Abstract
Herbal products are gaining popularity in dental and medical practice nowadays due to their biocompatibility, higher antimicrobial activity, antioxidant and anti-inflammatory properties. Herbal medicine has experienced rapid growth in recent years due to its beneficial properties, ease of availability, and lack of side effects. As pathogenic bacteria become more resistant to antibiotics and chemotherapeutic agents, researchers are becoming more interested in alternative products and treatment choices for oral diseases. As a result, natural phytochemicals separated from plants and utilized in traditional medicine are suitable substitutes for synthetic chemicals. The aim of this review article is to list and understand several herbal alternatives that are currently accessible for use as efficient endodontic medicaments. The herbal products used in endodontics have several advantages, including safety, ease of use, increased storability, low cost, and a lack of microbial tolerance. However, preclinical and clinical testing and interactions with other materials and adverse effects are required for these herbal products.
Keywords: herbal medications, endodontics application, root canal, natural products
1. Introduction
Herbal products are gaining popularity in dental and medical practice nowadays due to their biocompatibility, higher antimicrobial activity, antioxidant and anti-inflammatory properties [1]. Herbal medicine is defined by the World Health Organization as a plant-originated preparation or material that includes processed or raw components from one or more plants that have medicinal properties [2]. The use of herbal alternatives for root canal treatment is becoming more popular. “Phytotherapy, Phytomedicine, or Ethnopharmacology” is the term for using herbals to treat various ailments. Herbal medicine has experienced rapid growth in recent years due to its beneficial properties, ease of availability, and lack side effects [3].
As pathogenic bacteria become more resistant to antibiotics and chemotherapeutic agents, researchers are becoming more interested in alternative products and treatment choices for oral diseases. As a result, natural phytochemicals separated from plants and utilized in traditional medicine are suitable substitutes for synthetic chemicals [4]. Herbs could be a good substitute for conventional treatments for oral health issues, but there is a lack of knowledge about their effect on oral tissues, mechanisms of action, and side effects. As a result, more research is needed to investigate these traditional medicines [5].
Biofilms are microbial communities that adhere to a specific surface and are shielded by a polymeric matrix [6]. The infected root canal system contains all three elements required to form a microbial community: solid residue, fluid streams, and microorganisms [7]. Herbal medicines are now being incorporated into toothpaste to help prevent dental caries. Polyphenols’ anti-cariogenic properties are primarily due to a direct effect on S. mutans, and it interacts with microbial membrane proteins to prevent bacterial cells from adhering to the tooth surface [8]. The promise of using non-ionizing radiation diagnostic tests in dentistry, as well as the challenges associated with its application, has prompted scientific research in this area to produce intriguing discoveries that bode well for the future. According to a large body of evidence gathered in every department of dentistry from the implementation of these diagnostic examinations, magnetic resonance imaging (MRI) and ultrasonic imaging represent the most exciting developments in this field [9].
Herbs are primarily used in endodontics for root canal disinfection. Due to the adverse effects of most synthetic intracanal medications, there has been an increase in research into herbal irrigants. The bacteria E. faecalis is the most common cause of root canal treatment failure [10]. The unique behavior of microorganisms, resulting from their organization in complex microbial communities, necessitates extra caution when treating root canal infectious diseases [11]. The thorough cleaning of the root canal system is among the most essential goals of endodontic treatment, free of microbes and debris, and it also involves removing infected tissue from within the root canal in order to seal it with a microbial tight filling and to prevent infection of the peri-radicular tissues and aid in their healing [12,13]. This can be accomplished by chemically treating the root canal system. This treatment includes biomechanical preparation, irrigation, and medication administered between appointments [14].
However, large portions of the root canal are left untouched during instrumentation, resulting in failure of the root canal treatment. Irrigation aids in the proper disinfection and eradication of infected microbes. NaOCl, chlorhexidine, hydrogen peroxide, ethylenediaminetetraacetic acid (EDTA), citric acid, and other irritants are used [15]. The advantages of employing herbal alternatives include easy availability, prolonged storage life, cost-effectiveness, minimal toxicity, and the lack of microbial resistance recorded so far [16]. Therefore, the aim of this review article is to list and understand several herbal alternatives that are currently accessible for use as efficient endodontic medicaments.
2. Methodology
The literature search was conducted indefinitely using the Google scholar, MEDLINE, PubMed databases (Table 1). Reference lists of potentially relevant articles and review articles in the English language were screened. The following keywords were used in the search strategy: Herbal Intervention in Endodontics, Herbal Medications used in Endodontics, Natural Products used in Endodontics. Only studies on herbal products in endodontics were considered; studies on other dental specialties were not. Some of the articles were labelled as reviews. Two reviewers (AHA and MIK) screened the articles.
Table 1.
Information of sources and Search strategies using MeSH keywords.
| Database | Search Strategies | Results |
|---|---|---|
| PubMed | ((((((((((((Herbal[Title/Abstract]) OR (Natural[Title/Abstract])) OR (Herbal products[Title/Abstract])) OR (Natural products[Title/Abstract])) OR (Herbal products in endodontics[Title/Abstract])) OR (Natural products in endodontics[Title/Abstract])) OR (Herbal intervention in endodontics[Title/Abstract])) OR (Root canal[Title/Abstract])) OR (Dental applications[Title/Abstract])) OR (Herbal endodontics[Title/Abstract])) AND (((((((Herbal medication[Title/Abstract]) OR (Endodontics applications[Title/Abstract])) OR (Herbal medications in endodontics[Title/Abstract])) OR (Herbal endodontics[Title/Abstract])) | 586 |
| Google Scholar | Herbal OR Natural OR Endodontics OR Root canal OR Application OR Herbal products OR Natural products OR Root canal system OR Endodontics OR Herbal endodontics OR Herbal medications OR Natural medications OR Herbal intervention AND Herbal products in endodontics OR Application of herbal medicine in endodontics OR Natural products used in endodontics | 1048 |
| MEDLINE | Herbal OR Natural OR Endodontics OR Root canal OR Application OR Herbal products OR Natural products OR Root canal system OR Endodontics OR Herbal endodontics OR Herbal medications OR Natural medications OR Herbal intervention AND Herbal products in endodontics OR Application of herbal medicine in endodontics OR Natural products used in endodontics | 389 |
| Total | 2023 | |
3. Results
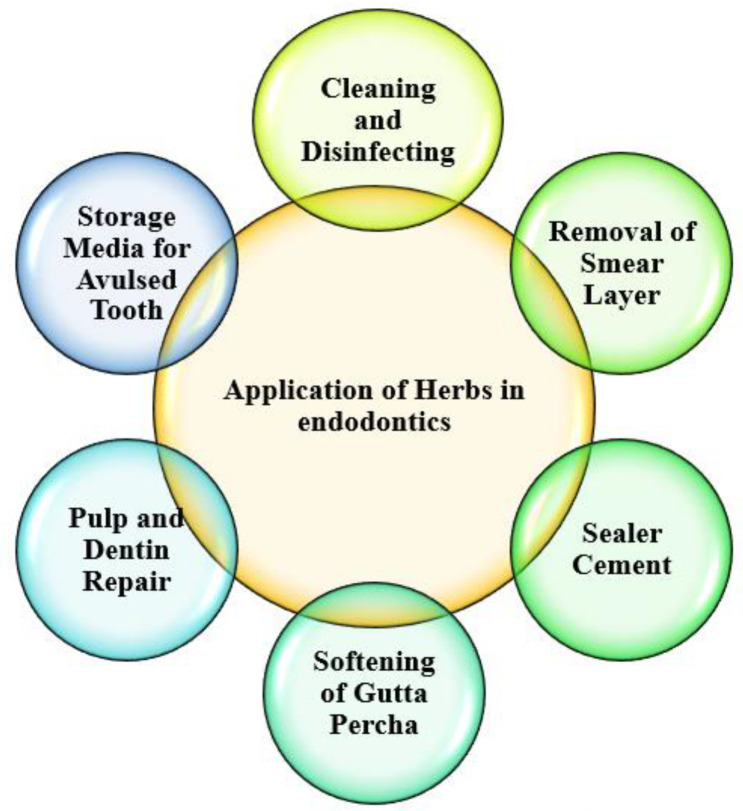
The search yielded 2023 articles, 1935 of which were eliminated due to non-compliance with the criteria of inclusion of the articles. Eighty-eight searched articles were selected as they met the inclusion criteria. Pulp and dentin repair, cleaning and disinfection, removal of smear layer, sealer cement for lubricating and aiding in the bonding of gutta-percha obturation material, elimination of obturation material by dissolving and softening it, and avulsed teeth storing media are all examples of herbal products used in endodontics. The different uses of herbal medications in endodontics are shown in Figure 1.
Figure 1.
Application of herbs in endodontics.
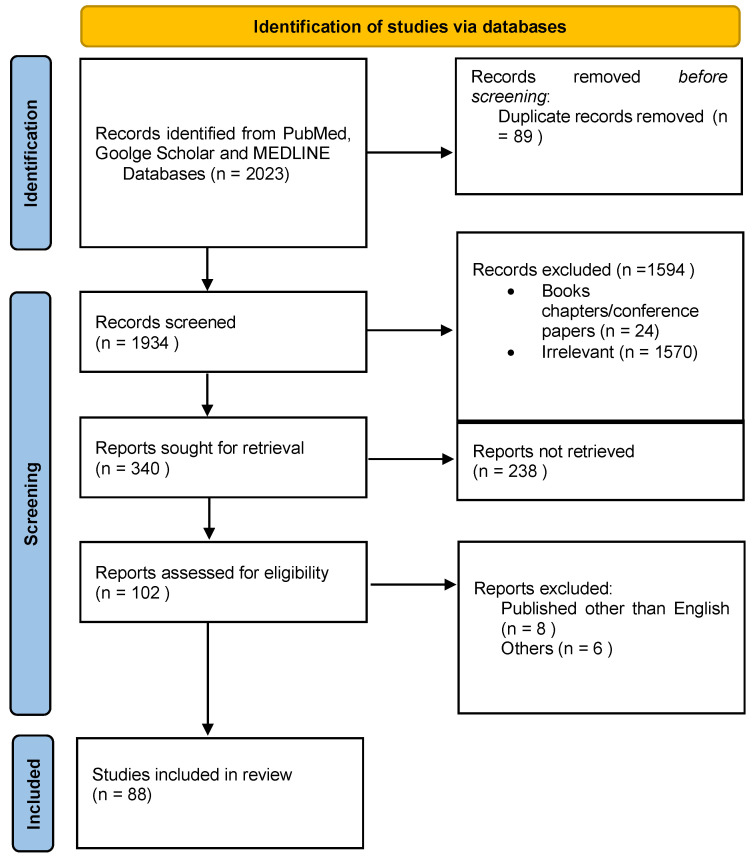
Figure 2 depicts the selection criteria as it follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. These 88 articles were examined for the current study based on the quality of the research studies.
Figure 2.
PRISMA flowchart showing the selection process of articles retrieved from different web sources.
4. Discussion
Over the past decade, there has been a significant surge of attention in preparations derived from medicinal plants. Many plants have been mentioned in the literature as a potential source of new endodontic therapies. In dentistry, phytomedicine has been utilized as an antibiotic, endodontic irrigant, anti-inflammatory, sedative, and analgesic [17]. Because most commercial intracanal medications cause cytotoxicity and are unable to eliminate microorganisms from dentinal tubules, biologic medications produced from natural plants have become popular in recent years [18].
4.1. Pulp and Dentin Repair
Pulp capping can be done with various materials to induce tissue repair dentinogenesis across the exposed pulp. The material chosen is calcium hydroxide, which is widely accepted. Although many researchers have advocated direct bonding of pulp exposure, it is still controversial [19]. Natural herbs could also be a feasible pulp capping option. According to a group of researchers, the natural product called baicalin, a flavonoid extracted from the plant’s root, enhanced the angiogenesis and odontoblastic differentiation of Human Dental Pulp Cells by promoting angiogenic factors, mineralization of alkaline phosphatase activity, and morphogenetic protein expression [20]. Lower amounts of ascorbic acid increased chondrogenesis and osteogenesis while decreased adipogenesis in stem cells from human exfoliated deciduous tooth. Ascorbic acid increased the release of growth factors, anti-inflammatory cytokines, and bone metabolism-related factors. Stem cells from human exfoliated deciduous tooth treated with ascorbic acid may offer novel therapy options for tooth and bone regeneration. It may be used in pediatric pulp capping, apexogenesis, cleft lip, and other congenital abnormalities [21]. Various studies related to the use of herbal medicine for the pulp and dentin repair used in endodontics are illustrated in Table 2
Table 2.
Studies related to the use of herbal medicine for pulp and dentin repair.
| Herbal Material | Characteristics | Reference |
|---|---|---|
| Propolis |
|
[22,23] |
| Baicalin |
|
[24,25] |
| Acemannan |
|
[26] |
| Galla Chinensis Extract |
|
[27] |
| Nigella stevia |
|
[28] |
| Genipin |
|
[29] |
| Green Tea Polyphenols |
|
[30,31] |
4.2. Cleaning and Disinfection
Irrigants have used a variety of synthetic antimicrobial agents to clean and disinfect root canals over the years. Because of rising resistance to antibiotics to certain antimicrobials, as well as the toxic and unpleasant side effects of a few prevalent antibacterial agents, there is a need for alternative agents that are nontoxic, inexpensive, and effective. Natural plant extracts have been discovered to clean and disinfect root canal irrigants [29] effectively. An endodontic irrigant should have the following properties: it should be nontoxic systemically, it should not cause an anaphylactic reaction, it should not harm periodontal tissues, it should have a robust antibacterial spectrum, it must be able to solubilize or prevent the development of a smear layer, as well as disintegrate the necrotic pulp tissue and deactivate endotoxins [15].
Sodium hypochlorite is amongst the most commonly utilized root canal irrigants because of its ability to kill a wide range of bacteria. Allergic potential, tissue toxicity, an unpleasant taste, and the failure to eradicate the smear layer are some of its disadvantages [32]. Because of its broad-spectrum antibacterial activity, chlorhexidine is another commonly used antimicrobial drug for irrigating root canals, disinfect infected root canals and biocompatibility [33]. However, it lacks tissue disintegrating properties and has some adverse side effects, such as discoloring the teeth, causing oral dryness, and even causing a burning sensation in the mouth [34]. Table 3 illustrates the studies related to herbal medicine for cleaning and disinfecting root canals.
Table 3.
Studies related to the use of herbal medicine for cleaning and disinfecting root canals.
| Herbal Material | Characteristics | References |
|---|---|---|
| Triphala |
|
[30,35] |
| Azadiratcha Indica (Neem) |
|
[36,37] |
| Propolis (Bee glue) |
|
[38] |
| Marticariarecutitia L (German chamomile) |
|
[39] |
| Melaleuca alternifolia (Tea tree oil) |
|
[1,40] |
| Curcuma longa (Turmeric) |
|
[41,42] |
| Salvadora persica (Miswak) |
|
[43,44] |
| Allium sativum (Garlic) |
|
[32,45] |
| Morinda citrifolia (Indian mulberry) |
|
[30] |
| Carvacrol |
|
[46] |
| Myrtus communis |
|
4.3. Removal of Smear Layer
Because microorganisms are found in all sections of the root canal system, notably in lateral canals, anastomoses, and dentinal tubules, mechanical preparation alone is ineffective in removing pulpal remains and microorganisms from root canals [47]. To eliminate the smear layer, chelating chemicals such as citric acid, ethylenediaminetetraacetic acid (EDTA), and maleic acid have been utilized. However, dentinal erosions, decreases in dentin microhardness, biocompatibility issues, and allergic reactions have all been reported as side effects of employing these chemical formulations [48,49]. The most commonly used smear layer remover is EDTA, which aids root canal cleaning by interacting with inorganic debris. Its contact with calcium ions in dentine promotes chelation of calcium, resulting in dentine demineralization in 5 min at 20–30 lm depths [50]. Table 4 shows the studies related to the use of herbal medicine for removal of smear layer.
Table 4.
Studies related to the use of herbal medicine for removal of smear layer.
| Herbal Material | Characteristics | References |
|---|---|---|
| Green tea extract |
|
[51] |
| Neem leaf extract |
|
[52] |
| Chitosan |
|
[50] |
| Oregano extract solution |
|
[53] |
| Triphala |
|
[54] |
| Morinda citrifolia juice |
|
[55] |
| Matricaria recutita extract |
|
[56] |
| Noni juice, citrus and carbonic acid juice |
|
[57] |
| Salvadora persica (Miswak) |
|
[58] |
4.4. Sealer Cement for Lubricating and Aiding in the Bonding of Gutta-Percha Obturation Material
The purpose of root canal therapy is to remove bacterial infections. Because the microorganisms persist in the dentinal tubules and root canal after the instrumentation, only chemo-mechanical canal preparation may not achieve the goal of root canal treatment [59]. It is desirable to employ sealers with the strong sealing ability and antimicrobial properties [60]. Because endodontic medications come into contact with tissues peri-apically, the sealers must be minimally cytotoxic [61]. Researchers are looking for natural alternatives to synthetic pharmaceuticals due to the ongoing rise in antibiotic resistance strains and adverse effects induced by synthetic drugs. Even though herbs have a wide range of applications in medicine, there have been few investigations in dentistry. Table 5 demonstrates the studies related to herbal medicine in combination with commercially available endodontic sealers.
Table 5.
Studies related to the use of herbal medicine in combination with commercially available endodontic sealers.
| Herbal Material | Characteristics | References |
|---|---|---|
| Licorice, Bakul and Guguchi added to the commercial root canal sealers |
|
[62] |
| Amla, Miswak and Nutmeg added to the commercial sealers |
|
[63] |
| Cinnamon oil |
|
[64] |
| Moringa root |
|
[65] |
| Tamarillo skin extract |
|
[66] |
| Propolis and Green tea |
|
[67] |
4.5. Removal of Obturation Material by Softening and Dissolving
When nonsurgical root canal therapy fails, an endodontically failed tooth may require treatment. The failure of nonsurgical endodontic therapy has been linked to several factors, including insufficient root canal system debridement and procedural mistakes. Gutta-percha is the most often used obturation substance [68]. A great deal of effort has developed a therapeutically viable procedure for removing this debris from the root canal. Thermal, mechanical, chemical, or a mix of these approaches are employed to remove the gutta-percha [69]. Although chemicals have been used for years to remove gutta-percha, the most successful chemicals are toxic or otherwise dangerous. Benzene, for example, has good solvent properties but is extremely flammable, with a flash point ranging from 0 to 12 degrees Celsius [70]. One of the most efficient solvents is chloroform. Alternative materials such as xylol, halothane, and tetrachloroethylene have arisen due to their possible carcinogenicity [71]. In dentistry, essential oils are utilized as gutta-percha solvents, smear layer removers, and antibacterial agents [71]. The intrinsic safety of essential oils encourages them to be searched for alternate solvents to chloroform. Table 6 illustrates the studies related to herbal medication for softening and dissolving gutta-percha.
Table 6.
Studies related to the use of herbal medication for softening and dissolving of gutta-percha.
| Herbal Material | Characteristics | References |
|---|---|---|
| Castor oil, Peppermint oil and Wintergreen oil |
|
[72] |
| Clove oil, Orange oil and Eucalyptus oil |
|
[73] |
| Cardamom seeds Oil and dry ginger rhizomes |
|
[71] |
| Tea tree oil |
|
[74] |
| Turmeric |
|
[75] |
| Tangerine, Grapefruit, Lemon Oils and Lime |
|
[76] |
4.6. Avulsed Teeth Storing Media
Traumatic injuries to the anterior teeth are most common in children aged 7 to 10, with tooth evulsion occurring in 0.5 per cent to 16 per cent of cases. One of the most severe types of dental trauma is avulsion injury [77]. The vitality of the periodontal ligament (PDL) cells persisting on the root surface, the quality of the root cementum, and minimum bacterial contamination determine the prognosis of a transplanted tooth and its preservation on the dental arch for the maximum time duration, which is directly connected to extra-alveolar time, storage form after avulsion, and root surface modifications [78]. Immediate tooth replantation improves PDL repair and dramatically minimizes the risk of root resorption. As a result, cutting the time between trauma and tooth replantation as short as feasible and keeping the avulsed tooth in a suitable transport medium might reduce the adverse effects of the extrabuccal period on the root surface and improve the prognosis significantly [79]. Numerous research has been conducted on various storage media that may aid in the vitality of periodontal ligament cells [80]. However, none of the currently employed media can meet all the ideal parameters for preserving cell viability. As a result, the search for an appropriate storage medium continues. Herbal products are readily available for the trauma site may benefit adequate storage capacity and cell viability [81]. Table 7 shows the studies related to herbal medicine for the storage of avulsed teeth.
Table 7.
Studies related to the use of herbal medication for storage of avulsed tooth.
| Herbal Material | Characteristics | References |
|---|---|---|
| Vaccinium macrocarpon, Punicia granatum, Psidium guajava, Prunus domestica and Camellia sinensis |
|
[81] |
| Propolis |
|
[82] |
| Green tea extract |
|
[83] |
| Morus rubra (red mulberry) |
|
[84] |
| Coconut water |
|
[85] |
| Aloe vera |
|
[86] |
| Rice water |
|
[87] |
| Honey |
|
[88] |
5. Future Implications and Recommendations
Herbs and other natural remedies are employed in endodontics to minimize inflammation and alleviate tissue irritation. As mentioned above and their products, the substances found in the herbs have been demonstrated to have antimicrobial activity against oral bacteria such as S. mutans, Candida albicans, and other pathogens in research. However, preclinical and clinical testing and interactions with other materials and adverse effects are required for these herbal products. There is still much room to learn about nature and its products to use in our profession. These herbal medications can be advantageous in nations where the bulk of the population cannot afford pricey therapies due to their advantages. However, further research is needed before being used in standard endodontic treatment.
6. Conclusions
Herbal products used in endodontics have several advantages, including safety, ease of use, increased storability, low cost, and a lack of microbial tolerance. Today is the era of scientific proof medicine, which means that any pharmaceutical intended for human use must undergo comprehensive in vitro and in vivo testing. Herbal products appear promising in vitro, but biocompatibility and safety must be evaluated in preclinical and clinical research before they can be definitively suggested to be used in endodontics. Herbs are generally harmless when appropriately used, but they can be hazardous if taken incorrectly.
Author Contributions
Conceptualization, M.I.K., A.H.A., T.Y.N. and G.A.S.; methodology, M.I.K.; software, A.H.A. and A.M.; validation, A.A.A., and S.N.B.; formal analysis, M.I.K. and A.M.P.; investigation, M.I.K. and A.H.A.; resources, A.A.A., A.M.P. and P.M.; data curation, M.I.K. and A.H.A.; writing—original draft preparation, M.I.K. and A.H.A.; writing—review and editing, A.A.A., S.N.B., A.M. and P.M.; supervision, M.I.K., T.Y.N. and G.A.S.; project administration, M.I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No associated data marked.
Conflicts of Interest
The authors declare no conflict of interest related to this article.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parle M., Bansal N. Herbal Medicines: Are They Safe? Natural Product Radiance, 2006. [(accessed on 10 April 2022)]. Volume 5. Available online: http://nopr.niscair.res.in/bitstream/123456789/7991/1/NPR%205(1)%206-14.pdf.
- 2.Murray P.E., Farber R.M., Namerow K.N., Kuttler S., Garcia-Godoy F. Evaluation of Morinda citrifolia as an endodontic irrigant. J. Endod. 2008;34:66–70. doi: 10.1016/j.joen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Sivakumar A., Ravi V., Prasad A., Sivakumar J. Herbendodontics–Phytotherapy in endodontics: A review. Biomed. Pharmacol. J. 2018;11:1073–1082. doi: 10.13005/bpj/1468. [DOI] [Google Scholar]
- 4.Badole G., Bahadure R., Kubde R. Herbal medicines in endodontics: An overview. J. Dent. Oral Disord. 2016;2:1046. [Google Scholar]
- 5.Taheri J.B., Azimi S., Rafieian N., Zanjani H.A. Herbs in dentistry. Int. Dent. J. 2011;61:287–296. doi: 10.1111/j.1875-595X.2011.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitchurch C.B., Tolker-Nielsen T., Ragas P.C., Mattick J.S. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 7.Haldal S., Arafath K.M.Y., Subair K., Joseph K. Biofilms in endodontics. J. Int. Oral Health. 2016;8:827. [Google Scholar]
- 8.Ferrazzano G.F., Amato I., Ingenito A., Zarrelli A., Pinto G., Pollio A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules. 2011;16:1486–1507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reda R., Zanza A., Cicconetti A., Bhandi S., Miccoli G., Gambarini G., Di Nardo D. Ultrasound imaging in dentistry: A literature overview. J. Imaging. 2021;7:238. doi: 10.3390/jimaging7110238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart C.H., Schwartz S.A., Beeson T.J., Owatz C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006;32:93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Voina-Țonea A., Văleanu M., Baciu S., Lazăr M., Voina C., Miron A., Vodnar D. Antimutagenic and antimicrobial effect of an experimental plant based extract for endodontic usage. Farmacia. 2018;66:107–114. [Google Scholar]
- 12.Abul N., Karthick K., Mathew S., Deepa N. Herbal extracts in endodontics. J. Indian Acad. Dent. Spec. Res. 2017;4:23–27. doi: 10.4103/jiadsr.jiadsr_18_17. [DOI] [Google Scholar]
- 13.Abedi-Amin A., Luzi A., Giovarruscio M., Paolone G., Darvizeh A., Agulló V.V., Sauro S. Innovative root-end filling materials based on calcium-silicates and calcium-phosphates. J. Mater. Sci. Mater. Med. 2017;28:31. doi: 10.1007/s10856-017-5847-1. [DOI] [PubMed] [Google Scholar]
- 14.Chandak M., Nikhade P., Chandak R., Bajaj P., Relan K., Chandak P., Rathi C., Chandak M. Herbal Sealers in Endodontics–A Systematic Review. Int. J. Res. Pharm. Sci. 2020;11:1278–1285. doi: 10.26452/ijrps.v11iSPL4.4289. [DOI] [Google Scholar]
- 15.Zehnder M. Root canal irrigants. J. Endod. 2006;32:389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Kaur K., Kumar T., Mittal S., Bansal R. Phytomedicine: Herbal venture in green endodontics. Endodontology. 2018;30:98. doi: 10.4103/endo.endo_115_17. [DOI] [Google Scholar]
- 17.Sinha D.J., Sinha A.A. Natural medicaments in dentistry. AYU. 2014;35:113. doi: 10.4103/0974-8520.146198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palombo E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid.-Based Complement. Altern. Med. 2011;2011:680354. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane L.E. Hard tissue barrier formation after pulp capping? Evid.-Based Dent. 2006;7:95. doi: 10.1038/sj.ebd.6400445. [DOI] [PubMed] [Google Scholar]
- 20.Almadi E.M., Almohaimede A.A. Natural products in endodontics. Saudi Med. J. 2018;39:124. doi: 10.15537/smj.2018.2.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhandi S., Alkahtani A., Mashyakhy M., Abumelha A.S., Albar N.H.M., Renugalakshmi A., Alkahtany M.F., Robaian A., Almeslet A.S., Patil V.R. Effect of Ascorbic Acid on Differentiation, Secretome and Stemness of Stem Cells from Human Exfoliated Deciduous Tooth (SHEDs) J. Pers. Med. 2021;11:589. doi: 10.3390/jpm11070589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabir A., Tabbu C.R., Agustiono P., Sosroseno W. Histological analysis of rat dental pulp tissue capped with propolis. J. Oral Sci. 2005;47:135–138. doi: 10.2334/josnusd.47.135. [DOI] [PubMed] [Google Scholar]
- 23.Parolia A., Kundabala M., Rao N., Acharya S., Agrawal P., Mohan M., Thomas M. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal. Aust. Dent. J. 2010;55:59–64. doi: 10.1111/j.1834-7819.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 24.Lei L., Tan W.-X., Wu K.-K., Wu L., Yu M., Zhan L.-H. The clinical application of bacailin in pulp capping in acute deep caries. J. Jinan Univ. (Med. Ed.) 2008;4 [Google Scholar]
- 25.Lee S.-I., Kim S.-Y., Park K.-R., Kim E.-C. Baicalein promotes angiogenesis and odontoblastic differentiation via the BMP and Wnt pathways in human dental pulp cells. Am. J. Chin. Med. 2016;44:1457–1472. doi: 10.1142/S0192415X16500816. [DOI] [PubMed] [Google Scholar]
- 26.Songsiripradubboon S., Banlunara W., Sangvanich P., Trairatvorakul C., Thunyakitpisal P. Clinical, radiographic, and histologic analysis of the effects of acemannan used in direct pulp capping of human primary teeth: Short-term outcomes. Odontology. 2016;104:329–337. doi: 10.1007/s10266-015-0215-4. [DOI] [PubMed] [Google Scholar]
- 27.Safy R.K., Ragab M.H. Comparative Histological Study of Two Different Pulp Capping Agents in Rabbits Teeth. Egypt. Dent. J. 2019;65:2699–2707. doi: 10.21608/edj.2019.72631. [DOI] [Google Scholar]
- 28.Omar O.M., Khattab N.M., Khater D.S. Nigella sativa oil as a pulp medicament for pulpotomized teeth: A histopathological evaluation. J. Clin. Pediatric Dent. 2012;36:335–341. doi: 10.17796/jcpd.36.4.n6674435856q86w8. [DOI] [PubMed] [Google Scholar]
- 29.Kwon Y.-S., Lim E.-S., Kim H.-M., Hwang Y.-C., Lee K.-W., Min K.-S. Genipin, a cross-linking agent, promotes odontogenic differentiation of human dental pulp cells. J. Endod. 2015;41:501–507. doi: 10.1016/j.joen.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Prabhakar J., Senthilkumar M., Priya M., Mahalakshmi K., Sehgal P., Sukumaran V. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: An in vitro study. J. Endod. 2010;36:83–86. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Taylor P.W., Hamilton-Miller J.M., Stapleton P.D. Antimicrobial properties of green tea catechins. Food Sci. Technol. Bull. 2005;2:71. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain P., Ranjan M. Role of herbs in root canal irrigation—A review. IOSR J. Pharm. Biol. Sci. 2014;9:6–10. doi: 10.9790/3008-09260610. [DOI] [Google Scholar]
- 33.Kanisavaran Z.M. Chlorhexidine gluconate in endodontics: An update review. Int. Dent. J. 2008;58:247–257. doi: 10.1111/j.1875-595X.2008.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 34.Kamat S., Rajeev K., Saraf P. Role of herbs in endodontics: An update. Endodontology. 2011;23:98–101. [Google Scholar]
- 35.Divya S., Sujatha S. Evaluation of Antimicrobial effect of Triphala versus conventional root canal irrigants in primary teeth-An In vivo study. Res. J. Pharm. Technol. 2019;12:655–659. doi: 10.5958/0974-360X.2019.00116.1. [DOI] [Google Scholar]
- 36.Dutta A., Kundabala M. Comparative anti-microbial efficacy of Azadirachta indica irrigant with standard endodontic irrigants: A preliminary study. J. Conserv. Dent. 2014;17:133. doi: 10.4103/0972-0707.128047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nayak A., Nayak R., Soumya B., Bhat K., Kudalkar M. Evaluation of antibacterial and anticandidial efficacy of aqueous and alcoholic extract of Neem (Azadirachta indica) an in vitro study. Int. J. Res. Ayurveda Pharm. 2011;2:230–235. [Google Scholar]
- 38.Tyagi S.P., Sinha D.J., Garg P., Singh U.P., Mishra C.C., Nagpal R. Comparison of antimicrobial efficacy of propolis, Morinda citrifolia, Azadirachta indica (Neem) and 5% sodium hypochlorite on Candida albicans biofilm formed on tooth substrate: An in-vitro study. J. Conserv. Dent. 2013;16:532. doi: 10.4103/0972-0707.120973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadr Lahijani M., Raoof Kateb H., Heady R., Yazdani D. The effect of German chamomile (Marticaria recutita L.) extract and tea tree (Melaleuca alternifolia L.) oil used as irrigants on removal of smear layer: A scanning electron microscopy study. Int. Endod. J. 2006;39:190–195. doi: 10.1111/j.1365-2591.2006.01073.x. [DOI] [PubMed] [Google Scholar]
- 40.Kamath U., Sheth H., Ramesh S., Singla K. Comparison of the antibacterial efficacy of tea tree oil with 3% sodium hypochlorite and 2% Chlorhexidine against E. faecalis: An in vitro study. J. Contemp. Dent. 2013;3:117–120. [Google Scholar]
- 41.Jori G., Coppellotti O. Inactivation of Pathogenic Microorganisms by Photodynamic Techniques: Mechanistic Aspects and Perspective Applications. Anti-Infect. Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Infect. Agents) 2007;6:119–131. [Google Scholar]
- 42.Neelakantan P., Subbarao C., Subbarao C.V. Analysis of antibacterial activity of curcumin against Enterococcus faecalis. Int. J. Curr. Res. Rev. 2011;3:37–42. [Google Scholar]
- 43.Al-Sabawi N., Al Sheikh Abdal A., Taha M.Y. The antimicrobial activity of Salvadora persica solution (miswak-siwak) as root canal irrigant (a comparative study) Univ. Sharjah J. Pure Appl. Sci. 2007;4:69–91. [Google Scholar]
- 44.Almas K. The effect of Salvadora persica extract (miswak) and chlorhexidine gluconate on human dentin: A SEM study. J. Contemp. Dent. Pract. 2002;3:27–35. doi: 10.5005/jcdp-3-3-27. [DOI] [PubMed] [Google Scholar]
- 45.Gopalakrishnan S., Rajesh S., Jotish R. A comparative evaluation of antimicrobial efficacy of cinnamon and garlic as endodontic irrigants against Enterococcus faecalis—An in vitro study. Endodontology. 2014;26:149–157. [Google Scholar]
- 46.Seghatoleslami S., Samadi N., Salehnia A., Azimi S. Antibacterial activity of endemic Satureja Khuzistanica Jamzad essential oil against oral pathogens. Iran. Endod. J. 2009;4:5. [PMC free article] [PubMed] [Google Scholar]
- 47.Siqueira Jr J.F., Lima K.C., Magalhães F.A., Lopes H.P., de Uzeda M. Mechanical reduction of the bacterial population in the root canal by three instrumentation techniques. J. Endod. 1999;25:332–335. doi: 10.1016/S0099-2399(06)81166-0. [DOI] [PubMed] [Google Scholar]
- 48.Teja K.V., Janani K., Srivastava K.C., Shrivastava D., Jose J., Marya A., Karobari M.I. Comparison of Herbal Agents with Sodium Hypochlorite as Root Canal Irrigant: A Systematic Review of In Vitro Studies. Evid.-Based Complement. Altern. Med. 2021;2021:8967219. doi: 10.1155/2021/8967219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim T., Wee T., Choi M., Koh W., Sae-Lim V. Light and scanning electron microscopic evaluation of Glyde™ File Prep in smear layer removal. Int. Endod. J. 2003;36:336–343. doi: 10.1046/j.1365-2591.2003.00648.x. [DOI] [PubMed] [Google Scholar]
- 50.Silva P., Guedes D., Nakadi F., Pécora J., Cruz-Filho A. Chitosan: A new solution for removal of smear layer after root canal instrumentation. Int. Endod. J. 2013;46:332–338. doi: 10.1111/j.1365-2591.2012.02119.x. [DOI] [PubMed] [Google Scholar]
- 51.Sebatni M., Kumar A. Smear layer removal efficacy of herbal extracts used as endodontic irrigants: An in vitro study. Endodontology. 2017;29:35. [Google Scholar]
- 52.Ghonmode W.N., Balsaraf O.D., Tambe V.H., Saujanya K., Patil A.K., Kakde D.D. Comparison of the antibacterial efficiency of neem leaf extracts, grape seed extracts and 3% sodium hypochlorite against E. feacalis–An in vitro study. J. Int. Oral Health. 2013;5:61. [PMC free article] [PubMed] [Google Scholar]
- 53.Ok E., Adanir N., Ozturk T. Antibacterial and smear layer removal capability of oregano extract solution. Eur. J. Dent. 2015;9:20–24. doi: 10.4103/1305-7456.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prabhakar J., Mensudar R., Geethapriya N., Prabhakar J. Cleaning Efficacy of Triphala (An Indian Herbal Medicine) and Green Tea Polyphenol Used as Irrigants on Removal of Smear Layer: A Sem Study. Biomed. Pharmacol. J. 2015;8:303. [Google Scholar]
- 55.Saghiri M.A., García-Godoy F., Asgar K., Lotfi M. The effect of Morinda Citrifolia juice as an endodontic irrigant on smear layer and microhardness of root canal dentin. Oral Sci. Int. 2013;10:53–57. doi: 10.1016/S1348-8643(12)00073-0. [DOI] [Google Scholar]
- 56.Saha S.G., Singh R., Bhardwaj A., Vijaywargiya P., Billore J., Saxena D. Efficacy of smear layer removal by two Ayurvedic herbal irrigants, using continuous vs. syringe and needle irrigation. Endodontology. 2019;31:72. [Google Scholar]
- 57.Chandrasekhar H. Evaluation of Natural Chelating Agents in Smear Layer Removal. Int. J. Res. Trends Innov. 2021;6:24–26. [Google Scholar]
- 58.Elkhashab R.A.M., Kataiab M.A., Kamel W.H., Shaaban M. Comparison of antibacterial effect and smear layer removal of herbal versus traditional irrigants—An in vitro study. Future Dent. J. Egypt. 2018;4:165–169. doi: 10.1016/j.fdj.2018.08.003. [DOI] [Google Scholar]
- 59.Saha S., Samadi F., Jaiswal J., Ghoshal U. Antimicrobial activity of different endodontic sealers: An in vitro evaluation. J. Indian Soc. Pedod. Prev. Dent. 2010;28:251. doi: 10.4103/0970-4388.76151. [DOI] [PubMed] [Google Scholar]
- 60.Kayaoglu G., Erten H., Alaçam T., ➢rstavik D. Short-term antibacterial activity of root canal sealers towards Enterococcus faecalis. Int. Endod. J. 2005;38:483–488. doi: 10.1111/j.1365-2591.2005.00981.x. [DOI] [PubMed] [Google Scholar]
- 61.Badr A., Omar N., Badria F. A laboratory evaluation of the antibacterial and cytotoxic effect of Liquorice when used as root canal medicament. Int. Endod. J. 2011;44:51–58. doi: 10.1111/j.1365-2591.2010.01794.x. [DOI] [PubMed] [Google Scholar]
- 62.Saha S., Dhinsa G., Ghoshal U., Hussain A.N.F.A., Nag S., Garg A. Influence of plant extracts mixed with endodontic sealers on the growth of oral pathogens in root canal: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2019;37:39. doi: 10.4103/JISPPD.JISPPD_66_18. [DOI] [PubMed] [Google Scholar]
- 63.Devi M.T., Saha S., Tripathi A.M., Dhinsa K., Kalra S.K., Ghoshal U. Evaluation of the Antimicrobial Efficacy of Herbal Extracts Added to Root Canal Sealers of Different Bases: An In Vitro Study. Int. J. Clin. Pediatric Dent. 2019;12:398. doi: 10.5005/jp-journals-10005-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cinthura C., Geetha R. Evaluation of antimicrobial activity of endodontic sealers in combination with cinnamon oil. Drug Invent. Today. 2018;10:2933–2936. [Google Scholar]
- 65.Kataia E.M., Omar N., Aly Y., ElShafei N. Assessment of Physical Properties of a ZnO/E~ Sealer Modified by Adding Moringa Oleifera: An Experimental in-vitro Study. [(accessed on 10 April 2022)];Dentistry. 2020 105 doi: 10.4103/jioh.jioh_347_19. Available online: https://buescholar.bue.edu.eg/dentistry/105. [DOI] [Google Scholar]
- 66.Siregar E., Susanto C. Effectiveness of Tamarillo Skin Extract (Solanum betaceum Cav.) with Sealer Combination in Inhibiting Growth of Enterococcus faecalis. Biomed. J. Indones. 2021;7:395–401. [Google Scholar]
- 67.Nour El Deen N., Kamel W., Sherief M., Rokaya M. A Comparative Study of the Antibacterial Efficacy of two Natural Irrigating Solutions with Two Different Root Canal Sealers on E-feacalis. Al-Azhar Dent. J. Girls. 2017;4:385–393. doi: 10.21608/adjg.2017.5286. [DOI] [Google Scholar]
- 68.Wourms D.J., Campbell A.D., Hicks M.L., Pelleu G.B. Alternative solvents to chloroform for gutta-percha removal. J. Endod. 1990;16:224–226. doi: 10.1016/S0099-2399(06)81675-4. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen T. Obturation of the root canal system. Pathw. Pulp. 1994;6:219–271. [Google Scholar]
- 70.Windholz M. The Merck Index. Merck and Company, Inc.; Rahway, NJ, USA: 1976. [Google Scholar]
- 71.El-Hawary S.S., Ezzat S.M., Eid G.E., Abd-El Rhman S.K. Effect of Certain Essential oils on Dissolution of Three Commercial Gutta-percha Brands. J. Essent. Oil Bear. Plants. 2015;18:1126–1137. doi: 10.1080/0972060X.2014.960278. [DOI] [Google Scholar]
- 72.Daokar S.G., Kagne K.S., Pawar K.S., Wahane K.D., Mahakale C.R., Thorat T.V. Comparative Evaluation of Efficacy of Three Natural Oils as Gutta-percha Solvents: In Vitro Study. J. Aesthetic Dent. Conserv. Dent. Endod. 2019;1:1–4. [Google Scholar]
- 73.Kulkarni G., Podar R., Singh S., Dadu S., Purba R., Babel S. Comparative evaluation of dissolution of a new resin-coated Gutta-percha, by three naturally available solvents. Endodontology. 2016;28:143. [Google Scholar]
- 74.Opačić-Galić V., Adžić S., Dželetović B., Vlajić T. In vitro study of essential oils efficacy as alternative solvents in endodontic retreatment. Stomatol. Glas. Srb. 2021;68:173–180. doi: 10.2298/SGS2104173O. [DOI] [Google Scholar]
- 75.Vahabi B.N.S.S., Rad M.M. Use of Herbs and Medicinal Plants in Dentistry: A Review. J. Dent. Sch. 2017;35:133–149. [Google Scholar]
- 76.Jantarat J., Malhotra W., Sutimuntanakul S. Efficacy of grapefruit, tangerine, lime, and lemon oils as solvents for softening gutta-percha in root canal retreatment procedures. J. Investig. Clin. Dent. 2013;4:60–63. doi: 10.1111/j.2041-1626.2012.00143.x. [DOI] [PubMed] [Google Scholar]
- 77.Andreasen J.O., Andreasen F.M., Andersson L. Textbook and Color Atlas of Traumatic Injuries to the Teeth. John Wiley & Sons; Hoboken, NJ, USA: 2018. [Google Scholar]
- 78.Caglar E., Sandalli N., Kuscu O., Durhan M., Pisiriciler R., Ak Calıskan E., Kargul B. Viability of fibroblasts in a novel probiotic storage media. Dent. Traumatol. 2010;26:383–387. doi: 10.1111/j.1600-9657.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 79.Poi W.R., Sonoda C.K., Martins C.M., Melo M.E., Pellizzer E.P., Mendonça M.R.D., Panzarini S.R. Storage media for avulsed teeth: A literature review. Braz. Dent. J. 2013;24:437–445. doi: 10.1590/0103-6440201302297. [DOI] [PubMed] [Google Scholar]
- 80.Udoye C.I., Jafarzadeh H., Abbott P.V. Transport media for avulsed teeth: A review. Aust. Endod. J. 2012;38:129–136. doi: 10.1111/j.1747-4477.2012.00356.x. [DOI] [PubMed] [Google Scholar]
- 81.Anegundi R.T., Paritosh S.N., Tavargeri A., Patil S., Trasad V., Battepati P. Novel herbal storage media for exarticulated teeth. Int. J. Contemp. Med. Res. 2016;3:729–732. [Google Scholar]
- 82.Casaroto A.R., Hidalgo M.M., Sell A.M., Franco S.L., Cuman R.K.N., Moreschi E., Victorino F.R., Steffens V.A., Bersani-Amado C.A. Study of the effectiveness of propolis extract as a storage medium for avulsed teeth. Dent. Traumatol. 2010;26:323–331. doi: 10.1111/j.1600-9657.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- 83.Hwang J.Y., Choi S.C., Park J.-H., Kang S.W. The use of green tea extract as a storage medium for the avulsed tooth. J. Endod. 2011;37:962–967. doi: 10.1016/j.joen.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 84.Özan F., Tepe B., Polat Z.A., Er K. Evaluation of in vitro effect of Morus rubra (red mulberry) on survival of periodontal ligament cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008;105:e66–e69. doi: 10.1016/j.tripleo.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Gopikrishna V., Baweja P.S., Venkateshbabu N., Thomas T., Kandaswamy D. RETRACTED: Comparison of Coconut Water, Propolis, HBSS, and Milk on PDL Cell Survival. Elsevier; Amsterdam, The Netherlands: 2008. [DOI] [PubMed] [Google Scholar]
- 86.Badakhsh S., Eskandarian T., Esmaeilpour T. The use of aloe vera extract as a novel storage media for the avulsed tooth. Iran. J. Med. Sci. 2014;39:327. [PMC free article] [PubMed] [Google Scholar]
- 87.Ashkenazi M., Marouni M., Sarnat H. In vitro viability, mitogenicity and clonogenic capacities of periodontal ligament fibroblasts after storage in four media supplemented with growth factors. Dent. Traumatol. 2001;17:27–35. doi: 10.1034/j.1600-9657.2001.170106.x. [DOI] [PubMed] [Google Scholar]
- 88.Ashkenazi M., Sarnat H., Keila S. In vitro viability, mitogenicity and clonogenic capacity of periodontal ligament cells after storage in six different media. Dent. Traumatol. 1999;15:149–156. doi: 10.1111/j.1600-9657.1999.tb00793.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No associated data marked.