Abstract
Background:
Limited English proficiency (LEP) is associated with adverse clinical outcomes. The clinical impact of LEP in hematopoietic stem cell transplant (HSCT) has not been studied. The objectives of this study were to compare HSCT outcomes and health care utilization of Hispanic pediatric patients with and without parental LEP.
Methods:
We conducted a retrospective review of Hispanic/Latino pediatric patients receiving HSCT at a single institution. Families were identified as LEP or English proficient (EP) based on clinicians’ notes, social work documentation or the signature of a Spanish interpreter on treatment consents.
Results:
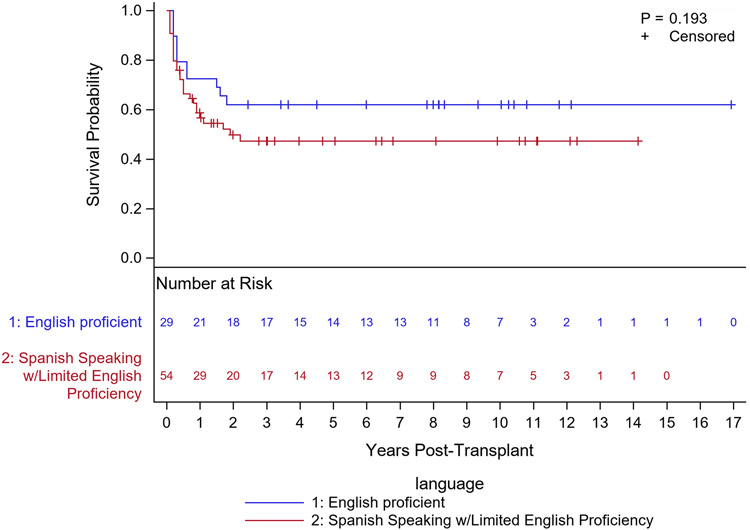
A total of 83 Hispanic/Latino patients were identified with 53 (65.1%) having parental LEP. More patients in the LEP group had a documented financial burden at pre-transplant psychosocial evaluation (72.2% vs 41.4%, p =0.009). LEP patients were more likely to have health insurance coverage through government-sponsored Medicaid (76.9% vs 27.6%, p <0.001). LEP patients were hospitalized on average 13 days longer than EP patients, and LEP patients were more likely to have pre-transplant CMV reactivity (67.3%) than EP patients (p=0.001). Overall survival was lower in LEP than EP but was not statistically significant (p=0.193). Multivariable Cox modeling suggested a potentially higher risk of death in LEP vs EP (hazard ratio=1.56, 95% CI: 0.38, 6.23).
Conclusions:
Parental LEP in HSCT is associated with prolonged hospitalization and pre-transplant CMV reactivity. These factors are associated with post-transplant complications and death. Our results suggest parental LEP is a risk factor for poor HSCT outcomes. Further study is warranted in a larger cohort.
Keywords: Limited English Proficiency, Pediatric Hematopoietic Stem Cell Transplant, Health Disparities
Introduction
The number of children in immigrant families represents a growing population with 25% of children in the United States living in a household with at least one immigrant parent.1,2 As this population has grown so has the number of pediatric medical encounters complicated by language barriers. According to the 2015 United States Census Bureau, more than 25 million Americans (9% of the population) speak English “less than very well” with most of these individuals having a preferred language of Spanish.3 Patients with limited English proficiency (LEP) are more likely than English-proficient (EP) patients to have poor health literacy, not understand their diagnoses, and experience increased rates of preventable morbidity and mortality.4-6 In pediatrics, parental LEP is associated with decreased satisfaction in care7,8, lack of insurance coverage9-11, decreased medication adherence12-15 and an increased risk for serious medical events during hospitalization.16 Although disparities are well documented in patients with LEP, the literature is limited regarding the effect of language barriers on objective pediatric clinical outcomes.17
Hematopoietic stem cell transplantation (HCST) is a potentially curative procedure for a variety of pediatric malignant and non-malignant diseases. However, HSCT poses a significant set of challenges for families. This process involves a prolonged hospitalization, requires a good understanding of the diagnosis, and demands excellent medication adherence to achieve good outcomes. Language discordance between clinicians and LEP families of pediatric patients undergoing HSCT may further complicate this process and potentially affect clinical outcomes. While racial and ethnic differences in adult HSCT clinical outcomes have been studied with mixed results,18-25 the relationship between language barriers and HSCT clinical outcomes has not been reported.
Our objectives in this study were to compare clinical outcomes and healthcare utilization among Spanish-speaking with LEP versus EP Hispanic patients and families that have received HSCT. We hypothesized that LEP would be associated with inferior clinical outcomes and increased healthcare resource utilization.
Methods
Study design
We conducted a retrospective cohort study of pediatric patients of Hispanic/Latino descent receiving HSCT at Duke University. Study approval was obtained from the Duke institutional review board with a waiver of informed consent. The existing Duke Pediatric Transplant and Cellular Therapy (PTCT) database was used to identify patients of Hispanic and/or Latino descent ages 0 to 21 years who had received HSCT by Duke PTCT department with date of transplant from January 1, 2000 through March 15, 2019. Patients were eligible for inclusion if they received hematopoietic transplantation of any type with a donor stem cell source of any type for any primary diagnosis. The study only included patients of Hispanic/Latino descent, to decrease the effect of ethnicity as a contributing factor for any detected differences in clinical outcomes. Patients with race, ethnicity or preferred language not documented and patients under pediatric bone marrow transplant care for therapies other than stem cell transplantation (e.g., CAR-T cell infusion, stem cell infusion for purposes other than HSCT, etc.) were excluded from this analysis.
Data were extracted from the Duke PTCT database and the electronic medical record, and entered into electronic case report forms. We collected data on baseline demographics and clinical information regarding the primary diagnosis for which HSCT was completed, transplant type and donor stem cell source, transplant co-morbidity index, and cytomegalovirus (CMV) seroreactivity status at the time of pre-transplant evaluation. Baseline demographics included sex, race, parental level of education (if available), insurance status, and note of family financial strain in the social work intake documentation. The presence of financial burden was defined as families self-identifying as having financial strain at the time of pre-transplant evaluation requiring assistance for housing, utilities, and/or food. Co-morbidity index (HCT-CI) was calculated for the patients based on their pre-transplant evaluation.26-28
Definitions
Parental LEP
The parents/guardians of patients undergoing HSCT were indicated to have LEP with Spanish language preference based on the presence of at least two of the following: (1) language barrier or use of interpreter noted in documentation of clinicians and ancillary staff, (2) Spanish as preferred language in electronic medical record, (3) social work intake documentation noting a preferred language of Spanish, and/or (4) the signature of a medical Spanish interpreter on the transplant treatment consents. The parents/guardians that did not meet these criteria were defined as EP.
CMV seroreactivity and reactivation
CMV positive status was defined as donor, recipient or both being CMV IgG positive at time of pre-transplant evaluation. Post-transplant CMV reactivation was defined as more than 600 CMV copies/milliliter on two consecutive polymerase chain reaction (PCR) analyses in serum within a 1-week interval.29, 30 Per institutional practice, each patient had weekly post-transplant surveillance with CMV PCR until at least day 100 after HSCT.
Statistical analysis
We evaluated the following clinical outcome measures: overall survival, rates of relapse of primary disease for those receiving transplant for malignant conditions, lengths of hospitalizations, time from engraftment to discharge, rates of re-hospitalizations, rates of complications including infections and graft versus host disease (GVHD) grades II-IV.
All statistical analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC). Patients were analyzed in two categories: those that were Spanish speaking with LEP and those that were EP. Continuous variables were summarized with mean, standard deviation, median and range and compared between groups using the Wilcoxon rank sum test. Fisher’s exact test was used to compare categorical variables between groups. Overall survival was estimated using the Kaplan-Meier method. Results for GVHD were reported using the cumulative incidence method described by Kalbfleisch and Prentice with death as a competing risk.
Results
Out of 1318 patients treated during the inclusion timeframe, three did not have race or ethnicity documented and 88 were identified as having an ethnicity of Hispanic and/or Latino. Five of these patients were excluded because three received treatment with CAR-T cells and two received stem cells for other experimental treatments than HSCT. A total of 83 patients met the inclusion criteria with 53 (65.1%) of these families identified as having LEP.
Baseline demographics are reported in Table 1. There were no significant differences in age at transplant, sex, and race between these groups. The parental educational levels were absent in the large majority of the patients so this information was not reported. More patients in the LEP group were self-identified as having financial strain at the time of pre-transplant psychosocial evaluation (72.2% vs 41.4%, p =0.009). LEP patients were more likely to have health insurance coverage through government-sponsored Medicaid (76.9% vs 27.6%, p <0.001).
TABLE 1.
Demographics
| Spanish Speaking w/Limited English Proficiency (N=54) |
English proficient (N=29) |
Total (N=83) |
P-value | |
|---|---|---|---|---|
| Age at transplant | 0.6471 | |||
| Mean (SD) | 7.71 (6.07) | 7.15 (5.86) | 7.51 (5.97) | |
| Median | 6.50 | 6.58 | 6.58 | |
| Range | (0.09-20.67) | (0.09-19.45) | (0.09-20.67) | |
| Sex | 0.8182 | |||
| Male | 27 (50.0%) | 16 (55.2%) | 43 (51.8%) | |
| Female | 27 (50.0%) | 13 (44.8%) | 40 (48.2%) | |
| Race3 | 0.4982 | |||
| White | 18 (34.0%) | 10 (34.5%) | 28 (34.1%) | |
| Black | 0 (0.0%) | 1 (3.4%) | 1 (1.2%) | |
| Other | 35 (66.0%) | 18 (62.1%) | 53 (64.6%) | |
| Health Insurance4 | <0.0012 | |||
| Government- sponsored Medicaid | 40 (76.9%) | 8 (27.6%) | 48 (59.3%) | |
| Private health insurance | 12 (23.1%) | 21 (72.4%) | 33 (40.7%) | |
| Financial strain at pre-transplant evaluation | 0.0092 | |||
| No | 15 (27.8%) | 17 (58.6%) | 32 (38.6%) | |
| Yes | 39 (72.2%) | 12 (41.4%) | 51 (61.4%) |
Wilcoxon rank sum test
Fisher’s exact test
Race is not known for 1 patient who was Spanish Speaking w/Limited English Proficiency
One patient who was Spanish Speaking w/Limited English Proficiency was not covered by insurance, and insurance status could not be determined for another patient in this group.
The diagnosis and transplant characteristics are listed in Table 2. The primary diagnosis for which HSCT was performed was similar between the two groups with the exception of more metabolic disorders in the EP group (34.5%) than LEP (14.8%). Type of transplant, donor, and stem cell product was similar in these groups. Patients with parental LEP were significantly more likely to have pre-transplant CMV positive reactivity status (73.6%) than EP patients (33.3%, p=0.001). In addition, patients in each group had similar calculated HCT-CI scores.
TABLE 2.
Diagnosis and Transplant Characteristics
| Spanish Speaking w/Limited English Proficiency (N=54) |
English proficient (N=29) |
Total (N=83) |
P-Value | |
|---|---|---|---|---|
| Year of Transplant | 0.0381 | |||
| Median | 2011 | 2008 | 2010 | |
| Range | (2001-2019) | (2000-2017) | (2000-2019) | |
| Diagnosis | 0.2202 | |||
| Leukemia | 28 (51.9%) | 13 (44.8%) | 41 (49.4%) | |
| Lymphoma | 0 (0.0%) | 1 (3.4%) | 1 (1.2%) | |
| Bone Marrow Failure/Myelodysplastic syndrome | 5 (9.3%) | 1 (3.4%) | 6 (7.2%) | |
| Immune Deficiency | 5 (9.3%) | 3 (10.3%) | 8 (9.6%) | |
| Metabolic Disorder | 8 (14.8%) | 10 (34.5%) | 18 (21.7%) | |
| Solid tumor | 6 (11.1%) | 1 (3.4%) | 7 (8.4%) | |
| Hemoglobinopathy | 2 (3.7%) | 0 (0.0%) | 2 (2.4%) | |
| Type of transplant | 0.8202 | |||
| Autologous | 7 (13.0%) | 2 (6.9%) | 9 (10.8%) | |
| Allogeneic, unrelated | 37 (68.5%) | 21 (72.4%) | 58 (69.9%) | |
| Allogeneic related | 10 (18.5%) | 6 (20.7%) | 16 (19.3%) | |
| Donor type3 | 0.8562 | |||
| Missing | 7 (.%) | 2 (.%) | 9 | |
| HLA-identical sibling | 9 (19.1%) | 6 (22.2%) | 15 (20.3%) | |
| HLA-mismatched relative | 1 (2.1%) | 0 (0.0%) | 1 (1.4%) | |
| Unrelated donor | 37 (78.7%) | 21 (77.8%) | 58 (78.4%) | |
| Product type | 0.2152 | |||
| Multiple UCB | 9 (16.7%) | 1 (3.4%) | 10 (12.0%) | |
| Single UCB | 25 (46.3%) | 20 (69.0%) | 45 (54.2%) | |
| PBSC | 7 (13.0%) | 2 (6.9%) | 9 (10.8%) | |
| PBSC + UCB | 1 (1.9%) | 0 (0.0%) | 1 (1.2%) | |
| BM | 12 (22.2%) | 6 (20.7%) | 18 (21.7%) | |
| CMV Seroreactivity Status4 | 0.0012 | |||
| Positive | 39 (73.6%) | 9 (33.3%) | 48 (60.0%) | |
| Negative | 14 (26.4%) | 18 (66.7%) | 32 (40.0%) | |
| HCT-CI5 | 0.5351 | |||
| 1-2 | 27 (79.4%) | 8 (88.9%) | 35 (81.4%) | |
| 3+ | 7 (20.6%) | 1 (11.1%) | 8 (18.6%) |
UCB = umbilical cord blood. PBSC = peripheral blood stem cells. BM = bone marrow. CMV = cytomegalovirus. HCT-CI = hematopoietic cell transplant comorbidity index.
Wilcoxon rank sum test
Fisher’s exact test
Donor type is not specified for autologous transplants
CMV status is missing for 1 patient who is Spanish Speaking w/Limited English Proficiency and 2 patients who are English Proficient
For patients with comorbidities only. A total of 20 patients in each group did not have any comorbidities at transplant.
Clinical outcomes (Table 3) revealed that the cumulative incidence of relapse, neutrophil engraftment, acute and chronic GVHD were similar in both groups, as were the frequency of post-transplant infections. Overall survival was lower in LEP than EP but this difference was not statistically significant (Fig. 1, p=0.193). Multivariable Cox modeling suggested a potentially higher risk of death in LEP vs EP (hazard ratio=1.56, 95% CI: 0.38, 6.23). Multivariate cox regression did not reveal a relationship between overall survival and other key covariates (year of transplant or prevalence of co-morbidities). Our cohort did not reveal a decreased overall survival associated with CMV seropositivity (hazard ratio= 0.48, 95% CI: 0.25, 0.92).
TABLE 3.
Transplant Outcomes
| Spanish Speaking w/Limited English Proficiency (N=54) |
English proficient (N=29) |
All (N=83) |
P-Value | |
|---|---|---|---|---|
| Time from transplant to discharge1 | 0.0374 | |||
| Mean (SD) | 46.66 (26.58) | 33.42 (20.67) | 41.77 (25.23) | |
| Median | 36.00 | 31.00 | 34.00 | |
| Range | (16.00-138.00) | (1.00-116.00) | (1.00-138.00) | |
| Post-transplantation CMV reactivation2 | 0.0685 | |||
| Yes | 23 (59.0%) | 2 (22.2%) | 25 (52.1%) | |
| No | 16 (41.0%) | 7 (77.8%) | 23 (47.9%) | |
| Neutrophil Engraftment @ Day 423 | 91 (78, 96) | 93 (71, 99) | 92 (83, 96) | 0.8086 |
| Relapse @ 1 Year3 | 29 (16, 45) | 19 (4, 41) | 26 (15, 39) | 0.1846 |
| Acute GVHD @ 100 Days3 | 44 (29, 57) | 50 (29, 68) | 46 (34, 57) | 0.3356 |
| Chronic GVHD @ 1 Year3 | 20 (11, 32) | 31 (15, 48) | 24 (16, 34) | 0.1786 |
Among patients discharged alive. A total of 5 English Proficient patients and 13 Spanish Speaking w/Limited English Proficiency died in the hospital.
Among patients who were seropositive prior to transplant
Cumulative incidence (with death as a competing risk) and 95% confidence intervals
Wilcoxon rank sum test
Fisher’s exact test
Gray’s test
Figure 1.
Overall Survival by Language Proficiency
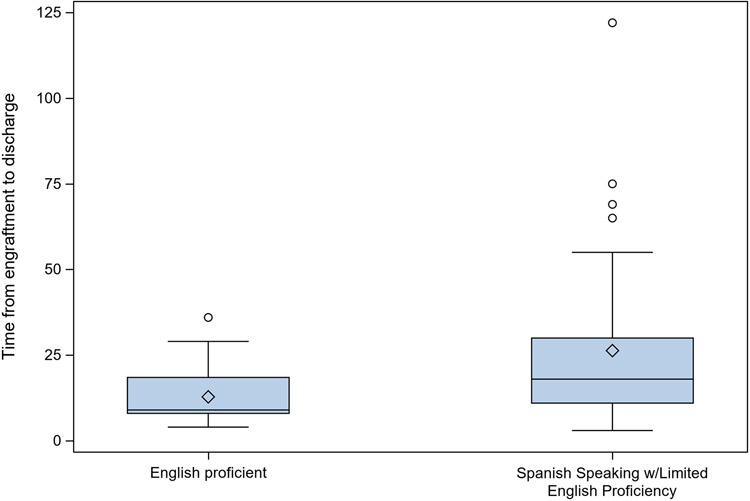
Patients with parental LEP were hospitalized on average 13 days longer than patients with EP (Table 3, p=0.037) and had a significantly longer time from engraftment to hospital discharge (Fig. 2, p=0.005). The rates of hospital readmissions were similar in both groups.
Figure 2.
Time from Neutrophil Engraftment to Discharge by Language Proficiency
Median (min, max; interquartile range) time from neutrophil engraftment to hospital discharge was 9 days (4, 36; 8, 18.5) for English Proficient Patients (N=24) and 18 days (3, 122; 11, 30) for Spanish Speaking w/Limited English Proficiency (N=41) (P=0.005, Wilcoxon Rank Sum Test). A total of 78/83 patients had neutrophil engraftment (28 English Proficient and 50 Spanish Speaking w/Limited English Proficiency). Time to discharge after neutrophil engraftment is not defined for 5 English Speaking patients and 9 Spanish Speaking w/Limited English Proficiency who died in the hospital after neutrophil engraftment.
Discussion
The results of this exploratory retrospective analysis reveal that parental LEP is associated with a higher risk of pre-transplant CMV seropositivity and prolonged hospitalizations for children receiving HSCT at our institution. Although there is evidence of LEP being a risk factor for serious adverse medical events,4,16 longer emergency department stays and lengths of hospitalizations,31-33 and higher rates of hospital readmissions34, there is little known about the effects of parental LEP on pediatric HSCT clinical outcomes. There is also no documented association of CMV seropositivity with language proficiency, thus we have reported two novel and important findings here.
CMV infection in the post-transplant period has been associated with an increased risk of overall mortality and prolonged length of hospitalizations.30,35 In a large European bone marrow transplant database analysis, CMV seropositive patients were found to have a lower overall survival, lower leukemia free survival and higher non-relapse mortality when compared to CMV seronegative patients receiving transplants from CMV seronegative donors.36 In our cohort, the trend of overall survival suggested a potentially higher risk of death in the LEP group. Multivariable analysis was completed to determine if this trend was in part secondary to the difference in CMV seropositivity but our analysis did not find a decreased overall survival to be associated with CMV seropositivity. While post-HSCT CMV infection is often viewed as risk factor for increased morbidity and mortality, there are reports of a potentially protective anti-leukemic effect which may explain why a negative relationship between overall survival and CMV seropositivity was not observed in our cohort.37,38 A larger study that is powered to these endpoints may be necessary to more definitively examine this relationship.
We suspected the difference in pre-transplant CMV seropositivity between the LEP and EP groups might be attributed to lower socioeconomic status, as the patients with parental LEP were more likely to have insurance through government-sponsored Medicaid and have financial burden documented on social work intake. An analysis of the National Health and Nutrition Examination Survey revealed CMV seropositivity in the United States was independently associated with foreign birthplace, low household income, high household crowding and low household education.39 Other groups have also observed an association with lower socioeconomic status and CMV seropositivity.40,41 Our results now show a similar association in the pediatric HSCT setting.
Parental LEP has been associated with increased health care resource utilization. In a retrospective cohort study of children admitted for infection requiring parenteral antibiotic therapy, parental LEP was associated with a 60% longer median hospital length of stay than pediatric patients with EP parents and a decreased number of home health referrals.32 Pediatric patients presenting to the emergency department with parental LEP are more likely to have diagnostic studies ordered and longer visit times than families with EP for similar presenting symptoms.42,43 In our cohort of Hispanic/Latino patients receiving HSCT, time from neutrophil engraftment to hospital discharge was significantly longer in patients with parental LEP, without apparent explanation by any measured clinical factors. An average extended hospitalization length of stay 13 days longer in LEP versus EP patients has considerable financial implications in a procedure that is already one of the most expensive medical procedures in the United States.44 It is not clear whether the increased length of stay is secondary to miscommunication due to LEP or whether this is secondary to other complications such as CMV reactivation. Further study is needed to better understand this marked difference.
The potential adverse effects of language barriers on clinical outcomes and health care resource utilization raises the question as to what interventions and resources can be allocated to prevent these differences in care. Medical interpreters have been an invaluable resource when caring for pediatric patients with parental LEP. A survey study of pediatricians across the United States revealed that most pediatricians report using family members to communicate with LEP patients and families but increased use of formal interpreters by pediatricians was observed in states that provided reimbursement for interpreter services.45 The underutilization of formal medical interpreters contributes to the health care disparities seen when language barriers are present and increased access and reimbursement to interpreter services may improve the care for these patients.46 However, formal medical interpreting services do not mitigate the challenges of communication across a language barrier as these encounters are also complicated by cultural differences.5,47,48 Improving the care for patients and families with LEP will require a multifaceted approach beyond increasing the access to formal medical interpreting services, and more dedicated research is needed to examine how to most effectively engage and communicate with LEP patients and families.
Our study has several notable limitations. It was a single-center retrospective study with a limited sample size of patients with parental LEP; findings may differ in other centers with a different demographic distribution of patients and clinicians and different interpreting services. Our institution’s PTCT department is composed of primarily English-speaking transplant physicians, nurse practitioners, and nursing staff. Therefore, most interactions with parents/guardians that are Spanish-speaking with LEP are complicated by language discordance between clinicians and families. Our institution offers the service of in-person Spanish interpreters that are available for both inpatient and outpatient encounters. If an in-person interpreter is not available, there is also the option of utilizing interpreters via telephone or via electronic tablet that features both audio and video interaction. While multiple options are available for interpreting services, we were not able to accurately document via our retrospective review whether these services were consistently utilized in every patient encounter with physicians and nurses. Accordingly, further studies on the relationship between frequency of interpreter service use, type of interpreter service used, and LEP patient and family experiences/outcomes are needed.
Another limitation was that we limited our cohort to Hispanic/Latino patients to decrease the effect of ethnicity as a contributing factor to differences between the LEP and EP groups. Consequently, the findings in this cohort may not be applicable to patients of other racial or ethnic backgrounds. Of note, we did not compare outcomes of our Hispanic/Latino cohort to the Non-Hispanic/Latino White or Black patients, as the focus of our study was to determine if there were differences on the basis of LEP, within the Hispanic/Latino cohort.
There were also multiple confounding variables including co-morbidities, CMV seroreactivity status, and socioeconomic status that may have contributed to the differences observed in our cohort. We did not have information regarding the family’s income or the parental educational level but it is possible that the differences observed in our cohort can be attributed to lower socioeconomic status, insurance status and decreased health literacy in the group of children with parental LEP. The parental LEP group were more likely to self-identify as having financial strain at the time of pre-transplant evaluation and these children were more likely to have government sponsored Medicaid, both of which suggest likely differences in socioeconomic status. A large pediatric transplant database analysis recently demonstrated inferior overall survival in children with Medicaid as compared to children with private insurance among pediatric patients receiving allogeneic transplant for malignant diseases.49
We also acknowledge that there are varying degrees of LEP. While we attempted to define criteria that would accurately identify parents/guardians as having LEP by using multiple factors, there is significant complexity to language preferences and proficiency making this variable difficult to fully and accurately define. It is possible that patients may have also met criteria as having parental LEP even if another parent or guardian had EP.
Despite these limitations, we believe that our study is an important addition to the literature as it highlights the unique challenges that children in immigrant families may face when they need a complex procedure such as HSCT, calling for an urgent need to investigate these issues further. In addition, language barriers have not been explored in HSCT and continued investigation of this topic may reveal whether differences in HSCT outcomes can truly be attributable to language preference. Regardless, ongoing investigation to describe the barriers for this patient population is needed, and will reveal opportunities for improvement and intervention to optimize clinical outcomes for this vulnerable pediatric population.
In our institution, this study has prompted us to perform an in-depth investigation into the underlying causes of the differences seen in this cohort to identify areas of targeted interventions in order to improve outcomes for this vulnerable population. As we expand this study, we will be collaborating with our International Patient Services Department and Office of Health Equity and Disparities to seek their expertise and guidance. Over the past years, our International Patient Services Department has made efforts to ensure access to medical interpreters that are available in person via telephone or via electronic tablets, which feature both audio and video interaction. While these efforts have improved ease of access to medical interpreting services, there is currently not an ideal process in place to ensure that clinicians, nurses, and ancillary staff are utilizing these services appropriately with each medical encounter. A provision of care and failure to use an interpreter category is now available in our safety reporting system but we suspect that this feature is underutilized in one-on-one encounters with parents with LEP without an interpreter. Examples of potential interventions that we hope to explore include, but are not limited to clinician education on appropriate use of medical interpreters and systematic processes to ensure the use of medical interpreters with all patients/families with LEP.
Conclusion
We found that parental LEP in pediatric patients undergoing HSCT is a risk factor for prolonged hospitalizations and pre-transplant CMV reactivity. These factors are known to be associated with post-transplant complications and death. Our results suggest LEP families are at higher risk for poor HSCT outcomes. Further study is warranted in a larger cohort to distinguish whether the differences seen in our cohort are attributable to language barriers or a reflection of differences in socioeconomic status among LEP and EP groups, and to inform interventions to close the gap in outcomes.
Abbreviations:
- CAR
Chimeric antigen receptor
- CMV
Cytomegalovirus
- EP
English proficient
- GVHD
Graft versus host disease
- HCT-CI
Hematopoietic cell transplantation comorbidity index
- HSCT
Hematopoietic stem cell transplantation
- Ig
Immunoglobulin
- LEP
Limited English proficiency
- PCR
Polymerase Chain Reaction
- PTCT
Pediatric Transplant and Cellular Therapy
Footnotes
Data presented as a poster meeting abstract: “The Effect of Limited-English Proficiency on Hematopoietic Stem Cell Transplantation Outcomes: A Retrospective Cohort Study of Hispanic Pediatric Transplant Patients at a Single Institution.” 2020 Transplantation & Cellular Therapy Meetings of ASTCT and CIBMTR. February 2020. TCT Journal.
Conflict of interest statement: The authors have the following conflict of interests to report:
- Dr. LeBlanc reports the following disclosures from the past 24 months: personal fees for consulting or advisory boards from AbbVie, Agios, AstraZeneca, Amgen, Astellas, CareVive, BMS/Celgene, Daiichi-Sankyo, Heron, Flatiron, Otsuka, Pfizer, and Seattle Genetics; royalties from UpToDate; speakers bureau fees from Agios, AbbVie, and BMS/Celgene; grants and/or research contracts from the American Cancer Society, AstraZeneca, BMS, Jazz Pharmaceuticals, the NINR / NIH, and Seattle Genetics.
- Dr. Troy reports the following disclosures from the past 24 months: Royalties from SinoCell, Research funding from Bristol Myers Squibb, Honorarium for serving on a DSMB for Gamida Cell, Honorarium for serving on a DSMB for Synthetic Biologics, Consulting services to Gamida Cell, Consulting services to The EMMES Corporation, Consulting services to The Community Data Roundtable, and Consulting services to AegisCN.
- The other authors (Dr. Robles, Dr. Schroeder and Dr. Martin) do not have any direct or indirect commercial financial incentive associated with publishing this article.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Foundation TAEC. Kids Count Data Center. Children in immigrant families in the United States. https://datacenter.kidscount.org/data/tables/115-children-in-immigrant-families?loc=1&loct=1#detailed/1/any/false/871,870,573,869,36,868,867,133,38,35/any/445,446. Published 2020. Updated January 2020. Accessed April 23, 2020. [Google Scholar]
- 2.Institute U. Children of immigrants data tool. http://webapp.urban.org/charts/datatool/pages.cfm. Published 2020. Updated 2020. Accessed April 23, 2020. [Google Scholar]
- 3.Bureau UC. Detailed Languages Spoken at Home and Ability to Speak English for the Population 5 Years and Over: 2009-2013. https://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Published 2015. Accessed2019. [Google Scholar]
- 4.Divi C, Koss RG, Schmaltz SP, Loeb JM. Language proficiency and adverse events in US hospitals: a pilot study. Int J Qual Health Care. 2007;19(2):60–67. [DOI] [PubMed] [Google Scholar]
- 5.Flores G, Rabke-Verani J, Pine W, Sabharwal A. The importance of cultural and linguistic issues in the emergency care of children. Pediatr Emerg Care. 2002;18(4):271–284. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The Association between Limited English Proficiency and Sepsis Mortality. J Hosp Med. 2019;14:E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerfeld CI, Hoffman JM, Ciol MA, Kartin D. Delayed or forgone care and dissatisfaction with care for children with special health care needs: the role of perceived cultural competency of health care providers. Matern Child Health J. 2011;15(4):487–496. [DOI] [PubMed] [Google Scholar]
- 8.Ngui EM, Flores G. Satisfaction with care and ease of using health care services among parents of children with special health care needs: the roles of race/ethnicity, insurance, language, and adequacy of family-centered care. Pediatrics. 2006;117(4):1184–1196. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg SJ, O'Connor KS, Kenney G. Unworried parents of well children: a look at uninsured children who reportedly do not need health insurance. Pediatrics. 2005;116(2):345–351. [DOI] [PubMed] [Google Scholar]
- 10.Yu SM, Singh GK. Household language use and health care access, unmet need, and family impact among CSHCN. Pediatrics. 2009;124 Suppl 4:S414–419. [DOI] [PubMed] [Google Scholar]
- 11.Yu SM, Nyman RM, Kogan MD, Huang ZJ, Schwalberg RH. Parent's language of interview and access to care for children with special health care needs. Ambul Pediatr. 2004;4(2):181–187. [DOI] [PubMed] [Google Scholar]
- 12.Inkelas M, Garro N, McQuaid EL, Ortega AN. Race/ethnicity, language, and asthma care: findings from a 4-state survey. Ann Allergy Asthma Immunol. 2008;100(2):120–127. [DOI] [PubMed] [Google Scholar]
- 13.Blumberg SJ, Read D, Avila RM, Bethell CD. Hispanic children with special health care needs from Spanish-language households. Pediatrics. 2010;126 Suppl 3:S120–128. [DOI] [PubMed] [Google Scholar]
- 14.Read D, Bethell C, Blumberg SJ, Abreu M, Molina C. An evaluation of the linguistic and cultural validity of the Spanish language version of the children with special health care needs screener. Matern Child Health J. 2007;11(6):568–585. [DOI] [PubMed] [Google Scholar]
- 15.Foster BA, Read D, Bethell C. An analysis of the association between parental acculturation and children's medication use. Pediatrics. 2009;124(4):1152–1161. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AL, Rivara F, Marcuse EK, McPhillips H, Davis R. Are language barriers associated with serious medical events in hospitalized pediatric patients? Pediatrics. 2005;116(3):575–579. [DOI] [PubMed] [Google Scholar]
- 17.Eneriz-Wiemer M, Sanders LM, Barr DA, Mendoza FS. Parental limited English proficiency and health outcomes for children with special health care needs: a systematic review. Acad Pediatr. 2014;14(2):128–136. [DOI] [PubMed] [Google Scholar]
- 18.Serna DS, Lee SJ, Zhang MJ, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21(20):3754–3760. [DOI] [PubMed] [Google Scholar]
- 19.Majhail NS, Nayyar S, Santibanez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47(11):1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(12):1543–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton BK, Rybicki L, Sekeres M, et al. Racial differences in allogeneic hematopoietic cell transplantation outcomes among African Americans and whites. Bone Marrow Transplant. 2015;50(6):834–839. [DOI] [PubMed] [Google Scholar]
- 22.Baker KS, Loberiza FR Jr., Yu H, et al. Outcome of ethnic minorities with acute or chronic leukemia treated with hematopoietic stem-cell transplantation in the United States. J Clin Oncol. 2005;23(28):7032–7042. [DOI] [PubMed] [Google Scholar]
- 23.Khera N, Chang YH, Slack J, et al. Impact of race and ethnicity on outcomes and health care utilization after allogeneic hematopoietic cell transplantation. Leuk Lymphoma. 2015;56(4):987–992. [DOI] [PubMed] [Google Scholar]
- 24.Eckrich MJ, Ahn KW, Champlin RE, et al. Effect of race on outcomes after allogeneic hematopoietic cell transplantation for severe aplastic anemia. Am J Hematol. 2014;89(2):125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwake CJ, Eapen M, Lee SJ, et al. Differences in characteristics of US hematopoietic stem cell transplantation centers by proportion of racial or ethnic minorities. Biol Blood Marrow Transplant. 2005;11(12):988–998. [DOI] [PubMed] [Google Scholar]
- 26.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorror ML, Logan BR, Zhu X, et al. Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant. 2015;21(8):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorror ML, Storer B, Storb RF. Validation of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in single and multiple institutions: limitations and inferences. Biol Blood Marrow Transplant. 2009;15(6):757–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64(1):87–91. [DOI] [PubMed] [Google Scholar]
- 30.Rastogi S, Ricci A, Jin Z, et al. Clinical and Economic Impact of Cytomegalovirus Infection among Children Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2019;25(6):1253–1259. [DOI] [PubMed] [Google Scholar]
- 31.John-Baptiste A, Naglie G, Tomlinson G, et al. The effect of English language proficiency on length of stay and in-hospital mortality. J Gen Intern Med. 2004;19(3):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levas MN, Cowden JD, Dowd MD. Effects of the limited English proficiency of parents on hospital length of stay and home health care referral for their home health care-eligible children with infections. Arch Pediatr Adolesc Med. 2011;165(9):831–836. [DOI] [PubMed] [Google Scholar]
- 33.Goldman RD, Amin P, Macpherson A. Language and length of stay in the pediatric emergency department. Pediatr Emerg Care. 2006;22(9):640–643. [DOI] [PubMed] [Google Scholar]
- 34.Ju M, Luna N, Park KT. The Effect of Limited English Proficiency on Pediatric Hospital Readmissions. Hosp Pediatr. 2017;7(1):1–8. [DOI] [PubMed] [Google Scholar]
- 35.Gimenez E, Torres I, Albert E, et al. Cytomegalovirus (CMV) infection and risk of mortality in allogeneic hematopoietic stem cell transplantation (Allo-HSCT): A systematic review, meta-analysis, and meta-regression analysis. Am J Transplant. 2019;19(9):2479–2494. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Hieber M, Labopin M, Beelen D, et al. CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013;122(19):3359–3364. [DOI] [PubMed] [Google Scholar]
- 37.Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122(7):1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–1412. [DOI] [PubMed] [Google Scholar]
- 39.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enders G, Daiminger A, Lindemann L, et al. Cytomegalovirus (CMV) seroprevalence in pregnant women, bone marrow donors and adolescents in Germany, 1996-2010. Med Microbiol Immunol. 2012;201(3):303–309. [DOI] [PubMed] [Google Scholar]
- 41.Voigt S, Schaffrath Rosario A, Mankertz A. Cytomegalovirus Seroprevalence Among Children and Adolescents in Germany: Data From the German Health Interview and Examination Survey for Children and Adolescents (KiGGS), 2003-2006. Open Forum Infect Dis. 2016;3(1):ofv193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hampers LC, Cha S, Gutglass DJ, Binns HJ, Krug SE. Language barriers and resource utilization in a pediatric emergency department. Pediatrics. 1999;103(6 Pt 1):1253–1256. [DOI] [PubMed] [Google Scholar]
- 43.Zamor R, Byczkowski T, Zhang Y, Vaughn L, Mahabee-Gittens EM. Language Barriers and the Management of Bronchiolitis in a Pediatric Emergency Department. Acad Pediatr. 2020;20(3):356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stranges E, Russo CA, Friedman B. Procedures with the Most Rapidly Increasing Hospital Costs, 2004-2007: Statistical Brief #82. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2006. [Google Scholar]
- 45.DeCamp LR, Kuo DZ, Flores G, O'Connor K, Minkovitz CS. Changes in language services use by US pediatricians. Pediatrics. 2013;132(2):e396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flores G, Laws MB, Mayo SJ, et al. Errors in medical interpretation and their potential clinical consequences in pediatric encounters. Pediatrics. 2003;111(1):6–14. [DOI] [PubMed] [Google Scholar]
- 47.Flores G, Abreu M, Schwartz I, Hill M. The importance of language and culture in pediatric care: case studies from the Latino community. J Pediatr. 2000;137(6):842–848. [DOI] [PubMed] [Google Scholar]
- 48.Flores G, Abreu M, Olivar MA, Kastner B. Access barriers to health care for Latino children. Arch Pediatr Adolesc Med. 1998;152(11):1119–1125. [DOI] [PubMed] [Google Scholar]
- 49.Bona K, Brazauskas R, He N, et al. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137(4):556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.