Abstract
This cohort study examines rates of gene testing in patients with urothelial cancer, uptake of gene-targeted therapy with erdafitinib, and overall survival outcomes.
In April 2019, after a single-arm phase 2 clinical trial,1 the Food and Drug Administration granted accelerated approval for erdafitinib, a pan–fibroblast growth factor receptor (FGFR) inhibitor, for treatment of advanced urothelial cancer with a susceptible FGFR3 alteration or FGFR2/3 fusion that progressed after platinum-containing chemotherapy. Because erdafitinib was the first gene-targeted therapy for urothelial cancer, we sought to describe uptake of FGFR molecular testing, erdafitinib use, and survival outcomes in routine care.
Methods
This cohort study used the Flatiron Health database derived from deidentified electronic health records of US patients with cancer.2 The University of Pennsylvania institutional review board approved the study, with a waiver of informed consent because deidentified retrospective data were used. Included patients were diagnosed with advanced urothelial cancer, received first-line platinum-based chemotherapy, and started a later line of therapy between May 1, 2016, and September 1, 2021. We followed the STROBE reporting guideline.
We calculated the number and proportion of eligible patients with a recorded FGFR test, susceptible FGFR alteration (FGFR3 alteration or FGFR2/3 fusion), and treatment start with erdafitinib. A linear probability model with robust SEs was used to estimate the rate of change in probability of initiating erdafitinib compared with standard second-line immunotherapy (atezolizumab, the first approved immune checkpoint inhibitor) in the 6-month interval after each drug’s approval (eMethods in the Supplement). To avoid selection bias in estimates of erdafitinib uptake among FGFR-tested patients, analyses were conducted among all comers after platinum therapy and in the eligible population expected to have susceptible FGFR alterations.
Overall survival (OS) among real-world patients receiving erdafitinib was analyzed using the Kaplan-Meier method. To compare OS in real-world patients vs those enrolled in a previously published trial,1 survival data reconstruction recreated individual-level survival data from the trial postchemotherapy Kaplan-Meier curve (eMethods in the Supplement); survival differences were tested using the log-rank test.3,4
Analyses were conducted using R, version 4.0.3; Microsoft Excel, version 16.59; and Stata, version 15.1. Two-sided P < .05 was considered significant.
Results
Among 761 patients (546 [71.7%] male; mean [SD] age, 70.26 [9.04] years) who received postplatinum therapy from April 1, 2019, to September 1, 2021, 343 (45.1%) had FGFR testing recorded, 71 of whom (20.7%) had a susceptible FGFR alteration. Thirty patients (42.3%) with susceptible FGFR alterations received erdafitinib. After erdafitinib approval, FGFR testing increased, although erdafitinib initiation remained low among patients with FGFR alterations (Figure 1A).
Figure 1. Real-world Use of Pan–Fibroblast Growth Factor Receptor (FGFR) Testing and Rate of Erdafitinib Uptake.
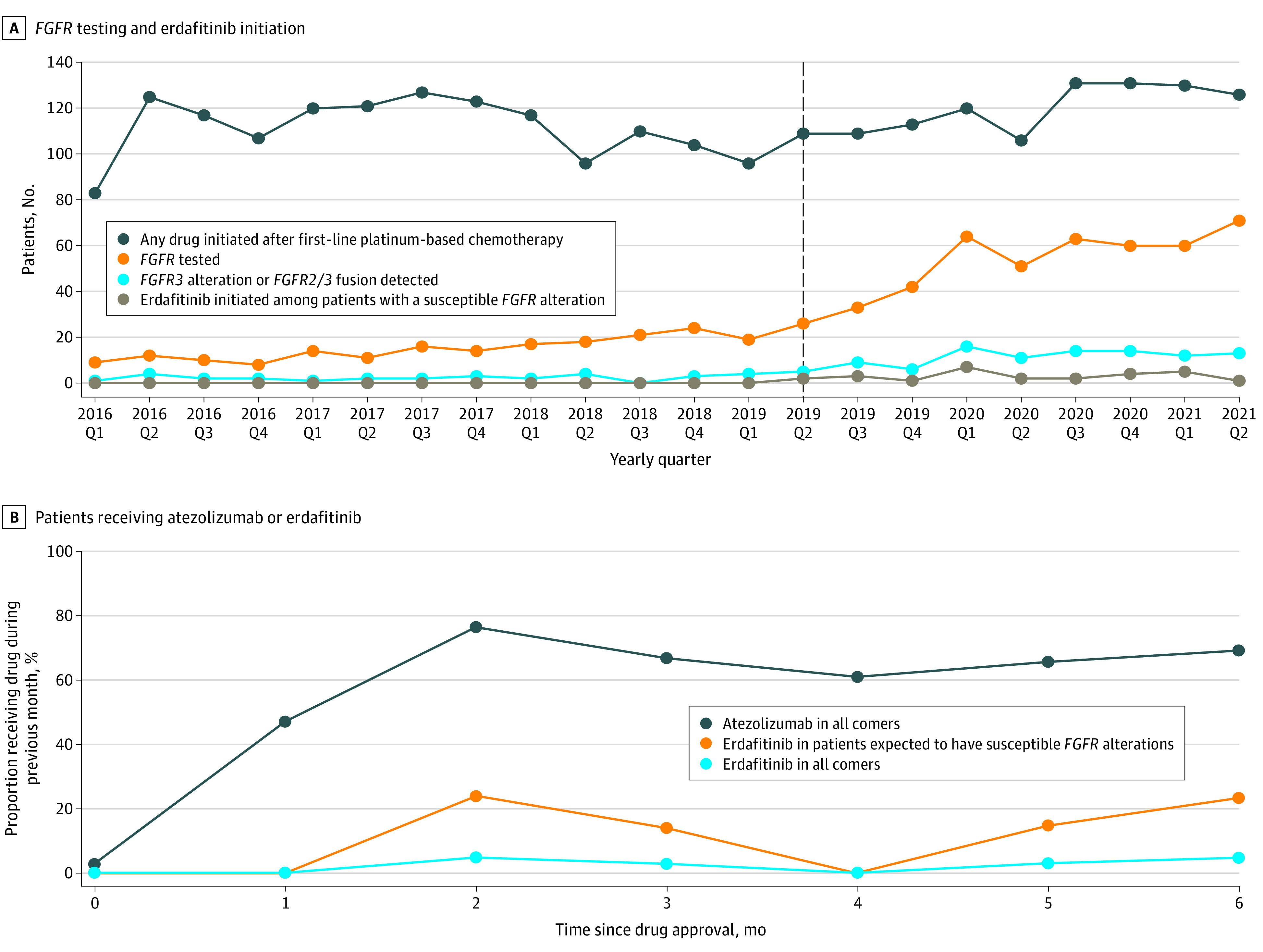
A, The dashed vertical line denotes the timing of approval of erdafitinib. B, The proportions of patients starting a later line of therapy who received atezolizumab and erdafitinib in the 6 months after each drug’s approval (April 12, 2019, to October 8, 2019 for erdafitinib and May 18, 2016, to November 13, 2016 for atezolizumab) are shown. The proportion of patients receiving erdafitinib in the population expected to have a susceptible FGFR alteration was calculated by dividing the proportion of patients starting erdafitinib among all comers by the literature-reported incidence of susceptible FGFR alterations (20%).1 Monthly uptake rates were determined using a linear probability model with robust SEs.
Among patients receiving FGFR testing, 305 (88.9%) had tissue-based testing and 74 (21.6%) had blood-based testing, with similar FGFR alteration detection rates (tissue, 19.3% [59 of 305]; blood,18.9% [14 of 74]). Among 36 patients with both tissue- and blood-based testing, 6 susceptible alterations were detected: 2 by tissue-based testing and 6 by blood-based testing.
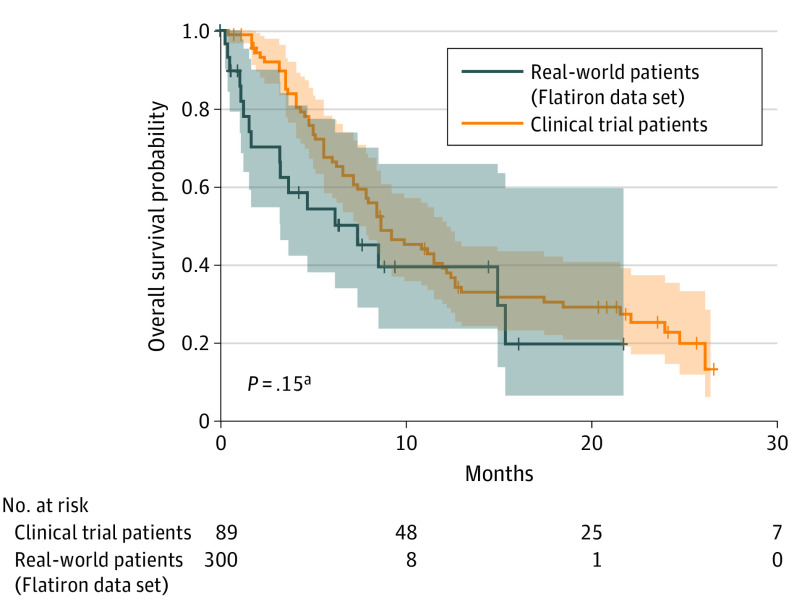
Monthly uptake rates of erdafitinib (all comers, 0.8% [95% CI, 0.1%-1.5%]; expected FGFR alteration, 3.9% [95% CI, 0.4%-7.5%]) were significantly lower than those of immunotherapy (16.8% [95% CI, 14.9%-18.7%]) (P < .001 for both) (Figure 1B). Kaplan-Meier survival estimates were similar between real-world and trial patients (median OS, 8.97 months [95% CI, 3.93 months to not reached] vs 10.5 months [95% CI, 8.97 to 14.8 months]; P = .15) (Figure 2).
Figure 2. Survival Outcomes.
Survival among patients receiving erdafitinib in the Flatiron Health data set compared with survival among patients receiving erdafitinib following chemotherapy after individual patient data were reconstructed from the BLC2001 trial.1 Shading represents 95% CIs.
aLog-rank test.
Discussion
In this study, uptake of the first gene-targeted therapy for urothelial carcinoma was limited despite real-world survival outcomes comparable with clinical trial data. Potential reasons include drug toxicity, drug cost limiting access, paucity of data on optimal treatment sequencing, and inadequate FGFR testing. Blood-based testing captured susceptible alterations at a rate similar to that of tissue testing and may identify alterations when tissue-testing does not.5 Limitations of this study include the possibility of uncaptured FGFR test results at sites outside the Flatiron Health Network. Further study is required to identify barriers to biomarker testing and erdafitinib use and to explore the potential for blood-based testing to augment identification of susceptible FGFR alterations and improve turnaround times, as seen for other tumors.6
eMethods.
References
- 1.Loriot Y, Necchi A, Park SH, et al. ; BLC2001 Study Group . Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381(4):338-348. doi: 10.1056/NEJMoa1817323 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res. 2021;56(6):1281-1287. doi: 10.1111/1475-6773.13669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siefker-Radtke AO, Necchi A, Park SH, et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol. Published online January 11, 2022. doi: 10.1016/S1470-2045(21)00660-4 [DOI] [PubMed]
- 4.Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2021;21(1):111. doi: 10.1186/s12874-021-01308-8 [DOI] [PMC free article] [PubMed]
- 5.Aggarwal C, Thompson JC, Black TA, et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell lung cancer. JAMA Oncol. 2019;5(2):173-180. doi: 10.1001/jamaoncol.2018.4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res. 2019;25(15):4691-4700. doi: 10.1158/1078-0432.CCR-19-0624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.