Abstract
This paper describes the production of isoprenoid wax esters during the aerobic degradation of 6,10,14-trimethylpentadecan-2-one and phytol by four bacteria (Acinetobacter sp. strain PHY9, Pseudomonas nautica [IP85/617], Marinobacter sp. strain CAB [DSMZ 11874], and Marinobacter hydrocarbonoclasticus [ATCC 49840]) isolated from the marine environment. Different pathways are proposed to explain the formation of these compounds. In the case of 6,10,14-trimethylpentadecan-2-one, these esters result from the condensation of some acidic and alcoholic metabolites produced during the biodegradation, while phytol constitutes the alcohol moiety of most of the esters produced during growth on this isoprenoid alcohol. The amount of these esters formed increased considerably in N-limited cultures, in which the ammonium concentration corresponds to conditions often found in marine sediments. This suggests that the bacterial formation of isoprenoid wax esters might be favored in such environments. Although conflicting evidence exists regarding the stability of these esters in sediments, it seems likely that, under some conditions, bacterial esterification can enhance the preservation potential of labile compounds such as phytol.
The C20 isoprenoid alcohol phytol usually occurs in an esterified form as the side chain of chlorophyll a; it is generally considered to be the most abundant acyclic isoprenoid compound in the biosphere (49). In sediments, the fate of most of the deposited phytol, as with other labile lipids, is remineralization to CO2. However, the ester bond between phytol and the tetrapyrrolic macrocycle can resist hydrolysis, as shown by the isolation of intact phytyl esters from sediments several million years old (5). Small amounts of free phytol are produced during early diagenesis in recent sediments (30, 42), and there are numerous reports of 6,10,14-trimethylpentadecan-2-one in sediments (17, 29, 48), which can be produced from free phytol, phytane, pristane, and chlorophyll by various pathways (43).
Phytol and some of its metabolites have been identified in both the alcohol and the fatty acid moieties of some naturally occurring wax esters (28). Phytyl esters occur in higher plants (16), bryophytes (12, 25), mosses (18), dinoflagellates (51) and marine zooplankton (45). In bacteria of the genus Acinetobacter, wax esters are generally considered to be energy storage components (3, 22). Esters containing phytol have also been detected in some marine (8) and lacustrine sediments (15), but their origin has not been satisfactorily explained.
Some incubation experiments (10, 11) have suggested that the esterification of phytol may be a significant process in microbially active sediments. However, the bacterial production of isoprenoid wax esters has not been reported. In this paper, we report the formation of isoprenoid wax esters during aerobic growth of four marine bacteria: Acinetobacter sp. strain PHY9, Pseudomonas nautica (IP85/617), Marinobacter sp. strain CAB (DSMZ 11874), and Marinobacter hydrocarbonoclasticus (ATCC 49840) when grown on free phytol and 6,10,14-trimethylpentadecan-2-one, which are two isoprenoid compounds widely distributed in marine sediments (43, 49).
MATERIALS AND METHODS
Description of the strains.
Acinetobacter sp. strain PHY9, P. nautica (IP85/617), M. hydrocarbonoclasticus (ATCC 49840), and Marinobacter sp. strain CAB (DSMZ 11874) were used in this study. These strains had been isolated in our laboratory from hydrocarbon-polluted marine coastal sediments and foams collected from different sites in Golf of Fos (Mediterranean Sea, France) and generally deposited in culture collections. The identification and description of these strains can be found elsewhere (7, 24, 39, 43). For our experiments, the strains were maintained at −270°C in the presence of glycerol (20% [vol/vol]). Bench cultures were made on solid tryptose blood agar medium (43).
Growth conditions.
The basic growth medium consisted of autoclaved artificial seawater (6) (ASW) supplemented with iron sulfate (0.1 mM), potassium phosphate (0.33 mM), and 6,10,14-trimethylpentadecan-2-one or phytol (3 mM) as the source of carbon and energy (Φ medium). For experiments in which growth was N limited, ammonium chloride was added to the Φ medium to a concentration of 0.1 mM instead of 60 mM. Aerobic cultures were maintained in 250-ml Erlenmeyer flasks containing 50 ml of medium and agitated on a reciprocal shaker at 96 rpm. For each experiment, two flasks were inoculated: the first for estimation of substrate degradation and identification of the metabolites and the second for monitoring bacterial growth. Anaerobic growth experiments were performed with 125-ml serum flasks containing 70 ml of Φ medium supplemented with KNO3 (20 mM). Anaerobic conditions were obtained by flushing nitrogen through the flask for 1 h. Experiments carried out in the presence of mercuric chloride (10 mg liter−1) served as abiotic controls. (Autoclaved sterilization was avoided, since phytol can be easily dehydrated during such a treatment.)
Bacterial numeration.
Cultures at stationary phase were fixed with formaldehyde to a final concentration of 2% and refrigerated until needed. The samples were then diluted with filtered (0.2-μm pore size) (Whatman, no. 7182002) ASW and gently sonicated in a Branson 2200 ultrasonic bath for 5 min. The samples were then vortexed for 15 s and incubated with DAPI (4′,6-diamidine-2′-phenylindole dihydrochloride) (Boehringer Mannheim) to a final concentration of 2 μg ml−1 in darkness for 15 min before filtration on prestained (Irgalen black) membrane filters (0.2-μm pore size) (Millipore, GTBP). Bacteria were counted immediately with an epifluorescence microscope equipped with a mercury lamp, on randomly selected fields, until 20 fields or about 500 bacteria were counted.
Isolation and characterization of bacterial metabolites.
At the end of the growth period, the contents of the flasks were acidified with hydrochloric acid (pH 1) and continuously extracted with chloroform overnight. The extracts thus obtained were then dried with anhydrous Na2SO4, filtered, and concentrated by means of rotary evaporation. After evaporation of the solvent, the residues were taken up in ethyl acetate (1 ml per mg of residue) containing BSTFA [N,O-bis(trimethylsilyl)trifluoroacetamide] (100 μl), allowed to silylate at 40°C for 30 min, and analyzed by gas chromatography-electron impact mass spectrometry.
Structural assignments were based on interpretation of mass spectrometric fragmentations and confirmed by comparison of retention times and mass spectra with those of authentic compounds. Quantitative determinations were based on integrator data that had been calibrated by using external standards.
Gas chromatography-electron impact mass spectrometry analyses were carried out with an HP 5890 (series II plus) gas chromatograph connected to an HP 5972 mass spectrometer (Hewlett-Packard). The following operating conditions were used: a capillary column 30 m by 0.25 mm (inner diameter) was coated with HP 5 (Hewlett-Packard), and the oven temperature was programmed from 60 to 130°C at 30°C min−1 and then from 130 to 300°C at 4°C min−1. The carrier gas (He) pressure was maintained at 1.05 × 105 Pa until the end of the temperature program and then programmed from 1.04 × 105 to 1.5 × 105 Pa at 0.04 × 105 Pa min−1. The injector (on column) temperature was 50°C, the electron energy was 70 eV, the source temperature was 170°C, and the cycle time was 1.5 s.
Chemicals.
(E)-Phytol was isolated from a commercially available mixture of (Z and E)-phytol (Aldrich) by column chromatography on silica gel with n-hexane–ethyl acetate (19/1 [vol/vol]) as the eluant. 6,10,14-Trimethylpentadecan-2-one was produced by oxidation of phytol with KMnO4 in acetone (14). The syntheses of 6,10,14-trimethylpentadecan-2-ol, 4,8,12-trimethyltridecan-1-ol, 4,8,12-trimethyltridecanoic acid, 5,9,13-trimethyltetradecanoic acid, (Z)-3,7,11-trimethyldodec-2-enoic acid, phytenals, and (Z and E)-phytenic acids have been described previously (41, 43). Phytanic acid was obtained by hydrogenation of phytenic acids with Pd-CaCO3 as a catalyst. Pristanic acid was synthesized from dihydrophytol according to the method of Cason and Graham (14). The synthesis of 4,8-dimethylnonanoic acid required four steps: (i) hydrogenation of geranylacetone (Aldrich) in methanol with Pd-CaCO3 as a catalyst, (ii) oxidation of the resultant 6,10-dimethylundecan-2-one with perbenzoic acid in CH2Cl2 (Baeyer-Villiger reaction), (iii) alkaline hydrolysis of the resulting ester to 4,8-dimethylnonan-1-ol, and (iv) oxidation of this alcohol to the corresponding acid with chromic anhydride in acetic acid (35). Acetylation of 4,8,12-trimethyltridecan-1-ol with a mixture of pyridine and acetic anhydride (2:1) gave 4,8,12-trimethyltridecan-1-ol acetate. 4,8,12-Trimethyltridecan-4-olide had been previously isolated from phytadiene photooxidation products and characterized (27).
Wax esters were prepared from alcohols and acids by the procedure described by Gellerman et al. (25).
Determination of double-bond position in monounsaturated acid metabolites.
The method used to determine double-bond position involved the formation of diols by stereospecific oxidation of double bonds with OsO4 in pyridine-dioxane (1:8 [vol/vol]) (33) and subsequent analyses by gas chromatography-electron impact mass spectrometry of the silylated {dimethyl sulfoxide-BSTFA [5:1 (vol/vol)] for 12 h at 60°C} diols. The double-bond position was obtained from the mass fragmentation patterns of these derivatized compounds.
RESULTS AND DISCUSSION
Metabolism of 6,10,14-trimethylpentadecan-2-one.
Like Marinobacter sp. strain CAB (DSMZ 11874) (43) and Acinetobacter sp. strain PHY9 (41), P. nautica (IP85/617) and M. hydrocarbonoclasticus (ATCC 49840) were able to grow on 6,10,14-trimethylpentadecan-2-one (compound 1) as the sole carbon and energy source under aerobic conditions. After 10 days of growth, the extent of degradation ranged from 50 to 95%, depending on the strain used. Several isoprenoid metabolites were detected (Table 1). These compounds (which were not found in sterile controls) were formally identified by comparison of their retention times and mass spectra with those of reference compounds.
TABLE 1.
Metabolites detected during growth of the different strains on 6,10,14-trimethylpentadecan-2-one (compound 1)
| Pseudomonas nautica | Marinobacter hydrocarbonoclasticus | Marinobacter sp. strain CAB | Acinetobacter sp. strain PHY9 | ||
|---|---|---|---|---|---|
| Biodegradation (%) | 58 | 50 | 68 | 95 | |
| Bacterial numeration (cells/ml) | 2.2 × 108 | 3.6 × 106 | 7.6 × 109 | 3.1 × 1011 | |
| Metabolite | Compound(s) | Relative percentage of metabolite by organisma | |||
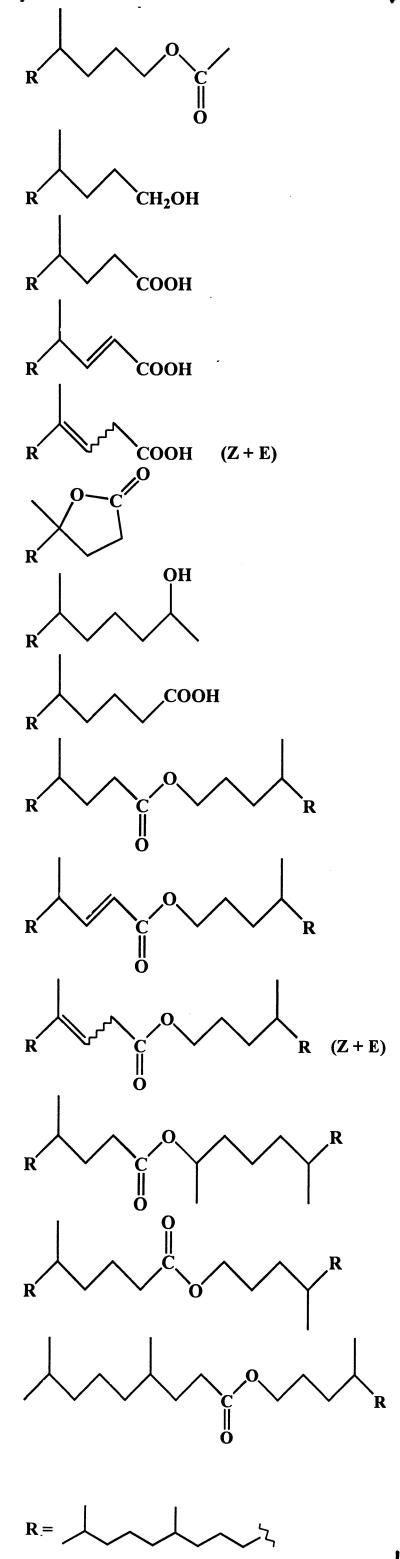 | |||||
| 2 | 1.78 | 0.30 | Traceb | 0.01 | |
| 3 | 0.53 | 2.30 | 0.06 | 0.16 | |
| 4 | 0.56 | 1.06 | 0.76 | 0.15 | |
| 5 | Trace | Trace | |||
| 6 and 7 | 0.02 | Trace | |||
| 8 | 0.61 | 0.90 | 0.60 | 0.05 | |
| 9 | Trace | Trace | Trace | ||
| 10 | Trace | 0.05 | |||
| 11 | 6.73 | 16.90 | 0.37 | 0.12 | |
| 12 | 0.41 | 0.58 | 0.02 | 0.01 | |
| 13 and 14 | 1.56 | 2.30 | 0.05 | 0.02 | |
| 15 | 0.43 | Trace | 0.10 | 0.01 | |
| 16 | 0.01 | ||||
| 17 | 0.30 | ||||
Relative percentages were based on the amount of degraded substrate (accuracy estimated to be ±0.01%).
Trace, relative percentage of <0.01.
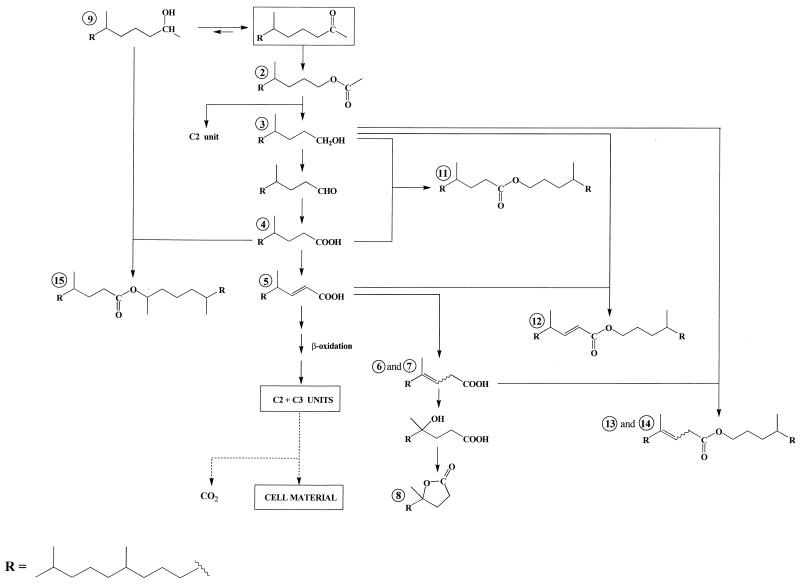
The production of compounds 2 to 5 can be attributed to an oxidation sequence involving the transformation of ketone 1 to 4,8,12-trimethyltridecan-1-ol acetate (compound 2). Subsequent hydrolysis of this ester affords 4,8,12-trimethyltridecan-1-ol (compound 3), which can be metabolized (after oxidation to the corresponding acid 4) via a classical β-oxidation sequence (Fig. 1). Such enzymatic oxidation of ketones to esters by microorganisms has been reported (9, 20, 37). This process is analogous to Baeyer-Villiger oxidation with peracids and seems to operate for each of the four strains studied (Table 1).
FIG. 1.
Proposed pathways for the production of isoprenoid wax esters during the metabolism of 6,10,14-trimethylpentadecan-2-one. (The processes for formation of esters 16 and 17 were omitted in order to simplify the scheme.)
If (E)-4,8,12-trimethyltridec-2-enoic acid (compound 5) constitutes a classical intermediate in the β-oxidation sequence, the presence of (Z and E)-4,8,12-trimethyltridec-3-enoic acids (compounds 6 and 7) is more surprising. The position of the double bonds of these acids was determined by gas chromatography-electron impact mass spectrometry after oxidation with OsO4 (33) and silylation. Subsequent hydration of these curious β,γ-unsaturated acids affords 4-hydroxy-4,8,12-trimethyltridecanoic acid, which lactonizes easily to 4,8,12-trimethyltridecan-4-olide (compound 8) (Fig. 1).
Small amounts of 6,10,14-trimethylpentadecan-2-ol (compound 9) were also formed in the experiments, probably by a dehydrogenase (38). The involvement of this “blind alley” pathway suggests that this process results from nonspecific enzyme activity that is not related specifically to 6,10,14-trimethylpentadecan-2-one (compound 1) degradation (32).
As previously described (41, 43), Acinetobacter sp. strain PHY9 and Marinobacter sp. strain CAB (DSMZ 11874) also produce 5,9,13-trimethyltetradecanoic acid (compound 10) after oxidation of the keto-terminal methyl group of the ketone 1 and subsequent decarboxylation of the resulting C18 α-keto acid.
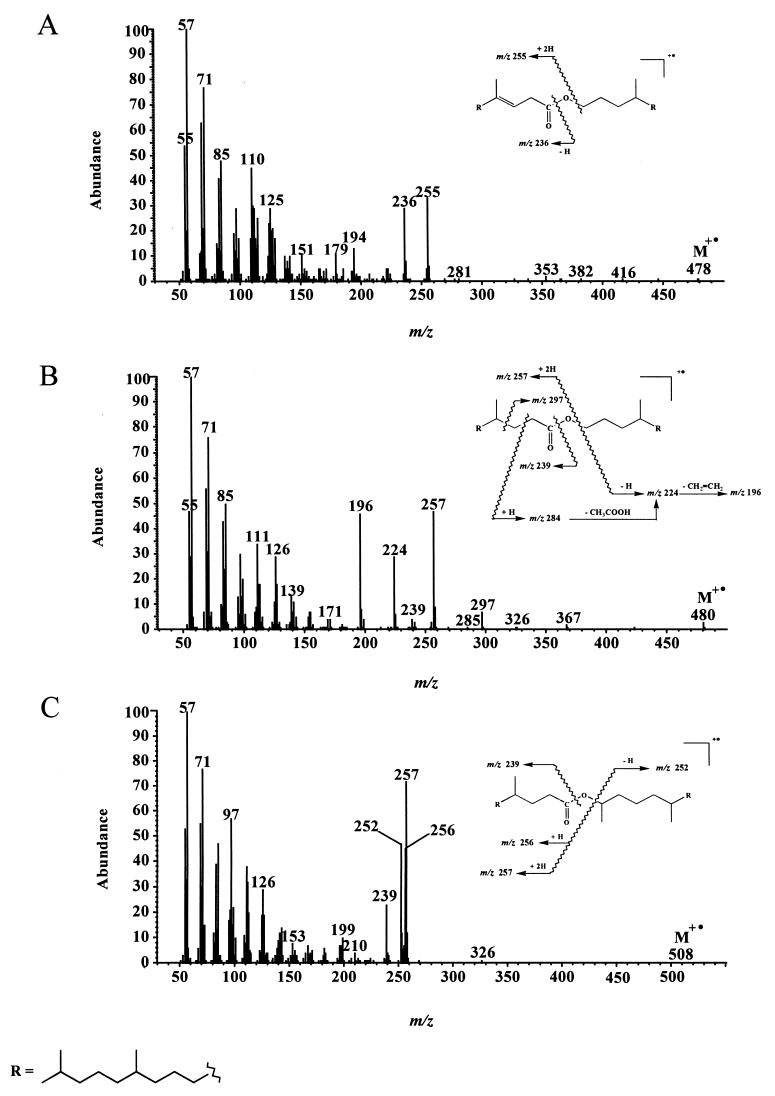
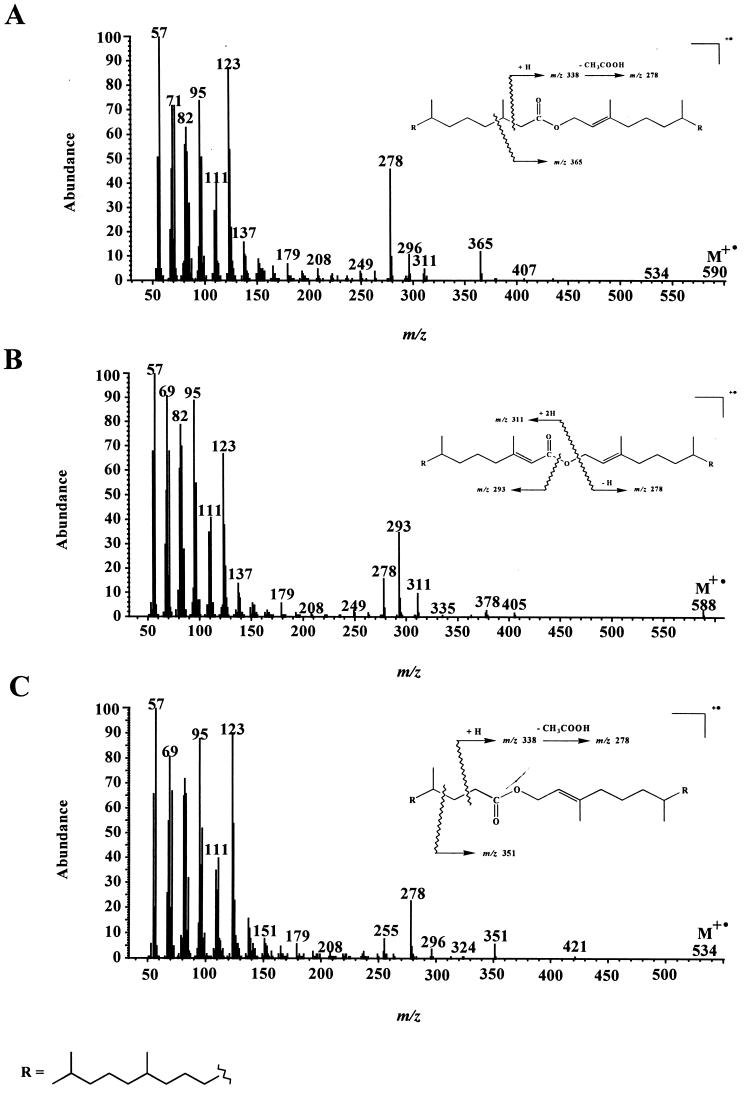
In addition to these different metabolites, we also detected several isoprenoid wax esters (compounds 11 to 17) (Table 1). Electron impact mass spectra of the most abundant esters are given in Fig. 2. Fragment ions of the general formula [RC(OH)⩵OH]+ formed after rearrangement of two hydrogen atoms (34) allow an easy characterization of the acid moiety of these esters, whereas the alcohol moiety generally gives [CnH2n]· + fragment ions (1). In contrast to compound 15, compounds 11 and 14 give notable molecular ions (M+ ·) (Fig. 2). The lack of M+ · in the electron impact mass spectrum of compound 15 can be attributed to the presence of a secondary carbon in the α position relative to the saturated oxygen atom, which strongly favored the cleavage of the molecule and thus decreased the abundance of the molecular ion. Identification of compounds 12 to 14, which were not synthesized, was based on the characterization of the respective isoprenoid alcohols and acids obtained after alkaline hydrolysis.
FIG. 2.
Electron impact mass spectra of (E)-4,8,12-trimethyltridecyl-4,8,12-trimethyltridec-3-enoate (compound 14) (A), 4,8,12-trimethyltridecyl-4,8,12-trimethyltridecanoate (compound 11) (B), and 6,10,14-trimethylpentadec-2-yl-4,8,12-trimethyl-tridecanoate (compound 15) (C).
Such wax esters had not been previously detected during studies of the aerobic biodegradation of 6,10,14-trimethylpentadecan-2-one (compound 1) (41, 43). This can be explained by (i) the relatively minor amounts of these compounds produced by Acinetobacter sp. strain PHY9 and Marinobacter sp. strain CAB (DSMZ 11874) (Table 1) and (ii) the use of a gas chromatograph having a heated splitless injector during the earlier studies, which strongly discriminates against such high-molecular-weight compounds. Previous attempts to analyze phytyl esters by gas chromatography have, in most cases, not been successful. Gellerman et al. (25) reported that phytyl esters decomposed when analyzed on OV-1 (methyl silicone)-packed columns, even though palmityl phytenate and esters of phytanic acid could be analyzed satisfactorily. Similar results were obtained by Withers and Nevenzel (51).
The structures of these esters suggest that they are formed by esterification between acids and alcohols produced during the metabolism of ketone 1 by the different bacterial strains (Fig. 1). The formation of these compounds implies the presence of a very active esterase system (especially in the case of P. nautica [IP85/617] and M. hydrocarbonoclasticus [ATCC 49840]), since the experimental conditions exclude a chemical esterification. Indeed, these esters were lacking in sterile controls, and they were not formed during the workup procedure. This active esterase system is probably the same as that involved during hydrolysis of 4,8,12-trimethyltricecan-1-ol acetate (compound 2) to 4,8,12-trimethyltridecan-1-ol (compound 3) and acetic acid. This hypothesis is supported by the fact that alcohol 3 constitutes the alcohol moiety of most of the wax esters identified (Table 1).
Although Marinobacter sp. strain CAB (DSMZ 11874) (43), P. nautica (IP85/617), and M. hydrocarbonoclasticus (ATCC 49840) are able to grow on 6,10,14-trimethylpentadecan-2-one (compound 1) under denitrifying conditions, they failed to produce isoprenoid wax esters under these conditions. This can be attributed to the fact that (i) the enzymatic oxidation of ketones by way of an ester intermediate cannot operate in the absence of oxygen, and (ii) the esterases involved during this process are generally inducible enzymes (9).
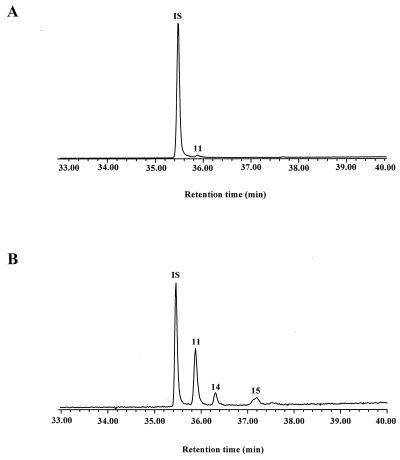
It is generally considered that wax esters constitute storage energy components of marine bacteria (3). Previous work (22) has demonstrated that the amount of wax esters produced increases considerably in N-limited cultures under conditions of low growth rate, where carbon and energy are in excess. In the case of P. nautica (IP85/617), the wax ester content increased by approximately 20-fold in the N-limited culture compared with that in the nonlimited one (Fig. 3), while the growth yield decreased slightly (9.5 × 106 cells/mg in the nonlimited culture and 3.3 × 106 cells/mg in the N-limited culture). It is interesting to note that the ammonium concentration used in the N-limited culture (0.1 mM) corresponds to conditions often found in marine sediments (36). We suggest, therefore, that the formation of isoprenoid wax esters might be favored in such environments when 6,10,14-trimethylpentadecan-2-one or phytol is available.
FIG. 3.
Partial total ion chromatograms showing the wax ester region of the extracts obtained after growth of P. nautica on 6,10,14-trimethylpentadecan-2-one (compound 1) with ammonium ion concentrations of 56 mM (A) and 0.1 mM (B). IS, internal standard (triacontane).
Metabolism of phytol.
All four strains were able to grow on phytol as the sole source of carbon and energy under aerobic conditions, but this compound appeared to be a poorer substrate for these organisms than 6,10,14-trimethylpentadecan-2-one. This relative biological recalcitrance can be attributed to the presence of a methyl group on the carbon 3 of phytol, which prevents classical β-oxidation, requiring an additional strategy, such as α-oxidation (26) or β-alkyl group removal (β-decarboxymethylation) (13, 46), to allow oxidation to proceed. Acinetobacter sp. strain PHY9 failed to produce detectable amounts of isoprenoid wax esters during growth on this substrate. This is in good agreement with the results described above, since this organism has the best cell yield and the lowest production of wax esters when grown on 6,10,14-trimethylpentadecan-2-one (Table 1). The first step of the bacterial degradation of phytol involves the transient production of the corresponding aldehyde (E)-3,7,11,15-tetramethylhexadec-2-enal (phytenal) (compound 18). This labile compound can be converted quickly and abiotically in seawater to 6,10,14-trimethylpentadecan-2-one (compound 1) (40). The production of this ketone involves addition of water to the activated double bond of phytenal followed by a retro-aldol reaction. In support of this view, we detected ketone 1 and some of its metabolites (compounds 3, 4, and 11 [described above]) after growth of the three strains on phytol (Table 2).
TABLE 2.
Metabolites detected during growth of the different strains on phytol
| Pseudomonas nautica | Marinobacter hydrocarbonoclasticus | Marinobacter sp. strain CAB | ||
|---|---|---|---|---|
| Biodegradation (%) | 26 | 75 | 63 | |
| Bacterial numeration (cells/ml) | <3 × 104 | <3 × 104 | 3.5 × 107 | |
| Metabolite | Compound(s) | Relative percentage of metabolite by organisma | ||
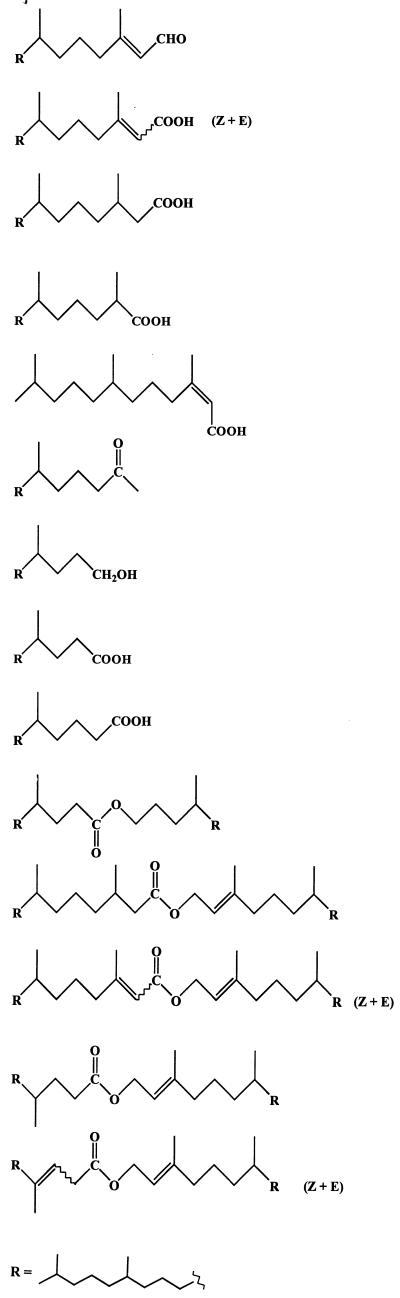 | ||||
| 18 | 5.89 | 1.16 | Traceb | |
| 19 and 20 | 0.25 | 0.13 | 0.14 | |
| 21 | 0.10 | 0.11 | 0.24 | |
| 22 | Trace | Trace | Trace | |
| 23 | 0.17 | Trace | ||
| 1 | 0.18 | 0.29 | 0.79 | |
| 3 | Trace | 0.26 | 0.62 | |
| 4 | 0.09 | 0.05 | 0.07 | |
| 10 | Trace | Trace | 0.01 | |
| 11 | 0.43 | 0.02 | 0.04 | |
| 24 | 3.71 | 0.39 | 0.25 | |
| 25 and 26 | Trace | 0.11 | 0.15 | |
| 27 | 0.41 | 0.43 | ||
| 28 and 29 | 0.05 | 0.12 | ||
Relative percentages were based on the amount of degraded substrate (accuracy estimated to be ±0.01%).
Trace, relative percentage of <0.01.
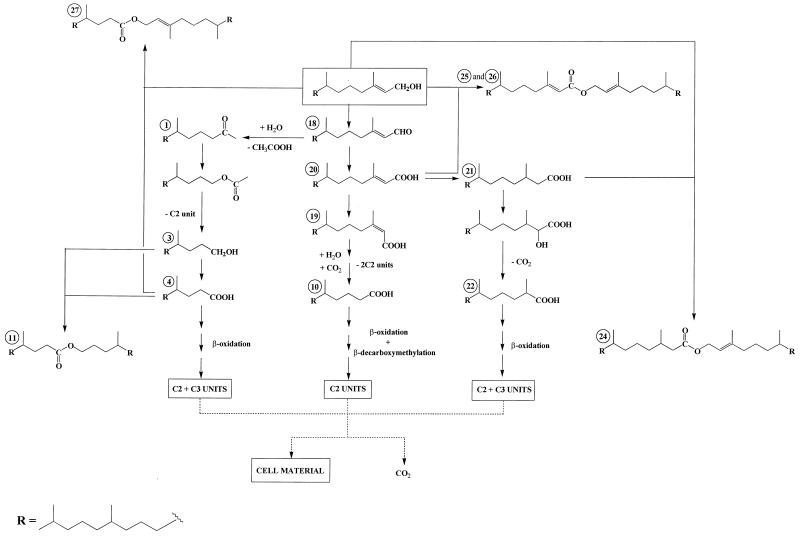
Concurrently, the phytol can be metabolized via (E)-phytenic acid (compound 20) by two different pathways (Fig. 4). The first pathway involves isomerization to (Z)-phytenic acid (compound 19) and subsequent alternating β-decarboxymethylation and β-oxidation sequences. The ability of microorganisms to carry out β-decarboxymethylation was originally established by Seubert (46). The net effect of this process is to replace a methyl substituent (which prevents β-oxidation) with a carbonyl oxygen (13). In the case of P. nautica (IP85/617) and Marinobacter sp. strain CAB (DSMZ 11874), the involvement of such a mechanism is supported by the detection of only the Z isomer of 3,7,11-trimethyldodec-2-enoic acid (compound 23) (Table 2). Activation of allylic methyl groups via carboxylation occurs only in the case of the Z isomers (13).
FIG. 4.
Proposed pathways for the production of isoprenoid wax esters during the metabolism of phytol. (The processes for formation of esters 28 and 29 were omitted in order to simplify the scheme.)
The second pathway consists of a hydrogenation to 3,7,11,15-tetramethylhexadecanoic acid (phytanic acid) (compound 21), followed by α-oxidation to 2-hydroxy-3,7,11,15-tetramethylhexadecanoic acid, which is then converted to 2,6,10,14-tetramethylpentadecanoic acid (pristanic acid) (compound 22) by decarboxylation. The pristanic acid (compound 22) thus formed may be subsequently metabolized via classical β-oxidation reactions. This pathway, which was previously proposed by Gillan et al. (26) during a study of the aerobic degradation of phytol by bacteria isolated from surface sediments, operates in the case of the three strains studied (Table 2).
After 10 days of growth, we also detected several isoprenoid wax esters (compounds 24 to 29 in Table 2) arising from the esterification of phytol with its acidic metabolites (Fig. 4). The electron impact mass spectra of some of these compounds are given in Fig. 5. The mass spectrometric characterization of the acid moiety of phytyl esters is not easy, since the double bond of phytol considerably decreases the intensity of the double hydrogen atom rearrangement. In contrast, the fragment ion at m/z 278 is of great diagnostic value for the characterization of the phytyl alcohol chain. The presence of a notable molecular peak only in the electron impact mass spectrum of compound 26 (Fig. 5) seems to indicate that the abundance of M· + of these wax esters increases with increased unsaturation. Esterification activity in these cultures was not confined to 4,8,12-trimethyltridecan-1-ol (compound 3) and 6,10,14-trimethylpentadecan-2-ol (compound 9); phytol appeared also to be an excellent substrate. This can be attributed to the well-known overlapping substrate selectivity of esterases involved during the enzymatic oxidation of ketones by way of an ester intermediate (47). The ability of phytol to give acyl esters is supported by the detection of phytyl esters in several plants (2, 12, 18, 51), which must also possess active esterase systems.
FIG. 5.
Electron impact mass spectra of (E)-3,7,11,15-tetramethylhexadec-2-enyl-3,7,11,15-tetramethylhexadecanoate (phytylphytanate) (compound 24) (A), (E,E)-3,7,11,15-tetramethylhexadec-2-enyl-3,7,11,15-tetramethylhexadec-2-enoate (phytylphytenate) (compound 26) (B), and 3,7,11,15-tetramethylhexadec-2-enyl-4,8,12-trimethyltridecanoate (compound 27) (C).
Phytyl esters have been detected in a few recent sediments (8, 15), but few data are available. Proposed sources have included mosses (18), bryophytes (12, 25), dinoflagellates (51), or zooplankton (45), but in most cases, no evidence was presented to substantiate this. The results obtained in the present study show that the aerobic bacterial degradation of phytol might constitute another potential source of these compounds in sediments. It is generally considered that the major proportion of phytol released from chlorophyll a is degraded before it can be incorporated into the anoxic region of the sediments (30). Consequently, aerobic bacterial degradation processes, which must play an important role in this disappearance, could produce significant amounts of phytyl esters in the water column and in the oxic zone of the sediments.
During incubation of [U-14C]phytol in sediments, Brooks and Maxwell (10) observed the formation of phytyl esters and suggested that esterification of the introduced phytol is brought about by enzymatic processes, possibly microbial. Our results strongly support this hypothesis and are also in good agreement with those of Volkman and Maxwell (49), who said in their review of acyclic isoprenoid compounds that esterification may be a significant process in microbially active sediments.
Phytyl esters have been reported in cultures of a few marine microalgae (21, 51), and small amounts are also present in senescent cultures of the green microalga Tetraselmis chui (48a). These limited examples suggest that microalgae are not likely to be major sources of phytyl or other wax esters in sediments. However, there are a variety of other sources, as discussed previously, and amounts can be large in some zooplankton. Zooplankton are a major source of wax esters in sedimenting particles in marine ecosystems (50), and even though phytyl esters have yet to be reported, one might still expect significant contributions to some sediments. In addition, our results suggest that they are also formed in sediments by bacterial action and that this process might be enhanced at low ammonium concentrations. Despite these observations, phytylphytenate and phytylphytanate are generally present in sediments at relatively low concentrations. We believe that this is probably due to their rapid hydrolysis during early diagenesis. Indeed, de Leeuw et al. (18) analyzed a lake sediment which received inputs of the moss Fontinalis antipyretica (in which phytylphytenate is a major wax ester) and failed to detect this ester. They concluded that a rapid hydrolysis of the ester occurred in the detritus, resulting in its absence in the underlying sediment. However, it is interesting to note that, despite the occurrence of these hydrolytic processes, Cranwell (15) detected phytylphytenate in lacustrine sediments up to 40,000 years old. Similarly, esters of sterols are thought to be more stable in sediments than the corresponding free compounds (19), so that, clearly under some conditions, esterification can enhance the preservation potential of labile compounds such as phytol.
Conclusions.
The production of linear wax esters in bacteria is well known (44) and has been demonstrated during the growth of bacteria on n-alkanes (4) and oleic acid (3, 22, 31). However, to our knowledge, this study is the first report of the production of wax esters when marine bacteria are grown on isoprenoid substrates. This property seems to be a characteristic of bacteria able to oxidize methyl ketones by way of an ester intermediate (9, 23).
There is a demonstrable need to identify bacterial metabolites that have sufficient structural specificity to act as biological markers for microbial degradation in the aquatic environment. Consequently, our results suggest that it would be useful to search for isoprenoid esters, such as compounds 11 to 17 and 27 to 29 (Tables 1 and 2, respectively), in marine sediments and particulate matter samples.
ACKNOWLEDGMENTS
This work was supported by grants from the Centre National de la Recherche Scientifique and the Elf Aquitaine Society (Research Groupment HYCAR 1123).
REFERENCES
- 1.Aasen A J, Hofstetter H H, Iyengar B T R, Holman R T. Identification and analysis of wax esters by mass spectrometry. Lipids. 1971;6:502–507. doi: 10.1007/BF02531236. [DOI] [PubMed] [Google Scholar]
- 2.Ahlquist L, Bergström G, Liljenberg C. Acyclic diterpene alcohols: occurrence and synthesis of geranylcitronellol, phytol and geranylgeraniol. Prog Chem Fats Other Lipids. 1977;16:231–255. doi: 10.1016/0079-6832(78)90046-0. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez H M, Pucci O H, Steinbüchel A. Lipid storage compounds in marine bacteria. Appl Microbiol Biotechnol. 1997;47:132–139. [Google Scholar]
- 4.Bacchin P, Robertiello A, Viglia A. Identification of n-decane oxidation products in Corynebacterium cultures by combined gas chromatography-mass spectrometry. Appl Microbiol. 1974;28:737–741. doi: 10.1128/am.28.5.737-741.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker E W, Smith G D. Pleistocene changes in chlorophyll pigments. In: Tissot B, Bienner F, editors. Advances in organic geochemistry 1973. Paris, France: Editions Technip; 1974. pp. 649–660. [Google Scholar]
- 6.Baumann P, Baumann L. The gram negative eubacteria genus Protobacterium, Beneckae, Alteromonas, Pseudomonas and Alcaligenes. In: Mortimer P S, et al., editors. The prokaryotes: a handbook on habitats, isolation and identification of bacteria. Berlin, Germany: Springer-Verlag; 1981. pp. 1302–1330. [Google Scholar]
- 7.Bonin P, Gilewicz M, Bertrand J-C. Denitrification by a marine bacterium, Pseudomonas nautica strain 617. Ann Inst Pasteur Microbiol. 1987;138:371–383. doi: 10.1016/0769-2609(87)90125-6. [DOI] [PubMed] [Google Scholar]
- 8.Boon J J, de Leeuw J W. The analysis of wax esters, very long mid-chain ketones and sterol ethers isolated from Walvis Bay diatomaceous ooze. Mar Chem. 1979;7:117–132. [Google Scholar]
- 9.Britton L N, Brand J M, Markovetz A J. Source of oxygen in the conversion of 2-tridecanone to undecyl acetate by Pseudomonas cepacia and Nocardia sp. Biochim Biophys Acta. 1974;369:45–49. doi: 10.1016/0005-2760(74)90190-8. [DOI] [PubMed] [Google Scholar]
- 10.Brooks P W, Maxwell J R. Early stage fate of phytol in a recently-deposited lacustrine sediment. In: Tissot B, Bienner F, editors. Advances in organic geochemistry 1973. Paris, France: Editions Technip; 1974. pp. 977–991. [Google Scholar]
- 11.Brooks P W, Maxwell J R, Patience R L. Stereochemical relationships between phytol and phytanic acid, dihydrophytol and C18 ketone in recent sediments. Geochim Cosmochim Acta. 1978;42:1175–1180. [Google Scholar]
- 12.Buchanan M S, Hashimoto T, Asakawa Y. Phytyl esters and phaeophytins from the hornwort Megaceros flagellaris. Phytochemistry. 1996;41:1373–1376. [Google Scholar]
- 13.Cantwell S G, Lau E P, Watt D S, Fall R R. Biodegradation of acyclic isoprenoids by Pseudomonas species. J Bacteriol. 1978;135:324–333. doi: 10.1128/jb.135.2.324-333.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cason J, Graham D W. Isolation of isoprenoid acids from a California petroleum. Tetrahedron. 1965;21:471–483. [Google Scholar]
- 15.Cranwell P A. Esters of acyclic and polycyclic isoprenoid alcohols: biochemical markers in lacustrine sediments. Org Geochem. 1986;10:891–896. [Google Scholar]
- 16.Csupor L. Das Phytol in vergilbten Blättern. Planta Med. 1971;19:37–41. doi: 10.1055/s-0028-1099802. [DOI] [PubMed] [Google Scholar]
- 17.de Leeuw J W, Correia V A, Schenck P A. On the decomposition of phytol under simulated geological conditions and in top layer of natural sediments. In: Tissot B, Bienner F, editors. Advances in organic geochemistry 1973. Paris, France: Editions Technip; 1974. pp. 993–1004. [Google Scholar]
- 18.de Leeuw J W, Rijpstra W I C, Boon J J, de Lange F, Schenck P A. The relationship between lipids from Fontinalis antipyretica, its detritus and the underlying sediment: the fate of wax esters and sterol esters. In: Golterman H L, editor. Interactions between sediments and freshwater. The Hague, The Netherlands: Junk; 1977. pp. 141–147. [Google Scholar]
- 19.de Leeuw J W, Rijpstra W I C, Schenck P A, Volkman J K. Free, esterified and residual bound sterols in Black Sea Unit I sediments. Geochim Cosmochim Acta. 1983;47:455–465. [Google Scholar]
- 20.Donoghue N A, Norris D B, Trudgill P W. The purification and properties of cyclohexanone oxygenase from Nocardia globurela CL1 and Acinetobacter NCIB 9871. Eur J Biochem. 1976;63:175–192. doi: 10.1111/j.1432-1033.1976.tb10220.x. [DOI] [PubMed] [Google Scholar]
- 21.Findlay J A, Patil A D. Antibacterial constituents of the diatom Navicula delognei. J Nat Prod. 1984;47:815–818. doi: 10.1021/np50035a010. [DOI] [PubMed] [Google Scholar]
- 22.Fixter L M, Nagi M N, McCormack J G, Fewson C A. Structure, distribution and function of wax esters in Acinetobacter calcoaceticus. J Gen Microbiol. 1986;132:3147–3157. [Google Scholar]
- 23.Forney F W, Markovetz A J. Subterminal oxidation of aliphatic hydrocarbons. J Bacteriol. 1970;102:281–282. doi: 10.1128/jb.102.1.281-282.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauthier M J, Lafay B, Christen R, Fernandez L, Acquaviva M, Bonin P, Bertrand J-C. Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. Int J Syst Bacteriol. 1992;42:568–576. doi: 10.1099/00207713-42-4-568. [DOI] [PubMed] [Google Scholar]
- 25.Gellerman J L, Anderson W H, Schlenk H. Synthesis and analysis of phytyl and phytenoyl wax esters. Lipids. 1975;10:656–661. doi: 10.1007/BF02532757. [DOI] [PubMed] [Google Scholar]
- 26.Gillan F T, Nichols P D, Johns R B, Bavor H J. Phytol degradation by marine bacteria. Appl Environ Microbiol. 1983;45:1423–1428. doi: 10.1128/aem.45.5.1423-1428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossi V. Formation et devenir des phytadiènes dans le milieu marin: implication de ces hydrocarbures dans les processus de dégradation des chlorophylles à chaîne phytyle. Ph.D. thesis. Marseille, France: University of Aix-Marseille II; 1996. [Google Scholar]
- 28.Hansen R P. Phytol: its metabolic products and their distribution. A review. N Z J Sci. 1980;23:259–275. [Google Scholar]
- 29.Ikan R, Baedecker M J, Kaplan I R. C18-isoprenoid ketone in recent marine sediment. Nature. 1973;244:154–155. [Google Scholar]
- 30.Johns R B, Gillan F T, Volkman J K. Early diagenesis of phytyl esters in a contemporary temperate intertidal sediment. Geochim Cosmochim Acta. 1980;44:183–188. [Google Scholar]
- 31.Kaneshiro T, Nakamura L K, Nicholson J J, Bagby M O. Oleyl oleate and homologous wax esters synthesized coordinately from oleic acid by Acinetobacter and coryneform strains. Curr Microbiol. 1996;32:336–342. doi: 10.1007/s002849900060. [DOI] [PubMed] [Google Scholar]
- 32.Klug M J, Markovetz A J. Utilization of aliphatic hydrocarbons by micro-organisms. Adv Microbiol Physiol. 1971;5:1–43. doi: 10.1016/s0065-2911(08)60404-x. [DOI] [PubMed] [Google Scholar]
- 33.McCloskey J A, McClelland M J. Mass spectra of O-isopropylidene derivatives of unsaturated fatty esters. J Am Chem Soc. 1965;87:5090–5093. [Google Scholar]
- 34.McLafferty F W. Mass spectra of ethyl and higher esters. In: Turro N J, editor. Interpretation of mass spectra. Mill Valley, Calif: University Science Books; 1980. pp. 203–205. [Google Scholar]
- 35.Mize C E, Avigan J, Steinberg D, Pittman R C, Fales H M, Milne G W A. A major pathway for the mammalian oxidative degradation of phytanic acid. Biochim Biophys Acta. 1969;176:720–739. doi: 10.1016/0005-2760(69)90253-7. [DOI] [PubMed] [Google Scholar]
- 36.Omnes P. Mise en évidence de la voie de réduction dissimilatrice du nitrate en ammonium. Application in situ aux systèmes particulaires et sédimentaires en Méditerranée. Ph.D. thesis. Marseille, France: University of Aix-Marseille II; 1996. [Google Scholar]
- 37.Patrick M A, Dugan P R. Influence of hydrocarbons and derivatives on the polar lipid fatty acids of an Acinetobacter isolate. J Bacteriol. 1974;119:76–81. doi: 10.1128/jb.119.1.76-81.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platen H, Schink B. Anaerobic degradation of acetone and higher ketones via carboxylation by newly isolated denitrifying bacteria. J Gen Microbiol. 1989;135:883–891. doi: 10.1099/00221287-135-4-883. [DOI] [PubMed] [Google Scholar]
- 39.Rambeloarisoa E, Rontani J-F, Giusti G, Duvnjak Z, Bertrand J-C. Degradation of crude oil by a mixed culture of bacteria isolated from surface foams. Mar Biol. 1984;83:69–81. [Google Scholar]
- 40.Rontani J-F, Combe I, Giral P J-P. Abiotic degradation of free phytol in the water column: a new pathway for the production of acyclic isoprenoids in the marine environment. Geochim Cosmochim Acta. 1990;54:1307–1313. [Google Scholar]
- 41.Rontani J-F, Acquaviva M. The aerobic bacterial metabolism of phytol in seawater: temperature dependence of an abiotic step and its consequences. Chemosphere. 1993;26:1513–1525. [Google Scholar]
- 42.Rontani J-F, Raphel D, Cuny P. Early diagenesis of intact and photooxidized chlorophyll phytyl chain in a recent temperate sediment. Org Geochem. 1996;24:825–832. [Google Scholar]
- 43.Rontani J-F, Gilewicz M J, Michotey V, Zheng T L, Bonin P C, Bertrand J-C. Aerobic and anaerobic metabolism of 6,10,14-trimethylpentadecan-2-one by a denitrifying bacterium isolated from marine sediments. Appl Environ Microbiol. 1997;63:636–643. doi: 10.1128/aem.63.2.636-643.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell N J, Volkman J K. The effect of growth temperature on wax ester composition in the psychrophilic bacterium Micrococcus cryophilis ATCC 15174. J Gen Microbiol. 1980;118:131–141. [Google Scholar]
- 45.Sargent J R, Falk-Petersen S. Ecological investigations on the zooplankton community in Balsfjorden, Northern Norway. Mar Biol. 1981;62:131–137. [Google Scholar]
- 46.Seubert W. Degradation of isoprenoid compounds by microorganisms. I. Isolation and characterization of an isoprenoid-degrading bacterium, Pseudomonas citronellolis n. sp. J Bacteriol. 1960;79:426–434. doi: 10.1128/jb.79.3.426-434.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shum A C, Markovetz A J. Specificity and induction of undecyl acetate esterase from Pseudomonas cepacia grown on 2-tridecanone. J Bacteriol. 1974;118:890–897. doi: 10.1128/jb.118.3.890-897.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simoneit B R T, Burlingame A L. Ketones derived from the oxidative degradation of Green River formation oil shale kerogen. In: Tissot B, Bienner F, editors. Advances in organic geochemistry 1973. Paris, France: Editions Technip; 1974. pp. 191–201. [Google Scholar]
- 48a.Volkmann, J. K. Unpublished results.
- 49.Volkman J K, Maxwell J R. Acyclic isoprenoids as biological markers. In: Johns R B, editor. Biological markers in the sedimentary record. Amsterdam, The Netherlands: Elsevier; 1986. pp. 1–46. [Google Scholar]
- 50.Wakeham S G, Farrington J W, Volkman J K. Fatty acids, wax esters, triacylglycerols and alkyldiacylglycerols associated with particles collected in sediment traps in the Peru upwelling. In: Bjorøy M, et al., editors. Advances in organic geochemistry 1981. Chichester, United Kingdom: John Wiley and Sons; 1983. pp. 185–197. [Google Scholar]
- 51.Withers N W, Nevenzel J C. Phytyl esters in a marine dinoflagellate. Lipids. 1977;12:989–993. [Google Scholar]