Abstract
Background Transorbital neuroendoscopic (TONES) approaches promise to open up new horizons for skull base surgery, offering alternative routes to reach the anterior and middle cranial fossa (ACF and MCF, respectively).
Objective The aim of this anatomical study is to acquire new surgical anatomy knowledge and exploit it for the refinement of TONES approaches, as an alternative to open surgery, to reduce the distance to the target, and the risk of neurovascular lesions in pathological conditions extending beyond the orbital cavity.
Materials and Methods Six head specimens (12 orbits) were studied/dissected. The orbit was approached and divided in a four clockwise quadrants manner to simulate three transconjunctival routes: the precaruncular (PC), preseptal (PS), and lateral retrocanthal (LRC), and one transpalpebral route—the superior eyelid crease (SLC). The boundaries and the most important anatomical landmarks were identified and are herein duly detailed with particular attention to the neurovascular structures encountered in each of those routes.
Results The dissections showed that the PC approach facilitates the treatment of optic nerve and frontal sinus pathologies, whereas LRC appears safer to reach ACF and MCF allowing for a free multiplanar working channel (up to 180 degrees) to the floor, roof, and lateral-to-medial walls.
Conclusion The plane of tendon lateral canthal's insertion and the sphenofrontal suture (SFS) were identified as the key anatomical landmarks for TONES approaches. Further studies are warranted to establish a practical clinical algorithm based on the anatomical four clockwise quadrants herein implemented/proposed, and the key surgical landmarks identified.
Keywords: orbit, four clockwise quadrants, skull base, transorbital neuroendoscopic approaches, anterior cranial fossa, middle cranial fossa, sphenofrontal suture
Introduction
Since the last two decades, skull base surgeons have been relying on innovative endoscopic technology to treat lesions that previously needed more complex and destructive transcranial approaches. 1 2 In the continuous path toward opening new routes, reducing the distance to the target and the risk related to retraction and/or manipulation of neurovascular structures, the introduction of the extended endoscopic endonasal approach (EEA) through a transnasal/transsphenoidal route represented an important step forward that has greatly enlarged the boundaries of skull base surgery in a 360-degree perspective. Several studies confirmed the safety and effectiveness of EEA to expose skull base pathologies by extensive bone drilling with minimal manipulation of neurovascular structures, such as cranial nerves, pituitary stalk and the circle of Willis. While EEA proved to be excellent for centrally located lesions, its major limitation seems to be represented by the difficulty to tackle lesional extension across the midline. 3 4 5
With the aim to offer new avenues to approach more laterally located lesions in anterior and middle cranial fossa (ACF and MCF, respectively), TONES approaches were proposed, and are nowadays in a stage of ongoing refinement. The main aims of those novel approaches are to achieve a shorter and safer surgical corridor to lesions located in close proximity to the Meckel's cave, the frontobasal region, and temporal pole. Such endoscopic techniques were partly inspired by surgical anatomy knowledge developed ever since the early 90s by other specialties, such as ophthalmology, ear–nose–tongue (ENT), and maxillofacial surgery, to either decompress the optic nerve (ON) or to reconstruct fractures of the floor, lateral and medial walls of the orbital cavity while preserving the other orbital structures. In 2010, Moe et al 6 started to introduce the use of an endoscope through the orbit with the intuition that also the ACF and MCF could be approached through this corridor. In the present study, we explore four different transorbital approaches, providing a detailed report of their anatomical boundaries, the most important surgical landmarks identified, and their proximity to the neurovascular structures encountered in each of those routes.
Materials and Methods
Endoscopic transorbital approaches were realized on six head specimens (12 orbits) at the Strasbourg University (ENT/Neurosurgery) Skull Base Laboratory. Specimens were positioned supine and fixed in a Mayfield's three-pin head holder. Fully endoscopic equipment (Karl Storz, Germany) with a 4-mm Ø and 0 to 30 degrees of optic (TELEPACK X LED system) was used during the study. For each orbit, three transconjunctival routes, namely, precaruncular (PC), preseptal (PS) and lateral retrocanthal (LRC), one transpalpebral route, and superior eyelid crease (SLC) were realized. All specimens underwent pre and post dissection computed tomography (CT) scans which were analyzed and reconstructed by OsiriX Dicom Viewer (Osirix Foundation, Geneva, Switzerland; Copyright ©2019 Pixmeo) to provide a quantitative analysis of the bone removal required by each of the surgical corridors developed/implemented with our conic model.
Results
Schematic description of each approach: we herein offer a description of the four types of approaches ( Table 1 ) performed during this laboratory dissection study.
Table 1. Description of the four types of approaches.
| SLC | PC | PS | LCR | |
|---|---|---|---|---|
| Target area | Orbital apex and roof, ACF, frontal sinus | ACF, lateral nasal cavity, CS, and ON | Inferior orbit | Lateral part of ACF; supraclinoid ICA, ON, temporal lobe, Sylvian fissure, petrous apex |
| Key landmarks | Superior orbicularis oculi muscle | Anterior and posterior ethmoid artery, ON, orbital roof | Inferior orbicularis oculi muscle; inferior orbital septum; orbital rim | Canthal lateral ligament; IOF, SFS, and SOF |
| Approach brief description | Incision at superior eyelid; dissection through orbicularis oculis muscle to obtain a skin-muscle flap turned toward the orbit, dissection is performed by scope in a preseptal plane | Incision starts at the junction of conjunctiva and skin, medial the caruncle, exposing the medial orbital wall in a top-bottom direction. Dissection follows the medial canthal tendon until the posterior lacrimal crest, where periosteum is incised allowing to lift superolaterally the periorbita up to the anterior ethmoid artery | Incision of the conjunctival surface of the lower eyelid, 2 mm below tarsus and 6 mm inferior to eyelid margin. Orbicularis oculi muscle is identified, and the dissection is performed; then periosteum is incised at the posterior aspect of the orbital rim, leaving the infraorbital nerve on the orbital floor | Incision starts posteriorly to canthal tendon that is retracted laterally. Periorbit is dissected medially allowing the endoscopic access; using a 30-degree scope, the SFS is identified; by drilling the bone area above and lateral, it is possible to access the lateral part of ACF, whereas by drilling the bone above and medial, it is possible to reach ICA and ON. By drilling bone area below to SFS, it is possible to access the MCF, Sylvian fissure, temporal lobe and cavernous sinus |
| Potential pathologies | ACF and frontal sinus lesions | Sellar, parasellar, CS, ON lesions | MCF, Meckel's cave, and lateral wall of cavernous sinus | Lateral ACF, middle fossa, Sylvian fissure and petrous apex regions lesions |
Abbreviations: ACF, anterior cranial fossa; CS, cavernous sinus; ICA, internal carotid artery; IOF, inferior orbital fissure; LCR, lateral retrocanthal approaches; MCF, middle cranial fossa; ON, optic nerve; SFS, sphenofrontal suture; SOF, superior orbital fissure.
SLC: the incision is made at the crease of the superior eyelid, and the dissection is performed through the orbicularis oculi muscle to obtain a skin-muscle flap turned toward the orbit and performed in a preseptal plane. Since this plane might be difficult to identify, the orbicularis muscle is usually used as an initial landmark, and the dissection is kept deeper to its plane ( Fig. 1 ). This allows for a surgical corridor to reach the orbital apex, which gives access to the orbital roof and eventually to the ACF and the frontal sinus.
PC: eyelids are retracted by stitches; the incision is started at the junction of the conjunctiva and skin, medial to the caruncle, using fine scissors to expose the medial orbital wall in a top-bottom direction. The dissection follows the medial canthal tendon until the posterior lacrimal crest, where the periosteum is incised allowing to lift the periorbita superolaterally up to the anterior ethmoid artery (AEA). The former constitutes the endoscopic start point to carry out the dissection from the floor up to the orbital roof until identification of the posterior ethmoid artery (PEA) and subsequently the ON, which is located at the most posterior aspect of the medial orbit, in the same plane of the ethmoid arteries ( Fig. 2A ). Once the orbital roof is well exposed, its medial part, as well as the cribriform plate, can be drilled off, always paying attention to preserve the lamina papyracea medially at the level of the foramina of the ethmoidal arteries ( Fig. 2B ). As shown above, the PC approach eventually gives full access to the ACF, lateral nasal cavity, CS, and ON.
PS: the approach starts with an incision on the conjunctival surface of the lower eyelid, roughly 2 mm below the tarsus and 6 mm inferior to the eyelid margin ( Fig. 3 ). The orbicularis oculi muscle is identified, and the dissection is performed immediately posterior to this structure, which is anterior to the inferior orbital septum. The inferior septum, as well as its superior counterpart, could be thin and difficult to identify; thus it may be better to use the overlying orbicularis muscle as a landmark and to follow it to the inferior orbital rim. Once the conjunctival flap is elevated it could be mobilized upwards by a horizontal passing suture; then the periosteum is incised at the posterior aspect of the orbital rim, exposing the infraorbital nerve on the orbital floor. With this approach, the entire inferior orbit is exposed and accessible to the surgeon.
LRC: key-landmarks for this approach are: canthal lateral ligament ( Fig. 4A ), inferior orbital fissure (IOF), sphenofrontal suture (SFS), superior orbital fissure (SOF; Fig. 4B ) and ON together with the internal carotid artery ( Fig. 4C ). The head is set in a neutral position and gentle retroflexed (∼15 degrees), allowing the brain to fall backward; the superior eyelid is retracted with a stitch, and the incision starts posteriorly to canthal tendon that is retracted laterally. 7 The periorbital fascia is dissected medially allowing for the endoscopic access; to carry out the dissection safely, it is important to work along the lateral orbital wall starting behind the insertion of the canthal tendon in a plane parallel to the Whitnall tubercle. Using a 30-degree scope through this plane, the SFS can be identified; at this stage, by drilling the bone area above and lateral to this suture, it is possible to access the lateral part of ACF, whereas by drilling the bone above and medial to this suture, it is possible to reach the supraclinoid internal carotid artery (ICA) and ON ( Fig. 4C ). Finally, by drilling the bone area below this suture, using a 0-degree scope, it is possible to access the MCF, Sylvian fissure, temporal lobe, and cavernous sinus. 8 Furthermore, through this same route and using the same endoscope, it is possible to retract posteriorly the temporal lobe and expose the first branch (entering the superior orbital fissure) and the second branch (entering the foramen rotundum) of the trigeminal nerve. 1
Fig. 1.
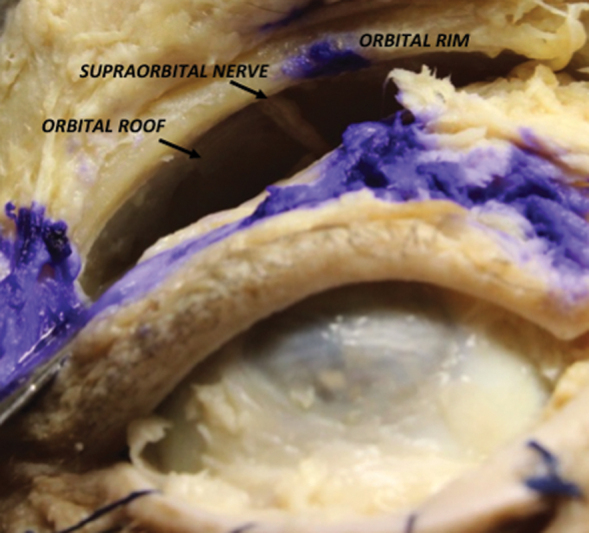
Right superior eyelid crease approach with exposition of the orbital rim, orbital roof and supraorbital nerve.
Fig. 2.
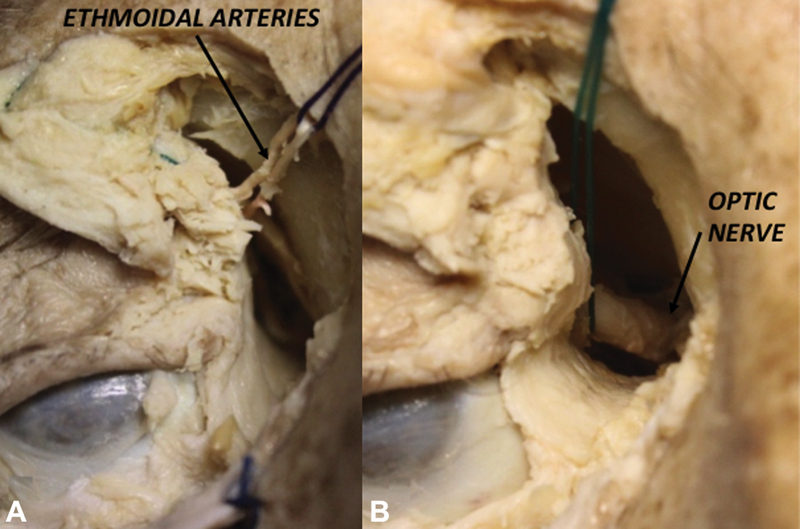
Right Precaruncular approach showing the exposition of ethmoidal arteries ( A ) and optic nerve ( B ).
Fig. 3.
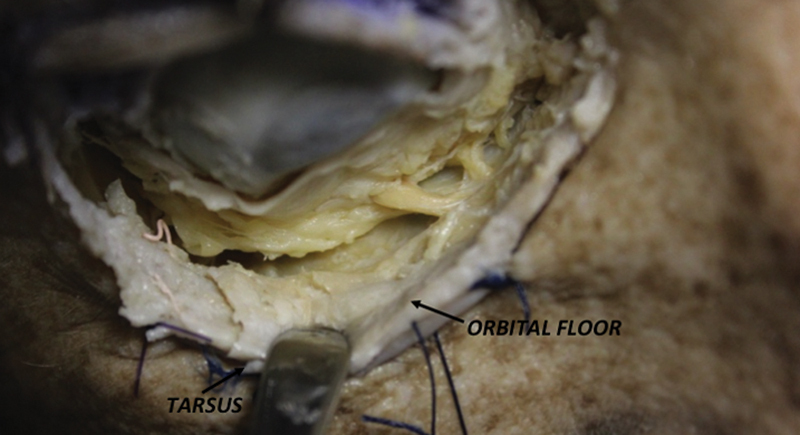
Right Preseptal approach with main landmarks.
Fig. 4.
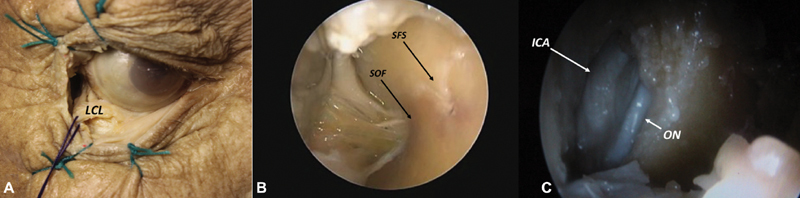
Right lateral retrocanthal approach macroscopic view: ( A ) showing lateral canthal ligament (LCL), and endoscopic view ( B ) showing superior orbital fissure (SOF), sphenofrontal suture (SFS) and also ( C ) internal carotid artery (ICA) and optic nerve (ON).
Use of a conic model for identification of endoscopic landmarks: when the orbit is considered as a conic model, split on a coronal plane at the mid-pupillary line, the main endoscopic landmarks that can be identified in a clockwise order are SFS, optic canal (OC), PEA, AEA, sphenoethmoid suture, optic strut, SFS, and lamina papyracea.
More specifically, the transorbital portal entry points were considered in four clockwise quadrants relating to the following four possible approaches ( Fig. 5 ):
Fig. 5.
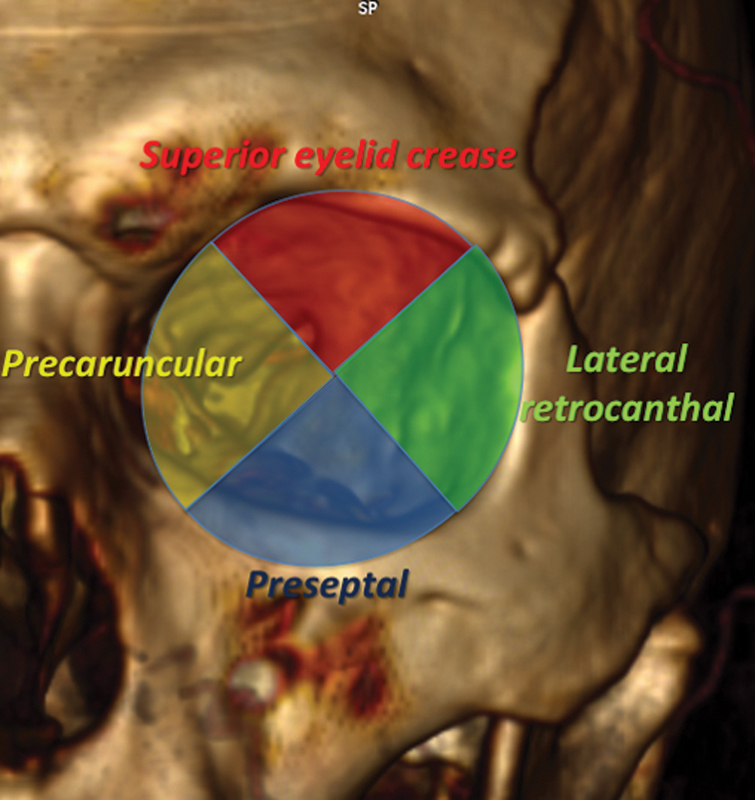
3D CT scan reconstruction of the left orbit showing the schematic four clockwise quadrants model implemented in TONES surgery guiding the choice of the right approach in relation to the lesion topography. 3D, three-dimensional; CT, computed tomography; TONES, transorbital neuroendoscopic.
Superior quadrant: SLC approach to access the ACF and orbital roof.
Medial quadrant: PC approach to access the ACF, lateral nasal cavity, CS, and ON.
Inferior quadrant: PS approach to access to the orbital floor, infraorbital nerve, inferior orbital septum (IOS), and middle fossa floor including the foramen rotundum.
Lateral quadrant: LRC approach to access the deep orbit, CS, MCF, and infratemporal fossa.
Many neurovascular structures can be encountered in each of these approaches, they are summarized as follows:
SLC: zygomatic-facial nerve and zygomatic-temporal nerve.
PC: infraorbital nerve, PEA, and AEA.
PS: infraorbital artery and meningolacrimal artery.
LRC: ON and CS.
Discussion
In 1943, Rae and McLean described the first case of combined transcranial and transorbital approach to the ACF to remove a retinoblastoma. 9
Fifty-five years later, the interest of TONES approaches was renewed by the potential advantages made possible by the diffusion of endoscopic techniques. A technical report by Luxenberger et al 10 on decompression, performed with this approach, represented the first step in this direction. The reported advantages included decreased morbidity with preservation of olfaction, faster recovery time, and more acceptable cosmetic results.
The first anatomical and clinical study on the use of transconjunctival routes was reported by Moe et al. 6 7 The authors performed a cadaveric dissection study and described relevant anatomical landmarks along possible surgical corridors through the orbit. Their seminal work led to the successful adoption of LRC in clinical settings, and to the proposition of other medial, superior, and inferior routes for the endoscopic management of orbital lesions, For the first time the acronym TONES was proposed to the surgical community. Since then, 10 more studies on various TONES approaches have been published. The majority of them were cadaveric investigations on a limited number of routes, mostly LRC and SLC. Nonetheless, few authors went beyond laboratory studies and presented also their clinical experience on the management of several conditions, including CSF leak, ON decompression, skull base fractures, and skull base tumors ( Table 2 ). More specifically, a relevant clinical experience regarding the development of these techniques was published in 2015 by Ramakrishna et al 11 on a surgical series of 40 patients treated between 2006 and 2013. The complications reported were two folds, cosmetic, and functional, including cases of diplopia, exophthalmos, epiphora, ptosis, and various other visual disturbances. However, the use of multiportal approaches and the possibility to combine them with EEA and/or supraorbital keyhole craniotomies to further reduce morbidity represented a great step forward.
Table 2. Clinical experience on the management of several conditions as presented by authors.
| Author | Year | Journal | Study type | N of pts | Approach | Complication | Mortality | FU (Months) |
|---|---|---|---|---|---|---|---|---|
| Moe et al | 2010 | Neurosurgery | Anatomic study | NA | PC; SLC; LRC; PS | No | NA | 9 |
| Moe et al | 2011 | Laryngoscope | Anatomic + prospective study | NA | PC; SLC; LRC; PS | Rhinorrea (1) | 0 | 7.4 |
| Lim et al | 2012 | J Neurol Surg B Skull Base | Prospective study | 13 | PC; SLC | No | 0 | 10 |
| Bly et al | 2014 | J Neurol Surg B Skull Base | Anatomic study | NA | NA | NA | NA | NA |
| Chen et al 14 | 2014 | J Neurosurg | Anatomic study | NA | NA | NA | NA | NA |
| Ramakrishna et al | 2016 | J Clin Neurosci | Retrospective study | 45 | PC; SLC; LRC; PS | enophthalmos (1), epiphora (1), ptosis (1) | 0 | 3 |
| Locatelli et al 17 | 2016 | J Neurosurg Sci | Review | NA | PC; SLC; LRC; PS | NA | NA | NA |
| Ciporen et al 15 | 2017 | J Neurol Surg B Skull Base | Anatomic study | NA | TOPA | NA | NA | NA |
| Di Somma et al 13 | 2018 | J Neurosurg | Anatomic study | NA | PC; SLC; LRC | NA | NA | NA |
| Zoia et al 16 | 2018 | Acta Neurochir | Technical note | 12 | PC; SLC; LRC; PS | 0 | 0 | NA |
| Golbin et al 18 | 2019 | Zh Vopr Neirokhir Im N N Burdenko | Prospective study | 12 | PC; SLC; LRC; PS | fifth and sixth nerves (1) | 0 | NA |
| Gerges et al 19 | 2019 | J Neurosurg | Anatomic study | NA | PC; SLC; LRC; PS | NA | NA | NA |
Abbreviations: LRC, lateral retrocathal approach; NA, not available; PC, pre-caruncular; PS, preseptal approach; SLC: superior eyelid crease; TOPA, transorbital precaruncular approach.
One limitation that emerged from the review of the literature was the heterogeneous nomenclature, for instance, Ferrari et al 12 proposed the use of a different technique called the inferolateral-transorbitary-endoscopic approach (ILTEA), an approach that similarly to the LRC that exploits the lateral orbital corridor. The authors concluded that the lateral orbital quadrant was safer than other transorbital routes. With the use of ILTEA, the authors showed four diverging corridors leading to (1) Meckel's cave, (2) ICA, (3) petrous bone, and (4) MCF. Our study confirmed some of their findings and clarified that the potential morbidity of using a lateral orbital corridor is mostly related to the following neurovascular structures: infraorbital nerve, zygomatic-facial nerve, zygomatic-temporal nerve, infraorbital artery, and meningolacrimal artery.
Making a rigorous analysis of our anatomical dissection study, we have realized that TONES approaches could schematically be performed through two main corridors based on a conic model of the orbit split on a coronal plane at the midpupillary line. The two half conic areas could easily be managed and dominated through the LRC (lateral) and the PC (medial) approaches which allow for a free multiplanar work corridor with ease of maneuvers and navigation.
In planning TONES approaches, few aspects should be considered to choose the best surgical trajectory with the shortest route as follows: (1) the position of the lesion in relation to the midpupillary line, and (2) the angle between the midpupillary line and a perpendicular line passing through the plane of the lesion. Furthermore, surgeons should bear in mind that a minimal ocular globe manipulation and displacement is the key to ensure the success of these approaches, although an ocular globe mobilization inferior or equal to 10 mm seems enough to avoid and/or reduce transitory or even permanent orbital injuries. 11 Additionally, it has been suggested that the removal of the soft orbital globe retractor every 15 to 20 minutes could represent a very simple and safe maneuver to avoid iatrogenic injuries.
The present study allowed us to identify the SFS as a key landmark to decide the exact site of the bone drilling; the suture is quite easily visible using a 0-degree endoscope, while a 30-degree scope can be used to orientate the surgeon during the bone drilling stage to access the ACF and MCF. Although mostly indicated to decompress the ON and canal, as well as to repair frontal sinus CSF leaks, the PC approach has a greater risk of ON and ethmoidal arteries damage, as well as a more limited working space and maneuverability (due to the neurovascular structures within the OC and IOF). Those aspects certainly limit its use compared with the LRC approach. While acquiring anatomical knowledge in a laboratory setting is fundamental, we do firmly believe that the systematic use of IGS, visual evoked potentials, micro-Doppler, and dedicated surgical instruments could further enhance the safety of endoscopic approaches and induce the consideration of TONES also in clinical settings.
Although it would be ambitious to consider that the TONES approaches will replace traditional open craniotomy or EEA, the development of transconjunctival routes expands our surgical options and offering an opportunity for combined transnasal/transorbital or transcranial/transorbital approaches. This is particularly valid for the supraorbital key-hole approach, which already constitutes a good option as a stand-alone technique, and whose indication can be broadened even further in combination with TONES.
Conclusion
In conclusion, we believe that TONES approaches are promising options that should be known to the neurosurgical community and included in the armamentarium of dedicated skull base surgeons. Those techniques, in fact, represent a minimally invasive alternative to avoid more extensive and destructive approaches. Agreeably avoiding brain retraction is important and a key component of minimal invasiveness, however, risks of visual disturbances following TONES are not negligible, hence a careful consideration of their pros and cons is warranted. For this, clinical studies are necessary to demonstrate the effectiveness, safety, and reproducibility of TONES approaches in managing ACF and MCF pathologies, as their current daily application is still very limited. Therefore by sharing this laboratory experience, our greatest ambition is to stimulate the debate among neurosurgeons with an interest in new routes and minimally invasive approaches and foster the gathering of new clinical insights.
Footnotes
Conflict of Interest None declared.
References
- 1.Maroon J C. Skull base surgery: past, present, and future trends. Neurosurg Focus. 2005;19(01):E1–E1. doi: 10.3171/foc.2005.19.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Snyderman C H, Kassam A B. Endoscopic techniques for pathology of the anterior cranial fossa and ventral skull base. J Am Coll Surg. 2006;202(03):563–563. doi: 10.1016/j.jamcollsurg.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Cappabianca P, Cavallo L M, Esposito F, De Divitiis O, Messina A, De Divitiis E. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151–199. doi: 10.1007/978-3-211-72283-1_4. [DOI] [PubMed] [Google Scholar]
- 4.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(01):E3. [PubMed] [Google Scholar]
- 5.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(01):E4. [PubMed] [Google Scholar]
- 6.Moe K S, Bergeron C M, Ellenbogen R G.Transorbital neuroendoscopic surgery Neurosurgery 201067(03, suppl operative):ons16–ons28. [DOI] [PubMed] [Google Scholar]
- 7.Moe K S, Jothi S, Stern R, Gassner H G. Lateral retrocanthal orbitotomy: a minimally invasive, canthus-sparing approach. Arch Facial Plast Surg. 2007;9(06):419–426. doi: 10.1001/archfaci.9.6.419. [DOI] [PubMed] [Google Scholar]
- 8.Bly R A, Ramakrishna R, Ferreira M, Moe K S. Lateral transorbital neuroendoscopic approach to the lateral cavernous sinus. J Neurol Surg B Skull Base. 2014;75(01):11–17. doi: 10.1055/s-0033-1353363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rae B S, Mc Lean J M. Combined intracranial and orbital operation for retinoblastoma. Arch Ophtalmol (Paris) 1943;30:437. [Google Scholar]
- 10.Luxenberger W, Stammberger H, Jebeles J A, Walch C. Endoscopic optic nerve decompression: the Graz experience. Laryngoscope. 1998;108(06):873–882. doi: 10.1097/00005537-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Ramakrishna R, Kim L J, Bly R A, Moe K, Ferreira M., Jr Transorbital neuroendoscopic surgery for the treatment of skull base lesions. J Clin Neurosci. 2016;24:99–104. doi: 10.1016/j.jocn.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari M, Schreiber A, Mattavelli D et al. The inferolateral transorbital endoscopic approach: a preclinical anatomic study. World Neurosurg. 2016;90:403–413. doi: 10.1016/j.wneu.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Di Somma A, Andaluz N, Cavallo LM, et al.Endoscopic transorbital superior eyelid approach: anatomical study from a neurosurgical perspective J Neurosurg 2018129051203–1216. [DOI] [PubMed] [Google Scholar]
- 14.Chen HI, Bohman LE, Loevner LA, Lucas TH.Transorbital endoscopic amygdalohippocampectomy: a feasibility investigation J Neurosurg 2014120061428–1436. [DOI] [PubMed] [Google Scholar]
- 15.Ciporen JN, Moe KS, Ramanathan D, et al.Multiportal endoscopic approaches to the central skull base: a cadaveric study World Neurosurg 20107306705–712. [DOI] [PubMed] [Google Scholar]
- 16.Zoia C, Bongetta D, Gaetani P.Endoscopic transorbital surgery for spheno-orbital lesions: how I do it Acta Neurochir (Wien) 2018160061231–1233. [DOI] [PubMed] [Google Scholar]
- 17.Locatelli D, Pozzi F, Turri-Zanoni M, et al.Transorbital endoscopic approaches to the skull base: current concepts and future perspectives J Neurosurg Sci 20166004514–525. [PubMed] [Google Scholar]
- 18.Golbin DA, Lasunin NV, Cherekaev VA, et al.Biopsiia i udalenie novoobrazovani? osnovaniia cherepa s primeneniem transorbital'nykh éndoskopicheskikh dostupov: pervye rezul'taty [Biopsy and resection of skull base tumors using transorbital endoscopic approaches: primary results] Zh Vopr Neirokhir Im N N Burdenko 2019830342–56. [DOI] [PubMed] [Google Scholar]
- 19.Gerges MM, Godil SS, Younus I, Rezk M, Schwartz TH.Endoscopic transorbital approach to the infratemporal fossa and parapharyngeal space: a cadaveric study [published online ahead of print, 2019 Nov 1] J Neurosurg 20191–12. [DOI] [PubMed] [Google Scholar]


