Abstract
The organic solvent tolerance of Escherichia coli was measured under conditions in which OmpF levels were controlled by various means as follows: alteration of NaCl concentration in the medium, transformation with a stress-responsive gene (marA, robA, or soxS), or disruption of the ompF gene. It was shown that solvent tolerance of E. coli did not depend upon OmpF levels in the membrane.
We previously constructed Escherichia coli mutants displaying improved organic solvent tolerance (3). E. coli JA300 (19), used as the parent, produced OmpF porin protein in the membrane even when grown in the presence of 1% NaCl. In contrast, the levels of OmpF protein were markedly decreased in the mutants because of mutations in marR (4, 9). It is reported that OprF porin protein is absent in a toluene-tolerant mutant of Pseudomonas aeruginosa (22). Hydrophobic β-lactam antibiotics pass through OmpF channels faster than through OmpC channels (28). It seemed likely that organic solvent molecules also could pass through the OmpF porin. Therefore, it was supposed that the decreased levels of OmpF or loss of OmpF might contribute to improvement of organic solvent tolerance in E. coli, as suggested for acquisition of multiple antibiotic resistance (2, 6). In this study, this possibility was explored by measuring the organic solvent tolerance of E. coli under conditions in which OmpF synthesis was controlled by various means.
First, we controlled OmpF synthesis in JA300 by growth under different salinity conditions. OmpF synthesis is repressed under conditions of high environmental osmolarity (13). This osmoregulation is mediated mainly by an increase in expression of micF RNA, an antisense RNA that inhibits OmpF translation. The extent of osmoregulation was evaluated for JA300 grown in the medium that we have usually used. A membrane fraction was prepared from sonicated lysate of the cells grown in modified Luria broth (LBGMg) (1% [wt/vol] Bacto Tryptone [Difco Laboratories, Detroit, Mich.], 0.5% Bacto Yeast Extract [Difco], 0.1% glucose, and 10 mM MgSO4) containing or not containing 1% (wt/vol) NaCl. JA300 produced a considerable amount of OmpF in the presence of NaCl, although the amount was less than that produced in the absence of NaCl (Fig. 1 and Table 1). The effect of NaCl on the OmpF level of JA300 carrying a vector plasmid, pBluescript II SK(+) (Toyobo Biochemical Inc., Osaka, Japan; hereafter pBS) was similar to that found in the host cell (results not shown).
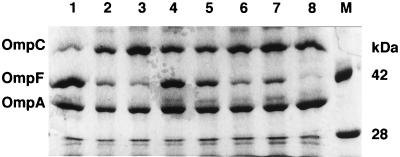
FIG. 1.
Sodium dodecyl sulfate-urea-polyacrylamide gel electrophoresis of envelope protein. JA300 cells transformed with the plasmids were grown in LBGMg medium. The cells were broken by sonication. Membrane fractions were extracted with 0.5% sodium N-lauroylsarcosine at room temperature (11). Insoluble proteins (45 μg) were electrophoresed on 0.1% sodium dodecyl sulfate–4 M urea–10% (wt/vol) polyacrylamide gels (1). Protein was stained with Coomassie Brilliant Blue R-250. Lanes: M, molecular size markers; 1 to 4, growth in the absence of NaCl; 5 to 8, growth in the presence of 1% (wt/vol) NaCl; 1 and 5, no plasmid; 2 and 6, pMarA; 3 and 7, pRob; 4 and 8, pSoxS. Bands containing OmpC, OmpF, and OmpA are shown.
TABLE 1.
Organic solvent tolerance levels of E. coli JA300 and its derivative
| Strain | Plasmid | 1% (wt/vol) NaCla | Growth on LBGMg agar overlaid withb:
|
OmpF levelc (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| DE 4.2 | nHex 3.9 | nHex/CH 3.7 | CH 3.4 | CH/pX 3.3 | pX 3.1 | ||||
| JA300 | None | − | + | + | − | − | − | − | 100 |
| + | + | + | − | − | − | − | 38 | ||
| pBS | − | + | − | − | − | − | − | NT | |
| + | + | + | − | − | − | − | NT | ||
| pMarA | − | + | + | + | + | − | − | 13 | |
| + | + | + | + | + | − | − | 11 | ||
| pRob | − | + | + | + | + | − | − | 7 | |
| + | + | + | + | + | − | − | 19 | ||
| pSoxS | − | + | + | + | + | − | − | 73 | |
| + | + | + | + | + | − | − | <1 | ||
| JOF501 | None | + | + | + | − | − | − | − | 0 |
The organisms were grown on LBGMg agar containing or not containing NaCl (1% [wt/vol]).
Abbreviations: DE, diphenyl ether; nHex, n-hexane; CH, cyclohexane; pX, p-xylene; nHex/CH and CH/pX, solvent mixtures consisting of n-hexane–cyclohexane and cyclohexane–p-xylene (1:1, vol/vol), respectively. The number shown under the solvent name represents the log POW value of the solvent. The value was calculated by the addition rule (21) with log POW calculation software, ClogP, version 1.0.3 (Bio Byte Corporation, Claremont, Calif.). The approximate values were estimated for solvent mixtures on the basis of the calculation rule (20).
The level of OmpF was estimated by image analysis with Bio Image Intelligent Quantifier version 2.1.2a software (Bio Image Systems Corporation, Ann Arbor, Mich.); NT, not tested.
LBGMg agar on which JA300 was plated was overlaid with a ca. 2-mm layer of an appropriate organic solvent and incubated at 37°C overnight. When JA300 grew confluently, it was considered that JA300 was tolerant of the organic solvent overlaying the agar (5). The toxicity of the organic solvent is reflected by its log POW value (5, 15), shown in Table 1. This value is inversely correlated with the toxicity of organic solvent. Here, log POW is the common logarithm of POW, the partition coefficient of the organic solvent between n-octanol and water. Growth without NaCl did not greatly lower the organic solvent tolerance level of JA300, although the OmpF level was high, as described above. We found that growth in the absence of NaCl reduced the organic solvent tolerance of JA300(pBS), compared with that grown in the presence of 1% (wt/vol) NaCl. This difference was observed probably because less growth occurred on the agar not containing NaCl.
Second, the OmpF levels were reduced by overexpression of marA, robA, or soxS. Transcriptional activators, MarA, Rob, and SoxS, positively regulate expression of mar-sox regulon genes including micF (8, 10, 17). Overproduction of these proteins triggered low production of OmpF. We intended to control OmpF synthesis through the repression brought about by overexpression of the stress-responsive genes and by growth at high osmolarity (salinity). The plasmids used here, pMarA, pRob, and pSoxS, were referred to previously as pHA105 (9), pOST42BR (26), and pHc3R (27), respectively.
The level of OmpF was reduced upon introduction of one of the plasmids (Fig. 1 and Table 1). The extent of repression differed depending on the plasmids carried and NaCl concentration. The repression caused by pMarA was independent of NaCl concentration. That caused by pRob was high in the absence of NaCl. In contrast, pSoxS severely repressed OmpF production in the presence of NaCl and slightly repressed it in the absence of NaCl. Consequently, various levels of OmpF production were achieved in JA300 cells.
We reported that overexpression of the genes improved the organic solvent tolerance of E. coli, based on the tolerance measured by monitoring growth in the presence of NaCl (9, 26, 27). The overexpression made JA300 grown without NaCl as highly tolerant as that grown with NaCl (Table 1). As far as examined, the organic solvent tolerance of each transformant did not differ between cells grown with NaCl and those grown without NaCl. It is particularly notable that JA300(pSoxS) grown without NaCl produced a high level of OmpF and was tolerant of cyclohexane, indicating that the increased level of OmpF did not reduce tolerance of cyclohexane. These results suggest that organic solvent tolerance levels are not directly related to OmpF levels.
The OmpF levels were controlled via micF expression in the experiments described above. These controls are indirect for OmpF production. In particular, growth of the organisms under different conditions such as salinity might cause unexpected effects on the cell membrane structures other than OmpF production. Finally, we constructed an ompF disruptant from JA300 and examined the solvent tolerance level. From E. coli RK4786 (14), ompF::Tn5 was transduced into JA300 by generalized transduction with P1kc (25). An OmpF-nonproducing transductant (JOF501) was selected from among kanamycin-resistant clones grown on LBGMg agar containing kanamycin (50 μg/ml). This P1 transductant did not display OmpF in the membrane at all, regardless of NaCl concentration (results not shown). The organic solvent tolerance level of the ompF disruptant, measured on LBGMg agar containing NaCl, was identical to that of JA300 (Table 1). The ompF disruption did not result in improvement of the organic solvent tolerance.
The organic solvent tolerance of JA300 was not altered to any detectable extent as a direct result of a decreased or increased level of OmpF in the membrane or its absence, except for JA300(pBS). The extremely low level of organic solvent tolerance of the tolC disruptant derived from JA300 is not improved by transformation with pMarA, pRob, or pSoxS (7), although the OmpF levels decreased in the transformants (results not shown). This fact supports the conclusion described above. It is likely that the solvent tolerance of the mutants or transformants is improved mainly by elevated expression of acrAB and tolC (7), not by repression of OmpF synthesis. Genes acrAB and tolC encode AcrA, AcrB, and TolC, consisting of a proton motive force-dependent efflux pump to extrude multiple antibiotics (12, 24). Recently, energy-dependent efflux systems extruding organic solvents have been reported to contribute to the organic solvent tolerances of E. coli (7, 32), P. aeruginosa (23, 29), and Pseudomonas putida (16, 18, 30, 31). Probably the AcrAB and TolC efflux system plays the most important role in organic solvent tolerance in E. coli.
REFERENCES
- 1.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, Sutton A, Silver R P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983;39:315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aono R, Aibe K, Inoue A, Horikoshi K. Preparation of organic solvent tolerant mutants from Escherichia coli K-12. Agric Biol Chem. 1991;55:1935–1938. [Google Scholar]
- 4.Aono R, Kobayashi H. Cell surface properties of organic solvent-tolerant mutants of Escherichia coli K-12. Appl Environ Microbiol. 1997;63:3637–3642. doi: 10.1128/aem.63.9.3637-3642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aono R, Kobayashi H, Joblin K N, Horikoshi K. Effects of organic solvents on growth of Escherichia coli K-12. Biosci Biotechnol Biochem. 1994;58:2009–2014. doi: 10.1271/bbb.58.1231. [DOI] [PubMed] [Google Scholar]
- 6.Aono R, Kobayashi M, Nakajima H, Kobayashi H. A close correlation between organic solvent tolerance and multiple antibiotic resistance systems. Biosci Biotechnol Biochem. 1995;59:213–218. doi: 10.1271/bbb.59.213. [DOI] [PubMed] [Google Scholar]
- 7.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance level of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariza R R, Li A, Ringstad N, Demple B. Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J Bacteriol. 1995;177:1655–1661. doi: 10.1128/jb.177.7.1655-1661.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asako H, Nakajima H, Kobayashi K, Kobayashi M, Aono R. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl Environ Microbiol. 1997;63:1428–1433. doi: 10.1128/aem.63.4.1428-1433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou J H, Greenberg J, Demple B. Posttranscriptional repression of Escherichia coli OmpF protein in response to redox stress: positive control of the micF antisense RNA by the soxRS locus. J Bacteriol. 1993;175:1026–1031. doi: 10.1128/jb.175.4.1026-1031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa Y, Yamada H, Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976;80:1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- 14.Heller K, Mann B J, Kadner R J. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985;161:896–903. doi: 10.1128/jb.161.3.896-903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;338:264–265. [Google Scholar]
- 16.Isken S, de Bont J A M. Active efflux of toluene in a solvent-tolerant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jair K-W, Martin R G, Rosner J L, Fujita N, Ishihama A, Wolf R E J. Purification and regulatory properties of MarA protein, a transcriptional activator of Escherichia coli multiple antibiotic and superoxide resistance promoters. J Bacteriol. 1995;177:7100–7104. doi: 10.1128/jb.177.24.7100-7104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 19.Kingsman A J, Clarke L, Mortimer R K, Carbon J. Replication in Saccharomyces cerevisiae of plasmid pBR313 carrying DNA from the yeast trp1 region. Gene. 1979;7:141–152. doi: 10.1016/0378-1119(79)90029-5. [DOI] [PubMed] [Google Scholar]
- 20.Laane C, Boeren S, Vos K, Veegar C. Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng. 1987;30:81–87. doi: 10.1002/bit.260300112. [DOI] [PubMed] [Google Scholar]
- 21.Leo A J. Calculating log Poct from structures. Chem Rev. 1993;63:1281–1306. [Google Scholar]
- 22.Li L, Komatsu T, Inoue A, Horikoshi K. A toluene-tolerant mutant of Pseudomonas aeruginosa lacking the outer membrane protein F. Biosci Biotechnol Biochem. 1995;59:2358–2359. doi: 10.1271/bbb.59.2358. [DOI] [PubMed] [Google Scholar]
- 23.Li X-Z, Li Z, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. doi: 10.1128/jb.180.11.2987-2991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 25.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. pp. 201–205. [Google Scholar]
- 26.Nakajima H, Kobayashi K, Kobayashi M, Asako H, Aono R. Overexpression of robA gene increases organic solvent tolerance and multiple antibiotic and heavy metal ion resistance in Escherichia coli. Appl Environ Microbiol. 1995;61:2302–2307. doi: 10.1128/aem.61.6.2302-2307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima H, Kobayashi M, Negishi T, Aono R. soxRS gene increases the level of organic solvent tolerance in Escherichia coli. Biosci Biotechnol Biochem. 1995;59:1323–1325. doi: 10.1271/bbb.59.1323. [DOI] [PubMed] [Google Scholar]
- 28.Nikaido H, Rosenberg E Y, Foulds J. Porin channels in Escherichia coli: studies with β-lactams in intact cells. J Bacteriol. 1983;153:232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi K, Nakajima H, Aono R. Effects of oxygen and nitrate on growth of Escherichia coli and Pseudomonas aeruginosa in the presence of organic solvents. Extremophiles. 1997;1:193–198. doi: 10.1007/s007920050033. [DOI] [PubMed] [Google Scholar]
- 30.Ramos J L, Duque E, Godoy P, Segura A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J Bacteriol. 1998;180:3323–3329. doi: 10.1128/jb.180.13.3323-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos J L, Duque E, Rodriguez H J J, Godoy P, Haidour A, Reyes F, Fernandez B A. Mechanisms for solvent tolerance in bacteria. J Biol Chem. 1997;272:3887–3890. doi: 10.1074/jbc.272.7.3887. [DOI] [PubMed] [Google Scholar]
- 32.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]