Abstract
It has been a challenge to analyze minute amounts of proteomic samples in a facile and robust manner. Herein we developed a quantitative proteomics workflow by integrating Suspension Trapping (S-Trap) based sample preparation and label-free data-independent acquisition (DIA) mass spectrometry and then applied it for the analysis of microgram and even nanogram amounts of exosome samples. S-Trap-based sample preparation outperformed the traditional in-solution digestion-based approach and the commonly used filter-aided sample preparation (FASP)-based approach with regards to the number of proteins and peptides identified. Moreover, S-Trap-based sample preparation coupled with DIA mass spectrometry also showed the highest reproducibility for protein quantification. In addition, this approach allowed for identification and quantification of exosome proteins with low starting amounts (down to 50~200 ng). Finally, the proposed method was successfully applied to label-free quantification of exosomal proteins extracted from MDA-MB-231 breast cancer cells and MCF-10A non-tumorigenic epithelial breast cells. Prospectively, we envision the integrated S-Trap sample preparation coupled with DIA quantification strategy as a promising alternative for highly efficient and sensitive analysis of trace amounts of proteomic samples (e.g., exosomal samples).
Keywords: Exosome, Suspension trapping (S-Trap), Filter-aided sample preparation (FASP), In-solution digestion, Data-independent acquisition (DIA), Quantitative proteomics
Introduction
Deep and precision proteomics play critical roles in the identification of molecular signatures associated with diseases, particularly those that emphasize protein pathways and signaling cascades. Due to its unique advantages, high-resolution mass spectrometry has become an indispensable tool for the identification and quantification of proteins and proteomes [1]. As an appealing approach, bottom-up proteomics requires proteins to be digested into peptides for further analysis [2]. Solubilization of proteins from samples of interest and efficient digestion of proteins into the corresponding peptides are critical for the success of bottom-up proteomics [3]. The depth of proteomic coverage and quantitative accuracy are largely dependent on the sample processing steps adopted. The traditional in-solution digestion-based sample preparation suffers from several inherent drawbacks (including tedious procedures and lengthy processing time), hindering sample processing throughput, analytical reproducibility, and sensitivity. To that end, a number of integrated sample processing strategies have been proposed, including immobilized enzymatic reactorbased digestion methods [4], improved in-solution digestion-based methods (e.g., filter-aided sample preparation (FASP) [5,6], in-StageTip [7], and nanoPOTS [8,9], and on-bead digestion-based methods (e.g., S-Trap [10,11], SP3 [12], and SISPROT [13]. Among them, FASP and STrap, which have been commercialized recently, have gained much attention. These methods allow processing of detergent-containing samples with substantially decreased manual-handling steps, showing great promise for robust and sensitive analysis of proteomic samples. Besides the upfront sample processing advantages, quantification is another key aspect of precision proteomics. Recently several powerful quantification techniques, including chemical or metabolic labeling strategies combined with data-dependent acquisition (DDA) [1,14], targeted quantification methods based on multiple-reaction monitoring (MRM) [15], and parallel reaction monitoring (PRM) [16] along with data-independent acquisition (DIA) [17–19] have been developed. Of note, the DIA method has both the breadth of DDA and the precision of MRM/PRM for reproducible identification and quantification of proteins (including those of low abundance), holding great promise for accurate proteomic quantification of trace biological samples.
Small extracellular-vesicles (small-EVs), also commonly referred to as exosomes, are phospholipid bi-layered nanosized particles (with diameter ranging from 30 to 120 nm) that are released by virtually all cells and that can mediate long-distance intercellular communication via transport of a variety of biomolecules including various important biological molecules (such as lipids, proteins, messenger RNAs, microRNAs, and noncoding RNAs) [20–22]. Proteomic analysis of exosomes is of great potential for elucidation of underlying molecular mechanisms of diseases and for the discovery of protein biomarkers (e.g. for tumor initiation and metastasis) [23,24]. Despite advances in the preparation of exosomes from cultured cells or from biofluids, the purification of large amounts of exosomes, which is required for proteomic analyses, is either a challenge or inherently limited by the biofluid volume output. The development of a highly sensitive and effective sample processing and quantification strategy would help facilitate exosomal proteomics and biomarker identification.
In this study, we aimed to evaluate several workflows and to develop a robust, sensitive, and accurate strategy for proteomic analysis. In comparison to the traditional in-solution digestion- and FASP-based approaches, S-Trap gave deeper proteomic coverage and enhanced detection of minute amounts of samples (down to 50 ng). Moreover, coupling S-Trap-based processing with DIA mass spectrometry improved quantification sensitivity and reproducibility of exosomal proteins. Besides exosomal proteomics, the proposed approach is readily applicable for the analysis of trace amounts of other biological samples.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Cultured cells were obtained from ATCC (Manassas, Virginia, USA). DTT (1,4-dithiothreitol) was purchased from Sigma-Aldrich (St. Louis, MO). Triethylammonium bicarbonate (TEABC) buffer (1 M, pH 8.4~8.6) was ordered from Fluka. Iodoacetamide (IAA) was ordered from VWR. Trifluoroacetic Acid (TFA, >99.5%), formic acid (FA, LC/MS grade) and acetonitrile (ACN, LC/MS grade) were purchased from Fisher Scientific (Waltham, MA). Nano UPLC mobile phase A: 0.1% formic acid in water (LC/MS grade) and mobile phase B: 100% ACN 0.1% formic acid (LC/MS grade) were ordered from Honeywell. Ultrafiltration filter (10-kDa cutoff) was purchased from Sartorius (Germany). S-Trap micro columns were purchased from Protifi (Huntington, NY). Lys-C and trypsin (MS grade) were purchased from Promega (Madison, WI, USA).
Exosome sample preparation
A sequential centrifugation approach was used for exosome isolation, as previously reported [25,26]. Briefly, centrifugation was performed at 300× g at 4 °C for 5 min to remove floating cells followed by 2,000× g, 4 °C for 10 min to remove dead cells and large growth cellular debris. The supernatant was centrifuged at 10,000× g at 4 °C for 30 min to remove additional debris. After being transferred to a clean ultracentrifuge tube, the supernatant was subjected to ultracentrifugation in a optima XE-90 ultracentrifuge equipped with a SW 41 Ti rotor (Beckman Coulter) at 100,000× g for 90 min at 4 °C. The supernatant was discarded and the exosome pellet was resuspended in 10 mL of sterile 1× PBS. The suspension was again ultracentrifuged at 100,000× g for 90 min at 4 °C. Exosome pellet was collected and stored at −80 °C for further experiments.
In-solution sample preparation
The in-solution digestion method was performed according to the previous protocol, with slight modifications [27]. Exosome samples were denatured in 8 M urea (50 mM TEABC, pH = 8), reduced in 10 mM DTT at 37 °C for 30 min, followed by alkylation in 30 mM IAA in darkness at room temperature for 30 min. Then the reaction was quenched in 10 mM DTT at 37 °C for 30 min. Subsequently, the samples were diluted 10 folds with 50 mM TEABC and digested with Lys-C/trypsin mixture at an enzyme-to-substrate ratio of 1:50 (w/w) at 37 °C overnight. After digestion, the resulting solution was acidified to stop proteolysis. The tryptic digests were desalted with C18 spin columns, dried by a SpeedVac (Fisher Scientific) and then kept at 20 °C before analysis.
Filter-aided sample preparation (FASP)
FASP filters were activated with 100 μL of water and centrifuged for 15 min at 14000 g. Exosome samples dissolved in 2% SDS buffer (50 mM TEABC) were diluted with 200 μL of UT buffer (8 M urea, 50 mM TEABC, 20 mM DTT, pH 8.0) and incubated at 37°C for 30 min. Then the samples were loaded onto pre-activated filters and centrifuged at 14000 g for 25 min. The filters were washed with 200 μL of UT buffer and centrifuged at 14000 g for 25 min. Then 100 μL of 50 mM IAA solution was added and incubated for 30 min at room temperature in the dark. Then the samples were washed twice with 200 μL of UT buffer, followed by washing with 200 μL of 50 mM TEABC solution for three times. Finally, 100 μL of Lys-C/trypsin mixture solution with an enzyme-to-substrate ratio of 1:50 (w/w) was added, with digestion performed at 37°C overnight. The filter unit was centrifuged at 14,000 × g for 15 min and washed twice with 50 μL of 50 mM TEABC solution. All eluents containing tryptic peptides were pooled and dried in a SpeedVac.
Suspension-trapping (S-Trap) sample preparation
S-Trap-based sample processing was performed according to the manufacturer instruction, with minor modifications. Exosome samples were suspended in 5% SDS buffer (50 mM TEABC) and reduced in 20 mM DTT by heating at 95 °C for 10 min. After cooling down, iodoacetamide was added to a final concentration of 40 mM for alkylation by incubating at room temperature for 30 min in the dark. The protein samples were acidified by aqueous phosphoric acid (a final concentration of ~1.2% phosphoric acid) and diluted by six volumes of the S-Trap buffer (90% aqueous methanol in 100 mM TEABC, pH = 7.1). The acidified proteins were transferred onto a micro S-Trap column followed by centrifugation at 2000 g for 1 min. After washing with the S-Trap buffer three times, proteins on the S-Trap column were first digested with Lys-C (an enzyme to substrate ratio of 1:50, w/w) at 37 °C for 2 h and then by trypsin at 37 °C overnight. The resulting peptides were eluted by adding 50 μL of 0.2% aqueous formic acid and 50% acetonitrile containing 0.2% formic acid subsequently. The elutes were combined and dried down with a SpeedVac.
NanoRPLC-ESI-MS/MS analysis
Peptides were analyzed with a nanoAcquity UPLC system (Waters) coupled with Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher) in either DDA mode or DIA mode. Samples were resuspended in 0.1% FA solution and loaded onto a C18 Trap column (Waters Acquity UPLC M-Class Trap, Symmetry C18, 100 Å, 5 μm, 180 μm × 20 mm) at 10 μL/min for 4 min. Peptides were then separated with an analytical column (Waters Acquity UPLC M-Class, peptide BEH C18 column, 300 Å, 1.7 μm, 75 μm × 150 mm) which was temperature controlled at 40°C. The flow rate was set as 400 nL/min. A 150-min gradient of buffer A (2% ACN, 0.1% formic acid) and buffer B (0.1% formic acid in ACN) was used for separation: 1% buffer B at 0 min, 5% buffer B at 1 min, 30% buffer B at 80 min, 50% buffer B at 120 min, 98% buffer B at 130 min, 98% buffer B at 133 min, 1% buffer B at 133.1 min, and 1% buffer B at 150 min. The MS data were acquired by Orbitap Fusion Lumos mass spectrometer using an ion spray voltage of 2.4 kV and an ion transfer temperature of 275°C. Mass spectra was recorded with Xcalibur 4.0. Advanced peak determination was on for MS analyses. DDA parameters were set as below: Detector Type: Orbitrap; Mass range: 375–1500 m/z; Orbitrap Resolution: 120,000; Scan Range: 375–1500 m/z; RF Lens: 30%; AGC Target: Standard; Maximum Injection Time Mode: Auto; Microscans: 1. Charge state: 2–7; Exclusion duration: 40 s; Cycle Time: 3 s. MS/MS parameters were set as below: Isolation Mode: Quadrupole; Isolation Window: 1.6 m/z; HCD normalized collision energy: 30%; Detector Type: Orbitrap; Resolution: 30,000; Normalized AGC Target: 200%. DIA data was acquired with the following parameters: scan range = 400–1250 m/z, MS resolution of 120,000 at m/z 200, an AGC target = 4e5, and maximum injection time = 50 ms. The DIA-MS/MS scan was performed in the HCD mode with the following parameters: isolation window of 20 Da with 1 Da overlap, precursor range = 400–1000 m/z, fragment scan range 110–1600 m/z; resolution = 30,000 with maximum injection time of 54 ms, AGC target = 5e4; normalized collision energy = 30%. All data were acquired in profile mode using positive polarity.
Data analysis
DDA raw files were processed in Proteome Discoverer (Thermo Fisher Scientific, version 2.4) with the Sequest HT database search engine. The SwissProt database with taxonomy as Homo sapiens (TaxID 9606) was downloaded from UniProt (Released on July 5, 2017, with 42253 protein sequences). The database searching parameters were set as below: full tryptic digestion and allowed up to two missed cleavages, the precursor mass tolerance was set at 10 ppm, whereas the fragment-mass tolerance was set at 0.02 Da. Carbamidomethylation of cysteines (+57.0215 Da) was set as a fixed modification, and variable modifications of methionine oxidation (+15.9949 Da) and acetyl (N-terminus, +42.011 Da) were allowed. The falsediscovery rate (FDR) was determined by using a target-decoy search strategy. The decoysequence database contains each sequence in reverse orientations, enabling FDR estimation. On the peptide level, the corresponding FDR was less than 1%.
DIA data analysis was performed with Spectronaut™ (Biognosys, v14) using standard settings with slight modifications. In brief, dynamic retention time prediction with local regression calibration was selected. Interference correction on MS and MS2 level was enabled. The Qvalue cutoff was set to 1% at peptide precursor and protein levels using scrambled decoy generation and dynamic size at 0.1 fraction of library size. MS2-based quantification by area was used, enabling local cross-run normalization.
Statistics and bioinformatics
Differentially expressed exosomal proteins in MDA-MB-231 breast tumor cells were identified by using unpaired two-tailed Student’s t-test with the thresholds of ± 2-fold change over MCF10a (i.e., fold change > 2 or < 0.5) and an adjusted p value < 0.05. Unsupervised hierarchical clustering of the identified differentially expressed proteins was performed based on their abundances in each sample with Euclidean distance measurement. The adjusted p values were calculated using Benjamini-Hochberg method [28]. Gene ontology (GO) enrichment analysis of the differentially expressed proteins was performed using DAVID [29,30], with the total list of human proteins used as the background. Enriched GO terms with Benjamini-Hochberg false discovery rate (FDR) < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Upfront sample processing is of vital improtance for sensitive and accurate MS-based proteomics. Herein we systematically evaulated three proteomic sample preparation workflows: 1- the traditional in-solution digestion approach; 2- FASP; and 3- S-Trap, by using either DDA-or DIA-based proteomics. We then applied our improved method (i.e., integrating S-Trapbased sample processing with DIA mass spectrometry) for the proteomics analysis of exosome samples from control and breast cancer cells.
Evaluation of three sample preparation methods
Three sample preparation methods (i.e., the traditional in-solution digestion approach, FASP, and S-Trap) were performed in parallel for analysis of the same pooled exosomal samples ultracentifugated from human breast cancer cells (Figure 1).
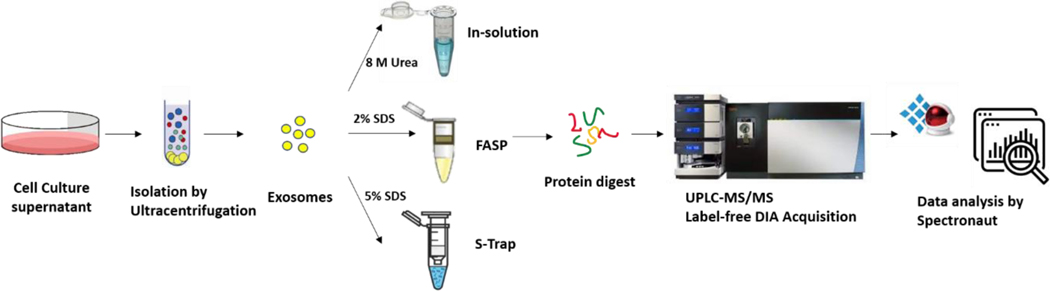
Figure 1.
Integrated workflow for exosomal proteomics. Exosomes were isolated from cell culture supernatant by ultracentrifugation, then processed with the traditional in-solution digestion, FASP, and S-Trap methods. All digests were analyzed by DIA acquisition via nanoUPLC-MS/MS, with the data files analyzed with Spectronaut.
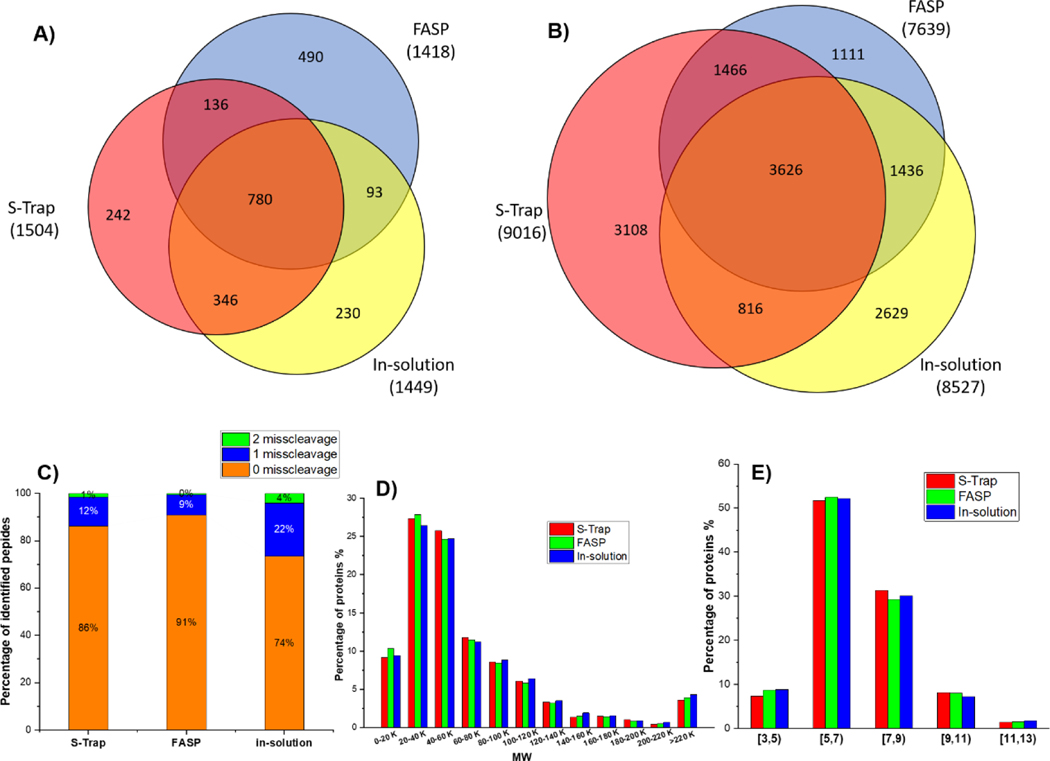
The performance of the three methods was first evaluated by processing an equal amount of starting material (i.e., five microgram of total protein from exosome samples) and analysis with DDA mass spectrometry. With the combination of identified proteins from triplicate analyses (see Supplementary Information (ESM) Table S1), a total number of 1449, 1418 and 1504 protein groups were identified (FDR less than 1%; matching with 8527, 7639 and 9016 peptides) from the traditional in-solution, FASP and S-Trap methods, respectively (Figure 2A and 2B). Overall, the S-Trap processing method yielded the highest number of proteins while the FASP method resulted in the fewest identifications, which was consistent with a previous report [31]. Among all identifications, 780 proteins and 3626 peptides were identified by all three methods. The presence of some unique proteins and peptides identified individually by each method suggests that the filter-based methods and in-solution based method may have certain preferential digestion characteristics for a small portion of the proteome (e.g., membrane proteins by filter-based methods). In addition, the missed cleavages of proteins of exosome samples were investigated to examine the trypsin efficiency in all digestion methods. The percentages of peptide containing 0 missed cleavages were 74%, 91% and 86% for insolution, FASP and S-Trap method, respectively (Figure 2C). In comparison to the traditional in-solution digestion-based method, FASP and S-Trap showed clearly superior trypsin digestion efficiency. Moreover, ~22% of peptides detected by the traditional in-solution digestion displayed one missed tryptic cleavage, while only 9–12% of peptides from FASP and S-Trap had one missed cleavage. The improved digestion procedure not only provides more fully tryptic peptides for protein identification but also benefits targeted studies that rely on quantification of specific peptides. Collectively, in comparison to the traditional in-solution digestion-based method, both FASP and S-Trap showed superior trypsin digestion efficiency. This substantial difference is likely due to the presence of relatively higher local trypsin concentrations when samples are digested on a filter such as with the S-trap column or with the membrane filter in FASP. Besides enhanced digestion, the S-Trap apporach has other advantages in contrast to the FASP approach, due to its unique processing principle [10]. STrap sample processing begins with sample lysis and solubilization in 5% SDS, which is a nearly universal protein solvent that dissolves even poorly-soluble molecules like membrane proteins (which are often discarded as pellets). In particular, SDS is required to create fine particles from the SDS-solubilized proteins and trapping the particles in the depth filter media. When protein particles are retained throughout the depth filter dimensions, SDS and other contaminants can be easily removed into the flow through. Proteins retained on the column are readily digested into peptides, without an additional desalting process. All these advantages help to further minimize sample loss and increase proteome coverage.
Figure 2.
Overlapping analyses of the peptides (A) and proteins (B) identified from exosomal samples processed by S-Trap, FASP, or the in-solution digestion-based methods (n=3); distribution of missed tryptic cleavage sites (C), MW (D) and pI (E) of the proteins identified from exosome samples.
The physiochemical characteristics of proteins identified by each method were further analyzed by evaluating the MW and pI distribution. As shown in Figure 2D and 2E, no distinct trends regarding MW and pI distribution of the identified proteins were observed for any of the methods, showing unbiased processing for proteins with different MW and pI distributions. Moreover, we used technical triplicates with each method to investigate the reproducibility of the sample processing and the nanoUPLC-MS/MS system. At the protein level, 82% of all the identified proteins from S-Trap method were identified by at least two independent runs, a value higher than that from the traditional in-solution digestion (78%) and FASP (79%) methods (Figure S1). At the peptide level, the S-Trap approach also yielded the highest overlap of up to 69% of peptides identified by at least in two runs, while the in-solution digestion method provided the least overlap (60%). These results further demonstrate that the S-Trap method affords the best reproducibility, which is of critical importance for the quantification of proteins.
The GO cellular component analysis for the proteins found in each dataset is shown in Figure S2. An overall similar pattern of proteins was observed when a relatively higher amount of proteins (e.g., 5 microgram) was processed by the three approaches (Figure S2A). However, it appears that the FASP and S-Trap methods provided a better coverage for plasma membrane proteins (Figure S2A)). Of special note, the traditional in-solution digestion method failed to detect any proteins when the starting amount of samples was decreased to one microgram. In comparison to the FASP approach, the S-Trap approach gave better identification of proteins from virtually all cellular compartments (Figure S2B), suggesting its superior performance for processing lower amounts of samples.
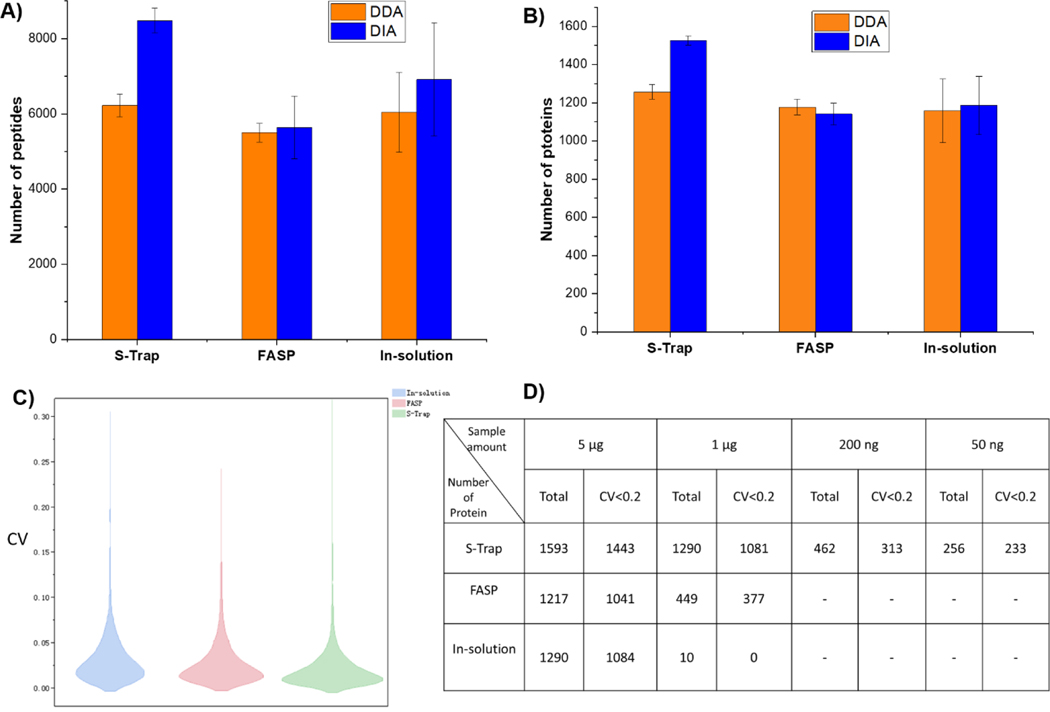
Besides sample preparation methods, we next explored and evaulated two mass spectrometry methods (i.e., DDA and DIA) for better detection and quantification of proteins. As shown in Figure 3, when setting the highest quality threshold on DIA-detected peptides (Spectronaut Q value < 0.01), DIA and DDA detected a comparable number of proteins and peptides for both the in-solution digestion and FASP procedures. Notably, when using DIA with the S-Trap method, the number of detectable proteins and peptides was increased by 21% and 36%, respetively, when compared to the DDA acquisition mode. Moreover, the S-Trap method displayed the lowest RSDs for the identified numbers of proteins and peptides (1.6% and 3.9%, respectively; n = 3), in comparison to that from FASP (RSDs of 4.9% and 14.8% for proteins and peptides, respectively) and the traditional in-solution digestion method (RSDs of 12.8% and 21.8% for proteins and peptides, respectively). Of note, ~86% of the proteins (i.e., 1368/1593 for the S-Trap method, 1057/1217 for the FASP method, and 1103/1290 for the in-solution processing method) were annotated as exosomal proteins (ESM Table S2), according to the ExoCarta database [32]. This high percentage of exosomal proteins further suggests the high quality of exosomal samples purified. For better visual evaluation, the variation of signal intensities of DIA data obtained from triplicate analyses (ESM Table S2) was expressed as coefficient of variation (CVs). The frequency of CVs in two dimensional distribution histograms (Figure 3C) shows that the CV likelihood maxima of all procedures were below 20%, while the S-Trap method led to the most reproducible results. Additionally, to further assess the performance of these methods when coupled with DIA mass spectrometry we carried out further evaluation by processing lower amouts of exosomal samples (e.g., 1 μg, 200 ng and 50 ng). Both the total numbers of proteins identified and the proteins with a CV < 0.2 were listed in Figure 3D. S-Trap coupled with DIA yielded robust identification of 1290 proteins with a starting amount of 1 μg, outperforming the FASP coupled with DIA when using the same material input (449). Remarkably, the traditional in-solution digestion coupled with the DIA method failed to identify proteins and peptides with a starting amount less than 1 μg, highlighting the severe limitations of this approach. In addition, the S-Trap approach coupled with the DIA method identified and quantified 462 proteins (with 313 proteins of CV < 0.2) and 256 proteins (with 233 proteins of CV < 0.2) from 200 ng and 50 ng of exosome sample input, respectively. Collectively, our analyses clearly highlight that coupling the S-Trap approach with the DIA method provides deep and reproducible identification and quantification of proteins and peptides when using small amounts of sample input, especially with exosomes.
Figure 3.
Comparison of numbers of peptides (A) and proteins (B) identified via DDA (n = 3, Error bars = +/− S.D.) and DIA acquisition from exosome samples processed by the S-Trap, FASP and in-solution digestion methods. (C) Comparison of coefficient of variation (CV) distribution for DIA quantification of exosome samples processed by the three different methods. (D) Table displaying the overall number of proteins and proteins with CV < 0.2 identified by each of the three methods when coupled with DIA mass spectrometry.
Coupling S-Trap and DIA for quantitative exosomal proteomics
For our additional analyses, we chose to further evaluate the coupling of the S-Trap approach with DIA mass spectrometry when applied to the label-free quantification of exosomal proteins and using two human breast cell lines, namely the MCF-10A (a non-tumorigenic epithelial breast cell line) and the MDA-MB-231 (a metastatic triple negative human breast cancer cell line). Our analyses were performed using three replicate of DIA runs, which yielded identification of 2048 proteins (including 1708 exosome proteins) and 1783 proteins (including 1573 exosome proteins) were identified from exosome samples purified from MCF-10A and MDA-MB-231 cells, respectively (ESM Table S3). To our knowledge, this is the most comprehensive dataset of exosomal proteins from these cells, compared with reports previously published (ESM Table S4).
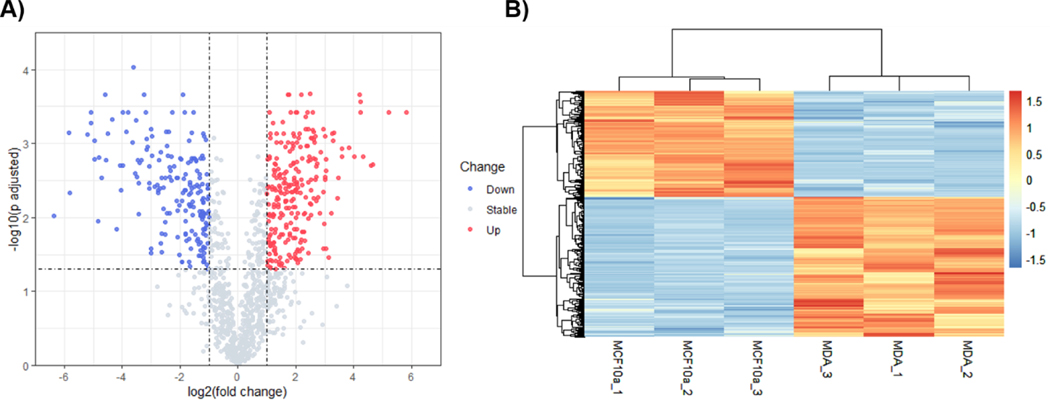
Among the identified proteins, 93 proteins were unique to the MDA-MB-231 breast cancer cell line, and 296 proteins were unique to the MCF-10A cell line. Moreover, 443 proteins were found to be differentially expressed between exosomes purified from MDA-MB-231 and MCF-10A cell lines (Figure 4). Specifically, 252 proteins were identified with increased levels and 191 proteins with decreased levels in the exosomes of MDA-MB-231 cells, which were derived from a lung cancer metastasis. Among them, several breast cancer biomarkers candidates (such as GSTP1 and SLC39A6) had decreased expression in the MDA-MB-231 cells, and several potential biomarkers (including FLNA, TFRC, ADAM10, PFN1 and SLC9A3R1) had increased expression [33,34]. The cytoskeletal proteins, β-actin, tubulin-β (TUBB) and keratin (KRT1), which have been associated with breast cancer metastasis [35,36], were over-represented in MDA-MD-231 exosomes. Interestingly, some exosomal integrins (including ITGB1, ITGA1, ITGA2, ITGA3 ITGA5) also had higher expression in MDA-MD-231 cells. These integrins may play important roles in metastasis since they have been found to activate the Src signaling pathway in the recipient cells and to induce the inflammatory response in cancer cells [37]. Furthermore, BASP1 was increased in MDA-MB-231 cells. Since BASP1 has already been demonstrated to promote cancer cell progression, [38], it may serve as a promising prognostic marker. High levels of α Enolase (ENO1) was also observed in exosomes from MDA-MB-231 cells. ENO1 has been associated with metastasis and drug resistance, and overall poor prognosis in breast cancer [39,40]. Additionally, many other exosomal proteins (including ALDOA, CALR, PSME1, PSME2, HLA-C and G6PD), were differentially expressed in our study. These proteins, which are involved in cancer immunesurveillance and dysregulation of energy synthesis in breast cancer microenvironment metastasis, may provide valuable study targets for understanding the molecular signaling pathways and contribution of exosomes during the process of metastasis [41,42].
Figure 4.
(A) Volcano plot analysis of differentially expressed exosomal proteins from MCF-10A and MDA-MB-231 cells, where significantly up-regulated and down regulated are displayed in red and blue, respectively. Proteins common to exosomes from the two different cell lines (MCF-10A and MDA-MD-231), which displayed no changes in abundance are displayed in grey. The X axis represents log2-transformed fold change values and the Y axis displays the -log10 (p-value adjusted) for multiple comparisons. (B) Hierarchical cluster analysis of exosomal proteome expression between MCF-10A and MDA-MB-231 cells. Increased protein expression/detection is displayed in red, while decreased expression/detection is displayed in blue (See gradient).
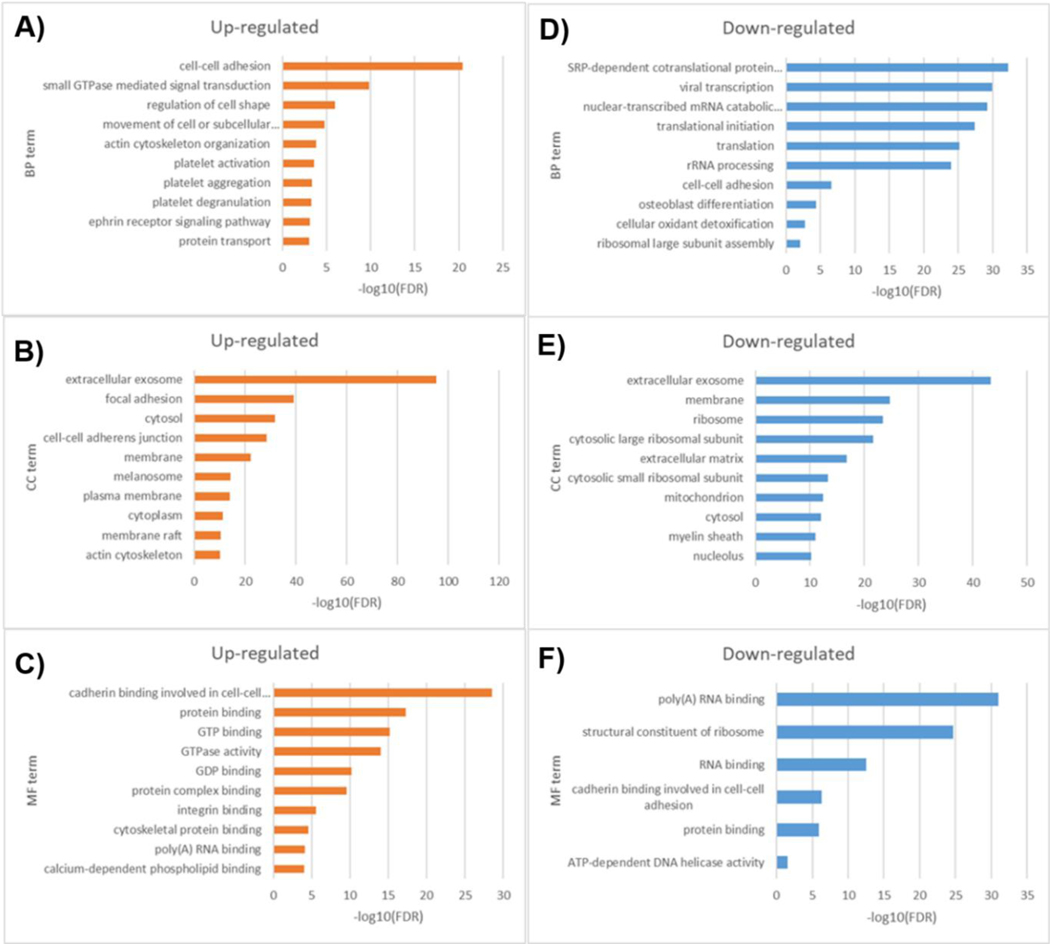
Globally, Gene Ontology (GO) enrichment analysis showed that proteins at high levels in exosomes from MDA-MB-231 cells were significantly enriched (Benjamini-Hochberg FDR < 0.05) in a number of biological processes that include cell-cell adhesion, small GTPase mediated signal transduction, regulation of cell shape, movement of cell or subcellular component, and actin cytoskeleton organization (Figure 5A and 5C), all functions that may be critical for exosomes to re-program recipient cells during establishment of the metastatic niche [43,44]. While down-regulated proteins were enriched in signal recognition particle (SRP)dependent co-translational protein targeting to membrane, viral transcription, nuclear-transcribed mRNA catabolic process, nonsense-mediated decay, translational initiation, translation and rRNA processing (Figure 5B and 5D). Moreover, it appears that the proteins which displayed the most significant changes in abundance in exosomes, were related to cell surface membrane (e.g., involved in focal adhesions), hence the functional advantage for intercellular communication and potential for modulation of cellular pathways. Since such proteins can be easily accessible for diagnostic sampling, they may potentially be used as biomarkers. Although further studies will be required to validate the potential of exosomal clinical markers, these proteins may have the potential to be used either alone or in combination with other biomarker panels, for tailored diagnostic of breast cancer and breast tumors.
Figure 5.
Gene ontology analysis of up- and down-regulated exosomal proteins from MAF10A and MDA-MB-231 cells.
CONCLUSIONS
We established a quantification proteomics workflow by integrating Suspension Trapping (S-Trap) sample preparation and label-free DIA-MS for analysis of microgram and even nanogram amounts of exosome samples. When compared to the traditional in-solution digestion and FASP methods, our proposed approach not only generated the highest numbers of proteins and peptides, but also demonstrated the highest sensitivity and quantification reproducibility when using decreased amounts of exosomes (as low as 50 ng of total protein input). S-Trap coupled with DIA acquisition was successfully applied to quantitative proteomics of exosomes from human breast cell lines and notably allowed identification of significantly differentially expressed proteins that may be associated with metastatic functions. Taken together, we present an integrated approach by coupling S-Trap sample processing with DIA quantification, that provides a promising workflow for highly efficient, sensitive, and robust quantitative proteomics when trace amounts of biological samples (e.g., circulating exosomes) are available.
Supplementary Material
Acknowledgement
This work is partially supported by NIH/NCI grant P30 CA051008 and GUMC institutional support. The Orbitrap Lumos Tribrid mass spectrometer is partially supported by Dekelbaum Foundation.
Footnotes
Supplementary Information
The online version contains supplementary material available at….
Statements and Declarations
The authors have declared no conflict of interest.
REFERENCES
- 1.Aebersold R, Mann M. Mass-spectrometric exploration of proteome structure and function. Nature. 2016. Sep;537(7620):347–55. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Fonslow BR, Shan B, Baek M-C, Yates JR. Protein Analysis by Shotgun/Bottom-up Proteomics. Chem Rev. 2013. Apr 10;113(4):2343–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye X, Tang J, Mao Y, Lu X, Yang Y, Chen W, et al. Integrated proteomics sample preparation and fractionation: Method development and applications. TrAC Trends Anal Chem. 2019. Nov;120:115667. [Google Scholar]
- 4.Ma J, Zhang L, Liang Z, Shan Y, Zhang Y. Immobilized enzyme reactors in proteomics. TrAC Trends Anal Chem. 2011. May;30(5):691–702. [Google Scholar]
- 5.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009. May;6(5):359–62. [DOI] [PubMed] [Google Scholar]
- 6.Liebler DC, Ham A-JL. Spin filter–based sample preparation for shotgun proteomics. Nat Methods. 2009. Nov;6(11):785–785. [DOI] [PubMed] [Google Scholar]
- 7.Kulak NA, Pichler G, Paron I, Nagaraj N, Mann M. Minimal, encapsulated proteomicsample processing applied to copy-number estimation in eukaryotic cells. Nat Methods. 2014. Mar;11(3):319–24. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Piehowski PD, Zhao R, Chen J, Shen Y, Moore RJ, et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10–100 mammalian cells. Nat Commun. 2018. Dec;9(1):882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi L, Piehowski PD, Shi T, Smith RD, Qian W-J. Advances in microscale separations towards nanoproteomics applications. J Chromatogr A. 2017. Nov;1523:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zougman A, Selby PJ, Banks RE. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. PROTEOMICS. 2014. May;14(9):1006–1000. [DOI] [PubMed] [Google Scholar]
- 11.HaileMariam M, Eguez RV, Singh H, Bekele S, Ameni G, Pieper R, et al. S-Trap, an Ultrafast Sample-Preparation Approach for Shotgun Proteomics. J Proteome Res. 2018. Sep 7;17(9):2917–24. [DOI] [PubMed] [Google Scholar]
- 12.Hughes CS, Moggridge S, Müller T, Sorensen PH, Morin GB, Krijgsveld J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat Protoc. 2019. Jan;14(1):68–85. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Wang S, Adhikari S, Deng Z, Wang L, Chen L, et al. Simple and Integrated Spintip-Based Technology Applied for Deep Proteome Profiling. Anal Chem. 2016. May 3;88(9):4864–71. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Fonslow BR, Shan B, Baek M-C, Yates JR. Protein Analysis by Shotgun/Bottom-up Proteomics. Chem Rev. 2013. Apr 10;113(4):2343–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol. 2008. Jan;4(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson AC, Russell JD, Bailey DJ, Westphall MS, Coon JJ. Parallel Reaction Monitoring for High Resolution and High Mass Accuracy Quantitative, Targeted Proteomics. Mol Cell Proteomics. 2012. Nov;11(11):1475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillet LC, Navarro P, Tate S, Röst H, Selevsek N, Reiter L, et al. Targeted Data Extraction of the MS/MS Spectra Generated by Data-independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol Cell Proteomics. 2012. Jun;11(6):O111.016717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig C, Gillet L, Rosenberger G, Amon S, Collins BC, Aebersold R. Data-independent acquisition-based SWATH-MS for quantitative proteomics: a tutorial. Mol Syst Biol. 2018. Aug;14(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li W, Chi H, Salovska B, Wu C, Sun L, Rosenberger G, et al. Assessing the Relationship Between Mass Window Width and Retention Time Scheduling on Protein Coverage for Data-Independent Acquisition. J Am Soc Mass Spectrom. 2019. Aug 1;30(8):1396–405. [DOI] [PubMed] [Google Scholar]
- 20.Mittelbrunn M, Sánchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012. May;13(5):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009. Aug;21(4):575–81. [DOI] [PubMed] [Google Scholar]
- 22.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002. Aug;2(8):569–79. [DOI] [PubMed] [Google Scholar]
- 23.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009. Jun;6(3):267–83. [DOI] [PubMed] [Google Scholar]
- 24.Hou R, Li Y, Sui Z, Yuan H, Yang K, Liang Z, et al. Advances in exosome isolation methods and their applications in proteomic analysis of biological samples. Anal Bioanal Chem. 2019. Aug;411(21):5351–61. [DOI] [PubMed] [Google Scholar]
- 25.Fricke F, Lee J, Michalak M, Warnken U, Hausser I, Suarez-Carmona M, et al. TGFBR2dependent alterations of exosomal cargo and functions in DNA mismatch repair-deficient HCT116 colorectal cancer cells. Cell Commun Signal. 2017. Dec;15(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell MI, Ben-Dov IZ, Liu C, Ye K, Chow K, Kramer Y, et al. Extracellular Vesicle Capture by AnTibody of CHoice and Enzymatic Release (EV-CATCHER): A customizable purification assay designed for small-RNA biomarker identification and evaluation of circulating small-EVs. J Extracell Vesicles. 2021. Jun;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldeghaither DS, Zahavi DJ, Murray JC, Fertig EJ, Graham GT, Zhang Y-W, et al. A Mechanism of Resistance to Antibody-Targeted Immune Attack. Cancer Immunol Res. 2019. Feb;7(2):230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995. Jan;57(1):289–300. [Google Scholar]
- 29.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009. Jan;4(1):44–57. [DOI] [PubMed] [Google Scholar]
- 30.Galperin MY, Cochrane GR. Nucleic Acids Research annual Database Issue and the NAR online Molecular Biology Database Collection in 2009. Nucleic Acids Res. 2009. Jan 1;37(Database):D1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig KR, Schroll MM, Hummon AB. Comparison of In-Solution, FASP, and S-Trap Based Digestion Methods for Bottom-Up Proteomic Studies. J Proteome Res. 2018. Jul 6;17(7):2480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keerthikumar S, Chisanga D, Ariyaratne D, Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: A web-based compendium of exosomal cargo. J Mol Biol. 2016. Feb 22; 428(4): 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risha Y, Minic Z, Ghobadloo SM, Berezovski MV. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci Rep. 2020. Dec;10(1):13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho K-C, Clark DJ, Schnaubelt M, Teo GC, Leprevost F da V, Bocik W, et al. Deep Proteomics Using Two Dimensional Data Independent Acquisition Mass Spectrometry. Anal Chem. 2020. Mar 17;92(6):4217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta BBA - Mol Cell Res. 2007. May;1773(5):642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanojia D, Morshed RA, Zhang L, Miska JM, Qiao J, Kim JW, et al. βIII-Tubulin Regulates Breast Cancer Metastases to the Brain. Mol Cancer Ther. 2015. May;14(5):1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015. Nov;527(7578):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang H, Wang Y, Zhang B, Xiong S, Liu L, Chen W, et al. High brain acid soluble protein 1(BASP1) is a poor prognostic factor for cervical cancer and promotes tumor growth. Cancer Cell Int. 2017. Dec;17(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsiao K-C, Shih N-Y, Fang H-L, Huang T-S, Kuo C-C, Chu P-Y, et al. Surface α-Enolase Promotes Extracellular Matrix Degradation and Tumor Metastasis and Represents a New Therapeutic Target. Karamanos NK, editor. PLoS ONE. 2013. Jul 19;8(7):e69354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tu S-H, Chang C-C, Chen C-S, Tam K-W, Wang Y-J, Lee C-H, et al. Increased expression of enolase α in human breast cancer confers tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010. Jun;121(3):539–53. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Li X, Yang S, Pi H, Li Z, Yao P, et al. Proteomic Landscape of Exosomes Reveals the Functional Contributions of CD151 in Triple-Negative Breast Cancer. Mol Cell Proteomics. 2021;20:100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C, et al. Increased Tumor Glycolysis Characterizes Immune Resistance to Adoptive T Cell Therapy. Cell Metab. 2018. May;27(5):977–987.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019. May 6;49(3):347–60. [DOI] [PubMed] [Google Scholar]
- 44.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015. Jun;17(6):816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.