Abstract
Cadmium (Cd) is a common environmental heavy metal contaminant of reproduction toxicity. Cd accumulation in animals leads to the damage of granulosa cells. However, its mechanism needs to be elucidated. This research found that treating granulosa cells with Cd resulted in reduced cell viability. The flow cytometry results showed that Cd increased the degree of apoptosis and level of superoxide anion (O2−) in granulosa cells. Further analysis showed that Cd treatment resulted in reduced expression levels of nuclear factor erythroid 2-related factor-2 (Nrf2), superoxide dismutase (SOD), catalase (CAT) and NAD(P)H: quinone oxidoreductase 1 (NQO1), and an increased expression level of malondialdehyde (MDA); the expression levels of Bcl-2 associated X (Bax) and caspase-3 increased, whereas that of B-cell lymphoma 2 (Bcl-2) decreased. Changes in m6A methylation-related enzymes were noted with Cd-induced damage to granulosa cells. The results of transcriptome and MeRIP sequencing revealed that the AKT pathway participated in Cd-induced damage in granulosa cells, and the MAX network transcriptional repressor (MNT) may be a potential target gene of fat mass and obesity-associated protein (FTO). FTO and YTH domain family member 2 (YTHDF2) regulated MNT expression through m6A modification. FTO overexpression alleviated Cd-induced apoptosis and oxidative stress through the activation of the AKT/Nrf2 pathway; this process could be reversed using siMNT. Overall, these findings associated m6A with Cd-induced damage to granulosa cells and provided insights into Cd-induced granulosa cell cytotoxicity from a new perspective centered on m6A modification.
Keywords: m6A, FTO, CdCl2, granulosa cells, apoptosis
1. Introduction
The development and maturation of oocytes determine the reproductive ability of female animals. Granulosa cells supply necessary nutrition and small molecules for the oocytes through gap junctions, which is crucial for oocyte maturation [1]. With the acceleration of global industrialization, Cd pollution has gradually spread worldwide. Pollution due to the heavy metal cadmium is particularly severe in developing countries. Several studies have focused on cadmium pollution; some of these studies have explored the alterations in the aquatic environment in Malaysia, agricultural soil in China, and water and food qualities in northern Vietnam with higher cadmium concentrations [2,3,4]. Various industrial activities lead to heavy metal-induced environmental pollution, including water, soil, and plant pollution; the heavy metals in soil and animal feed may accumulate into animal yields. The cadmium content in milk in industrial areas is significantly higher than that in unpolluted areas; the cadmium in milk may come from the soil in the following manner: from soil to silage to milk [5]. A significantly positive correlation has been reported between Cd concentration in cattle tissues and their feed [6]. Cadmium can accumulate in animal tissues, including ovaries, and endanger reproductive functions [7,8,9,10]. Studies have shown that granulosa cells may be one of the targets of Cd toxicity. Cd treatment can affect progesterone secretion in granulosa cells [11], thus increasing follicular atresia and apoptosis [12]. The use of cadmium chloride (CdCl2) to treat porcine granulosa cells demonstrated that Cd can increase reactive oxygen species (ROS) levels, and the degree of apoptosis increases in a dose- and time-dependent manner [13].
The Myc/Max/Mad network is involved in the regulation of various biological processes, including cell proliferation and apoptosis. Myc/Max and Mad/Max dimers play transcriptional activation and transcriptional repression effects, respectively, by binding to the E-box region of target genes. MNT is the most special member of the Mad family because it is the largest protein in the family and is widely expressed in human tissues, participating in a variety of biological processes [14]. MNT has an important role in development, and the deletion of MNT causes the death of mice shortly after birth [15,16]. MNT shares a basic helix-loop-helix-leucine zipper (bHLHLZ) domain and a SIN3-interacting domain (SID). bHLHLZ is used to interact with Max and bind the E-box of DNA, while SID is required for the inhibitory function of MNT [17,18]. Myc can induce apoptosis by inhibiting the expression of anti-apoptotic genes Bcl-X and Bcl-2. Mad/Max can compete with Myc/Max to bind to E-box and regulate the expression of target genes [19].
N6-methyladenosine (m6A) modification is widely present in various tissues and organs of animals and results in dynamic and reversible changes in various tissues across the developmental stages [20,21]. It mainly occurs in the consensus sequence 5’-RRACH-3’ (R = A or G, G > A; H = A, C, or U, U > A > C) and is highly conserved in several species [22,23,24,25]. The research map shows that m6A mainly occurs in the vicinity of stop codons and 3ʹ untranslated regions (3ʹUTRs) [26]. The m6A is influenced by multiple effectors, including the writer, eraser, and reader. The “writer”, as methyltransferases, mainly include methyltransferase-like protein 3 (METTL3), methyltransferase-like protein 14 (METTL14), and Wilms tumor 1-associating protein (WTAP). The “erasers”, as demethylases, mainly include AlkB Homolog 5 (ALKBH5) and fat mass and obesity-associated protein (FTO) [27]. The “readers”, as binding proteins, mainly include YTH domain-containing protein 1−2 (YTHDC1-2) and YTH domain family member 1−3 (YTHDF1-3). The reader can recognize m6A modifications and regulate the translation, shearing, transportation, positioning, and stability of RNA at the posttranscriptional level to ensure that m6A can perform its biological functions [26,28].
As a demethylase, FTO can reduce the m6A modification level of target genes. FTO is involved in a variety of biological processes and cell death. Overexpression of FTO inhibited the expression of pro-apoptotic proteins caspase-3, caspase-9 and Bax in vivo and in vitro experiments, thereby inhibiting mitochondria-dependent apoptosis in adipocytes [29]. FTO functions by recognizing m6A modification, and the inhibition of the FTO-induced m6A modification level may be related to MEHP-induced Leydig cell apoptosis [30]. FTO is involved in cell death, induced by a variety of toxicants. Cobalt is an environmental toxicant that can damage human health. CoCl2 induces the production of ROS in human neuroglioma H4 cells and affects the m6A modification level of apoptosis-related genes by reducing the expression of FTO, resulting in the activation of apoptosis [31]. It has been shown that the demethylase FTO can decrease the level of m6A on flotillin 2 (FLOT2) mRNA, increase the stability of FLOT2 mRNA, and induce the dysfunction of granulosa cells [32]. In human granulosa cells, FTO knockdown decelerates the decomposition of FOS-mRNA and upregulates FOS levels, ultimately leading to GC-mediated ovarian aging [33]. Several studies have reported that m6A methylation-related enzymes affect apoptosis in various cells, including those of the ovaries and testes [34,35,36].
However, the role of RNA m6A modification in Cd-induced apoptosis and oxidative stress in bovine ovarian granulosa cells remains to be investigated. Therefore, this study investigated how the m6A protects granulosa cells from Cd-induced apoptosis.
2. Results
2.1. Cd Increased the Degree of Apoptosis in Granulosa Cells
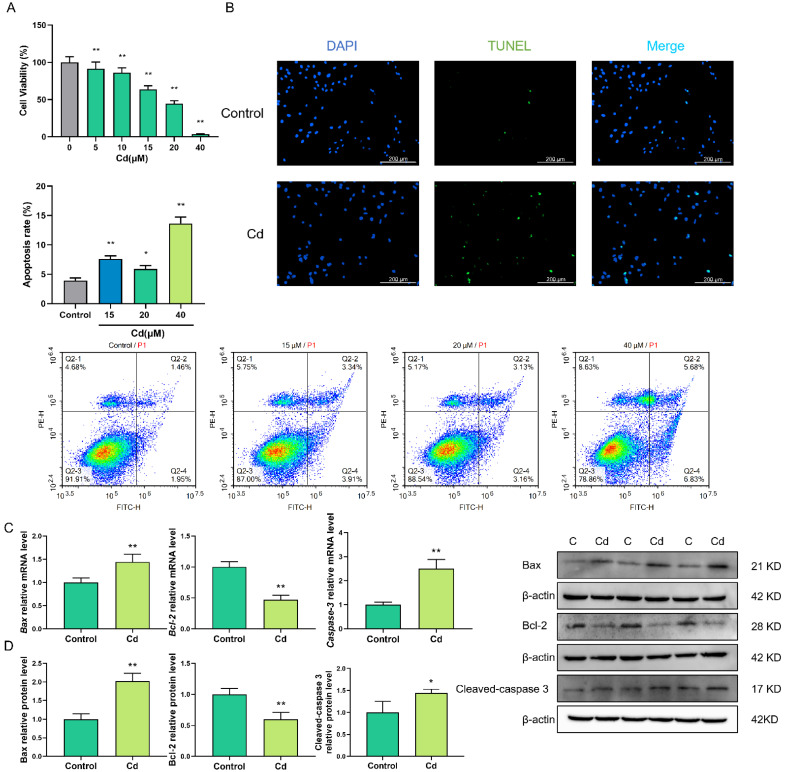
The viability of granulosa cells treated with Cd was assessed by CCK-8 (Figure 1A). Compared with the control group, 5 μM of Cd significantly reduced cell viability (p < 0.01), and cell viability decreased with an increasing Cd concentration. Flow cytometry analysis results indicated that 15−40 μM of Cd significantly increased the degree of apoptosis of the granulosa cells (p < 0.05; Figure 1B). A concentration of 15 μM was used as the subsequent treatment concentration. The TUNEL results indicated that the proportion of TUNEL-positive cells increased markedly after Cd treatment (Figure 1B). Subsequently, RT-qPCR and a Western assay indicated that Bax and caspase-3 expressions were significantly increased and Bcl-2 expression was significantly reduced after Cd treatment (p < 0.05; Figure 1C,D). In conclusion, Cd induces apoptosis in granulosa cells.
Figure 1.
Cd increased the degree of apoptosis in granulosa cells. (A) Analysis of the viability of cells exposed to Cd using CCK-8. (B) Flow cytometry analysis of the degree of apoptosis in the control and Cd groups. The TUNEL assay detected apoptotic cells in the control and Cd groups. Green fluorescence represents apoptotic cells. (C) RT-qPCR results revealed the transcript levels of these genes after Cd treatment. (D) Protein levels after Cd treatment were analyzed by Western blotting. * p < 0.05; ** p < 0.01.
2.2. Cd-Induced Oxidative Stress in Granulosa Cells
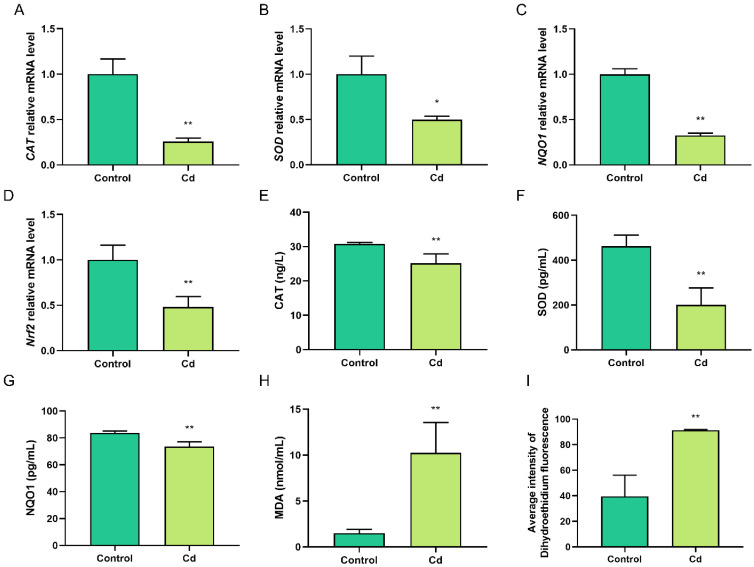
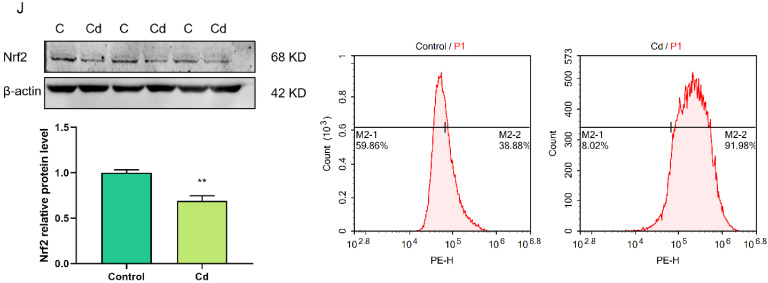
CAT, SOD, and NQO1 are the key components of the antioxidant gene, and the RT-qPCR analysis showed that the transcriptional levels of CAT, SOD, and NQO1 decreased following Cd treatment (p < 0.05; Figure 2A–C). In addition, the intracellular CAT, SOD, and NQO1 levels decreased significantly, which was accompanied by an increase in the MDA levels (p < 0.01; Figure 2E–H). Intracellular reactive oxygen species (ROS) was measured using flow cytometry analysis (DHE), and fluorescence intensity increased in the Cd group (p < 0.01; Figure 2I). Further testing found that the protein and mRNA expression of Nrf2 decreased significantly with Cd treatment (p < 0.01; Figure 2D,J). The above results suggested that Cd treatment leads to decreased antioxidant levels and increased ROS levels in granulosa cells, which induced oxidative stress.
Figure 2.
Cd-induced oxidative stress in granulosa cells. (A–C) RT-qPCR results revealed the transcript levels of CAT, SOD, and NQO1 after Cd treatment. (D) RT-qPCR results revealed the transcript levels of Nrf2 after Cd treatment. (E–H) ELISA results revealed the intracellular levels of CAT, SOD, NQO1, and MDA after Cd treatment. (I) Intracellular reactive oxygen species (ROS) was measured using flow cytometry analysis (DHE) in the control and Cd groups. (J) Nrf2 protein levels were analyzed by Western blotting after Cd treatment. * p < 0.05; ** p < 0.01.
2.3. Cd Alters the Expression of m6A Methylation-Regulated Genes in Granulosa Cells
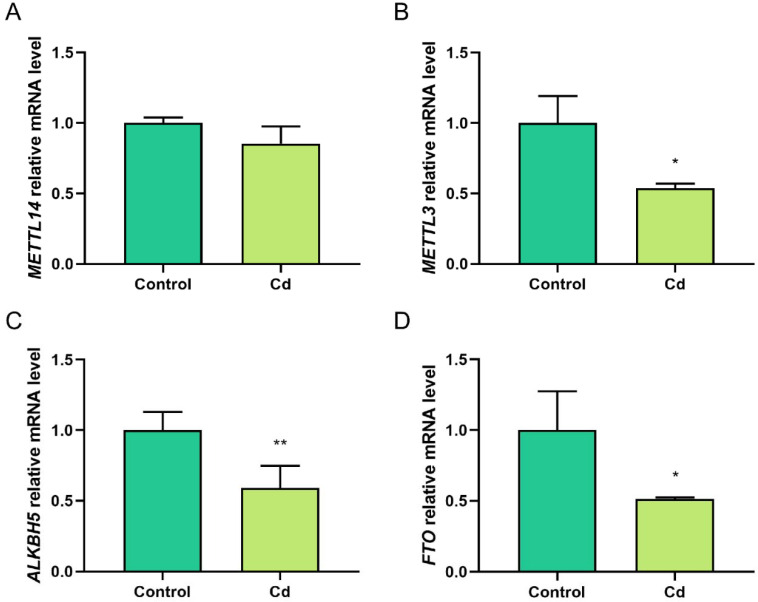
We conducted a determination of mRNA expression levels of essential genes that regulate m6A modification after Cd treatment by RT-qPCR. The results indicated that METTL3, ALKBH5, and FTO were significantly downregulated after Cd treatment (p < 0.05; Figure 3A–D).
Figure 3.
Expression of m6A methylation-regulated genes in Cd-treated granulosa cells. (A–D) RT-qPCR results revealed transcript levels of METTL14. METTL3, ALKBH5, and FTO after Cd treatment. * p < 0.05; ** p < 0.01.
2.4. Transcriptome and MeRIP Sequencing to Identified m6A modification Targets
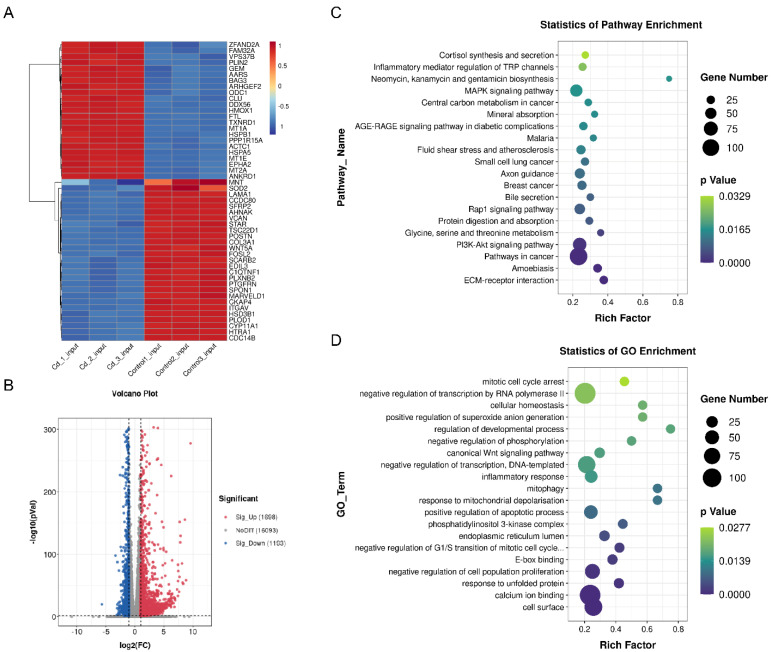
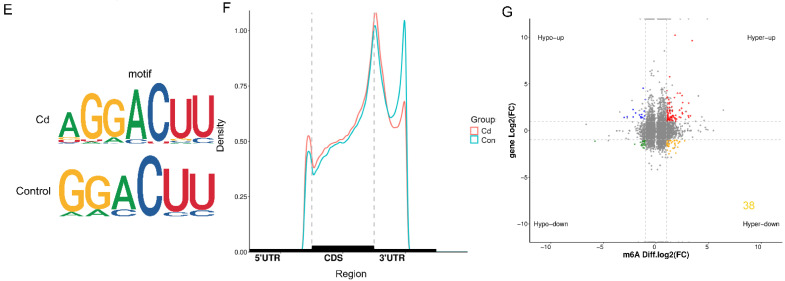
Differentially expressed genes were screened out via transcriptome sequencing under the conditions of |log2(FC)| of ≥1 and a p-value of <0.05. The heat and volcano map revealed significant differences between the two groups, and 2891 genes were screened for differential expression, 1698 of which were upregulated and 1193 were downregulated (Figure 4A,B). The different genes in the control and Cd groups were analyzed through GO and KEGG analyses. Under the standard of p < 0.05, 20 GO function enrichment and 20 KEGG pathways were screened out (Figure 4C,D). In the control and Cd groups, phosphatidylinositol 3-kinase complex (GO:0005942) and PI3K-Akt signaling pathway (ko04151) were enriched. In addition, positive regulations of O2− generation (GO:0032930) and apoptotic process (GO:0043065) were significantly enriched. MeRIP sequencing results confirmed that the m6A consensus motif was GGAC (Figure 4E). These methylation sites were primarily enriched in stop codons and 3′UTRs (Figure 4F). Finally, in the control and Cd groups, 165 significantly different genes from MeRIP-seq and mRNA-seq sequencing were screened out, of which 38 genes were upregulated in m6A modification and downregulated in mRNA expression (Figure 4G).
Figure 4.
Transcriptome and MeRIP sequencing of the control and Cd groups. (A) Heat map results revealed differential transcript levels after Cd treatment. (B) Volcano map showed the differentially expressed mRNAs in the control and Cd groups. (C,D) GO and KEGG analyses results revealed the enrichment of differential genes in the control and Cd groups. (E) The HOMER motif analysis results revealed the m6A motif in granulosa cells. (F) Density distribution of m6A enrichment across mRNA transcriptome in granulosa cells. (G) Distribution of peaks with significant differences in the RNA expression and m6A modification level in the control and Cd groups.
2.5. FTO Affects m6A Modification and Expression Level of the MNT mRNA
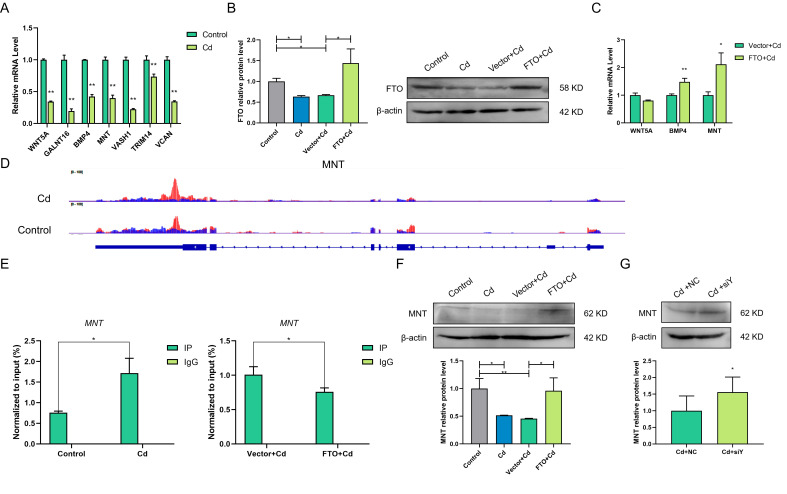
Based on the sequencing results, we preferentially screened seven highly expressed genes related to apoptosis from the hyper-down group (Figure 4G) for further verification. The results showed that the potential targets were significantly decreased after Cd treatment (Figure 5A). Among them, are WNT5A (GO:0043066, negative regulation of the apoptotic process), BMP4 (GO:0043066, negative regulation of the apoptotic process), and MNT (GO:2001234, negative regulation of the apoptotic signaling pathway) were involved in the negative apoptosis regulation. These genes were most likely FTO target genes. FTO overexpression treatment was performed, and FTO was highly expressed after transfection with pcDNA3.1-FTO (p < 0.05; Supplementary Figure S1 and Figure 5B). The results indicated that FTO overexpression increased BMP4 and MNT mRNA levels significantly (p < 0.05; Figure 5C). The distribution of m6A peaks of MNT was analyzed; the m6A modification of MNT increased after Cd treatment (Figure 5D). MeRIP-RT-qPCR results revealed that m6A modification of MNT was significantly increased after Cd treatment and it was significantly decreased when FTO was overexpressed (p < 0.05; Figure 5E), identifying MNT as a potential target of FTO. Next, Western blot results confirmed that FTO upregulated the Cd-induced decrease in MNT expression (p < 0.05; Figure 5F). YTHDF2 can recognize m6A modification and degrade target genes. We treated cells with siTYHDF2 (p < 0.05; Supplementary Figure S2) and found that a decreased YTHDF2 expression increased MNT expression (p < 0.05; Figure 5G).
Figure 5.
FTO affects m6A modification and the expression level of the MNT mRNA. (A) RT-qPCR results revealed the relative expression of seven potential target genes in the control and Cd groups. (B) Determination of FTO expression level after transfection with pcDNA3.1-FTO or pcDNA3.1-Vector. (C) RT-qPCR results revealed the mRNA expression of target genes after FTO overexpression. (D) Distribution of m6A peaks across MNT transcriptome. (E) Determination of m6A modification level of MNT mRNA after Cd treatment and FTO overexpression using MeRIP-RT-qPCR. (F) Western blotting results showed MNT protein expression after Cd treatment and FTO overexpression. (G) Western blotting results showed MNT protein expression after Cd and siYTHDF2 treatments. * p < 0.05; ** p < 0.01.
2.6. FTO Overexpression Suppressed Cd-Induced Apoptosis, Perhaps by Activating the AKT Pathway in Granulosa Cells
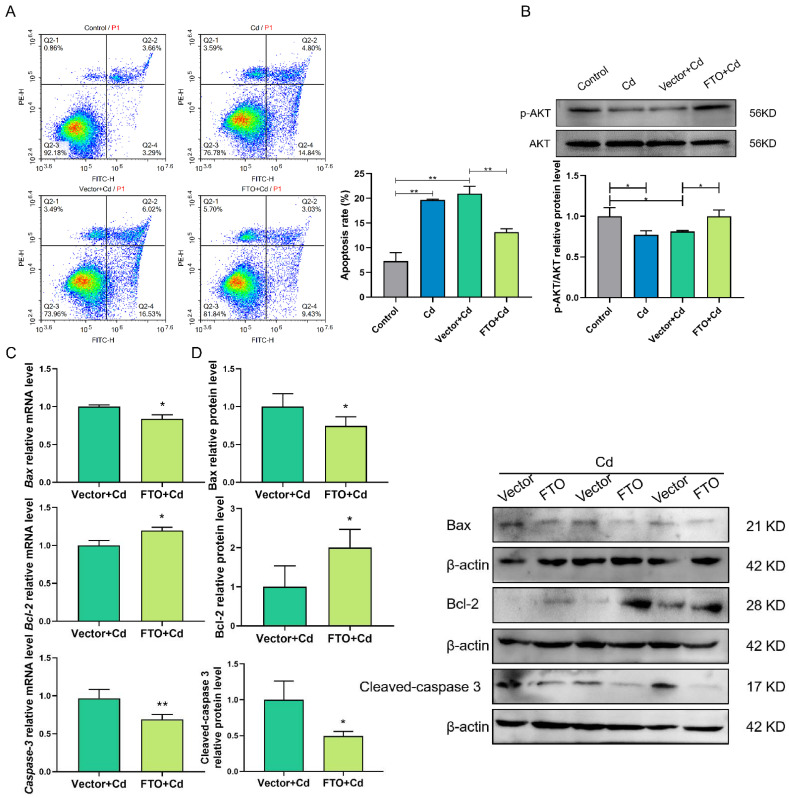
The role of FTO on Cd-induced apoptosis was researched. The degree of apoptosis assay showed that FTO overexpression suppressed Cd-induced apoptosis in granulosa cells (p < 0.01; Figure 6A). Further analysis showed that FTO overexpression reduced the expression levels of Bax and caspase-3 and increased that of Bcl-2 (p < 0.05; Figure 6C,D). Investigation of the associated pathways indicated that Cd significantly reduced the protein expression of p-AKT and FTO overexpression reversed this phenomenon (p < 0.05; Figure 6B). In short, the AKT pathway participates in the protective function of FTO on Cd-induced damage of granulosa cells.
Figure 6.
FTO overexpression suppressed Cd-induced apoptosis, perhaps by activating the AKT pathway in granulosa cells. (A) Flow cytometry analysis results detected apoptosis rate in the control, Cd, Vector + Cd, and FTO + Cd groups. (B) The p-AKT protein levels were analyzed by Western blotting after Cd and FTO treatments. (C,D) RT-qPCR and Western blotting results indicated the mRNA and protein levels of Bax, Bcl-2, and caspase-3 after FTO overexpression. * p < 0.05; ** p < 0.01.
2.7. FTO Overexpression Suppressed Cd-Induced Oxidative Stress
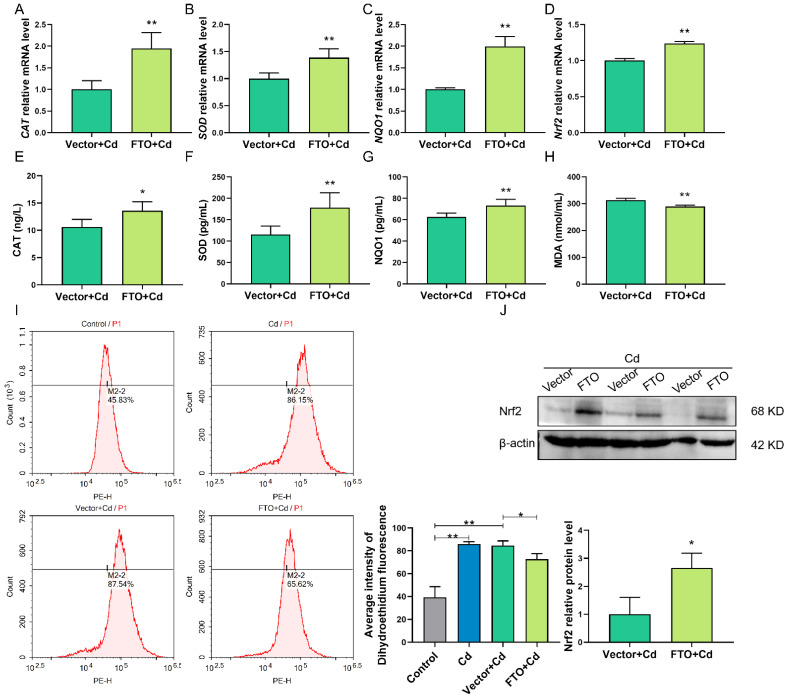
The role of FTO in Cd-induced oxidative stress was assessed. RT-qPCR indicated that increasing FTO expression increased the transcriptional levels of CAT, SOD, and NQO1 (p < 0.01; Figure 7A–C) and significantly increased the intracellular levels of CAT, SOD, and NQO1, which was accompanied by a decrease in the level of MDA (p < 0.05; Figure 7E–H). Further analysis using flow cytometry analysis (DHE) showed that FTO overexpression reduced the levels of intracellular ROS (p < 0.05; Figure 7I). These might be because of FTO overexpression, which increased the expression of Nrf2 mRNA and protein (p < 0.05; Figure 7D,J).
Figure 7.
FTO overexpression suppressed Cd-induced oxidative stress. (A–D) RT-qPCR revealed the mRNA levels of CAT, SOD, NQO1, and Nrf2 after Cd and FTO treatments. (E–H) ELISA results showed the intracellular levels of CAT, SOD, NQO1, and MDA after Cd and FTO treatments. (I) Intracellular ROS level was measured using flow cytometry analysis (DHE) in the control, Cd, Vector + Cd, and FTO + Cd groups. (J) Nrf2 protein levels were analyzed by Western blotting after Cd and FTO treatments. * p < 0.05; ** p < 0.01.
2.8. siMNT Reverses the Protective Effect of FTO during Cd Injury in Granulosa Cells
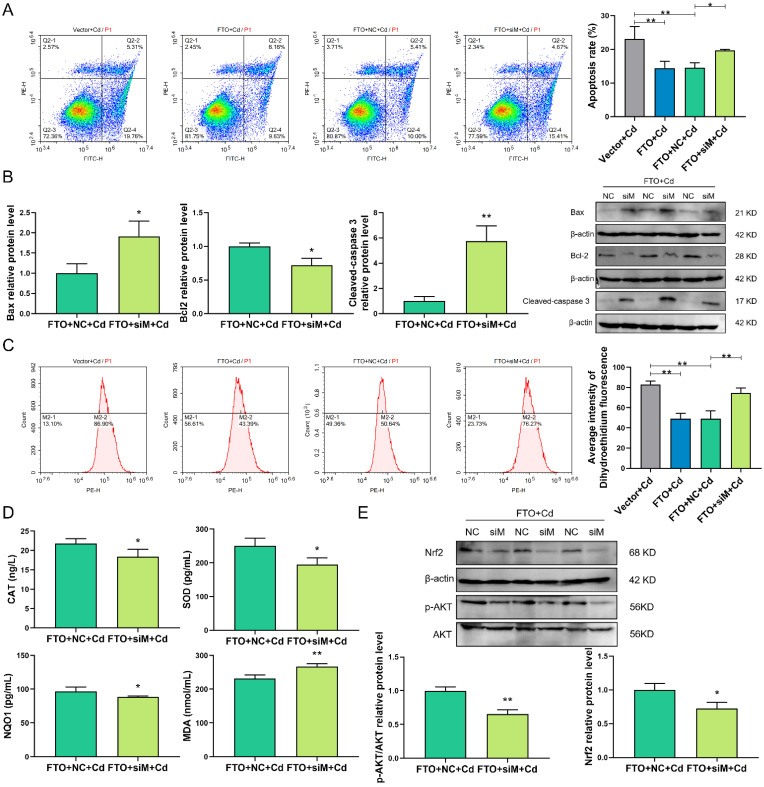
To explore the role of MNT in FTO protection of granulosa cells from Cd-induced damage, siMNT was transfected into granulosa cells co-treated with FTO and Cd. The interference efficiency of MNT is indicated in Supplementary Figure S3. In addition, FTO decreased Cd-induced apoptosis of granulosa cells, whereas the interference with MNT attenuated the effect of FTO (p < 0.05; Figure 8A). Similarly, interfering with MNT reduced the protein level of Bcl-2 and increased that of cleaved caspase-3 and Bax (p < 0.05; Figure 8B). Additionally, MNT was involved in protecting FTO against Cd-induced oxidative damage. Flow cytometry analysis (DHE) revealed that MNT interference leads to increased intracellular levels of ROS (p < 0.01; Figure 8C). In the MNT interference group, intracellular levels of CAT, SOD, and NQO1 were also significantly reduced, which was accompanied by an increase in the level of MDA (p < 0.05; Figure 8D). In detecting the Nrf2 and AKT pathways, it was found that MNT knockdown resulted in the downregulation of the protein levels of Nrf2 and AKT (p < 0.05; Figure 8E), which might be the reason why the protective effect of FTO on Cd-induced damage of granulosa cells was weakened after MNT interference.
Figure 8.
siMNT reverses the protective effect of FTO during Cd injury in granulosa cells. (A) Determination of the degree of apoptosis in the Vector + Cd, FTO + Cd, FTO + NC + Cd, and FTO + siM + Cd groups using flow cytometry analysis. (B) Western blotting results showed the protein expression levels of Bax, Bcl-2, and cleaved caspase-3 after siMNT and FTO treatments under Cd injury. (C) Intracellular level of ROS was measured using flow cytometry analysis (DHE) in Vector + Cd, FTO + Cd, FTO + NC + Cd, and FTO + siM + Cd groups. (D) ELISA results show the intracellular levels of CAT, SOD, NQO1, and MDA. (E) Nrf2 and AKT protein levels were analyzed by Western blotting after siMNT and FTO treatments under Cd injury. * p < 0.05; ** p < 0.01.
3. Discussion
As far as we know, the effect of Cd on the apoptosis and oxidative stress of bovine ovarian granulosa cells has not yet been reported. Our study indicated that m6A modification is involved in Cd-induced granulosa cell apoptosis and oxidative stress and identified FTO as a crucial factor affecting this process. In Cd injury, FTO regulates MNT expression through the m6A modification pathway. Additionally, FTO overexpression attenuates Cd-induced apoptosis and oxidative stress and activates the AKT and Nrf2 pathways, which were attenuated after MNT interference. In short, Cd modulates the expression of MNT in an m6A-modified manner, affecting apoptosis and oxidative stress in granulosa cells through the AKT/Nrf2 pathway.
Cd is reproductively toxic and affects the reproductive performance of animals. Several studies have reported the effect of Cd on germ cells. In the human granulosa cell line (KGN), Cd induces apoptosis in a dose- and time-dependent manner, and is accompanied by changes in the expression levels of Bax, BAK and Bcl-2. Cd may increase the intracellular ROS and Ca2+ levels and reduce mitochondrial membrane potential, thereby inducing oxidative stress in KGN cells [37]. Moreover, reported in chicken ovarian granulosa cells, Cd increases ROS production and apoptosis [38]. Cd also enhances ROS production and induces apoptosis in many other cells, including the thymus, hepatocytes, and leydig TM3 cells [39,40,41]. These findings are in line with our research, which revealed that Cd decreases the viability of granulosa cells in a concentration-dependent manner. Cd treatment promotes apoptosis, increases Bax and caspase-3 expression levels, and decreases Bcl-2 expression levels. Moreover, Cd induces oxidative stress in granulosa cells; particularly, the expression levels of CAT, SOD, and NQO1 decreased and those of MDA and O2− increased. In our study, Cd-induced damage of granulosa cells resulted in changes in m6A methylases, including the demethylase FTO. Previous studies have also reported that Cd leads to changes in the level of m6A modification [42,43], and FTO is involved in regulating ovarian aging and granulosa cell dysfunction [32,33].
The m6A could be participating in regulating Cd-induced damage in granulosa cells. Therefore, further analysis was performed through transcriptome and MeRIP sequencing. MeRIP sequencing indicated that the m6A consensus motif was consistent with 5’-RRACH-3’. Through the combined analysis of transcriptome and MeRIP sequencing, a potential target gene MNT was screened, which acts as a MAX network transcriptional repressor and is involved in the negative regulation of the apoptotic signaling pathway. MNT proteins use MAX as a cofactor for DNA binding and both MNT and MAX as a complex for the transcriptional repression [14]. MNT can also work independently, and it is highly expressed in the human ovary. MNT is associated with various cellular processes, including apoptosis [14]. MNT is expressed in the granulosa cell, and the lack thereof leads to embryonic growth defects and death in mice [15]. The deletion of MNT promotes apoptosis in mouse embryo fibroblasts [16]. The reduction in MNT expression enhances the induction of apoptosis by lithocholic acid in primary human hepatocytes [44]. In addition, the deletion of MNT promotes ROS generation [45]. Our results indicated that Cd increases the m6A modification level of MNT mRNA and decreases MNT expression, whereas siYTHDF2 increases MNT expression, which may be because YTHDF2 recognizes m6A modification to degrade MNT mRNA. Previous evidence shows that YTHDF2 recognizes m6A-modified degradation target genes [46]. Cd-induced reduction in the MNT level in an m6A-dependent manner may be responsible for granulosa cell damage. When FTO was overexpressed, the level of m6A modification of MNT mRNA decreased, whereas the expression level of MNT protein increased. These results suggest that, in Cd-treated granulosa cells, MNT expression is regulated via m6A modification involved in Cd injury. To our knowledge, this research is the first to explore the function of MNT in granulosa cell damage.
Transcriptome sequencing revealed that the PI3K-Akt signaling pathway was enriched in KEGG and GO analyses after Cd treatment, suggesting that the PI3K-Akt pathway participated in Cd-induced damage of granulosa cells. A study regarding the effect of Cd on germ cells found that the PI3K/Akt/Nrf2 pathway is involved in Cd-induced cell damage [13,47], and the PI3K-Akt pathway has an essential role in cell survival and regulates apoptosis by MNT [48]. These findings are consistent with our findings obtained from this study. In addition, positive regulation of O2− generation was significantly enriched in the GO analysis, and we confirmed that Cd treatment leads to an increased level of O2−.
Furthermore, m6A methylation is involved in the regulation of apoptosis and oxidative stress. Our results showed that FTO overexpression decreased Cd-induced granulosa cell apoptosis and oxidative stress; the AKT/Nrf2 pathway is activated in this process. siMNT partially reversed the effect of FTO. Although an AKT inhibitor was not used in this study, it is well known that the AKT/Nrf2 pathway is involved in anti-apoptotic and oxidative stress processes. As noted in previous studies, AKT/Nrf2 inhibited granulosa cell apoptosis and oxidative stress in different treatments [49,50,51,52]. The effect of m6A on the AKT/Nrf2 pathway has been studied previously. In ovarian cancer cells, FTO overexpression significantly reduced apoptosis and promoted AKT phosphorylation. FTO silencing exhibited contrasting results [34]. Another study found that the environmental endocrine disruptor di(2-ethylhexyl) phthalate leads to testicular damage in rats, inhibits the antioxidant function of Nrf2, and increases apoptosis and oxidative stress, which is accompanied by an increased level of m6A modification of the Nrf2 mRNA and decreased level of FTO [35]. The above studies suggest the reliability of our results. FTO may play a role in protecting granulosa cells from Cd-induced damage by regulating MNT expression and AKT/Nrf2 activity through m6A modification. Heavy metal poisoning is a chronic and long-term process, and the reproductive toxicity associated with Cd pollution should be further verified using in vivo experiments.
4. Materials and Methods
4.1. Primary Cell Culture
The ovaries of healthy cattle were collected from a slaughterhouse, stored in a container with 37 °C normal saline, and sent to the study laboratory within 4 h. The ovaries were washed five times with normal saline containing penicillin/streptomycin (Sangon Biotech, Shanghai, China) and were immediately transferred to the cell culture room for extraction. We used a 5 mL syringe to aspirate the follicular fluid from 3–8 mm follicles, centrifuged at 1000 rpm for 5 min to collect the cells, then washed with PBS containing penicillin/streptomycin for 2–3 times, and centrifuged again to collect the cells. Granulosa cells were cultured in DMEM/F-12 (Gibco, Waltham, MA, USA). The seeding density was 2 × 105 cells/well. The granulosa cells were cultured at 37 °C in the presence of 5% CO2. Granulosa cells were processed with 5, 10, 15, 20, and 40 μM of CdCl2 (MACKLIN, Shanghai, China). At least 40 ovaries were used at a time for the analysis.
4.2. Cell Viability
Granulosa cells were cultured in 96-well plates (2 × 104 cells/well) at 37 °C in the presence of 5% CO2 for 24 h. The cells were treated with 5, 10, 15, 20, and 40 μM of CdCl2 for 24 h, with six replicate wells for each concentration. Then, 10 μL of CCK-8 (APEXBIO, Houston, TX, USA) solution was added and incubated for 4 h in the dark. A microplate reader (BioTek, Winooski, VT, USA) was used to determine the absorbance at 450 nm.
4.3. Transient Cell Transfection
Transfection was performed when the granulosa cells reached 70% confluence. The siRNAs (Supplementary Table S1) were produced by GenePharma (GenePharma Biotechnology, Shanghai, China). The procedure was as previously described [53]. In short, to prepare the transfection solution for each group, 200 μL of DMEM/F-12 was used to dilute 9 μL of Lipofectamine™ 2000 and 3 μg of pcDNA3.1-FTO or pcDNA3.1-Vector and incubated for 5 min at room temperature. Subsequently, Lipofectamine™ 2000 and pcDNA3.1-FTO or pcDNA3.1-Vector dilution were mixed and incubated for 20 min at room temperature. The cells were washed twice or thrice with PBS, transferred to a 1.6 mL serum-free culture medium, and incubated for 6 h at 37 °C in the presence of 5% CO2. The complete culture medium was replaced to continue culture for 18 h. The amounts of silencing MNT (siMNT) and siYTHDF2 used were 200 pmol for both, and the operation steps were similar to the abovementioned steps. The cultured cells were obtained for subsequent experiments.
4.4. Real-Time Quantitative PCR (RT-qPCR)
Total RNA from the granulosa cells was extracted using TRIzol (Takara, Tokyo, Japan). The steps for RNA reverse transcription and RT-qPCR were the same as before [53]. PrimeScript™ RT reagent Kit (Takara, Tokyo, Japan) and SYBR® Premix Ex Taq™ II (Takara, Tokyo, Japan) for reverse transcription and RT-qPCR processes were used, respectively. The relative gene expression was analyzed by the 2−∆∆Ct comparison method. Primer sequences are provided in Supplementary Table S2.
4.5. Western Blot Analysis
Cells were lysed in RIPA buffer (Beyotime, Shanghai, China), and then centrifuged at 12,000 rpm for 15 min at 4 °C. The proteins were separated by 10–12% SDS-PAGE and transferred to nitrocellulose membranes (Merck Millipore, Darmstadt, Germany). After blocking with Intercept PBS blocking buffer (LI-COR Biosciences, Lincoln, NE, USA) for 1.5 h, they were incubated with the primary antibodies at 4 °C overnight. After washing with TBST (5 × 5 min), the secondary antibody was incubated for 1 h. ECL (NCM Biotech, Suzhou, China) was used for chemiluminescence detection. The antibodies are provided in Supplementary Table S3.
4.6. Apoptosis Assay
Cd-treated cells in dishes were collected with trypsin and resuspended in 200 μL of 1X buffer containing FITC-Annexin V (5 μL) and PI (5 μL) (BD Biosciences, San Jose, CA, USA) for staining. Then, incubated at 37 °C for 15 min in the dark. Subsequently, 200 μL of 1X buffer was added to each tube. Approximately 30,000 cells were examined for each sample. The apoptosis was determined by a flow cytometer (FITC and PerCP channels) (ACEA Biosciences, Hangzhou, China).
4.7. TUNEL Assay
Cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.2% Triton X-100, incubated with TUNEL reagent at 37 °C for 1 h, and stained with DAPI for 10 min (Beyotime, Shanghai, China). Finally, Gene5 software was used to acquire the images of apoptotic cells.
4.8. ELISA
After Cd treatment, the cells were disrupted by repeated freezing and thawing to obtain an intracellular suspension. They were centrifuged at 3000 rpm for 20 min at 4 °C, and the supernatant was collected carefully. The intracellular components were analyzed by ELISA kit (MEIMIAN, Jiangsu, China), following the producer’s instructions.
4.9. ROS Assay
Following the manufacturer’s instructions, detection of superoxide anion (O2−) in granulosa cells by dihydroethidium (DHE) fluorescent probe occurred (Beyotime, Shanghai, China). The procedure was as follows. The DHE powder was dissolved in DMSO to prepare a stock solution at a concentration of 10 mM. The cells were collected into a 1.5 mL centrifuge tube, DHE stock solution at a ratio of 1:1000 was added, and the final concentration of DHE was 10 μM. Incubate at 37 °C for 30 min, and then wash the cells with PBS 2–3 times for ROS detection.
4.10. Transcriptome Sequencing and MeRIP Sequencing Assay
This method is similar to the previous study in [30]. Briefly, total RNA from the granulosa cells was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). RNA quality and quantity were determined by NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA) and Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA), and a RIN number of >7.0. Purify Poly(A) RNA was purified from 50 μg of total RNA by Dynabeads Oligo (dT)25-61005 (Thermo Fisher, Lake Success, NY, USA) and then was fragmented into small pieces using the Magnesium RNA Fragmentation Module (NEB, Ipswich, MA, USA). Subsequently, the fragmented RNA was incubated for 2 h at 4 °C with an m6A antibody (No. 202003, Synaptic Systems, Gottingen, SN, Germany). The above products were reverse transcribed, and further screened and purified to construct a cDNA library with a fragment size of 300 bp ± 50 bp. Finally, 2 × 150 bp paired-end sequencing was performed using the Illumina Novaseq™ 6000 (LC-Bio, Hangzhou, China) according to the vendor’s protocol.
4.11. Bioinformatics
This method is similar to the previous study in [54]. Reads containing adapter contamination, low-quality bases, and undetermined bases were removed using the Fastp software [55] (https://github.com/OpenGene/fastp, accessed on 24 August 2021). Next, the sequence quality was confirmed using Fastp. HISAT2 [56] (http://daehwankimlab.github.io/hisat2, accessed on 24 August 2021) was used to map reads to the Bos taurus genome (version: v96). Mapped reads of IP and input libraries were provided for the R package exomePeak [57] (https://bioconductor.org/packages/exomePeak, accessed on 24 August 2021). This identifies m6A peaks using the bed or bigwig formats and is suitable for visualization with IGV software (http://www.igv.org, accessed on 11 January 2022). Motif analysis was conducted with MEME [58] (http://meme-suite.org, accessed on 11 January 2022) and HOMER (http://homer.ucsd.edu/homer/motif, accessed on 11 January 2022). Annotation of called peaks by intersection with gene structure was conducted using the R package ChIPseeker (https://bioconductor.org/packages/ChIPseeker, accessed on 24 August 2021) [59]. The gene assembly and quantification software were StringTie (https://ccb.jhu.edu/software/stringtie, accessed on 24 August 2021) [60], and FPKM was used for quantification. Differential analysis was conducted by using the R package edgeR (https://bioconductor.org/packages/edgeR, accessed on 24 August 2021) [61]. The screening of differentially expressed mRNAs was conducted according to the thresholds of a log2 (fold change) >1 or log2 (fold change) <−1 and a p-value of <0.05. The MeRIP-enriched regions (peaks) were visualized using IGV.
4.12. MeRIP-RT-qPCR
All operations were performed using the MeRIP™ m6A Kit (cat.A-P-9018, IVDSHOW), following the producer’s instructions. Primer sequences are provided in Supplementary Table S2.
4.13. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA, USA). Data were presented as means ± standard deviations. One-way analysis of variance (ANOVA) and Tukey’s post hoc test was used for the statistical analysis of more than two groups, while the Student’s t-test was used for the statistical analysis of two groups. Statistical significance was established when p < 0.05. Each experiment was repeated three times.
5. Conclusions
In conclusion, our study identified a possible epigenetic mechanism during Cd injury in granulosa cells. Cd induces apoptosis and oxidative stress in granulosa cells by reducing FTO expression to increase the degradation of MNT mRNA through m6A modification, thereby decreasing MNT expression. This mechanism may be mediated through the AKT/Nrf2 pathway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23094948/s1.
Author Contributions
Conceptualization, H.D. and Z.L.; data curation, Z.L., X.L. and X.Y.; formal analysis, H.L.; funding acquisition, W.L. and J.W.; investigation, J.Z.; methodology, H.D. and Z.L.; project administration, H.L., W.L. and J.W.; resources, J.Z. and H.L.; supervision, J.G.; validation, H.D.; visualization, J.Z.; writing—original draft, H.D. and Z.L.; writing—review and editing, H.D., H.L. and J.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by National Key Research and Development Program of China (2018YFE0122000), the National Natural Science Foundation of China International Cooperative Research and Exchange Program (31861143014), Key Research and Development Projects in Jilin Province (20200402050NC), Young and Middle-aged Scientific and Technological Innovation Leaders and Teams in Jilin Province (20200301031RQ), and China Agriculture Research System of MOF and MARA (CARS-37).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jaffe L.A., Egbert J.R. Regulation of Mammalian Oocyte Meiosis by Intercellular Communication within the Ovarian Follicle. Annu. Rev. Physiol. 2017;79:237–260. doi: 10.1146/annurev-physiol-022516-034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed M.F., Mokhtar M.B. Assessing Cadmium and Chromium Concentrations in Drinking Water to Predict Health Risk in Malaysia. Int. J. Environ. Res. Public Health. 2020;17:2966. doi: 10.3390/ijerph17082966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Teng Y., Lu S., Wang Y., Wang J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015;512:143–153. doi: 10.1016/j.scitotenv.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Ngoc N.T.M., Chuyen N.V., Thao N.T.T., Duc N.Q., Trang N.T.T., Binh N.T.T., Sa H.C., Tran N.B., Ba N.V., Khai N.V., et al. Chromium, Cadmium, Lead, and Arsenic Concentrations in Water, Vegetables, and Seafood Consumed in a Coastal Area in Northern Vietnam. Environ. Health Insights. 2020;14:1178630220921410. doi: 10.1177/1178630220921410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X., Zheng N., Su C., Wang J., Soyeurt H. Relationships between Pb, As, Cr, and Cd in individual cows’ milk and milk composition and heavy metal contents in water, silage, and soil. Environ. Pollut. 2019;255:113322. doi: 10.1016/j.envpol.2019.113322. [DOI] [PubMed] [Google Scholar]
- 6.Hashemi M. Heavy metal concentrations in bovine tissues (muscle, liver and kidney) and their relationship with heavy metal contents in consumed feed. Ecotoxicol. Environ. Saf. 2018;154:263–267. doi: 10.1016/j.ecoenv.2018.02.058. [DOI] [PubMed] [Google Scholar]
- 7.Suprewicz K., Kozikowska I., Chrobaczyńska-Dylag M., Gał A., Piekarz A., Sikora J., Sławska H., Stawarz R. Effects of the cigarette smoking on the newborn clinical parametrs and the accumulation of cadmium and lead in the placenta of women from Upper Silesia. Ginekol. Pol. 2013;84:776–780. doi: 10.17772/gp/1639. [DOI] [PubMed] [Google Scholar]
- 8.Richter P.A., Bishop E.E., Wang J., Swahn M.H. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: The National Health and Nutrition Examination Survey (NHANES) 1999–2004. Int. J. Environ. Res. Public Health. 2009;6:1930–1946. doi: 10.3390/ijerph6071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Luo L.F., Wang D.L., Wang W.X., Zhu J.L., Li Y.C., Chen N.Z., Huang H.L., Zhang W.C. Cadmium induces ovarian granulosa cell damage by activating PERK-eIF2α-ATF4 through endoplasmic reticulum stress. Biol. Reprod. 2019;100:292–299. doi: 10.1093/biolre/ioy169. [DOI] [PubMed] [Google Scholar]
- 10.Wan X., Zhu J., Zhu Y., Zhu Y., Ma X., Zheng Y., Wang F., Liu Z., Zhang T. Rat ovarian follicle bioassay reveals adverse effects of cadmium chloride (CdCl2) exposure on follicle development and oocyte maturation. Toxicol. Ind. Health. 2010;26:609–618. doi: 10.1177/0748233710375949. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W., Jia H. Effect and mechanism of cadmium on the progesterone synthesis of ovaries. Toxicology. 2007;239:204–212. doi: 10.1016/j.tox.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Weng S., Wang W., Li Y., Li H., Lu X., Xiao S., Wu T., Xie M., Zhang W. Continuous cadmium exposure from weaning to maturity induces downregulation of ovarian follicle development-related SCF/c-kit gene expression and the corresponding changes of DNA methylation/microRNA pattern. Toxicol. Lett. 2014;225:367–377. doi: 10.1016/j.toxlet.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang C., Ma W., Su Y. NF-κB pathway contributes to cadmium-induced apoptosis of porcine granulosa cells. Biol. Trace Elem. Res. 2013;153:403–410. doi: 10.1007/s12011-013-9650-7. [DOI] [PubMed] [Google Scholar]
- 14.Liaño-Pons J., Arsenian-Henriksson M., León J. The Multiple Faces of MNT and Its Role as a MYC Modulator. Cancers. 2021;13:4682. doi: 10.3390/cancers13184682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyo-oka K., Hirotsune S., Gambello M.J., Zhou Z.Q., Olson L., Rosenfeld M.G., Eisenman R., Hurlin P., Wynshaw-Boris A. Loss of the Max-interacting protein Mnt in mice results in decreased viability, defective embryonic growth and craniofacial defects: Relevance to Miller-Dieker syndrome. Hum. Mol. Genet. 2004;13:1057–1067. doi: 10.1093/hmg/ddh116. [DOI] [PubMed] [Google Scholar]
- 16.Hurlin P.J., Zhou Z.Q., Toyo-oka K., Ota S., Walker W.L., Hirotsune S., Wynshaw-Boris A. Deletion of Mnt leads to disrupted cell cycle control and tumorigenesis. EMBO J. 2003;22:4584–4596. doi: 10.1093/emboj/cdg442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurlin P.J., Quéva C., Eisenman R.N. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes Dev. 1997;11:44–58. doi: 10.1101/gad.11.1.44. [DOI] [PubMed] [Google Scholar]
- 18.Meroni G., Reymond A., Alcalay M., Borsani G., Tanigami A., Tonlorenzi R., Lo Nigro C., Messali S., Zollo M., Ledbetter D.H., et al. Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor. EMBO J. 1997;16:2892–2906. doi: 10.1093/emboj/16.10.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eischen C.M., Woo D., Roussel M.F., Cleveland J.L. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol. Cell. Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia G., Fu Y., Zhao X., Dai Q., Zheng G., Yang Y., Yi C., Lindahl T., Pan T., Yang Y.G., et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng G., Dahl J.A., Niu Y., Fedorcsak P., Huang C.M., Li C.J., Vågbø C.B., Shi Y., Wang W.L., Song S.H., et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rottman F.M., Desrosiers R.C., Friderici K. Nucleotide methylation patterns in eukaryotic mRNA. Prog. Nucleic Acid Res. Mol. Biol. 1976;19:21–38. doi: 10.1016/s0079-6603(08)60906-x. [DOI] [PubMed] [Google Scholar]
- 23.Schibler U., Kelley D.E., Perry R.P. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J. Mol. Biol. 1977;115:695–714. doi: 10.1016/0022-2836(77)90110-3. [DOI] [PubMed] [Google Scholar]
- 24.Dominissini D., Moshitch-Moshkovitz S., Schwartz S., Salmon-Divon M., Ungar L., Osenberg S., Cesarkas K., Jacob-Hirsch J., Amariglio N., Kupiec M., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 25.Batista P.J., Molinie B., Wang J., Qu K., Zhang J., Li L., Bouley D.M., Lujan E., Haddad B., Daneshvar K., et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y., Dominissini D., Rechavi G., He C. Gene expression regulation mediated through reversible m⁶A RNA methylation. Nat. Rev. Genet. 2014;15:293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 27.Wei J., Liu F., Lu Z., Fei Q., Ai Y., He P.C., Shi H., Cui X., Su R., Klungland A., et al. Differential m6A, m6A(m), and m1A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol. Cell. 2018;71:973–985.e5. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Śledź P., Jinek M. Structural insights into the molecular mechanism of the m6A writer complex. eLife. 2016;5:e18434. doi: 10.7554/eLife.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Z., Liu P., Sun Q., Li Y., Acharya R., Li X., Sun C. FTO inhibits UPR(mt)-induced apoptosis by activating JAK2/STAT3 pathway and reducing m6A level in adipocytes. Apoptosis Int. J. Program. Cell Death. 2021;26:474–487. doi: 10.1007/s10495-021-01683-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhao T., Wang J., Wu Y., Han L., Chen J., Wei Y., Shen L., Long C., Wu S., Wei G. Increased m6A modification of RNA methylation related to the inhibition of demethylase FTO contributes to MEHP-induced Leydig cell injury(☆) Environ. Pollut. 2021;268:115627. doi: 10.1016/j.envpol.2020.115627. [DOI] [PubMed] [Google Scholar]
- 31.Tang J., Su Q., Guo Z., Zhou J., Zheng F., Yu G., Shao W., Hu H., Wu S., Li H. N6-methyladenosine(m6A) demethylase FTO regulates cellular apoptosis following cobalt-induced oxidative stress. Environ. Pollut. 2022;297:118749. doi: 10.1016/j.envpol.2021.118749. [DOI] [PubMed] [Google Scholar]
- 32.Zhou L., Han X., Li W., Wang N., Yao L., Zhao Y., Zhang L. N6-methyladenosine Demethylase FTO Induces the Dysfunctions of Ovarian Granulosa Cells by Upregulating Flotillin 2. Reprod. Sci. 2022;29:1305–1315. doi: 10.1007/s43032-021-00664-6. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z.X., Wang Y.N., Li Z.Y., Dai Z.H., He Y., Chu K., Gu J.Y., Ji Y.X., Sun N.X., Yang F., et al. The m6A mRNA demethylase FTO in granulosa cells retards FOS-dependent ovarian aging. Cell Death Dis. 2021;12:744. doi: 10.1038/s41419-021-04016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L., Kong X., Zhong W., Wang Y., Li P. FTO accelerates ovarian cancer cell growth by promoting proliferation, inhibiting apoptosis, and activating autophagy. Pathol. Res. Pract. 2020;216:153042. doi: 10.1016/j.prp.2020.153042. [DOI] [PubMed] [Google Scholar]
- 35.Zhao T.X., Wang J.K., Shen L.J., Long C.L., Liu B., Wei Y., Han L.D., Wei Y.X., Wu S.D., Wei G.H. Increased m6A RNA modification is related to the inhibition of the Nrf2-mediated antioxidant response in di-(2-ethylhexyl) phthalate-induced prepubertal testicular injury. Environ. Pollut. 2020;259:113911. doi: 10.1016/j.envpol.2020.113911. [DOI] [PubMed] [Google Scholar]
- 36.Liu S., Zhuo L., Wang J., Zhang Q., Li Q., Li G., Yan L., Jin T., Pan T., Sui X., et al. METTL3 plays multiple functions in biological processes. Am. J. Cancer Res. 2020;10:1631–1646. [PMC free article] [PubMed] [Google Scholar]
- 37.Xu G., Liu S., Huang M., Jiang X., Yang M. Cadmium induces apoptosis of human granulosa cell line KGN via mitochondrial dysfunction-mediated pathways. Ecotoxicol. Environ. Saf. 2021;220:112341. doi: 10.1016/j.ecoenv.2021.112341. [DOI] [PubMed] [Google Scholar]
- 38.Zhu M., Miao S., Zhou W., Elnesr S.S., Dong X., Zou X. MAPK, AKT/FoxO3a and mTOR pathways are involved in cadmium regulating the cell cycle, proliferation and apoptosis of chicken follicular granulosa cells. Ecotoxicol. Environ. Saf. 2021;214:112091. doi: 10.1016/j.ecoenv.2021.112091. [DOI] [PubMed] [Google Scholar]
- 39.Ren X., Wang S., Zhang C., Hu X., Zhou L., Li Y., Xu L. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK /c-jun signaling pathway. Ecotoxicol. Environ. Saf. 2020;192:110266. doi: 10.1016/j.ecoenv.2020.110266. [DOI] [PubMed] [Google Scholar]
- 40.Chen X., Wang X., Yang L., Xu H., Wu Y., Wu J., Chen L., Xu C. Magnesium isoglycyrrhizinate prevents cadmium-induced activation of JNK and apoptotic hepatocyte death by reversing ROS-inactivated PP2A. J. Pharm. Pharmacol. 2021;73:1663–1674. doi: 10.1093/jpp/rgab125. [DOI] [PubMed] [Google Scholar]
- 41.Yiming Z., Zhaoyi L., Jing L., Jinliang W., Zhiqiang S., Guangliang S., Shu L. Cadmium induces the thymus apoptosis of pigs through ROS-dependent PTEN/PI3K/AKT signaling pathway. Environ. Sci. Pollut. Res. Int. 2021;28:39982–39992. doi: 10.1007/s11356-021-13517-1. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y., Zong C., Liu J., Zeng L., Li Q., Liu Z., Li Y., Zhu J., Li L., Zhang C., et al. C-myc promotes miR-92a-2-5p transcription in rat ovarian granulosa cells after cadmium exposure. Toxicol. Appl. Pharmacol. 2021;421:115536. doi: 10.1016/j.taap.2021.115536. [DOI] [PubMed] [Google Scholar]
- 43.Li L., Zhou M., Chen B., Wang Q., Pan S., Hou Y., Xia J., Zhou X. ALKBH5 promotes cadmium-induced transformation of human bronchial epithelial cells by regulating PTEN expression in an m6A-dependent manner. Ecotoxicol. Environ. Saf. 2021;224:112686. doi: 10.1016/j.ecoenv.2021.112686. [DOI] [PubMed] [Google Scholar]
- 44.Yang H., Li T.W., Ko K.S., Xia M., Lu S.C. Switch from Mnt-Max to Myc-Max induces p53 and cyclin D1 expression and apoptosis during cholestasis in mouse and human hepatocytes. Hepatology. 2009;49:860–870. doi: 10.1002/hep.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Link J.M., Ota S., Zhou Z.Q., Daniel C.J., Sears R.C., Hurlin P.J. A critical role for Mnt in Myc-driven T-cell proliferation and oncogenesis. Proc. Natl. Acad. Sci. USA. 2012;109:19685–19690. doi: 10.1073/pnas.1206406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du H., Zhao Y., He J., Zhang Y., Xi H., Liu M., Ma J., Wu L. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016;7:12626. doi: 10.1038/ncomms12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bashir N., Shagirtha K., Manoharan V., Miltonprabu S. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: Implication of PI3K/Akt/Nrf-2 signaling. Biosci. Rep. 2019;39:BSR20180515. doi: 10.1042/BSR20180515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terragni J., Nayak G., Banerjee S., Medrano J.L., Graham J.R., Brennan J.F., Sepulveda S., Cooper G.M. The E-box binding factors Max/Mnt, MITF, and USF1 act coordinately with FoxO to regulate expression of proapoptotic and cell cycle control genes by phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3 signaling. J. Biol. Chem. 2011;286:36215–36227. doi: 10.1074/jbc.M111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong Y., Luo S., Fan P., Zhu H., Li Y., Huang W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. RBE. 2020;18:121. doi: 10.1186/s12958-020-00677-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S., Lin S., Zhu M., Li C., Chen S., Pu L., Lin J., Cao L., Zhang Y. Acupuncture Reduces Apoptosis of Granulosa Cells in Rats with Premature Ovarian Failure Via Restoring the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2019;20:6311. doi: 10.3390/ijms20246311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akino N., Wada-Hiraike O., Terao H., Honjoh H., Isono W., Fu H., Hirano M., Miyamoto Y., Tanikawa M., Harada M., et al. Activation of Nrf2 might reduce oxidative stress in human granulosa cells. Mol. Cell. Endocrinol. 2018;470:96–104. doi: 10.1016/j.mce.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Khadrawy O., Gebremedhn S., Salilew-Wondim D., Taqi M.O., Neuhoff C., Tholen E., Hoelker M., Schellander K., Tesfaye D. Endogenous and Exogenous Modulation of Nrf2 Mediated Oxidative Stress Response in Bovine Granulosa Cells: Potential Implication for Ovarian Function. Int. J. Mol. Sci. 2019;20:1635. doi: 10.3390/ijms20071635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding H., Zhao J., Liu H., Wang J., Lu W. BMAL1 knockdown promoted apoptosis and reduced testosterone secretion in TM3 Leydig cell line. Gene. 2020;747:144672. doi: 10.1016/j.gene.2020.144672. [DOI] [PubMed] [Google Scholar]
- 54.Wang H.Q., Zhang J.B., Zheng Y., Zhang W.D., Guo H.X., Cong S., Ding Y., Yuan B. Comprehensive analysis of differences in N6-methyladenosine RNA methylomes in the rat adenohypophysis after GnRH treatment. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022;36:e22204. doi: 10.1096/fj.202101608R. [DOI] [PubMed] [Google Scholar]
- 55.Chen S., Zhou Y., Chen Y., Gu J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D., Langmead B., Salzberg S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng J., Lu Z., Liu H., Zhang L., Zhang S., Chen Y., Rao M.K., Huang Y. A protocol for RNA methylation differential analysis with MeRIP-Seq data and exomePeak R/Bioconductor package. Methods. 2014;69:274–281. doi: 10.1016/j.ymeth.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu G., Wang L.G., He Q.Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- 60.Pertea M., Pertea G.M., Antonescu C.M., Chang T.C., Mendell J.T., Salzberg S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.