Abstract
Objectives
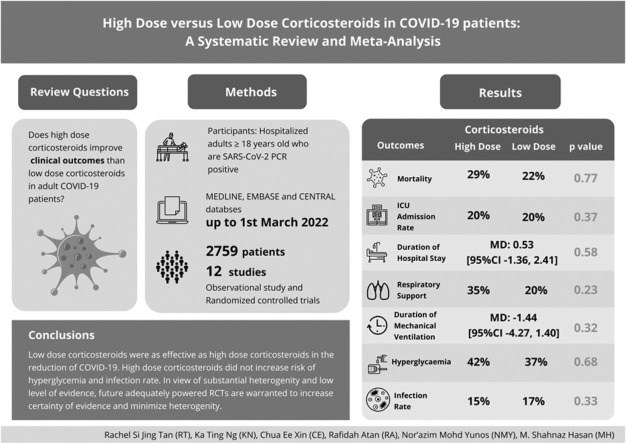
The clinical efficacy of corticosteroids remains unclear. The primary aim of this systematic review and meta-analysis was to evaluate the use of high-dose versus low- dose corticosteroids on the mortality rate of COVID-19 patients.
Design
Systematic review and meta-analysis.
Setting
Electronic search for randomized controlled trials and observational studies (MEDLINE, EMBASE, CENTRAL).
Participants
Hospitalized adults ≥ 18 years old who were SARS-CoV-2 PCR positive.
Interventions
High-dose and low-dose corticosteroids.
Measurements and Main Results
A total of twelve studies (n=2759 patients) were included in this review. The pooled analysis demonstrated no significant difference in mortality rate between the high-dose and low-dose corticosteroids groups (n=2632; OR: 1.07 [95%CI 0.67, 1.72], p=0.77, I2=76%, trial sequential analysis=inconclusive). No significant differences were observed in the incidence of intensive care unit (ICU) admission rate (n=1544; OR: 0.77[95%CI 0.43, 1.37], p=0.37, I2= 72%), duration of hospital stay (n=1615; MD: 0.53[95%CI -1.36, 2.41], p=0.58, I2=87%), respiratory support (n=1694; OR: 1.51[95%CI 0.77, 2.96], p=0.23, I2=84%), duration of mechanical ventilation (n=419; MD: -1.44[95%CI -4.27, 1.40], p=0.32, I2=93%), incidence of hyperglycemia (n=516, OR: 0.91[95%CI 0.58, 1.43], p=0.68, I2=0%) and infection rate (n=1485, OR: 0.86[95%CI 0.64, 1.16], p=0.33, I2=29%).
Conclusion
The meta-analysis demonstrated high-dose corticosteroids did not reduce mortality rate. However, high-dose corticosteroids did not pose higher risk of hyperglycemia and infection rate for COVID-19 patients. Due to the inconclusive trial sequential analysis, substantial heterogeneity and low level of evidence, future large-scale randomized clinical trials are warranted to improve the certainty of evidence for the use of high-dose compared to low-dose corticosteroids in COVID-19 patients.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Corticosteroids, Methylprednisolone, Dexamethasone
Graphical abstract
Introduction
In March 2020, the World Health Organization (WHO) had declared coronavirus disease 2019 (COVID-19) as a global pandemic.1 As of 1st February 2021, more than 100 million people have been diagnosed with COVID-19, and more than 2 million deaths were reported.2 It is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which belongs to the same family of human coronaviruses as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS).3 , 4
The severity of COVID-19 varies from asymptomatic to severe ARDS manifestation, which requires mechanical ventilator support, intensive care unit (ICU) admission, long duration of hospital stay and high mortality rate.5, 6, 7 Evidence suggests that COVID-19 is associated with dysregulated coagulation cascade and excessive release of proinflammatory cytokines,8, 9, 10 leading to diffuse pulmonary injury.4 Corticosteroids are antiinflammatory and immunomodulatory agents, which had been used as part of the treatment during the outbreak of Middle East Respiratory Syndrome coronavirus (MERS-CoV) in 2012 and Severe Acute Respiratory Syndrome coronavirus-1 (SARS-CoV-1) in 2002.11 , 12
In this current COVID-19 pandemic, corticosteroids use is believed to suppress the inflammatory response of COVID-19 on the lung tissues, leading to less severe lung injury and better recovery outcomes. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, one of the largest COVID-19 trials, demonstrated that dexamethasone reduced the 28-day mortality in COVID-19 patients who required supplemental oxygen.13 Subsequently, the updated guidelines from the World Health Organization (WHO) and the National Institute of Health (NIH) strongly recommended the use of corticosteroids for COVID-19 patients who require mechanical ventilation or extracorporeal membrane oxygenation (ECMO) or severe COVID-19 patients; albeit advising against its use in mild-to-moderate or nonsevere COVID-19 patients.14 , 15 However, the dosage of corticosteroids used among COVID-19 patients varied across different hospitals and countries.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Several clinical studies were conducted to investigate the role of high-dose versus low-dose corticosteroids as part of the treatment of COVID-19, with conflicting findings.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 At present, the efficacy and safety profile of high-dose versus low-dose corticosteroids remain unclear in the literature. Thus, a systematic review is warranted to synthesize evidence on the use of high-dose and low-dose corticosteroids in COVID-19 patients before any recommendations can be made.
The authors hypothesized that high-dose corticosteroids reduced the mortality rate in COVID-19 patients. The primary objective of this systematic review and meta-analysis was to investigate the clinical effects of high-dose and low-dose corticosteroids on the mortality rate of COVID-19 patients. Secondary aims were to examine the effect of high-dose and low-dose corticosteroids on the ICU admission rate, duration of hospital stay, and the number of patients needing ventilation.
Materials and Methods
Study Design
The protocol of this systematic review and meta-analysis was registered prospectively on a public database (PROSPERO- CRD42021249784). The systematic review was reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.28 The Population, Intervention, Control and Outcomes (PICO) framework was used to formulate the review questions (Online Supplementary Table A.1). The author-defined high-dose and low-dose corticosteroids were used as the inclusion criteria. The inclusion criteria of high-dose corticosteroids were methylprednisolone >100 mg/day or equivalent and dexamethasone >20 mg/day as high-dose corticosteroids; any dose lower than high-dose corticosteroids was considered as low-dose corticosteroids. Primary outcome of this review was mortality rate. Secondary outcomes included the incidence of ICU admission rate, duration of hospital stay, respiratory support, duration of mechanical ventilation, incidence of hyperglycemia and infection rate.
Search Strategy
A comprehensive literature search was conducted using Ovid MEDLINE, Ovid EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception until March 1, 2022. No language restriction was applied. The search terms and search strategies are listed in Online Supplementary Table A.2. The authors screened the reference lists of relevant articles to identify potential articles that could be included in the study. Given the data provided online are not extractable, the authors requested raw data from the authors of the potential articles.
Inclusion and Exclusion Criteria
Inclusion criteria were all randomized controlled trials (RCTs) and observational studies comparing high- dose versus low-dose corticosteroids in hospitalized COVID-19 adult patients (aged 18 years and older). Case reports, case series, reviews (nonsystematic), nonhuman studies, letters and conference abstracts were excluded.
Study Selection and Data Extraction
All titles, abstracts and full texts screening were independently conducted in accordance with the inclusion and exclusion criteria by two authors (RT and CE). A third author (KN) resolved any disagreements during the screening process. Data extraction was performed by two authors (RT and CE) independently using an online data collection sheet. Any median and interquartile range data were converted to mean and standard deviation.29 Along with all measured outcomes, other relevant data namely, authors’ name, year of publication, study design, sample size, dosage of corticosteroids and duration of corticosteroids used were extracted.
Quality Assessment
Risk of bias assessment of all included studies was conducted independently by two authors (RT and CE). Newcastle-Ottawa Scale (NOS) was used to assess the quality of observational studies. NOS comprises of three domains, namely selection of study groups, comparability of groups and ascertainment of outcomes in cohort studies.30 Articles were considered as high-risk bias and low- risk bias with a score of < 7 and 7, respectively. All the included RCTs were evaluated with the Cochrane Risk of Bias Assessment Tool, which included assessment of selection bias, performance bias, detection bias, attrition bias, reporting bias and other potential sources of bias.31 A third author (KN) adjudicated any discrepancy in the process of assessing the quality of the articles. The authors also adopted the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach to assess the quality of evidence for each measured outcomes based on several domains; namely, study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias.32
Data Analysis
Review Manager, version 5.4, was used for statistical analysis. Dichotomous and continuous data were presented in Odds Ratio (OR) and Mean Difference (MD), respectively, with 95% confidence interval (CI). Statistical heterogeneity was assessed using I-square (I2) test. I2 statistic < 40%, 40-60% and ≥60% were considered as low, moderate and high heterogeneity, respectively. A fixed-effect model was used for all the measured outcomes. If substantial heterogeneity was observed, a random-effect model was used for data analysis. Subgroup analysis was performed on the mortality rate based on the types of corticosteroids and respiratory support based on types of mechanical ventilation.
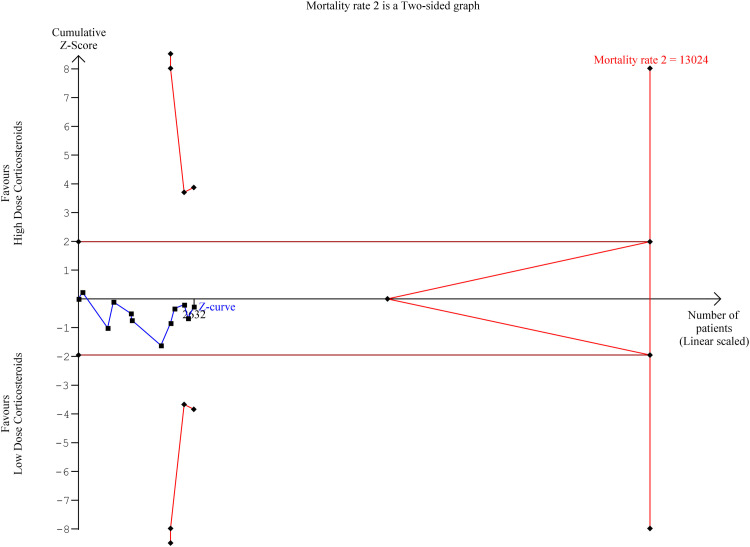
Trial sequential analysis was performed on the primary outcome (mortality) to assess the risk of random error and multiplicity phenomena due to repeated significant testing in meta-analyses33 (Figure 2). The required meta-analysis information size and adjusted significance thresholds were calculated based on a two-sided sequential analysis-adjusted random effects model, with 5% risk of type 1 error and power of 80%.
Figure 2.
Trial Sequential Analysis (TSA) for mortality rate
Results
Study Selection
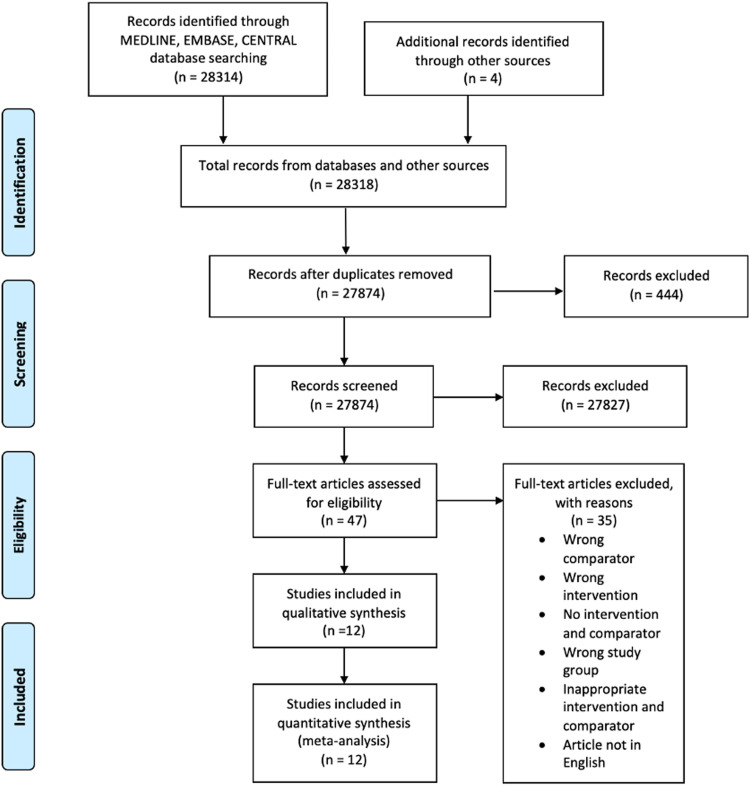
The search strategy yielded a total of 28,318 articles, and forty-seven articles fulfilled the inclusion and exclusion criteria for full-text screening (Figure 1 ). Of these, twelve studies (total sample size: 2759 patients) were eligible for qualitative synthesis and data pooling.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 The search identified one ongoing RCT (Online Supplementary Table A.3). Excluded studies are listed in Online Supplementary Table A.4.
Figure 1.
PRISMA Flow Diagram
Study Characteristics
The clinical characteristic of each study is tabulated in Table 1 . Of all included studies, there were eight observational studies,16 , 18, 19, 20, 21 , 23 , 25 , 26 one quasi-experimental study,17 and three RCTs.22 , 24 , 27 The types of corticosteroids used varied across studies; namely, methylprednisolone, dexamethasone, prednisolone, prednisone, and hydrocortisone. Three studies solely used one type of corticosteroids,16 , 24 , 27 eight studies used more than one type of corticosteroids,17 , 18 , 20, 21, 22, 23 , 25 , 26 and one study did not specify the type of corticosteroid used.19 The respective doses of each type of corticosteroids were methylprednisolone <0.5 mg/kg/day-to-500 mg/day, dexamethasone 6 mg/day-to-40 mg/day, hydrocortisone <200 mg/day-to-≥300 mg/day, and prednisone <0.5 mg/kg/day- to >0.5 mg/kg/day. The duration of low-dose and high-dose corticosteroids regimens varied from 3 days to day of hospital discharge.
Table 1.
Clinical characteristics of included studies
| No. | Author | Year | Sample size | Study Design | Types of Corticosteroids | Dosage | Duration of corticosteroids used (days) |
||
|---|---|---|---|---|---|---|---|---|---|
| Low Dose Corticosteroids | High Dose Corticosteroids | Low Dose Corticosteroids | High Dose Corticosteroids | ||||||
| 1 | Huang16 | 2020 | 28 | Observational study | Methylprednisolone | Methylprednisolone ≤ 1.5mg/kg/d |
Methylprednisolone > 1.5mg/kg/d |
6.8 ± 2.1 | 11.6 ± 6.3 |
| 2 | Fatima17 | 2020 | 100 | Quasi experimental, interventional study | Methylprednisolone, dexamethasone | Dexamethasone 8mg/day | Methylprednisolone 1mg/kg/day | 5 | 5 |
| 3 | Munoz18 | 2020 | 127 | Observational study | Methylprednisolone, dexamethasone | Dexamethasone 6mg/day | Methylprednisolone 125-250mg/day | 10 | 3 |
| 4 | Monreal19 | 2020 | 573 | Observational study | NR | 0.5-1.5mg/kg/day of methylprednisolone equivalent | 250-1000mg/day of methylprednisolone equivalent | NR | NR |
| 5 | Zúñiga20 | 2021 | 132 | Observational study | Methylprednisolone, dexamethasone | <1.5mg/kg/day of methylprednisolone or dexamethasone equivalent | ≥1.5mg/kg/day of methylprednisolone or dexamethasone equivalent | NR | 3 |
| 6 | Pinzón21 | 2021 | 216 | Observational study | Methylprednisolone, dexamethasone | Dexamethasone 6mg/day | Methylprednisolone 250-500mg/day | 7-10 | 3 |
| 7 | Ranjbar22 | 2021 | 93 | RCT | Methylprednisolone, dexamethasone | Dexamethasone 6mg/day | Methylprednisolone 2mg/kg/d | 10 | 5 |
| 8 | Gundogdu23 | 2021 | 400 | Observational study | Methylprednisolone, dexamethasone | Dexamethasone 8mg/day; Methylprednisolone 80mg/day |
Methylprednisolone 1g/day | NR | NR |
| 9 | Toroghi24 | 2021 | 93 | RCT | Dexamethasone | Dexamethasone 8mg/day | Dexamethasone 24mg/day | Up to 10 days or until hospital discharge | |
| 10 | Mora-Luján25 | 2021 | 682 | Observational study | Methylprednisolone, Dexamethasone | Dexamethasone 6mg/day | Methylprednisolone ≥100 mg/day |
10 | 3 |
| 11 | Batirel26 | 2021 | 126 | Observational study | Methylprednisolone, Dexamethasone | Dexamethasone 6mg/day equivalent | Methylprednisolone 250mg/day | NR | NR |
| 12 | Taboada27 | 2021 | 200 | RCT | Dexamethasone | Dexamethasone 6mg/day | Dexamethasone 20mg/day | 10 | 5 |
NR: Not Reported
RCT: Randomized controlled trial
Quality Assessment
The summary of risk of bias assessment (The Newcastle-Ottawa Scale and Cochrane Risk of Bias Tool) and GRADE approach are presented in Online Supplementary Table A.5 and Table 2 , respectively. All observational studies were overall low risk of bias.16 , 18, 19, 20, 21 , 23 , 26 Among all the included observational studies, four of them did not match of the clinical characteristics between high and low dose corticosteroids groups.18 , 20 , 21 , 23 Three RCT was assessed as low risk of bias22 , 24 , 27 while the other one RCT was high risk of bias due to lack of random allocation of participants into high dose and low dose corticosteroids groups, which constituted potential selection bias.17
Table 2.
Grading of Recommendations, Assessment, Development and Evaluations of each outcome
 |
CI: confidence interval; MD: mean difference; OR: odds ratio
Explanations
a.Substantial heterogeneity
bFunnel plot asymmetrical suggestive of publication bias
cDifferent dosages and types of corticosteroids used across all the included studies
d 1 high risk of bias study
Primary Outcome
Mortality Rate
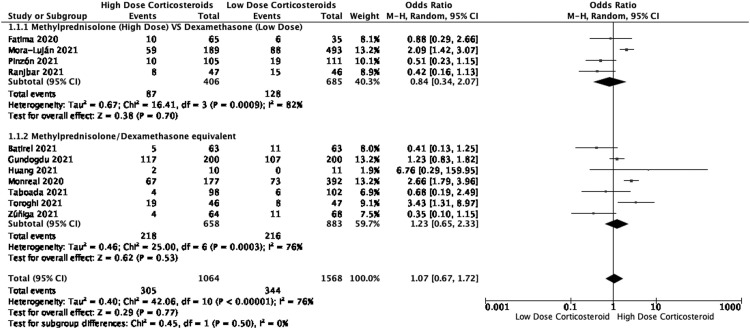
Eleven studies (n=2632) reported the mortality rate of COVID-19 patients after the administration of high-dose and low-dose corticosteroids (Table 3 ). The mortality rate for high-dose and low-dose groups were 29% and 22%, respectively. Among these studies, there were three RCTs,22 , 24 , 27 one quasi experimental study17 and seven observational studies.16 , 19, 20, 21 , 23 , 25 , 26 The pooled analysis demonstrated no significant difference in the reduction of mortality rate between high-dose and low-dose corticosteroids (OR: 1.07 [95%CI 0.67, 1.72], p=0.77, I2=76%, certainty of evidence= very low).
Table 3.
Summary findings for primary and secondary outcomes
| No. | Outcomes | Trials | N | I2 (%) | Effect Model | MD/OR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| 1 1.1 1.2 |
Mortality Subgroup analysis by type of corticosteroids Methylprednisolone (High Dose) VS Dexamethasone (Low Dose) Methylprednisolone/Dexamethasone equivalent Heterogeneity: Tau2 = 0.46; Chi2 = 42.06, df = 10 (P < 0.00001); I2 = 76% Test for overall effect: Z = 0.29 (P = 0.77) Test for subgroup differences: Chi2 = 0.45, df =1 (P = 0.50), I2 = 0% |
11 4 7 |
2632 1091 1541 |
76% 82 76 |
REM REM REM |
1.07 (0.67, 1.72) 0.84 (0.34, 2.07) 1.23 (0.65, 2.33) |
0.77 0.70 0.77 |
| 2 | ICU Admission Heterogeneity: Tau2 = 0.41; Chi2 = 21.51, df = 6 (P = 0.001); I2 = 72% Test for overall effect: Z = 0.89 (P = 0.37) |
7 |
1544 |
72 |
REM |
0.77 (0.43, 1.37) |
0.37 |
| 3 |
Duration of Hospital Stay Heterogeneity: Tau2 = 5.17, Chi2 = 44.54, df = 6 (P < 0.00001); I2 = 87% Test for overall effect: Z= 0.55 (P = 0.58) |
7 |
1215 | 87 |
REM | 0.53 (-1.36, 2.41) | 0.58 |
| 4 4.1 4.2 4.3 |
Respiratory Support Subgroup analysis by type of mechanical ventilations Mixed Mechanical Ventilation Non-invasive Mechanical Ventilation Invasive Mechanical Ventilation Heterogeneity: Tau2 = 0.64, Chi2= 38.09, df = 6 (P =0.00001); I2= 84% Test for overall effect: Z= 1.21 (P= 0.23) Test for subgroup differences: Chi2 = 2.04, df =2 (P = 0.36), I2 = 1.9% |
7 3 2 3 |
1694 393 219 1082 |
84 69 0 94 |
REM REM REM REM |
1.51 (0.77, 2.96) 0.95 (0.35, 2.57) 1.18 (0.61, 2.30) 2.78 (0.85, 9.08) |
0.23 0.93 0.62 0.09 |
| 5 | Duration of Mechanical Ventilation Heterogeneity: Tau2 = 5.81, Chi2= 27.17, df = 2 (P =0.00001); I2= 93% Test for overall effect: Z= 0.99 (P= 0.32) |
3 | 419 | 93 | REM | -1.44 (-4.27, 1.40) | 0.32 |
| 6 |
Hyperglycemia Heterogeneity: Chi2 = 0.31, df = 2 (P =0.86); I2 = 0% Test for overall effect: Z = 0.41 (P = 0.68) |
3 | 516 | 0 | FEM | 0.91 (0.58, 1.43) | 0.68 |
| 7 | Infection Rate Heterogeneity: Chi2 = 4.21, df = 3 (P =0.24); I2 = 29% Test for overall effect: Z = 0.97 (P = 0.33) |
5 | 1485 | 29 | FEM | 0.86 (0.64, 1.16) | 0.33 |
FEM: Fixed-Effect Model; REM: Random-Effect Model; MD: Mean Difference; OR: Odd Ratio; VS: Versus
In the subgroup analysis comparing between methylprednisolone (high-dose group) versus dexamethasone (low-dose group), four studies (n= 1091) were included in meta-analysis17 , 21 , 22 , 25 (Figure 3 ). Our result demonstrated no significant difference between high-dose methylprednisolone and low-dose dexamethasone (OR:0.84 [95 CI% 0.34, 2.07], p=0.70, I2 =82%). In the trial sequential analysis, the minimum required information size for mortality was 13024 patients. In the current meta-analysis, with 2632 patients, only 20% of the required information size was available to detect or reject a relative risk reduction of 20% mortality rate, based on a 5% risk of Type 1 error (two-sided), a power of 80%, and an incidence in the control arm of 24.5%, with a model variance-based heterogeneity correction.
Figure 3.
Forest Plots of Mortality Rate (Subgroup analysis based on different types of corticosteroids used)
Secondary outcomes
ICU Admission Rate
Data of seven studies (n= 1544) (two RCTs,24 , 27 one quasi-experimental study17 and four observational studies18 , 21 , 25 , 26 were pooled for meta-analysis (Table 3). The results revealed COVID-19 patients with high-dose corticosteroids were not associated with lower ICU admission rate than the low-dose corticosteroids group, as compared to low-dose corticosteroids (OR: 0.77[95%CI 0.43, 1.37], p=0.37, I2= 72%, certainty of evidence = very low).
Duration of Hospital Stay (days)
Seven studies (n=1215) (three RCT22 , 24 , 27 and four observational studies16 , 23 , 25 , 26) assessed the duration of hospital stay between the high-dose and low-dose corticosteroids groups (Table 3). No significant difference was observed (MD: 0.53[95%CI -1.36, 2.41], p=0.58, I2=87%, certainty of evidence = very low).
Respiratory Support
Seven studies (n=1694) (three RCTs,22 , 24 , 27 one quasi-experimental study17 and three observational studies23 , 25 , 26 reported the number of people requiring respiratory support (Table 3). There was no significant difference observed between high-dose and low-dose corticosteroids (OR: 1.51[95%CI 0.77, 2.96], p=0.23, I2=84%, certainty of evidence = very low).
Subgroup analysis was performed based on the type of mechanical ventilations, noninvasive mechanical ventilation (n=219) and mechanical ventilation (n=1082). The data analysis revealed no significant difference between high-dose and low-dose corticosteroids in the noninvasive mechanical ventilation subgroup (OR 1.18[95%CI 0.61, 2.30], p=0.62, I2=0%), as well as the invasive mechanical ventilation subgroup (OR 2.78[95%CI 0.85, 9.08], p=0.09, I2=94%).
Duration of Mechanical Ventilation
Three studies (n=419) (two RCTs24 , 27 and one observational study26 demonstrated no significant difference in the duration of mechanical ventilation between high-dose and low-dose corticosteroids groups (MD: -1.44[95%CI -4.27, 1.40], p=0.32, I2=93%, certainty of evidence = very low) (Table 3).
Hyperglycemia
Three studies (n=516) (one RCT,27 one quasiexperimental study17 and one observational study21), showed no significant difference in the incidence of hyperglycemia between the high-dose and low-dose corticosteroids groups (OR: 0.91[95%CI 0.58, 1.43], p=0.68, I2=0%, certainty of evidence = high) (Table 3).
Infection Rate
Five studies (n=1485) (one RCT,27 one quasiexperimental study17 and three observational studies19 , 21 , 23 were pooled for meta-analysis. The results revealed no significant difference between high-dose and low-dose corticosteroids in infection rate (OR: 0.86[95%CI 0.64, 1.16], p=0.33, I2=29%, certainty of evidence = low) (Table 3).
Discussion
To the best of the authors' knowledge, this was the first meta-analysis comparing the efficacy and safety profile of high-dose versus low-dose corticosteroids in COVID-19 patients. The meta-analysis demonstrated no significant differences in mortality rate, ICU admission rate, duration of hospital stays, number of patients requiring respiratory support, duration of mechanical ventilation, incidence of hyperglycemia and infection rate between the high-dose and low-dose corticosteroids groups among COVID-19 patients. The certainty of evidence for all measured outcomes ranged very low-to-high due to observational studies, inconsistency, publication bias and dose-response gradient.
In the meta-analysis, the pooled data demonstrated that high-dose corticosteroids were not superior to low-dose corticosteroids in the reduction of mortality rate in COVID-19 patients. The non-significant result could have been due to multifactorial causes; namely, different types, dosages and regimens (duration) of corticosteroids. In addition, the current meta-analysis did not achieve the minimum required information size for a conclusive finding. A recent study by the COVID STEROID 2 trial group involving COVID-19 patients with severe hypoxemia, 12 mg/d compared with 6 mg/d dexamethasone did not result in statistically significantly more days alive without life support at 28 days.34 However, in its preplanned Bayesian analysis of the trial, they found high probabilities of benefit with dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxemia on all outcomes, including the days alive without life support and mortality at days 28 and 90. They also found low probabilities of clinically important harm with 12 mg dexamethasone for all outcomes.35 In this study, the 2 doses used still were considered low-dose, but there was evidence showing lower mortality in the higher-dose group.
Corticosteroids inhibit proinflammatory protein synthesis (interleukin-6, interleukin-8, tumor necrosis factor, interferon γ) and induce the production of antiinflammatory proteins.36 , 37 However, it comes with some adverse events; namely, hyperglycemia, hypernatremia and neuromuscular weakness.36, 37, 38 While 7.5 mg of prednisone or equivalent slowly may activate the effect of corticosteroids, the use of >100 mg of prednisone equivalent and pulse therapy (≥ 250 mg prednisone equivalent) can rapidly saturate the cytoplasmic glucocorticoids receptors to activate fast onset of antiinflammatory action.36
A prospective study showed 3 days of high-dose pulsed corticosteroids therapy (at least 125 mg of methylprednisolone or its equivalent dexamethasone), significantly increased survival rate in COVID-19 patients.20 Others reported similar results with the use of 250 mg/day of methylprednisolone that reduced mortality rate and shortened duration of hospitalization in COVID-19-induced ARDS in patients.39 , 40 However, Tataki and colleagues reported that COVID-19 patients with an excessively high dose of pulsed corticosteroids therapy (1000 mg/day of methylprednisolone) were associated with a poorer prognosis.41 Thus, the optimal dosage of pulsed corticosteroids therapy for COVID-19 patients warrants future adequately powered randomized trials for more clarity and evidence.
The nonsignificant finding of mortality rate was similar to a meta-analysis by Cano et al. comparing two groups (high-dose corticosteroids vs no corticosteroids; low-dose corticosteroids vs no corticosteroids).42 In the authors' meta-analysis, they performed subgroup analysis based on different types of corticosteroids. They found that the mortality rate in the high-dose corticosteroids group (methylprednisolone group) was not significantly lower than the low-dose corticosteroids group (dexamethasone group). In other words, the mortality rate of COVID-19 patients did not improve with the use of methylprednisolone, while it is believed that methylprednisolone has better lung penetration.43 Ko and coworkers reported that methylprednisolone (1 mg/kg/d) had a significantly greater mortality benefit than dexamethasone (6mg/day) in COVID-19 mechanically ventilated patients.44 Ranjbar et al. compared methylprednisolone (2 mg/kg/day) and dexamethasone (6 mg/day) in hospitalized COVID-19 patients, and found that those who received methylprednisolone had better clinical outcomes22. Due to the limited number of studies comparing methylprednisolone and dexamethasone, it is premature to claim the superiority of either methylprednisolone or dexamethasone in treating pneumonia in COVID-19 patients. Therefore, future adequately powered studies can evaluate the efficacy of different types of corticosteroids in the treatment of COVID-19 pneumonia.
Despite the desirable antiinflammatory effect of corticosteroids, the safety profile of corticosteroids use in COVID-19 patients is inconclusive.17 , 19 , 21 , 23 , 27 , 45 , 46 The CoDEX trial demonstrated that 20 mg of dexamethasone did not significantly increase the risk of new infections, bacteremia, or any increased insulin use for hyperglycemia, as compared to a standard-care group.46 A meta-analysis revealed that the pulsed corticosteroids (>250 mg prednisone) were not associated with increased risk of side effects such as cardiac events, hyperglycemia, infections, hypertension, edema, gastrointestinal and psychiatric adverse events.47 Due to the limited data available, the authors only managed to pool the data for the incidence of hyperglycemia and infection rate between the high- and low-dose corticosteroids groups. The meta-analysis revealed no augmented risk of hyperglycemia and infection episodes in COVID-19 patients who received high-dose corticosteroids. Monedero and colleagues also reported moderate-to-high-dose corticosteroids (≥ 1 mg/kg/d methylprednisolone or ≥ 0.12 mg/kg/d dexamethasone) were not associated with higher risk of medical or infectious complications.45
The pooled meta-analysis demonstrated no significant reduction of the ICU admission rate in the high-dose corticosteroids group. Of note, many confounding factors; namely, underlying morbidities, obesity, baseline functional status, and severity of organ failure introduced bias to the findings. However, Pareja et al. found that high-dose corticosteroids (equivalent to ≥ 200 mg of methylprednisolone) were associated with lower mortality rate and ICU admission.48 Similarly, Pinzo'n and Munoz found that high-dose corticosteroids (125-500 mg/day of methylprednisolone) significantly reduced the ICU admission as compared to a low-dose group.18 , 21 This could have been due to the patients who received corticosteroids were those who required oxygen support or had more severe COVID-19 disease, which could denote more severe pulmonary involvement and inflammation. With higher-dose corticosteroids, it could activate faster onset of antiinflammatory action to achieve favorable outcomes.
In this review, there were no significant differences observed in the duration of hospital stay, the number of patients requiring respiratory support and the duration of mechanical ventilation. Different durations of corticosteroids regimens was used across all included studies, which introduced variances to the findings.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 In contrast, a triple-blinded RCT established that high-dose corticosteroids significantly reduced the length of hospital stay and the number of patients requiring respiratory support (2 mg/kg methylprednisolone) as compared to low-dose corticosteroids (6 mg dexamethasone).22 High-dose corticosteroids generally were used for fewer than 10 days in the reported studies, and were associated with a shorter duration of hospitalization and ventilation support.17 , 21 , 22 , 44 , 45 , 48
Low-dose corticosteroids commonly are used in surgery to minimize postoperative nausea and vomiting and as an adjunct to part of the multimodal analgesia. The dose-dependent use of corticosteroids for surgery comes with its adverse effects; namely, incidence of hyperglycemia, surgical site infection, myocardial infarction and mortality rate.49 The vaccination rate of the low-income countries remains low due to various challenges; namely, limited vaccine supply and vaccine hesitancy.50 With this, there are more and more COVID-19 patients presenting for surgery. In this review, subgroup analysis based on surgical and nonsurgical COVID-19 patients receiving high-dose versus low-dose corticosteroids was not conducted due to lack of data available for pooling. Thus, the findings may not generalize to COVID-19 patients undergoing surgery. Due to the paucity of evidence, the dosage of corticosteroids among surgical COVID-19 patients may be considered based on the severity of COVID-19 infection and types of surgery involving airway manipulation.
The review had several limitations. The main limitation was the inconsistency of the dosage, types and regimens of high-dose versus low-dose corticosteroids across all the reported studies,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 which may have contributed to a high degree of heterogeneity. Adverse events from high-dose and low-dose corticosteroids were not extensively reported in all the included studies. The outcomes of long- term follow up for high-dose and low-dose corticosteroids COVID-19 survivors are needed to determine any complication of corticosteroids in the relapse of ARDS or pneumonia. Subgroup analysis based on the severity of COVID-19 patients (ICU and non-ICU) was not performed due to limited data available for data pooling. In addition, the nature of observational studies with small sample sizes may introduce bias to the findings. Therefore, more adequately powered RCTs should be carried out to evaluate the efficacy of types, dosage, and duration of corticosteroids in the treatment of COVID-19.
In conclusion, this meta-analysis showed that low-dose corticosteroids were as effective as high-dose corticosteroids in the reduction of COVID-19 mortality rate. In view of substantial heterogeneity and low level of evidence, future adequately powered RCTs are warranted to increase certainty of evidence and minimize heterogeneity.
Acknowledgements
We thank Dr Enric Monreal for data sharing upon request.
Funding
This research did not receive specific grant from funding agencies in the public, commercial, or not-for profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1053/j.jvca.2022.05.011.
Appendix. Supplementary materials
References
- 1.Organización Mundial de la Salud. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Who.int. WHO Director General's speeches. 2020. Available from: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- 2.Wang C, Wang Z, Li W, et al. COVID-19 in early 2021: current status and looking forward. Sig Transduct Target Ther. 2021;6(1):114. doi: 10.1038/s41392-021-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.House NNC, Palissery S, Sebastian H. Corona Viruses: A Review on SARS, MERS and COVID-19. Microbiol Insights. 2021;14:1–8. doi: 10.1177/11786361211002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns M, George S, Taburyanskaya M, et al. A Review of the Evidence for Corticosteroids in COVID-19. J Pharm Pract. 2021:1–12. doi: 10.1177/0897190021998502. [DOI] [PubMed] [Google Scholar]
- 5.Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Paassen J, Vos JS, Hoekstra EM, et al. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24(1):1–22. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8(12):1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin Microbiol and Infect. 2021;27(2):244–252. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu T, Liu Y, Zhao M, et al. A comparison of COVID-19, SARS and MERS. PeerJ. 2020;8:e9725. doi: 10.7717/peerj.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed MH, Hassan A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): a Review. SN Compr Clin Med. 2020;2(12):1–10. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 202The New England journal of medicine [Internet] 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corticosteroids for COVID-19 [Internet]. World Health Organization; 2020 Sept 2. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1
- 15.Therapeutic Management of Hospitalized Adults with COVID-19 [Internet]. National Institutes of Health | COVID-19 Treatment Guidelines. [Updated 2022 Feb 24] Available from: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults–therapeutic-management/
- 16.Huang H, Song B, Xu Z, et al. Predictors of Coronavirus Disease 2019 Severity: A Retrospective Study of 64 Cases. Jpn J Infect Dis. 2021;74(1):54–60. doi: 10.7883/yoken.JJID.2020.298. [DOI] [PubMed] [Google Scholar]
- 17.Fatima SA, Asif M, Khan KA, et al. Comparison of efficacy of dexamethasone and methylprednisolone in moderate to severe covid 19 disease. Ann Med Surg. 2020;60:413–416. doi: 10.1016/j.amsu.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García MS, Santos SSM, Cebolla BT, et al. High-dose versus low-dose corticosteroid treatment strategy in patients hospitalised with COVID-19: effect on ICU admission rate. Rev OFIL ILAPHAR. 2021;31(1):13–17. https://ilaphar.org/wp-content/uploads/2021/02/Original-1-ILAPHAR-31-1.pdf Available from: [Google Scholar]
- 19.Monreal E, de la Maza SS, Natera-Villalba E, et al. High versus standard doses of corticosteroids in severe COVID-19: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2021;40(4):761–769. doi: 10.1007/s10096-020-04078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez Zuniga MA, Moreno-Moral A, Ocana-Granados A, et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinzón MA, Ortiz S, Holguín H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS one. 2021;16(5) doi: 10.1371/journal.pone.0252057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21(1):337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gundogdu O, Demir B, Coskun CO, et al. Efficacy of pulse steroid therapy in patients critically ill with COVID-19. Bratisl Lek Listy. 2021;122(11):793–798. doi: 10.4149/BLL_2021_126. [DOI] [PubMed] [Google Scholar]
- 24.Toroghi N, Abbasian L, Nourian A, et al. Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial. Pharmacol Rep. 2022;74(1):229–240. doi: 10.1007/s43440-021-00341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora-Luján JM, Tuells M, Montero A, et al. High-dose methylprednisolone pulses for 3 days vs. Low-dose dexamethasone for 10 days in severe, non-critical covid-19: A retrospective propensity score matched analysis. J Clin Med. 2021;10(19):4465. doi: 10.3390/jcm10194465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batirel A, Demirhan R, Eser N, et al. Pulse steroid treatment for hospitalized adults with COVID-19. Turk J Med Sci. 2021;51(5):2248–2255. doi: 10.3906/sag-2101-243. [DOI] [PubMed] [Google Scholar]
- 27.Taboada M, Rodríguez N, Varela PM, et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 Pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2021 doi: 10.1183/13993003.02518-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 31.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schünemann H, Brożek J, Guyatt G, et al. GRADE Handbook [Internet]. [Updated 2013 October]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html
- 33.Thorlund K, Engstrøm J, Wetterslev J, et al. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention research, Copenhagen, Denmark. 2011 [Updated 2017]. Available from: https://ctu.dk/tsa/
- 34.The COVID STEROID 2 Trial Group Effect of 12 mg vs 6 mg of Dexamethasone on the Number of Days Alive Without Life Support in Adults With COVID-19 and Severe Hypoxemia: The COVID STEROID 2 Randomized Trial. JAMA. 2021;326(18):1807–1817. doi: 10.1001/jama.2021.18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granholm A, Munch MW, Myatra SN, et al. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022;48(1):45–55. doi: 10.1007/s00134-021-06573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4(10):525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 37.Buttgereit F, Burmester G-R, Lipworth BJ. Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet. 2005;365(9461):801–803. doi: 10.1016/S0140-6736(05)17989-6. [DOI] [PubMed] [Google Scholar]
- 38.Johns M, George S, Taburyanskaya M, et al. A Review of the Evidence for Corticosteroids in COVID-19. J Pharm Pract. 2021 doi: 10.1177/0897190021998502. [DOI] [PubMed] [Google Scholar]
- 39.Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6) doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espinosa-Solano M, Gonzalez-Vergara D, Ferrer-Galvan M, et al. Repeated Pulses of Methyl-Prednisolone in Adults Hospitalized With COVID-19 Pneumonia and Acute Respiratory Distress Syndrome: A Preliminary Before–After Study (CortiCOVID Study). Open Respiratory Archives. 2021;3(2):100086, 10.1016/j.opresp.2021.100086 [DOI] [PMC free article] [PubMed]
- 41.Takaki M, Ichikado K, Kawamura K, et al. The negative effect of initial high-dose methylprednisolone and tapering regimen for acute respiratory distress syndrome: a retrospective propensity matched cohort study. Crit Care. 2017;21(1):1–7. doi: 10.1186/s13054-017-1723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cano EJ, Fuentes XF, Campioli CC, et al. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest J. 2021;159(3):1019–1040. doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vichyanond P, Irvin CG, Larsen GL, et al. Penetration of corticosteroids into the lung: Evidence for a difference between methylprednisolone and prednisolone. J Allergy Clin Immunol. 1989;84(6):867–873. doi: 10.1016/0091-6749(89)90381-3. [DOI] [PubMed] [Google Scholar]
- 44.Ko JJ, Wu C, Mehta N, et al. A Comparison of Methylprednisolone and Dexamethasone in Intensive Care Patients With COVID-19. J Intensive Care Med. 2021;36(6):673–680. doi: 10.1177/0885066621994057. [DOI] [PubMed] [Google Scholar]
- 45.Monedero P, Gea A, Castro P, et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care. 2021;25(1):2. doi: 10.1186/s13054-020-03422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edel Y, Avni T, Shepshelovich D, et al. The safety of pulse corticosteroid therapy- Systematic review and meta-analysis. Semin Arthritis Rheum. 2020;50(3):534–545. doi: 10.1016/j.semarthrit.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Pascual Pareja JF, García-Caballero R, Soler Rangel L, et al. Efectividad de los glucocorticoides en pacientes hospitalizados por neumonía grave por SARS-CoV-2. Med Clin (Barc) 2021;156(5):221–228. doi: 10.1016/j.medcli.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannah Ritchie, Edouard Mathieu, Lucas Rodés-Guirao, et al. "Coronavirus Pandemic (COVID-19)". Our World in Data. 2020 [Updated 2022 Apr]. Available from: https://ourworldindata.org/coronavirus
- 50.Ng KT, Van Paassen J, Langan C, et al. The efficacy and safety of prophylactic corticosteroids for the prevention of adverse outcomes in patients undergoing heart surgery using cardiopulmonary bypass: a systematic review and meta-analysis of randomized controlled trials. Eur J Cardiothorac Surg. 2020;57:620–627. doi: 10.1093/ejcts/ezz325. https://doi.org/10.1093.ejcts/ezz325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.