Abstract
Objective
To evaluate the utility of screening brain/orbital magnetic resonance imaging (MRI) in a large population of children with neurofibromatosis type 1 (NF1) over a 20-year period.
Study design
A retrospective analysis of clinical and imaging data from children with NF1 seen at a single center between 1990 and 2010 was performed.
Results
During the 20-year study period, 826 individuals with NF1 (402 females, 424 males) ages 1–9 years were screened for optic pathway gliomas (OPGs) using brain/orbital MRI; 18% were identified with OPGs with a median age at detection of 3 years. Fifteen percent of patients with OPGs had radiologic or clinical progression requiring therapy. Children with chiasmatic and postchiasmatic tumors were more likely to require therapy compared with patients with prechiasmatic OPGs (P < .0001). Patients with visual deficits at the time of diagnosis were more likely to experience visual decline despite therapy when compared with patients treated based on radiologic progression (P < .012).
Conclusions
Our findings confirm that chiasmatic and postchiasmatic OPG in children with NF1 have the highest risk for progression and vision loss. Early identification of OPG by screening MRI prior to the development of vision loss may lead to improved visual outcomes. Children with negative brain and orbital MRI screening at age 15 months or later did not develop symptomatic OPGs.
Neurofibromatosis type 1 (NF1) is a common autosomal dominant disorder with an incidence of 1 in 3000 individuals and which affects multiple systems of the body.1,2 Central nervous system (CNS) complications associated with NF1 include CNS tumors, learning disabilities, and attention deficit hyperactivity disorder. Optic pathway gliomas (OPGs) are the most common CNS tumors seen in NF1 and represent 3%−6% of all childhood brain tumors.3,4 They are found in 15%−21% of individuals with NF1 and are typically benign, low grade gliomas that predominantly occur in early childhood.1,5–8
OPGs in children with NF1 frequently remain indolent. This differs from OPGs in the general population, which are more aggressive tumors. However, when symptomatic, OPGs can lead to vision loss, hypothalamic abnormalities including precocious puberty, and account for significant morbidity in a subset of children with NF1.3 There is a lack of data regarding optimal imaging surveillance of OPGs. Most centers recommend annual ophthalmology examinations for young children with NF1, but there is no consensus on the utility of magnetic resonance imaging (MRI) in this population.4 Several authors have advocated that asymptomatic young children with NF1 should be screened with ophthalmologic examinations only and that brain MRI screening is unwarranted.9 However, many other physicians still routinely perform screening brain MRIs, and this has remained a controversial area within the NF1 field.
At present, treatment options for OPGs include surgery, radiotherapy, and chemotherapy. Surgical treatment of NF1 OPGs is generally to be avoided for these tumors.4,10 Radiotherapy causes unnecessary neurovascular, endocrinologic, and neuropsychological sequelae, particularly in young patients, and for the most part is not indicated for patients with NF1 and OPG.4,10 Chemotherapy has become the preferred treatment for OPGs, particularly in children under the age of 5 years,10 and avoids the long-term toxicities associated with surgery and radiotherapy.4
The objective of this study was to evaluate the utility of screening brain and orbital MRIs in a large population of children with NF1 over a 20-year period in a single neurofibromatosis (NF) center.
Methods
Children who met the National Institutes of Health NF1 consensus diagnostic criteria11 were identified from the NF Center of the Cincinnati Children’s Hospital Medical Center (CCHMC) by chart review from 1990–2010. During that time period, all children with NF1 at CCHMC underwent baseline MRI of brain and orbits with and without contrast at approximately 15 months of age or at the time an NF1 diagnosis was made, whichever was later. Those children identified with OPG were followed with detailed ophthalmologic examinations and repeat brain/orbital MRI every 3–6 months until stability of the OPG was documented. All other patients had annual ophthalmology examinations, with attention to visual acuity, afferent pupillary defect, color vision, and visual fields (in those old enough to cooperate). Patients were seen by members of the multidisciplinary NF team; they were initially seen by a geneticist and subsequently referred to a pediatric neuro-oncologist after diagnosis of OPG was made.
A retrospective analysis of data from clinical information, imaging data, and treatment history of this patient population was performed. The chart for each patient was reviewed with regards to age at NF1 diagnosis, age at OPG diagnosis, evidence of tumor progression, sex, ethnicity, ophthalmologic examination findings, and family history. MRI scans for each patient had been read by one of a group of neuroradiologists familiar with NF1; the images were not reinterpreted by a radiologist for the purposes of this study. When an OPG was identified, the location was recorded as prechiasmatic, chiasmatic, and/ or postchiasmatic; and as unilateral or bilateral. OPG location was classified according to the most posteriorly involved structure of the visual pathway. Information regarding type of chemotherapy, response, relapse, surgeries, endocrine abnormalities, and visual outcomes was obtained from the 22 patients who underwent treatment for symptomatic OPGs. This population was followed until December 2010. The study was approved by the CCHMC Institutional Review Board. Clinical data were abstracted from medical charts and entered into a password-protected database for analysis.
Statistical Analyses
Baseline clinical characteristics and treatment outcomes were analyzed in children with NF1 and OPGs. To characterize this population, basic descriptive statistics were used (frequencies for dichotomous measures and medians for continuous measures). To determine whether the frequencies of outcomes differed between groups, χ2 goodness of fit tests, and the 2-sample median test were performed as appropriate. Kaplan-Meier curves were calculated and log-rank tests were used to compare differences between recurrence-free survival curves based on tumor location.
Results
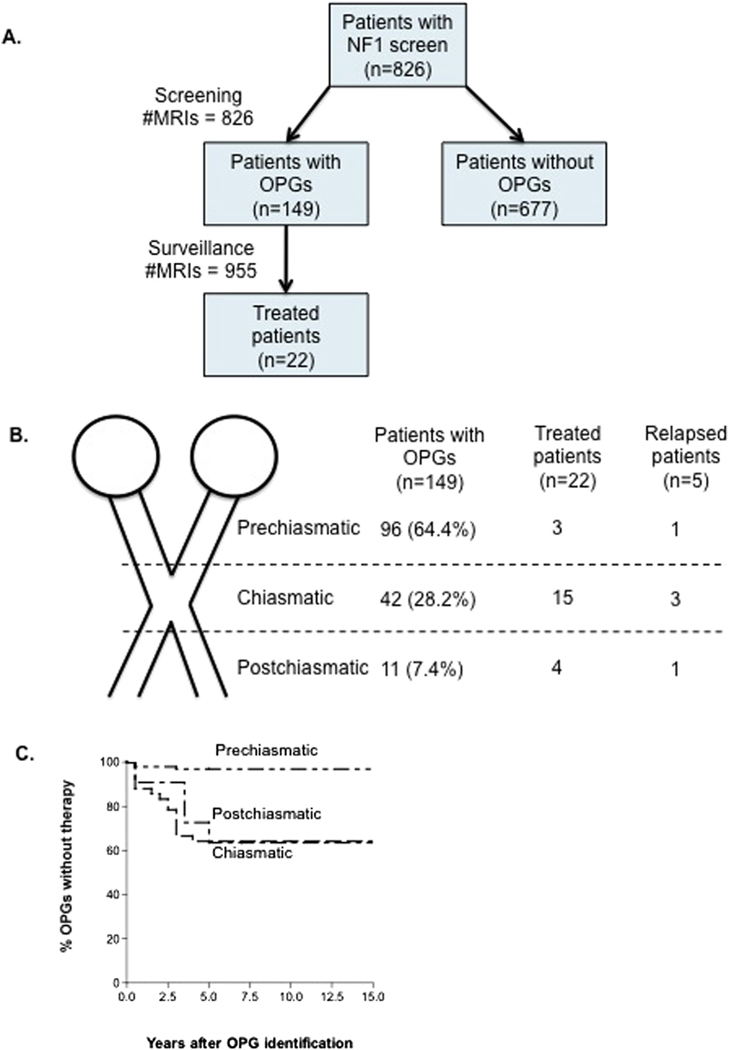
A total of 826 children with NF1 (402 females, 424 males) ages 1–9 years (median 2 years) were screened for OPGs using MRI of brain and orbits, with and without contrast (Figure, A). The majority of patients with NF1 were Caucasian (81.2%), followed by African American (12%), multiracial (3.4%), Hispanic (2.2%), and Asian (1.2%). OPGs were identified on brain/orbital MRI in a total of 149 children (18% of patients), and 22 patients were treated with chemotherapy for OPG (15% of those with OPG; 2.7% of total population). Decision for treatment was made based on a combination of ophthalmologic and MRI findings. OPGs were less likely to be identified in African American patients with NF1 compared with Caucasians (10.2% vs 17.5%) (P < .01) (Table I). Females more frequently had OPGs than did males (20.6% vs 15.6%), (P < .01). The majority (134/149, 90%) of OPGs were identified in patients less than 6 years of age. Median age at detection of OPGs was 3 years (range 1–12 years). An additional 955 surveillance brain/orbital MRI scans were performed in the subset of 149 patients with OPGs at established intervals to monitor tumor growth. Patients with chiasmatic (15/42) and postchiasmatic (4/11) tumors were more likely to need therapy compared with patients with isolated prechiasmatic OPGs (3/96) (P < .0001; Figure, B). The 3 patients with isolated prechiasmatic OPG who required therapy had bilateral lesions. Bilateral involvement was identified in 52 of the 149 patients (34.8%) with OPG; of the 22 treated patients, 11 (50%) had bilateral OPG involvement (P < .02). Hypothalamic involvement was seen in 5 of the 22 treated patients. None of the patients requiring treatment had an orbital plexiform neurofibroma, and proptosis was seen in only 2 patients. Only 1 of the 677 patients with a normal screening brain/orbital MRI performed after 15 months of age later developed an OPG. This was a girl who developed an enhancing unilateral prechiasmatic optic nerve glioma on imaging at age 11 years, which had not been present on earlier imaging at ages 17 months and 7 years. However, she remained asymptomatic and never required treatment.
Figure.
A, NF1 study population and results of brain/orbital MRI OPGs screening. B, Treatment by tumor location and need for therapy. C, Time to therapy after initial tumor identification by brain/orbital MRI.
Table I.
Baseline characteristics of the patients with NF1 with and without OPGs
| NF1 with OPGs | NF1 without OPGs | NF1 total population | |
|---|---|---|---|
|
| |||
| Number of patients (%) | 149 (18%) | 677 (82%) | 826 (100%) |
| Sex (male/female) | 66/83 | 358/319 | 424/402 |
| Median age at OPGs detection (y) | 3 (range 1–12) | N/A | N/A |
| Race | |||
| Caucasian | 117(17.5%) | 554 (82.5%) | 671 (81.2%) |
| African American | 10(10.2%)* | 89 (89.8%) | 99 (12%) |
| Other/biracial | 11 (39.2%) | 17(60.8%) | 28 (3.4%) |
| Hispanic | 8 (44%) | 10(56%) | 18(2.2%) |
| Asian | 3 (30%) | 7 (70%) | 10(1.2%) |
| Patients requiring treatment | 22 (14.8%) | N/A | N/A |
| Median age at OPGs treatment (y) | 5 (range 1.5–12) | N/A | N/A |
| Relapse | 7(31.8%) | N/A | N/A |
| Median time to relapse (y) | 3 (range 1–8) | N/A | N/A |
N/A, not applicable.
P < .01.
Therapeutic Interventions and Outcomes
Time to therapy after initial tumor identification by MRI ranged between 0.2 and 5 years (Figure, C). Vision loss and tumor growth were the most frequent reasons to initiate therapy. Twenty-two children (15%) with OPGs required therapeutic interventions, none of who were African American and 14 (63%) of whom were females (P < .01). Prior to therapy, 12 children had vision abnormalities and 10 children had normal ophthalmologic evaluations. Patients with postchiasmatic tumors (3/4) and chiasmatic tumors (8/15) were more likely to develop vision abnormalities compared with patients with isolated prechiasmatic OPGs (1/3) (P < .01). The most common ophthalmologic findings were decreased visual acuity (11/22), abnormal/atrophic optic disc (8/22), visual field defects (6/22), and unilateral abnormal pupillary response (1/22). The majority of children with OPGs received chemotherapy before 6 years of age (median 5 years; range 1.5–12 years). All patients initially received a regimen of vincristine and carboplatin, with the exception of 1 patient who subsequently received vincristine and dactinomycin after experiencing an allergic reaction to carboplatin. Seven patients relapsed after therapy (median 3 years; range 1–8 years). Surgical resections were performed in 2 patients who had progressive tumors despite chemotherapy, with severe vision abnormalities. Indications for OPG surgery were hydrocephalus (n = 1) and mass effect (n = 1).
Twelve children were treated during the first decade of the study and 10 children in the last decade. Treatment during the last decade was initiated earlier after diagnosis of OPG, at 1.5 years vs 2.2 years postdiagnosis in the first decade (P < .05). In addition, patients treated in the last decade maintained or improved vision more frequently (80%) compared with the first decade (33.3%) (P < .01). There were no differences in the chemotherapy regimen between the 2 decades. Surgical resections were only performed during the first decade.
A total of 12 children were treated based on a combination of brain MRI findings and ophthalmologic findings, such as afferent pupillary defect, visual field defect, reduced color vision, or reduced visual acuity; precocious puberty was present in 3 of these patients. Ten (10) children were treated with chemotherapy based on high-risk MRI findings, before showing any deficits on visual examination. High-risk MRI findings for this review were considered to be chiasmatic or postchiasmatic involvement, bilateral involvement, marked tumor progression, or tumor extending beyond the optic tracts. No patients with isolated unilateral prechiasmatic lesions required treatment, and this was considered a low risk group. Children with OPGs with visual findings prior to therapy were more likely to experience visual decline (10/12) when compared with children treated based on radiologic progression of OPGs (2/10) (P < .012). Fifty percent (50%) of patients with visual findings at diagnosis (6/12) progressed to vision loss (final vision of 20/200 or worse) in 1 or both eyes (5 unilateral, 1 bilateral). None of the patients treated based on radiologic progression had visual acuities of 20/200 or worse. Table II (available at www.jpeds.com) shows characteristics of children with NF1 who received therapy for OPGs (22 patients) and final visual outcomes after therapy. Children with chiasmatic (4/15), postchiasmatic (1/4), and isolated prechiasmatic (1/3) OPGs had the same risk for developing vision loss, when treatment was started after onset of visual symptoms.
Table II.
Clinical characteristics of children with NF1 who received therapy for OPGs and final visual outcomes after therapy
| Patient number | Age (y) at detection of OPGs | Optic pathway MRI findings | Hypothalamic involvement | Vision abnormalities prior to therapy | Age (y) at treatment | Therapy | Relapse | OPGs resection | Final vision | Final VA | Endocrine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||||||
| Ch | Pre-Ch | Post-Ch | VA | FD | PR | F | NC | I | W | |||||||||
|
| ||||||||||||||||||
| 1 | 1.5 | Yes | No | Yes | No | − | − | − | − | 4 | V, C | No | No | − | + | − | 20/20; 20/30 | Precocious puberty |
| 2 | 2.5 | Yes | Yes (U) | No | No | − | − | − | − | 5 | V, C | Yes | No | − | + | − | 20/20; 20/20 | No |
| 3 | 1.5 | Yes | Yes (U) | No | No | − | − | − | − | 2 | V, C | No | No | + | − | − | 20/20; 20/20 | No |
| 4 | 3 | Yes | No | No | Yes | − | − | − | + | 8 | V, C | No | No | − | + | − | 20/15; 20/15 | Precocious puberty + HP |
| 5 | 1.5 | Yes | Yes (B) | No | Yes | − | − | − | − | 2 | V, C | Yes | No | + | − | − | 20/25; 20/25 | No |
| 6 | 1 | Yes | Yes (U) | No | Yes | − | − | − | − | 2.5 | V, C | No | No | + | − | − | 20/30; 20/25 | No |
| 7 | 2 | No | Yes (B) | No | No | − | − | − | − | 4 | V, C | No | No | − | + | − | 20/20; 20/20 | No |
| 8 | 5 | Yes | No | No | No | − | − | − | − | 8 | V, C | No | No | − | − | + | 20/25; 20/40 | No |
| 9 | 3 | No | Yes (B) | No | No | − | − | − | − | 3.2 | V, C | No | No | − | − | + | 20/20; 20/40 | No |
| 10 | 2.5 | Yes | Yes (B) | No | No | − | − | − | − | 3 | V, C | No | No | + | − | − | 20/20; 20/20 | No |
| 11 | 3.2 | Yes | Yes (B) | No | No | + | − | − | + | 5 | V, C | Yes | No | − | − | + (U) | NLP; 20/25 | Precocious puberty |
| 12 | 2 | Yes | No | No | No | + | − | − | + | 3 | V, C | No | No | − | − | + (U) | NLP; 20/25 | HP |
| 13 | 8 | Yes | No | No | ND | − | + | − | − | 12 | V, C | No | No | + | − | − | 20/20; 20/20 | HP |
| 14 | 1 | Yes | Yes (B) | No | Yes | + | − | − | − | 3 | V, C | Yes | No | − | − | + (U) | 20/20; 20/125 | No |
| 15 | 9 | Yes | Yes (B) | Yes | No | + | + | − | + | 12 | V, C | Yes | No | − | − | + (B) | 20/160; 20/160 | HP |
| 16 | 5 | Yes | No | No | ND | + | − | − | + | 8 | V, C | No | No | − | − | + (U) | 20/400; 20/20 | No |
| 17 | 3 | No | Yes (B) | No | No | + | + | − | − | 3.4 | V, C | No | Yes | − | − | + (U) | NLP; 20/25 | No |
| 18 | 5 | Yes | Yes (U) | Yes | No | + | + | − | + | 5.2 | V, C | Yes | No | − | − | + (B) | 20/125; 20/125 | Precocious puberty + HP |
| 19 | 3 | Yes | No | No | ND | + | + | − | + | 5 | V, D | No | No | − | − | + (B) | 20/200; 20/200 | Precocious puberty |
| 20 | 1.5 | Yes | Yes (B) | Yes | ND | + | − | − | + | 1.8 | V, C | Yes | Yes | − | − | + (U) | NLP; 20/30 | HP |
| 21 | 1.5 | Yes | Yes (B) | No | No | + | + | − | + | 4 | V, C | No | No | + | − | − | 20/25; 20/25 | No |
| 22 | 4 | Yes | Yes (B) | No | Yes | + | − | + | − | 5.4 | V, C | No | No | − | − | + (U) | 20/200; 20/30 | No |
B, bilateral; C, carboplatin; Ch, chiasmatic; D, dactinomycin; F, fundoscopy; FD, field defect; HP, hypopituitarism; I, improved; NC, no change; ND, no data; NLP, no light perception; Post-Ch, postchiasmatic; PR, pupillary response; Pre-Ch, prechiasmatic; U, unilateral; V, vincristine; VA, visual acuity; W, worsening.
Additional Morbidity and Mortality
Vision deficits were the most common complications in children with OPGs (12/22), followed by endocrine abnormalities (9/22). Hypopituitarism (6/22) and precocious puberty (5/22) were common comorbidities of patients with chiasmatic and postchiasmatic OPGs and were not seen in children with isolated prechiasmatic tumors. One patient (patient 22, Table II) treated for OPG died at age 20 of an anaplastic astrocytoma during the time of this literature review.
Discussion
This study evaluated the clinical outcomes over a 20-year period of a large population of children with NF1 who underwent screening MRI of brain and orbits. No standard guidelines currently exist for the use of screening brain/orbital MRIs in the pediatric NF1 population and management of this relatively common NF1 complication is controversial in the field. Few studies have evaluated the utility of brain/orbital MRI screening in young children with NF1 in depth. Although the natural history of OPGs is not yet fully understood,12 it is known that they are often indolent and nonmetastatic in NF1.4 However, OPGs have the ability to disrupt vision and hypothalamic function in a subset of patients.13 The prevalence of OPGs detected by MRI in our pediatric NF1 population was 18%, comparable with previous reports of 15%−21%.1,10 Only a small percentage of our total NF1 pediatric population (2.7%) required chemotherapy for OPG.
In prior decades, many children with NF1 and asymptomatic optic gliomas received unnecessary treatment for what is often a very indolent lesion. Listernick et al14 were among the first to assert that OPGs in asymptomatic children with NF1 infrequently progress. In 1997, the OPG Task Force concluded early detection of tumors would not reduce the rate of loss of vision, and there was no compelling evidence to support OPG screening with neuroimaging.7 Blazo et al15 reported their results of brain MRI screening of 84 children with NF1, where 13 children were found to have OPG. They reported that 3 asymptomatic children with enlarging chiasmal lesions were treated with chemotherapy and had preservation of vision, whereas 5 children ascertained outside of screening guidelines had substantial vision loss, and suggested that routine surveillance for OPG could improve outcomes. Listernik and Charrow16 responded to the Blazo article, noting that 4 of the 5 tumors in the symptomatic patients were associated with proptosis and represented a biologically different group of tumors; they upheld their principle of screening only with ophthalmologic examinations in young asymptomatic children.
Our study suggests that MRI screening has the potential to improve and maintain visual outcome in young children with OPG. Supporting this assertion is that none of the children who were identified only with MRI (no visual symptoms) with progressive OPG, progressed to vision loss. However, 50% of children who presented with visual symptoms at the time of diagnosis of a progressive OPG demonstrated eventual vision loss in 1 or both eyes. This suggests that screening MRIs in patients with NF1 may identify aggressive lesions sooner and that this will lead to early treatment and, subsequently, better visual outcomes.
A potentially negative consequence of brain/orbital MRI screening is detection of lesions that would never progress or could resolve spontaneously. This could lead to unnecessary parental anxiety and high costs associated with frequent imaging. Our study suggests that the location of the OPGs is an important marker for tumor progression and need for therapy given that isolated prechiasmatic tumors are more likely to regress (25%) and to be indolent than chiasmatic and postchiasmatic OPGs (10%). It is also important to consider that chiasmatic and postchiasmatic OPGs are most likely to lead to visual symptoms, supporting consideration of therapy for this group if tumor growth is documented. Future studies will help to validate if frequency of neuroimaging surveillance could be modified based on glioma location.
Few studies have examined the effects of race and sex on NF1 clinical manifestations and mortality in patients with NF1.17–20 Most of these studies have been limited by their small sample size. King et al6 reported a lower prevalence of OPG in African American children compared with Caucasians; our study confirms that racial difference. Despite greater insight into the pathogenesis of NF1 and OPGs, little is known about the influences of race on NF1 disease phenotype. Our study also found an increased risk for need for therapy in females with NF1 and OPG, confirming recent studies suggesting that sex plays a role in the development of gliomas and neuronal dysfunction in patients with NF1.21,22
A recent large multicenter study of visual outcomes in children with NF1 and OPG showed that at completion of chemotherapy, visual acuity improved in 32% of subjects, remained stable in 40%, and declined in 28%.23
There are a number of limitations to this study. Although our program has performed surveillance brain/orbital MRI screening for the past 20 years, the data was reviewed retrospectively. We have no way of proving that the patients who had preservation of vision with chemotherapy might not have had spontaneous regression or stabilization of disease without therapy. A large, prospective study could likely better answer these questions, ideally one comparing outcomes between centers who perform routine MRI screening and those who do not.
In conclusion, our study found OPGs occurring in 18% of pediatric patients with NF1 occurring somewhat more frequently in females than males and less frequently in African Americans. Using conservative guidelines, only 14.8% of OPGs identified by MRI screening required treatment. OPGs with chiasmatic and postchiasmatic involvement had the highest risk for progression and need for therapy. This study also suggests that children who do not have an OPG detected on brain/orbital screening MRI at age 15 months or later, are at very low risk for this complication. In addition, this large study suggests that early treatment with chemotherapy prior to visual changes in a carefully selected group of patients can lead to better visual outcomes. Future studies are necessary to investigate if surveillance based on tumor location and newer visual screening methodologies can improve our screening strategy for OPGs, reduce high costs of neuroimaging, and improve visual outcomes.
Glossary
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CNS
Central nervous system
- MRI
Magnetic resonance imaging
- NF
Neurofibromatosis
- NF1
Neurofibromatosis type 1
- OPG
Optic pathway glioma
Footnotes
The authors declare no conflicts of interest.
References
- 1.Sharif S, Upadhyaya M, Ferner R, Majounie E, Shenton A, Baser M, et al. A molecular analysis of individuals with neurofibromatosis type 1 (NF1) and optic pathway gliomas (OPGs), and an assessment of genotype-phenotype correlations. J Med Genet 2011;48:256–60. [DOI] [PubMed] [Google Scholar]
- 2.Moharir M, London K, Howman-Giles R, North K. Utility of positron emission tomography for tumour surveillance in children with neurofibromatosis type 1. Eur J Nucl Med Mol Imaging 2010;37:1309–17. [DOI] [PubMed] [Google Scholar]
- 3.Binning MJ, Liu JK, Kestle JR, Brockmeyer DL, Walker ML. Optic pathway gliomas: a review. Neurosurg Focus 2007;23:E2. [DOI] [PubMed] [Google Scholar]
- 4.Jahraus CD, Tarbell NJ. Optic pathway gliomas. Pediatr Blood Cancer 2006;46:586–96. [DOI] [PubMed] [Google Scholar]
- 5.Nicolin G, Parkin P, Mabbott D, Hargrave D, Bartels U, Tabori U, et al. Natural history and outcome of optic pathway gliomas in children. Pediatr Blood Cancer 2009;53:1231–7. [DOI] [PubMed] [Google Scholar]
- 6.King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A 2003;122A:95–9. [DOI] [PubMed] [Google Scholar]
- 7.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol 1997;41:143–9. [DOI] [PubMed] [Google Scholar]
- 8.Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol 2006;24:2570–5. [DOI] [PubMed] [Google Scholar]
- 9.Listernick R, Charrow J, Gutmann DH. Intracranial gliomas in neurofibromatosis type 1. Am J Med Genet 1999;89:38–44. [DOI] [PubMed] [Google Scholar]
- 10.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol 2007;61:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institutes of Health Consensus Development Conference Statement: neurofibromatosis. Bethesda, Md., USA, July 13–15, 1987. Neurofibromatosis 1988;1:172–8. [PubMed] [Google Scholar]
- 12.Hernaiz Driever P, von Hornstein S, Pietsch T, Kortmann R, Warmuth-Metz M, Emser A, et al. Natural history and management of low-grade glioma in NF-1 children. J Neurooncol 2010;100:199–207. [DOI] [PubMed] [Google Scholar]
- 13.Pilling RF, Lloyd IC, Huson S. Utility of optic pathway glioma screening in young children with neurofibromatosis type I: questions generated by a clinical audit. Eye (Lond) 2010;24:1603–5. [DOI] [PubMed] [Google Scholar]
- 14.Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr 1994;125:63–6. [DOI] [PubMed] [Google Scholar]
- 15.Blazo MA, Lewis RA, Chintagumpala MM, Frazier M, McCluggage C, Plon SE. Outcomes of systematic screening for optic pathway tumors in children with neurofibromatosis type 1. Am J Med Genet A 2004; 127A:224–9. [DOI] [PubMed] [Google Scholar]
- 16.Listernick R, Charrow J. Knowledge without truth: screening for complications of neurofibromatosis type 1 in childhood. Am J Med Genet A 2004;127A:221–3. [DOI] [PubMed] [Google Scholar]
- 17.Mian A. Clinical predictors and risk of optic pathway glioma in neurofibromatosis type-1. Cincinnati, (OH): University of Cincinnati; 2006. [Google Scholar]
- 18.Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using US death certificates. Am J Hum Genet 2001;68:1110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman JM, Riccardi VM. Neurofibromatosis: Phenotype, natural history, and pathogenesis. Baltimore (MD): Johns Hopkins University Press; 1999. [Google Scholar]
- 20.Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet 1999;89:1–6. [PubMed] [Google Scholar]
- 21.Diggs-Andrews KA, Brown JA, Gianino SM, Rubin JB, Wozniak DF, Gutmann DH. Sex is a major determinant of neuronal dysfunction in neurofibromatosis type 1. Ann Neurol 2014;75:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amlin-Van Schaick JC, Kim S, DiFabio C, Lee MH, Broman KW, Reilly KM. Arlm1 is a male-specific modifier of astrocytoma resistance on mouse Chr 12. Neuro Oncol 2012;14:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher MJ, Loguidice M, Gutmann DH, Listernick R, Ferner RE, Ullrich NJ, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol 2012;14:790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]