Summary
Background:
Scant data exist on weight loss interventions for youth with intellectual disabilities (ID).
Objective:
To compare weight loss among youth with ID randomized to a 6-month, family-based behavioural intervention (FBBI) or a waitlist and to compare weight loss among youth who completed a 6-month maintenance (FBBI-M) intervention to a control group (FBBI-C).
Methods:
Youth with ID and overweight/obesity, aged 14–22 years, were randomized to the FBBI or to a waitlist and subsequently randomized to a maintenance intervention or a control group. Sessions were held weekly during the FBBI and biweekly during the FBBI-M. Using an intention-to-treat approach, we used linear mixed models to test differences in the change in weight and in BMI from the start of FBBI.
Results:
The 24 participants who received the FBBI lost, on average (SE), 5.1 (1.1) kg (P < .001) over 6 months. The 13 participants who were waitlisted gained, on average (SE), 1.2 (1.6) kg over the 6-month waiting period. At 12 months, those who received FBBI-M lost, on average (SE), 4.4 (1.7) kg more than those who received FBBI-C (−7.6 vs −3.2 kg, P-value = .008).
Conclusion:
Participation in an intensive FBBI for weight loss with ID was efficacious, and continued participation in a maintenance intervention yielded additional weight loss.
Keywords: family-based behavioural intervention, intellectual disabilities, randomized control trial, weight loss, youth
1 |. INTRODUCTION
Obesity prevalence among children and youth with intellectual disabilities (ID) exceeds that observed in children with typical development.1–4 Children with ID live in the same obesogenic environment as children with typical development but may have additional risk factors for obesity due to alterations in body composition,5 impairments in motor skills,6 barriers to physical activity,7,8 food selectivity9 and use of medications associated with weight gain.10 Furthermore, prevention of adult obesity and associated chronic disease is essential to ensure individuals with ID are able to live in the least restrictive environment possible.
Children with ID are typically excluded from paediatric weight loss trials, and weight loss studies that focus specifically on youth with ID are few. Two retrospective chart reviews in youth with developmental disabilities have reported improvements in BMI11,12 but are limited by the lack of a defined study protocol. Randomized controlled trials (RCT) of weight loss in children with ID are few13–15 and, with one exception,13 do not address weight maintenance following weight loss.
Given the high obesity prevalence and lack of weight loss trials for this vulnerable population, developing specifically tailored weight loss programs is imperative. Although multi-component family-based approaches that include diet, physical activity (PA) and behaviour modification have demonstrated their effectiveness in childhood obesity treatment among families of children with typical development,16–18 these interventions have not yet been adapted for youth with ID. One of the few controlled, multi-component weight loss interventions for young adults with ID found that those enrolled in a 14-week trial using behaviour modification techniques lost more weight than did waitlisted participants, with evidence for continued weight loss during a 5-week maintenance period.13 Two systematic reviews conducted in 201419 and 201820 identified only three additional controlled, multi-component interventions; however, one lacked a control group,21 and one compared the intervention to a historical control group.22 The third study, a small short-term RCT targeting PA and nutrition that leveraged technology, showed promising results.15 The 2014 systematic review of lifestyle interventions targeting body weight or body composition among youth with ID concluded that while there were some promising findings, selection factors and other methodologic deficiencies emphasize the need for further research.19
In our previous study of 21 youth with Down syndrome who were randomized to either a family-based behavioural intervention (FBBI) or a nutrition education-only program in a 6-month 16-session intervention, we found that youth who received the FBBI lost significantly more weight than those who received the education-only intervention.14 Considering the success of this trial, we sought to broaden the population to include a more heterogeneous sample of youth with ID and to evaluate the efficacy of a FBBI intervention and a weight maintenance program. Accordingly, the aims of the present study were to: (a) compare weight loss among youth with ID randomized to a 6-month FBBI to a waitlist control group and (b) compare weight loss among youth who completed a 6-month maintenance (FBBI-M) intervention to a control group (FBBI-C).
2 |. METHODS
2.1 |. Participants
This RCT, a family-based weight loss intervention for youth with intellectual disability (Health U) (NCT02033642), was conducted in two waves between February 2014 and January 2018. In the first wave, the intervention was carried out at two sites, one in Central Massachusetts and one in the Boston area. In the second wave, the intervention again took place at the same site in Central Massachusetts, and a second site was added in northeastern Massachusetts. Participants were recruited through mailed flyers, postings by disability-related organizations and physician referrals. Participants with ID met the following inclusion criteria: age 14–22 years; ID as defined by a score of ≤75 on standardized IQ and adaptive functioning assessments; overweight or obesity as indicated by body mass index (BMI) ≥85th percentile for age and sex (CDC) for those ages <20 years or BMI ≥25 for those ages ≥20 years and living at home with at least one caregiver who was willing to attend sessions. The age range was chosen to include youth who would have the cognitive, social, behavioural and verbal skills to participate in a group intervention with peers.
Exclusion criteria included orthopaedic or cardiac conditions that would preclude participation in PA, insulin-dependent diabetes, uncontrolled seizure disorder, chronic gastrointestinal disease, Prader-Willi syndrome, unwillingness to wear an accelerometer, and recent history of disruptive, inappropriate or dangerous behaviour. The protocol was approved by the University of Massachusetts Medical School Institutional Review Board.
2.2 |. Enrollment
Families were screened over the telephone and attended an eligibility visit which included height and weight measurements, IQ testing (Kaufman Brief Intelligence Test, second edition23), and a parent interview to assess adaptive behaviour (Vineland Adaptive Behavior Scales, second edition24). In approximately one-half of the cases, where adolescents had received an IQ and/or adaptive functioning assessment completed by a qualified provider within 3 years, we used these reports to confirm the presence of ID in lieu of additional testing to reduce participant burden. For the other participants that we assessed, the mean (SD) IQ Composite standard score was 53.1 (12.2) and the mean (SD) Adaptive Behavior Composite score was 63.4 (4.7). Eligible participants provided assent, and legal guardians provided written informed consent. At the subsequent pre-randomization enrollment visit, a registered dietitian nutritionist (RDN) met with each participant and parent to obtain a diet history and estimate of usual PA. The RDN developed an individualized picture-based Healthy Eating Plan (HEP) that was previously developed to promote gradual weight loss in our study of youth with Down syndrome14 and modified for this study to simplify the concept of discretionary calories.25
2.3 |. Randomization and follow-up
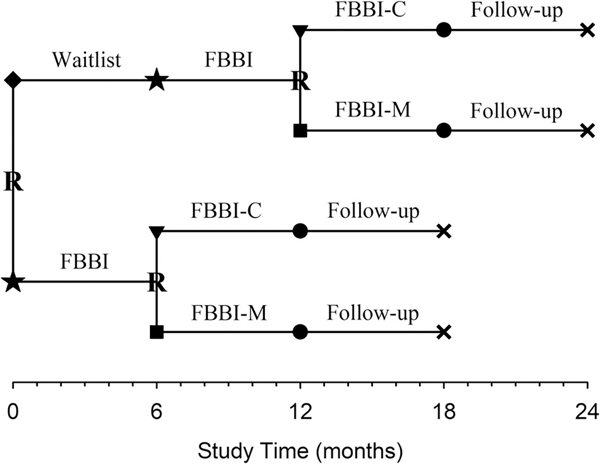
Following enrollment, participants were randomly assigned to receive FBBI immediately or were waitlisted to receive FBBI in 6 months (Figures 1 and 2). All study participants received the 6-month FBBI, and at its completion, were randomly assigned to receive either the 6-month maintenance intervention (FBBI-M) or no further treatment (FBBI-C). All participants were then followed for an additional 6 months. In sum, participants who were randomly assigned to receive FBBI immediately were scheduled to participate in the trial for 18 months; those who were waitlisted were scheduled to participate for 24 months. Intervention staff were blinded to the FBBI-M or FBBI-C assignments until the families were notified. The randomization schedule was prepared by the trial biostatistician in advance of the first eligible participant being enrolled into the trial. Randomization was stratified by wave and by site.
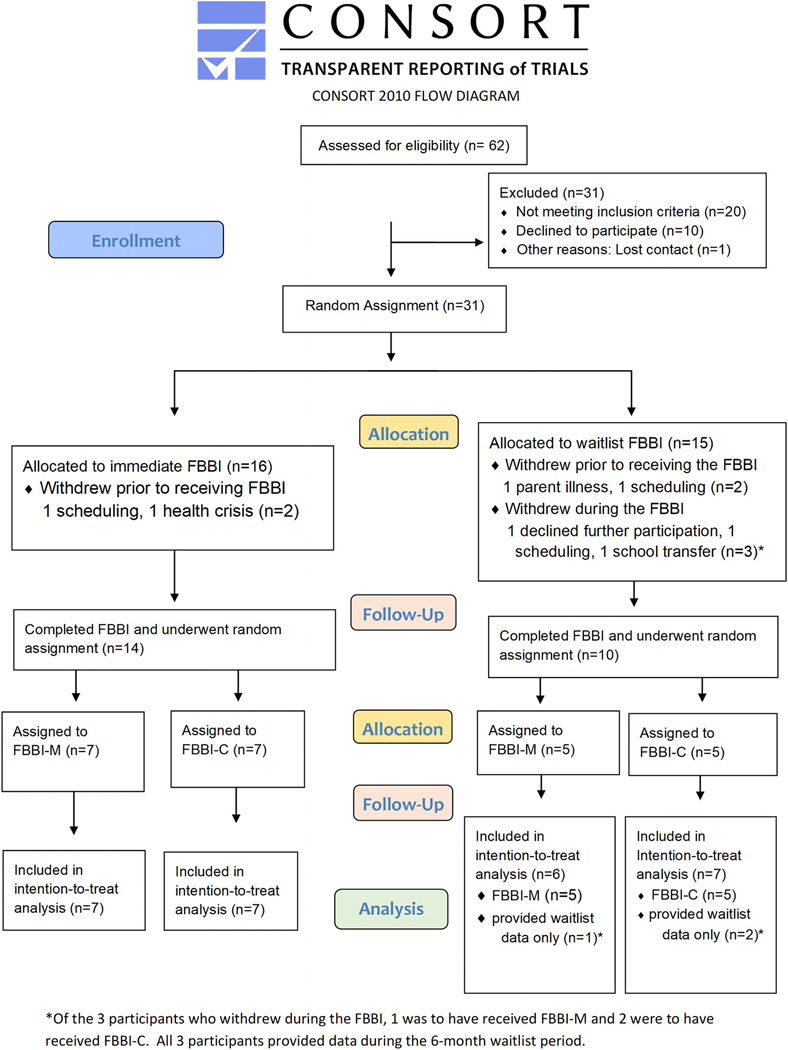
FIGURE 1.
Screening, randomization and follow-up. Participants were informed of their assignments (allocation) at two stages: after their enrollment visit (immediate FBBI or waitlist FBBI) and the week of their final counseling session during the FBBI (FBBI-M or FBBI-C [ie, no further treatment])
FIGURE 2.
Study timeline. Participants were randomly (R) assigned to FBBI (★) immediately or waitlisted (◆) for FBBI (★). At completion of their FBBI, participants were randomly (R) assigned to either FBBI-M (■) or no further treatment (FBBI-C [▼]). Subsequently, all participants were followed (●) for an additional 6 months
2.4 |. Family-based behavioural intervention (FBBI)
The FBBI was based upon evidence-based obesity interventions for children with typical development26,27 and was comprised of inperson group (ie, multiple families) nutrition and PA education sessions with separate parent-only training in behavioural strategies, individual counselling sessions with each family to monitor weight status and discuss dietary and lifestyle challenges and brief weekly phone calls with parents to address their questions and concerns. Educational strategies were adapted for youth with ID and included visual supports (eg, images of food items, food labels, physical activities, etc.), simplified language and messaging, consistent instructions and engaging hands-on activities.14 The behavioural strategies, based on social cognitive and behaviour theory, were used to help guide decision-making, generate positive expectations, provide motivation and direct pathways for change and increase child self-efficacy toward continued adherence to healthful dietary choices and diverse forms of PA. Participants were provided individualized tools, including a HEP and a tracking sheet to monitor daily food intake and PA. The HEP and tracking sheets displayed the number of servings from each food group recommended per day, using visuals to depict servings and food groups.
Delivered over 6 months, the FBBI consisted of eighteen 90-minute group educational sessions and six 30-minute individual counseling sessions (total weekly sessions = 24). Group sessions occurred 3 weeks per month, and individual sessions occurred once per month. Group sessions, each with two to five participants and their parent(s), provided: (a) RDN-led hands-on nutrition and PA education for participants and their parents together (45 minutes total); (b) lifestyle coach-led, parent-only behavioural training, supported by weekly home-based assignments to help parents translate knowledge into actual diet and PA behaviour change at home (45 minutes total) and (c) RDN- and research assistant-led participant-only PA games and taste-tests of healthy foods (45 minutes, while parents met with the lifestyle coach).
The parent-child nutrition and PA education sessions and materials were adapted to meet the learning and literacy needs of the youth with ID. Instructions were kept simple and short and lessons included demonstrations by staff and games. Materials were modified to meet the literacy needs of youth with ID and relied on visuals, such as food models and packages. The parent-only behavioural training sessions provided training on supportive evidence-based behavioural procedures, including tracking their child's daily servings of food and daily PA via pedometer step counts and PA duration, setting clearly quantifiable weekly goals to facilitate diet and PA improvements and reduce screen time, engaging in planning and decision-making with their child (eg, planning meals, making shopping lists, scheduling PA), altering stimulus control (eg, making physical changes to the home environment, posting schedules) to cue healthy behaviours and increasing motivation and providing praise and other forms of reinforcement for lifestyle change. The lifestyle coach called parents midweek to check on diet and PA tracking, weekly homework and to answer any questions that had arisen. While the parents were in behavioural training sessions with the lifestyle coach, the participants met with the RDN and research assistant to participate in games involving PA and a taste test of healthy foods and snacks, which were typically aligned with the nutrition lesson for the week.
One FBBI session each month was delivered as an individual session devoted to nutrition and behavioural counseling with participants and their parents. In these sessions, co-led by the RDN and lifestyle coach, the participant’s monthly weight status and progress were discussed. Minor changes to the servings of foods allocated on the HEP were made when appropriate. In addition, challenges solicited or identified by the participant or parent, such as difficulty using the HEP, selective eating and motivational difficulties, were discussed with suitable recommendations for change.
2.5 |. Maintenance intervention (FBBI-M)
The FBBI-M was implemented upon completion of the FBBI. Similar in format to the FBBI intervention, the FBBI-M was conducted with one to three participants and their parents in 12 bi-weekly sessions over a 6-month period. Sessions alternated between a 90-minute group session and a 30-minute individual session. Modelled after Wilfley et al,28 the behavioural training component was focussed on strategies to facilitate continued weight loss or weight maintenance. Parent training topics included relapse prevention and recovery methods, development of peer and community support networks and refresher content from the original FBBI intervention on selected behavioural skills. The alternating individual session provided nutrition and behavioural counseling to participants and their parents, as in the original FBBI intervention.
2.6 |. Control groups
The waitlist and the control (FBBI-C) groups had no contact with the project team during the 6-month waiting period and the 6-month follow-up period, respectively.
2.7 |. Fidelity of the Interventions
Intervention fidelity was assessed by checklists developed from the operations manual, as the percentage of correctly executed steps. Assessments were primarily through interventionist self-evaluation but included some independent observations of the sessions. The average fidelity was 98% (range, 60%-100%) for individual FBBI sessions, 98% (range, 67%-100%) for nutrition and PA education sessions and 99% (range, 83%-100%) for parent training sessions.
Infrequent departures from fidelity were primarily in the form of omitted protocol steps (eg, not collecting participant homework, etc.), rather than incorrect application of procedures. Low fidelity was scored in only three sessions in both the individual FBBI sessions and the nutrition and PA education sessions (0.9% and 1.8% of the sessions, respectively). During these sessions, the total protocol steps assessed ranged between three to six steps, thus, omitting one or two steps caused fidelity percentages to drop substantially. It is important to note that 92% of the sessions were scored at 100% fidelity.
2.8 |. Outcome Measures
The primary outcome measure of weight and the secondary outcome measure of BMI were recorded at the time of trial entry, at the start of the FBBI, at the time of randomization into FBBI-M or FBBI-C and subsequently at the start and end of the follow-up period. Weight was measured twice on a Seca digital scale (or more, if measures differed by 0.1 kg). Height was measured twice on a Seca stadiometer (or more, if measures differed >1.0 cm); participants wore light clothing and no shoes. In total, participants who were assigned to receive the FBBI immediately had four weight and height measurements, whereas those who were waitlisted had five weight and height measurements. Anthropometric measures were used to calculate BMI (kg/m2); we used BMI rather than BMI z-score because the participants included adolescents and young adults (with some adolescents turning 20 years of age over the course of the study), and a mixture of measures could not be analysed.
2.9 |. Statistical analyses
Using estimates from our pilot study,14 a sample size of 48 participants (including a 20% dropout rate) was determined to detect a difference in 6-month mean (SD) change in BMI between FBBI and waitlist of 1.5 kg/m2 [loss of 1.3(1.8) kg/m2 vs gain of 0.2(1.2) kg/m2] with 80% power at a two-sided 5% significance level. Analyses followed a modified intention-to-treat approach which included all eligible participants who had one or more post-randomization weight measures. The primary outcome measure was change in weight from the start of the FBBI. After verifying the appropriateness of distributional assumptions, linear mixed models estimated trial outcomes. The models included two fixed effects (assigned arm and target time point), the corresponding cross-product terms and three additional covariates (age, sex and weight) that were observed to be imbalanced between the arms at the start of the FBBI. An autoregressive (AR [1]) covariance structure was used to characterize the interdependence of the measurements over time as this structure yielded the smallest AIC and BIC relative to models with alternative covariance structures. Adjusted mean differences were assessed for statistical significance using linear contrasts. Change in BMI was analysed in an identical manner, except that BMI at the start of the FBBI was used as the covariate in place of weight. In subgroup analyses, changes in weight and in BMI were assessed separately in both females and males. All statistical analyses were carried out using SAS 9.4 (SAS Institute Inc., Cary, North Carolina), and results with P-values <.05 deemed statistically significant.
3 |. RESULTS
Participant enrollment began on February 20, 2014 and was completed on December 21, 2015. Of the 31 eligible participants who were randomized, four dropped out shortly after enrollment and yielded no follow-up data; the remaining 27 (87.1%) were included in the present analyses. Despite the trial not achieving its recruitment goal, the greater than expected weight loss among participants who received the FBBI resulted in the present trial having 87% statistical power for its change in weight comparison between the FBBI and waitlist groups. The average age of the participants was 18 years, ranging from 14 to 22 years. One-third were male, all were white and one identified as Hispanic or Latino. Fifteen of the 27 participants had Down syndrome. Over 75% of parents were married or living with a partner, and only one parent did not have some college or higher education. Two participants were taking atypical anti-psychotic medications: one in the delayed treatment group and one in the intervention group. The mean (SD) weight, height and BMI of the participants at the time of starting their FBBI were 80.8 (24.2) kg, 154.4 (14.7) cm and 33.5 (7.0) kg/m2, respectively. The distribution of participant characteristics in the four random assignment groups at the start of their FBBI is shown in Table 1. The average attendance rate was 89% for the FBBI, and the maintenance intervention had an average attendance rate of 94%.
TABLE 1.
Participant characteristics at the start of their FBBI
| Waitlist FBBI and FBBI-C(n = 7) | Waitlist FBBI and FBBI-M(n = 6) | Immediate FBBI and FBBI-C(n = 7) | Immediate FBBI and FBBI-M(n = 7) | |
|---|---|---|---|---|
| Age in years, mean (SD) | 19.7 (1.7) | 18.5 (2.6) | 17.7 (2.3) | 16.2 (1.3) |
| Male sex, n (%) | 2 (28.6) | 3 (50.0) | 2 (28.6) | 2 (28.6) |
| Hispanic or Latino, n (%) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) |
| Down syndrome, n (%) | 4 (57.1) | 3 (50.0) | 4 (57.1) | 4 (57.1) |
| Parent married or partnered, n (%) | 5 (71.4) | 5 (83.3) | 6 (85.7) | 5 (71.4) |
| Parent college or higher, n (%) | 7 (100.0) | 6 (100.0) | 6 (85.7) | 7 (100.0) |
| Weight (kg) at start of FBBI, mean (SD) | 78.8 (23.5) | 88.5 (31.7) | 75.3 (19.3) | 81.9 (26.2) |
| Height (cm) at start of FBBI, mean (SD) | 156.1 (17.9) | 156.8 (16.2) | 152.8 (9.9) | 152.3 (16.7) |
| BMI (kg/m2) at start of FBBI, mean (SD) | 31.7 (3.5) | 35.0 (5.5) | 31.8 (5.2) | 35.6 (11.7) |
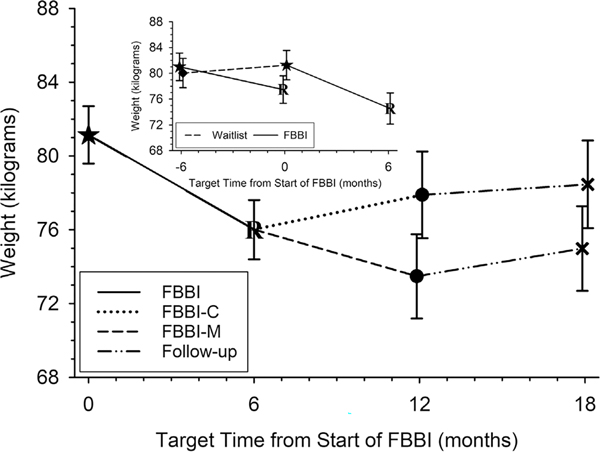
The 13 waitlisted participants increased their weight, on average (SE), by 1.2 (1.6) kg over their 6-month waiting period (P-value = .45, Figure 3 inset). During the same concurrent 6-month period, the 14 participants who received immediate FBBI significantly reduced their weight, on average (SE), by 3.5 (1.5) kg (P-value = .021, Figure 3 inset). This resulted in a significant net difference of 4.8 (2.2) kg in favour of the FBBI (P-value = .034). Despite their initial mean increase in weight, once the 10 remaining waitlisted participants received the FBBI; they significantly lost weight, which on average (SE) was 6.7 (1.7) kg (P-value <.001, Figure 3 inset). Three participants withdrew during the FBBI, one was to have received FBBI-M and two were to have received FBBI-C. Aggregating the data from the 24 participants, all of whom received FBBI, weight loss over 6 months was, on average (SE), 5.1(1.1) kg (P-value <.001, second and third rows in Table 2 and Figure 3).
FIGURE 3.
Adjusted mean weight (95% confidence interval) over time from start of FBBI. Shows significant weight loss from FBBI (—). Further loss from FBBI-M (---) compared to FBBI-C (.......). Inset shows weight gained among waitlisted (--- −6 to 0 months) and loss after FBBI (—0 to 6 months)
TABLE 2.
Change in weight and BMI at target time points
| Target Time from start of FBBI | 0 months | 6 months | 12 months | 18 months | |
|---|---|---|---|---|---|
| Milestones | Start of FBBI | End of FBBI | End of FBBI-C | End of Follow-up | |
| End of FBBI-M | End of Follow-up | ||||
| (n = 27) | (n = 24) | FBBI-C | (n = 12) | (n = 12) | |
| FBBI-M | (n = 12) | (n = 12) | |||
| Time interval (months) a | 0 (0 to 0) | 5.4 (4.8 to 6.0) | FBBI-C | 11.4 (10.8 to 12.2) | 17.2 (16.8 to 17.9) |
| FBBI-M | 11.3 (10.8 to 12.2) | 17.5 (16.8 to 19.4) | |||
| Weight (kg) b | 81.1 (0.8) | 76.0 (0.8); P < .001 | FBBI-C | 77.9 (1.2) | 78.5 (1.2) |
| P = .008 | P = .039 | ||||
| FBBI-M | 73.5 (1.2) | 75.0 (1.2) | |||
| Change in weight (kg) b | 0.0 (0.8) | −5.1 (0.8); P < .001 | FBBI-C | −3.2 (1.2) | −2.6 (1.2) |
| P = .008 | P = .039 | ||||
| FBBI-M | −7.6 (1.2) | −6.1 (1.2) | |||
| BMI (kg/m2) b | 33.5 (0.4) | 31.3 (0.4); P < .001 | FBBI-C | 32.0 (0.6) | 32.2 (0.6) |
| P = .020 | P = .043 | ||||
| FBBI-M | 30.2 (0.5) | 30.7 (0.5) | |||
| Change in BMI (kg/m2) b | 0.0 (0.4) | −2.2 (0.4); P < .001 | FBBI-C | −1.5 (0.6) | −1.3 (0.6) |
| P = 0.020 | P = .043 | ||||
| FBBI-M | −3.3 (0.5) | −2.8 (0.5) |
Results for Time Interval are reported as mean (minimum to maximum).
Results for Weight (and BMI) and Change reported as mean (SE), adjusted for age, sex, weight (or BMI) at Start of the FBBI. P-values at 6 months assess the statistical significance of measurements between 0 and 6 months. P-values at 12 and at 18 months assess the statistical significance of measurements between FBBI-C and FBBI-M groups.
The 12 participants who received the maintenance intervention (FBBI-M) exhibited further weight loss in comparison to the 12 participants who received no further treatment (FBBI-C) and whose mean weight increased (Figure 3). At 12 months, those who received the maintenance intervention (FBBI-M) lost, on average (SE), 4.4 (1.7) kg more than those who received no further treatment (−7.6 vs −3.2 kg, P-value = .008, Table 2). During the follow-up period, from 12 to 18 months, both groups gained approximately 1 kg, on average. However, relative to the start of the FBBI, participants who received the maintenance intervention (FBBI-M) lost an average (SE) of 6.1 (1.2) kg over 18 months compared to a 2.6 (1.2) kg loss among those who received no further treatment [mean (SE) difference of 3.5(1.7) kg, P-value = .039]. The BMI results closely mirrored the weight results (Table 2). In brief, the average (SE) BMI increased among those who waited to receive the FBBI but was significantly reduced by 2.8 (0.8) kg/m2 once they received the FBBI (P-value <.001). A further reduction in mean BMI was observed during the maintenance intervention in contrast to a mean increase in BMI among those who received no further treatment. The subgroup analyses revealed that, despite females weighing less and having lower BMI than males, both females and males experienced similar weight loss and changes in BMI (Tables S1A, B and S2A, B). No adverse events attributable to the intervention occurred.
4 |. DISCUSSION
Despite recommendations by the US Preventive Services Task Force that youth with overweight or obesity be referred to evidence-based multi-component weight loss interventions,29 few interventions have been adapted and evaluated to determine their efficacy for youth with ID. Our family-based intervention for weight loss for youth with ID was efficacious, and continued participation in a maintenance intervention yielded additional weight loss.
This RCT adds to a relatively small body of experimental research on weight loss in youth with ID. Over 40 years ago, Rotatori and Swtizky conducted one of the only controlled multi-component interventions for 12 young adults with ID and six waitlisted participants.13 Their 14-week intervention yielded higher rates of weight loss in the intervention group compared to the waitlist group. Continued weight loss was seen in a 5-week maintenance component that followed the primary intervention. Another study which included a 10-week study of 17 youth in New Zealand did not demonstrate any improvements in weight indicators.21 In a pilot study, Ptomey el al found that weight loss was achieved on both a conventional diet and enhanced stoplight intervention, both of which used computer tablets as a weight loss tool for tracking and engagement for adolescents with IDD.15
Family-based behavioural weight loss interventions have been used successfully to reduce obesity in children with typical development.26,30 Research on family-based interventions adapted for youth with ID is limited, but these adaptations are essential to meet the cognitive needs of the population.31 We previously demonstrated the efficacy of adding parent training in behavioural procedures designed to facilitate lifestyle change at home to a nutrition and PA education program adapted for youth with Down syndrome that included individually prescribed diet plans. The combined program showed promising levels of weight loss at 6 months and weight maintenance at 12 months follow-up, compared to the program without parent training.14
The results of the present trial support the translation of the family-based weight loss intervention strategies that were developed for children with typical development and their parents26,27,30,32 for adolescents with ID. As in our previous trial with youth with Down syndrome,14 the current intervention consisted of educational and behavioural programming with supporting materials tailored to the cognitive, literacy and behavioural needs of youth with ID. The present trial also evaluated an extended intervention aimed at promoting continued weight loss or weight maintenance, which resulted in additional weight loss, albeit less than the intensive portion of the intervention.
This intervention study had several noteworthy strengths. First, the low rate of attrition and high level of attendance suggest high levels of engagement of both participants and parents. Second, the program was adapted to address the unique needs of the population, including use of visual supports, clear and plain language, highly interactive small group activities, strategies to promote active participation and social interaction and reinforcement strategies to increase motivation for lifestyle change. Third, the HEP was based on servings from the food groups, allowing parents to track their child's food intake without monitoring calorie intake. Fourth, the intervention was designed to facilitate parent and participant transfer of session-based education to actual daily behaviour change at home. Finally, social support was established among parents in group activities during sessions, which may have reinforced some of their efforts.
A few limitations are also noted. Although we tracked weight loss, we did not collect information on metabolic health (eg, lipids, glucose, blood pressure, etc.), so we are unable to assess any benefits to cardiovascular risk factors. Further, the lack of participant diversity limits the generalizability of the results. In conducting this efficacy study, we actively recruited in two urban and suburban areas, each with ethnically and racially diverse populations of families with children with ID. The long process of recruiting and implementing the study with families was beneficial in that it revealed several barriers to what we had hoped would be broader participation. Important barriers included: (a) the requirement that families travel weekly to the study locations, which were not readily accessible by public transportation; (b) our lack of inclusion of ethnic foods in our handout materials and activities; (c) English-only oral presentations and written materials and (d) the in-session and at-home time commitments of the study. In future research, these barriers may be addressed by: (a) holding sessions in local community centres or via telehealth; (b) providing free transportation; (c) including traditional diets and foods in the nutrition sessions and supplemental materials; (d) employing dietitians and lifestyle coaches who are fluent in, and can present in, different languages; (e) translating written materials into other languages; (f) offering child care for siblings not in the study and (g) providing other resources for parents to have the time and support to participate in the study. Although many of these additions and modifications would have been difficult to implement, future studies should strive to meet these needs. Participant time commitment was substantial, which may also limit generalizability, as families who had the resources and time may have been more likely to participate. In addition, it is not clear if improved outcomes associated with the FBBI-M condition were due to the maintenance-tailored programming or due simply to extended intervention time. Finally, it is not clear the extent to which the social aspects of this in-person, group program influenced lifestyle change.
5 |. CONCLUSIONS
The present study provides evidence that a family-supported weight loss intervention can yield weight loss for youth with ID. Weight loss was improved further when participants underwent a 6-month maintenance intervention. Evidence-based weight loss interventions are urgently needed to address the unique cognitive, literacy and behavioural needs of youth with ID and to promote active transfer of learning from intervention sessions to home. Educational and behavioural strategies combined with the social support offered by bringing participants and their parents together to learn about healthy lifestyle change appear to be an effective approach for promoting weight loss in a healthy manner in youth with ID. In this study, the intervention was adapted for individuals with ID, which likely allowed participants of varying literacy and cognitive abilities to engage fully. Future adaptations could investigate the efficacy of such adaptations when applied to commercially available weight-loss programs. Our intervention included both participants and their parents; future research should evaluate whether outcomes would be similar if the youth were not present (ie, parent-only intervention),18 or when provided to adults with ID who live independently. The intervention should be tested with participants that represent diversity of socioeconomic status, race and ethnicity, with potential alterations made to the HEP to reflect foods and preferences of different cultures. The intervention could also be tested to assess feasibility for implementation in community, school, residential and other settings.
Supplementary Material
ACKNOWLEDGEMENTS
The study was presented at the International Association for the Scientific Study of Intellectual Disabilities Annual Meeting in Glasgow, Scotland in 2019 and at the Association of University Centers for Disabilities in Washington DC, 2019.
The authors wish to acknowledge the following members of the UMMS team for their contributions in carrying out the study: Barbara Fargnoli MS, RDN, Laura Jay MS, RDN, LDN Leslie Martell MS, RDN, LDN, and Maresa Weems MPH, RDN, LDN. We also wish to thank the research staff and the families who participated in the study.
Funding source: Supported by NIH grant HD072573, and IDDRC: 2P30HD004147-33A2, HRSA/MCHB Healthy Weight Research Network UA3MC25735-01-00 and UCEDD grant #: 90DD0564.
Funding information
Administration for Community Living, Grant/Award Number: 90DD0564; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: 2P30HD004147-33A2, HD072573; Maternal and Child Health Bureau, Grant/Award Number: UA3MC25735-01-00
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Bertapelli F, Pitetti K, Agiovlasitis S, Guerra-Junior G. Overweight and obesity in children and adolescents with down syndrome—prevalence, determinants, consequences, and interventions: a literature review. Res Dev Disabil. 2016;57:181–192. [DOI] [PubMed] [Google Scholar]
- 2.Maïano C. Prevalence and risk factors of overweight and obesity among children and adolescents with intellectual disabilities. Obes Rev. 2011;12(3):189–197. [DOI] [PubMed] [Google Scholar]
- 3.Segal M, Eliasziw M, Phillips S, et al. Intellectual disability is associated with increased risk for obesity in a nationally representative sample of U.S. children. Disabil Health J. 2016;9(3):392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Gao Y, Kwok HHM, Huang WYJ, Li S, Li L. Children with intellectual disability are vulnerable to overweight and obesity: a cross-sectional study among Chinese children. Child Obes. 2018;14(5):316–326. [DOI] [PubMed] [Google Scholar]
- 5.González-Agüero A, Ara I, Moreno LA, Vicente-Rodríguez G, Casajús JA. Fat and lean masses in youths with Down syndrome: gender differences. Res Dev Disabil. 2011;32(5):1685–1693. [DOI] [PubMed] [Google Scholar]
- 6.Vuijk PJ, Hartman E, Scherder E, Visscher C. Motor performance of children with mild intellectual disability and borderline intellectual functioning. J Intellect Disabil Res. 2010;54(11):955–965. [DOI] [PubMed] [Google Scholar]
- 7.Bossink LWM, van der Putten AA, Vlaskamp C. Understanding low levels of physical activity in people with intellectual disabilities: a systematic review to identify barriers and facilitators. Res Dev Disabil. 2017;68:95–110. [DOI] [PubMed] [Google Scholar]
- 8.Barr M, Shields N. Identifying the barriers and facilitators to participation in physical activity for children with Down syndrome. J Intellect Disabil Res. 2011;55(11):1020–1033. [DOI] [PubMed] [Google Scholar]
- 9.Bandini LG, Curtin C, Eliasziw M, et al. Food selectivity in a diverse sample of young children with and without intellectual disabilities. Appetite. 2019;133:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McQuire C, Hassiotis A, Bronwyn H, Pilling S. Pharmacological interventions for challenging behaviour in children with intellectual disabilities: a systematic review and meta-analysis. BMC Psychiatry. 2015; 15:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pona AA, Dreyer Gillette ML, Odar Stough C, Gerling JK, Sweeney BR. Long-term outcomes of a multidisciplinary weight management intervention for youth with disabilities. Child Obes. 2017;13 (6):455–461. [DOI] [PubMed] [Google Scholar]
- 12.Foster BA, Reynolds K, Callejo-Black A, Polensek N, Weill BC. Weight outcomes in children with developmental disabilities from a multidisciplinary clinic. Res Dev Disabil. 2021;108:103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotatori AF, Switzky H. A successful behavioral weight-loss program for moderately-retarded teenagers. Int J Obes (Lond). 1979;3(3):223–228. [PubMed] [Google Scholar]
- 14.Curtin C, Bandini LG, Must A, et al. Parent support improves weight loss in adolescents and young adults with down syndrome. J Pediatr. 2013;163(5):1402–1408.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ptomey LT, Sullivan DK, Lee J, Goetz JR, Gibson C, Donnelly JE. The use of technology for delivering a weight loss program for adolescents with intellectual and developmental disabilities. J Acad Nutr Diet. 2015;115(1):112–118. [DOI] [PubMed] [Google Scholar]
- 16.Janicke DM, Steele RG, Gayes LA, et al. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity. J Pediatr Psychol. 2014;39(8):809–825. [DOI] [PubMed] [Google Scholar]
- 17.Wilfley DE, Staiano AE, Altman M, et al. Improving access and systems of care for evidence-based childhood obesity treatment: conference key findings and next steps. Obesity (Silver Spring). 2017;25(1):16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boutelle KN, Rhee KE, Liang J, et al. Effect of attendance of the child on body weight, energy intake, and physical activity in childhood obesity treatment: a randomized clinical trial. JAMA Pediatr. 2017;171(7):622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maïano C, Normand CL, Aimé A, Bégarie J. Lifestyle interventions targeting changes in body weight and composition among youth with an intellectual disability: a systematic review. Res Dev Disabil. 2014;35(8):1914–1926. [DOI] [PubMed] [Google Scholar]
- 20.Conrad E, Knowlden AP. A systematic review of obesity interventions targeting anthropometric changes in youth with intellectual disabilities. J Intellect Disabil. 2020;24(3):398–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinckson EA, Dickinson A, Water T, Sands M, Penman L. Physical activity, dietary habits and overall health in overweight and obese children and youth with intellectual disability or autism. Res Dev Disabil. 2013;34(4):1170–1178. [DOI] [PubMed] [Google Scholar]
- 22.Wallén EF, Müllersdorf M, Christensson K, Marcus C. A school-based intervention associated with improvements in cardiometabolic risk profiles in young people with intellectual disabilities. J Intellect Disabil. 2013;17(1):38–50. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2nd ed. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- 24.Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2nd ed. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- 25.Weems M, Truex L, Scampini R, Fleming R, Curtin C, Bandini L. A novel weight-loss tool designed for adolescents with intellectual disabilities. J Acad Nutr Diet. 2017;117(10):1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA. 1990; 264(19):2519–2523. [PubMed] [Google Scholar]
- 27.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26(4):381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilfley DE, Stein RI, Saelens BE, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. J Am Med Assoc. 2007;298(14):1661–1673. [DOI] [PubMed] [Google Scholar]
- 29.Barton M Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125(2):361–367. [DOI] [PubMed] [Google Scholar]
- 30.Berge JM, Everts JC. Family-based interventions targeting childhood obesity: a meta-analysis. Child Obes. 2011;7(2):110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irby MB, Kolbash S, Garner-Edwards D, Skelton JA. Pediatric obesity treatment in children with neurodevelopmental disabilities: a case series and review of the literature. Infant Child Adolesc Nutr. 2012;4(4):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman M, Wilfley DE. Evidence update on the treatment of overweight and obesity in children and adolescents. J Clin Child Adolesc Psychol. 2015;44(4):521–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.