Abstract
Simple Summary
Second line anti-PD1 immunotherapy is effective in selected BRAF-mutated melanoma patients after BRAFi/MEKi immunotherapy failure. Nivolumab and pembrolizumab are of similar efficacy as a second line therapy. Worse performance status as well as high LDH levels are negative clinical biomarkers that correlated with shorter OS. The presence of brain and liver metastases correlates with shorter PFS and OS of these patients.
Abstract
(1) Background: BRAFi/MEKi are usually offered as a first line treatment for patients requiring rapid response; with elevated lactate dehydrogenase (LDH) activity, large tumor burden, and with brain metastases. The efficacy of second line therapies after BRAFi/MEKI failure is now well defined. (2) Methods: Patients treated with first line target BRAFi/MEKi therapy (vemurafenib plus cobimetinib, dabrafenib plus trametinib or encorafenib plus binimetinib); and for the second line treatment immunotherapy with programmed cell death 1 (PD-1) checkpoint inhibitors (nivolumab or pembrolizumab) with at least one cycle of second line were analyzed for survival and prognostic biomarkers. (3) Results: There were no statistically significant differences in ORR between the treatment groups with nivolumab and pembrolizumab, as well as median progression free-survival (PSF) and overall survival (OS) since the initiation of second line therapy; on nivolumab OS was 6.6 months, and on pembrolizumab 5.0 months. The greatest clinical benefit with second line immunotherapy was observed in patients with LDH ≤ ULN and <3 organ sites with metastasis at baseline. Longer OS was also noted in patients with time to PD >6 months in first line (slow progression). (4) Conclusions: Second line anti-PD1 immunotherapy is effective in BRAF-mutated melanoma patients after BRAFi/MEKi therapy failure.
Keywords: melanoma, immunotherapy, nivolumab, pembrolizumab, BRAF
1. Introduction
Currently, patients with BRAF (v-Raf murine sarcoma viral oncogene homolog B)-mutated melanoma patients are offered immunotherapy (pembrolizumab, nivolumab, or nivolumab and ipilimumab combination) or BRAF and MEK (Mitogen-Activated Protein Kinase Kinase 1, MAP2K1) inhibitors combination (BRAFi/MEKi) as a first line therapy [1,2,3,4]. Optimal first line therapy has not been clearly defined yet, although primary results of SECOMBIT and DREAMSeq trials suggest that in those individuals who qualify for combination immunotherapy, with nivolumab and ipilimumab, this could be a preferred choice [5,6,7]. At the same time, not all patients are good candidates for combination immunotherapy, and the toxicity of the doublet is relatively high with grade 5 also reported, as confirmed in phase III CheckMate069 and the expanded access program CheckMate 218 [8,9]. The choice of BRAFi/MEKi (vemurafenib and cobimetinib, dabrafenib and trametinib, or encorafenib and binimetinib) is favored by objective response rates (ORR) of more than 65%, oral administration, and low number of outpatient visits, which is of special interest in COVID pandemic [10,11,12,13]. Therefore BRAFi/MEKi anti-PD-1 immunotherapy sequential treatment is actually offered to large group of patients in routine clinical practice [14].
BRAF-mutated melanomas were shown as immunologically cold tumors with a downregulated expression of major histocompatibility complex (MHC) class I molecules, low effector T-cell infiltration, and high regulatory T-cells (Tregs), high myeloid-derived suppressor cells (MDSCs) infiltrates, as well as the accumulation of immunosuppressive interleukins (IL-6, IL-10) in the tumor niche, as well as impaired maturation of dendritic cells (DC) and their capacity to secrete proinflammatory cytokines (IL-12 and TNFα) [15,16,17]. On the contrary, multiple studies have shown that mutant BRAF protein epitopes may be recognized by host immunity and induce anti-melanoma immune responses [17,18,19]. Moreover, longitudinal studies have shown that BRAFi/MEKi treatment influence immune status of melanoma tumors. First molecular analyses have shown that initially treatment with either BRAF monotherapy or BRAF and MEK inhibitors combination (BRAFi/MEKi) initially promote the high expression of melanoma antigen recognized by T-cells 1 (MART-1, MLANA), tyrosinase-related protein 1 and 2 (TYRP-1 and TYRP-2), and melanocyte protein PMEL (GP100), namely melanoma antigens, and promote CD8+ T cell infiltration into the tumor niche. At the same time, the downregulation of interleukin-6 (IL-6)- and IL-8, namely immunosuppressive cytokines, and an increase of perforin and granzyme B expression, namely markers of T cell cytotoxicity in the tumors, was found. On the contrary, the expression of exhaustion markers, namely T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), and programmed cell death protein 1 (PD1), as well as of the immunosuppressive ligand programmed death-ligand 1 (PDL1), increase on BRAFi treatment. Further, at time of disease progression (PD), the downregulation of melanoma antigen expression and CD8 T cell infiltrate decrease was reported on BRAFi therapy [20]. During BRAFi treatment, in the PLX4720 model, melanoma gradually tumors developed immunosuppressive phenotype. The accumulation of regulatory T cells (Tregs) and CD11b(+)/Gr-1(+) myeloid cells as well as the loss of Th1 effector functions of CD4(+) tumor-infiltrating lymphocytes (TILs), including CD40L and interferon gamma (IFNɣ) expression, was observed [15]. Subsequently, BRAF inhibition results in paracrine suppressive activity of melanoma cells on dendritic cells by inhibiting their excretion of pro-inflammatory cytokines, namely IL-12 and tumor necrosis factor alpha (TNFα) [16].
At this point in time, limited data have been published concerning sequential treatment efficacy in patients with BRAF-mutant melanoma. Selected reports suggest lower activity of immunotherapy after PD on BRAFi/MEKi [21,22]. In the DREAMseq trial, response rates were similar for dabrafenib plus trametinib whether used in first or second line, while nivolumab with ipilimumab has shown lower ORR in second line, after PD on dabrafenib–trametinib first-line therapy [23]. Reported ipilimumab monotherapy ORR are lower after BRAFi failure than in the first line [24], while the efficacy of nivolumab after BRAFi or BRAFi/MEKi therapy was reported not to be changed [25]. In general, as it is often suggested that first line treatment with targeted therapy may select aggressive and low-immunogenic melanoma cells, second line immunotherapy may not be as effective [26]. Therefore, BRAFi/MEKi are usually offered as first line treatment for patients requiring rapid response; with elevated LDH activity, large tumor burden, and with brain metastases [27].
Real-world data analysis may provide information on the actual efficacy of nivolumab and pembrolizumab used as a second line treatment, after BRAFi/MEKi treatment. Analysis of regular clinical practice provides insight on the PFS, OS, and ORR in patients who did not qualify for clinical trials. In fact, a low number of analyses describe the long-term survival of BRAF mutated melanoma patients and the efficacy of second- or third-line therapy [14,28]. Moreover, second line treatment trials are currently rarely conducted in the melanoma field. Therefore, the analysis of patients who completed sequential treatment may help identify individuals who benefit from currently available melanoma BRAFi/MEKI–anti-PD1 sequential therapy. To date, no such analysis is available. The primary objectives of this study are to analyze the efficacy of anti-PD1 immunotherapy as a second line therapy in patients with unresectable and advanced melanoma. The secondary aim of the study is to characterized patients with advanced melanoma who may benefit from treatment with a targeted therapy -> immunotherapy sequence. Results of our analysis are expected to support the selection of sequential therapies in everyday practice.
2. Materials and Methods
2.1. Patients
For this observational study, we analyzed health record data of adult patients who started first-line therapy for advanced/metastatic BRAF-mutated melanoma between 1 December 2015 and 31 December 2020. The observation data cut off was 31 January 2022. We have included all consecutive sequentially treated patients from major oncology centers in Poland who have been treated with first line target BRAFi/MEKi therapy (vemurafenib plus cobimetinib, dabrafenib plus trametinib, or encorafenib plus binimetinib); and for the second line treatment immunotherapy with anti-PD-1 checkpoint inhibitors (nivolumab or pembrolizumab) with at least one cycle of second line therapy. Patients, if presented with brain metastases, were asymptomatic at treatment initiation and did not require steroid of >10 mg prednisone treatment when immunotherapy was started. All eligible patients had their diagnosis confirmed by pathologists experienced in skin cancer pathology and confirmed BRAF mutation. Patients were treated as described before [29,30] until RECIST 1.1 PD or death whichever comes first. Patients treated with neoadjuvant [31], adjuvant therapies, and within clinical trials were excluded from the study. Date of death was confirmed in the Polish National Cancer Registry via the personal identification number of all patients.
2.2. Data Analysis
Descriptive statistics were used to characterize baseline demographic features, including disease stage, metastases loci, as well as treatment duration and best response. Progression free survival (PFS) and OS were calculated with the Kaplan–Meier method, and a log-rank test was used for assessing differences between survival curves. The Cox proportional hazard model was used for multivariable analysis. All variables with a p-value < 0.1 in univariate analysis were included in the multivariable model, and 95% confidence intervals (CI) were reported. The differences were considered statistically significant if the p-values were <0.05 [32]. Patients without signs of PD were censored at the last follow-up visit. OS was calculated from the date of treatment start to death or last follow-up. Analysis was performed with Statistica version 13.3.
3. Results
3.1. Patients Treated
The enrolled patients included 106 (51%) females and 101 (49%) males (Table 1) with median age of 57 years (24–89 years old.). A majority (94%) of patients started treatment with stage IV disease, and among all patients almost 23% presented with asymptomatic brain metastases at treatment initiation. More than 61% of patients had elevated LDH at first line treatment start. Median follow-up time from initiation of first line therapy was over 16.8 months and after second line immunotherapy 5.1 months.
Table 1.
Baseline patients characteristics.
| Factor | Patients n = 207 | Percentage | |
|---|---|---|---|
| Sex | F | 106 | 51% |
| M | 101 | 49% | |
| Disease stage 1L TNM stage (AJCC 8th Edition) |
III Localy advanced | 13 | 6% |
| M1a | 30 | 14.5% | |
| M1b | 30 | 14.5% | |
| M1c | 87 | 42% | |
| M1d | 47 | 23% | |
| LDH 1L | Normal | 78 | 39% |
| Over ULN | 124 | 61% | |
| Less than 2× over ULN | 80 | 40% | |
| More than 2× over ULN | 44 | 21% | |
| No data | 5 | - | |
| ECOG 1L | 0 | 77 | 37% |
| 1 | 124 | 60% | |
| 2 | 6 | 3% | |
| Liver metastases 1L | No | 137 | 66% |
| Yes | 70 | 34% | |
| Brain metastases 1L | No | 160 | 77% |
| Yes | 47 | 23% | |
| First line treatment | Dabrafenib + trametinib | 141 | 68% |
| Vemurafenib + cobimetinib | 64 | 31% | |
| Encorafenib + binimetinib | 2 | 1% | |
| Second line treatment | Nivolumab | 130 | 63% |
| Pembrolizumab | 77 | 37% | |
| LDH 2L | Normal | 81 | 39% |
| Over ULN | 124 | 61% | |
| Less than 2× over ULN | 84 | 41% | |
| More than 2× over ULN | 40 | 20% | |
| No data | 2 | - | |
| ECOG 2L | 0 | 33 | 16% |
| 1 | 163 | 79% | |
| 2 | 10 | 5% | |
| No data | 1 | - | |
| Liver metastases 2L | No | 138 | 67% |
| Yes | 69 | 33% | |
| Brain metastases 2L | No | 135 | 65% |
| Yes | 72 | 35% | |
F, female; M, male; LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group (performance status); TNM, tumor, node, metastasis (staging system); AJCC, American Joint Committee on Cancer; 1L, first line treatment; 2L, second line treatment.
3.2. Sequential Treatment
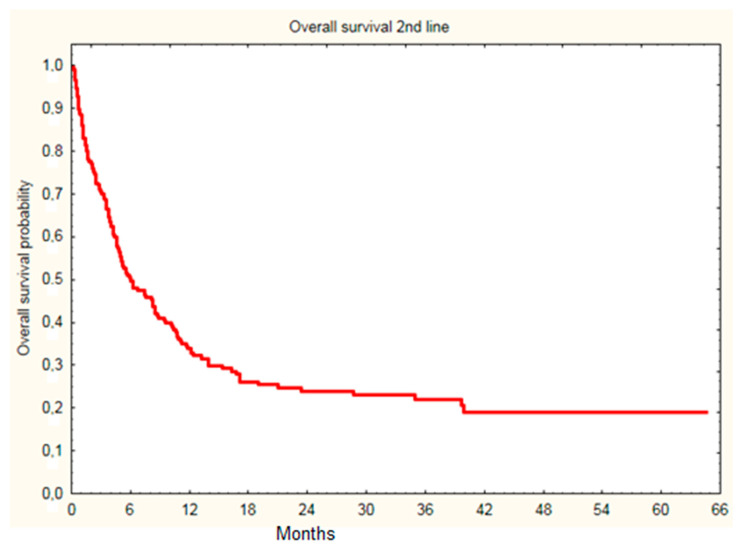
In the whole group, the most common first-line treatment used was dabrafenib and trametinib combination (141 cases, 68% of all treated patients), while encorafenib and binimetinib was used in only two patients. Among patients treated with immunotherapy in second line treatment, 33 patients continued treatment at the time of analysis. At data cut-off, 11 patients were treated with third line therapy, 11 patients were referred for BSC due to PD, and 148 patients died. Median OS since first line treatment initiation was 18.7 month, while 6.0 months since the initiation of immunotherapy (Figure 1).
Figure 1.
Overall survival of melanoma patients since initiation of second line immunotherapy (nivolumab or pembrolizumab) treatment (18.7 month median).
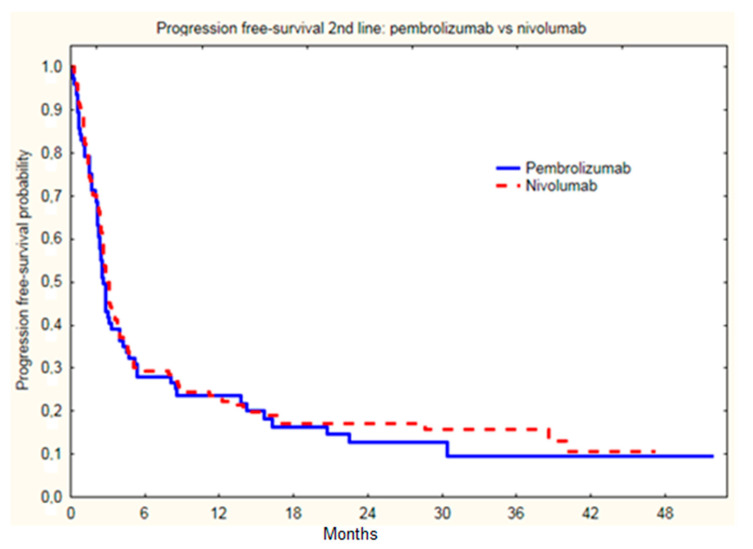
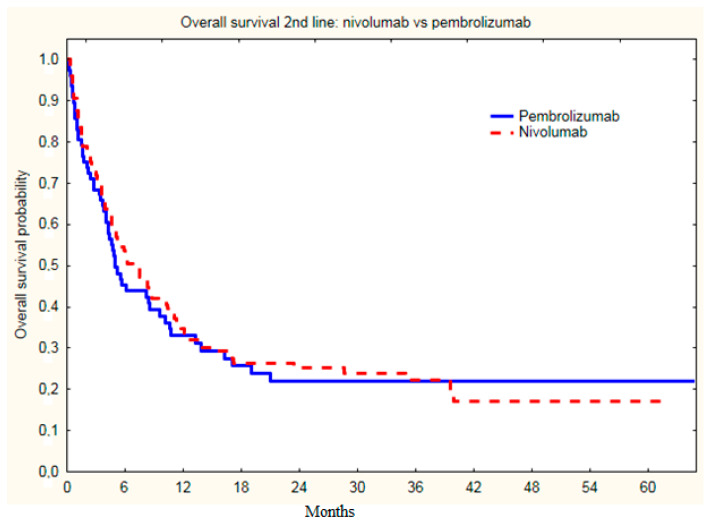
There were no statistically significant differences in ORR between the treatment groups with nivolumab and pembrolizumab (p = 0.90) (Table 2), as well as median PSF (p = 0.54), as PSF on nivolumab was 3.0 months, and on pembrolizumab 2.7 months (Figure 2); and OS on nivolumab was 6.6 months, and on pembrolizumab 5.0 months (Figure 3).
Table 2.
Best response on two lines of treatment.
| Treatment Response | Patients | Percentage | Patients | Percentage | p-Value | |
|---|---|---|---|---|---|---|
| Total | 1L | 2L | ||||
| Best response | PD | 21 | 10% | 123 | 61% | <0.0001 |
| SD | 71 | 34% | 39 | 19% | ||
| PR | 106 | 51% | 32 | 16% | ||
| CR | 9 | 5% | 9 | 4% | ||
| ORR | 196 | 56% | 41 | 20% | <0.0001 | |
| Not assessed | 0 | - | 4 | - | - | |
| Time to PD | >6 m | 137 | 66% | 57 | 28 | - |
| <6 m | 70 | 34% | 144 * | 72% | - | |
| 2L | Nivolumab | Pembrolizumab | ||||
| Best response | PD | 76 | 60% | 47 | 62% | 0.99 |
| SD | 25 | 20% | 14 | 18% | ||
| PR | 20 | 16% | 12 | 16% | ||
| CR | 6 | 4% | 3 | 4% | ||
| ORR | 26 | 20% | 15 | 20% | 0.90 | |
| Not assessed | 3 | - | 1 | - | - | |
CR, complete response; PR, partial response; SD, stable disease; PD, progression disease; ORR (CR + PR), overall response rate; PD, progressive disease; 1L, first line (treatment); 2L, second line (treatment). * 6 patient continuing treatment without progression < 6 months.
Figure 2.
Progression free survival of melanoma patients since initiation of second line nivolumab or pembrolizumab treatment (PSF on nivolumab was 3.0 months, and on pembrolizumab was 2.7 months; p = 0.54).
Figure 3.
Overall survival of melanoma patients since initiation of second line nivolumab or pembrolizumab treatment. (OS on nivolumab was 6.6 months, and on pembrolizumab was 5.0 months; p = 0.99).
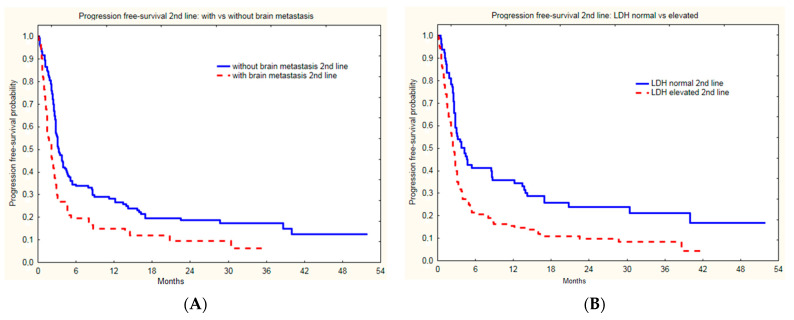
Groups treated with nivolumab and pembrolizumab did not differ in baseline subgroup characteristics based on LDH level (p = 0.99), gender (p = 0.19), age (p = 0.31), or presence of brain metastases (p = 0.59). The presence of liver metastases, lower performance status (ECOG1), and high LDH activity correlated with PSF on second line immunotherapy (p = 0.0208, p = 0.0179 and p = 0.0069, respectively) in the whole group (Table 3, Figure 4).
Table 3.
Factors that influence median PFS on second line immunotherapy in advanced metastatic melanoma patients.
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Factor | HR | CI 95% | p-Value | HR | CI 95% | p-Value |
| Age | 1.0 | 0.9–1.0 | 0.6772 | - | ||
| Sex | 0.91 | 0.7–1.2 | 0.5350 | - | ||
| LDH over ULN 2L | 0.57 | 0.4–0.8 | 0.0005 | 0.62 | 0.4–0.9 | 0.0069 |
| ECOG 0 2L | 0.24 | 0.1–0.5 | 0.0004 | 1.51 | 0.5–4.5 | 0.9237 |
| ECOG 1 2L | 0.42 | 0.2–0.8 | 0.4320 | 2.42 | 0.8–6.9 | 0.0179 |
| Brain metastases 2L | 0.58 | 0.4–0.8 | 0.0008 | 0.77 | 0.5–1.1 | 0.1807 |
| Liver metastases 2L | 0.55 | 0.4–0.8 | 0.0002 | 0.66 | 0.5–0.9 | 0.0208 |
| ORR 1L | 0.74 | 0.5–1.0 | 0.054 | 0.84 | 0.6–1.2 | 0.3408 |
| Time to PD 1L > 6 m | 1.49 | 1.1–2.0 | 0.0115 | 1.38 | 1.0–1.9 | 0.0666 |
LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group (performance status); PD, progression disease; ORR (CR + PR), overall response rate; PD, progressive disease; 1L, first line (treatment); 2L, second line (treatment); m, months, HR, hazard ratio; CI confidence interval.
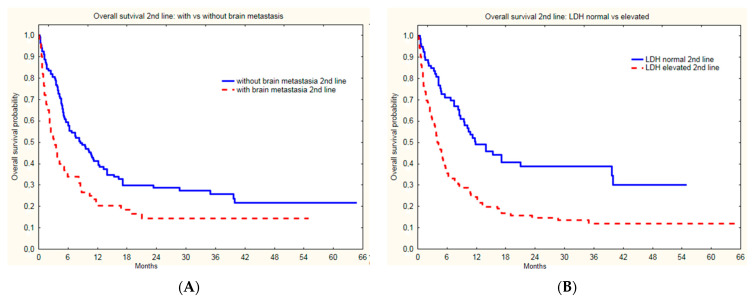
Figure 4.
Progression free survival of melanoma patients with and without brain metastases (A) and normal and elevated LDH level (B) since initiation of second line immunotherapy.
The presence of brain metastases (Figure 5A) and LDH level (Figure 5B) correlated with median OS (p < 0.0001 and p < 0.0001, respectively). Moreover, the presence of liver metastases at the initiation of second line therapy, i.e., immunotherapy, correlated with OS (Table 4).
Figure 5.
Overall survival of melanoma patients with and without brain metastases (A) and normal and elevated LDH level (B) since initiation of second line immunotherapy.
Table 4.
Factors that influence OS in metastatic melanoma patients treated with BRAFi/MEKi -> immunotherapy—treatment sequence.
| Factor | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | CI 95% | p-Value | HR | CI 95% | p-Value | |
| Age | 1.00 | 1.0–1.0 | 0.9848 | - | ||
| Sex | 1.14 | 0.8–1.6 | 0.4373 | - | ||
| Brain metastases 1L | 0.57 | 0.4–0.8 | 0.0310 | 0.62 | 0.4–0.9 | 0.0165 |
| Liver metastases 1L | 0.70 | 0.5–1.0 | 0.0406 | 0.88 | 0.6–1.3 | 0.4954 |
| Brain metastases 2L | 0.58 | 0.4–0.8 | 0.0011 | 0.44 | 0.3–0.6 | <0.0001 |
| Liver metastases 2L | 0.56 | 0.4–0.8 | 0.0007 | 0.69 | 0.5–1.0 | 0.0340 |
| LDH over ULN 1L | 0.61 | 0.4–0.9 | 0.0042 | 0.54 | 0.4–0.8 | 0.0018 |
| LDH over ULN 2L | 0.45 | 0.3–0.6 | <0.0001 | 0.49 | 0.3–0.7 | <0.0001 |
| Objective response in 1L | 0.79 | 0.6–1.1 | 0.1421 | 0.87 | 0.6–1.4 | 0.4326 |
| Objective response in 2L | 0.14 | 0.1–0.3 | <0.0001 | 0.13 | 0.1–0.2 | <0.0001 |
| Time to PD 1L > 6 m | 2.64 | 1.9–3.7 | <0.0001 | 2.8 | 1.9–4.1 | <0.0001 |
F, female; M, male; LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group (performance status); PD, progression disease; ORR (CR + PR), overall response rate; PD, progressive disease; 1L, first line (treatment); 2L, second line (treatment); m, months, HR, hazard ratio; CL, confidence interval.
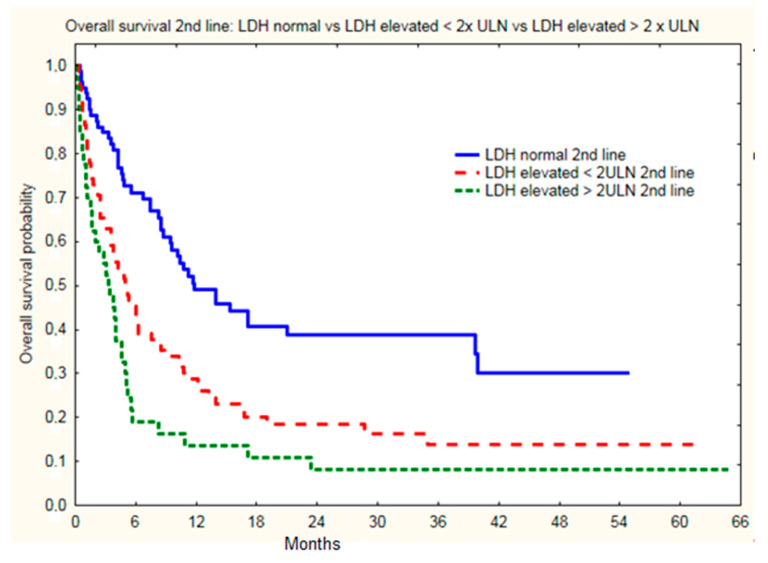
For anti-PD1 treatment, statistically significant differences were found in the OS of subgroups of patients with different LDH levels. The highest survival was observed in patients with normal LDH levels, where 24-month OS was 39% vs. 19% for patients with elevated LDH (<2ULN) vs. 8% for patients with LDH > 2ULN (p < 0.0001) (Figure 6). Other factors that correlated with OS achieved after initiation of second line treatment were presence with brain metastases both at treatment initiation as well as at second line therapy initiation (Table 4). None of the patients without brain and without liver metastases had normal LDH (Table 5). High LDH level also correlated with shorter survival in patients with liver (p = 0.004) metastases and in patients without brain metastases (p = 0.007). The greatest clinical benefit with second line immunotherapy and longest OS was observed in patients with LDH ≤ ULN and without liver or brain metastases at baseline (>2 years). Longer OS was also noted in patients with time to PD >6 months in first line (slow progression).
Figure 6.
Overall survival of melanoma patients with normal and elevated LDH level since initiation of second line immunotherapy.
Table 5.
LDH influence on OS in metastatic melanoma patients treated with BRAFi/MEKi -> immunotherapy—treatment sequence.
| LDH | Metastasis | Patients (n) | Median OS (Months) | |
|---|---|---|---|---|
| LDH normal | liver | without metastasis | 57 | 21.7 |
| with metastasis | 19 | 21.0 | ||
| brain | without metastasis | 69 | 25.2 | |
| with metastasis | 9 | 18.8 | ||
| liver and brain | without metastasis | 48 | 24.4 | |
| with metastasis | 0 | - | ||
| LDH elevated | liver | without metastasis | 68 | 16.2 |
| with metastasis | 50 | 12.2 | ||
| brain | without metastasis | 87 | 16.3 | |
| with metastasis | 37 | 9.0 | ||
| liver and brain | without metastasis | 49 | 19.0 | |
| with metastasis | 18 | 7.6 | ||
LDH, lactate dehydrogenase; OS, overall survival.
4. Discussion
In this analysis, the survival of unresectable and metastatic melanoma patients who received sequential therapy was investigated. Our real-world analysis utilizing nation-wide data from multiple reference melanoma reference centers confirms that treatment with second-line anti-PD-1 therapy prolongs overall survival in selected patients with advanced/metastatic BRAF-mutated melanoma, including patients with initially poor performance status. Patients with high tumor burden are likely to benefit from initial response to BRAFi/MEKi therapy and may continue treatment with anti-PD-1 as second line treatment. Our data is supported by other reports [33,34,35]. In the pooled analysis of phase 3 COMBI-d (NCT01584648) and COMBI-v (NCT01597908) trials that included 563 patients who received dabrafenib plus trametinib, 299 patients received subsequent anticancer therapies: 151 (51%) received an anti–CTLA-4 therapy and 102 (34%) received an anti–PD-1 therapy [34]. In a long-term KEYNOTE-006 trial follow up, BRAF-mutated melanoma patients who were not treated with a prior BRAFi lad longer PSF on pembrolizumab treatment (7.0 months) than those who were treated initially witb RAFi (2.8 months). Moreover, patients with BRAF V600E/K–mutant melanoma who received a previous BRAFi with or without MEKi had lower objective response rates (28.4% vs. 44.2%), four-year PFS (15.2% vs. 27.8%), and OS (26.9% vs. 49.3%), compared with those who had not received earlier targeted therapy [35]. In a phase IIa study (NCT02083354) of patients with unresectable or metastatic BRAF V600-mutant acral/cutaneous melanoma treated with dabrafenib (150 mg twice daily) with trametinib (2 mg once daily), treatment sequencing results were collected. Patients (n = 10/53) who were treated with a PD-1 inhibitor after PD on dabrafenib plus trametinib and achieved a median post progression survival (PPS) of 17.6 months (95%CI 16.9–28.3), which is similar to the OS reported by us [33].
Treatment beyond frontline therapy for patients with BRAF-mutated advanced/metastatic melanoma is currently under investigation in prospective trials. A first study that may provide more data is ImmunoCobiVem (NCT02902029), a German phase II trial comparing cobimetinib and vemurafenib followed by atezolizumab at PD versus atezolizumab in first line followed by targeted therapy [36]. In the phase III DREAMseq trial, also known as the ECOG-ACRIN EA6134 trial, treatment with nivolumab with ipilimumab resulted in an absolute 20% improvement in two-year OS over first line BRAFi/MEKi (dabrafenib plus trametinib). At the two-year analysis, the OS rate was 72% for patients treated with nivolumab with ipilimumab in first line and targeted therapy in second line. At the same time, the OS rate was only 52% for patients treated with dabrafenib plus trametinib as fist line therapy and with immunotherapy in second line. First line immunotherapy also led to durable responses. ORR for nivolumab with ipilimumab was 46% and for dabrafenib plus trametinib was 43%. After PD on first line therapy, response rates to second-line therapy were 48% for dabrafenib plus trametinib and 30% for nivolumab with ipilimumab. Response rates were similar for dabrafenib plus trametinib whether used first of second line. On the contrary, nivolumab with ipilimumab was less effective when used in second line. At a median follow-up of 27.7 months, 100 died, 38 patients who had first line of nivolumab with ipilimumab first and 62 in those treated with BRAFi/MEKi, which translates into a 20% difference in survival [23]. In the phase II Sequential Combo Immuno and Target Therapy (SECOMBIT, NCT02631447), treatment with nivolumab with ipilimumab followed by encorafenib plus binimetinib at PD (Arm B) and adopting the ‘sandwich’ strategy starting with encorafenib/binimetinib for eight weeks, then ipilimumab/nivolumab for eight weeks (arm C), improved survival rates over encorafenib plus binimetinib followed by nivolumab with ipilimumab were observed (Arm A). In this trial median OS was not yet reached in any of the arms, but the survival rate at two years was 73% for immunotherapy combination used first (arm B), 65% for the BRAFi/MEKi combination first (arm A), and 69% for the sandwich approach (arm C). At three years, it was 62%, 54%, and 60%, respectively. Moreover, the response rate to immunotherapy combination was 45% in the first line, but 25% in the second line, after PD on BRAFiMEKi [37,38]. Although data on immunotherapy combination use in first line treatment is interesting, we need to remember that not all patients will qualify for immunotherapy combination treatment and selected patients require fast response induction [39]. In the absence of known prognostic or predictive molecular biomarkers to determine the selection of BRAFi/MEKi as first line therapy, clinical factors are used to anticipate disease dynamics. We confirm that after PD, BRAFi/MEKi patients who are in good performance status still benefit from second line therapy. Patients who benefit with objective response on BRAFi/MEKi are expected to obtain additional OS benefit when offered second line therapy.
Real-word evidence, although of lower evidence level than randomized trials, helps to define the effectiveness of treatments in routine clinical practice, including subgroups of patients who are usually excluded or under-represented in the trials [40]. Limitations of real-world analysis could include patient selection bias due to the referral of patients in better performance status to immunotherapy or clinical trials. Moreover, in treatment outside of clinical trial, detailed patient data may be inconsistently collected during long-term observation or between different hospitals. In single cases, data on variables could be missed as a result of data collection bias. Nevertheless, due to the strict regulation of therapy reimbursement in Poland, data regarding melanoma patients (the description of regulations of national drug program is available here: https://www.gov.pl/web/zdrowie/choroby-onkologiczne—B59, accessed on 1 April 2022) must be recorded consistently in the whole country in a central electronic system. Our analysis may provide clinically significant information on second line immunotherapy in real-world practice in the melanoma field as well as prognostic biomarkers in these patients. Experienced researchers, data managers, and melanoma experts with interpretation skills were included in our research team in order to provide the proper interpretation of results. The treatment efficacy described by us complements the results reported by phase III registration trials and covers long-term observations of second line of therapy [40].
In our study, we have confirmed the significance of liver metastases as a negative biomarker for immunotherapy efficacy outside of clinical trials [41], which is expected due to their role in CD8+ T-cell elimination [42]. Moreover, our data support the role of LDH as negative prognostic biomarker in melanoma immunotherapy, namely in that a higher pre-treatment serum LDH level is predictive of shorter PFS [43]. High LDH levels are known to be inversely correlated with response to checkpoint inhibitors because LDH levels are associated with high tumor burden, enhanced glycolytic activity, and hypoxia-induced necrosis in the tumor niche. At the biochemical level, melanoma tumor-derived lactate increases the number of infiltrating MDSC in the tumors, as well as polarizes tumor-associated macrophages into immune suppressive M2 macrophages [44]. Most recent multi-omics analysis of metastatic melanoma patients identified no significant intra-tumoral molecular, immunological, or metabolic associations with serum LDH, including glycolysis, metabolism drivers, glucose metabolism, hypoxia, mTOR pathway, choline metabolism, checkpoint inhibitors expression, or genes of the adaptive and innate immune response. These authors have suggested that serum LDH serves as a surrogate of tumor burden, but not of tumor phenotype. Patients with elevated serum LDH levels had a significantly higher number of metastases in comparison to patients with normal serum LDH levels [45]. At the same time, intra-tumoral lactate dehydrogenase C isoform (LDHC) activation in melanoma cells provides a metabolic rescue pathway for these cells via the preference for lactate metabolism for ATP generation [46,47,48]. Clinical biomarkers seem to be surrogates of low immunity and potential immune-desert tumors, as also hypothesized by other authors [49]. Moreover, in the case of melanoma, more complex prognostic indexes could be developed in the future. At this point in time, the derived neutrophils/(leukocytes minus neutrophils) ratio, so called dNLR, along with LDH level, constitute an immune prognostic index that correlates with immunotherapy treatment outcome [47]. It may be expected that other clinical parameters, e.g., renal function or albumin levels, could be prognostic for immunotherapy, but not other types of treatment [47,48]. Additional translational analyses should be designed to directly measure immune signatures that could potentially correlate with anti-PD1 response after BRAFi/MEKi failure, as initial studies have not yet covered sequential treatment [50,51]. In terms of clinical practice, it may be expected that switching treatment to immunotherapy upon response to BRAFi/MEKi with LDH normalization could be an effective strategy to prolong OS. This is expected to be an effective approach in advanced melanoma patients with initial highly elevated serum LDH [52]. Others suggest that such patients may benefit from triple combination therapy [53,54].
5. Conclusions
Second line anti-PD1 immunotherapy is effective in BRAF-mutated melanoma patients after BRAFi/MEKi therapy failure. The presence of brain and liver metastases correlated with shorter PFS on second line treatment as well as the total OS of these patients. Poor performance status as well as high LDH levels are also clinical negative biomarkers that correlated with shorter survival. Patients with high tumor burden, including brain metastases, should be offered second line therapy, as about 20% may achieve an objective response, while another 20% may obtain some clinical benefit with disease stabilization. Further prospective studies of sequential treatment should include molecular and translational analysis to define additional predictive and prognostic biomarkers.
Acknowledgments
The authors thank Ewa Bartnik for text proofreading.
Author Contributions
Conceptualization and methodology, A.M.C., P.R. (Piotr Rutkowski), B.C.-S. and P.R. (Paweł Rogala); software, B.C.-S.; formal analysis, B.C.-S., P.R. (Paweł Rogala) and A.M.C.; investigation, all authors; resources, P.R. (Piotr Rutkowski); data curation, P.R. (Paweł Rogala), B.C.-S.; writing, A.M.C., P.R. (Paweł Rogala), B.C.-S. and P.R. (Piotr Rutkowski); writing—review and editing, A.M.C., P.R. (Paweł Rogala), B.C.-S. and P.R. (Piotr Rutkowski); visualization—P.R. (Paweł Rogala) and B.C.-S.; supervision, P.R. (Piotr Rutkowski) and A.M.C.; project administration, A.M.C. and B.C.-S.; funding acquisition, P.R. (Piotr Rutkowski). All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded with Maria Sklodowska-Curie National Research Institute of Oncology (Warsaw and Poland) statutory funding (Ministerstwo Edukacji i Nauki).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Maria Sklodowska-Curie National Research Institute of Oncology; ethical board approval: number KB/430-74/20.
Informed Consent Statement
This is retrospective non-interventional study and all the data analyzed were collected as a part of routine clinical practice for diagnosis and treatment. All the patients signed an informed consent form for treatment as per the standard operating procedures in our hospitals. In addition, the patients were diagnosed and treated following the national guidelines and policies. The treatment was covered according to the reimbursement regulations of National Health Fund (NFZ, Poland), based on the recommendations of the Polish Agency for Health Technology Assessment and Tariff System (AOTMiT).
Data Availability Statement
All data are available for research cooperation purposes from the PI of the study upon DTA approval.
Conflicts of Interest
PRu Financial Interests: Blueprint Medicines—advisory board; BMS—invited speaker, advisory board; Merck—advisory board, invited speaker; MSD—invited speaker, advisory board; Novartis—invited speaker; Pierre Fabre—invited speaker, advisory board; Sanofi—advisory board, invited speaker; BMS—institutional research grant; Pfizer—institutional research grant; Non-financial Interests: ASCO, Officer; Polish Society of Surgical Oncology, Member of Board of Directors., JM: advisory board—BMS, MSD; lectures—BMS; GSK, Roche, MSD, Novartis, Pierre-Fabre; travel reimbursement—BMS, GSK, Roche, MSD, Novartis, Pierre-Fabre. GKW: speakers’ bureau—Novartis, MSD, Pierre Fabre, Takeda, Recordati, Sanofi, BMS; advisory role: MSD, Recordati; travel reimbursement and fees—Pierre Fabre, Roche. MZ—BMS, MSD, Novartis—lectures and advisory boards outside of the scope of the study. Travel reimbursement and fees and speaker honoraria: AMC, BCS, RD, KK, PRo—BMS, MSD, Pierre Fabre, Novartis, Roche; RS, JS—BMS, MSD, Astellas Pharma; JM—BMS, GlaxoSmithKline, Roche, MSD, Novartis, Pierre Fabre; NKK —BMS, Roche. Others—none declared.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Keilholz U., Ascierto P., Dummer R., Robert C., Lorigan P., van Akkooi A., Arance A., Blank C., Sileni V.C., Donia M., et al. ESMO consensus conference recommendations on the management of metastatic melanoma: Under the auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020;31:1435–1448. doi: 10.1016/j.annonc.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Michielin O., van Akkooi A., Lorigan P., Ascierto P., Dummer R., Robert C., Arance A., Blank C., Sileni V.C., Donia M., et al. ESMO consensus conference recommendations on the management of locoregional melanoma: Under the auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020;31:1449–1461. doi: 10.1016/j.annonc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowski P., Wysocki P.J., Kozak K., Nasierowska-Guttmejer A., Jeziorski A., Wysocki W.M., Kalinka E., Świtaj T., Kamińska-Winciorek G., Czarnecka A.M., et al. Postępowanie diagnostyczno-terapeutyczne u chorych na czerniaki—zalecenia ekspertów. Onkol. W Prakt. Klin. Eduk. 2021 preprint . [Google Scholar]
- 4.Rutkowski P., Wysocki P.J., Nasierowska-Guttmejer A., Jeziorski A., Wysocki W.M., Kalinka E., Świtaj T., Kozak K., Kamińska-Winciorek G., Czarnecka A.M., et al. Czerniak Skóry. Onkol. W Prakt. Klin. Eduk. 2020;6:225–245. doi: 10.5603/OCP.2020.0021. [DOI] [Google Scholar]
- 5.Ascierto P.A., Dummer R., Melero I., Palmieri G., Giannarelli D., Abrami E., Curvietto M., Simeone E., Grimaldi A.M. SECOMBIT (sequential combo immuno and target therapy study): A three arms prospective, randomized phase II study to evaluate the best sequential approach with combo immunotherapy [ipilimumab (I)/nivolumab (N)] and combo target therapy [encorafenib (E)/binimetinib (B)] in patients with metastatic melanoma and BRAF mutation. J. Clin. Oncol. 2017;35:TPS9598. doi: 10.1200/jco.2017.35.15_suppl.tps9598. [DOI] [Google Scholar]
- 6.Atkins M.B., Lee S.J., Chmielowski B., Ribas A., Tarhini A.A., Truong T.-G., Davar D., O’Rourke M.A., Curti B.D., Brell J.M., et al. DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing): A phase III trial—ECOG-ACRIN EA6134. J. Clin. Oncol. 2021;39:356154. doi: 10.1200/JCO.2021.39.36_suppl.356154. [DOI] [Google Scholar]
- 7.Gibney G.T., Atkins M.B. Choice of first-line therapy in metastatic melanoma. Cancer. 2019;125:666–669. doi: 10.1002/cncr.31774. [DOI] [PubMed] [Google Scholar]
- 8.Hodi F.S., Chapman P.B., Sznol M., Lao C.D., Gonzalez R., Smylie M., Daniels G.A., Thompson J.A., Kudchadkar R., Sharfman W., et al. Safety and efficacy of combination nivolumab plus ipilimumab in patients with advanced melanoma: Results from a North American expanded access program (CheckMate 218) Melanoma Res. 2021;31:67–75. doi: 10.1097/CMR.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022;40:127–137. doi: 10.1200/JCO.21.02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascierto P.A., McArthur G.A., Dréno B., Atkinson V., Liszkay G., Di Giacomo A.M., Mandalà M., Demidov L., Stroyakovskiy D., Thomas L., et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 11.Dummer R., Ascierto P.A., Gogas H.J., Arance A., Mandala M., Liszkay G., Garbe C., Schadendorf D., Krajsova I., Gutzmer R., et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 12.Long G., Flaherty K., Stroyakovskiy D., Gogas H., Levchenko E., De Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2019;30:1848. doi: 10.1093/annonc/mdz221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutkowski P., Kozak K., Owczarek W., Świtaj T., Cybulska-Stopa B., Wysocki P., Wysocki W. Minimalne rekomendacje diagnostyczno-terapeutyczne u chorych na czerniaki w sytuacji ograniczonych zasobów i restrykcji związanych z pandemią SARS-CoV-2. Onkol. W Prakt. Klin. Eduk. 2020;6:69–73. [Google Scholar]
- 14.Czarnecka A.M., Teterycz P., Mariuk-Jarema A., Lugowska I., Rogala P., Dudzisz-Sledz M., Switaj T., Rutkowski P. Treatment Sequencing and Clinical Outcomes in BRAF-Positive and BRAF-Negative Unresectable and Metastatic Melanoma Patients Treated with New Systemic Therapies in Routine Practice. Target. Oncol. 2019;14:729–742. doi: 10.1007/s11523-019-00688-8. [DOI] [PubMed] [Google Scholar]
- 15.Ho P.C., Meeth K.M., Tsui Y.C., Srivastava B., Bosenberg M.W., Kaech S.M. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγ. Cancer Res. 2014;74:3205–3217. doi: 10.1158/0008-5472.CAN-13-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumimoto H., Imabayashi F., Iwata T., Kawakami Y. The BRAF–MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J. Exp. Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilieva K.M., Correa I., Josephs D.H., Karagiannis P., Egbuniwe I.U., Cafferkey M.J., Spicer J., Harries M., Nestle F.O., Lacy K.E., et al. Effects of BRAF Mutations and BRAF Inhibition on Immune Responses to Melanoma. Mol. Cancer Ther. 2014;13:2769–2783. doi: 10.1158/1535-7163.MCT-14-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharkey M.S., Lizée G., Gonzales M.I., Patel S., Topalian S.L. CD4+ T-Cell Recognition of Mutated B-RAF in Melanoma Patients Harboring the V599E Mutation. Cancer Res. 2004;64:1595–1599. doi: 10.1158/0008-5472.CAN-03-3231. [DOI] [PubMed] [Google Scholar]
- 19.Somasundaram R., Swoboda R., Caputo L., Otvos L., Weber B., Volpe P., Van Belle P., Hotz S., Elder D.E., Marincola F.M., et al. Human Leukocyte Antigen-A2–Restricted CTL Responses to Mutated BRAF Peptides in Melanoma Patients. Cancer Res. 2006;66:3287–3293. doi: 10.1158/0008-5472.CAN-05-1932. [DOI] [PubMed] [Google Scholar]
- 20.Frederick D.T., Piris A., Cogdill A., Cooper Z., Lezcano C., Ferrone C.R., Mitra D., Boni A., Newton L.P., Liu C., et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clin. Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia C.Y., Wang D.Y., Mason R., Smith J.L., Mckean M.A., Lo S., Guminski A.D., Long G.V., Carlino M.S., Atkinson V., et al. Activity of targeted therapy after failure of first-line immunotherapy in BRAF-mutant metastatic melanoma. J. Clin. Oncol. 2018;36:9532. doi: 10.1200/JCO.2018.36.15_suppl.9532. [DOI] [Google Scholar]
- 22.Rigo R., Doherty J., Koczka K., Kong S., Ding P.Q., Cheng T., Cheung W.Y., Monzon J.G. Real World Outcomes in Patients with Advanced Melanoma Treated in Alberta, Canada: A Time-Era Based Analysis. Curr. Oncol. 2021;28:3978–3986. doi: 10.3390/curroncol28050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogala P., Czarnecka A.M., Cybulska-Stopa B., Ostaszewski K., Piejko K., Ziętek M., Dziura R., Rutkowska E., Galus Ł., Kempa-Kamińska N., et al. Long-Term Outcomes of Targeted Therapy after First-Line Immunotherapy in BRAF-Mutated Advanced Cutaneous Melanoma Patients—Real-World Evidence. J. Clin. Med. 2022;11:2239. doi: 10.3390/jcm11082239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong B.Y., Carlino M.S., Menzies A.M. Biology and treatment of BRAF mutant metastatic melanoma. Melanoma Manag. 2016;3:33–45. doi: 10.2217/mmt.15.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin J., Lao C.D., Urba W.J., McDermott D.F., Horak C., Jiang J., Wolchok J.D. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: A pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1:433–440. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- 26.Simeone E., Grimaldi A., Festino L., Giannarelli D., Vanella V., Palla M., Curvietto M., Esposito A., Palmieri G., Mozzillo N., et al. Correlation between previous treatment with BRAF inhibitors and clinical response to pembrolizumab in patients with advanced melanoma. OncoImmunology. 2017;6:e1283462. doi: 10.1080/2162402X.2017.1283462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Audibert C., Stuntz M., Glass D. Treatment Sequencing in Advanced BRAF-Mutant Melanoma Patients: Current Practice in the United States. J. Pharm. Technol. 2017;34:17–23. doi: 10.1177/8755122517747089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cybulska-Stopa B., Rogala P., Czarnecka A.M., Galus Ł., Dziura R., Rajczykowski M., Rutkowski P. BRAF and MEK inhibitors rechallenge as effective treatment for patients with metastatic melanoma. Melanoma Res. 2020;30:465–471. doi: 10.1097/CMR.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 29.Czarnecka A.M., Sobczuk P., Rogala P., Świtaj T., Placzke J., Kozak K., Mariuk-Jarema A., Spałek M., Dudzisz-Śledź M., Teterycz P., et al. Efficacy of immunotherapy beyond RECIST progression in advanced melanoma: A real-world evidence. Cancer Immunol. Immunother. 2022:1–10. doi: 10.1007/s00262-021-03132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szatkowska L., Sieczek J., Tekiela K., Ziętek M., Stachyra-Strawa P., Cisek P., Matkowski R. Outcomes of Patients with Metastatic Melanoma—A Single-Institution Retrospective Analysis. Cancers. 2022;14:1672. doi: 10.3390/cancers14071672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czarnecka A.M., Ostaszewski K., Borkowska A., Szumera-Ciećkiewicz A., Kozak K., Świtaj T., Rogala P., Kalinowska I., Koseła-Paterczyk H., Zaborowski K., et al. Efficacy of Neoadjuvant Targeted Therapy for Borderline Resectable III B-D or IV Stage BRAF V600 Mutation-Positive Melanoma. Cancers. 2021;14:110. doi: 10.3390/cancers14010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 33.Mao L., Ding Y., Bai X., Sheng X., Dai J., Chi Z., Cui C., Kong Y., Fan Y., Xu Y., et al. Overall Survival of Patients With Unresectable or Metastatic BRAF V600-Mutant Acral/Cutaneous Melanoma Administered Dabrafenib Plus Trametinib: Long-Term Follow-Up of a Multicenter, Single-Arm Phase IIa Trial. Front. Oncol. 2021;11:3291. doi: 10.3389/fonc.2021.720044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nathan P.D., Robert C., Grob J.J., Stroyakovskiy D., Karaszewska B., Hauschild A., Levchenko E., Sileni V.C., Schachter J., Garbe C., et al. Five-year analysis on the long-term effects of dabrafenib plus trametinib (D + T) in patients with BRAF V600–mutant unresectable or metastatic melanoma. J. Clin. Oncol. 2019;37:9507. doi: 10.1200/JCO.2019.37.15_suppl.9507. [DOI] [Google Scholar]
- 35.Puzanov I., Ribas A., Robert C., Schachter J., Nyakas M., Daud A., Hamid O. Association of BRAF V600E/K mutation status and prior BRAF/MEK inhibition with pembrolizumab outcomes in advanced melanoma: Pooled analysis of 3 clinical trials. JAMA Oncol. 2020;8:1256–1264. doi: 10.1001/jamaoncol.2020.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karachaliou N., Gonzalez-Cao M., Sosa A., Berenguer J., Bracht J.W.P., Ito M., Rosell R. The combination of checkpoint immunotherapy and targeted therapy in cancer. Ann. Transl. Med. 2017;5:388. doi: 10.21037/atm.2017.06.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ascierto P., Mandala M., Ferrucci P., Rutkowski P., Guidoboni M., Fernandez A.A., Ferraresi V., Maiello E., Guida M., Del Vecchio M., et al. Lba45 First Report of Efficacy and Safety from the Phase Ii Study Secombit (Sequential Combo Immuno and Targeted Therapy Study) Ann. Oncol. 2020;31:S1173–S1174. doi: 10.1016/j.annonc.2020.08.2275. [DOI] [Google Scholar]
- 38.Ascierto P., Mandala M., Ferrucci P., Rutkowski P., Guidoboni M., Fernandez A.A., Ferraresi V., Maiello E., Guida M., Del Vecchio M., et al. LBA40 SECOMBIT: The best sequential approach with combo immunotherapy [ipilimumab (I) /nivolumab (N)] and combo target therapy [encorafenib (E)/binimetinib (B)] in patients with BRAF mutated metastatic melanoma: A phase II randomized study. Ann. Oncol. 2021;32:S1316–S1317. doi: 10.1016/j.annonc.2021.08.2118. [DOI] [Google Scholar]
- 39.Giugliano F., Crimini E., Tarantino P., Zagami P., Uliano J., Corti C., Ascierto P.A. Ascierto. First Line Treatment of Braf Mutated Advanced Melanoma: Does One Size Fit All? Cancer Treat. Rev. 2021;99:102253. doi: 10.1016/j.ctrv.2021.102253. [DOI] [PubMed] [Google Scholar]
- 40.Orlova K., Ledin E., Zhukova N., Orlova R., Karabina E., Volkonskiy M., Stroyakovskiy D., Yurchenkov A., Protsenko S., Novik A., et al. Real-World Experience with Targeted Therapy in BRAF Mutant Advanced Melanoma Patients: Results from a Multicenter Retrospective Observational Study Advanced Melanoma in Russia (Experience) (ADMIRE) Cancers. 2021;13:2529. doi: 10.3390/cancers13112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X., Ji Q., Yan X., Lian B., Si L., Chi Z., Sheng X., Kong Y., Mao L., Bai X., et al. The Impact of Liver Metastasis on Anti-PD-1 Monoclonal Antibody Monotherapy in Advanced Melanoma: Analysis of Five Clinical Studies. Front. Oncol. 2020;10:546604. doi: 10.3389/fonc.2020.546604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J., Green M.D., Li S., Sun Y., Journey S.N., Choi J.E., Rizvi S.M., Qin A., Waninger J.J., Lang X., et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J., Zhao J., Wang J., Sun C., Zhu X. Prognostic Value of Lactate Dehydrogenase for Melanoma Patients Receiving Anti-Pd-1/Pd-L1 Therapy: A Meta-Analysis. Medicine. 2021;100:e25318. doi: 10.1097/MD.0000000000025318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Wilpe S., Koornstra R., Den Brok M., De Groot J.W., Blank C., De Vries J., Gerritsen W., Mehra N. Lactate dehydrogenase: A marker of diminished antitumor immunity. OncoImmunology. 2020;9:1731942. doi: 10.1080/2162402X.2020.1731942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer G.M., Carapeto F.C.L., Joon A.Y., Haydu L.E., Chen H., Wang F., Van Arnam J.S., McQuade J.L., Wani K., Kirkwood J.M., et al. Molecular and immunological associations of elevated serum lactate dehydrogenase in metastatic melanoma patients: A fresh look at an old biomarker. Cancer Med. 2020;9:8650–8661. doi: 10.1002/cam4.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koslowski M., Türeci O., Bell C., Krause P., Lehr H.-A., Brunner J., Seitz G., Nestle F.O., Huber C., Sahin U. Multiple splice variants of lactate dehydrogenase C selectively expressed in human cancer. Cancer Res. 2002;62:6750–6755. [PubMed] [Google Scholar]
- 47.Mezquita L., Auclin E., Ferrara R., Charrier M., Remon J., Planchard D., Ponce S., Ares L.P., Leroy L., Audigier-Valette C., et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shouval R., Teper O., Fein J.A., Danylesko I., Tov N.S., Yerushalmi R., Avigdor A., Vasilev E., Magen H., Nagler A., et al. LDH and renal function are prognostic factors for long-term outcomes of multiple myeloma patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:1736–1743. doi: 10.1038/s41409-020-0829-1. [DOI] [PubMed] [Google Scholar]
- 49.Lindblad K.E., Lujambio A. Liver metastases inhibit immunotherapy efficacy. Nat. Med. 2021;27:25–27. doi: 10.1038/s41591-020-01190-9. [DOI] [PubMed] [Google Scholar]
- 50.Reschke R., Gussek P., Boldt A., Sack U., Köhl U., Lordick F., Gora T., Kreuz M., Reiche K., Simon J.-C., et al. Distinct Immune Signatures Indicative of Treatment Response and Immune-Related Adverse Events in Melanoma Patients under Immune Checkpoint Inhibitor Therapy. Int. J. Mol. Sci. 2021;22:8017. doi: 10.3390/ijms22158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scognamiglio G., Capone M., Sabbatino F., di Mauro A., Cantile M., Cerrone M., Madonna G., Grimaldi A.M., Mallardo D., Palla M., et al. The Ratio of Grzb(+)—Foxp3(+) over Cd3(+) T Cells as a Potential Predictor of Response to Nivolumab in Patients with Metastatic Melanoma. Cancers. 2021;13:2325. doi: 10.3390/cancers13102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schouwenburg M.G., Suijkerbuijk K.P., Koornstra R.H., Jochems A., Van Zeijl M.C., Eertwegh A.J.V.D., Haanen J.B., Aarts M.J., Van Akkooi A.C., Berkmortel F.W.V.D., et al. Switching to Immune Checkpoint Inhibitors upon Response to Targeted Therapy; The Road to Long-Term Survival in Advanced Melanoma Patients with Highly Elevated Serum LDH? Cancers. 2019;11:1940. doi: 10.3390/cancers11121940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dummer R., Long G.V., Robert C., Tawbi H.A., Flaherty K.T., Ascierto P.A., Nathan P.D., Rutkowski P., Leonov O., Dutriaux C., et al. Randomized Phase III Trial Evaluating Spartalizumab Plus Dabrafenib and Trametinib for BRAF V600–Mutant Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2022 doi: 10.1200/JCO.21.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitt A.M., Dumas L., Larkin J.M. Atezolizumab, cobimetinib, and vemurafenib as first-line treatment for unresectable metastatic BRAF V600 mutated melanoma. Expert Rev. Anticancer Ther. 2022;22:17–25. doi: 10.1080/14737140.2022.2017286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available for research cooperation purposes from the PI of the study upon DTA approval.