Abstract
In Italy, the West Nile Virus surveillance plan considers a multidisciplinary approach to identify the presence of the virus in the environment (entomological, ornithological, and equine surveillance) and to determine the risk of infections through potentially infected donors (blood and organ donors). The costs associated with the surveillance program for the Lombardy Region between 2014 and 2018 were estimated. The costs of the program were compared with a scenario in which the program was not implemented, requiring individual blood donation nucleic acid amplification tests (NAT) to detect the presence of WNV in human samples throughout the seasonal period of vector presence. Considering the five-year period, the application of the environmental/veterinary surveillance program allowed a reduction in costs incurred in the Lombardy Region of 7.7 million EUR. An integrated surveillance system, including birds, mosquito vectors, and dead-end hosts such as horses and humans, can prevent viral transmission to the human population, as well as anticipate the detection of WNV using NAT in blood and organ donors. The surveillance program within a One Health context has given the possibility to both document the expansion of the endemic area of WNV in northern Italy and avoid most of the NAT-related costs.
Keywords: blood donors, surveillance, mosquitoes, human, horses, NAT
1. Introduction
West Nile Virus (WNV) is a flavivirus transmitted by ornithophilic mosquitoes between avian hosts [1]. Virus amplification within avian and mosquito populations may lead to spillover to incidental mammalian hosts. In particular, humans and horses are considered incidental dead-end hosts for WNV; in fact, they are not part of the virus transmission cycle because they do not produce viremia sufficient to infect mosquitoes [2]. In humans, the WNV infection is mostly asymptomatic, although 20% of cases may develop flu-like symptoms and around 1% may develop neuro-invasive symptoms, potentially lethal for elderly and immunosuppressed individuals [3]. In addition to the direct risk to human health, asymptomatic blood donors represent a recognized problem for the safety of blood transfusions in affected areas [4]. In fact, transmission of the WND infection through blood transfusion as well as organ transplantations has been demonstrated [5,6].
The ecologic aspects of WNV infection were first described in the 1950s in Egypt [7] and it is now considered the most widespread arbovirus in the world [8]. In Europe, the first WNV outbreak in humans was reported in 1962–1963 in France, but the virus has been circulating in Europe since the 1950s, as shown by serological surveys [9]. Annual fluctuations in WNV activity have been assessed by the number of cases reported in European Union/European Economic Area (EU/EEA) countries and EU neighboring countries: 3067 cases (2018), 477 cases (2019), and 322 cases (2020). The 2018 spike in European cases could have been due to the unusually hot summer [10,11].
In addition, the economic impact of WNV infections might be relevant for health services around the world. Two published analyses, related to the context of the United States, reported costs for hospitalization equal to 778 million USD (US Dollar) between 1999 and 2012, with median initial costs of hospitalization (both direct and indirect) per case between 7501 and 82,542 USD, and median long-term costs (both direct and indirect) between 7025 and 76,747 USD among patients with fever, meningitis, encephalitis, and acute flaccid paralysis [12,13].
In Italy, the first outbreak of WNV infection was reported in 1998 in the Tuscany region [14], and then the virus re-emerged after 10 years in August 2008 involving eight provinces in three regions (Emilia Romagna, Veneto, and Lombardy).
Due to the re-emergence and the following geographical spread of WNV, the Directorate General for Prevention of the Italian Ministry of Health (MoH) issued in the spring of 2010 a national plan for WNND human surveillance that integrated human and veterinary surveillance [15].
Since then, WNV has been repeatedly identified through integrated surveillance programs conducted in Italian regions [16], including Lombardy [17] and Emilia Romagna [18]. In addition, human cases of WNND have been reported every year in Italy [19].
The occurrence of the WNV infection cycle is favored by geographic aspects (low altitude, presence of rivers) and climate (high temperature in the warmest month, high annual temperature range) [20]. These characteristics are those commonly present in the northern Italian regions of the Po basin, such as Piedmont, Lombardy, Veneto, and Emilia-Romagna. In these regions, a specific surveillance program has been active for more than 15 years to assess the presence of West Nile Disease infections [21] and consists of two distinct but complementary activities: epidemiological and environmental surveillance [22].
Epidemiological surveillance identifies human cases to quantify the disease burden and to identify seasonal, geographical, and demographic patterns of human morbidity and mortality, while environmental/veterinary surveillance monitors local WNV activity in vectors and nonhuman vertebrate hosts in advance of epidemic activity affecting humans. The aim of the surveillance program is to address and to maximize the response of healthcare providers and professionals via the early detection of the virus in the different epidemiological actors, as well as focus the interventions toward target areas and seasonal periods. The WNV surveillance plan considers a multidisciplinary approach to identify the presence of the virus in the environment (entomological, ornithological, and equine surveillance) and to determine the risk of infections through potentially infected donors (blood and organ donors) [23].
According to the indications coming from the environmental/veterinary surveillance, the analyses conducted to detect the presence of WNV in blood samples for transfusions, as well as all activities related to organ donations, are conducted starting from the date of the first positive sample identified in mosquitoes, birds, or horses, instead of being performed for a fixed period from the beginning of June to the end of November. Such an integrated and multidisciplinary WNV surveillance system, which includes, in addition to humans, wild birds, mosquitoes and horses, has been implemented in Lombardy Region since 2014. The main aims of this surveillance system are (1) early detection of the circulation of WNV in the environment, and (2) mitigation of the risk of viral transmission through blood and organ donations [24]. The detection of WNV in one of the target species of the surveillance system in a given province has led to the start of a systematic individual blood donation nucleic acid amplification testing (NAT) program in that province until the end of the annual transmission season (end of November), as required by the European regulation [25].
This One Health approach is based on the collaboration of different public institutions, in a network that connects humans, animals, and environmental health, under the coordination of the working group of the Regional Health and Welfare Unit.
There have been few studies establishing the economic efficiency of One Health approaches to disease mitigation [26]. However, such information is critical for the development of cost-effective control of zoonoses, including WNV. Therefore, the objective of this study was to assess the costs related to WNV surveillance in Lombardy Region between 2014 and 2018 and to estimate the costs avoided, particularly related to NAT not performed, thanks to the environmental/veterinary surveillance, by adopting the point of view of the Regional Health and Welfare Unit.
2. Materials and Methods
The analysis was conducted considering the costs associated with the environmental/veterinary surveillance program, the direct medical costs associated with the screening activity for blood donors and organ donations, and the costs associated with the management of WNV infections including hospitalization.
Furthermore, the costs of the program were compared with a scenario in which the program was not implemented, requiring only NAT analyses to detect the presence of WNV in human samples throughout the possible transmission period. The costs considered in the analysis are referred to each reference year.
2.1. Environmental and Veterinary Surveillance
With the objective of assessing the presence in the environment of WNV between May and October, veterinary surveillance was conducted in three areas of activity, targeting different species: Culex mosquitoes (entomological surveillance), birds (ornithological surveillance), and horses (equine surveillance).
Entomological surveillance was performed through fortnightly sampling with attraction traps, between May and October. The number of traps progressively increased from 38 at the beginning (2014) to 44 in total in 2020. They were placed in areas suitable for circulation of the virus vector [27] and distributed regularly in the region so as to cover the whole territory divided into 20 × 20 km2 areas.
Ornithological surveillance was performed through active surveillance (capture and killing of resident “reservoir” birds of selected species) and through passive surveillance (identifying episodes of abnormal wildlife mortality or analyzing animal death in wildlife refuge center). Carcasses of magpie (Pica pica), hooded crow (Corvus corone cornix), and Eurasian jays (Garrulus glandarius) were collected and delivered to the laboratory by rangers and hunters. These species are considered pests for crops and, therefore, are under population control programs, authorized yearly by the National Institute for Wildlife (ISPRA). The sampling from passive surveillance mainly involved birds belonging to the orders Accipitriformes, Charadiiformes, Columbiformes, Falconiformes, and Passeriformes.
Equine surveillance was performed through clinical assessment of neurologic symptoms in horses.
Table 1 provides summarized information of all cost items considered in the analysis.
Table 1.
Cost items included for the estimation of costs of the West Nile Virus (WNV) integrated surveillance system in Lombardy, 2014–2018.
| Item | Description | Details |
|---|---|---|
| Human surveillance |
|
Personnel cost not included for laboratory analysis and included for hospitalizations |
| Entomological surveillance |
|
Personnel cost included |
| Ornithological surveillance |
|
Personnel cost included |
| Equinesurveillance |
|
Personnel costnot included |
| Blood testing |
|
Personnel cost included |
To determine the costs related to the three types of environmental/veterinary surveillance (entomological, ornithological, and equine surveillance), a process analysis was performed identifying all the resources involved in the different actions, in terms of human resources, equipment, and consumables for both on-field and laboratory activities (Table 2).
Table 2.
Annual number of specimens and unit cost per analysis (including equipment and human resources for both collection of specimens and laboratory activities).
| Year | Entomological Surveillance | Ornithological Surveillance | Equine Surveillance | |||
|---|---|---|---|---|---|---|
| N° Samples | Unit Cost (EUR) | N° Samples | Unit Cost (EUR) | N° Samples | Unit Cost (EUR) | |
| 2014 | 1824 | 35.57 | 2638 | 10.67 | 898 | 3.20 |
| 2015 | 1817 | 35.15 | 2632 | 12.95 | 647 | 3.98 |
| 2016 | 2276 | 34.81 | 2962 | 12.27 | 760 | 2.96 |
| 2017 | 1992 | 34.21 | 2752 | 12.47 | 426 | 5.90 |
| 2018 | 2358 | 34.97 | 2082 | 17.13 | 1333 | 4.21 |
2.2. Blood Transfusion Costs
The screening of blood for transfusion has been one of the most important aspects of the WNV surveillance plan, since 2002, when the first WNV infection due to blood transfusion was detected in the United States [28,29].
Italian national guidelines mandate the screening of blood units between 1 June and 30 November for all blood donations in the provinces in which WNV was detected the previous year [30]. For all other provinces, the controls are activated only after the detection of a WNV-positive specimen (either human or animal—mosquitoes, birds, horses) in the laboratory test with confirmation through NAT [28].
Table 3 reports the number of blood donations tested yearly in Lombardy Region by the regional blood transfusion establishment during the period considered. The NAT cost was derived from the Lombardy Region equivalent, equal to 15.00 EUR [31]
Table 3.
Annual blood donations and NAT performed in Lombardy between 2014 and 2018.
| Year | Annual Number of Blood Donations | Number of Tests Performed |
|---|---|---|
| 2014 | 236,582 | 135,801 |
| 2015 | 235,991 | 168,942 |
| 2016 | 235,991 | 107,687 |
| 2017 | 227,192 | 117,812 |
| 2018 | 268,120 | 161,817 |
| Total | 1,203,976 | 692,059 |
2.3. Costs of West Nile Virus Infection in Human Cases
This analysis also considered direct medical costs related to human WNV infection cases confirmed by the Italian National Institute of Health (Istituto Superiore di Sanità—ISS) assuming the Regional Health Service perspective, in terms of inpatient activity and diagnostic activity. Costs were derived from a previously published analysis related to the Italian context [28]. A mean number of 21.3 inpatient days was considered per patient, as reported in the literature [28]. With a conservative approach, the costs considered excluded those related to intensive care units, due to a lack of this information. In Table 4, we report the numbers of annual confirmed human infections and inpatients and horses’ infection cases. The WNV infection in horses, as in humans, can involve neurologic disease and they are incidental hosts to WNV; therefore, they may share common epidemiological aspects regarding disease occurrence and spread. [32].
Table 4.
Confirmed cases of human and horse West Nile Virus infection.
| Year | Confirmed Cases of Human WNV Infection |
Confirmed Cases of Horses WNV Infection |
|---|---|---|
| 2014 | 13 | 11 |
| 2015 | 2 | 5 |
| 2016 | 15 | 4 |
| 2017 | 2 | 0 |
| 2018 | 23 | 10 |
3. Results
The multidisciplinary approach to WNV surveillance made it possible to identify the viral circulation in the study area early. In Table 5 we report the date on which the first positive case was reported by year and by type of surveillance of WNV, together with the date of the first human case of WNV infection detected in each year.
Table 5.
First positive cases of West Nile Virus detected by veterinary and human surveillance between 2014 and 2018.
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| Entomological surveillance | 16 July | 7 July | 5 July | 12 July | 3 July |
| Ornithological surveillance | 21 July | 20 May | 18 August | 5 August | 6 July |
| Equine surveillance | 18 July | 6 August | 5 August | 5 August | 31 July |
| Donor checking | 12 November | 6 July | 9 August | / | |
| First human WNV infection case diagnosed | 13 August | 28 July | 1–7 August | 1–7 August | 1–7 August |
The total cost of the surveillance activity for a five-year period was equal to 542,935 EUR. Costs were mainly related to entomological surveillance (358,574 EUR), followed by ornithological surveillance (168,535 EUR). Total NAT costs were equal to 10.38 million EUR, while the costs to manage WNV-related infections in terms of diagnostic activity and hospitalization were equal to 705,107 EUR. The costs per year for each activity are reported in Table 6, Figure 1 and Figure 2.
Table 6.
Costs related to surveillance activity, NATs, and human infection management.
| 2014 | 2015 | 2016 | 2017 | 2018 | Total | |
|---|---|---|---|---|---|---|
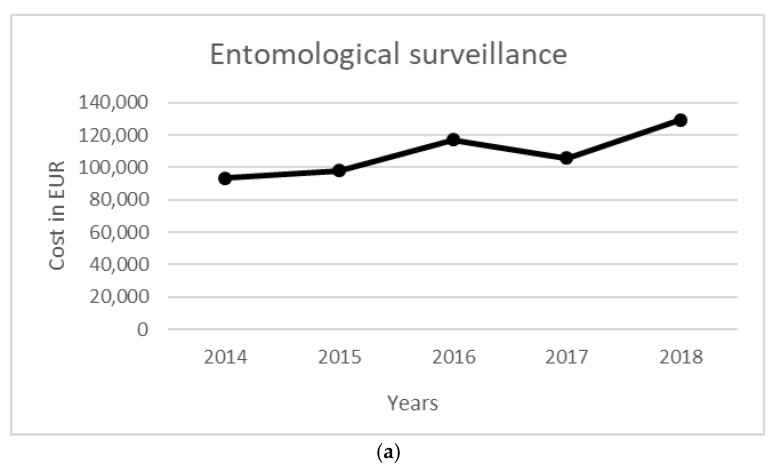
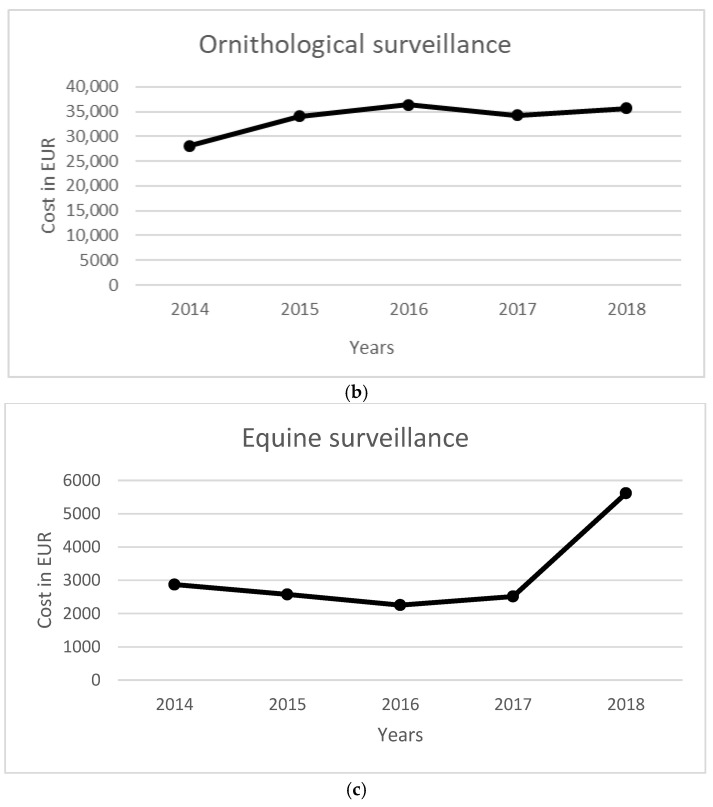
| Entomological surveillance (EUR) | 64,877 | 63,869 | 79,225 | 68,140 | 82,463 | 358,574 |
| Ornithological surveillance (EUR) | 28,150 | 34,077 | 36,343 | 34,304 | 35,661 | 168,535 |
| Equine surveillance (EUR) | 2872 | 2574 | 2253 | 2513 | 5614 | 15,826 |
| Total surveillance costs (EUR) | 95,899 | 100,520 | 117,821 | 104,957 | 123,739 | 542,935 |
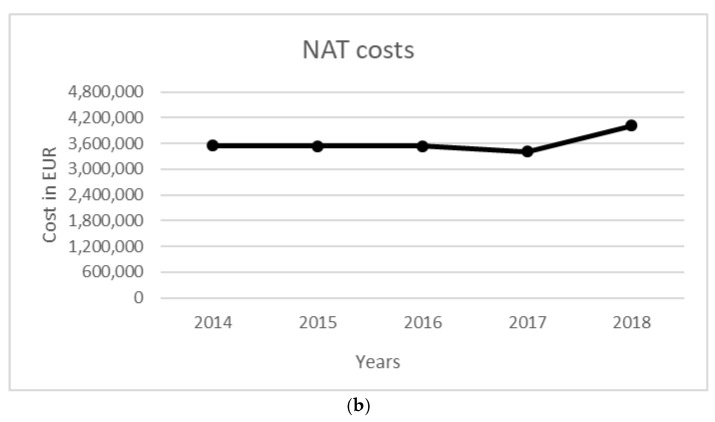
| Total NAT costs (EUR) | 2,037,015 | 2,534,130 | 1,615,305 | 1,767,180 | 2,427,255 | 10,380,885 |
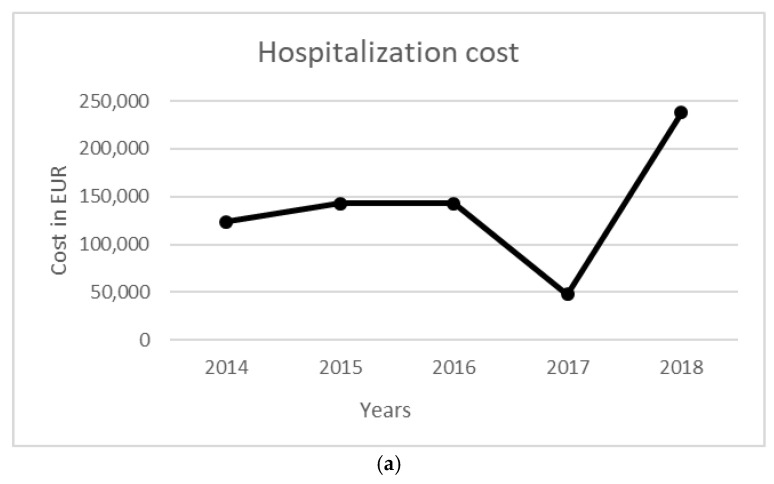
| Infection inpatient management costs (EUR) | 124,605 | 19,170 | 143,775 | 47,925 | 230,040 | 565,515 |
| Infection diagnostic costs (EUR) | 962 | 148 | 1110 | 370 | 1776 | 4366 |
| Total costs related to WNV and Usutu infections (EUR) | 125,567 | 19,315 | 144,885 | 48,295 | 231,816 | 569,881 |
| Total annual costs (EUR) | 2,258,481 | 2,653,968 | 1,878,011 | 1,920,432 | 2,782,809 | 11,493,701 |
Figure 1.
Annual costs per environmental/veterinary surveillance. (a) Entomological, (b) ornithological, and (c) equine surveillance.
Figure 2.
Annual costs per type of human surveillance (hospitalization (a) and NAT test (b)).
Considering the number of NATs for checking blood donations, performed starting from the first positive specimen detected by environmental/veterinary surveillance, mean annual avoided costs of 1,535,451 EUR were estimated due to delayed testing on blood units, compared with the scenario in which NATs were performed throughout the vectorial season, i.e., from 1 June to 30 November, as reported in Table 7. The higher estimated savings were related to the years 2016, 2017, and 2018, due to the higher annual number of samples tested as a result of a higher viral circulation.
Table 7.
Costs related to surveillance activity NATs.
| Year | Differential Number of NATs * | Differential Costs for NATs (EUR) |
|---|---|---|
| 2014 | 100,781 | 1,511,715 |
| 2015 | 67,049 | 1,005,735 |
| 2016 | 128,304 | 1,924,560 |
| 2017 | 109,380 | 1,640,700 |
| 2018 | 106,303 | 1,594,545 |
| Total | 511,817 | 7,677,255 |
* Cost difference between the surveillance program scenario and the scenario where NATs were performed for each case throughout the year.
Overall, considering the five-year period, application of the environmental/veterinary surveillance program allowed a reduction of approximately 7.7 million EUR in costs incurred in Lombardy Region.
4. Discussion
Application of the above-described surveillance system allowed the Regional Health and Welfare Unit of Lombardy Region to avoid costs representing more than 40% of the WNV monitoring plan costs. This was due to the possibility to postpone the beginning of the blood samples tests until the first positive specimen detected by environmental/veterinary surveillance.
Moreover, the sharing of information obtained from epidemiological and environmental/veterinary surveillance has enabled controlling the risk of WNV transmission via blood transfusion.
In accordance with White (2001) and Healy et al., (2015) [33,34], the surveillance system adopted was useful in providing important information on viral circulation in the reservoirs, as well as in vectors and the host population. In fact, the results collected from birds and mosquitoes informed the control bodies on the extent of circulation of the virus. Among vertebrate hosts, horses are particularly sensitive to WNV; thus, the detection of infection/disease in such species is highly pertinent and informative from a public health perspective and also gives an estimation of the population of infected vectors [35,36].
Our results also showed that viral activity was generally detected earlier in mosquitoes and birds than in humans [37]. In fact, in the observation period from 2014 to 2018, entomological surveillance revealed the presence of WNV on average 1 month in advance when compared with the detection of the first WNV clinical cases in humans or the detection of positivity in donors, except for 2015, when the first positive case was detected much earlier, i.e., on 21 May in a bird (as reported in Table 5) [38]. The proximity of the dates of the first positive specimen related to the entomological, ornithological, and equine surveillance in 2014 and 2018 may have been due to a higher virus circulation leading to a higher number of WNV infections and human clinical cases.
It should be noted that the cost of the personnel involved in the human and equine surveillance is not included for laboratory tests, since data available does not allow to isolate the costs of the tests related to WNV. This is due to the fact that the costs available for personnel (payroll) include a sum of activities performed as part of the normal routine activities expected of the specific role (physician or veterinary). Thus, it is not possible to identify the costs related dedicated to the sole aforementioned activities which are part of the surveillance systems.
In this context of West Nile Disease viral circulation control, savings in terms of resources were obtained by not activating NAT screening on blood transfusion or organ donors until circulation of the arbovirus was confirmed at the local (provincial) level. Indeed, studies, in some published cases [24], have demonstrated the sustainability of the system with variable deficit margins.
The costs associated with the management of infected patients vary widely depending on the clinical manifestation of the infection and on the course of the disease, being related to inpatient activity and considering severe cases, long inpatient days, and intensive care unit management.
In Italy, a cost–benefit analysis related to the implementation of the WNV surveillance program in Emilia-Romagna Region estimated 1.21 million EUR of costs saved by avoiding tests for blood donations and up to 2.98 million EUR of costs saved by avoiding hospitalization-related infections between 2009 and 2015 [25]. In this study, Paternoster and colleagues (2017) [25] considered further costs and epidemiological elements when compared to the analysis here presented, such as the lack of transmissions by transfusions and the related costs. Moreover, an additional constraint to the comparison between the results of the two analyses is related to relevant differences in the organization of the surveillance activities in terms of personnel involved in the management of traps. In fact, while in Emilia-Romagna this activity is managed by personnel specifically recruited, in Lombardy Region, it is performed by personnel already in charge at the Veterinary Services.
5. Conclusions
An integrated surveillance system, including birds as virus reservoirs, mosquito vectors, and dead-end hosts such as horses and humans, was used to identify the location and timing of viral activity in the Lombardy Region. The information gained through surveillance was used to prevent viral transmission to the human population, as well as to anticipate the detection of WNV in blood and organ donors using NAT [39,40]. At the same time, this plan allowed for the collection of data that could improve understanding of the epidemiology of WNV infection and identify possible risk factors related to human infections.
The above-described surveillance program highlighted the presence of WNV in Lombardy every year in the period 2014–2018, albeit with different intensities, and it allowed the possibility to both document the expansion of the endemic area of WNV in northern Italy and guarantee an avoidance of NAT-related costs. The latter was estimated to be 7.7 million EUR in the five-year period, with mean yearly avoided costs of 1.5 million EUR.
Acknowledgments
The authors would like to acknowledge all personnel supporting the activities of the integrated surveillance system.
Author Contributions
Conceptualization, F.D., D.L., M.C., M.F., U.R. and A.L.; methodology, F.D., S.C. and F.F.; data curation, F.D., M.P.C., M.D., A.L., D.C., R.C., A.M., E.S. and T.T.; writing—original draft preparation, F.D.; writing—review and editing, A.L. and U.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kramer L.D., Li J., Shi P.Y. West Nile virus. Lancet Neurol. 2007;6:171–181. doi: 10.1016/S1474-4422(07)70030-3. [DOI] [PubMed] [Google Scholar]
- 2.Taieb L., Ludwig A., Ogden N.H., Lindsay R.L., Iranpour M., Gagnon C.A., Bicout D.J. Bird species involved in West Nile virus epidemiological cycle in Southern Quebec. Int. J. Environ. Res. Public Health. 2020;17:4517. doi: 10.3390/ijerph17124517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am. J. Trop. Med. 1940;20:471–492. doi: 10.4269/ajtmh.1940.s1-20.471. [DOI] [Google Scholar]
- 4.Korves C.T., Goldie S.J., Murray M.B. Cost-Effectiveness of Alternative Blood-Screening Strategies for West Nile Virus in the United States. PLoS Med. 2006;3:e21. doi: 10.1371/journal.pmed.0030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto M., Jernigan D.B., Guasch A., Trepka M.J., Blackmore C.G., Hellinger W.C., Pham S.M., Zaki S., Lanciotti R.S., Lance-Parker S.E., et al. Transmission of West Nile Virus from an Organ Donor to Four Transplant Recipients. N. Engl. J. Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery S.P., Brown J.A., Kuehnert M., Smith T.L., Crall N., Lanciotti R.S., de Oliveira A.M., Boo T., Marfin A.A. Transfusion-associated transmission of West Nile virus, United States 2003 through 2005. Transfusion. 2006;46:2038–2046. doi: 10.1111/j.1537-2995.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 7.Weaver S.C., Reisen W.K. Present and future arboviral threats. Antivir. Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autorino G.L., Battisti A., Deubel V., Ferrari G., Forletta R., Giovannini A., Lelli R., Murri S., Scicluna M.T. West Nile virus Epidemic in Horses, Tuscany Region, Italy. Emerg. Infect. Dis. 2002;8:1372–1378. doi: 10.3201/eid0812.020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt E. West Nile virus spreads in Europe. Lancet Infect. Dis. 2018;18:1184. doi: 10.1016/S1473-3099(18)30616-9. [DOI] [PubMed] [Google Scholar]
- 10.Bakonyi T., Haussig J.M. West Nile virus keeps on moving up in Europe. Eurosurveillance. 2020;25:2001938. doi: 10.2807/1560-7917.ES.2020.25.46.2001938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burki T. Increase of West Nile virus cases in Europe for 2018. Lancet. 2018;392:1000. doi: 10.1016/S0140-6736(18)32286-4. [DOI] [PubMed] [Google Scholar]
- 12.Cantile C., Di Guardo G., Eleni C., Arispici M. Clinical and neuropathological features of West Nile virus equine encephalomyelitis in Italy. Equine Vet. J. 2000;32:31–35. doi: 10.2746/042516400777612080. [DOI] [PubMed] [Google Scholar]
- 13.García-Carrasco J.-M., Muñoz A.-R., Olivero J., Segura M., Real R. Predicting the spatio-temporal spread of West Nile virus in Europe. PLoS Negl. Trop. Dis. 2021;15:e0009022. doi: 10.1371/journal.pntd.0009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett A.D.T. Economic Burden of West Nile Virus in the United States. Am. J. Trop. Med. Hyg. 2014;90:389–390. doi: 10.4269/ajtmh.14-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Italian Ministry of Health . West Nile Disease Surveillance in Italy. Ministry of Health; Rome, Italy: 2010. [(accessed on 24 April 2022)]. Available online: http://www.normativasanitaria.it/normsan-pdf/0000/349231.pdf. (In Italian) [Google Scholar]
- 16.Rizzo C., Napoli C., Venturi G., Pupella S., Lombardini L., Calistri P., Monaco F., Cagarelli R., Angelini P., Bellini R., et al. West Nile virus transmission: Results from the integrated surveillance system in Italy, 2008 to 2015. Eurosurveillance. 2016;21:30340. doi: 10.2807/1560-7917.ES.2016.21.37.30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiari M., Prosperi A., Faccin F., Avisani D., Cerioli M., Zanoni M., Bertoletti M., Moreno A.M., Bruno R., Monaco F., et al. West Nile Virus Surveillance in the Lombardy Region, Northern Italy. Transbound. Emerg. Dis. 2015;62:343–349. doi: 10.1111/tbed.12375. [DOI] [PubMed] [Google Scholar]
- 18.Calzolari M., Angelini P., Bolzoni L., Bonilauri P., Cagarelli R., Canziani S., Cereda D., Cerioli M.P., Chiari M., Galletti G., et al. Enhanced West Nile Virus Circulation in the Emilia-Romagna and Lombardy Regions (Northern Italy) in 2018 Detected by Entomological Surveillance. Front. Vet. Sci. 2020;7:243. doi: 10.3389/fvets.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riccò M., Peruzzi S., Balzarini F. Epidemiology of West Nile Virus Infections in Humans, Italy, 2012–2020: A Summary of Available Evidences. Trop. Med. Infect. Dis. 2021;6:61. doi: 10.3390/tropicalmed6020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staples J.E., Shankar M.B., Fischer M., Meltzer M.I., Sejvar J.J. Initial and Long-Term Costs of Patients Hospitalized with West Nile Virus Disease. Am. J. Trop. Med. Hyg. 2014;90:402–409. doi: 10.4269/ajtmh.13-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancini G., Montarsi F., Calzolari M., Capelli G., Dottori M., Ravagnan S., Lelli D., Chiari M., Santilli A., Quaglia M., et al. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet. Ital. 2017;53:97–110. doi: 10.12834/VetIt.114.933.4764.2. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . West Nile Virus in the United States: Guidelines for Surveillance, Prevention, and Control. 4th ed. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases; Fort Collins, CO, USA: 2013. [(accessed on 20 November 2013)]. p. 69. Available online: http://www.cdc.gov/westnile/resources/pdfs/wnvguidelines.pdf. [Google Scholar]
- 23.Commission Directive 2014/110/EU of 17 December 2014 Amending Directive 2004/33/EC as Regards Temporary Deferral Criteria for Donors of Allogeneic Blood Donations. Official Journal of the European Union; Luxembourg: 2014. [Google Scholar]
- 24.Bellini R., Calzolari M., Mattivi A., Tamba M., Angelini P., Bonilauri P., Albieri A., Cagarelli R., Carrieri M., Dottori M., et al. The experience of West Nile virus integrated surveillance system in the Emilia-Romagna region: Five years of implementation, Italy, 2009 to 2013. Eurosurveillance. 2014;19:20953. doi: 10.2807/1560-7917.ES2014.19.44.20953. [DOI] [PubMed] [Google Scholar]
- 25.Paternoster G., Martins S.B., Mattivi A., Cagarelli R., Angelini P., Bellini R., Santi A., Galletti G., Pupella S., Marano G., et al. Economics of One Health: Costs and benefits of integrated West Nile virus surveillance in Emilia-Romagna. PLoS ONE. 2017;12:e0188156. doi: 10.1371/journal.pone.0188156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tisdell C. Economics of Controlling Livestock Diseases: Basic Theory. In: Rushton J., editor. Economics of Animal Health & Production. CABI; Wallingford, UK: 2009. pp. 46–49. [Google Scholar]
- 27.America’s Blood Centers . ABC Newsletter. America’s Blood Centers; Washington, DC, USA: 2003. Blood Centers Begin Implementing WNV Donor Screening Tests; pp. 1–3. [Google Scholar]
- 28.Pealer L.N., Marfin A.A., Petersen L.R., Lanciotti R.S., Page P.L., Stramer S.L., Stobierski M.G., Signs K., Newman B., Kapoor H., et al. Transmission of West Nile Virus through Blood Transfusion in the United States in 2002. N. Engl. J. Med. 2003;349:1236–1245. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 29.National Blood Center Guideline 2015. Indications for the Surveillance and Prevention of the Transmission of West Nile Virus (WNV) through the Transfusion of Labile Blood Components during the Summer Autumn 2015 Season. [(accessed on 25 January 2022)]. Available online: https://www.centronazionalesangue.it/wp-content/uploads/2017/07/Linee-Guida-per-il-Programma-di-Patient-Blood-Management_0.pdf.
- 30.Lombardy Region DGR N° X/7600 del 20/12/2017 “Determinazioni in Ordine Alla Gestione del Servizio Sociosanitario per l’esercizio 2018 (di concerto con gli Assessori Garavaglia e Brianza)”. [(accessed on 10 January 2022)]. Available online: https://allegati.aism.it/manager/UploadFile/2/20180102_221.pdf.
- 31.Aharonson-Raz K., Lichter-Peled A., Tal S., Gelman B., Cohen D., Klement E., Steinman A. Spatial and Temporal Distribution of West Nile Virus in Horses in Israel (1997–2013)—From Endemic to Epidemics. PLoS ONE. 2014;9:e113149. doi: 10.1371/journal.pone.0113149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White D.J. Vector surveillance for West Nile virus. Ann. N. Y. Acad. Sci. 2001;951:74–83. doi: 10.1111/j.1749-6632.2001.tb02686.x. [DOI] [PubMed] [Google Scholar]
- 33.Healy J.M., Reisen W.K., Kramer V.L., Fischer M., Lindsey N.P., Nasci R.S., Macedo P.A., White G., Takahashi R., Khang L., et al. Comparison of the Efficiency and Cost of West Nile Virus Surveillance Methods in California. Vector-Borne Zoonotic Dis. 2015;15:147–155. doi: 10.1089/vbz.2014.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen L.R., Roehrig J.T. West Nile Virus: A Reemerging Global Pathogen. Emerg. Infect. Dis. 2001;7:611–614. doi: 10.3201/eid0704.017401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leblond A., Hendrikx P., Sabatier P. West Nile Virus Outbreak Detection Using Syndromic Monitoring in Horses. Vector-Borne Zoonotic Dis. 2007;7:403–410. doi: 10.1089/vbz.2006.0593. [DOI] [PubMed] [Google Scholar]
- 36.Calzolari M., Gaibani P., Bellini R., Defilippo F., Pierro A., Albieri A., Maioli G., Luppi A., Rossini G., Balzani A., et al. Mosquito, Bird and Human Surveillance of West Nile and Usutu Viruses in Emilia-Romagna Region (Italy) in 2010. PLoS ONE. 2012;7:e38058. doi: 10.1371/journal.pone.0038058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Report West Nile Disease (WND) Year 2015. DG Welfare-Lombardy Region, IZLER and Regional Blood Center. Lombardy Region. 2016. [(accessed on 1 March 2022)]. Available online: https://www.regione.lombardia.it/wps/wcm/connect/32f70896-0d84-4b85-88b2-ed726cc5fa84/report_WND_2015.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-32f70896-0d84-4b85-88b2-ed726cc5fa84-lGjaM5.
- 38.Eldridge B.F. Strategies for Surveillance, Prevention, and Control of Arbovirus Diseases in Western North America. Am. J. Trop. Med. Hyg. 1987;37:77S–86S. doi: 10.4269/ajtmh.1987.37.77S. [DOI] [PubMed] [Google Scholar]
- 39.Gubler D.J., Campbell G.L., Nasci R., Komar N., Petersen L., Roehrig J. West Nile Virus in the United States: Guidelines for Detection, Prevention, and Control. Viral Immunol. 2000;13:469–475. doi: 10.1089/vim.2000.13.469. [DOI] [PubMed] [Google Scholar]
- 40.Petersen L.R. Epidemiology of West Nile Virus in the United States: Implications for Arbovirology and Public Health. J. Med. Èntomol. 2019;56:1456–1462. doi: 10.1093/jme/tjz085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.