Key Points
Question
Does combining an mTOR inhibitor (vistusertib) with anastrozole therapy help control disease in patients with recurrent or metastatic hormone receptor−positive endometrial cancer?
Findings
This multicenter open-label phase 1/2 randomized clinical trial of 75 women found that the progression-free rate at 8 weeks was 67.3% among the patients receiving vistusertib with anastrozole in the combination arm compared with 39.1% in the anastrozole alone arm.
Meaning
Treatment combining vistusertib with anastrozole demonstrated clinically meaningful improvement with manageable adverse events for patients with recurrent or metastatic endometrial cancer; further research on the selection of patients with endometrial cancer for endocrine treatment should be encouraged.
Abstract
Importance
Endometrial cancer is often hormone-dependent and treated with aromatase inhibitors. The PI3K-AKT-mTOR pathway deregulation observed in endometrial cancer drives hormonal resistance, thus supporting the rationale of combining mTOR inhibitor with endocrine therapy.
Objective
To evaluate the safety and efficacy of vistusertib in combination with anastrozole in the treatment of women with hormone receptor−positive recurrent or metastatic endometrial cancer.
Design, Settings, and Participants
The VICTORIA study was a multicenter, open-label, randomized clinical trial that accrued 75 patients with hormone receptor−positive recurrent or metastatic endometrial cancer from 12 cancer centers in France in April 2016 to October 2019. After a safety run-in period, a Simon 2-stage design was used. Data analyses were performed from December 11, 2020, to March 11, 2021.
Interventions
Patients were randomized in a 2:1 ratio to oral vistusertib (125 mg twice daily 2 days per week) and oral anastrozole (1 mg daily) in the combination vistusertib with anastrozole arm (V+A arm) or oral anastrozole alone (A arm).
Main Outcomes and Measures
The primary end point was serious adverse events for the safety run-in period and progression-free rate at 8 weeks (8wk-PFR)—assessed with a blinded independent central review in phase 2. The secondary end points were objective response rate, duration of response, progression-free survival (PFS), overall survival, and incidence of adverse events.
Results
Of the 75 patients who were randomized, 73 (median [range] age, 69.5 [37-88] y; all female) were treated: V+A arm, 49 patients; A arm, 24 patients. In the V+A arm, the 8wk-PFR was 67.3% (unilateral 95% CI, 54.7%) and in the A arm, 39.1% (unilateral 95% CI, 22.2%). No significant serious adverse events were reported during the safety run-in period (n = 6 in V+A arm). The overall response rate was 24.5% (95% CI, 13.3%-38.9%) in the V+A arm vs 17.4% (95% CI, 5.0%-38.8%) in the A arm. With a median follow-up of 27.7 months, median PFS was 5.2 (95% CI, 3.4-8.9) in the V+A arm and 1.9 (95% CI, 1.6-8.9) months in the A arm. Fatigue, lymphopenia, hyperglycemia, and diarrhea were the most common (grade ≥2) adverse events associated with vistusertib.
Conclusions and Relevance
This multicenter, open-label, phase 1/2 randomized clinical trial demonstrated that adding vistusertib to anastrozole improved 8wk-PFR, overall response rate, and PFS for patients with endometrial cancer and had manageable adverse events. Identification of molecular subgroups would allow for more precise selection of patients who may be most likely to experience favorable outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02730923
This randomized clinical trial of women with hormone receptor-positive recurrent or metastatic endometrial cancer examines adverse events and cancer recurrence among patients treated with vistusertib plus anastrozole and compares them with patients treated with anastrozole alone.
Introduction
Endometrial cancer is the most frequent gynecologic cancer in developed countries, with increasing incidence among the elderly population.1 Approximately 75% of endometrial cancers are diagnosed at a localized stage. For these patients, treatments used complex therapeutic strategy involving surgery, chemotherapy, and/or radiotherapy; however, 20% of the women relapse mainly in the first 5 years.2,3 After recurrence, response to systemic therapy is often limited, and prognosis is poor. Indeed, the 5-year survival rates in patients with advanced stage III and IV endometrial cancer are 60% and 25%, respectively.4
Hormone receptor-positive (HR+) endometrial cancer accounts for 65% of the endometrial cancers; it mainly belongs to endometrioid histologic subtype and frequently harbors mutations in phosphatase and tensin homologue (PTEN, 80%) and activates mutation in the phosphoinositide 3-kinases/protein kinase B/mechanistic target of rapamycin (PI3K/AKT/mTOR) pathway (PIK3CA, 36%-52%).5 The relevant threshold for HR positivity in endometrial cancer is less evident than in breast cancer. It is regularly accepted in clinical practice and clinical trials to retain a positivity threshold of 1%, analogous with breast cancer. The Cancer Genome Atlas Network refined endometrial cancer molecular characterization in 20136; it characterized 4 endometrial cancer subgroups according to molecular profiles with different prognoses—POLE ultramutated; MSI-hypermutated; copy number variation low group with PTEN (77%) and PIK3 (90%) mutations; and copy number variation high serous-like with TP53 mutation.
In patients with HR+ metastatic endometrial cancer, endocrine therapy allowed a tumor response in 15% to 30% of cases, predominantly including low-grade, endometrioid histologic subtype cancer, and correlation with HR expression level was identified.7,8 These responses are usually of short duration, but some long-term responses have been reported9 and most international guidelines include endocrine therapy.10 Although progestogens are cited more often, aromatase inhibitors are used more widely in clinical practice because they are better tolerated in daily practice and clinically and have a lower thromboembolic risk for this particular population and its comorbidities. Therefore, the PARAGON trial11 demonstrated a low response rate of 7% with anastrozole alone, but an interesting clinical benefit of 44% in women with recurrent HR+ endometrial cancer.
To avoid resistance to endocrine therapy, combining PI3K/AKT/mTOR inhibitor with endocrine treatment may improve outcomes in women with HR+ endometrial cancer. Indeed, the PI3K/AKT/mTOR pathway plays an important role in cancer cell survival, angiogenesis, and metastasis,12,13 and a critical role in endocrine resistance.14 Single-arm studies investigating PI3K/AKT/mTOR inhibition in recurrent or metastatic endometrial cancer in monotherapy or in combination with endocrine therapy have reported some encouraging results with improved progression-free survival (PFS), but no clear benefit in objective response rate (often <20%) or overall survival.15,16,17,18,19 Results obtained with a combination of endocrine therapy and mTOR inhibitors are roughly similar; however, any interpretation exclusively based on single-arm studies should be cautious.20,21
Vistusertib, an mTOR inhibitor, has a short half-life, inhibits both mTORC1 and mTORC2 complexes, and has already been evaluated in HR+ metastatic breast cancer.22 In vitro, vistusertib showed growth inhibition and cell death based on mTORC1 and mTORC2 inhibition, especially in HR+ breast cancer models.23 This randomized clinical trial aimed to evaluate the safety and efficacy of vistusertib in combination with an aromatase inhibitor, anastrozole, in patients with HR+ recurrent or metastatic endometrial cancer.
Methods
The Hormone Receptor−Positive Endometrial Carcinoma Treated by Dual mTORC1/mTORC2 Inhibitor and Anastrozole (VICTORIA) study was reviewed and approved by the ethics committee of Lyon Sud-Est IV and was conducted according to the Declaration of Helsinki and the International Conference on Harmonization on Good Clinical Practices. The full trial protocol is available in Supplement 1. Written informed consent was provided by each participant before enrollment. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patient Population
The VICTORIA study enrolled women with histologically confirmed estrogen receptor (ER) and/or progesterone receptor (PR) positive (immunohistochemistry threshold, 1%) recurrent or metastatic endometrial cancer not amenable to curative treatment. Patients with 1 prior chemotherapy regimen and/or 2 lines of endocrine therapy (except aromatase inhibitor) in a metastatic setting were accepted, as were those with previous adjuvant chemotherapy and/or radiotherapy. Patients with endometrial carcinosarcomas were excluded.
All patients had measurable lesions per the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.24 A tumor biopsy was mandatory at initiation and after 8 weeks of treatment. The main exclusion criteria were uncontrolled central nervous system metastases and previous mTOR inhibitor treatment.
Study Design and Treatment
The multicenter, noncomparative, open-label, randomized phase 1/2 VICTORIA was conducted in 12 comprehensive cancer centers in France. This trial assessed the safety (phase 1) and clinical activity (phase 2) of vistusertib plus anastrozole (V+A) and anastrozole (A) among patients randomly assigned in a 2:1 ratio to receive oral vistusertib (125 mg twice daily on 2 days per week) in combination with oral anastrozole (1 mg daily) or oral anastrozole alone. Random assignment used the centralized registration platform, and stratification was performed according to administration of 1 prior chemotherapy or not (1 vs 0; block design of block size 3 and 6 within each stratum). Clinician and patients were not masked to treatment group allocation. Study treatment was continued until disease progression (assessed by RECIST24), unacceptable adverse events, or consent withdrawal.
Tumor Assessments and Clinical Evaluations
Tumor evaluation using computed tomography (CT) scans of the chest, the abdomen, and the pelvis associated with magnetic resonance imaging (MRI; if relevant, for the affected region unless the disease was evaluable only by MRI) was performed every 8 weeks until disease progression. To assess safety, all adverse events were assessed using biological tests and physical examinations with monitoring of vital signs. Adverse events and abnormal laboratory results were graded according to the US National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.25
Study End Points
The phase 1 VICTORIA study evaluated the safety of the V+A combination therapy by monitoring the occurrence of serious adverse events (SAEs) during the first 8 weeks of treatment. The SAEs were defined as any grade of 4 or greater for a treatment-related adverse event and any grade of 3 or greater for a treatment-related adverse event with a duration of more than 7 days among the first 6 patients randomized to the V+A arm.
In phase 2 of the trial, the primary end point was to assess the clinical efficacy of the combination, based on the 8-week progression-free rate (8wk-PFR) per RECIST24 assessed by a blinded independent central review (BICR). Secondary end points were the investigator-assessed 8wk-PFR, overall response rate and duration of response, progression-free survival (PFS), overall survival, and safety.
Statistical Analysis
The sample size of the V+A combination arm was predefined by using a Simon optimal 2-stage design.26 The null and alternative hypotheses were defined considering an unpromising 40% 8wk-PFR and a 60% 8wk-PFR as the smallest nonprogression rate above which the combination would be considered of interest, respectively. Assuming a unilateral type I error rate of 5% and a power of 80%, enrollment of 46 evaluable patients was planned with 16 patients in phase 1 (expecting 8 nonprogression as a minimum to carry on the enrollment in phase 2) and 30 patients in stage 2. A cut-off of 24 of 46 evaluable patients not experiencing progression at 8 weeks was required to justify further investigation.
The planned size of the control arm (anastrozole) was 23 evaluable patients, given the 2:1 randomization ratio; however, the final number was 24 patients. No formal comparison (ie, statistical testing) between arms was scheduled. All analyzes in phase 2 were performed on the efficacy evaluable population—ie, patients with no major violations on eligibility criteria—with 1 dose or more of study drugs and with 1 or more tumor assessments after randomization. The study design provided results in terms of the 8wk-PFR for the control arm ensuring that the hypotheses was retained for the sample size calculation; randomization avoided patient selection biases.
The estimates of 8wk-PFR were provided with 1-sided 95% CIs, calculated according to the exact method. Duration of response was defined as the time from the date of the first confirmed objective response to the date of disease progression or death; PFS, as the time from the date of randomization to the date of disease progression or death from any cause; and overall survival , as the time from randomization to death from any cause. The overall survival, PFS, and duration of response were estimated with the Kaplan-Meier method. For secondary end points, 2-sided 95% CI were used. The duration of follow-up was estimated using the reverse Kaplan-Meier method. The date of data cutoff was September 11, 2020. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
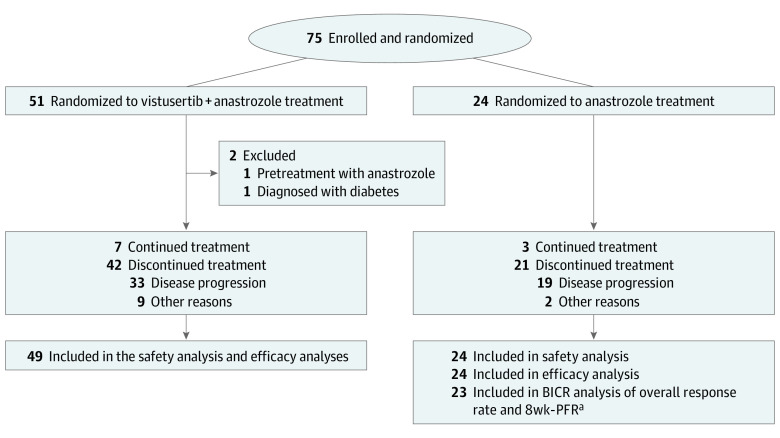
Of the 75 women enrolled from April 1, 2016, to October 31, 2019, by the 12 cancer centers, 73 eligible patients (median [range] age, 70 [37-88] years; 100% female; race and ethnicity information was not collected in accordance with the laws of France) comprised the study population; 2 patients in the V+A arm became ineligible after allocation (1 had pretreatment with A; the other was diagnosed with diabetes; Figure 1). Given the 2:1 ratio, the V+A arm comprised 51 patients and the A alone arm, 24 patients; however, 1 patient in the A arm was deemed not evaluable by the BICR, so the analysis for the primary objective and the response rate was performed with 23 patients. The baseline characteristics of the patients were balanced between study arms (Table 1), with 33 (45%) being obese or overweight with a body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 or higher.
Figure 1. CONSORT Diagram of Study Participants.
aOne patient was deemed not evaluable. BICR denotes a blinded independent central review; and 8wk-PFR, the 8-week progression-free rate.
Table 1. Characteristics of Patients With Hormone Receptor−Positive Endometrial Cancer, by Study Arm.
| Characteristic | No. (%) | |
|---|---|---|
| Vistusertib + anastrozole (n = 49) | Anastrozole (n = 24) | |
| Age, median (range), y | 70.8 (36.8-87.0) | 68.7 (40.5-87.8) |
| BMI | ||
| <25 | 15 (31) | 5 (21) |
| ≥25 and <30 | 15 (31) | 5 (21) |
| ≥30 | 19 (39) | 14 (58) |
| ECOG-PS | ||
| 0 | 26 (53) | 9 (38) |
| 1 | 23 (47) | 15 (63) |
| FIGO stage | ||
| III | 10 (20) | 4 (17) |
| IV | 39 (80) | 20 (83) |
| Endocrine receptors status | ||
| ER+ and PR+ | 36 (74) | 19 (83) |
| ER+ and PR– | 10 (20) | 3 (13) |
| ER– and PR+ | 3 (6) | 1 (4) |
| Grade | ||
| 1 | 10 (20) | 7 (29) |
| 2 | 17 (35) | 9 (38) |
| 3 | 12 (25) | 4 (17) |
| Unknown | 10 (20) | 4 (17) |
| Histologic subtype | ||
| Endometrioid | 43 (88) | 19 (79) |
| Other | 6 (12) | 5 (21) |
| Unknown | 1 (2) | 0 |
| Microsatellite status | ||
| Stable | 32 (65) | 15 (63) |
| Unstable | 13 (27) | 6 (25) |
| Unknown | 4 (8) | 3 (13) |
| Prior chemotherapy courses, No. | ||
| 0 | 22 (45) | 10 (42) |
| 1 | 27 (55) | 14 (58) |
| Prior endocrine therapy courses, No. | ||
| 0 | 42 (86) | 22 (92) |
| ≥1 | 7 (14) | 2 (8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ECOG-PS, Eastern Cooperative Oncology Group−performance status; ER, estrogen receptor; FIGO, International Federation of Gynecology and Obstetrics; PR, progesterone receptor.
By the end of the safety run-in period, 2 patients of 6 in the V+A arm had experienced a SAEs—lymphopenia grade 3 (n = 1) and grade 4 (n = 1)—that were determined to be clinically unimportant by the coordinating investigator (P.H.), the sponsor, and the Independent Data Monitoring Committee (IDMC) members. After validation by the IDMC, the V+A combination therapy was considered to be safe, and 15 additional patients were enrolled. At the end of Simon phase 1, 10 of the 16 patients in the V+A arm had not experienced cancer progression at 8 weeks; therefore, they continued to stage 2.
At the time of the data cutoff (November 9, 2020; median follow-up, 27.7 months), 7 patients were still receiving the study treatment in the V+A combination arm and 3 patients were still receiving treatment in the anastrozole arm. Low-grade tumor type was predominant (60% of grade I/II) and the International Federation of Gynecology and Obstetrics stage at inclusion in the clinical trial was III and IV in 14 (19%) and 59 (81%) patients, respectively. Pathology reports rarely mentioned mismatch repair deficiency and P53 status. Previous chemotherapy had been received by 41 (56%) patients, and only 9 (12%) had received a previous line of endocrine therapy. There was no significant difference between the patients’ general characteristics of the 2 treatment arms.
Efficacy Analysis
The VICTORIA trial met its primary end point with an 8wk-PFR assessed by a BICR in 33 of 49 evaluable patients (67.3%; unilateral 95% CI, 54.7%) in the V+A arm vs 9 of 23 patients (39.1%; unilateral 95% CI, 22.2%) in the anastrozole arm. Investigator-assessed 8wk-PFRs were similar with 34 of 49 patients (69.4%; unilateral 95% CI, 56.8%) in the V+A arm vs 11 of 24 patients (45.8%; unilateral 95% CI, 28.2%) in the A arm. The median (range) treatment duration was 3.4 (0.5-41) months in the combination V+A arm vs 2.9 (0.2-29) months in the A arm.
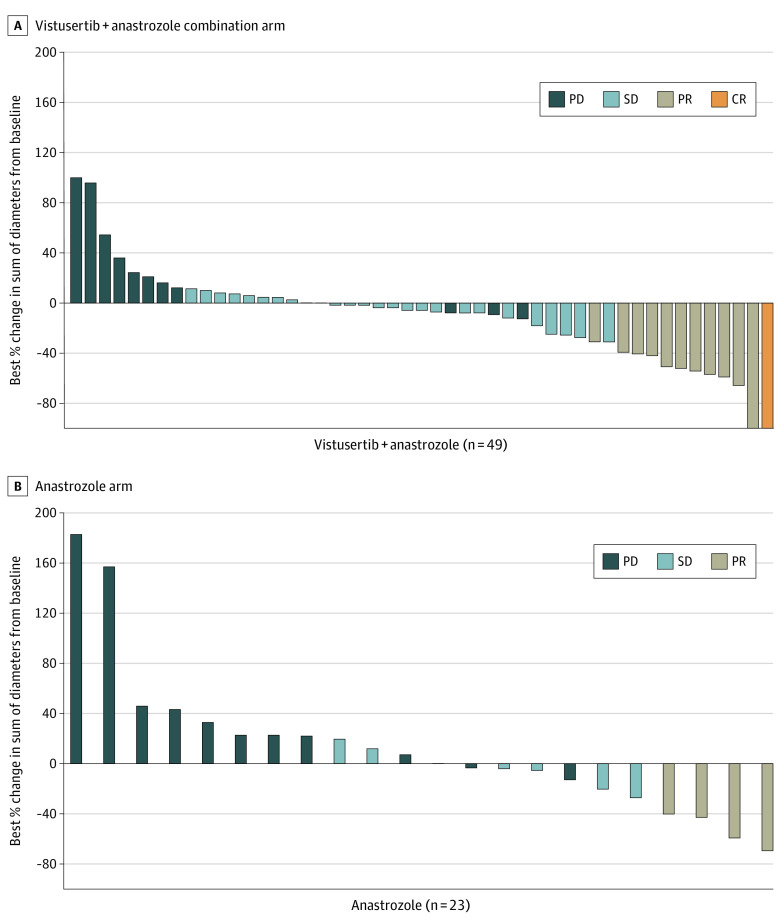
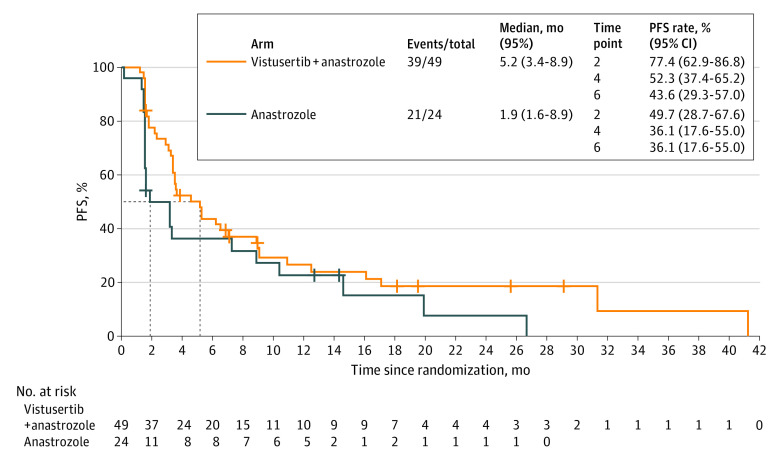
The overall response rate assessed in 12 of the 49 evaluable patients in the V+A arm was 24.5% (95% CI, 13.3%-38.9%) vs 17.4% (95% CI, 5.0%-38.8%) in 4 of the 23 patients in the A arm (Figure 2A). One complete response and 11 partial responses were observed in the V+A arm, and 4 partial responses were reported in the A arm (Figure 2B). Long responders, defined as patients with a PFS greater than 12 months, accounted for 9 patients in the V+A arm and 7 in the A arm (more details are available in eFigures 1 and 2 in Supplement 2). Among the 16 long-response patients, the median duration of response was 29.6 months (95% CI, 3.7-39.6) in the V+A arm and 7.5 months (95% CI, 5.6-18.3) in the A arm. The median PFS was statistically longer in the V+A arm (5.2 months [95% CI, 3.4-8.9] vs 1.9 months [95% CI, 1.6-8.9]; Figure 3). The overall survival rate was 70.8% (95% CI, 48.4%-84.9%) at 12 months and 53% (95% CI, 31.2%-70.8%) at 24 months in the A arm, and 71.3% (95% CI, 56.4%-81.9%) at 12 months and 38.1% (95% CI, 24.3%-51.7%) at 24 months in the V+A arm.
Figure 2. Waterfall Plots by Treatment Allocation, Vistusertib Plus Anastrozole Arm vs Anastrozole Alone Arm.
Two patients had 0% change from baseline of target lesion.
CR indicates complete response; PD, progressive disease; PR, partial response; and SD, stable disease.
Figure 3. Progression-Free Survival (PFS) for Vistusertib Plus Anastrozole Arm vs Anastrozole Alone Arm for Treatment of Endometrial Cancer.
Safety Evaluation
Among the 73 patients, 23 (32%) experienced 1 or more SAEs: in the V+A arm, 20 (41%) patients, including 11 (22%) who experienced a vistusertib-related SAE (according to the investigators) and in the A arm, 3 (13%) patients. Most of the adverse events were grade 1 or 2 (Table 2). In the V+A arm, the most common nonhematologic adverse events were fatigue (n = 34; 69%), nausea (n = 25; 51%), and diarrhea (n = 20; 41%), and in the A arm, fatigue (n = 7; 29%) and arthralgia (n = 7; 29%). The most common grade 3 and 4 adverse events in the V+A arm were lower lymphocyte count (20%), hyperglycemia (12%), and fatigue (8%); treatment was discontinued by 11 patients (22%), 4 of whom permanently discontinued vistusertib.
Table 2. Adverse Events Summary for Vistusertib Plus Anastrozole Arm vs Anastrozole Alone Arm for Treatment of Endometrial Cancer.
| Incidence and type of adverse event | No. (%) | |||
|---|---|---|---|---|
| Vistusertib + anastrozole (n = 49) | Anastrozole (n = 24) | |||
| Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | |
| Nausea | 25 (51) | 1 (2) | 2 (8) | 0 |
| Fatigue | 34 (69) | 4 (8) | 7 (29) | 0 |
| Vomiting | 11 (22) | 1 (2) | 1 (4) | 0 |
| Diarrhea | 20 (41) | 1 (2) | 3 (13) | 0 |
| Arthralgia | 11 (22) | 0 | 7 (29) | 0 |
| Decrease in lymphocytes count | 17 (35) | 10 (20) | 3 (13) | 2 (8) |
| Hyperglycemia | 15 (31) | 6 (12) | 2 (8) | 0 |
| Anemia | 13 (27) | 2 (4) | 1 (4) | 0 |
| ≥1 SAE | 20 (41) | NA | 3 (13) | NA |
| ≥1 Vistusertib-related SAE | 11 (22) | NA | NA | NA |
Abbreviations: NA, not applicable; SAE, serious adverse event.
Discussion
This multicenter, open-label, randomized clinical trial showed an 8wk-PFR of 67.3%, meaning that the combination of V+A met its primary end point. In addition, the 8wk-PFR of 39.1% in the A arm validated the initial hypotheses retained for sample-size calculation. The efficacy secondary end points—overall response rate, duration of response, and PFS—confirmed the clinical interest of the V+A combination with a manageable safety profile. However, these findings also underline a critical need for accurately determining the appropriate prognostic and predictive factors for endocrine therapy and mTOR-inhibitors responses in patients with HR+ recurrent or metastatic endometrial cancer.
The patient characteristics of this study population attest that most patients with endometrial cancer are older (>70 years) and obese. Despite an often-limited efficacy, the favorable tolerance profile of endocrine therapy makes it a good option for treatment among this frail population, specifically for patients with low tumor volume and/or indolent disease.
Histologic factors associated with response to endocrine therapy include low grade,27 endometrioid histologic subtype,20 and positive ER or PR status.28 However, with an overall response rate of only 17.4% and a PFS of less than 2 months in the anastrozole alone arm, it is clear that these criteria are not sufficient. Improved selection of patients who may better respond to endocrine therapy is still required, although tumor responses have been reported in patients with HR-negative endometrial cancer.29,30 In that respect, the choice of treatment according to the histologic characteristics is not sufficient, and highly selected molecular criteria are necessary.
Likewise, biological mechanisms underlying resistance to endocrine therapy have been well studied in hormone-dependent breast cancer.31 The mechanism of action of nonnuclear ER is mediated by a molecular interaction between ER pathways and intracellular signaling pathways.32 Estrogen−ER complexes directly interact with tyrosine kinase receptors and their downstream signaling pathways, specifically the MAP kinase and PI3K/AKT/mTOR pathways. Based on this biological rationale, the mTORC1 inhibitor everolimus became the first treatment targeting the PI3K/AKT/mTOR pathway used in clinical practice in ER-positive metastatic breast cancer.33,34 In metastatic HR+ endometrial cancer, the important clinical trials have investigated an mTOR inhibitor as monotherapy, with no specific requirement for cancer selection regarding hormone receptor status.20,21 Despite the absence of patient selection for the presence of HR, these studies reported an overall response rate of approximately 30% with the combination of mTOR inhibitor and endocrine therapy, results similar to those of the VICTORIA trial. These similarities highlight an inherent antineoplastic activity from these targeted therapies.
Indeed, to our knowledge, no predictive factor of response to mTOR inhibitors has been formally identified to date.35 Moreover, negative feedback loops downstream of mTORC1 would confer resistance to everolimus via an IGFR1-dependent over-activation of AKT.23 Development of vistusertib was issued from this scientific rationale, and in vitro studies have also demonstrated a significant inhibition of the intracellular signaling pathway PI3K/AKT/mTOR.23,36 Despite these biological data, the MANTA phase 2 trial failed to demonstrate a benefit of adding vistusertib to fulvestrant therapy compared with everolimus combined fulvestrant in patients with HR+ metastatic breast cancer that was progressive after an aromatase inhibitor.22 Taking these results into account, development of vistusertib has been definitively stopped during the VICTORIA inclusion period. However, these results show that there is a need for further research, as well as improved selection of patients with endometrial cancer that might best respond to endocrine therapy combined with an mTOR inhibitor.
Nevertheless, other research and development programs to improve the effectiveness of endocrine therapy exist. Recent phase 2 clinical trials investigating the combination of CDK4/6 inhibitors (ribociclib and palbociclib) with letrozole showed promising results, especially in terms of PFS.37,38 No details were provided on the existence of molecular biomarkers that may be predictive of a response to this combination.39 Several studies beyond HR+ endometrial cancer-specific disease have investigated various targeted therapies, such as anti-ERBB 2 targeted treatments,40 antiangiogenic agents,41,42 and agents targeting DNA repair43,44; however, their findings are premature or disappointing.
During the past few years, we have witnessed a major evolution in metastatic endometrial cancer treatments with promising immune checkpoint inhibitors that have made a paradigm shift in treatment for patients with high levels of microsatellite instability.45,46 Therefore, the challenge now is to identify this patient population, to evaluate new therapeutic combinations, and to propose benefits from these therapeutic innovations for the greatest number of patients. Thus, among the HR+ endometrial cancer population, even though histologically driven treatment may not be sufficient, molecular characteristics cannot guide treatment yet. The arrival of immune checkpoint inhibitors and the possibility of selecting patients based on molecular characteristics gives us hope for the future.
Limitations
This study had a number of limitations that should be considered. The relatively small number of patients included the absence of data on the level of expression of hormone receptors, and the predefined absence of statistical comparison between the 2 treatment arms are worth noting.
Conclusions
The results of the VICTORIA randomized clinical trial demonstrate that vistusertib combined with anastrozole improved overall response rate and PFS among patients with HR+ recurrent or metastatic endometrial cancer. Despite these important results, the classification of recurrent and/or metastatic endometrial cancer and the identification of predictive biomarkers of efficacy for targeted therapy remain fundamental challenges.
Trial Protocol
eFigure 1. Schematic Representation of the Randomized Patients According to Treatment Allocation.
eFigure 2. CONSORT with Screen Fail
Data Sharing Statement
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-2917. doi: 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363-385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(suppl 1):S105-S143. doi: 10.1016/S0020-7292(06)60031-3 [DOI] [PubMed] [Google Scholar]
- 5.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268-e278. doi: 10.1016/S1470-2045(13)70591-6 [DOI] [PubMed] [Google Scholar]
- 6.Kandoth C, Schultz N, Cherniack AD, et al. ; Cancer Genome Atlas Research Network . Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73. doi: 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thigpen JT, Blessing J, Disaia P. Oral medroxyprogesterone acetate in advanced or recurrent endometrial carcinoma: results of therapy and correlation with estrogen and progesterone receptor levels. In: Iacobelli S, ed. Endocrinology and malignancy: basic and clinical issues. Presented at the Proceedings of the First International Congress on Cancer and Hormones; 1986. [Google Scholar]
- 8.Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer. 2007;17(5):964-978. doi: 10.1111/j.1525-1438.2007.00897.x [DOI] [PubMed] [Google Scholar]
- 9.Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst Rev. 2010;(12):CD007926. doi: 10.1002/14651858.CD007926.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Concin N, Creutzberg CL, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Virchows Arch. 2021;478(2):153-190. doi: 10.1007/s00428-020-03007-z [DOI] [PubMed] [Google Scholar]
- 11.Mileshkin L, Edmondson R, O’Connell RL, et al. ; PARAGON study group . Phase 2 study of anastrozole in recurrent estrogen (ER)/progesterone (PR) positive endometrial cancer: The PARAGON trial−ANZGOG 0903. Gynecol Oncol. 2019;154(1):29-37. doi: 10.1016/j.ygyno.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 12.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605-635. doi: 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K inhibitors in cancer: clinical implications and adverse effects. Int J Mol Sci. 2021;22(7):3464. doi: 10.3390/ijms22073464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araki K, Miyoshi Y. Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2018;25(4):392-401. doi: 10.1007/s12282-017-0812-x [DOI] [PubMed] [Google Scholar]
- 15.Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010;116(23):5415-5419. doi: 10.1002/cncr.25515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombo N, McMeekin DS, Schwartz PE, et al. Ridaforolimus as a single agent in advanced endometrial cancer: results of a single-arm, phase 2 trial. Br J Cancer. 2013;108(5):1021-1026. doi: 10.1038/bjc.2013.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278-3285. doi: 10.1200/JCO.2010.34.1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray-Coquard I, Favier L, Weber B, et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. Br J Cancer. 2013;108(9):1771-1777. doi: 10.1038/bjc.2013.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heudel PE, Fabbro M, Roemer-Becuwe C, et al. Phase II study of the PI3K inhibitor BKM120 in patients with advanced or recurrent endometrial carcinoma: a stratified type I-type II study from the GINECO group. Br J Cancer. 2017;116(3):303-309. doi: 10.1038/bjc.2016.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soliman PT, Westin SN, Iglesias DA, et al. Everolimus, letrozole, and metformin in women with advanced or recurrent endometrioid endometrial cancer: a multi-center, single arm, phase II study. Clin Cancer Res. 2020;26(3):581-587. doi: 10.1158/1078-0432.CCR-19-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slomovitz BM, Jiang Y, Yates MS, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. 2015;33(8):930-936. doi: 10.1200/JCO.2014.58.3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid P, Zaiss M, Harper-Wynne C, et al. Fulvestrant plus vistusertib vs fulvestrant plus everolimus vs fulvestrant alone for women with hormone receptor-positive metastatic breast cancer: the MANTA phase 2 randomized clinical trial. JAMA Oncol. 2019;5(11):1556-1564. doi: 10.1001/jamaoncol.2019.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guichard SM, Curwen J, Bihani T, et al. AZD2014, an inhibitor of mTORC1 and mTORC2, is highly effective in ER+ breast cancer when administered using intermittent or continuous schedules. Mol Cancer Ther. 2015;14(11):2508-2518. doi: 10.1158/1535-7163.MCT-15-0365 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132-137. doi: 10.1016/j.ejca.2016.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US National Cancer Institute . Common Terminology Criteria for Adverse Events, version 4.03. Accessed April 7, 2022. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/
- 26.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1-10. doi: 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
- 27.Fiorica JV, Brunetto VL, Hanjani P, Lentz SS, Mannel R, Andersen W; Gynecologic Oncology Group study . Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(1):10-14. doi: 10.1016/j.ygyno.2003.11.008 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Zhao D, Gong C, et al. Prognostic role of hormone receptors in endometrial cancer: a systematic review and meta-analysis. World J Surg Oncol. 2015;13:208. doi: 10.1186/s12957-015-0619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, Zaino RJ, Filiaci VJ, Leslie KK. Relationship of estrogen and progesterone receptors to clinical outcome in metastatic endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2007;106(2):325-333. doi: 10.1016/j.ygyno.2007.03.042 [DOI] [PubMed] [Google Scholar]
- 30.Covens AL, Filiaci V, Gersell D, Lutman CV, Bonebrake A, Lee YC. Phase II study of fulvestrant in recurrent/metastatic endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;120(2):185-188. doi: 10.1016/j.ygyno.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 31.Schettini F, Buono G, Trivedi MV, De Placido S, Arpino G, Giuliano M. PI3K/mTOR Inhibitors in the treatment of luminal breast cancer. why, when and to whom? Breast Care (Basel). 2017;12(5):290-294. doi: 10.1159/000481657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233-247. doi: 10.1146/annurev-med-070909-182917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520-529. doi: 10.1056/NEJMoa1109653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30(22):2718-2724. doi: 10.1200/JCO.2011.39.0708 [DOI] [PubMed] [Google Scholar]
- 35.Dong C, Wu J, Chen Y, Nie J, Chen C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Front Pharmacol. 2021;12:628690. doi: 10.3389/fphar.2021.628690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pancholi S, Leal MF, Ribas R, et al. Combination of mTORC1/2 inhibitor vistusertib plus fulvestrant in vitro and in vivo targets oestrogen receptor-positive endocrine-resistant breast cancer. Breast Cancer Res. 2019;21(1):135. doi: 10.1186/s13058-019-1222-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirza MR, Bjørge L, Marmé FR, et al. A randomised double-blind placebo-controlled phase II trial of palbociclib combined with letrozole (L) in patients (pts) with oestrogen receptor-positive (ER+) advanced/recurrent endometrial cancer (EC): NSGO-PALEO/ENGOT-EN3 trial. Ann Oncol. 2020;31(S4):S1142-S1215. doi: 10.1016/j.annonc.2020.08.2258 [DOI] [Google Scholar]
- 38.Colon-Otero G, Zanfagnin V, Hou X, et al. Phase II trial of ribociclib and letrozole in patients with relapsed oestrogen receptor-positive ovarian or endometrial cancers. ESMO Open. 2020;5(5):e000926. doi: 10.1136/esmoopen-2020-000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoninger SF, Blain SW. The ongoing search for biomarkers of CDK4/6 inhibitor responsiveness in breast cancer. Mol Cancer Ther. 2020;19(1):3-12. doi: 10.1158/1535-7163.MCT-19-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fader AN, Roque DM, Siegel E, et al. Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol. 2018;36(20):2044-2051. doi: 10.1200/JCO.2017.76.5966 [DOI] [PubMed] [Google Scholar]
- 41.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259-2265. doi: 10.1200/JCO.2010.32.6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coleman RL, Sill MW, Lankes HA, et al. A phase II evaluation of aflibercept in the treatment of recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;127(3):538-543. doi: 10.1016/j.ygyno.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romero I, Rubio MJ, Medina M, et al. An olaparib window-of-opportunity trial in patients with early-stage endometrial carcinoma: POLEN study. Gynecol Oncol. 2020;159(3):721-731. doi: 10.1016/j.ygyno.2020.09.013 [DOI] [PubMed] [Google Scholar]
- 44.Shen K, Yang L, Li FY, et al. Research progress of PARP inhibitor monotherapy and combination therapy for endometrial cancer. Curr Drug Targets. 2021;23(2):145-155. doi: 10.2174/1389450122666210617111304 [DOI] [PubMed] [Google Scholar]
- 45.Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol. 2020;6(11):1766-1772. doi: 10.1001/jamaoncol.2020.4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38(26):2981-2992. doi: 10.1200/JCO.19.02627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Schematic Representation of the Randomized Patients According to Treatment Allocation.
eFigure 2. CONSORT with Screen Fail
Data Sharing Statement