Abstract
Glyphosate, a non-selective systemic biocide with broad-spectrum activity, is the most widely used herbicide in the world. It can persist in the environment for days or months, and its intensive and large-scale use can constitute a major environmental and health problem. In this systematic review, we investigate the current state of our knowledge related to the effects of this pesticide on the nervous system of various animal species and humans. The information provided indicates that exposure to glyphosate or its commercial formulations induces several neurotoxic effects. It has been shown that exposure to this pesticide during the early stages of life can seriously affect normal cell development by deregulating some of the signaling pathways involved in this process, leading to alterations in differentiation, neuronal growth, and myelination. Glyphosate also seems to exert a significant toxic effect on neurotransmission and to induce oxidative stress, neuroinflammation and mitochondrial dysfunction, processes that lead to neuronal death due to autophagy, necrosis, or apoptosis, as well as the appearance of behavioral and motor disorders. The doses of glyphosate that produce these neurotoxic effects vary widely but are lower than the limits set by regulatory agencies. Although there are important discrepancies between the analyzed findings, it is unequivocal that exposure to glyphosate produces important alterations in the structure and function of the nervous system of humans, rodents, fish, and invertebrates.
Keywords: glyphosate, glyphosate-based herbicides (GBH), neurotoxic effects, human, rodent, fish
1. Introduction
Glyphosate, N-(phosphonomethyl) glycine is the most widely used herbicide in the world [1]. It is a non-selective systemic biocide with broad-spectrum activity and was introduced in 1974 for the control of weeds in agricultural production fields [2]. The widespread use of glyphosate in agriculture and forestry has contributed to the development of numerous commercial formulations containing this compound. Herbicide formulations containing this active ingredient represent approximately 60% of the global market for non-selective herbicides [3].
In recent years, the use of glyphosate has spread worldwide due to the development of glyphosate-resistant crops. The main driver of the enormous success of this technology has been the economic benefits obtained in the agricultural sector after the introduction of genetically modified crops [4]. This has contributed to the positioning of glyphosate-based herbicides (GBH) as leaders in the global pesticide market. Thus, around 600.000 to 750.000 tons of glyphosate are used each year, and it is estimated that its use will increase reaching between 740.000 and 920.000 tons by 2025 [5].
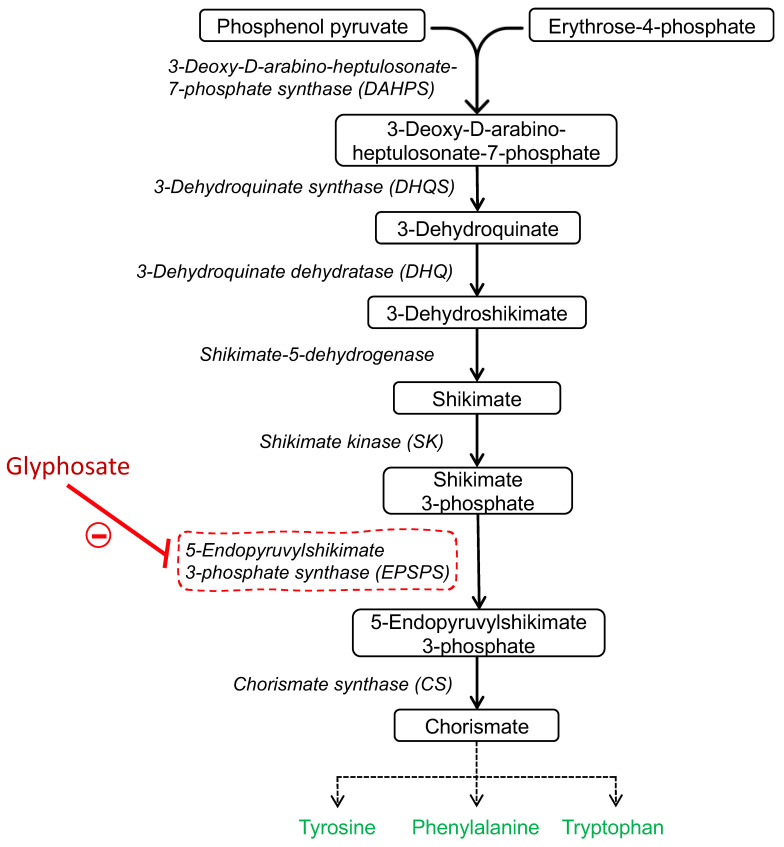
The mechanism of action of glyphosate is associated with its ability to block the shikimic acid pathway, which is involved in the synthesis of aromatic amino acids in plants, fungi, and some microorganisms [6]. Glyphosate inhibits the enzyme 5-enolpyruvilshikimate-3-phosphate synthase, which is the penultimate step in the shikimate pathway [7]. This inhibition leads to a reduction in the synthesis of the aromatic amino acids tyrosine, phenylalanine, and tryptophan, as well as a decrease in protein synthesis [8] (Figure 1). Therefore, blocking this metabolic pathway eventually causes the death of the target organism within a few days [9].
Figure 1.
Glyphosate inhibits the enzymatic activity of the 5-endopyruvylshikimate 3-phosphate synthase (EPSPS) in the shikimic acid pathway, preventing the synthesis of the aromatic amino acids tyrosine, phenylalanine, and tryptophan.
The absence of the shikimate pathway in animals has led to the conclusion that GBH does not pose a health risk to animals and humans [10]. Moreover, many investigations on glyphosate toxicity in animals have suggested the low toxicity of this compound, the adverse effects of which have only been observed after exposure to relatively high doses [8,11,12]. These data led to the classification of glyphosate in the least toxic category (category IV, practically non-toxic and non-irritating) by the United States Environmental Protection Agency (EPA) [13,14].
In general, in the different environmental compartments, glyphosate is mainly degraded by microorganisms, so that its persistence is considered to be low to moderate, although it is considerably variable. On the one hand, although glyphosate is assumed to be readily degraded in soil, its biodegradation is influenced by numerous factors, including physico-chemical, biological properties and soil composition [15]. Thus, the half-life of glyphosate in soil can range from 1 to 280 days, while that of aminomethylphosphonic acid (AMPA), its main metabolite, ranges from 23 to 958 days [16,17,18]. In soil, glyphosate can bind strongly to its constituent particles and remain biologically inactive, or it can reach groundwater, due to its high water solubility [19]. However, repeated applications of glyphosate have been shown to result in a gradual difficulty for its biodegradation in the soil, which could increase the risk of groundwater contamination [20,21]. In water, the permanence of glyphosate is also widely variable and depends on factors such as light and temperature, being more persistent and toxic under conditions of darkness and higher water temperatures [22]. In general, the half-life of glyphosate in water varies from a few days to 91 days [23,24], although it has been found to remain for up to 315 days in marine waters [25]. On the other hand, while the persistence of glyphosate in vegetation may be only days, several studies have detected its presence in many foods and crops even a year after application [26,27].
Therefore, although the concentrations of glyphosate residues that persist over time are relatively low, it is possible that due to extensive use on a large scale they may accumulate and become a risk to animal and human health, as they are chronically exposed to residues in the water and food they consume [23,28,29]. This has been confirmed by the detection of glyphosate in the organs and urine of a high proportion of farm animals and farmers [30,31,32,33]. In addition, residues were also found in the urine of 60–80% of the general population in the United States at medium and maximum concentrations of 2–3 and 233 μg/L, respectively. In Europe, residues were also detected in the urine of 44% of the population, although their average and maximum concentrations were lower: <1 and 5 μg/L, respectively [9,28].
Recently, data on glyphosate contamination in the environment suggest that acute toxicity may not be as relevant as toxicity from chronic exposure to lower concentrations of this compound. Therefore, the number of publications demonstrating the chronic toxicity of glyphosate in animals and humans has increased considerably. This has led to increased concern about the potential harmful side effects that chronic exposure to glyphosate could have on animal and human health. Glyphosate has recently received increasing attention from the scientific community and from national and international regulatory agencies. Based on research on the chronic side effects of glyphosate, in 2015, the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) reclassified glyphosate as probably carcinogenic to humans. Nevertheless, IARC’s conclusion has not been confirmed by European Union assessment or the recent joint assessment by the Food and Agriculture Organization of the United Nations (FAO)/OMS [34]. This highlights the existence of significant disagreement between regulatory agencies, as well as the need to reach consensus and update safety standards for glyphosate in order to protect public health and the environment.
Numerous commercial formulations of glyphosate contain several adjuvants, which improve the penetration of the active ingredient into the target plants and increase their efficacy [35,36]. It has been postulated that the activity of GBH is not exclusively due to the active ingredient but could be due to the intrinsic toxicity of the adjuvants or the possible synergy between glyphosate and the other ingredients of the formulation [37]. In fact, polyethoxylated tallow amine, the predominant surfactant in several commercial formulations, has been found to increase glyphosate-induced toxicity by facilitating its penetration through plasma membranes [38,39,40,41,42]. Therefore, it is important for research to evaluate the toxicity of both commercial formulations and pure glyphosate [43].
Taken together, above information shows that the intensive and widescale use of glyphosate can constitute a major environmental and health problem. In recent years, there has been an increase in the number of scientific publications dealing with the possible side effects of glyphosate and its commercial formulations. Nevertheless, a full review of research articles on the effects of glyphosate on the nervous system is still needed.
Therefore, in the present study, we carry out a systematic review of the scientific literature available on the effects and mechanisms of action of glyphosate and its commercial formulations on the nervous system of various animal species and humans. The main objective of this review is to understand the risks arising from increasing exposure to glyphosate residues in the environment and in food.
2. Methodology
The present review was carried out with the aim of unifying the results of the most recent studies on the effects of glyphosate and its commercial formulations on animal health. For this purpose, a systemic review was performed following the guidelines established by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [44]. Searches were carried out in the specialized databases PubMed, Scopus, and Web of Science in February 2022, with a time restriction limited to studies published in the last ten years.
The following terms referring to glyphosate and its commercial formulations were used to select the scientific articles to be included in this review: “glyphosate”, “Roundup”, and “GBH”. Each of the three terms mentioned above was entered into the databases according to the following search strategies: “((glyphosate OR Roundup OR GBH) AND (nervous system))”; “((glyphosate OR Roundup OR GBH) AND (neurotoxicity))”; “((glyphosate OR Roundup OR GBH) AND (nervous system) AND (effects))”; ((glyphosate OR Roundup OR GBH) AND (nervous system) AND (toxicity))”.
Exclusion and Inclusion Criteria
The articles included in this review met the following inclusion criteria: (1) original studies in article format; (2) in English or Spanish; and (3) studying the effects of the pesticide on the nervous system. Articles were excluded according to the following exclusion criteria: (1) theoretical articles or reviews; (2) studies in which glyphosate was administered in combination with other pesticides; (3) studies evaluating the effects of pesticides other than glyphosate; (4) case studies; and (5) studies that used glyphosate doses above the no-observed-adverse-effect levels (NOAEL) established by regulatory agencies.
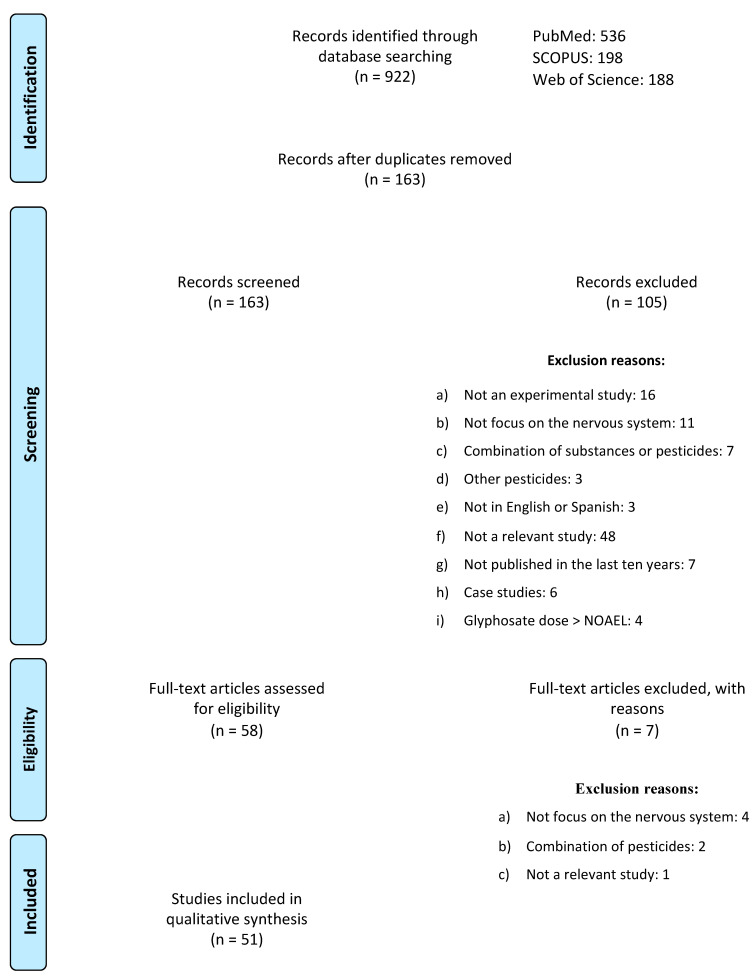
As a result of the searches carried out in the three databases, 922 articles published in the last ten years were identified. The articles were then exported to Refworks to eliminate duplicates. In accordance with the inclusion and exclusion criteria, 163 titles and abstracts were screened to verify whether they met the previously mentioned criteria. After this procedure, 112 articles were excluded for the reasons summarized in Figure 2. Ultimately, 51 articles were included in the present systematic review.
Figure 2.
Flow diagram of the systematic search process.
3. Results
3.1. Effects of Glyphosate in Humans
A series of studies show that glyphosate and its commercial formulations can produce detrimental effects on the human nervous system. These investigations have shown that glyphosate can cross and affect the blood–brain barrier (BBB) and cause various types of short-term or long-term disturbances in the human nervous system (Table 1).
Table 1.
Studies on the effects of glyphosate and/or its commercial formulations in humans.
| Type of Study | Toxic Agent | Exposure Mode/Objetives | Results | Reference |
|---|---|---|---|---|
| Transversal study | GBH | Occupational exposure |
|
[45] |
| Prospective cohort study | GBH | Not specified |
|
[46] |
| Population-based case-control study | GBH | Occupational exposure |
|
[47] |
| Cohort study | GBH | Occupational exposure |
|
[48] |
| Cohort study | GBH | Occupational exposure |
|
[49] |
| In vitro SH-SY5Y cell line |
GLY alone or mixed with other formulants: 5.33 to 3.200 μg/mL for 24 h | Investigate whether GBH toxicity is related to formulants |
|
[50] |
| In vitro IMR90-c4 iPSCs line |
GLY, AMPA: 0.1 to 1000 μM for 24 or 48 h | Investigate the effect of GLY on the BBB in vitro and compare it with that of AMPA and glycine |
|
[51] |
| In vitro SH-SY5Y cell line |
GLY, AMPA: 0.1 to 20 mM for 48 h | Investigate the effects of GLY and AMPA on oxidative stress, neurodevelopment, and cell death. |
|
[52] |
Abbreviations: GBH, glyphosate-based herbicide; ↑, increase; S100B, S100 calcium-binding protein B; GLY, glyphosate; iPSCs, induced pluripotent stem cells; AMPA, aminomethylphosphonic acid; BBB, blood–brain barrier; ↓, decrease; LDH, lactate dehydrogenase; MDA, malondialdehyde; NO, nitric oxide; ROS, reactive oxygen species; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; CAMK2, Ca2+/calmodulin-dependent protein kinase 2.
3.1.1. Descriptive and Analytical Studies
Although most of studies in humans mainly describe the consequences of glyphosate poisoning after suicide attempts, it appears that occupational or chronic exposure to this pesticide (via inhalation and dermal routes) may also cause neurotoxic effects. In a study by Fuhrimann et al. [45], the authors describe that glyphosate exposure has been associated with the development of visual memory impairment in Ugandan smallholder farmers. However, other studies have not found an association between occupational exposure to glyphosate and increased risk of health problems, such as nerve conduction abnormalities [48,49]. These results have led some authors to postulate that glyphosate is less toxic to farmers’ health than other pesticides. Therefore, future research is needed to follow up and compare the potential toxic effects that agricultural use of different pesticides may exert on human health.
Another consideration about the effects of glyphosate is the fact that its effects do not appear immediately after exposure but one or two days later. In this sense, Lee et al. [46] found that S100 calcium-binding protein B (S100B) could be an important predictor of neurological complications in patients poisoned with glyphosate because its levels were increased in the group that was exposed to this substance and that presented neurological alterations. S100B levels peaked on the second day after exposure, indicating that glyphosate can reach the brain parenchyma and cause maximum brain damage after some time [46].
3.1.2. In Vitro Studies with Human Line Cells
The ability of glyphosate to cross the BBB was also reported in an in vitro study by Martínez and Al-Ahmad [51]. In this study, the authors observed that both glyphosate and its metabolite AMPA can increase BBB permeability, possibly by interfering with the proteins that mediate the hermetic junctions between the endothelial cells that comprise the BBB. This study also showed that glucose uptake by brain endothelial cells increased after exposure to high doses of glyphosate [51]. Glucose is the main source of energy for the brain, and its entry into the central nervous system (CNS) is mediated by microvascular endothelial cells. Therefore, increased availability of glucose after exposure to glyphosate could alter the metabolic activity of neurons, as seen in a study by Martínez and Al-Ahmad [51]. This investigation also documented a decrease in cellular metabolism that did not appear to be related to neurotoxicity, as neurite density and formation were not affected by treatment with glyphosate.
Exposure to glyphosate appears to affect neuronal development in the human CNS, altering the expression of molecules involved in the growth and maturation of neurons. GBH administration has been shown to alter the proliferation of cells in culture, although this effect did not occur when glyphosate was administered alone [50]. In addition, exposure to glyphosate and its metabolite induced a negative regulation in the expression of the TUBB3 and GAP43 genes, which are responsible for the synthesis of neuronal cytoskeleton proteins and axonal growth cones, respectively [52].
Likewise, exposure to glyphosate induced an increase in the levels of mRNA-Wnt3a, -Wnt5a, and -Wnt7a, which are also related to the regulation of neuronal development [52]. Given that the components of the Wnt signaling pathways are expressed in a strictly controlled manner during development, their deregulation by glyphosate could promote neuronal morphological defects and cause changes in the correct neuronal development [53,54].
In line with this, there is evidence of the participation of Wnt signaling in various neurocognitive developmental disorders, such as autism [55,56]. Thus, the deregulation of this pathway by glyphosate in human cells in vitro could be related to a higher incidence of developmental and autism spectrum disorders in children whose mothers were exposed to pesticides [57,58,59], including glyphosate, during pregnancy [47].
Martínez and colleagues [52] also showed that glyphosate increased the mRNA expression of the two isoforms of calcium-calmodulin-dependent protein kinase 2 (CAMK2A and CAMK2B). The product of these genes appears to have a dual role, as, although they are related to neuronal development and survival, they also contribute to regulation of neuronal death in response to a variety of insults [60,61]. Thus, the increased expression of this glyphosate-induced mRNA could be related to neuronal apoptosis triggered in response to oxidative stress. However, according to a study by Martínez et al. [52], AMPA decreased the expression levels of this mRNA, which led the authors to suggest that glyphosate metabolism could partially prevent neuronal death.
Another effect observed in a study by Martínez et al. [52] is an increase in oxidative stress, evidenced as an increase in the production of reactive oxygen species (ROS) and nitric oxide (NO), as well as lipid peroxidation (LPO). In addition, glyphosate and its metabolite also potentiated an inflammatory response by upregulating the expression of the proinflammatory cytokine interleukin 6 (IL-6) genes and tumor necrosis factor-alpha (TNF-α).
As mentioned previously, injuries caused by glyphosate, such as neuroinflammation or oxidative stress, can cause neuronal death. It was shown that both glyphosate and AMPA reduced the viability of human cells and increased the leakage of lactate dehydrogenase (LDH) [52]. LDH is involved in energy production, is found in almost every organ in the body (including the brain), and, when an organ or tissue is damaged, is released into the blood. Thus, glyphosate-induced LDH increases could be indicative of damage caused by the herbicide in the CNS. Likewise, there was also an upregulation in the gene expression of the different pathways of cell death (apoptosis, autophagy, and necrosis), including caspases 3 and 7, involved in the execution of apoptosis [52]. These results corroborate the possible involvement of mechanisms of apoptosis, autophagy, and necrosis in glyphosate-induced cell death in humans.
3.2. Effects of Glyphosate in Rodents
Several studies show that exposure to glyphosate or GBH produces many toxic effects on both the CNS and peripheral nervous system (PNS) of rodents. The main effects observed include changes in the development of the nervous system and in the neurotransmission systems, as well as oxidative stress and neuroinflammation, processes that lead to neuronal death and the appearance of behavioral changes (Table 2 and Table 3). Most of these studies show the neurotoxic effects of glyphosate administered at early ages during the intrauterine period and lactation, although chronic or acute exposure in adulthood also causes important alterations in the function and structure of the nervous system.
Table 2.
In vivo effects of glyphosate and/or its commercial formulations in rodents.
| Species | Dose and Exposition | Time Exposition | Objectives | Results | Reference |
|---|---|---|---|---|---|
| Swiss mice | Roundup®: 250 or 500 mg/kg/day orally | Subchronic exposition: 6 weeks Chronic exposition: 12 weeks |
Assess the effects of acute or repeated GBH exposure on the developing brain of young and adult mice | Chronic/subchronic exposure:
|
[62] |
| Swiss mice | Roundup®: 250 or 500 mg/kg/day orally | Subchronic exposition: 6 weeks Chronic exposition: 12 weeks |
Evaluate the effects of GBH on learning and memory functions, AChE activity, and oxidation/antioxidation homeostasis | Chronic/subchronic exposure:
|
[63] |
| Swiss mice | Roundup®: 250 or 500 mg/kg/day orally | From GD0 to PND21 | Evaluate the behavioral (PND5-PND25) and biochemical (PND60) effects of gestational and lactational exposure to GBH on offspring |
|
[64] |
| CF-1 mice | Glifloglex®: 50 mg/kg/day intranasally | Three days a week for four weeks | Assess the neurobehavioral effects of repeated intranasal administration of a GBH |
|
[65] |
| Wistar rats | Roundup®: 70 mg/kg/day orally | Chronic exposition: from GD5 to PND15. Acute exposition: 30 min in vitro |
Determine the neurotoxic effects of GBH on the hippocampal function of immature rats after chronic exposure (pregnancy and lactation) and after acute in vitro exposition. | Acute in vitro exposition:
|
[37] |
| Wistar rats | Roundup®: 1% in drinking water (0.38% GLY) | Subchronic exposition: from GD5 to PND21. Chronic exposition: from GD5 to PND60. |
Investigate the effects of subchronic exposure to GBH on neurochemical and behavioral parameters in immature and adult offspring |
|
[3] |
| Wistar rats | Roundup®: 70 mg/kg/day orally | Subchronic exposition: from GD5 to PND15. | Investigate possible biochemical and cell-persistent effects in the brain of adult rats following perinatal exposure to GBH |
|
[66] |
| Wistar rats | GLY: 24 or 35 mg/kg intraperitoneally | Dams received injections every 48 h from GD8 to GD20, totaling seven injections over two weeks | Evaluate the neurobehavioral effects of GLY in neonate rats after gestational exposure |
|
[67] |
| Sprague-Dawley rats | GLY, Roundup®: 5 mg/kg/day orally | From GD10 to PND22 | Compare the potential effects of a low dose of GLY and GBH on maternal behavior and maternal neuroplasticity, focusing on the hippocampus and cingulate gyrus |
|
[68] |
| CF-1 mice | Glifloglex®: 50 mg/kg/day intranasally | Four weeks (three injections per week) | Elucidate the mechanisms by which the intranasal administration of a GBH exerts its neuropathological effects |
|
[69] |
| Sprague-Dawley rats | GLY: 50, 100, or 150 mg/kg intraperitoneally | Two weeks (three injections per week) | Assess the integrity of the nigrostriatal and mesolimbic dopaminergic systems and their relationship with spontaneous locomotor activity after repeated or acute exposure to GLY |
|
[70] |
| ICR mice | Roundup®: 50 mg/kg/day orally | From GD14 to PND7 | Assess the miRNA expression patterns in the PFC of mouse offspring after exposure to GBH during pregnancy and lactation |
|
[71] |
| Balb/c mice | Roundup®: 25, 50 or 100 mg/kg orally | Acute exposure | Investigate the behavioral effects induced by acute exposure to a GBH in increasing doses |
|
[72] |
| Wistar rats | GBH: 2.5, 5, 10, 20 or 40 mM | Single dose | Assess the inhibitory potency of a GBH on AChE activity in rat tissues |
|
[73] |
| Wistar rats | GLY: 35 or 70 mg/kg subcutaneous injection | From PND7 to PND27 | Evaluate the effects of glyphosate on hippocampal synapses and cognitive functioning |
|
[74] |
| Wistar rats | GLY: 35, 75, 150 or 800 mg/kg/day orally | Six days | Determine the effects of GLY on the levels of DA, NE, and 5-HT and their metabolites, as well as the turnover in striatum, hippocampus, PFC, hypothalamus, and midbrain. |
|
[13] |
Abbreviations: GBH, glyphosate-based herbicide; ↓, decrease; ↑, increase; 5-HT, serotonin; TH, tyrosine hydroxylase; AChE, acetylcholinesterase; SOD, superoxide dismutase; GD, gestational day; PND, postnatal day; TNF-α, tumor necrosis factor alpha; BDNF, brain-derived neurotrophic factor; TrkB, tyrosine-related kinase receptor B; NMDAR: N-methyl-D-aspartate receptor; L-VDCC, voltage-dependent calcium channels; CaMKII, Ca2+/calmodulin-dependent protein kinase II; ERK, extracellular signal-regulated kinases; LPO, lipid peroxidation; GGT, gamma-glutamyl transferase; G6PD, glucose-6-phosphate dehydrogenase; GSH, glutathione; C-MeAIB, C-methylaminoisobutyric acid; GLY, glyphosate; GST, glutathione S-transferase; NF-kB, nuclear factor-kB; S100B, S100 calcium-binding protein B; SN, substantia nigra; CAT, catalase; ChAT, choline acetyltransferase; nAChRs, nicotinic acetylcholine receptors; GPT, glutamate-pyruvate transaminase; GOT, glutamate-oxaloacetate transaminase; PFC, prefrontal cortex; DA, dopamine; NE, noradrenaline; 5-HIAA, 5-hydroxyindoleacetic acid; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; MHPG, methoxy-4-hydroxyphenylglycol.
Table 3.
In vitro effects of glyphosate and/or its commercial formulations in rodents.
| Cellular Line | Dose and Time of Exposure | Objectives | Results | Reference |
|---|---|---|---|---|
| PC12 cells | GLY: 0, 5, 10, 20, or 40 mM for 12, 24, 48, or 72 h | Investigate the neurotoxicity of GLY in differentiated rat PC12 cells and explore the role of apoptosis and autophagy pathways in toxicity |
|
[75] |
| Hippocampal pyramidal cells | GLY: 0.5 or 1 mg/mL for five or ten days | Examine the effects of glyphosate on synapse formation and maturation in the hippocampus |
|
[74] |
| NSC | GLY: 0.1, 700, 7000, or 36,000 μg/L for 24 h | Understand the effects of two maximum permissible concentrations of GLY on the basic processes of neurogenesis in NSCs of the postnatal mouse subventricular zone. |
|
[76] |
| Astroglioma (C6) | GLY: concentrations from 0 to 160 mM for 24 h | Determine the activity of enzymes related to energy metabolism, as well as parameters of oxidative stress, mitochondrial mass, nuclear area, and autophagy in astrocytes treated with GBH |
|
[77] |
| Embryonic DRG and pure Schwann cells | GLY, Roundup®: 0.0005% and 0.005% for ten days (DRG) or 72 h (Schwann cells) | Investigate the effects of pure GLY and GBH in murine embryonic DRG cultures |
|
[78] |
| Embryonic DRG and pure Schwann cells | GLY, Roundup®: doses not specified for ten days (DRG) or 72 h (Schwann cells) | Study and compare the effects of pure GLY and GBH in murine embryonic DRG explant cultures |
|
[79] |
Abbreviations: PC-12, pheochromocytoma; GLY, glyphosate; ↓, decrease; NSC, neural stem cells; ↑, increase; SOD, superoxide dismutase; GBH, glyphosate-based herbicide; CK, creatine kinase; DRG, dorsal root ganglia; TNF-α, tumor necrosis factor alpha; NO, nitric oxide.
3.2.1. Development of Nervous System
Data from in vitro and ex vivo studies show that exposure to glyphosate in the early stages of neuronal development induces dysregulation of various signaling pathways and cascades, leading to delayed neuronal differentiation, growth, migration, and myelination processes in both the CNS and PNS during this period [76,78,79]. Coullery et al. [67] showed that glyphosate downregulates the Wnt5a/CaMKII signaling pathway in embryonic hippocampal neurons, a cascade that controls neuronal circuit formation and integration. Similarly, Luna et al. [74] found that glyphosate administered in the early postnatal stage reduced the expression of CAMKII in the hippocampus. Because of this deregulation, glyphosate would alter the process of neuronal differentiation and the subsequent formation of synaptic connections between hippocampal neurons [80]. This was verified by the team of Luna et al. [74], who observed that glyphosate administration was associated with a decrease in dendritic complexity and synapse formation in hippocampal neurons. However, Cattani et al. [37] observed a different effect, as GBH exposure activated the CaMKII pathway. These authors related CaMKII activation to the increase in glutamate-induced Ca2+ influx in the hippocampus of GBH-treated animals. The difference between studies may be related to the time of exposure, as Coullery et al. [67] and Luna et al. [74] applied a subchronic treatment with glyphosate, whereas Cattani and colleagues [37] applied a single exposure to GBH in vitro.
It was also observed that GBH downregulated the expression of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex (PFC) and hippocampus [64]. BDNF plays an essential role in the processes of neurogenesis, growth, survival, and neuronal plasticity. In the brain, the BDNF binds to tyrosine-related kinase receptor B (TrkB) and activates an intracellular signaling cascade to promote synaptic survival and plasticity [81,82]. In this regard, GBH exposure upregulated TrkB expression in the PFC, which could reflect a compensatory mechanism to balance the reduction in BDNF levels [64].
Exposure to GBH also modified the expression of S100B. In vitro exposure to GBH induced a downregulation of this protein during development, but its levels increased in adult offspring after chronic exposure [3]. The S100B protein is mainly found in the glial cells (predominantly astrocytes) and exerts trophic or toxic effects, depending on its concentration [83]. At nanomolar concentrations, S100B acts as a neurotrophic factor that promotes neuronal growth and survival in the developing or injured nervous system. Conversely, at micromolar levels, S100B is thought to stimulate the expression of inflammatory cytokines that cause cell death. Thus, increased levels of this protein are considered a marker of nervous system damage. Down-regulation of S100B levels in the early stages of development could reflect one of the mechanisms by which glyphosate alters proper neuronal development, whereas upregulation of this protein during adulthood could be indicative of damage to the CNS induced by glyphosate.
Nuclear factor kappa B (NF-kB) expression is another parameter modified after GBH exposure [3]. NF-kB proteins represent a family of transcription factors that are expressed in both neuronal and non-neuronal cells. These proteins are involved in a wide variety of functions, including differentiation, cell survival, synaptic plasticity, or adult neurogenesis, among many others. Exposure to glyphosate during the periods of pregnancy and lactation induced a decrease in NF-kB activation in the hippocampus of immature rats, indicating that early exposure to this compound could affect neurogenesis and therefore the correct development of CNS [3].
Likewise, Ji et al. [71] demonstrated that GBH exposure modified the expression of numerous microRNAs that are implicated in brain development and in the pathogenesis of various diseases. MicroRNAs are small, non-coding RNAs that are involved in gene silencing and thus can shape the landscape of post-transcriptional gene expression [84]. They are also involved in numerous biological processes, such as early neurogenesis, circuit development, or synaptic plasticity, and their dysregulation is linked to the onset of numerous diseases. In an in vitro study, Masood et al. [76] observed the ability of glyphosate to modify gene expression, as its administration negatively regulated the expression of the cytoprotective CYP1A1 gene. These results reflect the fact that altered gene expression patterns could be another mechanism underlying glyphosate-induced neurotoxicity.
GBH-induced neurotoxicity in the hippocampus has also been linked to the recruitment of signal transduction pathways, leading to the activation of kinase cascades, including extracellular signal-regulated kinase (ERK) [3,37]. The ERK cascade is involved in the regulation of a wide variety of cellular functions, such as proliferation, differentiation, neuronal survival, and synaptic plasticity [85,86]. However, previous studies have shown that ERK activation can also mediate cell death [87,88]. Specifically, Jiang et al. [89] and Satoh et al. [90] found that activation of this pathway was involved in glutamate-induced neuronal apoptosis. Given that in a study by Cattani et al. [37], it was shown that GBH, in addition to ERK overactivation, also increased glutamate levels, the authors linked the activation of this pathway to increases in glutamate levels and subsequent neuronal death by apoptosis.
The glutamate excitotoxicity and calcium overload observed by Cattani et al. [37] may be related to the decrease in the expression of proteins of the dynorphin family observed later by the same authors [66]. This is because dynorphins are opioid receptor agonists [91], but they can also have other effects, such as the modulation of N-methyl-D-aspartate glutamatergic receptors (NMDAR) in the hippocampus and voltage-gated calcium channels (VDCCs) [92,93,94], and a decrease in their levels can trigger the previously observed effects on neurotransmission. Furthermore, dynorphins act as protectors of dopaminergic neurons and reduce the progression of Parkinson’s disease [95]. Thus, the decrease in dynorphin levels observed by Cattani et al. [66] in the substantia nigra could also be related to increased vulnerability of dopaminergic neurons to environmental pollutants, such as glyphosate.
The previously mentioned alterations could be implicated in the delay in sensorimotor development observed after GBH exposure in a study by Ait-Bali et al. [64]. Specifically, GBH exposure caused a delay in the development of innate reflexes and a deficit in the motor development of the offspring.
However, glyphosate not only appears to affect neural development in immature offspring but could also alter neurogenesis during adulthood. Various brain regions in adult females undergo remodeling during the peripartum period to adapt their behavior to the needs of the offspring [96,97]. One such region is the hippocampus, which has received much attention due to its persistent ability to generate new neurons even in adulthood [98]. It has been shown that glyphosate alone or in formulation affected aspects of neurogenesis in the maternal hippocampus during the postnatal period [68].
3.2.2. Effects on Neurotransmission
Neurotransmission is another fundamental process in the functioning of the nervous system on which glyphosate seems to exert a toxic effect, the glutamatergic system being one of the most affected after exposure to the pesticide. Glutamate is the most abundant excitatory neurotransmitter in the nervous system and is involved in various cognitive functions, such as learning and memory. Under physiological conditions, glutamate is maintained mainly in the intracellular medium, and only a small fraction exists outside the cells, as an increase in the extracellular levels of this neurotransmitter and its interaction with pre- or postsynaptic receptors can induce neuronal death by excitotoxicity [99,100,101].
Studies by Ait-Bali et al. [64] and Cattani et al. [3,37] show the effects of early GBH exposure on glutamatergic neurotransmission in immature or adult offspring. Acute or chronic treatment with GBH increased in vitro glutamate release, decreased its reuptake by astrocytes, and increased Ca2+ influx into hippocampal terminals. Furthermore, GBH increased the expression of NMDAR in both the hippocampus and the PFC [64] and activated these receptors and L-type voltage-dependent calcium channels (L-VDCC) in the hippocampus [3,37]. On the other hand, it was also demonstrated that GBH may alter glutamate metabolism by decreasing the activity of glutamic-pyruvic transaminase (GPT) and glutamic-oxaloacetic transaminase (GOT) [69]. Furthermore, an increase in glutamine transport into neurons was observed after chronic GBH exposure [37].
On the other hand, both early and late exposure to glyphosate seems to also affect cholinergic neurotransmission. Acetylcholine is a neurotransmitter that plays a central role in learning, attention, and synaptic plasticity [102,103]. In a study by Gallegos et al. [69], it was shown that GBH exposure reduced the number of cholinergic neurons in the medial septum, which was evidenced by a decrease in the expression of choline acetyltransferase (ChAT), the enzyme responsible for the synthesis of acetylcholine. GBH also reduced levels of the α7-type nicotinic acetylcholine receptor in the hippocampus. These data suggest that exposure to glyphosate alters the functioning of the septo-hippocampal cholinergic pathway, which is involved in the processing of memory [104,105,106].
Several of the studies reviewed here show that glyphosate or GBH was able to induce a slight decrease in acetylcholinesterase (AChE) activity in different brain areas [3,63,64,69,73]. The effect of glyphosate on AChE activity differs considerably depending on treatment and brain area analyzed, but in all cases, it is observed that, unlike other organophosphate pesticides, glyphosate is a weak inhibitor of AChE. Therefore, concentrations much higher than ambient levels of glyphosate that would be required to produce an effective inhibition of the activity of this enzyme in the brain [73].
Another neurotransmitter system affected by glyphosate exposure is the dopaminergic system. Dopamine is a biogenic amine involved in the modulation of locomotor activity, affectivity, and neuroendocrine communication in the CNS, and its alteration can lead to the development of neurodegenerative disorders, such as Parkinson’s disease [106]. Studies by Ait-Bali et al. [62,64], Hernández-Plata et al. [70], and Martínez et al. [13] show the effects of glyphosate on mesocorticolimbic and nigrostriatal dopaminergic neurotransmission. Ait-Bali et al. [62,64] have shown that early or adult exposure to GBH caused a decrease in the number of dopaminergic neurons, observed as a decrease in immunoreactivity for the enzyme tyrosine hydroxylase (TH) in the substantia nigra pars compacta and ventral tegmental area.
Furthermore, in studies by Hernández-Plata et al. [70] and Martínez et al. [13], the authors documented that systemic administration of glyphosate in adult rats significantly decreased total dopamine content, especially in the striatum, PFC, and hippocampus, in addition to increasing dopamine turnover in the PFC and hippocampus. Likewise, Hernández-Plata et al. [70] demonstrated that glyphosate decreased extracellular levels of dopamine and its metabolites in the striatum, as well as the specific binding of dopamine to its D1-type receptor in the nucleus accumbens. Thus, glyphosate could affect mechanisms that regulate the density or affinity of dopaminergic D1 receptors. However, these alterations in dopaminergic neurotransmission appear to occur when a critical concentration of glyphosate is present in the system but disappear over time. Taken together, the results of these studies seem to indicate that glyphosate exposure produces short-term effects on dopaminergic neurotransmission in various brain regions.
Other alterations in neurotransmitter processes documented after exposure to glyphosate or GBH include a decrease in the number of serotonergic neurons and in the contents of noradrenaline and serotonin in various brain areas [13,62]. Both serotonin and noradrenaline play a fundamental role in the modulation of mood and emotions, so alterations in these systems induced by glyphosate could favor the onset of depression or anxiety, according to observations in some studies analyzed herein [3,62,64,65].
Another effect observed on neurotransmitter processes is the alteration of synaptophysin expression after perinatal exposure to glyphosate or GBH. Specifically, an increase in synaptophysin expression was observed in the dentate gyrus and CA3 regions of the hippocampus, while its expression was downregulated in the cingulate gyrus [68]. Synaptophysin is a membrane protein present on the surface of synaptic vesicles that is expressed in most CNS neurons [107]. Glyphosate-induced changes in the expression of this protein could indicate its ability to alter neurotransmitter release and metabolism in various brain regions.
3.2.3. Effects on Behavior
Exposure to glyphosate also induces important changes in rodent behavior, possibly because of alterations in neurotransmission. Early or late exposure to glyphosate or GBH has been shown to cause a decrease in locomotion, which could be associated with the changes in the dopaminergic system discussed previously [62,64,65,67,70]. Furthermore, an increase in anxiety levels and depression-like behavior of the animals was also observed [3,62,64,65]. In general, alterations in mood could arise because of the decrease in serotonin and noradrenaline levels caused by glyphosate. Likewise, cognitive functioning also seems to be affected by the action of this compound. In this regard, it was shown that exposure to GBH or glyphosate caused an impairment in learning and memory processes, which could be due to alterations in the functioning of the cholinergic system [63,65,67,74].
However, early exposure to glyphosate not only affects offspring behavior but also maternal behavior. Glyphosate and GBH treatments altered maternal licking behavior toward pups [68]. The changes induced by glyphosate in maternal behavior could favor the subsequent development of behavioral alterations in the offspring, such as the decreased social activity in adult mice observed in a study by Ait-Bali et al. [64].
3.2.4. Induction of Oxidative Stress and Inflammation
Many of the most widely used pesticides worldwide exert their neurotoxic effects through oxidative stress mechanisms. Oxidative stress occurs when there is an imbalance between the production of ROS and the antioxidant capacity of the system responsible for detoxifying these reactive products. Consequently, oxidative damage of essential biomolecules, such as proteins, lipids, and DNA, occurs. The nervous system is particularly vulnerable to oxidative damage, mainly due to its low level of antioxidant activity, high oxygen requirement, and high lipid composition [108,109].
Glyphosate has been shown to induce oxidative stress immediately after administration, evidenced by an increase in LPO, which is induced by free radical action [37]. Oxidative stress induced by glyphosate exposure was also evidenced by alterations in protein and non-protein thiol concentrations [69,77]. Thiols are powerful antioxidants with the capacity to protect the organism from oxidative attack, and changes in their levels are used as indicators of the antioxidant status of organisms [110,111].
The enzymatic antioxidant defense system of living cells constitutes an adaptive mechanism in which the activities of the enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) stand out [108,112]. Studies have shown that glyphosate can alter the activity of these enzyme systems in the nervous system of intoxicated animals. Decreases in SOD, CAT, and peroxidase activity [3,63,69], as well as increases in GPx activity and SOD expression [76] have been observed. In this regard, it is important to note that any change in the expression or activity of antioxidant enzymes, whether increased or decreased, is indicative of oxidative stress [113].
The oxidative damage induced by glyphosate was also confirmed by the inhibition of the enzymatic activity of gamma-glutamyl transferase (GGT) and glucose-6-phosphate dehydrogenase (G6PD), which are involved in the synthesis and reduction of glutathione (GSH), respectively [3,37]. GSH is one of the most efficient intrinsic antioxidants in the brain, and its decreased availability markedly promotes the production of free radicals that enhance oxidative damage [114,115]. The ability of GBH to decrease GSH content after acute exposure has been documented, although the change was not sustained over time [3,37]. This inhibitory effect was also observed for glutathione-S-transferases (GSTs), which are involved in catalyzing the conjugation of GSH to various hydrophobic and electrophilic substrates, with the aim of protecting cells from oxidative attack [116]. Therefore, it is possible that after the initial depletion of GSH, the organism undergoes various adaptive modifications to restore the levels of this antioxidant to normal concentrations in order to mitigate the neurotoxic consequences of exposition to glyphosate.
On the other hand, the main source of ROS inside cells is the mitochondria, organelles whose function is also severely altered under oxidative stress conditions. In this regard, Da Silva et al. [77] showed that in vitro exposure to GBH in astrogliomas inhibited the activity of mitochondrial respiratory chain enzymes and creatine kinase (CK), an enzyme related to energy metabolism in nervous tissue. The effect of glyphosate on mitochondrial functioning and cell survival was previously demonstrated in a study by Astiz et al. [117]. These authors demonstrated that glyphosate alone or in combination with other pesticides induced loss of mitochondrial membrane potential and reduced concentrations of cardiolipin, a phospholipid involved in the electron transport chain that is highly vulnerable to oxidative stress due to its richness in fatty acids, leading to increased LPO and neuronal death in the substantia nigra. Taken together, these results suggest that glyphosate and GBH can severely alter the functioning of mitochondria and consequently cause their elimination by mitophagy, as evidenced by the loss of mitochondrial mass observed by Da Silva et al. [77]. It would be interesting for future research to clarify whether mitochondrial dysfunction is a cause or a consequence of oxidative stress caused by glyphosate.
Neuroinflammation appears to be another process contributing to neurotoxicity induced by glyphosate. The inflammatory reaction plays a healthy role in helping the immune system cope with certain pathological conditions, but when this reaction becomes unbalanced or prolonged over time, it can damage the CNS [118]. During the inflammatory process, activation of microglia and astrocytes occurs, which release a variety of molecular signals, such as TNF-α, that contribute to the inflammatory state of the CNS [119,120]. Furthermore, during the process, activated glial cells expressing the enzyme nitric oxide synthase (NOS) can generate excessive amounts of NO, a molecule that leads to the formation of reactive nitrogen species that promote oxidative damage in the brain [121].
In this regard, the data analyzed in the present review show that exposure to glyphosate or GBH produces some proinflammatory effects measured as increases in the number and activation of microglia and astrocytes and increased expression of TNF-α in the CNS of mice [64,69], as well as increased concentrations of NO in murine PNS [78,79].
3.2.5. Induction of Apoptosis and Autophagy
All of the neurotoxicity mechanisms triggered by glyphosate discussed previously could ultimately lead to neuronal death. Several studies have shown that exposure to glyphosate at any life stage reduces cell viability [37,75,76,77]. Specifically, it has been shown that both apoptosis and autophagy processes could be involved in the glyphosate-induced decrease in neuronal viability and neuronal death in rodents.
Apoptosis is a form of programmed cell death in which cells induce their own death through different pathways when subjected to certain types of stimuli. When the mitochondrial apoptotic pathway is activated, the Bax protein, which promotes neuronal death, inserts into the mitochondrial membrane and enables the release of cytochrome C into the cytoplasm, which ultimately leads to cell death [122]. In line with this, it has been observed that glyphosate treatment led to an increase in Bax protein expression while reducing levels of the anti-apoptotic protein Bcl-2 [75]. Bcl-2 promotes cell survival and inhibits the action of apoptotic proteins [123,124,125]. These data show the pro-apoptotic action of glyphosate in rat PC12 cells.
On the other hand, autophagy constitutes a mechanism for the turnover or destruction of dysfunctional or unnecessary cytoplasmic components within cells [126]. In a study by Gui et al. [75], it was observed that glyphosate treatment increased the concentration of the lipidated form of microtubule-associated protein 1A/1B light chain 3 (LC3-II), which is used as a marker for autophagosomes. The authors hypothesize that it is possible that glyphosate-induced autophagy is related to the need to remove cellular components damaged by this compound, such as mitochondria. Furthermore, these results were corroborated by the glyphosate-induced increase in the expression of the gene encoding Beclin-1, a protein that acts as a potential regulator of both [75].
3.3. Effects of Glyphosate in Fish
The zebrafish (Danio rerio) is a small freshwater teleost fish classically used as an experimental model for biomedical research and aquaculture breeding. After the mouse, the zebrafish has become one of the most widely used animal models for the study of various types of alterations in the nervous system, such as neurodegenerative and motor diseases, as well as for the study of neurotoxicity of environmental pollutants [127,128,129]. The main advantages of zebrafish, besides the high fecundity rate, rapid development, and low cost, are the organization of its nervous system, which is very similar to that of the human nervous system, and the similarity between the neurotransmitter systems [130,131].
Concerning the effects of glyphosate on fish, many of the studies included in this review describe the toxic effects of glyphosate or GBH on the nervous system of zebrafish, although the effects on other fish species have also been analyzed. Consistent with observations in for rodents, the analyzed studies show that glyphosate mainly affects nervous system development, neurotransmission, behavior, and energy metabolism, as well as producing oxidative stress and inflammation. The effects of glyphosate on fish are described in Table 4.
Table 4.
Effects of glyphosate and/or its commercial formulations in fish.
| Species | Dose and Time Exposure | Objectives | Results | Reference |
|---|---|---|---|---|
|
Cnesterodon decemmaculatus (Ten-spotted livebearer) |
GLY: 1 or 10 mg/L for 96 h | Assess the effect of seasonal variability on AChE activity in fish exposed to chlorpyrifos and GLY |
|
[132] |
|
Colossoma macropomum (Blackfin pacu) |
Roundup®: 10 or 15 mg/L for 96 h | Investigate the effects of GBH on gill morphology and function, hematological parameters, biotransformation enzymes, the antioxidant system in the gills and liver, as well as on both neurological and erythrocytic DNA damage |
|
[133] |
| Danio rerio (Zebrafish) | Roundup®, GLY: 0.01, 0.065, or 0.5 mg/L for 96 h | Evaluate the effects of GLY and GBH on morphological and behavioral parameters in larvae and adult zebrafish | GLY and GBH caused:
|
[134] |
| Danio rerio (Zebrafish) | GLY: 5, 10, or 50 μg/mL for 96 h | Identify a possible mechanism of toxicity for GLY related to changes in microtubule stability, which could alter the distribution and dynamics of cytoskeletal components |
|
[135] |
| Pintado da Amazônia | Roundup®: 0.37, 0.75, 2.25, 4.5, 7.5, 11.25, 15, 22.5, or 30 mg/L for 24, 48, 72, or 96 h | Evaluate the lethal concentration of the GLY and the oxidative stress parameters in tests with sublethal concentrations |
|
[136] |
| Danio rerio (Zebrafish) | GLY: 0.3 or 3 μg/L for 2 weeks | Analyze the neurotoxicity of GLY in adult zebrafish after exposure through water to environmentally relevant concentrations |
|
[130] |
| Danio rerio (Zebrafish) | GLY: 0.05 to 10.000 μg/L for a period of 1.5 to 120 h after fertilization | Explore the effects of the use of different concentrations of GLY on anatomy and behavior of fish | High concentrations of GLY (≥1000 μg/L) caused:
|
[137] |
|
Hypomesus transpacificus (Delta smelt) |
Roundup®: 0.064, 0.64, 6.4, 64, or 640 mg/L for 6 h | Compare the sublethal toxicity of four herbicides (penoxsulam, imazamox, fluridone, and GBH). |
|
[138] |
|
Cyprinus carpio (European carp) |
GLY: 0.02, 0.05, 0.07, or 0.1 mg/L for 24, 48, 72, or 96 h | Analyze the effect of GLY and atrazine on the hematological and biochemical parameters of blood and on behavioral aspects |
|
[139] |
| Danio rerio (Zebrafish) | Roundup®: 2, 5, or 8.5 μg/mL for 72 h | Investigate the lethal and sublethal developmental effects, neurotoxic potential, and oxidative stress responses after GBH exposure | High concentrations of GBH caused:
|
[140] |
| Danio rerio (Zebrafish) | Roundup®: 1, 2, or 5 μg/mL for 72 h | Assess GBH effects at environmentally relevant concentrations through a set of behavioral patterns |
|
[141] |
|
Carassius auratus (Goldenfish) |
Nongteshi®: 0.22, 0.44, or 0.88 mmol/L for 96 h | Investigate the toxic effects of GBH exposure using a metabolomic approach supplemented with histological inspection and hematological evaluation |
|
[142] |
| Carassius auratus | Nongteshi®: 0.2 mmol/L for 90 days | Assess GBH toxicity after prolonged exposure | Results in the brain:
|
[143] |
| Danio rerio (Zebrafish) | GLY: 5 or 10 mg/L for 24 and 96 h | Evaluate oxidative stress parameters, as well as the activity and expression of AChE |
|
[144] |
| Cnesterodon decemmaculatus | GLY: 1, 17.5, or 35 mg/L for 96 h | Assess the toxic effect of acute exposure to sublethal GLY concentrations on AChE activity in different parts of the body |
|
[145] |
|
Odontesthes bonariensis (Argentinian silverside) |
GBH: 1 or 10 mg/L for 15 days | Determine the basal levels of adenylates, phosphagens, and the AEC index in the brain, muscle, and liver, as well as the impact of exposure to sublethal GBH on the subcellular energy balance |
|
[146] |
| Danio rerio (Zebrafish) | GBH, GLY: 0.065, 1, 10, 160, 1.6 × 103, 4 × 103, or 8 × 103 mg/L for 3 h (in vitro) or for 7 days (in vivo) | Investigate the neurotoxic effects of GBH by focusing on acute toxicity, activity, and transcription levels of mitochondrial respiratory chain complexes, mitochondrial membrane potential, reactive species formation, and behavioral repertoire | In vivo exposure to GBH (7 days) caused:
|
[147] |
| Danio rerio (Zebrafish) | Roundup®, GLY: 50 μg/mL for 24 h | Investigate the neurotoxic effects of GBH and GLY exposure on the developing brain | Both GBH and GLY caused:
|
[148] |
|
Jenynsia multidentate (Onesided livebearer) |
Roundup® (Original, Transorb or WG): 0.5 mg/L for 96 h | Evaluate and compare the effects of three GBH formulations on behavior patterns | Roundup WG® was the most harmful formulation and negatively affected:
|
[149] |
|
Rhamdia quelen (Silver catfish) |
GLY: 6.5 mg/L for 12, 24, 48, or 72 h | Investigate the effects of GLY on the antioxidant system, as well as the neurotoxic effects on eggs and larvae |
|
[150] |
| Danio rerio (Zebrafish) | GLY: 0.01, 0.1, 0.5, 1, 5, 10, 100, 200, 400, or 600 mg/L from 3 hpf until 96 hpf | Assess the developmental, morphological, and genetic effects of GLY in zebrafish embryos |
|
[151] |
Abbreviations: GLY, glyphosate; ↓, decrease; AChE: acetylcholinesterase; GBH, glyphosate-based herbicide; ↑, increase; LPO, lipid peroxidation; 5-HT, serotonin; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA, homovanillic acid; CAT, catalase; SOD, superoxide dismutase; GSH, glutathione; GABA, gamma-aminobutyric acid; NAA, N-acetyl-L-aspartate; AMP, adenosine monophosphate; ROS, reactive oxygen species; AEC, adenylate energy charge; NADH, nicotinamide adenine dinucleotide dehydrogenase; GST, glutathione S-transferase; GR, glutathione reductase; hpf, hours post fertilization.
3.3.1. Development of Nervous System
Early exposure to glyphosate or GBH causes dysregulation of genetic pathways directly involved in neuronal physiology and synaptic transmission in zebrafish, especially affecting the development of the forebrain, midbrain, and eye structure [137,148]. Dysregulation of these pathways can lead to the appearance of different alterations and malformations in the brain, such as those observed in the studies of Lanzarin et al. [140], Roy et al. [148], and Zhang et al. [151].
Likewise, it has also been shown that treatment with high doses of glyphosate during development can alter the dynamics and structure of the components of the cell cytoskeleton [135]. Specifically, exposure to glyphosate reduced the levels of acetylated α-tubulin in the polymeric fraction of zebrafish embryos, suggesting a decrease in microtubule stability.
3.3.2. Effects on Behavior
The use of the zebrafish model to evaluate behavioral alterations provides a series of very relevant information for the investigation of the effects of environmental toxicants on the nervous system. Half of the studies reviewed herein reported glyphosate-induced behavioral alterations in these animals. In a study by Zhang et al. [151], an increase in locomotor activity of larvae during the day was observed, which, according to the authors, could be due to damage in the axons of primary motor neurons caused by glyphosate. In contrast, in the investigations of Forner-Piquer et al. [137] and Bridi et al. [134], early exposure to glyphosate or GBH caused a decrease in locomotion in both larvae and adults. It is possible that this apparent contrast is due to the difference in doses and exposure times used in each study. An increase in locomotion was documented by Zhang et al. [151] when exposing animals to the highest doses used (100–600 mg/L), whereas Forner-Piquer et al. [137] and Bridi et al. [134] used doses in the 0.05 to 10 mg/L range.
Another change observed after treatment with glyphosate and GBH was a decrease in aggressive behavior [134], which could be related to alterations in serotonergic neurotransmission [152]. Likewise, in a study by Faria et al. [130], glyphosate exposure caused impairment of exploratory and social behavior consistent with increased anxiety. However, the team of Lanzarin et al. [141] did not find such an association between GBH treatment and alterations in exploratory behavior and anxiety, the latter measured by alterations in hormone cortisol. Exposure to glyphosate also induced avoidance in the presence of aversive stimulus [134,141]. These results are likely related to the decreased exploratory ability observed in fish after glyphosate exposure. Finally, the occurrence of memory impairment in zebrafish after exposure to GBH was also documented, which could be related to an incorrect functioning of the cholinergic system in these animals [134].
3.3.3. Effects on Neurotransmission
Several of the studies included herein investigated glyphosate-induced changes in AChE activity in various species, and the data reflect a heterogeneity of effects. Sobjak et al. [150] observed that exposure of eggs and larvae of silver catfish (Rhamdia quelen) to glyphosate caused an unexpected increase in AChE activity at 48 h after exposure, followed by a decline at 96 h. These results suggest that exposure to glyphosate during early developmental stages may cause an early induction of AChE activity and a subsequent difficulty in maintaining elevated AChE activity at later times.
On the other hand, regarding the effects of glyphosate on AChE in young and adult fish, several studies support its inhibitory potential against the activity of this enzyme [132,133,145]. This enzymatic inhibition would affect the anterior and middle regions of the fish body and seems to depend on the concentration and time of year, being more potent in autumn [132,145]. In contrast, in a study by Teixeira et al. [136], an increase in brain AChE activity was observed, whereas Jin et al. [138] and Lopes et al. [144] did not detect any alteration in its activity level. The considerable variability of these results is probably due to the wide diversity of species and doses of glyphosate used in each of the investigations.
Another of the changes observed is an alteration of the balance between glutamine, glutamate, and the amino acid gamma-aminobutyric acid (GABA), which are fundamental components of brain metabolism and function. Glutamine is produced from glutamate reuptake by astrocytes, and although it does not fulfill a neurotransmitter function, it is the main precursor for glutamate and GABA synthesis [153]. In this regard, Li et al. [142] observed that GBH exposure reduced both GABA and glutamate, resulting in an elevation in brain glutamine levels and the subsequent appearance of various behavioral alterations.
Furthermore, Faria et al. [130] documented an increase in the content of dopamine and its acidic metabolites (3,4-dihydroxyphenylacetic acid, DOPAC, and homovanillic acid, HVA) and in dopamine turnover in the forebrain. However, no variations were found in the levels of 3-methoxytyramine, an extracellular metabolite of dopamine. Therefore, because DOPAC is an intracellular metabolite and HVA reflects both intra- and extraneuronal metabolism, the changes observed after glyphosate exposure suggest that it could increase the intraneuronal metabolism of dopamine but without inducing increased release of the neurotransmitter. Glyphosate also downregulated the expression of several genes involved in dopamine synthesis, degradation, and transport [130]. In this same study, the authors also reported that glyphosate exposure increased serotonin levels in the forebrain, which could influence the mood and behavior of the animals.
The alterations in neurotransmission could explain the behavioral alterations observed by Sánchez et al. [149] in one-sided livebearer (Jenysia multidentate). In this study, exposure to 0.5 mg/L GBH for 96 h was associated with a decrease in spatial exploration and swimming performance, as well as impaired long-term memory consolidation. Furthermore, these authors also observed a negative effect of GBH on social interaction and sexual behavior of fish.
3.3.4. Induction of Oxidative Stress and Inflammation
As previously discussed, many of the most widely used pesticides exert their neurotoxicity by altering the balance between ROS production and the ability of the organism’s antioxidant system to neutralize them. In this regard, Faria et al. [130], Pereira et al. [147], and Sobjak et al. [150] have documented the ability of glyphosate and GBH to increase ROS concentrations in the nervous system of fish, as well as the LPO caused by them. The exception is the work of Lopes et al. [144], wherein a decrease in LPO levels was observed in zebrafish after exposure to glyphosate. Although the team of Teixeira et al. [136] found no changes in LPO levels after treatment of pintado da Amazônia fish with sublethal doses of GBH, they did observe an increase in carbonyl protein content. These data suggest the existence of an alteration in normal protein metabolism that can be used as a marker of protein oxidative damage.
Mitochondria are cellular organelles that contribute considerably to ROS production and thereby to oxidative stress. In a study by Pereira et al. [147], in vivo exposure to GBH induced mitochondrial dysfunction in brain cells after 7 days of treatment, evidenced by a decrease in the activity of the nicotinamide adenine dinucleotide (NADH) dehydrogenase and cytochrome C, enzymes of mitochondrial complexes I and IV, respectively. These alterations were also associated with changes in the transcription of genes encoding electron transfer proteins in the mitochondrial respiratory chain and a subsequent hyperpolarization of the mitochondrial membrane potential. In this regard, when the mitochondrial membrane potential is high, the mitochondrial respiratory chain becomes a producer of ROS, which poses a serious risk to cell integrity [154].
GBH exposure also caused a decrease in the levels of choline; its derivative, phosphocholine; and its metabolite, betaine, in the brain. Choline constitutes an essential component of different phospholipids of lipid bilayers and is necessary for the maintenance of structural integrity of cell membranes [155]. Therefore, because cell membrane phospholipids are the main target of oxidative attack, the decrease in levels of choline and its derivatives after glyphosate exposure could be indicative of an accelerated use of these components to repair cell membranes impaired by free radicals.
Another marker of oxidative stress induced by exposure to neurotoxic agents is the alteration in the activity of enzymes responsible for scavenging free radicals and restoring the normal antioxidant status of nerve tissue. It has been documented that exposure to glyphosate induced an increase in the activity of the antioxidant enzymes CAT, SOD, and glutathione reductase (GR), with a concomitant decrease in GSH reserves, which could reflect an attempt by the cells to neutralize the excessive levels of ROS caused by glyphosate [130]. However, Sobjak et al. [150] found no changes in CAT and GST activity in the brain of silver catfish after exposure to glyphosate.
Glyphosate exposure also appears to induce an inflammatory reaction in the nervous system of zebrafish. This was demonstrated by a study by Forner-Piquer et al. [137], who administered concentrations of 0.05 and 10.000 μg/L glyphosate and detected the presence of amoeboid cells, suggesting microglial activation and therefore activation of the inflammatory process. In addition, transcriptomic analysis revealed dysregulation of genetic pathways involved in inflammation. In contrast, Li et al. [142] demonstrated that exposure of goldfish (Carassius auratus) to 0.22, 0.44, or 0.88 mmol/L of GBH induced a decrease in the levels of myoinositol, a compound that is mainly present in glial cells and is therefore considered a glial marker [156].
3.3.5. Effects on Energy Metabolism
The effect of glyphosate on brain energy metabolism is an aspect not addressed in studies in other species analyzed so far, although the available evidence supports the potential of pesticides to interfere with energy production [157]. Glucose is the main source of energy in the brain, and its catabolism via glycolysis results in pyruvate, which is subsequently transferred to mitochondria to produce adenosine triphosphate (ATP) via the tricarboxylic acid (TCA) cycle and the mitochondrial respiratory chain [158]. GBH exposure was shown to deregulate glucose metabolism in the goldfish brain, which was evidenced by decreased succinate and citrate concentrations [143]. Because both are intermediates of the TCA cycle, the reduction in their levels suggests that glyphosate may block the TCA cycle and thereby generate an insufficient amount of ATP.
To survive this energy crisis, the body can use additional resources of energy production. For example, creatine and phosphocreatine are important energy metabolites because phosphocreatine hydrolysis donates a phosphate group to adenosine diphosphate (ADP) to synthesize ATP [159]. In this regard, a decrease in creatine and phosphocreatine levels was observed in the brain of fish dosed with GBH, suggesting the activation of the phosphocreatine reaction with ADP in order to supply the need of ATP [142]. Likewise, Li et al. [143] observed increased levels of ketone 3-hydroxybutyrate in the brain tissue of GBH-treated goldfish. In the brain, ketones are the main alternative fuel to glucose, so they could be used to meet the insufficient energy supply caused by glyphosate exposure [160].
When glucose is unable to maintain brain homeostasis, other substrates, such as acetate, can also be used as metabolic precursors [161]. In line with this, Li et al. [142] found that GBH exposure induced a considerable reduction in brain levels of the amino acid N-acetyl-L-aspartate (NAA), the second most abundant amino acid in the CNS, which can be degraded to aspartate for energy production [162].
On the other hand, adenosine monophosphate (AMP)-activated kinase is the main sensor and regulator of cellular energy homeostasis. Under low-energy conditions, this kinase is activated and inhibits ATP-using (anabolic) processes while stimulating ATP-producing (catabolic) processes [163]. Therefore, the reduction in AMP levels in brain tissues of fish exposed to GBH described by Li et al. [143] may affect the AMP-activated kinase pathway and thus alter downstream metabolic processes. In contrast to these results, in a study by Menéndez-Helman et al. [146], GBH treatment did not produce alterations in adenylate energy charge (AEC), a measure reflecting the amount of energy available from the adenylate pool (ATP, ADP, and AMP).
Overall, the changes at the molecular level induced by glyphosate discussed previously can lead to the manifestation of important alterations both in structure and in brain function. This is clearly reflected in the destruction of the microscopic structure of the brain or in the appearance of abnormal and variable brain activity in the midbrain of fish exposed to glyphosate alone or in formulation [137,141]. Nevertheless, when considering these studies, the heterogeneity of the obtained results is evident, which, as mentioned above, may be due to the use of different species, as well as to treatment with doses and exposure periods that differ considerably. This makes it difficult to draw rigorous conclusions and demonstrates the need for more research in this area.
3.4. Effects of Glyphosate on Invertebrates
The number of recent studies on the effects of glyphosate on the nervous system of invertebrates is limited. Two studies were identified with the worm Caenorhabditis elegans describing that GBH also produces neurotoxicity in this species, which was evidenced by altered neuronal development, mitochondrial damage, oxidative stress, and in behavioral patterns.
On the one hand, it has been shown that GBH treatment affected cell development in worms, which was evidenced by a decrease in the number and size of dopaminergic neurons from the fourth larval stage onwards [164]. These authors also observed that GBH caused a marked increase in superoxide levels during the fourth larval stage [164] and hydrogen peroxide in treated worms [165]. As has been observed in rodents, GBH exposure also affects mitochondrial function in these animals. In addition, Burchfield et al. [165] showed that glyphosate induced inhibition of mitochondrial complex II, and consequently, a decrease in ATP levels occurred.
4. Discussion
4.1. Overview of the Main Mechanisms of Action of Glyphosate on the Nervous System
A wide variety of studies show the correlation between pesticide exposure and the development of various types of diseases. Organophosphate exposure has been reported to be associated with various human conditions, such as mood disorders, attention deficit hyperactivity disorder, cancer, kidney damage, and autism, among others [166,167,168,169]. Furthermore, it has been postulated that pesticides may be the main environmental factor associated with the etiology of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease [59,170].
Regarding glyphosate, some previous studies have reported DNA damage in human and rodent cells after exposure to this compound and GBH [171,172,173]. The teratogenic action of GBH in vertebrates was also described [174]. Likewise, human clinical reports on the effects of intoxication with glyphosate formulations have described harmful effects on the nervous system, including parkinsonism [175].
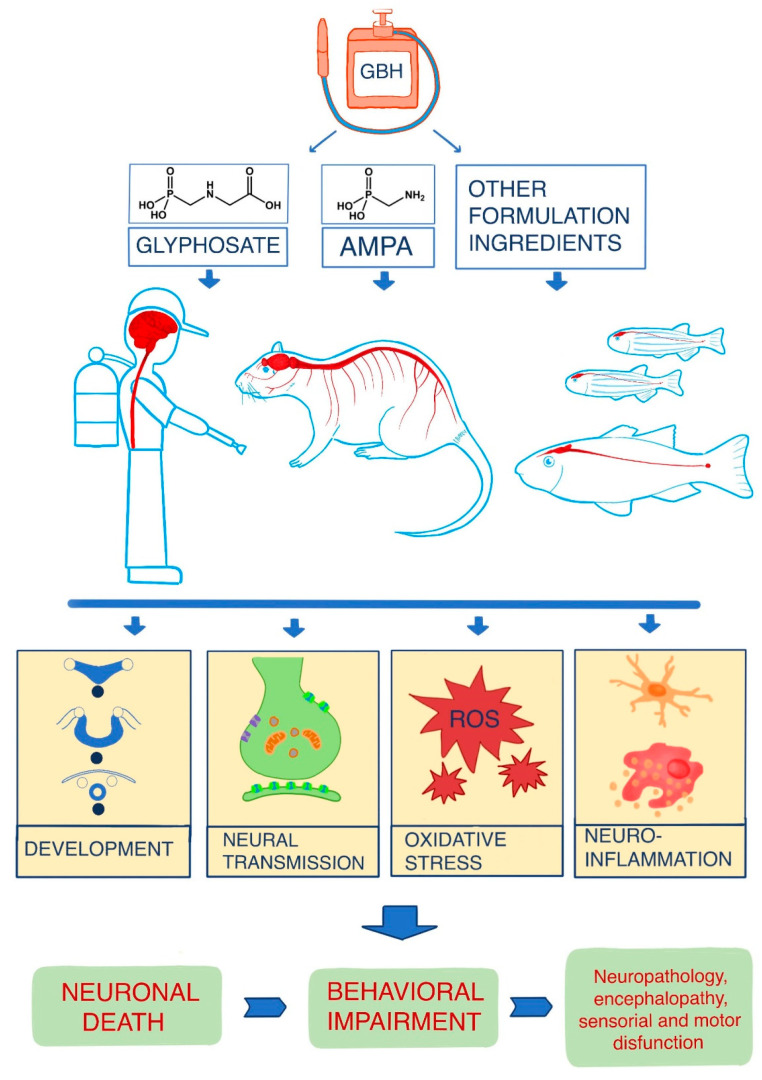
The data analyzed in the present review show that exposure to glyphosate or GBH generates various types of neurotoxic effects in all the species studied (Figure 3). The study of glyphosate effects in humans is based on the observation of clinical signs and symptoms in people accidentally or intentionally exposed to the pesticide or from in vitro studies with human cell lines. Although these in vitro studies are quite heterogeneous, in general, the results corroborate those described for the other species studied. Some of the findings of these investigations corroborate the ability of glyphosate to cross the human BBB and produce neurotoxic effects in the CNS. This ability of glyphosate to cross both the placental barrier and the BBB in humans was also observed in other previous studies that detected this compound in the brain and cerebrospinal fluid of individuals who had been exposed to GBH [176,177,178].
Figure 3.
Exposition to glyphosate; its main metabolite, AMPA (aminomethylphosphonic acid); or its commercial formulations induces neurotoxic effects in all studied species. The main modes of action include changes in the development of the nervous system and in the neurotransmission systems, oxidative stress, neuroinflammation, processes that lead to neuronal death, and the appearance of behavioral changes. Changes in the structure and function of neurons lead to the development of neuropathology, encephalopathology, and sensory and motor dysfunctions.
Nevertheless, it appears that in adult humans, glyphosate does not produce toxic effects immediately after exposure but takes one or two days to do so. It is possible that this delay is due, at least in part, to the fact that the pesticide takes time to alter the integrity of the BBB, cross it, and subsequently distribute itself in the CNS. Clinical observations also show that the adverse effects caused by glyphosate disappear as the concentration of the compound in the body decreases, although there are certain sequelae that seem to be maintained over time, such as memory disturbances.
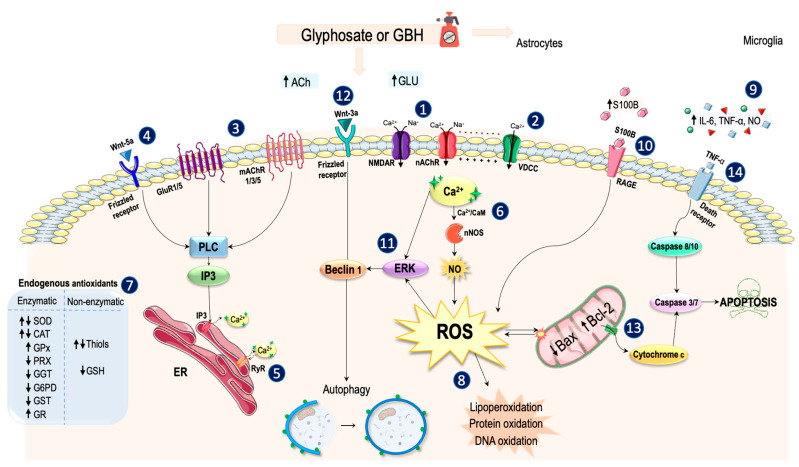
Figure 4 summarizes the main mechanisms by which glyphosate produces its toxic effects on the nervous system. However, the existence of important discrepancies between the results obtained in studies carried out with humans, rodents, and fish must be considered. The data analyzed suggest that glyphosate induces the entry of Na+ and Ca2+ from the extracellular medium through different mechanisms. Glyphosate causes an overstimulation of NMDAR, which leads to an influx of these ions and membrane depolarization. On the other hand, the inhibition of AChE by glyphosate produces an increase in acetylcholine levels and the stimulation of its receptors, including the α7 and α4β2 receptors. The opening of these channels also induces an Na+ and Ca2+ influx. Furthermore, although α4β2 receptors are rapidly desensitized, α7 receptors can remain open for longer in the presence of agonists, further increasing Ca2+ influx [179]. It is possible that the sustained activity of these receptors induced by chronic exposure to glyphosate eventually leads to a decrease in their expression. Taken together, depolarization resulting from activation of glutamatergic and cholinergic receptors can cause VDCCs to open, allowing more Ca2+ to enter the cell. Some studies have shown that because of excess glutamate and acetylcholine, their metabotropic receptors are also activated [37,180,181]. The mGluR1 and mGluR5 receptors and mAChRs 1, 3, and 5 act on the same signaling pathway by activating associated G proteins, which in turn activate phospholipase C and the production of inositol triphosphate (IP3). The resulting IP3 diffuses through the cytoplasm and binds to specific receptors in the endoplasmic reticulum (ER), causing the release of Ca2+ to the cytosol [182].
Figure 4.
Possible mechanism of action of glyphosate or GBH in the nervous system. The presence of glyphosate induces several changes, including (1) opening of nAChRs and NMDA receptors, as well as entry of Na+ and Ca2+ into the cell due to increased levels of ACh and GLU and/or the direct binding of glyphosate to the cavities of NMDAR; (2) opening of the VDCCs by cellular depolarization and entry of Ca2+; (3) activation of the metabotropic GLU and ACh receptors, which stimulate the PLC to generate IP3, which causes the release of Ca2+ from inside the ER; (4) increase in the levels of Wnt-5a, which binds to Frizzled receptors and triggers the generation of IP3, with the consequential release of Ca2+ from the interior of the ER; (5) Ca2+ binding to ryanodine receptors and Ca2+ release from inside the ER; (6) binding of Ca2+ to calmodulin and activation of nNOs, which releases NO; (7) modification of the activity and/or concentrations of endogenous antioxidants; (8) excessive levels of ROS, leading to oxidation of lipids, proteins, and DNA; (9) activation of glial cells, which release inflammatory cytokines and NO; (10) release of S100B protein, which binds to neuronal RAGEs and increases ROS overproduction; (11) activation of ERK due to excessive levels of Ca2+ and ROS, which activates Beclin 1 and induces autophagy; (12) increased levels of Wnt-3a, which binds to Frizzled receptors and induces autophagy; (13) mitochondrial dysfunction, leading to activation of the intrinsic apoptosis pathway; and (14) binding of the ligand TNF-α to the death receptor, activating the extrinsic apoptosis pathway. Parts of the figure were created using templates from Servier Medical Art, which are licensed under a Creative Commons Attribution 3.0 Unported License (http://smart.servier.com/ accessed on 17 February 2022). Abbreviations: GBH: glyphosate-based herbicide; GLU: glutamate; nAChR: nicotinic acetylcholine receptor; NMDAR: N-methyl-D-aspartate receptor; VDCC: voltage-dependent calcium channel; PLC: phospholipase C; IP3: inositol trisphosphate; ER: endoplasmic reticulum; CaM: calmodulin; nNOS: neuronal nitric oxide synthase; NO: nitric oxide; SOD: superoxide dismutase; CAT: catalase; GPx: glutathione peroxidase; PRX: peroxidase; GGT: gamma-glutamyl transferase; G6PD: glucose-6-phosphate dehydrogenase; GST: glutathione S-transferase; GR: glutathione reductase; GSH: glutathione; ROS: reactive oxygen species; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; S100B: S100 calcium-binding protein B; RAGE: receptor for advanced glycation end products; ERK: extracellular signal-regulated kinase; ↑, increase; ↓, decrease.
Similarly, exposure of human cells to glyphosate has been shown to induce an increase in the expression of Wnt-5a mRNA, although this increase was not observed in rodents. Wnt-5a can interact with the Frizzled receptor, the activation of which also leads to an increase in IP3 levels and the consequential release of Ca2+ to the cytoplasm [183]. Likewise, Ca2+ ions inside the cell can bind to ryanodine receptors, Ca2+ channels present in the ER, the opening of which allows for the exit of this cation into the cytoplasm [182]. Ca2+ is an intracellular messenger involved in multiple signaling pathways, the concentrations of which are strictly controlled. Thus, this increase causes numerous changes in the cell. One such change is its binding to calmodulin, which leads to the activation of neuronal nitric oxide synthase (nNOS), which contributes to oxidative stress through the release of large amounts of NO [184].
As has been amply demonstrated, glyphosate produces a marked increase in intracellular ROS concentrations, which induce the activation of a series of mechanisms to neutralize oxidative stress conditions. Among these mechanisms is the activity of endogenous antioxidants, both enzymatic (SOD, CAT, GPx, peroxidase (PRX), GST, GGT, and G6PD) and non-enzymatic (GSH and thiols). However, these mechanisms appear to be insufficient to counteract the large amount of free radicals resulting from exposure to the pesticide. ROS act on the membranes of organelles, proteins, and DNA, causing their oxidation. In this situation, the cell can adopt various strategies to repair its components, such as increasing the activity of alkaline phosphatase to repair its DNA.
On the other hand, glyphosate has been shown to induce neuroinflammation characterized by the activation of both microglia and astrocytes. Because of their activation, these cells release large amounts of inflammatory cytokines, such as TNF-α and IL-6. In addition, exposure to glyphosate could also promote the release of the S100B protein by astrocytes, reaching its maximum levels one day after intoxication. At micromolar concentrations, the S100B protein can act on the receptor for advanced glycation end products (RAGE) at the neuronal membrane, favoring the overproduction of ROS [185]. Furthermore, this protein also binds to RAGEs present in the glial membrane, where it amplifies the inflammatory response [186]. In line with this, both the S100B protein and some inflammatory cytokines can trigger inducible nitric oxide synthase (iNOS) activation in astrocytes [187]. Consequently, these glial cells produce large amounts of NO, which further enhances oxidative stress.
To eliminate the organelles damaged by the action of ROS and obtain energy, the cells can induce the process of autophagy. Thus, the increase in Ca2+ and ROS concentrations caused by glyphosate has been related to increased activation of ERK, which can activate the Beclin 1 protein and induce autophagy [188]. In addition, this natural recycling process can also be induced by other mechanisms observed in studies with glyphosate, such as the binding of the Wnt-3a ligand to the Frizzled receptor or through the deficient production of ATP in mitochondria [189].
Although autophagy favors cell survival in stressful situations or under nutrient deprivation, excessive induction of this mechanism can be destructive to the cell and lead to apoptosis [190]. The conditions of oxidative stress induced by glyphosate can trigger the intrinsic or mitochondrial apoptotic pathway because the ability of these organelles to produce energy and maintain Ca2+ homeostasis is severely affected. Therefore, the increase in the intracellular Ca2+ also leads to a marked increase in its levels in the mitochondria. Consequently, activation of the Bax protein occurs. This activated protein forms pores in the mitochondrial membrane and allows for the release of cytochrome C, which activates the caspases responsible for orchestrating apoptosis [191].
Exposure to glyphosate can also activate the extrinsic apoptotic pathway. As previously mentioned, glial activation by the pesticide causes the release of inflammatory cytokines, such as TNF-α, which can act as a ligand for the death receptors at the membrane. As a result of the activation of these receptors, the activation of caspase 8 occurs, which clears other caspases and finally causes neuronal death by apoptosis [192].
These findings also suggest that when exposure occurs early in life, the pesticide can induce serious disturbances in the development of the nervous system, possibly by deregulating some of the signaling pathways involved in this process. On the other hand, a common aspect that can be drawn from in vivo and in vitro studies with rodents and humans is that both in early age and in adulthood, the hippocampus is especially vulnerable to the action of glyphosate. As a result of this damage, exposure to different concentrations of glyphosate has usually been associated with severe memory impairment that is sometimes irreversible.
4.2. Relationship between Glyphosate Doses and Neurotoxic Effects in Rodents and Humans
The findings reported in this review coincide in pointing out the neurotoxic potential of glyphosate in the different animal species studied. Although many of the studies used doses higher than those commonly found in the environment, the main objective of these studies was the evaluation of the neurotoxic mechanisms of action of the pesticide on the nervous system. Therefore, to assess the real toxic risk that exposure to glyphosate may pose in animals, it is necessary to consider environmental concentrations of the pesticide.
The first point to consider is that there are several discrepancies with respect to the values established as NOAEL. Several previous investigations have established a NOAEL for glyphosate at considerably high values, such as 1000 mg/kg/day, which established for maternal and developmental toxicity in rats [14]. However, these guideline values were established considering carcinogenicity and toxicity in organs other than the brain. Therefore, a considerable number of regulatory toxicity studies found no evidence that high doses of glyphosate caused neurotoxic effects in rodents. This has led to some subsequent studies to establish a NOAEL for neurotoxicity in rats ranging from 2000 to 20.000 mg/kg/day of glyphosate [193]. Finally, more recent research established a more moderate NOAEL value of 617 mg/kg/day [34].
Most of studies of rodents analyzed in this review used doses of glyphosate or GBH that did not exceed the current NOAEL and ranged from 5 mg/kg [68] to 800 mg/kg [13]. These doses are not representative of human environmental exposures, which are in the range of μg/kg/day. However, the data provided by these studies suggest that even oral treatment with the lowest used dose of glyphosate or GBH (5 mg/kg) was associated with alterations in behavior and neuroplasticity in rats [68]. Likewise, the dose of glyphosate most used in the studies with rodents was 50 mg/kg, which is also much lower than the established NOAEL, and it was associated with important alterations in the structure and functioning of the CNS. Therefore, although it appears that more conservative guideline values for the neurotoxicity of glyphosate and GBH have been adopted in recent years, the data discussed herein suggest that these values may still be too high. The results analyzed in this review suggest that the ability of glyphosate, alone or in formulation, to produce neurotoxic effects in rodents has been underestimated.
The current evidence analyzed in this review raises concern and indicates the need for more research, mainly in rodents. This is because rodent studies provide essential information for the establishment of reference values for humans. In line with this, the reference dose of glyphosate established by the EPA (USA) is 1.75 mg/kg/day, whereas the JMPR/WHO is more restrictive and limits the maximum dose to 1 mg/kg/day [194]. The EPA has estimated that the exposure of the general population to glyphosate through food and water is 0.088 mg/kg/day (range 0.058–0.230 mg/kg/day) [195], some studies have indicated that glyphosate is habitually found in urine in concentrations corresponding to a daily intake of approximately 0.1–0.3 μg/kg/day [28]. These data indicate that the general population is exposed to glyphosate levels lower than the reference dose and the acceptable daily intakes proposed by several regulatory agencies, not representing an immediate risk to health. However, under certain circumstances, the established reference values can be clearly exceeded, as in the case of some accidental or intentional exposures. Thus, the most serious neurotoxic effects reported have been observed after suicide attempts, with exposures to doses of glyphosate much higher than those normally found in the environment.
Studies on occupational toxicity in rural populations are of particular relevance. In such studies, glyphosate concentrations commonly used in agricultural practice or those found in residential environments close to growing areas were considered. These studies reveal a possible relationship between exposure to glyphosate during prenatal and childhood and an increased risk of developing diseases such as autism spectrum disorder [47]. Likewise, peripheral neuropathy and memory impairment have also been reported in farmers occupationally exposed to the pesticide [45,196]. These data suggest that pesticide concentrations found in agricultural settings could represent a health risk to children and adults.
4.3. Exposure Levels and Neurotoxic Effects in Fish
When GBH is applied to crops, it can remain in the soil until it is degraded by microorganisms or mobilized by wind, rain, or irrigation. These factors increase infiltration and surface runoff, favoring the arrival of glyphosate in aquatic environments [146]. Varied concentrations of glyphosate have been reported in aquatic environments, such as concentrations of 0.1–1.48 mg/L detected in waters of Argentina and Brazil, or the maximum concentration of 0.65 mg/L detected in waters in Europe [149,197]. During the glyphosate use season, its concentration reaches 10 mg/L in Taihu Lake (China) [48].
Therefore, Roundup® concentrations below 10 mg/L can be considered environmentally realistic considering that with current application rates, a bare water body can have a maximum glyphosate concentration of 3.7 mg/L, which corresponds to 9 mg/L of Roundup® [198]. Almost all the fish studies analyzed in this review used doses that ranged from 0.064 to 10 mg/L of GBH or glyphosate. Exposure to these environmentally relevant doses of glyphosate was associated with a wide range of neurotoxic effects, including alterations in the development of the nervous system and the appearance of alterations in behavior. In addition, although, given the physicochemical characteristics of glyphosate, it is not expected that it can bioaccumulate in the tissues of animals, the available evidence supports that this compound can accumulate in the tissues of some aquatic species, which could result in an increase in its neurotoxicity [199,200,201,202].
Although elevated concentrations of glyphosate have been detected in some aquatic environments, some countries have adopted more restrictive limits. Thus, the maximum level of glyphosate accepted in the United States is 0.7 mg/L, whereas in Europe, the maximum accepted for a standard environmental quality is 0.028 mg/L [43,203]. In line with this, Bridi et al. [134] showed that exposure of zebrafish to glyphosate or GBH (0.01–0.5 mg/L) caused alterations in locomotion, aversive and aggressive behavior, as well as memory impairment. In a study by Khan et al. [139], treatment with 0.02–0.1 mg/L of glyphosate also altered the behavior in European carp (Cyprinus carpio). Similarly, Faria et al. [130] found that the exposure of zebrafish to concentrations of 0.3 or 3 μg/L of glyphosate for two weeks caused a deterioration in exploratory and social behavior, as well as an increase in anxiety. Likewise, the team of Sánchez et al. [149] observed that treatment of one-sided livebearer (Jenynsia multidentate) with 0.5 mg/L GBH reduced social interaction, spatial exploration, swimming performance, and long-term memory consolidation and even caused severe alterations in sexual behavior.
These results suggest that exposure to glyphosate or GBH, although in doses that are within legally accepted limits, can result in neurotoxicity in fish and alter behaviors essential for the survival of these species. Ultimately, this could have important ecological consequences, especially in agricultural areas where agrochemicals are applied regularly. Therefore, these results suggest the need to adopt more conservative contamination limits that consider the neurotoxicity induced by glyphosate.
5. Limitations
This systematic review is not free of limitations, which should be considered when generalizing the results. First, most of the studies analyzed used commercial formulations of glyphosate, which are mixtures of glyphosate (active ingredient) with other adjuvants. Thus, it is not possible to determine precisely which component(s) of the formulation was (or were) responsible for the observed neurotoxic effects. In addition, the results of various studies support the greater toxicity of commercial glyphosate formulations compared to glyphosate administered alone [147,204,205,206]. For this reason, the results analyzed herein cannot be attributed exclusively to glyphosate, as they could have been caused by other components of the formulation or even by possible synergy between these components and glyphosate [65,207,208,209].
On the other hand, most research has studied the effects of higher doses of glyphosate than the concentrations to which the general population is routinely exposed. Thus, it is possible that exposure to environmental concentrations of glyphosate does not result in the wide range of neurotoxic effects documented here. Furthermore, when analyzing many studies, a great heterogeneity in the results obtained is observed; the various discrepancies identified when comparing the studies could be due to differences in their experimental conditions, such as the route of exposure, the compound used (glyphosate, AMPA, or GBH), the total duration of treatment, and the time at which the evaluations were performed since the end of the treatment.
6. Conclusions
The information summarized in the present review indicates that exposure to glyphosate, AMPA, or GBH could induce several toxic effects on the nervous system of all species studied. Exposure to glyphosate during the early stages of life can severely affect normal cell development by deregulating some of the signaling pathways involved in this process, leading to alterations in differentiation, neuronal growth, migration, and myelination. Glyphosate also seems to exert a significant toxic effect on neurotransmission, with the glutamatergic system being one of the most affected systems. Glyphosate was found to increase glutamate release and decreased its reuptake, in addition to activating NMDAR and L-VDCC, thus increasing the influx of Ca2+ into neurons. Likewise, the results analyzed herein reflect the capacity of glyphosate to induce oxidative stress, neuroinflammation, and mitochondrial dysfunction, processes that lead to neuronal death by autophagia, necrosis, or apoptosis, as well as the appearance of behavioral and motor disorders. Although there are important discrepancies between the findings analyzed in this review, it is unequivocal that exposure to glyphosate, alone or in commercial formulations, can produce important alterations in the structure and function of the nervous system of humans, rodents, fish, and invertebrate animals.
Acknowledgments
The authors thank Elena Durán, Vigo, Spain, for illustrations.
Author Contributions
C.C.-F. performed research, wrote the paper, critically revised, and finalized the manuscript; R.D. designed the study and critically revised the manuscript; L.R.F.F. designed the study, wrote the paper, critically revised, and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Xunta de Galicia for the financial support (grant ED431B 2019/33).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest, financial or otherwise.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dayan F.E., Barker A., Takano H., Bough R., Ortiz M., Duke S.O. Herbicide mechanism of action and resistance. In: Moo-Young M., editor. Comprehensive Biotechnology. 3rd ed. Volume 4. Elsevier; Amsterdam, The Netherlands: 2020. p. 4826. [Google Scholar]
- 2.Duke S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018;74:1027–1034. doi: 10.1002/ps.4652. [DOI] [PubMed] [Google Scholar]
- 3.Cattani D., Cesconetto P.A., Tavares M.K., Parisotto E.B., Oliveira P.V., Rieg C.E.H., Leite M.C., Prediger R., Wendt N., Razzera G., et al. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology. 2017;387:67–80. doi: 10.1016/j.tox.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Klümper W., Qaim M. A Meta-Analysis of the Impacts of Genetically Modified Crops. PLoS ONE. 2014;9:e111629. doi: 10.1371/journal.pone.0111629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maggi F., la Cecilia D., Tang F.H., McBratney A. The global environmental hazard of glyphosate use. Sci. Total Environ. 2020;717:137167. doi: 10.1016/j.scitotenv.2020.137167. [DOI] [PubMed] [Google Scholar]
- 6.Saunders L.E., Pezeshki R. Glyphosate in Runoff Waters and in the Root-Zone: A Review. Toxics. 2015;3:462–480. doi: 10.3390/toxics3040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funke T., Han H., Healy-Fried M.L., Fischer M., Schönbrunn E. Molecular basis for the herbicide resistance of Roundup Ready crops. Proc. Natl. Acad. Sci. USA. 2006;103:13010–13015. doi: 10.1073/pnas.0603638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meftaul I.M., Venkateswarlu K., Dharmarajan R., Annamalai P., Asaduzzaman, Parven A., Megharaj M. Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environ. Pollut. 2020;263:114372. doi: 10.1016/j.envpol.2020.114372. [DOI] [PubMed] [Google Scholar]
- 9.Van Bruggen A.H.C., He M.M., Shin K., Mai V., Jeong K.C., Finckh M.R., Morris J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018;616–617:255–268. doi: 10.1016/j.scitotenv.2017.10.309. [DOI] [PubMed] [Google Scholar]
- 10.Cox C. Glyphosate (roundup) J. Pestic. Reform. 1998;18:3–17. [Google Scholar]
- 11.Kier L.D., Kirkland D.J. Review of genotoxicity studies of glyphosate and glyphosate-based formulations. Crit. Rev. Toxicol. 2013;43:283–315. doi: 10.3109/10408444.2013.770820. [DOI] [PubMed] [Google Scholar]
- 12.Williams G.M., Aardema M., Acquavella J., Berry S.C., Brusick D., Burns M.M., De Camargo J.L.V., Garabrant D., Greim H.A., Kier L.D., et al. A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Crit. Rev. Toxicol. 2016;46:3–20. doi: 10.1080/10408444.2016.1214677. [DOI] [PubMed] [Google Scholar]
- 13.Martínez M.-A., Ares I., Rodríguez J.-L., Martínez M., Martínez-Larrañaga M.-R., Anadón A. Neurotransmitter changes in rat brain regions following glyphosate exposure. Environ. Res. 2018;161:212–219. doi: 10.1016/j.envres.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 14.Williams G.M., Kroes R., Munro I.C. Safety Evaluation and Risk Assessment of the Herbicide Roundup and Its Active Ingredient, Glyphosate, for Humans. Regul. Toxicol. Pharmacol. 2000;31:117–165. doi: 10.1006/rtph.1999.1371. [DOI] [PubMed] [Google Scholar]
- 15.Kanissery R., Gairhe B., Kadyampakeni D., Batuman O., Alferez F. Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition. Plants. 2019;8:499. doi: 10.3390/plants8110499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bento C.P.M., Yang X., Gort G., Xue S., van Dam R., Zomer P., Mol H.G.J., Ritsema C.J., Geissen V. Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci. Total Environ. 2016;572:301–311. doi: 10.1016/j.scitotenv.2016.07.215. [DOI] [PubMed] [Google Scholar]
- 17.Bergström L., Börjesson E., Stenström J. Laboratory and Lysimeter Studies of Glyphosate and Aminomethylphosphonic Acid in a Sand and a Clay Soil. J. Environ. Qual. 2011;40:98–108. doi: 10.2134/jeq2010.0179. [DOI] [PubMed] [Google Scholar]
- 18.Laitinen P., Siimes K., Eronen L., Rämö S., Welling L., Oinonen S., Mattsoff L., Ruohonen-Lehto M. Fate of the herbicides glyphosate, glufosinate-ammonium, phenmedipham, ethofumesate and metamitron in two Finnish arable soils. Pest Manag. Sci. 2006;62:473–491. doi: 10.1002/ps.1186. [DOI] [PubMed] [Google Scholar]
- 19.Tzanetou E., Karasali H. Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production. IntechOpen; London, UK: 2020. Glyphosate Residues in Soil and Air: An Integrated Review. [DOI] [Google Scholar]
- 20.De Andrea M.M., Peres T.B., Luchini L.C., Bazarin S., Papini S., Matallo M.B., Savoy V.L.T. Influence of repeated applications of glyphosate on its persistence and soil bioactivity. Pesqui. Agropecuária Bras. 2003;38:1329–1335. doi: 10.1590/S0100-204X2003001100012. [DOI] [Google Scholar]
- 21.Carretta L., Cardinali A., Onofri A., Masin R., Zanin G. Dynamics of Glyphosate and Aminomethylphosphonic Acid in Soil Under Conventional and Conservation Tillage. Int. J. Environ. Res. 2021;15:1037–1055. doi: 10.1007/s41742-021-00369-3. [DOI] [Google Scholar]
- 22.Leoci R., Ruberti M. Glyphosate in Agriculture: Environmental Persistence and Effects on Animals. A Review. J. Agric. Environ. Int. Dev. 2020;114:99–122. doi: 10.12895/JAEID.20201.1167. [DOI] [Google Scholar]
- 23.Battaglin W.A., Meyer M.T., Kuivila K.M., Dietze J.E. Glyphosate and Its Degradation Product AMPA Occur Frequently and Widely in U.S. Soils, Surface Water, Groundwater, and Precipitation. JAWRA J. Am. Water Resour. Assoc. 2014;50:275–290. doi: 10.1111/jawr.12159. [DOI] [Google Scholar]
- 24.Berman M.C., Marino D., Quiroga M.V., Zagarese H. Occurrence and levels of glyphosate and AMPA in shallow lakes from the Pampean and Patagonian regions of Argentina. Chemosphere. 2018;200:513–522. doi: 10.1016/j.chemosphere.2018.02.103. [DOI] [PubMed] [Google Scholar]
- 25.Mercurio P., Flores F., Mueller J.F., Carter S., Negri A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014;85:385–390. doi: 10.1016/j.marpolbul.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2018;16 doi: 10.2903/j.efsa.2018.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edge C., Brown M., Heartz S., Thompson D., Ritter L., Ramadoss M. The Persistence of Glyphosate in Vegetation One Year after Application. Forests. 2021;12:601. doi: 10.3390/f12050601. [DOI] [Google Scholar]
- 28.Niemann L., Sieke C., Pfeil R., Solecki R. A critical review of glyphosate findings in human urine samples and comparison with the exposure of operators and consumers. J. Verbr. Lebensm. 2015;10:3–12. doi: 10.1007/s00003-014-0927-3. [DOI] [Google Scholar]
- 29.Reddy K.N., Rimando A.M., Duke S.O., Nandula V.K. Aminomethylphosphonic Acid Accumulation in Plant Species Treated with Glyphosate. J. Agric. Food Chem. 2008;56:2125–2130. doi: 10.1021/jf072954f. [DOI] [PubMed] [Google Scholar]
- 30.Acquavella J.F., Alexander B.H., Mandel J.S., Gustin C., Baker B., Chapman P., Bleeke M. Glyphosate biomonitoring for farmers and their families: Results from the Farm Family Exposure Study. Environ. Health Perspect. 2004;112:321–326. doi: 10.1289/ehp.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conrad A., Schröter-Kermani C., Hoppe H.-W., Rüther M., Pieper S., Kolossa-Gehring M. Glyphosate in German adults—Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health. 2017;220:8–16. doi: 10.1016/j.ijheh.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Krüger M., Schledorn P., Schrödl W., Hoppe H.W., Lutz W., Shehata A.A. Detection of Glyphosate Residues in Animals and Humans. J. Environ. Anal. Toxicol. 2014;4:1000210. doi: 10.4172/2161-0525.1000210. [DOI] [Google Scholar]
- 33.Krüger M., Schrödl W., Pedersen I. Detection of Glyphosate in Malformed Piglets. J. Environ. Anal. Toxicol. 2014;4:1000230. doi: 10.4172/2161-0525.1000230. [DOI] [Google Scholar]
- 34.Tarazona J.V., Court-Marques D., Tiramani M., Reich H., Pfeil R., Istace F., Crivellente F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017;91:2723–2743. doi: 10.1007/s00204-017-1962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Currie Z., Prosser R.S., Rodriguez-Gil J.L., Mahon K., Poirier D., Solomon K.R. Toxicity of Cúspide 480SL® spray mixture formulation of glyphosate to aquatic organisms. Environ. Toxicol. Chem. 2015;34:1178–1184. doi: 10.1002/etc.2913. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Smeda R.J., Sellers B.A., Johnson W.G. Influence of formulation and glyphosate salt on absorption and translocation in three annual weeds. Weed Sci. 2005;53:153–159. doi: 10.1614/WS-03-075R1. [DOI] [Google Scholar]
- 37.Cattani D., Cavalli V.L.D.L.O., Rieg C.E.H., Domingues J.T., Dal-Cim T., Tasca C.I., Silva F.R.M.B., Zamoner A. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: Involvement of glutamate excitotoxicity. Toxicology. 2014;320:34–45. doi: 10.1016/j.tox.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Bringolf R.B., Cope W.G., Mosher S., Barnhart M.C., Shea D. Acute and chronic toxicity of glyphosate compounds to glochidia and juveniles of lampsilis siliquoidea (unionidae) Environ. Toxicol. Chem. 2007;26:2094–2100. doi: 10.1897/06-519R1.1. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues L.D.B., de Oliveira R., Abe F.R., Brito L.B., Moura D.S., Valadares M.C., Grisolia C.K., de Oliveira D.P., de Oliveira G.A.R. Ecotoxicological assessment of glyphosate-based herbicides: Effects on different organisms. Environ. Toxicol. Chem. 2016;36:1755–1763. doi: 10.1002/etc.3580. [DOI] [PubMed] [Google Scholar]
- 40.Mesnage R., Bernay B., Séralini G.-E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. 2013;313:122–128. doi: 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Nobels I., Spanoghe P., Haesaert G., Robbens J., Blust R. Toxicity Ranking and Toxic Mode of Action Evaluation of Commonly Used Agricultural Adjuvants on the Basis of Bacterial Gene Expression Profiles. PLoS ONE. 2011;6:e24139. doi: 10.1371/journal.pone.0024139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prosser R.S., Rodriguez-Gil J.L., Solomon K.R., Sibley P.K., Poirier D.G. Effects of the herbicide surfactant MON 0818 on oviposition and viability of eggs of the ramshorn snail (Planorbella pilsbryi) Environ. Toxicol. Chem. 2016;36:522–531. doi: 10.1002/etc.3571. [DOI] [PubMed] [Google Scholar]
- 43.Bai S.H., Ogbourne S. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016;23:18988–19001. doi: 10.1007/s11356-016-7425-3. [DOI] [PubMed] [Google Scholar]
- 44.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuhrimann S., Farnham A., Staudacher P., Atuhaire A., Manfioletti T., Niwagaba C.B., Namirembe S., Mugweri J., Winkler M.S., Portengen L., et al. Exposure to multiple pesticides and neurobehavioral outcomes among smallholder farmers in Uganda. Environ. Int. 2021;152:106477. doi: 10.1016/j.envint.2021.106477. [DOI] [PubMed] [Google Scholar]
- 46.Lee J.-W., Choi Y.-J., Park S., Gil H.-W., Song H.-Y., Hong S.-Y. Serum S100 protein could predict altered consciousness in glyphosate or glufosinate poisoning patients. Clin. Toxicol. 2017;55:357–359. doi: 10.1080/15563650.2017.1286013. [DOI] [PubMed] [Google Scholar]
- 47.Von Ehrenstein O.S., Ling C., Cui X., Cockburn M., Park A.S., Yu F., Wu J., Ritz B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: Population based case-control study. BMJ. 2019;364:l962. doi: 10.1136/bmj.l962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C., Hu R., Huang J., Huang X., Shi G., Li Y., Yin Y., Chen Z. Health effect of agricultural pesticide use in China: Implications for the development of GM crops. Sci. Rep. 2016;6:34918. doi: 10.1038/srep34918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C., Sun Y., Hu R., Huang J., Huang X., Li Y., Yin Y., Chen Z. A comparison of the effects of agricultural pesticide uses on peripheral nerve conduction in China. Sci. Rep. 2018;8:9621. doi: 10.1038/s41598-018-27713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao Y., Zhang Y., Ni H., Gao J., Yang Y., Xu W., Tao L. Evaluation of the cytotoxic effects of glyphosate herbicides in human liver, lung, and nerve. J. Environ. Sci. Health Part B. 2019;54:737–744. doi: 10.1080/03601234.2019.1633215. [DOI] [PubMed] [Google Scholar]
- 51.Martinez A., Al-Ahmad A.J. Effects of glyphosate and aminomethylphosphonic acid on an isogeneic model of the human blood-brain barrier. Toxicol. Lett. 2018;304:39–49. doi: 10.1016/j.toxlet.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Martínez M.-A., Rodriguez-Gutierrez J.-L., Torres B.L., Martínez M., Martínez-Larrañaga M.-R., Maximiliano J.-E., Anadón A., Ares I. Use of human neuroblastoma SH-SY5Y cells to evaluate glyphosate-induced effects on oxidative stress, neuronal development and cell death signaling pathways. Environ. Int. 2019;135:105414. doi: 10.1016/j.envint.2019.105414. [DOI] [PubMed] [Google Scholar]
- 53.Luo J., Chen J., Deng Z.-L., Luo X., Song W.-X., Sharff K.A., Tang N., Haydon R.C., Luu H.H., He T.-C. Wnt signaling and human diseases: What are the therapeutic implications? Lab. Investig. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- 54.Okerlund N.D., Cheyette B.N.R. Synaptic Wnt signaling—a contributor to major psychiatric disorders? J. Neurodev. Disord. 2011;3:162–174. doi: 10.1007/s11689-011-9083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwan V., Unda B.K., Singh K.K. Wnt signaling networks in autism spectrum disorder and intellectual disability. J. Neurodev. Disord. 2016;8:45. doi: 10.1186/s11689-016-9176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Yuan X.-S., Wang Z., Li R. The canonical Wnt signaling pathway in autism. CNS Neurol. Disord.—Drug Targets. 2014;13:765–770. doi: 10.2174/1871527312666131223114149. [DOI] [PubMed] [Google Scholar]
- 57.Good P. Evidence the U.S. autism epidemic initiated by acetaminophen (Tylenol) is aggravated by oral antibiotic amoxicillin/clavulanate (Augmentin) and now exponentially by herbicide glyphosate (Roundup) Clin. Nutr. ESPEN. 2018;23:171–183. doi: 10.1016/j.clnesp.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Pu Y., Yang J., Chang L., Qu Y., Wang S., Zhang K., Xiong Z., Zhang J., Tan Y., Wang X., et al. Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA. 2020;117:11753–11759. doi: 10.1073/pnas.1922287117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson N.L., Leu A., Abrahamson J., Wallet B. Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. JOS. 2014;9:6–37. [Google Scholar]
- 60.Coultrap S.J., Vest R.S., Ashpole N.M., Hudmon A., Bayer K.U. CaMKII in cerebral ischemia. Acta Pharmacol. Sin. 2011;32:861–872. doi: 10.1038/aps.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X., Connelly J., Levitan E.S., Sun D., Wang J.Q. Calcium/Calmodulin–Dependent Protein Kinase II in Cerebrovascular Diseases. Transl. Stroke Res. 2021;12:513–529. doi: 10.1007/s12975-021-00901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bali Y.A., Ba-Mhamed S., Bennis M. Behavioral and Immunohistochemical Study of the Effects of Subchronic and Chronic Exposure to Glyphosate in Mice. Front. Behav. Neurosci. 2017;11:146. doi: 10.3389/fnbeh.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bali Y.A., Kaikai N.-E., Ba-M’Hamed S., Bennis M. Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology. 2019;415:18–25. doi: 10.1016/j.tox.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Ait-Bali Y., Ba-M’Hamed S., Gambarotta G., Sassoè-Pognetto M., Giustetto M., Bennis M. Pre- and postnatal exposure to glyphosate-based herbicide causes behavioral and cognitive impairments in adult mice: Evidence of cortical ad hippocampal dysfunction. Arch. Toxicol. 2020;94:1703–1723. doi: 10.1007/s00204-020-02677-7. [DOI] [PubMed] [Google Scholar]
- 65.Baier C.J., Gallegos C.E., Raisman-Vozari R., Minetti A. Behavioral impairments following repeated intranasal glyphosate-based herbicide administration in mice. Neurotoxicology Teratol. 2017;64:63–72. doi: 10.1016/j.ntt.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Cattani D., Struyf N., Steffensen V., Bergquist J., Zamoner A., Brittebo E., Andersson M. Perinatal exposure to a glyphosate-based herbicide causes dysregulation of dynorphins and an increase of neural precursor cells in the brain of adult male rats. Toxicology. 2021;461:152922. doi: 10.1016/j.tox.2021.152922. [DOI] [PubMed] [Google Scholar]
- 67.Coullery R., Pacchioni A.M., Rosso S.B. Exposure to glyphosate during pregnancy induces neurobehavioral alterations and downregulation of Wnt5a-CaMKII pathway. Reprod. Toxicol. 2020;96:390–398. doi: 10.1016/j.reprotox.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Dechartres J., Pawluski J.L., Gueguen M., Jablaoui A., Maguin E., Rhimi M., Charlier T.D. Glyphosate and glyphosate-based herbicide exposure during the peripartum period affects maternal brain plasticity, maternal behaviour and microbiome. J. Neuroendocr. 2019;31:e12731. doi: 10.1111/jne.12731. [DOI] [PubMed] [Google Scholar]
- 69.Gallegos C.E., Bartos M., Gumilar F., Raisman-Vozari R., Minetti A., Baier C.J. Intranasal glyphosate-based herbicide administration alters the redox balance and the cholinergic system in the mouse brain. NeuroToxicology. 2020;77:205–215. doi: 10.1016/j.neuro.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Hernández-Plata I., Giordano M., Díaz-Muñoz M., Rodríguez V.M. The herbicide glyphosate causes behavioral changes and alterations in dopaminergic markers in male Sprague-Dawley rat. NeuroToxicology. 2015;46:79–91. doi: 10.1016/j.neuro.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Ji H., Xu L., Wang Z., Fan X., Wu L. Differential microRNA expression in the prefrontal cortex of mouse offspring induced by glyphosate exposure during pregnancy and lactation. Exp. Ther. Med. 2017;15:2457–2467. doi: 10.3892/etm.2017.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joaquim A., Spinosa H., Macrini D.J., Rodrigues P.A., Ricci E.L., Artiolli T.S., Moreira N., Suffredini I.B., Bernardi M.M. Behavioral effects of acute glyphosate exposure in male and female Balb/c mice. Braz. J. Vet. Res. Anim. Sci. 2012;49:367–376. doi: 10.11606/issn.2318-3659.v49i5p367-376. [DOI] [Google Scholar]
- 73.Larsen K.E., Lifschitz A.L., Lanusse C.E., Virkel G.L. The herbicide glyphosate is a weak inhibitor of acetylcholinesterase in rats. Environ. Toxicol. Pharmacol. 2016;45:41–44. doi: 10.1016/j.etap.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Luna S., Neila L.P., Vena R., Borgatello C., Rosso S.B. Glyphosate exposure induces synaptic impairment in hippocampal neurons and cognitive deficits in developing rats. Arch. Toxicol. 2021;95:2137–2150. doi: 10.1007/s00204-021-03046-8. [DOI] [PubMed] [Google Scholar]
- 75.Gui Y.-X., Fan X.-N., Wang H.-M., Wang G., Chen S.-D. Glyphosate induced cell death through apoptotic and autophagic mechanisms. Neurotoxicol. Teratol. 2012;34:344–349. doi: 10.1016/j.ntt.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Masood M.I., Naseem M., Warda S.A., Tapia-Laliena M., Rehman H.U., Nasim M.J., Schäfer K.H. Environment permissible concentrations of glyphosate in drinking water can influence the fate of neural stem cells from the subventricular zone of the postnatal mouse. Environ. Pollut. 2020;270:116179. doi: 10.1016/j.envpol.2020.116179. [DOI] [PubMed] [Google Scholar]
- 77.Da Silva K.N., Cappellaro L.G., Ueda C.N., Rodrigues L., Remor A.P., Martins R.D.P., Latini A., Glaser V. Glyphosate-based herbicide impairs energy metabolism and increases autophagy in C6 astroglioma cell line. J. Toxicol. Environ. Health Part A. 2020;83:153–167. doi: 10.1080/15287394.2020.1731897. [DOI] [PubMed] [Google Scholar]
- 78.Szepanowski F., Szepanowski L.-P., Mausberg A.K., Albrecht P., Kleinschnitz C., Kieseier B.C., Stettner M. Differential impact of pure glyphosate and glyphosate-based herbicide in a model of peripheral nervous system myelination. Acta Neuropathol. 2018;136:979–982. doi: 10.1007/s00401-018-1938-4. [DOI] [PubMed] [Google Scholar]
- 79.Szepanowski F., Kleinschnitz C., Stettner M. Glyphosate-based herbicide: A risk factor for demyelinating conditions of the peripheral nervous system? Neural Regen. Res. 2019;14:2079–2080. doi: 10.4103/1673-5374.262579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arredondo S.B., Guerrero F.G., Herrera-Soto A., Jensen-Flores J., Bustamante D.B., Oñate-Ponce A., Henny P., Varas-Godoy M., Inestrosa N.C., Varela-Nallar L. Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem Cells. 2019;38:422–436. doi: 10.1002/stem.3121. [DOI] [PubMed] [Google Scholar]
- 81.Habtemariam S. The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: New pharmacological concepts for old and new drugs. Neural Regen. Res. 2018;13:983–984. doi: 10.4103/1673-5374.233438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murray P.S., Holmes P.V. An Overview of Brain-Derived Neurotrophic Factor and Implications for Excitotoxic Vulnerability in the Hippocampus. Int. J. Pept. 2011;2011:1–12. doi: 10.1155/2011/654085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rothermundt M., Peters M., Prehn J., Arolt V. S100B in brain damage and neurodegeneration. Microsc. Res. Tech. 2003;60:614–632. doi: 10.1002/jemt.10303. [DOI] [PubMed] [Google Scholar]
- 84.Pomper N., Liu Y., Hoye M.L., Dougherty J.D., Miller T.M. CNS microRNA profiles: A database for cell type enriched microRNA expression across the mouse central nervous system. Sci. Rep. 2020;10:4921. doi: 10.1038/s41598-020-61307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cruz C.D., Cruz F. The ERK 1 and 2 Pathway in the Nervous System: From Basic Aspects to Possible Clinical Applications in Pain and Visceral Dysfunction. Curr. Neuropharmacol. 2007;5:244–252. doi: 10.2174/157015907782793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun J., Nan G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target (Review) Int. J. Mol. Med. 2017;39:1338–1346. doi: 10.3892/ijmm.2017.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Subramaniam S., Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2009;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y., Luo P., Guo Q., Li S., Zhang L., Zhao M., Xu H., Yang Y., Poon W., Fei Z. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp. Neurol. 2012;237:489–498. doi: 10.1016/j.expneurol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 89.Jiang Q., Gu Z., Zhang G., Jing G. Diphosphorylation and involvement of extracellular signal-regulated kinases (ERK1/2) in glutamate-induced apoptotic-like death in cultured rat cortical neurons. Brain Res. 2000;857:71–77. doi: 10.1016/S0006-8993(99)02364-1. [DOI] [PubMed] [Google Scholar]
- 90.Satoh T., Nakatsuka D., Watanabe Y., Nagata I., Kikuchi H., Namura S. Neuroprotection by MAPK/ERK kinase inhibition with U0126 against oxidative stress in a mouse neuronal cell line and rat primary cultured cortical neurons. Neurosci. Lett. 2000;288:163–166. doi: 10.1016/S0304-3940(00)01229-5. [DOI] [PubMed] [Google Scholar]
- 91.Schwarzer C. 30 years of dynorphins—New insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 2009;123:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Albillos A., Carbone E., Gandia L., Garcia A.G., Polio A. Opioid Inhibition of Ca 2+ Channel Subtypes in Bovine Chromaffin Cells: Selectivity of Action and Voltage-dependence. Eur. J. Neurosci. 1996;8:1561–1570. doi: 10.1111/j.1460-9568.1996.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 93.Rittase W.B., Dong Y., Barksdale D., Galdzicki Z., Bausch S.B. Dynorphin up-regulation in the dentate granule cell mossy fiber pathway following chronic inhibition of GluN2B-containing NMDAR is associated with increased CREB (Ser 133) phosphorylation, but is independent of BDNF/TrkB signaling pathways. Mol. Cell. Neurosci. 2014;60:63–71. doi: 10.1016/j.mcn.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagner J.J., Terman G.W., Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nat. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sgroi S., Tonini R. Opioidergic Modulation of Striatal Circuits, Implications in Parkinson’s Disease and Levodopa Induced Dyskinesia. Front. Neurol. 2018;9:524. doi: 10.3389/fneur.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bridges R.S. Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Horm. Behav. 2015;77:193–203. doi: 10.1016/j.yhbeh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kohl J., Autry A.E., Dulac C. The neurobiology of parenting: A neural circuit perspective. BioEssays. 2016;39:e201600159-11. doi: 10.1002/bies.201600159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pawluski J.L., Lambert K.G., Kinsley C.H. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Horm. Behav. 2016;77:86–97. doi: 10.1016/j.yhbeh.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 99.Lau A., Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch.-Eur. J. Physiol. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 100.Moussawi K., Riegel A., Nair S., Kalivas P.W. Extracellular Glutamate: Functional Compartments Operate in Different Concentration Ranges. Front. Syst. Neurosci. 2011;5:94. doi: 10.3389/fnsys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simões A.P., Silva C.S.G., Marques J., Pochmann D., Porciúncula L.O., Ferreira S., Oses J.P., Beleza R.O., Real J.I., Köfalvi A., et al. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018;9:297. doi: 10.1038/s41419-018-0351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maurer S.V., Williams C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017;8:1489. doi: 10.3389/fimmu.2017.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Picciotto M.R., Higley M.J., Mineur Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jaffard R., Galey D., Micheau J., Durkin T. The cholinergic septo-hippocampal pathway, learning and memory. In: Will B.E., Schmitt P., Dalrymple-Alford J.C., editors. Brain Plasticity, Learning, and Memory. Springer; Boston, MA, USA: 1985. pp. 167–181. [Google Scholar]
- 105.Nicoll R.A. The septo-hippocampal projection: A model cholinergic pathway. Trends Neurosci. 1985;8:533–536. doi: 10.1016/0166-2236(85)90190-0. [DOI] [Google Scholar]
- 106.Bahena-Trujillo R., Flores G., Arias-Montaño J.A. Dopamina: Síntesis, liberación y receptores en el Sistema Nervioso Central. Rev. Biomed. 2000;11:39–60. doi: 10.32776/REVBIOMED.V11I1.218. [DOI] [Google Scholar]
- 107.Gudi V., Gai L., Herder V., Tejedor L.S., Kipp M., Amor S., Sühs K.-W., Hansmann F., Beineke A., Baumgärtner W., et al. Synaptophysin Is a Reliable Marker for Axonal Damage. J. Neuropathol. Exp. Neurol. 2017;76:109–125. doi: 10.1093/jnen/nlw114. [DOI] [PubMed] [Google Scholar]
- 108.Salim S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017;360:201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baba S.P., Bhatnagar A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018;7:133–139. doi: 10.1016/j.cotox.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harris C., Hansen J.M. Oxidative stress, thiols, and redox profiles. In: Harris C., Hansen J., editors. Developmental Toxicology. Humana Press; Totowa, NJ, USA: 2012. pp. 325–346. [DOI] [PubMed] [Google Scholar]
- 112.Grant R.S., Guest J.A. Effects of dietary derived antioxidants on the central nervous system. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012;2:185. doi: 10.4103/2231-0738.99470. [DOI] [Google Scholar]
- 113.Peng L. Mice Brain Tissue Injury Induced by Diisononyl Phthalate Exposure and the Protective Application of Vitamin, E.J. Biochem. Mol. Toxicol. 2015;29:311–320. doi: 10.1002/jbt.21700. [DOI] [PubMed] [Google Scholar]
- 114.Kwon D.H., Cha H.-J., Lee H., Hong S.-H., Park C., Park S.-H., Kim G.-Y., Kim S., Kim H.-S., Hwang H.-J., et al. Protective Effect of Glutathione against Oxidative Stress-induced Cytotoxicity in RAW 264.7 Macrophages through Activating the Nuclear Factor Erythroid 2-Related Factor-2/Heme Oxygenase-1 Pathway. Antioxidants. 2019;8:82. doi: 10.3390/antiox8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tan B.L., Norhaizan M.E., Liew W.-P.-P., Rahman H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018;9:1162. doi: 10.3389/fphar.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar S., Trivedi P.K. Glutathione S-Transferases: Role in Combating Abiotic Stresses Including Arsenic Detoxification in Plants. Front. Plant Sci. 2018;9:751. doi: 10.3389/fpls.2018.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Astiz M., de Alaniz M.J., Marra C.A. Effect of pesticides on cell survival in liver and brain rat tissues. Ecotoxicol. Environ. Saf. 2009;72:2025–2032. doi: 10.1016/j.ecoenv.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Yang Q., Zhou J. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia. 2018;67:1017–1035. doi: 10.1002/glia.23571. [DOI] [PubMed] [Google Scholar]
- 119.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yuste J.E., Tarragon E., Campuzano C.M., Cros E.T. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015;9:322. doi: 10.3389/fncel.2015.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saraste A., Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000;45:528–537. doi: 10.1016/S0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 123.Badadani M. Autophagy Mechanism, Regulation, Functions, and Disorders. ISRN Cell Biol. 2012;2012:927064. doi: 10.5402/2012/927064. [DOI] [Google Scholar]
- 124.Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem. Biophys. Res. Commun. 2018;500:26–34. doi: 10.1016/j.bbrc.2017.06.190. [DOI] [PubMed] [Google Scholar]
- 125.Mizushima N. Autophagy: Process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 126.Stavoe A., Holzbaur E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019;35:477–500. doi: 10.1146/annurev-cellbio-100818-125242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Babin P.J., Goizet C., Raldúa D. Zebrafish models of human motor neuron diseases: Advantages and limitations. Prog. Neurobiol. 2014;118:36–58. doi: 10.1016/j.pneurobio.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 128.Fitzgerald J.A., Könemann S., Krümpelmann L., Županič A., Berg C.V. Approaches to Test the Neurotoxicity of Environmental Contaminants in the Zebrafish Model: From Behavior to Molecular Mechanisms. Environ. Toxicol. Chem. 2020;40:989–1006. doi: 10.1002/etc.4951. [DOI] [PubMed] [Google Scholar]
- 129.Flinn L., Bretaud S., Lo C., Ingham P.W., Bandmann O. Zebrafish as a new animal model for movement disorders. J. Neurochem. 2008;106:1991–1997. doi: 10.1111/j.1471-4159.2008.05463.x. [DOI] [PubMed] [Google Scholar]
- 130.Faria M., Bedrossiantz J., Ramírez J.R.R., Mayol M., García G.H., Bellot M., Prats E., Garcia-Reyero N., Gómez-Canela C., Gómez-Oliván L.M., et al. Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environ. Int. 2020;146:106253. doi: 10.1016/j.envint.2020.106253. [DOI] [PubMed] [Google Scholar]
- 131.Horzmann K.A., Freeman J.L. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics. 2016;4:19. doi: 10.3390/toxics4030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bernal-Rey D.L., Cantera C.G., Afonso M.D.S., Menéndez-Helman R.J. Seasonal variations in the dose-response relationship of acetylcholinesterase activity in freshwater fish exposed to chlorpyrifos and glyphosate. Ecotoxicol. Environ. Saf. 2019;187:109673. doi: 10.1016/j.ecoenv.2019.109673. [DOI] [PubMed] [Google Scholar]
- 133.Braz-Mota S., Sadauskas-Henrique H., Duarte R., Val A., Almeida-Val V.M. Roundup® exposure promotes gills and liver impairments, DNA damage and inhibition of brain cholinergic activity in the Amazon teleost fish Colossoma macropomum. Chemosphere. 2015;135:53–60. doi: 10.1016/j.chemosphere.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 134.Bridi D., Altenhofen S., Gonzalez J.B., Reolon G.K., Bonan C.D. Glyphosate and Roundup® alter morphology and behavior in zebrafish. Toxicology. 2017;392:32–39. doi: 10.1016/j.tox.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 135.Díaz-Martín R.D., Valencia-Hernández J.D., Betancourt-Lozano M., Yáñez-Rivera B. Changes in microtubule stability in zebrafish (Danio rerio) embryos after glyphosate exposure. Heliyon. 2021;7:e06027. doi: 10.1016/j.heliyon.2021.e06027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Teixeira J.M.D.S., Lima V.D.S., De Moura F.R., Marisco P.D.C., Sinhorin A.P., Sinhorin V.D.G. Acute toxicity and effects of Roundup Original® on pintado da Amazônia. Environ. Sci. Pollut. Res. 2018;25:25383–25389. doi: 10.1007/s11356-018-2630-x. [DOI] [PubMed] [Google Scholar]
- 137.Forner-Piquer I., Faucherre A., Byram J., Blaquiere M., de Bock F., Gamet-Payrastre L., Ellero-Simatos S., Audinat E., Jopling C., Marchi N. Differential impact of dose-range glyphosate on locomotor behavior, neuronal activity, glio-cerebrovascular structures, and transcript regulations in zebrafish larvae. Chemosphere. 2020;267:128986. doi: 10.1016/j.chemosphere.2020.128986. [DOI] [PubMed] [Google Scholar]
- 138.Jin J., Kurobe T., Ramírez-Duarte W.F., Bolotaolo M.B., Lam C.H., Pandey P.K., Hung T.-C., Stillway M.E., Zweig L., Caudill J., et al. Sub-lethal effects of herbicides penoxsulam, imazamox, fluridone and glyphosate on Delta Smelt (Hypomesus transpacificus) Aquat. Toxicol. 2018;197:79–88. doi: 10.1016/j.aquatox.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 139.Khan A.K., Shah N., Gul A., Sahar N.U., Ismail A., Muhammad M., Aziz F., Farooq M., Adnan M., Rizwan M. Comparative Study of Toxicological Impinge of Glyphosate and Atrazine (Herbicide) on Stress Biomarkers; Blood Biochemical and Hematological Parameters of the Freshwater Common Carp (Cyprinus carpio) Pol. J. Environ. Stud. 2016;25:1995–2001. doi: 10.15244/pjoes/62698. [DOI] [Google Scholar]
- 140.Lanzarin G.A.B., Félix L.M., Santos D., Venâncio C.A.S., Monteiro S.M. Dose-dependent effects of a glyphosate commercial formulation–Roundup® UltraMax-on the early zebrafish embryogenesis. Chemosphere. 2019;223:514–522. doi: 10.1016/j.chemosphere.2019.02.071. [DOI] [PubMed] [Google Scholar]
- 141.Lanzarin G.A., Venâncio C., Monteiro S.M., Félix L.M. Behavioural toxicity of environmental relevant concentrations of a glyphosate commercial formulation-RoundUp® UltraMax-During zebrafish embryogenesis. Chemosphere. 2020;253:126636. doi: 10.1016/j.chemosphere.2020.126636. [DOI] [PubMed] [Google Scholar]
- 142.Li M.-H., Xu H.-D., Liu Y., Chen T., Jiang L., Fu Y.-H., Wang J.-S. Multi-tissue metabolic responses of goldfish (Carassius auratus) exposed to glyphosate-based herbicide. Toxicol. Res. 2016;5:1039–1052. doi: 10.1039/C6TX00011H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li M., Ruan L.-Y., Zhou J.-W., Fu Y.-H., Jiang L., Zhao H., Wang J.-S. Metabolic profiling of goldfish (Carassius auratis) after long-term glyphosate-based herbicide exposure. Aquat. Toxicol. 2017;188:159–169. doi: 10.1016/j.aquatox.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 144.Lopes F.M., Caldas S.S., Primel E.G., da Rosa C.E. Glyphosate Adversely Affects Danio rerio Males: Acetylcholinesterase Modulation and Oxidative Stress. Zebrafish. 2017;14:97–105. doi: 10.1089/zeb.2016.1341. [DOI] [PubMed] [Google Scholar]
- 145.Menéndez-Helman R.J., Ferreyroa G.V., Afonso M.D.S., Salibián A. Glyphosate as an Acetylcholinesterase Inhibitor in Cnesterodon decemmaculatus. Bull. Environ. Contam. Toxicol. 2011;88:6–9. doi: 10.1007/s00128-011-0423-8. [DOI] [PubMed] [Google Scholar]
- 146.Menéndez-Helman R.J., Miranda L.A., Afonso M.D.S., Salibián A. Subcellular energy balance of Odontesthes bonariensis exposed to a glyphosate-based herbicide. Ecotoxicol. Environ. Saf. 2015;114:157–163. doi: 10.1016/j.ecoenv.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 147.Pereira A.G., Jaramillo M.L., Remor A.P., Latini A., Davico C.E., da Silva M.L., Müller Y.M., Ammar D., Nazari E.M. Low-concentration exposure to glyphosate-based herbicide modulates the complexes of the mitochondrial respiratory chain and induces mitochondrial hyperpolarization in the Danio rerio brain. Chemosphere. 2018;209:353–362. doi: 10.1016/j.chemosphere.2018.06.075. [DOI] [PubMed] [Google Scholar]
- 148.Roy N.M., Carneiro B., Ochs J. Glyphosate induces neurotoxicity in zebrafish. Environ. Toxicol. Pharmacol. 2016;42:45–54. doi: 10.1016/j.etap.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 149.Sánchez J.A.A., Barros D.M., Bistoni M.D.L.A., Ballesteros M.L., Roggio M.A., Martins C.D.G.M. Glyphosate-based herbicides affect behavioural patterns of the livebearer Jenynsia multidentata. Environ. Sci. Pollut. Res. 2021;28:29958–29970. doi: 10.1007/s11356-020-11958-8. [DOI] [PubMed] [Google Scholar]
- 150.Sobjak T.M., Romão S., Nascimento C.Z.D., dos Santos A.F.P., Vogel L., Guimarães A.T.B. Assessment of the oxidative and neurotoxic effects of glyphosate pesticide on the larvae of Rhamdia quelen fish. Chemosphere. 2017;182:267–275. doi: 10.1016/j.chemosphere.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 151.Zhang S., Xu J., Kuang X., Li S., Li X., Chen D., Zhao X., Feng X. Biological impacts of glyphosate on morphology, embryo biomechanics and larval behavior in zebrafish ( Danio rerio ) Chemosphere. 2017;181:270–280. doi: 10.1016/j.chemosphere.2017.04.094. [DOI] [PubMed] [Google Scholar]
- 152.Carhart-Harris R.L., Nutt D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017;31:1091–1120. doi: 10.1177/0269881117725915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Strużyńska L., Sulkowski G. Relationships between glutamine, glutamate, and GABA in nerve endings under Pb-toxicity conditions. J. Inorg. Biochem. 2004;98:951–958. doi: 10.1016/j.jinorgbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 154.Zorova L.D., Popkov V.A., Plotnikov E.Y., Silachev D.N., Pevzner I.B., Jankauskas S.S., Babenko V.A., Zorov S.D., Balakireva A.V., Juhaszova M., et al. Mitochondrial membrane potential. Anal. Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tayebati S., Marucci G., Santinelli C., Buccioni M., Amenta F. Choline-Containing Phospholipids: Structure-Activity Relationships Versus Therapeutic Applications. Curr. Med. Chem. 2015;22:4328–4340. doi: 10.2174/0929867322666151029104152. [DOI] [PubMed] [Google Scholar]
- 156.Zahr N.M., Mayer D., Rohlfing T., Sullivan E.V., Pfefferbaum A. Imaging Neuroinflammation? A Perspective from MR Spectroscopy. Brain Pathol. 2014;24:654–664. doi: 10.1111/bpa.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He B., Ni Y., Jin Y., Fu Z. Pesticides-induced energy metabolic disorders. Sci. Total Environ. 2020;729:139033. doi: 10.1016/j.scitotenv.2020.139033. [DOI] [PubMed] [Google Scholar]
- 158.Dienel G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019;99:949–1045. doi: 10.1152/physrev.00062.2017. [DOI] [PubMed] [Google Scholar]
- 159.Holtzman D. Brain Creatine Kinases and Phosphocreatine: An Update. Dev. Neurosci. 1996;18:522–523. doi: 10.1159/000111449. [DOI] [PubMed] [Google Scholar]
- 160.Cunnane S.C., Courchesne-Loyer A., Vandenberghe C., St-Pierre V., Fortier M., Hennebelle M., Croteau E., Bocti C., Fulop T., Castellano C.-A. Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer’s Disease. Front. Mol. Neurosci. 2016;9:53. doi: 10.3389/fnmol.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tovar-Franco J.A., Ortiz M.J.S., Morales L., Espíndola C. Regulación de la utilización del aspartato por los astrocitos durante la prelactancia. Univ. Sci. 2009;14:151–163. doi: 10.11144/javeriana.SC14-2-3.rdlu. [DOI] [Google Scholar]
- 162.Moffett J.R., Arun P., Ariyannur P.S., Namboodiri A.M. N-Acetylaspartate reductions in brain injury: Impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Front. Neuroenergetics. 2013;5:11. doi: 10.3389/fnene.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Domise M., Sauvé F., Didier S., Caillerez R., Bégard S., Carrier S., Colin M., Marinangeli C., Buée L., Vingtdeux V. Neuronal AMP-activated protein kinase hyper-activation induces synaptic loss by an autophagy-mediated process. Cell Death Dis. 2019;10:221. doi: 10.1038/s41419-019-1464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McVey K.A., Snapp I.B., Johnson M.B., Negga R., Pressley A.S., Fitsanakis V.A. Exposure of C. elegans eggs to a glyphosate-containing herbicide leads to abnormal neuronal morphology. Neurotoxicol. Teratol. 2016;55:23–31. doi: 10.1016/j.ntt.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burchfield S.L., Bailey D.C., Todt C.E., Denney R.D., Negga R., Fitsanakis V.A. Acute exposure to a glyphosate-containing herbicide formulation inhibits Complex II and increases hydrogen peroxide in the model organism Caenorhabditis elegans. Environ. Toxicol. Pharmacol. 2018;66:36–42. doi: 10.1016/j.etap.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fortes C., Mastroeni S., Segatto M.M., Hohmann C., Miligi L., Bakos L., Bonamigo R. Occupational Exposure to Pesticides With Occupational Sun Exposure Increases the Risk for Cutaneous Melanoma. J. Occup. Environ. Med. 2016;58:370–375. doi: 10.1097/JOM.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 167.Jayasumana C., Gunatilake S., Senanayake P. Glyphosate, Hard Water and Nephrotoxic Metals: Are They the Culprits Behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka? Int. J. Environ. Res. Public Health. 2014;11:2125–2147. doi: 10.3390/ijerph110202125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kamel F., Hoppin J.A. Association of Pesticide Exposure with Neurologic Dysfunction and Disease. Environ. Health Perspect. 2004;112:950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee W.J., Alavanja M.C., Hoppin J., Rusiecki J.A., Kamel F., Blair A., Sandler D.P. Mortality among Pesticide Applicators Exposed to Chlorpyrifos in the Agricultural Health Study. Environ. Health Perspect. 2007;115:528–534. doi: 10.1289/ehp.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Le Couteur D., McLean A., Taylor M., Woodham B., Board P. Pesticides and Parkinson’s disease. Biomed. Pharmacother. 1999;53:122–130. doi: 10.1016/S0753-3322(99)80077-8. [DOI] [PubMed] [Google Scholar]
- 171.Gasnier C., Dumont C., Benachour N., Clair E., Chagnon M.-C., Séralini G.-E. Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology. 2009;262:184–191. doi: 10.1016/j.tox.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 172.Koller V.J., Fürhacker M., Nersesyan A., Mišík M., Eisenbauer M., Knasmueller S. Cytotoxic and DNA-damaging properties of glyphosate and Roundup in human-derived buccal epithelial cells. Arch. Toxicol. 2012;86:805–813. doi: 10.1007/s00204-012-0804-8. [DOI] [PubMed] [Google Scholar]
- 173.Prasad S., Srivastava S., Singh M., Shukla Y. Clastogenic Effects of Glyphosate in Bone Marrow Cells of Swiss Albino Mice. J. Toxicol. 2009;2009:308985. doi: 10.1155/2009/308985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lajmanovich R.C., Sandoval M.T., Peltzer P.M. Induction of Mortality and Malformation in Scinax nasicus Tadpoles Exposed to Glyphosate Formulations. Bull. Environ. Contam. Toxicol. 2003;70:612–618. doi: 10.1007/s00128-003-0029-x. [DOI] [PubMed] [Google Scholar]
- 175.Barbosa E.R., da Costa M.D.L., Bacheschi L.A., Scaff M., Leite C.C. Parkinsonism after glycine-derivate exposure. Mov. Disord. 2001;16:565–568. doi: 10.1002/mds.1105. [DOI] [PubMed] [Google Scholar]
- 176.Menkes D., Temple W., Edwards I. Intentional Self-Poisoning with Glyphosate-Containing Herbicides. Hum. Exp. Toxicol. 1991;10:103–107. doi: 10.1177/096032719101000202. [DOI] [PubMed] [Google Scholar]
- 177.Mose T., Kjaerstad M.B., Mathiesen L., Nielsen J.B., Edelfors S., Knudsen L.E. Placental Passage of Benzoic acid, Caffeine, and Glyphosate in an Ex Vivo Human Perfusion System. J. Toxicol. Environ. Health Part A. 2008;71:984–991. doi: 10.1080/01932690801934513. [DOI] [PubMed] [Google Scholar]
- 178.Poulsen M.S., Rytting E., Mose T., Knudsen L.E. Modeling placental transport: Correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol. Vitr. 2009;23:1380–1386. doi: 10.1016/j.tiv.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 179.Wooltorton J., Pidoplichko V.I., Broide R.S., Dani J.A. Differential Desensitization and Distribution of Nicotinic Acetylcholine Receptor Subtypes in Midbrain Dopamine Areas. J. Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Balali-Mood M., Saber H. Recent Advances in the Treatment of Organophosphorous Poisonings. Iran. J. Med Sci. 2012;37:74–91. [PMC free article] [PubMed] [Google Scholar]
- 181.Scheffel C., Niessen K.V., Rappenglück S., Wanner K., Thiermann H., Worek F., Seeger T. Counteracting desensitization of human α7-nicotinic acetylcholine receptors with bispyridinium compounds as an approach against organophosphorus poisoning. Toxicol. Lett. 2018;293:149–156. doi: 10.1016/j.toxlet.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 182.Wojda U., Salinska E., Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;60:575–590. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- 183.De A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Biophys. Sin. 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 184.McMurry J.L., Chrestensen C.A., Scott I.M., Lee E.W., Rahn A.M., Johansen A.M., Forsberg B.J., Harris K.D., Salerno J.C. Rate, affinity and calcium dependence of nitric oxide synthase isoform binding to the primary physiological regulator calmodulin. FEBS J. 2011;278:4943–4954. doi: 10.1111/j.1742-4658.2011.08395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Donato R., Sorci G., Riuzzi F., Arcuri C., Bianchi R., Brozzi F., Tubaro C., Giambanco I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta. 2009;1793:1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 186.Bianchi R., Giambanco I., Donato R. S100B/RAGE-dependent activation of microglia via NF-κB and AP-1: Co-regulation of COX-2 expression by S100B, IL-1β and TNF-α. Neurobiol. Aging. 2010;31:665–677. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 187.Petrova T.V., Hu J., Van Eldik L.J. Modulation of glial activation by astrocyte-derived protein S100B: Differential responses of astrocyte and microglial cultures. Brain Res. 2000;853:74–80. doi: 10.1016/S0006-8993(99)02251-9. [DOI] [PubMed] [Google Scholar]
- 188.Sun F., Xu X., Wang X., Zhang B. Regulation of autophagy by Ca2+ Tumor Biol. 2016;37:15467–15476. doi: 10.1007/s13277-016-5353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ríos J.A., Godoy J.A., Inestrosa N.C. Wnt3a ligand facilitates autophagy in hippocampal neurons by modulating a novel GSK-3β-AMPK axis. Cell Commun. Signal. 2018;16:15. doi: 10.1186/s12964-018-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lorzadeh S., Kohan L., Ghavami S., Azarpira N. Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochim. Biophys. Acta. 2020;1868:118926. doi: 10.1016/j.bbamcr.2020.118926. [DOI] [PubMed] [Google Scholar]
- 191.Hollville E., Romero S.E., Deshmukh M. Apoptotic cell death regulation in neurons. FEBS J. 2019;286:3276–3298. doi: 10.1111/febs.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fricker M., Tolkovsky A.M., Borutaite V., Coleman M., Brown G.C. Neuronal Cell Death. Physiol. Rev. Am. Physiol. Soc. 2018;98:813–880. doi: 10.1152/physrev.00011.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.World Health Organization Pesticide Residues in Food-2004: Evaluations 2004: Part II-Toxicological (Vol. 178). World Health Organization. 2006. [(accessed on 30 July 2021)]. Available online: http://apps.who.int/iris/bitstream/handle/10665/43624/9241665203_eng.pdf;jsessionid=98409D614439EBC6C58392A3809839C4?sequence=1.
- 194.Solomon K.R. Glyphosate in the general population and in applicators: A critical review of studies on exposures. Crit. Rev. Toxicol. 2016;46:21–27. doi: 10.1080/10408444.2016.1214678. [DOI] [PubMed] [Google Scholar]
- 195.Paumgartten F.J.R. To be or not to be a carcinogen; delving into the glyphosate classification controversy. Braz. J. Pharm. Sci. 2019;55:2175-97902019000118217. doi: 10.1590/s2175-97902019000118217. [DOI] [Google Scholar]
- 196.Kawagashira Y., Koike H., Kawabata K., Takahashi M., Ohyama K., Hashimoto R., Iijima M., Katsuno M., Sobue G. Vasculitic Neuropathy Following Exposure to a Glyphosate-based Herbicide. Intern. Med. 2017;56:1431–1434. doi: 10.2169/internalmedicine.56.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ramwell C.T., Heather A.I.J., Shepherd A.J. Herbicide loss following application to a roadside. Pest Manag. Sci. 2002;58:695–701. doi: 10.1002/ps.506. [DOI] [PubMed] [Google Scholar]
- 198.Langiano V.D.C., Martinez C. Toxicity and effects of a glyphosate-based herbicide on the Neotropical fish Prochilodus lineatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008;147:222–231. doi: 10.1016/j.cbpc.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 199.Contardo-Jara V., Klingelmann E., Wiegand C. Bioaccumulation of glyphosate and its formulation Roundup Ultra in Lumbriculus variegatus and its effects on biotransformation and antioxidant enzymes. Environ. Pollut. 2008;157:57–63. doi: 10.1016/j.envpol.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 200.Dey S., Samanta P., Pal S., Mukherjee A.K., Kole D., Ghosh A.R. Integrative assessment of biomarker responses in teleostean fishes exposed to glyphosate-based herbicide (Excel Mera 71) Emerg. Contam. 2016;2:191–203. doi: 10.1016/j.emcon.2016.12.002. [DOI] [Google Scholar]
- 201.El-Sheshtawy S.M., Nada M.M., Elhafeez M.S.A., Samak D.H. Protective Effect of Supplementation with Powdered Mulberry Leaves on Glyphosate-Induced Toxicity in Catfish (Clarias gariepinus) Adv. Anim. Vet.-Sci. 2021;9:1718–1731. doi: 10.17582/journal.aavs/2021/9.10.1718.1731. [DOI] [Google Scholar]
- 202.Rossi A.S., Fantón N., Michlig M.P., Repetti M.R., Cazenave J. Fish inhabiting rice fields: Bioaccumulation, oxidative stress and neurotoxic effects after pesticides application. Ecol. Indic. 2020;113:106186. doi: 10.1016/j.ecolind.2020.106186. [DOI] [Google Scholar]
- 203.Fiorino E., Sehonova P., Plhalova L., Blahova J., Svobodova Z., Faggio C. Effects of glyphosate on early life stages: Comparison between Cyprinus carpio and Danio rerio. Environ. Sci. Pollut. Res. 2018;25:8542–8549. doi: 10.1007/s11356-017-1141-5. [DOI] [PubMed] [Google Scholar]
- 204.Benachour N., Séralini G.-E. Glyphosate Formulations Induce Apoptosis and Necrosis in Human Umbilical, Embryonic, and Placental Cells. Chem. Res. Toxicol. 2009;22:97–105. doi: 10.1021/tx800218n. [DOI] [PubMed] [Google Scholar]
- 205.Mesnage R., Ibragim M., Mandrioli D., Falcioni L., Tibaldi E., Belpoggi F., Brandsma I., Bourne E., Savage E., Mein C.A., et al. Comparative Toxicogenomics of Glyphosate and Roundup Herbicides by Mammalian Stem Cell-Based Genotoxicity Assays and Molecular Profiling in Sprague-Dawley Rats. Toxicol. Sci. 2021;186:83–101. doi: 10.1093/toxsci/kfab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsui M.T., Chu L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere. 2003;52:1189–1197. doi: 10.1016/S0045-6535(03)00306-0. [DOI] [PubMed] [Google Scholar]
- 207.El-Shenawy N.S. Oxidative stress responses of rats exposed to Roundup and its active ingredient glyphosate. Environ. Toxicol. Pharmacol. 2009;28:379–385. doi: 10.1016/j.etap.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 208.Peixoto F. Comparative effects of the Roundup and glyphosate on mitochondrial oxidative phosphorylation. Chemosphere. 2005;61:1115–1122. doi: 10.1016/j.chemosphere.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 209.Richard S., Moslemi S., Sipahutar H., Benachour N., Seralini G.-E. Differential Effects of Glyphosate and Roundup on Human Placental Cells and Aromatase. Environ. Health Perspect. 2005;113:716–720. doi: 10.1289/ehp.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.