivin, an Env glycan-specific lectin devoid of T-cell mitogenic activity. PLoS ONE. 2010;5:e11143. doi: 10.1371/journal.pone.0011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tanaka H, Chiba H, Inokoshi J, Kuno A, Sugai T, Takahashi A, et al. Mechanism by which the lectin actinohivin blocks HIV infection of target cells. Proc Natl Acad Sci. 2009;106:15633–15638. doi: 10.1073/pnas.0907572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Siqueira AS, Lima ARJ, de Souza RC, Santos AS, Vianez JLdSG, Gonçalves EC. In silico analysis of the cyanobacterial lectin scytovirin: new insights into binding properties. Mol Biol Rep. 2017;44:353–358. doi: 10.1007/s11033-017-4116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coelho LCBB, Silva PMdS, Lima VLdM, Pontual EV, Paiva PMG, Napoleao TH, et al. Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evid-Based Complement Altern Med. 2017;2017.
- 165.Stewart-Jones GB, Soto C, Lemmin T, Chuang G-Y, Druz A, Kong R, et al. Trimeric HIV-1-Env structures define glycan shields from clades A, B, and G. Cell. 2016;165:813–826. doi: 10.1016/j.cell.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Swanson MD, Winter HC, Goldstein IJ, Markovitz DM. A lectin isolated from bananas is a potent inhibitor of HIV replication. J Biol Chem. 2010;285:8646–8655. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Khan JM, Qadeer A, Ahmad E, Ashraf R, Bhushan B, Chaturvedi SK, et al. Monomeric banana lectin at acidic pH overrules conformational stability of its native dimeric form. PLoS ONE. 2013;8:e62428. doi: 10.1371/journal.pone.0062428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bodora P, Szokeb A, Toth-Lencsesb K, Veresb A, Deaka T, Kozmac P, et al. Agriculture and environmental biotechnology. 2014.
- 169.Singh SS, Devi SK, Ng TB. Banana lectin: a brief review. Molecules. 2014;19:18817–18827. doi: 10.3390/molecules191118817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hopper JT, Ambrose S, Grant OC, Krumm SA, Allison TM, Degiacomi MT, et al. The tetrameric plant lectin BanLec neutralizes HIV through bidentate binding to specific viral glycans. Structure. 2017;25(773–82):e5. [Google Scholar]
- 171.Covés-Datson EM, King SR, Legendre M, Gupta A, Chan SM, Gitlin E, et al. A molecularly engineered antiviral banana lectin inhibits fusion and is efficacious against influenza virus infection in vivo. Proc Natl Acad Sci. 2020;117:2122–2132. doi: 10.1073/pnas.1915152117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Covés-Datson EM, Dyall J, DeWald LE, King SR, Dube D, Legendre M, et al. Inhibition of Ebola virus by a molecularly engineered banana lectin. PLoS Negl Trop Dis. 2019;13:e0007595. doi: 10.1371/journal.pntd.0007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lopandić Z, Dragačević L, Popović D, Andjelković U, Minić R, Gavrović-Jankulović M. BanLec-eGFP chimera as a tool for evaluation of lectin binding to high-mannose glycans on microorganisms. Biomolecules. 2021;11:180. doi: 10.3390/biom11020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Swanson MD, Boudreaux DM, Salmon L, Chugh J, Winter HC, Meagher JL, et al. Engineering a therapeutic lectin by uncoupling mitogenicity from antiviral activity. Cell. 2015;163:746–758. doi: 10.1016/j.cell.2015.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jaakkonen A, Volkmann G, Iwaï H. An off-the-shelf approach for the production of fc fusion proteins by protein trans-splicing towards generating a lectibody in vitro. Int J Mol Sci. 2020;21:4011. doi: 10.3390/ijms21114011. [DOI] [Google Scholar]
- 176.Bewley CA, Cai M, Ray S, Ghirlando R, Yamaguchi M, Muramoto K. New carbohydrate specificity and HIV-1 fusion blocking activity of the cyanobacterial protein MVL: NMR, ITC and sedimentation equilibrium studies. J Mol Biol. 2004;339:901–914. doi: 10.1016/j.jmb.2004.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shahzad-ul-Hussan S, Cai M, Bewley CA. Unprecedented glycosidase activity at a lectin carbohydrate-binding site exemplified by the cyanobacterial lectin MVL. J Am Chem Soc. 2009;131:16500–16508. doi: 10.1021/ja905929c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ishag HZ, Li C, Huang L, Sun M-X, Wang F, Ni B, et al. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch Virol. 2013;158:349–358. doi: 10.1007/s00705-012-1489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.An J, Liu JZ, Wu CF, Li J, Dai L, Van Damme E, et al. Anti-HIV I/II activity and molecular cloning of a novel mannose/sialic acid-binding lectin from rhizome of Polygonatum cyrtonema Hua. Acta Biochim Biophys Sin. 2006;38:70–78. doi: 10.1111/j.1745-7270.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 180.Ding J-J, Bao J-K, Zhu D-Y, Zhang Y, Wang D-C. Crystallization and preliminary x-ray diffraction analysis of a novel mannose-binding lectin with antiretroviral properties from Polygonatum cyrtonema Hua. Protein Pept Lett. 2008;15:411–414. doi: 10.2174/092986608784246551. [DOI] [PubMed] [Google Scholar]
- 181.Ding J, Bao J, Zhu D, Zhang Y, Wang D-C. Crystal structures of a novel anti-HIV mannose-binding lectin from Polygonatum cyrtonema Hua with unique ligand-binding property and super-structure. J Struct Biol. 2010;171:309–317. doi: 10.1016/j.jsb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 182.Le Moal M, Colle J-H, Galelli A, Truffa-Bachi P. Mouse T-lymphocyte activation by Urtica dioica agglutinin: I—delineation of two lymphocyte subsets. Res Immunol. 1992;143:691–700. doi: 10.1016/0923-2494(92)80007-8. [DOI] [PubMed] [Google Scholar]
- 183.Balzarini J, Neyts J, Schols D, Hosoya M, Van Damme E, Peumans W, et al. The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine) n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antiviral Res. 1992;18:191–207. doi: 10.1016/0166-3542(92)90038-7. [DOI] [PubMed] [Google Scholar]
- 184.Does MP, Ng DK, Dekker HL, Peumans WJ, Houterman PM, Van Damme EJ, et al. Characterization of Urtica dioica agglutinin isolectins and the encoding gene family. Plant Mol Biol. 1999;39:335–347. doi: 10.1023/A:1006134932290. [DOI] [PubMed] [Google Scholar]
- 185.Lannoo N, Peumans WJ, Van Pamel E, Alvarez R, Xiong T-C, Hause G, et al. Localization and in vitro binding studies suggest that the cytoplasmic/nuclear tobacco lectin can interact in situ with high-mannose and complex N-glycans. FEBS Lett. 2006;580:6329–6337. doi: 10.1016/j.febslet.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 186.Schouppe D, Rougé P, Lasanajak Y, Barre A, Smith DF, Proost P, et al. Mutational analysis of the carbohydrate binding activity of the tobacco lectin. Glycoconj J. 2010;27:613–623. doi: 10.1007/s10719-010-9305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Delporte A, Van Holle S, Lannoo N, Van Damme E. The tobacco lectin, prototype of the family of Nictaba-related proteins. Curr Protein Pept Sci. 2015;16:5–16. doi: 10.2174/1389203716666150213154107. [DOI] [PubMed] [Google Scholar]
- 188.Li Y, Liao X, Chen G, Yap Y, Zhang X. Cloning, expression and purification of Microcystis viridis lectin in Escherichia coli. Mol Biotechnol. 2011;47:105–110. doi: 10.1007/s12033-010-9315-0. [DOI] [PubMed] [Google Scholar]
- 189.Kachko A, Loesgen S, Shahzad-ul-Hussan S, Tan W, Zubkova I, Takeda K, et al. Inhibition of hepatitis C virus by the cyanobacterial protein Microcystis viridis lectin: mechanistic differences between the high-mannose specific lectins MVL, CV-N, and GNA. Mol Pharm. 2013;10:4590–4602. doi: 10.1021/mp400399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Moulaei T, Stuchlik O, Reed M, Yuan W, Pohl J, Lu W, et al. Topology of the disulfide bonds in the antiviral lectin scytovirin. Protein Sci. 2010;19:1649–1661. doi: 10.1002/pro.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, et al. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology. 2010;402:187–196. doi: 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Garrison AR, Giomarelli BG, Lear-Rooney CM, Saucedo CJ, Yellayi S, Krumpe LR, et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antiviral Res. 2014;112:1–7. doi: 10.1016/j.antiviral.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Martínez-Alarcón D, Blanco-Labra A, García-Gasca T. Expression of lectins in heterologous systems. Int J Mol Sci. 2018;19:616. doi: 10.3390/ijms19020616. [DOI] [Google Scholar]
- 194.Janahi EMA, Haque S, Akhter N, Wahid M, Jawed A, Mandal RK, et al. Bioengineered intravaginal isolate of Lactobacillus plantarum expresses algal lectin scytovirin demonstrating anti-HIV-1 activity. Microb Pathog. 2018;122:1–6. doi: 10.1016/j.micpath.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 195.Moulaei T, Alexandre KB, Shenoy SR, Meyerson JR, Krumpe LR, Constantine B, et al. Griffithsin tandemers: flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology. 2015;12:1–14. doi: 10.1186/s12977-014-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Levendosky K, Mizenina O, Martinelli E, Jean-Pierre N, Kizima L, Rodriguez A, et al. Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. Antimicrob Agents Chemother. 2015;59:7290–7298. doi: 10.1128/AAC.01816-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chen Y, Lu K, Li J, Liang D, Luo H, Wang X, et al. Structure and function analysis of Polygonatum cyrtonema lectin by site-directed mutagenesis. Acta Biochim Biophys Sin. 2017;49:1099–1111. doi: 10.1093/abbs/gmx116. [DOI] [PubMed] [Google Scholar]
- 198.Saul FA, Rovira P, Boulot G, Van Damme EJ, Peumans WJ, Truffa-Bachi P, et al. Crystal structure of Urtica dioica agglutinin, a superantigen presented by MHC molecules of class I and class II. Structure. 2000;8:593–603. doi: 10.1016/S0969-2126(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 199.Harata K, Muraki M. Crystal structures of Urtica dioica agglutinin and its complex with tri-N-acetylchitotriose. J Mol Biol. 2000;297:673–681. doi: 10.1006/jmbi.2000.3594. [DOI] [PubMed] [Google Scholar]
- 200.François KO, Auwerx J, Schols D, Balzarini J. Simian immunodeficiency virus is susceptible to inhibition by carbohydrate-binding agents in a manner similar to that of HIV: implications for further preclinical drug development. Mol Pharmacol. 2008;74:330–337. doi: 10.1124/mol.108.047621. [DOI] [PubMed] [Google Scholar]
- 201.Kumaki Y, Wandersee MK, Smith AJ, Zhou Y, Simmons G, Nelson NM, et al. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antiviral Res. 2011;90:22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gordts SC, Renders M, Férir G, Huskens D, Van Damme EJ, Peumans W, et al. NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J Antimicrob Chemother. 2015;70:1674–1685. doi: 10.1093/jac/dkv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Itakura Y, Nakamura-Tsuruta S, Kominami J, Tateno H, Hirabayashi J. Sugar-binding profiles of chitin-binding lectins from the hevein family: a comprehensive study. Int J Mol Sci. 2017;18:1160. doi: 10.3390/ijms18061160. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.