Abstract
Based on data from The Global Burden of Disease Study in 2016, dental and oral health problems, especially dental caries, are a disease experienced by almost half of the world’s population (3.58 billion people). One of the main causes of dental caries is the pathogenesis of Streptococcus mutans. Prevention can be achieved by controlling S. mutans using an antibacterial agent. The most commonly used antibacterial for the treatment of dental caries is chlorhexidine. However, long-term use of chlorhexidine has been reported to cause resistance and some side effects. Therefore, the discovery of a natural antibacterial agent is an urgent need. A natural antibacterial agent that can be used are herbal medicines derived from medicinal plants. Piper crocatum Ruiz and Pav has the potential to be used as a natural antibacterial agent for treating dental and oral health problems. Several studies reported that the leaves of P. crocatum Ruiz and Pav contain secondary metabolites such as essential oils, flavonoids, alkaloids, terpenoids, tannins, and phenolic compounds that are active against S. mutans. This review summarizes some information about P. crocatum Ruiz and Pav, various isolation methods, bioactivity, S. mutans bacteria that cause dental caries, biofilm formation mechanism, antibacterial properties, and the antibacterial mechanism of secondary metabolites in P. crocatum Ruiz and Pav.
Keywords: red betel leaf, Piper crocatum Ruiz and Pav, antibacterial, Streptococcus mutans, phytochemical profiling
1. Introduction
The oral cavity is a place of growth for more than 700 species of microorganisms, which ultimately has many impacts on the health of the teeth and oral cavity. One of the health problems experienced globally is oral infectious diseases such as dental caries [1,2,3]. In 2017, the prevalence of dental caries in permanent teeth per 100,000 population in each country reached 20% to more than 50% [4]. The cause is the synergistic interaction of bacteria such as Streptococcus sanguinis and S. mutans to form a biofilm on the tooth surface [5,6,7,8,9]. The high prevalence of dental caries and the weakness of the strategies used today indicate an urgent need to identify alternative treatment options that are more effective and efficient, one of which is the use of medicinal plants [10].
Some studies reported that red betel leaf has the potential to be used as a natural antibacterial agent in treating dental and oral health problems. Red betel leaf contains secondary metabolites such as essential oils, flavonoids, alkaloids, and phenolic compounds that actively inhibit S. mutans [11,12]. Based on this, this review focuses on the antibacterial activity found in red betel leaf (P. crocatum Ruiz and Pav) which has been studied extensively [13]. This review will also discuss the relationship between antibacterial activity and the structure of several compounds contained in red betel leaf extract.
2. Gram-Positive and Negative Bacteria Cause Dental Caries
2.1. Gram-Positive Bacteria
2.1.1. Streptococcus mutans
S. mutans is a Gram-positive bacterium that is considered to be the microorganism that most often plays a role in tooth decay [14]. These bacteria are able to organize themselves in the bacterial community through cell–cell interactions and connections with other components present in the medium such as polysaccharides, proteins, and DNA to form biofilms [15,16]. Biofilm is a structured and organized community of microbial cells in a dynamic environment, enclosed and embedded in a three-dimensional (3D) extracellular matrix [17,18,19]. The cariogenic biofilm matrix formed by S. mutans is rich in exopolysaccharides and contains extracellular DNA (eDNA) and lipoteichoic acid (LTA) [20,21,22,23]. Microbial species are found in oral biofilms such as Candida albicans, Candida glabrata, Enterococcus faecalis, S. mutans, Veillonella dispar, Fusobacterium nucleatum, and many others [24].
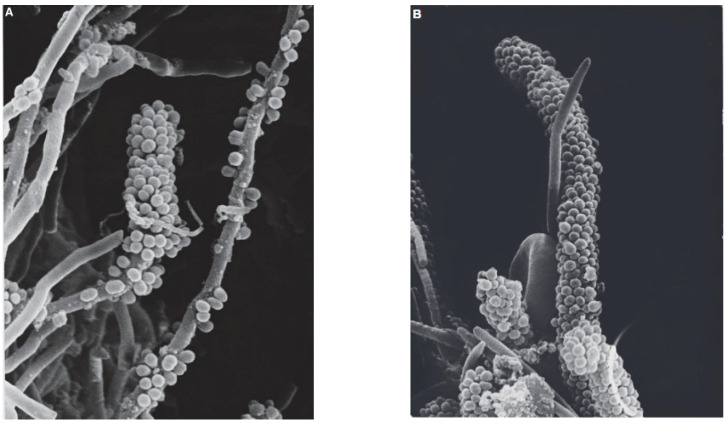
One of the diseases caused by S. mutans is dental caries. There are several factors that cause dental caries to get worse including sugar, saliva, and also putrefactive bacteria [25,26,27]. In addition, the growth of bacteria in the mouth and forming biofilms is caused by several factors, namely saliva which plays a role in modulating the plaque layer on the teeth, the temperature in the environment around the mouth in the range of 35–36 °C, and pH 6.75–7.25 [28,29]. The mechanism of biofilm formation on teeth is followed by five stages, namely initial adhesion which produces extracellular polymeric substances, initial attachment where cell division occurs, formation of young biofilms, mature biofilms, and dispersed biofilms which cause cell autolysis [30] (Figure 1).
Figure 1.
(A) Co-aggregation between S. mutans and filaments in developing dental biofilm; (B) typical corncob formation [30].
The pathogenesis of S. mutans begins after consuming something containing sugar, especially sucrose, a sticky glycoprotein (a combination of protein and carbohydrate molecules) that is retained on the teeth to initiate plaque formation on the teeth [31,32]. At the same time, millions of bacteria, including S. mutans, also survive on the glycoprotein. S. mutans has an enzyme called glucosyl transferase on its surface which is involved in glycolysis [25,33,34]. Glycolysis is the breaking down of glucose in sucrose that is carried out to obtain energy.
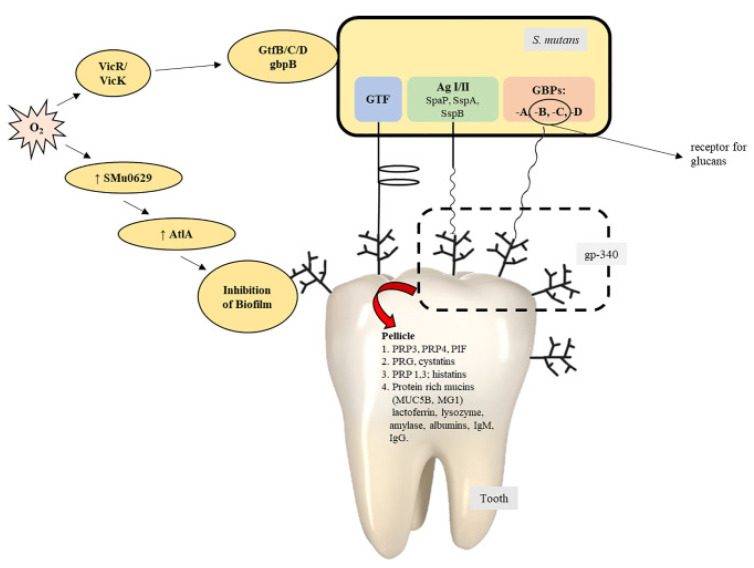
The glucosyltransferase enzyme continues to work, namely, to add more glucose molecules to form dextran which has a structure very similar to amylase in starch. Dextran together with other bacteria adheres tightly to the tooth enamel and subsequently forms plaque on the teeth [35,36]. In addition, glycolysis under anaerobic conditions also produces lactic acid. This lactic acid causes a decrease in pH to a certain extent so that it can destroy hydroxyapatite in the tooth enamel and cause the formation of a cavity or hole in the tooth [37,38] (Figure 2).
Figure 2.
Contribution of S. mutans in the process of biofilm formation [39].
2.1.2. Streptococcus sanguinis
Streptococcus sanguinis is a type of Gram-positive bacteria that does not have spores and is a facultative anaerobe. Cell division in S. sanguinis occurs along a single axis and produces chains or pairs of cocci. The genome sequence of S. sanguinis SK36 isolated from dental plaque in humans has a circular DNA molecule consisting of 2,388,435 base pairs, with 2274 predicted protein codes. In tRNA, there are 61 genes that are predicted to be able to produce 20 amino acids and 50 carbohydrate transporters, including the phosphotransferase enzyme which functions to transport glucose, fructose, mannose, cellobiose, glucoside, lactose, trehalose, galactitiol, and maltose. S. sanguinis is able to utilize various carbohydrate sources to survive [39].
Oral biofilm formation begins with the attachment of S. sanguinis and other pioneering colonists to a macromolecular complex formed on the saliva-coated tooth surface [22,40,41,42]. S. sanguinis was the first bacterium to bind to the biofilm and a species that plays an important role in the oral biofilm ecosystem [43,44,45,46]. However, these bacteria also have a positive role, namely producing H2O2 as a means to produce excess oxygen and working as a non-specific antimicrobial agent that can trigger the growth of S. mutans and other anaerobic periodontal pathogens [47,48,49].
The negatively charged residue and electrostatic interactions with hydrophilic regions in salivary proteins facilitate the attachment of bacteria to the tooth surface to form the Acquired Enamel Pellicle (AEP). Although S. sanguinis can directly adhere to saliva-free hydroxyapatite, the major mineral found in tooth enamel, the initial attachment process is most likely driven by the interaction of the streptococcal surface with salivary components. Binding to salivary proteins is mediated through protein–protein or protein–carbohydrate interactions with receptors exposed on the bacterial surface. Amylase is the most abundant salivary protein and is present both in AEP and in dental plaque. S. sanguinis specifically binds to amylase via long filamentous pili [50,51].
2.2. Gram-Negative Bacteria
Veillonella parvula
Veillonella parvula is an anaerobic Gram-negative coccus that is part of the normal flora found in the human mouth and digestive tract [52]. Human oral Veillonella species include V. parvula, V. dispar, V. atypica, V. denticariosi, V. rogosae, V. tobetsuensis, V. infantium, and V. nakazawae [53,54,55]. Lactate and malate are the preferred carbon sources by Veillonellae spp. These carbon sources will be metabolized into propionate, acetate, CO2, and H2 [56,57]. Pyruvate, fumarate, and oxaloacetate can also be metabolized, but citrate, iso-citrate, and malonate are not. Succinate catabolism has been reported to have not resulted in suboptimal growth [58]. The balanced stoichiometry of lactate catabolism is (Equation (1)) [59]:
| 8 Lactate → 5 Propionate + 3 Acetate + 3 CO2 + H2 | (1) |
Evidence that Veillonellae spp. acts as a linking species in biofilm development has been demonstrated in both in vivo and in vitro studies. Human epidemiological studies have shown Veillonellae spp. to be very abundant in both supra and sub-gingival plaques as well as on the tongue and in saliva [60,61,62,63,64]. Veillonella spp. (especially V. parvula) was found to be associated with dental caries in children [58,65]. Besides that, it was also found in adults. V. parvula was also one of the most abundant and prevalent bacteria in all samples of both healthy and carious teeth. However the abundance of V. parvula in carious tooth samples appears to be higher [66]. The physiological relationship between Veillonellae (as lactate users) and S. mutans (as lactate producers) has prompted many clinical studies on the relationship of Veillonellae with caries. Research conducted by Aas et al. [67] also demonstrated the association of the genera Veillonella with caries development. Belstrom et al. reported that Streptococcus spp. and Veillonella spp. were the most dominant genera among all saliva samples from 292 participants with mild to moderate dental caries [68].
It can be argued that the observed association between cariogenic bacteria and Veillonella stems from the metabolic need to produce organic acids which are indeed found in higher concentrations in active caries. Therefore, the presence of Veillonellae can be an indication of, and prediction of, a local decrease in pH. Bradshaw and Marsh reported that the number and proportion of S. mutans and Lactobacillus spp. increases as the pH decreases, especially below low pH [65]. Similarly in another clinical study, Gross et al. found the proportion of Veillonellae spp. increased commensurate with the proportion of Streptococcus spp. [69]. In other words, Veillonellae can be a risk factor for caries initiation, whereas S. mutans are a risk factor for caries development.
3. Antibacterial
3.1. Definition
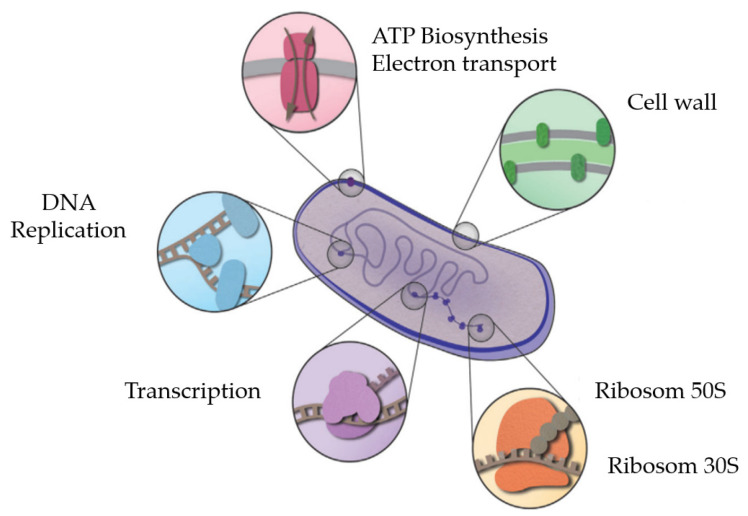
An antibacterial is a substance that can inhibit the growth of bacteria and will kill pathogenic bacteria [70]. Antibacterial substances are divided into two types, namely bacteriostatic which suppresses bacterial growth and bactericidal which can kill bacteria [71]. Bacteria have evolved a lot to be able to survive in various environments and can develop resistance to various antibacterial reagents quickly [72]. Inhibition of bacteria can be through several synthesis pathways in bacteria, namely the bacterial cell wall biogenesis pathway, DNA replication pathway, transcription pathway, and protein biosynthesis pathway [73]. The cell wall structure consists of peptidoglycan which provides a mechanical effect on bacteria to maintain morphology. The peptidoglycan layer is formed from N-acetyl glucosamine and N-acetylmuramic acid linked by 1,4-glycosidic bonds [74].
3.2. Antibacterial Mechanism of Secondary Metabolic Compounds
Several secondary metabolites that are isolated from plants can be natural antibacterial agents. Each compound has their own antibacterial mechanism in inhibiting bacteria. Their mechanism will be explained in the following:
3.2.1. Phenol
The mechanism of phenol as an antibacterial agent acts as a toxin in the protoplasm, damaging and penetrating the wall, causing the function of selective permeability, active transport, and protein composition control, so that bacterial cells become deformed and lysed [75,76,77].
3.2.2. Flavonoids
Flavonoids work to inhibit bacterial growth by inhibiting nucleic acid synthesis, changing cytoplasmic membrane function, inhibiting energy metabolism, reducing cell attachment and biofilm formation, inhibiting porin in cell membranes, and disrupting permeability of cell walls and membranes to cause bacterial cell lysis [38,78,79,80,81]. In addition, flavonoids also act as inhibitors of the FabZ enzyme and inhibit the production of fimbriae [82].
3.2.3. Saponins
Meanwhile, the saponins themselves work as antibacterial agents by disrupting the stability of the bacterial cell membrane, causing bacterial cell lysis [75,83,84,85].
3.2.4. Terpenoids
Terpenoids work as antibacterials by disrupting the function of cell membranes to cause damage to bacterial cell membranes, interfering with glucosyltransferase activity, inactivating thiol-containing enzymes and causing bacterial death [86,87,88,89,90,91,92,93,94,95,96,97].
3.2.5. Alkaloids
Alkaloids inhibit growth and kill bacteria by interfering with the permeability of cell walls and membranes, inhibiting of nucleic acid and protein synthesis, and inhibiting bacterial cell metabolism to cause lysis. Moreover, alkaloids can also act as inhibitors in the protein biosynthesis process in bacterial cells [98,99,100].
3.2.6. Tannins
Tannins work by coagulating bacterial protoplasm, precipitating proteins, and binding proteins to inhibit the formation of bacterial cell walls [101,102,103] (Figure 3).
Figure 3.
Pathway of inhibition of bacteria by antibacterial agents [73].
3.3. Antibacterial Mechanism with MurA Enzyme
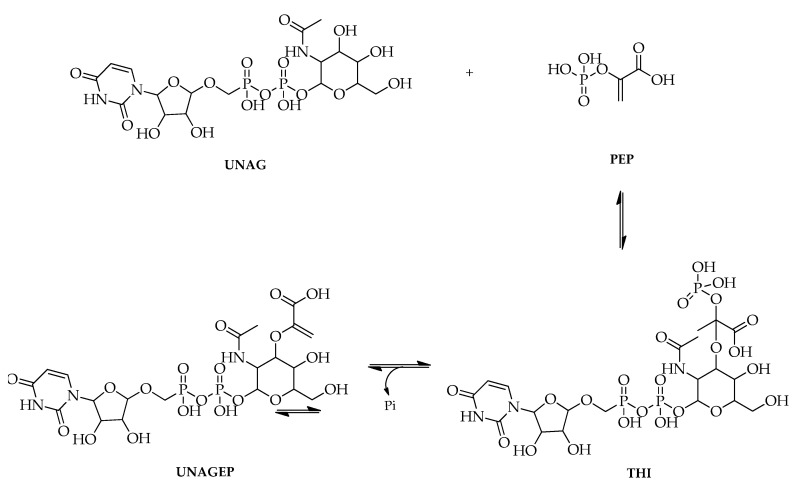
In addition, the antibacterial mechanism can be carried out by inhibiting the action of the MurA enzyme that catalyzes the first step of bacterial cell wall biosynthesis. Therefore, the inhibition of the activity of oral pathogenic bacteria can be undertaken by inhibiting the enzyme MurA [104]. In cell wall peptidoglycan biosynthesis, the enzyme MurA involves the transfer of the enolpyruvate group from phosphoenolpyruvate (PEP) to UDP-N-acetylglucosamine (UNAG) to form UDP-N-acetylglucosamine enolpyruvate (UNAGEP) [90,91].
Based on the performance of fosfomycin, the inhibition of the MurA enzyme is competitive. Antibiotics act as PEP analogues and form covalent bonds with the active cysteine residue of the enzyme as shown in the figure below. Antibiotics interact with enzymes and UDP-N-acetylglucosamine and then form hydrogen bonds with different segments of the polypeptide chain. In addition, hydrogen bonds can be formed between the hydroxyl group of phosphomycin and the C-3 hydroxyl of the sugar ring UDP-N-acetylglucosamine and between one of its phosphonate oxygen atoms and the nitrogen amide of UDP-N-acetylglucosamine [105] (Figure 4).
Figure 4.
Catalytic reaction on the MurA enzyme [106].
3.4. Commonly Used Dental Caries Antibiotics
To control caries mediated by pathogenic bacteria, dental and oral hygiene products are widely used which consist of chemical compounds, such as fluoride, chlorhexidine, triclosan, cetylpyridinium chloride, and chlorophyll.
3.4.1. Fluoride
Fluoride is the most effective caries prevention agent. Since the 1940s, it has been added to water supplies and oral care products, such as toothpaste, mouthwash, and dental floss [107]. In fact, the use of oral hygiene products containing fluoride reduced the prevalence of caries by 24–26% in permanent teeth. Water fluoridation in the range of 0.50–1.00 mg/L−1 is a cost-effective method for moderating caries potential [108]. In addition, the combination of nicomethanol hydrofluoride with siliglycol further enhances fluoride uptake by teeth and controls or inhibits dental biofilm development and strengthens tooth structure [109]. However, the use of fluoride for oral health also causes side effects, such as the emergence of fluoride-resistant strains [110,111]
3.4.2. AIK(SO4)2
AIK(SO4)2 was found to be able to reduce fissure caries, both smooth surface and sulcus caries. The mechanism of dental caries treatment of alum may be almost the same as the mechanism of dental caries treatment using fluoride [112].
3.4.3. Chlorhexidine (CHX)
Dental and oral hygiene products consist of another chemical compound, namely chlorhexidine (CHX). Chlorhexidine is a symmetric bis-biguanide agent consisting of two chloroguanide chains linked by a central hexamethylene chain and has diverse medical applications as a surface disinfectant and as an antiseptic for topical application. Chlorhexidine carryes two positive charges at physiological pH which can interact electrostatically with negatively charged phospholipids (CHX) and has been used to control dental caries caused by acid-tolerant bacteria such as S. mutans since the 1970s [113]. However, the use of chlorhexidine also causes certain disadvantages with long-term use such as tooth staining and taste changes [114]. It is also believed that the continued and increasing use of chlorhexidine can lead to the emergence of new strains of mycobacteria with lower susceptibility
High prevalence of dental caries and the weakness of the strategies used today indicate an urgent need to identify alternative treatment options that are more effective, efficient, and non-toxic, one of which is by utilizing herbal medicines derived from medicinal plants [115]. In recent decades, research focus has also shifted to herbal medicines due to increasing bacterial resistance and side effects of antimicrobial agents. Extracts of plant origin can enhance antibiotic efficacy when used in combination against bacterial pathogens [10]. In addition, the use of medicinal plants or natural products is indeed a safe approach for rapid clinical translation because they are generally recognized as safe by the United States Food and Drug Administration.
4. Piper crocatum Ruiz and Pav
Based on some research literature, it has been reported that red betel leaf has the potential to be used as a natural antibacterial agent in treating dental and oral health problems. Red betel leaf (P. crocatum Ruiz and Pav) is a plant that grows in the tropics and was previously known as an ornamental plant, but was later used as a medicinal plant [116]. P. crocatum Ruiz and Pav is a natural ingredient that has the potential to treat dental caries and the leaf contains secondary metabolites such as essential oils, flavonoids, alkaloids, and phenolic compounds which may be active against S. mutans that plays a role in caries formation. The use of red P. crocatum Ruiz and Pav is traditionally useful in curing diseases such as canker sores and toothache. The red betel leaf decoction which is an antiseptic can act as a mouthwash, preventing bad breath. From chromatography it is known that P. crocatum Ruiz and Pav leaf contains flavonoid compounds, polyphenol compounds, tannins, and essential oils, where flavonoids are known to be inhibitors of the growth of S. mutans [11,50].
4.1. Isolation of Secondary Metabolites of Piper crocatum Ruiz and Pav
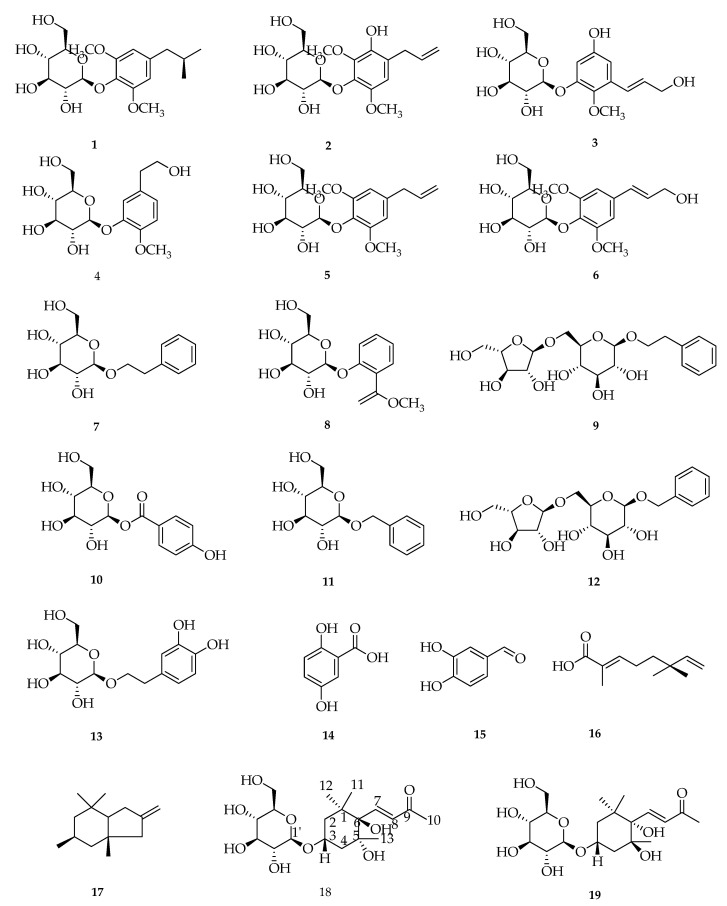
Several studies reported the isolation of P. crocatum Ruiz and Pav by many methods. Li et al., 2019 isolated 2.60 kg of dried red betel leaf samples, then extracted by reflux method using methanolic solvent (5 L × 3 times). The results of the isolation of P. crocatum Ruiz and Pav leaves revealed 23 compounds including 15 phenolic compounds (1–15), two monoterpenes (16 and 17), three sesquiterpene compounds (19–21), phenolic amide glycosides (22), neolignans (23), and the flavonoid compound C-glycoside (24). The structure of the compounds obtained was identified through spectroscopic methods and compared with the literature. Seven compounds (7, 11, 13, 14, 17, 20, and 24) of the species P. crocatum Ruiz and Pav and 17 others (1–6, 8–10, 12, 15–16, 18–19, and 21–23) from the genus Piper and the family Piperaceae were isolated and reported for the first time [117] (Figure 5).
Figure 5.
Compounds obtained from the methanol extract of red betel leaf. (1) (8R)-8-(4-hydroxy-3,5-dimethoxy)-propane-8-ol-4-O-β-D-glucopyranoside; (2) 4-Allyl-2,6-dimethoxy-3-hydroxy-1-D-glucopyranoside; (3) 3-[(1E)-3-hydroxy-1-propen-1-yl]-2,5-dimethoxyphenyl-D-glucopyranoside; (4) Cimidahurinin; (5) Erigeside II; (6) Syringe; (7) β-phenylethyl-β-D-glucoside; (8) Methylsalicylate-2-O-β-D-glucopyranoside; (9) Icariside D1; (10) 4-Hydroxybenzoic acid-D-glucosylester; (11) Benzyl-β-D-glucoside; (12) Phenylmethyl-6-O-α-L-arabinofuranosyl-β-D-glucopyranoside; (13) Hydroxytyrosol-1glucopyranoside (14) Gentisic acid; (15) Catechaldehyde; (16) (S)-Menthiafolic acid; (17) Ioliolide; (18) 5β,6β-dihydroxy-3α-(β-D-glucopyranosyloxy)-7E-Megastigmen-9-one; (19) (3E)-4-[(1S,2S,4S)-4-(β-D-glucopyranosyloxy)-1,2-dihydroxy-2,6,6-tri-methylcyclohexyl]3-buten-2-one; (20) (6S,9S)-roseoside; (21) Cuneataside E (22) N-trans-feruloyltyramine-4′-O-β-D-glucopyranoside; (23) Syringaresinol-β-D-glucoside; and (24) Vitexin 2″-O-rhamnoside.
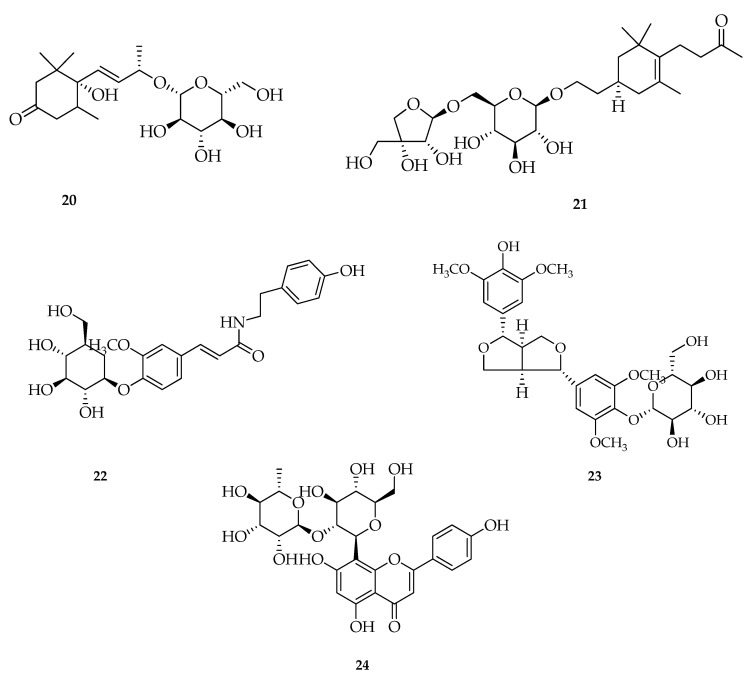
Another isolation method was carried out by Emrizal et al., 2014 for P. crocatum Ruiz and Pav, as much as 0.84 kg were extracted at room temperature with methanolic solvent to obtain a crude methanolic extract of 253.27 g (30.11%) after which the extract was evaporated, and they proceeded to separate the components of the compound. The results of the isolation obtained two compounds from the P. crocatum Ruiz and Pav plant which were then identified based on literature data and spectroscopic analysis. It was concluded that the two compounds were β-sitosterol and 2-(5′,6′-dimethoxy-3′,4′-methylenedioxyphenyl)-6-(3″,4″,5-trimethoxyphenyl)-dioxabiclo [3,3,0] octane. In addition, the two compounds were also reported to have antitumor activity with an IC50 value of 2.04; 1.34, 2.08, and 27.40 g/mL in the fractions of n-hexane, ethyl acetate, buthanolic, and methanolic extract, respectively [118] (Figure 6).
Figure 6.
Compounds obtained from the methanolic extract of red betel leaf (P. crocatum Ruiz and Pav). (25) β-sitosterol and (26) 2-(5′,6′-dimethoxy-3′,4′-methylenedioxyphenyl)-6-(3″,4″,5-trimethoxyphenyl)-dioxabiclo [3,3,0] octane.
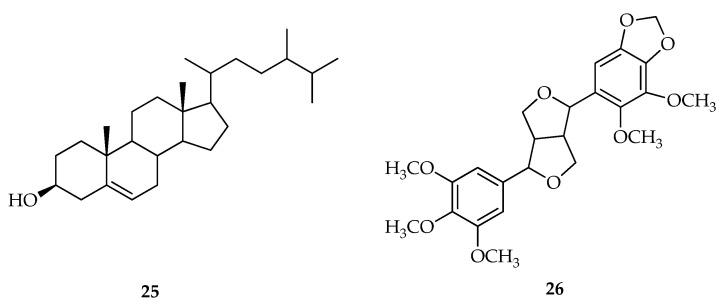
Arbain et al., 2018 isolated a 1.10 kg sample of P. crocatum Ruiz and Pav by using the maceration extraction method twice with methanolic solvent (5 L) for 48 h. Two new bicyclo [3.2.1] octanoid neolignans of the guianine type, crocatin A and crocatin B, together with the known compounds pachypodol and 1-triacontanol isolated from Indonesian P. crocatum Ruiz and Pav leaf. Its structure and configuration were determined by 1D- and 2D-NMR, MS spectroscopy, and single-crystal X-ray diffraction analysis [119] (Figure 7).
Figure 7.
Compounds obtained from the methanolic extract of red betel leaf (P. crocatum Ruiz and Pav). (27) Crocatin A; (28) Crocatin B; (29) Pachypodol [4′,5-dihydroxy-3,3′,7-trimethoxyflavone]; and (30) 1-Triacontanol.
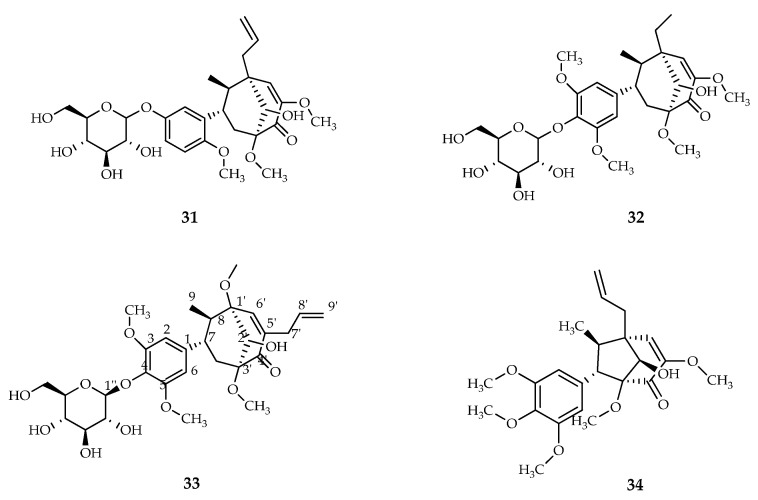
In a study conducted by Chai et al. (2021), 2.60 kg of dried leaves of P. crocatum Ruiz and Pav were isolated which were then extracted using the reflux method using methanol (5 L × 3 times) as a solvent. The isolation results reported that four bicyclo [3.2.1] octanoid neolignans were isolated from the methanolic extract of P. crocatum Ruiz and Pav. Neolignans were identified as pipcroside A, pipcroside B, pipcroside C, and crocatin B. In addition, this study by Chai et al., 2021 also provides the basis for further exploration of P. crocatum Ruiz and Pav and bicyclo [3.2.1] octanoid neolignans from the Piper plant as a new source of natural antineoplastic agents [120] (Figure 8).
Figure 8.
Compounds obtained from the methanolic extract of red betel leaf. (31) Pipcroside A; (32) Pipcroside B; (33) Pipcroside C; and (34) Bicyclo [3.2.1] octanoid neolignans.
4.2. Bioactivity of Piper crocatum Ruiz and Pav
The Piperaceae family is one type of plant that is often found in the surrounding environment and several types of plants in that family are classified as dicotyledonous plants. One of them that is often used by the community as a traditional medicinal plant is the Piper genus. It has more than 700 species spread throughout the world and commercial, economic, and medicinal importance. Many plant species of this genus have high potential for local and industrial uses, as well as applications in botanical pharmacy, pharmacognosy, and traditional medicine. The efficacy of the drug basically comes from several secondary metabolite compounds contained in the plant.
Secondary metabolites of the Piper genus, in addition to their unique structure, are also reported to have potential as bioactive compounds. Tests for the bioactivity of this genus have been carried out on both extracts and pure compounds. The isolation results support its use in traditional medicine (Table 1).
Table 1.
Bioactivity of isolated Piper genus.
| No. | Species | Secondary Metabolites | Plant Parts | Bioactivity | References |
|---|---|---|---|---|---|
| 1 | P. betle | Phenylpropanoid | Leaf | Antioxidant | Atiya et al., 2018 [121] |
| 2 | P. terminaliflorum tseng | Furfuran Lignan | All parts of plant | Anticancer | T. Liu et al., 2018 [122] |
| 3 | P. chimonantifolium | Flavonoids Steroids |
Leaf | Antifungal | Lago et al., 2012 [123] |
| 4 | P. montealegreanum | Monoterpens Seskuiterpens |
Twig | Da S. Alves et al., 2011 [124] | |
| 5 | P. hispidum | Chalcones, Flavanone |
Leaf | Antileishmanial | Ruiz et al., 2011 [125] |
| 6 | P. maingayi | Amida | Twig | Antibacterial | Hashim et al., 2019 [126] |
| 7 | P. officinarum | Phenylpropanoid Alkaloids Triterpene |
Twig | Antioxidant | Salleh et al., 2014 [127] |
| 8 | P. taiwanense | Amida | Aerial | Antioxidant | Chen et al., 2017 [128] |
| 9 | P. sarmentosum | Flavonoids | Leaf | Antioxidant | Ugusman et al., 2011 [129] |
| 10 | P. solmsianum C. | Flavonoids | Twig | Antifungal | De Campos et al., 2005 [130] |
| 11 | P. betle L. | Terpenoid | Leaf | Antibacterial | Batubara et al., 2011 [131] |
| 12 | P. betle L. | Phenolic | Leaf | Antibacterial | Kurnia et al., 2020 [132] |
| 13 | P. ningrum | Alkaloid-piperidine | Fruit | Anticancer | Reshmi et al., 2010 [133] |
Like plants from other Piper genera, P. crocatum Ruiz and Pav also has some bioactivity, both from the level of extract, fraction and isolation results, and several instances of bioactivity of red betel have been reported. In the table below are some studies of isolation of P. crocatum Ruiz and Pav with various kinds of bioactivity of each (Table 2).
Table 2.
Bioactivity of isolated P. crocatum Ruiz and Pav leaves.
| No. | Secondary Metabolites | Plant Parts | Bioactivity | References |
|---|---|---|---|---|
| 1 | Flavonoids Terpenoids Steroids |
Leaf | Antitumor | Emrizal et al., 2014 [118] |
| 2 | 2 flavonoids 2 monoterpenes 3 seskuiterpenes 17 Glucoside |
Leaf | Anti-inflammatory | Li et al., 2019 [117] |
| 3 | 12 Phenolic | Leaf | Hypoallergenic | Li et al., 2019 [134] |
| 4 | Bicyclo[3,2,1]Octanoid Neolignane | Leaf | Pyruvate dehydrogenase inhibitors | Chai et al., 2021 [120] |
| 5 | Essential Oil | Leaf | Antibacterial | Rizkita et al., 2017 [13] |
4.3. Antibacterial Activity of Red Betel Extract
One of the examples of bioactivity of P. crocatum Ruiz and Pav, which is the topic of this review, is antibacterial activity. Especially, the antibacterial activity of red betel against the bacteria S. mutans, S. sangguinis, V. parvula, and other bacteria found in the oral cavity that cause dental and oral health problems, one of which is dental caries. Therefore, the potential of red betel as an antibacterial agent can be understood by looking at several studies that have been reported. The table below shows data from previous research reports that reported the antibacterial ability of red betel leaf extract (Table 3).
Table 3.
Antibacterial activity methods of red betel extract (P. crocatum Ruiz and Pav).
| No. | Compounds | Types of Bacteria | Methods | References |
|---|---|---|---|---|
| 1 | Flavonol Chalcone Anthocyanins |
S. mutans | The Kirby–Bauer method of the disc diffusion test combined with UV irradiating treatment was used. The results showed the diameter of the inhibition zone (15.00 ± 0.05) mm for 10 watt and (15.96 ± 0.05) mm for 15 watt. | Dyah Astuti et al., 2020 [135] |
| 2 | Alkaloids Steroids Tannins |
B. subtilis
P. aeuruginosa |
Antibacterial activity was tested using the well method. Inhibited the growth of B. substilis and P. aeruginosa bacteria but the activity was weak, the inhibition zone was < 5 mm. | Puspita et al., 2019 [136] |
| 3 | Flavonoid Saponin Tannins Phenolic |
Staphylococcus epidermidis | Bacterial test was carried out using the well method, extract concentrations of 50 and 100% could inhibit the growth of S. epidermidis. | Januarti et al., 2019 [137] |
| 4 | Tannins | Staphylococcus aureus | Tests using the well method can inhibit S. aureus bacteria. Maceration extraction technique to get the average inhibition zone of 12.30 mm. | Soleha, 2018 [138] |
| 5 | Flavonoids Alkaloids Tannins Essential oil |
Porphyromonas gingivalis
S. viridians |
The antibacterial test was carried out using the well method, the inhibition zone on P. gingivalis was 10.34 mm while S. viridians was 8.42 mm. | Pujiastuti et al., 2015 [139] |
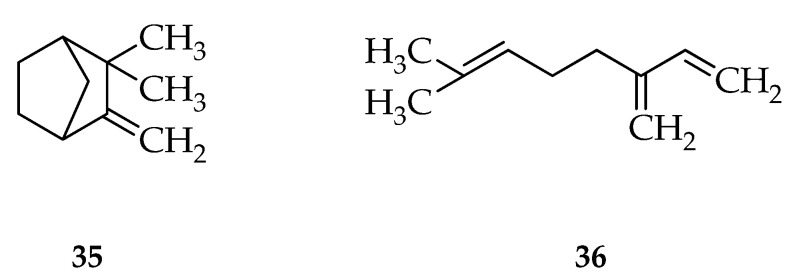
In research conducted by Rizkita et al. (2017), the research procedure includes four stages, namely plant determination, betel leaf oil refining, identification of betel oil components, and betel oil activity test, then the two oils are compared. Further component identification was carried out by mass spectrometry. The results of mass spectrometry will obtain the mass spectrum of each peak detected on the GC chromatogram. The mass spectra analysis was based on the value of Similarity Index (SI), base peak, and the fractional trend of the mass spectra compared to the library mass spectra, namely WILEY229.LIB. It was reported that the isolation results from P. betle L. and P. crocatum Ruiz and Pav contain essential oils which consist of five main active compounds that have antibacterial properties. The test was carried out by applying the disc method. The media used was Mueller Hinton Agar media because in this medium S. mutants bacteria lived optimally. The agar media that had been planted with the test bacteria were filled with samples of green betel oil and red betel oil with concentration variations (100, 75, 50, and 25%), propylene glycol solvent as a negative control, and amoxicillin as a positive control (Figure 9) [13].
Figure 9.
Structure of compounds of isolated red betel leaf oil. (35) Camphene and (36) Myrcene [13].
These compounds are terpenoid group compounds including camphene, sabinene, cariophilene, humulena, and germakron in green betel while the terpenoid compounds in red betel leaf include sabinene and mirsen. The antibacterial activity test of these compounds proved that there was an inhibition of the growth of S. mutans bacteria. Antibacterial compounds are thought to be able to inhibit the growth of Gram-positive bacteria by penetrating the cell wall, the cell wall of Gram-positive bacteria has a simple composition consisting of 60–100% peptidoglycan, which is made of N-acetyl glucosamine and N-acetyl muramate. The simple arrangement of the cell wall and the absence of an outer membrane causes antibacterial compounds to penetrate the cell wall and interfere with the cell wall biosynthesis process.
Sesquiterpene compounds have hydrophobic properties that cause disruption of the integrity of bacterial cells by reducing intracellular ATP reserves, lowering cell pH, being absorbed and penetrated into bacterial cells, then bacteria will experience precipitation and protein denaturation, and will lyse bacterial cell membranes. The difference in the concentration of the content contained in green betel leaf and red betel leaf contains 1.00–4.20% (w/v) essential oil yield, chavicol 7.20–16.70%, cavibetol 2.70–6.70%, and eugenol 26.80–42.50%. Meanwhile, the yield of red betel leaf was 0.73 (w/v), chavicol 5.10–8.20%, and eugenol 26.10–42.50%.
5. Conclusions
Medicinal plants of P. crocatum Ruiz and Pav have a significant role in applications of ethno-medicine. They contain secondary metabolites that have several examples of bioactivity, such as antioxidant, antimicrobial, antibacterial, antifungal, anti-inflammatory, and others. The bioactivity is influenced by the structure and functional groups of each secondary metabolite compound contained therein. Based on several research reports, it can be seen that P. crocatum Ruiz and Pav has considerable potential as an antibacterial agent in the treatment of oral health problems such as dental caries with several different methods. Secondary metabolites contained in P. crocatum Ruiz and Pav have their own mechanism to inhibit bacteria. This scientific finding is useful information for further drug research and development to find new potential antimicrobial agents.
Acknowledgments
The authors are grateful to Indonesian Ministry of Research, Technology and Higher Education for Grant of PDUPT—DIKTI 2022, and to Universitas Pakuan for all research facilities.
Author Contributions
Conceptualization, L.H., S.L. and D.K.; methodology, L.H. and D.A.; software, L.H.; validation, L.H. and D.A.; formal analysis, D.K. and U.H.; investigation, L.H.; resources, L.H., and S.L.; data curation, D.K.; writing—original draft preparation, L.H., S.L. and D.A.; writing—review and editing, L.H. and D.A.; visualization, D.A.; supervision, D.K.; project administration, D.K.; funding acquisition, L.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuang X., Chen V., Xu X. Novel Approaches to the Control of Oral Microbial Biofilms. Biomed. Res. Int. 2018;2018:6498932. doi: 10.1155/2018/6498932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiao Y., Tay F.R., Niu L.-N., Chen J. Hua Advancing Antimicrobial Strategies for Managing Oral Biofilm Infections. Int. J. Oral Sci. 2019;11:28. doi: 10.1038/s41368-019-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma D., Garg P.K., Dubey A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018;200:525–540. doi: 10.1007/s00203-018-1505-3. [DOI] [PubMed] [Google Scholar]
- 4.Peres M.A., Macpherson L.M.D., Weyant R.J., Daly B., Venturelli R., Mathur M.R., Listl S., Celeste R.K., Guarnizo-Herreño C.C., Kearns C., et al. Oral Diseases: A Global Public Health Challenge. Lancet. 2019;394:249–260. doi: 10.1016/S0140-6736(19)31146-8. [DOI] [PubMed] [Google Scholar]
- 5.Irani S. Oral Health and Related Factors: An Update. J. Int. Oral Health. 2016;8:1140–1144. doi: 10.2047/Jioh-08-12-19. [DOI] [Google Scholar]
- 6.Huang R., Li M., Gregory R.L. Bacterial Interactions in Dental Biofilm. Virulence. 2011;2:435–444. doi: 10.4161/viru.2.5.16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolenbrander P.E. Multispecies Communities: Interspecies Interactions Influence Growth on Saliva as Sole Nutritional Source. Int. J. Oral Sci. 2011;3:49–54. doi: 10.4248/IJOS11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang R., Li M., Gregory R.L. Effect of Nicotine on Growth and Metabolism of Streptococcus mutans. Eur. J. Oral Sci. 2012;120:319–325. doi: 10.1111/j.1600-0722.2012.00971.x. [DOI] [PubMed] [Google Scholar]
- 9.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global Burden of Untreated Caries: A Systematic Review and Metaregression. J. Dent. Res. 2015;94:650–658. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 10.Saquib S.A., Alqahtani N.A., Ahmad I., Kader M.A., Al Shahrani S.S., Asiri E.A. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal Pathobionts: An In Vitro Microbiological Study. Antibiotics. 2019;8:89. doi: 10.3390/antibiotics8030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suri M.A., Azizah Z., Asra R. A Review: Traditional Use, Phytochemical and Pharmacological Review of Red Betel Leaves (Piper crocatum Ruiz & Amp; Pav) Asian J. Pharm. Res. Dev. 2021;9:159–163. doi: 10.22270/Ajprd.V9i1.926. [DOI] [Google Scholar]
- 12.Gurning K., Lumbangaol S., Situmorang R.F.R., Silaban S. Determination of Phenolic Contents and Antioxidant Activity Test of Ethanol Extract of Sirih Merah (Piper crocatum Ruiz & Pav.) Leaves Using the DPPH Method. J. Pendidik. Kim. 2021;13:137–142. doi: 10.24114/Jpkim.V13i2.26984. [DOI] [Google Scholar]
- 13.Rizkita A.D., Cahyono E., Mursiti S. Isolasi dan Uji Antibakteri Minyak Daun Sirih Hijau dan Merah Terhadap Streptococcus mutans. J. Chem. Sci. 2017;6:279–286. [Google Scholar]
- 14.Larsen T., Fiehn N. Dental Biofilm Infections—An Update. APMIS. 2017;125:376–384. doi: 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- 15.Amin M., Ain N., Mohd A., Sharaf A., Al-Hammadi S. Materials Science & Engineering C Organic and Inorganic Antibacterial Approaches In Combating Bacterial Infection for Biomedical Application. Mater. Sci. Eng. C. 2021;118:111382. doi: 10.1016/J.Msec.2020.111382. [DOI] [PubMed] [Google Scholar]
- 16.Cui T., Luo W., Xu L., Yang B., Zhao W., Cang H. Progress of Antimicrobial Discovery Against The Major Cariogenic Pathogen Streptococcus mutans. Curr. Issues Mol. Biol. 2019;32:601–644. doi: 10.21775/cimb.032.601. [DOI] [PubMed] [Google Scholar]
- 17.Erviana R. Active Compounds Isolated from Red Betel (Piper crocatum Ruiz & Pav) Leaves Active Against Streptococcus mutans through Its Inhibition Effect on Glucosyltransferase Activity. J. Med. Sci. 2011;43:71–78. [Google Scholar]
- 18.Vaillancourt K., Lebel G., Pellerin G., Lagha A.B., Grenier D. Effects of the Licorice Isoflavans Licoricidin and Glabridin on the Growth, Adherence Properties, and Acid Production of Streptococcus mutans, and Assessment of Their Biocompatibility. Antibiotics. 2021;10:163. doi: 10.3390/antibiotics10020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avilés-Reyes A., Miller J.H., Simpson-Haidaris P.J., Lemos J.A., Abranches J. Cnm Is A Major Virulence Factor of Invasive Streptococcus mutans and Part of A Conserved Three-Gene Locus. Mol. Oral Microbiol. 2014;29:11–23. doi: 10.1111/omi.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi N., Nyvad B. The Role of Bacteria in the Caries Process: Ecological Perspectives. J. Dent. Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 21.Xiao J., Klein M.I., Falsetta M.L., Lu B., Delahunty C.M., Yates J.R., Heydorn A., Koo H. The Exopolysaccharide Matrix Modulates The Interaction between 3D Architecture and Virulence of A Mixed-Species Oral Biofilm. PLoS Pathog. 2012;8:7–9. doi: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palareti G., Legnani C., Cosmi B., Antonucci E., Erba N., Poli D., Testa S., Tosetto A., De Micheli V., Ghirarduzzi A., et al. Comparison between Different Imer Cutoff Values to Assess the Individual Risk of Recurrent Venous Thromboembolism: Analysis of Results Obtained in the DULCIS Study. Int. J. Lab. Hematol. 2016;38:42–49. doi: 10.1111/ijlh.12426. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Van Der Mei H.C., Busscher H.J., Ren Y. Streptococcus mutans Adhesion Force Sensing in Multi-Species Oral Biofilms. npj Biofilms Microbiomes. 2020;6:25. doi: 10.1038/s41522-020-0135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger D., Rakhamimova A., Pollack A., Loewy Z. Oral Biofilms: Development, Control, and Analysis. High-Throughput. 2018;7:24. doi: 10.3390/Ht7030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Oliveira R.V.D., Bonafé F.S.S., Spolidorio D.M.P., Koga-Ito C.Y., De Farias A.L., Kirker K.R., James G.A., Brighenti F.L. Streptococcus mutans and Actinomyces Naeslundii Interaction in Dual-Species Biofilm. Microorganisms. 2020;8:194. doi: 10.3390/microorganisms8020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marin L.M., Xiao Y., Cury J.A., Siqueira W.L. Modulation of Streptococcus mutans Adherence To Hydroxyapatite By Engineered Salivary Peptides. Microorganisms. 2022;10:223. doi: 10.3390/microorganisms10020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paqué P.N., Herz C., Wiedemeier D.B., Mitsakakis K., Attin T., Bao K., Belibasakis G.N., Hays J.P., Jenzer J.S., Kaman W.E., et al. Salivary Biomarkers For Dental Caries Detection and Personalized Monitoring. J. Pers. Med. 2021;11:235. doi: 10.3390/jpm11030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Supiandi M.I., Ege B., Julung H., Zubaidah S., Mahanal S. Ethnobotany of Traditional Medicine In Dayak Jangkang Tribe, Sanggau District, West Kalimantan, Indonesia. Biodiversitas J. Biol. Divers. 2021;22:5417–5424. doi: 10.13057/biodiv/d221224. [DOI] [Google Scholar]
- 29.Vyas T., Bhatt G., Gaur A., Sharma C., Sharma A., Nagi R. Chemical Plaque Control—A Brief Review. J. Fam. Med. Prim. Care. 2021;10:1562. doi: 10.4103/jfmpc.jfmpc_2216_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thi M.T.T., Wibowo D., Rehm B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020;21:8671. doi: 10.3390/ijms21228671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosterd C., Moineau S. Characterization of A Type II-A CRISPR-Cas System in Streptococcus mutans. Msphere. Asm. Org. 2020;9:e00235-20. doi: 10.1128/mSphere.00235-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agung I.G., Ambarawati D., Sukrama I.D.M., Putu I.W., Yasa S. Deteksi Gen Gtf-B Streptococcus mutans Dalam Plak Dengan Gigi Karies Pada Siswa Di SD N 29 Dangin Puri. Discoversys. 2020;11:1049–1055. doi: 10.15562/Ism.V11i3.337. [DOI] [Google Scholar]
- 33.Castillo Pedraza M.C., Novais T.F., Faustoferri R.C., Quivey R.G., Terekhov A., Hamaker B.R., Klein M.I. Extracellular DNA and Lipoteichoic Acids Interact With Exopolysaccharides in the Extracellular Matrix of Streptococcus mutans Biofilms. Biofouling. 2017;33:722–740. doi: 10.1080/08927014.2017.1361412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castillo Pedraza M.C., Rosalen P.L., De Castilho A.R.F., Freires I.D.A., De Sales Leite L., Faustoferri R.C., Quivey R.G., Klein M.I. Inactivation of Streptococcus mutans Genes Lytst and Dltad Impairs Its Pathogenicity In Vivo. J. Oral Microbiol. 2019;11:1607505. doi: 10.1080/20002297.2019.1607505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juntarachot N., Sirilun S., Kantachote D., Sittiprapaporn P., Tongpong P., Peerajan S., Chaiyasut C. Anti- Streptococcus mutans and Anti-Biofilm Activities of Dextranase and Its Encapsulation in Alginate Beads for Application In Toothpaste. PeerJ. 2020;8:E10165. doi: 10.7717/peerj.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwabuchi Y., Nakamura T., Kusumoto Y., Nakao R., Iwamoto T., Shinozuka O., Senpuku H. Effects Of Ph on the Properties of Membrane Vesicles Including Glucosyltransferase in Streptococcus mutans. Microorganisms. 2021;9:2308. doi: 10.3390/microorganisms9112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q., Ma Q., Wang Y., Wu H., Zou J. Molecular Mechanisms of Inhibiting Glucosyltransferases for Biofilm Formation In Streptococcus mutans. Int. J. Oral Sci. 2021;13:30. doi: 10.1038/s41368-021-00137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong T., He X., Chen J., Tang B., Zheng T., Jing M., Lin Y., Pan Y., Ma Q., Li Y., et al. Transcriptional Profiling Reveals the Importance of Rcrr in the Regulation of Multiple Sugar Transportation and Biofilm Formation in Streptococcus mutans. Msystems. 2021;6:e00788-21. doi: 10.1128/mSystems.00788-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bin Z., Lorna C.M., Todd K., Ping X. Streptococcus sanguinis Biofilm Formation & Interaction with Oral Pathogens. Future Microbiol. 2018;13:915–932. doi: 10.2217/fmb-2018-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz P.I., Chalmers N.I., Rickard A.H., Kong C., Milburn C.L., Palmer R.J., Kolenbrander P.E. Molecular Characterization of Subject-Specific Oral Microflora During Initial Colonization of Enamel. Appl. Environ. Microbiol. 2006;72:2837–2848. doi: 10.1128/AEM.72.4.2837-2848.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giacaman R.A., Torres S., Gómez Y., Muñoz-Sandoval C., Kreth J. Correlation of Streptococcus mutans and Streptococcus sanguinis Colonization and Ex Vivo Hydrogen Peroxide Production in Carious Lesion-Free and High Caries Adults. Arch. Oral Biol. 2015;60:154–159. doi: 10.1016/j.archoralbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Valdebenito B., Tullume-Vergara P.O., González W., Kreth J., Giacaman R.A. In Silico Analysis of The Competition Between Streptococcus sanguinis and Streptococcus mutans in The Dental Biofilm. Mol. Oral Microbiol. 2017;33:168–181. doi: 10.1111/omi.12209. [DOI] [PubMed] [Google Scholar]
- 43.Inagaki S., Fujita K., Takashima Y., Nagayama K., Ardin A.C., Matsumi Y., Matsumoto-Nakano M. Regulation of Recombination between Gtfb/Gtfc Genes in Streptococcus mutans By Recombinase A. Sci. World J. 2013;2013:405075. doi: 10.1155/2013/405075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozmos M., Virant P., Rojko F., Abram A., Rudolf R., Raspor P., Zore A., Bohinc K. Bacterial Adhesion of Streptococcus Mutans to Dental Material Surfaces. Molecules. 2021;26:1152. doi: 10.3390/molecules26041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumi Y., Fujita K., Takashima Y., Yanagida K., Morikawa Y., Matsumoto-Nakano M. Contribution of Glucan-Binding Protein A to Firm and Stable Biofilm Formation by Streptococcus mutans. Mol. Oral Microbiol. 2015;30:217–226. doi: 10.1111/omi.12085. [DOI] [PubMed] [Google Scholar]
- 46.Rinaudo C.D., Rosini R., Galeotti C.L., Berti F., Necchi F., Reguzzi V., Ghezzo C., Telford J.L., Grandi G., Maione D. Specific Involvement of Pilus Type 2a in Biofilm Formation in Group B Streptococcus. PLoS ONE. 2010;5:e9216. doi: 10.1371/journal.pone.0009216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redanz S., Cheng X., Giacaman R.A., Pfeifer C.S., Merritt J., Kreth J. Live and Let Die: Hydrogen Peroxide Production by the Commensal Flora and Its Role in Maintaining A Symbiotic Microbiome. Mol. Oral Microbiol. 2018;33:337–352. doi: 10.1111/omi.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redanz S., Masilamani R., Cullin N., Giacaman R.A., Merritt J., Kreth J. Distinct Regulatory Role of Carbon Catabolite Protein A (Ccpa) in Oral Streptococcal Spxb Expression. J. Bacteriol. 2018;200:e00619-17. doi: 10.1128/JB.00619-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng X., Redanz S., Cullin N., Zhou X., Xu X., Joshi V., Koley D., Merritt J., Kreth J. Plasticity of The Pyruvate Node Modulates Hydrogen Peroxide Production and Acid Tolerance in Multiple Oral Streptococci. Appl. Environ. Microbiol. 2018;84:e01697-17. doi: 10.1128/AEM.01697-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liao S., Klein M.I., Heim K.P., Fan Y., Bitoun J.P., Ahn S.J., Burne R.A., Koo H., Brady L.J., Wen Z.T. Streptococcus mutans Extracellular DNA Is Upregulated During Growth in Biofilms, Actively Released via Membrane Vesicles, And Influenced By Components of The Protein Secretion Machinery. J. Bacteriol. 2014;196:2355–2366. doi: 10.1128/JB.01493-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein M.I., Hwang G., Santos P.H.S., Campanella O.H., Koo H. Streptococcus mutans-Derived Extracellular Matrix in Cariogenic Oral Biofilms. Front. Cell. Infect. Microbiol. 2015;5:10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matera G., Muto V., Vinci M., Zicca E., Abdollahi-Roodsaz S., Van De Veerdonk F.L., Kullberg B.J., Liberto M.C., Van Der Meer J.W.M., Focà A., et al. Receptor Recognition of and Immune Intracellular Pathways for Veillonella parvula Lipopolysaccharide. Clin. Vaccine Immunol. 2009;16:1804–1809. doi: 10.1128/CVI.00310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mashima I., Liao Y., Miyakawa H., Theodorea C.F., Thawboon B., Thaweboon S., Scannapieco F.A., Nakazawa F. Veillonella infantium sp. Nov., An Anaerobic, Gram-Stain-Negative Coccus Isolated from Tongue Biofilm of A Thai Child. Int. J. Syst. Evol. Microbiol. 2018;68:1101–1106. doi: 10.1099/ijsem.0.002632. [DOI] [PubMed] [Google Scholar]
- 54.Mashima I., Theodorea C.F., Djais A.A., Kunihiro T., Kawamura Y., Otomo M. Veillonella nakazawae sp. Nov., An Anaerobic Gram-negative Coccus Isolated from the Oral Cavity of Japanese Children. Int. J. Syst. Evol. Microbiol. 2021;71:004583. doi: 10.1099/ijsem.0.004583. [DOI] [PubMed] [Google Scholar]
- 55.Hamidou A., Des C., Bonnet M., Fournier P., Raoult D., Million M. Veillonella Massiliensis, A New Anaerobic Species Isolated from Human Colostrum. Hum. Microbiome J. 2017;4:20–21. doi: 10.1016/J.Humic.2017.05.003. [DOI] [Google Scholar]
- 56.Kreth J., Herzberg M.C. The Root Canal Biofilm. Vol. 9. Springer; Berlin/Heidelberg, Germany: 2015. Molecular Principles of Adhesion and Biofilm Formation; pp. 23–53. [DOI] [Google Scholar]
- 57.Lahiri D., Nag M., Banerjee R., Mukherjee D., Garai S., Sarkar T., Dey A., Sheikh H.I., Pathak S.K., Edinur H.A., et al. Amylases: Biofilm Inducer or Biofilm Inhibitor? Front. Cell. Infect. Microbiol. 2021;11:1–13. doi: 10.3389/fcimb.2021.660048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denger K., Schink B. Energy Conservation by Succinate Decarboxylation in Veillonella parvula. J. Gen. Microbiol. 1992;138:967–971. doi: 10.1099/00221287-138-5-967. [DOI] [PubMed] [Google Scholar]
- 59.Ng S.K.C., Hamilton I.R. Lactate Metabolism by Veillonella parvula. J. Bacteriol. 1971;105:999–1005. doi: 10.1128/jb.105.3.999-1005.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aas J.A., Paster B.J., Stokes L.N., Olsen I., Dewhirst F.E. Defining The Normal Bacterial Flora of The Oral Cavity. J. Clin. Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker M.R., Paster B.J., Leys E.J., Moeschberger M.L., Kenyon S.G., Galvin J.L., Boches S.K., Dewhirst F.E., Griffen A.L. Molecular Analysis of Bacterial Species Associated with Childhood Caries. J. Clin. Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mashima I., Theodorea C.F., Thaweboon B., Thaweboon S., Scannapieco F.A., Nakazawa F. Exploring the Salivary Microbiome of Children Stratified by the Oral Hygiene Index. PLoS ONE. 2017;12:E0185274. doi: 10.1371/journal.pone.0185274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Djais A.A., Theodorea C.F., Mashima I., Otomo M., Saitoh M., Nakazawa F. Identification and Phylogenetic Analysis of Oral Veillonella Species Isolated from the Saliva of Japanese Children. F1000Research. 2019;8:616. doi: 10.12688/f1000research.18506.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashima I., Theodorea C.F., Thaweboon B., Thaweboon S., Nakazawa F. Identification of Veillonella Species in the Tongue Biofilm by Using A Novel One-Step Polymerase Chain Reaction Method. PLoS ONE. 2016;11:E0157516. doi: 10.1371/journal.pone.0157516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Distler W., Kröncke A. The Lactate Metabolism of the Oral Bacterium Veillonella from Human Saliva. Arch. Oral Biol. 1981;26:657–661. doi: 10.1016/0003-9969(81)90162-X. [DOI] [PubMed] [Google Scholar]
- 66.Preza D., Olsen I., Aas J.A., Willumsen T., Grinde B., Paster B.J. Bacterial Profiles of Root Caries in Elderly Patients. J. Clin. Microbiol. 2008;46:2015–2021. doi: 10.1128/JCM.02411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aas J.A., Griffen A.L., Dardis S.R., Lee A.M., Olsen I., Dewhirst F.E., Leys E.J., Paster B.J. Bacteria of Dental Caries in Primary and Permanent Teeth in Children And Young Adults. J. Clin. Microbiol. 2008;46:1407–1417. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belstrøm D., Holmstrup P., Nielsen C.H., Kirkby N., Twetman S., Heitmann B.L., Klepac-Ceraj V., Paster B.J., Fiehn N.E. Bacterial Profiles of Saliva in Relation to Diet, Lifestyle Factors, and Socioeconomic Status. J. Oral Microbiol. 2014;6:23609. doi: 10.3402/jom.v6.23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gross E.L., Beall C.J., Kutsch S.R., Firestone N.D., Leys E.J., Griffen A.L. Beyond Streptococcus mutans: Dental Caries Onset Linked to Multiple Species by 16S Rrna Community Analysis. PLoS ONE. 2012;7:e47722. doi: 10.1371/journal.pone.0047722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paju N., Yamlean P.V.Y., Kojong N. Uji Efektivitas Salep Ekstrak Daun Binahong (Anredera cordifolia (Ten.) Steenis) Pada Kelinci (Oryctolagus cuniculus) Yang Terinfeksi Bakteri Staphylococcus aureus. Pharmacon. 2013;2:51–62. doi: 10.35799/Pha.2.2013.885. [DOI] [Google Scholar]
- 71.Safitri S., Rofiza Y., Eti M. Studi Etnobotani Tumbuhan Obat di Kecamatan Rambah Kabupaten Rokan Hulu. Ejournal. 2015;2:2–3. doi: 10.1182/Blood-2014-01-551671. [DOI] [Google Scholar]
- 72.Liu Y., Breukink E. The Membrane Steps of Bacterial Cell Wall Synthesis as Antibiotic Targets. Antibiotics. 2016;5:28. doi: 10.3390/antibiotics5030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stokes J.M., Lopatkin A.J., Lobritz M.A., Collins J.J. Bacterial Metabolism and Antibiotic Efficacy. Cell Metab. 2019;30:251–259. doi: 10.1016/j.cmet.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu H., Si Z., Luo Y., Feng P., Wu X., Hou W., Zhu Y., Chan-Park M.B., Xu L., Huang D. The Mechanisms and the Applications of Antibacterial Polymers in Surface Modification on Medical Devices. Front. Bioeng. Biotechnol. 2020;8:910. doi: 10.3389/fbioe.2020.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tenda P.E., Lenggu M.Y., Ngale M.S., Farmasi J., Kupang P.K., Activity A. Antibacterial Activity Test of Ethanol Extract of Faloak Tree Skin (Sterculia sp.) on Staphylococcus aureus Bacteria. J. Info. Kesehat. 2017;15:227–239. doi: 10.31965/Infokes.V15i1.143. [DOI] [Google Scholar]
- 76.Dohude G.A., Ginting F.C. The Effectivity of Binahong (Anredera Cordifolia (Ten.) Steenis) Leaves Extract for Growth Inhibition of Streptococcus mutans in Oral Cavity. J. Dentomaxillofacial Sci. 2021;6:151. doi: 10.15562/jdmfs.v6i3.1040. [DOI] [Google Scholar]
- 77.Kurek-Górecka A., Walczyńska-Dragon K., Felitti R., Baron S., Olczyk P. Propolis and Diet Rich in Polyphenols as Cariostatic Agents Reducing Accumulation of Dental Plaque. Molecules. 2022;27:271. doi: 10.3390/molecules27010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanasi E., Dewhirst F.E., Chalmers N.I., Kent R., Jr., Moore A., Hughes C.V., Pradhan N., Loo C.Y., Tanner A.C.R. Clonal Analysis of the Microbiota of Severe Early Childhood Caries. Caries Res. 2010;44:485–497. doi: 10.1159/000320158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kusuma S.A.F., Manan W.S., Budiman F. Inhibitory Effect of Red Piper betel Leaf Ethanol Extract (Piper crocatum Ruiz and Pav.) against Trichomonas Vaginalis Trophozoites In Vitro. Asian J. Pharm. Clin. Res. 2017;10:311–314. doi: 10.22159/ajpcr.2017.v10i11.20778. [DOI] [Google Scholar]
- 80.Farhadi F., Khameneh B., Iranshahi M., Iranshahy M. Antibacterial Activity of Flavonoids and Their Structure-Activity Relationship: An Update Review. Phyther. Res. 2019;33:13–40. doi: 10.1002/ptr.6208. [DOI] [PubMed] [Google Scholar]
- 81.Adamczak A., Ożarowski M., Karpiński T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019;9:109. doi: 10.3390/jcm9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Divaris K., Shungin D., Rodríguez-Cortés A., Basta P.V., Roach J., Cho H., Wu D., Ferreira Zandoná A.G., Ginnis J., Ramamoorthy S., et al. The Supragingival Biofilm in Early Childhood Caries: Clinical and Laboratory Protocols and Bioinformatics Pipelines Supporting Metagenomics, Metatranscriptomics, and Metabolomics Studies of the Oral Microbiome. Methods Mol. Biol. 2019;1922:525–548. doi: 10.1007/978-1-4939-9012-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuhrotun R.K.B., Subarkah A.R., Dhana Rizkita A., Cahyono E., Sri Mursiti D.F., Rana J., Campos J.L.S., Poggianella M., Bestagno M. Antioxidant Activity, Phenolic, Flavonoid and Tannin Content of Piper betle and Leucosyke Capitella Murni. Int. J. Infect. Dis. 2019;8:60. doi: 10.4314/Tjpr.V13i10.6. [DOI] [Google Scholar]
- 84.Dong S., Yang X., Zhao L., Zhang F., Hou Z., Xue P. Industrial Crops & Products Antibacterial Activity and Mechanism of Action Saponins from Chenopodium Quinoa Willd. Husks Against Foodborne Pathogenic Bacteria. Ind. Crop. Prod. 2020;149:112350. doi: 10.1016/J.Indcrop.2020.112350. [DOI] [Google Scholar]
- 85.Zhao Y., Su R., Zhang W., Yao G., Chen J. Industrial Crops & Products Antibacterial Activity of Tea Saponin from Camellia Oleifera Shell by Novel Extraction Method. Ind. Crop. Prod. 2020;153:112604. doi: 10.1016/J.Indcrop.2020.112604. [DOI] [Google Scholar]
- 86.Yang W., Chen X., Li Y., Guo S., Wang Z., Yu X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020;15:1934578X2090355. doi: 10.1177/1934578X20903555. [DOI] [Google Scholar]
- 87.Banerjee M., Parai D., Chattopadhyay S. Andrographolide: Antibacterial Activity Against Common Bacteria of Human Health Concern and Possible Mechanism of Action. Folia Microbiol. (Praha). 2017;62:237–244. doi: 10.1007/s12223-017-0496-9. [DOI] [PubMed] [Google Scholar]
- 88.He N., Wang P., Wang P., Ma C., Kang W. Antibacterial Mechanism of Chelerythrine Isolated from Root Of Toddalia Asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018;18:261. doi: 10.1186/s12906-018-2317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khin M., Jones A.M., Cech N.B., Caesar L.K. Phytochemical Analysis and Antimicrobial Efficacy of Macleaya Cordata Against Extensively Drug-Resistant Staphylococcus aureus. Nat. Prod. Commun. 2018;13:1934578X1801301. doi: 10.1177/1934578X1801301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gutiérrez-Del-Río I., Fernández J., Lombó F. Plant Nutraceuticals As Antimicrobial Agents in Food Preservation: Terpenoids, Polyphenols and Thiols. Int. J. Antimicrob. Agents. 2018;52:309–315. doi: 10.1016/j.ijantimicag.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 91.Guimarães A.C., Meireles L.M., Lemos M.F., Guimarães M.C.C., Endringer D.C., Fronza M., Scherer R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules. 2019;24:2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahizan N.A., Yang S.-K., Moo C.-L., Song A.A.-L., Chong C.-M., Chong C.-W., Abushelaibi A., Lim S.-H.E., Lai K.-S. Terpene Derivatives As A Potential Agent Against Antimicrobial Resistance (AMR) Pathogens. Molecules. 2019;24:2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cox-Georgian D., Ramadoss N., Dona C., Basu C. Therapeutic and Medicinal Uses of Terpenes. Med. Plants Farm Pharm. 2019;15:333–359. doi: 10.1007/978-3-030-31269-5_15. [DOI] [Google Scholar]
- 94.Cappiello F., Loffredo M.R., Del Plato C., Cammarone S., Casciaro B., Quaglio D., Mangoni M.L., Botta B., Ghirga F. The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics. 2020;9:325. doi: 10.3390/antibiotics9060325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ortega-Ramirez L.A., Gutiérrez-Pacheco M.M., Vargas-Arispuro I., González-Aguilar G.A., Martínez-Téllez M.A., Fernando Ayala-Zavala J. Inhibition of Glucosyltransferase Activity and Glucan Production as An Antibiofilm Mechanism of Lemongrass Essential Oil Against Escherichia Coli O157:H7. Antibiotics. 2020;9:102. doi: 10.3390/antibiotics9030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Presentato A., Piacenza E., Scurria A., Albanese L., Zabini F., Meneguzzo F., Nuzzo D., Pagliaro M., Martino D.C., Alduina R., et al. A New Water-Soluble Bactericidal Agent for the Treatment of Infections Caused by Gram-Positive and Gram-Negative Bacterial Strains. Antibiotics. 2020;9:586. doi: 10.3390/antibiotics9090586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alibi S., Crespo D., Navas J. Plant-Derivatives Small Molecules with Antibacterial Activity. Antibiotics. 2021;10:231. doi: 10.3390/antibiotics10030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Casciaro B., Mangiardi L., Cappiello F., Romeo I., Loffredo M.R., Iazzetti A., Calcaterra A., Goggiamani A., Ghirga F., Mangoni M.L., et al. Naturally-Occurring Alkaloids of Plant Origin as Potential Antimicrobials Against Antibiotic-Resistant Infections. Molecules. 2020;25:3619. doi: 10.3390/molecules25163619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jain A., Parihar D.K. Antibacterial, Biofilm Dispersal and Antibiofilm Potential of Alkaloids and Flavonoids of Curcuma. Biocatal. Agric. Biotechnol. 2018;16:677–682. doi: 10.1016/j.bcab.2018.09.023. [DOI] [Google Scholar]
- 100.Yan Y., Li X., Zhang C., Lv L., Gao B., Li M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics. 2021;10:318. doi: 10.3390/antibiotics10030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Othman T.A.M., Hanafiah R.M., Nam N.A., Mohd-Said S., Adnan S.N.A. Chemical Composition and In Vitro Antimicrobial Properties of Phyllanthus columnaris Stem Bark Tannins Against Oral Pathogens. J. Int. Dent. Med. Res. 2018;12:848–853. [Google Scholar]
- 102.Kabeer A., Yang Q., Kim G., Li H., Zhu F., Liu H., Gan R., Corke H. Food Bioscience Tannins as an Alternative to Antibiotics. Food Biosci. 2020;38:100751. doi: 10.1016/J.Fbio.2020.100751. [DOI] [Google Scholar]
- 103.Juniarti D.E., Kusumaningsih T., Juliastuti W.S., Soetojo A., Wungsu N.D. Phytochemical Analysis and Antibacterial Activity of Purple Leaf Extract [Graptophyllum pictum (L.) Griff] Against Streptococcus mutans. Acta Med. Philipp. 2021;55:802–806. doi: 10.47895/amp.v55i8.2125. [DOI] [Google Scholar]
- 104.Khan M.F., Karim M.A. Alum: Role In Dentistry As A Potent Anti-Plaque and Anti-Caries Agent. Int. J. 2019;3:6–8. [Google Scholar]
- 105.El Zoeiby A., Sanschagrin F., Levesque R.C. Structure and Function of The Mur Enzymes: Development of Novel Inhibitors. Mol. Microbiol. 2003;47:1–12. doi: 10.1046/j.1365-2958.2003.03289.x. [DOI] [PubMed] [Google Scholar]
- 106.Mihalovits L.M., Ferenczy G.G., Keserű G.M. Catalytic Mechanism and Covalent Inhibition of UDP-N-Acetylglucosamine Enolpyruvyl Transferase (MurA): Implications to the Design of Novel Antibacterials. J. Chem. Inf. Model. 2019;59:5161–5173. doi: 10.1021/acs.jcim.9b00691. [DOI] [PubMed] [Google Scholar]
- 107.Autio-Gold J. The Role of Chlorhexidine in Caries Prevention. Oper. Dent. 2008;33:710–716. doi: 10.2341/08-3. [DOI] [PubMed] [Google Scholar]
- 108.Mahajan R., Khinda P.K., Gill A.S., Kaur J., Saravanan S.P., Shewale A., Taneja M., Joshi V. Comparison of Efficacy of 0.2% Chlorhexidine Gluconate and Herbal Mouthrinses on Dental Plaque: An In Vitro Comparative Study. Eur. J. Med. Plants. 2016;13:1–11. doi: 10.9734/EJMP/2016/23318. [DOI] [Google Scholar]
- 109.Vinod K.S., Sunil K.S., Sethi P., Bandla R.C., Singh S., Patel D. A Novel Herbal Formulation Versus Chlorhexidine Mouthwash in Efficacy Against Oral Microflora. Orig. Artic. 2018;8:184. doi: 10.4103/jispcd.JISPCD_59_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng L., Li J., He L., Zhou X. Natural Products and Caries Prevention. Caries Res. 2015;49:38–45. doi: 10.1159/000377734. [DOI] [PubMed] [Google Scholar]
- 111.Lee H.J., Song J., Kim J.N. Genetic Mutations That Confer Fluoride Resistance Modify Gene Expression and Virulence Traits of Streptococcus mutans. Microorganisms. 2021;9:849. doi: 10.3390/microorganisms9040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Astuti I.P., Munawaroh E. Karakteristik Morfologi Daun Sirih Merah: Piper crocatum Ruitz & Pav dan Piper porphyrophyllum N.E.Br. Koleksi Kebun Raya Bogor. Berk. Penel. Hayati Ed. Khusus. 2011;7A:83–85. [Google Scholar]
- 113.Gong Y., Li H.X., Guo R.H., Widowati W., Kim Y.H., Yang S.Y., Kim Y.R. Anti-Allergic Inflammatory Components from the Leaves of Piper crocatum Ruiz & Pav. Biol. Pharm. Bull. 2021;44:245–250. doi: 10.1248/Bpb.B20-00726. [DOI] [PubMed] [Google Scholar]
- 114.Wardhana Y.W., Warya S., Trisnawaty A. Formulation of Toothpaste Gel Containing Mixture of Aloe Vera (Aloe Barbadensis Mill.) and Red Betel (Piper crocatum) Extract in Prevention of Dental Caries. J. Pharm. Sci. Res. 2017;9:2172–2174. [Google Scholar]
- 115.Al-Shamahy H.A. Efficacy of Some Antibiotics Against Streptococcus mutans Associated with Tooth Decay in Children and Their Mothers. Online J. Dent. Oral Health. 2019;2:1–4. doi: 10.33552/OJDOH.2019.02.000530. [DOI] [Google Scholar]
- 116.Safithri M., Fahma F. Potency of Piper crocatum Decoction as an Antihiperglycemia in Rat Strain Sprague Dawley. HAYATI J. Biosci. 2008;15:45. doi: 10.4308/hjb.15.1.45. [DOI] [Google Scholar]
- 117.Li H.X., Widowati W., Azis R., Yang S.Y., Kim Y.H., Li W. Chemical Constituents of the Piper crocatum Leaves and Their Chemotaxonomic Significance. Biochem. Syst. Ecol. 2019;86:103905. doi: 10.1016/j.bse.2019.05.013. [DOI] [Google Scholar]
- 118.Emrizal, Fernando A., Yuliandari R., Rullah K., Indrayani N.R., Susanty A., Yerti R., Ahmad F., Sirat H.M., Arbain D. Cytotoxic Activities of Fractions and Two Isolated Compounds from Sirih Merah (Indonesian Red Betel), Piper crocatum Ruiz & Pav. Procedia Chem. 2014;13:79–84. doi: 10.1016/J.Proche.2014.12.009. [DOI] [Google Scholar]
- 119.Arbain D., Nofrizal, Syafni N., Ismed F., Yousuf S., Choudhary M.I. Bicyclo[3.2.1]Octanoid Neolignans from Indonesian Red Betle Leaves (Piper Crocatum Ruiz & Pav.) Phytochem. Lett. 2018;24:163–166. doi: 10.1016/J.Phytol.2018.02.006. [DOI] [Google Scholar]
- 120.Chai Y.J., Go Y., Zhou H.Q., Li H.X., Lee S.J., Park Y.J., Widowatib W., Rizal R., Kim Y.H., Yang S.Y., et al. Unusual Bicyclo[3.2.1]Octanoid Neolignans from Leaves of Piper crocatum and Their Effect on Pyruvate Dehydrogenase Activity. Plants. 2021;10:1855. doi: 10.3390/plants10091855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Atiya A., Sinha B.N., Ranjan Lal U. New Chemical Constituents from the Piper betle Linn. (Piperaceae) Nat. Prod. Res. 2018;32:1080–1087. doi: 10.1080/14786419.2017.1380018. [DOI] [PubMed] [Google Scholar]
- 122.Liu T., Liang Q., Zhang X.M., Huang S.Y., Xu W.H. A New Furofuran Lignan from Piper terminaliflorum tseng. Nat. Prod. Res. 2018;32:335–340. doi: 10.1080/14786419.2017.1350671. [DOI] [PubMed] [Google Scholar]
- 123.Lago J.H.G., Ito A.T., Fernandes C.M., Young M.C.M., Kato M.J. Secondary Metabolites Isolated from Piper Chimonantifolium and Their Antifungal Activity. Nat. Prod. Res. 2012;26:770–773. doi: 10.1080/14786419.2011.561435. [DOI] [PubMed] [Google Scholar]
- 124.Alves H.D.S., De Souza M.D.F.V., Chaves M.C.D.O. Three New Compounds form Piper montealegreanum Yuncker (Piperaceae) J. Braz. Chem. Soc. 2011;22:1610–1615. doi: 10.1590/S0103-50532011000800026. [DOI] [Google Scholar]
- 125.Ruiz C., Haddad M., Alban J., Bourdy G., Reategui R., Castillo D., Sauvain M., Deharo E., Estevez Y., Arevalo J., et al. Activity-Guided Isolation of Antielishmanial Compounds from Piper hispidum. Phytochem. Lett. 2011;4:363–366. doi: 10.1016/j.phytol.2011.08.001. [DOI] [Google Scholar]
- 126.Hashim N.A., Ahmad F., Salleh W.M.N.H.W., Khamis S. A New Amide from Piper maingayi Hk.F. (Piperaceae) Nat. Prod. Commun. 2019;14:3–7. doi: 10.1177/1934578X19855826. [DOI] [Google Scholar]
- 127.Salleh W.M.N.H.W., Ahmad F., Yen K.H. Antioxidant and Anti-Tyrosinase Activities from Piper officinarum C.DC (Piperaceae) J. Appl. Pharm. Sci. 2014;4:87–91. doi: 10.7324/JAPS.2014.40516. [DOI] [Google Scholar]
- 128.Chen J.J., Wang S.W., Chen C.L., Kuo Y.H., Cheng M.J., Chang T.H., Sung P.J., Kuo W.L., Lim Y.P. A New Amide and Antioxidant Constituents of Piper taiwanense. Chem. Nat. Compd. 2017;53:1117–1121. doi: 10.1007/s10600-017-2213-y. [DOI] [Google Scholar]
- 129.Ugusman A., Zakaria Z., Hui C.K., Nordin N.A.M.M., Mahdy Z.A. Flavonoids of Piper sarmentosum and Its Cyto- Protective Effects Against Oxidative Stress. EXCLI J. 2011;4:10–19. [PMC free article] [PubMed] [Google Scholar]
- 130.De Campos M.P., Cechinel Filho V., Da Silva R.Z., Yunes R.A., Zacchino S., Juarez S., Bella Cruz R.C., Bella Cruz A. Evaluation of Antifungal Activity of Piper solmsianum C. DC. Var. Solmsianum (Piperaceae) Biol. Pharm. Bull. 2005;28:1527–1530. doi: 10.1248/bpb.28.1527. [DOI] [PubMed] [Google Scholar]
- 131.Batubara I., Rahminiwati M., Darusman L.K., Mitsunaga T. Tyrosinase Activity of Piper Betle and Piper crocatum Essential Oil. Oral. 2011:50–53. [Google Scholar]
- 132.Kurnia D., Hutabarat G.S., Windaryanti D., Herlina T., Herdiyati Y., Satari M.H. Potential Allylpyrocatechol Derivatives as Antibacterial Agent Against Oral Pathogen of S. sanguinis ATCC 10,556 and As Inhibitor of Mura Enzymes: In Vitro and In Silico Study. Drug Des. Devel. Ther. 2020;14:2977–2985. doi: 10.2147/DDDT.S255269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reshmi S.K., Sathya E., Devi P.S. Isolation of Piperdine from Piper Nigrum and Its Antiproliferative Activity. African J. Pharm. Pharmacol. 2010;4:562–573. doi: 10.5897/JMPR10.033. [DOI] [Google Scholar]
- 134.Li H.X., Yang S.Y., Kim Y.H., Li W. Isolation of Two New Compounds and Other Constituents from Leaves of Piper crocatum and Study of Their Soluble Epoxide Hydrolase Activities. Molecules. 2019;24:489. doi: 10.3390/molecules24030489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dyah Astuti S., Dysan Tirtana R., Fitriana Mahmud A., Mawaddah A., Abdurachman, Yasin M. Ultraviolet (UV) Activation Effect on Antibacterial Agents of Red Betel (Piper crocatum) Extract To Streptococcus Mutans. J. Phys. Conf. Ser. 2020;1445:012004. doi: 10.1088/1742-6596/1445/1/012004. [DOI] [Google Scholar]
- 136.Puspita P.J., Safithri M., Sugiharti N.P. Antibacterial Activitieso of Sirih Merah (Piper crocatum) Leaf Extracts. Curr. Biochem. 2019;5:1–10. doi: 10.29244/cb.5.3.1-10. [DOI] [Google Scholar]
- 137.Januarti I.B., Wijayanti R., Wahyuningsih S., Nisa Z. Potensi Ekstrak Terpurifikasi Daun Sirih Merah (Piper crocatum Ruiz & Pav) Sebagai Antioksidan dan Antibakteri. JPSCR J. Pharm. Sci. Clin. Res. 2019;4:60. doi: 10.20961/Jpscr.V4i2.27206. [DOI] [Google Scholar]
- 138.Soleha F. Pengaruh Metode Ekstraksi Maserasi Terhadap Aktivitas Antibakteri Daun Sirih Merah (Piper crocatum Ruiz & Pav) pada Bakteri Staphylococcus Aureus Menggunakan Metode Sumur Difusi. J. Anal. Farm. 2018;3:62–70. doi: 10.33024/Jaf.V3i1.2778. [DOI] [Google Scholar]
- 139.Pujiastuti P., Lestari S., Fakultas P., Gigi K., Jember U., Konservasi B., Kedokteran F., Universitas G. Perbedaan Efektifitas Antibakteri Ekstrak Daun Sirih Merah (Piper Crocatum) pada Porphyromonas Gingivalis dan Streptococcus Viridans. JKG Unej. 2015;12:1–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study did not report any data.