Abstract
Intrauterine growth restriction (IUGR) refers to the slow growth and development of a mammalian embryo/fetus or fetal organs during pregnancy, which is popular in swine production and causes considerable economic losses. Nutritional strategies have been reported to improve the health status and growth performance of IUGR piglets, among which dietary curcumin supplementation is an efficient alternative. Curcumin is a natural lipophilic polyphenol derived from the rhizome of Curcuma longa with many biological activities. It has been demonstrated that curcumin promotes intestinal development and alleviates intestinal oxidative damage. However, due to its low bioavailability caused by poor solubility, chemical instability, and rapid degradation, the application of curcumin in animal production is rare. In this manuscript, the structural-activity relationship to enhance the bioavailability, and the nutritional effects of curcumin on intestinal health from the aspect of protecting piglets from IUGR associated intestinal oxidative damage were summarized to provide new insight into the application of curcumin in animal production.
Keywords: curcumin, intestinal health, intrauterine growth retardation, oxidative stress, pigs
Introduction
Intrauterine growth restriction (IUGR), is defined as the slow growth and development of a mammalian embryo/fetus or fetal organs during pregnancy, which has become a difficult problem in human medicine and animal husbandry (1, 2). Pig is a kind of mammal animal with multiple pregnancies, it has a high incidence of IUGR, which would not only reduce the survival rate of the newborn piglets but also affect the growth and development and health status of piglets in a longer period after birth (3–5). Therefore, it is of great significance for the economic benefits of pig production to improve the health status of IUGR piglets, improve their survival rate and growth performance through nutritional strategies. Meanwhile, due to the high similarities between pigs and humans in anatomy, physiology, and nutrient metabolism, the IUGR pigs can be used as an ideal animal model to study human diseases (6, 7).
The intestinal tract is the direct place for the communication between the internal environment and the external environment and is an important defense line for animals to maintain the homeostasis of the internal environment (8–10). Optimum intestinal health is of prime importance to animal growth as well as animal health. Previous studies have revealed that IUGR caused a significant negative effect on the growth and development of the gastrointestinal tract of piglets, manifested by the decreased intestinal length and weight, decreased villus height (VH) and increased crypt depth (CD), increased apoptosis of intestinal epithelial cells, and increased oxidative damage (11–14). The impaired development of the gastrointestinal tract is likely to be the main reason for retard growth and the poor health status of IUGR piglets (6, 15–17). The growing body of evidence has shown that the health status and growth performance of IUGR piglets can be improved through nutritional strategies (7, 15–17). For example, the addition of functional additives, such as functional amino acids (18), nucleotides (19), probiotics (7) as well as curcumin (15–17, 20) in the diet can promote intestinal improve the intestinal antioxidant capacity and immunity, and improve gut health of IUGR piglets.
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], as a natural lipophilic polyphenol derived from the rhizome of Curcuma longa, has been used for centuries in traditional Asian medicine and food additives (21–23). Nowadays, curcumin has received considerable attention in animal husbandry because of its diverse pharmacological activities including antioxidant (16), anti-microbial (24), and anti-inflammatory properties (25). Research evidence showed that curcumin supplementation can effectively improve the antioxidant capacity, improve digestion and absorption and promote the development and repair of the damaged intestinal tract, and enhance the growth performance of IUGR piglets (15–17, 20). However, the application of curcumin in animal production is limited due to its low bioavailability caused by poor solubility, chemical instability, and rapid degradation. A good understanding of the characteristics of curcumin is the precondition to improve its application. The purpose of this paper is to review the physical and chemical properties of curcumin and its metabolites and its nutritional effects on intestinal health from the aspect of protecting IUGR piglets from oxidative damage. This review provides a theoretical basis for the application of curcumin in animals and humans with IUGR.
Overview of Curcumin
Curcumin is mainly derived from the rhizome of Curcuma longa (turmeric), a kind of plant belongs to Zingiberaceae which contains more than 12 active components (26). Commercially, curcumin is one of the main active components in turmeric, which accounted for 77% of active components besides two other related compounds, demethoxycurcumin and bis-demethoxycurcumin (Figure 1) (27). Curcumin is a kind of natural polyphenol that possess a wide spectrum of biological and pharmacological activities, including anti-inflammatory (28–30), antioxidant (31–33), anti-tumor (34, 35), anti-cancer (36, 37), antiangiogenic (38), anti-aging (39), anti-microbial (24), and wound healing (40) activities, which confirmed by in vitro and in vivo studies. Chemically, curcumin is a bis-α,β-unsaturated β-diketone with two benzene rings that have phenolic hydroxyl and the methoxy, respectively (Figure 1). The molecular formula of curcumin is C21H20O6 with a molecular weight of 368.37 g/mol, and a melting point of 183°C (41).
FIGURE 1.

Chemical structures of curcuminoids and their main biological function.
Curcumin is insoluble in water while it is easily soluble in organic solvents, alkali and extremely acidic solvents (27, 42). It has been reported that under acidic and neutral conditions, curcumin is stable, while under alkaline conditions, curcumin is unstable and easily degrades into other organic substances, including ferulic acid, feruloyl methane, vanillin, vanillic acid, ferulic aldehyde, 4-vinyl guaiacol, p-hydroxybenzaldehyde, and p-hydroxybenzoic acid, suggesting that pH-dependent stability (27, 43).
The absorption, distribution, metabolism, and excretion of curcumin are critical for its bioavailability. The poor solubility, chemical instability, and rapid degradation have been reported as a cause for the low bioavailability of curcumin (44, 45), which limits its application in animal production. Previous studies have demonstrated that curcumin is poorly absorbed by intestinal cells, rapidly metabolized by the liver, and rapidly eliminated from organism (39, 46, 47), Thus, structural characteristics should be considered to improve its bioavailability and enhance its biological activities. Hence, different strategies were tested to improve its bioavailability, e.g., curcumin nanoparticles, curcumin nanospheres, and emulsion or microsphere preparations of curcumin (48–52). Encapsulation of curcumin into water-soluble proteins or water-insoluble proteins seems to be an effective manner to enhance its antioxidant capacity. Tapal and Tiku et al. (53) reported that the binding of curcumin to soy protein isolate improved its water solubility, stability, and antioxidant activity of curcumin. Moghadam et al. (54) showed that the encapsulation of curcumin by pH-driven method into walnut proteins improved its water solubility, free radical [1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic) acid (ABTS)] scavenging activity as well as reducing power. Similarly, Mohammadian et al. (55) also reported that complexed curcumin with whey protein nanofibrils could drastically improve DPPH radical scavenging activity and reduce power. Structural modification is another way to improve the antioxidant capacity of curcumin. With the great potential of nanotechnology, modification of curcumin with colloidal nanoparticles has been shown to improve biological activities (56). In this regard, Chen et al. (57) demonstrated that the supplementation of nanobubble curcumin could help mice to overcome physical fatigue by altering the gut microbiota composition. Research by Shaikh et al. (58) reported that structural modification of curcumin to its isoxazole (CI) and pyrazole (CP) showed high reactivity toward a variety of free radicals. However, in recent years, researchers found that the potential biological function of curcumin may not depend on its bioavailability, but may come from its positive impact on gastrointestinal health and function (59). For example, dietary supplementation with curcumin would regulate the intestinal permeability, influence of intestinal flora structure, reduce gastrointestinal inflammation and oxidative stress, and reduce the intestinal pathogens infection (23, 45, 59–61). What else, curcumin’s main metabolites may have stronger pharmacological activity and higher bioavailability than curcumin, which are involved in the biological activities of curcumin (59). However, the biological activities of curcumin’s main metabolites differed among different studies. For example, Luo et al. (62) indicated that compared with curcumin, tetrahydrocurcumin and octahydrocurcumin (two important metabolites of curcumin) can bind to the active site of cytochrome enzyme CYP2E1 to inhibit its activity and simultaneously activate the antioxidant signaling pathway. Zhang et al. (63) showed that tetrahydrocurcumin and octahydroturmeric exerted more effect than curcumin in selectively inhibiting the expression of cyclooxygenase 2 (COX-2) and suppressing nuclear factor-κB (NF-κB) pathways; while, Zhao et al. (29) indicated that curcumin exerted a more potent effect on lipopolysaccharide (LPS)-challenged RAW 264.7 cells compared to that of its three metabolites, tetrahydrocurcumin, hexahydrocurcumin, and octahydroturmeric. Thus, whether the metabolites of curcumin can explain the biological activities is yet to be validated.
Curcumin Promotes Growth and Intestinal Development of Intrauterine Growth Restriction Pigs
In addition to its anti-inflammatory, antioxidant, immunomodulatory, and other biological functions, curcumin has been reported to promote growth performance and intestinal development of animals. Nowadays, curcumin was widely applied in poultry (64–69), ruminant (70, 71), aquatic animals (72–75), and swine production (76–79).
Curcumin Promotes Growth Performance of Pigs With Intrauterine Growth Restriction
Intrauterine growth restriction, defined as fetal weight less than the 10th percentile for gestational age, has adverse effects on animal’s growth and development (17, 80). In actual production, IUGR occurs in 15–20% of newborn piglets, which causes considerable economic losses in swine production (81). IUGR has a significant negative effect on the growth and health status of piglets, and IUGR pig neonates manifest retard growth, weak immunity, and poor feed efficiency (1, 82). Xiong et al. (81) showed that compared to normal-birth-weight (NBW) pigs, IUGR pigs had lower initial (1.86 kg vs. 0.96 kg), weaned (6.57 kg vs. 3.66 kg), and final body weight (105.40 kg vs. 81.71 kg); Niu et al. (17) showed that the body weight of IUGR piglets were lower than those of the NBW piglets at 0, 7, 14, and 26 days of age. In brief, IUGR have an adverse effect on growth performance of pigs. Previous studies reported that these conditions can be attenuated by the supplementation of curcumin in the diets of IUGR piglets because of its affordability and safety, with no known toxic side effects (16, 17, 20, 78). Wang et al. (83) showed that the total weight gain and total feed intake of piglets with IUGR were significantly lower than that of NBW piglets in a 24-day experiment, while IUGR piglets fed a diet containing 400 mg/kg curcumin significantly increased the total weight gain and total feed intake. Similarly, Niu et al. (16, 17) reported that dietary curcumin supplementation (400 mg/kg diet) significantly improved the body weight gain and feed intake of IUGR piglets compared with IUGR piglets fed only basal diet. These studies demonstrated that curcumin can promote the growth of piglets with IUGR. In contrast, the results from Zhang et al. showed that dietary supplementation with 200 mg/kg curcumin did not affect the body weight of IUGR piglets on day 0, 26, 56, and 115 of the experimental period when compared with IUGR piglets without curcumin supplementation; and it also recorded a lower ADFI of IUGR piglets fed a diet containing curcumin from day 56 to day 115, while observed improvement in the redox status and meat quality of leg muscles (78). The difference among these studies may be related to the different doses of curcumin used. Since the bioavailability of curcumin is very low due to its poor solubility (44, 45), high doses are required to achieve detectable levels in serum, which can exert its biological function.
Curcumin Promotes Intestinal Development of Pigs With Intrauterine Growth Restriction
The intestinal tract is not only the direct place for nutrient digestion and absorption but also provided an important barrier to protect the body from antigens, toxins, and pathogens and maintain the stability of the internal environment (8, 9). Therefore, well-developed and healthy intestines are linked with the overall health status of animals. IUGR is a common problem in the pig industry, and a change in intestinal morphology between IUGR piglets and NBW piglets was observed (84). Several studies have reported that IUGR piglets had a lower digestive and absorptive function and an impaired intestinal barrier function (5, 6, 84). It showed that IUGR piglets had a decreased intestinal length and weight, shorty VH, increased apoptosis of intestinal crypt cells as well as reduced activity of brush border enzymes, which leads to an increase in the occurrence of diarrhea and high morbidity and mortality after birth (5, 14).
As a natural polyphenol with a variety of biological activities, curcumin can promote intestinal development and health (67, 76). For example, adding 300 mg/kg or 400 mg/kg curcumin to diet can significantly increase villus height to crypt depth ratio (VCR), improve the morphology of ileum epithelial mucosa, and repair the intestinal injury in Escherichia coli (E. coli) induced intestinal injury piglets model (76). Curcumin can also promote the intestinal development of animals with IUGR including piglets. The intestinal VH, CD, and VCR are commonly used to reflect intestinal development and function (85). Wang et al. (83) showed that IUGR piglets have a poor intestinal morphology, which manifested by a decreased VH and VCR, and increased CD in duodenum, jejunum, and ileum; while, dietary curcumin supplementation (400 mg/kg diet) significantly increased the VH and VCR, which indicated that curcumin has a positive protective effect on improving intestinal morphological damage caused by IUGR in piglets. Similarly, Yan et al. (15) indicated that curcumin can alleviate the jejunum injury in IUGR piglets by increasing the antioxidant capacity.
Disaccharidase (lactase, maltase, and sucrase) activities are important indicators of intestinal functional development (86, 87). In a rabbit model, the authors found that both lactase and maltase activities were depressed in IUGR fetuses compared with NBW ones (86). Likewise, the maltase and lactase in the jejunum and the maltase and sucrase in the ileum were significantly decreased when piglets suffered from IUGR (83). It means that IUGR affects the secretion and activity of intestinal digestive enzymes and hinders the digestion and utilization of nutrients in weaned piglets. Curcumin can reverse this adverse effect caused by IUGR which was indicated by Wang et al. (83) who reported that diet supplemented with 400 mg/kg curcumin significantly improved the ileum lactase activity of IUGR weaned piglets.
Curcumin and Intestinal Antioxidant Function of Intrauterine Growth Restriction Pigs
Curcumin is a polyphenol, characterized by the inclusion of two aromatic rings, and its phenolic hydrogens are responsible for its ability to react with reactive species and are believed to impart antioxidant activity to the molecule (88). So far, data from in vivo and in vitro studies have shown the antioxidant activity of curcumin in different pathological conditions through different pathways (89, 90). The antioxidant activity of curcumin mainly from two aspects: one is curcumin as a free radical scavenger (91, 92); and the other is curcumin as inducers of antioxidant signaling pathways in cells, by enhancing the activity of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and phase II metabolizing enzymes, heme oxygenase (HO-1) and quinone oxidoreductase (NQO1) (33, 67, 93, 94). Hence, curcumin may be a beneficial antioxidant to prevent oxidative damage.
The Ability of Curcumin to Scavenge Free Radicals
High reactive oxygen species (ROS) and reactive nitrogen species (RNS) are devastating for cells, and therefore free radical scavenging is important for preventing some diseases (9, 89). The antioxidant activity of a substance is evaluated by the ability to scavenge nitric oxide (NO), DPPH, ABTS, superoxide radical (O2–), hydrogen peroxide (H2O2) (9, 95–97). Previous studies have demonstrated that curcumin has a strong free radical scavenging activity, thereby protecting against oxidative damage (94, 98). For example, Borra et al. (98) showed that curcumin could efficiently scavenge DPPH, H2O2, NO, superoxide anion in a dose-dependent manner. Ferric-reducing antioxidant power (FRAP) and reducing power assay represent their ability to reduce the ferric (Fe3+) form to the ferrous (Fe2+) form (9, 99). Curcumin also could efficiently scavenge the peroxy radicals, which can induce hemolysis in erythrocytes and inhibit the erythrocyte membrane lipid peroxidation (94). Barzegar et al. (100) showed that curcumin exhibited scavenging intracellular smaller oxidative molecules including H2O2, HO–, ROO–, and can readily transfer electrons or easily donate H-atom from two phenolic sites to scavenge free radicals. These studies indicated that curcumin can be used as an effective antioxidant for ROS protection within the polar cytoplasm due to its superb intracellular ROS scavenging activity.
In vivo and in vitro Antioxidant Activity of Curcumin
Curcumin is a natural phenolic compound with impressive antioxidant properties which has gained increasing attention owing to its beneficial health properties (31, 101). Previous studies showed that curcumin can relieve oxidative stress caused by many unfavorable factors (102–104). In vitro and in vivo studies showed that curcumin is an important inducer of nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant signaling pathways (15, 105). Nrf2 is the main regulator of mammalian cell redox response and plays a vital role in maintaining cellular homeostasis (106, 107). Under normal physiological conditions, kelch-like ECH-associated protein-1 (Keap1) binds to Nrf2 in the cytoplasm and facilitates Nrf2 ubiquitination which can prevent Nrf2 translocation into the nucleus (107). But under oxidative stress conditions, Nrf2 was isolated from Keap1 and transferred to the nucleus, and bound with antioxidant response elements (ARE) to activate the expression of its downstream antioxidant enzymes (SOD, CAT, and GSH-Px), and phase II metabolic enzymes (HO-1 and NQO1) to protect cells from oxidative damage (106–109). For example, Wu et al. (110) reported that the chicken fibroblast cells suffered from heat stress stimulate ROS and malondialdehyde (MDA) production, and it decreased the antioxidant enzymes including CAT, SOD, and GSH-Px; curcumin administration reversed these heat stress-induced oxidative damage by activating Nrf2 signaling pathway. Similarly, Li et al. (111) reported that dietary 300 mg/kg diet curcumin supplementation to broilers alleviates aflatoxin B1 induced liver oxidative stress by activating the Nrf2 pathway. The in vitro and in vivo antioxidant effects of curcumin are summarized in Tables 1, 2, respectively. All these studies revealed that curcumin plays an important role in relieving oxidative stress by improving antioxidant activities.
TABLE 1.
Summary of the in vitro studies investigating the antioxidant effect of curcumin.
| Cell lines | Injure model | Doses | Outcomes | References |
| RAW264.7 cells | Hydrogen peroxide- induced oxidative injure | 5, 10, 20 μM | Low- and middle-dose of curcumin decreased MDA and ROS levels; increased activity of CAT, SOD and GSH-Px; upregulated Nrf2 and HO-1 expression | (33) |
| Bovine fetal hepatocyte-derived cell line (BFH12) | Aflatoxin B1-induced hepatic toxicity | 2.5, 5, 10 μM | Reduced the MDA content, increased the NQO1 enzymatic activity | (70) |
| Porcine intestinal epithelial cells (IPEC-J2) | Hydrogen peroxide- induced oxidative stress | 10 μM | Reduced MDA and ROS production, increased the expression of Cu/Zn-SOD, Mn-SOD, GPX-1 and GPX-4 | (93) |
| Bovine Mammary Epithelial Cells | Lipopolysaccharide – induced oxidative stress | 10 μM | Decreased production of ROS and MDA; increased the activities of T-SOD, T-AOC and GSH; increased the levels of Nrf2 and HO-1 and NQO1 | (105) |
| Primary spinal cord astrocytes | Hydrogen peroxide- induced oxidative injure | 10 μM | Decreased the level of intracellular ROS, and inhibited oxidative stress via the Nrf2/ARE pathway | (108) |
| Chicken embryonic fibroblasts cells | Heat-induced oxidative stress | 5 μM – 40 μM, | Decreased ROS and MDA content; increased antioxidant enzymes and Nrf2 expression | (110) |
| Human trophoblast HTR8/SVneo cells | H2O2-induced oxidative stress | 2.5 or 5 μM | Reduced ROS accumulation, upregulated the activities of the antioxidant enzymes CAT and GSH-Px, increased antioxidant transcription factor Nrf2 | (112) |
| SH-SY5Y cells | Copper-induced neurotoxicity | 5 μM | Decreased the production of ROS and MDA; increased the activities of SOD and CAT; up-regulated pro-caspase 3, pro-caspase 9, and downregulated the Bax/Bcl-2 ratio | (113) |
| Leydig cells | Zearalenone-induced oxidative stress | 20 μM | Reduced MDA content; increased the GSH content and the activities of GSH-Px; increased nuclear Nrf2 and HO-1 protein expression | (114) |
| Human retinal pigment epithelium cell lines (ARPE-19) | Hydrogen peroxide- induced oxidative stress | 15 μM | Reduced ROS production and increased HO-1 expression | (115) |
| Bone marrow mesenchymal stem cells (BMSCs) | Hydrogen peroxide- induced oxidative stress | 1, 5, 10 or 20 μM | Curcumin pretreatment can inhibit reactive oxygen species accumulation in BMSCs | (116) |
| Bone marrow mesenchymal stem cells (BMSCs) | Hypoxia and reoxygenation triggered injury | 1, 5, 10 or 20 μM | Curcumin pretreatment prevented hypoxia and reoxygenation-induced mitochondrial dysfunction through suppressing reactive oxygen species accumulation | (117) |
| Porcine granulosa cells | Zearalenone -induced oxidative stress | 20 μM | Pre-treated with curcumin decreased the ROS production, and increased the expression of SOD1 and CAT | (118) |
| Tilapia hepatocytes | Hydrogen peroxide- induced oxidative injure | 5, 10, 20, 40 μM | Reduced MDA levels, and increased SOD activity; upregulate the Nrf2-Keap1 signaling pathway at the transcriptional level | (119) |
| Min-6 mouse pancreatic beta cells | High glucose – induced oxidative stress | 10 μM | Decreased MDA and ROS levels; increased SOD activity | (120) |
| Porcine TM cells | Hydrogen peroxide- induced oxidative injure | 1–20 μM | Curcumin treatment at concentrations between 1 and 20 μM reduced the production of intracellular ROS | (121) |
| INS-1 cells | High glucose/palmitate- induced cell damage | 20 μM | Reduced the production of ROS Increased SOD and CAT activity | (122) |
| Human hepatocyte L02 cells | Quinocetone-induced hepatic toxicity | 2.5, 5 μM | Attenuated ROS formation; increased SOD activity and GSH level | (123) |
| Human intestinal epithelial cells (Caco2) | Hydrogen peroxide- induced oxidative injure | 5, 20, 80 μM | Decreased MDA release; increased SOD activity; increased HO-1 expression | (124) |
| SH-SY5Y cells | Paraquat-induced cell death | 5 μM | Curcumin reduced ROS levels and increased expression of the antioxidant genes, SOD and GSH-Px | (125) |
ARE, antioxidant response elements; CAT, catalase; GSH, glutathione; GSH-Px, glutathion peroxidase; HO-1, heme oxygenase; Keap1, kelch-like ECH-associated protein 1; MDA, malonaldehyde; NQO1, quinone oxidoreductase; Nrf2, nuclear factor erythroid 2-related factor 2; ROS, reactive oxygen species; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
TABLE 2.
Summary of the in vivo studies investigating the antioxidant effect of curcumin.
| Animals | Damaged model | Doses | Outcomes | References |
| Pigs | Intrauterine growth retardation | 200 mg/kg | Increased the gene expression of Nrf2, GCLC, SOD1, GCLM and NQO1, and the protein expression of Nrf2 and NQO1 | (15) |
| Pigs | Intrauterine growth retardation | 400 mg/kg | Reduced the levels of MDA and H2O2; improved serum and liver antioxidant enzymes as well as up-regulated Nrf2 and HO-1 expression | (17) |
| Pigs | Intrauterine growth retardation | 400 mg/kg | Reduced PC, 8-OHdG, increased T-AOC, CAT, SOD, Nrf2, NQO1 expression | (20) |
| Pigs | Intrauterine growth retardation | 200 mg/kg | Increased mRNA expressions of GSH-ST, HO-1 and CAT, increased NQO1 protein expression of leg muscles | (78) |
| Pigs | Diquat -induced oxidative stress | 200 mg/kg | Reduced the MDA level, and increased the SOD, CAT activity in the intestinal mucosa | (93) |
| Rats | Intestinal ischemia reperfusion | 100 mg/kg | Decreased the MDA levels, and increased of SOD and GSH-Px enzyme activities | (60) |
| Rats | Dimethylnitrosamine-induced liver injury | 200 mg/kg | Enhanced antioxidant transcription and ARE-binding of Nrf2; increased HO-1 protein expression as well as activity in rat liver | (102) |
| Rats | Lipopolysaccharide/diclofenac-induced liver injury | 200 mg/kg/d | Decreased the MDA levels; increased GSH content and SOD enzyme activities; increased expression of HO-1 | (103) |
| Rats | Intestinal ischemia reperfusion | 200 mg/kg | Decreased the MDA levels, and increased SOD enzyme activities | (126) |
| Rats | Renal ischemia reperfusion | 15 mg/kg, 30 mg/kg, 60 mg/kg | Decreased MDA; increased the level of SOD, CAT, GSH-Px, GSH | (127) |
| Rats | Ochratoxin A-induced Hepatotoxicity | 100 mg/kg | antioxidant enzymes SOD, CAT and GSH-Px increased; MDA level decreased | (128) |
| Rats | Streptozoticin -induced diabetic | 100 mg/kg/d | The activity of SOD increased and the amount of MDA reduced; the expression of NQO1 and Nrf2 was increased | (129) |
| Rats | Intrauterine growth retardation | 400 mg/kg | Decreased the MDA, PC and 8-OHDG contents, improved the hepatic glutathione redox cycle | (130) |
| Rats | Aluminum chloride-induced oxidative stress | 10 mg/kg BW | Decreased the MDA levels, and increased SOD and CAT activities in liver tissue | (131) |
| Mice | arsenic-induced hepatotoxicity and oxidative injuries | 200 mg/kg | Decreased hepatic MDA level, increased hepatic GSH level, and up-regulated Nrf2 protein, NQO1 and HO-1 expression | (31) |
| Mice | Cadmium-induced histopathological damages | 100 mg/kg | Increased serum CAT, SOD, and GSH-Px activities; decreased the serum MDA and H2O2 level | (132) |
| Mice | Cadmium induced lung oxidative stress | 100 mg/kg | Decreased MDA levels; increased CAT, GSH-Px,SOD activities | (133) |
| Mice | Ethanol-induced oxidative stress | 50 mg/kg | Reduced ROS and lipid peroxidation (LPO) generation, and increased Nrf2/HO-1 expression in the experimental mice brains | (134) |
| Ducks | Ochratoxin A induced liver oxidative injury | 400 mg/kg | Increased liver CAT activity | (24) |
| Ducks | Aflatoxin B1-induced intestinal injure | 500 mg/kg | Enhanced the activities of SOD, GSH-Px, GSH-ST; decreased the concentrations of MDA in the ileum | (67) |
| Ducks | Ochratoxin A–induced intestinal injure | 400 mg/kg | Decreased the concentrations of MDA; increased the activity of GSH-Px in the jejunal mucosa | (135) |
| Broilers | Aflatoxin B1-induced liver injury | 300 mg/kg | Inhibited the generation of ROS, MDA and 8-OHdG; increased the activities of GSH, SOD and CAT; increased the expression of Nrf2 and HO-1 | (111) |
| Broilers | Aflatoxin B1-induced liver injury | 300 mg/kg | Decreased the content of MDA and the level of ROS; increased the contents of GSH and activities of SOD and CAT | (136) |
| Broilers | Aflatoxin B1-induced liver injury | 450 mg/kg | Decreased the MDA levels, and increased GSH-Px and SOD activity; up-regulated Nrf2 protein expression | (137) |
| Broilers | Aflatoxin B1-induced liver injury | 300 mg/kg | Improved Nrf2 expression, and Enhanced phase-II metabolizing enzymes expressions and activity | (138) |
| Laying hens | Heat-induced oxidative stress | 100 to 300 mg/kg | Decreased the MDA levels; increased T-AOC, CAT, SOD and GSH-Px activities | (139) |
8-OHdG, 8-hydroxy-2′-deoxyguanosine; ARE, antioxidant response elements; CAT, catalase; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; GSH, glutathione; GSH-Px, glutathion peroxidase; GSH-ST, glutathione S-transferase; HO-1, heme oxygenase; H2O2, hydrogen peroxide; Keap1, kelch-like ECH-associated protein 1; MDA, malonaldehyde; NQO1, quinone oxidoreductase; Nrf2, nuclear factor erythroid 2-related factor 2; PC, protein carbonyl; ROS, reactive oxygen species; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Curcumin Alleviates Intestinal Oxidative Stress in Intrauterine Growth Restriction Pigs
Oxidative stress, recognized as a state of imbalance between the production of free radicals and antioxidant defenses, plays a crucial role in the development of numerous human and animal diseases (107, 140, 141). In cells, free radicals are unstable compounds that readily bind to oxygen to become reactive species such as ROS and RNS, causing cytotoxic effects (85, 142). Free radicals are a double-edged sword, on the one hand, physiological levels of ROS and RNS are required for some enzymatic, cell signaling, and cellular adaptive responses; while on the other hand the excessive production of free radicals, which in turn, induce oxidative damage to cellular biomolecules, including proteins, lipids, and nucleic acids (128, 143). Oxidative stress is associated with IUGR (86, 144, 145). Previous studies have revealed that IUGR offsprings tend to have increased ROS, 8-OHdG, protein carbonyl (PC), MDA, and H2O2, and decreased levels of antioxidant enzymes (SOD, CAT, GSH-Px), and phase II metabolizing enzymes (HO-1 and NQO1) (15, 17–20, 130, 146). IUGR is associated with intestinal oxidative stress in weaned piglets (15, 20). Substantial evidence has indicated that oxidative stress triggered the onset and development of intestinal diseases as well as implicated in the pathophysiology of IUGR-associated intestinal injury (15, 147, 148). It is believed that oxidative stress is involved in intestinal barrier dysfunction and various digestive tract diseases (107, 149). At present many natural oxidation products have been used to alleviate oxidative stress in IUGR pigs (146, 148), in which curcumin has been mentioned as a remedy. Wang et al. (20) showed that IUGR stimulated jejunum PC and 8-OHdG, and ileum PC, MDA, and H2O2 production, and it decreased the total antioxidant capacity (T-AOC), CAT activity, and glutathione (GSH) content in the jejunum, and CAT activity in the ileum, which suggested that IUGR caused oxidative stress in the intestinal tract. The authors further reported that administration of curcumin at a dose of 400 mg/kg reversed IUGR associated intestinal damage by activating the Nrf2 signaling pathway and stimulating antioxidant enzymes secretion (SOD and CAT), and phase II metabolic enzyme, NQO1 expression. Similarly, Yan et al. (15) showed that the IUGR growing pigs fed a diet containing 200 mg/kg curcumin had significantly lower MDA content and higher total SOD activity in the jejunum, and upregulated Nrf2, NQO1, and SOD expression. These studies suggested that curcumin can alleviate intestinal oxidative stress caused by IUGR and improve intestinal antioxidant status through activating Nrf2/ARE signaling pathway.
Conclusion
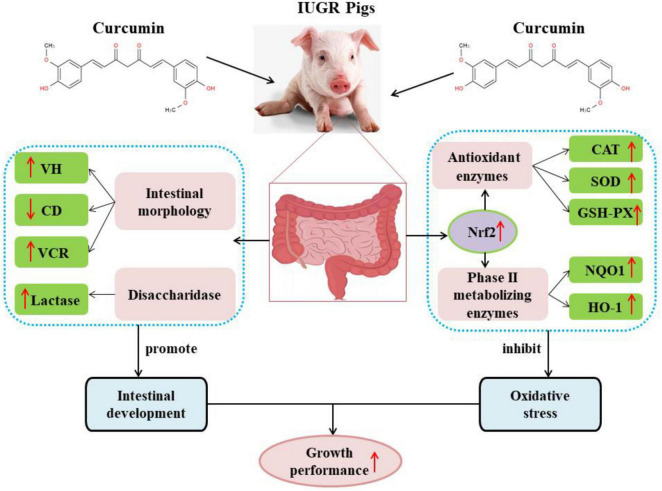
In conclusion, curcumin has a good antioxidant capacity with a strong free radical scavenging activity and can effectively improve intestinal development and alleviate intestinal oxidative stress caused by IUGR, thereby improving the growth performance and health status of pigs with IUGR (Figure 2), however, the mechanism of curcumin in relieving intestinal oxidative stress and intestinal dysplasia in IUGR piglets is yet to be investigated. Curcumin exhibited low bioavailability due to poor solubility, chemical instability and rapid degradation, and those will limited its application in animal production. Therefore, further studies should focus on how to improve the bioavailability of curcumin to enhance biological activities.
FIGURE 2.
Curcumin improved the growth performance of pigs with IUGR by improving intestinal development and alleviating intestinal oxidative stress. CAT, catalase; CD, crypt depth; GSH-Px, glutathion peroxidase; HO-1, heme oxygenase; NQO1, quinone oxidoreductase; Nrf2, nuclear factor erythroid 2-related factor 2; SOD, superoxide dismutase. VCR, the ratio of villus height to crypt depth; VH, villus height.
Author Contributions
XT, KX, and XW advocated writing this review and reviewed. XT collected literature and wrote the manuscript. TW revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This research was funded by grants from the Key Project of Science and Technology Program of Guizhou Province (No. 5411 2017 Qiankehe Pingtai Rencai), the World Top Discipline Program of Guizhou Province (No. 125 2019 Qianjiao Keyan Fa), and the Key Collaborative Research Program of the Alliance of International Science Organizations (Grant No. ANSO-CR-KP-2021-10).
References
- 1.Gondret F, Lefaucheur L, Louveau I, Lebret B, Pichodo X, Cozler YL. Influence of piglet birth weight on postnatal growth performance, tissue lipogenic capacity and muscle histological traits at market weight. Livest Prod Sci. (2005) 93:137–46. 10.1016/j.livprodsci.2004.09.009 [DOI] [Google Scholar]
- 2.Wang J, Chen L, Li D, Yin Y, Wang X, Li P, et al. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr. (2008) 138:60–6. 10.1093/jn/138.1.60 [DOI] [PubMed] [Google Scholar]
- 3.Gaccioli F, Aye ILMH, Sovio U, Charnock-Jones DS, Smith GCS. Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers. Am J Obstet Gynecol. (2018) 218:S725–37. 10.1016/j.ajog.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Li T, Huang S, Lei L, Tao S, Xiong Y, Wu G, et al. Intrauterine growth restriction alters nutrient metabolism in the intestine of porcine offspring. J Anim Sci Biotechnol. (2021) 12:15. 10.1186/s40104-020-00538-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olszewski J, Zabielski R, Skrzypek T, Matyba P, Wierzbicka M, Adamski A, et al. Differences in intestinal barrier development between intrauterine growth restricted and normal birth weight piglets. Animals. (2021) 11:990. 10.3390/ani11040990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferenc K, Pietrzak P, Godlewski MM, Piwowarski J, Kiliañczyk R, Guilloteau P, et al. Intrauterine growth retarded piglet as a model for humans–studies on the perinatal development of the gut structure and function. Reprod Biol. (2014) 14:51–60. 10.1016/j.repbio.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 7.Hu L, Peng X, Chen H, Yan C, Liu Y, Xu Q, et al. Effects of intrauterine growth retardation and Bacillus subtilis PB6 supplementation on growth performance, intestinal development and immune function of piglets during the suckling period. Eur J Nutr. (2017) 56:1753–65. 10.1007/s00394-016-1223-z [DOI] [PubMed] [Google Scholar]
- 8.Tang X, Liu H, Yang S, Li Z, Zhong J, Fang R. Epidermal growth factor and intestinal barrier function. Mediators Inflamm. (2016) 2016:1927348. 10.1155/2016/192734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang X, Liu X, Zhong J, Fang R. Potential application of Lonicera japonica extracts in animal production: from the perspective of intestinal health. Front. Microbiol. (2021) 12:719877. 10.3389/fmicb.2021.719877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang X, Xiong K. Effects of epidermal growth factor on glutamine and glucose absorption by IPEC-j2 cells challenged by lipopolysaccharide using the ussing chamber system. Pak J Zool. (2021) 53:417–22. 10.17582/journal.pjz/20200117080156 [DOI] [Google Scholar]
- 11.Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ. Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Biol Neonate. (2005) 88:66–72. 10.1159/000084645 [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Li Y, Li H, Wang Y, Zhao K. Nano-selenium and Macleaya cordata extracts improved immune functions of intrauterine growth retardation piglets under maternal oxidation stress. Biol Trace Elem Res. (2021). 10.1007/s12011-021-03009-1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.D’Inca R, Che L, Thymann T, Sangild PT, Le Huërou-Luron I. Intrauterine growth restriction reduces intestinal structure and modifies the response to colostrum in preterm and term piglets. Livest Sci. (2010) 133:20–2. 10.1016/j.livsci.2010.06.015 [DOI] [Google Scholar]
- 14.Liu J, Chen D, Mao X, Yu B. Effects of maternal folic acid supplementation on morphology and apoptosis-related gene expression in jejunum of newborn intrauterine growth retarded piglets. Arch Anim Nutr. (2011) 65:376–85. 10.1080/1745039x.2011.594352 [DOI] [PubMed] [Google Scholar]
- 15.Yan E, Zhang J, Han H, Wu J, Gan Z, Wei C, et al. Curcumin alleviates IUGR jejunum damage by increasing antioxidant capacity through Nrf2/Keap1 pathway in growing pigs. Animals. (2019) 10:41. 10.3390/ani10010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu Y, He J, Ahmad H, Shen M, Zhao Y, Gan Z, et al. Dietary curcumin supplementation increases antioxidant capacity, upregulates Nrf2 and hmox1 levels in the liver of piglet model with intrauterine growth retardation. Nutrients. (2019) 11:2978. 10.3390/nu11122978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu Y, He J, Zhao Y, Shen M, Zhang L, Zhong X, et al. Effect of curcumin on growth performance, inflammation, insulin level, and lipid metabolism in weaned piglets with IUGR. Animals. (2019) 9:1098. 10.3390/ani912109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su W, Zhang H, Ying Z, Li Y, Zhou L, Wang F, et al. Effects of dietary L-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur J Nutr. (2018) 57:2735–45. 10.1007/s00394-017-1539-3 [DOI] [PubMed] [Google Scholar]
- 19.Hu L, Peng X, Qin L, Wang R, Fang Z, Lin Y, et al. Dietary nucleotides supplementation during the suckling period improves the antioxidative ability of neonates with intrauterine growth retardation when using a pig model. RSC Adv. (2018) 8:16152–60. 10.1039/C8RA00701B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, He J, Shen M, Hang H, Niu Y, Zhang L, et al. Effect of curcumin supplementation on intestinal antioxidant function in weaning piglets with intrauterine growth retardation. Food Sci. (2019) 40:177–83. 10.7506/spkx1002-6630-20180814-145 [DOI] [Google Scholar]
- 21.Ayati Z, Ramezani M, Amiri MS, Moghadam AT, Rahimi H, Abdollahzade A, et al. Ethnobotany, phytochemistry and traditional uses of curcuma spp. and pharmacological profile of two important species (C. longa and C. zedoaria): a review. Curr Pharm Des. (2019) 25:871–935. 10.2174/1381612825666190402163940 [DOI] [PubMed] [Google Scholar]
- 22.Willenbacher E, Khan SZ, Mujica SCA, Trapani D, Hussain S, Wolf D, et al. Curcumin: new insights into an ancient ingredient against cancer. Int J Mol Sci. (2019) 20:1808. 10.3390/ijms20081808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scazzocchio B, Minghetti L, D’Archivio M. Interaction between gut microbiota and curcumin: a new key of understanding for the health effects of curcumin. Nutrients. (2020) 12:2499. 10.3390/nu12092499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai SS, Ruan D, Zhu YW, Li MC, Ye H, Wang WC, et al. Protective effect of curcumin on ochratoxin A-induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult Sci. (2020) 99:1124–34. 10.1016/j.psj.2019.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohno M, Nishida A, Sugitani Y, Nishino K, Inatomi O, Sugimoto M, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS One. (2017) 12:e0185999. 10.1371/journal.pone.0185999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyagi AK, Prasad S, Yuan W, Li S, Aggarwal BB. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: comparison with curcumin. Invest New Drugs. (2015) 33:1175–86. 10.1007/s10637-015-0296-5 [DOI] [PubMed] [Google Scholar]
- 27.Lestari ML, Indrayanto G. Curcumin. Profiles Drug Subst Excip Relat Methodol. (2014) 39:113–204. 10.1016/B978-0-12-800173-8.00003-9 [DOI] [PubMed] [Google Scholar]
- 28.Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol. (2011) 11:331–41. 10.1016/j.intimp.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 29.Zhao F, Gong Y, Hu Y, Lu M, Wang J, Dong J, et al. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: translocation of nuclear factor-κB as potential target. Mol Med Rep. (2015) 11:3087–93. 10.3892/mmr.2014.3079 [DOI] [PubMed] [Google Scholar]
- 30.Edwards RL, Luis PB, Varuzza PV, Joseph AI, Presley SH, Chaturvedi R, et al. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J Biol Chem. (2017) 292:21243–52. 10.1074/jbc.RA117.000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao S, Duan X, Wang X, Dong D, Liu D, Li X, et al. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food Chem Toxicol. (2013) 59:739–47. 10.1016/j.fct.2013.07.032 [DOI] [PubMed] [Google Scholar]
- 32.Aftab N, Vieira A. Antioxidant activities of curcumin and combinations of this curcuminoid with other phytochemicals. Phytother Res. (2010) 24:500–2. 10.1002/ptr.2960 [DOI] [PubMed] [Google Scholar]
- 33.Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H, et al. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. (2019) 14:e0216711. 10.1371/journal.pone.0216711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Wang C, Tao Z, Zhao L, Zhu Z, Wu W, et al. Curcumin derivative WZ35 inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in breast cancer. J Exp Clin Cancer Res. (2019) 38:460. 10.1186/s13046-019-1424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jahanbakhshi F, Maleki Dana P, Badehnoosh B, Yousefi B, Mansournia MA, Jahanshahi M, et al. Curcumin anti-tumor effects on endometrial cancer with focus on its molecular targets. Cancer Cell Int. (2021) 21:120. 10.1186/s12935-021-01832-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abd Wahab NA, Lajis NH, Abas F, Othman I, Naidu R. Mechanism of anti-cancer activity of curcumin on androgen-dependent and androgen-independent prostate cancer. Nutrients. (2020) 12:679. 10.3390/nu12030679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirzaei H, Bagheri H, Ghasemi F, Khoi JM, Pourhanifeh MH, Heyden YV, et al. Anti-cancer activity of curcumin on multiple myeloma. Anticancer Agents Med Chem. (2021) 21:575–86. 10.2174/1871520620666200918113625 [DOI] [PubMed] [Google Scholar]
- 38.Mendes TS, Novais EA, Badaró E, de Oliveira Dias JR, Kniggendorf V, Lima-Filho AAS, et al. Antiangiogenic effect of intravitreal curcumin in experimental model of proliferative retinopathy. Acta Ophthalmol. (2020) 98:e132–3. 10.1111/aos.14179 [DOI] [PubMed] [Google Scholar]
- 39.Bielak-Zmijewska A, Grabowska W, Ciolko A, Bojko A, Mosieniak G, Bijoch Ł, et al. The role of curcumin in the modulation of ageing. Int J Mol Sci. (2019) 20:1239. 10.3390/ijms20051239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barchitta M, Maugeri A, Favara G, Magnano San Lio R, Evola G, Agodi A, et al. Nutrition and wound healing: an overview focusing on the beneficial effects of curcumin. Int J Mol Sci. (2019) 20:1119. 10.3390/ijms20051119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. (2008) 75:787–809. 10.1016/j.bcp.2007.08.016 [DOI] [PubMed] [Google Scholar]
- 42.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. (1997) 15:1867–76. 10.1016/S0731-7085(96)02024-9 [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Sanidad KZ, Sukamtoh E, Zhang G. Potential roles of chemical degradation in the biological activities of curcumin. Food Funct. (2017) 8:907–14. 10.1039/c6fo01770c [DOI] [PubMed] [Google Scholar]
- 44.Payton F, Sandusky P, Alworth WL. NMR study of the solution structure of curcumin. J Nat Prod. (2007) 70:143–6. 10.1021/np060263s [DOI] [PubMed] [Google Scholar]
- 45.Lopresti AL. The problem of curcumin and its bioavailability: could its gastrointestinal influence contribute to its overall health-enhancing effects? Adv Nutr. (2018) 9:41–50. 10.1093/advances/nmx011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, et al. Oral bioavailability of curcumin: problems and advancements. J Drug Target. (2016) 24:694–702. 10.3109/1061186X.2016.1157883 [DOI] [PubMed] [Google Scholar]
- 47.Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. (2018) 9:705–14. 10.1039/c7fo01242j [DOI] [PubMed] [Google Scholar]
- 48.Shinde RL, Devarajan PV. Docosahexaenoic acid-mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. (2017) 24:152–61. 10.1080/10717544.2016.1233593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaguezeski AM, Gündel SS, Favarin FR, Gündel A, Souza CF, Baldissera MD, et al. Low-dose curcumin-loaded Eudragit L-100-nanocapsules in the diet of dairy sheep increases antioxidant levels and reduces lipid peroxidation in milk. J Food Biochem. (2019) 43:e12942. 10.1111/jfbc.12942 [DOI] [PubMed] [Google Scholar]
- 50.Baldissera MD, Souza CF, Zeppenfeld CC, Velho MC, Klein B, Abbad LB, et al. Dietary supplementation with nerolidol nanospheres improves growth, antioxidant status and fillet fatty acid profiles in Nile tilapia: benefits of nanotechnology for fish health and meat quality. Aquaculture. (2020) 516:734635. 10.1016/j.aquaculture.2019.734635 [DOI] [Google Scholar]
- 51.Yadav YC, Pattnaik S, Swain K. Curcumin loaded mesoporous silica nanoparticles: assessment of bioavailability and cardioprotective effect. Drug Dev Ind Pharm. (2019) 45:1889–95. 10.1080/03639045.2019.1672717 [DOI] [PubMed] [Google Scholar]
- 52.Mohammed HS, Hosny EN, Khadrawy YA, Magdy M, Attia YS, Sayed OA, et al. Protective effect of curcumin nanoparticles against cardiotoxicity induced by doxorubicin in rat. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165665. 10.1016/j.bbadis.2020.165665 [DOI] [PubMed] [Google Scholar]
- 53.Tapal A, Tiku PK. Complexation of curcumin with soy protein isolate and its implications on solubility and stability of curcumin. Food Chem. (2012) 130:960–5. 10.1016/j.foodchem.2011.08.025 [DOI] [Google Scholar]
- 54.Moghadam M, Salami M, Mohammadian M, Delphi L, Sepehri H, Emam-Djomeh Z, et al. Walnut protein–curcumin complexes: fabrication, structural characterization, antioxidant properties, and in vitro anticancer activity. J Food Meas Charact. (2020) 14:876–85. 10.1007/s11694-019-00336-9 [DOI] [Google Scholar]
- 55.Mohammadian M, Salami M, Momen S, Alavi F, Emam-Djomeh Z, Moosavi-Movahedi AA. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocolloid. (2019) 87:902–14. 10.1016/j.foodhyd.2018.09.001 [DOI] [Google Scholar]
- 56.Kato M, Nishikawa S, Ikehata A, Dochi K, Tani T, Takahashi T, et al. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol Nutr Food Res. (2017) 61:1600471. 10.1002/mnfr.201600471 [DOI] [PubMed] [Google Scholar]
- 57.Chen YM, Chiu WC, Chiu YS, Li T, Sung HC, Hsiao CY. Supplementation of nano-bubble curcumin extract improves gut microbiota composition and exercise performance in mice. Food Funct. (2020) 11:3574–84. 10.1039/c9fo02487e [DOI] [PubMed] [Google Scholar]
- 58.Shaikh SAM, Singh BG, Barik A, Balaji N, Subbaraju GV, Naik DB, et al. Unravelling the effect of β-diketo group modification on the antioxidant mechanism of curcumin derivatives: a combined experimental and DFT approach. J Mol Struct. (2019) 1193:166–76. 10.1016/j.molstruc.2019.05.029 [DOI] [Google Scholar]
- 59.Ruan D, Wang Y, Jiang S, Zheng C. Biological activity of curcumin and its molecular mechanism regulating intestinal mucosal barrier function of animals. Chin J Anim Nutri. (2021) 33:1801–10. 10.3969/j.issn.006-267x.2021.04.001 [DOI] [Google Scholar]
- 60.Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol. (2011) 42:579–87. 10.1007/s10735-011-9364-0 [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z, Chen Y, Xiang L, Wang Z, Xiao GG, Hu J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients. (2017) 9:1146. 10.3390/nu9101146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo DD, Chen JF, Liu JJ, Xie JH, Zhang ZB, Gu JY, et al. Tetrahydrocurcumin and octahydrocurcumin, the primary and final hydrogenated metabolites of curcumin, possess superior hepatic-protective effect against acetaminophen-induced liver injury: role of CYP2E1 and Keap1-Nrf2 pathway. Food Chem Toxicol. (2019) 123:349–62. 10.1016/j.fct.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 63.Zhang ZB, Luo DD, Xie JH, Xian YF, Lai ZQ, Liu YH, et al. Curcumin’s metabolites, tetrahydrocurcumin and octahydrocurcumin, possess superior anti-inflammatory effects in vivo through suppression of TAK1-NF-κB pathway. Front Pharmacol. (2018) 9:1181. 10.3389/fphar.2018.01181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galli GM, Da Silva AS, Biazus AH, Reis JH, Boiago MM, Topazio JP, et al. Feed addition of curcumin to laying hens showed anticoccidial effect, and improved egg quality and animal health. Res Vet Sci. (2018) 118:101–6. 10.1016/j.rvsc.2018.01.022 [DOI] [PubMed] [Google Scholar]
- 65.Xie Z, Shen G, Wang Y, Wu C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult Sci. (2019) 98:422–9. 10.3382/ps/pey315 [DOI] [PubMed] [Google Scholar]
- 66.Salah AS, Mahmoud MA, Ahmed-Farid OA, El-Tarabany MS. Effects of dietary curcumin and acetylsalicylic acid supplements on performance, muscle amino acid and fatty acid profiles, antioxidant biomarkers and blood chemistry of heat-stressed broiler chickens. J Therm Biol. (2019) 84:259–65. 10.1016/j.jtherbio.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 67.Jin S, Yang H, Jiao Y, Pang Q, Wang Y, Wang M, et al. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods. (2021) 10:1370. 10.3390/foods10061370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salah AS, Ahmed-Farid OA, Nassan MA, El-Tarabany MS. Dietary curcumin improves energy metabolism, brain monoamines, carcass traits, muscle oxidative stability and fatty acid profile in heat-stressed broiler chickens. Antioxidants. (2021) 10:1265. 10.3390/antiox10081265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajput N, Naeem M, Ali S, Zhang JF, Zhang L, Wang T. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on broiler pigmentation and immunity. Poult Sci. (2013) 92:1177–85. 10.3382/ps.2012-02853 [DOI] [PubMed] [Google Scholar]
- 70.Pauletto M, Giantin M, Tolosi R, Bassan I, Barbarossa A, Zaghini A, et al. Curcumin mitigates AFB1-induced hepatic toxicity by triggering cattle antioxidant and anti-inflammatory pathways: a whole transcriptomic in vitro study. Antioxidants. (2020) 9:1059. 10.3390/antiox9111059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh H, Purohit SD, Bhaskar R, Yadav I, Bhushan S, Gupta MK, et al. Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J Biomed Mater Res B Appl Biomater. (2022) 110:210–9. 10.1002/jbm.b.34903 [DOI] [PubMed] [Google Scholar]
- 72.Baldissera MD, Souza CF, Zeppenfeld CC, Descovi S, Machado VS, Santos RCV, et al. Efficacy of dietary curcumin supplementation as bactericidal for silver catfish against Streptococcus agalactiae. Microb Pathog. (2018) 116:237–40. 10.1016/j.micpath.2018.01.044 [DOI] [PubMed] [Google Scholar]
- 73.Mohamed AA, El-Houseiny W, El-Murr AE, Ebraheim LLM, Ahmed AI, El-Hakim YMA. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: role of curcumin supplemented diet. Ecotoxicol Environ Saf. (2020) 188:109890. 10.1016/j.ecoenv.2019.109890 [DOI] [PubMed] [Google Scholar]
- 74.Giri SS, Kim MJ, Kim SG, Kim SW, Kang JW, Kwon J, et al. Role of dietary curcumin against waterborne lead toxicity in common carp Cyprinus carpio. Ecotoxicol Environ Saf. (2021) 219:112318. 10.1016/j.ecoenv.2021.112318 [DOI] [PubMed] [Google Scholar]
- 75.Ming J, Ye J, Zhang Y, Yang X, Shao X, Qiang J, et al. Effects of curcumin on growth performance, oxidative stress resistance and expression of nuclear factor erythroid 2-related factor 2/antioxidant responsive element signaling pathway-related genes in grass carp (Ctenopharyngodon idella). Chin J Anim Nutr. (2019) 31:809–23. 10.3369/j.issn.1006-267x.2019.02.037 [DOI] [Google Scholar]
- 76.Xun W, Shi L, Zhou H, Hou G, Cao T, Zhao C. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int Immunopharmacol. (2015) 27:46–52. 10.1016/j.intimp.2015.04.038 [DOI] [PubMed] [Google Scholar]
- 77.Gan Z, Wei W, Li Y, Wu J, Zhao Y, Zhang L, et al. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules. (2019) 24:1220. 10.3390/molecules24071220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang L, Zhang J, Yan E, He J, Zhong X, Zhang L, et al. Dietary supplemented curcumin improves meat quality and antioxidant status of intrauterine growth retardation growing pigs via Nrf2 signal pathway. Animals. (2020) 10:539. 10.3390/ani10030539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi L, Xun W, Peng W, Hu H, Cao T, Hou G. Effect of the single and combined use of curcumin and piperine on growth performance, intestinal barrier function, and antioxidant capacity of weaned wuzhishan piglets. Front Vet Sci. (2020) 7:418. 10.3389/fvets.2020.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moniruzzaman M, Min T. Curcumin, curcumin nanoparticles and curcumin nanospheres: a review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics. (2020) 12:447. 10.3390/pharmaceutics12050447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong L, You J, Zhang W, Zhu Q, Blachier F, Yin Y, et al. Intrauterine growth restriction alters growth performance, plasma hormones, and small intestinal microbial communities in growing-finishing pigs. J Anim Sci Biotechnol. (2020) 11:86. 10.1186/s40104-020-00490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, et al. Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr. (2013) 110:1819–27. 10.1017/S0007114513001232 [DOI] [PubMed] [Google Scholar]
- 83.Wang F, He J, Shen M, Gan Z, Niu Y, Zhang L, et al. Effects of curcumin supplementation on growth performance and intestinal histomorphology of weaned piglets with intrauterine growth retardation. Anim Husb Vet Med. (2019) 51:23–30. [Google Scholar]
- 84.Ferenc K, Pilżys T, Skrzypek T, Garbicz D, Marcinkowski M, Dylewska M, et al. Structure and function of enterocyte in intrauterine growth retarded pig neonates. Dis Markers. (2017) 2017:5238134. 10.1155/2017/5238134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou L, Xiong X, Liu H, Zhou J, Liu Y, Yin Y. Effects of dietary lysozyme levels on growth performance, intestinal morphology, immunity response and microbiota community of growing pigs. J Sci Food Agric. (2019) 99:1643–50. 10.1002/jsfa.9348 [DOI] [PubMed] [Google Scholar]
- 86.Buchmiller-Crair TL, Gregg JP, Rivera FA, Jr, Choi RS, Diamond JM, Fonkalsrud EW. Delayed disaccharidase development in a rabbit model of intrauterine growth retardation. Pediatr Res. (2001) 50:520–4. 10.1203/00006450-200110000-00016 [DOI] [PubMed] [Google Scholar]
- 87.Tsukahara T, Inoue R, Nakatani M, Fukuta K, Kishino E, Ito T, et al. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine. Anim Sci J. (2016) 87:67–75. 10.1111/asj.12399 [DOI] [PubMed] [Google Scholar]
- 88.Mazieiro R, Frizon RR, Barbalho SM, Goulart RA. Is Curcumin a possibility to treat inflammatory bowel diseases? J Med Food. (2018) 21:1077–85. 10.1089/jmf.2017.0146 [DOI] [PubMed] [Google Scholar]
- 89.Abrahams S, Haylett WL, Johnson G, Carr JA, Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: a review. Neuroscience. (2019) 406:1–21. 10.1016/j.neuroscience.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 90.Naemi M, Farahani Z, Norooznezhad AH, Khodarahmi R, Hantoushzadeh S, Ahangari R, et al. Possible potentials of curcumin for pregnancies complicated by intra-uterine growth restriction: role of inflammation, angiogenesis, and oxidative stress. Heliyon. (2021) 7:e08034. 10.1016/j.heliyon.2021.e08034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. (2008) 174:27–37. 10.1016/j.cbi.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 92.Gupta N, Verma K, Nalla S, Kulshreshtha A, Lall R, Prasad S. Free radicals as a double-edged sword: the cancer preventive and therapeutic roles of curcumin. Molecules. (2020) 25:5390. 10.3390/molecules25225390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao S, Wang C, Yan J, Li X, Wen J, Hu C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic Biol Med. (2020) 147:8–22. 10.1016/j.freeradbiomed.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 94.Zhang JF, Bai KW, Su WP, Wang AA, Zhang LL, Huang KH, et al. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult Sci. (2018) 97:1209–19. 10.3382/ps/pex408 [DOI] [PubMed] [Google Scholar]
- 95.Kong D, Li Y, Bai M, Deng NY, Liang G, Wu H. A comparative study of the dynamic accumulation of polyphenol components and the changes in their antioxidant activities in diploid and tetraploid Lonicera japonica. Plant Physiol Biochem. (2017) 112:87–96. 10.1016/j.plaphy.2016.12.027 [DOI] [PubMed] [Google Scholar]
- 96.Liu Q, Jing Y, Han C, Zhang H, Tian Y. Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocolloid. (2019) 93:432–42. 10.1016/j.foodhyd.2019.02.003 [DOI] [Google Scholar]
- 97.Ng ZX, Koick YTT, Yong PH. Comparative analyses on radical scavenging and cytotoxic activity of phenolic and flavonoid content from selected medicinal plants. Nat Prod Res. (2021) 35:5271–6. 10.1080/14786419.2020.1749617 [DOI] [PubMed] [Google Scholar]
- 98.Borra SK, Gurumurthy P, Mahendra J, Jayamathi KM, Cherian CN, Chand R. Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J Med Plants Res. (2013) 7:2680–90. 10.5897/JMPR2013.5094 [DOI] [Google Scholar]
- 99.Lee J, Park G, Chang YH. Nutraceuticals and antioxidant properties of Lonicera japonica Thunb. as affected by heating time. Int J Food Prop. (2019) 22:630–45. 10.1080/10942912.2019.1599389 [DOI] [Google Scholar]
- 100.Barzegar A, Moosavi-Movahedi AA. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS One. (2011) 6:e26012. 10.1371/journal.pone.0026012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zieliñska A, Alves H, Marques V, Durazzo A, Lucarini M, Alves TF, et al. Properties, extraction methods, and delivery systems for curcumin as a natural source of beneficial health effects. Medicina (Kaunas). (2020) 56:336. 10.3390/medicina56070336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. (2008) 46:1279–87. 10.1016/j.fct.2007.09.095 [DOI] [PubMed] [Google Scholar]
- 103.Al-Dossari MH, Fadda LM, Attia HA, Hasan IH, Mahmoud AM. Curcumin and selenium prevent lipopolysaccharide/diclofenac-induced liver injury by suppressing inflammation and oxidative stress. Biol Trace Elem Res. (2020) 196:173–83. 10.1007/s12011-019-01910-4 [DOI] [PubMed] [Google Scholar]
- 104.Tan L, Cao Z, Chen H, Xie Y, Yu L, Fu C, et al. Curcumin reduces apoptosis and promotes osteogenesis of human periodontal ligament stem cells under oxidative stress in vitro and in vivo. Life Sci. (2021) 270:119125. 10.1016/j.lfs.2021.119125 [DOI] [PubMed] [Google Scholar]
- 105.Li R, Fang H, Shen J, Jin Y, Zhao Y, Wang R, et al. Curcumin alleviates LPS-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells via the NFE2L2 signaling pathway. Toxins. (2021) 13:208. 10.3390/toxins13030208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu X, Li M, Chen W, Yu H, Yang Y, Hang L. Apigenin attenuates oxidative injury in arpe-19 cells thorough activation of Nrf2 pathway. Oxid Med Cell Longev. (2016) 2016:4378461. 10.1155/2016/4378461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang X, Liu B, Wang X, Yu Q, Fang R. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int J Mol Sci. (2018) 19:848. 10.3390/ijms19030848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang H, Tian X, Guo Y, Duan W, Bu H, Li C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biol Pharm Bull. (2011) 34:1194–7. 10.1248/bpb.34.1194 [DOI] [PubMed] [Google Scholar]
- 109.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. (2018) 1865:721–33. 10.1016/j.bbamcr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 110.Wu J, Ibtisham F, Niu YF, Wang Z, Li GH, Zhao Y, et al. Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2 / ARE signaling pathway in chicken fibroblasts cells. J Therm Biol. (2019) 79:112–9. 10.1016/j.jtherbio.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 111.Li S, Muhammad I, Yu H, Sun X, Zhang X. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol Environ Saf. (2019) 176:137–45. 10.1016/j.ecoenv.2019.03.089 [DOI] [PubMed] [Google Scholar]
- 112.Qi L, Jiang J, Zhang J, Zhang L, Wang T. Curcumin protects human trophoblast HTR8/SVneo cells from H2O2-induced oxidative stress by activating Nrf2 signaling pathway. Antioxidants. (2020) 9:121. 10.3390/antiox9020121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiang B, Li D, Chen Y, Li M, Zhang Y, Sun T, et al. Curcumin ameliorates copper-induced neurotoxicity through inhibiting oxidative stress and mitochondrial apoptosis in SH-SY5Y cells. Neurochem Res. (2021) 46:367–78. 10.1007/s11064-020-03173-1 [DOI] [PubMed] [Google Scholar]
- 114.Chen S, Yang S, Wang M, Chen J, Huang S, Wei Z, et al. Curcumin inhibits zearalenone-induced apoptosis and oxidative stress in Leydig cells via modulation of the PTEN/Nrf2/Bip signaling pathway. Food Chem Toxicol. (2020) 141:111385. 10.1016/j.fct.2020.111385 [DOI] [PubMed] [Google Scholar]
- 115.Woo JM, Shin DY, Lee SJ, Joe Y, Zheng M, Yim JH, et al. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol Vis. (2012) 18:901–8. [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X, Gao J, Wang Y, Zhao B, Zhang Y, Han F, et al. Curcumin pretreatment prevents hydrogen peroxide-induced oxidative stress through enhanced mitochondrial function and deactivation of Akt/Erk signaling pathways in rat bone marrow mesenchymal stem cells. Mol Cell Biochem. (2018) 443:37–45. 10.1007/s11010-017-3208-5 [DOI] [PubMed] [Google Scholar]
- 117.Wang X, Zhang Y, Yang Y, Zhang W, Luo L, Han F, et al. Curcumin pretreatment protects against hypoxia/reoxgenation injury via improvement of mitochondrial function, destabilization of HIF-1α and activation of Epac1-Akt pathway in rat bone marrow mesenchymal stem cells. Biomed Pharmacother. (2019) 109:1268–75. 10.1016/j.biopha.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 118.Qin X, Cao M, Lai F, Yang F, Ge W, Zhang X, et al. Oxidative stress induced by zearalenone in porcine granulosa cells and its rescue by curcumin in vitro. PLoS One. (2015) 10:e0127551. 10.1371/journal.pone.0127551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li L, Zhang Z, Huang Y. Integrative transcriptome analysis and discovery of signaling pathways involved in the protective effects of curcumin against oxidative stress in tilapia hepatocytes. Aquat Toxicol. (2020) 224:105516. 10.1016/j.aquatox.2020.105516 [DOI] [PubMed] [Google Scholar]
- 120.Hou K, Chen Y, Zhu D, Chen G, Chen F, Xu N, et al. Curcumin inhibits high glucose oxidative stress and apoptosis in pancreatic beta cells via CHOP/PCG-1a and pERK1/2. Front Biosci (Landmark Ed). (2020) 25:1974–84. 10.2741/4887 [DOI] [PubMed] [Google Scholar]
- 121.Lin C, Wu X. Curcumin protects trabecular meshwork cells from oxidative stress. Invest Ophthalmol Vis Sci. (2016) 57:4327–32. 10.1167/iovs.16-19883 [DOI] [PubMed] [Google Scholar]
- 122.Li J, Wu N, Chen X, Chen H, Yang X, Liu C. Curcumin protects islet cells from glucolipotoxicity by inhibiting oxidative stress and NADPH oxidase activity both in vitro and in vivo. Islets. (2019) 11:152–64. 10.1080/19382014.2019.1690944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai C, Tang S, Li D, Zhao K, Xiao X. Curcumin attenuates quinocetone-induced oxidative stress and genotoxicity in human hepatocyte L02 cells. Toxicol Mech Methods. (2015) 25:340–6. 10.3109/15376516.2015.1045659 [DOI] [PubMed] [Google Scholar]
- 124.Wang N, Wang G, Hao J, Ma J, Wang Y, Jiang X, et al. Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Dig Dis Sci. (2012) 57:1792–801. 10.1007/s10620-012-2094-7 [DOI] [PubMed] [Google Scholar]
- 125.Jaroonwitchawan T, Chaicharoenaudomrung N, Namkaew J, Noisa P. Curcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagy. Neurosci Lett. (2017) 636:40–7. 10.1016/j.neulet.2016.10.050 [DOI] [PubMed] [Google Scholar]
- 126.Okudan N, Belviranlı M, Gökbel H, Oz M, Kumak A. Protective effects of curcumin supplementation on intestinal ischemia reperfusion injury. Phytomedicine. (2013) 20:844–8. 10.1016/j.phymed.2013.03.022 [DOI] [PubMed] [Google Scholar]
- 127.Cui X, Lin L, Sun X, Wang L, Shen R. Curcumin protects against renal ischemia/reperfusion injury by regulating oxidative stress and inflammatory response. Evid Based Complement Alternat Med. (2021) 2021:8490772. 10.1155/2021/8490772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Damiano S, Longobardi C, Andretta E, Prisco F, Piegari G, Squillacioti C, et al. Antioxidative effects of curcumin on the hepatotoxicity induced by ochratoxin a in rats. Antioxidants. (2021) 10:125. 10.3390/antiox10010125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med. (2020) 24:12355–67. 10.1111/jcmm.15725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He J, Niu Y, Wang F, Wang C, Cui T, Bai K, et al. Dietary curcumin supplementation attenuates inflammation, hepatic injury and oxidative damage in a rat model of intra-uterine growth retardation. Br J Nutr. (2018) 120:537–48. 10.1017/S0007114518001630 [DOI] [PubMed] [Google Scholar]
- 131.Cheraghi E, Roshanaei K. The protective effect of curcumin against aluminum chloride-induced oxidative stress and hepatotoxicity in rats. Pharm Biomed Res. (2019) 5:11–8. 10.18502/pbr.v5i1.761 [DOI] [Google Scholar]
- 132.Momeni HR, Eskandari N. Curcumin protects the testis against cadmium-induced histopathological damages and oxidative stress in mice. Hum Exp Toxicol. (2020) 39:653–61. 10.1177/0960327119895564 [DOI] [PubMed] [Google Scholar]
- 133.Sharma S, Kumari A. Amelioration of curcumin against cadmium induced oxidative stress in lung of albino mice. Int J Adv Res. (2016) 4:1020–6. 10.21474/IJAR01/455 [DOI] [Google Scholar]
- 134.Ikram M, Saeed K, Khan A, Muhammad T, Khan MS, Jo MG, et al. Natural dietary supplementation of curcumin protects mice brains against ethanol-induced oxidative stress-mediated neurodegeneration and memory impairment via Nrf2/TLR4/RAGE signaling. Nutrients. (2019) 11:1082. 10.3390/nu11051082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ruan D, Wang WC, Lin CX, Fouad AM, Chen W, Xia WG, et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal. (2019) 13:42–52. 10.1017/S1751731118000678 [DOI] [PubMed] [Google Scholar]
- 136.Li S, Liu R, Wei G, Guo G, Yu H, Zhang Y, et al. Curcumin protects against Aflatoxin B1-induced liver injury in broilers via the modulation of long non-coding RNA expression. Ecotoxicol Environ Saf. (2021) 208:111725. 10.1016/j.ecoenv.2020.111725 [DOI] [PubMed] [Google Scholar]
- 137.Muhammad I, Wang H, Sun X, Wang X, Han M, Lu Z, et al. Dual role of dietary curcumin through attenuating AFB1-induced oxidative stress and liver injury via modulating liver phase-i and phase-ii enzymes involved in AFB1 bioactivation and detoxification. Front Pharmacol. (2018) 9:554. 10.3389/fphar.2018.00554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang H, Muhammad I, Li W, Sun X, Cheng P, Zhang X. Sensitivity of Arbor Acres broilers and chemoprevention of aflatoxin B1-induced liver injury by curcumin, a natural potent inducer of phase-II enzymes and Nrf2. Environ Toxicol Pharmacol. (2018) 59:94–104. 10.1016/j.etap.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 139.Nawab A, Li G, Liu W, Lan R, Wu J, Zhao Y, et al. Effect of dietary curcumin on the antioxidant status of laying hens under high- temperature condition. J Therm Biol. (2019) 86:102449. 10.1016/j.jtherbio.2019.102449 [DOI] [PubMed] [Google Scholar]
- 140.Ashrafizadeh M, Ahmadi Z, Mohammadinejad R, Farkhondeh T, Samarghandian S. Curcumin activates the Nrf2 pathway and induces cellular protection against oxidative injury. Curr Mol Med. (2020) 20:116–33. 10.2174/1566524019666191016150757 [DOI] [PubMed] [Google Scholar]
- 141.Tan BL, Norhaizan ME, Liew WP. Nutrients and oxidative stress: friend or foe? Oxid Med Cell Longev. (2018) 2018:9719584. 10.1155/2018/9719584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gebicki JM. Oxidative stress, free radicals and protein peroxides. Arch Biochem Biophys. (2016) 595:33–9. 10.1016/j.abb.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 143.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. (2007) 39:44–84. 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 144.Rashid CS, Bansal A, Simmons RA. Oxidative stress, intrauterine growth restriction, and developmental programming of type 2 diabetes. Physiology (Bethesda). (2018) 33:348–59. 10.1152/physiol.00023.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oke SL, Hardy DB. The role of cellular stress in intrauterine growth restriction and postnatal dysmetabolism. Int J Mol Sci. (2021) 22:6986. 10.3390/ijms22136986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang H, Chen Y, Chen Y, Ji S, Jia P, Xu J, et al. Pterostilbene attenuates liver injury and oxidative stress in intrauterine growth-retarded weanling piglets. Nutrition. (2021) 81:110940. 10.1016/j.nut.2020.110940 [DOI] [PubMed] [Google Scholar]
- 147.Aw TY. Molecular and cellular responses to oxidative stress and changes in oxidation-reduction imbalance in the intestine. Am J Clin Nutr. (1999) 70:557–65. 10.1093/ajcn/70.4.557 [DOI] [PubMed] [Google Scholar]
- 148.Chen Y, Zhang H, Chen Y, Jia P, Ji S, Zhang Y, et al. Resveratrol and its derivative pterostilbene ameliorate intestine injury in intrauterine growth-retarded weanling piglets by modulating redox status and gut microbiota. J Anim Sci Biotechnol. (2021) 12:70. 10.1186/s40104-021-00589-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. (2008) 13:7210–26. 10.2741/3223 [DOI] [PMC free article] [PubMed] [Google Scholar]