Abstract
Simple Summary
The presence of lymph neck node metastasis is the most important clinical prognostic factor in patients with head and neck squamous cell carcinoma. The expression of the semaphorin-3F and neuropilin-2 proteins is involved in the regulation of lymphangiogenesis. We analized the transcriptional expression of semaphorin-3F and neuropilin-2 in head and neck squamous cell carcinoma tumors of patients without clinical or radiology evidence of lymph node involvement treated with elective neck dissection. We found that patients with high semaphorin-3F and low neuropilin-2 had a lower risk of occult lymph node metastasis than the ones who had low semaphorin-3F or high semaphorin-3F and high neuropilin-2. If our results are validated, the expression of the SEMA3F-NRP2 axis could be used as a biomarker predicting the risk of occult lymph node metastasis, with elective neck dissections being indicated exclusively for patients at higher risk. The availability of a biomarker with the capacity to predict the presence of occult lymph node metastases would make it possible to avoid elective neck dissections in low-risk patients, with the consequent decrease in surgical time and morbidity of the treatment without impairing the oncologic outcome.
Abstract
The expression of the semaphorin-3F (SEMA3F) and neuropilin-2 (NRP2) is involved in the regulation of lymphangiogenesis. The present study analyzes the relationship between the transcriptional expression of the SEMA3F-NRP2 genes and the presence of occult lymph node metastases in patients with cN0 head and neck squamous cell carcinomas. We analyzed the transcriptional expression of SEMA3F and NRP2 in a cohort of 53 patients with cN0 squamous cell carcinoma treated with an elective neck dissection. Occult lymph node metastases were found in 37.7% of the patients. Patients with occult lymph node metastases (cN0/pN+) had significantly lower SEMA3F expression values than patients without lymph node involvement (cN0/pN0). Considering the expression of the SEMA3F-NRP2 genes, patients were classified into two groups according to the risk of occult nodal metastasis: Group 1 (n = 34), high SEMA3F/low NRP2 expression, with a low risk of occult nodal involvement (14.7% cN0/pN+); Group 2 (n = 19), low SEMA3F or high SEMA3F/high NRP2 expression, with a high risk of occult nodal involvement (78.9% cN0/pN+). Multivariate analysis showed that patients in Group 2 had a 26.2 higher risk of lymph node involvement than patients in Group 1. There was a significant relationship between the transcriptional expression values of the SEMA3F-NRP2 genes and the risk of occult nodal metastases.
Keywords: semaphorin-3F, neuropilin-2, occult lymph node metastases, elective neck dissection, head and neck squamous cell carcinoma, HNSCC, lymphangiogenesis
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer type by incidence and mortality, with 890,000 new cases and 450,000 deaths worldwide in 2018 [1]. The presence of lymph neck node metastasis is the most important clinical prognostic factor in HNSCC patients.
Depending on the location and extent of the primary tumor, a variable proportion of patients with head and neck squamous cell carcinoma (HNSCC) without clinical or radiological evidence of lymph node involvement at diagnosis (cN0) have occult lymph node metastases (cN0/pN+) [2,3]. The presence of occult lymph node metastases justifies elective neck dissections in these patients and has a significant impact on survival [4].
The availability of biomarkers with the ability to predict the presence of occult lymph node metastases would make it possible to select patients who are candidates for elective treatment of the lymph node areas, limiting the intensity of treatment at the regional level in low-risk patients, with the consequent reduction in morbidity and impairment of quality of life.
Tumor lymphangiogenesis is a complex process involving interactions between endothelial cells, tumor cells, and the tumor microenvironment. One of the factors with the highest capacity to promote lymphangiogenesis is the vascular endothelial growth factor-C (VEGF-C), which appears overexpressed in many carcinoma models, correlating with the tumor lymphangiogenesis and the appearance of lymph node metastasis [5].
One of the receptors targeted by VEGF-C is neuropilin-2 (NRP2), which has been implicated in the process of VEGF-C-mediated lymphangiogenesis [6]. NRP2 was first identified as a receptor for semaphorin-3F (SEMA3F). Semaphorins are a family of proteins initially described in relation to processes of neuronal orientation. Semaphorins are involved also in the regulation of the angiogenesis process. Specifically, it has been described that SEMA3F could inhibit tumor growth and angiogenesis after binding with NRP2 in diverse human cell types [7,8].
Several studies have shown that the expression of SEMA3F and its receptors fulfill important regulatory roles in multiple forms of cancer [9], and that the use of treatments aimed at modifying the activity of the SEMA3F-NRP2 axis would have therapeutic potential [10,11].
High immunohistochemical expression of NRP2 in patients with HNSCC has been observed to increase lymphatic vascular density and lymphovascular invasion, with a higher risk of regional involvement and decreased survival [12,13]. Conversely, high expression of SEMA3F has been associated with a reduction in the degree of tumor lymphovascular infiltration and increased survival [12,14].
Considering these regulatory roles played by the SEMA3F-NRP2 axis in the lymphovascular infiltration process and lymph node involvement, we hypothesized that there is a relationship between the expression of these genes and the risk of occult lymph node metastasis in patients with HNSCC. The aim of the present study is to analyze the predictive capacity of the transcriptional expression of SEMA3F and NRP2 in determining the presence of occult lymph node metastases in patients with HNSCC.
2. Materials and Methods
2.1. Patients
With the exception of patients with a glottic cT1N0 tumor, our center’s treatment protocol includes the use of an elective neck dissection for all patients with HNSCC without clinical or radiological evidence of lymph node involvement at the time of diagnosis (cN0) and who received a surgical treatment of the primary location of the tumor.
The current study was carried out retrospectively by analyzing biopsies obtained from the primary location of the tumor prior to any treatment from patients with a pathologically confirmed squamous cell carcinoma located in the oral cavity, larynx or hypopharynx, without clinical or radiological evidence of lymph node involvement at the time of diagnosis (cN0) and treated with an elective uni- or bilateral neck dissection. Fifty-three patients met the inclusion criteria and were the basis for the present study. The clinical information was obtained from a database that prospectively collects information of all patients with malignant head and neck tumors treated in our center since 1985 [15].
All patients included in the study were assessed by the Oncology Board of our institution, which determined the convenience of carrying out an elective neck dissection. Table 1 shows the characteristics of the patients included in the study.
Table 1.
Characteristics of the patients included in the study.
| Characteristics | N Patients (%) | |
|---|---|---|
| Age | Mean 66.7 years/Range 31.1–87.3 years | |
| Gender | Men | 44 (83.0%) |
| Women | 9 (17.0%) | |
| Location | Oral cavity | 15 (28.3%) |
| Hypopharynx | 3 (5.7%) | |
| Larynx | 35 (66.0%) | |
| Toxic consumption | No | 7 (13.2%) |
| Moderate | 13 (24.5%) | |
| Severe | 33 (62.3%) | |
| Local extension | cT1–2 | 4 (7.5%) |
| cT3 | 22 (41.5%) | |
| cT4 | 27 (50.9%) | |
| Histologic grade | Well differentiated | 3 (5.7%) |
| Moderately differentiated | 45 (84.9%) | |
| Poorly differentiated | 5 (9.4%) | |
| Adjuvant treatment | No | 22 (41.5%) |
| Radiotherapy | 21 (39.6%) | |
| Chemoradiotherapy | 10 (18.9%) | |
All the patients were treated with surgical resection of the primary location of the tumor combined with an elective uni- (n = 15, 28.3%) or bilateral (n = 38, 71.7%) neck dissection. In 31 patients the surgery was followed by adjuvant treatment with radiotherapy (n = 21) or chemoradiotherapy (n = 10). The criteria for adjuvant treatment were pT4 tumor extension, positive or close resection margins, three or more metastatic lymph nodes, or the presence of lymph node metastases with extracapsular spread. Given the interaction between tobacco and alcohol consumption, a combined variable of toxic consumption was created with three categories: no consumption; moderate consumption (<20 cigarettes/day and/or <80 g alcohol/day); and severe consumption (≥20 cigarettes/day or ≥80 g alcohol/day).
Based on the analysis of pathologic findings, we classified the patients according to the absence (cN0/pN0) or presence (cN0/pN+) of occult neck node metastases. For cN0/pN+ patients, we considered the uni- or bilateral involvement, as well as the presence of metastatic nodes with extracapsular spread. The mean number of dissected nodes per patient was 34.0 (SD = 17.3, range = 10–84).
The design of the study was approved by the Institutional Review Board of our center (IIBSP-CCC-14-93) and was performed in accordance with the principles outlined in the Declaration of Helsinki.
2.2. Transcriptional Analysis
Samples obtained from each patient were immediately suspended in RNA-later (Quiagen GmbH, Hilden, Germany) to prevent mRNA degradation, and stored at −80 °C until processing. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cDNA was obtained by reverse transcription of 1 g of RNA with High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) and transcriptional expression of NRP2, SEMA3F, and beta-actin as an endogenous control was assessed by RT-PCR on an ABI Prism 7000 using predesigned validated assays (TaqMan Gene Expression Assays; Applied Biosystems).
2.3. External Validation Sutyd: The Cancer Genome Atlas Database
The Cancer Genome Atlas (TCGA) [16] is a public data base that includes clinical information and the transcriptome of 528 patients with HNSCC and 43 samples of healthy mucosa. In order to obtain an external validation cohort, we conducted a study including 176 patients of the TCGA with a cN0 HNSCC for which information on the pathologic results of the elective neck dissection was available. Table S1 of the Supplementary Material shows the characteristics of the patients included in the validation study.
2.4. Statistical Analyses
The distribution of the transcriptional expression values of SEMA3F did not meet normality criteria (Kolmogorov–Smirnov test, p = 0.008), so the measures of central tendency were expressed using the median value, and nonparametric techniques were used in the comparison between the expression values. SEMA3F and NRP2 expression values were compared according to the location of the primary tumor, toxic consumption, histological grade, clinical category of local tumor extension (cT), and the presence of occult lymph node metastases (pN0 versus pN+). Comparisons were performed using the Mann–Whitney U test or the Kruskal–Wallis test depending on the application conditions. We proceeded to classify patients considering the presence of occult lymph node metastases as the dependent variable according to SEMA3F and NRP2 transcriptional expression values with a classification and regression tree (CRT) analysis. The hazard ratio for the presence of occult lymph node metastases was analyzed as a function of the categories obtained with the CRT with a logistic regression model. Disease-specific survival was estimated according to the categories obtained with the CRT analysis with the Kaplan–Meier method, using the log-rank test in the comparison of the survival curves. Finally, a multivariate analysis (logistic regression) was performed considering the presence of occult lymph node metastases as the dependent variable and including in the model the location of the primary tumor, the local extension of the tumor (cT), and the categories obtained with the CRT. Comparison between the transcriptional expression of SEMA3F and NRP2 between normal and tumor mucosa samples from patients included in TCGA was performed with a paired sample t-test. Statistical analysis was performed with the SPSS 26.0.0.1 software.
3. Results
Twenty patients included in the study (37.7%) presented occult lymph node metastases (cN0/pN+) in the elective neck dissections, 17 unilaterally and 3 bilaterally. Of the cN0/pN+ patients, in three cases one of the positive lymph nodes presented extracapsular spread.
3.1. SEMA3F/NRP2 Expression According to Clinical Variables
When analyzing the levels of NRP2 transcriptional expression depending on the clinical variables, no significant differences appeared according to the location of the primary tumor (p = 0.449), toxics consumption (p = 0.284), category of local extension of the tumor (p = 0.255), or histological grade (p = 0.921). Similarly, no significant differences appeared in the SEMA3F transcriptional expression according to the primary tumor location (p = 0.394), toxic consumption (p = 0.770), local extension of the tumor (p = 0.794), or histological grade (p = 0.647).
There were no significant differences in NRP2 transcriptional expression according to the presence of occult lymph node metastases (p = 0.545), whereas patients with occult lymph node metastases (cN0/pN+) had significantly lower SEMA3F expression values than patients without lymph node involvement (cN0/pN0) (p = 0.006). Figure S1 of the Supplementary Material shows the distribution of NRP2 and SEMA3F values as a function of the pathological status of the neck dissections. Table S2 of the Supplementary Material shows the median NRP2 and SEMA3F expression values as a function of the clinical variables analyzed.
3.2. Classification Depending on the SEMA3F/NRP2 Expression
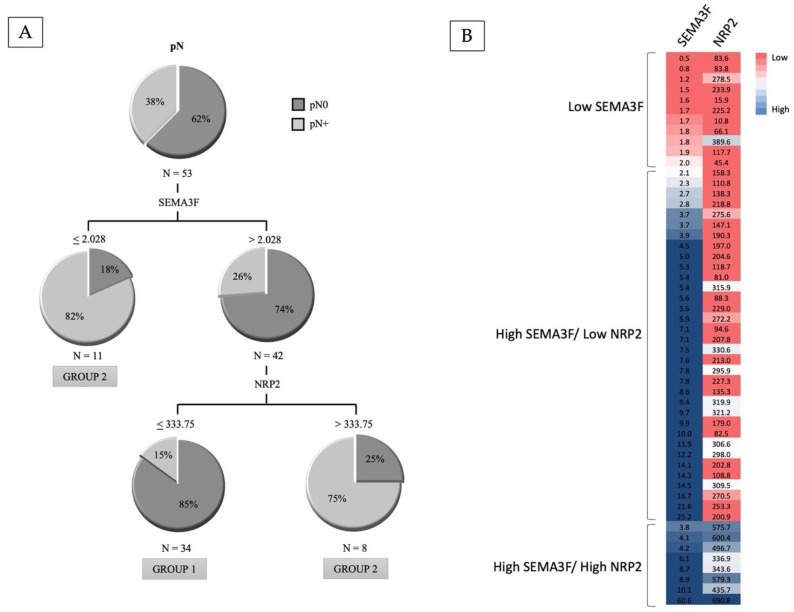
Considering the presence of occult lymph node metastases as the dependent variable, the CRT classified the patients into three categories, with a first partition according to SEMA3F expression values, and a second partition level that only affected cases with high SEMA3F expression depending on the NRP2 expression values (Figure 1A). Figure 1B shows the heatmap of SEMA3F and NRP2 according to the results obtained in the classification tree. Three terminal nodes were obtained: patients with low SEMA3F expression (n = 11, 20.8%; 81.8% pN+); patients with high SEMA3F/high NRP2 expression (n = 8, 15.1%; 75% pN+); and patients with high SEMA3F/low NRP2 expression (n = 34, 64.2%; 14.7% pN+). The two terminal nodes with a high risk of occult lymph nodal metastases were grouped, classifying patients into two groups: Group 1, high SEMA3F/low NRP2 expression (n = 34), with a low risk of occult nodal involvement (14.7% cN0/pN+); Group 2, low SEMA3F expression or high SEMA3F/high NRP2 expression (n = 19), with a high risk of occult nodal involvement (78.9% cN0/pN+). Relative to Group 1, the hazard ratio of the presence of occult lymph node metastases for patients included in Group 2 was 21.7 (95% CI, HR = 5.0–93.1).
Figure 1.
(A): Classification tree according to the transcriptional expression values of SEMA3F and NRP2 considering the presence of occult lymph node metastases as the dependent variable. (B): Heatmap of SEMA3F and NRP2 according to the results obtained in the classification tree.
According to the result of the multivariate analysis (Table 2), the only variable that was significantly related to the risk of occult lymph node metastasis was the classification obtained from the SEMA3F-NRP2 expression values. Relative to patients with a low risk of occult nodal involvement (Group 1), patients included in Group 2 had a 26.2-fold increased risk of nodal involvement (95% CI 5.1–132.4, p < 0.001). Neither the location of the primary tumor nor the local extension of the tumor was related to the risk of occult nodal involvement.
Table 2.
Results of a multivariate analysis considering the appearance of occult lymph node metastases as the dependent variable (HR: hazard ratio).
| Variation | HR | CI 95% HR | p | |
|---|---|---|---|---|
| Location | Oral cavity | 1 | ||
| Hypopharynx | 0.34 | 0.010–12.30 | 0.558 | |
| Larynx | 0.25 | 0.04–1.44 | 0.121 | |
| Local extension | cT1-2 | 1 | ||
| cT3 | 0.14 | 0.06–3.41 | 0.233 | |
| cT4 | 0.18 | 0.08–4.14 | 0.286 | |
| SEMA3F-NRP2 | Group 1 | 1 | ||
| Group 2 | 26.21 | 5.19–132.42 | 0.000 | |
The transcriptional profile of the SEMA3F-NRP2 genes was reflected in the patients’ disease-free survival. Five-year disease-free survival rate of patients included in Group 1 (high SEMA3F/low NRP2 expression, n = 34) was 74.6% (95% CI 57.6–91.6%), significantly higher than that of those patients included in Group 2 (low SEMA3F or high SEMA3F/high NRP2 expression, n = 19), whose rate was 27.1% (95% CI 3.6–50.6%) (p = 0.0001). Figure S2 of the Supplementary Material shows the disease-free survival curves according to the SEMA3F-NRP2 transcriptional profile.
3.3. Results of the External Validation Study with the TCGA Data
In the external validation study, occult lymph node metastases were found in 56 (31.8%) of the 176 patients of the TCGA. The CRT analysis classified the patients into three groups considering the transcriptional expression of the SEMA3F-NRP2 genes. A first partition was obtained according to the SEMA3F expression values, and a second partition only affected cases with low SEMA3F expression depending on the NRP2 expression values. Figure S3 of the Supplementary Material shows the resulting classification tree. Patients with high SEMA3F expression (n = 46) had a low risk of occult lymph node metastases (15.2%). Patients with low SEMA3F/low NRP2 expression (n = 72) had an intermediate risk of occult lymph node metastases (30.6%). Finally, patients with low SEMA3F/high NRP2 expression (n = 58) were the group that had the highest risk of occult lymph node metastases (46.6%).
We compared the transcriptional expression of the tumor and the healthy mucosa of the patients of the TCGA. The expression of SEMA3F was higher in healthy mucosa than in the tumor (p = 0.015) and, in contrast, the expression of NRP2 was higher in the tumor than in healthy mucosa (p = 0.001). Figure S4 of the Supplementary Material shows the distribution of the transcriptional expression values of SEMA3F and NRP2 in the samples of healthy mucosa and tumor.
4. Discussion
According to our results, the transcriptional expression of the SEMA3F-NRP2 genes was significantly associated with the risk of occult lymph node metastases in neck dissections carried out electively in patients with HNSCC. The result of multivariate analysis showed that tumors with low SEMA3F expression or high SEMA3F expression associated with high NRP2 expression had a more than 25-fold higher risk of occult lymph node metastasis than tumors with high SEMA3F and low NRP2 expression.
The SEMA3F gene is located on 3p21, a frequently deleted loci in HNSCC, being one of the most commonly under-expressed genes [17]. Immunohistochemical and transcriptional studies in patients with HNSCC have shown a reduction in the expression of SEMA3F relative to healthy mucosa [14,18]. In agreement with these findings, when analyzing the results included in TCGA we could observe that the normal mucosal samples had higher transcriptional expression values than those observed in the tumor sample, while NRP2 expression behaved in the opposite way, with a higher expression in the tumor samples. All these findings lead us to suppose that the reduction in the expression of SEMA3F and the increase in NRP2 should be considered as elements that favor the carcinogenesis process. Moreover, in vitro studies have found an increase in cell proliferation, migration, invasion capacity, and higher vascular recruitment when SEMA3F expression was suppressed, thus facilitating the angiogenesis process through its interaction with NRP2. Additionally, in vivo studies established that the induction of SEMA3F expression resulted in the inhibition of the tumor growth, as well as a significant reduction in tumor lymphangiogenesis and lymph node involvement [14,18]. Zhang et al. [12] found a relationship between the decrease in SEMA3F expression and an increase in the risk of regional involvement with a significant decrease in survival in patients with oral squamous cell carcinomas. Similarly, Xie et al. [19] established an association between the loss of SEMA3F expression and a significant increase in the risk of lymph node involvement in patients with esophageal squamous cell carcinoma, a tumor model that shares many characteristics with HNSCC.
In patients with cN0 oral cavity carcinomas, Ong et al. evidenced a direct relationship between NRP2 immunopositivity and regional involvement, with high NRP2 expression being associated with a significant reduction in survival [13]. The inhibition of NRP2 expression induced a significant reduction in proliferation, migration, and invasion capacity. In the same way, Zhang et al. also described a significant correlation between a high NRP2 expression at the oral tumor primary location and a high lymphatic density and lymphatic vascular invasion, entailing an increased frequency of lymph node involvement and a significant decrease in survival [12].
The increased risk of lymph node involvement in patients with reduced SEMA3F expression seems to be related to the loss of the ability to directly suppress the lymphangiogenesis process as well as the decrease in NRP2 receptor occupation, facilitating the competitive activity of other specific ligands with the capacity to promote lymph node metastasis such as VEGF-C [12,14]. On the other hand, even in the presence of SEMA3F, the high expression of NRP2 would allow its activity as a potent promoter of tumor-associated lymphangiogenesis [13]. However, we did not conduct mechanistic studies to demonstrate these changes in enzyme activity or its downstream effects, nor its potential relationship with VEGF-C. Although the predictive ability of the SEMA3F/NRP2 transcriptional expression is clearly demonstrated in our study, the involvement of this pathway in the progression of HNSCC requires further study.
In addition to the increased risk of lymphatic metastasis, high NRP2 and low SEMA3F expression has been associated with an increase in tumor aggressiveness, evidenced as an increase in the capacity for proliferation and infiltration [12,13,14,18]. Taken together, these findings support the significant relationship between the pattern in the transcriptional expression of the SEMA3F-NRP and the patient survival observed in our study.
Comparing the results obtained with the patients of the TCGA with our sample, we can observe that predictive capacity of the transcriptional expression of the SEMA3F/NRP2 genes in detecting the presence of occult lymph node metastases was maintained. Both expressions of low SEMA3F and a high NRP2 were associated with an increased risk of occult lymph node metastases.
According to the results obtained in various meta-analyses [20,21] and clinical trials [22], elective neck dissections can significantly reduce the rate of regional nodal recurrence and improve the disease-free survival in patients with HNSCC without clinical or radiologic evidence of lymph node metastasis in the neck (cN0). However, these neck dissections involve carrying out neck surgery in a proportion of patients who will not obtain therapeutic benefit from the procedure. One of the approaches to minimize the proportion of patients who do not benefit from the elective neck dissection is the sentinel lymph node biopsy. Another strategy is to have biomarkers with the capacity of predicting the risk of occult lymph node metastases in cN0 patients.
If our results are validated, the expression of the SEMA3F-NRP2 axis could be used as a biomarker predicting the risk of occult lymph node metastasis, with elective neck dissections being indicated exclusively for patients at higher risk. Patients with a low risk could be candidates for observation or sentinel node biopsy. The availability of a biomarker with the capacity to predict the presence of occult lymph node metastases would make it possible to avoid elective neck dissections in low-risk patients, with the consequent decrease in surgical time and morbidity of the treatment without impairing the oncologic outcome.
Several authors have studied the relationship between the expression of molecular biomarkers and the risk of occult lymph node metastases in patients with HNSCC. Table 3 shows a summary of the studies performed. Most of the studies have analyzed the different markers by determining immunohistochemical expression, mainly in patients with tumors located in the oral cavity, obtaining variable results in terms of prognostic capacity. The results of our study are among those that achieved the highest accuracy rate (83% of cases correctly classified), with a sensitivity of 75% and relatively high specificity and negative predictive values (87.7% and 85.2%, respectively).
Table 3.
Results obtained in studies that have analyzed the relationship of molecular biomarkers with the presence of occult lymph node metastases in patients with HNSCC (S, sensitivity; E, specificity; PPV, positive predictive value; NPV, negative predictive value; AI, accuracy index; OC, oral cavity; ORF, oropharynx; HNSCC, head and neck squamous cell carcinoma; IHC, immuno-histochemistry). * Numerical aberrations of CCND1; ** Serum MYO5A levels.
| Author (Year) | Biomarker | Location | n | % pN+ | Determination | Related to | S | E | PPV | NPV | AI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Franchi (1996) [23] | PCNA | Larynx | 60 | 50% | IHQ | pN+ | 80.0% | 80.0% | 80.0% | 80.0% | 80.0% |
| MIB-1 | pN+ | 56.6% | 83.3% | 77.2% | 65.7% | 70.0% | |||||
| E-cadherin | pN0 | 50.0% | 86.6% | 78.9% | 63.4% | 68.3% | |||||
| Capaccio (2000) [24] | Cyclin D1 | HNSCC | 96 | 33.3% | IHQ | pN+ | 68.7% | 68.7% | 52.3% | 81.4% | 68.7% |
| Myo (2005) [25] | Cyclin D1 * | OC | 45 | 37.7% | FISH | pN+ | 70.5% | 89.2% | 80.0% | 83.3% | 82.2% |
| Huber (2011) [26] | E-cadherin | OC, ORF | 120 | 37.5% | IHQ | pN0 | 82.2% | 44.0% | 46.8% | 80.4% | 58.3% |
| Zullig (2013) [27] | SOX2 | OC | 120 | 37.5% | IHQ | pN0 | 95.6% | 32.0% | 45.7% | 92.3% | 55.8% |
| Kelner (2014) [28] | Activin A | OC | 110 | 26.35 | IHQ | pN+ | 74.0% | 56.4% | 37.0% | 86.2% | 58.2% |
| Noorlag (2016) [29] | Cyclin D1 | OC | 152 | 25.0% | IHQ | pN+ | 63.1% | 66.6% | 38.7% | 84.4% | 65.7% |
| Mermod (2016) [30] | PROX1 | OC, ORF | 52 | 19.2% | IHQ | pN+ | 60.0% | 98.0% | 86.0% | 91.0% | 88.0% |
| Mermod (2018) [31] | CD31 | OC, ORF | 56 | 19.6% | IHQ | pN+ | 91.0% | 65.0% | 40.0% | 97.0% | 71.0% |
| Zhao (2018) [32] | MYO5A ** | Larynx | 103 | 31.0% | ELISA | pN+ | 77.8% | 75.4% | - | - | - |
| Boeve (2021) [33] | Cortactin | OC | 33 | 18.1% | IHQ | pN+ | 66.7% | 88.8% | 57.1% | 92.3% | 84.8% |
| Current study | SEMA3F | CECC | 53 | 37.7% | PCR | pN0 | 75.0% | 87.8% | 78.9% | 85.2% | 83.0% |
| NRP2 | pN+ |
Moreover, the presence of occult lymph node metastases has been associated with certain histological features of the tumor such as the degree of lymphatic invasion [34], an aggressive pattern of the tumor infiltration margin [35], the depth of infiltration [22,34,36], the presence of perineural invasion [35], the presence of myofibroblasts in the tumor stroma [37], the eosinophilic infiltration of the tumor [38], or the appearance of hybrid circulating cells [39].
Further studies evaluating in conjunction the predictive capacity of two or more of these molecular and/or histological markers are needed in order to find a combination of biomarkers with sufficient sensitivity and specificity to safely select cN0 patients who are not candidates for elective neck dissection.
The present study has limitations inherent to its retrospective nature and a small sample of patients. Future prospective external validation studies in large cohorts of patients are needed before we can consider that the transcriptional activity of the SEMA3F-NRP2 genes has sufficient predictive capacity to avoid elective neck dissections in patients with HNSCC.
5. Conclusions
According to the results obtained in our study, there is a significant relationship between the transcriptional expression of the SEMA3F-NRP2 genes and the risk of occult lymph node metastasis in patients with HNSCC. The risk of occult lymph node metastasis was low for the group of patients with high SEMA3F/low NRP2 expression, while it was high for patients with low SEMA3F or high SEMA3F/high NRP2 expression.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14092259/s1, Figure S1: Distribution of the transcriptional expression values of NRP2 and SEMA3F according to the pathologic status of the neck; Figure S2: Disease-free survival according to the expression categories of the SEMA3F-NRP2 genes; Figure S3: Classification tree according to the transcriptional expression values of SEMA3F and NRP2 of the patients included in The Cancer Genome Atlas considering the presence of occult lymph node metastases as the dependent variable; Figure S4: Distribution of the transcriptional expression values of NRP2 and SEMA3F in the samples of the healthy mucosa and the tumor of the patients included in The Cancer Genome Atlas; Table S1: Characteristics of the patients of The Cancer Genome Atlas included in the validation study; Table S2: Median of the transcriptional expression values of SEMA3F and NRP2 according to the characteristics of the patients included in the study.
Author Contributions
Conceptualization, X.L.V.; Data curation, Gemma Fuster; Formal analysis, F.-X.A.-J. and X.L.V.; Funding acquisition, F.-X.A.-J., X.T. and X.L.V.; Investigation, C.M.-C., X.T., P.B., G.F. and M.C.; Methodology, P.B.; Project administration, X.L.V.; Resources, X.T. and X.L.V.; Supervision, F.-X.A.-J., X.T. and X.L.V.; Validation, I.V.; Writing—original draft, C.M.-C. and X.L.V.; Writing—review and editing, C.M.-C., F.-X.A.-J. and I.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. The study was approved by the Hospital de Sant Pau Ethics Committee for Scientific Research (IIBSP-CCC-14-93).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Nacional Cancer Institute. The Cancer Genome Atlas Program. Available online: https://tcga-data.nci.nih.gov/tcga (accessed on 22 February 2022).
Conflicts of Interest
The authors declare that they do not have any conflict of interest.
Funding Statement
This research was funded by a grant from Plan Estatal de I+D+I of the Instituto de Salud Carlos III (FIS PI15/02047 and FIS PI18/0844 to F.X.A.-J., and FIS PI19/01661 to X.L.V.) and a grant from the Spanish Ministry of Economy and Competitiveness (PID2019-104991RB-I00 to P.B.). Fondo Europeo de Desarrollo Regional (FEDER), A Way to Build Europe. X.T. is a Serra-Húnter fellow. Asociación Española contra el Cáncer (LABAE18025AVIL).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020;70:313. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Massey C., Dharmarajan A., Bannuru R.R., Rebeiz E. Management of N0 neck in early oral squamous cell carcinoma: A systematic review and meta-analysis. Laryngoscope. 2019;129:E284–E298. doi: 10.1002/lary.27627. [DOI] [PubMed] [Google Scholar]
- 3.Sharbel D.D., Abkemeier M., Groves M.W., Albergotti W.G., Byrd J.K., Reyes-Gelves C. Occult Metastasis in Laryngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Ann. Otol. Rhinol. Laryngol. 2021;130:67–77. doi: 10.1177/0003489420937744. [DOI] [PubMed] [Google Scholar]
- 4.Gourin C.G., Conger B.T., Porubsky E.S., Sheils W.C., Bilodeau P.A., Coleman T.A. The Effect of Occult Nodal Metastases on Survival and Regional Control in Patients With Head and Neck Squamous Cell Carcinoma. Laryngoscope. 2008;118:1191–1194. doi: 10.1097/MLG.0b013e31816e2eb7. [DOI] [PubMed] [Google Scholar]
- 5.Achen M.G., Stacker S.A. Molecular Control of Lymphatic Metastasis. Ann. N. Y. Acad. Sci. 2008;1131:225–234. doi: 10.1196/annals.1413.020. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y., Yuan L., Mak J., Pardanaud L., Caunt M., Kasman I., Larrivée B., Del Toro R., Suchting S., Medvinsky A., et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 2010;188:115–130. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao B., Liu S., Tan X., Lu P., Wang D., Xu H. Class-3 semaphorins: Potent multifunctional modulators for angiogenesis-associated diseases. Biomed. Pharmacother. 2021;137:111329. doi: 10.1016/j.biopha.2021.111329. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama H., Bruneau S., Kochupurakkal N., Coma S., Briscoe D.M., Klagsbrun M. Regulation of mTOR Signaling by Semaphorin 3F-Neuropilin 2 Interactions In Vitro and In Vivo. Sci. Rep. 2015;5:11789. doi: 10.1038/srep11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toledano S., Nir-Zvi I., Engelman R., Kessler O., Neufeld G. Class-3 Semaphorins and Their Receptors: Potent Multifunctional Modulators of Tumor Progression. Int. J. Mol. Sci. 2019;20:556. doi: 10.3390/ijms20030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Klamer B., Li J., Fernandez S., Li L. A pan-cancer study of class-3 semaphorins as therapeutic targets in cancer. BMC Med. Genom. 2020;13((Suppl. S5)):45. doi: 10.1186/s12920-020-0682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama H., Kusumoto C., Nakahara M., Fujiwara A., Higashiyama S. Semaphorin 3F and Netrin-1: The Novel Function as a Regulator of Tumor Microenvironment. Front. Physiol. 2018;9:1662. doi: 10.3389/fphys.2018.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B., Gao Z., Sun M., Li H., Fan H., Chen D., Zheng J. Prognostic significance of VEGF-C, semaphorin 3F, and neuropilin-2 expression in oral squamous cell carcinomas and their relationship with lymphangiogenesis. J. Surg. Oncol. 2015;111:382–388. doi: 10.1002/jso.23842. [DOI] [PubMed] [Google Scholar]
- 13.Ong H., Gokavarapu S., Xu Q., Tian Z., Li J., Ji T., Zhang C. Cytoplasmic neuropilin 2 is associated with metastasis and a poor prognosis in early tongue cancer patients. Int. J. Oral Maxillofac. Surg. 2017;46:1205–1219. doi: 10.1016/j.ijom.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Doçi C.L., Mikelis C.M., Lionakis M.S., Molinolo A.A., Gutkind J.S. Genetic Identification of SEMA3F as an Antilymphangiogenic Metastasis Suppressor Gene in Head and Neck Squamous Carcinoma. Cancer Res. 2015;75:2937–2948. doi: 10.1158/0008-5472.CAN-14-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.León X., Orús C., Quer M. Diseño, mantenimiento y explotación de una base de datos oncológica para pacientes con tumores malignos de cabeza y cuello [Design, maintenance, and exploitation of an oncologic database for patients with malignant tumors of the head and neck] Acta Otorrinolaringol. Esp. 2002;53:185–190. doi: 10.1016/S0001-6519(02)78299-X. [DOI] [PubMed] [Google Scholar]
- 16.Nacional Cancer Institute The Cancer Genome Atlas Program. [(accessed on 22 February 2022)]; Available online: https://tcga-data.nci.nih.gov/tcga.
- 17.Shaikh M.H., Barrett J.W., Khan M.I., Kim H.A., Zeng P.Y., Mymryk J.S., Nichols A.C. Chromosome 3p loss in the progression and prognosis of head and neck cancer. Oral Oncol. 2020;109:104944. doi: 10.1016/j.oraloncology.2020.104944. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Li R., Yin K., Ren G., Zhang Y. The crucial role of SEMA3F in suppressing the progression of oral squamous cell carcinoma. Cell. Mol. Biol. Lett. 2017;2:32. doi: 10.1186/s11658-017-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Z., Li T., Huang B., Liu S., Zhang L., Zhang Q. Semaphorin 3F Serves as a Tumor Suppressor in Esophageal Squamous Cell Carcinoma and is Associated With Lymph Node Metastasis in Disease Progression. Technol. Cancer Res. Treat. 2020;19:1533033820928117. doi: 10.1177/1533033820928117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Ghanem S., Yehuda M., Carmel N.-N., Leshno M., Abergel A., Gutfeld O., Fliss D.M. Elective Neck Dissection vs Observation in Early-Stage Squamous Cell Carcinoma of the Oral Tongue With No Clinically Apparent Lymph Node Metastasis in the Neck: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Neck Surg. 2016;142:857–865. doi: 10.1001/jamaoto.2016.1281. [DOI] [PubMed] [Google Scholar]
- 21.Oh L., Phan K., Kim S., Low T., Gupta R., Clark J. Elective neck dissection versus observation for early-stage oral squamous cell carcinoma: Systematic review and meta-analysis. Oral Oncol. 2020;105:104661. doi: 10.1016/j.oraloncology.2020.104661. [DOI] [PubMed] [Google Scholar]
- 22.D’Cruz A.K., Vaish R., Kapre N., Dandekar M., Gupta S., Hawaldar R., Agarwal J.P., Pantvaidya G., Chaukar D., Deshmukh A., et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015;373:521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 23.Franchi A., Gallo O., Boddi V., Santucci M. Prediction of occult neck metastases in laryngeal carcinoma: Role of proliferating cell nuclear antigen, MIB-1, and E-cadherin immunohistochemical determination. Clin. Cancer Res. 1996;2:1801–1808. [PubMed] [Google Scholar]
- 24.Capaccio P., Pruneri G., Carboni N., Pagliari A.V., Quatela M., Cesana B.M., Pignataro L. Cyclin D1 expression is predictive of occult metastases in head and neck cancer patients with clinically negative cervical lymph nodes. Head Neck. 2000;22:234–240. doi: 10.1002/(SICI)1097-0347(200005)22:3<234::AID-HED5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Myo K., Uzawa N., Miyamoto R., Sonoda I., Yuki Y., Amagasa T. Cyclin D1 gene numerical aberration is a predictive marker for occult cervical lymph node metastasis in TNM Stage I and II squamous cell carcinoma of the oral cavity. Cancer. 2005;104:2709–2716. doi: 10.1002/cncr.21491. [DOI] [PubMed] [Google Scholar]
- 26.Huber G.F., Züllig L., Soltermann A., Roessle M., Graf N., Haerle S.K., Studer G., Jochum W., Moch H., Stoeckli S.J. Down regulation of E-Cadherin (ECAD)-a predictor for occult metastatic disease in sentinel node biopsy of early squamous cell carcinomas of the oral cavity and oropharynx. BMC Cancer. 2011;11:1–8. doi: 10.1186/1471-2407-11-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Züllig L., Roessle M., Weber C., Graf N., Haerle S., Jochum W., Stoeckli S., Moch H., Huber G. High sex determining region Y-box 2 expression is a negative predictor of occult lymph node metastasis in early squamous cell carcinomas of the oral cavity. Eur. J. Cancer. 2013;49:1915–1922. doi: 10.1016/j.ejca.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Kelner N., Rodrigues P.C., Bufalino A., Fonseca F.P., dos Dds A.R.S.-S., Dds M.C.C.M., Pinto C.A.L., Dds A.F.P.L., Graner E., Salo T., et al. Activin A immunoexpression as predictor of occult lymph node metastasis and overall survival in oral tongue squamous cell carcinoma. Head Neck. 2015;37:479–486. doi: 10.1002/hed.23627. [DOI] [PubMed] [Google Scholar]
- 29.Noorlag R., Boeve K., Witjes M., Koole R., Peeters T.L.M., Schuuring E., Willems S.M., van Es R.J.J. Amplification and protein overexpression of cyclin D1: Predictor of occult nodal metastasis in early oral cancer. Head Neck. 2016;39:326–333. doi: 10.1002/hed.24584. [DOI] [PubMed] [Google Scholar]
- 30.Mermod M., Bongiovanni M., Petrova T.V., Dubikovskaya E.A., Simon C., Tolstonog G., Monnier Y. Prediction of occult lymph node metastasis in squamous cell carcinoma of the oral cavity and the oropharynx using peritumoral Prospero homeobox protein 1 lymphatic nuclear quantification. Head Neck. 2016;38:1407–1415. doi: 10.1002/hed.24452. [DOI] [PubMed] [Google Scholar]
- 31.Mermod M., Bongiovanni M., Petrova T., Goun E., Simon C., Tolstonog G., Monnier Y. Prediction of Occult Lymph Node Metastasis in Head and Neck Cancer with CD31 Vessel Quantification. Otolaryngol. Neck Surg. 2019;160:277–283. doi: 10.1177/0194599818791779. [DOI] [PubMed] [Google Scholar]
- 32.Zhao X., Zhang W., Ji W. MYO5A inhibition by miR-145 acts as a predictive marker of occult neck lymph node metastasis in human laryngeal squamous cell carcinoma. OncoTargets Ther. 2018;11:3619–3635. doi: 10.2147/OTT.S164597. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Boeve K., Mastik M.F., Slagter-Menkema L., van Dijk B.A.C., Dds J.L.N.R., Laan B.F.A.M., Witjes M.J.H., Vegt B., Schuuring E. Cortactin expression assessment improves patient selection for a watchful waiting strategy in pT1cN0- staged oral squamous cell carcinomas with a tumor infiltration depth below 4 mm. Head Neck. 2021;43:2688–2697. doi: 10.1002/hed.26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozdek A., Sarac S., Akyol M.U., Unal O.F., Sungur A. Histopathological predictors of occult lymph node metastases in supraglottic squamous cell carcinomas. Eur. Arch. Oto-Rhino-Laryngol. 2000;257:389–392. doi: 10.1007/s004050000231. [DOI] [PubMed] [Google Scholar]
- 35.Sparano A., Weinstein G., Chalian A., Yodul M., Weber R. Multivariate Predictors of Occult Neck Metastasis in Early Oral Tongue Cancer. Otolaryngol. Neck Surg. 2004;131:472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Bittar R.F., Ferraro H.P., Ribas M.H., Lehn C.N. Predictive factors of occult neck metastasis in patients with oral squamous cell carcinoma. Braz. J. Otorhinolaryngol. 2016;82:543–547. doi: 10.1016/j.bjorl.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luksic I., Suton P., Manojlovic S., Virag M., Petrovecki M., Macan D. Significance of myofibroblast appearance in squamous cell carcinoma of the oral cavity on the occurrence of occult regional metastases, distant metastases, and survival. Int. J. Oral Maxillofac. Surg. 2015;44:1075–1080. doi: 10.1016/j.ijom.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira D.T., Biassi T.P., Faustino S.E.S., Carvalho A.L., Landman G., Kowalski L.P. Eosinophils may predict occult lymph node metastasis in early oral cancer. Clin. Oral Investig. 2011;16:1523–1528. doi: 10.1007/s00784-011-0651-7. [DOI] [PubMed] [Google Scholar]
- 39.Henn T.E., Bs A.N.A., Hollett Y.R., Sutton T.L., Walker B.S., Swain J.R., Sauer D.A., Clayburgh D.R., Wong M.H. Circulating hybrid cells predict presence of occult nodal metastases in oral cavity carcinoma. Head Neck. 2021;43:2193–2201. doi: 10.1002/hed.26692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Nacional Cancer Institute. The Cancer Genome Atlas Program. Available online: https://tcga-data.nci.nih.gov/tcga (accessed on 22 February 2022).