Abstract
BACKGROUND
The role of multidetector computed tomography (MDCT) in patients with acute infectious colitis is still unclear.
AIM
To examine the usefulness of MDCT in distinguishing the etiology of acute infectious colitis.
METHODS
Overall, 244 patients who met the criteria for acute infectious colitis and visited the Hospital from February 2015 to 2018 were retrospectively enrolled and divided into two groups (bacterial: 204, viral: 40) according to causes of acute colitis, based on stool PCR. Eleven MDCT parameters, including wall thickening, submucosal edema, mucosal enhancement, serosa involvement, empty colon sign, small bowel involvement, comb sign, continuous distribution, accordion sign, mucosal thickening, and lymph node enlargement, were constructed in a blinded fashion.
RESULTS
MDCT parameters of wall thickening (OR: 13.60; 95%CI: 5.80–31.88; P < 0.001), submucosal edema (OR: 36.08; 95%CI: 13.54–96.13; P < 0.001), mucosal enhancement (OR: 22.55; 95%CI: 9.28–54.81; P < 0.001), serosal involvement (OR: 14.50; 95%CI: 3.33–63.23; P < 0.001), empty colon sign (OR: 6.68; 95%CI: 2.44–18.32; P < 0.001), continuous distribution (OR: 24.09; 95%CI: 9.38–61.90; P < 0.001), accordion sign (OR: 9.02; 95%CI: 1.12–72.35; P = 0.038), mucosal thickening (OR: 46.41; 95%CI: 10.38–207.51; P < 0.001), and lymph node enlargement (OR: 4.39; 95%CI: 1.22–15.72; P = 0.023) were significantly associated with bacterial colitis. At least one positive finding in four CT outcomes (submucosal edema, mucosal enhancement, continuous distribution, mucosal thickening) in summer showed a high probability of bacterial colitis (sensitivity, 41.67; specificity, 92.50; OR: 24.95).
CONCLUSION
MDCT provides many clues that can be useful in suggesting a specific etiology of acute infectious colitis.
Keywords: Colitis, Multidetector computed tomography, Differential diagnosis, Bacterial infections, Viral infections
Core Tip: The main purpose of this study was to investigate if computed tomography (CT) is valuable in discriminating bacterial colitis from viral colitis. Multidetector CT (MDCT) parameters of wall thickening, submucosal edema, mucosal enhancement, serosal involvement, empty colon sign, continuous distribution, accordion sign, mucosal thickening, and lymph node enlargement may be suggestive of bacterial colitis. At least one positive finding in four MDCT parameters (submucosal edema, mucosal enhancement, continuous distribution, mucosal thickening) in the summer season is suggestive of a bacterial infection.
INTRODUCTION
Acute infectious colitis is a common health problem globally. Although many cases of colitis are self-limiting and can be managed by adequate conservative treatment, such as hydration or electrolyte correction, antibiotics may be applied in certain situations (i.e., sepsis with enteric fever, immunocompromised patient, internationally-travelled patient)[1]. Additionally, it is difficult to diagnose and manage some cases complicated with inflammatory bowel disease, ischemic colitis, or medication-related colitis. Therapy customized to the etiology of colitis should be applied as soon as possible. The earlier customized therapy starts, the better the expected prognosis[1,2].
Stool culture is generally the definitive diagnostic tool for bacterial colitis. Because most viruses that cause infectious colitis cannot be cultured, electron microscopy (EM) examination and immunoassay have been used for viruses, such as norovirus, rotavirus, and adenovirus. Microscopic examination is still routinely used for parasite identification. However, these examination methods require considerable expertise, are labor intensive, and are very time-consuming[3,4]. Multiplex molecular PCR panels have recently been developed for the detection of gastrointestinal pathogens directly from clinical stool samples. They have a high diagnostic performance (sensitivity = 94.5%, specificity = 99%) and take only several days[5-8].
Acute infectious colitis usually shows non-specific clinical manifestations and physical signs. Definitive pathogens of infectious colitis can be proven by culture, EM, immunoassay, and microscopy.
However, clinicians need blood and imaging tests to investigate the severity and extent, as well as to exclude other causes of abdominal pain. For blood tests, C-reactive protein and WBC, which can detect infection or inflammation, are mainly used. In particular, C-reactive protein may help differentiate pathogens[9]. Multidetector CT (MDCT) is now being universally used as the first imaging modality for the evaluation of patients with non-specific abdominal pain, including colitis. As a result, many cases of colitis have been diagnosed using MDCT. CT has the merits of broad availability and ease of performance[10].
A radiologist can help to confirm the diagnosis and evaluate the severity, as well as to assess distributing pattern, morphology of the wall, pericolonic soft tissues and adjacent structures, often the key for differentiating specific causes[11]. The purpose of this study is to discuss and illustrate MDCT features that offer important diagnostic clues for suggesting a specific etiology of acute colitis. In particular, we focus on infectious colitis, with the aim to differentiate the diagnosis of bacterial colitis from viral colitis. We examined the correlation between MDCT findings and PCR results.
MATERIALS AND METHODS
MDCT technique
MDCT examinations were conducted on the following two multidetector devices: MDCT 64 sections (Brilliance64, Philips) and MDCT 64 sections (LightSpeed volume CT, General Electric Medical Systems).
All patients were examined in the supine position, from the dome of the liver to the level of the perineum, to cover the entire course of the gastrointestinal tract. We routinely administered 1.5 mL of contrast matter (Optiray, Ultravist) per kg of body weight in patients without renal disorder. Non-ionic iodinated contrast matter at a concentration of 30 g of iodine per 100 mL was administered at a rate of 3 mL/s through an 18-gauge (G) venous catheter. Scanning was started 70 s after the beginning of contrast matter injection. This interval was consistent with the mesenteric phase of abdominal imaging and allowed us to evaluate the status of the colon wall in the best condition. This phase also offered comprehensive information about the abdomen and pelvis, including abdominal vessels.
MDCT results were retrospectively analyzed and evaluated independently by two residents in training in the radiology department. One board-certified radiologist with specialty in abdominal imaging reviewed and confirmed the CT images. All contrast-enhanced CT (CECT) images were evaluated in the context of the hospital’s picture archiving and communication system (PACS). Measurements were conducted using an electronic ruler. The colon was divided into four anatomic segments: Rectosigmoid colon, descending colon, transverse colon, and ascending colon including cecum.
The reviewers were blinded to the patients’ clinical histories and final diagnoses. For further analysis, disagreements were resolved through discussions until a consensus was achieved.
MDCT image analysis
The following 11 MDCT parameters were evaluated and described: bowel wall thickening, submucosal edema, mucosal hyperenhancement, serosal involvement (fat stranding), small bowel involvement, comb sign, continuous distribution (≥ 6 cm), accordion sign, mucosal thickening, lymph node enlargement (short diameter > 10 mm), and empty colon sign. We recoded all CT findings to binary values.
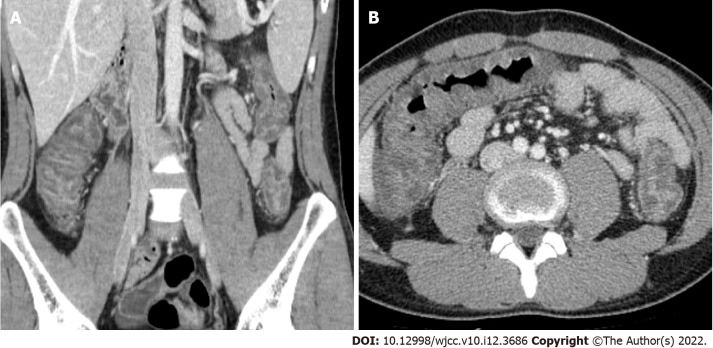
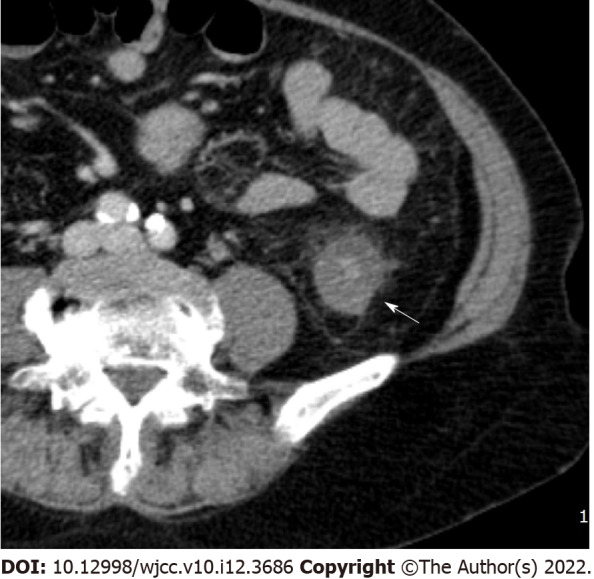
Bowel wall thickening was classified into normal (< 5 mm) and abnormal (≥ 5 mm)[12,13]. Submucosal edema and mucosal hyperenhancement indicate inflammatory changes. The target sign means stratification of the three layers: mucosa, submucosa, and serosa. The mucosa and serosa were enhanced, whereas the submucosa remained hypoattenuated because of edema[14]. Serosal involvement (fat stranding) (Figure 1) indicates an acute inflammatory process and serous fluid leakage. Small bowel involvement was defined as positive when any segment of the small bowel wall was thickened. The comb sign (Figure 2) refers to the hypervascular appearance of the mesentery because of fibro-fatty proliferation and perivascular inflammatory infiltration in the distended intestinal arcades[15]. Continuous distribution indicates that bowel wall and pericolonic change invade continuously without skipping areas. The accordion sign (Figure 3) comprises of markedly thickened haustral folds, low attenuation from mucosal and submucosal edema, and irregular mucosal contour with polypoid protrusions[16]. The appearance of the colon may resemble that of an accordion. Mucosal thickening and lymph node enlargement (short diameter > 10 mm) (Figure 4) were also evaluated as present or absent. The empty colon sign (Figure 5) which represents the complete emptiness of the colonic lumen, such as absence of gas, fluid or feces, was graded as either absent or present[17]. Small bowel involvement was checked when small bowel wall thickening, dilatation, or both were seen[18].
Figure 1.
Serosal involvement. Enhanced multidetector computed tomography axial image in portal venous phase shows wall thickening with submucosal edema and pericolic fat stranding (arrow) in descending colon.
Figure 2.
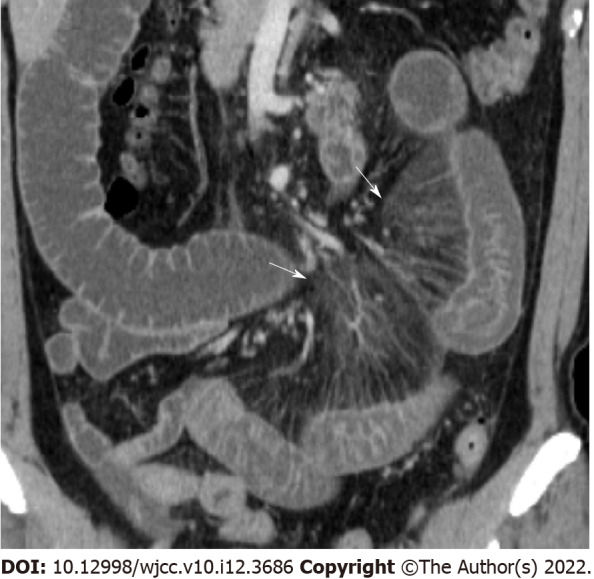
Comb sign. Coronal reconstructed image shows perivascular inflammatory infiltration (arrow) that forms linear densities on the mesenteric side of the affected segments of left small bowel. Fluid distended bowel is also noted.
Figure 3.
Accordion sign. Contrast-enhanced multidetector computed tomography coronal reconstructed image (A) and axial image (B) show hyperemic enhancing mucosa stretched over markedly thickened submucosal folds with irregular mucosal contour with polypoid protrusions.
Figure 4.
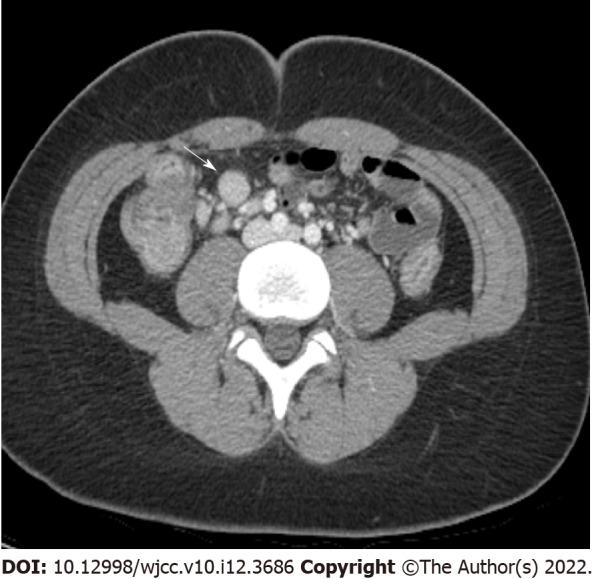
Enlarged lymph node. Enhanced multidetector computed tomography axial image in portal venous phase shows enlarged lymph node (arrow, short axis diameter is measured as 12 mm) with strong enhancement adjacent to ascending colon.
Figure 5.
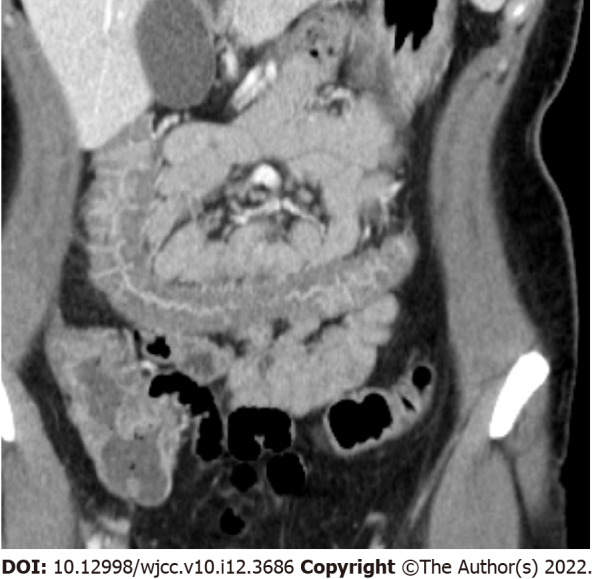
Empty colon sign. Coronal reconstructed image shows complete emptiness (no gas, fluid, or feces) of the transverse colon. Marked wall thickening with mucosal hyperenhancement is also seen.
C-reactive protein and WBC analysis
The mean values of C-reactive protein and WBC were calculated to compare the numerical differences between bacterial and viral colitis. In addition, by classifying the patient groups based on the number of presumed infection or inflammation, the difference between bacterial and viral colitis was analyzed. C-reactive protein was determined to be 0.3 mg/dL or higher and WBC was 10000 x 109/L or higher to determine the presence of infection or inflammation. In addition, in order to find out whether there are differences in the clinical symptoms of colitis according to the etiology, the patient groups were classified and compared according to the three symptoms: fever, diarrhea, and abdominal pain.
Statistical analysis
To evaluate the utility of MDCT in infectious colitis, patients were categorized into groups based on stool PCR (bacterial colitis and viral colitis). Fisher’s exact test was used for examining the relationship of MDCT parameters and baseline demographics with the two groups of bacterial and viral colitis. We then computed sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each MDCT parameter by bacterial and viral colitis. We also evaluated diagnostic performance for each group of colitis by estimating odds ratios of colitis for each MDCT parameter using logistic regression models after adjusting for age, season, diabetes, cardiovascular diseases, and cancer history. A P value of < 0.05 was considered statistically significant. All statistical analyses were conducted in R version 3.6.2 (The R Foundation for Statistical Computing; https://www.r-project.org/). The statistical methods of this study were reviewed by Kim SY from Department of Cancer Control and Population Health, National Cancer Center, Goyang.
RESULTS
Subjects
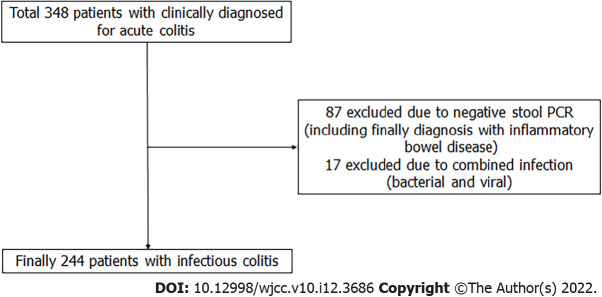
Figure 6 showes study design and patient selection. From February 2015 to December 2018, 348 patients were clinically diagnosed with acute colitis. However 87 patients were excluded due to PCR-negative, and 17 patients were excluded by the bacterial and viral combined infection, So Finally 244 patients was enrolled to study. Table 1 presents the baseline characteristics of the study population. The patients were 134 women and 110 men, with an average age of 45.1 years. The cause of colitis was bacterial for 84% patients (n = 204) and viral for 16% (n = 40). Bacterial colitis was more common in summer than in other three seasons (P = 0.001). The other baseline characteristics did not differ between the two groups of colitis.
Figure 6.
Flow diagram showing study design and patient selection.
Table 1.
Acute colitis prevalence and summary statistics of baseline characteristics by COI
|
|
Value
|
Total
|
Viral
|
Bacterial
|
P
value1
|
| Prevalence (%) | 16 | 84 | |||
| Season (%) | Spring | 24 | 35 | 22 | 0.001 |
| Summer | 39 | 15 | 44 | ||
| Autumn | 22 | 23 | 22 | ||
| Winter | 15 | 28 | 13 | ||
| Sex (%) | Male | 45 | 45 | 45 | 1.000 |
| DM (%) | Yes | 14 | 15 | 13 | 0.801 |
| CVD (%) | Yes | 11 | 10 | 11 | 1.000 |
| MALIG (%) | Yes | 3 | 3 | 3 | 1.000 |
| Age (mean ± SD) | 45.1 ± 20.9 | 41.8 ± 21.5 | 45.7 ± 20.8 | 0.271 | |
| Height (mean ± SD) | 165.4 ± 9.6 | 166.5 ± 9.4 | 165.2 ± 9.6 | 0.487 | |
| Weight (mean ± SD) | 65.1 ± 14.6 | 67.8 ± 17.4 | 64.6 ± 14.1 | 0.271 | |
| BMI (mean ± SD) | 23.7 ± 4.1 | 24.1 ± 4.6 | 23.6 ± 4.1 | 0.492 |
Fisher's exact test for season, sex, diabetes mellitus, cardiovascular disease;, and history of malignancy, and ANOVA test otherwise.
DM: Diabetes mellitus; CVD: Cardiovascular disease; MALIG: History of malignancy; BMI: Body mass index.
MDCT analysis results
Table 2 shows the prevalence of acute colitis for 11 MDCT parameters. Acute colitis was more prevalent in the bacterial colitis group than in the viral group for all MDCT parameters; the relationship with the cause of colitis was significant for all MDCT parameters except for small bowel involvement.
Table 2.
Acute colitis prevalence for 11 computed tomography parameters by COI
|
|
Value
|
Total
|
Viral
|
Bacterial
|
P
value1
|
| Prevalence (%) | 16 | 84 | |||
| Wall thickening (%)2 | Yes | 82 | 40 | 90 | 0.000 |
| Submucosal edema (%) | Yes | 82 | 30 | 93 | 0.000 |
| Mucosal enhancement (%) | Yes | 79 | 28 | 89 | 0.000 |
| Serosal involvement (%) | Yes | 39 | 5 | 46 | 0.000 |
| Empty colon sign (%)2 | Yes | 43 | 13 | 49 | 0.000 |
| Small bowel involvement (%) | Yes | 38 | 30 | 40 | 0.288 |
| Comb sign (%) | Yes | 13 | 3 | 15 | 0.036 |
| Continuous distribution (%) | Yes | 75 | 25 | 85 | 0.000 |
| Accordion sign (%) | Yes | 15 | 3 | 17 | 0.014 |
| Mucosal thickening (%) | Yes | 57 | 5 | 67 | 0.000 |
| Lymph node enlargement (%) | Yes | 18 | 8 | 21 | 0.072 |
Fisher's exact test.
Recategorized to binary values of normal vs non-normal.
Wall thickening was present in 90% of bacterial colitis cases vs 40% of viral colitis cases (P < 0.001). Submucosal edema was encountered in 93% of bacterial colitis cases vs 30% of viral colitis cases (P < 0.001). Mucosal enhancement was found in 89% of bacterial colitis cases vs 28% of viral colitis cases (P < 0.001). Serosal involvement was detected in 46% of bacterial colitis cases vs 5% of viral colitis cases (P < 0.001). Empty colon sign was found in 49% of bacterial colitis cases vs 13% of viral colitis cases (P < 0.001). Continuous distribution was detected in 85% of bacterial colitis cases vs 25% of viral colitis cases (P < 0.001). Accordion sign was found in 17% of bacterial colitis cases vs 3% of viral colitis cases (P = 0.038). Mucosal thickening was found in 67% of bacterial colitis cases vs 5% of viral colitis cases (P < 0.001). Lymph node enlargement was found in 21% of bacterial colitis cases vs 8% of viral colitis cases (P = 0.023).
Table 3 summarizes the diagnostic performances of MDCT by bacterial and viral colitis. Bacterial colitis showed better diagnostic performance than viral colitis for all MDCT parameters. Sensitivity, specificity, PPV, and NPV of wall thickening were 89.7%, 60.0%, 92.0%, 89.5%, and 53.3%, respectively. Submucosal edema, mucosal enhancement, and continuous distribution had higher sensitivity (84.8%–92.6%), whereas serosal involvement, empty colon sign, comb sign, accordion sign, mucosal thickening, and lymph node enlargement had higher specificity (87.5%–97.5%). Viral colitis showed particularly low sensitivity and specificity. Sensitivity of each MDCT findings for viral colitis ranged from 2.5% to 40%.
Table 3.
Sensitivity and specificity of the 11 computed tomography parameters for predicting viral or bacterial acute colitis prevalence
| CT parameter |
Viral
|
Bacterial
|
||||||
|
Se (%)
|
Sp (%)
|
PPV (%)
|
NPV (%)
|
Se (%)
|
Sp (%)
|
PPV (%)
|
NPV (%)
|
|
| Wall thickening1 | 40.0 | 10.3 | 8.0 | 46.7 | 89.7 | 60.0 | 92.0 | 53.3 |
| Submucosal edema | 30.0 | 7.4 | 6.0 | 34.9 | 92.6 | 70.0 | 94.0 | 65.1 |
| Mucosal enhancement | 27.5 | 11.3 | 5.7 | 44.2 | 88.7 | 72.5 | 94.3 | 55.8 |
| Serosal involvement | 5.0 | 53.9 | 2.1 | 74.3 | 46.1 | 95.0 | 97.9 | 25.7 |
| Empty colon sign1 | 12.5 | 51.0 | 4.8 | 74.8 | 49.0 | 87.5 | 95.2 | 25.2 |
| Small bowel involvement | 30.0 | 60.3 | 12.9 | 81.5 | 39.7 | 70.0 | 87.1 | 18.5 |
| Comb sign | 2.5 | 85.3 | 3.2 | 81.7 | 14.7 | 97.5 | 96.8 | 18.3 |
| Continuous distribution | 25.0 | 15.2 | 5.5 | 50.8 | 84.8 | 75.0 | 94.5 | 49.2 |
| Accordion sign | 2.5 | 82.8 | 2.8 | 81.3 | 17.2 | 97.5 | 97.2 | 18.8 |
| Mucosal thickening | 5.0 | 33.3 | 1.4 | 64.2 | 66.7 | 95.0 | 98.6 | 35.8 |
| Lymph node enlargement | 7.5 | 79.4 | 6.7 | 81.4 | 20.6 | 92.5 | 93.3 | 18.6 |
Recategorized to binary values of normal vs non-normal.
CT: Computed tomography; Se: Sensitivity; Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value.
Table 4 shows odds ratios (ORs) of relevant MDCT parameters by the two groups of acute colitis. For bacterial colitis, OR of wall thickening was 13.60 (95% confidence interval: 5.80–31.88). In 11 MDCT parameters, submucosal edema, mucosal enhancement, continuous distribution, and mucosal thickening had especially high ORs [36.08 (13.54–96.13), 22.55 (19.28–54.81), 24.09 (9.38–61.90), and 46.41 (10.38–207.51), respectively]. For viral colitis, all ORs were below 1.
Table 4.
Odds ratios of viral or bacterial acute colitis prevalence for each of the 11 computed tomography parameters
| CT parameter |
Viral
|
Bacterial
|
||||||
|
OR
|
95%CI
|
P
value
|
OR
|
95%CI
|
P
value
|
|||
| Wall thickening1 | 0.07 | 0.03 | 0.17 | 0.000 | 13.60 | 5.80 | 31.88 | 0.000 |
| Submucosal edema | 0.03 | 0.01 | 0.07 | 0.000 | 36.08 | 13.54 | 96.13 | 0.000 |
| Mucosal enhancement | 0.04 | 0.02 | 0.11 | 0.000 | 22.55 | 9.28 | 54.81 | 0.000 |
| Serosal involvement | 0.07 | 0.02 | 0.30 | 0.000 | 14.50 | 3.33 | 63.23 | 0.000 |
| Empty colon sign1 | 0.15 | 0.05 | 0.41 | 0.000 | 6.68 | 2.44 | 18.32 | 0.000 |
| Small bowel involvement | 0.69 | 0.32 | 1.50 | 0.353 | 1.44 | 0.67 | 3.13 | 0.353 |
| Comb sign | 0.19 | 0.02 | 1.50 | 0.115 | 5.25 | 0.67 | 41.32 | 0.115 |
| Continuous distribution | 0.04 | 0.02 | 0.11 | 0.000 | 24.09 | 9.38 | 61.90 | 0.000 |
| Accordion sign | 0.11 | 0.01 | 0.89 | 0.038 | 9.02 | 1.12 | 72.35 | 0.038 |
| Mucosal thickening | 0.02 | 0.00 | 0.10 | 0.000 | 46.41 | 10.38 | 207.51 | 0.000 |
| Lymph node enlargement | 0.23 | 0.06 | 0.82 | 0.023 | 4.39 | 1.22 | 15.72 | 0.023 |
Recategorized to binary values of normal vs non-normal.
Adjusted for age, season, sex, diabetes, cardiovascular diseases, and cancer history. CT: Computed tomography.
Tables 5 and 6 show diagnostic statistics of four CT results (submucosal edema, mucosal enhancement, continuous distribution, and mucosal thickening) with seasonal consideration. The incidence of colitis was the highest in the summer season (39%). While relying upon at least one of four MDCT parameters in summer, sensitivity and specificity for bacterial colitis was found to be particularly high (41.67 and 92.50, respectively). OR of four combined MDCT parameters was higher for bacterial colitis than for viral colitis.
Table 5.
Sensitivity and specificity of the four combined computed tomography parameters1 for predicting viral or bacterial acute colitis prevalence
| CT parameter |
Viral
|
Bacterial
|
||||||
|
Se (%)
|
Sp (%)
|
PPV (%)
|
NPV (%)
|
Se (%)
|
Sp (%)
|
PPV (%)
|
NPV (%)
|
|
| Summer and all four | 2.50 | 74.02 | 1.85 | 79.47 | 25.98 | 97.50 | 98.15 | 20.53 |
| Summer and at least one | 7.50 | 58.33 | 3.41 | 76.28 | 41.67 | 92.50 | 96.59 | 23.72 |
| Winter and all four | 2.50 | 90.69 | 5.00 | 82.59 | 9.31 | 97.50 | 95.00 | 17.41 |
| Winter and at least one | 15.00 | 87.75 | 19.35 | 84.04 | 12.25 | 85.00 | 80.65 | 15.96 |
Submucosal edema, mucosal enhancement, continuous distribution, and mucosal thickening.
CT: Computed tomography; Se: Sensitivity; Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value.
Table 6.
Odds ratios of viral or bacterial acute colitis prevalence for each of the four combined computed tomography parameters1
| CT parameter |
Viral
|
Bacterial
|
||||||
|
OR
|
95%CI
|
P
value
|
OR
|
95%CI
|
P
value
|
|||
| Summer and all four | 0.13 | 0.01 | 1.16 | 0.07 | 7.75 | 0.86 | 69.93 | 0.07 |
| Summer and at least one | 0.04 | 0.01 | 0.28 | 0.00 | 24.95 | 3.59 | 173.43 | 0.00 |
| Winter and all four | 0.04 | 0.00 | 0.35 | 0.00 | 26.90 | 2.82 | 256.26 | 0.00 |
| Winter and at least one | 0.04 | 0.00 | 0.40 | 0.01 | 28.51 | 2.48 | 327.72 | 0.01 |
Submucosal edema, mucosal enhancement, continuous distribution, mucosal thickening. Adjusted for age, season, sex, diabetes, cardiovascular diseases, and cancer history.
CT: Computed tomography.
C-reactive protein and WBC analysis
Comparing the mean values of C-reactive protein, Bacterial colitis was higher than viral colitis, which was statistically significant. However, there was no difference in the prevalence of bacterial and viral colitis classified according to the reference values of WBC and C-reactive protein and there was no statistical significance in differentiating the etiology by clinical symptoms.
DISCUSSION
The main purpose of this study was to investigate if CT is valuable in discriminating bacterial colitis from viral colitis. There were a few previous studies on underlying etiology in a large patient cohort[17,19]. However, no studies reported on subdividing the etiology of infectious colitis into bacterial or viral causes. Plastaras et al[17] investigated the usefulness of MDCT in examining the underlying cause of acute colitis. They classified bowel wall thickening on the basis of 3 mm. This thickening was found in most cases (97%) of colitis; however, it was not useful in distinguishing the causes. However, this study classified bowel wall thickening on the basis of 5 mm. Increasing thickness was suggestive of bacterial colitis. Additionally, submucosal edema was associated with bacterial colitis, suggesting that edematous changes in the submucosal layer had a significant effect on bowel wall thickness.
Plastaras et al[17] first suggested the empty colon sign, which was defined as a complete emptiness of the colonic lumen. They reported that the empty colon sign was a finding suggestive of infectious colitis. In our study, the empty colon sign was particularly related to bacterial colitis.
The Comb sign, which refers to a hypervascular appearance of the mesentery with vascular dilatation and engorgement of the vasa recta, can be frequently seen in inflammatory bowel disease. It is not pathognomonic of Crohn’s disease; however, it can be seen in other causes of acute colitis[20,21] or vasculitis[22].
Distribution of colonic abnormality was more associated with inflammatory bowel disease and ischemic colitis than with infectious colitis[23-25]. The probability of the specific location according to the organisms was mentioned (i.e., right colon for Yersinia and Salmonella, diffuse involvement for cytomegalovirus and Escherichia coli, left colon for Shigella), but there could be considerable overlap. In addition, there was no significant segmental predominance[26,27]. Therefore, in this study, the distribution was simply divided into continuous or skipped involvement, and continuous involvement of colonic abnormality had significant difference in bacterial colitis.
In the past, the accordion sign was thought to be specific for pseudomembranous colitis. Now, it is indicative of severe colonic edema or inflammation, and not specific for Clostridium difficile colitis[28]. This study has demonstrated a statistically significant difference between the accordion sign and bacterial colitis.
Lymph node enlargement was a discriminating factor in the diagnosis of bacterial causes. There were many endoscopy-proven infectious cases which showed bowel wall thickening with fat stranding and lymph node enlargement on CT[29]. In the case of a wall thickening associated with lymph node enlargement, it was necessary to rule out a malignancy. Heterogeneous and asymmetric focal thickening suggested malignancy, while symmetric regular and homogeneous thickening were associated with benign conditions[18,30].
Plastaras et al[17] suggest that the presence of the empty colon sign, continuous distribution of colonic abnormality, and the absence of enlarged lymph nodes are discriminating CT signs for infectious colitis[1]. However, in this study, lymph node enlargement was found in 18% of total colitis and the empty colon sign was present in 43%, while continuous distribution was detected in 75% of total colitis. Thus, continuous distribution is considered appropriate for distinguishing infectious colitis, but there are considerable exceptions in which the absence of lymph node enlargement is highly likely to discriminate infectious enteritis.
It was statistically significant that C-reactive protein was higher in bacterial colitis than in viral colitis, but it was not clear how high the threshold should be for suspicion. We took non-normal values as a reference point, but no statistical significance was found. However, in the case of bacterial colitis, since the sensitivity of C-reactive protein was 93%, if it is elevated, it is necessary to consider Bacterial colitis as an exclusion diagnosis.
If it is summertime and there is at least one positive finding in four CT parameters (submucosal edema, mucosal enhancement, continuous distribution, mucosal thickening), a bacterial infection may be suspected with a sensitivity of 41.67 and a specificity of 92.50. Although the sensitivity is a little low and the specificity is high, this may be helpful in clinical use, such as antibiotics administration, when bacterial infection is suspected.
Limits of the study
The present study has several limitations. First, this study was performed in a retrospective, observational, and single center manner. Second, there was no control group of patients without colitis. Also, there were more patients in the bacterial colitis group (84%) compared to too few patients in the viral colitis group (16%). This may be considered a selection bias. Thus, a large prospective and well-designed study, including protocols—such as MDCT, serology/bacteriology, and endoscopy result—is necessary. Third, this present study only dealt with infectious colitis. Other causes of colitis (i.e., inflammatory or ischemic colitis) and colitis with unexplained causes were excluded. Finally, three radiologists participated in the MDCT readout. Therefore, there could be inter-observer differences. However, we did our best to minimize the discrepancy with enthusiastic results for equivocal MDCT findings.
CONCLUSION
MDCT provides many clues that can be useful in suggesting a specific diagnosis of acute infectious colitis. MDCT parameters of wall thickening, submucosal edema, mucosal enhancement, serosal involvement, empty colon sign, continuous distribution, accordion sign, mucosal thickening, and lymph node enlargement may be suggestive of bacterial colitis. At least one positive finding in four MDCT parameters (submucosal edema, mucosal enhancement, continuous distribution, mucosal thickening) in the summer season is suggestive of a bacterial infection.
ARTICLE HIGHLIGHTS
Research background
Multidetector computed tomography (MDCT) findings are well known depending on the etiology of colitis such as ischemic colitis, infectious colitis, and pseudomembranous colitis, but comparison of MDCT findings between bactria and virus infection in infectious colitis has not been studied.
Research motivation
If we can better differentiate between bacterial colitis and viral colitis, we can get help in deciding on a treatment method, such as the use of antibiotics.
Research objectives
This study's objective is to examine the usefulness of MDCT in distinguishing the etiology of acute infectious colitis.
Research methods
The cause of infectious colitis was defined using stool polymerase chain reaction (PCR) test for 244 infectious colitis patients for 4 years, and CT findings according to the cause were compared by using 11 parameters.
Research results
The parameters of bacterial colitis, except for small bowel involvement and comb sign had a significantly higher odds ratio than parameters of viral coltitis.
Research conclusions
MDCT provides many clues that can be useful in suggesting a specific etiology of acute infectious colitis.
Research perspectives
This study had limitations such as retrospective study, selection bias and exception of other cause of colitis, so a large prospective and well-designed study, including protocols—such as MDCT, serology/bacteriology, and endoscopy result—is necessary.
Footnotes
Institutional review board statement: Ethics committee approval was received for this study from Institutional Review Board of the Myongji hospital (Decision No. MJH 2018-08-020).
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Korean Association of Internal Medicine, No. 12991; The Korean Society of Gastroenterology, No. 1-13-2095.
Peer-review started: May 31, 2021
First decision: July 1, 2021
Article in press: March 4, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Carannante F, Italy; Kitamura K, Japan; Sipos F, Hungary S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
Contributor Information
Seung Jung Yu, Department of Internal Medicine, Inje University Busan Paik Hospital, Busan 47392, South Korea.
Jae Hyuk Heo, Department of Internal Medicine, Inje University Busan Paik Hospital, Busan 47392, South Korea.
Eun Jeong Choi, Department of Internal Medicine, Inje University Busan Paik Hospital, Busan 47392, South Korea.
Jong Hyuk Kim, Department of Radiology, Myongji Hospital, Hanyang University College of Medicine, Goyang 10475, South Korea.
Hong Sub Lee, Department of Internal Medicine, Inje University Busan Paik Hospital, Busan 47392, South Korea. hslee@paik.ac.kr.
Sun Young Kim, Department of Cancer Control and Population Health, National Cancer Center, Goyang 10408, South Korea.
Jae Hoon Lim, Department of Radiology, Myongji Hospital, Hanyang University College of Medicine, Goyang 10475, South Korea.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at hslee@paik.ac.kr
References
- 1.Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, Langley JM, Wanke C, Warren CA, Cheng AC, Cantey J, Pickering LK. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017;65:1963–1973. doi: 10.1093/cid/cix959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical Guideline for the Diagnosis and Treatment of Gastrointestinal Infections. Infect Chemother. 2010;42:323–361. [Google Scholar]
- 3.Lee JM, Lee KM. Endoscopic Diagnosis and Differentiation of Inflammatory Bowel Disease. Clin Endosc. 2016;49:370–375. doi: 10.5946/ce.2016.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantzaris GJ. Endoscopic diagnosis of infectious colitis. Ann Gastroenterol. 2007;20 [Google Scholar]
- 5.Zhang H, Morrison S, Tang YW. Multiplex polymerase chain reaction tests for detection of pathogens associated with gastroenteritis. Clin Lab Med. 2015;35:461–486. doi: 10.1016/j.cll.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binnicker MJ. Multiplex Molecular Panels for Diagnosis of Gastrointestinal Infection: Performance, Result Interpretation, and Cost-Effectiveness. J Clin Microbiol. 2015;53:3723–3728. doi: 10.1128/JCM.02103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halligan E, Edgeworth J, Bisnauthsing K, Bible J, Cliff P, Aarons E, Klein J, Patel A, Goldenberg S. Multiplex molecular testing for management of infectious gastroenteritis in a hospital setting: a comparative diagnostic and clinical utility study. Clin Microbiol Infect. 2014;20:O460–O467. doi: 10.1111/1469-0691.12476. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg SD, Bacelar M, Brazier P, Bisnauthsing K, Edgeworth JD. A cost benefit analysis of the Luminex xTAG Gastrointestinal Pathogen Panel for detection of infectious gastroenteritis in hospitalised patients. J Infect. 2015;70:504–511. doi: 10.1016/j.jinf.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki K, Fujita I, Hamasaki Y, Miyazaki S. Differentiating between bacterial and viral infection by measuring both C-reactive protein and 2'-5'-oligoadenylate synthetase as inflammatory markers. J Infect Chemother. 2002;8:76–80. doi: 10.1007/s101560200010. [DOI] [PubMed] [Google Scholar]
- 10.Thoeni RF, Cello JP. CT imaging of colitis. Radiology. 2006;240:623–638. doi: 10.1148/radiol.2403050818. [DOI] [PubMed] [Google Scholar]
- 11.Delabrousse E, Ferreira F, Badet N, Martin M, Zins M. Coping with the problems of diagnosis of acute colitis. Diagn Interv Imaging. 2013;94:793–804. doi: 10.1016/j.diii.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JK. Abnormal colonic wall thickening on computed tomography. J Comput Assist Tomogr. 1983;7:90–97. doi: 10.1097/00004728-198302000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khowaiter SS, Brahmania M, Kim E, Madden M, Harris A, Yoshida EM, Gray JR. Clinical and endoscopic significance of bowel-wall thickening reported on abdominal computed tomographies in symptomatic patients with no history of gastrointestinal disease. Can Assoc Radiol J. 2014;65:67–70. doi: 10.1016/j.carj.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Barral M, Boudiaf M, Dohan A, Hoeffel C, Camus M, Pautrat K, Fishman EK, Cohen S, Soyer P. MDCT of acute colitis in adults: an update in current imaging features. Diagn Interv Imaging. 2015;96:133–149. doi: 10.1016/j.diii.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Meyers MA, McGuire PV. Spiral CT demonstration of hypervascularity in Crohn disease: "vascular jejunization of the ileum" or the "comb sign". Abdom Imaging. 1995;20:327–332. doi: 10.1007/BF00203365. [DOI] [PubMed] [Google Scholar]
- 16.Macari M, Balthazar EJ, Megibow AJ. The accordion sign at CT: a nonspecific finding in patients with colonic edema. Radiology. 1999;211:743–746. doi: 10.1148/radiology.211.3.r99jn32743. [DOI] [PubMed] [Google Scholar]
- 17.Plastaras L, Vuitton L, Badet N, Koch S, Di Martino V, Delabrousse E. Acute colitis: differential diagnosis using multidetector CT. Clin Radiol. 2015;70:262–269. doi: 10.1016/j.crad.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes T, Oliveira MI, Castro R, Araújo B, Viamonte B, Cunha R. Bowel wall thickening at CT: simplifying the diagnosis. Insights Imaging. 2014;5:195–208. doi: 10.1007/s13244-013-0308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philpotts LE, Heiken JP, Westcott MA, Gore RM. Colitis: use of CT findings in differential diagnosis. Radiology. 1994;190:445–449. doi: 10.1148/radiology.190.2.8284397. [DOI] [PubMed] [Google Scholar]
- 20.Madureira AJ. The comb sign. Radiology. 2004;230:783–784. doi: 10.1148/radiol.2303020645. [DOI] [PubMed] [Google Scholar]
- 21.Panizza PS, Viana PC, Horvat N, Dos Santos VR Júnior, de Araújo DA, Yamanari TR, Leite CD, Cerri GG. Inflammatory Bowel Disease: Current Role of Imaging in Diagnosis and Detection of Complications: Gastrointestinal Imaging. Radiographics. 2017;37:701–702. doi: 10.1148/rg.2017160050. [DOI] [PubMed] [Google Scholar]
- 22.Ju JH, Min JK, Jung CK, Oh SN, Kwok SK, Kang KY, Park KS, Ko HJ, Yoon CH, Park SH, Cho CS, Kim HY. Lupus mesenteric vasculitis can cause acute abdominal pain in patients with SLE. Nat Rev Rheumatol. 2009;5:273–281. doi: 10.1038/nrrheum.2009.53. [DOI] [PubMed] [Google Scholar]
- 23.Iacobellis F, Berritto D, Fleischmann D, Gagliardi G, Brillantino A, Mazzei MA, Grassi R. CT findings in acute, subacute, and chronic ischemic colitis: suggestions for diagnosis. Biomed Res Int. 2014;2014:895248. doi: 10.1155/2014/895248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilcoyne A, Kaplan JL, Gee MS. Inflammatory bowel disease imaging: Current practice and future directions. World J Gastroenterol. 2016;22:917–932. doi: 10.3748/wjg.v22.i3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berritto D, Iacobellis F, Mazzei MA, Volterrani L, Guglielmi G, Brunese L, Grassi R. MDCT in ischaemic colitis: how to define the aetiology and acute, subacute and chronic phase of damage in the emergency setting. Br J Radiol. 2016;89:20150821. doi: 10.1259/bjr.20150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horton KM, Corl FM, Fishman EK. CT evaluation of the colon: inflammatory disease. Radiographics. 2000;20:399–418. doi: 10.1148/radiographics.20.2.g00mc15399. [DOI] [PubMed] [Google Scholar]
- 27.Jung JY, Park YS, Baek DH, Choi JH, Jo YJ, Kim SH, Son BK, Chae JD, Kim DH, Jung YY. The prevalence of Yersinia infection in adult patients with acute right lower quadrant pain. Korean J Gastroenterol. 2011;57:14–18. doi: 10.4166/kjg.2011.57.1.14. [DOI] [PubMed] [Google Scholar]
- 28.Ash L, Baker ME, O'Malley CM Jr, Gordon SM, Delaney CP, Obuchowski NA. Colonic abnormalities on CT in adult hospitalized patients with Clostridium difficile colitis: prevalence and significance of findings. AJR Am J Roentgenol. 2006;186:1393–1400. doi: 10.2214/AJR.04.1697. [DOI] [PubMed] [Google Scholar]
- 29.Lee JS, Cho JH, Kim KO, Lee SH, Jang BI. Clinical significance of the large intestinal wall thickening detected by abdominal computed tomography. Korean J Gastroenterol. 2012;60:300–305. doi: 10.4166/kjg.2012.60.5.300. [DOI] [PubMed] [Google Scholar]
- 30.Tapasvi C, Prajapati N, Madhok R, Gupta AK, Taneja V, Aggarwal A. Evaluation of bowel wall thickening by computed tomography to differentiate benign from malignant lesions. J Clin Diagn Res. 2014;8:RC09–RC12. doi: 10.7860/JCDR/2014/10601.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at hslee@paik.ac.kr