Abstract
Photocatalytic hydrogen evolution is considered one of the promising routes to solve the energy and environmental crises. However, developing efficient and low-cost photocatalysts remains an unsolved challenge. In this work, ultrathin 2D g-C3N4 nanosheets are coupled with flat TiO2 nanoparticles as face-to-face 2D/2D heterojunction photocatalysts through a simple electrostatic self-assembly method. Compared with g-C3N4 and pure TiO2 nanosheets, 2D/2D TiO2/g-C3N4 heterojunctions exhibit effective charge separation and transport properties that translate into outstanding photocatalytic performances. With the optimized heterostructure composition, stable hydrogen evolution activities are threefold and fourfold higher than those of pure TiO2, and g-C3N4 are consistently obtained. Benefiting from the favorable 2D/2D heterojunction structure, the TiO2/g-C3N4 photocatalyst yields H2 evolution rates up to 3875 μmol·g−1·h−1 with an AQE of 7.16% at 380 nm.
Keywords: hydrogen evolution, 2D/2D heterojunction, charge separation
1. Introduction
Owing to the abundance of low-cost solar energy, the numerous uses of hydrogen and its advantages as an energy carrier, the photocatalytic generation of hydrogen is a highly appealing process [1,2]. However, the cost-effective photogeneration of hydrogen requires high activity and stable photocatalysts, development of which has been a long-standing goal. Over the past decades, numerous semiconductors have been tested as photocatalysts for hydrogen evolution. Among them, titanium dioxide (TiO2) has received special attention owing to its stability, high abundance, low toxicity, being the earliest to be discovered and becoming the first to be industrialized [3]. Nevertheless, due to its wide bandgap and relatively fast charge recombination rate, its applicability has been strongly limited. Numerous strategies have been proposed to improve the photocatalytic performance of TiO2, facilitating charge separation and promoting efficiency and activity, [4,5,6,7] including the control of its particle facets and morphology [8,9,10,11], its modification with cocatalysts [12,13,14,15] and its coupling with other semiconductors to form heterostructures [16,17,18,19,20,21,22,23,24].
Graphite carbonitride (g-C3N4) with a layered structure similar to graphite, high chemical stability and low cost has received increasing interest in recent years [25,26,27]. In particular, as a polymeric semiconductor, g-C3N4 has been recently reported as a promising candidate photocatalyst due to its unique structure and electronic characteristics, with a 2.7 eV bandgap that allows absorbing part of the visible spectrum [28,29]. Additionally, two-dimensional (2D) g-C3N4 nanosheets, benefiting from a huge specific surface area and a suitable band structure, have shown especially interesting properties and offer an excellent platform to produce heterojunctions with other semiconductors [30,31,32,33].
Recently, 2D/2D heterojunctions have been demonstrated to provide great advantages to improve charge separation [34,35]. 2D/2D heterojunctions simultaneously maximize the interface and surface areas, i.e., the charge transfer between the two materials and the interaction with the media, which can potentially improve photocatalytic activities.
In the present work, we target improving photocatalytic hydrogen production using 2D/2D heterojunctions. In this direction, we report the first synthesis of 2D/2D TiO2/g-C3N4 heterostructures. Such composite materials are produced from the electrostatic assembly of 2D anatase TiO2 flat nanoparticles synthesized through a simple colloidal method with 2D ultrathin g-C3N4. The produced heterostructures are tested as photocatalysts for hydrogen evolution under simulated solar light irradiation. The excellent hydrogen evolution performance obtained after optimizing the weight contents of TiO2 and g-C3N4 within 2D/2D heterojunction are rationalized using photoluminescence, photocurrent and impedance spectroscopy analysis.
2. Experiment
Synthesis of bulk g-C3N4 (bCN) and ultrathin g-C3N4 (uCN): Bulk g-C3N4 powder was synthesized by thermal polymerization of urea. Briefly, 10 g of urea (99%, Acros Organics) was placed into a ceramic crucible. The crucible was covered and heated to 550 °C at a ramp rate of 2 °C min−1 for 4 h under air atmosphere. After cooling to room temperature, the resulting light-yellow solid was ground with the mortar to obtain the bulk g-C3N4 powder. To obtain ultrathin g-C3N4 (uCN), bulk g-C3N4 (2.0 g) was placed in a covered ceramic crucible, and it was heated to 520 °C with a ramp rate of 5 °C min−1 for 2 h under air atmosphere to obtain a light-yellow powder.
Synthesis of TiO2 nanosheets: Titanium dioxide nanoparticles were prepared using a colloidal method. All the syntheses were performed using standard airless techniques [36,37]. Typically, 10 mL of oleylamine (OAm, 80–90%, Acros Organics, Geel, Belgium), 10 mL of octadecene (ODE, 90%, Sigma-Aldrich, Burlington, MA, USA) and 1 mL of oleic acid (OAc, 90%, Sigma-Aldrich, Burlington, MA, USA) were loaded in a three-neck flask and degassed under vacuum at 120 °C for 1 h while being strongly stirred using a magnetic bar. Then, 300 mg of TiF4 (99%, Sigma, Burlington, MA, USA) was added in a mixed solution of 2 mL OAm, 3 mL OAc and 6 mL ODE and sonicated for 0.5 h to prepare a precursor solution. Subsequently, under nitrogen atmosphere, 10 mL of the precursor solution were slowly added to the reaction flask, which was then heated to 290 °C at a rate of 5 °C min−1 and maintained for 1 h. The solid product was centrifuged and washed with acetone and hexane three times. The particles were finally dispersed in hexane at a concentration of 10 mg/mL.
Ligand removal from TiO2 nanoparticles: In a typical process, 10 mL of a TiO2 dispersion in hexane (2 mg/mL) was combined with 10 mL acetonitrile to form a two-phase mixture. Then, 1 mL of a HBF4 solution (48%, Sigma-Aldrich, Burlington, MA, USA) was added. The resulting solution was sonicated until the particles transferred from the upper to the bottom layer. The surface-modified particles were washed with ethanol and a 1 mol/L sodium hydroxide (85%, Sigma-Aldrich, Burlington, MA, USA) aqueous solution three times to remove the residual fluoride ions and ligands. The particles were then washed with water to adjust the PH close to neutral. Finally, the particles were dispersed in 10 mL of water with a small amount of DMF.
Synthesis of 2D/2D TiO2/ultrathin g-C3N4 (TiO2/uCN) composite: TiO2/uCN heterojunctions were produced by an electrostatic self-assembly method. Briefly, 20 mg of as prepared ultrathin g-C3N4 was dissolved in 10 mL of ultrapure water and sonicated for 1 h. The solution was then mixed with an ethanol solution of ligand-removed TiO2 nanoparticles with a weight ratio of 1:2, 1:1 and 2:1. The mixed solution was stirred for 24 h after 1 h of sonication. The obtained composite was collected by centrifuging, it was washed with ethanol 2 times, and it was finally dried at 60 °C for 12 h. The collected materials were named T1/uCN2, T1/uCN1 and T2/uCN1 based on the different TiO2/ultrathin g-C3N4 weight ratios. TiO2/bulk g-C3N4 (T/bCN) samples were prepared using the same procedure. For photocatalytic measurements, 1 wt% of Pt was loaded on the surface of the photocatalysts by a photoreduction method.
Photocatalytic Hydrogen Evolution Procedure
The photocatalytic hydrogen evolution experiments were carried out in a Perfect Light Labsolar-III (AG) photoreactor (Pyrex glass) connected to a closed-loop gas circulation system. In a typical experiment, 20 mg photocatalyst was dispersed in 100 mL aqueous solution containing 10 mL methanol and 1 wt% Pt cocatalyst (40 uL 25.625 mmol/L H2PtCl6 aqueous solution). The mixed solution was bubbled with N2 for 30 min to ensure anaerobic state and illuminated 30 min with UV light before simulated solar light irradiation to ensure the complete loading of Pt. The incident light was provided by a 300 W Xe lamp with an AM 1.5 filter, and the reaction conditions were kept at room temperature. The resulting gas was analyzed by a Labsolar-III (AG) gas chromatograph equipped with a thermal conductivity detector, with high-purity argon as the carrier gas.
3. Result and Discussion
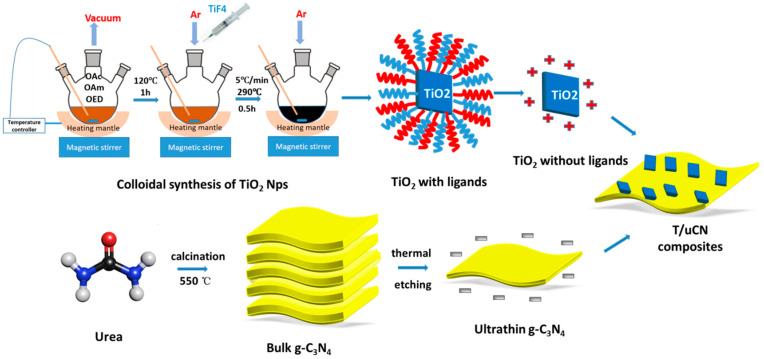
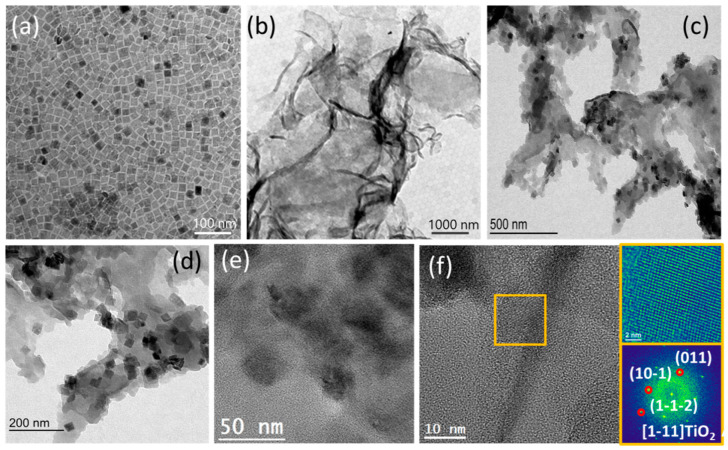
TiO2/g-C3N4 heterostructures were obtained by the electrostatic assembly of TiO2 nanoparticles and ultrathin g-C3N4 nanosheets (Figure 1, see Experimental section for details). Colloidal TiO2 nanoparticles were produced in the presence of OAm and OAc using TiF4 as the Ti precursor. As shown in Figure 2a, low-resolution TEM images exhibited the TiO2 particles to have a flat square morphology with a side length of 30–50 nm and a thickness of about 5–10 nm. g-C3N4 nanosheets were produced by the thermal etching of bulk g-C3N4. As observed by scanning electron microscopy (SEM, Figure S1a,b) and transmission electron microscopy (TEM, Figure 2b) characterization, bCN and uCN displayed significantly different morphologies. The uCN showed a thin nanosheet-based structure pointing at the occurrence of a layer etching during the thermal process. Figure S1c displays the nitrogen adsorption–desorption isotherms of bCN and uCN, which further proved uCN (85.7 m2/g) to be characterized by a larger specific surface area than bCN (46.3 m2/g).
Figure 1.
Schematic illustration of the process used to produce 2D/2D TiO2/uCN composite.
Figure 2.
Representative TEM images of (a) TiO2 nanoparticles; (b) g-C3N4 nanosheets and T1/uCN1 composite with representative (c) low and (d) high magnification. (e,f) HRTEM images of T1/uCN1. A magnified detail (top right) of the orange squared region in the HRTEM image and its corresponding indexed power spectrum (bottom right) is shown, revealing the TiO2 anatase phase (space group = I41/amd) with a = b = 3.7840 Å, and c = 9.5000 Å. TiO2 lattice fringe distances were measured to be 0.233 nm, 0.352 nm and 0.348 nm at 41.30° and 139.38°, which could be interpreted as the anatase TiO2 phase, visualized along its [1–11] zone axis.
To positively charge the surface of the TiO2 particles, enable their dispersion in an aqueous solution and promote charge transfer with the media; the organic ligands attached to the particle surface were removed using HBF4 (Figure S2). As observed by zeta-potential analysis, while the g-C3N4 nanosheets were negatively charged (V = −33.8 mV), after ligands removal the TiO2 particles were positively charged (V = +18.6 mV), which enabled the electrostatic self-assembly of the two components [38]. Indeed, when combining solutions of the two types of material, a light-yellow precipitate was formed. The precipitate was composed of large uCN nanosheets containing numerous nanoparticles attached to their surface. TEM analyses showed these nanoparticles lie flat on the surface of uCN, forming 2D/2D heterostructures (Figure 2c,d). High resolution TEM (HRTEM) further confirmed these nanoparticles are TiO2 with good crystallinity (Figure 2e,f).
SEM-EDS elemental maps (Figure S4) displayed a homogeneous distribution of C, N, O and Ti, demonstrating a uniform distribution of TiO2 particles on the uCN surface at the microscale. On the other hand, quantitative EDX analyses showed the TiO2:CN weight ratio to be close to that of the nominal combination of each phase: TiO2:CN = 0.47 for T1/uCN2; TiO2:CN = 1.1 for T1/uCN1 and TiO2:CN = 1.9 for T2/uCN1, obtained from mixing 1:2, 1:1 and 2:1 mass ratios of particles, respectively (Figures S5–S7).
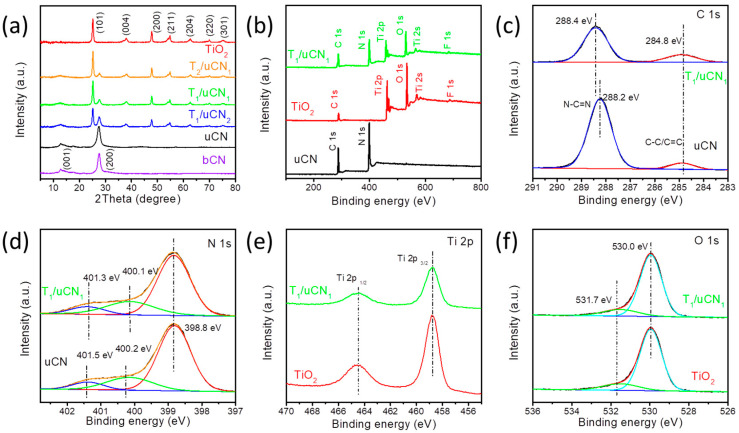
Figure 3a displays the X-ray diffraction (XRD) patterns of bCN, uCN, TiO2 and T/uCN samples. The XRD peaks at 25.2° (101), 38.0° (004), 47.7° (200) and 54.8° (211) are associated with the anatase TiO2 phase (JCPDS No. 21-1272) [39]. Additonally, the characteristic diffraction peaks at 13.1° and 27.4° correspond to the (002) and (100) planes of g-C3N4 (JCPDS No. 87-1526) [40]. The characteristic diffraction peaks of both TiO2 and g-C3N4 can be observed in all the composites samples, confirming the coexistence of anatase TiO2 and g-C3N4.
Figure 3.
(a) XRD patterns of TiO2, uCN and T/uCN. (b) XPS survey spectrum of TiO2, uCN and T/uCN; high-resolution XPS spectra at the regions (c) C 1s, (d) N 1s, (e) Ti 2p and (f) O 1s.
The X-ray photoelectron spectroscopy spectra of TiO2, uCN and T/uCN are displayed in Figure 3b–f. As observed from the survey XPS spectrum, besides Ti, C, O and N, a residual amount of F from the TiF4 precursor used to prepare the TiO2 particles was also present in the final material (Figure 3b). The high-resolution C 1s XPS spectrum of uCN showed two main contributions at 288.2 eV and 284.8 eV, which were assigned to C-(N3) and C–C/C=C, respectively (Figure 3c). Compared with pure uCN, the peak for C-(N3) of the T1/uCN1 sample was slightly shifted to 288.2 eV. The high-resolution N 1s XPS spectra were deconvoluted using three contributions at binding energies of 398.1 eV, 499.4 eV and 400.5 eV for uCN and 398.1 eV, 499.6 eV and 400.7 eV for T/uCN (Figure 3d). These three contributions were assigned to N-(C2), N-(C3) and N-Hx groups of the heptazine framework. The small shifts detected for C and some of the N components might be related to a certain degree of charge between the TiO2 and the CN phases. Figure 3e displays the high-resolution Ti 2p XPS spectra of TiO2 and T/uCN. Both samples show two strong peaks at approximately 458.7 and 464.5 eV, which are assigned to the Ti 2p3/2 and Ti 2p1/2 levels of Ti within a TiO2 environment. The high-resolution O 1s XPS spectra of TiO2 and T/uCN were fitted with two peaks at 530.4 eV and 531.8 eV, which were associated with oxygen within the TiO2 lattice and oxygen-containing surface adsorption groups such as surface hydroxyl, respectively (Figure 3f).
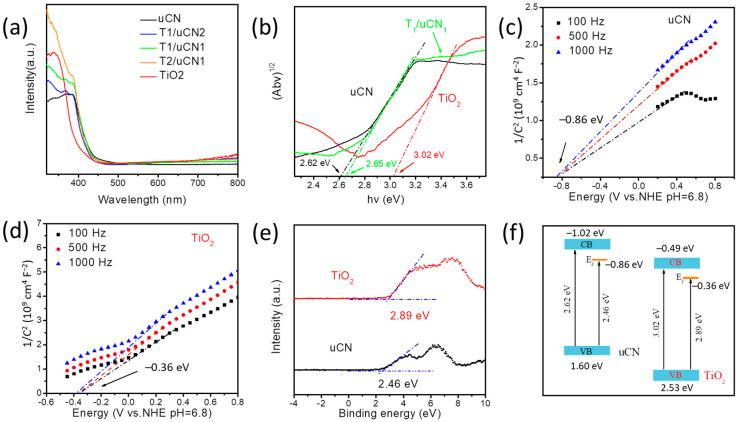
The UV-vis spectra showed the UV absorption edge of TiO2 particles and uCN nanosheets at about 390 nm and 445 nm, respectively (Figure 4a). T/uCN composites showed a similar onset absorption edge as uCN but an increased absorption below 400 nm related to the presence of the TiO2 component. All TiO2 and T/uCN samples presented a small absorption in the range 500–800 nm related to a small amount of F ion doping. According to the Kubelk–Munk function, the band gaps of TiO2, uCN and T1/Ucn1 samples were calculated at about 3.02 eV, 2.62 eV and 2.65 eV, respectively (Figure 4b).
Figure 4.
(a) UV-vis absorption spectra. (b) Kubelka–Munk-transformed function of TiO2, uCN and T1/uCN1. (c,d) Mott–Schottky plots of uCN (c) and TiO2 (d). (e) Valence band XPS spectrum of TiO2 and uCN. (f) Diagram of the band structure of TiO2 and uCN.
According to Mott–Schottky analyses (Figure 4c,d and Figure S3), the flat band potentials of TiO2 and uCN were −0.36 V and −0.86 V vs. the normal hydrogen electrode (NHE). The valence band (VB) XPS spectra of TiO2 and uCN showed the valence band maximum (VBM) to be located at 2.89 eV and 2.46 eV from the Fermi level, respectively. Since the flat band potentials are approximately equal to the Fermi level [41,42], the VBM was located at 2.53 eV and 1.60 eV with respect to the NHE for TiO2 and uCN, respectively. Then, taking into account the calculated band gaps (Eg = Evb − Ecb) [43], the conduction band minimum (CBM) was located at 0.49 and −1.02 for TiO2 and uCN, respectively. Figure 4f displays the energy-level diagram calculated for TiO2 and uCN samples. According to this scheme, when combining uCN with TiO2, a type II heterojunction is formed, involving electron transfer from the uCN to the TiO2 particles. Besides, it is predicted that within such heterostructure, photogenerated electrons move toward the TiO2 phase and photogenerated holes toward the uCN, respectively.
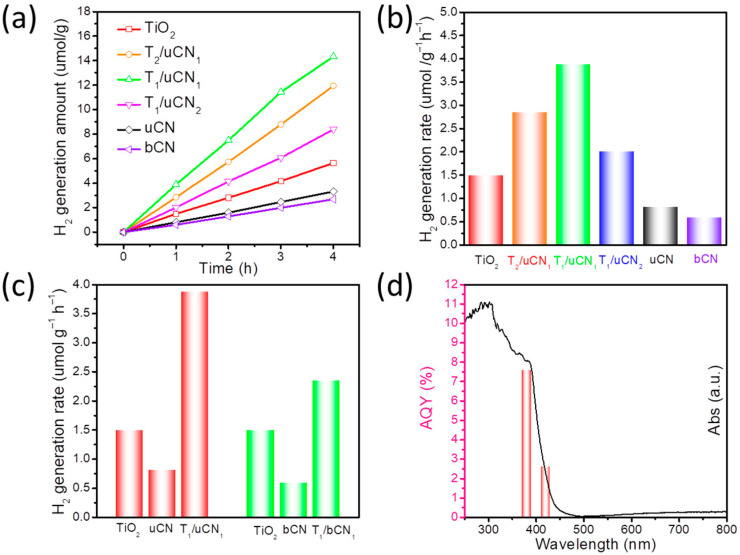
To analyze the photocatalytic activity towards hydrogen generation, all the samples were loaded with 1 wt% platinum as cocatalyst. Figure 5 displays the photocatalytic hydrogen generation from bCN, uCN, TiO2 and TiO2/uCN composites for 4 h under simulated solar light and using methanol as a sacrificial agent. Figures S8 and S9 and Table S2 show the chromatogram plots and the linear fitting of the standard hydrogen curve for gas chromatography, which show our measurement error is less than 0.2%.
Figure 5.
(a) Photocatalytic hydrogen generation on bCN, TiO2 and T/uCN samples during four hours under simulated solar light illumination. (b) Photocatalytic hydrogen peroxide generation rate of bCN, TiO2 and T/uCN samples. (c) H2 production rate contrast between T1/uCN1 and T1/bCN1. (d) Wavelength-dependent AQY of T1/uCN1.
For TiO2, a high hydrogen evolution rate (HER) up to 1449 μmol·g−1·h−1 was obtained. Additionally, a notable HER was also obtained from uCN (801 μmol·g−1·h−1), well above that of bCN (599 μmol·g−1·h−1), which is consistent with the larger surface area provided by the thin-layered structure of uCN. All the TiO2/uCN composites displayed a significant HER improvement with respect to pure TiO2 or uCN. The highest HERs were obtained with the TiO2/uCN composites having a 1:1 weight ratio of the two components, reaching a HER of 3875 μmol·g−1·h−1, which is 2.7 and 4.8 times higher than that of TiO2 and uCN, respectively. The observed synergistic effect obtained when mixing both materials is related to the transfer and thus separation of photogenerated carriers at the 2D/2D heterojunctions, which prevents their recombination. Table S3 provides a comparison of the activity obtained here with those of previous published works, demonstrating the outstanding activity provided by the 2D/2D TiO2/uCN heterojunction.
As a reference, we also measured the HER of TiO2/bCN composites with the optimized weight ratio 1:1 (T1/bCN1). As observed in Figure 5c and Figure S7, the HER of T1/bCN1 also showed an obvious improvement with respect to that of pure TiO2 and bCN, but the highest HER values were well below those of 2D/2D T/uCN heterojunctions having extended surface and interface areas.
The apparent quantum yield (AQY) of the process was evaluated under 380 nm (4.51 mW·cm−2) and 420 nm (12.14 mW·cm−2) irradiation (Table S4, see details in the SI). For T1/uCN1, the AQY at 380 nm and 420 nm was estimated at 7.61% and 2.64%, respectively, which is consistent with UV-vis spectroscopy results (Figure 5d).
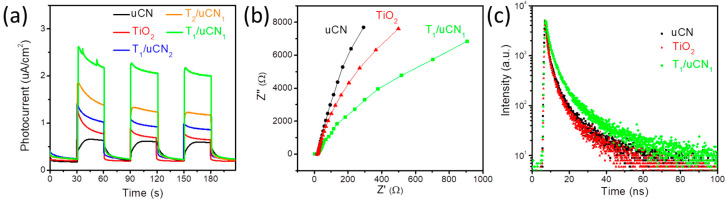
Figure 6a displays the positive photocurrents measured from uCN, TiO2 and TiO2/uCN samples under simulated solar irradiation. All the composite T/uCN, electrodes displayed significantly higher photocurrents than pure TiO2 and uCN, especially the T1/uCN1 electrode that showed the highest photocurrents, fourfold higher than those of uCN and TiO2. This result further confirms an improvement of the charge separation/transport with the formation of the 2D/2D heterojunction.
Figure 6.
(a) Photocurrent response curves of bCN, TiO2 and T/uCN samples; (b) electrochemical impedance spectroscopy (EIS) Nyquist plots of bCN,TiO2 and T1/uCN1 sample; (c) TRPL decay of bCN, TiO2 and T/uCN samples.
Electrochemical impedance spectroscopy (EIS) was further employed to identify the charge transport dynamics. Figure 6b displays the Nyquist plot of the impedance spectra of TiO2, uCN and T1/uCN1. Consistent with previous results, the T1/uCN1 electrode presented a much smaller arc radius than the other two samples, confirming a much lower charge transfer resistance with the formation of the 2D/2D TiO2/uCN heterojunction [44].
A strong photoluminescence (PL) peak was obtained under 370 nm light excitation from the uCN sample at about 455 nm, which is ascribed to the radiative band-to-band recombination of photogenerated charge carriers. When incorporating increasing amounts of TiO2, the PL intensity of T/uCN was progressively quenched (Figure S10). Additional time-resolved PL (TRPL) spectra under 365 nm light excitation (Figure 6c) allowed calculating significantly longer PL lifetimes (4.72 ns) for T1/uCN1 samples than for TiO2 (3.15 ns) and uCN (3.51 ns), which points at an effective separation of photogenerated charge carriers within the TiO2/uCN heterostructures [45].
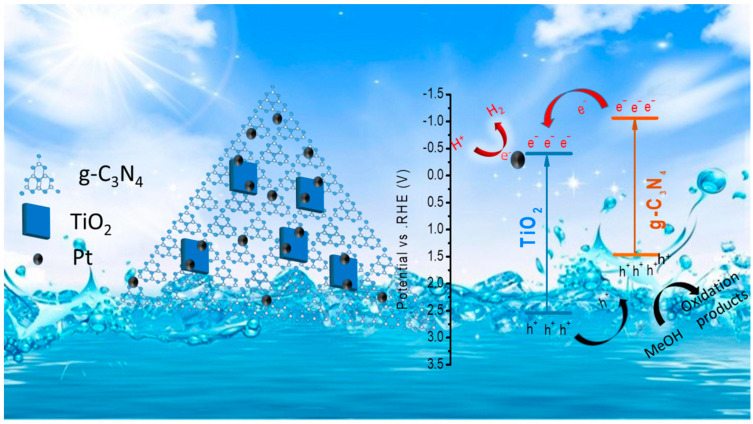
Based on the above results, the photocatalytic mechanism displayed in Figure 7 is proposed for hydrogen generation in T/uCN heterojunction photocatalysts. While both TiO2 and uCN can generate electrons and holes under simulated solar light irradiation, the photogenerated electron–hole pairs in pure TiO2 and uCN rapidly recombine, resulting in moderate HERs. Through the formation of a 2D/2D T/uCN heterostructure, the photogenerated electrons remain or are transferred to the TiO2 CB because the TiO2 CBM is located 0.53 eV below that of CN. Similarly, photogenerated holes remain or are driven to the uCN VB, which is located 0.93 eV above that of TiO2. Electrons at the TiO2 CB migrate to the platinum, which has a larger work function, thus a lower Fermi level, from where they are transferred to adsorbed H+ to produce H2. On the other hand, holes react with sacrificial methanol at the CN surface. Consequently, the photocatalytic hydrogen evolution process using sacrificial methanol can be described as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Figure 7.
Schematic diagram of photocatalytic hydrogen production over T/uCN photocatalyst.
Finally, the stability of the T1/uCN1 photocatalyst in hydrogen evolution conditions under simulated solar light irradiation was measured through five four-hour cycles. As shown in Figure S11a, after this 20 h of reaction, the photocatalytic performance was hardly reduced, proving the excellent stability and reusability of the T1/uCN1 photocatalyst. Additionally, as displayed in Figure S11b,c, SEM and XRD analysis of the catalyst after 20 h photocatalytic hydrogen generation reaction demonstrated the morphology and crystallographic structure of the material to be stable under photocatalytic reaction conditions.
4. Conclusions
In summary, we detailed the synthesis of 2D/2D T/uCN heterojunctions from ultrathin g-C3N4 (uCN) and colloidal TiO2 nanosheets through an electrostatic self-assembly approach. The highest hydrogen generation rate was achieved from T/uCN composites with a 1:1 mass ratio of the two components. The photocatalytic performance for H2 production was increased in the following order: bCN < uCN < TiO2 < T1/uCN2 < T2/uCN1 < T1/uCN1. The enhanced performance was attributed to the unique 2D/2D type II heterojunction architecture that simultaneously maximized the surface area to interact with the media and the interface between the two materials. The face-to-face interfacial contact between ultrathin layers of g-C3N4 and the faceted TiO2 provided fast separation of photogenerated charges inside the composites, reducing recombination and thus increasing the apparent quantum yield.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano12091557/s1, Figure S1: SEM image of (a) bulk g-C3N4 and (b) ultrathin g-C3N4, (c) N2 adsorption-desorption isotherms of bCN and uCN; Figure S2: FTIR spectra of OAC, OLMA and TiO2 before and after ligands remove; Figure S3: Zeta potential distribution spectrum of TiO2 after ligands removal (a) and uCN (b); Figure S4: SEM image and EDS compositional maps of a T1/uCN1 composite; Figure S5: SEM image of T1/uCN2 and corresponding EDS spectrum; Figure S6: SEM image of T1/uCN2 and corresponding EDS spectrum; Figure S7: SEM image of T1/uCN2 and corresponding EDS spectrum; Figure S8: Chromatogram plots for 0.5 ml of standard hydrogen injected every half hour; Table S1: Gas Chromatography Peak Processing Data based on figure S8; Figure S9: Standard hydrogen curve for gas chromatography; Table S2: Exponential decay-fitted parameters of fluorescence lifetime of uCN, TiO2 and T1/uCN1; Figure S10: Photocatalytic hydrogen generation amount on bCN, TiO2 and T1/bCN1 during 4 h under simulated solar light irradiation; Table S3: Photocatalytic hydrogen production about TiO2/g-C3N4 based catalysts; Table S4: The AQE values with different incident light wavelengths for T1/uCN1; Figure S11: (a) Stability cycles of the T1/uCN1 for H2 evolution under simulated solar light irradiation; (b) TEM image of T1/uCN1 after 20 h photocatalytic H2 evolution reaction and (c) XRD pattern of T1/uCN1 before and after 20 h photocatalytic H2O2 evolution reaction.
Author Contributions
In this work, R.D. designed the experiment and the nanocomposites, prepared all the materials and conducted XRD, SEM, ZEM-EDS, TEM characterization, photoelectrochemical measurements and wrote the first draft of the manuscript. B.L. conducted the photocatalytic hydrogen evolution test and TRPL test. K.X., C.Z. and X.W. significantly contributed to the result discussion. X.H. and J.A. participated in high-resolution TEM characterization. A.C. conceived and guided the project and supervised the work. The manuscript was corrected and improved by all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available on reasonable request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
R.D.: K.X., X.H., X.W. and C.Z. thank the China Scholarship Council for the scholarship support. IREC and ICN2 acknowledge funding from Generalitat de Catalunya, projects 2017 SGR 1246 and 2017 SGR 327, respectively. The authors thank the support from the project NANOGEN (PID2020-116093RB-C43), funded by MCIN/AEI/10.13039/501100011033/ and the project COMBENERGY (PID2019-105490RB-C32) from the Spanish Ministerio de Ciencia e Innovación. ICN2 is supported by the Severo Ochoa program from Spanish MINECO (Grant No. SEV-2017-0706) and is funded by the CERCAProgramme / Generalitat de Catalunya. Baoying Li greatly appreciates the financial support from the National Natural Science Foundation of China (Nos. 22171154 & 21801144), the Youth Innovative Talents Recruitment and the Cultivation Program of Shandong Higher Education. This study was supported by MCIN with funding from the European Union NextGenerationEU (PRTR-C17.I1), Generalitat de Catalunya and by “ERDF A way of making Europe” by the “European Union”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campos-Martin J.M., Blanco-Brieva G., Fierro J.L.G. Hydrogen peroxide synthesis: An outlook beyond the anthraquinone process. Angew. Chem. Int. Ed. 2006;45:6962–6984. doi: 10.1002/anie.200503779. [DOI] [PubMed] [Google Scholar]
- 2.Xiang Q., Cheng B., Yu J. Hierarchical porous CdS nanosheet-assembled flowers with enhanced visible-light photocatalytic H2-production performance. Appl. Catal. B Environ. 2013;138:299–303. doi: 10.1016/j.apcatb.2013.03.005. [DOI] [Google Scholar]
- 3.Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J., Xu Q., Feng Z., Li M., Li C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. 2008;120:1790–1793. doi: 10.1002/ange.200704788. [DOI] [PubMed] [Google Scholar]
- 5.Irie H., Watanabe Y., Hashimoto K. Carbon-doped anatase TiO2 powders as a visible-light sensitive photocatalyst. Chem. Lett. 2003;32:772–773. doi: 10.1246/cl.2003.772. [DOI] [Google Scholar]
- 6.Zhang Y., Tang Z.-R., Fu X., Xu Y.-J. TiO2− graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2− graphene truly different from other TiO2− carbon composite materials? ACS Nano. 2010;4:7303–7314. doi: 10.1021/nn1024219. [DOI] [PubMed] [Google Scholar]
- 7.Thompson T.L., Yates J.T. Surface science studies of the photoactivation of TiO2 new photochemical processes. Chem. Rev. 2006;106:4428–4453. doi: 10.1021/cr050172k. [DOI] [PubMed] [Google Scholar]
- 8.Hamad S., Catlow C.R.A., Woodley S.M., Lago S., Mejias J.A. Structure and stability of small TiO2 nanoparticles. J. Phys. Chem. B. 2005;109:15741–15748. doi: 10.1021/jp0521914. [DOI] [PubMed] [Google Scholar]
- 9.Sang L., Zhao Y., Burda C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014;114:9283–9318. doi: 10.1021/cr400629p. [DOI] [PubMed] [Google Scholar]
- 10.Chen J.S., Lou X.W. Anatase TiO2 nanosheet: An ideal host structure for fast and efficient lithium insertion/extraction. Electrochem. Commun. 2009;11:2332–2335. doi: 10.1016/j.elecom.2009.10.024. [DOI] [Google Scholar]
- 11.Zhang Y.X., Li G.H., Jin Y.X., Zhang Y., Zhang J., Zhang L.D. Hydrothermal synthesis and photoluminescence of TiO2 nanowires. Chem. Phys. Lett. 2002;365:300–304. doi: 10.1016/S0009-2614(02)01499-9. [DOI] [Google Scholar]
- 12.Wang P., Wang J., Wang X., Yu H., Yu J., Lei M., Wang Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013;132:452–459. doi: 10.1016/j.apcatb.2012.12.009. [DOI] [Google Scholar]
- 13.Zhou W., Yin Z., Du Y., Huang X., Zeng Z., Fan Z., Liu H., Wang J., Zhang H. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small. 2013;9:140–147. doi: 10.1002/smll.201201161. [DOI] [PubMed] [Google Scholar]
- 14.Ou Y., Lin J., Fang S., Liao D. MWNT–TiO2: Ni composite catalyst: A new class of catalyst for photocatalytic H2 evolution from water under visible light illumination. Chem. Phys. Lett. 2006;429:199–203. doi: 10.1016/j.cplett.2006.08.024. [DOI] [Google Scholar]
- 15.Liu Q., Huang J., Tang H., Yu X., Shen J. Construction 0D TiO2 nanoparticles/2D CoP nanosheets heterojunctions for enhanced photocatalytic H2 evolution activity. J. Mater. Sci. Technol. 2020;56:196–205. doi: 10.1016/j.jmst.2020.04.026. [DOI] [Google Scholar]
- 16.Meng A., Zhu B., Zhong B., Zhang L., Cheng B. Direct Z-scheme TiO2/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Surf. Sci. 2017;422:518–527. doi: 10.1016/j.apsusc.2017.06.028. [DOI] [Google Scholar]
- 17.Cheng C., Amini A., Zhu C., Xu Z., Song H., Wang N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014;4:4181. doi: 10.1038/srep04181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J., Wu H., Chen H., Zhang Y., Zhang F., Liu S.F. Fabrication of TiO2/C3N4 heterostructure for enhanced photocatalytic Z-scheme overall water splitting. Appl. Catal. B Environ. 2016;191:130–137. doi: 10.1016/j.apcatb.2016.03.026. [DOI] [Google Scholar]
- 19.Xie M., Fu X., Jing L., Luan P., Feng Y., Fu H. Long-lived, visible-light-excited charge carriers of TiO2/BiVO4 nanocomposites and their unexpected photoactivity for water splitting. Adv. Energy Mater. 2014;4:1300995. doi: 10.1002/aenm.201300995. [DOI] [Google Scholar]
- 20.Wang Y., Zhu C., Zuo G., Guo Y., Xiao W., Dai Y., Kong J., Xu X., Zhou Y., Xie A. 0D/2D Co3O4/TiO2 Z-Scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. B Environ. 2020;278:119298. doi: 10.1016/j.apcatb.2020.119298. [DOI] [Google Scholar]
- 21.Yuan L., Weng B., Colmenares J.C., Sun Y., Xu Y. Multichannel charge transfer and mechanistic insight in metal decorated 2D–2D Bi2WO6–TiO2 cascade with enhanced photocatalytic performance. Small. 2017;13:1702253. doi: 10.1002/smll.201702253. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Xu J., Mei J., Sarina S., Wu Z., Liao T., Yan C., Sun Z. Strongly interfacial-coupled 2D-2D TiO2/g-C3N4 heterostructure for enhanced visible-light induced synthesis and conversion. J. Hazard. Mater. 2020;394:122529. doi: 10.1016/j.jhazmat.2020.122529. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q., Lu H., Shi Z., Wu F., Guo J., Deng K., Li L. 2D ZnIn2S4 nanosheet/1D TiO2 nanorod heterostructure arrays for improved photoelectrochemical water splitting. ACS Appl. Mater. Interfaces. 2014;6:17200–17207. doi: 10.1021/am505015j. [DOI] [PubMed] [Google Scholar]
- 24.Chava R.K., Son N., Kang M. Surface engineering of CdS with ternary Bi/Bi2MoO6-MoS2 heterojunctions for enhanced photoexcited charge separation in solar-driven hydrogen evolution reaction. Appl. Surf. Sci. 2021;565:150601. doi: 10.1016/j.apsusc.2021.150601. [DOI] [Google Scholar]
- 25.Fu J., Yu J., Jiang C., Cheng B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018;8:1701503. doi: 10.1002/aenm.201701503. [DOI] [Google Scholar]
- 26.Wen J., Xie J., Chen X., Li X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017;391:72–123. doi: 10.1016/j.apsusc.2016.07.030. [DOI] [Google Scholar]
- 27.Chava R.K., Do J., Kang M. Strategy for improving the visible photocatalytic H2 evolution activity of 2D graphitic carbon nitride nanosheets through the modification with metal and metal oxide nanocomponents. Appl. Catal. B Environ. 2019;248:538–551. doi: 10.1016/j.apcatb.2019.01.075. [DOI] [Google Scholar]
- 28.Ran Y., Cui Y., Zhang Y., Fang Y., Zhang W., Yu X., Lan H., An X. Assembly-synthesis of puff pastry-like g-C3N4/CdS heterostructure as S-junctions for efficient photocatalytic water splitting. Chem. Eng. J. 2022;431:133348. doi: 10.1016/j.cej.2021.133348. [DOI] [Google Scholar]
- 29.Cabot A., Du R., Xiao K., Li B., Han X., Zhang C., Wang X., Zuo Y., Pablo G., Li J., et al. Controlled Oxygen Doping in Highly Dispersed Ni-Loaded G-C3N4 Nanotubes for Efficient Photocatalytic H2O2 Production. SSRN Electron. J. 2022;441:135999. doi: 10.2139/ssrn.4018808. [DOI] [Google Scholar]
- 30.Zhang X., Yuan X., Jiang L., Zhang J., Yu H., Wang H., Zeng G. Powerful combination of 2D g-C3N4 and 2D nanomaterials for photocatalysis: Recent advances. Chem. Eng. J. 2020;390:124475. doi: 10.1016/j.cej.2020.124475. [DOI] [Google Scholar]
- 31.Zuo Y., Xu X., Zhang C., Li J., Du R., Wang X., Han X., Arbiol J., Llorca J., Liu J., et al. SnS2/g-C3N4/graphite nanocomposites as durable lithium-ion battery anode with high pseudocapacitance contribution. Electrochim. Acta. 2020;349:136369. doi: 10.1016/j.electacta.2020.136369. [DOI] [Google Scholar]
- 32.Fu J., Xu Q., Low J., Jiang C., Yu J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019;243:556–565. doi: 10.1016/j.apcatb.2018.11.011. [DOI] [Google Scholar]
- 33.Qin Y., Li H., Lu J., Feng Y., Meng F., Ma C., Yan Y., Meng M. Synergy between van der waals heterojunction and vacancy in ZnIn2S4/g-C3N4 2D/2D photocatalysts for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020;277:119254. doi: 10.1016/j.apcatb.2020.119254. [DOI] [Google Scholar]
- 34.Cao S., Shen B., Tong T., Fu J., Yu J. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 2018;28:1800136. doi: 10.1002/adfm.201800136. [DOI] [Google Scholar]
- 35.Su J., Li G., Li X., Chen J. 2D/2D heterojunctions for catalysis. Adv. Sci. 2019;6:1801702. doi: 10.1002/advs.201801702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbain F., Du R., Tang P., Smirnov V., Andreu T., Finger F., Divins N.J., Llorca J., Arbiol J., Cabot A. Upscaling high activity oxygen evolution catalysts based on CoFe2O4 nanoparticles supported on nickel foam for power-to-gas electrochemical conversion with energy efficiencies above 80% Appl. Catal. B Environ. 2019;259:118055. doi: 10.1016/j.apcatb.2019.118055. [DOI] [Google Scholar]
- 37.Zuo Y., Liu Y., Li J., Du R., Yu X., Xing C., Zhang T., Yao L., Arbiol J., Llorca J. Solution-processed ultrathin SnS2–Pt nanoplates for photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces. 2019;11:6918–6926. doi: 10.1021/acsami.8b17622. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C., Du R., Biendicho J.J., Yi M., Xiao K., Yang D., Zhang T., Wang X., Arbiol J., Llorca J., et al. Tubular CoFeP@CN as a Mott–Schottky Catalyst with Multiple Adsorption Sites for Robust Lithium−Sulfur Batteries. Adv. Energy Mater. 2021;11:2100432. doi: 10.1002/aenm.202100432. [DOI] [Google Scholar]
- 39.Liu M., Piao L., Lu W., Ju S., Zhao L., Zhou C., Li H., Wang W. Flower-like TiO2 nanostructures with exposed {001} facets: Facile synthesis and enhanced photocatalysis. Nanoscale. 2010;2:1115–1117. doi: 10.1039/c0nr00050g. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Lv K., Ho W., Dong F., Wu X., Xia Y. Hybridization of rutile TiO2 (rTiO2) with g-C3N4 quantum dots (CN QDs): An efficient visible-light-driven Z-scheme hybridized photocatalyst. Appl. Catal. B Environ. 2017;202:611–619. doi: 10.1016/j.apcatb.2016.09.055. [DOI] [Google Scholar]
- 41.Tian N., Zhang Y., Li X., Xiao K., Du X., Dong F., Waterhouse G.I.N., Zhang T., Huang H. Precursor-reforming protocol to 3D mesoporous g-C3N4 established by ultrathin self-doped nanosheets for superior hydrogen evolution. Nano Energy. 2017;38:72–81. doi: 10.1016/j.nanoen.2017.05.038. [DOI] [Google Scholar]
- 42.Tian N., Huang H., Wang S., Zhang T., Du X., Zhang Y. Facet-charge-induced coupling dependent interfacial photocharge separation: A case of BiOI/g-C3N4 pn junction. Appl. Catal. B Environ. 2020;267:118697. doi: 10.1016/j.apcatb.2020.118697. [DOI] [Google Scholar]
- 43.Yu X., Du R., Li B., Zhang Y., Liu H., Qu J., An X. Biomolecule-assisted self-assembly of CdS/MoS2/graphene hollow spheres as high-efficiency photocatalysts for hydrogen evolution without noble metals. Appl. Catal. B Environ. 2016;182:504–512. doi: 10.1016/j.apcatb.2015.09.003. [DOI] [Google Scholar]
- 44.Du R., Zhang Y., Li B., Yu X., Liu H., An X., Qu J. Biomolecule-assisted synthesis of defect-mediated Cd1−xZnxS/MoS2/graphene hollow spheres for highly efficient hydrogen evolution. Phys. Chem. Chem. Phys. 2016;18:16208–16215. doi: 10.1039/C6CP01322H. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y., Liu X., Hou L., Guo X., Fu R., Sun J. Construction of covalent bonding oxygen-doped carbon nitride/graphitic carbon nitride Z-scheme heterojunction for enhanced visible-light-driven H2 evolution. Chem. Eng. J. 2020;383:123132. doi: 10.1016/j.cej.2019.123132. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available on reasonable request from the corresponding authors.