Abstract
Glycogen synthase kinase-3 (GSK-3), a serine/threonine kinase, is a vital glycogen synthase regulator controlling glycogen synthesis, glucose metabolism, and insulin signaling. GSK-3 is widely expressed in different types of cells, and its abundant roles in cellular bioregulation have been speculated. Abnormal GSK-3 activation and inactivation may affect its original bioactivity. Moreover, active and inactive GSK-3 can regulate several cytosolic factors and modulate their diverse cellular functional roles. Studies in experimental liver disease models have illustrated the possible pathological role of GSK-3 in facilitating acute hepatic injury. Pharmacologically targeting GSK-3 is therefore suggested as a therapeutic strategy for liver protection. Furthermore, while the signaling transduction of GSK-3 facilitates proinflammatory interferon (IFN)-γ in vitro and in vivo, the blockade of GSK-3 can be protective, as shown by an IFN-γ-induced immune hepatitis model. In this study, we explored the possible regulation of GSK-3 and the potential relevance of GSK-3 blockade in IFN-γ-mediated immune hepatitis.
Keywords: glycogen synthase kinase-3, immune hepatitis, interferon-γ, liver
1. Multiple Roles of Glycogen Synthase Kinase-3 (GSK-3) in Human Diseases
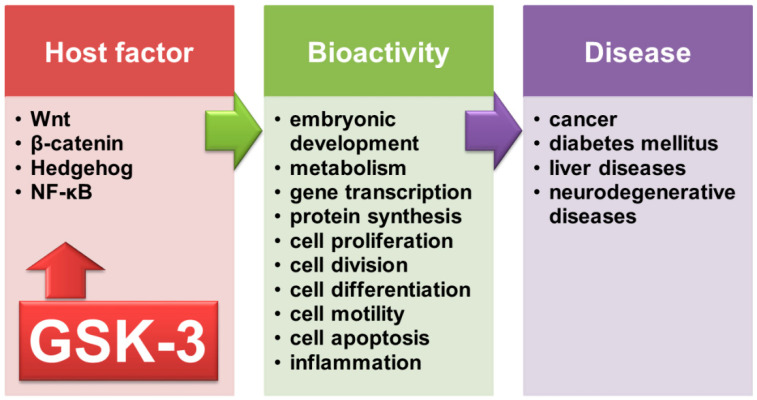
Glycogen synthase kinase-3 (GSK-3) was first recognized as a critical glycogen synthase and glycogen regulator responding to insulin signaling and glucose metabolism [1]. With regard to glycogen being made and stored primarily in the liver, particularly in hepatocytes, controlling glycogen by glycogen synthase is essential. GSK-3 consists of GSK-3α and GSK-3β [2] and is primarily expressed in the cytosol and nucleus in response to stimuli [3]. In response to growth factor withdrawal and starvation, GSK-3 is activated and then phosphorylates glycogen synthase to deactivate its enzymatic activity. In contrast, in response to blood glucose, insulin, and insulin-like growth factor (IGF) 1, GSK-3 is generally inactivated, and glycogen synthase is next activated to process glycogen biosynthesis. In addition, nuclear GSK-3 facilitates the phosphorylation of nuclear cyclin D1 in the S phase of the cell cycle [4]. However, GSK-3α and GSK-3β have different biological roles; the induction of embryonic lethality has been shown in GSK-3β but not GSK-3α knockout mice [5]. In an early study, GSK-3 was also found to participate in various biological processes by modulating Wnt/β-catenin, Hedgehog, and nuclear factor κB (NF-κB) signaling [6]. The multifactorial actions of GSK-3 are exhibited by its multiple intracellular substrates involving signaling, structure, and transcription [7] and regulate several cellular processes, including embryonic development, metabolism, gene transcription, protein synthesis, cell proliferation and division, differentiation, motility, apoptosis, and inflammation [1,8]. Hence, aberrant activation and inactivation of GSK-3 have been implicated in cancer, diabetes mellitus, liver diseases, and neurodegenerative diseases [9,10]. As an important regulator in response to diverse stimuli, the possible roles of GSK-3 are therefore summarized in Figure 1.
Figure 1.
The various roles of GSK-3 contribute to diverse bioactivities and human diseases.
2. Regulation of GSK-3 in Facilitating Proapoptosis and Proinflammation
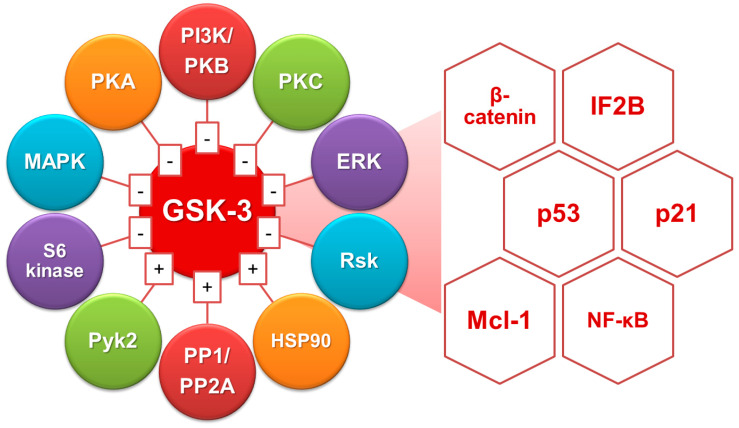
Regulation of GSK-3 activation is suggested to be necessary for controlling many vital intracellular factors (Figure 2). First, GSK-3 inhibition by phosphorylation is regulated at the N-terminal serine 9 residue through phosphatidylinositol 3-kinase (PI3K)-Akt (protein kinase B, PKB) [11]. Pharmacological blockade of PI3K-Akt signaling causes GSK-3β dephosphorylation and activation followed by cell apoptosis in a GSK-3β-regulated manner [12,13]. Furthermore, activation of protein phosphatases, including protein phosphatase (PP) 1 and PP2A, can directly or indirectly dephosphorylate GSK-3β for activation by causing Akt dephosphorylation [14]. Additionally, the signaling pathways of the extracellular signal-regulated kinase (ERK), PKA, PKC, mitogen-activated protein kinase (MAPK)-activated protein kinase-1 (also known as p90rsk), p70 ribosomal S6 kinase, and Wnt activation also promote GSK-3 inactivation [7]. Alternatively, tyrosine kinases such as proline-rich tyrosine kinase (Pyk) 2 [15], MAPK/ERK kinase, and Src-like kinase regulate GSK-3 activity [7]. Moreover, a heat shock protein 90-mediated autophosphorylation mechanism has been suggested as a regulatory factor [16].
Figure 2.
Molecular regulation of GSK-3 for activating the diverse intracellular factors.
The proapoptotic role of GSK-3 is suggested in Alzheimer’s disease [17]. GSK-3 overexpression in target cells induces apoptosis [13,18]. Therefore, GSK-3 activation has been reported in apoptotic stimuli, including endoplasmic reticulum (ER) stress, growth factor withdrawal, heat shock, hypoxia, staurosporine administration, and mitochondrial complex I inhibition [12,18,19,20]. GSK-3 exerts its multiple regulatory actions on apoptosis through different mechanisms. Interactions of GSK-3β with β-catenin, initiation factor 2B, p21Cip1, and p53 translation may modulate cell fate in survival and apoptosis [7,13]. The current study demonstrated the novel proapoptotic role of GSK-3 by negatively regulating myeloid cell leukemia (Mcl)-1 protein followed by triggering mitochondrial damage [21]. PP2A and PI3K-Akt modulate GSK-3β activity, and GSK-3β, in turn, regulates mitochondrial permeability in ceramide-induced apoptosis [22]. In response to the ER stressor tunicamycin, GSK-3 is essential for cell apoptosis [23]. These molecular regulations show the proapoptotic role of GSK-3.
Disrupting the GSK-3β gene causes embryonic lethality [5]. In GSK-3β-deficient mice, severe liver degeneration results from excessive tumor necrosis factor-α (TNF-α) cytotoxicity. Significantly, GSK-3β can affect the early stage of NF-κB activation by interfering with cytosolic IκB degradation and nuclear translocation of NF-κB. The data indicate that GSK-3β regulates NF-κB signaling at the transcriptional complex. The potential regulation of NF-κB activation by GSK-3 was demonstrated in lipopolysaccharide (LPS)/Toll-like receptor (TLR)-4 and TNF-α/TNF receptor signaling. Further studies demonstrated that inhibiting GSK-3β protects cells from inflammatory stimuli, including endotoxemia [24], experimental autoimmune encephalomyelitis [25], experimental colitis [26], TNF-α [27], type II collagen-induced arthritis [28], TLR-mediated inflammatory responses [29,30], and OVA-induced asthma. Furthermore, GSK-3 regulates the expression of nitric oxide (NO), inducible NO synthase (iNOS), and regulated on activation, normal T-cell expressed and secreted (RANTES) in LPS-activated macrophages and endotoxemia-induced acute renal failure [31,32]. Furthermore, inhibiting GSK-3 results in an anti-inflammatory effect in LPS/interferon (IFN)-γ- and heat-inactivated staphylococcal aureus-activated macrophages and microglia [33,34]. A study on the therapeutic mechanisms of GSK-3 inhibition will help to understand the proinflammatory role of GSK-3. Because the activation of NF-κB is involved in various immune responses, GSK-3 is speculated to be proinflammatory and could be a therapeutic target for anti-inflammation [5,24,31,32].
3. Targeting GSK-3 as a Protective Strategy against Hepatic Injury
The therapeutic effects of GSK-3 blockade on hepatic protection have been demonstrated in TLR-mediated systemic inflammation involving multiorgan failure, including the lungs, liver, pancreatic injury, and renal dysfunction [35]. In diseased mice with GSK-3 inhibitor treatment, proinflammatory and proapoptotic molecules such as iNOS, nitrotyrosine, poly(ADP-ribose), CD30, CD30 ligand, and Fas ligand are markedly reduced. In a murine model of liver partial warm ischemia/reperfusion injury (IRI), active GSK-3 favors the development of liver pathology, while GSK-3 inhibitor ameliorates the hepatocellular injury as indicated by the presence of aspartate aminotransferase and histopathological examination [36]. Therefore, the findings on the pathogenic role of active GSK-3 are essential for explaining how carbon monoxide works to protect the IRI liver [37]. Carbon monoxide treatment causes activation of PI3K-Akt signaling to deactivate GSK-3. Notably, in these diseased mice with GSK-3 inhibitor treatment [36], the induction of anti-inflammatory interleukin (IL)-10 is essential for liver protection while neutralizing IL-10 overcomes the therapeutic effects. It is suggested that the blockade of GSK-3 confers an indirect intercellular regulation. However, the IL-10-producing cells required for hepatic inflammatory resolution need further investigation. Targeting GSK-3 as a therapeutic strategy against liver injury is therefore suggested.
Upon TLR stimuli, regulation of IL-10 production is generally critical for immune resolution [30]. Tight regulation of GSK-3-mediated IL-10 generation has been previously reported since a critical transcriptional factor cAMP-response element-binding protein (CREB) required for IL-10 gene transactivation is suggested for use in GSK-3 regulation [38]. CREB is deactivated by active GSK-3 at the acute phase of TLR-mediated inflammatory responses. Therefore, suppressing IL-10 production is necessary for early activation of proinflammation, while active GSK-3 is also vital to sustaining TLR-induced NF-κB activation. Therefore, targeting GSK-3 could be anti-inflammatory directly by interfering with NF-κB-regulated inflammatory factor expression and indirectly causing CREB-mediated IL-10 induction. The IL-10-regulating effects raised by the blockade of GSK-3 have been widely shown in the models of liver protection [36,39]. Similar to the GSK-3 blockade, the exogenous administration and expression of IL-10 are protective in acute liver injury, including allograft liver transplantation [40], liver fibrosis [41], and immune hepatitis [42].
In a murine acute liver injury model induced by LPS and D-galactosamine (D-GalN), administrating the blocker of ER stress, 4-phenylbutyric acid, effectively rescues mice from hepatic injury and inflammation [43]. Upon ER stress, GSK-3 is activated for mediating cellular activation toward proinflammatory and proapoptotic responses [23,44]. It has been demonstrated that the blockade of ER stress also inhibits GSK-3 activation and GSK-3-mediated cell death and inflammatory activation. In brief, the inhibition of GSK-3 also confers protection from LPS- [24,45] and cecal ligation and puncture-induced liver injury [46], hemorrhagic shock [47], liver ischemia-reperfusion [36,48], and LPS/D-GalN-induced acute hepatic injury [49]. For anti-inflammation, inhibiting GSK-3 promotes autophagy to increase the expression of peroxisome proliferator-activated receptor (PPAR) α [49]. Additionally, active GSK-3 mediates ER stress to facilitate LPS-triggered hepatic inflammation [43]. Additional data have shown that in the same acute hepatic injury, the blockade of GSK-3 reduces ER stress-triggered [44] and oxidative stress-induced [50] apoptosis in hepatocytes. In studies of supplementation, including methane-rich saline [39], suberoylanilide hydroxamic acid [51], curcumin [52], and l-carnitine [53], on liver protection, all of the treatments inhibit several models of acute hepatic injury by suppressing inflammation as well as hepatocyte apoptosis. Notably, targeting GSK-3 signaling pathways for anti-inflammation and anti-apoptosis are the main effects of these liver-associated protective agents.
In addition to modulating hepatic inflammation and hepatic cell death, pharmacologically inhibiting GSK-3 by using lithium in patients with chronic hepatitis C confers antioxidant responses to avoid the progression of hepatic injury [54]. As shown in liver biopsy specimens from these patients with GSK-3 inhibition, an inactive phosphorylated GSK-3 is significantly increased and positively correlated with antioxidant Nrf2 expression. Nrf2 acts as a significant suppressor of cellular oxidative responsive pathways in the hepatic cells [55]. In saturated free fatty acid-induced hepatocyte lipoapoptosis, palmitate treatment causes GSK-3 activation, while pharmacologically inhibiting GSK-3 significantly reduced palmitate-mediated lipoapoptosis in an experimental cell culture model of Huh-7 cells. The short hairpin RNA technique to knock down GSK-3 showed that GSK-3 facilitates palmitate-induced JNK activation followed by the induction of the proapoptotic effector p53-upregulated modulator of apoptosis (PUMA) [56]. The potential treatment by targeting GSK-3 in experimental models of hepatic injury is summarized in Table 1.
Table 1.
GSK-3 in liver diseases and hepatic cell injury.
| Hepatic Injury Model | The Blockade of GSK-3 | References |
|---|---|---|
| Zymosan | 4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8) | [35] |
| IRI | SB216763/TDZD-8/Carbon monoxide | [36,37,48] |
| Carbon tetrachloride | Methane | [39] |
| LPS/D-GalN | 4-Phenylbutyric acid/SB216763 | [43,44,49,50] |
| LPS | Lithium chloride (LiCl) | [45] |
| CLP | SB216763 | [46] |
| Hemorrhagic shock | TDZD-8 | [47] |
| Transplantation | Suberoylanilide hydroxamic acid | [51] |
| Lead | Curcumin/l-carnitine | [52,53] |
| HCV | LiCl | [54] |
| Palmitate | GSK-3 inhibitor IX/Enzastaurin | [56] |
4. Generation of IFN-γ and Its Multiple Proinflammatory Roles
IFN-γ is primarily produced by T cells, natural killer (NK) cells, and NKT cells [57,58]. Previous studies proved that the T-box transcription factor Tbx21 (T-bet) is required for IFN-γ production [59,60,61,62]. In Th1 differentiation, IFN-γ-signal transducer and activator of transcription (STAT) 1 signaling activates T-bet and then sustains the positive feedback loop to produce more IFN-γ [59,63]. T-bet may also be important in many kinds of immune cells, including CD8+ T cells [61,64], dendritic cells [65], B cells [66], NK cells, and NKT cells [62,67]. In general, NK and NKT cells express IFN-γ in response to infection [61,68]. Therefore, NK- and NKT-driven IFN-γ production plays a proinflammatory role in the immune hepatitis model [69]. However, the regulation of IFN-γ production by T-bet is still unclear. Following T-bet activation, T-bet (Ser508), which is phosphorylated by casein kinase I and GSK-3, is required for controlling cytokine production in developing Th1 cells [70].
IFN-γ generally and positively affects the production of the proinflammatory cytokine TNF-α and chemokines, including IFN-inducible protein-10, monocyte chemoattractant protein-1, monokine induced by IFN-γ, macrophage inflammatory protein-1α/β, and RANTES [58], but decreases the expression of the anti-inflammatory cytokine IL-10 [57]. In addition, IFN-γ synergizes with LPS-stimulated iNOS/NO biosynthesis [71]. Furthermore, it has been reported that IFN-γ may trigger the full activation of a variety of signaling factors, including NF-κB [72], MAPK [73], STAT1 [71], and interferon regulatory factor-1 (IRF-1) [74], to modulate its proinflammatory activation. In addition, IFN-γ induces immune cell chemotaxis into sites of inflammation through the upregulation of adhesion molecules, including intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, and chemokines [75]. In brief, IFN-γ is a potent cytokine that promotes antigen processing and presentation, microbial killing, and proinflammatory cytokine production [58,68].
5. IFN-γ Signaling and Its Regulation
IFN-γ receptor (IFNGR) is composed of IFNGR1 and IFNGR2, which bind to Janus kinase (Jak) 1 and Jak2, respectively [58,76]. Following IFN-γ stimulation, Jak2 is autophosphorylated and activated to cause Jak1 transphosphorylation. Through Jak1-mediated IFNGR1 phosphorylation, activated IFNGR1 creates a docking site for STAT1 recruitment, followed by Jak2-mediated phosphorylation at a tyrosine residue (Tyr701) [58,76]. Furthermore, IFN-γ-activated MAPKs, such as ERK and p38 MAPK, subsequently phosphorylate Ser727 of STAT1 (Tyr701) to facilitate its dimerization, nuclear translocation, and DNA binding stability [77]. Beurel and Jope [78] further demonstrated the requirement of GSK-3β in facilitating IFN-γ-activated STAT3 and STAT5. This finding suggests a novel role of GSK-3β in IFN-γ signaling, but the complete regulation of GSK-3 in IFN-γ signaling remains unclear.
Critical signal components, including Jak1, Jak2, and IFNGR1, are rapidly phosphorylated within one minute of IFN-γ treatment in HeLa cells [79]. The time required for full IFN-γ-induced STAT1-IRF-1 activation and nuclear translocation is approximately thirty minutes [80]. Notably, STAT1 activation is then inhibited within one hour of IFN-γ treatment [80], and three families of proteins, SH2-containing phosphatase (SHP) 2, protein inhibitors of activated STATs, and suppressor of cytokine signaling (SOCS), have been reported to show negative inhibition of IFN-γ signaling [81,82]. SOCS proteins, including SOCS1–SOCS7, are identified as inducible negative regulators of cytokine signaling. SOCS proteins contain an SH2 domain and a carboxy-terminal SOCS box [83]. It is now known that Jak-STAT-induced SOCS1 and SOCS3 proteins subsequently interfere with Jak by repressing its activity after ligand binding [83,84]. In addition to SOCSs, dual phosphatase SHP2 can cause the dephosphorylation of Jak1, Jak2, IFNGR1, and STAT1 [85]. SHP2 becomes phosphorylated at Tyr542 and Tyr580 residues in response to growth factor stimulation [86]. However, the in-depth molecular mechanisms of SHP2 activation remain largely unclear.
6. GSK-3 Is Involved in IFN-γ Signaling Pathways
Targeting GSK-3 expression and activity suppresses TLR-mediated inflammation but increases anti-inflammatory cytokine IL-10 production [29,30,36]. Active GSK-3β negatively regulates the IL-10-regulating transcription factor cyclic AMP responsive element binding protein [29,87]. With a dysregulation of GSK-3-mediated excessive proinflammatory cytokine production and IL-10 downregulation, cirrhotic patients show a high risk of developing sepsis under endotoxin exposure [88]. While GSK-3 regulates the expression of NO, iNOS, and RANTES in LPS-activated macrophages, pharmacologically inhibiting GSK-3 increases IL-10 production to relieve anti-inflammation [31,88]. Accordingly, treatment with GSK-3 inhibitors comprehensively improves the survival of endotoxemic C3H/HeN mice. An advanced study demonstrated that IFN-γ treatment synergizes with TLR2-mediated IκB degradation and NF-κB activation, while TNF-α production is effectively induced by suppressing IL-10-dependent phosphorylation of STAT3 in a GSK-3-regulated manner [87,89]. In GSK-3β-deficient fetal liver cells, IFN-γ increases GSK-3β activity to reduce IL-10 expression in TLR2-stimulated cells [90]. This finding suggests that GSK-3β plays a decisive signaling role in transducing the proinflammatory activity of IFN-γ.
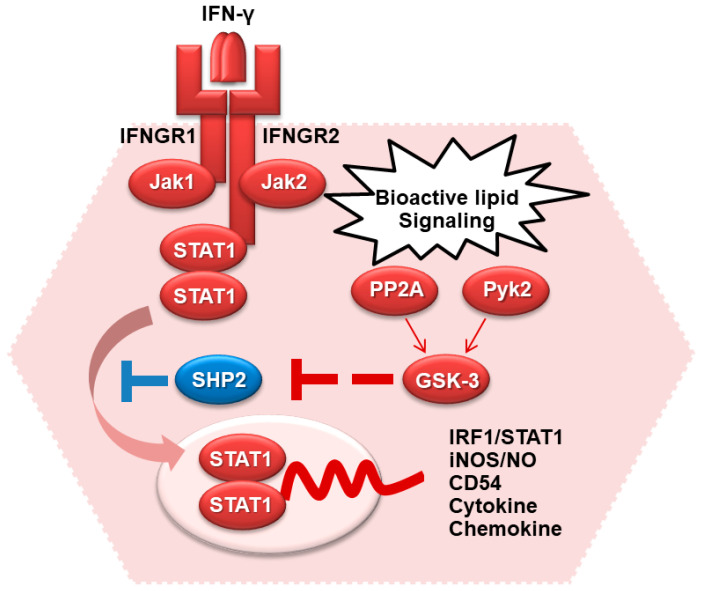
Following the generation of bioactive lipid signaling, treatment of IFN-γ activates phosphatidylcholine-specific phospholipase C and PKC to cause Pyk2- and PP2A-regulated GSK-3 activation [91]. Inhibiting GSK-3 activates SHP2 to prevent STAT1 activation. Among the signaling pathways, a calcium-dependent tyrosine kinase, Pyk2, causes GSK-3β phosphorylation (Tyr216) and activation [34,38,92]. The involvement of GSK-3β in facilitating IFN-γ signaling has been widely investigated [34,78,87,89]; however, the mechanisms for IFN-γ-regulated GSK-3β activation remain undecided. Pyk2 can act as a downstream kinase of immunoreceptor tyrosine-based activation motif-associated receptors and causes the regulation of the IFN-induced activation of Jak-STAT [38]. Therefore, Pyk2 is involved in the regulation of Jak-STAT signaling. Moreover, Pyk2 is constitutively bound to Jak2 and undergoes tyrosine phosphorylation and activation caused by IFN-γ [93]. In response to IFN-γ-induced iNOS/NO biosynthesis, diacylglycerol is generated to activate PKC. The activations of PKC-mediated Src, Pyk2, and GSK-3β are essential for regulating IFN-γ signaling [91,94]. Importantly, our previous work [91] demonstrated the possible inhibitory effects of GSK-3 on SHP2 activation, an inhibitor of STAT1 signaling. The possible regulation of GSK-3 in facilitating IFN-γ-activated STAT1 signaling and bioactivity is summarized in Figure 3.
Figure 3.
The involvement of GSK-3 in IFN-γ signaling.
7. Immune Hepatitis
Immune-mediated hepatic injury, also called immune hepatitis, is caused by many agents, such as infectious pathogens and chemical and metal drugs [95]. Following stimulation, the condition is further induced by adverse hepatic immune responses, including activating local and infiltrated immune cells, resulting in hepatocytes undergoing apoptosis [96]. In addition, in the liver, T, NK, and NKT cells, sinusoid endothelial cells, Kupffer cells, and stellate cells are involved in hepatic immunity [97]. Therefore, advances in understanding hepatic immunopathogenesis will improve the treatment of immune hepatitis.
Many viral infections can cause chronic diseases in the liver. To mimic acute immune hepatitis, lymphocyte mitogen concanavalin A (ConA)-induced immune hepatitis closely resembles the pathology of viral-, drug-, and autoimmune-induced immune hepatitis [98]. Intravenous injection of ConA can induce immune cell infiltration in the liver and can elevate the serum alanine aminotransferase and serum aspartate aminotransferase level, followed by hepatocyte death [98]. Activated immune cells, such as T, NK, NKT, and Kupffer cells, may exhibit direct cytotoxicity or may release procytotoxic and proinflammatory cytokines to mediate liver damage [99]. NKT cells, which express invariant T-cell receptors, are an abundant cell population in the liver and play a pathogenic role in immune responses in ConA-induced immune-mediated hepatic injury [100]. In general, activated NKT cell-mediated excessive inflammatory responses may cause hepatocellular apoptosis. It has been shown that liver injury in this model depends on IFN-γ and TNF-α overproduction since administering neutralizing antibodies that recognize either cytokine effectively protects against ConA-induced immune hepatitis [101,102].
Hepatocellular apoptosis is the primary cause of hepatic injury [95]. Hepatocyte apoptosis is caused by excessive inflammation resulting from activated T cells, NKT cells, polymorphonuclear granulocytes (PMNs), and cytokine responses [96,103]. Additionally, it has been reported that ConA-induced immune hepatitis is fully protected by using macrophage depletion, T-cell depletion, and T-cell-deficient mice [98]. NKT cells increase the production of proinflammatory cytokines and procytotoxic factors, leading to hepatic injury [100,104,105,106]. Further studies showed the suppression of ConA-induced immune hepatitis in CD4+ neutralized mice, while the CD8+ neutralized mice showed no significant change [107]. PMNs are also reported to modulate the generation of IFN-γ in ConA-induced hepatic injury [103,108]. Kupffer cells are resident hepatic macrophages and can facilitate neutrophil infiltration. In Kupffer cell-depleted mice, hepatic cell apoptosis and inflammatory responses in ConA-induced immune hepatitis are reduced [109]. Upon ConA stimulation, a variety of hepatic immune cells are involved in the pathogenesis of immune hepatitis.
Several cytokine- and apoptosis-related effector molecules, including IFN-γ [101,106,110], CD95 Ligand (CD95L) [111], TNF-α [102,112], and IL-4 [104], take part in ConA-induced T cell- or NKT-mediated hepatic injury [96]. T cells are generally activated, followed by the immediate secretion of IFN-γ and TNF-α, causing cellular activation and cytotoxicity in ConA-induced hepatic injury [101]. IFN-γ-deficient mice show significant resistance to ConA-induced hepatocyte apoptosis, suggesting the proapoptotic role of IFN-γ in immune-mediated hepatic injury [113]. Hepatocytes, sinusoidal endothelial cells, stellate cells, and Kupffer cells express CD95 [114], and CD95L is generally expressed on cytotoxic T cells, NK cells, NKT cells, and hepatic macrophages [115]. Notably, the induction of CD95 expression on hepatocytes and CD95L expression on cytotoxic NKT cells after treatment with ConA is mediated by IFN-γ, and this elevated expression of CD95 causes apoptosis [113]. Furthermore, IFN-γ signaling determines the induction of multiple chemokines and adhesion molecules in ConA-induced immune hepatitis [69]. The pathogenesis of ConA-induced immune hepatitis is generally regulated by T cells, NKT cells, PMNs, cytokines, chemokines, adhesion molecules, and apoptosis.
8. GSK-3 in IFN-γ-Mediated Hepatic Immune Hepatitis and Its Therapeutic Efficacy
Active GSK-3 facilitates the signal transduction of IFN-γ to modulate IFN-γ-induced proinflammatory responses [34,78,87,89]. Pharmacological inhibition of GSK-3 provides anti-inflammation and cytoprotection against IFN-γ- [34,91,116], LPS- [29,31,117], and TNF-α-induced inflammation in vitro [27] and endotoxemic multiple organ failure in vivo [24,32,117,118]. In addition, the blockade of GSK-3 also has a protective effect in several IFN-γ-related autoimmune mouse models, including experimental autoimmune encephalomyelitis [25], experimental colitis [26], and type II collagen-induced arthritis [28]. Evidence has shown that IFN-γ-deficient and STAT1 mice are resistant to ConA-induced immune hepatitis [60,106,113]. It is speculated that IFN-γ-activated Jak-STAT signaling is required for ConA-induced immune hepatitis by increasing CD95/CD95L-mediated apoptosis, and GSK-3 is essential in ConA-induced IFN-γ-mediated immune hepatitis by modulating IFN-γ signaling. Previous work [106] showed that exogenous administration of ConA caused GSK-3 activation in NKT cells and hepatocytes in an in vitro cell culture model and an in vivo model of experimental immune hepatitis. The activation of GSK-3 in these cells is speculated to be important in controlling the downstream signaling of ConA-activated hepatic NKT cells as well as IFN-γ-activated hepatocytes. In the ConA-treated liver, the loss of glycogen could be observed to be accompanied by the decrease in glycogen synthase and the increase in active GSK-3 in the hepatocytes. As shown by the blockade of GSK-3 using selective inhibitors of GSK-3, the loss of glycogen is restored. While a ConA-induced liver injury is an appropriate model of glycogen deregulated disorder, our other results demonstrate that GSK-3 causes dual effects on T-bet-dependent IFN-γ production in hepatic NKT cells and IFN-γ-activated Jak2/STAT1 for proinflammatory as well as procytotoxic effects in hepatocytes. The downstream effects of GSK-3 activation are necessary for promoting IFN-γ-mediated ConA-induced immune hepatitis.
There are multiple causes of hepatic cell apoptosis in immune hepatitis. Hepatocyte apoptosis may be caused by mechanisms other than those mediated by the CD95-CD95L system because lpr/lpr mice showed only partial resistance against ConA-hepatitis [113,119]. Indeed, other results have shown IFN-γ-induced CD95-independent apoptosis of mouse hepatocytes in vitro [120]. Interestingly, stimulating IFN-γ effectively triggers primary hepatocyte apoptosis, probably in an IRF-1-dependent manner [121,122]. Additionally, IFN-γ-induced iNOS, a potent inducer of apoptosis [123,124], is known to be induced by IFN-γ. LPS/D-GalN-induced hepatocyte apoptosis is mediated by iNOS/NO biosynthesis [125]. IFN-γ synergizes with LPS [34] or TLR2 [87] to increase iNOS/NO biosynthesis by involving GSK-3 activation followed by inhibiting IL-10. The requirement of GSK-3 is indispensable in IFN-γ-induced iNOS expression in primary hepatocytes or Huh7 cells. Therefore, GSK-3 contributes to ConA/IFN-γ-induced iNOS/NO-mediated hepatocyte apoptosis.
The roles of GSK-3 in regulating bioactivities are diverse depending on its protein expression, activation, intracellular location, interacting molecules, and cell types [1,2,8]. This review shows the benefits of GSK-3 blockade in many acute and chronic liver diseases; however, GSK-3 may also protect hepatocytes from TNF-α-induced hepatocyte apoptosis [126]. Initially and importantly, GSK-3β deficiency causes embryonic lethality in mice since GSK-3 is required for TNF-α-activated p65 phosphorylation and upregulation of NF-κB transactivation [5]. Furthermore, during the stage of liver generation in the embryo, TNF-α-activated NF-κB is essential for hepatocyte survival by upregulating antiapoptotic protein expression [5,126] as well as iNOS/NO biosynthesis [127]. According to these findings, it is controversial in GSK-3-involved liver diseases whether targeting GSK-3 may be protective or pathogenic [10].
Furthermore, studies have shown the potential implications of inhibiting GSK-3 against septic shock and multiorgan failure [9,118]. Patients with liver cirrhosis have a high risk of developing sepsis due to excessive inflammation resulting from the deregulation of GSK-3-modulated inflammation and anti-inflammation [88]. Therefore, GSK-3 is an attractive therapeutic target of pharmacologic intervention that has become indispensable for investigation, particularly in acute liver diseases [10]. To stretch the blockade of GSK-3, inhibitors of GSK-3 are approached by using metal ions (such as lithium), which are used to block the enzymatic activity. Additionally, GSK-3 inhibitors are developed by three main classes, including ATP-competitive (such as BIO, SB216763, and SB415286), non-ATP-competitive (such as TDZD-8), and substrate competitive (such as L803) [117,128]. Additionally, modulating the upstream signaling pathways of GSK-3 activation and inactivation are suggested to be functionally regulated for controlling GSK-3. The selectivity of GSK-3 inhibitors used to suppress its intracellular activation is therefore crucial for further investigation.
9. Conclusions
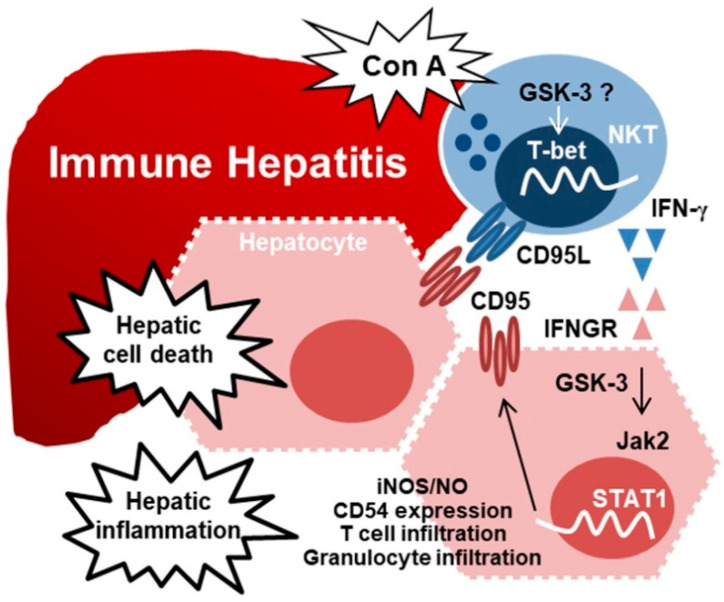
In summary (Figure 4), in an experimental model of ConA-induced immune hepatitis [106], activating GSK-3 by ConA determines IFN-γ generation in NKT cells and synergistically facilitates IFN-γ-activated Jak-STAT, inflammatory responses (such as CD54 expression, iNOS/NO biosynthesis, and immune cell infiltration), and proapoptotic effects (such as CD95L/CD95 signaling) in the liver, particularly in hepatocytes. GSK-3 inhibition has been used to prevent inflammatory disorders, including neurodegenerative disorders, infectious pathogens, endotoxemia, trauma, and asthma [128,129,130]. Therefore, GSK-3 inhibition represents a potential therapeutic strategy to prevent or reduce disease progression, probably through anti-inflammation and anti-apoptosis. Based on the essential roles of GSK-3 in immune hepatitis and IFN-γ signaling, drug targeting of GSK-3 and its upstream or downstream signaling can provide strategies for anti-inflammation and anti-apoptosis in immune-mediated hepatic injury.
Figure 4.
A hypothetical model for GSK-3-facilitated IFN-γ immune hepatitis. Treatment of ConA causes immune hepatitis through a mechanism involving NKT activation, hepatic cell apoptosis, and inflammatory activation. In activated NKT cells, in addition to CD95L induction, ConA induces GSK-3 activation to facilitate T-bet-modulated IFN-γ generation. Furthermore, signaling of IFN-γ and its receptor IFNGR may cause GSK-3-regulated Jak2/STAT1 signaling in hepatocytes to facilitate IFN-γ-activated Jak2-STAT1 signaling. IFN-γ is essential for inducing hepatic injury, including CD95-mediated hepatic cell death and hepatic inflammatory responses such as iNOS/NO biosynthesis, CD54 induction, and immune T cell and granulocyte infiltration. These findings illustrate a pathogenic role of GSK-3 in guiding ConA-induced immune hepatitis by facilitating IFN-γ expression, signaling, hepatic injury, and inflammation.
Abbreviations
| ConA | concanavalin A |
| CREB | cAMP-response element-binding protein |
| D-GalN | D-galactosamine |
| ER | endoplasmic reticulum |
| ERK | extracellular signal-regulated kinase |
| GSK | glycogen synthase kinase |
| IFN | interferon |
| IFNGR | IFN-γ receptor |
| IL | interleukin |
| iNOS | inducible NO synthase |
| IRF | interferon regulatory factor |
| IRI | ischemia/reperfusion injury |
| LiCl | lithium chloride |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor κB |
| NK | natural killer |
| NO | nitric oxide |
| PI3K | phosphatidylinositol 3-kinase |
| PK | protein kinase |
| PMNs | polymorphonuclear granulocytes |
| PP | protein phosphatase |
| Pyk | proline-rich tyrosine kinase |
| RANTES | regulated on activation, normal T-cell expressed and secreted |
| SHP | SH2-containing phosphatase |
| SOCS | suppressor of cytokine signaling |
| STAT | signal transducer and activator of transcription |
| T-bet | T-box transcription factor Tbx21 |
| TDZD-8 | 4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
Author Contributions
C.-L.C., P.-C.T., C.-C.T. and C.-F.L. conceived the idea. P.-C.T., R.D.S. and T.T.N. collected the references. C.-L.C., C.-C.T. and C.-F.L. wrote and edited the manuscript. All authors contributed clarifications and guidance to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology (MOST109-2320-B-038-050, 109-2327-B-006-010, 110-2327-B-006-005, and 110-2320-B-038-064-MY3), Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this manuscript are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frame S., Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001;359:1–16. doi: 10.1042/bj3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doble B.W., Woodgett J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijur G.N., Jope R.S. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport. 2003;14:2415–2419. doi: 10.1097/00001756-200312190-00025. [DOI] [PubMed] [Google Scholar]
- 4.Diehl J.A., Cheng M., Roussel M.F., Sherr C.J. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeflich K.P., Luo J., Rubie E.A., Tsao M.S., Jin O., Woodgett J.R. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 6.Kockeritz L., Doble B., Patel S., Woodgett J.R. Glycogen synthase kinase-3—An overview of an over-achieving protein kinase. Curr. Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 7.Jope R.S., Johnson G.V. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P., Frame S. The renaissance of GSK3. Nat. Rev. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 9.Jope R.S., Yuskaitis C.J., Beurel E. Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emma M.R., Augello G., Cusimano A., Azzolina A., Montalto G., McCubrey J.A., Cervello M. GSK-3 in liver diseases: Friend or foe? Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118743. doi: 10.1016/j.bbamcr.2020.118743. [DOI] [PubMed] [Google Scholar]
- 11.Cross D.A., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 12.Hetman M., Hsuan S.L., Habas A., Higgins M.J., Xia Z. ERK1/2 antagonizes glycogen synthase kinase-3beta-induced apoptosis in cortical neurons. J. Biol. Chem. 2002;277:49577–49584. doi: 10.1074/jbc.M111227200. [DOI] [PubMed] [Google Scholar]
- 13.Pap M., Cooper G.M. Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta signaling pathway. Mol. Cell. Biol. 2002;22:578–586. doi: 10.1128/MCB.22.2.578-586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivaska J., Nissinen L., Immonen N., Eriksson J.E., Kahari V.M., Heino J. Integrin alpha 2 beta 1 promotes activation of protein phosphatase 2A and dephosphorylation of Akt and glycogen synthase kinase 3 beta. Mol. Cell. Biol. 2002;22:1352–1359. doi: 10.1128/MCB.22.5.1352-1359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartigan J.A., Xiong W.C., Johnson G.V. Glycogen synthase kinase 3beta is tyrosine phosphorylated by PYK2. Biochem. Biophys. Res. Commun. 2001;284:485–489. doi: 10.1006/bbrc.2001.4986. [DOI] [PubMed] [Google Scholar]
- 16.Cole A., Frame S., Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004;377:249–255. doi: 10.1042/bj20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimes C.A., Jope R.S. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 2001;65:391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 18.Bijur G.N., De Sarno P., Jope R.S. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J. Biol. Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 19.Somervaille T.C., Linch D.C., Khwaja A. Growth factor withdrawal from primary human erythroid progenitors induces apoptosis through a pathway involving glycogen synthase kinase-3 and Bax. Blood. 2001;98:1374–1381. doi: 10.1182/blood.V98.5.1374. [DOI] [PubMed] [Google Scholar]
- 20.Song L., De Sarno P., Jope R.S. Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J. Biol. Chem. 2002;277:44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- 21.Maurer U., Charvet C., Wagman A.S., Dejardin E., Green D.R. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Lin C.F., Chen C.L., Chiang C.W., Jan M.S., Huang W.C., Lin Y.S. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J. Cell Sci. 2007;120:2935–2943. doi: 10.1242/jcs.03473. [DOI] [PubMed] [Google Scholar]
- 23.Huang W.C., Lin Y.S., Chen C.L., Wang C.Y., Chiu W.H., Lin C.F. Glycogen synthase kinase-3beta mediates endoplasmic reticulum stress-induced lysosomal apoptosis in leukemia. J. Pharmacol. Exp. Ther. 2009;329:524–531. doi: 10.1124/jpet.108.148122. [DOI] [PubMed] [Google Scholar]
- 24.Dugo L., Collin M., Allen D.A., Patel N.S., Bauer I., Mervaala E.M., Louhelainen M., Foster S.J., Yaqoob M.M., Thiemermann C. GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit. Care Med. 2005;33:1903–1912. doi: 10.1097/01.CCM.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- 25.De Sarno P., Axtell R.C., Raman C., Roth K.A., Alessi D.R., Jope R.S. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whittle B.J., Varga C., Posa A., Molnar A., Collin M., Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta. Br. J. Pharmacol. 2006;147:575–582. doi: 10.1038/sj.bjp.0706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takada Y., Fang X., Jamaluddin M.S., Boyd D.D., Aggarwal B.B. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J. Biol. Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- 28.Cuzzocrea S., Mazzon E., Di Paola R., Muia C., Crisafulli C., Dugo L., Collin M., Britti D., Caputi A.P., Thiemermann C. Glycogen synthase kinase-3beta inhibition attenuates the degree of arthritis caused by type II collagen in the mouse. Clin. Immunol. 2006;120:57–67. doi: 10.1016/j.clim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Martin M., Rehani K., Jope R.S., Michalek S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodgett J.R., Ohashi P.S. GSK3: An in-Toll-erant protein kinase? Nat. Immunol. 2005;6:751–752. doi: 10.1038/ni0805-751. [DOI] [PubMed] [Google Scholar]
- 31.Huang W.C., Lin Y.S., Wang C.Y., Tsai C.C., Tseng H.C., Chen C.L., Lu P.J., Chen P.S., Qian L., Hong J.S., et al. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology. 2009;128:e275–e286. doi: 10.1111/j.1365-2567.2008.02959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Huang W.C., Wang C.Y., Tsai C.C., Chen C.L., Chang Y.T., Kai J.I., Lin C.F. Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. Br. J. Pharmacol. 2009;157:1004–1013. doi: 10.1111/j.1476-5381.2009.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Y.L., Wang C.Y., Huang W.C., Tsai C.C., Chen C.L., Shen C.F., Chi C.Y., Lin C.F. Staphylococcus aureus induces microglial inflammation via a glycogen synthase kinase 3beta-regulated pathway. Infect. Immun. 2009;77:4002–4008. doi: 10.1128/IAI.00176-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C.F., Tsai C.C., Huang W.C., Wang C.Y., Tseng H.C., Wang Y., Kai J.I., Wang S.W., Cheng Y.L. IFN-gamma synergizes with LPS to induce nitric oxide biosynthesis through glycogen synthase kinase-3-inhibited IL-10. J. Cell. Biochem. 2008;105:746–755. doi: 10.1002/jcb.21868. [DOI] [PubMed] [Google Scholar]
- 35.Cuzzocrea S., Di Paola R., Mazzon E., Crisafulli C., Genovese T., Muia C., Abdelrahman M., Esposito E., Thiemermann C. Glycogen synthase kinase 3beta inhibition reduces the development of nonseptic shock induced by zymosan in mice. Shock. 2007;27:97–107. doi: 10.1097/01.shk.0000235084.56100.71. [DOI] [PubMed] [Google Scholar]
- 36.Ren F., Duan Z., Cheng Q., Shen X., Gao F., Bai L., Liu J., Busuttil R.W., Kupiec-Weglinski J.W., Zhai Y. Inhibition of glycogen synthase kinase 3 beta ameliorates liver ischemia reperfusion injury by way of an interleukin-10-mediated immune regulatory mechanism. Hepatology. 2011;54:687–696. doi: 10.1002/hep.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.J., Joe Y., Kong J.S., Jeong S.O., Cho G.J., Ryter S.W., Chung H.T. Carbon monoxide protects against hepatic ischemia/reperfusion injury via ROS-dependent Akt signaling and inhibition of glycogen synthase kinase 3beta. Oxid. Med. Cell. Longev. 2013;2013:306421. doi: 10.1155/2013/306421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L., Tassiulas I., Park-Min K.H., Reid A.C., Gil-Henn H., Schlessinger J., Baron R., Zhang J.J., Ivashkiv L.B. ‘Tuning’ of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat. Immunol. 2008;9:186–193. doi: 10.1038/ni1548. [DOI] [PubMed] [Google Scholar]
- 39.Yao Y., Wang L., Jin P., Li N., Meng Y., Wang C., Xu M., Zhang Y., Bian J., Deng X. Methane alleviates carbon tetrachloride induced liver injury in mice: Anti-inflammatory action demonstrated by increased PI3K/Akt/GSK-3beta-mediated IL-10 expression. J. Mol. Histol. 2017;48:301–310. doi: 10.1007/s10735-017-9728-1. [DOI] [PubMed] [Google Scholar]
- 40.Shinozaki K., Yahata H., Tanji H., Sakaguchi T., Ito H., Dohi K. Allograft transduction of IL-10 prolongs survival following orthotopic liver transplantation. Gene Ther. 1999;6:816–822. doi: 10.1038/sj.gt.3300881. [DOI] [PubMed] [Google Scholar]
- 41.Choi J.S., Jeong I.S., Han J.H., Cheon S.H., Kim S.W. IL-10-secreting human MSCs generated by TALEN gene editing ameliorate liver fibrosis through enhanced anti-fibrotic activity. Biomater. Sci. 2019;7:1078–1087. doi: 10.1039/C8BM01347K. [DOI] [PubMed] [Google Scholar]
- 42.Louis H., Le Moine O., Peny M.O., Quertinmont E., Fokan D., Goldman M., Deviere J. Production and role of interleukin-10 in concanavalin A-induced hepatitis in mice. Hepatology. 1997;25:1382–1389. doi: 10.1002/hep.510250614. [DOI] [PubMed] [Google Scholar]
- 43.Ren F., Zhou L., Zhang X., Wen T., Shi H., Xie B., Li Z., Chen D., Wang Z., Duan Z. Endoplasmic reticulum stress-activated glycogen synthase kinase 3beta aggravates liver inflammation and hepatotoxicity in mice with acute liver failure. Inflammation. 2015;38:1151–1165. doi: 10.1007/s10753-014-0080-2. [DOI] [PubMed] [Google Scholar]
- 44.Chen L., Ren F., Zhang H., Wen T., Piao Z., Zhou L., Zheng S., Zhang J., Chen Y., Han Y., et al. Inhibition of glycogen synthase kinase 3beta ameliorates D-GalN/LPS-induced liver injury by reducing endoplasmic reticulum stress-triggered apoptosis. PLoS ONE. 2012;7:e45202. doi: 10.1371/journal.pone.0045202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong J.H., Gong J.P., Li J.Z., He K., Li P.Z., Jiang X.W. Glycogen synthase kinase 3 inhibitor attenuates endotoxin-induced liver injury. J. Surg. Res. 2013;184:1035–1044. doi: 10.1016/j.jss.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H., Wang W., Fang H., Yang Y., Li X., He J., Jiang X., Wang W., Liu S., Hu J., et al. GSK-3beta inhibition attenuates CLP-induced liver injury by reducing inflammation and hepatic cell apoptosis. Mediat. Inflamm. 2014;2014:629507. doi: 10.1155/2014/629507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jellestad L., Fink T., Pradarutti S., Kubulus D., Wolf B., Bauer I., Thiemermann C., Rensing H. Inhibition of glycogen synthase kinase (GSK)-3-beta improves liver microcirculation and hepatocellular function after hemorrhagic shock. Eur. J. Pharmacol. 2014;724:175–184. doi: 10.1016/j.ejphar.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 48.Rocha J., Figueira M.E., Barateiro A., Fernandes A., Brites D., Pinto R., Freitas M., Fernandes E., Mota-Filipe H., Sepodes B. Inhibition of glycogen synthase kinase-3beta attenuates organ injury and dysfunction associated with liver ischemia-reperfusion and thermal injury in the rat. Shock. 2015;43:369–378. doi: 10.1097/SHK.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 49.Ren F., Zhang L., Zhang X., Shi H., Wen T., Bai L., Zheng S., Chen Y., Chen D., Li L., et al. Inhibition of glycogen synthase kinase 3beta promotes autophagy to protect mice from acute liver failure mediated by peroxisome proliferator-activated receptor alpha. Cell Death Dis. 2016;7:e2151. doi: 10.1038/cddis.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei L., Ren F., Zhang X., Wen T., Shi H., Zheng S., Zhang J., Chen Y., Han Y., Duan Z. Oxidative stress promotes D-GalN/LPS-induced acute hepatotoxicity by increasing glycogen synthase kinase 3 beta activity. Inflamm. Res. 2014;63:485–494. doi: 10.1007/s00011-014-0720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Deng M., Wu H., Wang M., Gong J., Bai H., Wu Y., Pan J., Chen Y., Li S. Suberoylanilide hydroxamic acid alleviates orthotopic liver transplantationinduced hepatic ischemiareperfusion injury by regulating the AKT/GSK3beta/NFkappaB and AKT/mTOR pathways in rat Kupffer cells. Int. J. Mol. Med. 2020;45:1875–1887. doi: 10.3892/ijmm.2020.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alhusaini A., Fadda L., Hasan I.H., Zakaria E., Alenazi A.M., Mahmoud A.M. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating Akt/GSK-3 beta signaling pathway. Biomolecules. 2019;9:703. doi: 10.3390/biom9110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdel-Emam R.A., Ali M.F. Effect of l-carnitine supplementation on lead acetate-induced liver cell apoptosis and inflammation: Role of caspase-3 and glycogen synthase kinase-3beta enzymes. Life Sci. 2022;291:120277. doi: 10.1016/j.lfs.2021.120277. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Y., Bao H., Ge Y., Tang W., Cheng D., Luo K., Gong G., Gong R. Therapeutic targeting of GSK3beta enhances the Nrf2 antioxidant response and confers hepatic cytoprotection in hepatitis C. Gut. 2015;64:168–179. doi: 10.1136/gutjnl-2013-306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang W., Jiang Y.F., Ponnusamy M., Diallo M. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. 2014;20:13079–13087. doi: 10.3748/wjg.v20.i36.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim S.H., Akazawa Y., Cazanave S.C., Bronk S.F., Elmi N.A., Werneburg N.W., Billadeau D.D., Gores G.J. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J. Hepatol. 2011;54:765–772. doi: 10.1016/j.jhep.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrero C., Hu X., Li W.P., Samuels S., Sharif M.N., Kotenko S., Ivashkiv L.B. Reprogramming of IL-10 activity and signaling by IFN-gamma. J. Immunol. 2003;171:5034–5041. doi: 10.4049/jimmunol.171.10.5034. [DOI] [PubMed] [Google Scholar]
- 58.Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 59.Boothby M. The calculus of integrating differentiation: Timing control of T-bet. Immunity. 2009;30:666–668. doi: 10.1016/j.immuni.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Siebler J., Wirtz S., Klein S., Protschka M., Blessing M., Galle P.R., Neurath M.F. A key pathogenic role for the STAT1/T-bet signaling pathway in T-cell-mediated liver inflammation. Hepatology. 2003;38:1573–1580. doi: 10.1016/j.hep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 61.Szabo S.J., Sullivan B.M., Stemmann C., Satoskar A.R., Sleckman B.P., Glimcher L.H. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 62.Townsend M.J., Weinmann A.S., Matsuda J.L., Salomon R., Farnham P.J., Biron C.A., Gapin L., Glimcher L.H. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/S1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 63.Schulz E.G., Mariani L., Radbruch A., Hofer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Shier P., Hofstra C.L., Ma X.J., Wu Y., Ngo K., Fung-Leung W.P. Tbt-1, a new T-box transcription factor induced in activated Th1 and CD8+ T cells. Immunogenetics. 2000;51:771–778. doi: 10.1007/s002510000212. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Fathman J.W., Lugo-Villarino G., Scimone L., von Andrian U., Dorfman D.M., Glimcher L.H. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J. Clin. Investig. 2006;116:414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng S.L., Szabo S.J., Glimcher L.H. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl. Acad. Sci. USA. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuda J.L., George T.C., Hagman J., Gapin L. Temporal dissection of T-bet functions. J. Immunol. 2007;178:3457–3465. doi: 10.4049/jimmunol.178.6.3457. [DOI] [PubMed] [Google Scholar]
- 68.Schoenborn J.R., Wilson C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 69.Jaruga B., Hong F., Kim W.H., Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: A critical role of IRF-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G1044–G1052. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 70.Hwang E.S., Hong J.H., Glimcher L.H. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J. Exp. Med. 2005;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang H., Rose J.L., Hoyt D.G. p38 Mitogen-activated protein kinase mediates synergistic induction of inducible nitric-oxide synthase by lipopolysaccharide and interferon-gamma through signal transducer and activator of transcription 1 Ser727 phosphorylation in murine aortic endothelial cells. Mol. Pharmacol. 2004;66:302–311. doi: 10.1124/mol.66.2.302. [DOI] [PubMed] [Google Scholar]
- 72.Held T.K., Weihua X., Yuan L., Kalvakolanu D.V., Cross A.S. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect. Immun. 1999;67:206–212. doi: 10.1128/IAI.67.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan E.D., Riches D.W. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 2001;280:C441–C450. doi: 10.1152/ajpcell.2001.280.3.C441. [DOI] [PubMed] [Google Scholar]
- 74.Koide N., Mu M.M., Hassan F., Islam S., Tumurkhuu G., Dagvadorj J., Naiki Y., Mori I., Yoshida T., Yokochi T. Lipopolysaccharide enhances interferon-gamma-induced nitric oxide (NO) production in murine vascular endothelial cells via augmentation of interferon regulatory factor-1 activation. J. Endotoxin Res. 2007;13:167–175. doi: 10.1177/0968051907080894. [DOI] [PubMed] [Google Scholar]
- 75.Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 76.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 77.Decker T., Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 78.Beurel E., Jope R.S. Differential regulation of STAT family members by glycogen synthase kinase-3. J. Biol. Chem. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Igarashi K., Garotta G., Ozmen L., Ziemiecki A., Wilks A.F., Harpur A.G., Larner A.C., Finbloom D.S. Interferon-gamma induces tyrosine phosphorylation of interferon-gamma receptor and regulated association of protein tyrosine kinases, Jak1 and Jak2, with its receptor. J. Biol. Chem. 1994;269:14333–14336. doi: 10.1016/S0021-9258(17)36621-8. [DOI] [PubMed] [Google Scholar]
- 80.Darnell J.E., Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 81.Wormald S., Hilton D.J. Inhibitors of cytokine signal transduction. J. Biol. Chem. 2004;279:821–824. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- 82.Yamada S., Shiono S., Joo A., Yoshimura A. Control mechanism of JAK/STAT signal transduction pathway. FEBS Lett. 2003;534:190–196. doi: 10.1016/S0014-5793(02)03842-5. [DOI] [PubMed] [Google Scholar]
- 83.Krebs D.L., Hilton D.J. SOCS: Physiological suppressors of cytokine signaling. Pt 16J. Cell Sci. 2000;113:2813–2819. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- 84.Yasukawa H., Sasaki A., Yoshimura A. Negative regulation of cytokine signaling pathways. Annu. Rev. Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 85.You M., Yu D.H., Feng G.S. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol. Cell. Biol. 1999;19:2416–2424. doi: 10.1128/MCB.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bennett A.M., Tang T.L., Sugimoto S., Walsh C.T., Neel B.G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc. Natl. Acad. Sci. USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu X., Paik P.K., Chen J., Yarilina A., Kockeritz L., Lu T.T., Woodgett J.R., Ivashkiv L.B. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 88.Coant N., Simon-Rudler M., Gustot T., Fasseu M., Gandoura S., Ragot K., Abdel-Razek W., Thabut D., Letteron P., Ogier-Denis E., et al. Glycogen synthase kinase 3 involvement in the excessive proinflammatory response to LPS in patients with decompensated cirrhosis. J. Hepatol. 2011;55:784–793. doi: 10.1016/j.jhep.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 89.Hu X., Chen J., Wang L., Ivashkiv L.B. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 2007;82:237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 90.Wang H., Brown J., Martin M. Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine. 2011;53:130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsai C.C., Kai J.I., Huang W.C., Wang C.Y., Wang Y., Chen C.L., Fang Y.T., Lin Y.S., Anderson R., Chen S.H., et al. Glycogen synthase kinase-3beta facilitates IFN-gamma-induced STAT1 activation by regulating Src homology-2 domain-containing phosphatase 2. J. Immunol. 2009;183:856–864. doi: 10.4049/jimmunol.0804033. [DOI] [PubMed] [Google Scholar]
- 92.Sayas C.L., Ariaens A., Ponsioen B., Moolenaar W.H. GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol. Biol. Cell. 2006;17:1834–1844. doi: 10.1091/mbc.e05-07-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takaoka A., Tanaka N., Mitani Y., Miyazaki T., Fujii H., Sato M., Kovarik P., Decker T., Schlessinger J., Taniguchi T. Protein tyrosine kinase Pyk2 mediates the Jak-dependent activation of MAPK and Stat1 in IFN-gamma, but not IFN-alpha, signaling. EMBO J. 1999;18:2480–2488. doi: 10.1093/emboj/18.9.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gough D.J., Levy D.E., Johnstone R.W., Clarke C.J. IFNgamma signaling-does it mean JAK-STAT? Cytokine Growth Factor Rev. 2008;19:383–394. doi: 10.1016/j.cytogfr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Patel T. Apoptosis in hepatic pathophysiology. Clin. Liver Dis. 2000;4:295–317. doi: 10.1016/S1089-3261(05)70112-4. [DOI] [PubMed] [Google Scholar]
- 96.Dong Z., Wei H., Sun R., Tian Z. The roles of innate immune cells in liver injury and regeneration. Cell. Mol. Immunol. 2007;4:241–252. [PubMed] [Google Scholar]
- 97.Zheng Z.Y., Weng S.Y., Yu Y. Signal molecule-mediated hepatic cell communication during liver regeneration. World J. Gastroenterol. WJG. 2009;15:5776–5783. doi: 10.3748/wjg.15.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tiegs G., Hentschel J., Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sass G., Heinlein S., Agli A., Bang R., Schumann J., Tiegs G. Cytokine expression in three mouse models of experimental hepatitis. Cytokine. 2002;19:115–120. doi: 10.1006/cyto.2002.1948. [DOI] [PubMed] [Google Scholar]
- 100.Takeda K., Hayakawa Y., Van Kaer L., Matsuda H., Yagita H., Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc. Natl. Acad. Sci. USA. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kusters S., Gantner F., Kunstle G., Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology. 1996;111:462–471. doi: 10.1053/gast.1996.v111.pm8690213. [DOI] [PubMed] [Google Scholar]
- 102.Mizuhara H., O’Neill E., Seki N., Ogawa T., Kusunoki C., Otsuka K., Satoh S., Niwa M., Senoh H., Fujiwara H. T cell activation-associated hepatic injury: Mediation by tumor necrosis factors and protection by interleukin 6. J. Exp. Med. 1994;179:1529–1537. doi: 10.1084/jem.179.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hatada S., Ohta T., Shiratsuchi Y., Hatano M., Kobayashi Y. A novel accessory role of neutrophils in concanavalin A-induced hepatitis. Cell. Immunol. 2005;233:23–29. doi: 10.1016/j.cellimm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 104.Kaneko Y., Harada M., Kawano T., Yamashita M., Shibata Y., Gejyo F., Nakayama T., Taniguchi M. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J. Exp. Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toyabe S., Seki S., Iiai T., Takeda K., Shirai K., Watanabe H., Hiraide H., Uchiyama M., Abo T. Requirement of IL-4 and liver NK1+ T cells for concanavalin A-induced hepatic injury in mice. J. Immunol. 1997;159:1537–1542. [PubMed] [Google Scholar]
- 106.Tsai C.C., Huang W.C., Chen C.L., Hsieh C.Y., Lin Y.S., Chen S.H., Yang K.C., Lin C.F. Glycogen synthase kinase-3 facilitates con a-induced IFN-gamma—Mediated immune hepatic injury. J. Immunol. 2011;187:3867–3877. doi: 10.4049/jimmunol.1100770. [DOI] [PubMed] [Google Scholar]
- 107.Carambia A., Herkel J. CD4 T cells in hepatic immune tolerance. J. Autoimmun. 2010;34:23–28. doi: 10.1016/j.jaut.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 108.Bonder C.S., Ajuebor M.N., Zbytnuik L.D., Kubes P., Swain M.G. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 2004;172:45–53. doi: 10.4049/jimmunol.172.1.45. [DOI] [PubMed] [Google Scholar]
- 109.Hatano M., Sasaki S., Ohata S., Shiratsuchi Y., Yamazaki T., Nagata K., Kobayashi Y. Effects of Kupffer cell-depletion on concanavalin A-induced hepatitis. Cell. Immunol. 2008;251:25–30. doi: 10.1016/j.cellimm.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 110.Tiegs G., Gantner F. Immunotoxicology of T cell-dependent experimental liver injury. Exp. Toxicol. Pathol. 1996;48:471–476. doi: 10.1016/S0940-2993(96)80058-3. [DOI] [PubMed] [Google Scholar]
- 111.Tagawa Y., Kakuta S., Iwakura Y. Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. Eur. J. Immunol. 1998;28:4105–4113. doi: 10.1002/(SICI)1521-4141(199812)28:12<4105::AID-IMMU4105>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 112.Gantner F., Leist M., Lohse A.W., Germann P.G., Tiegs G. Concanavalin A-induced T-cell-mediated hepatic injury in mice: The role of tumor necrosis factor. Hepatology. 1995;21:190–198. doi: 10.1016/0270-9139(95)90428-x. [DOI] [PubMed] [Google Scholar]
- 113.Tagawa Y., Sekikawa K., Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(-/-) mice, but not in TNF-alpha(-/-) mice: Role for IFN-gamma in activating apoptosis of hepatocytes. J. Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 114.Wu Z., Han M., Chen T., Yan W., Ning Q. Acute liver failure: Mechanisms of immune-mediated liver injury. Liver Int. 2010;30:782–794. doi: 10.1111/j.1478-3231.2010.02262.x. [DOI] [PubMed] [Google Scholar]
- 115.Mita A., Hashikura Y., Tagawa Y., Nakayama J., Kawakubo M., Miyagawa S. Expression of Fas ligand by hepatic macrophages in patients with fulminant hepatic failure. Am. J. Gastroenterol. 2005;100:2551–2559. doi: 10.1111/j.1572-0241.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 116.Kai J.I., Huang W.C., Tsai C.C., Chang W.T., Chen C.L., Lin C.F. Glycogen synthase kinase-3beta indirectly facilitates interferon-gamma-induced nuclear factor-kappaB activation and nitric oxide biosynthesis. J. Cell. Biochem. 2010;111:1522–1530. doi: 10.1002/jcb.22881. [DOI] [PubMed] [Google Scholar]
- 117.Eldar-Finkelman H. Glycogen synthase kinase 3: An emerging therapeutic target. Trends Mol. Med. 2002;8:126–132. doi: 10.1016/S1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- 118.Dugo L., Collin M., Thiemermann C. Glycogen synthase kinase 3beta as a target for the therapy of shock and inflammation. Shock. 2007;27:113–123. doi: 10.1097/01.shk.0000238059.23837.68. [DOI] [PubMed] [Google Scholar]
- 119.Seino K., Kayagaki N., Takeda K., Fukao K., Okumura K., Yagita H. Contribution of Fas ligand to T cell-mediated hepatic injury in mice. Gastroenterology. 1997;113:1315–1322. doi: 10.1053/gast.1997.v113.pm9322527. [DOI] [PubMed] [Google Scholar]
- 120.Morita M., Watanabe Y., Akaike T. Protective effect of hepatocyte growth factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology. 1995;21:1585–1593. [PubMed] [Google Scholar]
- 121.Kano A., Haruyama T., Akaike T., Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem. Biophys. Res. Commun. 1999;257:672–677. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 122.Kano A., Watanabe Y., Takeda N., Aizawa S., Akaike T. Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. J. Biochem. 1997;121:677–683. doi: 10.1093/oxfordjournals.jbchem.a021639. [DOI] [PubMed] [Google Scholar]
- 123.Horras C.J., Lamb C.L., Mitchell K.A. Regulation of hepatocyte fate by interferon-gamma. Cytokine Growth Factor Rev. 2011;22:35–43. doi: 10.1016/j.cytogfr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vodovotz Y., Kim P.K., Bagci E.Z., Ermentrout G.B., Chow C.C., Bahar I., Billiar T.R. Inflammatory modulation of hepatocyte apoptosis by nitric oxide: In vivo, in vitro, and in silico studies. Curr. Mol. Med. 2004;4:753–762. doi: 10.2174/1566524043359944. [DOI] [PubMed] [Google Scholar]
- 125.Lee H.J., Oh Y.K., Rhee M., Lim J.Y., Hwang J.Y., Park Y.S., Kwon Y., Choi K.H., Jo I., Park S.I., et al. The role of STAT1/IRF-1 on synergistic ROS production and loss of mitochondrial transmembrane potential during hepatic cell death induced by LPS/d-GalN. J. Mol. Biol. 2007;369:967–984. doi: 10.1016/j.jmb.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 126.Schwabe R.F., Brenner D.A. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- 127.Hatano E., Bennett B.L., Manning A.M., Qian T., Lemasters J.J., Brenner D.A. NF-kappaB stimulates inducible nitric oxide synthase to protect mouse hepatocytes from TNF-alpha- and Fas-mediated apoptosis. Gastroenterology. 2001;120:1251–1262. doi: 10.1053/gast.2001.23239. [DOI] [PubMed] [Google Scholar]
- 128.Kandar C.C., Sen D., Maity A. Anti-inflammatory potential of GSK-3 inhibitors. Curr. Drug Targets. 2021;22:1464–1476. doi: 10.2174/1389450122666210118150313. [DOI] [PubMed] [Google Scholar]
- 129.Cortes-Vieyra R., Silva-Garcia O., Gomez-Garcia A., Gutierrez-Castellanos S., Alvarez-Aguilar C., Baizabal-Aguirre V.M. Glycogen synthase kinase 3 beta modulates the inflammatory response activated by bacteria, viruses, and parasites. Front. Immunol. 2021;12:675751. doi: 10.3389/fimmu.2021.675751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roca C., Campillo N.E. Glycogen synthase kinase 3 (GSK-3) inhibitors: A patent update (2016–2019) Expert Opin. Ther. Patents. 2020;30:863–872. doi: 10.1080/13543776.2020.1815706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this manuscript are available from the corresponding author upon reasonable request.