Abstract
Objective Despite its technical feasibility, anterior skull base surgery still carries the risk of severe postoperative complications, morbidity, and mortality. The reported rate of complications has diminished over the past two decades, but they continue to pose various challenges. This study aims to report late complications in a relatively large series of patients who underwent open anterior skull base surgery, and to propose methods for averting such complications.
Methods Retrospective chart review of all patients who underwent anterior open skull base surgery between 2000 and 2016 in a university-affiliated tertiary referral cancer center.
Results There were 301 operations, of which 198 (65.8%) were for benign disease and 103 (34.2%) were for malignant tumors. The male-to-female ratio was 1.4:1, and the mean age was 44.8 years. Delayed complications occurred in 85 patients (28.2%): 31 (10.3%) involved wounds, 18 (13.9%) involved the central nervous system, and 14 (4.6%) involved the orbit. Multivariate analysis found malignant pathology, intracranial extension, and previous radiochemotherapy as predictors for the development of a delayed complication. The patients who were operated in the later study period (after 2007) had lower rates of all three types of complications compared with the earlier study period.
Conclusion Delayed complications following skull base surgery are in decline. This is mainly due to the advancement in imaging studies, surgical techniques, development of sophisticated reconstructive procedures, and the cooperation of multidisciplinary teams. We attribute the reduction in our department to our revised treatment protocol which is presented herein, with emphasis on averting the occurrence of these complications.
Level of Evidence The level of evidence is 4.
Keywords: skull base surgery, craniofacial, surgery, complications, protocol
Introduction
Lesions of the skull base, which were considered inoperable at the beginning of the last century, can now be reached by means of various open and endoscopic approaches. Ever since Dandy's description of a craniofacial technique in 1941 and Ketcham's first description of open approaches to the skull base in 1963, ongoing efforts for improvement and refinement of the technique have led to reduction in complication rates. 1 2 3 The skull base region is complex, and it harbors a unique structure with great functional and esthetical significance. Its composition of various types of tissues that are subject to defects that are often large, taken together with anticipated adjuvant chemoradiation, requires meticulous ablation and assorted methods of reconstruction. Given the proximity of these lesions to vital structures, both the primary pathology as well as its treatment can be associated with a variety of complications. Accumulating experience has led to a better understanding of this unique region. Multidisciplinary treatment approaches that evolved during the past decades have led to considerably improved treatment of these patients. Despite such improvement, however, surgery in this region still carries a high risk for complications which occur in up to 50% of the cases. 4 5 6
The purpose of this study was to present 16 years of experience of a single Israeli tertiary referral cancer center. We compared a recent cohort to a historic cohort to identify trends and risk factors for the development of delayed complications after surgery by open approaches to the anterior skull base and to offer a strategy for averting their occurrence.
Patients and Methods
This study was approved by the Institutional Review Board (IRB TLV-0730-14) of Tel Aviv Sourasky Medical Center, and patient consent was waived. A computer-assisted search performed by the institutional operation registry identified all patients who were operated for skull base lesions at all ages. We then reviewed the medical records of all patients who were operated for anterior skull base lesions at the medical center between 2000 and 2016.
A total of 301 patients underwent open anterior skull base surgery. Their medical charts were reviewed to retrieve the following data: demographics, imaging studies, comorbidities, tumor histology, disease characteristics, surgical approach and extension, reconstruction method, surgical pathology, postoperative morbidity, and mortality. Follow-up data were obtained from the clinical notes, imaging studies, and histopathological results for all patients.
We focused specifically on delayed complications (> 30 days postsurgery), and they were divided into three categories: wounds (local infection, wound dehiscence, seroma, fistula, and osteonecrosis), the central nervous system (CNS, cerebrospinal fluid [CSF] leak, meningitis, hemorrhage, pneumocephalus, cerebral edema, and seizures), and the orbit (infection, hematoma, optic nerve or retinal injury, globe injury, muscular injury, epiphora, ectropion, telecanthus and diplopia, and enophthalmos).
First, we sought to identify the predictors for delayed complications. Demographics, tumor characteristics, and delayed complication rates were then compared between the group of patients who were operated in the early period of the study (2000–2007) and the group of patients who were operated in the later period (2008–2016).
Statistical Analyses
Categorical variables were described using frequency and percentage. Continuous variables were evaluated for normal distribution using histograms and Q–Q plots. Continuous variables were expressed as median and interquartile range. Categorical variables were compared between categories using the chi-square test or Fisher's exact test. The Mann–Whitney's test was used to compare continuous variables between age categories. A stratified Cox's regression was used to compare between groups. All statistical tests were two tailed, and a p- value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (IBM Corp. Released 2014. IBM SPSS Statistics for Windows, Version 22.0, Armonk, New York, United States: IBM Corp.).
Results
A total of 301 open anterior skull base surgeries were performed throughout the 16-year study period. The patients' mean age was 44.8 years (range from 3 months to 88 years). The male-to-female ratio was 1.4:1. One-hundred and seven (35.5%) patients had major comorbidities, 103 patients (34.2%) had malignant pathology, 111 (32.9%) had undergone previous surgery of the anterior skull base, and 78 (25.9%) had undergone preoperative chemoradiotherapy. The overall rate of delayed complications was 28%: 31 patients (10.3%) had wound complications, 42 (13.9%) had CNS complications, and 14 (4.6%) had orbital complications.
The univariate analysis revealed that age older than 50 years, major medical comorbidities, previous surgery, previous radiochemotherapy, malignant pathology, dural tumor extension, intracranial tumor extension, lumbar drainage insertion, and operation before 2007 were predictors for the development of complications. Reconstruction with a vascularized flap was found to reduce complications ( Table 1 ). The multivariate analysis revealed that previous radiochemotherapy, intracranial tumor extension, and malignant pathology were predictors for the development of complications ( Table 2 ).
Table 1. Univariate analysis of complications.
| Variable | Complication rate | p -Value |
|---|---|---|
| Age, y | ||
| < 50 | 38/185 (20.5%) | <0.001 |
| ≥ 50 | 47/116 (40.5%) | |
| Gender | ||
| Female | 30/125 (24%) | 0.168 |
| Male | 55/176 (31.5%) | |
| Pathology | ||
| Malignant | 60/103 (58.2%) | <0.001 |
| Benign | 25/198 (12.6%) | |
| Major medical comorbidities | ||
| Absent | 39/194 (20.1%) | <0.001 |
| Present | 48/107 (44.9%) | |
| Previous surgery | ||
| Absent | 33/181 (18.2%) | <0.001 |
| Present | 55/120 (43.3%) | |
| Previous radiochemotherapy | ||
| Absent | 45/223 (20.2%) | <0.001 |
| Present | 40/78 (51.3%) | |
| Adjuvant radiochemotherapy | ||
| Absent | 39/231 (16.9%) | <0.001 |
| Present | 46/70 (65.7%) | |
| Intracranial extension | ||
| Absent | 22/176 (12.5%) | <0.001 |
| Present | 63/125 (50.4%) | |
| Dural extension | ||
| Absent | 11/134 (8.2%) | <0.001 |
| Present | 74/167 (44.3%) | |
| Orbital extension | ||
| Absent | 40/162 (24.7%) | 0.061 |
| Present | 48/139 (32.4%) | |
| Lumbar drainage | ||
| Absent | 21/180 (11.7%) | <0.001 |
| Present | 64/121 (52.9%) | |
| Vascularized flap reconstruction | ||
| Absent | 56/167 (33.5%) | 0.012 |
| Present | 64/134 (21.6%) | |
| Year of surgery | ||
| 2000–2006 | 59/130 (45.4%) | <0.001 |
| 2007–2016 | 26/171 (15.2%) | |
Note: Bold indicates significance.
Table 2. Multivariate analysis of complications.
| Variable | Odds ratio | 95% Confidence interval | p -Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Malignant pathology | 148.341 | 3.865 | 5,692.737 | 0.007 |
| Intracranial extension | 18.305 | 1.874 | 178.799 | 0.012 |
| Previous radiochemotherapy | 0.047 | 0.003 | 0.79 | 0.034 |
| Previous surgery | 3.057 | 0.508 | 18.404 | 0.222 |
| Lumbar drainage | 0.327 | 0.035 | 3.042 | 0.326 |
| Operation >2007 | 1.91 | 0.412 | 8.864 | 0.409 |
| Orbital extension | 1.79 | 0.422 | 7.599 | 0.43 |
| Major comorbidities | 1.897 | 0.372 | 9.667 | 0.441 |
| Dural extension | 0.519 | 0.063 | 4.28 | 0.543 |
| Age ≥50 y | 1.288 | 0.246 | 6.749 | 0.764 |
| Vascularized flap reconstruction | 0.857 | 0.217 | 3.381 | 0.826 |
Note: Bold indicates significance.
The patient and tumor characteristics of the group of patients who were operated in the earlier period (2000–2007) were similar to those of the patients who were operated in the later period (2008–2016), with the exception of a higher rate of vascularized flap reconstructions in the latter group ( Table 3 ). There were significant reductions in the CNS (from 23.8 to 6.4%, p < 0.01) and wound (from 14.6 to 7%, p = 0.03) complications rates, as well as a reduction in the orbital complication rate (from 6.9 to 2.9%, p = 0.1) between the earlier period and the later period ( Fig. 1 ).
Table 3. Time-related demographics and tumor characteristics (univariate analysis).
| Variable | 2000–2007 n = 130 |
2008–2016 n = 171 |
p -Value |
|---|---|---|---|
| Age, mean, y | 39.7 ± 19 | 45.5 ± 17.9 | |
| Age ≥50 (%) | 49 (37.7) | 67 (39.2) | 0.793 |
| Gender, male (%) | 78 (60) | 98 (57.3) | 0.639 |
| Major medical comorbidities (%) | 43 (33.1) | 64 (37.4) | 0.435 |
| Previous surgery (%) | 53 (40.8) | 67 (39.2) | 0.78 |
| Previous radiochemotherapy (%) | 34 (26.1) | 44 (25.7) | 0.934 |
| Adjuvant radiochemotherapy (%) | 33 (25.4) | 37 (21.6) | 0.446 |
| Malignant pathology (%) | 49 (37.7) | 54 (31.6) | 0.268 |
| Intracranial extension (%) | 58 (44.6) | 67 (39.2) | 0.343 |
| Dural extension (%) | 65 (50) | 102 (59.6) | 0.095 |
| Orbital extension (%) | 60 (46.15) | 79 (46.2) | 0.994 |
| Vascularized flap reconstruction (%) | 44 (33.8) | 90 (52.6) | 0.001 |
Note: Bold indicates significance.
Fig. 1.
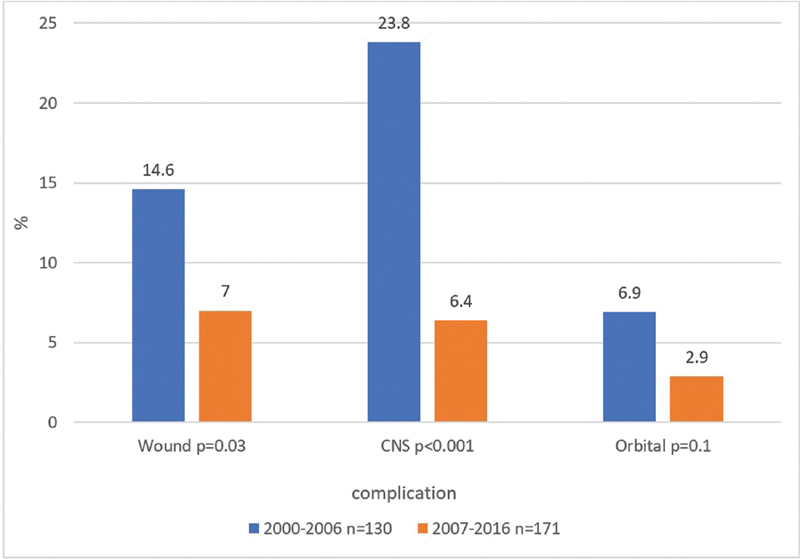
Time-related complications (univariate analysis). CNS, central nervous system.
Discussion
Delayed Complications
Advancements in imaging studies, surgical techniques, and reconstruction methods have led to an increasing number of operable skull base lesions and a decrease in the associated complication rates. 4 7 8 9 Craniofacial and subcranial approaches to the anterior skull base are known to provide good tumor control. Nevertheless, this complex region involves neurovascular and other structures, whose preservation is important for maintaining function and for obtaining a good aesthetic result. In addition to the resultant anatomy following surgical treatment, the chemoradiation therapy that is given to the majority of patients with malignant tumors in this region also increases the likelihood of tissue insult.
In light of the considerable progress and refinements in surgical capabilities as well as the better understanding of this region that had been gained through experience, we sought to examine the trends over time in postoperative morbidity in our single tertiary cancer center over a 16-year period. This study's main purpose was to identify the factors that affected the complication rate throughout this period, and to trace time-related changes in patient and tumor characteristic and complications. At the beginning of the later period of this study (2007), we established an institutional treatment protocol based on methods of treatment described in the literature and our own accumulated experience. We then compared the outcomes of delayed complications of open anterior skull base surgery before and after its implementation.
Although patients in both the earlier and later groups were comparable in demographics and tumor characteristics ( Table 3 ), the later group had significantly lower overall complications rates, significantly lower CNS and wound rates, and a trend toward lower orbital complication rates ( Fig. 1 ). Of note, they also had a higher rate of vascular flap reconstructions. We attribute this reduction to our department's open skull base treatment protocol (see later).
Averting Complications
We established a departmental treatment protocol for open approach skull base surgery in 2007 ( Fig. 2 ). 10 Its basis is the cooperation of a multidisciplinary team, which includes otolaryngologic and maxillofacial surgeons, neurosurgeons, plastic surgeons, ophthalmologists, anesthesiologists, neuroradiologists, head and neck oncologists, radiation oncologists, pathologists, nurses, physiotherapists, speech therapists, nutritionists, psychologists, and social workers. Selection of the appropriate treatment method is based on thorough assessment of each of our patients by members of this expert team. Their cooperative efforts were highly instrumental in providing superior treatment of patients with skull base lesions. In the current study, the univariate analysis found that age older than 50 years, major comorbidities, previous surgery, previous radiochemotherapy, and malignant pathology were some of the predictors for the development of late complications, whereas the multivariate analysis found that previous radiochemotherapy, intracranial extension, and malignant pathology were predictors for delayed complications ( Table 2 ). This is in concordance with earlier studies and one recent publication which proved the feasibility of skull base surgery in the elderly and even in octogenarians. 4 8 11
Fig. 2.
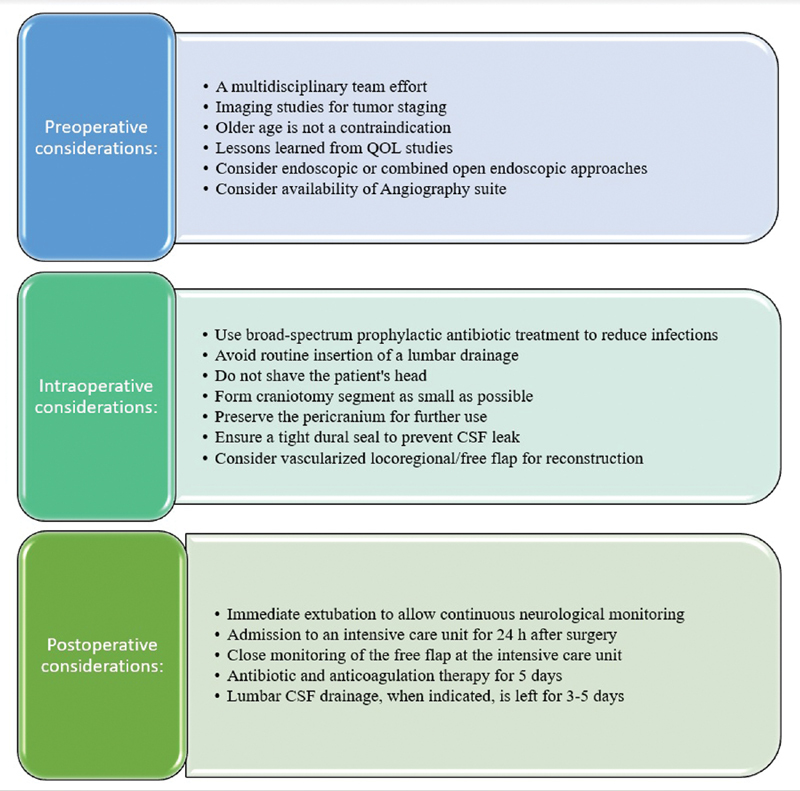
Proposed open skull base treatment protocol. CSF, cerebrospinal fluid; QOL, quality of life.
The evolution of imaging studies allowed better staging and delineation of the tumor extension to involved structures. 12 In the current study, the multivariate analysis found that intracranial tumor extension was a predictor for complication development. We routinely use magnetic resonance imaging, computed tomography (CT), and positron emission tomography-CT studies to assess tumor extension and to select the preferred reconstruction method. We recently started printing three-dimensional models based on imaging studies, and found it to be a valuable tool for treatment planning and simulation for better ablative and reconstruction outcomes.
The results of the current study demonstrated that reconstruction with vascularized flaps lowered the complication rate in the univariate analysis but not in the multivariate analysis. Reconstruction with vascularized locoregional or free flaps carries many advantages. The ablation sometimes results in a large defect with complex spatial structures, and the sterile cerebral cavity needs to be sealed, isolated, and separated from the nasal cavity. Some of the patients are planned for adjuvant radiation which bears an increased risk of tissue insult that results in fistulas, osteoradionecrosis, and other wound complications. Although it extends surgical time and requires an experienced reconstruction team, we prefer reconstruction with vascularized flaps rather than primary closure. For subcranial approaches, it is important to use as small a craniotomy segment as possible, and to preserve the distal third of the nasal bone to reduce facial deformity. The vascularized pericranium flap is used to wrap the bony craniotomy segment, and the fascia lata graft is used for dural reconstruction when there are large dural defects, as we have described in depth elsewhere. 13 14 15
Methods for the prevention of infection include avoidance of shaving the patient's head, which was shown to increase infections as a result of breakage of the skin barrier, while also improving patient satisfaction during the early postoperative period. 16 Prophylactic broad-spectrum antibiotic treatment is part of our routine, as had been noted by ourselves and others, it might enable better healing and reduce the risk of chronic contamination of pathogenic flora. 17 18 19 In 2007, we abandoned the routine use of continuous lumbar CSF drainage, having observed that it increased early CNS complications. 20 When a high-flow CSF leak is anticipated, a lumbar drain may be left for the first 3–5 postoperative days.
The postoperative section of our protocol includes immediate extubation to allow immediate and subsequent neurological assessment. A 3- to 5-day stay in an intensive care unit is mandatory for neurological, wound, and free flap monitoring. Part of our routine includes the use of pneumatic compression devices and anticoagulation therapy for the prevention of deep vein thrombosis, 5 days of antibiotic therapy as a method for preventing infection, daily physiotherapy and nutritional status assessment, and close follow-up by a psychologist and a social worker. In addition, ongoing quality of life assessment and lessons learned from questionnaires and previous studies enable better understating of these unique patients and serve to personalize their treatment. 21 22 23 24
The evolving possibility of reaching various sites at the base of the skull via an endoscopic, and now by means of robots, is highly promising by yielding lower morbidity and better outcomes in some cases. Nevertheless, the open approach or the combined open–endoscopic approach is sometimes unavoidable, for better tumor control. Limitations to this study include its retrospective nature and the heterogeneity of the tumor histologies and characteristics. In addition, changes in treatment strategies over the 16-year period might have led to a patient selection bias.
Conclusion
Open surgery for the ablation of anterior skull base lesions is effective and safe. This study identified the predictors for the development of delayed complications as being malignant pathology, intracranial extension, and previous radiochemotherapy to the anterior skull base. Patients who were operated before implantation of a herein proposed treatment protocol in our department in 2007 had more CNS, wound, and orbital complications compared with patients who were operated in the later period. We suggest that our accumulating experience and current treatment protocol led to the reductions of the rate of late complications following open skull base surgery, rather than any single change in management. We offer our treatment protocol as a possible strategy for averting late complications of anterior skull base surgery.
Acknowledgment
Esther Eshkol, MA, the institutional medical and scientific copy editor (Tel-Aviv Sourasky Medical Center), provided editorial assistance.
Footnotes
Conflict of Interest None declared.
References
- 1.Ketcham A S, Wilkins R H, Vanburen J M, Smith R R. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963;106(05):698–703. doi: 10.1016/0002-9610(63)90387-8. [DOI] [PubMed] [Google Scholar]
- 2.Ketcham A S, Van Buren J M. Tumors of the paranasal sinuses: a therapeutic challenge. Am J Surg. 1985;150(04):406–413. doi: 10.1016/0002-9610(85)90145-x. [DOI] [PubMed] [Google Scholar]
- 3.Dandy W E. Results following transcranial operative attack on orbital tumors. Arch Ophthalmol. 1941;25(02):191–216. [Google Scholar]
- 4.Ganly I, Patel S G, Singh B et al. Complications of craniofacial resection for malignant tumors of the skull base: report of an International Collaborative Study. Head Neck. 2005;27(06):445–451. doi: 10.1002/hed.20166. [DOI] [PubMed] [Google Scholar]
- 5.Miller J D, Taylor R J, Ambrose E C, Laux J P, Ebert C S, Zanation A M. Complications of open approaches to the skull base in the endoscopic era. J Neurol Surg B Skull Base. 2017;78(01):11–17. doi: 10.1055/s-0036-1583948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray S T, Lin A, Curry W T et al. Delayed complications after anterior craniofacial resection of malignant skull base tumors. J Neurol Surg B Skull Base. 2014;75(02):110–116. doi: 10.1055/s-0033-1359306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Malley B W, Jr, Janecka I P. Evolution of outcomes in cranial base surgery. Semin Surg Oncol. 1995;11(03):221–227. doi: 10.1002/ssu.2980110307. [DOI] [PubMed] [Google Scholar]
- 8.Patel S G, Singh B, Polluri A et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98(06):1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- 9.Janecka I P, Sen C, Sekhar L N et al. Cranial base surgery: results in 183 patients. Otolaryngol Head Neck Surg. 1994;110(06):539–546. doi: 10.1177/019459989411000611. [DOI] [PubMed] [Google Scholar]
- 10.Fliss D M, Gil Z. Berlin, Germany: Springer; 2016. Atlas of Surgical Approaches to Paranasal Sinuses and the Skull Base. [Google Scholar]
- 11.Ringel B, Carmel-Neiderman N N, Ben-Ner D et al. Outcomes of craniofacial open surgery in octogenarians. J Neurol Surg B Skull Base. 2018;79(06):515–521. doi: 10.1055/s-0038-1635077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirsch C FE. Advances in magnetic resonance imaging of the skull base. Int Arch Otorhinolaryngol. 2014;18 02:S127–S135. doi: 10.1055/s-0034-1390013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil Z, Abergel A, Leider-Trejo L et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17(01):25–37. doi: 10.1055/s-2006-959333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amir A, Gatot A, Zucker G, Sagi A, Fliss D M. Harvesting large fascia lata sheaths: a rational approach. Skull Base Surg. 2000;10(01):29–34. [PMC free article] [PubMed] [Google Scholar]
- 15.Gil Z, Fliss D M.Pericranial wrapping of the frontal bone after anterior skull base tumor resection Plast Reconstr Surg 200511602395–398., discussion 399 [DOI] [PubMed] [Google Scholar]
- 16.Gil Z, Cohen J T, Spektor S, Fliss D M. The role of hair shaving in skull base surgery. Otolaryngol Head Neck Surg. 2003;128(01):43–47. doi: 10.1067/mhn.2003.14. [DOI] [PubMed] [Google Scholar]
- 17.Carrau R L, Snyderman C, Janecka I P, Sekhar L, Sen C, D'Amico F. Antibiotic prophylaxis in cranial base surgery. Head Neck. 1991;13(04):311–317. doi: 10.1002/hed.2880130407. [DOI] [PubMed] [Google Scholar]
- 18.Kraus D H, Gonen M, Mener D, Brown A E, Bilsky M H, Shah J P. A standardized regimen of antibiotics prevents infectious complications in skull base surgery. Laryngoscope. 2005;115(08):1347–1357. doi: 10.1097/01.mlg.0000172201.61487.69. [DOI] [PubMed] [Google Scholar]
- 19.Gil Z, Patel S G, Bilsky M, Shah J P, Kraus D H. Complications after craniofacial resection for malignant tumors: are complication trends changing? Otolaryngol Head Neck Surg. 2009;140(02):218–223. doi: 10.1016/j.otohns.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 20.Ringel B, Carmel-Neiderman N N, Peri A et al. Continuous lumbar drainage and the postoperative complication rate of open anterior skull base surgery. Laryngoscope. 2018;128(12):2702–2706. doi: 10.1002/lary.27266. [DOI] [PubMed] [Google Scholar]
- 21.Gil Z, Abergel A, Spektor S et al. Quality of life following surgery for anterior skull base tumors. Arch Otolaryngol Head Neck Surg. 2003;129(12):1303–1309. doi: 10.1001/archotol.129.12.1303. [DOI] [PubMed] [Google Scholar]
- 22.Abergel A, Cavel O, Margalit N, Fliss D M, Gil Z. Comparison of quality of life after transnasal endoscopic vs open skull base tumor resection. Arch Otolaryngol Head Neck Surg. 2012;138(02):142–147. doi: 10.1001/archoto.2011.1146. [DOI] [PubMed] [Google Scholar]
- 23.de Almeida J R, Vescan A D, Gullane P J et al. Development of a disease-specific quality-of-life questionnaire for anterior and central skull base pathology--the skull base inventory. Laryngoscope. 2012;122(09):1933–1942. doi: 10.1002/lary.23426. [DOI] [PubMed] [Google Scholar]
- 24.Abergel A, Fliss D M, Margalit N, Gil Z. A prospective evaluation of short-term health-related quality of life in patients undergoing anterior skull base surgery. Skull Base. 2010;20(01):27–33. doi: 10.1055/s-0029-1242982. [DOI] [PMC free article] [PubMed] [Google Scholar]


