Abstract
Since the development of indica hybrid rice in the 1970s, great success has been achieved in hybrid rice production in China and around the world. The utilization of inter-subspecific indica–japonica hybrid rice has always been considered due to its stronger heterosis characteristics. However, indica–japonica hybrids face a serious problem of sterility, which hinders the exploitation of their heterosis. In the past decades, the genetic basis of indica–japonica hybrid sterility has been well studied. It was found that in sterile indica–japonica hybrids, female sterility was mainly controlled by the S5 locus and male sterility by the Sa, Sb, Sc, Sd, and Se loci. In this study, we developed wide-compatible indica lines (WCILs) by pyramiding multiple neutral (n) alleles of the hybrid sterility loci. First, we identified Sn alleles of the loci in single-segment substitution lines (SSSLs) in the genetic background of indica Huajingxian 74 (HJX74). Then, the Sn alleles of S5, Sb, Sc, Sd, and Se loci in SSSLs were pyramided in the HJX74 genetic background. The WCILs carrying Sn alleles at the S5, Sb, Sc, Sd, and Se loci showed wide compatibility with indica and japonica rice varieties. Therefore, the WCILs will be used to develop inter-subspecific indica–japonica hybrid rice with normal fertility.
Keywords: hybrid rice, heterosis, hybrid sterility, neutral allele, breeding by design
Introduction
Asian cultivated rice (Oryza sativa L.) is the staple food for more than half of the world’s population (Fukagawa and Ziska, 2019). The breeding of high-yielding varieties is essential for maintaining global food security (Peng et al., 2008; Khush, 2013). Since the 1970s, indica hybrid rice has been successfully developed in China and around the world (Yuan and Virmani, 1988; Cheng et al., 2007). However, the heterosis of intra-subspecific hybrid rice is limited, resulting in a yield plateau for production of hybrid rice (Peng et al., 2004; Cheng et al., 2007). There is great heterosis in inter-subspecific hybrids, and exploiting this heterosis has long been considered a promising approach to further increase the yield potential of rice (Khush, 2013; Zhang et al., 2021). However, the severe sterility associated with indica–japonica hybrid hinders the utilization of heterosis (Ikehashi and Araki, 1986; Ouyang and Zhang, 2018; Zhang, 2020, 2022).
The sterility of hybrids produced by crossing indica and japonica rice varieties can be attributed to female or embryo sac sterility and male or pollen sterility. The female sterility in hybrid is mainly controlled by the S5 locus, which was mapped on chromosome 6 (Ikehashi and Araki, 1986; Yanagihara et al., 1995; Ji et al., 2005; Qiu et al., 2005). The male sterility in hybrid is mainly controlled by Sa, Sb, Sc, Sd, and Se loci (Zhang and Lu, 1989, 1993; Zhang et al., 1993, 1994). Using molecular markers, Sa was found to be located on chromosome 1 (Zhuang et al., 1999; Su and Liu, 2003), Sb on chromosome 5 (Zhuang et al., 2002; Li et al., 2006), Sc on chromosome 3 (Zhang and Zhang, 2001; Yang et al., 2004), Sd on chromosome 1 (Li et al., 2008), and Se on chromosome 12 (Zhu et al., 2008). The S5, Sa, and Sc genes were then cloned and functionally analyzed (Chen et al., 2008; Long et al., 2008; Yang et al., 2012; Shen et al., 2017). The genetic model of hybrid sterility is the one-locus sporo-gametophytic interaction model (Ikehashi and Araki, 1986; Zhang and Lu, 1993; Zhang, 2020). In this genetic model, it is assumed that indica varieties have Si allele, and japonica varieties have Sj allele at the loci. At the S5 locus, the interaction between Si and Sj causes the abortion of female gametes carrying Sj allele (Ikehashi and Araki, 1986). At the Sa, Sb, Sc, Sd, and Se loci, the interaction between Si and Sj causes the abortion of male gametes carrying Sj allele (Zhang and Lu, 1993). At these loci, some varieties carry Sn, a neutral allele, and the allelic interaction between Si/Sn and Sj/Sn cannot cause the abortion of any gamete (Ikehashi and Araki, 1986; Zhang and Lu, 1993; Yang et al., 2012; Shen et al., 2017; Xie et al., 2017). The understanding of the genetic and molecular mechanisms of sterility in indica–japonica hybrids has laid the foundation for overcoming hybrid sterility.
With the development of molecular breeding technology, the concept of “breeding by design” was proposed (Peleman and van der Voort, 2003). To implement the strategy of rice breeding by design, a library of single-segment substitution lines (SSSLs) in rice was constructed by using 43 accessions from seven species of AA genome as donors of chromosome substitution segments in the genetic background of Huajingxian 74 (HJX74), an elite indica variety from south China. A total of 2,360 HJX74-SSSLs have been included in the library, which contains rich genetic resources for rice breeding techniques (Zhang et al., 2004; Xi et al., 2006; He et al., 2017; Zhao et al., 2019; Zhang, 2021). The HJX74-SSSL library was used as a platform for designing new rice cultivars, and several cytoplasmic male sterility (CMS), maintainer, and restorer lines were developed (Dai et al., 2015, 2016; Luan et al., 2019). Therefore, target chromosome-segment substitution is a way to breeding by design in rice (Zhang, 2021).
With the understanding of the genetic and molecular mechanisms of indica–japonica hybrid sterility and the development of molecular breeding techniques, the breeding strategies for developing inter-subspecific indica–japonica hybrid rice were proposed (Zhang, 2020, 2022). One strategy for overcoming the hybrid sterility of indica–japonica rice is to develop indica-compatible japonica lines (ICJLs) (Zhang and Lu, 1999; Zhang, 2020). Recently, the ICJLs were developed by pyramiding Si allele at the Sb, Sc, Sd, and Se loci and Sn allele at the S5 locus in japonica genetic background by marker-assisted selection (MAS). The ICJLs are compatible with indica but incompatible with japonica in pollen fertility and spikelet fertility (Guo et al., 2016). Another strategy for overcoming the hybrid sterility of indica–japonica rice is to develop wide-compatible indica lines (WCILs) (Zhang, 2020, 2022). Herein, we report the development of WCILs using the HJX74-SSSL library. By pyramiding Sn allele at the S5, Sb, Sc, Sd, and Se loci in the HJX74 genetic background, the obtained WCILs were compatible with both indica and japonica rice in pollen fertility and spikelet fertility. The breeding of WCILs provides a technique to develop inter-subspecific indica–japonica hybrid rice.
Materials and Methods
Plant Materials and Field Trials
Seven SSSLs carrying the Sc gene for hybrid male sterility in their chromosome substitution segments and seven SSSLs carrying the S5 gene for hybrid female sterility in their chromosome substitution segments were selected from the HXJ74-SSSL library (Supplementary Table 1). A set of indica and japonica varieties were used as testers to test the hybrid fertility. The genotypes of Sa, Sb, Sc, Sd, and Se loci for hybrid male sterility and S5 locus for hybrid female sterility have been identified in some of the testers. It was found that at these six loci, the indica variety Guang-lu-ai 4 (GLA4) carried the Si alleles, while the japonica variety Taichung 65 (T65) carried the Sj alleles (Zhang et al., 1994; Guo et al., 2016). All the study samples were planted from 2008 to 2019 at the farm of South China Agricultural University, Guangzhou (23°07′N, 113°15′E). These plants were planted in two cropping seasons each year, with the first cropping season (FCS) running from late February to mid-July and the second cropping season (SCS) running from late July to mid-November. Seeds were sown in seedbeds, and seedlings were transplanted into the field. Field management, including irrigation, fertilization, and pest control, followed normal agricultural practices.
Genotyping by Molecular Markers
The SSR markers were selected on the rice microsatellite maps (McCouch et al., 2002; Zhang et al., 2007). The functional markers of the S5 gene were selected to identify the genotypes at the S5 loci (Sundaram et al., 2010; Du et al., 2011; Yang et al., 2012; Guo et al., 2016). Markers linked with the Sa, Sb, Sc, Sd, Se, and S5 loci were selected from the published studies (Yang et al., 2004, 2012; Li et al., 2006, 2008; Chen et al., 2008; Long et al., 2008; Zhu et al., 2008). New molecular markers were developed in this study (Supplementary Table 2). The PCR products were separated into 6% non-denaturing polyacrylamide gels (Panaud et al., 1996; Li et al., 2006).
Phenotyping of Fertility and Agronomic Traits
To check pollen fertility, nine mature flowers were collected from the upper third of panicles during the flowering stage and fixed in FAA solution. Pollens were stained with the 1% I2-KI solution containing 0.1% (w/v) iodine and 1% (w/v) potassium iodide. Pollens were divided into normal pollens and sterile pollens, which were further divided into stained abortive pollens (stained but small size) and empty abortive pollens (small size and empty) (Zhang and Lu, 1989). Three panicles per plant and 10–12 plants per line were used to examine the spikelet fertility, and 20–40 plants per line were used to investigate the agronomic traits.
Statistical Analysis
For statistical analysis, the percentage data were converted to the square root of the arcsine values. Student’s t-test was used to compare the data between the two groups. The Dunnett t-test was used to compare multiple groups with the control group. The least significance range (LSR) was used for the multiple range test among the multiple groups. The chi-square (χ2) test was performed to detect the distorted segregation of three genotypes in F2 populations according to the Mendelian ratio of 1:2:1. SPSS statistics 23.0 and Origin Pro 9.0 were used for data analysis and charting1.
Results
Genotypes of Sa, Sb, Sc, Sd, Se, and S5 Loci in Huajingxian 74
To identify the genotypes of Sa, Sb, Sc, Sd, Se, and S5 loci associated with hybrid sterility, HJX74 was test crossed with T65, a japonica variety with Sj alleles at these six loci, and GLA4, an indica variety with Si alleles at these six loci (Zhang et al., 1994; Guo et al., 2016). The F1 hybrids obtained from the cross of T65/GLA4 showed severe sterility, where the pollen fertility was only 20.51% and spikelet fertility was only 5.89%. In contrast, the F1 hybrid of the HJX74/GLA4 cross showed normal pollen fertility and spikelet fertility of 92.39% and 93.39%, respectively. In the F1 hybrids obtained from the cross of HJX74/T65, the pollen fertility was 72.89% and the spikelet fertility was 61.58%, which were significantly higher than those of T65/GLA4 and significantly lower than those of HJX74/GLA4 hybrids (Figure 1A). The results showed that the hybrid of HJX74/T65 exhibited partial pollen sterility and partial spikelet sterility.
FIGURE 1.
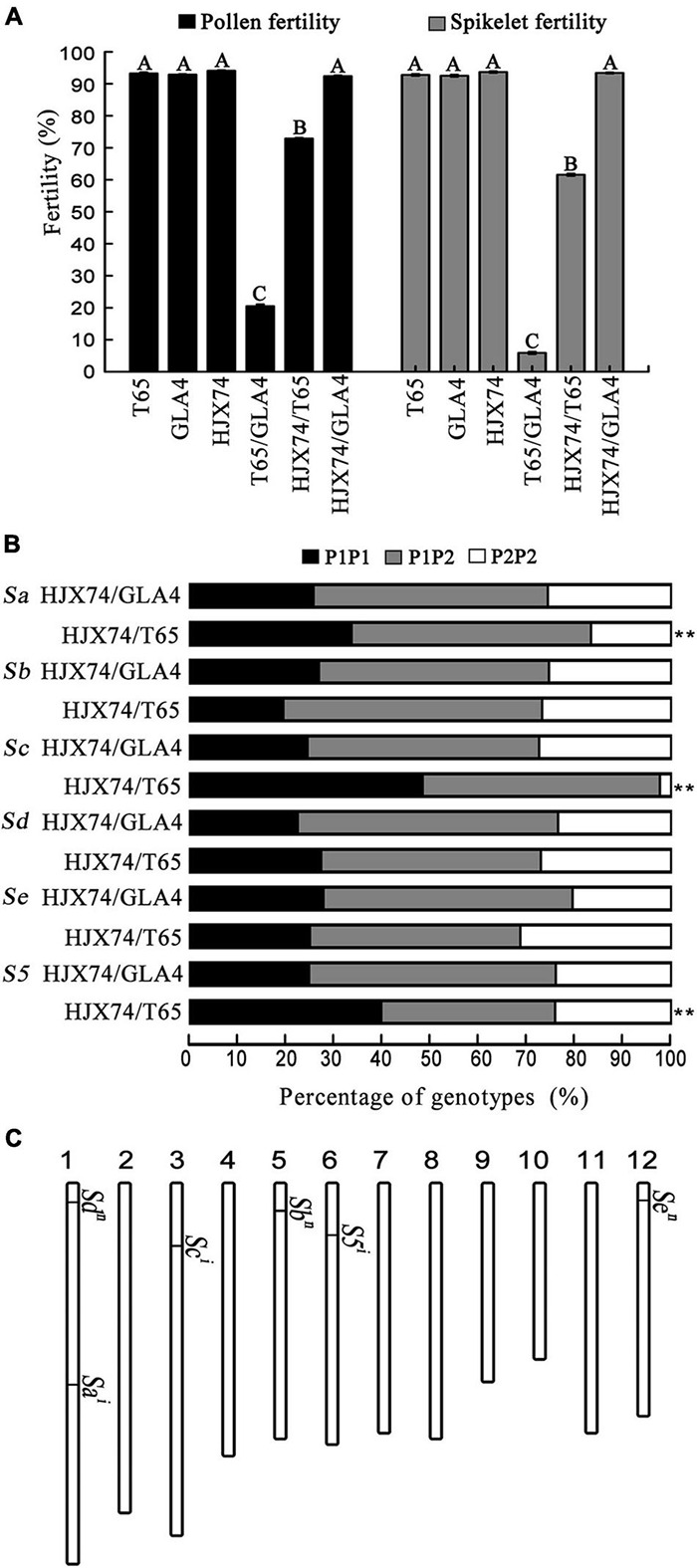
Genotypes of hybrid sterility loci Sa, Sb, Sc, Sd, Se, and S5 in HJX74. (A) Pollen and spikelet fertility of three parents and their F1 hybrids. (B) Ratios of genotypes at Sa, Sb, Sc, Sd, Se, and S5 loci in the F2 populations from the crosses between HJX74 and testers Taichung 65 (T65) and Guang-lu-ai 4 (GLA4). (C) Chromosome location and genotypes of the genes at the Sa, Sb, Sc, Sd, Se, and S5 loci in HJX74. Vertical bars represent rice chromosomes. P1P1, Genotypes of HJX74; P1P2, Heterozygous genotype; P2P2, Genotype of testers T65 (Sj/Sj) or GLA4 (Si/Si). Capital letters indicate statistical differences at the 0.01 probability level.
The molecular markers linked to the Sa, Sb, Sc, Sd, Se, and S5 loci were used to investigate genotype segregation in the F2 populations obtained from the crosses of HJX74/GLA4 and HJX74/T65. At the Sb, Sd, and Se loci, the genotype segregation of F2 populations from both crosses fit the Mendelian ratio of 1:2:1. At the Sa, Sc, and S5 loci, distorted segregation of the genotypes was detected in the F2 population of HJX74/T65 but not in the genotypes of HJX74/GLA4. At the Sa locus, the genotype ratios of SaHJX74/SaHJX74, SaHJX74/SaT65, and SaT65/SaT65 were 68:100:33, which significantly distorted from the Mendelian ratio of 1:2:1. At the Sc locus, the genotype ratios of ScHJX74/ScHJX74, ScHJX74/ScT65, and ScT65/ScT65 were 69:70:3, which significantly distorted from the Mendelian ratio. Distorted segregation was also detected at the S5 locus, where the genotype ratios of S5HJX74/S5HJX74, S5HJX74/S5T65, and S5T65/S5T65 were found to be 72:65:43 (Figure 1B). In addition, HJX74 was tested using a group of indica and japonica testers. The results showed that distorted segregation was detected only at the Sa, Sc, and S5 loci in the crosses of HJX74/japonica testers (Supplementary Table 3).
These results indicated that HJX74 carried Si/Si at the Sa, Sc, and S5 loci and Sn/Sn at the Sb, Sd, and Se loci (Figure 1C). At the Sa and Sc loci, the allele interaction between Si of HJX74 and Sj of japonica testers caused the abortion of male gametes carrying Sj in hybrids, resulting in the significant reduction of plants with Sj/Sj in the F2 populations. At the S5 locus, the allele interaction between S5i of HJX74 and S5j of japonica testers caused the abortion of female gametes carrying S5j in hybrids, resulting in the significant reduction of plants with S5j/S5j in the F2 populations. At the Sb, Sd, and Se loci, allele interaction between Sn of HJX74 and Sj of japonica testers or Si of indica testers could not cause the abortion of any gamete in hybrids, and genotype segregation in the F2 populations fit the Mendelian ratio of 1:2:1 (Supplementary Table 3). In addition, compared with the Sc locus, the Sa locus showed weak distorted segregation, where χ2(1:2:1) = 34.00–62.27 in the five segregation populations of the Sc locus, while χ2(1:2:1) = 9.36–12.19 in the three segregation populations of the Sa locus (Supplementary Table 3). The results showed that the hybrid male sterility caused by the interaction between Si and Sj at the Sa locus was weaker than that at the Sc locus.
Genotypes of the Sc Locus in the Substitution Segments of Single-Segment Substitution Lines
To screen the Scn gene, seven SSSLs carrying the Sc locus on the substitution segments obtained from different donors were selected from the HJX74-SSSL library (Supplementary Table 1). The pollen fertility of F1 hybrids from the crosses between the SSSLs and HJX74 was over 90% (Figure 2A). The SSSLs were then tested with three indica testers and three japonica testers. Four SSSLs (01-03, 05-03, 06-03, and 14-03) and HJX74 showed significantly higher pollen fertility in their F1 hybrids with indica testers than those obtained with japonica testers. In contrast, the other three SSSLs (11-03, 22-03, and 27-03) did not show a significant difference in the pollen fertility of F1 hybrids between the crosses with indica and japonica testers (Figure 2B). Two SSSLs (06-03 and 27-03) were then selected to detect the segregation of Sc genotypes in F2 populations obtained from the crosses with T65. In the F2 population of the T65/27-03 cross, the Sc genotypes of T65/T65, T65/27-03, and 27-03/27-03 segregated in the ratios of 40:83:53, which fit the Mendelian ratio of 1:2:1. In contrast, in the F2 population of the T65/06-03 cross, the genotype ratios of T65/T65, T65/06-03, and 06-03/06-03 were 23:64:69, which significantly distorted from the Mendelian ratio (Figure 2C). These results indicated that at the Sc locus, SSSLs 11-03, 22-03, and 27-03 carried the Sn allele, while 01-03, 05-03, 06-03, and 14-03 carried the Si allele.
FIGURE 2.
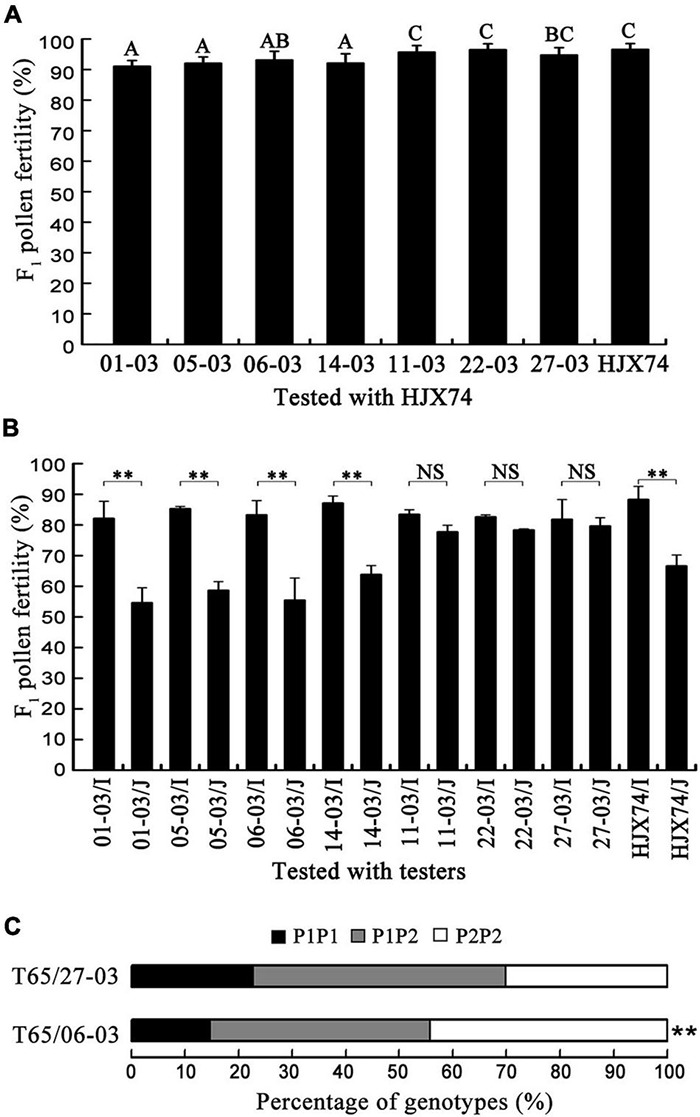
Genotypes and genetic effects of the Sc gene on chromosomal substitution segments of SSSLs with the HJX74 genetic background. (A) Pollen fertility of F1 hybrids from crosses between SSSLs and HJX74. (B) Pollen fertility of F1 hybrids from crosses between SSSLs and testers. I, indica testers; J, japonica testers. (C) Segregation ratios of Sc genotypes in F2 populations from crosses between SSSLs and T65. P1P1, Genotype of T65 (Sj/Sj); P1P2, Heterozygous genotypes; P2P2, Genotypes of SSSLs. **, Significant difference at 0.01 probability level. NS, No significance.
Genotypes of the S5 Locus in the Substitution Segments of Single-Segment Substitution Lines
To screen the S5n gene, seven SSSLs carrying the S5 locus in the substitution segments obtained from different donors were selected from the HJX74-SSSL library (Supplementary Table 1). The genotypes of the S5 locus in the SSSLs were detected by functional markers. The results showed that in the substitution segments, three SSSLs (04-06, 13-06, and 14-06) carried S5i, one SSSL (10-06) carried S5j, and the other three SSSLs (21-06, 23-06, and 27-06) carried S5n (Supplementary Table 4).
Five genotypes of the S5 locus were obtained from the F1 hybrids crossed by seven SSSLs (Supplementary Table 5). The pollen fertility of hybrids was normal in all crosses, ranging from 93.04 to 94.42%. The spikelet fertility of S5i/S5i, S5i/S5n, S5n/S5j, and S5n/S5n genotypes was normal (from 89.84% to 91.28%), but that of S5i/S5j genotype from the crosses between 10-06 carrying S5j/S5j and SSSLs carrying S5i/S5i was only 68.34%, which was significantly lower than the spikelet fertility of the other four genotypes (Figure 3A and Supplementary Table 5). The segregation of S5 genotypes in F2 populations obtained from three heterozygous genotypes, S5n/S5j, S5n/S5i, and S5j/S5i, was detected by using the functional markers of the S5 gene. Distorted segregation was detected in the S5j/S5i segregation population produced from the crosses between 10-06 carrying S5j/S5j and SSSLs carrying S5i/S5i, but was not detected in the segregation populations of S5n/S5j from 21-06/10-06 and of S5n/S5i from 21-06/13-06 (Figure 3B).
FIGURE 3.
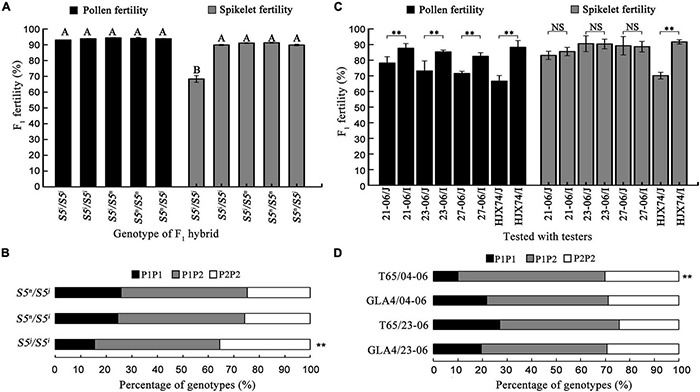
Genotypes and genetic effects of the S5 gene on chromosomal substitution segments of SSSLs with the HJX74 genetic background. (A) Pollen fertility and spikelet fertility in F1 hybrids from the crosses between SSSLs and different S5 genotypes. (B) Segregation ratios of S5 genotypes in F2 populations from different crosses. (C) Pollen fertility and spikelet fertility in F1 hybrids from crosses between SSSLs (21-06, 23-06, and 27-06) and testers. I, indica testers; J, japonica testers. Capital letters indicate statistical differences at the 0.01 probability level. (D) Segregation ratios of S5 genotypes in F2 populations from crosses between SSSLs and testers. P1P1, Genotype of tester; P1P2, Heterozygous genotypes; P2P2, Genotypes of SSSLs. **, Significant difference at 0.01 probability level. NS, No significance.
The three SSSLs with S5n, 21-06, 23-06, and 27-06, were tested for their wide compatibility by crossing with indica and japonica testers. The F1 hybrids from all crosses showed high spikelet fertility, from 80.28% to 95.05%. As a control, the spikelet fertility of F1 hybrids in HJX74/japonica testers was 70.13% (Figure 3C). In addition, distorted segregation of the S5 locus was detected in the F2 population of T65/04-06, but was not detected in the F2 populations of the other three crosses, that is, GLA4/04-06, T65/23-06, and GLA4/23-06 (Figure 3D). These results showed that the three SSSLs (21-06, 23-06, and 27-06) were compatible with indica testers and japonica testers in spikelet fertility as a result of their carrying S5n locus.
Pyramiding of Sn Alleles at the Sc and S5 Loci in the Huajingxian 74 Genetic Background
Three SSSLs (11-03, 22-03, and 27-03) with the Scn gene and three SSSLs (21-06, 23-06, and 27-06) with the S5n gene were selected to pyramid the two Sn genes in the HJX74 genetic background. Three SSSLs with Scn were crossed with three SSSLs with S5n, respectively. In the segregating populations, the plants carrying Scn and S5n loci were selected. Nine pyramiding lines were developed, which carried Scn and S5n loci from different donors and Sbn, Sdn, and Sen in the HJX74 genetic background (Figure 4A and Supplementary Table 6). Therefore, the nine pyramiding lines thus obtained were WCILs.
FIGURE 4.
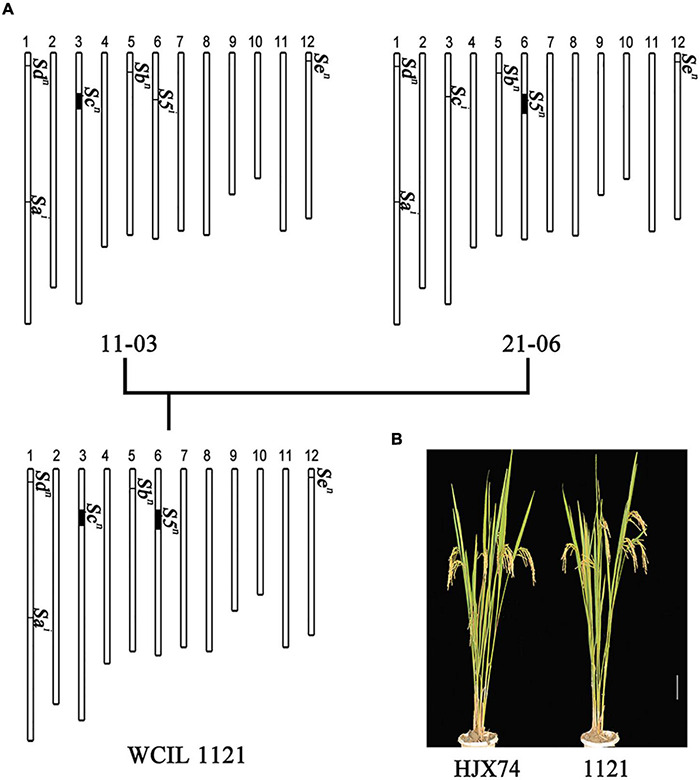
Pyramiding of Sn genes at the Sc and S5 loci in the HJX74 genetic background. (A) Development of WCIL 1121 by pyramiding of Scn in the substitution segment of SSSL 11-03 and S5n in the substitution segment of SSSL 21-06. Scale bar, 2 cm. Vertical bars represent rice chromosomes. Deep parts represent the substitution segments from donors and light parts represent the genetic background of HJX74. (B) Plant types of WCIL 1121 and HJX74. Scale bar, 10 cm.
In the nine WCILs, the plant type was similar to HJX74 (Figure 4B). In addition, no significant difference between HJX74 and WCILs was found in the majority of the investigated traits, including heading date, plant height, width of flag leaf, length of flag leaf, grain length, grain width, and grain weight (Supplementary Table 7).
Compatibility of Wide-Compatible indica Lines
To evaluate the compatibility of nine WCILs, the WCILs were test crossed with six indica testers and five japonica testers (Supplementary Tables 8–11). When tested with indica tester group, F1 hybrids of nine WCILs showed normal pollen fertility and spikelet fertility, with no significant difference when compared to HJX74. When tested with the japonica tester group, nine WCILs showed significantly higher F1 pollen fertility and spikelet fertility when compared to HJX74 (Figure 5). These results indicated that the WCILs showed wide compatibility, producing high pollen fertility and spikelet fertility in their F1 hybrids with both indica and japonica rice varieties.
FIGURE 5.
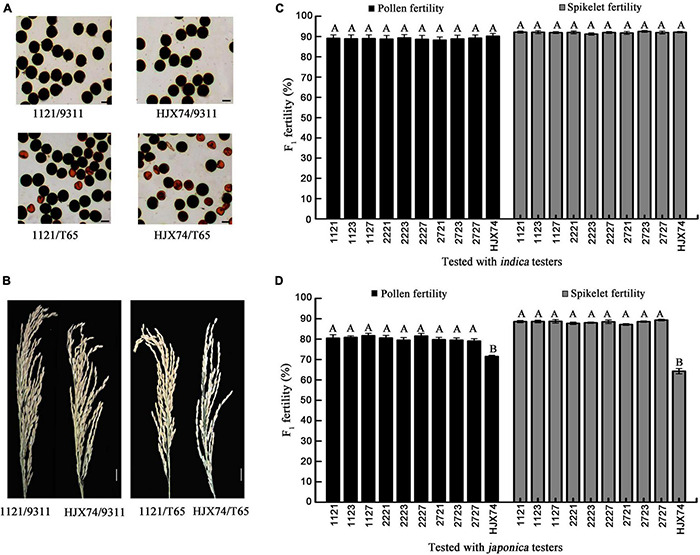
Compatibility of WCILs. (A) Pollen grains stained by I2-KI solution in F1 hybrids of four crosses. Scale bar, 30 μm. (B) Spikelet fertility of the panicles in F1 hybrids. Scale bar, 2 cm. (C) Pollen fertility and spikelet fertility of the F1 hybrids from the crosses between WCILs (HJX74 as control) and indica testers (See Supplementary Tables 8–9). (D) Pollen fertility and spikelet fertility of the F1 hybrids from the crosses between WCILs (HJX74 as control) and japonica testers (See Supplementary Tables 10–11). The information about nine WCILs (1121, 1123, 1127, 2221, 2223, 2227, 2721, 2723, and 2727) is given in Supplementary Table 6. HJX74 is a recipient of SSSLs. The 9311 is an indica tester. T65 is a japonica tester. Capital letters indicate statistical differences at the 0.01 probability level.
Although the WCILs showed significantly higher F1 pollen fertility when tested with japonica testers, the F1 pollen fertility was still lower when tested with indica testers (Figures 5C,D). To identify the problem, the genotype segregation at the Sa, Sb, Sc, Sd, Se, and S5 loci in F2 populations was examined with molecular markers linked with these loci. No distorted segregation at the Sb, Sc, Sd, Se, and S5 loci was found in all the detected F2 populations, further confirming the fact that the alleles of the Sb, Sc, Sd, Se, and S5 loci in WCILs were Sn. However, significantly distorted segregation was detected at the Sa locus in all four populations (Supplementary Table 12). These results verified that WCILs carried the Sai gene in the HJX74 genetic background, and the interaction between Sai from WCILs and Saj from japonica testers caused some male gametes with Saj to become abortive in F1 hybrids obtained from the crosses of WCILs with japonica testers.
Three indica lines, GLA4 carrying the genotype of Sai, Sbi, Sci, Sdi, Sei, and S5i, HJX74 carrying the genotype of Sai, Sbn, Sci, Sdn, Sen, and S5i, and WCIL 2223 carrying the genotype of Sai, Sbn, Scn, Sdn, Sen, and S5n, were selected to test their compatibility with eight japonica varieties of different ecotypes. In the F1 hybrids of GLA4 with eight japonica varieties, pollen fertility was 13.24–90.68% with an average of 46.94%, and spikelet fertility was 5.89–92.95% with an average of 44.40%. In the F1 hybrids of HJX74 with eight japonica varieties, pollen fertility was 75.19–95.74% with an average of 85.37%, and spikelet fertility was 58.34–93.90% with an average of 74.69%. On comparison of data, pollen fertility was 82.35–95.79% with an average of 88.82%, and spikelet fertility was 89.19–94.29% with an average of 91.73% in the F1 hybrids of WCIL 2223 with the eight japonica varieties (Supplementary Table 13). The results showed that WCIL 2223 had higher and wider compatibility with japonica varieties than GLA4 and HJX74. The pollen fertility and spikelet fertility in F1 hybrids of WCIL with various japonica varieties were normal or near normal.
Discussion
Sterility or Compatibility of Hybrids Between indica and japonica Subspecies Is a Complex Trait
In the past decades, the genetic basis of indica–japonica hybrid sterility has been understood. In indica–japonica hybrid sterility, the S5 locus was found to be responsible for female sterility, and the Sa, Sb, Sc, Sd, and Se loci were responsible for male sterility. Following the tri-allele pattern and the one-locus sporo-gametophytic interaction model, the allele interaction between Si and Sj leads to the abortion of male or female gametes carrying Sj, whereas the allele interaction between Sn and Si or Sj does not lead to the abortion of any gamete (Zhang, 2020, 2022). Thus, the sterility or compatibility of hybrids between indica and japonica subspecies is a complex trait that is controlled by multiple genes. Due to the diversity of indica and japonica rice varieties, the genotypes of hybrid sterility vary greatly among different varieties, particularly modern varieties, resulting in different crossing combinations with different degrees of hybrid sterility. In addition, the effects of alleles obtained from different donors are quantitatively different, resulting in the continuous variation of fertility at a single locus (Zhang et al., 1993, 1994). The molecular basis of allele diversity has been revealed by the cloned genes of S5 (Chen et al., 2008; Yang et al., 2012), Sa (Long et al., 2008; Xie et al., 2017), and Sc (Shen et al., 2017). In this study, we found that HJX74, the recipient of SSSLs, carried the Sn allele at the Sb, Sd, and Se loci but the Si allele at the S5, Sa, and Sc loci (Figure 1). In addition, the effect of Sai was weaker than that of Sci in HJX74 (Supplementary Table 3). The identification of genotypes that lead to hybrid sterility provided a prerequisite for improving the compatibility of HJX74.
Hybrid Sterility in indica–japonica Rice Can Be Overcome by Developing ICJLs and Wide-Compatible indica Lines
Based on the tri-allele pattern and the one-locus sporo-gametophytic interaction model, the indica–japonica hybrid sterility can be overcome by developing ICJLs and WCILs (Zhang and Lu, 1999; Zhang, 2020, 2022). ICJLs can be developed by transferring the Si allele from indica to japonica rice. In hybrids between indica varieties having Si allele and ICJLs having Si allele in japonica genetic background, the Si/Si genotype cannot cause the abortion of any gamete. In a previous study, we transferred the Si allele from indica donors to the japonica T65 variety to develop ICJLs, which carry the Si allele at hybrid sterility loci in the japonica genetic background. The result was that ICJLs were compatible with indica but incompatible with japonica rice (Guo et al., 2016). In another method, WCILs can be developed by transferring the Sn allele from donors to indica rice. In hybrids between WCILs having Sn allele in indica genetic background and japonica varieties having Sj allele, the Sn/Sj genotype cannot cause the abortion of any gamete. In this study, we pyramided the Sn allele of SSSLs to develop WCILs, which carry Sn allele in indica HJX74 genetic background. The result was that WCILs showed wide compatibility, which was compatible with both indica and japonica rice varieties (Figures 4, 5). These results showed that the breeding of ICJLs and WCILs is practicable and that the indica–japonica hybrid sterility could be overcome by using ICJLs and WCILs.
The Single-Segment Substitution Line Library Is a Powerful Platform for Developing Wide-Compatible indica Lines
The development of WCILs requires pyramiding Sn alleles of multiple hybrid sterility loci to improve compatibility. The breeding of WCILs is a challenging task because it is a time-consuming and laborious technique. First, the Sn alleles of the S5, Sa, Sb, Sc, Sd, and Se loci need to be identified and selected from a wide range of genetic resources. Second, Sn alleles of multiple loci need to be pyramided in an indica genetic background by MAS. In addition, WCILs need to have improved traits to be used as parents of indica–japonica hybrid rice. Over the past two decades, we have constructed a HJX74-SSSL library, which is used as a platform for rice design (Zhang, 2021). Using this platform, a series of CMS, maintainer, and restorer lines were developed (Dai et al., 2015, 2016; Luan et al., 2019). In this study, we identified Sn alleles at the S5, Sb, Sc, Sd, and Se loci from the HJX74-SSSL library. Since HJX74, the recipient of SSSLs, carried the Sn alleles at Sb, Sd, and Se loci, but the Si alleles at S5, Sa, and Sc loci, the SSSLs carrying S5n or Scn alleles were selected from the HJX74-SSSL library (Figures 2, 3). The Scn and S5n of the SSSLs were then pyramided in the HJX74 genetic background. Nine WCILs carrying Sn alleles at the S5, Sb, Sc, Sd, and Se loci in the HJX74 genetic background were developed (Figures 4, 5). The results show that the HJX74-SSSL library is a powerful platform for developing WCILs possessing the complex trait of wide compatibility.
Wide-Compatible indica Lines Will Be Used to Develop indica–japonica Hybrid Rice
It is believed that inter-subspecific hybrids have stronger heterosis than intra-subspecific hybrids (Fu et al., 2014; Birchler, 2015). Therefore, the exploitation of inter-subspecific heterosis for the production of improved rice varieties has long been considered (Cheng et al., 2007; Zhang, 2020). The main obstacle in utilizing inter-subspecific heterosis in rice is the indica–japonica hybrid sterility. In this study, WCILs were developed using the HJX74-SSSL platform. The WCILs had compatibility with a wide range of japonica varieties (Figure 5 and Supplementary Table 13). Therefore, the development of WCILs is an effective approach to overcoming the problem of indica–japonica hybrid sterility in breeding practice. By further improving their fertility restoration ability, WCILs can be improved to produce wide-compatible indica restorer lines (WCIRLs). Using the HJX74-SSSL platform, a series of WCIRLs is being developed and will be used to develop indica–japonica hybrid rice by crossing with japonica male sterile lines. Therefore, it is expected that indica–japonica hybrid rice will be the rice of next generation (Zhang, 2022).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
GZ designed and supervised the work, analyzed the data and wrote the manuscript. JG, YL, and LX performed most of the experiments and compiled the experimental data. TY, JZ, ZD, GT, KS, XL, WY, and QT conducted a part of the experiments. HZ, RZ, and SW prepared the experimental materials and supervised some experiments. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Funding
This work was supported by grants from the major program of transgenic new variety breeding in China (grant no. 2009ZX08009005) and the National Natural Science Foundation of China (grant nos. 91435207 and 31801449).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.890568/full#supplementary-material
References
- Birchler J. A. (2015). Heterosis: the genetic basis of hybrid vigour. Nat. Plants 1:15020. 10.1038/nplants.2015.20 [DOI] [PubMed] [Google Scholar]
- Chen J., Ding J., Ouyang Y., Du H., Yang J., Cheng K., et al. (2008). A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica-japonica hybrids in rice. Proc. Natl. Acad. Sci. U.S.A. 105 11436–11441. 10.1073/pnas.0804761105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Cao L., Zhuang J., Chen S., Zhan X., Fan Y., et al. (2007). Super hybrid rice breeding in China: achievements and prospects. J. Integr. Plant Biol. 49 805–810. 10.1111/j.1744-7909.2007.00514.x [DOI] [Google Scholar]
- Dai Z., Lu Q., Luan X., Cai J., Zhu H., Liu Z., et al. (2015). Development of a platform for breeding by design of CMS lines based on an SSSL library in rice (Oryza sativa L.). Euphytica 205 63–72. 10.1007/s10681-015-1384-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z., Lu Q., Luan X., Ouyang L., Guo J., Liang J., et al. (2016). Development of a platform for breeding by design of CMS restorer lines based on an SSSL library in rice (Oryza sativa L.). Breed. Sci. 66 768–775. 10.1270/jsbbs.16044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Ouyang Y., Zhang C., Zhang Q. (2011). Complex evolution of S5, a major reproductive barrier regulator, in the cultivated rice Oryza sativa and its wild relatives. New Phytol. 191 275–287. 10.1111/j.1469-8137.2011.03691.x [DOI] [PubMed] [Google Scholar]
- Fu D., Xiao M., Hayward A., Fu Y., Liu G., Jiang G., et al. (2014). Utilization of crop heterosis: a review. Euphytica 197 161–173. 10.1007/s10681-014-1103-7 [DOI] [Google Scholar]
- Fukagawa N. K., Ziska L. H. (2019). Rice: importance for global nutrition. J. Nutr. Sci. Vitaminol. 65 S2–S3. 10.3177/jnsv.65.S2 [DOI] [PubMed] [Google Scholar]
- Guo J., Xu X., Li W., Zhu W., Zhu H., Liu Z., et al. (2016). Overcoming inter-subspecific hybrid sterility in rice by developing indica-compatible japonica lines. Sci. Rep. 6:26878. 10.1038/srep.26878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N., Wu R., Pan X., Peng L., Sun K., Zou T., et al. (2017). Development and trait evaluation of chromosome single-segment substitution lines of O. meridionalis in the background of O. sativa. Euphytica 213:281. 10.1007/s10681-017-2072-4 [DOI] [Google Scholar]
- Ikehashi H., Araki H. (1986). “Genetics of F1 sterility in remote crosses in rice,” in Proceedings of the 1st Rice Genetics Symposium: Rice Genetics (Manila: International Rice Research Institute; ), 119–130. 10.1142/9789812814265_0011 [DOI] [Google Scholar]
- Ji Q., Lu J., Chao Q., Gu M., Xu M. (2005). Delimiting a rice wide-compatibility gene S5n to a 50 kb region. Theor. Appl. Genet. 111 1495–1503. 10.1007/s00122-005-0078-0 [DOI] [PubMed] [Google Scholar]
- Khush G. S. (2013). Strategies for increasing the yield potential of cereals: case of rice as an example. Plant Breed. 132 433–436. 10.1111/pbr.1991 [DOI] [Google Scholar]
- Li W., Zeng R., Zhang Z., Ding X., Zhang G. (2006). Fine mapping of locus S-b for F1 pollen sterility in rice (Oryza sativa L.). Chin. Sci. Bull. 51 675–680. 10.1007/s11434-006-0675-6 [DOI] [Google Scholar]
- Li W., Zeng R., Zhang Z., Ding X., Zhang G. (2008). Identification and fine mapping of S-d, a new locus conferring the partial pollen sterility of intersubspecific F1 hybrids in rice (Oryza sativa L.). Theor. Appl. Genet. 116 915–922. 10.1007/s00122-008-0723-5 [DOI] [PubMed] [Google Scholar]
- Long Y., Zhao L., Niu B., Su J., Wu H., Chen Y., et al. (2008). Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl. Acad. Sci. U.S.A. 105 18871–18876. 10.1073/pnas.0810108105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan X., Dai Z., Yang W., Tan Q., Lu Q., Guo J., et al. (2019). Breeding by design of CMS lines on the platform of SSSL library in rice. Mol. Breed. 39:126. 10.1007/s11032-019-1028-x [DOI] [Google Scholar]
- McCouch S. R., Teytelman L., Xu Y., Lobos K. B., Clare K., Walton M., et al. (2002). Development and mapping of 2240 new SSR markers for rice (Oryza sativa L.). DNA Res. 9 199–207. 10.1093/dnares/9.6.199 [DOI] [PubMed] [Google Scholar]
- Ouyang Y., Zhang Q. (2018). The molecular and evolutionary basis of reproductive isolation in plants. J. Genet. Genomics 45 613–620. 10.1016/j.jgg.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Panaud O., Chen X., McCouch S. R. (1996). Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol. Gen. Genet. 252 597–607. 10.1007/bf02172406 [DOI] [PubMed] [Google Scholar]
- Peleman J. D., van der Voort J. R. (2003). Breeding by design. Trends Plant Sci. 8 330–334. 10.1016/S1360-1385(03)00134-1 [DOI] [PubMed] [Google Scholar]
- Peng S., Khush G. S., Virk P., Tang Q., Zou Y. (2008). Progress in ideotype breeding to increase rice yield potential. Field Crops Res. 108 32–38. 10.1016/j.fcr.2008.04.001 [DOI] [Google Scholar]
- Peng S., Laza R. C., Visperas R. M., Khush G. S., Virk P., Zhu D. (2004). “Rice: progress in breaking the yield ceiling “new directions for a diverse planet”,” in Proceedings of the 4th International Crop Science Congress, Brisbane, Qld. [Google Scholar]
- Qiu S. Q., Liu K., Jiang J. X., Song X., Xu C. G., Li X. H., et al. (2005). Delimitation of the rice wide compatibility gene S5n to a 40-kb DNA fragment. Theor. Appl. Genet. 111 1080–1086. 10.1007/s00122-005-0033-0 [DOI] [PubMed] [Google Scholar]
- Shen R., Wang L., Liu X., Wu J., Jin W., Zhao X., et al. (2017). Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat. Commun. 8:1310. 10.1038/s41467-017-01400-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Liu Y. (2003). Fine mapping and cloning of the gene S-a for F1 pollen sterility in cultivated rice (Oryza sativa L.). Mol. Plant Breed. 1 757–758. [Google Scholar]
- Sundaram R. M., Sakthivel K., Hariprasad A. S., Ramesha M. S., Viraktamath B. C., Neeraja C. N., et al. (2010). Development and validation of a PCR-based functional marker system for the major wide-compatible gene locus S5 in rice. Mol. Breed. 26 719–727. 10.1007/s11032-010-9482-5 [DOI] [Google Scholar]
- Xi Z., He F., Zeng R., Zhang Z., Ding X., Li W., et al. (2006). Development of a wide population of chromosome single-segment substitution lines in the genetic background of an elite cultivar of rice (Oryza sativa L.). Genome 49 476–484. 10.1139/g06-005 [DOI] [PubMed] [Google Scholar]
- Xie Y., Niu B., Long Y., Li G., Tang J., Zhang Y., et al. (2017). Suppression or knockout of SaF/SaM overcomes the Sa-mediated hybrid male sterility in rice. J. Integr. Plant Biol. 59 669–679. 10.1111/jipb.12564 [DOI] [PubMed] [Google Scholar]
- Yanagihara S., Mccouch S. R., Ishikawa K., Ogi Y., Maruyama K., Ikehashi H. (1995). Molecular analysis of the inheritance of the S-5 locus, conferring wide compatibility in indica/japonica hybrids of rice (Oryza sativa L.). Theor. Appl. Genet. 90 182–188. 10.1007/BF00222200 [DOI] [PubMed] [Google Scholar]
- Yang C., Chen Z., Zhuang C., Mei M., Liu Y. (2004). Genetic and physical fine-mapping of the Sc locus conferring indica-japonica hybrid sterility in rice (Oryza sativa L.). Chin. Sci. Bull. 49 1718–1721. 10.1007/BF03184305 [DOI] [Google Scholar]
- Yang J., Zhao X., Cheng K., Du H., Ouyang Y., Chen J., et al. (2012). A killer-protector system regulates both hybrid sterility and segregation distortion in rice. Science 337 1336–1340. 10.1126/science.1223702 [DOI] [PubMed] [Google Scholar]
- Yuan L., Virmani S. (1988). “Status of hybrid rice research and development,” in Hybrid Rice (Manila: Internationa Rice Research Institute; ), 7–24. [Google Scholar]
- Zhang G. (2020). Prospects of utilization of inter-subspecific heterosis between indica and japonica rice. J. Integr. Agr. 19 1–10. 10.1016/S2095-3119(19)62843-1 [DOI] [Google Scholar]
- Zhang G. (2021). Target chromosome-segment substitution: a way to breeding by design in rice. Crop J. 9 658–668. 10.1016/j.cj.2021.03.001 [DOI] [Google Scholar]
- Zhang G. (2022). The next generation of rice: inter-subspecific indica-japonica hybrid rice. Front. Plant Sci. 13:857896. 10.3389/fpls.2022.857896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Lu Y. (1989). Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). I. Diallel analysis of the hybrid sterility among isogenic F1 sterile lines. Chin. J. Rice Sci. 3 97–101. [Google Scholar]
- Zhang G., Lu Y. (1993). Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). II. A genic model for F1 pollen sterility. Acta Genet. Sin. 20 222–228. [Google Scholar]
- Zhang G., Lu Y. (1999). Breeding of the indica-compatible japonica lines and their use in the breeding of super-high-yield hybrid rice. Hybrid Rice 14 3–5. [Google Scholar]
- Zhang G., Lu Y., Liu G., Yang J., Zhang H. (1993). Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa). III. Allele differentiation of F1 pollen sterility in different types of varieties. Acta Genet. Sin. 20 541–551. [Google Scholar]
- Zhang G., Lu Y., Zhang H., Yang J., Liu G. (1994). Genetic studies on the hybrid sterility in cultivated rice (Oryza sativa). IV. Genotypes for F1 pollen sterility. Acta Genet. Sin. 21 34–41. [Google Scholar]
- Zhang G., Zeng R., Zhang Z., Ding X., Li W., Liu G., et al. (2004). The construction of a library of single segment substitution lines in rice (Oryza sativa L.). Rice Genet. Newsl. 21 85–87. [Google Scholar]
- Zhang S., Huang X., Han B. (2021). Understanding the genetic basis of rice heterosis: advances and prospects. Crop J. 9 688–692. 10.1016/j.cj.2021.03.011 [DOI] [Google Scholar]
- Zhang Z., Deng Y., Tan J., Hu S., Yu J., Xue Q. (2007). A genome-wide microsatellite polymorphism database for the indica and japonica rice. DNA Res. 14 37–45. 10.1093/dnares/dsm005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang G. (2001). Fine mapping of the S-c locus and marker-assisted selection using PCR markers in rice. Acta Agron. Sin. 27 704–709. [Google Scholar]
- Zhao H., Sun L., Xiong T., Wang Z., Liao Y., Zou T., et al. (2019). Genetic characterization of the chromosome single-segment substitution lines of O. glumaepatula and O. barthii and identification of QTLs for yield-related traits. Mol. Breed. 39:51. 10.1007/s11032-019-0960-0 [DOI] [Google Scholar]
- Zhu W., Li W., Ding X., Zhang Z., Zeng R., Zhu H., et al. (2008). Preliminary identification of F1 pollen sterility gene S-e in Oryza sativa. J. South China Agri. Univ. 29 1–5. [Google Scholar]
- Zhuang C., Mei M., Zhang G., Lu Y. (2002). Chromosome mapping of the S-b locus for F1 pollen sterility in cultivated rice (Oryza sativa L.) with RAPD markers. Acta Genet. Sin. 29 700–705. 10.1088/1009-1963/11/5/313 [DOI] [PubMed] [Google Scholar]
- Zhuang C., Zhang G., Mei M., Lu Y. (1999). Molecular mapping of the S-a locus for F1 pollen sterility in cultivated rice (Oryza sativa L.). Acta Genet. Sin. 26 213–218. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.


