Abstract
It has been hypothesized that, by specifically lysing numerically dominant host strains, the virioplankton may play a role in maintaining clonal diversity of heterotrophic bacteria and phytoplankton populations. If viruses selectively lyse only those host species that are numerically dominant, then the number of a specific virus within the virioplankton would be expected to change dramatically over time and space, in coordination with changes in abundance of the host. In this study, the abundances of specific viruses in Chesapeake Bay water samples were monitored, using nucleic acid probes and hybridization analysis. Total virioplankton in a water sample was separated by pulsed-field gel electrophoresis and hybridized with nucleic acid probes specific to either single viral strains or a group of viruses with similar genome sizes. The abundances of specific viruses were inferred from the intensity of the hybridization signal. By using this technique, a virus comprising 1/1,000 of the total virioplankton abundance (ca. 104 PFU/ml) could be detected. Titers of either a single virus species or a group of viruses changed over time, increasing to peak abundance and then declining to low or undetectable levels, and were geographically localized in the bay. Peak signal intensities, i.e., peak abundances of virus strains, were 10-fold greater than the low background level. Furthermore, virus species were found to be restricted to a particular depth, since probes specific to viruses from bottom water did not hybridize with virus genomes from surface water at the same geographical location. Overall, changes in abundances of specific viruses within the virioplankton were episodic, supporting the hypothesis that viral infection influences, if not controls, clonal diversity within heterotrophic bacteria and phytoplankton communities.
Since the relatively recent discovery that very large numbers of viruses are present in marine and estuarine environments, field studies have shown that aquatic virus populations are a dynamic component of aquatic microbial communities. Virioplankton abundance is now recognized to be responsive not only to seasonal changes in a geographical region (27, 69) but also to physiochemical changes associated with depth (12, 52) and along trophic gradients (64). Evidence of the dynamic nature of virioplankton abundance prompted debate on the impact of viral infection and lysis on bacterial and phytoplankton communities. Two broad hypotheses concerning the importance of viral infection in maintenance of aquatic microbial communities have been developed. The first is that viruses directly limit productivity of the host community by lysis of host bacteria and phytoplankton (21, 22). To date, there is significant evidence indicating that viral infection and lysis account for between 10 and 20% of heterotrophic bacterial mortality in marine environments (53). The second is that viral lysis is important in maintaining clonal and genetic diversity of the host populations (63). The mechanisms by which viral lysis influences host clonal diversity are hypothesized to be reduction of the number of a particular bacterial host when that host becomes dominant (“blooms”) (59) and transduction (49). These theories are not mutually exclusive; therefore, in addition to the impact of viral infection on host population mortality, viruses may play an important role in influencing the clonal compositions of bacterial and algal host communities.
A strong argument against geographically widespread and numerically significant levels of viral infections in aquatic microbial communities is the tendency for fast-growing clonal organisms, like bacteria, to acquire resistance to cooccurring parasites. The observation that newly isolated Synechococcus clones were resistant to cyanophages occurring within the same environment led Waterbury and Valois (63) to conclude that cyanophage-induced lysis would have little impact on Synechococcus concentrations in situ. Similarly, a collection of activated-sludge bacteria was found to comprise largely those bacterial strains which were phage resistant (24). By extension, bacterioplankton may be resistant to cooccurring bacteriophages. The ability of bacteria to develop resistance to a virulent phage has been demonstrated in long-term chemostat studies showing that after acquisition of resistance, the number of host cells is 10 to 100 times greater than the number of virulent phage, yet the virulent phage population is not eliminated (31). An explanation for stable coexistence may be that virulent phage survive by scavenging the few sensitive cells within a clonal host population (63). In turn, sensitive host strains are maintained because of a growth advantage over resistant hosts (7, 23, 33, 51).
There is very little information on the in situ temporal dynamics of specific virus-host systems. Observations of high titers of virus-like particles associated with the decline of monospecific phytoplankton blooms (4, 37, 39, 40, 50) have led many investigators to conclude that viruses control the population sizes of specific planktonic hosts. Empirical demonstration of a bacteriophage selectively controlling the population size of a susceptible bacterial strain within the bacterioplankton was recently provided through a mesocosm experiment in which a phage-susceptible bacterial strain, PWH3a, was added to a natural planktonic community. Hennes et al. (25) observed that titers of phage infecting PWH3a increased dramatically and simultaneously with a decline in the number of PWH3a cells. If viruses selectively eliminate host strains when the latter are at population peaks, then the population sizes of a given host and the virus to which it is susceptible would best be described as episodic, i.e., occurring in short, highly localized blooms. In this study, using molecular genetic techniques, we examined temporal and spatial changes in the abundances of specific viruses or groups of viruses with similar-size genomes. Our working hypothesis was that high abundances of particular viruses within the virioplankton would be spatially limited and short-lived. The results of this study are discussed in reference to a conceptual model for the impact of viral infection on aquatic bacterial community structure.
MATERIALS AND METHODS
Virioplankton concentration.
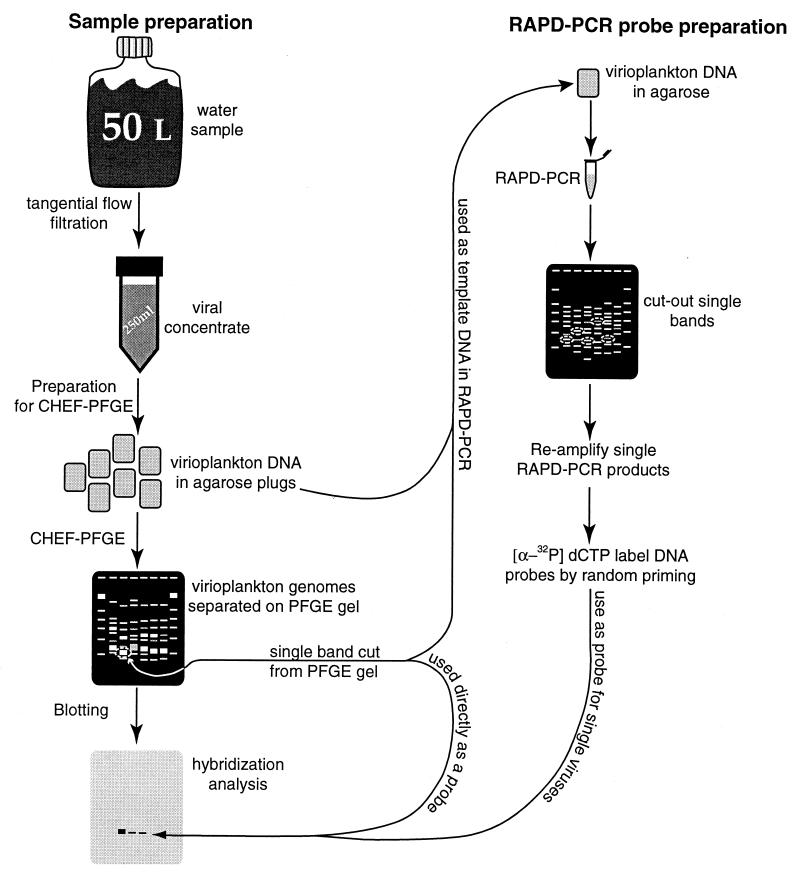
Water samples were collected in 10-liter Niskin bottles mounted on an instrument rosette. Samples were collected at stations located in the main stem of the Chesapeake Bay (69). The sites of the sampling stations were located on a transect of the bay, from the Patapsco River to the York River. Station designations and locations are 908 (39°08′N, 76°20′W), 858 (38°58′N, 76°23′W), 845 (38°45′N, 76°26′W), 834 (38°34′N, 76°24′W), 818 (38°18′N, 76°26′W), 744 (37°44′N, 76°11′W), and 724 (37°24′N, 76°05′W). The letter B following the station number indicates that the water sample was collected 1 m above the sediment-water interface. The locations of the stations used in this study are similar to those used in an earlier virioplankton enumeration study (69). A total of 27 water samples were collected on four cruises during 21 to 23 August 1995, 9 to 11 May 1996, 31 May to 2 June 1996, and 5 to 9 July 1996. Figure 1 illustrates the steps involved in hybridization analysis of Chesapeake Bay virioplankton.
FIG. 1.
Flow chart of methods used in the hybridization analysis of Chesapeake Bay virioplankton.
Sample processing was conducted aboard ship immediately following collection. During the 1996 research cruises, Chesapeake Bay virioplankton were concentrated by using the spiral-cartridge filtration method of Suttle et al. (57). Bacteria and larger plankton were removed by two-stage filtration, using 142-mm-diameter filters mounted in stainless steel filter holders. Each 50-liter water sample was passed through a glass fiber filter (GF-D; nominal pore size, 1.2 μm, Gelman, Ann Arbor, Mich.) under low vacuum (<300 mm Hg), collected, and subsequently passed through a 0.2-μm-pore-size polycarbonate filter (Poretics, Livermore, Calif.). Viruses in the 50-liter filtrates were concentrated by using the CH 2 system and SIY30 filter (Amicon, Bedford, Mass.) (50 liters reduced to 250 ml) (57) to a 200 to 250× final concentration of in situ virioplankton. Final water sample concentrates contained particulates of between 30,000 Da (approximately 2 nm) and 0.22 μm in size.
During the August 1995 cruise, virioplankton were concentrated as described by Wommack et al. (68) to a 10× final concentration of in situ virioplankton. The August 1995 virioplankton concentrates contained particulates of between 10,000 Da (approximately 1 nm) and 0.22 μm in size. Volume measurements were recorded to ensure accurate calculation of sample concentration.
Preparation of viral concentrates for pulsed-field gel electrophoresis (PFGE).
Approximately 30 ml of virioplankton concentrate in 32-ml ultracentrifuge tubes was centrifuged for 3.5 h at 100,000 × g. The supernatant was gently decanted, and the virus pellet was resuspended and incubated overnight at 4°C in 450 μl of SM buffer (0.1 M NaCl, 8 mM MgSO4 · 7H2O, 50 mM Tris-HCl, and 0.005% [wt/vol] glycerol) with gentle shaking. Equal volumes of the viral concentrate and molten (50°C) 1.5% InCert agarose (FMC, Rockland, Maine) were mixed, vortexed, and dispensed into plug molds. After solidification of the gel, plugs were punched from the molds into a small volume of buffer (250 mM EDTA, 1% sodium dodecyl sulfate [SDS], pH 8.0) containing 1 mg of proteinase K (Fisher Scientific, Pittsburgh, Pa.) per ml. The plugs were incubated at room temperature overnight in the dark. The proteinase K digestion buffer was decanted, and the plugs were washed three times for 30 min each in 10 mM Tris–1 mM EDTA, pH 8.0. Virioplankton agarose plugs were stored at 4°C in 20 mM Tris–50 mM EDTA, pH 8.0.
PFGE.
Optimal conditions for electrophoresis were determined empirically. Virioplankton plugs and plugs containing phage lambda concatamers (Promega, Madison, Wis.), serving as molecular size markers, were placed into wells of a 1% SeaKem GTG agarose (FMC) gel with an overlay of molten 1% agarose. PFGE of samples collected in 1996 was performed with the contour-clamped homogeneous electric field DR-II Cell (Bio-Rad, Richmond, Calif.) under the following conditions: 1× TBE gel buffer (90 mM Tris-borate and 1 mM EDTA, pH 8.0), 0.5× TBE tank buffer, 1- to 15-s pulse ramp, 200 V, 14°C, and 22 h. Virioplankton preparations of the August 1995 samples were analyzed under identical electrophoretic conditions for 24 h. After electrophoresis, the gels were stained for 30 min in SYBR Green I (Molecular Probes, Eugene, Oreg.) according to the manufacturer’s instructions and digitally scanned for fluorescence by using a laser fluoroimager (FluorImager; Molecular Dynamics, Sunnyvale, Calif.).
RAPD-PCR of virioplankton DNA.
A single PFGE plug containing total virioplankton DNA from a Chesapeake Bay viral concentrate was melted at 65°C, and 5 μl of the molten DNA-agarose mixture was added to the PCR mix. PCR was carried out in a 25-μl volume containing 2 mM MgCl2; 0.16 mM each dCTP, dATP, dGTP, and dTTP; 0.8 μM primer; a 1× final concentration of Taq polymerase reaction buffer; and 0.5 U of Taq polymerase (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). The primer used in the randomly amplified polymorphic DNA PCR (RAPD-PCR) was either CRA-22 (5′-CCGCAGCCAA-3′) or OPA-13 (5′-CAGCACCCAC-3′) (41). Reaction conditions were as follows: (i) 94°C for 1.5 min, (ii) 50°C for 3.5 min (with 0.5 U of Taq polymerase added), (iii) 35°C for 3 min, (iv) 72°C for 1 min, (v) 94°C for 30 s, (vi) repeat of steps ii to v for 29 cycles, (vii) 35°C for 3 min, and (viii) 72°C for 10 min.
PCR products were separated electrophoretically on a 40 mM Tris-acetate–0.2 mM EDTA (pH 8.0)–2% NuSieve GTG agarose (FMC) gel, and single bands, 200 to 600 bp in size, were cut from the gel. DNAs within the gel slices of single RAPD-PCR bands served as templates for a second round of PCR amplification. Gel slices were melted at 65°C, and 5 μl of the molten DNA-agarose mixture was added for a second PCR with the same conditions and primers. Ten microliters of the second reaction mixture was loaded onto a 0.5× TBE–1.8% Metaphor agarose (FMC) gel and tested for purity. DNA within the remaining 15 μl of the reamplification reaction mixture was quantified, labeled, and tested for use as a probe. In addition, virioplankton DNA within a single band cut from a low-melting-point pulsed-field gel was used as a template for RAPD-PCR probe generation. Details of probes used in this study are presented in Table 1.
TABLE 1.
Virioplankton probes used in this study
| Probe | Size | PCR template or sample used to generate probe | Primer | Primer sequence |
|---|---|---|---|---|
| Band 1a | ca. 40 kb | June 1996, station 818T | NAb | NA |
| RAPD 1 | 220 bp | June 1996, station 834B | CRA-22 | 5′ CCGCAGCCAA 3′ |
| RAPD 2 | 300 bp | June 1996, station 834B | CRA-22 | 5′ CCGCAGCCAA 3′ |
| RAPD 7 | 370 bp | July 1996, station 724T | OPA-13 | 5′ CAGCACCCAC 3′ |
| RAPD 10 | 520 bp | July 1996, station 724T | OPA-13 | 5′ CAGCACCCAC 3′ |
| RAPD E | 400 bp | Band 1 DNA | OPA-13 | 5′ CAGCACCCAC 3′ |
Cut directly from virioplankton PFGE fingerprint.
NA, not applicable.
Hybridization analysis.
Virioplankton DNA within pulsed-field gels was transferred to a nylon membrane (Zeta-Probe; Bio-Rad) by capillary transfer under alkaline conditions (46). Briefly, gels were exposed to 1,200 μJ of UV radiation cm−2 per side in a UV oven (UV Crosslinker; Fisher Scientific), after which they were soaked twice for 30 min, with shaking, in a bath of 0.5 N NaOH and 1.5 M NaCl. Arrangement of the membrane, gel, and blotting pads was according to the manufacturer’s instructions. Upward capillary transfer continued for at least 48 h, after which the membrane was removed from the gel, neutralized for 5 min in 0.5 M Tris (pH 7.0), and rinsed in 2× SSC (0.3 M NaCl and 30 mM Na3C6H5O7). After the rinse, DNA was covalently linked to the membrane by exposure to 1,200 μJ of UV radiation cm−2, followed by drying in a vacuum oven.
Tests of hybridization efficiency were carried out with slot blots of bacteriophage genomic DNA prepared from three Chesapeake Bay bacteriophage isolates: CB 7Φ, CB 38Φ, and CB 45Φ (70). Genomic DNA was prepared from known concentrations of bacteriophage (suspended in 0.9% saline) added to alkaline lysis buffer (0.4 M NaOH, 10 mM EDTA [final concentrations]), heated to 100°C for 10 min, and rapidly cooled on ice. Bacteriophage DNA was deposited onto a Zeta-Probe membrane by using a vacuum blotting device (Life Technologies, Gaithersburg, Md.). The membrane was neutralized, rinsed, cross-linked, and dried, as described above. DNA prepared from the same Chesapeake Bay bacteriophages was radiolabeled by random priming with [α-32P]dCTP (Amersham, Arlington Heights, Ill.) according to the manufacturer’s instructions (Ready-to-Go DNA labeling beads; Pharmacia Biotech Inc., Piscataway, N.J.) and used as a probe against bacteriophage DNA on the nylon membranes. All probes used in the study were radiolabeled by the same random-priming method.
Radiolabeled probes designed for specific viruses consisted of either virioplankton DNA purified from a single band of a pulsed-field gel or a single amplification product of RAPD-PCR, using a total virioplankton DNA template. Preparation of single-band probes was as follows. A virioplankton PFGE plug was electrophoresed, as described above; however, the gel consisted of 1.2% SeaPlaque (FMC) low-melting-point agarose. After SYBR Green I staining, DNA within the gel was visualized on a UV light box, and a single band was cut from the pulsed-field gel and placed in 10 mM Tris–1 mM EDTA (pH 8.0) buffer. Virioplankton DNA within the gel slice was released from the gel matrix by β-agarase digestion (Boehringer Mannheim Biochemicals) and subsequently purified according to the manufacturer’s instructions.
Membranes were incubated in an hybridization oven (Amersham) for at least 30 min at 65°C in hybridization buffer (0.5 M Na2HPO4 [pH 7.2], 7% [wt/vol] SDS, 1 mM EDTA, 10% dextran sulfate). After prehybridization, 32P-radiolabeled probe was added to the hybridization buffer at a final concentration of ca. 2 × 106 cpm ml−1, and the membranes were incubated for at least 20 h. After hybridization, the hybridization buffer was decanted, and membranes were washed twice for 30 min each in buffer 1 (40 mM Na2HPO4 [pH 7.2], 5% SDS, 1 mM EDTA). If blots contained high background levels of radioactivity, the membranes were washed once for 30 min in buffer 2 (40 mM Na2HPO4 [pH 7.2], 1% SDS, 1 mM EDTA).
Densitometric analysis of autoradiograms.
Autoradiographic imaging of virioplankton PFGE Southern blots and Chesapeake Bay bacteriophage slot blots was done with a phosphorescent screen and a phosphorimager (Storm; Molecular Dynamics). The hybridization intensity (relative fluorescence units) was used as a measure of the concentration of a specific viral strain within the virioplankton sample. The software programs Image Quant (Molecular Dynamics) and Photo Shop (Adobe Systems Inc., Mountain View, Calif.) were used to analyze and crop, respectively, the digital images on a Power Macintosh computer (Apple Computer, Cupertino, Calif.).
RESULTS
Detection of single virus strains.
Known titers of bacteriophage CB 45Φ were loaded onto a PFGE gel, electrophoresed, transferred to a nylon membrane, and probed with radiolabeled CB 45Φ DNA. The detection limits for hybridization analysis and SYBR Green I staining of CB 45Φ DNA were ca. 105 and 106 phage particles, respectively (data shown elsewhere [66]). For comparison, known quantities of DNAs prepared from three Chesapeake Bay bacteriophage isolates, CB 45Φ, CB 38Φ, and CB 7Φ, were deposited onto a nylon membrane by vacuum blotting and probed with homologous bacteriophage genomic DNA probes. In each case, the detection limit for a single bacteriophage strain on the slot blot was 105 phage particles (data shown elsewhere [66]), indicating that capillary transfer of viral genomic DNA from virioplankton PFGE fingerprints was as efficient as direct vacuum blotting of viral genomic DNA.
Generation of virioplankton probes by RAPD-PCR.
Total virioplankton DNA or virioplankton DNA recovered from a small region of a virioplankton PFGE fingerprint served as a DNA template in RAPD-PCR. Candidate DNA amplicons for single-virus probes were cut from an electrophoretic gel of RAPD-PCR products and purified. A second round of RAPD-PCR was conducted to test the purity of the single products and to provide DNA suitable for radiolabeling. Candidate probes were hybridized to Southern blots of the original virioplankton template DNA. On average, ca. 20% of probes generated from templates of total virioplankton DNA could be used successfully to detect a single band in the virioplankton PFGE fingerprint. However, all probes generated from RAPD-PCR of virioplankton DNA from a small subregion of a virioplankton PFGE fingerprint detected single bands in the virioplankton PFGE fingerprints.
Hybridization analysis of virioplankton PFGE fingerprints.
Since an objective of the study was to determine temporal and spatial dynamics of viruses within Chesapeake Bay virioplankton communities, a specific virus or group of viruses autochthonous to the Chesapeake Bay was selected. Initial trials, using genomic DNA probes of bacteriophages previously isolated from water samples collected from the Chesapeake Bay (67), were not successful in detecting homologous viral DNA within the virioplankton PFGE fingerprints. Therefore, virioplankton DNA harvested from a small subregion of a virioplankton PFGE fingerprint was utilized to determine the prevalence of this group of virioplankton strains in the Chesapeake Bay at different locations and times. That is, virioplankton DNA, ca. 40 kb in molecular size, was cut from a virioplankton PFGE fingerprint of a water sample collected at station 818 in June 1996 (Fig. 2A). This DNA, designated band 1, was radiolabeled and probed against virioplankton PFGE fingerprints from all other water samples. As shown in Fig. 2, homologous virioplankton DNA was detected in water samples collected during June 1996 from the middle to lower bay (stations 845, 818, and 744). In every case, hybridization of band 1 DNA with virioplankton PFGE fingerprint DNA occurred within the same molecular size region from which the band 1 probe originated. Not surprisingly, June 1996 water samples yielded the strongest signals, notably for stations 744 and 818. However, significant hybridization was also detected in the May and July 1996 water samples. Thus, virioplankton strains comprising band 1 DNA were most abundant in the lower bay during May and June 1996, especially at stations 845 and 724, and were less abundant in the upper-bay water samples (stations 908 and 858). Interestingly, the larger amount of band 1 virus DNA was detected in the June 1996 water sample collected at station 744 and not in the water sample from which band 1 DNA was obtained, i.e., June 1996 from station 818.
FIG. 2.
Autoradiograms and hybridization intensity data. DNA from the rectangle in gel A was radiolabeled and hybridized against virioplankton PFGE fingerprints of Chesapeake Bay water samples. (A) Virioplankton PFGE of water samples from station 818. Lanes: 1, lambda marker; 2, August 1995; 3 to 5, May, June, and July 1996, respectively. (B to G) Autoradiograms of virioplankton PFGE fingerprints of water samples collected at stations 908, 858, 845, 818, 744, and 724, respectively. Lanes 1 to 3, May, June, and July 1996, respectively. The box in gel A outlines the subregion from which probe DNA was harvested. *, water sample from which the probe was generated.
Because band 1 DNA was harvested directly from a virioplankton PFGE fingerprint, it is likely comprised of DNAs from several virus strains. To determine the prevalence of a single virus within the group of viruses represented in band 1, a RAPD-PCR probe, RAPD E (Table 1), was generated by using band 1 as template DNA. As shown in Fig. 3, the pattern of RAPD E hybridization signals was similar to that of band 1 (Fig. 2), and, like band 1, only DNA within the ca. 40-kb region of the virioplankton PFGE fingerprints was detected. The highest abundance of the virus represented by RAPD E occurred in the June 1996 water sample from station 818, the same sample from which the RAPD-PCR template DNA was taken. The RAPD E virus strain was less widespread than its parent group, band 1 viruses, with the largest amounts at stations 845, 818, and 744 during June 1996.
FIG. 3.
Autoradiograms and hybridization data obtained by using RAPD-PCR-generated probe RAPD E. (A to F) Autoradiograms of virioplankton PFGE fingerprints of water samples collected at stations 908, 858, 845, 818, 744, and 724, respectively. Lanes 1 to 3, May, June, and July 1996, respectively. *, water sample from which the probe was generated.
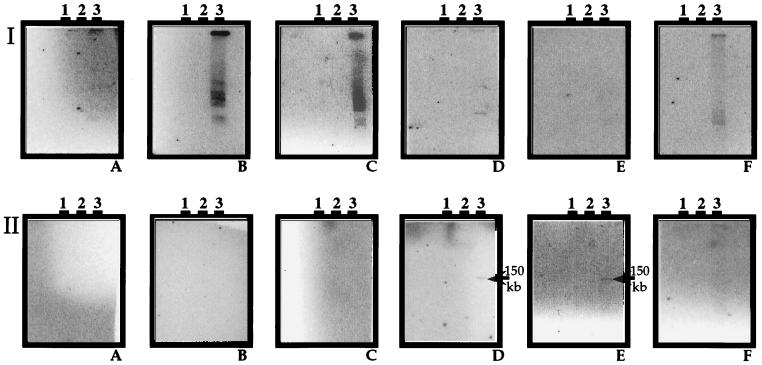
To avoid having to use DNA from a subregion of a virioplankton PFGE fingerprint as either a probe or template DNA in RAPD-PCR, total virioplankton DNA within a PFGE agarose plug was employed directly in RAPD-PCR. As noted above, only 20% of the candidate probes generated from RAPD-PCR of total virioplankton DNA were found to be suitable. Hybridization results with two RAPD-PCR probes, RAPD 7 and RAPD 10 (Table 1), generated from total virioplankton DNA in the July 1996 water sample from station 724, are shown in Fig. 4. Both the RAPD 7 and RAPD 10 probes detected only those virioplankton in water samples collected during July 1996. Unlike other virioplankton probes, RAPD 10 hybridized over a broad range of molecular sizes in the virioplankton PFGE fingerprints, suggesting that the RAPD 10 amplicon may be a common genetic element in Chesapeake Bay virioplankton. RAPD 10-like viruses were not found throughout the Chesapeake Bay but were localized to stations 858, 845, and 724. Probe RAPD 7 demonstrated high specificity, hybridizing to a single virioplankton genome of ca. 150 kb located at stations 818 and 744.
FIG. 4.
Autoradiograms of virioplankton PFGE fingerprints probed with RAPD 10 and RAPD 7. Probes were generated from virioplankton DNA of the water sample from station 724 in July 1996. (Series I) Southern blots probed with RAPD 10. (Series II) Southern blots probed with RAPD 7. (A to F) Water samples from stations 908, 858, 845, 818, 744, and 724, respectively. Lanes 1 to 3, May, June, and July 1996 samples, respectively.
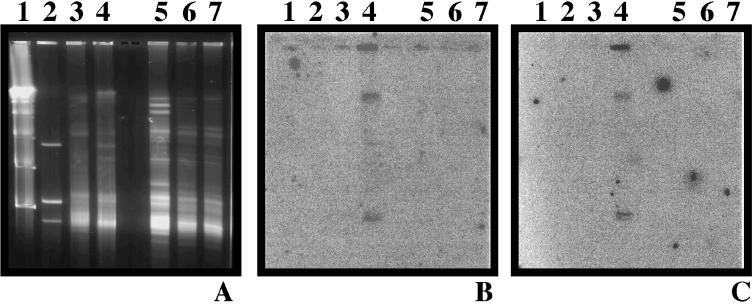
RAPD-PCR probes from total virioplankton DNA were used to detect the occurrence of a virus throughout the water column. That is, RAPD-PCR probes were generated from total virioplankton DNA in a water sample collected 1 m above the bottom, i.e., the sediment-water interface, at station 834 during June 1996. These two probes, RAPD 1 and RAPD 2 (Table 1), were hybridized against virioplankton PFGE fingerprints of surface and bottom water samples collected in June 1996 at station 834 and in July 1996 at station 818. As shown in Fig. 5, RAPD 1 and RAPD 2 revealed identical patterns of hybridization, showing a positive signal only in the bottom water sample from station 834 in June 1996. Specifically, probes RAPD 1 and RAPD 2 hybridized to three bands within the virioplankton fingerprint of the June 1996 sample from station 834, at molecular sizes of ca. 40 and 200 kb and the plug well. The three bands appear to represent a single virus genome, most likely the 40-kb band, concatemerized to form the larger 200-kb fragment, and the fragment retained in the plug well during electrophoresis. Yet, the specificity of RAPD 1 and RAPD 2 for a virus or viruses found only in the water sample collected at the bottom indicates that in a stratified water column, significant differences can exist among and between virus populations in surface and bottom waters.
FIG. 5.
Autoradiograms and virioplankton PFGE fingerprints of virioplankton communities in surface and bottom waters. (A) Virioplankton PFGE fingerprint. (B) Autoradiogram of panel A probed with RAPD 1. (C) Autoradiogram of panel A probed with RAPD 2. Lanes: 1, molecular size markers of lambda phage concatamers; 2, 108 PFU each of genomic DNAs from three Chesapeake Bay bacteriophages; 3, virioplankton PFGE of water sample from station 834 in June 1996; 4, virioplankton PFGE of water sample from station 834B in June 1996; 5, 6, and 7, virioplankton PFGE of water samples at various depths from station 818 in July 1996.
DISCUSSION
Detection and quantification of specific viruses within the virioplankton can be achieved, thus allowing for observations of changes in abundances of virioplankton strains with time and space. In each instance, the abundance of a specific virus or group of viruses with similar genome sizes was localized to a specific region of the bay or depth in the water column. When examined over time, titers of a specific virus(es) in water samples peaked and then declined to a low, background level. Overall, the abundance of a virus(es) within the virioplankton changed in an episodic, bloom-like fashion. These observations have important implications for understanding the population dynamics and function of virioplankton communities. If episodic blooms in abundance are typical of most viruses within Chesapeake Bay virioplankton, then the overall composition of the virioplankton community should change dramatically over time and space. Indeed, the findings of this study, with data demonstrating by PFGE analysis that the overall composition of the Chesapeake Bay virioplankton changes significantly with time and geographic location (72), support this conclusion. Furthermore, temporal and spatial dynamics related to abundances of individual viruses in Chesapeake Bay, as observed in this study, suggest that viral infection and lysis, by selective elimination of numerically dominant hosts, can affect the clonal composition of plankton host communities.
By using a radiolabeled probe, it was found that the detection limit for a specific bacteriophage on a PFGE gel was ca. 105 virus particles. At this level of sensitivity, a virus comprising 1/1,000 of the total virioplankton population can be detected. In the Chesapeake Bay, the total virioplankton abundance is ca. 107 viruses ml−1 (69). By employing the viral concentration methods described in this study, it is possible to detect a single virus species at an in situ abundance of 104 ml−1. This compares well with results of previous studies using hybridization methods to measure abundances of bacteriophages of Pseudomonas aeruginosa in lake water (42, 43).
The most sensitive assays for specific viruses in water samples are those based on titers of infectious viruses (14, 54, 63). However, these methods (plaque assay or most probable number) require isolation and culture of specific host strains. Such approaches are inappropriate for viral ecology because of bias towards viruses infecting host strains that can be cultured, as is the case for those bacteriophages infecting heterotrophic bacterial species, a group that comprises the majority of the bacterioplankton biomass (16). Overall, little is known about the community structure of heterotrophs, principally because only a small proportion (1 to 2%) of autochthonous heterotrophic aquatic bacteria can be grown in culture (34). Recent findings suggest that the low culturability of many autochthonous heterotrophic bacteria may be related to low plating efficiency on bacteriological culture media (47). Nevertheless, given that hundreds, if not thousands, of bacterial species are believed to comprise the bacterioplankton (13), it is not yet feasible to determine whether a given bacterial isolate is actually a dominant and/or important member of the bacterioplankton community, at least with methods currently available.
Prior to this study, the distributions and abundances of phages infecting four autochthonous bacterial hosts in the Chesapeake Bay were examined by plaque assay (71). The results illustrate the difficulty of the culture-based approach. For example, of 36 water samples collected at six stations during different times of the year, only 10 samples contained bacteriophages infecting one of the host bacterial strains. Among the 10 successful bacteriophage isolations, only two of the titers exceeded the detection limit, i.e., 1 PFU. After taking into account concentration of 10- to 100-fold prior to plaque assay, the final estimate of phage abundance in the positive water samples was 7 PFU liter−1. In our experience, it is extremely difficult to investigate the population dynamics of specific viruses infecting heterotrophic bacteria by plaque assay. There are cases where titer determination methods have been successful in viral ecology, most notably in studies of the distributions and abundances of viruses of the photosynthetic marine picoflagellate Micromonas pusilla (14) and marine Synechococcus spp. (22, 54, 55, 63).
Virioplankton probe design.
Studies utilizing nucleic acid probes to examine either the taxonomy of autochthonous viral strains or occurrence of specific viruses in environmental samples have generally relied on probes constructed by utilizing genetic information gathered for specific viruses. These probes have consisted of total genomic DNA prepared from a bacteriophage isolate (15, 42, 43), a single restriction fragment from a bacteriophage isolate (30), or a PCR amplicon of a conserved viral gene (8–11). While these approaches are important in providing taxonomic information, they require isolation and culture. In the case of PCR, detection sensitivity is often gained at the expense of quantitative information concerning in situ viral abundance. For studies of the occurrence of bacterial species in the environment, the availability of taxonomically conserved genetic elements has provided options for design of nucleic acid probes specific to functional or phylogenetic bacterial groups. Overwhelmingly, the molecule of choice for ecological studies of bacterial consortia has been 16S rRNA (2).
Because viruses are metabolically inactive and possess reduced genomes, viruses might be considered to be less likely to contain taxonomically conserved genetic elements useful in designing a strain- or group-specific probe. An exception is the DNA polymerase gene (pol) of algal viruses, which was used by Chen and colleagues to examine diversity of algal viruses in the Gulf of Mexico (8–11).
Our initial approach for examining the dynamics of viruses comprising the virioplankton was to develop probes from Chesapeake Bay bacteriophage isolates. In several trials we were unable to detect any of our isolates within the virioplankton DNA on a pulsed-field gel. Therefore, by utilizing molecular approaches, probes to viruses known to be present within the virioplankton consortia were developed, utilizing either viral DNA harvested from a pulsed-field gel or a RAPD-PCR amplicon of virioplankton DNA as a nucleic acid probe. These virioplankton probes hybridized to single bands within virioplankton PFGE fingerprints; a good example is the band 1 and RAPD E probes, which hybridized only to DNA within the molecular size region from which the probe was generated. Intuitively, each RAPD-PCR product from a virioplankton DNA template should be derived from a single virus strain. However, it is possible that in PCR of complex genomic mixtures, such as total virioplankton DNA, chimeric amplification products can result, thus lowering the specificities of amplicons used as probes. This is especially true in the case of highly conserved genes, such as that for 16S rRNA, where PCR has been recently shown to yield a high incidence (up to 32%) of chimeras (62). To avoid the possibility of enriching RAPD-PCR with chimeras, PCR of virioplankton DNA was restricted to 30 cycles. Lower numbers of amplification cycles also prevented overproduction of rare amplicons. Nevertheless, a majority of RAPD-PCR amplification products were probably specific for viruses at a low in situ density, as only 20% of RAPD-PCR probes generated from total virioplankton DNA hybridized to DNA within virioplankton PFGE fingerprints. It is interesting that for studies of bacterial consortia, probes based on 16S rRNA sequences cannot discriminate between bacterial strains within a species. Two recent studies examining the prevalence of bacterial strains within a complex bacterial community utilized probes generated from either RAPD-PCR (20) or repetitive-sequence PCR (36) to overcome this deficiency of 16S rRNA-based probing.
Viral infection and the maintenance of host community diversity.
Many investigators have speculated that viruses in aquatic microbial communities influence the diversity and clonal composition of bacterio- and phytoplankton populations (4, 6, 21, 22, 32, 44, 52, 53, 56, 58, 63). Indeed, the levels of in situ virus-mediated mortality estimated in the literature indicate that this mortality may be an incidental outcome of selective viral control over the densities of specific hosts. It has been proposed that widespread and universal viral infection within aquatic microbial communities may serve as an explanation for the paradoxical situation noted by Hutchinson (26) more than 35 years ago, i.e., the question of why, in a relatively homogenous aquatic environment, instances of diverse assemblages of coexisting phytoplankton species (and by extension bacterioplankton strains) competing for similar resources occur, when theory would predict only a few dominant species (4, 22). The specific nature of viral predation, combined with the importance of host cell density to viral propagation, can be envisioned to exert selective mortality on those species which exceed a defined stoichiometric density limit.
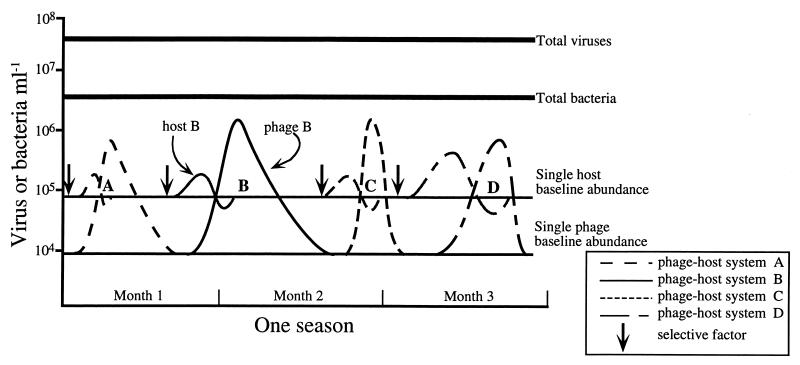
A conceptual model which depicts changes in the abundances of four phage-host systems within a consortium of virio- and bacterioplankton (Fig. 6) illustrates how the data presented on changes in the abundance of a specific virus(es) fit the hypothesis that viral lysis is involved in maintenance of host community diversity. It is assumed that within a given season of a calendar year, the total viral and total bacterial abundances remain relatively stable, with the former usually exceeding the latter by a factor of 10 (69), and that within a typical aquatic microbial community, there is a subset of active bacterial species accounting for the majority of the total bacterial abundance (47). The model given in Fig. 6 represents eutrophic estuarine waters, where total bacterial and viral abundances are ca. 106 and 107 ml−1, respectively. This community is hypothesized to contain 10 to 50 different bacterial species, with an average abundance of each individual species of ca. 105 ml−1, and 100 to 300 different bacteriophage strains, with an average abundance of a single phage type of ca. 104 ml−1. The degree to which the number and abundance of a specific phage and bacterial species used in this hypothetical model reflect a typical microbial consortium in estuarine waters is not known at this time. It has been speculated that a coastal seawater sample could be expected to contain between 100 and 300 different phage-host systems (3, 5), and the actual number of bacterial species within the consortium could be much higher if many species are at a low density (ca. 103 ml−1). The strength of assumptions concerning the constitution of specific host and viral abundances cannot be judged, as suitable techniques for unbiased assessment of the diversity and abundance of individual bacterial and bacteriophage species within a complex natural community are only now being developed.
FIG. 6.
Conceptual model of virioplankton regulation of host community diversity. For each phage-host system, a selective factor stimulates growth of a specific host. An epidemic of phage infection begins at a critical threshold host cell density, and the abundance of a specific phage increases; thereafter, phage lysis causes the abundance of host cells to decline to background levels, preventing overdominance of a single host species. At the end of the epidemic, numbers of infective phage decline to a baseline level at a decay rate specific for each phage. It is also possible that the phage-host systems are temperate. Stimulation of host growth by a selective event causes curing of lysogeny and thus a release of phage. While abundances of specific hosts and phages change rapidly, the overall abundance of virio- and bacterioplankton is stable over longer, seasonal scales. A and D, moderate burst size of 10 to 50; B and C, large burst size of 100 to 500; A and B, low decay rate; C and D, high decay rate.
The proposed mechanism by which viral infection influences host community diversity is selective destruction of only those bacterial strains at high concentration and undergoing fast growth. This process of selectively “killing the winner populations,” recently modeled by Thingstad and Lignell (59), begins with an event, such as the influx of a particular limiting nutrient, which creates favorable growth conditions for a single bacterial species (species A). In response to increased nutrient availability, species A undergoes rapid growth and quickly increases in population density. Eventually, the density of species A reaches a critical threshold at which an epidemic of phage (ΦA) infection ensues. A dramatic increase in the titer of ΦA occurs, and eventually the microepidemic of phage infection reduces the number of sensitive host cells to an abundance well below the threshold necessary to maintain phage production. At the height of the epidemic, ΦA could reach a concentration 10 to 50 times greater than that of its host and possibly 100 times greater than its usual, nonepidemic concentration. Eventually, the microepidemic of ΦA infection ceases once the frequency of ΦA-strain A encounters no longer supports viral production at a level necessary to replace the loss of infectious virions.
These bloom-like changes in the abundance of a single virus, predicted in Fig. 6, may explain the dramatic changes in abundance observed for the virus(es) hybridizing to probes band 1 and RAPD E. Indeed, it is plausible that increases in the abundances of these viruses resulted from lysis of particular bacterioplankton strains. The question remains, however, whether the abundances of the bacterial strains responsible for producing these viruses were significantly reduced. The model (Fig. 6) addresses the possibility that during a microepidemic, free phage are produced either directly through infection and lysis (virulent bacteriophages) or via induction of prophage from lysogenic bacteria (temperate bacteriophages). However, it does not include viral production via leakage or budding, a process limited to a few rare bacteriophage groups (1). It has been demonstrated that, in some instances, lysogens (17–19) and phage-susceptible strains (7, 23, 51) possess a competitive growth advantage over nonlysogens and phage-resistant strains. It is also known that sudden changes in the growth rate of a lysogen from improved growth conditions can cause induction of prophage and subsequent host cell lysis (35). Therefore, the bacterial microblooms depicted in Fig. 6 could easily represent subpopulations of lysogenic bacteria or phage-susceptible bacteria which, upon reaching high growth rates, are lysed through induction of prophage or by direct phage infection. The phenomenon of bursts in phage production, with changes in nutrient status or environmental quality, is perhaps best demonstrated by those phage-host systems identified as pseudolysogenic (43, 48, 60, 65). Indeed, if many aquatic bacteriophages are capable of pseudolysogeny, as has been suggested (1, 38), this would support many aspects of the model presented in Fig. 6.
The suggestion that selective events can lead to monospecific bacterial blooms within aquatic microbial communities is supported by the frequent observations of natural phytoplankton blooms. Heterotrophic bacterial communities undergo similar bursts in the abundance of a single strain. Early autecology studies of Vibrio parahaemolyticus in the Chesapeake Bay (28, 29), as well as recent data (47, 61), corroborate this view. Involvement of viral infection in phytoplankton bloom dynamics has been suspected for some time (see reviews by Padan and Shilo [45] and Zingone [73]), and there is strong circumstantial evidence implicating viral lysis in bloom collapse. Concomitant increases in the numbers of both free and intracellular viruses with the decline of natural or stimulated monospecific algal blooms has been documented for several important bloom-forming algae (4, 37, 39, 40, 50).
The most promising approaches for investigating effects of viruses on host community diversity are those which utilize sensitive and nonselective methods, as in this study, to examine in situ temporal changes in abundances of specific virio- or bacterioplankton. The results of this study support predictions (Fig. 6) concerning changes in the abundances of specific viruses with time. The abundance of a virus(es) hybridizing to the probes band 1 and RAPD E changed significantly over a 2-month period, i.e., between the first weeks of May and July 1996 (Fig. 1 and 2). In both cases, viral abundance was low in May and July 1996 water samples and high in June 1996 water samples. When hybridized against the range of virioplankton PFGE fingerprints, all virioplankton probes demonstrated a specificity for viruses found in specific geographical locations in the Chesapeake Bay. Probes RAPD 1 and RAPD 2 hybridized only to viruses in bottom water samples collected at station 834, suggesting that physiochemical changes occurring with depth influence the structure of virioplankton populations. These observations indicate that Chesapeake Bay virioplankton should be viewed as a highly dynamic and active community in which those viruses comprising the most abundant members are constantly changing. At present, it is not clear whether virioplankton populations from other aquatic environments, such as the oligotrophic ocean, will demonstrate similar dynamics. Together, results showing the Chesapeake Bay virioplankton community structure to be temporally and spatially variable (72), coupled with findings of this study, support the hypothesis that viral infection can affect significantly the clonal diversity of bacterio- and phytoplankton communities.
ACKNOWLEDGMENTS
We acknowledge the excellent cooperation of Wayne Coates and Diane Stoecker, permitting K.E.W. to participate in research cruises, and the assistance of the crew of the R/V Cape Henlopen.
Footnotes
Contribution no. 316 from the Center of Marine Biotechnology; contribution no. 913 from the Australian Institute of Marine Science.
REFERENCES
- 1.Ackermann H W, DuBow M S. Viruses of prokaryotes: general properties of bacteriophages. Boca Raton, Fla: CRC Press, Inc.; 1987. [Google Scholar]
- 2.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergh O, Borsheim K Y, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 4.Bratbak G, Egge J K, Heldal M. Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar Ecol Prog Ser. 1993;93:39–48. [Google Scholar]
- 5.Bratbak G, Heldal M, Thingstad T F, Tuomi P. Dynamics of virus abundance in coastal seawater. FEMS Microbiol Ecol. 1996;19:263–269. [Google Scholar]
- 6.Bratbak G, Thingstad F, Heldal M. Viruses and the microbial loop. Microb Ecol. 1994;28:209–221. doi: 10.1007/BF00166811. [DOI] [PubMed] [Google Scholar]
- 7.Chao L, Levin B R, Stewart F M. A complex community in a simple habitat: an experimental study with bacteria and phage. Ecology. 1977;58:369–378. [Google Scholar]
- 8.Chen F, Suttle C A. Amplification of DNA polymerase gene fragments from viruses infecting microalgae. Appl Environ Microbiol. 1995;61:1274–1278. doi: 10.1128/aem.61.4.1274-1278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Suttle C A. Evolutionary relationships among large double-stranded DNA viruses that infect microalgae and other organisms as inferred from DNA polymerase genes. Virology. 1996;219:170–178. doi: 10.1006/viro.1996.0234. [DOI] [PubMed] [Google Scholar]
- 10.Chen F, Suttle C A. Nested PCR with three highly degenerate primers for amplification and identification of DNA from related organisms. BioTechniques. 1995;18:609–612. [PubMed] [Google Scholar]
- 11.Chen F, Suttle C A, Short S M. Genetic diversity in marine algal virus communities as revealed by sequence analysis of DNA polymerase genes. Appl Environ Microbiol. 1996;62:2869–2874. doi: 10.1128/aem.62.8.2869-2874.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochlan W P, Wikner J, Steward G F, Smith D C, Azam F. Spatial distribution of viruses, bacteria, and chlorophyll a in neritic, oceanic and estuarine environments. Mar Ecol Prog Ser. 1993;92:77–87. [Google Scholar]
- 13.Colwell R R. Microbial diversity: the importance of exploration and conservation. J Ind Microbiol Biotechnol. 1997;18:302–307. doi: 10.1038/sj.jim.2900390. [DOI] [PubMed] [Google Scholar]
- 14.Cottrell M T, Suttle C A. Dynamics of a lytic virus infecting the photosynthetic marine picoflagellate Micromonas pusilla. Limnol Oceanogr. 1995;40:730–739. [Google Scholar]
- 15.Cottrell M T, Suttle C A. Genetic diversity of algal viruses which lyse the photosynthetic picoflagellate Micromonas pusilla (Prasinophyceae) Appl Environ Microbiol. 1995;61:3088–3091. doi: 10.1128/aem.61.8.3088-3091.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducklow H W, Purdie D A, Williams P J LeB, Davies J M. Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science. 1986;232:863–867. doi: 10.1126/science.232.4752.865. [DOI] [PubMed] [Google Scholar]
- 17.Edlin G. Alteration of Escherichia coli outer membrane proteins by prophages. A model for benevolent virus-cell interaction. In: Stevens J G, Todaro G J, Fox C F, editors. Persistent viruses. Vol. 11. New York, N.Y: Academic Press; 1978. pp. 1–14. [Google Scholar]
- 18.Edlin G, Lin L, Bitner R. Reproductive fitness of phage P-1 lysogen, phage P-2 lysogen, and phage Mu lysogen of Escherichia coli. J Virol. 1977;21:560–564. doi: 10.1128/jvi.21.2.560-564.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edlin G, Lin L, Kudrna R. Lambda lysogens of Escherichia coli reproduce more rapidly than non-lysogens. Nature. 1975;255:735–737. doi: 10.1038/255735a0. [DOI] [PubMed] [Google Scholar]
- 20.Erlandson K, Batt C A. Strain-specific differentiation of lactococci in mixed starter culture populations by using randomly amplified polymorphic DNA-derived probes. Appl Environ Microbiol. 1997;63:2702–2707. doi: 10.1128/aem.63.7.2702-2707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuhrman J. Bacterioplankton roles in cycling of organic matter: the microbial food web. In: Falkowski P G, Woodhead A D, editors. Primary productivity and biogeochemical cycles in the sea. New York, N.Y: Plenum Press; 1992. pp. 361–383. [Google Scholar]
- 22.Fuhrman J A, Suttle C A. Viruses in marine planktonic systems. Oceanography. 1993;6:50–62. [Google Scholar]
- 23.Gill M L, Nealson K. Isolation and host range studies of marine bacteriophage. Biol Bull. 1972;143:463–464. [Google Scholar]
- 24.Hantula J, Kurki A, Vuoriranta P, Bamford D H. Ecology of bacteriophages infecting activated sludge bacteria. Appl Environ Microbiol. 1991;57:2147–2151. doi: 10.1128/aem.57.8.2147-2151.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hennes K P, Suttle C A, Chan A M. Fluorescently labeled virus probes show that natural virus populations can control the structure of marine microbial communities. Appl Environ Microbiol. 1995;61:3623–3627. doi: 10.1128/aem.61.10.3623-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson G E. The paradox of the plankton. Am Nat. 1961;95:137–145. [Google Scholar]
- 27.Jiang S C, Paul J H. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar Ecol Prog Ser. 1994;104:163–172. [Google Scholar]
- 28.Kaneko T, Colwell R R. Distribution of Vibrio parahaemolyticus and related organisms in the Atlantic Ocean off South Carolina and Georgia. Appl Microbiol. 1975;28:1009–1017. doi: 10.1128/am.28.6.1009-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko T, Colwell R R. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J Bacteriol. 1973;113:24–32. doi: 10.1128/jb.113.1.24-32.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellogg C A, Rose J B, Jiang S C, Thurmond J M, Paul J H. Genetic diversity of related vibriophages isolated from marine environments around Florida and Hawaii, USA. Mar Ecol Prog Ser. 1995;120:89–98. [Google Scholar]
- 31.Lenski R E. Dynamics of interactions between bacteria and virulent bacteriophage. Adv Microb Ecol. 1988;10:1–44. [Google Scholar]
- 32.Lenski R E, Levin B R. Constraints on the coevolution of bacteria and virulent phage: a model, some experiments, and predictions for natural communities. Am Nat. 1985;125:585–602. [Google Scholar]
- 33.Levin B R, Stewart F M, Chao L. Resource limited growth, competition, and predation: a model and experimental studies with bacteria and bacteriophage. Am Nat. 1977;111:3–24. [Google Scholar]
- 34.MacLeod R A. The question of the existence of specific marine bacteria. Bacteriol Rev. 1965;29:2–23. [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh P, Wellington E M H. Phage-host interactions in soil. FEMS Microbiol Ecol. 1994;15:99–107. [Google Scholar]
- 36.Matheson V G, Munakata-Marr J, Hopkins G D, McCarty P L, Tiedje J M, Forney L J. A novel means to develop strain-specific DNA probes for detecting bacteria in the environment. Appl Environ Microbiol. 1997;63:2863–2869. doi: 10.1128/aem.63.7.2863-2869.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milligan K L D, Cosper E M. Isolation of virus capable of lysing the brown tide microalga, Aureococcus anophagefferens. Science. 1994;266:805–807. doi: 10.1126/science.266.5186.805. [DOI] [PubMed] [Google Scholar]
- 38.Moebus K. Marine bacteriophage reproduction under nutrient-limited growth of host bacteria. 2. Investigations with phage-host system [H3:H3/1] Mar Ecol Prog Ser. 1996;144:13–22. [Google Scholar]
- 39.Nagasaki K, Ando M, Imai I, Itakura S, Ishida Y. Virus-like particles in Heterosigma akashiwo (Raphidophyceae): a possible red tide disintegration mechanism. Mar Biol. 1994;119:307–312. [Google Scholar]
- 40.Nagasaki K, Ando M, Itakura S, Imai I, Ishida Y. Viral mortality in the final stage of Heterosigma akashiwo (Raphidophyceae) red tide. J Plankton Res. 1994;16:1595–1599. [Google Scholar]
- 41.Neilan B A. Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Appl Environ Microbiol. 1995;61:2286–2291. doi: 10.1128/aem.61.6.2286-2291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogunseitan O A, Sayler G S, Miller R V. Application of DNA probes to analysis of bacteriophage distribution patterns in the environment. Appl Environ Microbiol. 1992;58:2046–2052. doi: 10.1128/aem.58.6.2046-2052.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogunseitan O A, Sayler G S, Miller R V. Dynamic interactions of Pseudomonas aeruginosa and bacteriophages in lake water. Microb Ecol. 1990;19:171–185. doi: 10.1007/BF02012098. [DOI] [PubMed] [Google Scholar]
- 44.Olofsson S, Kjelleberg S. Virus ecology. Nature. 1991;351:612–613. [Google Scholar]
- 45.Padan E, Shilo M. Cyanophages—viruses attacking blue-green algae. Bacteriol Rev. 1973;37:343–370. doi: 10.1128/br.37.3.343-370.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reed K C, Mann D A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985;13:7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rehnstam A S, Bäckman S, Smith D C, Azam F, Hagström Å. Blooms of sequence-specific culturable bacteria in the sea. FEMS Microbiol Ecol. 1993;102:161–166. [Google Scholar]
- 48.Ripp S, Miller R V. The role of pseudolysogeny in bacteriophage-host interactions in a natural freshwater environment. Microbiology. 1997;143:2065–2070. doi: 10.1099/00221287-143-6-2065. [DOI] [PubMed] [Google Scholar]
- 49.Ripp S, Ogunseitan O A, Miller R V. Transduction of a freshwater microbial community by a new Pseudomonas aeruginosa generalized transducing phage, UT1. Mol Ecol. 1994;3:121–126. doi: 10.1111/j.1365-294x.1994.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 50.Sieburth J M, Johnson P W, Hargraves P E. Ultrastructure and ecology of Aureococcus anophagefferens new genus new species Chrysophyceae, the dominant picoplankter during a bloom in Narragansett Bay, Rhode Island, USA, summer 1985. J Phycol. 1988;24:416–425. [Google Scholar]
- 51.Spanakis E, Horne M T. Co-adaptation of Escherichia coli and coliphage lambda vir in continuous culture. J Gen Microbiol. 1987;133:353–360. doi: 10.1099/00221287-133-2-353. [DOI] [PubMed] [Google Scholar]
- 52.Steward G F, Smith D C, Azam F. Abundance and production of bacteria and viruses in the Bering and Chukchi seas. Mar Ecol Prog Ser. 1996;131:287–300. [Google Scholar]
- 53.Suttle C A. The significance of viruses to mortality in aquatic microbial communities. Microb Ecol. 1994;28:237–243. doi: 10.1007/BF00166813. [DOI] [PubMed] [Google Scholar]
- 54.Suttle C A, Chan A M. Dynamics and distribution of cyanophages and their effect on marine Synechococcus spp. Appl Environ Microbiol. 1994;60:3167–3174. doi: 10.1128/aem.60.9.3167-3174.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suttle C A, Chan A M. Marine cyanophages infecting oceanic and coastal strains of Synechococcus: abundance, morphology, cross-reactivity and growth characteristics. Mar Ecol Prog Ser. 1993;92:99–109. [Google Scholar]
- 56.Suttle C A, Chan A M, Cottrell M T. Infection of phytoplankton by viruses and reduction of primary productivity. Nature. 1990;347:467–469. [Google Scholar]
- 57.Suttle C A, Chan A M, Cottrell M T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991;57:721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thingstad T F, Heldal M, Bratbak G, Dundas I. Are viruses important partners in pelagic food webs? Trends Ecol Evol. 1993;8:209–212. doi: 10.1016/0169-5347(93)90101-T. [DOI] [PubMed] [Google Scholar]
- 59.Thingstad T F, Lignell R. Theoretical models for the control of bacterial growth rate, abundance, diversity and carbon demand. Aquat Microb Ecol. 1997;13:19–27. [Google Scholar]
- 60.Torsvik T, Dundas I D. Persisting phage infection in Halobacterium salinarium str. 1. J Gen Virol. 1980;47:29–36. [Google Scholar]
- 61.Tuomi P, Torsvik T, Heldal M, Bratbak G. Bacterial population dynamics in a meromictic lake. Appl Environ Microbiol. 1997;63:2181–2188. doi: 10.1128/aem.63.6.2181-2188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang G C-Y, Wang Y. Frequency of formation of chimeric molecules as a consequence of PCR coamplification of 16S rRNA genes from mixed bacterial genomes. Appl Environ Microbiol. 1997;63:4645–4650. doi: 10.1128/aem.63.12.4645-4650.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waterbury J B, Valois F W. Resistance to cooccurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol. 1993;59:3393–3399. doi: 10.1128/aem.59.10.3393-3399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weinbauer M G, Fuks D, Peuzzi P. Distribution of viruses and dissolved DNA along a coastal trophic gradient in the northern Adriatic Sea. Appl Environ Microbiol. 1993;59:4074–4082. doi: 10.1128/aem.59.12.4074-4082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson W H, Carr N G, Mann N H. The effect of phosphate status on the kinetics of cyanophage infection in the oceanic cyanobacterium Synechococcus sp. WH7803. J Phycol. 1996;32:506–516. [Google Scholar]
- 66.Wommack K E. Aspects of the ecological role of bacteriophages. Ph.D. dissertation. College Park: University of Maryland; 1998. [Google Scholar]
- 67.Wommack K E, Hill R T, Colwell R R. Abstracts of the 93rd General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Ecological studies on natural and cultured estuarine bacteriophages, abstr. Q-288; p. 399. [Google Scholar]
- 68.Wommack K E, Hill R T, Colwell R R. A simple method for the concentration of viruses from natural water samples. J Microbiol Methods. 1995;22:57–67. [Google Scholar]
- 69.Wommack K E, Hill R T, Kessel M, Russek-Cohen E, Colwell R R. Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol. 1992;58:2965–2970. doi: 10.1128/aem.58.9.2965-2970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wommack K E, Hill R T, Muller T A, Colwell R R. Effects of sunlight on bacteriophage viability and structure. Appl Environ Microbiol. 1996;62:1336–1341. doi: 10.1128/aem.62.4.1336-1341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wommack K E, Hill R T, Ravel J, Colwell R R. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Analysis of bacteriophage distribution patterns in Chesapeake Bay utilizing classical and molecular approaches, abstr. N-23; p. 159. [Google Scholar]
- 72.Wommack K E, Ravel J, Hill R T, Chun J, Colwell R R. Population dynamics of Chesapeake Bay virioplankton: total-community analysis by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1999;65:231–240. doi: 10.1128/aem.65.1.231-240.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zingone A. The role of viruses in the dynamics of phytoplankton blooms. Giornale Botanico Italiano. 1995;129:415–423. [Google Scholar]